Abstract
Idiopathic pulmonary fibrosis (IPF) is a debilitating, incurable, and life-threatening disease. A cardinal feature of the pathogenesis of IPF is excessive extracellular matrix deposition attributable to proliferation of activated fibrotic lung fibroblasts (fLfs). To assess the underlying mechanism, we analyzed the status of the tumor suppressor protein p53 in fLfs from the lungs of IPF patients or mice with bleomycin-induced established PF. We report that basal expression of p53 is markedly reduced in fLfs. Forced expression of caveolin-1 in fLfs increased basal p53 and reduced profibrogenic proteins, including collagen-1. Transduction of fLfs with adenovirus expressing p53 reduced expression of these proteins. Conversely, inhibition of baseline p53 in control lung fibroblasts from lung tissues increased profibrogenic protein expression. Lung transduction of adenovirus expressing p53 reduced bleomycin-induced PF in wild-type or caveolin-1–deficient mice. Furthermore, treatment of fLfs or fibrotic lung tissues with caveolin-1 scaffolding domain peptide (CSP) or its fragment, CSP7, restored p53 and reduced profibrogenic proteins. Treatment of wild-type mice with i.p. CSP or CSP7 resolved bleomycin-induced PF. These peptides failed to resolve PF in inducible conditional knockout mice lacking p53 in fLfs, indicating the induction of baseline fLf p53 as the basis of the antifibrotic effects.
Progressive fibrotic remodeling is a pathologic process that can affect virtually every vital organ, including the lungs.1, 2 Pulmonary fibrosis (PF) is a restrictive lung disease typified by a progressive destruction of lung architecture and gas exchange, leading to a decline in lung function.3, 4, 5 Recent studies indicate that PF may occur secondary to repeated injuries to lung progenitor type II alveolar epithelial cells (AECs) by chronic exposure to cigarette smoke, pollutants, and radiation; acute lung injury/acute respiratory distress syndrome; and defective surfactant protein-C expression.6, 7, 8 The etiology of idiopathic PF (IPF) remains enigmatic, although 10% of cases are categorized as familial.9 IPF is a fatal disease, with a median survival of 3 years after diagnosis.10, 11 Recently, pirfenidone and nintedanib have been approved by the US Food and Drug Administration for the treatment of IPF and have been shown to slow disease progression.12, 13 Their mechanism of action is incompletely understood.14, 15 These drugs also have adverse effects, have poor pharmacologic properties, and do not clearly offer a durable survival benefit.16, 17 Thus, no pharmacologic cure is available, and lung transplantation is used, when available, to improve survival in patients with end-stage disease. These circumstances underline the urgency to understand fundamental mechanisms contributing to the progression of PF and to identify new targets and interventions. Delineation of the mechanisms underlying activation and differentiation of myofibroblasts or fibrotic lung fibroblasts (fLfs) thereby assumes importance.
The molecular mechanisms leading to aberrant activation of lung fibroblasts and their differentiation into fLfs are unclear.18 However, AEC injury promotes fibrotic repair in the lungs of patients with IPF and in animal models of PF.19, 20, 21 In IPF lungs, activated fLfs accumulate in heterogeneous structures called fibrotic foci, which synthesize and deposit collagen-rich extracellular matrix (ECM) and alter lung architecture.22, 23 Fibrosing lung injury releases a cascade of proinflammatory and profibrotic cytokines, such as transforming growth factor-β, IL-13, and IL-17A.24, 25, 26 Transforming growth factor-β levels are elevated in fibrotic lung diseases, including human IPF and animal models of PF, which promotes activation and differentiation of lung fibroblasts into fLfs.22, 27, 28 This transition results in the secretion of ECM proteins and α-smooth muscle actin (α-SMA) expressed by fLfs, which leads to architectural and physiological impairment.18, 29
In this study, we show that the expression of the tumor suppressor protein p53 is reduced in fLfs from the lungs of patients with IPF or mice with bleomycin (BLM)–induced PF compared with its expression in normal lung fibroblasts (nLfs) from control lungs without apparent PF. Given the loss of basal p53 in fLfs, we determined whether the inhibition of baseline p53 activates nLfs to produce excessive ECM proteins. It was further tested whether restoration of p53 in fLfs mitigates their activation and differentiation. Last, it was evaluated whether treatment of fLfs with caveolin-1 (Cav1), the Cav1 scaffolding domain peptide (CSP) or CSP7, a 7-mer residing within CSP, restores p53 and mitigates ECM deposition. Our studies reveal that restoration of p53 expression in fLfs inhibits production of ECM proteins, whereas its ablation in fLfs abolishes the antifibrotic effects of CSP or CSP7 in vitro and in mice with BLM-induced PF. These studies demonstrate a novel, pivotal role for p53 in the regulation of lung remodeling associated with PF.
Materials and Methods
Human Lung Tissues
Deidentified lung tissues were obtained from surgical biopsy specimens or harvested at the time of transplantation or autopsy of patients with IPF or control donors without known lung disease.
BLM Model of Established PF
All animal experiments reported in this study were approved by the University of Texas Health Science Center at Tyler Animal Care and Use Committee. Wild-type (WT) and Cav1- or p53-deficient mice (aged 6 to 8 weeks) of C57BL/6 background, weighing 20 to 22 g, were purchased from The Jackson Laboratory (Bar Harbor, ME). Homozygous p53 floxed (p53fl/fl) and collagen Cre (ColCre) mice were exposed once to freshly prepared BLM (8 U/kg of body weight) in 50 μL of saline or only saline via intranasal instillation.
Isolation of Human and Mouse Lung Fibroblasts
Human nLfs and fLfs (IPF) were purchased from Dr. Cory Hogaboam (University of Michigan, Ann Arbor), provided by Dr. Ganesh Raghu (University of Washington, Seattle), or isolated locally from control and IPF lung tissues. All human fLfs used in the study were well characterized and derived from patients with usual interstitial pneumonia, as described elsewhere.30 Human nLfs were derived from control donors without apparent interstitial or other lung diseases. Mouse nLfs were isolated from the lungs of mice without any lung injury and fLfs from the fibrotic lungs of mice 21 days after exposure to BLM. These cells were cultured in Dulbecco’s modified Eagle’s medium containing 10% fetal bovine serum (FBS) and 1% penicillin and streptomycin at 37°C and used within three to five passages of initial isolation. nLfs and fLfs were switched to 0.5% FBS media overnight and subjected to different treatments. The fibroblast culture media and lysates were analyzed for Col1, α-SMA, p53, and β-actin by Western blotting. RNA from these cells were analyzed for Col1, α-SMA, p53, and β-actin mRNA by real-time quantitative RT-PCR.
Overexpression of Cav1 or p53 in fLfs and Inhibition of p53 Expression in nLfs
Human or mouse fLfs were transduced with adenovirus expressing Cav1 (Ad-Cav1) or adenovirus expressing p53 (Ad-p53). Naïve fLfs and fLfs transduced with empty adenovirus vector (Ad-Ev) were used as controls. In a separate experiment, nLfs in culture dishes were treated with lentivirus expressing p53 (Lv-p53) shRNA to suppress its baseline expression. Naïve nLfs and nLfs exposed to nonspecific control (Ctrl)–shRNA were used as controls.
Cell Proliferation, Migration, and Invasion Assays
Equal numbers of fLfs were seeded in 60-mm dishes and subjected to various treatments. Five days later, cells were counted to assess the rate of proliferation. For fibroblast migration, transwell assays were performed in 6.5-mm–diameter Boyden chambers with a pore size of 8.0 mm (Corning Life Sciences, Corning, NY). All group lung fibroblasts (1.0 × 105 cells per well) were resuspended in the Dulbecco’s modified Eagle’s medium without FBS and placed in the upper compartment of transwell chambers. The lower compartment was filled with 600 μL Dulbecco’s modified Eagle’s medium containing 10% FBS as a chemoattractant. After incubation for 12 hours, fibroblasts on the lower surface of the filter were fixed in 4% formaldehyde and stained with 0.1% crystal violet. Five random fields were counted for each filter. For invasion assays, cell invasion (1 × 105 cells per well) was measured in 24-well Matrigel-coated invasion chambers (Cell Biolabs, Inc., San Diego, CA) over an incubation period of 15 hours.
Treatment of fLfs with CSP or Its Deletion Fragments
Peptides-CSP, deletion fragment of CSP, and control peptide of scrambled sequence (CP; 3 mg each) were dissolved separately in 50 μL of dimethyl sulfoxide. Five microliters of stock solution was dissolved in 2 mL of Hanks’ balanced salt solution to provide the peptide working solution (150 μg/mL). nLfs and fLfs were switched to Dulbecco’s modified Eagle’s medium containing 0.5% FBS overnight and later treated with PBS or 10 μmol/L CSP, nutlin-3a (N3a) (Cayman Chemical, Ann Arbor, MI), or CP for 48 hours in vitro. The conditioned media and the cell lysates were subjected to Western blotting. Fibrotic lung tissues from mice with PF 21 days after BLM injury were treated with PBS, N3a, CSP7, or CP for 48 hours ex vivo. Lung homogenates were tested for changes in Col1 and α-SMA by Western blotting.
Resolution of Single-Dose BLM-Induced PF by Ad-p53, CSP, or CSP7
Fourteen days after WT and Cav1- or p53-deficient mice were exposed to BLM, as described above, they were transduced with Ad-p53 and then euthanized 21 days post-BLM exposure. Mice exposed to saline and mice with BLM-induced PF either remained untreated or were transduced with Ad-Ev and were used as controls.
Inhibition of p53 Expression in Fibroblasts
To inhibit p53 expression, p53fl/fl mice were exposed to BLM. After 21 days, these mice were euthanized and fLfs were isolated from the fibrotic lung tissues. These fLfs were later transduced with Lv-Cre to inhibit p53 expression. fLfs that transduced Lv-Ev were used as controls.
For generation of inducible conditional knockout (p53cKO) mice lacking expression in fibroblasts, p53fl/fl mice were cross-bred with ColCre mice. First-generation heterozygous p53fl and ColCre mice were back crossed with homozygous p53fl/fl mice. The pups carrying only mutant p53 and ColCre alleles were consecutively given an i.p. injection of tamoxifen (75 mg/kg body weight) for 5 days to inhibit p53 expression in fibroblasts. The p53fl/fl and p53cKO mice were exposed to BLM to induce PF in vivo.
Ex Vivo Treatments of Fibrotic Lung Explants
WT mice (aged 6 to 8 weeks) were exposed once to freshly prepared BLM (8 U/kg of body weight) in 50 μL of saline via intranasal instillation. These mice were euthanized 21 days after initial exposure to BLM, and the fibrotic lungs were harvested. The fibrotic lung tissue explants prepared from BLM-exposed mice (n = 3) were chopped into small pieces and treated with 10 μmol/L CSP7, CP, or N3a for 48 hours ex vivo, as described earlier.31, 32 The chopped fibrotic lung tissue explants from mice treated with PBS ex vivo were used as vehicle controls for comparison.
Statistical Analysis
Differences between two groups were analyzed by t-test, and differences for multiple groups were analyzed by one-way analysis of variance (with Tukey's multiple-comparison) tests.
Results
Disparate p53 Levels in fLfs versus Apoptotic AECs in IPF Lungs
Hematoxylin and eosin (H&E) and Masson's trichrome staining showed heterogeneous structures of varying sizes in the IPF-lung sections (Figure 1A). These honeycomb-like structures were absent in control lung sections. Furthermore, immunohistochemistry (IHC) analyses of IPF-lung sections showed increased staining for Col1, α-SMA, and fibronectin (FN) antigens in these structures, suggesting accumulation of fLfs. Interestingly, IHC of p53 antigens showed little staining in the areas of fibrotic foci in IPF lungs. However, relatively intense staining for Ki-67 and S100A4 antigens was observed around fibrotic foci (Figure 1B), consistent with proliferation of fLfs. IHC detected less Cav1 antigens in the IPF-lung sections; however, their staining was robust in lung epithelial cells of control lung sections. Immunofluorescence staining for p53 and surfactant protein-C and colocalization revealed that 55% of p53-positive cells are surfactant protein-C positive, and 24% of surfactant protein-C–positive cells are positive for p53 in IPF lung sections (Supplemental Figure S1A). Moreover, 66.6% of p53-positive cells are also positive for active caspase-3, and 33% of caspase-3–positive cells are p53 positive (Supplemental Figure S1B). In addition, p53 and plasminogen activator inhibitor-1 (PAI-1) staining colocalized with AECs surrounding the fibrotic foci (Supplemental Figure S1C). This indicates increased p53 expression in AECs bordering fibrotic foci, with most of these cells undergoing apoptosis. However, fLfs that accumulated in the fibrotic foci show minimal p53 and PAI-1 expression and increased expression of Ki-67 antigen.
Figure 1.
Disparate expression of p53 by fibroblasts from idiopathic pulmonary fibrosis (IPF) and normal lungs. A: Lung sections from patients with IPF and control normal subjects (nLs) were subjected to hematoxylin and eosin (H&E) or trichrome staining and immunohistochemistry (IHC) for collagen 1 (Col1), α-smooth muscle actin (α-SMA), and fibronectin (FN) antigens to assess fibrotic foci containing myofibroblasts. B: nL and IPF lung sections were subjected to IHC using anti-p53, caveolin-1 (Cav1), S100A4, or Ki-67 antibody. A and B: One representative example of 10 to 15 fields/specimen from four lung tissue is shown. Arrows indicate fibrotic foci. Boxed areas are shown at higher magnification below. n = 5 per group (A). Scale bars: 500 μm (A and B, top rows); 100 μm (A and B, bottom rows). Original magnifications: ×4 (A and B, top rows); ×20 (A and B, bottom rows).
Differential Expression of p53 in nLfs and fLfs
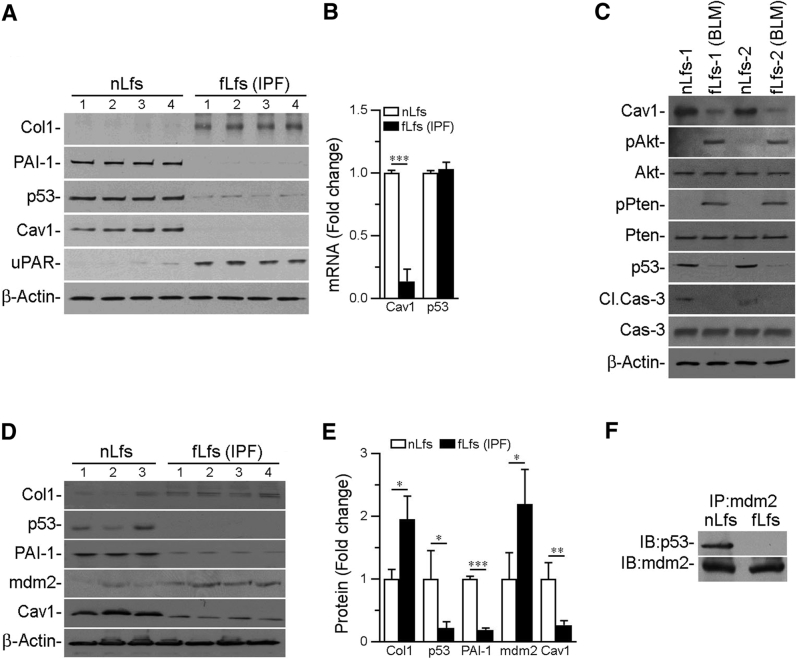
Next, it was determined whether p53 expression was altered in fLfs from IPF lungs versus nLfs of control lungs. There was a reduction in p53 in fLfs isolated from IPF lungs compared with p53 levels in nLfs (Figure 2A). fLfs also expressed minimal Cav1 and PAI-1, whereas basal Col1 and urokinase plasminogen activator (uPA) receptor (uPAR) levels were increased in fLfs. Real-time quantitative RT-PCR revealed that there was no significant difference in steady-state levels of p53 mRNA between nLfs and fLfs, whereas Cav1 mRNA was significantly reduced in fLfs (Figure 2B). This suggests that differential expression of p53 between nLfs and fLfs was regulated at the posttranslational level. fLfs were isolated from the fibrotic lungs of mice with PF (21 days after BLM injury), and minimal levels of p53 and Cav1 expression were found in fLfs compared with nLfs from control mice (Figure 2C). There were no significant differences in p53 mRNA levels between the two cell types (data not shown). Serine phosphorylation of Akt and phosphatase and tensin homolog increased in murine fLfs, indicating involvement of Akt signaling. Both human and mouse fLfs expressed elevated levels of α-SMA and Col1 proteins and mRNAs and also showed significant increases in basal rate of proliferation compared with nLfs.
Figure 2.
Suppression of caveolin-1 (Cav1) and p53 by fibrotic lung fibroblasts (fLfs) from the lungs of patients with idiopathic pulmonary fibrosis (IPF) and mice with bleomycin (BLM)–induced established PF. A: Control normal lung fibroblasts (nLfs) isolated from healthy donors and fLfs from patients with IPF were immunoblotted for changes in collagen 1 (Col1), plasminogen activator inhibitor-1 (PAI-1), p53, Cav1, urokinase plasminogen activator receptor (uPAR), and β-actin. B: RNA from human nLfs or fLfs was analyzed for Cav1 and p53 mRNA by real-time quantitative RT-PCR and normalized to the corresponding levels of β-actin mRNA. C: fLfs isolated from the lungs of mice with established PF 21 days after BLM injury or nLfs from saline-treated control mice were immunoblotted for Cav1, phosphorylated/total Akt (pAkt/Akt), phosphorylated/total Pten (pPten/Pten), p53, and cleaved/total caspase-3 (Cl.Cas-3/Cas-3). D: Lysates from human nLfs and fLfs were immunoblotted for Col1, p53, PAI-1, mdm2, Cav1, and β-actin. E: Bar graph represents fold change in the densities of the Col1, p53, PAI-1, mdm2, and Cav1 after normalization with β-actin controls. F: Lysates from human nLfs and fLfs immunoprecipitated (IP) for mdm2 using 2 μg of anti-mdm2 antibody and immunocomplexes were immunoblotted (IB) for p53 and mdm2 to assess their interactions. All data are representative of two independent experiments. Data are expressed as means ± SD (B and E). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance with Tukey's multiple-comparison test). mdm2, mouse double minute 2 homolog; Pten, phosphatase and tensin homolog.
Because no significant difference was detected in p53 mRNA levels between fLfs and nLfs, we postulated that mouse double minute 2 homolog (mdm2)-mediated ubiquitination regulated its steady-state expression. Therefore, nLfs and fLfs were analyzed for mdm2. mdm2 levels were elevated in fLfs compared with nLfs (Figure 2, D and E). Consistent with earlier data, fLfs expressed greater Col1 with reduction in p53, Cav1, and PAI-1. Because the mdm2 interaction with p53 contributes to its degradation, nLf and fLf lysates were immunoprecipitated for mdm2, and mdm2-immune complexes were immunoblotted for the presence of p53. p53 protein was detected in mdm2 immunoprecipitates with nLf lysates (Figure 2F). p53 was absent in fLf lysates, despite elevated basal levels of mdm2 in these cells (Figure 2D). This suggests that degradation of p53 and the low steady-state expression of p53 in fLfs is attributable to elevated levels of mdm2.
Inhibition of Profibrotic Phenotype by Forced Expression of Cav1 or p53 in fLfs
Cav1-deficient mice are highly susceptible to PF,33 despite resistance to AEC senescence.34 Furthermore, overexpression of Cav1 mitigates PF in these mice. Because Cav1 and p53 are markedly reduced in fLfs (Figure 2, A and D), it was investigated whether the antifibrotic effects of Cav1 involve restoration of p53 expression. fLfs were transduced with Ad-Cav1, and the cell lysates were tested for p53 and changes in Col1 and α-SMA. A marked increase was found in baseline Col1 and α-SMA levels in fLfs, and transduction of fLfs with Ad-Cav1 reduced their expression compared with naïve fLfs or fLfs transduced with Ad-Ev (Figure 3A). Transduction of Ad-Cav1 not only induced Cav1 but also increased p53, which was otherwise reduced in fLfs. Transduction of fLfs with Ad-Cav1 also induced PAI-1 and decreased uPA, indicative of p53-mediated downstream changes in the uPA-fibrinolytic system.
Figure 3.
Reversal of profibrogenic markers by overexpression of p53 or caveolin-1 (Cav1) in fibrotic lung fibroblasts (fLfs). A: fLfs isolated from patients with idiopathic pulmonary fibrosis (IPF) were transduced with empty adenovirus vector (Ad-Ev) or adenovirus expressing Cav1 (Ad-Cav1). Naïve fLfs were controls. After 48 hours, conditioned media (CM) and cell lysates (CLs) were analyzed for collagen 1 (Col1), plasminogen activator inhibitor-1 (PAI-1), urokinase plasminogen activator (uPA), p53, Cav1, and β-actin. B: fLfs from IPF lungs were transduced with Ad-Ev or Ad-p53 as in A. The CM and CLs from naïve fLfs or fLfs exposed to Ad-Ev or Ad-p53 were immunoblotted for Col1, PAI-1, uPA, uPA receptor (uPAR), p53, Cav1, cleaved caspase 3 (Cl.Cas-3)/Cas-3, β-galactosidase (β-Gal), and β-actin. C: RNA from naïve fLfs or fLfs transduced with Ad-Ev or Ad-p53 was tested for profibrogenic marker mRNA. P values were generated by one-way analysis of variance with Tukey's multiple-comparison test. D: Mouse fLfs were transduced with Ad-Ev or Ad-p53 as in B. Cell lysates were immunoblotted for proteins in B. E: RNA from naïve mouse fLfs or those transduced with Ad-Ev or Ad-p53 were tested for profibrogenic marker mRNA. F: Equal numbers of human fLfs in 60-mm dishes remained untreated (naïve fLfs) or transduced with Ad-Ev or Ad-p53. Five days later, cells were counted to assess the rate of proliferation. G: Equal numbers of human fLfs were suspended in the upper chamber of transwell plates. The cells were later transduced with or without Ad-Ev or Ad-p53. Cells that migrated to the lower surface of the filter were fixed, stained, and counted. Invasion of naïve human fLfs or fLfs transduced with Ad-Ev or Ad-p53 was determined 15 hours after incubation in Matrigel-coated chambers. All data are representative of two to three replications. Mean values, P values, and SDs are shown. Data are expressed as means ± SD (C and E–G). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance with Tukey's multiple-comparison test). BLM, bleomycin; FN, fibronectin; α-SMA, α-smooth muscle actin; TN-C, tenascin-C.
To determine whether the antifibrotic effect of Cav1 involves p53, fLfs were transduced with Ad-p53 and the outcome was compared with naïve fLfs and fLfs transduced with Ad-Ev. Transduction of fLfs with Ad-p53 increased p53 and PAI-1 and reduced Col1 without affecting low Cav1 (Figure 3B). The changes occurred with induction of β-galactosidase and active caspase-3 and inhibition of uPA and uPAR in fLfs, which are indicative of reduced viability. Real-time quantitative RT-PCR showed inhibition of profibrogenic marker transcripts in human fLfs exposed to Ad-p53 compared with their elevated levels in control cells (Figure 3C). Transduction of mouse fLfs with Ad-p53 reduced Col1, α-SMA, FN, tenascin-C (TN-C), and uPAR and increased β-galactosidase and caspase-3 activation. The findings indicate that antifibrotic effects and reduced viability are causally linked to the baseline expression of p53 in these fLfs (Figure 3D). Consistent with inhibition of profibrogenic markers, transduction of Ad-p53 reduced their transcripts in murine fLfs (Figure 3E). These changes were associated with increased PAI-1 mRNA that occurred with reduced levels of uPA and uPAR mRNA (data not shown), indicating p53 and uPA-system cross talk. Consistent with inhibition of profibrogenic markers, transduction with Ad-p53 reduced fLf proliferation (Figure 3F), migration, or invasion (Figure 3G). However, these functions in naïve fLfs and fLfs treated with Ad-Ev were maintained, indicating that phenotype changes were attributable to relatively low levels of p53 in fLfs.
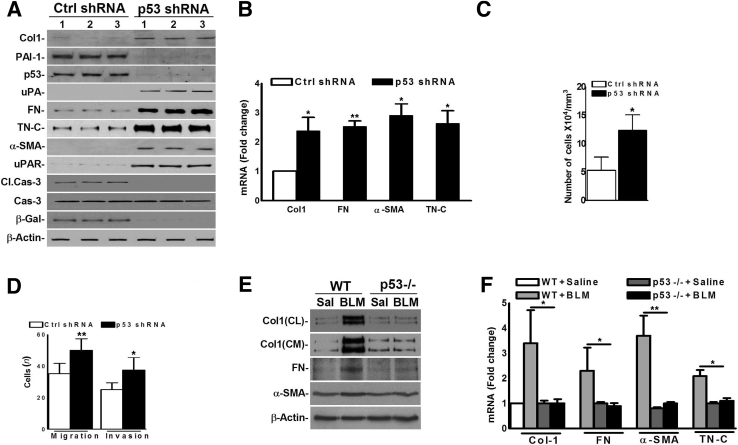
Inhibition of Baseline p53 Generates Profibrogenic Responses in Lung Fibroblasts
To test whether inhibition of p53 in nLfs elicits a profibrogenic phenotype, nLfs were transduced with p53-shRNA. nLfs transduced with nonspecific shRNA (Ctrl-shRNA) served as controls. Treatment of nLfs with p53-shRNA reduced p53 compared with nLfs exposed to Ctrl-shRNA. Inhibition of p53 increased profibrogenic proteins, uPA and uPAR, with reduced baseline PAI-1, activated caspase-3, and β-galactosidase, indicative of the increased viability and profibrogenic features acquired by these cells (Figure 4A). Inhibition of p53 increased profibrogenic marker mRNAs (Figure 4B). Consistent with reduction in PAI-1, inhibition of p53 reduced PAI-1 mRNA while inducing uPA and uPAR mRNA. Inhibition of p53 increased the rate of cell proliferation (Figure 4C). Migration and invasion were likewise increased compared with Ctrl-shRNA–treated nLfs (Figure 4D). The WT and p53-deficient mice were exposed to saline or BLM, fibroblasts were isolated from the lungs 21 days later, and their expression of profibrogenic proteins was tested. Consistent with previous data,30 fLfs from BLM-treated WT mice expressed greater levels of profibrogenic proteins than nLfs from saline-treated mice. However, BLM failed to induce profibrogenic proteins (Figure 4E) or mRNA (Figure 4F) in fLfs isolated from p53-deficient mice, consistent with resistance of these mice to PF.
Figure 4.
Dedifferentiation of control normal lung fibroblasts (nLfs) by suppression of baseline p53 expression. A: Human nLfs were transduced with Lv-expressing control (Ctrl) shRNA or p53 shRNA. After 48 hours, lysates were immunoblotted for listed proteins. B: RNA from nLfs, treated as in A, were tested for profibrogenic marker mRNAs by real-time quantitative RT-PCR. C: Equal numbers of nLfs in 60-mm dishes were transduced with Lv-Ctrl shRNA or Lv-p53 shRNA. Five days later, cells were counted. D: Equal numbers of nLfs were suspended in the upper chamber of transwell plates. The cells were later transduced with or without Lv-Ctrl shRNA or Lv-p53 shRNA. The number of migrating cells was fixed, stained, and counted. Invasion of nLfs transduced with Lv-Ctrl shRNA or Lv-p53 shRNA was determined 15 hours later. P values were generated by one-way analysis of variance with Tukey's multiple-comparison test. E: Lung fibroblasts isolated from wild-type (WT) and p53-deficient mice 21 days after saline (Sal) or bleomycin (BLM) exposure were tested for profibrogenic marker proteins. F: Lung fibroblast RNA from saline- or BLM-exposed WT and p53-deficient mice, as in E, was tested for profibrogenic marker mRNAs. Data are representative of two to three independent experiments. Mean values, P values, and SDs are shown. Data are expressed as means ± SD (B–D and F). ∗P < 0.05, ∗∗P < 0.01 versus corresponding control (one-way analysis of variance with Tukey's multiple-comparison test). Cas-3, caspase 3; CL, cell lysate; Cl.Cas-3, cleaved Cas-3; CM, conditioned media; Col1, collagen 1; FN, fibronectin; β-Gal, β-galactosidase; Lv, lentiviral; PAI-1, plasminogen activator inhibitor-1; α-SMA, α-smooth muscle actin; TN-C, tenascin-C; uPA, urokinase plasminogen activator; uPAR, uPA receptor.
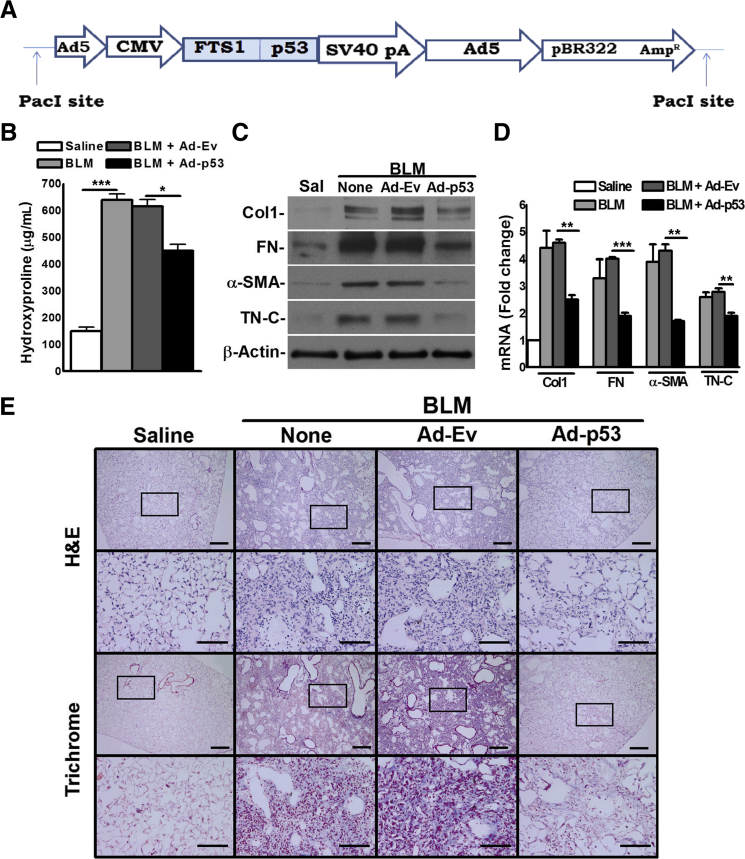
Inhibition of PF by Forced p53 Expression in WT and Cav1-Deficient Mice
fLfs express low levels of p53, and overexpression of p53 attenuates the profibrogenic properties of these cells. An adenoviral vector harboring fibroblast-specific protein promoter and p53 cDNA (Ad-p53) was therefore constructed (Figure 5A) to express p53 in fibroblasts. WT mice with BLM-induced established PF were then transduced with this vector. IHC of lung sections showed increase in both p53 and PAI-1 (Supplemental Figure S2A) after transduction of WT mice with Ad-p53 compared with controls exposed to Ad-Ev. IHC and confocal microscopy revealed a striking reduction in S100A4 expression in mice transduced with Ad-p53. Most p53-expressing cells also colocalized with S100A4-positive cells (Supplemental Figure S2B). Whole lung homogenates were next tested, and it was found that lung fibroblast–specific expression of p53 reduced total lung hydroxyproline content compared with control WT mice with established PF exposed to vehicle or Ad-Ev (Figure 5B). Analyses of lung homogenates for profibrogenic proteins (Figure 5C) or mRNA (Figure 5D) confirmed inhibition after overexpression of p53 in BLM mice. H&E- and trichrome-stained lung sections confirmed that overexpression of p53 in lung fibroblasts resulted in a remarkable suppression of BLM-induced PF in WT mice (Figure 5E).
Figure 5.
Overexpression of p53 in fibrotic lung fibroblasts inhibits bleomycin (BLM)–induced pulmonary fibrosis in wild-type (WT) mice. A: Schematic diagram showing adenoviral (Ad) vector harboring fibroblast-specific protein (FTS1) promoter and p53 cDNA (Ad-p53). WT mice were exposed to saline (Sal) or BLM. After 14 days, mice exposed to BLM were transduced with Ad-p53 or empty adenovirus vector (Ad-Ev) via the airway. Control mice were treated with saline or BLM alone. Four days after transduction, mice were euthanized and lungs were extracted. B: Whole lung homogenates were analyzed for hydroxyproline content. C and D: Whole lung protein and RNA were analyzed for profibrogenic marker proteins (C) and mRNA (D), respectively. E: Lung sections from saline, BLM, BLM+Ad-Ev, and BLM+Ad-p53 mice were subjected to hematoxylin and eosin and trichrome staining. A representative histologic example from four lung specimens tested is shown. All other data are representative of two independent experiments. In all mouse experiments, five mice were used per group. Data are expressed as means ± SD (B and D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance with Tukey's multiple-comparison test). Scale bars: 300 μm (E, first and third rows); 100 μm (E, second and fourth rows). Original magnifications: ×4 (E, first and third rows); ×20 (E, second and fourth rows). CMV, cytomegalovirus; Col1, collagen 1; FN, fibronectin; H&E, hematoxylin and eosin; α-SMA, α-smooth muscle actin; TN-C, tensacin-C.
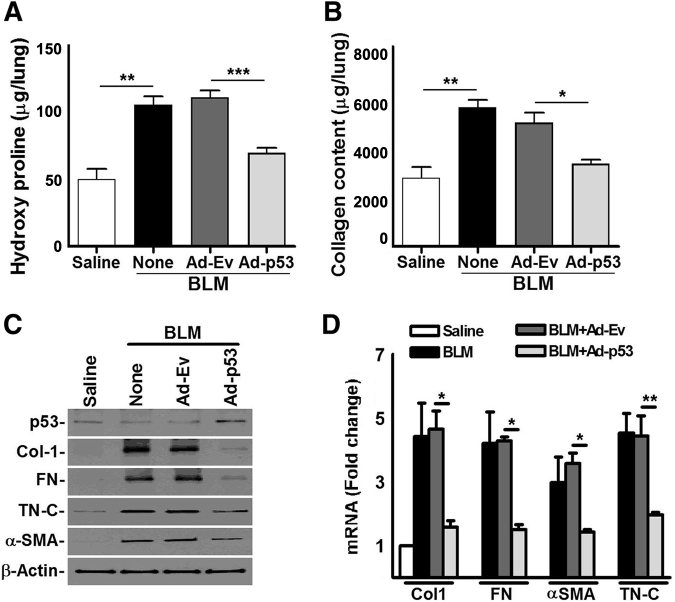
Cav1-deficient mice are highly susceptible to BLM injury,33 and transduction of fLfs with Ad-Cav1 inhibits profibrogenic protein expression (Figure 3A). We therefore investigated whether restoration of p53 expression in lung fibroblasts by transducing Cav1-deficient mice can inhibit BLM-induced PF. BLM exposure increased total lung hydroxyproline (Figure 6A) and soluble collagen (Figure 6B), indicating robust PF by day 21. However, transduction of Cav1-deficent mice with BLM-induced PF with Ad-p53 decreased hydroxyproline and soluble Col levels in the lung tissues. Cav1-deficient mice with BLM-induced PF exposed to vehicle or Ad-Ev showed elevated total hydroxyproline or collagen in the lung tissues. Whole lung homogenates from Cav1-deficient mice with established PF were next analyzed, and that steady-state levels of Col1, α-SMA, FN, and TN-C protein were found (Figure 6C); their mRNAs (Figure 6D) were found to be decreased after transduction with Ad-p53. H&E and trichrome staining of lung sections further showed considerable reduction in Col and other ECM protein deposition compared with control untreated Cav1-deficient mice or those treated with Ad-Ev (Supplemental Figure S3), demonstrating the antifibrotic effects of lung fibroblast p53 in Cav1-deficient mice.
Figure 6.
Overexpression of p53 in fibrotic lung fibroblasts inhibits bleomycin (BLM)–induced established pulmonary fibrosis in caveolin-1 (Cav1)–deficient mice. Cav1-deficient mice were exposed to saline or BLM. Fourteen days later, BLM-exposed mice were transduced with empty adenovirus vector (Ad-Ev) or Ad-p53 by intranasal instillation. Cav1-deficient mice treated with saline or BLM alone were used as additional controls. A and B: Whole lung homogenates from Cav1-deficient mice exposed to saline, BLM, BLM+Ad-Ev, or BLM+Ad-p53 were analyzed for hydroxyproline (A) and soluble collagen (Col) content using Sircol soluble collagen assay kit (Biocolor, Inc., County Antrim, UK) (B). P values were generated by one-way analysis of variance with Tukey's multiple-comparison test. C: Lung homogenates were immunoblotted for p53, profibrogenic marker protein, and β-actin. D: Total lung RNA was evaluated for profibrogenic marker mRNA. All data are representative of two independent experiments. In all mouse experiments, five mice were used per group. Mean values, P values, and SDs are shown. Data are expressed as means ± SD (A, B, and D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001. FN, fibronectin; α-SMA, α-smooth muscle actin; TN-C, tenascin-C.
Inhibition of Profibrotic Phenotypes by CSP and CSP7-Mediated Restoration of p53 in fLfs
Transduction of fLfs with Ad-Cav1 reduced Col1 and α-SMA and induced p53 from its low baseline level of expression. It was therefore studied whether CSP, a scaffolding domain fragment of Cav1, can mitigate Col1 and α-SMA in fLfs and whether the process involves restoration of p53 in these cells. Treatment of fLfs from IPF lungs with CSP markedly reduced Col1 and α-SMA, while restoring baseline p53 (Figure 7A). CSP reduced basal uPA and restored PAI-1 expression. Naïve fLfs and fLfs treated with CP had no effect. Treatment of mouse fLfs with CSP reduced Col1 and α-SMA, while inducing p53 (Figure 7B). Because increased mdm2-mediated degradation of p53 contributes to low p53 levels in fLfs, human fLfs were treated with the mdm2 inhibitor N3a. Treatment of mouse fLfs with N3a reduced Col1, α-SMA, and uPA expression, while restoring baseline p53 and PAI-1 (Figure 7C). Reduced Col1 and α-SMA mRNA were also found in fLfs treated with N3a, confirming that the antifibrotic effects occurred through blockade of mdm2-mediated degradation of p53 (Figure 7D). Human nLfs and fLfs were next treated with these agents to determine whether they differentially induce p53 in these two cell types. nLfs failed to respond to CSP or N3a, whereas these agents increased p53 from the low levels in fLfs (Figure 7E). Treatment of fLfs also induced PAI-1 and reduced uPA expression, indicating p53-uPA system cross talk. A series of overlapping deletions were made, and fLfs from IPF lungs were treated with deletion peptides to identify the minimal number of amino acid residues contributing to the antifibrotic effects of CSP. A seven amino acid sequence (FTTFTVT), CSP7, mimicked the antifibrotic activity of CSP in fLfs by restoring baseline p53 level in these cells (Supplemental Figure S4A). Furthermore, CSP7 induced baseline PAI-1 while inhibiting uPA. Four tandem repeats of CSP7 failed to enhance the activity of CSP7. New fLf lines isolated from the lungs of different patients with IPF were treated with CSP7 or N3a, and similar reductions in Col1 and uPA were found with induction of p53 and PAI-1 (Supplemental Figure S4B). Treatment of fLfs with either N3a or CSP7 also reduced the rate of fLf proliferation compared with naïve fLfs or fLfs exposed to CP (Supplemental Figure S4C). Human nLf and fLf lines were treated with CSP or CSP7, and changes in Col1, α-SMA, and p53 expression were analyzed. Both CSP and CSP7 inhibited Col1 and α-SMA, while restoring baseline p53 (Figure 7F). Interestingly, both peptides failed to suppress baseline Col1 or α-SMA or induce p53 in nLfs. Further analyses of lysates revealed marked inhibition of phosphorylation of Akt and phosphatase and tensin homolog with increase in baseline activation of caspase-3 after treatment of fLfs with CSP or CSP7 (Figure 7G). These results indicate their high selectivity toward target cells, where the p53 and mdm2 balance is dysregulated, as in fLfs. Fibrotic lung tissues from mice 21 days after BLM were treated with CSP7 or N3a ex vivo (Figure 7H). Treatment of fibrotic lung tissues from BLM mice with CSP7 or N3a ex vivo reduced Col1 and α-SMA, which were otherwise increased in fibrotic lung tissues treated with or without CP.
Figure 7.
Restoration of p53 by caveolin-1 scaffolding domain peptide (CSP) and CSP7 inhibits profibrogenic properties of fibrotic lung fibroblasts (fLfs) in vitro. A and B: fLfs from the lungs of patients with idiopathic pulmonary fibrosis (IPF) (A) or mice with bleomycin (BLM)–induced PF (B) were treated with phosphate-buffered saline (PBS) or 10 μmol/L of CSP or control peptide of scrambled sequence (CP) for 48 hours. The cell lysates were immunoblotted for listed proteins. C: Mouse control normal lung fibroblasts (nLfs) and fLfs treated with PBS or 10 μmol/L nutlin-3a (N3a) for 48 hours were analyzed for above proteins. D: RNA from the mouse fLfs, treated as in C, were analyzed for collagen 1 (Col1) and α-smooth muscle actin (α-SMA) mRNA. P values were generated by one-way analysis of variance with Tukey's multiple-comparison test. E: Human nLfs and fLfs treated with PBS, CSP, or N3a were immunoblotted for p53 and β-actin. F: Human nLfs and fLfs treated with PBS, CSP, CSP7, or CP for 48 hours were tested for Col1, α-SMA, p53, and cleaved caspase-3 (Cl.Cas-3)/Cas-3. G: fLfs treated with PBS, CSP, CP, or CSP7 were immunoblotted for pAkt/Akt, pPten/Pten, and Cl.Cas-3. H: Chopped mouse fibrotic lung (mfL) tissues from mice 21 days after BLM injury were treated with PBS or 10 μmol/L CSP7, CP, or N3a for 48 hours ex vivo. These samples were tested for Col1 and α-SMA. All data are representative of two to three independent experiments. Data are expressed as means ± SD (D). n = 3 (H). ∗P < 0.05, ∗∗P < 0.01. PAI-1, plasminogen activator inhibitor-1; Pten, phosphatase and tensin homolog; uPA, urokinase plasminogen activator.
Inhibition of p53 Abrogates Antifibrotic Effects of CSP and CSP7 in fLfs
WT and p53-deficient mice were exposed to BLM. Fourteen days later, they were treated with vehicle, CSP7, or CP. BLM exposure caused a significant increase in the total lung hydroxyproline content of WT mice, indicating increased PF (Figure 8A). Lung fibrosis was significantly reduced after BLM-exposed WT mice were treated with CSP7 and not with CP. p53-Deficient mice exposed to BLM resisted PF, and CSP7 showed minimal effect on baseline levels of total lung hydroxyproline. H&E or trichrome staining (Supplemental Figure S5A), or IHC for Col1, FN, and α-SMA (Supplemental Figure S5B), showed mitigation of BLM-induced PF in WT mice treated with CSP7, whereas BLM failed to induce PF in p53-deficient mice. Analyses of lung homogenates and lung RNA for profibrogenic marker proteins (Figure 8B) and mRNA (Figure 8C) confirmed the antifibrotic activity of CSP7 in WT mice.
Figure 8.
p53 expression and mitigation of pulmonary fibrosis (PF). Wild-type (WT) or p53-deficient mice were intraperitoneally injected with caveolin-1 scaffolding domain peptide fragment (CSP7) or control peptide of scrambled sequence (CP) 14 days after bleomycin (BLM). Controls were treated with saline or BLM alone. Lung homogenates were analyzed for total hydroxyproline (A), profibrogenic marker protein (B), and mRNA (C). Human fibrotic lung fibroblasts (fLfs) transduced with lentiviral (Lv)-p53 shRNA or Lv-control (Ctrl) shRNA and treated with phosphate-buffered saline (PBS), CSP7, or CP were subjected to immunoblotting (D) and real-time quantitative RT-PCR (E). fLfs from p53fl/fl mice with BLM-induced PF exposed to Lv-Ev or Lv-Cre were treated with or without CSP7 or CP. Lysates were analyzed by immunoblotting (F) or real-time quantitative RT-PCR (G). All data are representative of two to three replications. In all mouse experiments, five mice were used per group. Data are expressed as means ± SD (A, C, E, and G). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance with Tukey's multiple-comparison test). Cas-3, caspase-3; Cl.Cas-3, cleaved Cas-3; Col1, collagen 1; FN, fibronectin; IPF, idiopathic PF; PAI-1, plasminogen activator inhibitor-1; α-SMA, α-smooth muscle actin; TN-C, tenascin-C.
Cav1-, CSP-, and CSP7-mediated antifibrotic effects are associated with parallel restoration of basal p53 in fLfs. p53-Deficient mice resist PF, whereas forced expression of p53 in fLfs alone mimics the antifibrotic effects of Cav1, CSP, or CSP7. To confirm p53 involvement in the antifibrotic effects, fLfs were transduced with p53-shRNA and later these cells were treated with CSP7 or CP. The responses were compared with the naïve fLfs or fLfs treated with p53-shRNA or Ctrl-shRNA alone or Ctrl-shRNA and CSP7 or CP. CSP7 increased p53 and PAI-1 (Figure 8D) and reduced expression of profibrogenic proteins, indicating antifibrotic function in naïve fLfs or fLfs only exposed to Ctrl-shRNA. CSP7 also caused activation of caspase-3, suggesting apoptosis of fLfs. These changes were not found in fLfs treated with p53-shRNA. This was confirmed at the Col1, FN, TN-C, and α-SMA mRNA levels (Figure 8E). Suppression of baseline p53 also abolished the ability of CSP7 to reduce proliferation (Supplemental Figure S5C) or migration and invasion of fLfs (Supplemental Figure S5D). These results emphasize the importance of baseline p53 in regulating fLf phenotypes.
To independently confirm that p53 in fLfs is required for resolution of PF, p53fl/fl mice were exposed to BLM to induce PF. Twenty-one days later, the fLfs were isolated and transduced with Lv-Cre to suppress p53 expression. Naïve fLfs and fLfs transduced with Lv-Ev were used as controls. These cells were treated with or without CSP7 or CP. In fLfs transduced with Lv-Cre, CSP7 failed to suppress baseline levels of Col1, α-SMA, FN, and TN-C in protein (Figure 8F) or mRNA (Figure 8G). However, control fLfs from p53fl/fl mice exposed to Lv-Ev responded to CSP treatment and showed increased activation of caspase-3 because of induction of baseline p53. Treatment of fLfs isolated from p53fl/fl mice transduced with Lv-Cre revealed that CSP7 failed to inhibit baseline proliferation, whereas it caused a significant reduction in proliferation in those cells exposed to Lv-Ev (Supplemental Figure S5E). Suppression of baseline p53 in p53fl/fl fLfs also abolished CSP7-mediated suppression of fLf migration and invasion (Supplemental Figure S5F). In a separate experiment, Ad-p53–transduced fLfs were treated with CSP7 to test whether CSP7 had supplemental effects or functions independent of p53. Treatment of fLfs with either Ad-p53 or CSP7 induced PAI-1 and activation of caspase-3 and reduced otherwise elevated baseline profibrogenic protein expression (Supplemental Figure S5G). The antifibrotic activities in fLfs treated with Ad-p53 alone, Ad-p53+CSP7, or Ad-p53+CP were similar without an additive or antagonizing effect, suggesting that a common mechanism was involved.
Resistance of p53cKO Mice Lacking p53 Expression in Fibroblasts to CSP- or CSP7-Mediated Resolution of PF
p53fl/ColCre (p53cKO) mice (Supplemental Figure S6A) were generated by cross-breeding p53fl/fl mice with Col1Cre mice. These mice were later intraperitoneally injected with tamoxifen to suppress expression of p53 in fibroblasts through induction of Cre-recombinase. Tamoxifen-inducible p53cKO mice lacking p53 expression in fibroblasts were exposed to BLM, and 14 days later, these mice were exposed to vehicle, CSP, CSP7, or CP. p53fl/fl Mice with PF and treated with or without CSP, CSP7, or CP were used as controls. BLM induced PF in p53fl/fl and p53cKO mice, as indicated by the increased total hydroxyproline (Figure 9A) and soluble Col (Figure 9B) in whole lung homogenates. Interestingly, neither CSP nor its deletion fragment, CSP7, reduced BLM-induced total hydroxyproline or soluble Col in p53cKO mice, whereas p53fl/fl mice responded to peptide treatment. This indicates that resolution of established PF requires CSP7-mediated restoration of p53 in fLfs. Further analyses of lung homogenates for profibrogenic marker proteins (Figure 9C) and mRNA (Figure 9D) confirmed the antifibrotic effect of CSP or CSP7 against established PF in p53fl/fl mice. These findings were independently confirmed by H&E (Supplemental Figure S6B) and trichrome (Supplemental Figure S6C) staining of lung sections. The inability of CSP or CSP7 to resolve PF in BLM-treated p53cKO mice established that restoration of baseline expression of p53 is required for the salutary effects.
Figure 9.
p53cKO mice lacking p53 in fibrotic lung fibroblasts fail to respond to caveolin-1 scaffolding domain peptide (CSP) or CSP7. A: Generation of p53cKO mice by breeding p53fl/fl and Col1Cre mice. p53fl/fl and p53cKO mice were treated with tamoxifen, as described in Materials and Methods, to induce Cre recombinase. These mice were later exposed to saline or bleomycin (BLM). After 14 days, BLM-treated p53fl/fl and p53cKO mice were intraperitoneally injected with or without CSP, CSP7, or control peptide of scrambled sequence (CP). Twenty-one days after BLM, mice were euthanized. A and B: Lung homogenates were analyzed for hydroxyproline (A) and soluble collagen (Col; B). C: Lung homogenates from p53fl/fl and p53cKO mice, treated as above, were immunoblotted for profibrogenic marker proteins. D: Total RNA was evaluated for profibrogenic marker mRNA. All other data are representative of two to three replications. In all mouse experiments, five mice were used per group. Data are expressed as means ± SD (A, B, and D). ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001 (one-way analysis of variance with Tukey's multiple-comparison test). FN, fibronectin; α-SMA, α-smooth muscle actin; TN-C, tenascin-C.
Discussion
The pathogenesis of interstitial lung diseases, including IPF, is characterized by AEC apoptosis, proliferation, accumulation of activated fLfs, ECM deposition, and PF, resulting in loss of lung function.35, 36, 37 Characteristic morphologic lesions include spatial and temporal heterogeneity incorporating areas of normal lung adjacent to areas of evolving fibrosis, containing fLfs.30, 38, 39 p53 and Cav1 expression levels are increased in AECs from the lungs of patients with IPF and in mice with early injury or with established PF.40, 41 Increased p53 contributes to AEC apoptosis, which leads to development of PF, and suppression of excessive p53 inhibits AEC apoptosis and prevents PF.35, 41 Chronic lung epithelial injury initiates PF and can be prevented by inhibiting AEC apoptosis through control of excessive p53.35 However, fLfs populate, continue to expand, and replace epithelial cells in the fibrotic foci of IPF lungs, leading to progressive destruction of lung architecture and loss of lung function.17, 42, 43 Therefore, there is thus a strong premise for the present study, which is to understand the mechanism by which perturbations of p53 in fLfs influence the pathogenesis of PF.
Using fLfs from patients with IPF and mice with BLM-induced PF, we demonstrate, for the first time, that p53 is a key regulator of profibrogenic properties of lung fibroblasts. The loss of p53 imparts profibrogenic functions and the ability of fLfs to drive fibrosis.30, 40, 44 These data further reveal that the mechanism involves increased mdm2-mediated ubiquitination of p53 because of a disproportionate increase in mdm2 and consequent activation and proliferation of fLfs. Cav1 expression is reduced in fLfs, and transduction with Ad-Cav1 mitigates ECM deposition. The antifibrotic effect of Ad-Cav1 is associated with restoration of basal expression of p53, which is decreased in fLfs. Our studies using human and mouse nLfs and fLfs show that loss of baseline p53 potentiates fibrogenic phenotypes. We further show that expression of p53 in fLfs in vitro induces antifibrotic responses. Forced expression of p53 in lung fibroblasts of Cav1-deficient mice mitigates established PF. These findings provide proof of concept that suppression of fibrotic properties in fLfs after expression of Cav1 is causally associated with concurrent induction of p53. Depletion of Cav1 promotes degradation of p53 by mdm2, whereas Cav1, via direct interaction with mdm2, protects p53 from proteosomal degradation in nLfs. The ability of p53 to limit profibrogenic responses is lost because of the presence of excessive mdm2 and loss of Cav1 expression in fLfs.
Cav1-deficient mice unexpectedly were protected against BLM-induced PF, and the effect was attributed to early protection of the lung epithelium against stress-induced apoptosis and senescence.34 By contrast, Cav1 deficiency promotes mesenchymal transition of lung fibroblasts, leading to increased matrix deposition.33, 45 Therefore, therapeutic strategies to target Cav1 should take into account cell-specific effects, including those on the lung epithelium and fibroblasts. In this study, we found that CSP, comprising the amino acid sequence 82 to 101 of Cav1, mimics Cav1 function in fLfs. On the basis of our data, CSP concurrently targets Cav1 in injured AECs, because of its increased expression in AECs,46 and mdm2 in fLfs, because of a relative overexpression of mdm2 and low Cav1 levels in fLfs. These novel findings extend our recent report that the expression of PAI-1, a downstream mediator of p53, was significantly reduced in fLfs and that restoration of its expression inhibits the viability of fLfs and ECM deposition. CSP-based intervention controls viability and proliferation of fLfs through restoration of basal p53 in human and murine fLfs, which are opposite those on AECs, and involves reversal of mdm2 catabolism of p53. The changes in p53 are associated with coordinate changes in survival signals; uPA and uPAR, which promote viability, are suppressed as p53 levels increase in CSP-treated fLfs, and proapoptotic PAI-1 levels increase. These changes block fLf proliferation, phenotypic mesenchymal transition, and expression of matrix proteins. These observations indicate that CSP not only prevents apoptosis and senescence of the lung epithelium, but blocks fLf expansion as well. Marked inhibition of PF after transduction of lung fibroblasts with Ad-p53 was also found in WT or Cav1-deficient mice. This indicates that restoration of fLf p53 expression plays an important role in the resolution of PF.
This work also demonstrates that CSP7 mimics the antifibrotic effects of full-length CSP, supporting the therapeutic potential of this peptide and mitigating potential off-target effects attributable to its shorter sequence. CSP/CSP7 is internalized in lung fibroblasts and in AECs.20 These data further show that CSP7 prevents fLf expansion. CSP inhibits the Cav1 interaction with protein phosphatase 2A, an ataxia-telangiectasia mutated kinase inhibitor leading to reduction in serine-15 phosphorylation of p53 in injured AECs.46 This increases degradation of p53 by mdm2, with inhibition of p53 and AEC apoptosis and protection against development of PF. However, unlike injured AECs, basal expression of p53 in fLfs is low, even when compared with nLfs attributable to low Cav1, elevated mdm2, and increased mdm2-mediated degradation of p53. In fLfs, CSP7 binds to mdm2 and inhibits degradation of p53. This, in turn, restores p53 and restrains proliferation of fLfs and ECM deposition without affecting nLfs. Collectively, these effects lead to mitigation of established PF.
BLM-induced PF was inhibited in WT and Cav1-deficient mice with forced expression of p53 in lung. The inability of CSP or CSP7 to induce antifibrotic responses in p53-shRNA– or Lv-Cre–transduced WT or p53fl/fl fLfs or to mitigate BLM-induced established PF in p53cKO mice lacking p53 expression in fibroblasts provides further proof that baseline p53 expression regulates fibroblast activation and progression of lung fibrosis. However, consistent with our and others’ earlier observations,40, 47, 48 unlike WT mice, p53 global knockout mice exposed to BLM are protected from development of PF. The resistance of p53-deficient mice to BLM-induced fibrogenesis is attributable to the resistance of these mice to AEC apoptosis because of lack of induction of p53.35, 40, 47, 48 This underlines the importance of AEC apoptosis and early alveolar epithelial injury to activation and transformation of lung fibroblasts via loss of baseline p53 in these cells, which in the aggregate predisposes to PF. The salutary effect of CSP7 in a selected cell-specific manner both in vitro and in a mouse model of established PF in vivo indicates that this peptide represents a promising therapeutic intervention for PF. Its potential will need to be assessed in future interventional studies in humans. The current study provides a novel interventional target, p53, that may be intervened with CSP or CSP7, with differential effects in AECs and fLfs that are salutary and mitigate PF. On the basis of the present study, we posit that p53-targeted intervention by CSP/CSP7 represents a unique approach for treatment of IPF and related forms of PF.
Acknowledgments
M.R.N., N.T., S.K.S., A.S.M., L.F., V.G., V.R., and S.S. performed the experiments and analyzed the data; R.S.O. and J.F. provided unique reagents; S.I. reviewed all of the data and edited the manuscript; S.S. conceived and designed the experiments and wrote the manuscript.
Footnotes
Supported in part by NIH grants R01HL133067-01 (S.S.) and R21ES025815 (S.S.) and Flight Attendant Medical Research Institute, Clinical Investigator Award 150063 (S.S.).
M.R.N., N.T., and S.K.S. contributed equally to this work.
Disclosures: S.S. and S.I. have patents issued for the use of the caveolin-1 spanning domain and its fragments for the treatment of pulmonary fibrosis (869,784,0B2 and 9,630,990); S.I. is an unpaid member of the board of directors and Chief Scientific Officer of Lung Therapeutics, Inc., a University of Texas start-up biotechnology firm that is commercializing caveolin-1 scaffolding domain peptide 7 for the treatment of idiopathic pulmonary fibrosis; S.I. has an equity position in the firm as a founder and investor; S.S. is a consultant for Lung Therapeutics, Inc.
Current address of S.K.S., Department of Biochemistry, University of Iowa Carver College of Medicine, Iowa City, IA.
Supplemental material for this article can be found at https://doi.org/10.1016/j.ajpath.2018.07.005.
Supplemental Data
Supplemental Figure S1.
Disparate p53 and plasminogen activator inhibitor-1 (PAI-1) expression, and apoptosis by alveolar epithelial cells from idiopathic pulmonary fibrosis (IPF) and normal lungs. Lung sections from patients with IPF and control normal subjects (nLs) were subjected to immunofluorescence for p53 and surfactant protein-C (SP-C; A), p53 and cleaved caspase-3 (Cl.Cas-3; B), and p53 and PAI-1 (C) to assess colocalization. One representative example of a total of four lung specimens tested is shown. n = 5 per group (A–C). Scale bars: 100 μm (A–C). Original magnification, ×20 (A–C). fL, fibrotic lung.
Supplemental Figure S2.
Overexpression of p53 in fibrotic lung fibroblasts inhibits bleomycin (BLM)–induced pulmonary fibrosis (PF) in wild-type (WT) mice. WT mice were exposed to saline or BLM to induce PF. After 14 days, mice exposed to BLM were transduced with adenoviral (Ad) vector harboring fibroblast-specific protein promoter and p53 cDNA (Ad-p53) or empty adenovirus vector (Ad-Ev) via the airway. Control mice were treated with saline or BLM alone. Four days after transduction, mice were euthanized and lungs were extracted. Lung sections were subjected to immunohistochemistry for p53 and plasminogen activator inhibitor-1 (PAI-1; A), and colocalization of fluorescent anti-S100A4 and anti-p53 antibodies was performed (B). Scale bar = 100 μm (A and B). Original magnification, ×20 (A and B).
Supplemental Figure S3.

Overexpression of p53 in fibrotic lung fibroblasts inhibits bleomycin (BLM)–induced established pulmonary fibrosis (PF) in caveolin-1 (Cav1)–deficient mice. Cav1-deficient mice were exposed to saline or BLM to cause PF. Fourteen days later, BLM-exposed mice were transduced with empty adenovirus vector (Ad-Ev) or Ad-p53 by intranasal instillation. Cav1-deficient mice treated with saline or BLM alone were used as additional controls. Sections of inflated lungs of mice were subjected to hematoxylin and eosin (H&E) and trichrome staining. Boxed areas are shown at higher magnification below. Scale bars: 300 μm (first and third rows); 100 μm (second and fourth rows). Original magnifications: ×4 (first and third rows); ×20 (second and fourth rows).
Supplemental Figure S4.
Restoration of p53 by caveolin-1 scaffolding domain peptide (CSP) and CSP7 inhibits profibrogenic properties of fibrotic lung fibroblasts (fLfs) in vitro. A: A series of overlapping deletions were made in CSP, and human fLfs were treated with these peptides. Control fLfs were exposed to phosphate-buffered saline (PBS) or control peptide of scrambled sequence (CP). Lysates were tested for collagen 1 (Col1), p53, plasminogen activator inhibitor-1 (PAI-1), urokinase plasminogen activator (uPA), and β-actin. Blue box shows the seven amino acid deletion fragment of CSP, CSP7. B: fLfs from idiopathic pulmonary fibrosis (IPF) lungs treated with PBS, nutlin-3a (N3a), CSP7, or CP for 48 hours were tested for above proteins. C: fLfs cultured in 12-well plates were exposed to PBS, N3a, CSP7, or CP in Dulbecco’s modified Eagle’s medium. After 5 days, cells were detached and counted. P values were generated by one-way analysis of variance. All experiments were repeated two to four times. Data are expressed as means ± SD (C). ∗P < 0.05, ∗∗P < 0.01 versus control.
Supplemental Figure S5.
p53 Expression and mitigation of pulmonary fibrosis. Wild-type (WT) or p53-deficient mice were intraperitoneally injected with caveolin-1 scaffolding domain peptide fragment (CSP7) or control peptide of scrambled sequence (CP) 14 days after bleomycin (BLM). Controls were treated with saline or BLM alone. A and B: Lung sections were subjected to hematoxylin and eosin (H&E) and trichrome staining (A) or immunohistochemistry (B). C: Human fibrotic lung fibroblasts (fLfs) exposed to Lv-p53 shRNA or control (Ctrl)-shRNA with or without CSP7 or CP for 5 days were assessed for cell proliferation. D: fLfs suspended in the upper chamber of transwell plates were treated with or without Lv-Ctrl shRNA or Lv-p53 shRNA and CSP7 or CP. Invasion of fLfs was determined 15 hours after incubation in Matrigel-coated chambers. fLfs from p53fl/fl mice with BLM-induced pulmonary fibrosis (PF) exposed to Lv-Ev or Lv-Cre were treated with or without CSP7 or CP. E: Proliferation was assessed by counting the total number of cells. F: Invasion and migration were assessed using Matrigel-coated chambers. P values were generated by one-way analysis of variance with Tukey's multiple-comparison test. G: Lysates of naïve human fLfs and fLfs exposed to adenovirus expressing p53 with or without CSP7 or CP were subjected to immunoblotting. Boxed areas are shown at higher magnification below. All data are representative of two to three replications. In all mouse experiments, five mice were used per group. Data are expressed as means ± SD (C–F). ∗P < 0.05, ∗∗P < 0.01. Scale bars: 300 μm (A, first and third rows); 100 μm (A, second and fourth rows, and B). Original magnifications: ×4 (A, first and third rows); ×20 (A, second and fourth rows, and B). Cas-3, caspase-3; Cl.Cas-3, cleaved Cas-3; Col1, collagen 1; FN, fibronectin; IPF, idiopathic PF; PAI-1, plasminogen activator inhibitor-1; α-SMA, α-smooth muscle actin; TN-C, tenascin-C.
Supplemental Figure S6.
p53cKO mice lacking p53 in fibrotic lung fibroblasts fail to respond to caveolin-1 scaffolding domain peptide (CSP) or CSP7. A: Generation of p53fl/ColCre (p53cKO) mice (lane 4) by breeding p53fl/fl and Col1Cre mice. p53fl/fl and p53cKO mice were treated with tamoxifen, as described in Materials and Methods, to induce Cre recombinase. These mice were later exposed to saline or bleomycin (BLM). After 14 days, BLM-treated p53fl/fl and p53cKO mice were intraperitoneally injected with or without CSP, CSP7, or control peptide of scrambled sequence (CP). Twenty-one days after BLM, mice were euthanized. Boxed areas are shown at higher magnification below. B and C: Lung sections were subjected to hematoxylin and eosin (B) and trichrome (C) staining. Scale bars: 300 μm (B and C, first and third rows); 100 μm (B and C, second and fourth rows). Original magnifications: ×4 (B and C, first and third rows); ×20 (B and C, second and fourth rows). M, size markers.
References
- 1.Hinz B., Phan S.H., Thannickal V.J., Prunotto M., Desmoulière A., Varga J., De Wever O., Mareel M., Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–1355. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rockey D.C., Bell P.D., Hill J.A. Fibrosis: a common pathway to organ injury and failure. N Engl J Med. 2015;372:1138–1149. doi: 10.1056/NEJMra1300575. [DOI] [PubMed] [Google Scholar]
- 3.Chapman H.A. Disorders of lung matrix remodeling. J Clin Invest. 2004;113:148–157. doi: 10.1172/JCI20729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kalluri R., Neilson E.G. Epithelial-mesenchymal transition and its implications for fibrosis. J Clin Invest. 2003;112:1776–1784. doi: 10.1172/JCI20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K.K., Kugler M.C., Wolters P.J., Robillard L., Galvez M.G., Brumwell A.N., Sheppard D., Chapman H.A. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc Natl Acad Sci U S A. 2006;103:13180–13185. doi: 10.1073/pnas.0605669103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aoshiba K., Nagai A. Oxidative stress, cell death, and other damage to alveolar epithelial cells induced by cigarette smoke. Tob Induc Dis. 2003;1:21. doi: 10.1186/1617-9625-1-3-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter R., Gottlieb D.J., O'Connor G.T. Environmental and genetic risk factors and gene-environment interactions in the pathogenesis of chronic obstructive lung disease. Environ Health Perspect. 2000;108:733–742. doi: 10.1289/ehp.00108s4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson L.N., Koval M. Cross-talk between pulmonary injury, oxidant stress, and gap junctional communication. Antioxid Redox Signal. 2009;11:355–367. doi: 10.1089/ars.2008.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lawson W.E., Loyd J.E., Degryse A.L. Genetics in pulmonary fibrosis: familial cases provide clues to the pathogenesis of IPF. Am J Med Sci. 2011;341:439–443. doi: 10.1097/MAJ.0b013e31821a9d7a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lehtonen S.T., Veijola A., Karvonen H., Lappi-Blanco E., Sormunen R., Korpela S., Zagai U., Sköld M.C., Kaarteenaho R. Pirfenidone and nintedanib modulate properties of fibroblasts and myofibroblasts in idiopathic pulmonary fibrosis. Respir Res. 2016;17:14. doi: 10.1186/s12931-016-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maher T.M. Beyond the diagnosis of idiopathic pulmonary fibrosis: the growing role of systems biology and stratified medicine. Curr Opin Pulm Med. 2013;19:460–465. doi: 10.1097/MCP.0b013e328363f4b7. [DOI] [PubMed] [Google Scholar]
- 12.Margaritopoulos G.A., Vasarmidi E., Antoniou K.M. Pirfenidone in the treatment of idiopathic pulmonary fibrosis: an evidence-based review of its place in therapy. Core Evid. 2016;11:11–22. doi: 10.2147/CE.S76549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raghu G., Selman M. Nintedanib and pirfenidone: new antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med. 2015;191:252–254. doi: 10.1164/rccm.201411-2044ED. [DOI] [PubMed] [Google Scholar]
- 14.Myllärniemi M., Kaarteenaho R. Pharmacological treatment of idiopathic pulmonary fibrosis: preclinical and clinical studies of pirfenidone, nintedanib, and N-acetylcysteine. Eur Clin Respir J. 2015;2 doi: 10.3402/ecrj.v2.26385. 10.3402/ecrj.v2.26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollin L., Wex E., Pautsch A., Schnapp G., Hostettler K.E., Stowasser S., Kolb M. Mode of action of nintedanib in the treatment of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45:1434–1445. doi: 10.1183/09031936.00174914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes G., Toellner H., Morris H., Leonard C., Chaudhuri N. Real world experiences: pirfenidone and nintedanib are effective and well tolerated treatments for idiopathic pulmonary fibrosis. J Clin Med. 2016;5:78. doi: 10.3390/jcm5090078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakai N., Tager A.M. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013;1832:911–921. doi: 10.1016/j.bbadis.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendall R.T., Feghali-Bostwick C.A. Fibroblasts in fibrosis: novel roles and mediators. Front Pharmacol. 2014;5:123. doi: 10.3389/fphar.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore B., Lawson W.E., Oury T.D., Sisson T.H., Raghavendran K., Hogaboam C.M. Animal models of fibrotic lung disease. Am J Respir Cell Mol Biol. 2013;49:167–179. doi: 10.1165/rcmb.2013-0094TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marudamuthu A.S., Bhandary Y.P., Shetty S.K., Fu J., Sathish V., Prakash Y., Shetty S. Role of the urokinase-fibrinolytic system in epithelial-mesenchymal transition during lung injury. Am J Pathol. 2015;185:55–68. doi: 10.1016/j.ajpath.2014.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borensztajn K., Crestani B., Kolb M. Idiopathic pulmonary fibrosis: from epithelial injury to biomarkers - insights from the bench side. Respiration. 2013;86:441–452. doi: 10.1159/000357598. [DOI] [PubMed] [Google Scholar]
- 22.Todd N.W., Luzina I.G., Atamas S.P. Molecular and cellular mechanisms of pulmonary fibrosis. Fibrogenesis Tissue Repair. 2012;5:11. doi: 10.1186/1755-1536-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimbori C., Gauldie J., Kolb M. Extracellular matrix microenvironment contributes actively to pulmonary fibrosis. Curr Opin Pulm Med. 2013;19:446–452. doi: 10.1097/MCP.0b013e328363f4de. [DOI] [PubMed] [Google Scholar]
- 24.Wynn T.A., Ramalingam T.R. Mechanisms of fibrosis: therapeutic translation for fibrotic disease. Nat Med. 2012;18:1028–1040. doi: 10.1038/nm.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wynn T.A. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–1350. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S.-H., Chen W.-C., Esmaeil N., Lucas B., Marsh L.M., Reibman J., Grunig G. Interleukin 13– and interleukin 17A–induced pulmonary hypertension phenotype due to inhalation of antigen and fine particles from air pollution. Pulm Circ. 2014;4:654–668. doi: 10.1086/678511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khalil N., Greenberg A.H. The role of TGF-beta in pulmonary fibrosis. Ciba Found Symp. 1991;157:194–207. doi: 10.1002/9780470514061.ch13. discussion 207–211. [DOI] [PubMed] [Google Scholar]
- 28.Fernandez I.E., Eickelberg O. The impact of TGF-β on lung fibrosis: from targeting to biomarkers. Proc Am Thorac Soc. 2012;9:111–116. doi: 10.1513/pats.201203-023AW. [DOI] [PubMed] [Google Scholar]
- 29.Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 1990;259:L159–L184. doi: 10.1152/ajplung.1990.259.4.L159. [DOI] [PubMed] [Google Scholar]
- 30.Marudamuthu A.S., Shetty S.K., Bhandary Y.P., Karandashova S., Thompson M., Sathish V., Florova G., Hogan T.B., Pabelick C.M., Prakash Y.S., Tsukasaki Y., Fu J., Ikebe M., Idell S., Shetty S. Plasminogen activator inhibitor-1 suppresses profibrotic responses in fibroblasts from fibrotic lungs. J Biol Chem. 2015;290:9428–9441. doi: 10.1074/jbc.M114.601815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Markowski D.N., Helmke B.M., Radtke A., Froeb J., Belge G., Bartnitzke S., Wosniok W., Czybulka-Jachertz I., Deichert U., Bullerdiek J. Fibroid explants reveal a higher sensitivity against MDM2-inhibitor nutlin-3 than matching myometrium. BMC Womens Health. 2012;12:2. doi: 10.1186/1472-6874-12-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markowski D.N., Helmke B.M., Belge G., Nimzyk R., Bartnitzke S., Deichert U., Bullerdiek J. HMGA2 and p14Arf: major roles in cellular senescence of fibroids and therapeutic implications. Anticancer Res. 2011;31:753–761. [PubMed] [Google Scholar]
- 33.Wang X.M., Zhang Y., Kim H.P., Zhou Z., Feghali-Bostwick C.A., Liu F., Ifedigbo E., Xu X., Oury T.D., Kaminski N., Choi A.M.K. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med. 2006;203:2895–2906. doi: 10.1084/jem.20061536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivshankar P., Brampton C., Miyasato S., Kasper M., Thannickal V.J., Le Saux C.J. Caveolin-1 deficiency protects from pulmonary fibrosis by modulating epithelial cell senescence in mice. Am J Respir Cell Mol Biol. 2012;47:28–36. doi: 10.1165/rcmb.2011-0349OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shetty S.K., Tiwari N., Marudamuthu A.S., Puthusseri B., Bhandary Y.P., Fu J., Levin J., Idell S., Shetty S. p53 and miR-34a feedback promotes lung epithelial injury and pulmonary fibrosis. Am J Pathol. 2017;187:1016–1034. doi: 10.1016/j.ajpath.2016.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Noble P.W., Homer R.J. Idiopathic pulmonary fibrosis: new insights into pathogenesis. Clin Chest Med. 2004;25:749–758. doi: 10.1016/j.ccm.2004.04.003. vii. [DOI] [PubMed] [Google Scholar]
- 37.Wilson M., Wynn T. Pulmonary fibrosis: pathogenesis, etiology and regulation. Mucosal Immunol. 2009;2:103–121. doi: 10.1038/mi.2008.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pardo A., Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc. 2016;13:S417–S421. doi: 10.1513/AnnalsATS.201605-341AW. [DOI] [PubMed] [Google Scholar]
- 39.Ramos C., Montaño M., García-Alvarez J., Ruiz V., Uhal B.D., Selman M., Pardo A. Fibroblasts from idiopathic pulmonary fibrosis and normal lungs differ in growth rate, apoptosis, and tissue inhibitor of metalloproteinases expression. Am J Respir Cell Mol Biol. 2001;24:591–598. doi: 10.1165/ajrcmb.24.5.4333. [DOI] [PubMed] [Google Scholar]
- 40.Bhandary Y.P., Shetty S.K., Marudamuthu A.S., Ji H.-L., Neuenschwander P.F., Boggaram V., Morris G.F., Fu J., Idell S., Shetty S. Regulation of lung injury and fibrosis by p53-mediated changes in urokinase and plasminogen activator inhibitor-1. Am J Pathol. 2013;183:131–143. doi: 10.1016/j.ajpath.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puthusseri B., Marudamuthu A., Tiwari N., Fu J., Idell S., Shetty S. Regulation of p53-mediated changes in the uPA-fibrinolytic system and in lung injury by loss of surfactant protein C expression in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2017;312:L783–L796. doi: 10.1152/ajplung.00291.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prasad R., Gupta N., Singh A., Gupta P. Diagnosis of idiopathic pulmonary fibrosis: current issues. Intractable Rare Dis Res. 2015;4:65–69. doi: 10.5582/irdr.2015.01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meltzer E.B., Noble P.W. Idiopathic pulmonary fibrosis. Orphanet J Rare Dis. 2008;3:8. doi: 10.1186/1750-1172-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kodama T., Takehara T., Hikita H., Shimizu S., Shigekawa M., Tsunematsu H., Li W., Miyagi T., Hosui A., Tatsumi T., Ishida H., Kanto T., Hiramatsu N., Kubota S., Takigawa M., Tomimaru Y., Tomokuni A., Nagano H., Doki Y., Mori M., Hayashi N. Increases in p53 expression induce CTGF synthesis by mouse and human hepatocytes and result in liver fibrosis in mice. J Clin Invest. 2011;121:3343–3356. doi: 10.1172/JCI44957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Strippoli R., Loureiro J., Moreno V., Benedicto I., Pérez Lozano M.L., Barreiro O., Pellinen T., Minguet S., Foronda M., Osteso M.T., Calvo E., Vázquez J., López Cabrera M., del Pozo M.A. Caveolin-1 deficiency induces a MEK-ERK1/2-Snail-1-dependent epithelial–mesenchymal transition and fibrosis during peritoneal dialysis. EMBO Mol Med. 2015;7:102–123. doi: 10.15252/emmm.201404127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhandary Y.P., Shetty S.K., Marudamuthu A.S., Fu J., Pinson B.M., Levin J., Shetty S. Role of p53–fibrinolytic system cross-talk in the regulation of quartz-induced lung injury. Toxicol Appl Pharmacol. 2015;283:92–98. doi: 10.1016/j.taap.2015.01.007. [DOI] [PubMed] [Google Scholar]
- 47.Bhandary Y.P., Shetty S.K., Marudamuthu A.S., Gyetko M.R., Idell S., Gharaee-Kermani M., Shetty R.S., Starcher B.C., Shetty S. Regulation of alveolar epithelial cell apoptosis and pulmonary fibrosis by coordinate expression of components of the fibrinolytic system. Am J Physiol Lung Cell Mol Physiol. 2012;302:L463–L473. doi: 10.1152/ajplung.00099.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Okudela K., Ito T., Mitsui H., Hayashi H., Udaka N., Kanisawa M., Kitamura H. The role of p53 in bleomycin-induced DNA damage in the lung: a comparative study with the small intestine. Am J Pathol. 1999;155:1341–1351. doi: 10.1016/S0002-9440(10)65236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.