Abstract
Whilst the role of the Disrupted-in-Schizophrenia 1 (DISC1) gene in the aetiology of major mental illnesses is debated, the characterization of its function lends it credibility as a candidate. A key aspect of this functional characterization is the determination of the role of common non-synonymous polymorphisms on normal variation within these functions. The common allele (A) of the DISC1 single-nucleotide polymorphism (SNP) rs821616 encodes a serine (ser) at the Ser704Cys polymorphism, and has been shown to increase the phosphorylation of extracellular signal-regulated protein Kinases 1 and 2 (ERK1/2) that stimulate the phosphorylation of tyrosine hydroxylase, the rate-limiting enzyme for dopamine biosynthesis. We therefore set out to test the hypothesis that human ser (A) homozygotes would show elevated dopamine synthesis capacity compared with cysteine (cys) homozygotes and heterozygotes (TT and AT) for rs821616. [18F]-DOPA positron emission tomography (PET) was used to index striatal dopamine synthesis capacity as the influx rate constant Kicer in healthy volunteers DISC1 rs821616 ser homozygotes (N = 46) and healthy volunteers DISC1 rs821616 cys homozygotes and heterozygotes (N = 56), matched for age, gender, ethnicity and using three scanners. We found DISC1 rs821616 ser homozygotes exhibited a significantly higher striatal Kicer compared with cys homozygotes and heterozygotes (P = 0.012) explaining 6.4% of the variance (partial η2 = 0.064). Our finding is consistent with its previous association with heightened activation of ERK1/2, which stimulates tyrosine hydroxylase activity for dopamine synthesis. This could be a potential mechanism mediating risk for psychosis, lending further credibility to the fact that DISC1 is of functional interest in the aetiology of major mental illness.
Introduction
The dopamine hypothesis has been a leading theory underlying the neurobiology of schizophrenia for the last four decades (1,2). The hypothesis was initially based on evidence showing that antipsychotic medications block dopamine receptors (3–5) and that drugs increasing dopamine levels elicit psychotic symptoms in healthy people (6–8) and people with schizophrenia (9,10). Using [18F] fluoro-3,4-dihydroxyphenyl-l-alanine (F-DOPA) positron emission tomography (PET), increased presynaptic dopamine synthesis capacity has been found in schizophrenia (11), people with prodromal psychotic symptoms (12,13) and those with clinical progression to psychosis (14). Whilst a substantial body of evidence supports the role of increased presynaptic dopamine synthesis capacity in the pathoaetiology of psychosis, little is known about how genetic factors affect the implicated dopamine system(s) (15).
The Disrupted-in-Schizophrenia 1 (DISC1) gene was originally discovered at the breakpoint of a balanced t(1;11) (q42; q14.3) translocation in a Scottish family with a high-prevalence of psychiatric disorders including schizophrenia (16–18). Further evidence for a link between DISC1 and psychotic and affective disorders emerged from the follow-up of families displaying rare DISC1 mutations (19,20) and large family-based studies in the population isolate of Finland (21–23) although a large meta-analysis of families did not observe linkage at this region (24). Furthermore, evidence from individual population-based cohorts has been inconsistent (25,26) leading to ongoing debate on its involvement in schizophrenia (27,28). Whilst this controversy remains unresolved, there is value in seeking convergent evidence via studies elucidating the functional impact of the gene and its variations (29–32). DISC1 is a scaffold protein involved in a wide range of neuronal functions including neuro-signalling (30,33). Preclinical studies show that DISC1 variant models exhibit increased amphetamine-induced dopamine release in the ventral striatum [see (34–37) reviewed in (38)], indicating that DISC1 variations might affect presynaptic dopamine synthesis capacity.
One of the most studied DISC1 single-nucleotide polymorphisms (SNPs) is rs821616 which is a non-synonymous mutation leading to the translation of a serine (ser) (A allele) or a cysteine (cys) (T allele) at codon 704 in exon 11 (39). Importantly, this polymorphism represents therefore not only a variation at the genetic sequence level but also at the protein sequence level of DISC1. At a molecular level, Hashimoto et al. (47) found that overexpression of the ser variant of codon 704 by viral transduction resulted in a significant increase in phosphorylated Extracellular signal-regulated protein Kinases 1 and 2 (ERK1/2), the more biologically active form (40). ERK1/2 in turn regulates the state of phosphorylation of tyrosine hydroxylase, the rate-limiting enzyme for dopamine biosynthesis, to increase its activity and subsequent dopamine synthesis by up to 2-fold (41–44). Dopamine is synthesized by converting first tyrosine into dihydroxyphenyl-l-alanine (l-DOPA) by tyrosine hydroxylase, and second dihydroxyphenyl-l-alanine (l-DOPA) into dopamine by aromatic acid decarboxylase (AADC) (45). [18F]-DOPA PET signal reflects AADC function and dopamine storage capacity (45), but not directly tyrosine hydroxylase function. However, it should be noted that (i) tyrosine hydroxylase is the rate limiting step for dopamine synthesis capacity (43) and (ii) the topological distribution of the [18F]-DOPA signal correlates highly with tyrosine hydroxylase immunostaining in unilaterally 6-hydroxydopamine (6-OHDA)-lesioned rats, thus indicating that the [18F]-DOPA signal is strongly influenced by endogenous dopamine formed by tyrosine hydroxylase (46).
In summary, preclinical findings suggest that the Ser704Cys variation affects dopamine synthesis by regulating ERK1/2 and its control over tyrosine hydroxylase activity. However, it remains unknown whether the Ser704Cys variation is associated with altered dopamine synthesis in humans. The aim of this study was therefore to test the hypothesis that ser homozygotes would exhibit increased striatal dopamine synthesis capacity relative to cys homozygotes and heterozygotes.
Results
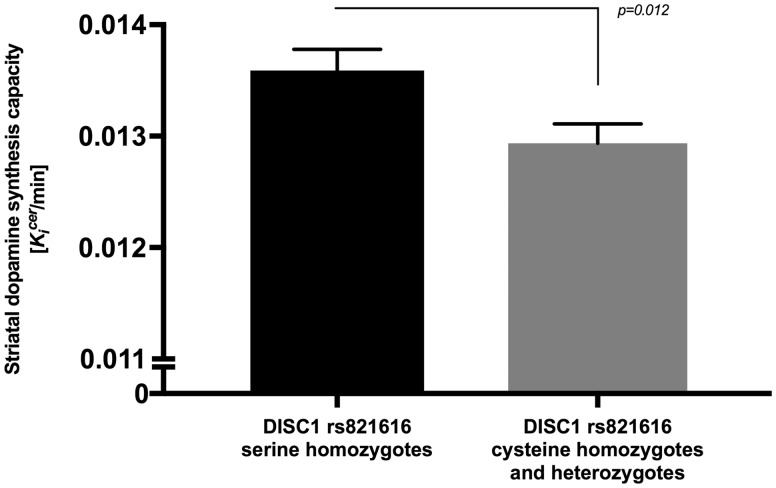
Demographics, scan parameters including the injected dose and substance use characteristics are shown in Table 1. A total of 46 ser homozygotes and 56 cys homozygotes and heterozygotes (which encompass 45 heterozygotes and 11 cys homozygotes) were included in the study. The genotype frequencies (shown in Table 1) did not significantly deviate from Hardy–Weinberg equilibrium (χ2 = 1.422 with P = 0.233), with a Minor Allele Frequency (T allele) of 0.335. Age (year) and Kicer (1 min−1) in the striatum were normally distributed across the two groups whereas injected dose (MBq) was not. There was no significant difference in age between groups t(100) = 1.588, P = 0.115 (independent t test) and no significant difference in injected dose P = 0.408 (Mann–Whitney U test). Levene’s test indicated no difference between the variances in the two groups, F = 0.398, P = 0.529. The univariate analysis of covariance (ANCOVA) showed that the main effect of the DISC1 SNP rs821616 on the dopamine synthesis capacity in the striatum was significant, F(1, 96) = 6.555, P = 0.012, partial η2 = 0.064 (Fig. 1). The effects of the covariates were: for scanner, F(1, 96) = 16.573, P < 0.01, age, F(1, 96)=1.056, P = 0.307, gender, F(1, 96)=0.114, P = 0.736 and ethnicity, F(1, 96)=0.061, P = 0.805.
Table 1.
Sample characteristics and scan parameters
|
DISC1 SNP rs821616 |
||||
|---|---|---|---|---|
| Total | Cysteine homozygotes and heterozygotes | Serine homozygotes | P value | |
| Total genotype counts | 102 | 45 (AT) and 11 (TT) | 46 (AA) | |
| Females | 46 | 21 | 25 | |
| PET scanner 1 | 35 | 19 | 16 | 0.549a |
| PET scanner 2 | 33 | 16 | 17 | |
| PET scanner 3 | 34 | 21 | 13 | |
| Age | 30.2 (9.3) | 31.5 (9.9) | 28.6 (8.4) | 0.115b |
| Tobacco smoking status (non-smoker) | 75 | 43 | 32 | 0.411c |
| Tobacco smoking status (smoker) | 27 | 13 | 14 | |
| Radioactivity injected (MBq) | 157.7 (16.2) | 156.6 (16.2) | 159.2 (16.4) | 0.529c |
| White European | 70 | 35 | 35 | 0.503a |
| Black British/other | 22 | 15 | 7 | |
| Asian British/other | 5 | 3 | 2 | |
| Mixed ethnicity | 5 | 3 | 2 | |
All data±SD.
aPearson Chi-Square.
bIndependent t test.
cMann–Whitney U test.
Figure 1.
Mean (SEM) striatal dopamine synthesis capacity (Kicer value, min−1) in DISC1 rs821616 cys homozygotes and heterozygotes (TT and TA, N = 56) and DISC1 rs821616 ser homozygotes (AA, N = 46). Dopamine synthesis capacity was significantly increased in ser homozygotes compared with cys homozygotes and heterozygotes [F(1, 96)=6.555, P = 0.012].
Discussion
In line with our hypothesis, we found that participants ser homozygotes (AA genotype) for the Ser704Cys functional DISC1 polymorphism exhibited a significantly greater Kicer value in the striatum, indicating greater dopamine synthesis capacity compared with cys homozygotes and heterozygotes (AT or TT genotype). This result is in accordance with preclinical evidence showing that the ser 704 DISC1 variant increases the activity of ERK1/2, which in turn enhances the phosphorylation of tyrosine hydroxylase, the rate limiting step in dopamine synthesis (41,47).
Limitations
The main limitation of this study was that we used data from three different PET scanners, which could add error variance. However, scanner was included as a covariate to adjust for this. Furthermore, the effect of the Ser704Cys polymorphism remained significant when we only included subjects from PET scanner 2 [F(1, 28) = 5.273, P = 0.029 (N = 16 cys homozygotes and heterozygotes, N = 17 ser homozygotes)], but not PET scanner 1 only [F(1, 30) = 0.766, P = 0.388 (N = 19 cys homozygotes and heterozygotes, N = 16 ser homozygotes)] and PET scanner 3 only [F(1, 29) =0.426, P = 0.519 (N = 21 cys homozygotes and heterozygotes, N = 13 ser homozygotes)]. It is important to recognize that we measured the final step in the synthesis of dopamine, the conversion of l-DOPA into dopamine via AADC. However, the parameter measured could be affected by other variables including the uptake of l-DOPA into the brain, although this should be controlled for by the reference region and there is no a priori reason to consider that this should be affected by the DISC1 protein. Importantly, this polymorphism was chosen based on a specific prior hypothesis. Although there was evidence to reject the null hypothesis, the P-value would not survive genome-wide correction and therefore the result requires replication.
Implications for mental disorders
The Ser704Cys polymorphism has been associated with schizophrenia with an odds ratio in the range of 1.3–4.18 in various populations including European (48), mixed European/African-American (49) and Chinese Han (50–52). Inconsistencies have been found, with some studies indicating increased risk associated with the ser (A) allele (48,51), whilst others the cys (T) (allele) (50,52) and no association found (25) mainly in the Japanese population (53–55). A recent meta-analysis has also reported association of the ser allele with schizophrenia in Chinese (OR = 1.338) and Japanese populations (OR = 1.524), as well as in the overall mixed race sample (56). The inconsistencies in these results might be owing to different ethnic populations. It should be noted that ever expanding studies of European ancestry population level genetic variants in schizophrenia continually demonstrate no significant associations at the entire DISC1 locus (57,58), although there is evidence implicating the DISC1 interactor phosphodiesterase 4B as a genome-wide significant single gene locus in a recent large schizophrenia genome-wide association study (GWAS) (58). Whilst GWAS have made crucial advances in the understanding of the genetic of schizophrenia, the biological mechanisms directly underlying the disorder remain yet poorly elucidated (59–61). In this context, the DISC1 protein has been suggested as a biological candidate of interest for investigating molecular mechanisms of mental illnesses at the protein levels (33,62). Beyond studies of dichotomous diagnoses, the ser allele has also been associated with increased risk for poor concentration among Korean patients with schizophrenia (63), increased severity of positive symptoms and hallucinations in European patients with First-Episode Psychosis (64) and increased lifetime severity of delusions in European patients with schizophrenia (65). A potential mechanism for the increased risk could be by dysregulating the control of dopamine to lead to increased dopamine synthesis. Findings in prodromal populations show that increased dopamine synthesis is associated with increased risk for psychosis (12,13). The difference in dopamine synthesis capacity we observe here between ser homozygotes and carriers of the alternative allele is much smaller than the differences seen in at risk subjects (14,66). It is therefore likely that the Ser704Cys variant interacts with other genetic changes to mediate risk, potentially by affecting dopamine synthesis.
The fact that the common ser allele has been described as the risk allele is compatible with schizophrenia GWAS, in which ∼50% of the implicated index SNPs are the more common alleles (67). At the population level, the genetic susceptibility to schizophrenia is caused by a few rare variants of high penetrance (mainly copy number variants and translocations) and many common variants of small penetrance (SNPs and variable number of tandem repeats) (68). As each SNP very minimally impacts schizophrenia risk and is compatible with modern models of natural selection (67), it is expected that other genetic factors are needed, in the same individual, to increase the liability to a point of schizophrenia onset. For example, the Ser704Cys site affects interaction with nuclear distribution element-like 1 (NDEL1) and its homolog Nuclear Distribution Element 1 (NDE1, also known as NudE) (69,70), and there is evidence for an interaction between NDEL1 rs1391768 and the Ser704 allele and the NDE1 rs3784859 and the Cys704 allele on the risk for schizophrenia in European participants (71). Ser704Cys is also the binding site for proteins such as kendrin [also known as pericentrin (PCNT)] and Pericentriolar material 1 (72), which have been both described as risk factor genes for schizophrenia (73). Furthermore, environmental factors such as exposure to psychosocial stress may also interact with the Ser704Cys polymorphism to affect dopamine function and mediate risk for schizophrenia (15). Interestingly, using a transgenic expression of truncated human Disc1 protein with dominant-negative effect, Niwa et al. have shown that an interaction between DISC1 and stress exposure, as a 3-week social isolation paradigm, increased dopamine release after amphetamine challenge (34) and induced alterations in DNA methylation of the tyrosine hydroxylase gene (74).
Evidence also suggests that the Ser704Cys polymorphism is a risk factor for affective disorders. The cys allele has been associated with major depression in Japanese population (47), and shown to form a protective haplotype for bipolar spectrum disorder with two others DISC1 SNPs (rs1411771 and rs980989) in Finnish population (75), whereas a higher ser allele rate has been found in South Indian population with bipolar disorder (76). Interestingly, increased dopamine synthesis capacity is seen in both mania (77) and bipolar psychosis (78), whilst major depression with affective flattening is characterized by a decreased synthesis capacity (79,80).
The Ser704Cys SNP has also been shown to have a functional impact at the brain level (39). Compared with healthy cys homozygotes and heterozygotes, ser homozygotes display increased (for the same level of performance, thus putatively inefficient) prefrontal cortex activation in the left middle and left superior frontal gyri and in the homologous right superior frontal gyrus, the left inferior frontal and cingulate cortex, the thalamus and the caudate nucleus in a verbal fluency task (81), as well as an effect on thalamic-prefrontal connectivity (82). Ser704Cys SNP has also been shown to affect activation during declarative memory task with inconsistent findings. Callicott et al. (48) found decreased activation bilaterally in the hippocampal formation during a declarative memory task and increased activation bilaterally in the hippocampal formation in an N-back task in ser704 homozygotes controls compared with cys homozygotes and heterozygotes, whereas Di Giorgio et al. (83) found increased hippocampal formation/dorsolateral prefrontal cortex coupling during memory encoding in a declarative memory task in ser homozygotes compared with healthy cys homozygotes and heterozygotes.
In summary, our results provide unprecedented preliminary evidence that DISC1 Ser704Cys has an impact on the dopamine synthesis capacity, in a large sample of 102 healthy volunteers. Further studies should aim at (i) replicating this result in different cohorts; (ii) investigating potential epistatic interactions with DISC1 and other risk genes. Genetic studies based on molecular evidence could help identify the molecular mechanism that underlies the pathoaetiology of dopamine-related disorders such as psychotic disorders, and help identify novel potential treatment targets (15).
Conclusion
We found that the ser allele of DISC1 Ser704Cys (rs821616) was associated with significantly higher striatal dopamine synthesis capacity, consistently with its previous association with heightened activation of ERK1/2 that stimulates tyrosine hydroxylase activity for dopamine synthesis. This implicates the DISC1 polymorphism in altering a psychosis relevant mechanism in the brain, i.e. the facilitation of greater dopamine synthesis capacity. Although, this effect of rs821616 may be of too small effect to be identified in population-based studies of end state diagnoses at their current large size, it continues to implicate the functional role of DISC1. Firstly by highlighting the role of this polymorphism at this gene in creating variation within the normal functioning of the brain, but also by indicating this function as a potential mechanism through which other rare or familial mutations for major mental illnesses could disrupt functioning and increase risk to these devastating disorders.
Materials and Methods
Overview
All participants gave informed written consent to take part after full description of the study. All studies were approved by the institutional review board and the local research ethics committee.
Participants
Participants were recruited via advertisement in local media based in London. One hundred and twenty-three participants underwent a [18F]-DOPA PET scan. For all participants the inclusion criteria were (i) age above 18 years; (ii) capacity to give written informed consent. The exclusion criteria were (i) any current medical conditions or history of medical condition (past minor self-limiting conditions were permitted); (ii) history of a psychiatric disorder as determined by the Structured Clinical Interview for DSM-IV Axis 1 Disorders, Clinician Version (SCID-CV) (84); (iii) history of substance abuse/dependence as determined by the Structured Clinical Interview for DSM-IV Axis 1 Disorders, Clinician Version (SCID-CV) (84); (iv) history of head injury with a loss of consciousness; (v) a family history of any psychotic disorder in first- or second-degree relatives; (vi) contraindications to PET scanning (significant prior exposure to radiation, pregnancy or breast feeding). All participants provided urine samples prior to the scan to screen for drug use and pregnancy test in women. Six participants were excluded owing to positive urine THC screening, 12 participants were excluded to contamination of samples and 3 participants were excluded owing to current psychotropic medication use. This resulted in the final inclusion of 102 participants (46 females/56 males, age: 30.2 ± 9.3 years, mean ± Standard Deviation). Both scanning and imaging analysis were done blind to the genotype status.
[18F]-FDOPA PET
PET data were acquired using three different PET scanners. PET scanner 1 was an ECAT HR+ 962 PET scanner (CTI/Siemens, Knoxville, TN, USA). The dynamic images were acquired in 3D mode with an axial field of view of 15.5 cm and reconstructed using filterback projection. PET scanners 2 and 3 were two Siemens Biograph HiRez XVI PET-CT scanner (Siemens Healthcare, Erlangen, Germany) at Imanova, Centre for Imaging Sciences. PET scanner 1 and PET scanner 2–3 were identical with the only exception of the axial field of view: 16.2 versus 21.6 cm, respectively. The dynamic images were also reconstructed using a 3D filtered back-projection algorithm (discrete inverse Fourier transform, DIFT) with a 128 matrix, a zoom of 2.6 and a 5 mm isotropic Gaussian smoothing. Participants were scanned at various times of the day. Some of the imaging data has been included in prior reports but not for genetic analysis (85–88). For attenuation and model-based scatter correction, a 10 min transmission scan was performed using a 150-MBq cesium-137 rotating point source for the ECAT HR+ 962 PET scanner and a computed tomography scan (effective dose = 0.36 mSv) for the Siemens Biograph HiRez XVI PET-CT scanners were acquired prior to each PET scan. Experimental protocol was consistent for all the participants (85). Participants were asked to fast and abstain from smoking from midnight on the day of the scan as tobacco use has been associated with increased striatal dopamine synthesis capacity (89) although this has not been replicated (85). Oral doses of carbidopa (150 mg) and entacapone (400 mg) were administrated 1 h before scanning. While the first reduces the peripheral metabolism of the tracer (90), the latter minimizes the formation of radiolabelled [18F]-FDOPA metabolites, which can cross the blood–brain barrier (91). Head movement was monitored and minimized with a light head strap. If participants moved extensively during the acquisition or got out of the scanner a second attenuation correction image was acquired at the end of the acquisition. PET data were acquired dynamically during 95 min after bolus injection of the radioactive tracer [18F]-DOPA through a cannula inserted into a vein. Dynamic data were binned into 26 frames (PET scanner 1) and 32 frames (PET scanner 2 and 3).
Image analysis
Head movement was corrected using a frame-by-frame realignment and denoizing algorithm (92) with a level 2 order 64 Battle–Lemarie wavelet filter applied on the non-attenuation-corrected dynamic images. These images were used because they include a significant scalp signal compared with attenuation-corrected images (93). Frames were realigned to a reference frame corresponding to the frame with the highest number of counts, i.e. obtained 7 min (for the ECAT HR+ 962 PET scanner-CTI/Siemens) and 17 min (for the Siemens Biograph HiRez XVI PET-CT scanners, Siemens Healthcare) after the radiotracer injection using a mutual information algorithm (94). The transformation parameters were then applied to the corresponding attenuation-corrected dynamic images. These realigned frames were summated, creating a movement-corrected dynamic image from which to extract the time activity curves for graphical analysis quantification. Standardized regions in Montreal Neurologic Institute space were defined in the striatum delineated as previously described to create a region of interest (ROI) map (95) and in the cerebellum using the probabilistic Martinez atlas (95,96). The cerebellum was used as a reference region as it is largely devoid of dopaminergic neurons or projections (45). A non-linear transformation procedure on SPM8 (http://www.fil.ion.ucl.ac.uk/spm) was used to normalize the ROI map together with the [18F]-DOPA template to each individual PET summation image, in order to place the ROI automatically on individual [18F]-DOPA PET dynamic images. Influx constant Kicer value (min−1) for the striatum was calculated relative to uptake in the reference region using a graphical approach (97), a method which has been shown to have good reliability (95).
Genetic analysis
DNA was extracted from blood or cheek swabs using standard methods (98). Genotyping of the rs821616 A>T SNP, was performed by KBioscience (Herts, UK, http://www.kbioscience.co.uk) using a competitive allele-specific polymerase chain reaction system. Quality control procedures included negative control (water) wells and duplicate wells.
Statistical analysis
The normality of the distribution for all variables was examined using the Shapiro–Wilk test, inspection of Q–Q plots and skewness and kurtosis values within range of ±2. Homogeneity of variance was assessed with Levene’s Test for Equality of Variances. An alpha threshold was set at 0.05 (two-tailed) for significance for all statistical comparisons. Statistical Package for the Social Sciences version 24 was used for all statistical analysis (IBM, Armonk, NY, USA). All data are shown as mean ± SD. An univariate ANCOVA was performed on 102 healthy controls, with the DISC1 SNP Ser704Cys variation (ser homozygotes versus cys homozygotes and heterozygotes) as the independent variable, Kicer in the striatum as the dependent variable and age, gender, ethnicity (Table 1) and the three PET scanners separately as covariates as these variables have been previously found to influence dopamine synthesis capacity (99,100). Effect sizes are reported as partial η2. Independent t test and Mann–Whitney U test were used to compare age and injected dose.
Acknowledgements
We thank participants, all the staff at GE Imanet and Imanova for their assistance with this study and Lucinda Hopkins for assistance with genotyping.
Conflict of Interest statement. D.P. is a co-founder of the neuroimaging services company NeuroPsyAI, Ltd. O.D.H. has received investigator-initiated research funding from and/or participated in advisory/speaker meetings organised by Angellini, Astra-Zeneca, Autifony, Biogen, BMS, Eli Lilly, Heptares, Jansenn, Lundbeck, Lyden-Delta, Otsuka, Servier, Sunovion, Rand and Roche. Neither Dr Howes nor his family have been employed by or have holdings/a financial stake in any biomedical company. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. All other authors do not declare any conflict of interest.
Funding
This work was supported by an EU-FP7 MC-ITN IN-SENS grant (grant number 607616) to T.D., O.D.H., C.K., W.H. T.D. was supported by the National Institute for Health Research (NIHR) at Oxford Health NHS Foundation Trust. A.F.P. and J.W in Cardiff University were supported by funding from the Medical Research Council (MRC) Centre (MR/L010305/1), Program Grant (G0800509) Project Grant (MR/L011794/1). M.V. was supported by the NIHR Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. C.K. was supported by a grant from the Research Commission of the Medical Faculty of the Heinrich Heine University Düsseldorf (#9772651). C.N. was supported by March of Dimes Foundation (MC-A656-5DD30) and Medical Research Council (MR/K004867/1). M.A.P.B. was supported by the NIHR, British Medical Association (BMA) and the UCL Hospitals Neurosciences Biomedical Research Centre. W.H. was supported by an Academy of Finland Grant (No. 259589). D.P. was supported by a UK National Institute for Health Research Fellowship (NIHR, PDF-2010–03-047), a Marie Curie Career Integration grant (FP7-PEOPLE-2013-CIG-631952) and a Fundação para Ciência e Tecnologia (FCT) Investigator grant (IF/00787/2014). O.D.H was supported by Medical Research Council-UK (No. MC-A656-5QD30), Maudsley Charity (No. 666), Brain and Behavior Research Foundation, and Wellcome Trust (No. 094849/Z/10/Z) grants and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London.
References
- 1. Meltzer H.Y., Stahl S.M. (1976) The dopamine hypothesis of schizophrenia: a review. Schizophr. Bull., 2, 19–76. [DOI] [PubMed] [Google Scholar]
- 2. Howes O.D., McCutcheon R., Stone J. (2015) Glutamate and dopamine in schizophrenia: an update for the 21st century. J. Psychopharmacol., 29, 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seeman P., Lee T., Chau-Wong M., Wong K. (1976) Antipsychotic drug doses and neuroleptic/dopamine receptors. Nature, 261, 717–719. [DOI] [PubMed] [Google Scholar]
- 4. Creese I., Burt D.R., Snyder S.H. (1976) Dopamine receptors and average clinical doses. Science, 194, 546.. [DOI] [PubMed] [Google Scholar]
- 5. van Rossum J.M. (1966) The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch. Int. Pharmacodyn. Ther., 160, 492–494. [PubMed] [Google Scholar]
- 6. Berman S.M., Kuczenski R., McCracken J.T., London E.D. (2009) Potential adverse effects of amphetamine treatment on brain and behavior: a review. Mol. Psychiatry, 14, 123–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grant K.M., LeVan T.D., Wells S.M., Li M., Stoltenberg S.F., Gendelman H.E., Carlo G., Bevins R.A. (2012) Methamphetamine-associated psychosis. J. Neuroimmune Pharmacol., 7, 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Connell P.H. (1957) Amphetamine psychosis. Br. Med. J., 1, 582. [Google Scholar]
- 9. Curran C., Byrappa N., McBride A. (2004) Stimulant psychosis: systematic review. Br. J. Psychiatry, 185, 196–204. [DOI] [PubMed] [Google Scholar]
- 10. Lieberman J.A., Kane J.M., Alvir J. (1987) Provocative tests with psychostimulant drugs in schizophrenia. Psychopharmacology, 91, 415–433. [DOI] [PubMed] [Google Scholar]
- 11. Howes O.D., Kambeitz J., Kim E., Stahl D., Slifstein M., Abi-Dargham A., Kapur S. (2012) The nature of dopamine dysfunction in schizophrenia and what this means for treatment. Arch. Gen. Psychiatry, 69, 776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Howes O.D., Montgomery A.J., Asselin M.C., Murray R.M., Valli I., Tabraham P., Bramon-Bosch E., Valmaggia L., Johns L., Broome M.. et al. (2009) Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch. Gen. Psychiatry, 66, 13–20. [DOI] [PubMed] [Google Scholar]
- 13. Egerton A., Chaddock C.A., Winton-Brown T.T., Bloomfield M.A., Bhattacharyya S., Allen P., McGuire P.K., Howes O.D. (2013) Presynaptic striatal dopamine dysfunction in people at ultra-high risk for psychosis: findings in a second cohort. Biol. Psychiatry, 74, 106–112. [DOI] [PubMed] [Google Scholar]
- 14. Howes O.D., Bose S., Turkheimer F., Valli I., Egerton A., Stahl D., Valmaggia L., Allen P., Murray R., McGuire P. (2011) Progressive increase in striatal dopamine synthesis capacity as patients develop psychosis: a PET study. Mol. Psychiatry, 16, 885–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howes O.D., McCutcheon R., Owen M.J., Murray R.M. (2017) The role of genes, stress, and dopamine in the development of schizophrenia. Biol. Psychiatry, 81, 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. St Clair D., Blackwood D., Muir W., Walker M., St Clair D., Muir W., Carothers A., Spowart G., Gosden C., Evans H.J. (1990) Association within a family of a balanced autosomal translocation with major mental illness. Lancet, 336, 13–16. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs P., Brunton M., Frackiewicz A., Newton M., Cook P., Robson E. (1970) Studies on a family with three cytogenetic markers. Ann. Hum. Genet. (Lond.), 33, 325–336. [Google Scholar]
- 18. Millar J.K., Wilson-Annan J.C., Anderson S., Christie S., Taylor M.S., Semple C.A., Devon R.S., St Clair D.M., Muir W.J., Blackwood D.H., Porteous D.J. (2000) Disruption of two novel genes by a translocation co-segregating with schizophrenia. Hum. Mol. Genet., 9, 1415–1423. [DOI] [PubMed] [Google Scholar]
- 19. Blackwood D.H., Fordyce A., Walker M.T., St Clair D.M., Porteous D.J., Muir W.J. (2001) Schizophrenia and affective disorders–cosegregation with a translocation at chromosome 1q42 that directly disrupts brain-expressed genes: clinical and P300 findings in a family. Am. J. Hum. Genet., 69, 428–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sachs N.A., Sawa A., Holmes S.E., Ross C.A., DeLisi L.E., Margolis R.L. (2005) A frameshift mutation in disrupted in Schizophrenia 1 in an American family with schizophrenia and schizoaffective disorder. Mol. Psychiatry, 10, 758–764. [DOI] [PubMed] [Google Scholar]
- 21. Ekelund J., Hennah W., Hiekkalinna T., Parker A., Meyer J., Lonnqvist J., Peltonen L. (2004) Replication of 1q42 linkage in Finnish schizophrenia pedigrees. Mol. Psychiatry, 9, 1037–1041. [DOI] [PubMed] [Google Scholar]
- 22. Ekelund J., Hovatta I., Parker A., Paunio T., Varilo T., Martin R., Suhonen J., Ellonen P., Chan G., Sinsheimer J.S.. et al. (2001) Chromosome 1 loci in Finnish schizophrenia families. Hum. Mol. Genet., 10, 1611–1617. [DOI] [PubMed] [Google Scholar]
- 23. Hennah W., Varilo T., Kestila M., Paunio T., Arajarvi R., Haukka J., Parker A., Martin R., Levitzky S., Partonen T.. et al. (2003) Haplotype transmission analysis provides evidence of association for DISC1 to schizophrenia and suggests sex-dependent effects. Hum. Mol. Genet., 12, 3151–3159. [DOI] [PubMed] [Google Scholar]
- 24. Lewis C.M., Levinson D.F., Wise L.H., DeLisi L.E., Straub R.E., Hovatta I., Williams N.M., Schwab S.G., Pulver A.E., Faraone S.V.. et al. (2003) Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: schizophrenia. Am. J. Hum. Genet., 73, 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathieson I., Munafo M.R., Flint J. (2012) Meta-analysis indicates that common variants at the DISC1 locus are not associated with schizophrenia. Mol. Psychiatry, 17, 634–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Farrell M.S., Werge T., Sklar P., Owen M.J., Ophoff R.A., O'Donovan M.C., Corvin A., Cichon S., Sullivan P.F. (2015) Evaluating historical candidate genes for schizophrenia. Mol. Psychiatry, 20, 555–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sullivan P.F. (2013) Questions about DISC1 as a genetic risk factor for schizophrenia. Mol. Psychiatry, 18, 1050–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Porteous D.J., Thomson P.A., Millar J.K., Evans K.L., Hennah W., Soares D.C., McCarthy S., McCombie W.R., Clapcote S.J., Korth C.. et al. (2014) DISC1 as a genetic risk factor for schizophrenia and related major mental illness: response to Sullivan. Mol. Psychiatry, 19, 141–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brandon N.J., Millar J.K., Korth C., Sive H., Singh K.K., Sawa A. (2009) Understanding the role of DISC1 in psychiatric disease and during normal development. J. Neurosci., 29, 12768–12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Porteous D.J., Millar J.K., Brandon N.J., Sawa A. (2011) DISC1 at 10: connecting psychiatric genetics and neuroscience. Trends Mol. Med., 17, 699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hennah W., Thomson P., McQuillin A., Bass N., Loukola A., Anjorin A., Blackwood D., Curtis D., Deary I.J., Harris S.E.. et al. (2009) DISC1 association, heterogeneity and interplay in schizophrenia and bipolar disorder. Mol. Psychiatry, 14, 865–873. [DOI] [PubMed] [Google Scholar]
- 32. Tomppo L., Hennah W., Miettunen J., Jarvelin M.R., Veijola J., Ripatti S., Lahermo P., Lichtermann D., Peltonen L., Ekelund J. (2009) Association of variants in DISC1 with psychosis-related traits in a large population cohort. Arch. Gen. Psychiatry, 66, 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Brandon N.J., Sawa A. (2011) Linking neurodevelopmental and synaptic theories of mental illness through DISC1. Nat. Rev. Neurosci., 12, 707–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Niwa M., Jaaro-Peled H., Tankou S., Seshadri S., Hikida T., Matsumoto Y., Cascella N.G., Kano S., Ozaki N., Nabeshima T.. et al. (2013) Adolescent stress-induced epigenetic control of dopaminergic neurons via glucocorticoids. Science, 339, 335–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Jaaro-Peled H., Niwa M., Foss C.A., Murai R., de Los Reyes S., Kamiya A., Mateo Y., O'Donnell P., Cascella N.G., Nabeshima T.. et al. (2013) Subcortical dopaminergic deficits in a DISC1 mutant model: a study in direct reference to human molecular brain imaging. Hum. Mol. Genet., 22, 1574–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Niwa M., Kamiya A., Murai R., Kubo K., Gruber A.J., Tomita K., Lu L., Tomisato S., Jaaro-Peled H., Seshadri S.. et al. (2010) Knockdown of DISC1 by in utero gene transfer disturbs postnatal dopaminergic maturation in the frontal cortex and leads to adult behavioral deficits. Neuron, 65, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Nakai T., Nagai T., Wang R., Yamada S., Kuroda K., Kaibuchi K., Yamada K. (2014) Alterations of GABAergic and dopaminergic systems in mutant mice with disruption of exons 2 and 3 of the Disc1 gene. Neurochem. Int., 74, 74–83. [DOI] [PubMed] [Google Scholar]
- 38. Dahoun T., Trossbach S.V., Brandon N.J., Korth C., Howes O.D. (2017) The impact of Disrupted-in-Schizophrenia 1 (DISC1) on the dopaminergic system: a systematic review. Transl. Psychiatry, 7, e1015.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duff B.J., Macritchie K.A., Moorhead T.W., Lawrie S.M., Blackwood D.H. (2013) Human brain imaging studies of DISC1 in schizophrenia, bipolar disorder and depression: a systematic review. Schizophr. Res., 147, 1–13. [DOI] [PubMed] [Google Scholar]
- 40. Roskoski R., Jr (2012) ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol. Res., 66, 105–143. [DOI] [PubMed] [Google Scholar]
- 41. Lindgren N., Goiny M., Herrera-Marschitz M., Haycock J.W., Hokfelt T., Fisone G. (2002) Activation of extracellular signal-regulated kinases 1 and 2 by depolarization stimulates tyrosine hydroxylase phosphorylation and dopamine synthesis in rat brain. Eur. J. Neurosci., 15, 769–773. [DOI] [PubMed] [Google Scholar]
- 42. Guo Z., Du X., Iacovitti L. (1998) Regulation of tyrosine hydroxylase gene expression during transdifferentiation of striatal neurons: changes in transcription factors binding the AP-1 site. J. Neurosci., 18, 8163–8174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Daubner S.C., Le T., Wang S. (2011) Tyrosine hydroxylase and regulation of dopamine synthesis. Arch. Biochem. Biophys., 508, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Haycock J.W. (2002) Peptide substrates for ERK1/2: structure-function studies of serine 31 in tyrosine hydroxylase. J. Neurosci. Methods, 116, 29–34. [DOI] [PubMed] [Google Scholar]
- 45. Kumakura Y., Cumming P. (2009) PET studies of cerebral levodopa metabolism: a review of clinical findings and modeling approaches. Neuroscientist, 15, 635–650. [DOI] [PubMed] [Google Scholar]
- 46. Kyono K., Takashima T., Katayama Y., Kawasaki T., Zochi R., Gouda M., Kuwahara Y., Takahashi K., Wada Y., Onoe H.. et al. (2011) Use of [18F]FDOPA-PET for in vivo evaluation of dopaminergic dysfunction in unilaterally 6-OHDA-lesioned rats. EJNMMI Res., 1, 25.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hashimoto R., Numakawa T., Ohnishi T., Kumamaru E., Yagasaki Y., Ishimoto T., Mori T., Nemoto K., Adachi N., Izumi A.. et al. (2006) Impact of the DISC1 Ser704Cys polymorphism on risk for major depression, brain morphology and ERK signaling. Hum. Mol. Genet., 15, 3024–3033. [DOI] [PubMed] [Google Scholar]
- 48. Callicott J.H., Straub R.E., Pezawas L., Egan M.F., Mattay V.S., Hariri A.R., Verchinski B.A., Meyer-Lindenberg A., Balkissoon R., Kolachana B.. et al. (2005) Variation in DISC1 affects hippocampal structure and function and increases risk for schizophrenia. Proc. Natl. Acad. Sci. U.S.A., 102, 8627–8632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Song W., Li W., Feng J., Heston L.L., Scaringe W.A., Sommer S.S. (2008) Identification of high risk DISC1 structural variants with a 2% attributable risk for schizophrenia. Biochem. Biophys. Res. Commun., 367, 700–706. [DOI] [PubMed] [Google Scholar]
- 50. Qu M., Tang F., Yue W., Ruan Y., Lu T., Liu Z., Zhang H., Han Y., Zhang D., Wang F.. et al. (2007) Positive association of the Disrupted-in-Schizophrenia-1 gene (DISC1) with schizophrenia in the Chinese Han population. Am. J. Med. Genet. B Neuropsychiatric Genet., 144B, 266–270. [DOI] [PubMed] [Google Scholar]
- 51. Luo X., Jin C., Zhou Z., Liu X., Zhang F., Zhang F., Zhu J., Wang Y., Cheng Z., Shugart Y.Y. (2015) New findings support the association of DISC1 genetic variants with susceptibility to schizophrenia in the Han Chinese population. Psychiatry Res., 228, 966–968. [DOI] [PubMed] [Google Scholar]
- 52. He B.S., Zhang L.Y., Pan Y.Q., Lin K., Zhang L.L., Sun H.L., Gao T.Y., Su T.Q., Wang S.K., Zhu C.B. (2016) Association of the DISC1 and NRG1 genetic polymorphisms with schizophrenia in a Chinese population. Gene, 590, 293–297. [DOI] [PubMed] [Google Scholar]
- 53. Schumacher J., Laje G., Abou Jamra R., Becker T., Muhleisen T.W., Vasilescu C., Mattheisen M., Herms S., Hoffmann P., Hillmer A.M.. et al. (2009) The DISC locus and schizophrenia: evidence from an association study in a central European sample and from a meta-analysis across different European populations. Hum. Mol. Genet., 18, 2719–2727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kinoshita M., Numata S., Tajima A., Ohi K., Hashimoto R., Shimodera S., Imoto I., Itakura M., Takeda M., Ohmori T. (2012) Meta-analysis of association studies between DISC1 missense variants and schizophrenia in the Japanese population. Schizophr. Res., 141, 271–273. [DOI] [PubMed] [Google Scholar]
- 55. Ratta-Apha W., Hishimoto A., Mouri K., Shiroiwa K., Sasada T., Yoshida M., Supriyanto I., Ueno Y., Asano M., Shirakawa O.. et al. (2013) Association analysis of the DISC1 gene with schizophrenia in the Japanese population and DISC1 immunoreactivity in the postmortem brain. Neurosci. Res., 77, 222–227. [DOI] [PubMed] [Google Scholar]
- 56. Wang H.Y., Liu Y., Yan J.W., Hu X.L., Zhu D.M., Xu X.T., Li X.S. (2018) Gene polymorphisms of DISC1 is associated with schizophrenia: evidence from a meta-analysis. Prog. Neuropsychopharmacol. Biol. Psychiatry, 81, 64–73. [DOI] [PubMed] [Google Scholar]
- 57. Schizophrenia Working Group of the Psychiatric Genomics C. (2014) Biological insights from 108 schizophrenia-associated genetic loci. Nature, 511, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Pardiñas A.F., Holmans P., Pocklington A.J., Escott-Price V., Ripke S., Carrera N., Legge S.E., Bishop S., Cameron D., Hamshere M.L.. et al. (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and maintained by background selection Nat. Genet., 50, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Harrison P.J. (2014) Recent genetic findings in schizophrenia and their therapeutic relevance. J. Psychopharmacol, 29, 85–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Corvin A., Sullivan P.F. (2016) What next in Schizophrenia genetics for the psychiatric genomics consortium? Schizophr. Bull., 42, 538–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McClellan J., King M.C. (2010) Genomic analysis of mental illness: a changing landscape. JAMA, 303, 2523–2524. [DOI] [PubMed] [Google Scholar]
- 62. Niwa M., Cash-Padgett T., Kubo K.I., Saito A., Ishii K., Sumitomo A., Taniguchi Y., Ishizuka K., Jaaro-Peled H., Tomoda T.. et al. (2016) DISC1 a key molecular lead in psychiatry and neurodevelopment: no-More Disrupted-in-Schizophrenia 1. Mol. Psychiatry, 21, 1488–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kim H.J., Park H.J., Jung K.H., Ban J.Y., Ra J., Kim J.W., Park J.K., Choe B.K., Yim S.V., Kwon Y.K.. et al. (2008) Association study of polymorphisms between DISC1 and schizophrenia in a Korean population. Neurosci. Lett., 430, 60–63. [DOI] [PubMed] [Google Scholar]
- 64. Vazquez-Bourgon J., Mata I., Roiz-Santianez R., Ayesa-Arriola R., Suarez Pinilla P., Tordesillas-Gutierrez D., Vazquez-Barquero J.L., Crespo-Facorro B. (2014) A disrupted-in-Schizophrenia 1 gene variant is associated with clinical symptomatology in patients with first-episode psychosis. Psychiatry Invest., 11, 186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. DeRosse P., Hodgkinson C.A., Lencz T., Burdick K.E., Kane J.M., Goldman D., Malhotra A.K. (2007) Disrupted in schizophrenia 1 genotype and positive symptoms in schizophrenia. Biol. Psychiatry, 61, 1208–1210. [DOI] [PubMed] [Google Scholar]
- 66. Howes O.D., Bose S.K., Turkheimer F., Valli I., Egerton A., Valmaggia L.R., Murray R.M., McGuire P. (2011) Dopamine synthesis capacity before onset of psychosis: a prospective [18F]-DOPA PET imaging study. Am. J. Psychiatry, 168, 1311–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pardinas A.F., Holmans P., Pocklington A.J., Escott-Price V., Ripke S., Carrera N., Legge S.E., Bishop S., Cameron D., Hamshere M.L.. et al. (2018) Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat. Genet., 50, 381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Owen M.J., Sawa A., Mortensen P.B. (2016) Schizophrenia. Lancet, 388, 86–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Leliveld S.R., Hendriks P., Michel M., Sajnani G., Bader V., Trossbach S., Prikulis I., Hartmann R., Jonas E., Willbold D.. et al. (2009) Oligomer assembly of the C-terminal DISC1 domain (640-854) is controlled by self-association motifs and disease-associated polymorphism S704C. Biochemistry, 48, 7746–7755. [DOI] [PubMed] [Google Scholar]
- 70. Kamiya A., Tomoda T., Chang J., Takaki M., Zhan C., Morita M., Cascio M.B., Elashvili S., Koizumi H., Takanezawa Y.. et al. (2006) DISC1-NDEL1/NUDEL protein interaction, an essential component for neurite outgrowth, is modulated by genetic variations of DISC1. Hum. Mol. Genet., 15, 3313–3323. [DOI] [PubMed] [Google Scholar]
- 71. Burdick K.E., Kamiya A., Hodgkinson C.A., Lencz T., DeRosse P., Ishizuka K., Elashvili S., Arai H., Goldman D., Sawa A.. et al. (2008) Elucidating the relationship between DISC1, NDEL1 and NDE1 and the risk for schizophrenia: evidence of epistasis and competitive binding. Hum. Mol. Genet., 17, 2462–2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soares D.C., Carlyle B.C., Bradshaw N.J., Porteous D.J. (2011) DISC1: structure, function, and therapeutic potential for major mental illness. ACS Chem. Neurosci., 2, 609–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bradshaw N.J., Porteous D.J. (2012) DISC1-binding proteins in neural development, signalling and schizophrenia. Neuropharmacology, 62, 1230–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Niwa M., Lee R.S., Tanaka T., Okada K., Kano S., Sawa A. (2016) A critical period of vulnerability to adolescent stress: epigenetic mediators in mesocortical dopaminergic neurons. Hum. Mol. Genet., 25, 1370–1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Palo O.M., Antila M., Silander K., Hennah W., Kilpinen H., Soronen P., Tuulio-Henriksson A., Kieseppa T., Partonen T., Lonnqvist J.. et al. (2007) Association of distinct allelic haplotypes of DISC1 with psychotic and bipolar spectrum disorders and with underlying cognitive impairments. Hum. Mol. Genet., 16, 2517–2528. [DOI] [PubMed] [Google Scholar]
- 76. Ram Murthy A., Purushottam M., Kiran Kumar H.B., ValliKiran M., Krishna N., Jayramu Sriharsha K., Janardhan Reddy Y.C., Ghosh S., Jain S. (2012) Gender-specific association of TSNAX/DISC1 locus for schizophrenia and bipolar affective disorder in South Indian population. J. Hum. Genet., 57, 523–530. [DOI] [PubMed] [Google Scholar]
- 77. Ashok A.H., Marques T.R., Jauhar S., Nour M.M., Goodwin G.M., Young A.H., Howes O.D. (2017) The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol. Psychiatry, 22, 666–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jauhar S., Nour M.M., Veronese M., Rogdaki M., Bonoldi I., Azis M., Turkheimer F., McGuire P., Young A.H., Howes O.D. (2017) A test of the transdiagnostic dopamine hypothesis of psychosis using positron emission tomographic imaging in bipolar affective disorder and schizophrenia. JAMA Psychiatry, 74, 1206–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Bragulat V., Paillere-Martinot M.L., Artiges E., Frouin V., Poline J.B., Martinot J.L. (2007) Dopaminergic function in depressed patients with affective flattening or with impulsivity: [18F]fluoro-L-dopa positron emission tomography study with voxel-based analysis. Psychiatry Res., 154, 115–124. [DOI] [PubMed] [Google Scholar]
- 80. Martinot M., Bragulat V., Artiges E., Dolle F., Hinnen F., Jouvent R., Martinot J. (2001) Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am. J. Psychiatry, 158, 314–316. [DOI] [PubMed] [Google Scholar]
- 81. Prata D.P., Mechelli A., Fu C.H., Picchioni M., Kane F., Kalidindi S., McDonald C., Kravariti E., Toulopoulou T., Miorelli A.. et al. (2008) Effect of disrupted-in-schizophrenia-1 on pre-frontal cortical function. Mol. Psychiatry, 13, 915–917, 909. [DOI] [PubMed] [Google Scholar]
- 82. Liu B., Fan L., Cui Y., Zhang X., Hou B., Li Y., Qin W., Wang D., Yu C., Jiang T. (2015) DISC1 Ser704Cys impacts thalamic-prefrontal connectivity. Brain Struct. Funct., 220, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Di Giorgio A., Blasi G., Sambataro F., Rampino A., Papazacharias A., Gambi F., Romano R., Caforio G., Rizzo M., Latorre V.. et al. (2008) Association of the SerCys DISC1 polymorphism with human hippocampal formation gray matter and function during memory encoding. Eur. J. Neurosci., 28, 2129–2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. First M.B., Spitzer R.L., Miriam G., Williams J.B.W. (1996) Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press, Inc, Washington, D.C. [Google Scholar]
- 85. Bloomfield M.A., Pepper F., Egerton A., Demjaha A., Tomasi G., Mouchlianitis E., Maximen L., Veronese M., Turkheimer F., Selvaraj S.. et al. (2014) Dopamine function in cigarette smokers: an [(1)(8)F]-DOPA PET study. Neuropsychopharmacology, 39, 2397–2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bloomfield M.A., Morgan C.J., Egerton A., Kapur S., Curran H.V., Howes O.D. (2014) Dopaminergic function in cannabis users and its relationship to cannabis-induced psychotic symptoms. Biol. Psychiatry, 75, 470–478. [DOI] [PubMed] [Google Scholar]
- 87. Jauhar S., Veronese M., Rogdaki M., Bloomfield M., Natesan S., Turkheimer F., Kapur S., Howes O.D. (2017) Regulation of dopaminergic function: an [18F]-DOPA PET apomorphine challenge study in humans. Transl. Psychiatry, 7, e1027.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Froudist-Walsh S., Bloomfield M.A., Veronese M., Kroll J., Karolis V.R., Jauhar S., Bonoldi I., McGuire P.K., Kapur S., Murray R.M.. et al. (2017) The effect of perinatal brain injury on dopaminergic function and hippocampal volume in adult life. eLife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Salokangas R.K., Vilkman H., Ilonen T., Taiminen T., Bergman J., Haaparanta M., Solin O., Alanen A., Syvalahti E., Hietala J. (2000) High levels of dopamine activity in the basal ganglia of cigarette smokers. Am. J. Psychiatry, 157, 632–634. [DOI] [PubMed] [Google Scholar]
- 90. Garnett E.S., Firnau G., Nahmias C. (1983) Dopamine visualized in the basal ganglia of living man. Nature, 305, 137–138. [DOI] [PubMed] [Google Scholar]
- 91. Sawle G.V., Burn D.J., Morrish P.K., Lammertsma A.A., Snow B.J., Luthra S., Osman S., Brooks D.J. (1994) The effect of entacapone (OR-611) on brain [18F]-6-L-fluorodopa metabolism: implications for levodopa therapy of Parkinson's disease. Neurology, 44, 1292–1297. [DOI] [PubMed] [Google Scholar]
- 92. Turkheimer F.E., Brett M., Visvikis D., Cunningham V.J. (1999) Multiresolution analysis of emission tomography images in the wavelet domain. J. Cerebr. Blood Flow Metab., 19, 1189–1208. [DOI] [PubMed] [Google Scholar]
- 93. Bose S.K., Turkheimer F.E., Howes O.D., Mehta M.A., Cunliffe R., Stokes P.R., Grasby P.M. (2008) Classification of schizophrenic patients and healthy controls using [18F] fluorodopa PET imaging. Schizophr. Res., 106, 148–155. [DOI] [PubMed] [Google Scholar]
- 94. Studholme C., Hill D.L., Hawkes D.J. (1997) Automated three-dimensional registration of magnetic resonance and positron emission tomography brain images by multiresolution optimization of voxel similarity measures. Med. Phys., 24, 25–35. [DOI] [PubMed] [Google Scholar]
- 95. Egerton A., Demjaha A., McGuire P., Mehta M.A., Howes O.D. (2010) The test-retest reliability of 18F-DOPA PET in assessing striatal and extrastriatal presynaptic dopaminergic function. NeuroImage, 50, 524–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Martinez D., Slifstein M., Broft A., Mawlawi O., Hwang D.R., Huang Y., Cooper T., Kegeles L., Zarahn E., Abi-Dargham A.. et al. (2003) Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cerebr. Blood Flow Metab., 23, 285–300. [DOI] [PubMed] [Google Scholar]
- 97. Patlak C.S., Blasberg R.G. (1985) Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J. Cerebr. Blood Flow Metab., 5, 584–590. [DOI] [PubMed] [Google Scholar]
- 98. Freeman B., Smith N., Curtis C., Huckett L., Mill J., Craig I.W. (2003) DNA from buccal swabs recruited by mail: evaluation of storage effects on long-term stability and suitability for multiplex polymerase chain reaction genotyping. Behav. Genet., 33, 67–72. [DOI] [PubMed] [Google Scholar]
- 99. Kumakura Y., Vernaleken I., Buchholz H.G., Borghammer P., Danielsen E., Grunder G., Heinz A., Bartenstein P., Cumming P. (2010) Age-dependent decline of steady state dopamine storage capacity of human brain: an FDOPA PET study. Neurobiol. Aging, 31, 447–463. [DOI] [PubMed] [Google Scholar]
- 100. Egerton A., Howes O.D., Houle S., McKenzie K., Valmaggia L.R., Bagby M.R., Tseng H.H., Bloomfield M.A., Kenk M., Bhattacharyya S.. et al. (2017) Elevated striatal dopamine function in immigrants and their children: a risk mechanism for psychosis. Schizophr. Bull., 43, 293–301. [DOI] [PMC free article] [PubMed] [Google Scholar]