Abstract
Objective:
Invasive coronary interventions can fail due to intimal hyperplasia and restenosis. Endothelial cell (EC) seeding to the vessel lumen, accelerating re-endothelialization, or local release of mTOR pathway inhibitors have helped reduce intimal hyperplasia after vessel injury. While animal models are powerful tools, they are complex and expensive, and not always reflective of human physiology. Therefore, we developed an in vitro 3D vascular model validating previous in vivo animal models and utilizing isolated human arteries to study vascular remodeling after injury. Approach: We utilized a bioreactor that enables the control of intramural pressure and shear stress in vessel conduits to investigate the vascular response in both rat and human arteries to intraluminal injury.
Results:
Culturing rat aorta segments in vitro, we show that vigorous removal of luminal ECs results in vessel injury, causing medial proliferation by Day-4 and neointima formation, with the observation of SCA1+ cells (stem cell antigen-1) in the intima by Day-7, in the absence of flow. Conversely, when endothelial-denuded rat aortae and human umbilical arteries were subjected to arterial shear stress, pre-seeding with human umbilical ECs decreased the number and proliferation of smooth muscle cell (SMC) significantly in the media of both rat and human vessels.
Conclusion:
Our bioreactor system provides a novel platform for correlating ex vivo findings with vascular outcomes in vivo. The present in vitro human arterial injury model can be helpful in the study of EC-SMC interactions and vascular remodeling, by allowing for the separation of mechanical, cellular, and soluble factors.
Keywords: human vessel, bioreactor, vessel injury, endothelial cell, neointima, SCA1
Introduction
Cardiovascular diseases, affecting one-third of the US population, are the leading cause of mortality globally1,2. Balloon angioplasty, stenting, and autologous coronary bypass grafting are the most commonly used methods to treat coronary occlusion3. These interventions result in partial or complete endothelium denudation (due to the mechanical trauma of vessel harvest or manipulation) and smooth muscle injuries, which cause short- and long-term complications such as inflammation, thrombosis or excessive smooth muscle cell (SMC) growth, or intimal hyperplasia4–7. Furthermore, endothelial cells (ECs) of vein grafts cannot adapt to arterial flow after coronary bypass8,9. In the absence of a functional endothelium, platelet adhesion and activation on the vessel lumen is followed by localization of monocytes, neutrophils, and T-lymphocytes in the subendothelium10,11. Foam cells derived from monocytes, and other immune cells, accumulate to form plaques and fatty streaks, which result in endothelial damage and, subsequently, intimal hyperplasia and the vessel occlusion11–13.
In addition, during the surgical preparation of vein grafts (pressurizing vein grafts to overcome vasospasm or to check the competence of side branches, etc.), the vessel wall is distended and stretched14–17. Mechanical injury results in SMC migration and proliferation through increase of matrix metalloproteinases production18, and local secretion of growth factors such as basic fibroblast growth factor (bFGF)19, platelet derived growth factor (PDGF)20,21 and transforming growth factor-β (TGF-β)22. Moreover, some ex vivo vessel culture studies have shown that the disruption of extracellular matrix in the media triggers SMC proliferation by degradation of cyclin-dependent kinase inhibitors23. In addition to medial SMCs, the migration and proliferation of adventitial cells can contribute to formation of intimal hyperplasia24–26.
Therapeutic use of ECs, or elements of their secretome, may be an effective biological approach to treat vascular injuries caused by the aforementioned interventions. In animal models, local delivery of endothelial or endothelial progenitor cells to the injury site27–31, injection of intra-arterial VEGF32 or VEGF gene delivery33, all result in reduction of intimal hyperplasia after balloon injury in animal models. However, all of these studies employ animal models that are not ideally representative of human physiology. The ability to mimic vessel injury on human tissues, and to re-endothelialize these vessels ex vivo to study the EC-SMC interaction and vessel remodeling, would be a useful tool for enhancing our understanding of arterial injury and repair processes. Perfusion bioreactors have been used previously to study cell viability and proliferation in ex vivo vessel cultures34–36. Although most of the reported data is in animal tissues, recent studies have used human saphenous veins in bioreactors to assess vein adaptation to arterial pressure37–39.
Here, we created an ex vivo vessel culture system that is able to validate the previous in vivo works and to isolate different factors affecting vessel remodeling. To this aim, we studied the effect of vessel wall injury and pre-endothelilalization of injured vessels, where we applied arterial shear and pressure in perfusion bioreactors. We first validated the ex vivo bioreactor system for creating vessel injury and remodeling in rats, and then studied human vessel injury and remodeling using umbilical arteries. Our results show that culture of human umbilical arteries in perfusion bioreactors is an effective in vitro model for EC-SMC co-culture, or for drug testing studies, and that arterial shear stress and the presence of endothelium interact to impact the extent of SMC remodeling ex vivo after injury.
Materials and Methods
This study was approved by the Yale University Institutional Animal Care and Use Committee. All animal care complied with the Guide for the Care and Use of Laboratory Animals. Human tissues and cell populations were obtained using protocols approved by the Yale University Human Investigation Committee, and were discarded tissues.
Perfusion bioreactor design
In order to create an in vitro system that mimics arterial pressure and flow, we designed a bioreactor as shown in Fig. 1A. The main body of the bioreactor is a glass medium reservoir fitted with a silicone stopper. The vessels are cannulated with glass pipettes, secured with silk sutures, and placed into the bioreactor. PharMed silicone tubing (Westlake, OH, USA) was attached to the glass pipettes, reservoir inlet and outlet to complete the perfusion loop. Rat aortas and umbilical arteries were isolated under sterile conditions. Each bioreactor and tubing component was autoclaved and handled with sterile gloves in the hood. Following mounting of the vessel to bioreactor and assembling all components, each connection was sealed with 70% ethanol-soaked parafilm (Fig. 2). Culture medium was drawn from the media reservoir and pumped through the vessel and then drained back to the reservoir via a Masterflex L/S roller pump (Vernon Hills, IL, USA). Lengths of the tubing and the attachment configurations were optimized to achieve 50–70 mmHg diastolic pressures, and 90–130 mmHg systolic pressures, at a target shear stress of 20 dyne/cm2. The intraluminal shear was calculated according to Equation 1, where Q, µ, and r are the volumetric flow rate, dynamic viscosity of the fluid, and the lumen radius, respectively40.
| 1 |
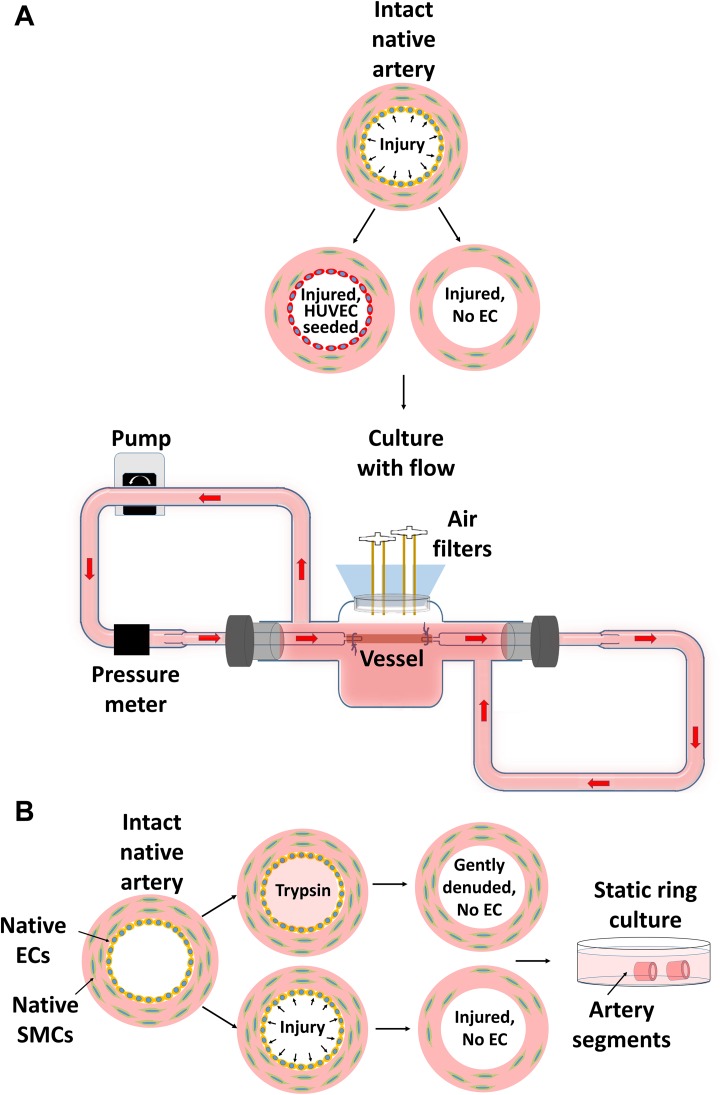
Fig. 1.
Intact vessels were either gently denuded via flushing with trypsin or injured by inserting 14-gauge needle into lumen. A: Injured vessels (5 cm in length), either seeded with HUVECs onto luminal surface or without ECs, were cultured with flow in perfusion bioreactors. B: Segments of both gently-denuded and injured arteries were cultured statically in 6-well plates.
Fig. 2.
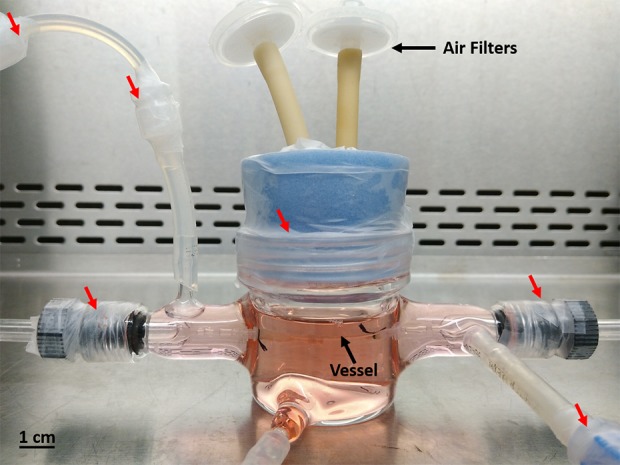
Vessel in the bioreactor. Every connection and cap are sealed with ethanol soaked parafilm (red arrows) to prevent contamination.
This simple bioreactor system enables us to tune the flow rate, intramural pressure and wall shear stress effectively by adjusting pump speed, tubing resistance and media viscosity. We were able to create similar shear stress (20 dyne/cm2) and intramural pressure (50–70 mmHg diastolic and 90–130 mmHg systolic) in both rat and human vessels. Compared to other bioreactor systems, where a separate media reservoir is used37,38,41, our system utilized a close loop system that circulates the same media in and out of the vessels, thereby conserving reagents and cost.
Rat aorta preparation
Sprague-Dawley rats weighing 250–300 g were euthanized via intraperitoneal injection of 150 mg/kg sodium pentobarbital (Sigma, St. Louis, MO, USA). Only male rats were used due to larger aorta sizes. The descending thoracic aorta was dissected free and placed in ice-cold Dulbecco’s modified eagle’s medium (DMEM) with 10% FBS (Hyclone, Marlborough, MA, USA), and then the surrounding connective tissues and fat were removed. For gentle removal of luminal ECs without inducing extensive medial injury, arteries were flushed with 0.25% trypsin EDTA (Life Technologies, Grand Island, NY, USA), PBS, (Invitrogen, Carlsbad, CA, USA) and DMEM with 10% FBS to deactivate trypsin, consecutively. Medial injury was created by inserting a 14-gauge sterile needle into the vessel. Aortas were then stored in ice-cold DMEM with 10% FBS until being mounted to perfusion bioreactors (less than 6 h) or cut into segments of 0.3–0.5 cm length for static ring culture.
Human umbilical artery preparation
Anonymized human umbilical cords were obtained from Yale–New Haven Children’s Hospital (New Haven, CT, USA). Arteries were isolated from human umbilical cords (20–30 cm in length) using sharp dissection in a sterile hood immediately after delivery as described42. Briefly, a pair of Metzenbaum scissors were inserted into the Wharton’s jelly surrounding the arteries, and the tissue was dissected from the arterial vessels. Intact arteries were separated from the umbilical vein and then cut into segments of 5 cm length. Gentle denudation and medial injury induction were performed as described for rat aortas.
Static vessel segment culture
Gently denuded and medially-injured rat aorta segments were cultured in 6-well tissue culture plates with DMEM: Vasculife (1:1) containing 5% FBS for up to 14 days (Vasculife, Lifeline Technologies, Frederick, MD, USA). The medium was changed every 2 days. Samples were fixed with 10% neutral buffered formalin at indicated times and processed for immunofluorescence staining analysis.
Culture under arterial flow in perfusion bioreactor
Human umbilical vein endothelial cells (HUVECs) were isolated from umbilical veins using 0.1% collagenase, and were cultured in Vasculife at 37 °C and 5% CO2 until sub-confluence. Cells at passage 3–4 were used in all experiments in this study. To seed endothelium onto denuded arteries in vitro, fibronectin in PBS (50 µg/mL) was delivered through the glass cannulae in the lumen of vessels mounted in perfusion bioreactors. Fibronectin was applied for 1 h at 37 °C and then replaced with HUVECs at 3 x 106 cell/mL. HUVECs were allowed to adhere for 3 h at 37 °C and 5% CO2, after which the non-adhered cells were flushed and vessels were perfused with co-culture medium: DMEM: Vasculife (1:1) with 5% FBS. The viscosity of the medium was brought to 3.8 cp by addition of 3% dextran (MW 1.5–2.8 x 106, Sigma, St. Louis, MO, USA)43. The intraluminal shear was increased gradually from 1.5 dyne/cm2 to 20 dyne/cm2 over 80 h. The vessels were cultured with flow for 4 or 7 days. To assure uniform shear stress application, we analyzed only the 1.5-cm-long central part of the vessels to avoid the effect of flow distortion in the inlet and outlet regions.
Labeling adventitial cells
To track possible adventitial cell migration, the cells in this regions were stained with FastDil dye (Invitrogen, D7756, Carlsbad, CA, USA). Freshly isolated intact rat aortas were ligated from both ends to isolate intraluminal space from staining. FastDil dye was diluted in culture media with 12 μg/mL final concentration and applied gently to the outer surface of the intact vessel by a sterile cotton swab. The vessels were incubated at 37 °C for 5 min, and then briefly rinsed with PBS. Ligated ends were cut and the vessels were injured, cut into segments and cultured statically as described above. Non-injured vessels were also cultured as control. Samples were frozen in optimum cutting temperature (OCT) compound at Day-1, Day-4, and Day-7 time points, cut in 5-μm-thick sections and imaged with 555 nm fluorescent excitation.
Histology and immunostaining
Vessels were fixed with 10% neutral buffered formalin, embedded in paraffin, and sectioned at 5 µm thickness. Tissue slides were stained for hematoxylin and eosin (H&E) as described previously42. Cell death was by labeling DNA strand breaks (TUNEL stain, Sigma, St. Louis, MO, USA) according to the manufacturer’s instructions. For immunofluorescence, sections were incubated in sodium citrate buffer (pH 6) at 75 °C for 20 min for antigen retrieval, permeabilized, and blocked with PBS containing 5% BSA, 0.75% glycine, and 0.2% Triton X-100 for 1 h, and subsequently incubated in primary antibodies against ki67 (Abcam, ab16667, rabbit monoclonal, 0.5 μg/mL, Cambridge, MA, USA), alpha-smooth muscle actin (αSMA) (Dako M081, mouse monoclonal, 70 μg/mL, Agilent Technologies, Santa Clara, CA, USA), platelet endothelial cell adhesion molecule (CD31) (Abcam ab28364, rabbit polyclonal, 20 μg/mL, Cambridge, MA, USA and Santa Cruz, sc-1506, goat polyclonal 4μg/mL, Dallas, TX, USA), von Willebrand factor (vWF) (Abcam, ab6994, rabbit polyclonal, 20 μg/mL, Cambridge, MA, USA), stem cell antigen-1 (SCA1) (Millipore, ab4336, rabbit polyclonal, 2.5 μg/mL Burlington, MA, USA) overnight at 4 °C. After washing slides with 0.2% Triton x-100 in PBS, secondary antibodies (Alexafluor 555, 647, 546or 488) were applied at 1:500 dilution for 1 h. For negative control, secondary antibodies were applied without incubating samples with primary antibodies (Supplementary Fig. 1). The slides were visualized using a Zeiss Axiovert 200 m fluorescence microscope. Cell number was quantified by counting DAPI stain in nuclei. Proliferating and apoptotic cells were quantified by counting nuclei that are co-localized with ki67+ and TUNEL+ stain, respectively. Cells with cytoplasmic SCA1+ stain was counted to quantify SCA1 expression. Cells expressing the related markers were counted in five randomly taken 20× magnification images of each vessel section, and the numbers normalized to the average number of total nuclei in freshly isolated vessel sections.
Statistical analysis
Data were expressed as mean ± SD and analyzed using one-way ANOVA test with Tukey’s multiple comparison using GraphPad Prism-7.01 (GraphPad Software, Inc., La Jolla, CA, USA). A p value of <0.05 was considered statistically significant.
Results
Effect of gentle denudation and medial injury on EC and SMC viability in rat aortas
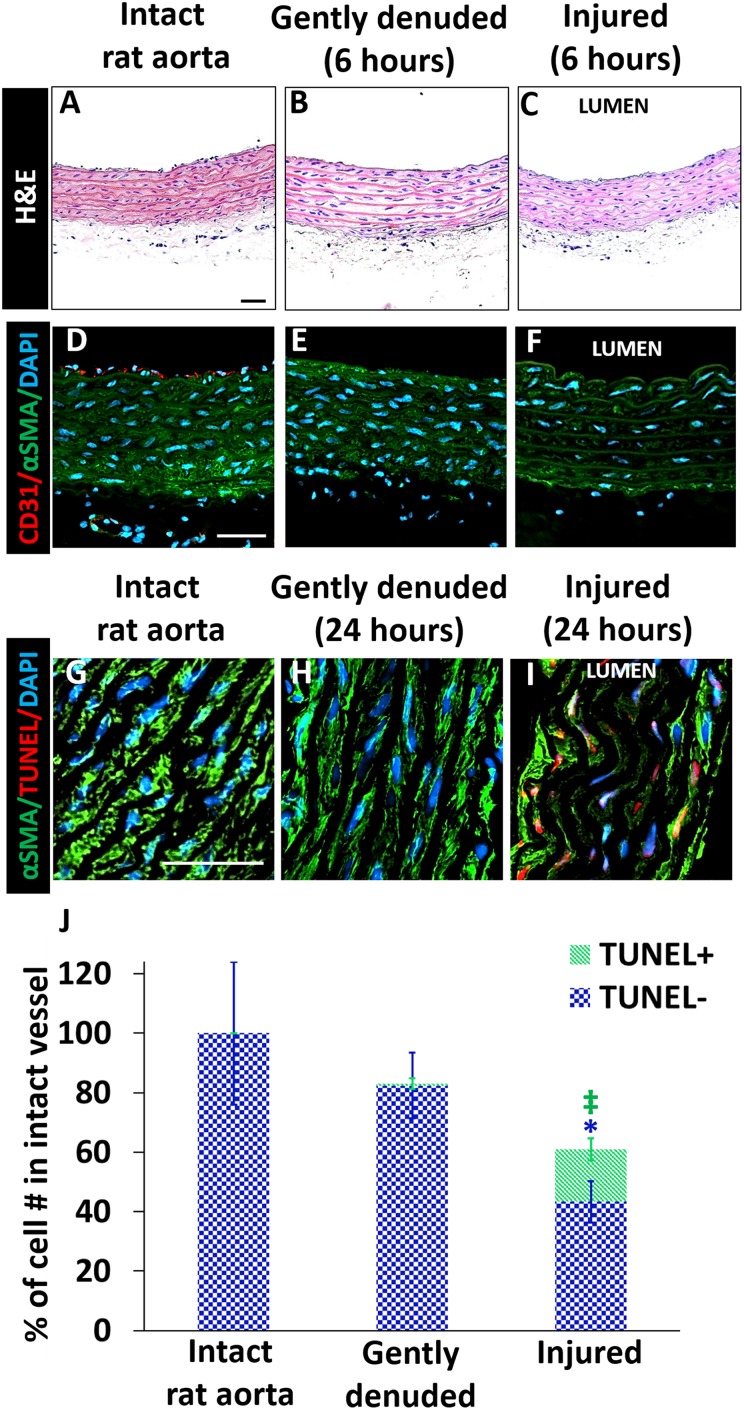
To differentiate the effect of EC denudation with and without medial injury, freshly isolated rat aortas were either gently flushed with trypsin to remove native ECs, or injured by inserting a 14-gauge (2.05 mm) needle to the lumen (that mimics balloon injury) and moving back and forth three times. The needle diameter is 1.5-fold larger than that of the intact rat aorta (1.4 ± 0.2 mm). The aortas shrank to their initial diameter immediately after needle withdrawal. In the native vessels, there was a confluent layer of ECs shown by CD31 and vWF staining, which was lost after trypsin or needle injury (Fig. 3 A–F, Supplementary Fig. 2), indicating that both of the treatments removed the native ECs successfully. The treated vessels were then cultured in 6-well culture plates for 24 h, and cell apoptosis was evaluated by TUNEL staining. SMC death in freshly isolated aortas was less than 0.5% of total nuclei, consistent with many prior observations44,45. As expected, medial injury induced by stretching the vessel wall caused significantly higher SMC death than did gentle EC denudation (40 ± 8% vs. 0.75 ± 2%, n = 4, p < 0.01) (Fig. 3 G–J). In addition, the αSMA intracellular staining appeared to be altered in needle-treated group (Fig. 3 D–F). These results indicate that the treatment with trypsin could remove ECs without extensive SMC injury, while needle treatment could mimic the vessel wall injury in ways similar to invasive percutaneous interventions.
Fig. 3.
Characterization of intact, gently denuded and injured rat aortas. (A–C): H&E; (D–F): αSMA (green)/CD31 (red); (G–I): αSMA (green)/TUNEL stain (red). (J): Average number of nuclei and TUNEL+ cells per area was normalized to average cell number in the intact rat aorta. The total bar height represents the total cell number. The total number of cells in the media+intima of intact native vessel was set as 100%. n = 4 for each group, one-way ANOVA with Tukey test for multiple comparison. *p < 0.01, ‡ p < 0.0001. Scale bars: 50 µm.
Effect of gentle denudation and medial injury on proliferation, SCA1+ cells and neointima formation in rat aortas
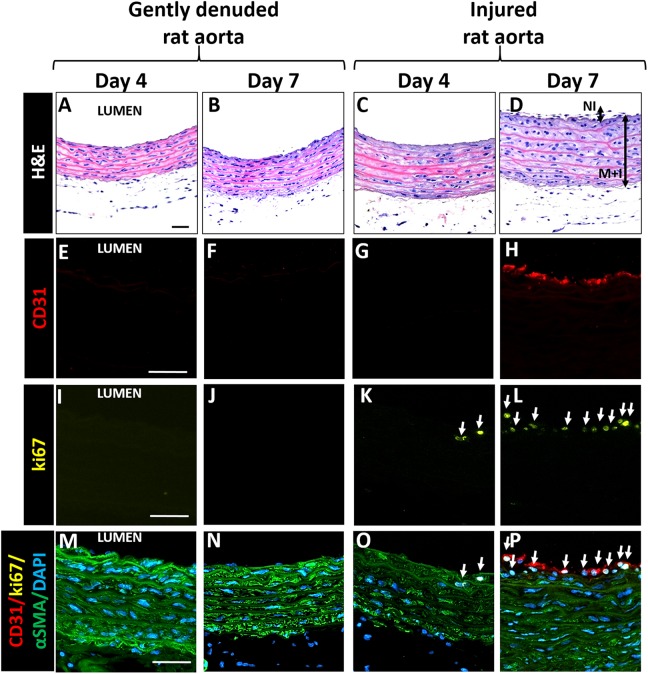
To probe cellular proliferation and migration in response to EC removal and medial injury, we cultured gently denuded and medially-injured rat aorta segments in 6-well culture plates for 4 and 7 days. As shown by H&E staining (Fig. 4 A–D), the wall thickness was 0.16 ± 0.02 mm in the freshly isolated vessel, but decreased to 0.10 ± 0.01 mm following both procedures. The wall thickness of the injured rat aorta at days 4 and 7 (0.12 ± 0.02 mm and 0.14 ± 0.02 mm, respectively) was greater than that of the gently denuded aorta (0.10 ± 0.02 mm and 0.10 ± 0.01 mm, respectively). The vessel walls in injured aortas started to thicken as de novo cell layers formed over the original vessel wall, which was bound by native connective tissue, indicating very early stages of intimal hyperplasia.
Fig. 4.
Characterization of statically cultured vessel segments 4–7 days following either gently denuding or injury of rat aortas. A–D: H&E; E– H: CD31 (red); I–L: ki67 (yellow), M–P: Merged: CD31 (red)/ki67 (yellow)/aSMA (green)/DAPI (blue). White arrows indicate ki67þ cells (K, L, O and P). Injury but not gentle denudation causes formation of neointima (D), where some of the cells are both proliferatingand CD31þ by day 7 (white stain in H). Scale bars: 50 mm. NI: Neointima, MþI: MediaþIntima.
To assess the degree of SMC damage in the media, the endothelial recovery and recruitment of progenitor cells in vessel repair, we stained tissues for αSMA, CD31, and SCA1, respectively. The αSMA staining was altered in injured rat aorta as compared to gently denuded aortas, and, interestingly, CD31 immunostaining was evident after 7 days in the injured rat aortas (Fig. 4 D and E) but not in the gently denuded aortas. Most of the CD31+ cells seen in the injured aortas were also positive for SCA1 (SCA1+) (Fig. 5 I, J, N, and O).
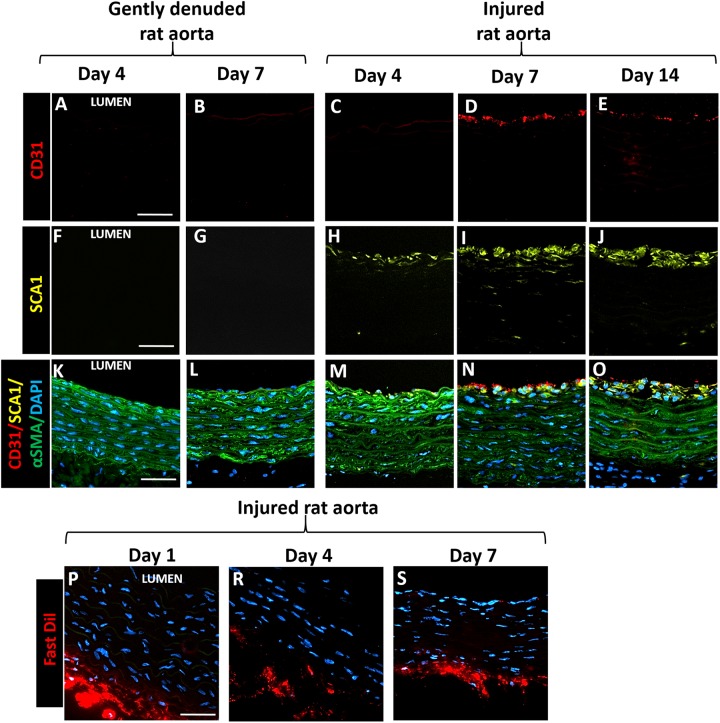
Fig. 5.
Distribution of SCA1þ cells in statically cultured rat aorta segments 4–14 days following either gently denuding or injury. A–E: CD31 (red); F–J: SCA1 (yellow), K–O: Merged, CD31 (red)/SCA1 (yellow)/aSMA (green)/DAPI (blue). SCA1þ cells appeared in the media layer of injured rat aortas 4 days after injury (H). By day 7 SCA1þ cells formed neointima-like layer, and started to express CD31 but not aSMA on the lumen surface (N). By day 14, neointima layer became thicker (O). P–S: FastDil (red) labeled adventitia cells. Labeled adventitial cells did not migrate toward lumen. Scale bars: 50 mm.
In native fresh aortas, 14% cells were SCA1+, and were mostly located in the near-adventitia region. Four days later, medial injury increased the percentage of the SCA1+ cells in the media of rat aortas to 29.9%, while in gently denuded group the fraction of SCA1+ cells was 4% (Fig. 5). By day 7, the neointma cells in the injured rat aortas were mostly SCA1+/CD31+ but αSMA–/vWF– (Fig. 5 D, E, I, J, N, and O and Supplementary Fig. 2). We labeled the adventitial cells with FastDil dye to investigate the possibility of adventitial cell migration toward lumen after injury. As shown in Fig. 5, labeled cells stayed in the adventitia during the entire culture period, but new SCA1+ cells appeared in the media 4 days, and in the neointima 7 days, after the injury. These results imply that the medial injury trigger the activation and proliferation of SCA1+ cells, which might contribute to ongoing vessel wall repair during ex vivo culture.
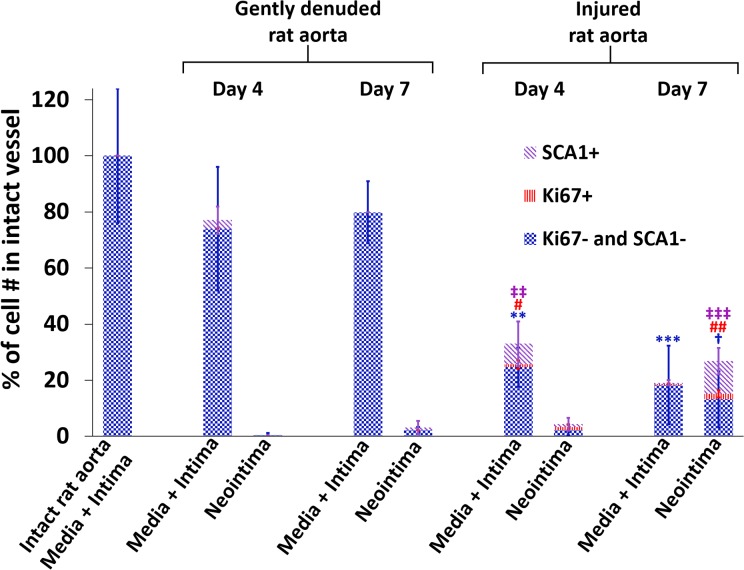
There was an apparent decrease in cell number in the media sections of the injured rat aortas as compared to those of the gently denuded aortas. In the injured rat aortas, the total cell number in the media layer reduced to 28 ± 7% of that in the intact rat aortas on day 4 of culture, and to 18 ± 4% on day 7 of culture, while cell number in the media layer of gently denuded aortas did not change (Fig. 6, n = 4–6). To quantify cell proliferation, vessel segments were stained for ki67. In the medially-injured group, 6.6% of the cells in media layer were proliferating by day 4 (Fig. 5 K) while both in gently denuded aortas and freshly isolated aortas the number of ki67+ cells in the media region were less than 0.5%. By day 7, neointima-like cell layers on the luminal surface were observed in the injured but not the gently denuded rat aortas, and 14.8% of the neointimal cells were ki67+ (Fig. 6). Thus, the medial injury was necessary to trigger medial proliferation, along with neointima-like cell accumulation on the lumen.
Fig. 6.
Distribution of ki67+ and SCA1+ cells at day 4 and day 7 as a response to gently denuding or injury. Numbers were normalized to the average cell number in intact rat aorta. The total bar height represents the total cell number. The total number of cells in the media+intima of intact native vessel was set as 100%. n = 4–6 per group. **p < 0.01, ***p < 0.0001; total number of the cells in the media+intima was significantly less than gently denuded groups. † p < 0.05; significantly more cells in neointima than any other group. # p < 0.05; significantly more ki67+ cells in the media+intima than any other group, ## p < 0.001; significantly more ki67+ cells in the neointima than any other group. ‡‡ p < 0.01; significantly more SCA1+ cells in the media+intima than any other group. ‡‡‡ p < 0.0001 significantly more SCA1+ cells in the neointima than any other group (one-way ANOVA with Tukey test for multiple comparison).
Effect of arterial flow on remodeling of injured rat aortas
To determine whether exogenously-seeded and arterial-shear stimulated ECs can reduce medial cell proliferation and migration to the lumen22,26, 5-cm-long injured rat aortas were cultured in perfusion bioreactors at 20 dyne/cm2 (arterial shear) for 4 and 7 days46, with and without HUVECs seeded onto the vessel lumen. After 7 days of culture under arterial shear, the diameter of the rat aortas remained similar as that of freshly isolated ones (1.5 ± 0.3 mm), either with or without seeded HUVECs. Regardless of HUVEC seeding, arterial flow did not change the wall thickness, which was 0.10 ± 0.02 mm immediately after vessel injury.
In contrast to static culture, neither proliferation nor neointima formation was observed in aortas cultured with arterial flow, either in the absence or the presence of ECs (Fig. 7). These results suggest that the cyclic distension created by intramural pressure alone might inhibit SMC proliferation47 and migration in medially-injured rat aortas whereas adventitial cells can leak into the lumen through severely damaged vessel walls.
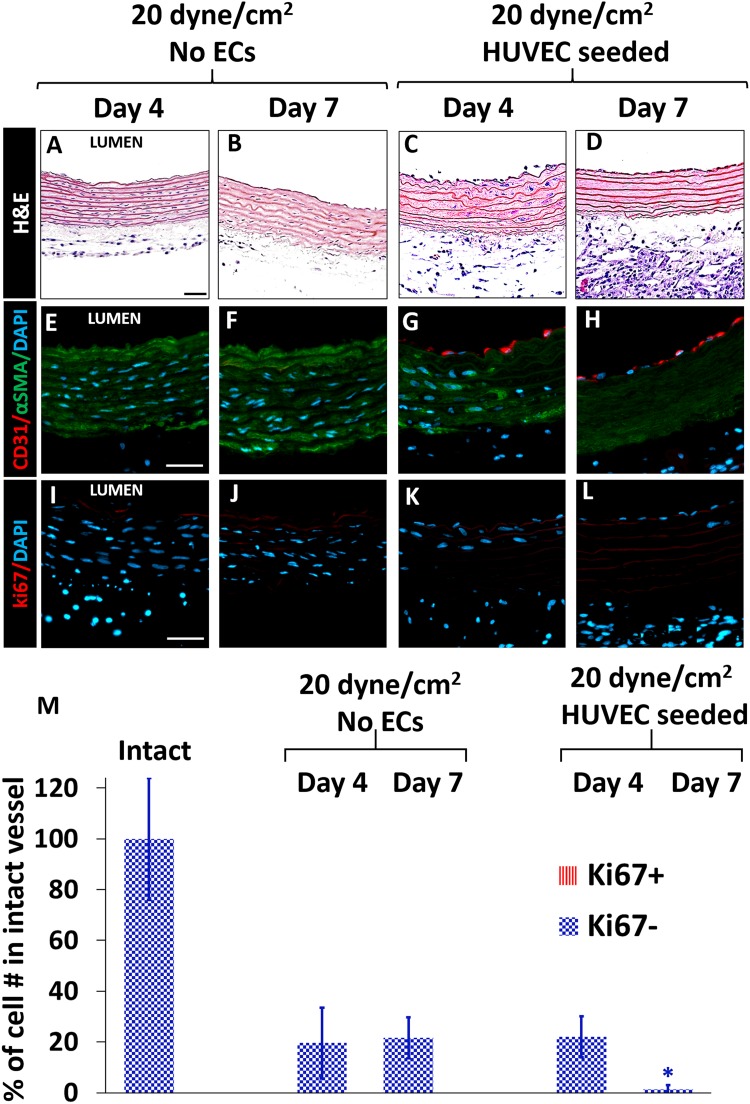
Fig. 7.
Injured rat aortas cultured under arterial shear either with or without HUVECs seeded in the lumen for 4 and 7 days. A–D: H&E; E–H: αSMA (green), CD31 (red); I–L: ki67 (red). Neither proliferation in the media layer nor neointima formation was observed in culture with flow. In the HUVEC-seeded groups, number of SMCs in the media layer was less than that in No-EC group on day 4 and day 7, respectively. Scale bars: 50 µm. M: The total bar height represents the total cell number. The total number of cells in the media+intima of intact native vessel was set as 100%. *p < 0.05; significantly less number of cells in media+intima than no-EC groups. (n = 3–7 for each rat group, one-way ANOVA with Tukey test for multiple comparison).
Under arterial shear stress, the total cell number decreased to 20% of that of freshly isolated aortas on the day-4 of culture, regardless of HUVEC seeding (Fig. 7). In aortas without HUVEC seeding, cell number did not significantly change until day-7 of culture, but in HUVEC-seeded rat aortas, severe SMC loss was observed (98.4 ± 1.4%) (Fig. 7 M). These results suggest that ECs might trigger SMC death in injured rat aortas under arterial flow.
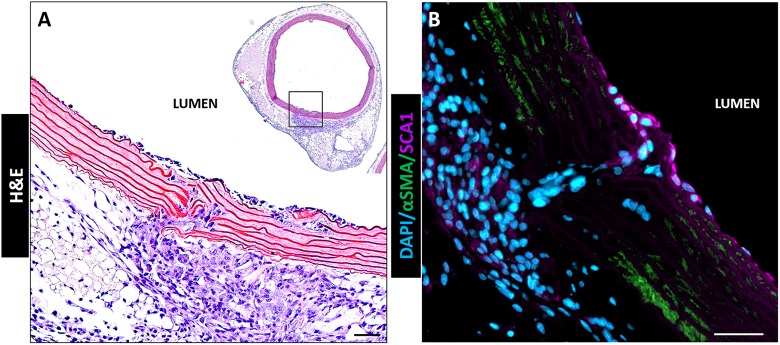
Interestingly, when the vessel wall was broken by medial injury, adventitial cells migrated through the gap in the vessel matrix and invaded toward the lumen as shown in Fig. 8. These invading cells were stained negative for αSMA, but positive for SCA1 (Fig. 8 B).
Fig. 8.
Medially-injured rat aorta in perfusion bioreactor after 7 days of culture. Adventitial cells migrated through broken vessel wall and invaded luminal surface. The cells invading the lumen stained SCA1 but not αSMA (B). Scale bars: 50 μm.
Remodeling of injured human umbilical arteries cultured in perfusion bioreactor with flow
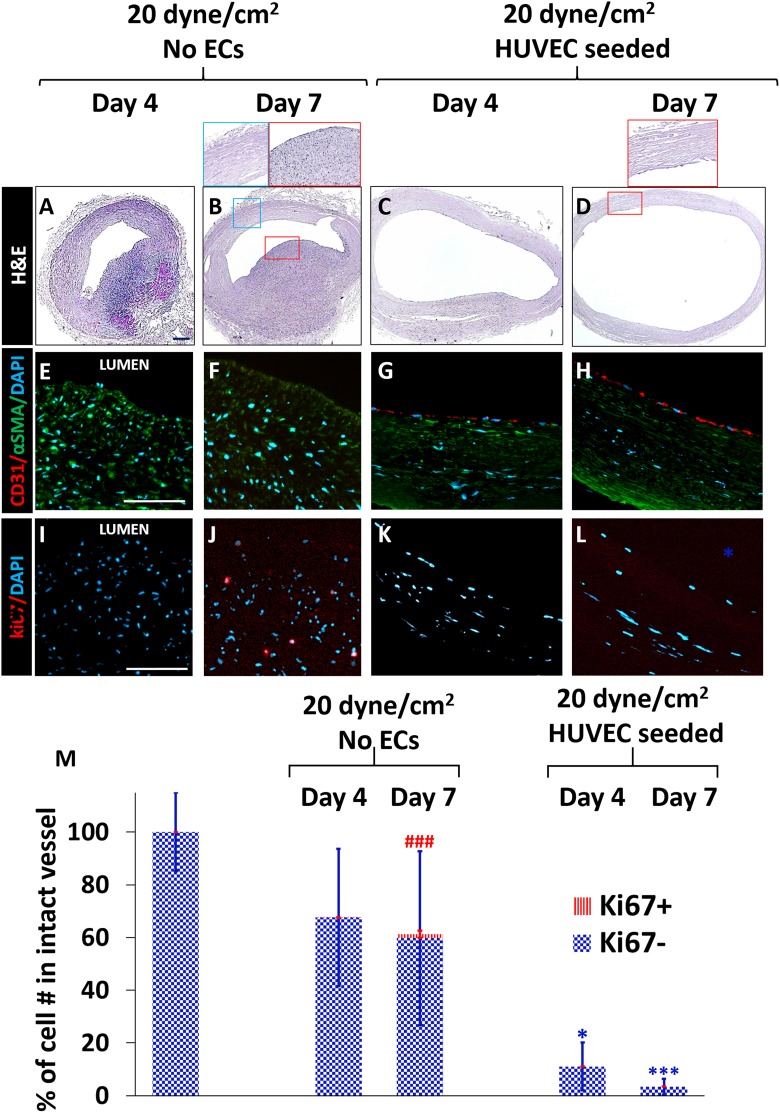
We have demonstrated that our perfusion bioreactor was able to generate arterial shear stress and pressure, and culture rat aortae ex vivo. In order to determine whether this bioreactor model can be used to provide information on human vessel injury and remodeling ex vivo, we cultured injured human umbilical arteries in these bioreactors for 4 and 7 days. To understand the contribution of ECs in vascular remodeling after injury in human vessels, human umbilical arteries were used for proof-of-concept testing. We cultured 5-cm-long injured human umbilical arteries under 20 dyne/cm2 shear stress, with and without HUVECs seeded onto the luminal surface for 4 and 7 days. The medial injury created with a 14-gauge needle (2.05 mm) increased the inner diameters of the umbilical arteries from 0.59 ± 0.88 mm to 0.92 ± 0.12 mm. Arterial flow did not significantly increase the inner diameter without ECs (1.01 ± 0.1 mm). However, in the presence of seeded HUVECs, the inner diameters of the umbilical arteries were significantly larger than those of non-EC-seeded arteries: 1.42 ± 0.14 mm on day 4 and 2.03 ± 0.17 mm on day 7 (Fig. 9). These results showed that seeded ECs caused significant arterial dilatation under flow conditions.
Fig. 9.
Injured human umbilical arteries cultured under arterial shear either with or without HUVECs seeded in the lumen for 4 and 7 days. A–D: H&E; E–H: αSMA (green), CD31 (red); I–L: ki67 (red). In the absence of HUVECs, ki67+ cells were observed on day 4 and day 7. In the HUVEC-seeded group, similar to the rat aorta, the number of SMCs in the media layer was decreased significantly compared to No-EC group on day 4 (*p < 0.01) and Day 7 (***p < 0.001), respectively. Scale bars; A–D: 200 µm, E–L: 100 µm. M: The total bar height represents the total cell number. The total number of cells in the media+intima of intact native vessel was set as 100%. ### p < 0.0001; significantly more ki67+ cells than other groups in media+intima. (n = 3–8, one-way ANOVA with Tukey test for multiple comparison).
Similar to rat aortas cultured with arterial flow, we did not observe de novo cell layers at the luminal surface in umbilical arteries that were cultured in bioreactors. The wall thickness of freshly isolated umbilical arteries was almost uniform along the circumference (0.61 ± 0.1 mm), and seeded HUVECs decreased the wall thickness circumferentially to 0.22 ± 0.07 mm while dilating the vessel wall as described above (Fig. 9 C and D). In No-EC-seeded groups, however, the wall thickness increased to 1.08 ± 0.1 mm on one-half of the vessel, and decreased to 0.24 ± 0.02 mm on the other half (Fig. 9 A and B). These results indicate that two vessel types remodeled differently as a response to in vitro arterial flow.
To determine cell proliferation in umbilical arteries cultured under arterial flow, we stained cells with Ki67. There were significantly more proliferating cells (6.9%) in vessels without ECs, as compared to the HUVEC-seeded group on day 7 (Fig. 9 J and Fig. 9 M). In HUVEC-seeded arteries, the total cell number decreased significantly (to levels of 2% of original native vessels) after 7 days (Fig. 9 C, D, H, G and M). Therefore, these results indicate that exogenously-seeded ECs caused severe SMC loss and significant vessel thinning in injured human umbilical arteries cultured with arterial flow.
Discussion
In the present study, we built an ex vivo bioreactor system that can create arterial flow and pressure. Most of our findings regarding the cellular responses to either vascular wall damage or endothelial denudation were reported in early studies. Here, we show the ability of our bioreactor culture method to validate previous findings, and, also, to isolate the effect of different factors by adding or withdrawing components like flow, pressure, growth factor, or ECs. Rat aortas and human umbilical arteries were injured (via needle application) or gently denuded (with trypsin), and cultured ex vivo to study the early stages of vessel remodeling after injury. We assessed the effect of arterial flow, as well as exogenously-seeded ECs, on the cellular response to injury in the ex vivo bioreactor system.
The vascular injury created by needle application, which mimics wall distention upon stenting or balloon injury, successfully triggered proliferation in the media region of the rat aorta segments within 4 days post injury. Without injury, gentle removal of native ECs did not result in media proliferation, even in the presence of 30% FBS (Supplementary Fig. 3). These observations are consistent with studies reporting that application of high serum or exogenous growth factors is not enough to trigger SMC dedifferentiation and proliferation in vessel segments cultured ex vivo, unless medial injury is created20,48. Following endothelial denudation in vivo, PDGF release from adhered platelets and vascular SMCs acts as a chemoattractant for SMC migration to the lumen10,20,48,49 but early medial proliferation is caused by bFGF released from injured SMCs and ECs19. It has been reported that SMC proliferation in the rat balloon injury model is proportional with the degree of balloon injury, since degradation of arterial heparan sulfate proteoglycans and cyclin-dependent kinase inhibitors surrounding the SMC surface are required for SMCs to enter the cell cycle16,23,50.
In this study, we also found that the number of SCA1+ cells in the media was significantly higher than that in gently denuded group in the rat vessels. By day 7, we observed neointima-like cell accumulation on the luminal surface of the medially-injured rat aortas. The majority of these cells were SCA1+ and CD31+ and 14.8% were proliferating. Majesky and other investigators have reported that SCA1+ cells are important mediators of vascular repair24,26,51. An “outside-in” model of vascular repair suggests that inflammation originates from the adventitia, and moves inward toward the intima24,26,51,52. Mesenchymal stem/progenitor cells (SCA1+ /Gli-1+) residing in the adventitia can migrate into the media 3–4 days after injury, and thence to the intima after 7–14 days22,51. However, in our study, the labeled adventitial cells did not migrate through the vessel wall, except leaking through the broken vessel wall. Therefore, SCA1+ cells residing in the medial region might become activated and migrate to the lumen upon injury, and contribute to the restoration of the endothelium, as well as formation of neointima. This observation is in agreement with the recent findings showing that mature SMCs can serve as a source of adventitial SCA1 cells53. Formation of neointima 7 days after medial injury has been reported frequently in in vitro and in vivo studies19,51,54,55. In this study, cells in neointima were also CD31+ at 2 weeks following the injury, indicating that SCA1+ cells might assume EC phenotype to repair endothelium22. Further research is needed to clarify the factors (such as growth factors) causing SCA1+ cell activation and migration to the luminal surface for vascular remodeling/repair, which could be elucidated using our ex vivo bioreactor system. Our observations using the ex vivo injury model are very similar to results reported from in vivo balloon injury studies.
Physiological shear stress is known to inhibit vascular SMC proliferation and neointimal thickening56,57. In this study, when injured rat aortas and human umbilical arteries were cultured under arterial shear stress in the bioreactors, unlike static culture, there was no SMC proliferation or neointima formation 7 days after the injury, we attribute this difference to the effect of shear stress and the cyclic strain on the SMC proliferation. Interestingly, we observed some proliferating SMCs in the media layer in umbilical arteries on day 4. Since rat and human vessels were exposed to similar shear stress, this difference might arise mainly from the difference in the mechanical properties of the vessels (aorta versus umbilical artery), and species. Even for the same species, vessels from different regions of the body would have different mechanotransduction properties, and show different responses to ECs and the applied shear. For instance, the remodeling of human coronary artery and human iliac artery in the same bioreactor settings might be dramatically different58.
Mature EC or EC progenitor cell transplantation to the injured vessel lumen27–31, or accelerating re-endothelialization by VEGF or VEGF gene delivery32,33, may be promising to combat restenosis after balloon injury or in-stent stenosis. To compare the effect of ECs and arterial flow on vascular remodeling, we seeded HUVECs in the lumen of rat aortas and human umbilical arteries immediately after injury. We observed severe SMC death in both rat and human arteries, but dramatic dilation only in umbilical arteries in the presence of HUVECs and arterial shear. In the absence of ECs, SMCs were viable in both species, while proliferating only in umbilical arteries. Interestingly, when HUVEC-seeded and injured vessels were cultured without arterial shear, SMC death was not observed (Supplementary Fig. 4). Also, we did not observe SMC death when we cultured intact or gently-denuded (non-injured) and HUVEC-seeded rat aortas with arterial flow (Supplementary Fig. 5). Neither ECs without flow nor flow without ECs caused SMC death, suggesting a synergistic effect of ECs and the flow/stretch imposed on SMCs of the injured arteries but not of the non-injured arteries, resulting in significant SMC death. It is known that shear stress increases nitric oxide production in ECs59. Thus, it can be speculated that cyclic stretch and nitric oxide production enhanced by shear stress on ECs can trigger apoptosis in injured SMCs in a synergistic fashion. Unlike in vivo balloon injury models, our vessels in perfusion bioreactors lacked surrounding connective tissue to prevent excessive periodic distension caused by pulsatile arterial flow. It is known that cyclic stretch enhances p53 upregulated modulator of apoptosis (PUMA) protein and gene expression in vascular SMCs60, and that p53 is activated by nitric oxide61. Furthermore, the p53 gene promotes SMC apoptosis in mechanically injured vessels but has no effect on native atherosclerotic vessels62. These findings are parallel to our observations that only injured SMCs were sensitive to flow/EC combination. Although elucidation of this mechanism grants further research focusing on nitric oxide production and apoptosis pathways, the present ex vivo vessel injury model can be a very useful platform to isolate factors triggering excessive SMC proliferation or apoptosis, since it enables us to separate components such as shear, stretch, endothelium, and soluble factors.
Conclusions
In the present ex vivo vessel injury model, we successfully cultured rat and human arteries ex vivo, to study the effects of seeded ECs under arterial flow conditions created in perfusion bioreactors. Although our system does not fully represent an ideal model of arterial injury, it was able to validate the well-established findings from previous in vivo studies. In addition, we observed interesting vascular response, which revealed the complexity of the problem that was not known before, and opens additional investigations in the future. The present ex vivo vessel culture model might be very useful for future studies focusing on the effects of different shear levels, EC types (arterial versus venous), growth factors on remodeling of different vessel types.
Supplemental Material
Supplemental Material, Kural_Supplementary_Figures_-_R for An Ex Vivo Vessel Injury Model to Study Remodeling by Mehmet H. Kural, Guohao Dai, Laura E. Niklason, and Liqiong Gui in Cell Transplantation
Acknowledgments
The authors thank Dr. Yibing Qyang for his valuable feedback, Dr. Andrew Le and Mr. Alex Engler for their help with isolation of rat aortas, and Mr. Liping Zhao and Ms. Pavlina Baevova for their technical support.
Footnotes
Ethical Approval: This study was approved by the Yale University Institutional Animal Care and Use Committee.
Statement of Human and Animal Rights: All animal care complied with the Guide for the Care and Use of Laboratory Animals. Human tissues and cell populations were obtained using protocols approved by the Yale University Human Investigation Committee, and were discarded tissues.
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Disclosures: L.E.N. is a founder and shareholder in Humacyte, Inc., which is a regenerative medicine company. Humacyte produces engineered blood vessels from allogeneic smooth muscle cells for vascular surgery. L.E.N.’s spouse has equity in Humacyte, and L.E.N. serves on Humacyte’s Board of Directors. L.E.N. is an inventor on patents that are licensed to Humacyte and that produce royalties for L.E.N. L.E.N. has received an unrestricted research gift to support research in her laboratory at Yale. Humacyte did not influence the conduct, description, or interpretation of the findings in this report. The other authors report no conflicts.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH R01 HL118245 (to L.G. and G.D.), and by an unrestricted Research Gift from Humacyte, Inc. (to L.E.N).
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics–2008 update. Circulation. 2008;117(4):e25–e146. [DOI] [PubMed] [Google Scholar]
- 2. Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics—2009 Update. Circulation. 2009;119(3):480–486. [DOI] [PubMed] [Google Scholar]
- 3. Michaels AD, Chatterjee K. Angioplasty versus bypass surgery for coronary artery disease. Circulation. 2002;106(23):e187–e190. [DOI] [PubMed] [Google Scholar]
- 4. Steffel J, Luscher TF, Tanner FC. Tissue factor in cardiovascular diseases: molecular mechanisms and clinical implications. Circulation. 2006;113(5):722–731. [DOI] [PubMed] [Google Scholar]
- 5. Wyttenbach R, Corti R, Alerci M, Cozzi L, Di Valentino M, Segatto JM, Badimon JJ, Fuster V, Gallino A. Effects of percutaneous transluminal angioplasty and endovascular brachytherapy on vascular remodeling of human femoropopliteal artery: 2 years follow-up by noninvasive magnetic resonance imaging. Eur J Vasc Endovasc Surg. 2007;34(4):416–423. [DOI] [PubMed] [Google Scholar]
- 6. Lau GT, Ridley LJ, Bannon PG, Wong LA, Trieu J, Brieger DB, Lowe HC, Freedman BS, Kritharides L. Lumen loss in the first year in saphenous vein grafts is predominantly a result of negative remodeling of the whole vessel rather than a result of changes in wall thickness. Circulation. 2006;114(1 Suppl):I435–I440. [DOI] [PubMed] [Google Scholar]
- 7. Kaneda H, Terashima M, Takahashi T, Iversen S, Felderhoff T, Grube E, Yock PG, Honda Y, Fitzgerald PJ. Mechanisms of lumen narrowing of saphenous vein bypass grafts 12 months after implantation: an intravascular ultrasound study. Am Heart J. 2006;151(3):726–729. [DOI] [PubMed] [Google Scholar]
- 8. Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, Westvik TS, Frattini JC, Breuer CK, Cha CH, Nishibe T, Tellides G, Sessa WC, Dardik A. Venous identity is lost but arterial identity is not gained during vein graft adaptation. Arterioscler Thromb Vasc Biol. 2007;27(7):1562–1571. [DOI] [PubMed] [Google Scholar]
- 9. Otsuka F, Yahagi K, Sakakura K, Virmani R. Why is the mammary artery so special and what protects it from atherosclerosis? Ann Cardiothorac Surg. 2013;2(4):519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fingerle J, Au YP, Clowes AW, Reidy MA. Intimal lesion formation in rat carotid arteries after endothelial denudation in absence of medial injury. Arteriosclerosis. 1990;10(6):1082–1087. [DOI] [PubMed] [Google Scholar]
- 11. Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. [DOI] [PubMed] [Google Scholar]
- 12. Perrins CJ, Bobryshev YV. Current advances in understanding of immunopathology of atherosclerosis. Virchows Arch. 2011;458(2):117–123. [DOI] [PubMed] [Google Scholar]
- 13. Clowes AW, Schwartz SM. Significance of quiescent smooth muscle migration in the injured rat carotid artery. Circ Res 1985;56(1):139–145. [DOI] [PubMed] [Google Scholar]
- 14. Zwolak RM, Adams MC, Clowes AW. Kinetics of vein graft hyperplasia: association with tangential stress. J Vasc Surg. 1987;5(1):126–136. [PubMed] [Google Scholar]
- 15. Soyombo AA, Angelini GD, Bryan AJ, Newby AC. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc Res. 1993;27(11):1961–1967. [DOI] [PubMed] [Google Scholar]
- 16. Indolfi C, Esposito G, Di Lorenzo E, Rapacciuolo A, Feliciello A, Porcellini A, Avvedimento VE, Condorelli M, Chiariello M. Smooth muscle cell proliferation is proportional to the degree of balloon injury in a rat model of angioplasty. Circulation. 1995;92(5):1230–1235. [DOI] [PubMed] [Google Scholar]
- 17. Curcio A, Torella D, Indolfi C. Mechanisms of smooth muscle cell proliferation and endothelial regeneration after vascular injury and stenting; approach to therapy. Circ J. 2011;75(6):1287–1296. [DOI] [PubMed] [Google Scholar]
- 18. Zempo N, Kenagy RD, Au YPT, Bendeck M, Clowes MM, Reidy MA, Clowes AW. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994;20(2):209–217. [DOI] [PubMed] [Google Scholar]
- 19. Lindner V, Reidy MA. Proliferation of smooth muscle cells after vascular injury is inhibited by an antibody against basic fibroblast growth factor. Proc Natl Acad Sci U S A. 1991;88(9):3739–3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jawien A, Bowen-Pope DF, Lindner V, Schwartz SM, Clowes AW. Platelet-derived growth factor promotes smooth muscle migration and intimal thickening in a rat model of balloon angioplasty. J Clin Invest. 1992;89(2):507–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Crowley ST, Ray CJ, Nawaz D, Majack RA, Horwitz LD. Multiple growth factors are released from mechanically injured vascular smooth muscle cells. Am J Physiol 1995;269(5 Pt 2):H1641–H1647. [DOI] [PubMed] [Google Scholar]
- 22. Wan M, Li C, Zhen G, Jiao K, He W, Jia X, Wang W, Shi C, Xing Q, Chen YF, Jan De Beur S, Yu B, Cao X. Injury-activated TGFβ controls mobilization of MSCs for tissue remodeling. Stem Cells. 2012;30(11):2498–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Izzard TD, Taylor C, Birkett SD, Jackson CL, Newby AC. Mechanisms underlying maintenance of smooth muscle cell quiescence in rat aorta: role of the cyclin dependent kinases and their inhibitors. Cardiovasc Res. 2002;53(1):242–252. [DOI] [PubMed] [Google Scholar]
- 24. Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovasc Res. 2007;75(4):640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kramann R, Goettsch C, Wongboonsin J, Iwata H, Schneider RK, Kuppe C, Kaesler N, Chang-Panesso M, Machado FG, Gratwohl S, Madhurima K, Hutcheson JD, Jain S, Aikawa E, Humphreys BD. Adventitial MSC-like cells are progenitors of vascular smooth muscle cells and drive vascular calcification in chronic kidney disease. Cell Stem Cell. 2016;19(5):628–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Majesky MW. Adventitia and perivascular cells. Arterioscler Thromb Vasc Biol. 2015;35(8):e31–e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Griese DP, Ehsan A, Melo LG, Kong D, Zhang L, Mann MJ, Pratt RE, Mulligan RC, Dzau VJ. Isolation and transplantation of autologous circulating endothelial cells into denuded vessels and prosthetic grafts: implications for cell-based vascular therapy. Circulation. 2003;108(21):2710–2715. [DOI] [PubMed] [Google Scholar]
- 28. Gulati R, Lerman A, Simari RD. Therapeutic uses of autologous endothelial cells for vascular disease. Clin Sci. 2005;109(1):27–37. [DOI] [PubMed] [Google Scholar]
- 29. He T, Smith LA, Harrington S, Nath KA, Caplice NM, Katusic ZS. Transplantation of circulating endothelial progenitor cells restores endothelial function of denuded rabbit carotid arteries. Stroke. 2004;35(10):2378–2384. [DOI] [PubMed] [Google Scholar]
- 30. Kong D, Melo LG, Mangi AA, Zhang L, Lopez-Ilasaca M, Perrella MA, Liew CC, Pratt RE, Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109(14):1769–1775. [DOI] [PubMed] [Google Scholar]
- 31. Froehlich H, Gulati R, Boilson B, Witt T, Harbuzariu A, Kleppe L, Dietz AB, Lerman A, Simari RD. Carotid repair using autologous adipose-derived endothelial cells. Stroke. 2009;40(5):1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xie H, Yang J, Han Y, Zhu X, Fang Q. Inhibition of intimal hyperplasia via local delivery of vascular endothelial growth factor cDNA nanoparticles in a rabbit model of restenosis induced by abdominal aorta balloon injury. Exp Ther Med. 2015;10(1):55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang T, Qu G. Magnetic nanosphere-guided site-specific delivery of vascular endothelial growth factor gene attenuates restenosis in rabbit balloon-injured artery. J Vasc Surg. 2016;63(1):226–233.e1. [DOI] [PubMed] [Google Scholar]
- 34. Clerin V, Gusic RJ, O’Brien J, Kirshbom PM, Myung RJ, Gaynor JW, Gooch KJ. Mechanical environment, donor age, and presence of endothelium interact to modulate porcine artery viability ex vivo. Ann Biomed Eng. 2002;30(9):1117–1127. [DOI] [PubMed] [Google Scholar]
- 35. Conklin BS, Zhong DS, Zhao W, Lin PH, Chen C. Shear stress regulates occludin and VEGF expression in porcine arterial endothelial cells. J Surg Res. 2002;102(1):13–21. [DOI] [PubMed] [Google Scholar]
- 36. Bardy N, Karillon GJ, Merval R, Samuel JL, Tedgui A. Differential effects of pressure and flow on DNA and protein synthesis and on fibronectin expression by arteries in a novel organ culture system. Circ Res. 1995;77(4):684–694. [DOI] [PubMed] [Google Scholar]
- 37. Piola M, Soncini M, Prandi F, Polvani G, Beniamino Fiore G, Pesce M. Tools and procedures for ex vivo vein arterialization, preconditioning and tissue engineering: a step forward to translation to combat the consequences of vascular graft remodeling. Recent Pat Cardiovasc Drug Discov. 2012;7(3):186–195. [DOI] [PubMed] [Google Scholar]
- 38. Prandi F, Piola M, Soncini M, Colussi C, D’Alessandra Y, Penza E, Agrifoglio M, Vinci MC, Polvani G, Gaetano C, Fiore GB, Pesce M. Adventitial vessel growth and progenitor cells activation in an ex vivo culture system mimicking human saphenous vein wall strain after coronary artery bypass grafting. PLoS One. 2015;10(2):e0117409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piola M, Ruiter M, Vismara R, Mastrullo V, Agrifoglio M, Zanobini M, Pesce M, Soncini M, Fiore GB. Full mimicking of coronary hemodynamics for ex-vivo stimulation of human saphenous veins. Ann Biomed Eng. 2017;45(4):884–897. [DOI] [PubMed] [Google Scholar]
- 40. Papaioannou TG, Stefanadis C. Vascular wall shear stress: basic principles and methods. Hellenic J Cardiol. 2005;46(1):9–15. [PubMed] [Google Scholar]
- 41. McFetridge PS, Bodamyali T, Horrocks M, Chaudhuri JB. Endothelial and smooth muscle cell seeding onto processed ex vivo arterial scaffolds using 3D vascular bioreactors. ASAIO J. 2004;50(6):591–600. [DOI] [PubMed] [Google Scholar]
- 42. Gui L, Muto A, Chan SA, Breuer CK, Niklason LE. Development of decellularized human umbilical arteries as small-diameter vascular grafts. Tissue Eng Part A. 2009;15(9):2665–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rinker KD, Prabhakar V, Truskey GA. Effect of contact time and force on monocyte adhesion to vascular endothelium. Biophys J. 2001;80(4):1722–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. von Wnuck Lipinski K, Keul P, Ferri N, Lucke S, Heusch G, Fischer JW, Levkau B. Integrin-mediated transcriptional activation of inhibitor of apoptosis proteins protects smooth muscle cells against apoptosis induced by degraded collagen. Circ Res. 2006;98(12):1490–1497. [DOI] [PubMed] [Google Scholar]
- 45. Riascos-Bernal DF, Chinnasamy P, Gross JN, Almonte V, Egana-Gorrono L, Parikh D, Jayakumar S, Guo L, Sibinga NES. Inhibition of smooth muscle beta-catenin hinders neointima formation after vascular injury. Arterioscler Thromb Vasc Biol. 2017;37(5):879–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S. Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res. 1983;53(4):502–514. [DOI] [PubMed] [Google Scholar]
- 47. Mills I, Cohen CR, Kamal K, Li G, Shin T, Du W, Sumpio BE. Strain activation of bovine aortic smooth muscle cell proliferation and alignment: study of strain dependency and the role of protein kinase A and C signaling pathways. J Cell Physiol. 1997;170(3):228–234. [DOI] [PubMed] [Google Scholar]
- 48. Fingerle J, Johnson R, Clowes AW, Majesky MW, Reidy MA. Role of platelets in smooth muscle cell proliferation and migration after vascular injury in rat carotid artery. Proc Natl Acad Sci U S A. 1989;86(21):8412–8416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. George SJ, Williams A, Newby AC. An essential role for platelet-derived growth factor in neointima formation in human saphenous vein in vitro. Atherosclerosis. 1996;120(1):227–240. [DOI] [PubMed] [Google Scholar]
- 50. Bingley JA, Hayward IP, Campbell JH, Campbell GR. Arterial heparan sulfate proteoglycans inhibit vascular smooth muscle cell proliferation and phenotype change in vitro and neointimal formation in vivo. J Vasc Surg. 1998;28(2):308–318. [DOI] [PubMed] [Google Scholar]
- 51. Hoglund VJ, Dong XR, Majesky MW. Neointima formation: a local affair. Arterioscle Thromb Vasc Biol. 2010;30(10):1877–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hu Y, Xu Q. Adventitial biology: differentiation and function. Arterioscler Thromb Vasc Biol. 2011;31(7):1523–1529. [DOI] [PubMed] [Google Scholar]
- 53. Majesky MW, Horita H, Ostriker A, Lu S, Regan JN, Bagchi A, Dong XR, Poczobutt J, Nemenoff RA, Weiser-Evans MC. Differentiated smooth muscle cells generate a subpopulation of resident vascular progenitor cells in the adventitia regulated by Klf4. Circ Res. 2017;120(2):296–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Louis H, Lacolley P, Kakou A, Cattan V, Daret D, Safar M, Bonnet J, Daniel Lamaziere JM. Early activation of internal medial smooth muscle cells in the rabbit aorta after mechanical injury: relationship with intimal thickening and pharmacological applications. Clin Exp Pharmacol Physiol. 2006;33(1–2):131–138. [DOI] [PubMed] [Google Scholar]
- 55. Zhao W, Wang C, Liu R, Wei C, Duan J, Liu K, Li S, Zou H, Zhao J, Wang L, Qi Y, Liang W, Jiang J, Zhang W, Pang L, Li F. Effect of TGF-β1 on the migration and recruitment of mesenchymal stem cells after vascular balloon injury: involvement of matrix metalloproteinase-14. Sci Rep. 2016;6:21176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kraiss LW, Kirkman TR, Kohler TR, Zierler B, Clowes AW. Shear stress regulates smooth muscle proliferation and neointimal thickening in porous polytetrafluoroethylene grafts. Arterioscler Thromb. 1991;11(6):1844–1852. [DOI] [PubMed] [Google Scholar]
- 57. Qiu J, Zheng Y, Hu J, Liao D, Gregersen H, Deng X, Fan Y, Wang G. Biomechanical regulation of vascular smooth muscle cell functions: from in vitro to in vivo understanding. J R Soc Interface. 2014;11(90):20130852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ward MR, Kanellakis P, Ramsey D, Jennings GL, Bobik A. Response to balloon injury is vascular bed specific: a consequence of de novo vessel structure? Atherosclerosis. 2000;151(2):407–414. [DOI] [PubMed] [Google Scholar]
- 59. Davis ME, Grumbach IM, Fukai T, Cutchins A, Harrison DG. Shear stress regulates endothelial nitric-oxide synthase promoter activity through nuclear factor kappaB binding. J Biol Chem. 2004;279(1):163–168. [DOI] [PubMed] [Google Scholar]
- 60. Cheng WP, Wang BW, Chen SC, Chang H, Shyu KG. Mechanical stretch induces the apoptosis regulator PUMA in vascular smooth muscle cells. Cardiovasc Res. 2012;93(1):181–189. [DOI] [PubMed] [Google Scholar]
- 61. Messmer UK, Ankarcrona M, Nicotera P, Brune B. p53 expression in nitric oxide-induced apoptosis. FEBS Lett. 1994;355(1):23–26. [DOI] [PubMed] [Google Scholar]
- 62. Sanz-Gonzalez SM, Barquin L, Garcia-Cao I, Roque M, Gonzalez JM, Fuster JJ, Castells MT, Flores JM, Serrano M, Andres V. Increased p53 gene dosage reduces neointimal thickening induced by mechanical injury but has no effect on native atherosclerosis. Cardiovasc Res. 2007;75(4):803–812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Kural_Supplementary_Figures_-_R for An Ex Vivo Vessel Injury Model to Study Remodeling by Mehmet H. Kural, Guohao Dai, Laura E. Niklason, and Liqiong Gui in Cell Transplantation