Abstract.
Wolbachia bacteria are known to cause deviations from random mating and affect sperm competition (SC) in some of their arthropod hosts. Because these effects could influence the effectiveness of Wolbachia in mosquito population replacement and suppression programs, we developed a theoretical framework to investigate them and we collected relevant data for the wMel infection in Aedes aegypti. Using incompatibility patterns as a measure of mating success of infected versus uninfected mosquitoes, we found some evidence that uninfected males sire more offspring than infected males. However, our theoretical framework suggests that this effect is unlikely to hamper Wolbachia invasion and has only minor effects on population suppression programs. Nevertheless, we suggest that mating effects and SC need to be monitored in an ongoing manner in release programs, given the possibility of ongoing selection for altered mating patterns.
INTRODUCTION
In many arthropods infected with Wolbachia, the maternally inherited endosymbiotic bacterium can cause cytoplasmic incompatibility (CI), which is observed as infertility in uninfected females when mated only to infected males (reviewed in Hoffmann and Turelli1 and Engelstädter and Telschow2). Wolbachia-infected females can produce viable, infected offspring when mated with uninfected or infected males, which equates to a fitness advantage over uninfected females. The fitness advantage gained by Wolbachia-infected females gives Wolbachia infection an edge in invading a population, and this can contribute to the rapid spread of Wolbachia in natural populations.3,4 However, uninfected females could be selected to reduce the effect of incompatibility. This may occur through direct selection for alleles in host genomes that increase compatibility between crosses of uninfected females to infected males and indirect selection on Wolbachia alleles to decrease deleterious fitness effects,5 or else by selection for mating isolation such that there is lower probability of uninfected eggs being fertilized by sperm from infected males than by sperm from uninfected males. Such mating isolation may be due to uninfected females preferentially mating with uninfected males6 and/or sperm competition (SC) such that sperm from infected males is less likely to fertilize the egg when compared with sperm from uninfected males.7,8 By reducing the effective level of incompatibility, these factors have potential to reduce the likelihood of Wolbachia infections spreading in host populations.1
Wolbachia introduced into Aedes aegypti block transmission of dengue and other viruses.9–12 As the Wolbachia also cause CI in Ae. aegypti,13–15 Wolbachia infections are good candidates for dengue control by means of mosquito population replacement. Wolbachia infections have also been proposed for population suppression using release of infected male mosquitoes to induce CI16 akin to the sterile insect technique (SIT). Wolbachia-infected mosquitoes have now been used in field releases involving different strains but particularly wMel,17 as well as in trial SIT releases in Singapore (http://www.nea.gov.sg/public-health/environmental-public-health-research/wolbachia-technology) and elsewhere. Quality control is essential to understand the challenges that may impact Wolbachia utility in achieving population replacement or suppression. Quality control includes activities from laboratory fitness studies15,18–20 to field evaluation21 and evaluating patterns of genetic variation in Wolbachia and mtDNA.22 Only a small number of mating isolation studies have been performed within the context of Wolbachia infection for population control,23,24 and a greater understanding of mosquito mating behavior is needed.
Aedes aegypti females are thought to be monandrous (inseminated once),25–28 whereas the males seek multiple mates.29,30 During insemination, which usually occurs in a copulation event of > 6 seconds,27 a substance within the male accessory gland appears to render females refractory to further insemination,25,26 but females can still be inseminated within 24 hours after exposure to the male accessory gland.25 There is also molecular evidence for some multiple insemination in the field.31 This raises the question whether Wolbachia dynamics could be altered through SC and interrupted mating. Evidence for Wolbachia effects on SC of infected male relative to uninfected male range from a positive effect,32 or no significant effect,7,33 to a negative effect.34
Deviations from random mating may occur in Ae. aegypti for a number of reasons. Female size can affect male mate choice,35 with mature males preferring larger females. Wing beat frequency, which allows some level of species recognition36 during mating, appears to exhibit harmonic convergence when males and females mate.37 With regard to mating success, Cator et al.38 found that the ability to converge harmonically in wing beat frequencies could be crucial and represents a heritable trait that also increases mating success in male offspring. Each of these factors could be influenced by Wolbachia, given that there are known size and behavioral effects on Ae. aegypti associated with this infection.20,39
Here, we define a deterministic framework for investigating the effects of nonrandom mating and SC. We performed mating assays, exposing uninfected females to a mixture of infected and uninfected mosquitoes. Egg hatch rates were measured as a proxy for assessing whether females were mated to an uninfected (compatible) male or infected (incompatible) male. We use the framework and data to derive estimates of the magnitude of nonrandom mating and SC. Results are used to predict potential effects on population replacement and population suppression outcomes when releasing Wolbachia-infected mosquitoes.
MATERIALS AND METHODS
We first describe our empirical work testing for nonrandom mating at different Wolbachia infection frequencies. We then outline a model for nonrandom mating and SC and consider the implications for the mosquito control strategies before linking the empirical data to these predicted effects.
Mosquito lines.
A laboratory-uninfected line and a wMel-infected line of Ae. aegypti on a genetic background from Cairns, Far North Queensland, Australia, were used in this experiment. The uninfected line came from several hundred eggs collected around the Cairns region that were subsequently maintained as a mass bred population of several hundred adults. The wMel-infected line was obtained from Gordonvale near Cairns where a field release of wMel-infected mosquitoes had successfully led to Wolbachia invasion in the region.17 The line had been held for three to seven generations in the laboratory at the time of the experiments. All cultures and experiments were maintained at 26°C, with a relative humidity of 70–80%, and 12:12 (hours) light to dark cycle, with about an hour of dim light before and after the light phase, to simulate dawn and dusk.
Mosquito rearing.
Eggs were hatched in 3 L of reverse osmosis (RO) water with yeast (∼0.09 mg) and one crushed tablet (∼300 mg) of TetraMin® Tropical Fish Food tablet Rich Mix (Tetra Holdings Inc., Blacksburg, VA) in plastic trays (20 cm × 28.5 cm × 9 cm). Two days later, density was controlled to 225 larvae at approximately second instar in 4 L of RO water in plastic trays (42.7 cm × 31.2 cm × 7.2 cm) (Modulab Systems, Gratnell Ltd., Harlow, United Kingdom). Each density-controlled tray of larvae was supplied with one tablet of TetraMin® (300 mg). Further food was added in the next 5 days whenever food became scarce. Care was taken to make sure there was always some food left in the trays to prevent starvation. Under this regimen, the size of males and females does not differ significantly between wMel-infected and uninfected mosquitoes.40
Seven days after the day of hatching, approximately 80–90% of the larvae were expected to have pupated, with a small percentage of males being expected to have eclosed. Each tray of 225 mosquitoes was transferred into 500 mL round containers with approximately 400 mL RO water. Pupae were sexed by size (females are larger than males) (cf.41). Virgin females and males were separated into several temporary cages (12.5 L space) for at least 3 days before the experimental setup. Each cage was supplied with a wick connected to 25 mL 10% sucrose solution.
Mating test.
Because the number of females required to be assessed would be too large if individual females were used, we pooled 25 females at a time. To further increase the power of detection, we competitively mated uninfected females with a male population with a range of Wolbachia infection proportions, namely, 0, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, and 1, with 0.1 omitted because of logistical constraints. We replicated the controls (0 and 1) three times, treatments 0.5 and 0.6 seven times, and the other treatments eight times (see next section for relevant power analysis). By using a range of infection proportions, we could use regression to assess patterns (described in the next section) rather than pairwise comparison of treatments. Competitive mating was performed in 3 L rectangle containers housing 25 uninfected females and 30 males with the aforementioned infection proportions. The density of mosquitoes used in such containers was higher than that usually observed in the field (e.g., < 10 females per house42), but the density in the field varies locally and can be high during the wet season.43 We chose to include more males as it has been suggested that the operational sex ratios (OSRs) (sexually mature males to female ratios) are male biased.44
Adult females were exposed to males for 10–14 days before egg collection. Adult females were provided blood from human volunteers 7–10 days after exposure to males. Eggs were collected four times over four consecutive days on filter papers from a pool of 25 females for each replicate. Filter papers with eggs were kept wet for 3 days and then partially dried (moist to touch). Photos of eggs were taken and eggs were then counted using the ImageJ program (https://imagej.net) by placing marks on each egg (although where egg counts were low (< 50), they were counted directly by scanning the paper). Within a week of drying, filter papers with eggs were submerged in 1 L of water in a plastic container with excess (300 g per 225 larvae) Tetramin® fish food tablets. Eggs collected on different days were hatched separately to avoid overcrowding. Although we did not control for density, care was taken to avoid food depletion (or an oversupply of food which promotes anaerobic conditions) by monitoring containers and adding additional food as required. We did not detect an effect of larval density on hatch rate (see Results). Hatch rates were estimated from the total number of third instar larvae (5–6 days after hatching) divided by the total egg count. Larvae were counted with the help of a glass dropper pipette with a rubber bulb, by taking up a small number of larvae and releasing these into a container of water before counting the larvae with a manual counter as they exited the pipette. Before the regression analysis, we checked for fecundity bias by testing whether fecundity was associated with proportion of infected males in the experiment based on a pilot experiment (see Supplemental Material 1). We validated results by also using a linear model to test whether egg numbers in this experiment was associated with frequency of infected males. We assessed whether density of larvae hatched in the container affected the hatch rate by running a linear model with density, replicate, and treatment (frequency of infected males) as the independent variable and arcsine-transformed hatch rate as the dependent variable.
Estimating relative fitness of infected males.
We let 1) β be the relative fitness of infected males to uninfected males, 2) Hobs be the observed hatch rate of eggs from uninfected females when exposed to Wolbachia-infected and uninfected males, 3) pI be the Wolbachia infection frequency in males, 4) H be the hatch rate of eggs resulting from incompatible crossing (Wolbachia-infected male crossed with uninfected female) to account for incomplete CI, and 5) h be the average hatch rate of eggs from completely compatible crosses, to account for absolute fecundity/hatch rate that was not 100%. From the mating experiment, Hobs was estimated via the larvae counts divided by egg counts, H was the hatch rate of the control with proportion infected males = 1, and h was the hatch rate of the control with proportion infected males = 0. β was the parameter that we were interested in estimating. All parameters are probability values except β (β ≥ 0). β < 1 signifies lower fitness in infected males, whereas β > 1 signifies greater fitness.
| (1) |
The numerator of equation (1) signifies all the possible viable offspring, whereas the denominator is the total probability of all possible matings, given the relative fitness β. We rearranged equation (1) such that those parameters could be treated as the slope of a linear regression model as described in equation (2).
| (2) |
where
| (3) |
The linear regression model treats (Hobs − h)*(1 − pI) as the y axis and pI *(H − Hobs) as the x axis, forcing the y intercept to zero. We ran Shapiro–Wilk’s tests on those axis values to test the normality assumption.
Mating isolation in the context of Wolbachia-infected and uninfected males could be either attributed to 1) nonrandom mating due to a fitness difference between infected and uninfected males (NR); 2) nonrandom mating due to assortative mating (AM), or the nonrandom mating of similar individuals, that is, infected × infected or uninfected × uninfected; or 3) SC where sperm from a male with one infection status is more successful at fertilizing the egg. If only one of the three types of mating isolation occurs, the estimate of β would be reflective of the strength of that particular mating isolation (see Table 1), but these effects are otherwise difficult to separate in Ae. aegypti. Nonrandom mating could be evaluated by observing the frequency of different combinations of mating pairs, but this was difficult in Ae. aegypti because of their short copula duration. It might be possible to approximate nonrandom mating effects if a separate experiment using sequential mating could be performed, but to mate female Ae. aegypti to two males in sequence can be challenging because, following formation of the first mating pairing, the second copulation might not lead to successful sperm transfer. As we were unable to disentangle nonrandom mating and SC effects, the estimate of β is the composite effect of the relative fitness of infected males to uninfected males.
Table 1.
Mating isolation scenarios, experimental method to estimate their effect via equations (1)–(3), associated assumptions, and what β means in each context
| Mating isolation scenario | Experimental method | Assumptions | What does β signify? |
|---|---|---|---|
| Nonrandom mating due to fitness difference between infected and uninfected males | Via measuring the hatch rate of eggs from uninfected females that were placed in an arena of infected and uninfected males | Females mate only once, probability of encountering males reflects infected/uninfected frequencies | Relative fitness of infected males to uninfected males |
| Nonrandom mating due to assortative mating | Via measuring the hatch rate of eggs from uninfected females that were placed in an arena of infected and uninfected males | Females mate only once, probability of encountering males reflects infected/uninfected frequencies | Relative preference of females for males of different infection status to males of the same infection status |
| Sperm competition | Via measuring the hatch rate of eggs from uninfected females that were sequentially force-mated to two males of different infection status, pI = 0.5 | Each male contributes the same number of sperm. No effect of order of mating | Relative fitness of sperm from infected males to sperm from uninfected males |
Under random mating and no SC, the average reduction in the hatch rate observed in uninfected females would reflect the frequency of infection among males. We performed a power analysis to determine the sample size required to estimate β using equations (1)–(3). The power analysis was based on the null hypothesis that the hypothetical reduction in the hatch rate is equivalent to the infection frequency (as in the complement of equation [1]), whereas with the alternate hypothesis, we were interested in detecting β = 0.5, that is, the effective frequency of infected males is halved. For example, if h = 1 and H = 0, and if the null hypothesis, Ho, states that if pI = 0.75, the average reduction in hatch rate = 0.75, then, the alternate hypothesis, H1, should be that the average reduction in hatch rate = 0.6 (the complement of equation [1], with β = 0.5).
For uninfected female sample sizes, n of 100, 120, 130,150, 180, and 200, we simulated the reduction in the hatch rate due to incompatible mating (at infection frequencies, pI of 0.05–0.95). We simulated n individual females assuming normally distributed egg numbers with mean and standard deviation determined in a fecundity assay of uninfected females (see Supplemental Material 1). We then simulated for each individual whether they would encounter an infected male with frequency, pI (0.05–0.95). If they encounter an infected male, the female will have no offspring. Then, the overall hatch rate of these n individuals was computed. These n individuals were simulated 10,000 times, resulting in 10,000 computations of simulated overall hatch rate. The overall hatch rates are partitioned into quantiles 0–100% at an interval of 5%. Say H0: average reduction in hatch rate = pI(1), the 5% quantile would mean that there is only a 5% chance that the observed reduction in hatch rate is due to an effective infection frequency, pI(1). This 5% quantile is the critical region for rejection (α = 0.05) of the null hypothesis. If (say) under an alternative hypothesis, the true effective reduction in hatch rate is pI(2) < pI(1) and the 5% quantile for pI(1) fell within the 90–95% quantile of pI(2), then the power of detection (accepting the alternative hypothesis) would be in the range of 90–95%. Using this, we found that we needed to assess egg hatch from 150 to 200 uninfected females if we wanted more than 90% power of detecting that infected males were only half as likely as uninfected males to mate successfully with uninfected females (β < 0.5) (see Supplemental Material 2).
We benchmarked the analytical method to further evaluate the type 1 and 2 errors of the experimental design (25 uninfected females in each treatment, three replicates of each control, 0% and 100% infected males, seven replicates of 50% and 60% infected males and eight replicates of 20%, 30%, 40%, 70%, 80%, and 90%). The experimental design used in this study was simulated 10,000 times and each of the simulated datasets was subjected to the regression analysis described in equations (1)–(3), testing the null hypothesis that the relative fitness of infected to uninfected males is equal or β = 1. From this, we found that we needed to adjust the P value to 5 × 10−5 to have less than 5% type 1 error and this adjusted P value had > 95% power (type 2 error < 5%) of detecting a relative fitness of infected to uninfected, β ≤ 0.8 (see Supplemental Material 3)
Model of mating.
Nonrandom mating (in females) due to a fitness difference between infected versus uninfected males (NR), and SC could be considered as a bias for or against males (and their sperm) of different Wolbachia infection status, whereas nonrandom mating due to AM could be considered a bias for or against mating events involving male and female pairs with a different infection status. We let βnr be the ratio of preference for Wolbachia-infected males to uninfected males, βam be the ratio of mating pair with dissimilar mating pair to mating pair where both males/females have the similar infection status, and βsc be the ratio of preference for sperm of Wolbachia-infected males to uninfected males. These parameters can take values from 0 toward positive infinity. Where 1) βnr, βam, and βsc values = 1, there are no NR, AM, and SC, respectively; thus, the effective infection frequency is the same as the actual infection frequency. 2) Where βnr and βsc < 1, there is a bias against infected males, and while βam < 1 implies females having greater preference for males of the same infection status, 3) βnr and βsc > 1 implies a bias for infected males, or βam > 1 implies a bias for males of dissimilar infection status. Under conditions where male preference is the same regardless of female infection status, we can estimate the effective infection frequency in males under NR, pnr:
| (4) |
where pI is the actual infection frequency in males. When considering nonrandom mating due to AM, it is not possible to estimate effective male infection frequency as the effect is also dependent on female infection status (see Table 2), unless we were looking at only one female infection state. The effective male infection frequency under SC, psc, is defined as
Table 2.
Punnett squares with proportion of each cross resulting in viable offspring under different models
| Male | Female | ||
|---|---|---|---|
| 1 − pI (uninfected) | pI (infected) | ||
| Uninfected offspring | Uninfected offspring | Infected offspring | |
| Nonrandom mating due to difference in male fitness | |||
| 1 − pI (uninfected) | 1 | μ*(1 − sf) | (1 − sf)*(1 − μ) |
| pI (infected) | β*(1 − sh) | β*μ*(1 − sf) | β*(1 − sf)*(1 − μ) |
| Nonrandom mating due to assortative mating | |||
| 1 − pI (uninfected) | 1 | β*μ*(1 − sf) | β*(1 − sf)*(1 − μ) |
| pI (infected) | β*(1 − sh) | μ*(1 − sf) | (1 − sf)*(1 − μ) |
| Sperm competition | |||
| 1 − psc (uninfected) | 1 | μ*(1 − sf) | (1 − sf)*(1 − μ) |
| psc (infected) | (1 − sh) | μ*(1 − sf) | (1 – sf)*(1 − μ) |
psc is defined in equation (5).
| (5) |
where pm is the remating frequency. For simplicity, we consider a maximum of two matings in polyandrous females. The effective infection frequency under SC, psc in (2), assumes equal sperm contribution from all males mated to the polyandrous females. It also assumes that each remating event is independent of the previous mating, and all males have an equal opportunity to mate, but SC is modified by the βsc parameter.
Effect of nonrandom mating and SC on population replacement.
To evaluate the impact of nonrandom mating and SC on population replacement, it was necessary to evaluate the infection unstable equilibrium frequency, as this is the frequency that defines whether the infection will increase (when infection frequency is above the equilibrium) or decrease (below the equilibrium). The formulation of the infection frequency difference equation can easily be derived from the Punnett square (Table 2). The next-generation probability of each cell is given by first multiplying the corresponding female and the male frequencies and the relative viability within the given cell. This is then divided by the summed value across all cells. The sh term refers to the reduction in viability due to infected sperm fertilizing an uninfected egg, sf is the relative reduction in fecundity in infected females to uninfected females, and μ is the probability of offspring of infected females not acquiring Wolbachia infection, that is, transmission leakage. For nonrandom mating due to the difference in male fitness (Table 2), it should be apparent if all cells were divided by βp + (1 − p), the β parameter could be absorbed into the male frequencies; hence, the model can be derived with the effective male infection frequency described in equation (4). It is not possible to factor out the β parameter into one of male/female frequencies in the AM model (Table 2). We derived the difference equation assuming discrete generations so that the unstable equilibria are mathematically tractable. We assumed there was no maternal transmission leakage, μ = 0. Although there has been a reported case of maternal transmission leakage in the field45 and transmission leakage can be induced under certain stressful temperature conditions,19 under constant rearing temperature of 26°C, maternal transmission leakage has not been detected for this infection14,19 (see Discussion).
Effect on Incompatible Insect Technique.
Akin to SIT, IIT is the inundation of the population with infected (incompatible) males rendering uninfected females sterile by CI. As the basis of IIT is male-only release, the impact of nonrandom mating effects and SC could be evaluated by looking at the effective male infection frequency. The effective male infection frequency in this case can be considered as the infection frequency when there is random mating and no SC. Thus, the infection frequency required to achieve the same effect as a given infection frequency under the random mating, and no SC, is given by making pI the subject in equations (4) and (5).
The infection frequency required to achieve effective male infection frequency because of nonrandom mating effects is given by
| (6) |
Because IIT is a male-only release, the effect of nonrandom mating due to a difference in male fitness or AM should be the same because there are no infected females. The infection frequency required to achieve effective male infection frequency due to SC can be obtained by solving
| (7) |
To better reflect the magnitude of the release, we transformed the estimates of infection frequency, pI, into release ratios as it provides a more direct view of how many incompatible males with respect to uninfected males will be required. For example, pI = 0.5 equates to a ratio of one released Wolbachia-infected male to one uninfected wild male. The relationship of infection frequency, p, to the release ratio of infected factory produced males to uninfected wild males, R, is given by
| (8) |
We can use equations (6) and (7) to determine the infection frequency required to achieve the same effect as when there is no NR, AM, or SC (reflected in pnr and psc). Then, the release ratio, R, can be determined by equation (8). To estimate the burden of NR, AM, and SC on the release program, we calculate the percentage increase, Δ, in number required to achieve the same effect as when there is no NR, AM, or SC, defined by
| (9) |
In fact, under the NR or AM model, equation (9) reduces into a constant independent of pI.
| (10) |
However, under the SC model, equation (9) is still dependent on pI. In fact, increasing pI under the SC model decreases the estimate of Δ when pm and βsc are held constant. With this model framework, we made an empirical estimation of the values of β under various nonrandom mating and SC effects to evaluate wMel-infected Ae. aegypti as a candidate for population replacement and IIT.
RESULTS
Mating test.
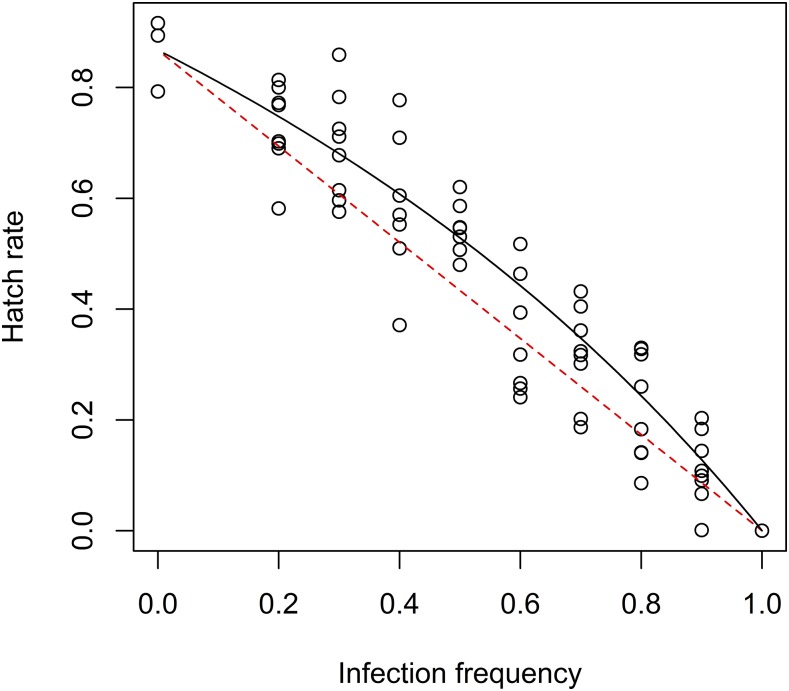
Based on incompatible control crosses (100% wMel-infected males crossed with uninfected females), we estimated H = 0 or complete CI. Using the compatible cross-controls (0% wMel-infected males), the average hatch rate of eggs, h, is around 0.8676. From a previous pilot experiment, there was no evidence that female fecundity was affected by the male infection status (see Supplemental Material 1). We further found no association between fecundity of uninfected females with proportion of infected males (F1,65 = 0.014, P = 0.9069). We found no association between hatch rates with either replicate (F7,226 = 1.467, P = 0.1800) or larval density (F1,226 = 0.1138, P = 0.7362). More specifically, when looked at treatments with the least infected males (0 and 0.2), there was no evidence that larval density affected the hatch rate (F1,38 = 0.686, P = 0.4129). We then ran a linear regression based on equation (2) and found that the estimate (and 95% confidence interval) for β was 0.6410 ± 0.0907 (Figure 1). The test for the null hypothesis of β = 1 yielded an unadjusted P value of 1.97 × 10−11 (which is far lower than threshold 5 × 10−5) based on a t test with 66 degrees of freedom (there were 67 pools of females tested).
Figure 1.
Hatch rate of eggs from replicate pools of 25 uninfected female Aedes aegypti exposed to a corresponding frequency of wMel-infected males. Each circle represents a single replicate pool of 25 uninfected females. The dotted line is β = 1 (no difference in infected and uninfected male contribution) and β = 0.6410 is the estimated value from the regression. This figure appears in color at www.ajtmh.org.
Effect of nonrandom mating and SC on population replacement.
The unstable equilibrium under nonrandom mating due to difference in male fitness and nonrandom mating due to AM was
| (11) |
| (12) |
The unstable equilibrium under the NR model is nonzero and less than one if and only if sh > sf, and for the AM model, if and only if βam < (1 − sf)−1 and sh > sf*(2 − sf) or βam < (1 − sf)/(1 − sh) and sh < sf*(2 − sf).
Under SC with maximum two mates only, the unstable equilibrium is the positive solution to equation (13) bounded by 0 and 1, where sh > sf,
| (13) |
As should be expected, when βnr = βam = βsc = 1, that is, random mating or no SC, the unstable equilibrium reduces to sf/sh which is the unstable equilibrium under random mating and no SC. In all cases, the stable equilibrium frequencies are 0 and 1. Decreasing the value of β increases the estimate of the unstable equilibrium. Under the two nonrandom mating scenarios, it can be shown that βnr = βam = sf/(sh − sf) when the unstable equilibrium is 0.5 (Figure 2) for any scenario of sf and sh (see Supplemental Material 4). The unstable equilibrium under the NR and SC models (equations [11] and [13]) is in fact the same as applying equations (6) and (7), replacing pnr and psc with the unstable equilibrium under random mating and no SC (see Supplemental Material 5). This means that the effect of βnr and βsc will be the same for any combination of sf and sh which gives rise to the same values of an unstable equilibrium under random mating and no SC, that is, sf/sh. This, however, only works when we assume no maternal transmission leakage, μ = 0 (see Supplemental Material 6).
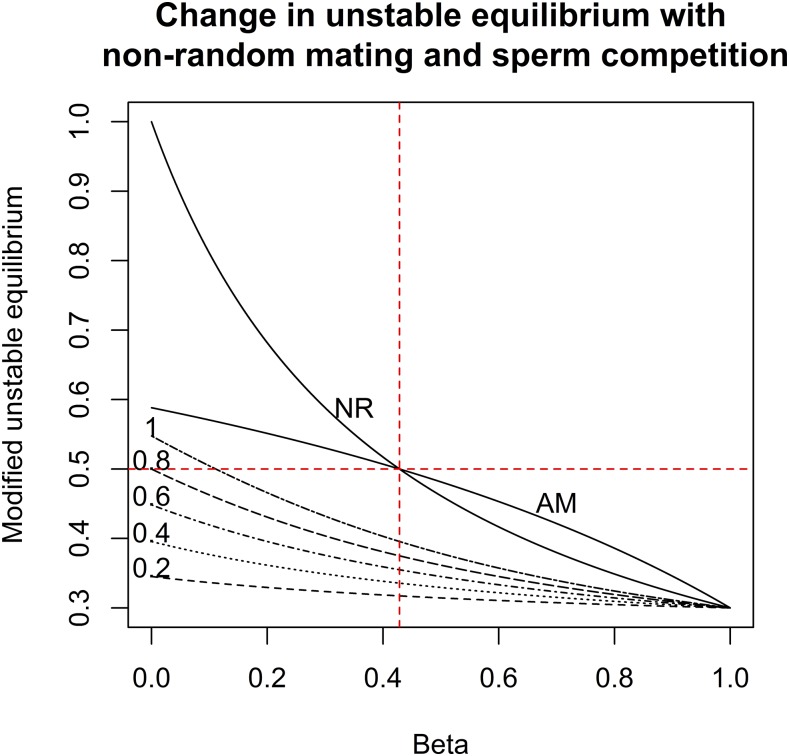
Figure 2.
Modifications to the unstable equilibrium under nonrandom mating due to difference in male fitness (NR), assortative mating (AM), and sperm competition (SC) (with remating frequency, pm, labeled on the graph). The estimate of unstable equilibrium when there is random mating and no SC was assumed to be 0.3, given by sf and sh of 0.3 and 1, respectively. β in general (except for AM) refers to relative fitness of infected to uninfected males (Aedes aegypti) under NR, βnr, or SC, βsc. NR occurs under the scenario when infected males are βnr times likely than uninfected males to mate with females (infected or uninfected). The lines labeled with values (just above the line) are under the SC only model for which the values equate to the remating frequency. Under the AM model (note that the line above the number 1 is part of the AM model), β is the relative frequency of mate pairing between nonidentical infection states, to mate pairing between identical infection states. At β = 0.4286 (vertical dotted line), both NR and AM models modify the unstable equilibrium to 0.5. This figure appears in color at www.ajtmh.org.
Say if sf and sh were 0.3 and 1, respectively, the unstable equilibrium under random mating and no SC is 0.3. If β was less than 0.5 (or more specifically < 0.4286) for both nonrandom mating scenarios (NR and AM), the unstable equilibrium would increase beyond 0.5. However, the effect of SC appears to be less than that of NR, as it depends on the remating rate (Figure 2, see Supplemental Material 7) and also on the number of male mates, n. We showed numerically that when remating frequency is 1 and n is large, the effect of SC will be similar to that of AM for the same β values (see Supplemental Material 8). Given it is biologically unforeseeable that a female could have very large numbers of mates especially if reproductive lifespan is short, it is safe to say that SC will never surpass the effect of nonrandom mating due to difference in male fitness, given the same relative fitness of infected to uninfected types. It is important to reiterate that this assumes equal contribution of male mates without any sperm exclusion strategy.
Effect on IIT.
It has been suggested that release ratios of sterile males to wild female numbers be in the magnitude of 1.7 up to 150 times46–49 to achieve suppression of the target insect. IIT in Aedes species is being based on a relative ratio of 5–20 (Zhiyong Xi, personal communication). As stated in the previous section, the effect of SC was likely lower than that of NR/AM; thus, we use the effect of NR on the release ratios as the upper bound which is a constant dependent only on β, as in equation (10).
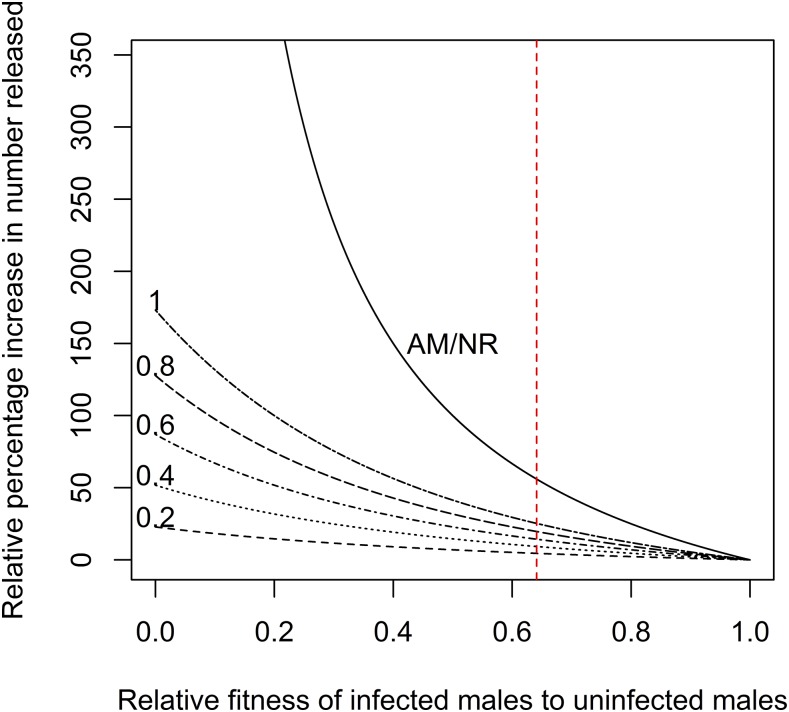
It was apparent that if β = 0.5, then the release ratio would need to be increased by at most 100% (i.e., twice the effort) (Figure 3). This meant that if the ratio of release was forecast to be 5:1 when AM/NR is not considered, then for β = 0.5, the ratio of release needs to be increased to 10:1 to achieve the same suppression effect. If a release ratio of 5:1 was necessary and the production line was able to tolerate an increase up to 20:1 (increase of 300%), then based on equation (10), the lowest possible β is 0.25.
Figure 3.
Percentage increase in number of infected male Aedes aegypti required to achieve the same suppression levels as when there is assortative mating or sperm competition (SC) effects, β = 1. Assortative mating/NR is the curve for the effects of nonrandom mating, whereas the other curves are for SC effects under varying remating frequencies (given on lines). This figure appears in color at www.ajtmh.org.
Linking models to empirical data.
Because it was not possible to make an empirical estimate of βsc, the value of β = 0.6410 ± 0.0907 represents the composite effect of nonrandom mating and SC. As we observed that the nonrandom mating due to difference in male fitness model reflected the greatest change to the effective infection frequency and also the unstable equilibrium (see also Supplemental Materials 7 and 8), we evaluated the results based on that model.
The unstable equilibrium frequency for the wMel infection in Ae. aegypti based on previously estimated fitness parameters was around 0.3.17 Assuming no maternal transmission leakage, the result in Figure 2 will be reflective of the wMel infection for all values of βnr between 0 and 1. The estimated value β should not lead to an unstable equilibrium that will exceed 0.5. In the IIT context, the recommended increase in production of wMel-infected males was 56.0% to achieve the same effect as the initial estimates when AM/NR was not considered (Figure 3, and equation [10]). If the maximum capacity of the production facility was also the same as the recommended release ratio when AM/NR was not considered, say 20 infected males to one wild uninfected male, we found that the extra time required to achieve the same level of suppression was only at most 17.9% longer (see Supplemental Material 9).
DISCUSSION
Here, we defined three separate contexts for nonrandom mating (difference in male fitness, AM, and SC). We used these contexts to estimate relative fitness of Wolbachia-infected males to uninfected males based on hatch rates of eggs from uninfected females that were exposed to different proportions of infected and uninfected males. We defined a separate framework for assessing SC based on the assumption of no sperm displacement. As this represents postmating isolation, it is dependent on the females mating multiple times. Disentangling the effects of nonrandom mating (premating effects) and SC required remating frequencies to be estimated along with the incidence of forced remating to estimate SC effects separately. However, it was impossible to estimate remating frequency using only offspring hatch rate data, which required the assumption of no AM. Also, forced remating may not be testable when cage environments restrict the ability of females to counter males by moving away. This led to estimates based on the composite effect of nonrandom mating and SC.
The empirical method we used to estimate the composite relative fitness of infected to uninfected males is identical to Fried’s competitive index50 or similarly the competitive index based on the relative sterility index (CRSI), which was proposed as a measure to evaluate sterile male quality. The Fried competitive index, I, is given by
| (14) |
where N and S are the number of non-sterile and sterile males, respectively, whereas HN, Ho, and HS are the average hatch rates of non-sterile crosses, observed hatch rate, and average hatch rate of eggs of sterile males crossed with wild females. When compared with the relative sterility index (RSI), which is also often used in sterile male evaluation, RSI can be computed by taking I/(I + 1). Values of RSI, and CRSI in the context of sterile tephritid fruit flies, are directly estimated from identifying capturing mating pairs. However, in the context of Ae. aegypti, studying mating couples is practically impossible because of short copula duration,27 whereas mating isolation studying egg hatch rate is actually well established in Ae. aegypti, and has been used in monitoring releases of Wolbachia-infected mosquitoes.17,51
When compared with equation (3), it should be apparent that N/S is the same as (1 − pI)/pI, and h and H are analogous to HN and HS, respectively. The composite effect should provide the most pessimistic view of the effect of nonrandom mating and SC because it is based on the nonrandom mating model which provides the worst-case scenario for the relative fitness of infected to uninfected males. In general, we were most interested in knowing if nonrandom mating causes the unstable equilibrium to exceed 0.5, as this was the conservative estimate at which the infection will not spatially spread.52 Both models were identical when determining the nonrandom mating parameter values (βnr and βam) at which the unstable equilibrium exceeds 0.5. However, in most other cases, both models affected the unstable equilibrium slightly differently. To fully disentangle the type of nonrandom mating, it was, therefore, necessary to study mating pairing probability for all combinations of infected/uninfected males and females.
In our scenario, we had a male-biased OSR in swarms which is thought to be the norm for mosquitoes,44 although Ae. aegypti exhibits variation in swarming behavior.53,54 Male-biased OSR and monandrous females may tend to select for more aggressive males that can encounter more virgin females and also select for female choice because females have only one chance to maximize their fitness. Laboratory experiments may not relate to field conditions well but offer a way of studying fitness across multiple treatments. In a confined space such as a cage, females may be unable to escape as easily from males as in the field, reducing the opportunity for mate choice. Also, less aggressive males are less likely to be disadvantageous compared with aggressive males in confined spaces. This means that inferences about relative fitness in the context of AM based on cage studies need to be made with caution. Results from larger tent enclosures considering the relative fitness of Wolbachia-infected males at one male density are consistent with those obtained from cages23 but it is not easy to scale-up such experiments with the type of replication required to establish frequency dependent patterns.
In this study, we have omitted Wolbachia maternal transmission leakage from the models, which is normally low for wMel even under field conditions45 except under hot conditions.19 The estimation of relative fitness of infected males to uninfected males does not require any knowledge of maternal transmission leakage. However, it does affect the estimation of the unstable equilibrium under the population replacement scenario. If we assume that relative fitness of males is independent of maternal transmission leakage, then transmission leakage of Wolbachia will lead to a higher unstable equilibrium, as in worst outcome for population replacement. Say if the lower relative fitness of infected males to uninfected males was due to a mitochondrial defect that hitchhiked with the Wolbachia infection, the resultant unstable equilibrium may decrease as lower fitness uninfected males are generated, but this is unlikely to be any worse than in the previous example for the Wolbachia infection. In terms of population suppression with infected males, maternal transmission leakage will not have any effect because there are no infected females, unless the females that gave rise to the infected males were reared under suboptimal conditions.
In the experiments, we showed that we needed to measure at least 175 females to detect βam < 0.5 with 90% power when rejection probability is < 0.05. Moreover, we formulated a linear model in the form of equation (2) to allow a large amount of replicated data with a spectrum of infection frequencies to be analyzed and avoid multiple comparison concerns when estimating β, increasing the power of detection. Because of logistics of observing hatch rates from a large number of individual females, pooling females into equal pool sizes was performed. This may be prone to bias contribution from females that were more fecund; for example, if more fecund females by chance mated to a particular infected male, then the pool hatch rate will likely become lower than expected. However, the total number of females tested (1,550 across all treatments and 75 for each control, i.e., 0% and 100% infected male) should overcome any stochastic effects of infection status of male mates (see Supplemental Material 3). It was necessary to ensure that uninfected female fecundity was not associated with infection status of the male mate (see Supplemental Material 1). Because hatch rates were determined at a later juvenile stage, we needed to ensure that the estimation of the hatch rate was not affected by larval competition (due to varying densities) and we did not detect confounding effects of larval density. However, in future experiments it may be possible to control larval density at an earlier instar stage by placing larvae in a larger volume of water.
In our case study using the wMel infection in Ae. aegypti, we found that there was a statistically significant disadvantage in the composite effect of AM and SC. However, the effect on the unstable equilibrium for population replacement programs and relative release ratios for IIT programs was relatively minor and unlikely to affect efficacy of those programs. In the context of population replacement programs, the unstable equilibrium did not exceed 0.5 when including the composite effect, which meant that spatial spread of the infection is unlikely to be affected.52 The increase in the production rate required to achieve the desired suppression effects is unlikely to tip the balance of the economic benefit from releases. Still, in concert with other fitness costs that were not considered in the evaluation of wMel infection, such as temperature-dependent mortality19 and larval performance under starvation conditions,18 any disadvantage for infected males can complicate release programs.
Although we did not find large effects of the infection on mating or SC in this study, it is possible that such effects might evolve over time. Evolutionary changes are unlikely in cases where the infection rapidly increases to a high frequency following releases because there will be little opportunity for evolutionary changes to occur before the entire population becomes infected. However, if there is a high degree of maternal transmission leakage, uninfected females that mate with uninfected males will have a massive fitness advantage over randomly mating uninfected females, creating a strong selection pressure for mate recognition (or SC). Strong selection pressures may also exist following releases when adjacent areas with high and low infection frequencies occur side by side because of dispersal barriers, as seen in areas of North Queensland following Wolbachia releases.55,56 For this reason, it may be prudent to monitor mating behavior across time.
CONCLUSION
We developed a way to evaluate the efficacy of Wolbachia infection for population replacement or IIT when hatch rate data are easy to obtain. In the context of population control, studying the composite effects of nonrandom mating and SC may be sufficient to assess the effects of mating isolation. In the context of population replacement or IIT using releases of both sexes, knowing male relative fitness might be sufficient to evaluate spatial spread capability of the infection. If possible, it is advisable to study mating isolation in both sexes (i.e., all possible crosses within one enclosure replicated many times). In the wMel infection, there was no strong evidence to suggest that the estimated reduction in efficacy of infected versus uninfected males would significantly impair population replacement and IIT programs.
Supplementary Material
Acknowledgment:
The authors thank Ashley G. Callahan, Kelly M. Richardson, Anjali Goundar, Perran A. Ross and Jason K. Axford for assisting significantly in the mating test bioassay.
Note: Supplemental materials appear at www.ajtmh.org.
REFERENCES
- 1.Hoffmann AA, Turelli M, 1997. Cytoplasmic incompatibility in insects. O’Neill S, Hoffmann A, Werren J, eds. Influential Passengers. Oxford, United Kingdom: Oxford University Press, 42–80. [Google Scholar]
- 2.Engelstädter J, Telschow A, 2009. Cytoplasmic incompatibility and host population structure. Heredity (Edinb) 103: 196–207. [DOI] [PubMed] [Google Scholar]
- 3.Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR, 2013. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog 9: e1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turelli M, Hoffmann AA, 1991. Rapid spread of an inherited incompatibility factor in California Drosophila. Nature 353: 440–442. [DOI] [PubMed] [Google Scholar]
- 5.Turelli M, 1994. Evolution of incompatibility-inducing microbes and their hosts. Evolution 48: 1500–1513. [DOI] [PubMed] [Google Scholar]
- 6.Vala F, Egas M, Breeuwer JAJ, Sabelis MW, 2004. Wolbachia affects oviposition and mating behaviour of its spider mite host. J Evol Biol 17: 692–700. [DOI] [PubMed] [Google Scholar]
- 7.Hoffmann AA, Turelli M, Harshman LG, 1990. Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics 126: 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Champion de Crespigny FE, Hurst LD, Wedell N, 2008. Do Wolbachia-associated incompatibilities promote polyandry? Evolution 62: 107–122. [DOI] [PubMed] [Google Scholar]
- 9.Bian GW, Xu Y, Lu P, Xie Y, Xi ZY, 2010. The endosymbiotic bacterium Wolbachia induces resistance to dengue virus in Aedes aegypti. PLoS Pathog 6: e1000833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Hurk AF, Hall-Mendelin S, Pyke AT, Frentiu FD, McElroy K, Day A, Higgs S, O’Neill SL, 2012. Impact of Wolbachia on infection with chikungunya and yellow fever viruses in the mosquito vector Aedes aegypti. PLoS Negl Trop Dis 6: e1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kambris Z, Cook PE, Phuc HK, Sinkins SP, 2009. Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science 326: 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moreira LA, et al. 2009. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139: 1268–1278. [DOI] [PubMed] [Google Scholar]
- 13.McMeniman CJ, Lane RV, Cass BN, Fong AWC, Sidhu M, Wang YF, O’Neill SL, 2009. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323: 141–144. [DOI] [PubMed] [Google Scholar]
- 14.Walker T, et al. 2011. The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature 476: 450–453. [DOI] [PubMed] [Google Scholar]
- 15.Yeap HL, et al. 2011. Dynamics of the “popcorn” Wolbachia infection in outbred Aedes aegypti informs prospects for mosquito vector control. Genetics 187: 583–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laven H, 1967. Eradication of Culex pipiens fatigans through cytoplasmic incompatibility. Nature 216: 383–384. [DOI] [PubMed] [Google Scholar]
- 17.Hoffmann AA, et al. 2011. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476: 454–457. [DOI] [PubMed] [Google Scholar]
- 18.Ross PA, Endersby NM, Hoffmann AA, 2016. Costs of three Wolbachia infections on the survival of Aedes aegypti larvae under starvation conditions. PLoS Negl Trop Dis 10: e0004320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross PA, Wiwatanaratanabutr I, Axford JK, White VL, Endersby-Harshman NM, Hoffmann AA, 2017. Wolbachia infections in Aedes aegypti differ markedly in their response to cyclical heat stress. PLoS Pathog 13: e1006006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turley AP, Zalucki MP, O’Neill SL, McGraw EA, 2013. Transinfected Wolbachia have minimal effects on male reproductive success in Aedes aegypti. Parasit Vectors 6: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeap HL, Axford JK, Popovici J, Endersby NM, Iturbe-Ormaetxe I, Ritchie SA, Hoffmann AA, 2014. Assessing quality of life-shortening Wolbachia-infected Aedes aegypti mosquitoes in the field based on capture rates and morphometric assessments. Parasit Vectors 7: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yeap HL, Rasic G, Endersby-Harshman NM, Lee SF, Arguni E, Le Nguyen H, Hoffmann AA, 2016. Mitochondrial DNA variants help monitor the dynamics of Wolbachia invasion into host populations. Heredity (Edinb) 116: 265–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segoli M, Hoffmann AA, Lloyd J, Omodei GJ, Ritchie SA, 2014. The effect of virus-blocking Wolbachia on male competitiveness of the dengue vector mosquito, Aedes aegypti. PLoS Negl Trop Dis 8: e3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Axford JK, Ross PA, Yeap HL, Callahan AG, Hoffmann AA, 2016. Fitness of wAlbB Wolbachia infection in Aedes aegypti: parameter estimates in an outcrossed background and potential for population invasion. Am J Trop Med Hyg 94: 507–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Craig GB, Jr., 1967. Mosquitoes: female monogamy induced by male accessory gland substance. Science 156: 1499–1501. [DOI] [PubMed] [Google Scholar]
- 26.Fuchs MS, Craig GB, Jr., Despommi DD, 1969. The protein nature of the substance inducing female monogamy in Aedes aegypti. J Insect Physiol 15: 701–709. [Google Scholar]
- 27.Jones JC, 1973. Are mosquitos monogamous? Nature 242: 343–344. [DOI] [PubMed] [Google Scholar]
- 28.Spielman A, Leahy MG, Skaff V, 1967. Seminal loss in repeatedly mated female Aedes aegypti. Biol Bull 132: 404–412. [Google Scholar]
- 29.Christophers SSR, 1960. Aedes aegypti (L.) the Yellow Fever Mosquito; Its Life History, Bionomics and Structure. New York, NY: Cambridge University Press. [Google Scholar]
- 30.Roth LM, 1948. A study of mosquito behavior—an experimental laboratory study of the sexual behavior of Aedes aegypti (Linnaeus). Am Midl Nat 40: 265–352. [Google Scholar]
- 31.Richardson JB, Jameson SB, Gloria-Soria A, Wesson DM, Powell J, 2015. Evidence of limited polyandry in a natural population of Aedes aegypti. Am J Trop Med Hyg 93: 189–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wade MJ, Chang NW, 1995. Increased male-fertility in Tribolium confusum beetles after infection with the intracellular parasite Wolbachia. Nature 373: 72–74. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann AA, Hercus M, Dagher H, 1998. Population dynamics of the Wolbachia infection causing cytoplasmic incompatibility in Drosophila melanogaster. Genetics 148: 221–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Crespigny FEC, Pitt TD, Wedell N, 2006. Increased male mating rate in Drosophila is associated with Wolbachia infection. J Evol Biol 19: 1964–1972. [DOI] [PubMed] [Google Scholar]
- 35.Ponlawat A, Harrington LC, 2009. Factors associated with male mating success of the dengue vector mosquito, Aedes aegypti. Am J Trop Med Hyg 80: 395–400. [PubMed] [Google Scholar]
- 36.Duhrkopf RE, Hartberg WK, 1992. Differences in male mating response and female flight sounds in Aedes aegypti and Ae. albopictus (Diptera, Culicidae). J Med Entomol 29: 796–801. [DOI] [PubMed] [Google Scholar]
- 37.Cator LJ, Arthur BJ, Harrington LC, Hoy RR, 2009. Harmonic convergence in the love songs of the dengue vector mosquito. Science 323: 1077–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cator LJ, Harrington LC, 2011. The harmonic convergence of fathers predicts the mating success of sons in Aedes aegypti. Anim Behav 82: 627–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ross PA, Endersby NM, Yeap HL, Hoffmann AA, 2014. Larval competition extends developmental time and decreases adult size of wMelPop Wolbachia-infected Aedes aegypti. Am J Trop Med Hyg 91: 198–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yeap HL, Endersby NM, Johnson PH, Ritchie SA, Hoffmann AA, 2013. Body size and wing shape measurements as quality indicators of Aedes aegypti mosquitoes destined for field release. Am J Trop Med Hyg 89: 78–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koenraadt CJ, 2008. Pupal dimensions as predictors of adult size in fitness studies of Aedes aegypti (Diptera: Culicidae). J Med Entomol 45: 331–336. [DOI] [PubMed] [Google Scholar]
- 42.Ritchie SA, Montgomery BL, Hoffmann AA, 2013. Novel estimates of Aedes aegypti (Diptera: Culicidae) population size and adult survival based on Wolbachia releases. J Med Entomol 50: 624–631. [DOI] [PubMed] [Google Scholar]
- 43.Ritchie SA, Pyke AT, Hall-Mendelin S, Day A, Mores CN, Christofferson RC, Gubler DJ, Bennett SN, van den Hurk AF, 2013. An explosive epidemic of DENV-3 in Cairns, Australia. PLoS One 8: e68137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.South A, Catteruccia F, 2016. Sexual selection and the evolution of mating systems in mosquitoes. Adv In Insect Phys 51: 67–92. [Google Scholar]
- 45.Schmidt T, Filipović IA, Hoffmann A, Rašić G, 2018. Fine-scale landscape genomics helps explain the slow spatial spread of Wolbachia through the Aedes aegypti population in Cairns, Australia. Heredity 120: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Atkinson MP, Su Z, Alphey N, Alphey LS, Coleman PG, Wein LM, 2007. Analyzing the control of mosquito-borne diseases by a dominant lethal genetic system. Proc Natl Acad Sci U S A 104: 9540–9545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alphey L, Benedict M, Bellini R, Clark GG, Dame DA, Service MW, Dobson SL, 2010. Sterile-insect methods for control of mosquito-borne diseases: an analysis. Vector Borne Zoonotic Dis 10: 295–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hendrichs J, Vreysen MJB, Enkerlin WR, Cayol JP, 2005. Strategic options in using sterile insects for area-wide integrated pest management. Dyck VA, Hendrichs J, Robinson AS, eds. Sterile Insect Technique: Principles and Practice in Area-Wide Integrated Pest Management. Dordrecht, The Netherlands: Springer Netherlands, 563–600. [Google Scholar]
- 49.Phuc HK, et al. 2007. Late-acting dominant lethal genetic systems and mosquito control. BMC Biol 5: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fried M, 1971. Determination of sterile-insect competitiveness. J Econ Entomol 64: 869. [Google Scholar]
- 51.Hoffmann AA, Iturbe-Ormaetxe I, Callahan AG, Phillips BL, Billington K, Axford JK, Montgomery B, Turley AP, O’Neill SL, 2014. Stability of the wMel Wolbachia infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 8: e3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Turelli M, 2010. Cytoplasmic incompatibility in populations with overlapping generations. Evolution 64: 232–241. [DOI] [PubMed] [Google Scholar]
- 53.Cabrera M, Jaffe K, 2007. An aggregation pheromone modulates lekking behavior in the vector mosquito Aedes aegypti (Diptera: Culicidae). J Am Mosq Control Assoc 23: 1–10. [DOI] [PubMed] [Google Scholar]
- 54.Oliva CF, Damiens D, Benedict MQ, 2014. Male reproductive biology of Aedes mosquitoes. Acta Trop 132 (Suppl): S12–S19. [DOI] [PubMed] [Google Scholar]
- 55.Schmidt TL, et al. 2017. Local introduction and heterogeneous spatial spread of dengue-suppressing Wolbachia through an urban population of Aedes aegypti. PLoS Biol 15: e2001894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Turelli M, Barton NH, 2017. Deploying dengue-suppressing Wolbachia: Robust models predict slow but effective spatial spread in Aedes aegypti. Theor Popul Biol 115: 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.