Abstract.
Flaviviruses such as Zika, dengue, and yellow fever cause epidemics throughout the tropics and account for substantial global morbidity and mortality. Although malaria and other vector-borne diseases have long been appreciated in Africa, flavivirus epidemiology is incompletely understood. Despite the existence of an effective vaccine, yellow fever continues to cause outbreaks and deaths, including at least 42 fatalities in the Democratic Republic of the Congo (DRC) in 2016. Here, we leveraged biospecimens collected as part of the nationally representative 2013–2014 Demographic and Health Survey in the DRC to examine serological evidence of flavivirus infection or vaccination in children aged 6 months to 5 years. Even in this young stratum of the Congolese population, we find evidence of infection by dengue and Zika viruses based on results from enzyme-linked immunosorbent assay and neutralization assay. Surprisingly, there was remarkable discordance between reported yellow fever vaccination status and results of serological assays. The estimated seroprevalences of neutralizing antibodies against each virus are yellow fever, 6.0% (95% confidence interval [CI] = 4.6–7.5%); dengue, 0.4% (0.1–0.9%); and Zika, 0.1% (0.0–0.5%). These results merit targeted, prospective studies to assess effectiveness of yellow fever vaccination programs, determine flavivirus seroprevalence across a broader age range, and investigate how these emerging diseases contribute to the burden of acute febrile illness in the DRC.
INTRODUCTION
Arthropod-borne flaviviruses cause hundreds of millions of infections in humans each year, with manifestations ranging from fever to birth defects, hemorrhage, shock, encephalitis, and even death. The public health importance of this group of pathogens is immense and growing. Dengue virus (DENV) has steadily expanded in both case number and geographical range over recent decades, leading to approximately 400 million infections annually.1 Zika virus (ZIKV) dramatically emerged in Latin America in 2015, becoming an international public health emergency because of the adverse fetal outcomes that can occur when ZIKV is transmitted from an infected mother to her developing fetus.2 Finally, yellow fever virus (YFV) has caused hundreds of epidemics dating back to at least the 17th century.3
Risk for transmission of all three of these viruses exists wherever competent mosquito vectors, predominantly Aedes aegypti, are found, and ZIKV can also be sexually transmitted.4 The epidemiology of DENV and ZIKV in Asia and the Americas is well described, but their epidemiology in Africa is incompletely understood. Although there have been several serosurveys for DENV and ZIKV in Africa, most of the studies are outdated and confounded by the serological cross-reactivity between flaviviruses.5,6 Infections in travelers returning from Africa make up many of the more recent reports of DENV in this region, hinting that prevalence in the African population is likely largely underestimated.6 Similarly, reports of exported ZIKV in travelers are common and these data are being leveraged to identify areas of endemicity, including in Africa.7
On the other hand, the threat posed by YFV in Africa is well recognized. A recent large outbreak in 2016 in Angola and surrounding countries demonstrated that YFV represents a constant hazard that can move quickly through susceptible populations.8 Furthermore, despite the existence of an effective vaccine since the 1930s, substantial barriers remain to successful implementation of this tool. Neglect of vaccination programs, exacerbated by increasing, disorganized urbanization in recent decades, has resulted in the resurgence of the disease.9,10
The Democratic Republic of the Congo (DRC) is a large, mostly rural country in central sub-Saharan Africa. Following decades without reports of YFV transmission in the DRC, the virus reemerged at the beginning of the 21st century, and at least 42 deaths were confirmed in the country in 2016 alone after the Angolan outbreak crossed country borders.11,12 These outbreaks occurred despite the YFV vaccine being recommended by the Expanded Program on Immunization since 2003.13
Because of the risk for YFV outbreaks in the DRC and sparse data on other flavivirus infections such as DENV and ZIKV, we conducted a seroepidemiological survey of these three flaviviruses. We tested dried blood spots (DBSs) collected from children during the 2013–2014 Demographic and Health Survey (DHS), a nation-wide, population representative survey, using both enzyme-linked immunosorbent assay (ELISA) and neutralization assays to detect past flavivirus exposure, analyzing the results in relation to geographical distribution and documented yellow fever vaccination history.
MATERIALS AND METHODS
Subjects and specimens.
The DHS program (Rockville, MD) employs household surveys and the collection of blood samples to amass extensive, country-wide health and population data, including information about disease prevalence, vaccination practices, nutrition, and beliefs and behaviors related to the spread of disease. All data and biospecimens are collected with informed consent under Institutional Review Board approval maintained by the Kinshasa School of Public Health. In this study, we accessed de-identified archived DBSs, which were collected between 2013 and 2014 during the second DHS in the DRC. Each DBS was spotted with approximately 45 μL of blood collected from participants in their homes during the survey.14 From 9,790 DBSs collected from children aged 6 months to 5 years in representative household clusters, 10% (978) were randomly selected for inclusion in the present study.
Elution of blood from DBSs.
Eluted sera and DBSs were stored at −80°C until use. Sera were stored in sealed 96-well plates and DBSs in sealed plastic bags with desiccant. For initial ELISAs, archived eluted sera were used. One 6-mm hole punch of each DBS was previously eluted in 1 mL phosphate-buffered saline, 0.05% w/v Tween 20%, and 5% w/v nonfat dried milk powder, with elution by shaking for 1 hour at room temperature.
For neutralization assays, DBSs were freshly eluted in Dulbecco’s modified Eagle medium (DMEM, Gibco, 11330-032) supplemented with 2% fetal bovine serum (Millipore, TM-013-B), 1% L-Glut (Gibco, 25030-081), 1% Anti-Anti (Gibco, 15240-062), and 1% non-essential amino acids solution (Gibco, 11140-50) at a concentration of 1:20 (one 6-mm hole punch of dried blood per 150 μL medium). Sera were eluted by rocking for 2 hours at 37°C and then centrifuged to remove the supernatant from the pelleted filter paper. The sera were heat inactivated for 30 minutes in a 56°C water bath. The samples were centrifuged again to pellet proteinaceous debris and only the supernatant was transferred to a final tube. The eluted sera were stored at 4°C and tested within 3 days.
Virus strains.
The following World Health Organization (WHO) reference strains of viruses were used: DENV1, West Pac 74; DENV2, S-16803; DENV3, CH-54389; DENV4, TVP-360; ZIKV, H/PF2013; and YFV, 17D.
ELISA.
As described previously, each of the serum samples was tested for flavivirus-binding antibodies in three separate immunoglobulin G (IgG) antigen-capture ELISAs to the following antigens: DENV serotypes 1–4 mix, ZIKV, and YFV.15 ELISA plates were coated overnight at 4°C with 100 ng of the mouse mAb 4G2 (ATCC, HB-112). Each well was washed with tris-buffered saline containing 0.2% v/v Tween and then blocked for 2 hours at 37°C with blocking buffer containing 3% w/v nonfat dry milk. Viral antigens were captured for 1 hour at 37°C and ELISA plates were washed. Fifty microliters of eluted sera were added in singlicate to the ELISA plate and incubated for 1 hour at 37°C. The plates were washed and alkaline phosphatase–conjugated goat antihuman IgG (A9544; Sigma, St. Louis, MO) was added and incubated for an additional hour at 37°C and then washed again before the addition of p-nitrophenyl phosphate substrate. Absorbance was measured at 405 nm on an Epoch microplate spectrophotometer. Readings were taken every 5 minutes for 25 minutes. A positive control (serum from an individual with known previous exposure to the virus) and normal human serum (NHS) negative control were included in duplicate on each plate. The last plate reading at which the difference between the positive control optical density (OD) and negative control OD was less than 1.5 (DENV and ZIKV) or less than 0.5 (YFV) was analyzed. Because the NHS control often exhibited higher background than many of the test samples, the background signal for each plate was defined as the average of the eight lowest ODs from the test samples on the plate. The threshold OD for positives was defined as the mean of the eight lowest ODs + 3 standard deviations + 0.1 (DENV and ZIKV) or + 0.05 (YFV).16,17 The correction factor of 0.1 was reduced to 0.05 for the YFV assay because the OD value exhibited by the positive control and the general distribution of OD values on each plate were consistently approximately 50% of those in the DENV assay.
Micro-neutralization assay.
A standard focus reduction neutralization test (FRNT) was modified to accommodate limited specimen availability.15 Briefly, four serial 3-fold dilutions were made of each serum sample and mixed with equal volume of virus at a concentration of ∼40–140 focus-forming units, incubated for 1 hour at 37°C, and then transferred to 96-well plates seeded with Vero-81 cells. After 1 hour, overlay medium was added and the infected cells were incubated for 40 hours (ZIKV), 48 hours (DENV2 and DENV4), or 51–52 hours (DENV1, DENV3, and YFV) at 37°C. The cells were fixed with 4% paraformaldehyde for 30 minutes and then permeabilization buffer was added for 10 minutes followed by blocking buffer (3% normal goat serum) and left overnight at 4°C. A mixture of primary antibodies 4G2 and 2H2 (ATCC, HB-114) were added to the plates and incubated for 1 hour at 37°C. The cells were washed followed by the addition of goat antimouse secondary antibody conjugated with horseradish peroxidase (Kirkegaard and Perry Laboratories, 074-1806) for 1 hour at 37°C. The plates were washed again and then foci were stained with TrueBlue (Kirkegaard and Perry Laboratories, 5510-0030) and counted. Two NHS controls were included on every plate to define 100% infection. Sera with > 50% neutralization at the first dilution point were further analyzed in Prism 7 (GraphPad, La Jolla, CA, www.graphpad.com). Focus reduction neutralization test 50 values (the serum dilution effecting a 50% reduction in virus infection) were determined by a nonlinear regression model using the sigmoidal dose response (variable slope) equation. Because of some nonspecific inhibition of infection by many ELISA-negative samples and negative controls, an FRNT50 < 100 was considered background and sera with FRNT50 > 100 were considered positive for neutralizing antibodies.
Data visualization and mapping.
Euler diagrams were constructed using EulerAPE v3.0 and Venn diagrams using BioVenn.18,19 Episheet was used to calculate exact confidence intervals (CIs).20 Samples were linked to their geographical location using the DRC 2013–2014 Global Positioning System dataset from the DHS, and ArcGIS 10.4 was used to map the data and examine spatial patterns.
RESULTS
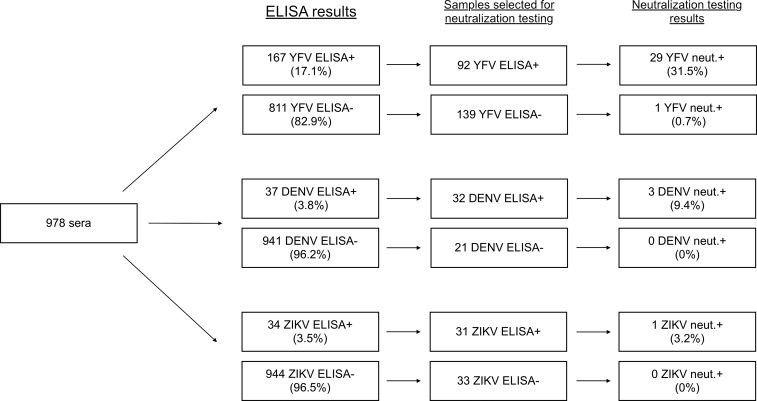
All 978 serum samples were tested by ELISA and a subset of ELISA-positive and ELISA-negative sera were tested by neutralization assay (stratified by virus, Figure 1; across all viruses tested, Supplemental Figure 1).
Figure 1.
Enzyme-linked immunosorbent assay (ELISA) and neutralization testing flowchart. Of the total study population of 978 samples, the breakdown by ELISA positivity, selection for neutralization testing, and neutralization results are shown. Percentages of positive samples out of the total number tested using each assay are shown in parentheses. Results are stratified by virus.
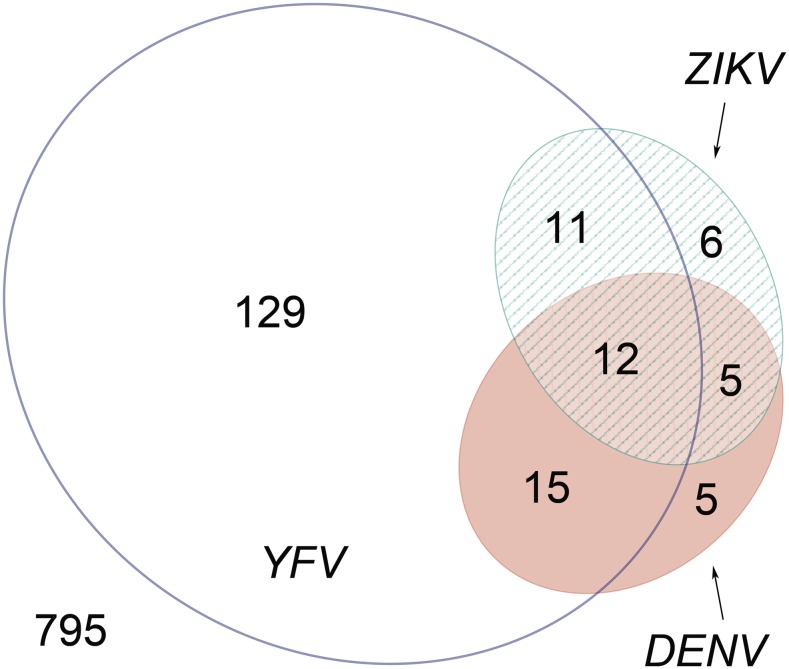
Of the 978 sera tested, 183 (18.7%) were positive for binding antibodies to one or more of the three flaviviruses tested. One hundred and sixty-seven (17.1%) reacted with YFV, 34 (3.5%) with ZIKV, and 37 (3.8%) with DENV. Of the ELISA-positive samples, 43 (23.5%) had antibodies that reacted with more than one of the viruses tested (Figure 2), which could be due to antibody cross-reactivity among flaviviruses or prior infection by or vaccination against more than one virus. The numbers (and percent of total ELISA-positive specimens) uniquely positive for YFV, DENV, or ZIKV were 129 (70.5%), five (2.7%), and six (3.3%), respectively.
Figure 2.
Distribution of sera with cross-reactive binding antibodies against dengue virus (DENV), Zika virus (ZIKV), and yellow fever virus (YFV). The number of sera positive by enzyme-linked immunosorbent assay (ELISA) for IgG against each virus is shown (DENV = pink, ZIKV = green, and YFV = transparent). Sera with reactive antibodies against two or three viruses are shown in the overlapping areas. The number of children negative by ELISA against all three viruses is shown in the bottom left. Each number refers to a distinct section, for example, 129 sera were uniquely positive for anti-YFV antibodies and 15 sera had antibodies against both YFV and DENV but not ZIKV. This figure appears in color at www.ajtmh.org.
To address the risk of false-positive ELISA results due to cross-reactive antibodies, we tested samples from a subset of subjects for the presence of neutralizing antibodies.21 Subjects were selected for neutralization testing based on sample availability, initial ELISA results, and YFV vaccine status, with the goal of testing both ELISA-positive and ELISA-negative and YFV-vaccinated and YFV-unvaccinated subjects. Of note, sera were not randomly selected for YFV neutralization testing; rather, sera with the most remaining sample and/or highest ELISA ODs were preferentially tested. Because sample volume was limiting, a modified FRNT approach was used (see Materials and Methods).22
Few samples were neutralization positive for ZIKV or DENV (Figure 1 and Table 1). Of 32 DENV ELISA-positive sera tested by neutralization assay, three (9.4%) were positive for neutralizing antibodies against DENV; of 21 DENV ELISA-negative sera tested, none were positive for neutralizing antibodies against DENV; of 31 ZIKV ELISA-positive sera tested, one (3.2%) was positive for neutralizing antibodies against ZIKV; and of 33 ZIKV ELISA-negative sera tested, none were positive for neutralizing antibodies against ZIKV (Figure 1).
Table 1.
Sera positive for DENV- or ZIKV-neutralizing antibodies
| Sample ID | ELISA positive | Neutralization positive | Neutralization negative |
|---|---|---|---|
| 223 | DENV, ZIKV, and YFV | DENV1, DENV2, and DENV3 | DENV4 |
| 2759 | DENV and ZIKV | DENV1 | DENV2–4, ZIKV, and YFV |
| 7787 | DENV, ZIKV, and YFV | DENV2 and YFV | DENV1, 3, and 4 and ZIKV |
| 4787 | ZIKV | ZIKV | YFV |
ELISA = enzyme-linked immunosorbent assay; DENV = dengue virus; YFV = yellow fever virus; ZIKV = Zika virus.
One of the specimens exhibited neutralization against DENV1, DENV2, and DENV3. This is likely due to two or more past DENV infections or cross-neutralizing antibodies from a recent primary infection.17 A second specimen neutralized DENV2 and YFV, and a third neutralized only DENV1. The ZIKV neutralization-positive specimen did not exhibit cross-neutralizing antibodies (Table 1).
More subjects were positive for YFV-neutralizing antibodies (Figure 1). Of 92 YFV ELISA-positive and 139 ELISA-negative sera tested by neutralization assay, 29 (31.5%) and one (0.7%) were positive for neutralizing antibodies against YFV, respectively. The single YFV ELISA-negative subject with detectable neutralizing antibodies reported a history of YFV vaccination.
Based on the number of subjects with neutralizing antibodies in each subset of ELISA-positive and ELISA-negative sera tested for each virus, we can approximate the seroprevalences in the whole cohort to be as follows: YFV, 6.0% (95% CI = 4.6–7.5%); DENV, 0.4% (0.1–0.9%); and ZIKV, 0.1% (0.0–0.5%).
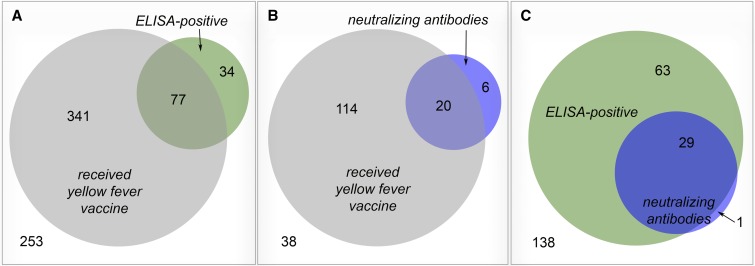
Overall, YFV ELISA and neutralization results correlated poorly with reported vaccination history (Figure 3). Among the 705 subjects with known vaccination history, 418 reported receiving YFV vaccination but only 77 of these (18.4%) were positive by ELISA (Figure 3A). In the subset of 178 sera from children with known vaccination history that also underwent neutralization testing, 134 received vaccination, but only 20 of these (14.9%) had neutralizing antibodies (Figure 3B). Similar proportions of YFV-unvaccinated individuals were found to be ELISA positive (34 of 287, 11.8%; Figure 3A) and have neutralizing antibodies (6 of 44, 13.6%; Figure 3B). Of note, 29 of 30 (96.7%) of specimens testing positive for YFV neutralization antibodies were also ELISA positive, which lends credence to the laboratory methods used here (Figure 3C).
Figure 3.
Overlap between yellow fever virus enzyme-linked immunosorbent assay (ELISA) results, neutralization assay results, and reported vaccination status. The number of children with each condition or combination of conditions is shown in the corresponding section (reported yellow fever vaccination = gray, ELISA positivity = green, and neutralizing antibodies = blue). The number of children negative for all conditions is shown in the bottom left of each panel. (A) Yellow fever vaccination history vs. ELISA positivity and includes all children with known vaccination history. (B) Yellow fever vaccination history vs. neutralization testing results and includes all children with both known vaccination history and neutralization testing results. (C) ELISA positivity vs. neutralization assay results and includes all children with neutralization testing results. This figure appears in color at www.ajtmh.org.
To confirm the discordance between reported vaccination and YFV serology results, we compared the FRNT50 values of 110 sera tested by neutralization assay from children who either had vaccine cards that confirmed YFV vaccine administration or reported not receiving the vaccine. This excludes children whose mothers affirmed YFV vaccination history status but lacked vaccine cards so as to minimize the impact of recall bias on our findings. There was no difference in the distributions of FRNT50 values between the two groups (Mann–Whitney test, P = 0.3107; Supplemental Figure 2).
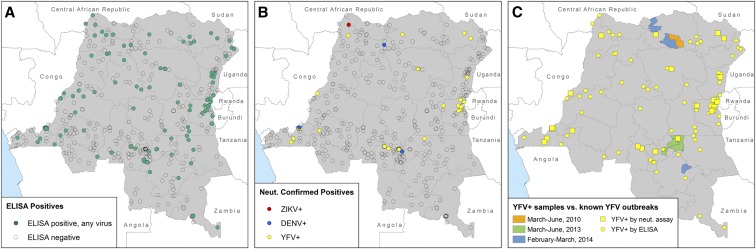
Results from both the ELISA and neutralization assay were spatially mapped by DHS sampling location to visualize the geographical distribution of flavivirus seropositivity across the DRC. There was no spatial clustering of ELISA-positive samples (Figure 4A). Samples with neutralizing antibodies against any of the three flaviviruses were observed more frequently near the border regions of the country (Figure 4B), although there was no significant spatial clustering according to the Moran’s I test of spatial autocorrelation. In addition, the locations of children who tested positive for yellow fever binding or neutralizing antibodies were compared with the locations of yellow fever outbreaks reported by WHO in the 5 years preceding the DHS (Figure 4C).23 There was no clear spatial relationship between yellow fever–positive samples and known yellow fever outbreaks; however, clusters of children with neutralizing antibodies against yellow fever are seen along the border with Rwanda and near the capital city of Kinshasa. These clusters could be sites of undetected outbreaks, represent areas with higher vaccine coverage, or higher seroconversion following vaccination.
Figure 4.
Geographical distribution of samples and serology testing results. Each point on the map represents a Demographic and Health Survey sampling cluster; clusters with no children testing positive are transparent. (A) Sampling clusters with children positive by enzyme-linked immunosorbent assay (ELISA) for antibodies against dengue virus (DENV), Zika virus (ZIKV), and/or yellow fever virus (YFV). (B) Sampling clusters with children positive for neutralizing antibodies against each of the three viruses. (C) Sampling clusters with children positive for antibodies against YFV by ELISA and neutralization assay overlaid on locations of known YFV outbreaks as reported by the World Health Organization.61 This figure appears in color at www.ajtmh.org.
DISCUSSION
This study provides evidence that flaviviruses, including DENV, ZIKV, and YFV are circulating in the DRC and suggests a lack of seroconversion in many Congolese children with histories of vaccination against YFV. To our knowledge, this study presents the first evidence of ZIKV in the DRC. Neutralizing antibodies against ZIKV were only reported in a single sample, and this sample was not tested by neutralization assay against YFV and DENV (although it failed to react to these viruses when tested by ELISA); therefore, this finding remains inconclusive and should be confirmed in future studies. Our data confirm previous reports that DENV is present in the DRC at a prevalence < 1% in children younger than 5 years. The prevalence of malaria parasites reported in the same age range is around 38%, suggesting that malaria continues to be a more common cause of febrile illness in the DRC.24
The detection of antibodies against ZIKV is not surprising, given that the virus was originally isolated in Uganda and has been reported in many countries bordering the DRC.5 Dengue virus was known to have a presence in the DRC based on sporadic detection of virus or antibodies in the Congolese population, returning travelers, and wild game animals.6,25,26 Our data further indicate that DENV serotypes 1 and 2 are co-circulating and suggest the virus is not localized to any one area of the country. In dengue-hyperendemic areas of Asia and Latin America, seroprevalence in children younger than 5 years ranges from 34% to 60%, and well over half of children have been exposed by age 10.16,27–29 Although few data are available for ZIKV seroprevalence in other countries in this age group, we can reasonably assume that children living in urban areas of Latin America and Asia where ZIKV has recently emerged have had ample opportunity for infection. Seroprevalence among schoolchildren aged 6–16 years was 66% in an area of French Polynesia in 2014–2015.30 In Bolivia, where the virus arrived as recently as 2016, seroprevalence in adults in some regions already reaches around 20–40%.31 We hypothesize that DENV and ZIKV infections in the DRC are likely sporadic spillover events, a distinct ecology from the urban cycle that drives high transmission of these viruses in other countries. If so, children would have little opportunity for exposure, but seroprevalence may be significantly higher in other population subgroups (e.g., men working near heavily forested areas who are at risk of exposure to the sylvatic cycle). A better understanding of flavivirus epidemiology in countries such as the DRC is needed to optimize surveillance, identify populations most at risk, and improve diagnosis and treatment of non-malarial febrile illness. More generally, the myriad etiologies of acute febrile illness in developing countries is increasingly appreciated, and enhanced detection of flaviviruses will ideally be but one component of a greater effort to improve diagnostic laboratory capacity.32
For all three flaviviruses tested, there were a large number of sera positive by ELISA but negative by neutralization assay. This raises the possibility that other closely related flaviviruses are present in the DRC. A recent study detected flavivirus nucleic acids, but not DENV or YFV, in mosquitoes in Kinshasa.33 At least 10 other flaviviruses have been reported in Africa.34 This combined with the results reported here suggests that the diversity of flaviviruses circulating in the DRC may be underappreciated. In addition, infection with malaria can also cause false-positive serologic test results for a number of pathogens as recently reiterated in reports of false-positive ZIKV testing with malaria infection.35,36
Maternal antibodies in the sera of the youngest subjects in this study may explain a small proportion of the positive ELISA and neutralization results. Anti-DENV maternal antibodies begin to decay substantially by 6 months of age and completely disappear in most children by 12 months.37–39 Previous studies have reported that maternal antibodies fall to subneutralizing levels during their decay and potentially become disease enhancing.40,41 The presence of binding, non-neutralizing maternal antibodies may explain some of the lack of correlation between the ELISA and neutralization results reported here.
A striking finding of our study was the discovery that most children reported to have received the YFV vaccine failed to show evidence of seroconversion. This finding held true even when only children with vaccine cards were included in the analysis (i.e., excluding children whose vaccine history is only verbally reported by their mothers without a vaccine card), making misreporting an unlikely explanation. This phenomenon is worrisome and without precedent in the literature. The YFV vaccine is highly immunogenic, and both the WHO and the U.S. Centers for Disease Control and Prevention recently concluded that a single dose of the vaccine is sufficient for lifelong immunity in most populations, although this statement is not universally accepted.42–48 There is some evidence that children are less likely to seroconvert than adults; however, the rates of seroconversion in children are nevertheless consistently reported to be above 80%.44,49–51
Duration of immunity in children receiving the YFV vaccine must also be considered in addition to early convalescent seroconversion; but unfortunately, data to that point are notably deficient in the literature over the past half century. Moreover, it has been suggested that a proportion of children lose seropositivity following vaccination, which could be consistent with our findings.42,45,49 However, the very low seroconversion rate observed in the present study may not be entirely attributable to the young age of vaccinees. A second possible explanation is that coadministration of the YFV vaccine with another live-virus vaccine is contributing to lower seroconversion. Several studies have investigated decreased immunogenicity of the YFV vaccine because of interference by coadministration of the measles vaccine, with conflicting results.52–55 Notably, the vast majority of children in the present study who had dated vaccination cards received the YFV vaccine on the same day as the measles vaccine.
The high prevalence of malnutrition in children in the DRC could also be contributing to impaired immune responses to the vaccine. In the 2013–2014 DHS, 43% of children surveyed were chronically malnourished.56 Malnutrition has been reported to variably diminish antibody production following a number of vaccinations, and studies in the 1960s specifically detected an impaired immune response to the YFV vaccine in children with protein malnutrition.57–59
It is also possible that the sensitivity of the ELISA and neutralization assays performed in this study were insufficient to detect low levels of antibodies that are sufficient for protection, although the vaccine is generally thought to evoke high neutralizing antibody titers, well over the limits of detection of the assays used in the present study.60 It should be noted that there is no established correlate of protection against yellow fever in humans; therefore, neither an ELISA nor a neutralization assay can definitively indicate who is and is not immune to disease caused by yellow fever infection following vaccination.42
The fact that the DRC continues to experience major yellow fever outbreaks indicates that a substantial portion of the Congolese population remains susceptible to infection despite renewed efforts to integrate the YFV vaccine into routine childhood vaccination programs.12 The issue with the vaccine may be as much programmatic as biological, that is, due to difficulties in accessing remote areas, poor surveillance, and/or suboptimal transport and storage of the vaccine. Prospective studies to evaluate seroconversion following vaccination and monitor for vaccine failures could bolster public health initiatives to protect the Congolese population from yellow fever.
Strengths of this study include the use of specimens from a nationally representative survey, which allowed for the estimation of country-wide prevalence and access to geographic location and vaccine history linked to each sample. The study was limited by sample availability, which only allowed for testing samples in singlicate and prohibited neutralization testing of some ELISA-positive sera; selection bias in which sera were tested by neutralization assay; recall bias in reporting vaccination history; testing of a single age group; and potentially low sensitivity of the YFV ELISA assay based on modest OD values exhibited by the positive controls.
Overall, the results presented here suggest that flavivirus epidemiology in the DRC and throughout Africa warrants further study. Additional research is required to corroborate and investigate the reasons for the low rates of seroconversion observed in vaccinated Congolese children. Moreover, future surveillance undertakings should investigate flavivirus seroprevalence across a larger population of all ages. Distinct studies should explore flavivirus infection as an etiology of acute febrile illness, especially in patients who test negative for malaria. These combined activities will characterize the health burden of flavivirus infections in the DRC and improve standard practices for their diagnosis and treatment. This type of study would also facilitate the isolation, sequencing, and phylotyping of wild-type virus, which would improve knowledge of the history and evolution of these viruses on the African continent. Ultimately, a better understanding of global flavivirus epidemiology will also help to identify populations at highest risk for these pathogens with epidemic potential and provide early opportunities for their detection and prevention.
Supplementary Material
Acknowledgments:
We thank the participants in the 2013-14 DRC DHS, without whom this work would not have been possible. We also thank Lauren Levitz and Stephanie Doctor for their help with sample preparation and Alice Liou for advice on laboratory methods.
Note: Supplemental figures appear at www.ajtmh.org.
REFERENCES
- 1.Guzman MG, Harris E, 2015. Dengue. Lancet 385: 453–465. [DOI] [PubMed] [Google Scholar]
- 2.Krauer F, Riesen M, Reveiz L, Oladapo OT, Martínez-Vega R, Porgo TV, Haefliger A, Broutet NJ, Low N; WHO Zika Causality Working Group , 2017. Zika virus infection as a cause of congenital brain abnormalities and Guillain–Barré Syndrome: systematic review. PLoS Med 14: e1002203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brès PL, 1986. A century of progress in combating yellow fever. Bull World Health Organ 64: 775–786. [PMC free article] [PubMed] [Google Scholar]
- 4.Hills SL, Russell K, Hennessey M, Williams C, Oster AM, Fischer M, Mead P, 2016. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 65: 215–216. [DOI] [PubMed] [Google Scholar]
- 5.Musso D, Gubler DJ, 2016. Zika virus. Clin Microbiol Rev 29: 487–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amarasinghe A, Kuritsk JN, Letson GW, Margolis HS, 2011. Dengue virus infection in Africa. Emerg Infect Dis 17: 1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leder K, et al. 2017. Zika beyond the Americas: travelers as sentinels of Zika virus transmission. A GeoSentinel analysis, 2012 to 2016. PLoS One 12: e0185689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization , 2016. Yellow Fever Situation Report Available at: http://www.who.int/emergencies/yellow-fever/situation-reports/30-september-2016/en/. Accessed December 1, 2017.
- 9.Robertson SE, Hull BP, Tomori O, Bele O, LeDuc JW, Esteves K, 1996. Yellow fever: a decade of reemergence. JAMA 276: 1157–1162. [PubMed] [Google Scholar]
- 10.Gardner CL, Ryman KD, 2010. Yellow fever: a reemerging threat. Clin Lab Med 30: 237–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization , 2017. WHO Vaccine-Preventable Diseases: Monitoring System. 2017 Global Summary Available at: http://apps.who.int/immunization_monitoring/globalsummary. Accessed December 1, 2017.
- 12.Otshudiema JO, et al. 2017. Yellow fever outbreak—Kongo Central Province, Democratic Republic of the Congo, August 2016. MMWR Morb Mortal Wkly Rep 66: 335–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.World Health Organization , 2010. Yellow Fever in the Democratic Republic of the Congo Available at: http://www.who.int/csr/don/2010_07_19a/en/. Accessed December 1, 2017.
- 14.ICF International , 2012. MEASURE DHS Biomarker Field Manual. Calverton, MD: ICF International. [Google Scholar]
- 15.Collins MH, McGowan E, Jadi R, Young E, Lopez CA, Baric RS, Lazear HM, de Silva AM, 2017. Lack of durable cross-neutralizing antibodies against Zika virus from dengue virus infection. Emerg Infect Dis 23: 773–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tissera H, Amarasinghe A, De Silva AD, Kariyawasam P, Corbett KS, Katzelnick L, Tam C, Letson GW, Margolis HS, de Silva AM, 2014. Burden of dengue infection and disease in a pediatric cohort in urban Sri Lanka. Am J Trop Med Hyg 91: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Corbett KS, Katzelnick L, Tissera H, Amerasinghe A, De Silva AD, de Silva AM, 2015. Preexisting neutralizing antibody responses distinguish clinically inapparent and apparent dengue virus infections in a Sri Lankan pediatric cohort. J Infect Dis 211: 590–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Micallef L, Rodgers P, 2014. eulerAPE: drawing area-proportional 3-Venn diagrams using ellipses. PLoS One 9: e101717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hulsen T, de Vlieg J, Alkema W, 2008. BioVenn—a web application for the comparison and visualization of biological lists using area-proportional Venn diagrams. BMC Genomics 9: 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rothman KJ, 2015. Episheet: Spreadsheets for the Analysis of Epidemiologic Data Available at: http://www.krothman.org/episheet.xls. Accessed January 21, 2018.
- 21.Roehrig JT, Hombach J, Barrett AD, 2008. Guidelines for plaque-reduction neutralization testing of human antibodies to dengue viruses. Viral Immunol 21: 123–132. [DOI] [PubMed] [Google Scholar]
- 22.Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS, 2016. Dengue virus envelope dimer epitope monoclonal antibodies isolated from dengue patients are protective against Zika virus. MBio 7: e01123–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.World Health Organization , 2014. Disease Outbreak News Archive, Yellow Fever Available at: http://www.who.int/csr/don/archive/disease/yellow_fever/en/. Accessed July 1, 2017.
- 24.Doctor SM, et al. 2016. Malaria surveillance in the Democratic Republic of the Congo: comparison of microscopy, PCR, and rapid diagnostic test. Diagn Microbiol Infect Dis 85: 16–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nur YA, Groen J, Heuvelmans H, Tuynman W, Copra C, Osterhaus AD, 1999. An outbreak of West Nile fever among migrants in Kisangani, Democratic Republic of Congo. Am J Trop Med Hyg 61: 885–888. [DOI] [PubMed] [Google Scholar]
- 26.Kading RC, Borland EM, Cranfield M, Powers AM, 2013. Prevalence of antibodies to alphaviruses and flaviviruses in free-ranging game animals and nonhuman primates in the greater Congo Basin. J Wildl Dis 49: 587–599. [DOI] [PubMed] [Google Scholar]
- 27.Braga C, Luna CF, Martelli CM, de Souza WV, Cordeiro MT, Alexander N, de Albuquerque Mde F, Júnior JC, Marques ET, 2010. Seroprevalence and risk factors for dengue infection in socio-economically distinct areas of Recife, Brazil. Acta Trop 113: 234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amaya-Larios IY, Martínez-Vega RA, Mayer SV, Galeana-Hernández M, Comas-García A, Sepúlveda-Salinas KJ, Falcón-Lezama JA, Vasilakis N, Ramos-Castañeda J, 2014. Seroprevalence of neutralizing antibodies against dengue virus in two localities in the state of Morelos, Mexico. Am J Trop Med Hyg 91: 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prayitno A, et al. 2017. Dengue seroprevalence and force of primary infection in a representative population of urban dwelling Indonesian children. PLoS Negl Trop Dis 11: e0005621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aubry M, et al. 2017. Zika virus seroprevalence, French Polynesia, 2014–2015. Emerg Infect Dis 23: 669–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saba Villarroel PM, et al. 2018. Zika virus epidemiology in Bolivia: a seroprevalence study in volunteer blood donors. PLoS Negl Trop Dis 12: e0006239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Prasad N, Murdoch DR, Reyburn H, Crump JA, 2015. Etiology of severe febrile illness in low- and middle-income countries: a systematic review. PLoS One 10: e0127962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mbanzulu KM, Wumba R, Mukendi JK, Zanga JK, Shija F, Bobanga TL, Aloni MN, Misinzo G, 2017. Mosquito-borne viruses circulating in Kinshasa, Democratic Republic of the Congo. Int J Infect Dis 57: 32–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braack L, Gouveia De Almeida AP, Cornel AJ, Swanepoel R, de Jager C, 2018. Mosquito-borne arboviruses of African origin: review of key viruses and vectors. Parasit Vectors 11: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schwarz NG, et al. 2017. No serological evidence for zika virus infection and low specificity for anti-Zika virus ELISA in malaria positive individuals among pregnant women from Madagascar in 2010. PLoS One 12: e0176708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Esbroeck M, Meersman K, Michiels J, Ariën K, Van den Bossche D, 2016. Letter to the editor: specificity of Zika virus ELISA: interference with malaria. Euro Surveill 21: pii=30237. [DOI] [PubMed] [Google Scholar]
- 37.Watanaveeradej V, Endy TP, Samakoses R, Kerdpanich A, Simasathien S, Polprasert N, Aree C, Vaughn DW, Ho C, Nisalak A, 2003. Transplacentally transferred maternal-infant antibodies to dengue virus. Am J Trop Med Hyg 69: 123–128. [PubMed] [Google Scholar]
- 38.Pengsaa K, et al. 2006. Dengue virus infections in the first 2 years of life and the kinetics of transplacentally transferred dengue neutralizing antibodies in Thai children. J Infect Dis 194: 1570–1576. [DOI] [PubMed] [Google Scholar]
- 39.van Panhuis WG, Luxemburger C, Pengsaa K, Limkittikul K, Sabchareon A, Lang J, Durbin AP, Cummings DA, 2011. Decay and persistence of maternal dengue antibodies among infants in Bangkok. Am J Trop Med Hyg 85: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halstead SB, Lan NT, Myint TT, Shwe TN, Nisalak A, Kalyanarooj S, Nimmannitya S, Soegijanto S, Vaughn DW, Endy TP, 2002. Dengue hemorrhagic fever in infants: research opportunities ignored. Emerg Infect Dis 8: 1474–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee PX, Ong LC, Libau EA, Alonso S, 2016. Relative contribution of dengue IgG antibodies acquired during gestation or breastfeeding in mediating dengue disease enhancement and protection in type I interferon receptor-deficient mice. PLoS Negl Trop Dis 10: e0004805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gotuzzo E, Yactayo S, Córdova E, 2013. Review article: efficacy and duration of immunity after yellow fever vaccination: systematic review on the need for a booster every 10 years. Am J Trop Med Hyg 89: 434–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.World Health Organization , 2013. Meeting of the strategic advisory group of experts on immunization, April 2013—conclusions and recommendations. Wkly Epidemiol Rec 88: 201–206. [PubMed] [Google Scholar]
- 44.Staples JE, Bocchini JA, Jr., Rubin L, Fischer M, 2015. Yellow fever vaccine booster doses: recommendations of the advisory committee on immunization practices, 2015. MMWR Morb Mortal Wkly Rep 64: 647–650. [PMC free article] [PubMed] [Google Scholar]
- 45.Amanna IJ, Slifka MK, 2016. Questions regarding the safety and duration of immunity following live yellow fever vaccination. Expert Rev Vaccines 15: 1519–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grobusch MP, Goorhuis A, Wieten RW, Verberk JD, Jonker EF, van Genderen PJ, Visser LG, 2013. Yellow fever revaccination guidelines change—a decision too feverish? Clin Microbiol Infect 19: 885–886. [DOI] [PubMed] [Google Scholar]
- 47.Collaborative Group for Studies on Yellow Fever Vaccines , 2014. Duration of post-vaccination immunity against yellow fever in adults. Vaccine 32: 4977–4984. [DOI] [PubMed] [Google Scholar]
- 48.Patel D, Simons H, 2013. Yellow fever vaccination: is one dose always enough? Travel Med Infect Dis 11: 266–273. [DOI] [PubMed] [Google Scholar]
- 49.Belmusto-Worn VE, et al. 2005. Randomized, double-blind, phase III. Pivotal field trial of the comparative immunogenicity, safety, and tolerability of two yellow fever 17D vaccines (ARILVAX™ and YF-VAX®) in healthy infants and children in Peru. Am J Trop Med Hyg 72: 189–197. [PubMed] [Google Scholar]
- 50.Osei-Kwasi M, Dunyo SK, Koram KA, Afari EA, Odoom JK, Nkrumah FK, 2001. Antibody response to 17D yellow fever vaccine in Ghanaian infants. Bull World Health Organ 79: 1056–1059. [PMC free article] [PubMed] [Google Scholar]
- 51.Collaborative Group for Studies of Yellow Fever Vaccine , 2015. A randomised double-blind clinical trial of two yellow fever vaccines prepared with substrains 17DD and 17D-213/77 in children nine-23 months old. Mem Inst Oswaldo Cruz 110: 771–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nascimento Silva JR, Camacho LA, Siqueira MM, Freire Mde S, Castro YP, Maia Mde L, Yamamura AM, Martins RM, Leal Mde L; Collaborative Group for the Study of Yellow Fever Vaccines , 2011. Mutual interference on the immune response to yellow fever vaccine and a combined vaccine against measles, mumps and rubella. Vaccine 29: 6327–6334. [DOI] [PubMed] [Google Scholar]
- 53.Lhuillier M, Mazzariol MJ, Zadi S, Le Cam N, Bentejac MC, Adamowicz L, Marie FN, Fritzell B, 1989. Study of combined vaccination against yellow fever and measles in infants from six to nine months. J Biol Stand 17: 9–15. [DOI] [PubMed] [Google Scholar]
- 54.Stefano I, et al. 1999. Recent immunization against measles does not interfere with the sero-response to yellow fever vaccine. Vaccine 17: 1042–1046. [DOI] [PubMed] [Google Scholar]
- 55.Michel R, et al. 2015. Observational study on immune response to yellow fever and measles vaccines in 9 to 15-month old children. Is it necessary to wait 4 weeks between two live attenuated vaccines? Vaccine 33: 2301–2306. [DOI] [PubMed] [Google Scholar]
- 56.Ministère du Plan et Suivi de la Mise en oeuvre de la Révolution de la Modernité (MPSMRM), Ministère de la Santé Publique (MSP), ICF International , 2014. Enquête Démographique et de Santé en République Démocratique du Congo 2013–2014. Rockville, MD: MPSMRM, MSP and ICF International.
- 57.Rytter MJ, Kolte L, Briend A, Friis H, Christensen VB, 2014. The immune system in children with malnutrition—a systematic review. PLoS One 9: e105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown RE, Katz M, 1966. Failure of antibody production to yellow fever vaccine in children with Kwashiorkor. Trop Geogr Med 18: 125–128. [PubMed] [Google Scholar]
- 59.Anonymous , 1967. Effects of malnutrition on smallpox and yellow fever vaccination. Nutr Rev 25: 108–110. [DOI] [PubMed] [Google Scholar]
- 60.Monath TP, Vasconcelos PF, 2015. Yellow fever. J Clin Virol 64: 160–173. [DOI] [PubMed] [Google Scholar]
- 61.World Health Organization , 2014. Disease Outbreak News Archive, Yellow Fever Available at: http://www.who.int/csr/don/archive/disease/yellow_fever/en/. Accessed July 1, 2017.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.