Abstract
Kedrostis africana, is a tuberous plant commonly used by traditional healers in the Eastern Cape Province of South Africa for the management of obesity. The aim of this study was to investigate the antiobesity and cytotoxic effects of Kedrostis africana extracts in vitro The α-amylase, α-glucosidase and lipase inhibitory activities of aqueous and ethanol extracts of Kedrostis africana tuber were investigated while the cytotoxic effects of these extracts were analyzed using Hoechst 33342 and propidium iodide (PI) dual staining in combination with Molecular Devices ImageXpress Micro XLS Widefield microscope for high content analysis on human cervical (HeLa) cell line. The ethanol extract exhibited the strongest inhibitory effect on pancreatic lipase (IC50 = 381.86 μg/ml) and on α-glucosidase (IC50 = 157.99 μg/mL) while the aqueous extract has strongest α-amylase (IC50 = 439.45 μg/ml). Both tuber extracts were found nontoxic at tested concentrations on HeLa cell lines as confirmed by the Hoechst 33342 and propidium iodide dual staining respectively. This study revealed that both the aqueous and ethanol tuber extract of K. africana exerts a certain degree of inhibitory effect on α-amylase, α-glucosidase and lipase and were also nontoxic to HeLa cell line at tested concentrations.
Keywords: Biochemistry, Biotechnology
1. Introduction
Obesity is a metabolic disorder which results from excessive accumulation of body fat, and it is associated with a myriad of comorbidities, such as the onset of cardiovascular diseases (especially heart diseases and stroke), that caused most death in 2012; diverse types of cancer (colon, kidney, liver, breast, pancreatic, ovarian, prostate, and endometrial cancers); osteoarthritis, hypertension and type 2 diabetes mellitus are responsible for most deaths (Wanderley and Ferreira, 2010; Kitahara et al., 2014; Gallagher and LeRoith, 2015; WHO, 2015).
Within the space of 1980 and 2014, the world's obesity prevalence has increased yearly. According to data from the World Health Organization report of 2014, more than 1.9 billion adults were overweight and, among them, more than 600 million were obese (WHO, 2015). The solution to obesity epidemic requires a multifaceted approach. Some obese people have difficulty losing weight through diet and exercise alone. Therefore the use of medications is employed, they control body weight by restricting energy absorption and cause weight loss (Boniglia et al., 2008). However, effect is noticed after a long while (Kaukua et al., 2003) and their weight are regained when treatment is discontinued. Also, some of these anti-obesity drugs approved and marketed have now been withdrawn due to adverse effects such as abdominal pain, bloating, flatulence, oily stools, diarrhea, and decreasing in fat soluble vitamins absorption (Kang and Park, 2012). It is, therefore, necessary to develop an alternative source of medicine from natural products or plant based sources as this would help in the discovery and development of new drugs with no side effects. A number of natural products possess the ability to induce body weight reduction and prevent diet induced obesity (Birari and Bhutani, 2007; Mohamed et al., 2014; Yun, 2010). These natural products possess inhibitors of digestive enzymes that interfere with the hydrolysis and absorption of dietary carbohydrates and lipids (Wang et al., 2010; Garza et al., 2011; Gu et al., 2011). The main enzymes involved in the hydrolysis of carbohydrates and fat are α-amylase, α-glucosidase and pancreatic lipase (Gu et al., 2011; Nair et al., 2013). There is a possibility that plants with strong α-amylase, α-glucosidase and lipase inhibitory activities may have potential toxic and carcinogenic effects, thus rendering them unsuitable for therapeutic applications (Fennell et al., 2004). Hence, it is imperative to examine the potential cytotoxic activity in order ascertain the safety limits of medicinal plant extracts. According to Ahmad et al. (2006), some plants' extracts possess bioactivity, but these activities are neutralized by their cytotoxicity activity; therefore is necessary to critically evaluate plant extracts so as to determine their overall efficacy.
Kedrostis africana (L.) Cogn (Cucurbitaceae) commonly called baboon's cucumber is indigenous to Nambia and South Africa (Eastern Cape, Free State, Gauteng, Kwazulu-Natal, Limpopo, Mpumalanga, Northern Cape, North West and Western Cape) (Eggli, 2002). Locally, it is called “uthuvana/uthuvishe” by the Xhosa-speaking South Africans (Dold and Cocks, 1999). The plant is traditionally used by the Khoi-San and Cape Dutch medicine as an emetic, purgative, diuretic and against dropsy (van Wyk, 2008). In the Eastern Cape of South Africa, the crushed fresh bulb is used for the management of obesity in folklore (Afolayan and Mbaebie, 2010; George and Nimmi, 2011).
According to Unuofin et al. (2017a), this plant serves as a rich source of minerals and proximate contents. Also, certain biological activities such as antioxidant, antibacterial, fungicidal and larvicidal activities have been ascribed to different solvent extracts of K. africana (Unuofin et al., 2017b,c).
Kedrostis africana has been used in folk medicine for the management of obesity, but scientific evaluation is still lacking in this regard. Therefore, in this study, α-amylase, α-glucosidase, pancreatic lipase inhibitory activities were screened in vitro, and cytotoxicity was evaluated against HeLa cell line of aqueous and ethanol extracts of K. africana.
2. Materials and methods
2.1. Location and collection of sample
K. africana tuber used for this study was collected in August 2015 in Fort Beaufort in the Amathole District Municipality, Eastern Cape, South Africa. It was authenticated by Mr Tony Dold, Selmar Schonland Herbarium, Rhodes University, South Africa, and a voucher specimen (Unuofin Med, 2015/2) was prepared and deposited in the Giffen Herbarium, University of Fort Hare.
2.2. Preparation of extracts
The oven-dried powder of the tuber were extracted using ethanol and water and shaken in an orbital shaker (Orbital Incubator Shaker, Gallenkamp) for 48 hours. The extracts were filtered using a Whatman No. 1 filter paper. The ethanol extract was concentrated under vacuum to dryness while the aqueous extract was concentrated using a freeze dryer (Vir Tis benchtop K, Vir Tis Co., Gardiner, NY).
2.3. Chemicals
Melphalan, RPMI1640 medium, 10% fetal bovine serum, dimethyl sulfoxide (DMSO), Hoechst 33342, Propidium iodide, Phospahte Buffer Saline (PBS), para-Nitrophenol palmitate (pNPP), gum arabic, sodium deoxycholate, Triton X-100, Tris-HCl buffer (pH 8.0), monosodium and disodium phosphate, p-nitrophenyl-α-D-glucopyranoside (PNP-GLUC), sodium carbonate, epigallocatechin gallate (EGCG), α-glucosidase, α-amylase, isopropanol, Triton X-100, Tris-HCl buffer, porcine pancreatic lipase, orlistat.
2.4. In vitro cytotoxic activity of plant extracts (Hoechst/PI staining)
The plant extracts were solubilised in 1% DMSO to a final concentration of 50 000 μg/mL. Samples were then stored at 4 °C until required. HeLa (cervical cancer) were used for the screening process. Cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum. Cells were seeded in 200 μL aliquots at a cell density of 3 × 104 cells/mL (i.e. 6 000 cells per well) in 96 well plates and left overnight to attach. For treatment of the cell line, the medium was replaced with fresh medium containing three concentrations (50, 100 and 200 μg/mL) of extract. Treated cells were incubated at 37 °C in a humidified 5% CO2 incubator for 48 hours. Treatment medium was gently removed prior to the addition of the Hoechst/PI staining and replaced with 100 μl PBS containing bisBenzamide H 33342 trihydrochloride (Hoechst 33342) at a concentration of 5 μg/mL. Cells were stained for 20 minutes at 37°C. Propidium Iodide was added at a concentration of 10 μg/mL using 10 μL per well of a 110 μg/mL stock directly before imaging. Image acquisition was performed using an ImageXpress Micro XLS Widefield High-Content Analysis System (Molecular Devices®). This microscope is Africa's first high content analysis system and thus the accuracy and sensitivity were compared to the crystal violet assay, a more traditional method of quantifying cell density. The images were analyzed using the MetaXpress® High-Content Image Acquisition & Analysis Software and Multi-wavelength Cell Scoring analysis module.
The average cell number was calculated using MetaXpress® software.
| % cell density = A570 nm of treated cells/A570 nm of untreated cells X 100. |
2.5. Alpha-amylase inhibitory activity of plant extracts
The α-amylase inhibitory activity of the extract was carried out according to the standard method with minor modification (Ademiluyi and Oboh, 2013). Ten microliter of porcine pancreatic amylase solution (0.1 mg/mL) were mixed with 30 μL of plant extracts or phosphate buffer (the control), or positive control (acarbose, 64 μg/mL) and pre-incubated at 37 °C for 10 minutes. Then, 40 μL starch solution was added to initiate reaction and incubation was done at 37 °C for 30 minutes, then 20 μL of 1 M HCl and 75 μL iodine reagent were added to the 96-well plate. The absorbance of the mixture was measured at 580 nm.
α-amylase inhibitory activity was measured using the formula:
| % α-amylase inhibition = [1- (A/B)] X 100 |
Where A = absorbance of test well (plant extract or acarbose)B = absorbance of enzyme control
Two controls (without enzyme and substrate) were used to ascertain if any reaction took place in their absent. This was done to exclude false positive results, as some plants extracts have been reported to contain traces of α-amylase or starch.
2.6. Alpha-glucosidase inhibitory activity of plant extracts
The α-glucosidase inhibitory activity was determined by a colorimetric assay utilizing a well established protocol (You et al., 2011) with some modifications. Forty microliter of α-glucosidase solution (50 μg/mL) were mixed with 10 μL of plant extract or Epigallocatechin gallate (EGCG) as positive control and pre-incubated at 37 °C for 5 minutes. Then, 10 μL of PNP-GLUC was then added to initiate reaction and incubation was done at 37 °C for 30 min, after which 50 μL of sodium carbonate solution were added to the 96-well plate to terminate the reaction. The absorbance of the mixture was measured at 405 nm.
α-glucosidase inhibitory activity was measured using the formula:
| % α-glucosidase inhibition = [1- (A/B)] X 100. |
Where A = absorbance of test well (plant extract or positive control)B = absorbance of enzyme control
2.7. Pancreatic lipase inhibitory activity of plant extracts
The inhibitory activity against pancreatic lipase was measured using p-nitrophenyl butyrate (p-NPB) as a substrate with a modified method from Zhang et al. (2008). 10 μL of extracts (prepared at concentrations of 50, 100 and 200 μg/mL), positive control (Orlistat, 100 μM) and DMSO (negative control vehicle used to dissolve the extracts) were pipetted into respective wells of a 96-well plate. Freshly prepared porcine pancreatic lipase was added at four times the volume of the test samples, positive and negative controls (40 μL). The plates were initially incubated at 37 °C for 15 minutes. Thereafter, 170 μL of the substrate solution was added to the wells. The plate was then incubated at 37 °C for 25 minutes and the absorbance was read at 405 nm.
Percentage lipase inhibition was calculated as:
| % Lipase inhibition = [1- (A/B)] X 100. |
Where A = absorbance of test well (plant extract or orlistat)B = absorbance of enzyme control
2.8. Data analysis
The statistical analysis was done on MINITAB version 17 for Windows. Data were expressed as mean ± SD of five replicates and were subjected to one-way analysis of variance (ANOVA) followed by Fischer's Least Significant Difference (LSD) to determine significant differences in all the parameters. Values were considered statistically significant at p < 0.05.
3. Result
3.1. Cytotoxicity activity of extracts on HeLa cell lines
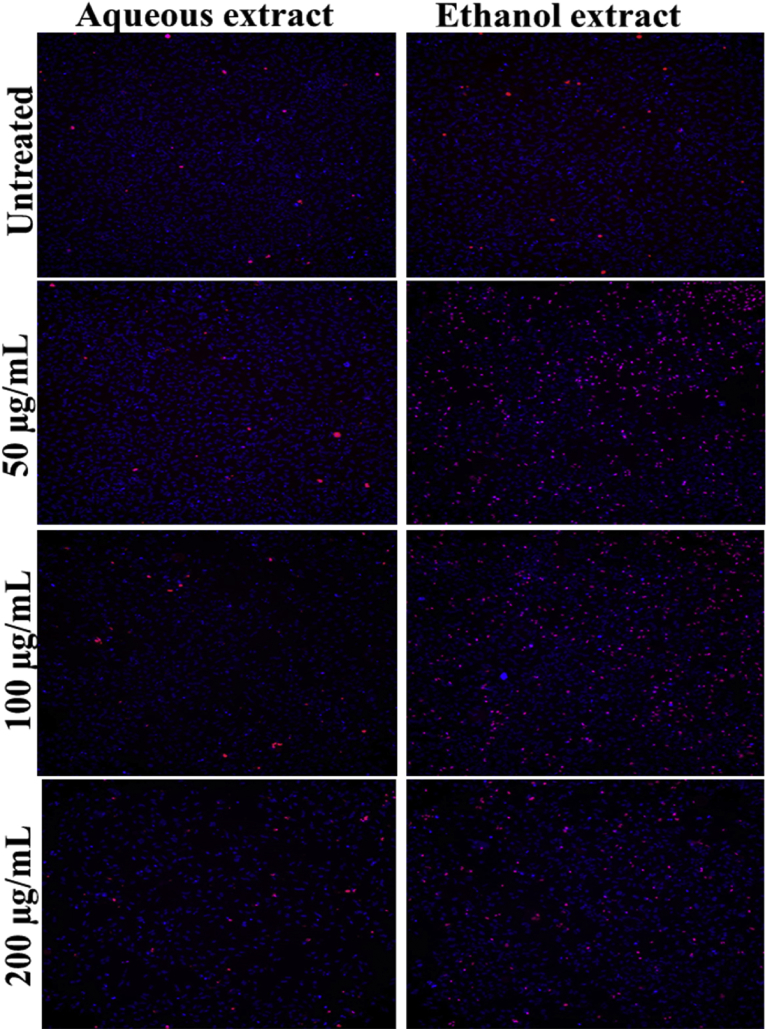
Cytotoxicity screening was performed on HeLa cell line. Cell numbers were determined using Hoechst 33342/PI staining. Fig. 1 shows the fluorescent micrographs that were captured during this experiment. The ethanol extract had more cell death due to the cytotoxic nature, this was depicted with the cell numbers greatly reducing (blue colour) and the number of dead cells stained positive (red colour) for PI is increased when compared with the aqueous extract and the untreated sample.
Fig. 1.
Fluorescent micrographs captured during the cytotoxicity screening assay comparing the untreated sample with the aqueous and ethanol extract of K. africana for HeLa cells using Hoechst 33342 (blue) and propidium iodide (red).
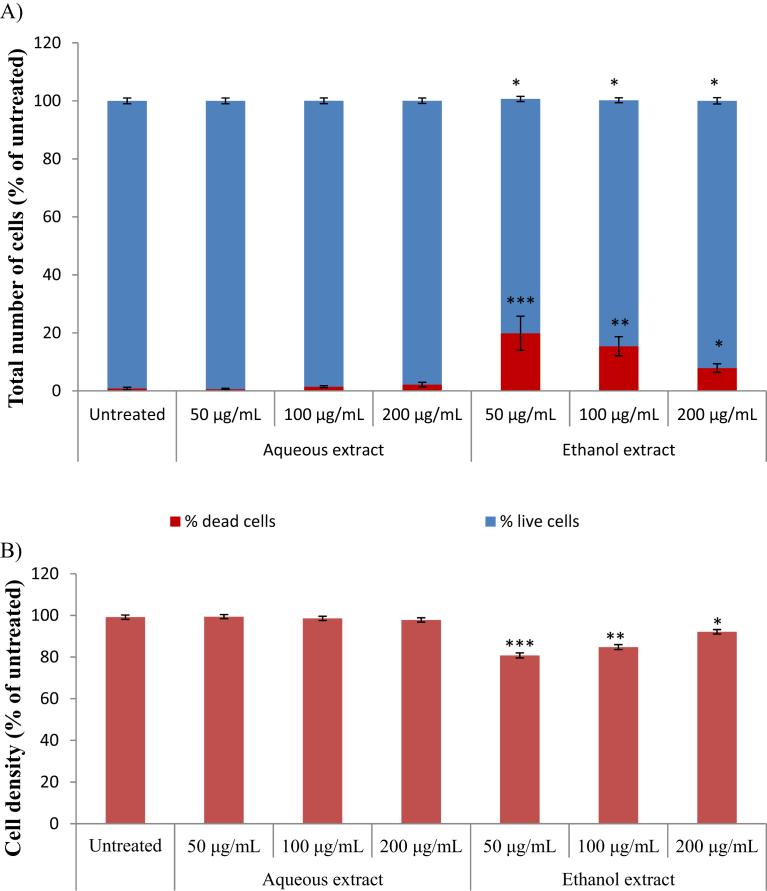
Fig. 2 displays the cytotoxicity activity of K. africana extracts on HeLa cell line as confirmed by Hoechst 33342/PI staining. As indicated in Fig. 2, the aqueous extract of K. africana did not cause any significant decrease in cell number across all tested concentrations (50–200 μg/mL). However, the ethanol extract displayed a noticeable increase in cell death at all the test concentration with a more significant (p < 0.005) cell death at the lowest concentration (50 μg/mL). The IC50 values for both the aqueous and ethanol extracts are greater than 200 μg/mL which is the highest concentration tested (Table 1).
Fig. 2.
Cytotoxicity screening of Kedrostis africana (A) cell number with (B) cell density for HeLa cells; live- and dead cell numbers were obtained from Hoechst 33342 and PI staining, respectively and cell density from crystal violet staining. Results are reported as means ± SD where each experiment was performed three times, each in triplicate (*p < 0.05; **p < 0.01; ***p < 0.005 compared to untreated sample).
Table 1.
IC50 values (μg/ml) for cytotoxic activity of Kedrostis africana extracts on HeLa cells after 48 hours exposure.
| Name of extract | Cytotoxicity IC50 (μg/mL) |
|---|---|
| Aqueous | >200 |
| Ethanol | >200 |
Data are presented as mean ± SD values of triplicate determinations.
3.2. α-amylase inhibitory activity
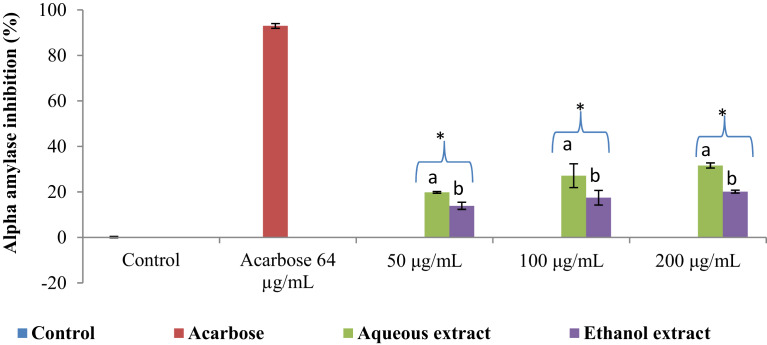
Both the aqueous and ethanol extracts of K. africana exhibited weak α-amylase inhibitory activity at the tested concentrations (50, 100, or 200 μg/mL) with inhibition in a concentration-dependent manner (Fig. 3). Although, the percentage inhibition of the positive control (acarbose) was 92.98 ± 1.37% at 64 μg/mL, it ranged from 19.85 ± 0.37% to 31.64 ± 1.11% and 13.91 ± 1.55% to 20.14 ± 0.63% for the aqueous and ethanol extracts, respectively. However, both extracts (aqueous and ethanol) had lower activities than acarbose. The IC50 values are depicted in Table 2.
Fig. 3.
α-amylase inhibitory activity of the aqueous and ethanol extracts of Kedrostis africana. Values are mean ± SD (n = 3). Mean separation by LSD (p < 0.05). Set of bars (the same concentration) with different alphabets are different. *Lower than acarbose (positive control). SD: Standard deviation; LSD: Least Significant Difference.
Table 2.
IC50 values for α-amylase, α-glucosidase and lipase inhibition activity of Kedrostis africana extracts.
| Name of extract | IC50 values (μg/mL) |
||
|---|---|---|---|
| α-amylase | α-glucosidase | Lipase | |
| Aqueous | 439.45 ± 1.95 | 486.92 ± 4.64 | 842.48 ± 2.24 |
| Ethanol | 949.75 ± 3.68 | 157.99 ± 5.41 | 381.86 ± 3.48 |
Data are presented as mean ± SD values of triplicate determinations.
3.3. α-Glucosidase inhibitory activity
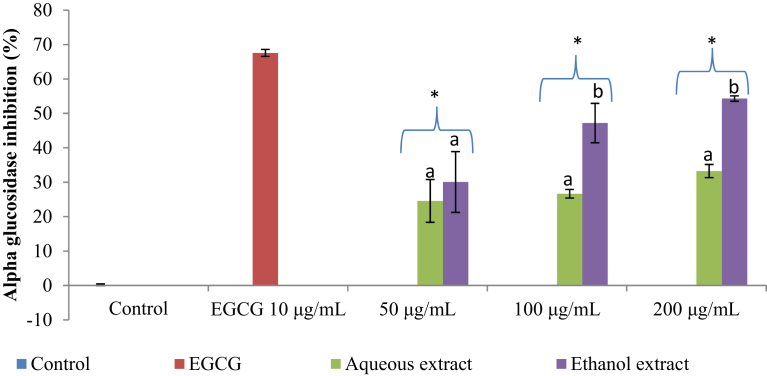
The alpha-glucosidase inhibitory activity of the aqueous and ethanol extracts of K. africana was observed to be in a concentration-dependent manner. A stronger inhibition was observed when compared with α-amylase inhibition (Fig. 4). The percentage α-glucosidase inhibition ranged from 24.57 ± 6.22% to 33.25 ± 1.91% in the aqueous extract and from 30.07 ± 8.84% to 54.30 ± 0.79% in the ethanol extract. The ethanol extract had a better α-glucosidase inhibitory activity with IC50 values of 157.99 ± 5.41 μg/mL while the aqueous extract IC50 value was 486 ± 4.64 μg/mL (Table 2).
Fig. 4.
α-Glucosidase inhibitory activity of the aqueous and ethanol extracts of Kedrostis africana. Values are mean ± SD (n = 3). Mean separation by LSD (p < 0.05). Set of bars (the same concentration) with different alphabets are different. ∗Lower than the positive control EGCG: Epigallocatechin gallate; SD: Standard deviation; LSD: Least Significant Difference.
3.4. Porcine pancreatic lipase (PPL) inhibitory activity
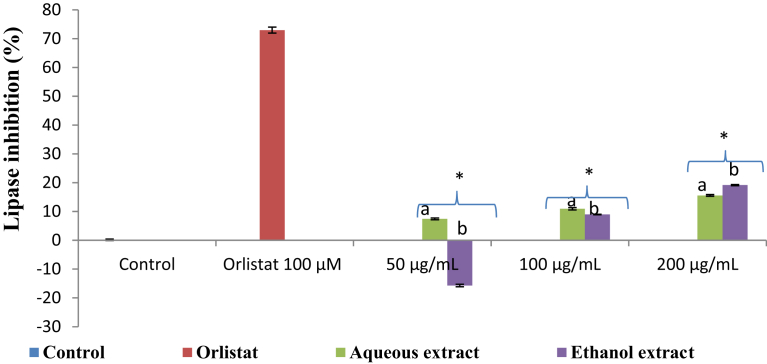
Lipase inhibitory activity of the aqueous and ethanol extracts of K. africana exhibited inhibitory activity at the tested concentrations (50, 100, or 200 μg/mL) in a concentration-dependent manner (Fig. 5). Although, the percentage inhibition of the positive control (acarbose) was 72.98 ± 1.37% at 64 μg/mL, it ranged from 7.43 ± 0.32% to 15.58 ± 0.31% and -15.69 ± 0.43% to 19.17 ± 0.21% for the aqueous and ethanol extracts, respectively. However, both extracts (aqueous and ethanol) had lower activities than orlistat. The ethanol extract had a better lipase inhibitory activity with IC50 values of 381.86 ± 3.48 μg/mL while the aqueous extract IC50 value was 842.48 ± 2.24 μg/mL (Table 2).
Fig. 5.
Lipase inhibitory activity of the aqueous and ethanol extracts of Kedrostis africana. Values are mean ± SD (n = 3). Mean separation by LSD (p < 0.05). Set of bars (the same concentration) with different alphabets are different. ∗Lower than the positive control (Orlistat). SD: Standard deviation; LSD: Least Significant Difference.
4. Discussion
Over the years, there has been an increasing search for natural products having potent bioactive compounds with low toxicity and possess ability to oxidize fats, control appetite, regulate levels of hormones related to obesity and inhibit digestive enzymes involved in the absorption of carbohydrates and lipids (Cho et al., 2010; Rains et al., 2011) when compared with synthetic compounds.
In this study, we evaluated the in vitro cytotoxicity and inhibitory activities against α-amylase, α-glucosidase and lipase of aqueous and ethanol extracts of K. africana. Although a number of studies have reported that some medicinal plants possess α-amylase, α-glucosidase and lipase inhibitory activities (Shirwaikar et al., 2005; Ortiz-Andrade et al., 2007) an attribute similar to that of K. africana, a wild plant used in folklorically for the management of obesity in the Eastern Cape, South Africa. However, we observed that there is still lacking of information on K. africana cytotoxicity and its abilities to inhibit α-amylase, α-glucosidase and lipase activities in vitro.
Different scientific reports have shown that there is a positive correlation between the effects of flavonoids and polyphenols content on inhibitory potentials of alpha-amylase and alpha-glucosidase (Pereira et al., 2011; Adisakwattana et al., 2012; Kam et al., 2013). Flavonoids and their derivatives such as luteolin and kaempferol possess the ability of blocking glucose absorption, inhibiting sodium dependent glucose transporter-1 which improves glucose tolerance (Thouri et al. 2017). Also, high levels of polyphenolic compounds have been shown to reduce the potency of alpha-amylase and alpha-glucosidase by either interacting or inhibiting specific position of the enzyme (Rohn et al., 2002). According to Unuofin et al. (2017b), the tuber extracts of K. africana have strong free radical scavenging potential and high polyphenolic contents which have been implicated in enzyme inhibitory capacity. The aqueous extract showed higher inhibition against α-amylase and weaker inhibition against glucosidase while the ethanol extract had a low inhibitory effect against α-amylase and stronger inhibitory activity against α-glucosidase, thence the consumption of both extracts could serve as an antidiabetic agent for the treatment of postprandial hyperglycemia with little or no side effects. The lack of correlation in the result with the above statement could be attributed to the differences in the mechanisms involved and also the inference of other phytochemicals. Our findings are in line with previous reports which stated that mild α-amylase inhibition is permitted in order to prevent the abnormal bacterial fermentation which results from the presence of undigested carbohydrates in the colon and thus promote flatulence and diarrhoea (Etxeberria et al., 2012; Lordana et al., 2013; Nagmoti and Juvekar, 2013). According to Chen et al. (2008), the inhibition of α-glucosidase aids gastric emptying, which brings about satiety and weight loss, which are useful in the treatment of obesity. Thus, the strong alpha-glucosidase activity of the extracts could aid in bringing about satiety and weight loss in obese subjects. Hence, the ability of natural products/medicinal plants in inhibiting α-amylase and α-glucosidase could serve as an alternative therapy for the treatment of obesity in place of synthetic drugs (Mc Dougall et al., 2005).
Pancreatic lipase is the most important enzyme responsible for digestion of dietary fat, slowing down the deposition of fat into adipose tissue and suppression of weight gain which is of beneficial effects to overweight and obesity (Podsędek et al., 2014; Dechakhamphu and Wongchum, 2015). In this study, both crude extracts (aqueous and ethanol) exhibited moderate inhibitory activity against pancreatic lipase. It is noteworthy that at 50 μg/mL of the ethanol extract, their was a stimulatory effect against lipase. Orlistat was more potent than the crude extracts may due to the presence of both active and non active components. Reports have shown that plants rich in phytochemicals such saponins and polyphenol can inhibit pancreatic lipase and lighten weight gain in high-fat diets induced murine models (Han et al., 2002, 2005; Li et al., 2007).
Natural products/medicinal plants are usually thought to be safer than pharmaceuticals. According to the United States America National Cancer Institute of Plant Screening Program, any plant extract with an IC50 value equal to or lesser than 20 μg/mL after an incubation period 48 hrs is generally considered to have an active cytotoxic effect (Lee and Houghton, 2005; Malek et al., 2009). From the above statement, it can be deduced that both extracts screened using HeLa cells are not cytotoxic because their IC50 values are far greater than 20 μg/mL. These relatively low toxicity result of all the extracts from this study suggests that these extracts may safely use for anti-obesity treatment.
5. Conclusion
Data obtained from this study suggest that both the aqueous and ethanol tuber extract of K. africana exerts mild inhibitory effect on α-amylase, α-glucosidase and lipase with nontoxic effect to HeLa cell line. Further, the results revealed that both extracts could be a good candidate for anti-obesity evaluations in experimental animals. In addition, these results support the traditional use of K. africana in the management of obesity.
Declarations
Author contribution statement
Jeremiah Oshiomame Unuofin: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Gloria Aderonke Otunola: Conceived and designed the experiments; Wrote the paper.
Anthony Jide Afolayan: Conceived and designed the experiments; Wrote the paper.
Funding statement
This work was supported by Govan Mbeki Research Development Centre (GMRDC), University of Fort Hare, South Africa (grant number C127).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- Ademiluyi A.O., Oboh G. Aqueous extracts of Roselle (Hibiscus sabdariffa Linn.) varieties inhibit α-amylase and α-glucosidase activities in vitro. J. Med. Food. 2013;16(1):88–93. doi: 10.1089/jmf.2012.0004. [DOI] [PubMed] [Google Scholar]
- Adisakwattana S., Ruengsamran T., Kampa P., Sompong W. In vitro inhibitory effects of plant based foods and their combinations on intestinal α-glucosidase and pancreatic α-amylase. BMC Compl. Alternative Med. 2012;12:110. doi: 10.1186/1472-6882-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afolayan A.J., Mbaebie B.O. Ethnobotanical study of medicinal plants used as anti-obesity remedies in Nkonkobe Municipality of South Africa. Phcog. J. 2010;2(11):368–373. [Google Scholar]
- Ahmad I.A., Farrukh F., Amad F., Owais M. Herbal medicines: prospects and constraints. In: Ahmad I., Aqil F., Owais M., editors. Modern Phytomedicine: Turning Medicinal Plants into Drugs. Wiley-VCH Verlag GmbH & Co.; Germany: 2006. pp. 59–78. 2006. [Google Scholar]
- Birari R.B., Bhutani K.K. Pancreatic lipase inhibitors from natural sources: unexplored potential. Drug Discov. Today. 2007;12(19–20):879–889. doi: 10.1016/j.drudis.2007.07.024. [DOI] [PubMed] [Google Scholar]
- Boniglia C., Carratù B., Di Stefano S., Giammarioli S., Mosca M., Sanzini E. Lectins, trypsin and α-amylase inhibitors in dietary supplements containing Phaseolus vulgaris. Eur. Food Res. Technol. 2008;227(3):689–693. [Google Scholar]
- Chen X., Xu G., Li X., Li Z., Ying H. Purification of an α-amylase inhibitor in a polyethylene glycol/fructose-1,6-bisphosphate trisodium salt aqueous twophase system. Process Biochem. 2008;43:765–768. [Google Scholar]
- Cho A.S., Jeon S.M., Kim M.J., Yeo J., Seo K.L., Choi M.S., Lee M.K. Chlorogenic acid exhibits antiobesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010;48:937–943. doi: 10.1016/j.fct.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Dechakhamphu A., Wongchum N. Screening for anti-pancreatic lipase properties of 28 traditional Thai medicinal herbs. Asian Pac J Trop Biomed. 2015;5:1042–1045. [Google Scholar]
- Dold A.P., Cocks M.L. Preliminary list of Xhosa plant names from the Eastern Cape, South Africa. Bothalia. 1999;29(2):267–292. [Google Scholar]
- Eggli Urs. Springer; Germany: 2002. Illustrated Handbook of Succulent Plants: Dicotyledons. [Google Scholar]
- Etxeberria U., de la Garza A.L., Campión J., Martínez J.A., Milagro F.I. Anti-diabetic effects of natural plant extracts via inhibition of carbohydrate hydrolysis enzymes with emphasis on pancreatic alpha amylase. Expert Opin. Ther. Targets. 2012;16:269–297. doi: 10.1517/14728222.2012.664134. [DOI] [PubMed] [Google Scholar]
- Fennell C.W., Lindsey K.L., McGaw L.J., Sparg L.G., Stafford G.I., Elgorashi E.E., Grace O.M., van Staden J. Assessing African medicinal plants for efficacy and safety: pharmacological screening and toxicology. J. Ethnopharmacol. 2004;94:205–217. doi: 10.1016/j.jep.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Gallagher E.J., LeRoith D. Obesity and diabetes: the increased risk of cancer and cancer-related mortality. Physiol. Rev. 2015;95(3):727–748. doi: 10.1152/physrev.00030.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza A.L., Milagro F.I., Boque N., Campión J., Martínez J.A. Natural inhibitors of pancreatic lipase as new players in obesity treatment. Planta Med. 2011;77(8):773–785. doi: 10.1055/s-0030-1270924. [DOI] [PubMed] [Google Scholar]
- George P., Nimmi O.S. Cent percent safe centum plants for antiobesity. Int J Innov Technol Cre Eng. 2011;1(3):1–19. [Google Scholar]
- Gu Y., Hurst W.J., Stuart D.A., Lambert J.D. Inhibition of key digestive enzymes by cocoa extracts 1 and procyanidins. J. Agric. Food Chem. 2011;59(10):5305–5311. doi: 10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han L.K., Zheng Y.N., Xu B.J., Okuda H., Kimura Y. Saponins from Platycodi radix ameliorate high fat diet-induced obesity in mice. J. Nutr. 2002;132:2241–2245. doi: 10.1093/jn/132.8.2241. [DOI] [PubMed] [Google Scholar]
- Han L.K., Zheng Y.N., Yoshikawa M., Okuda H., Kimura Y. Anti-obesity effects of chikusetsusaponins isolated from Panax japonicus rhizomes. BMC Compl. Alternative Med. 2005;5:9–18. doi: 10.1186/1472-6882-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kam A., Li K.M., Razmovshi-Naumovshi V., Nammi S., Shi J., Chan K., Li G.Q. A comparative study on the inhibitory effects of different parts and chemical constituents of pomegranate on α-amylase and α-glucosidase. Phytother Res. 2013;27:1614–1620. doi: 10.1002/ptr.4913. [DOI] [PubMed] [Google Scholar]
- Kang J., Park C. Anti-obesity drugs: a review about their effects and safety. Diabetes Metab. J. 2012;36:13–25. doi: 10.4093/dmj.2012.36.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukua J., Pekkarinen T., Sane T., Mustajoki P. Health-related quality of life in obese outpatients losing weight with very-low-energy diet and behaviour modification: a 2-y follow-up study. Int. J. Obes. Relat. Metab. Disord. 2003;27(9):1072–1080. doi: 10.1038/sj.ijo.0802366. [DOI] [PubMed] [Google Scholar]
- Kitahara C.M., Flint A.J., de Gonzalez A.B., Bernstein L., Brotzman M., MacInnis R.J., Moore S.C., Robien K., Rosenberg P.S., Singh P.N., Weiderpass E., Adami H.O., Anton-Culver H., Ballard-Barbash R., Buring J.E., Freedman M., Fraser G.E., Freeman L.E.B., Gapstur S.M., Gaziano J.M., Giles G.G., Håkansson N., Hoppin J.A., Hu F.B., Koenig K., Linet M.S., Park Y., Pate A.V., Purdue M.P., Schairer C., Sesso H.D., Visvanathan K., White E., Wolk A., Zeleniuch-Jacquotte A., Hartge P. Association between class III obesity (BMI of 40-59 kg/m2) and mortality: a pooled analysis of 20 prospective studies. PLoS Med. 2014;11(7) doi: 10.1371/journal.pmed.1001673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C.C., Houghton P. Cytotoxicity of plants from Malaysia and Thailand used traditionally to treat cancer. J. Ethnopharmacol. 2005;100(3):237–243. doi: 10.1016/j.jep.2005.01.064. [DOI] [PubMed] [Google Scholar]
- Li F., Li W., Fu H., Zhang Q., Koike K. Pancreatic lipase-inhibiting triterpenoid saponins from fruits of Acanthopanax senticosus. Chem. Pharm. Bull. (Tokyo) 2007;55:1087–1089. doi: 10.1248/cpb.55.1087. [DOI] [PubMed] [Google Scholar]
- Lordana S., Smyth T.J., Soler-Vila A., Stanton C., Rossa R.P. The a-amylase and a-glucosidase inhibitory effects of Irish seaweed Extracts. Food Chem. 2013;141:2170–2176. doi: 10.1016/j.foodchem.2013.04.123. [DOI] [PubMed] [Google Scholar]
- Malek S.N.A., Shin S.K., Wahab N.A., Yaacob H. Cytotoxic components of pereskia bleo (Kunth) DC. (Cactaceae) leaves. Molecules. 2009;14(5):1713–1724. doi: 10.3390/molecules14051713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mc Dougall G.J., Shpiro F., Dobson P., Smith P., Blake A., Stewart D. Different polyphenolic components of soft fruits inhibit α-amylase and α-glucosidase. J. Agric. Food Chem. 2005;53:2760–2766. doi: 10.1021/jf0489926. [DOI] [PubMed] [Google Scholar]
- Mohamed G.A., Ibrahim S.R.M., Elkhayat E.S., El Dine R.S. Natural anti-obesity agents. Bull. Fac. Pharm. Cairo Univ. 2014;52:269–284. [Google Scholar]
- Nagmoti D.M., Juvekar A.R. In vitro inhibitory effects of Pithecellobium dulce (Roxb.) Benth seeds on intestinal α-glucosidase and pancreatic α-amylase. J. Biochem. Technol. 2013;4(3):616–621. [Google Scholar]
- Nair S.S., Kavrekar V., Mishra A. In vitro studies on alpha amylase and alpha glucosidase inhibitory activities of selected plant extracts. Eur. J. Exp. Biol. 2013;3(1):128–132. [Google Scholar]
- Ortiz-Andrade R.R., García-Jiménez S., Castillo-España P., Ramírez-Avila G., Villalobos-Molina R., Estrada-Soto S. alpha-Glucosidase inhibitory activity of the methanolic extract from Tournefortia hartwegiana: an anti-hyperglycemic agent. J. Ethnopharmacol. 2007;109:48–53. doi: 10.1016/j.jep.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Pereira D.F., Cazarolli L.H., Lavado C., Mengatto V., Figueiredo M.S., Guedes A., Pizzolatti M.G., Silva F.R. Effect of flavonoids on α-glucosidase activity: potential targets for glucose homeostasis. Nutrition. 2011;27:1161–1167. doi: 10.1016/j.nut.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Podsędek A., Majewska I., Redzynia M., Sosnowska D., Koziołkiewicz M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014;62(20):4610–4617. doi: 10.1021/jf5008264. [DOI] [PubMed] [Google Scholar]
- Rains T.M., Agarwal S., Maki K.C. Antiobesity effects of green tea catechins: a mechanistic review. J. Nutr. Biochem. 2011;22:1–7. doi: 10.1016/j.jnutbio.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Rohn S., Rawel H.M., Kroll J. Inhibitory effects of plant phenols on the activity of selected enzymes. J. Agric. Food Chem. 2002;50:3566–3571. doi: 10.1021/jf011714b. [DOI] [PubMed] [Google Scholar]
- Shirwaikar A., Rajendran K., Punitha I.S. Antidiabetic activity of alcoholic stem extract of Coscinium fenestratum in streptozotocin-nicotinamide induced type 2 diabetic rats. J. Ethnopharmacol. 2005;97:369–374. doi: 10.1016/j.jep.2004.11.034. [DOI] [PubMed] [Google Scholar]
- Thouri A., Chahdoura H., El Arem A., Omri Hichri A., Ben Hassin R., Achour L. Effect of solvents extraction on phytochemical components and biological activities of Tunisian date seeds (var. Korkobbi and Arechti) BMC Complement Altern. Med. 2017;17(1):248–257. doi: 10.1186/s12906-017-1751-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unuofin J.O., Otunola G.A., Afolayan A.J. Nutritional evaluation of Kedrostis africana (L.) Cogn: an edible wild plant of South Africa. Asian Pac. J. Trop. Biomed. 2017;7(5):443–449. [Google Scholar]
- Unuofin J.O., Otunola G.A., Afolayan A.J. Phytochemical screening and in vitro evaluation of antioxidant and antimicrobial activities of Kedrostis africana (L.) Cogn. Asian Pac. J. Trop. Biomed. 2017;7(10):901–908. [Google Scholar]
- Unuofin J.O., Otunola G.A., Afolayan A.J. Toxicity assessment of Kedrostis africana Cogn: a medicinal plant used in the management of obesity in South Africa using brine shrimp (Artemia salina Linn.) assay. Int. J. Pharm Sci. Res. 2017;8(9):3719–3725. [Google Scholar]
- van Wyk B.E. A review of Khoi-San and Cape Dutch medical ethnobotany. J. Ethnopharmacol. 2008;119(3):331–341. doi: 10.1016/j.jep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Wanderley E.M., Ferreira V.A. Obesity: a plural perspective. Ciên. Saúde. Colet. 2010;15:185–194. doi: 10.1590/s1413-81232010000100024. [DOI] [PubMed] [Google Scholar]
- Wang H., Du Y., Song H. α-Glucosidase and α-amylase inhibitory activities of guava leaves. Food Chem. 2010;123(1):6–13. [Google Scholar]
- WHO . World Health Organization; 2015. Obesity and Overweight.http://www.who.int/mediacentre/factsheets/fs311/en/ (accessed March 2015) [Google Scholar]
- You Q., Chen F., Wang X., Luo P.G., Jiang Y. Inhibitory effects of muscadine anthocyanins on alpha-glucosidase and pancreatic lipase activities. J. Agric. Food Chem. 2011;59(17):9506–9511. doi: 10.1021/jf201452v. [DOI] [PubMed] [Google Scholar]
- Yun J.W. Possible anti-obesity therapeutics from nature-a review. Phytochemistry. 2010;71(14-15):1625–1641. doi: 10.1016/j.phytochem.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Zhang J., Kang M.J., Kim M.J., Kim M.E., Song J.H., Lee Y.M., Kim J.I. Pancreatic lipase inhibitory activity of taraxacum officinale in vitro and in vivo. Nutr. Res. Pract. 2008;2(4):200–203. doi: 10.4162/nrp.2008.2.4.200. [DOI] [PMC free article] [PubMed] [Google Scholar]