Abstract
Background
Poor subpleural perfusion (PSP) in the capillary phase of pulmonary angiography predicts worse outcomes following pulmonary endarterectomy in operable chronic thromboembolic pulmonary hypertension (CTEPH). Balloon pulmonary angioplasty (BPA) has emerged as a treatment for nonoperable CTEPH. The goal of the present article was to assess the association between PSP and BPA failure.
Methods
Subpleural perfusion was classified as poor (defined as subpleural spaces either not perfused or minimally perfused in all segments) or normal. We retrospectively reviewed PSP and hemodynamic variables of 101 consecutive patients who underwent BPA from February 2014 to August 2016. The total cross-sectional area of bronchial arteries was also measured by using CT scanning. Patients were categorized according to hemodynamic results after the last BPA: a failure group (defined as mean pulmonary arterial pressure > 30 mm Hg and a decrease in pulmonary vascular resistance < 30% [n = 15]) or a success group (n = 86).
Results
Although baseline hemodynamic variables were similar between the two groups, PSP was observed in 46.7% of patients in the failure group vs 13.9% in the success group (P = .003). Multivariate analysis revealed that PSP was the only predictor of BPA failure (OR, 4.02 [95% CI, 1.17-13.89]; P = .028). Patients with PSP exhibited poorly developed bronchial arteries compared with patients with normal perfusion (7.0 [5.8-9.6] mm2 vs 8.7 [6.9-11.3] mm2; P = .032).
Conclusions
PSP in the capillary phase, suggesting the presence of small vessel disease with diffuse distal thrombosis, is a predictor of BPA failure. PSP was also associated with less developed bronchial arteries, which suggests a key role of bronchial-pulmonary anastomoses in maintaining the pulmonary capillary bed open downstream of the pulmonary arterial obstruction. PSP affected approximately 15% of patients with nonoperable CTEPH who underwent BPA.
Key Words: balloon pulmonary angioplasty, chronic thromboembolic pulmonary hypertension, poor subpleural perfusion, predictive factor
Abbreviations: BPA, balloon pulmonary angioplasty; CTEPH, chronic thromboembolic pulmonary hypertension; Dlco, diffusing capacity for carbon monoxide; DSA, digital subtraction angiography; NYHA, New York Heart Association; PAP, pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PEA, pulmonary endarterectomy; PH, pulmonary hypertension; PSP, poor subpleural perfusion; PVR, pulmonary vascular resistance; RHC, right heart catheterization
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by stenosis and obstruction of the pulmonary arteries with nonresolving, organized thromboemboli leading to elevated pulmonary vascular resistance (PVR), severe pulmonary hypertension (PH), right heart failure, and, ultimately, death.1, 2, 3 Without treatment, the prognosis of patients with CTEPH is poor, with a 5-year survival rate of 10% in patients with a mean pulmonary arterial pressure (PAP) > 50 mm Hg.4 In a recent meta-analysis including 4,047 patients with pulmonary embolism who were followed up for > 2 years, it was reported that the CTEPH incidence was at least 0.56%.5, 6 It has been also reported that 25% to 40% of patients had no clinically apparent history of acute pulmonary embolism.7, 8
Various factors are involved in the development of PH in patients with CTEPH. Studies have revealed that not only mechanical obstruction with organized thrombus in large and/or middle-sized pulmonary arteries but also peripheral microvasculopathy (small pulmonary vessel disease) likely contribute to the development and progression of the disease.2, 9, 10 The histologic changes of small vessel disease mainly affect the wall of distal muscular pulmonary arteries, but they also affect arterioles; these changes are similar to those observed in idiopathic pulmonary arterial hypertension (PAH), including intimal thickening, intimal fibromuscular proliferation, and eccentric intimal fibrosis. These changes may develop in nonoccluded areas as a result of shear stress secondary to chronic exposure to high PAP, leading to endothelial dysfunction.11, 12 However, microvasculopathy is also found distally to pulmonary arteries occluded by fibrotic thrombi, possibly because of collateral blood supply from bronchial and systemic arteries that maintain perfusion in obstructed territories.10, 12
In addition, small vessel disease in CTEPH also consists of diffuse distal thrombosis when bronchial arteries fail to develop.10 Poor subpleural perfusion (PSP) in the capillary phase of pulmonary angiography is believed to reflect the existence of small pulmonary vessel disease with diffuse distal thrombosis.10 Tanabe et al13 reported that PSP was related to worse outcomes and higher mortality for patients with CTEPH who underwent pulmonary endarterectomy (PEA).
PEA remains the gold standard treatment of CTEPH.1, 2, 3 However, about 40% of patients with CTEPH are judged as nonoperable due to distal lesions or the presence of comorbidities.8, 14 Recently, balloon pulmonary angioplasty (BPA), an endovascular procedure to widen narrowed or obstructed pulmonary arteries that are surgically inaccessible, has emerged as an alternative treatment option for patients with nonoperable CTEPH. In the first case series reported by Feinstein et al15 in 2001, the efficacy and safety of the procedure were not satisfactory. With refinements in the technique, several reports, mainly from Japan, have described the efficacy and safety of BPA.16, 17, 18, 19
BPA is a promising treatment strategy for most nonoperable patients because segmental and subsegmental pulmonary arteries are accessible with BPA. However, as with patients who underwent PEA, some patients exhibited residual PH despite repeated BPA sessions. BPA is an emerging procedure, and there are no clear criteria for indications or contraindications. Furthermore, the underlying predictors for poor clinical outcomes after BPA are still unknown.
The goal of the present study was to assess the association between PSP and outcomes after BPA in patients with nonoperable CTEPH. We hypothesized that PSP would be associated with worse hemodynamic responses to BPA, due to its relation with small pulmonary vessel disease with diffuse distal thrombosis.
Patients and Methods
This retrospective study complied with the Declaration of Helsinki. Although French law does not require ethics committee approval or informed consent for retrospective data collection, the data were anonymized and complied according to the requirements of the Commission Nationale Informatique et Liberté, the organization dedicated to privacy, information technology, and civil rights in France. The committee approved the methods used to collect and analyze data on May 24, 2003 (approval number 842063).
Patients/Study Design
This observational study was conducted in all consecutive patients who underwent BPA in the hospitals affiliated with Université Paris-Sud (Hôpital Marie Lannelongue, Le Plessis-Robinson, and Hôpital Bicêtre) from February 2014 (commencement of our BPA program) to August 2016. All patients were diagnosed with CTEPH according to established clinical guidelines20 in the French reference center for pulmonary hypertension (Université Paris-Sud) and judged as nonoperable in a multidisciplinary meeting that included expert physicians, interventional cardiologists, radiologists, and PEA surgeons. Baseline hemodynamic variables by right heart catheterization (RHC), status with the New York Heart Association (NYHA) functional class, lung function test results, and exercise capacity with the 6-min walk distance were assessed within 3 months prior to the first BPA session. Re-evaluation by using RHC was performed 3 months following the last BPA session. Data were collected from hospital medical records.
Exclusion criteria were as follows: (1) rescue or emergency BPA for life support; (2) patients with unclear or absent baseline pulmonary angiography; and (3) patients without baseline or follow-up (3 months after the last BPA) hemodynamic evaluation by using RHC.
Pulmonary Angiography and CT ImaginG
Digital subtraction angiography (DSA) of the pulmonary arteries was performed at the time of CTEPH diagnosis by using a 5F pigtail catheter selectively in the right- and left-sided pulmonary arteries from an anterior-posterior view and lateral view of each lung. In total, 20 to 25 mL of contrast media was injected with a flow rate of 12 to 15 mL/s for each of the four series. DSA arteriograms were taken at 3.75 frames per second. DSA images were digitally recorded in the hospital PACS (Picture Archiving and Communication System) workstation.
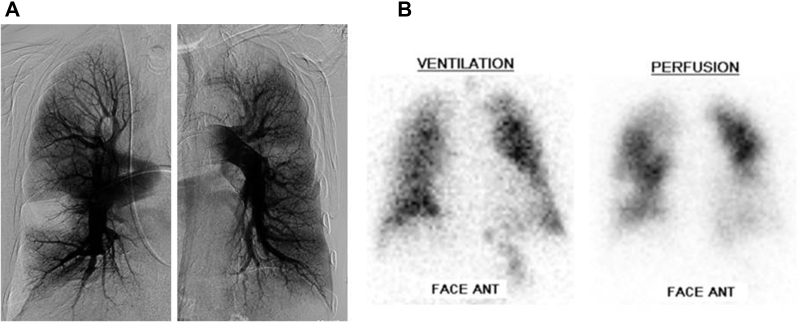
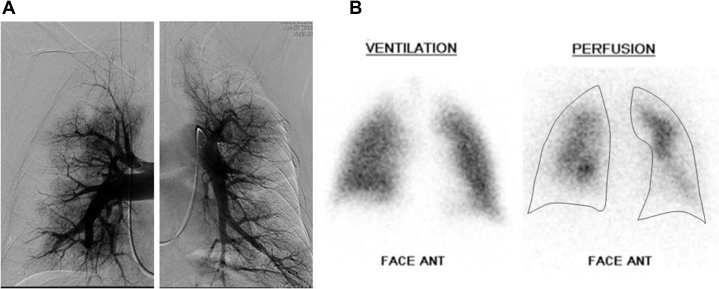
We assessed perfusion of the subpleural areas with the same method as previously described by Tanabe et al.13 The subpleural area was defined as ≤ 1.5 cm (approximately one rib width) from the lateral pleura in the capillary phase of selective pulmonary angiography on the posterior-anterior views and by lateral views of the dorsal area. Patients were classified into either a normally perfused group or a poorly perfused group. The normally perfused group was defined as having normal perfusion of the subpleural space in at least one segment (Fig 1). The poorly perfused group was defined by subpleural spaces that were unperfused or minimally perfused in all segments13 (Fig 2).
Figure 1.
A, Capillary phase of pulmonary angiograms and (B) ventilation-perfusion scintigram in a case with normal subpleural perfusion (pulmonary arterial pressure: 79/25 mm Hg [mean: 45 mm Hg]; cardiac index: 3.33 L/min/m2; pulmonary vascular resistance: 7.47 Wood units).
Figure 2.
A, Capillary phase of pulmonary angiograms and (B) ventilation-perfusion scintigram in a case with poor subpleural perfusion (pulmonary arterial pressure: 79/27 mm Hg [mean: 45 mm Hg]; cardiac index: 2.19 L/min/m2; pulmonary vascular resistance: 10.0 Wood unit).
The development of bronchial arteries was also assessed with a 64-row multidetector enhanced CT scanner during the arterial phase with the same method described by Shimizu et al.21 Bronchial arteries were identified arising from the descending aorta or branches of the aorta, and running together with a bronchus. Their diameter was measured at the proximal site where they originated from the aorta. The total bronchial artery area was calculated as the sum of the cross-sectional area of each bronchial artery.
The assessment of pulmonary angiograms and CT images was performed blinded to the patients' identity and hemodynamic information. In cases in which PSP and bronchial artery anatomy assessment were difficult, a final consensus was reached by consultation with two experienced radiologists (P. B. and O. P.).
BPA Procedure
BPA was performed by using techniques similar to those previously described.16, 17 We approached the pulmonary arteries through the right femoral vein using a peripheral guiding sheath (6F Destination 65 cm [Terumo]; 7F ArrowFlex 80 cm [Teleflex]). A 6F guide catheter (Launcher, Multipurpose, or Judkins right and left 4.0; Medtronic) was inserted through the peripheral guiding sheath and was advanced to the target vessels. Based on selective pulmonary angiography, a 0.014-inch guidewire (Whisper MS; Abbott Vascular) was passed across the target lesion. While selecting the target vessels, the lower lobe lesions were preferentially dilated because the pulmonary blood flow at this site is relatively high, thus lowering mean PAP. In cases of severe stenosis, abrupt narrowing, or complete obstruction, a 2.0-mm balloon catheter was initially used to dilate the lesions. Subsequently, the lesions were dilated to an appropriate size by using 2.0- to 9.0-mm balloon catheters depending on vessel diameter (NC TREK or Viatrac 14 Plus; Abbott Vascular). We treated two to six segmental or subsegmental arteries in each procedure session according to patient severity, time of procedure (< 2 h), and the amount of contrast media given. Two BPA sessions were performed at 2- or 3-day intervals during one hospital admission, and a total of two to 10 sessions were completed. If patients were symptomatic (hemoptysis, worsening of dyspnea, and/or hypoxemia), chest radiography or CT imaging of the chest was performed for detection of pulmonary opacities indicative of lung injury.
Definition of BPA Success and Failure
We classified patients into a “BPA success” group and a “BPA failure” group according to the results of RHC re-evaluation 3 months following the last BPA session. There is no consensus regarding the definition of BPA success or failure. Tsuji et al22 defined BPA responders as patients who achieved a mean PAP ≤ 30 mm Hg at follow-up. However, the mean PAP value following BPA procedures cannot be used alone to identify responders and nonresponders. Indeed, a patient who has, for instance, a pre-BPA mean PAP of 60 mm Hg and who achieves a post-BPA mean PAP just above 30 mm Hg cannot be considered as a poor responder. Thus, the use of two hemodynamic parameters, including the mean PAP value and the decrease in PVR after BPA, seems more appropriate to classify patients as responders (BPA success) or poor responders (BPA failure).
Considering the results of randomized controlled trials that assessed the efficacy of targeted medical therapies in nonoperable CTEPH, a decrease in PVR by at least 30% seems relevant to define a significant improvement in pulmonary hemodynamic variables.23, 24, 25 We therefore defined the BPA failure group as patients who met both of the following criteria: (1) mean PAP > 30 mm Hg; and (2) PVR decrease < 30% at re-evaluation despite a sufficient number of BPA sessions. If mean PAP at re-evaluation was > 30 mm Hg but PVR decreased ≥ 30%, or if mean PAP was ≤ 30 mm Hg but PVR decreased < 30%, BPA was classified as successful.
Statistical Analysis
Data were stored in a personal computer-based spreadsheet. All statistical analyses were performed by using GraphPad Prism version 5 (GraphPad Software) and SPSS Statistics 17.0 (IBM SPSS Statistics, IBM Corporation). Continuous variables are expressed as mean ± SD or median and interquartile range according to variable distribution. Differences in continuous variables of patients’ age, 6-min walk distance, total area of bronchial arteries, and hemodynamic characteristics were compared by using independent Student’s t tests for normally distributed variables and the Mann-Whitney U test for nonnormally distributed variables. Categorical data of patient sex, subpleural perfusion, NYHA functional class, and use of PAH-targeted medications were expressed as number and percentage and were compared by using the χ2 test for independence.
Univariate and multivariate analyses based on the logistic regression model were used to examine the association of each variable (baseline clinical and hemodynamic characteristics) with failure of BPA. For all analyses, the level of statistical significance was set at P < .05.
Results
BPA Success Group vs Failure Group
During the study period, 147 patients underwent BPA. Among these, 46 patients were excluded: rescue or emergency BPA (n = 4), absent or unclear baseline pulmonary angiography (n = 11), and no baseline or re-evaluation after BPA (n = 31). Of the 101 patients included in the analysis, 86 were classified in the BPA success group and 15 patients in the BPA failure group. The median interval from baseline RHC to first BPA session was 2.1 months (interquartile range, 1.0-3.7 months), and the median interval from last BPA session to re-evaluation RHC was 3.5 months (interquartile range, 3.2-4.5 months).
Baseline hemodynamic variables and characteristics of the BPA success and failure groups are summarized in Table 1. Four patients had baseline mean PAP ≤ 30 mm Hg. Baseline hemodynamic variables in both groups were similar. However, the BPA success group had a higher diffusing capacity for carbon monoxide (Dlco) (63.1 ± 12.8% vs 54.9 ± 15.7%; P = .035) and had more developed bronchial arteries with a larger total cross-sectional area (8.7 [6.9 to 11.3] mm2 vs 6.7 [5.3 to 10.0] mm2; P = .043) compared with the BPA failure group. PSP was observed in 13.9% of patients in the BPA success group compared with 46.7% of patients in the BPA failure group (P = .003).
Table 1.
Baseline Characteristics of the Patient Population
| Variable | Overall Population (N = 101) |
BPA Success Group (n = 86) |
BPA Failure Group (n = 15) |
P Valuea |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age, y | 63.4 ± 13.8 | 62.9 ± 13.8 | 66.1 ± 13.8 | .392 |
| Male | 54 (53.5%) | 45 (52.3%) | 9 (60.0%) | .582 |
| BAA, mm2 | 8.4 [6.4-11.2] | 8.7 [6.9-11.3] | 6.7 [5.3-10.0] | .043 |
| NYHA functional class (I, II/III, IV), % | 26.6/73.4 | 27.9/72.1 | 20.0/80.0 | .753 |
| 6MWD, m | 399 ± 116 | 408 ± 111 | 376 ± 79 | .359 |
| Subpleural perfusion (poorly perfused) | 19 (18.8%) | 12 (13.9%) | 7 (46.7%) | .003 |
| Dlco, % | 61.8 ± 13.6 | 63.1 ± 12.8 | 54.9 ± 15.7 | .035 |
| Baseline hemodynamics | ||||
| Mean RAP, mm Hg | 8.1 ± 3.7 | 8.1 ± 3.6 | 8.4 ± 4.4 | .747 |
| Systolic PAP, mm Hg | 78.4 ± 17.4 | 78.7 ± 18.1 | 76.3 ± 12.4 | .633 |
| Diastolic PAP, mm Hg | 25.3 ± 7.9 | 24.9 ± 7.8 | 27.4 ± 8.1 | .267 |
| Mean PAP, mm Hg | 45.6 ± 10.3 | 45.5 ± 10.4 | 46.1 ± 10.2 | .827 |
| PAWP, mm Hg | 9.5 ± 3.6 | 9.6 ± 3.7 | 8.7 ± 2.8 | .375 |
| DPG, mm Hg (diastolic PAP-PCWP) | 15.8 ± 8.3 | 15.3 ± 8.5 | 18.7 ± 6.4 | .147 |
| Cardiac output, L/min | 4.65 ± 1.22 | 4.61 ± 1.15 | 4.88 ± 1.60 | .425 |
| Cardiac index, L/min/m2 | 2.53 ± 0.51 | 2.52 ± 0.49 | 2.60 ± 0.69 | .582 |
| PVR, Wood unit | 8.30 ± 3.16 | 8.32 ± 3.27 | 8.21 ± 2.47 | .886 |
| Svo2, % | 61.7 ± 7.2 | 62.3 ± 7.2 (n = 62) |
58.1 ± 6.2 (n = 11) |
.072 |
| PH medical treatment at baseline | ||||
| No treatment/monotherapy/double combination/triple combination therapy, % | 46/23/27/4 | 48/22/26/4 | 33/27/33/7 | .585 |
Data are presented as mean SD or median [interquartile range] unless otherwise indicated. 6MWD = 6-min walk distance; BAA = bronchial arterial area; BPA = balloon pulmonary angioplasty; DPG = diastolic pressure gradient; Dlco = diffusing capacity for carbon monoxide; NYHA = New York Heart Association; PAP = pulmonary arterial pressure; PAWP = pulmonary artery wedge pressure; PCWP = pulmonary capillary wedge pressure; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; RAP = right atrial pressure; Svo2 = mixed venous oxygen saturation.
Comparison between operable and nonoperable patient populations.
Hemodynamic results of both groups after BPA procedures are summarized in Table 2. Fifty-three patients (62%) in the BPA success group achieved a mean PAP ≤ 30 mm Hg and 24 patients (28%) in the BPA success group achieved a mean PAP ≤ 25 mm Hg following BPA.
Table 2.
Hemodynamic Results Between BPA Success Group and BPA Failure Group
| Variable | BPA Success Group (n = 86) |
BPA Failure Group (n = 15) |
P Value |
|---|---|---|---|
| No. of BPA sessions | 6.1 ± 2.4 | 5.3 ± 2.2 | .203 |
| After BPA | |||
| NYHA functional class (I, II/III, IV), % | 91.3/8.7 | 58.3/41.7 | .009 |
| 6MWD, m | 444 ± 124 | 376 ± 96 | .076 |
| Hemodynamics after BPA | |||
| Mean RAP, mm Hg | 6.4 ± 2.8 | 7.5 ± 3.5 | .167 |
| Systolic PAP, mm Hg | 49.1 ± 13.7 | 72.0 ± 14.9 | < .0001 |
| Diastolic PAP, mm Hg | 16.6 ± 5.2 | 26.5 ± 8.7 | < .0001 |
| Mean PAP, mm Hg | 29.1 ± 7.1 | 43.1 ± 8.7 | < .0001 |
| PAWP, mm Hg | 10.7 ± 3.5 | 9.5 ± 3.3 | .227 |
| Cardiac output, L/min | 5.62 ± 1.45 | 4.87 ± 1.46 | .070 |
| Cardiac index, L/min/m2 | 3.07 ± 0.70 | 2.54 ± 0.55 | .007 |
| PVR, Wood unit | 3.46 ± 1.39 | 7.35 ± 2.63 | < .0001 |
| Svo2, % | 69.5 ± 5.8 | 62.9 ± 4.9 | .001 |
| % decrease of PVR | –54.6 ± 18.6 | –15.2 ± 10.8 | < .0001 |
| % decrease of mean PAP | –34.0 ± 17.2 | –5.3 ± 13.4 | < .0001 |
| No. of patients (mean PAP ≤ 30 mm Hg) | 53 | 0 | < .001 |
Data are presented as mean ± SD unless otherwise indicated. See Table 1 legend for expansion of abbreviations.
Hemodynamic Results According to Subpleural Perfusion
Eighty-two patients were classified in the normally perfused group and 19 in the poorly perfused group. At baseline, the poorly perfused group had higher diastolic PAP (29.1 ± 6.9 mm Hg vs 24.4 ± 7.9 mm Hg; P = .019), lower Dlco (55.9 ± 11.4% vs 63.3 ± 13.7%; P = .037), lower mixed oxygen saturation (57.9 ± 6.2% vs 62.4 ± 7.2%; P = .046), a higher proportion of NYHA functional class III and IV (94.7% vs 68.0%; P = .018), and less well developed bronchial arteries (total area of bronchial arteries: 7.0 [5.8-9.6] mm2 vs 8.7 [6.9-11.3] mm2; P = .032) compared with those in the normally perfused group. Other baseline hemodynamic variables and medical treatments did not differ significantly between groups (Table 3). There was no significant difference in number of BPA sessions between the two groups.
Table 3.
Baseline Characteristics and Treatments in Patients From the Normally Perfused Group and the Poorly Perfused Group
| Variable | Normally Perfused (n = 82) |
Poorly Perfused (n = 19) |
P Value |
|---|---|---|---|
| Baseline characteristics | |||
| Age, y | 63.6 ± 13.9 | 62.5 ± 13.8 | .765 |
| Male | 42 (51.2%) | 12 (63.2%) | .374 |
| BAA, mm2 | 8.7 [6.9-11.3] | 7.0 [5.8-9.6] | .032 |
| NYHA functional class (I, II/III, IV), % | 32.0/68.0 | 5.3/94.7 | .018 |
| 6MWD, m | 404 ± 114 | 378 ± 174 | .421 |
| Dlco, % | 63.3 ± 13.7 | 55.9 ± 11.4 | .037 |
| Baseline hemodynamic variables | |||
| Mean RAP, mm Hg | 8.0 ± 3.6 | 8.7 ± 4.1 | .458 |
| Systolic PAP, mm Hg | 77.8 ± 17.5 | 80.6 ± 17.1 | .543 |
| Diastolic PAP, mm Hg | 24.4 ± 7.9 | 29.1 ± 6.9 | .019 |
| Mean PAP, mm Hg | 44.9± 10.3 | 48.6 ± 9.7 | .161 |
| PAWP, mm Hg | 9.4 ± 3.8 | 9.8 ± 2.8 | .707 |
| DPG, mm Hg (diastolic PAP-PCWP) | 15.0 ± 8.4 | 19.3 ± 6.9 | .042 |
| Cardiac output, L/min | 4.71 ± 1.26 | 4.34 ± 1.03 | .246 |
| Cardiac index, L/min/m2 | 2.57 ± 0.52 | 2.34 ± 0.47 | .083 |
| PVR, Wood unit | 8.12 ± 3.20 | 9.03 ± 3.0 | .271 |
| Svo2, % | 62.4 ± 7.2 (n = 61) |
57.9 ± 6.2 (n = 12) |
.046 |
| PH medical treatment at baseline | |||
| No treatment/monotherapy/ double combination therapy/ triple combination therapy, % | 48/25/23/4 | 37/16/42/5 | .348 |
Data are presented as mean ± SD or median [interquartile range] unless otherwise indicated. See Table 1 legend for expansion of abbreviations.
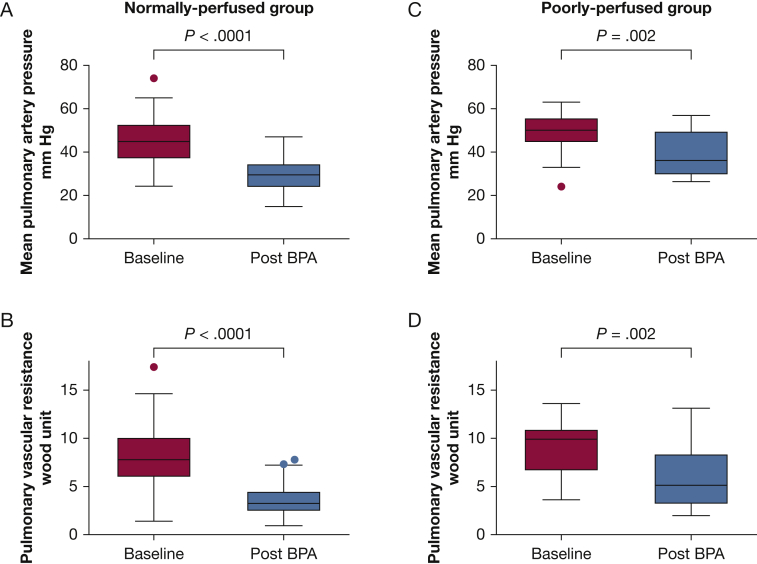
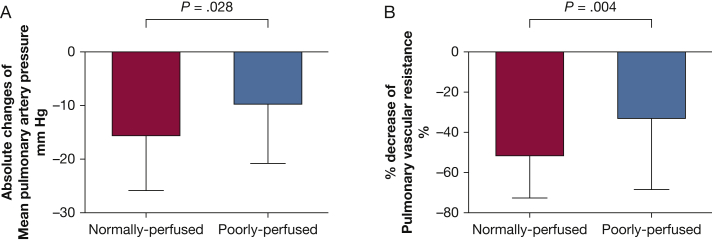
Figure 3 shows the hemodynamic results following BPA in the normal and PSP groups. In the normally perfused group, mean PAP and PVR markedly decreased (44.9 ± 10.3 mm Hg to 29.4 ± 7.5 mm Hg [P < .0001] and 8.12 ± 3.20 Wood unit to 3.61 ± 1.57 Wood unit [P < .0001], respectively). In the poorly perfused group, mean PAP and PVR also improved (48.6 ± 9.7 mm Hg to 39.0 ± 10.2 mm Hg [P = .002] and 9.03 ± 2.99 Wood unit to 5.89 ± 3.10 Wood unit [P = .002]). The decrease in mean PAP and percent decrease in PVR in the poorly perfused group were less than those observed in the normally perfused group (mean PAP: –9.6 ± 11.1 mm Hg vs –15.5 ± 10.3 mm Hg [P = .028]; mean PVR: –33.1 ± 35.2% vs –51.5 ± 21.0% [P = .004]) (Fig 4).
Figure 3.
A-D, Hemodynamic results in the normally perfused and poorly perfused groups. Mean (A) PAP and (B) PVR in the normally perfused group. Mean (C) PAP and (D) PVR in the poorly perfused group. PAP = pulmonary arterial pressure; PVR = pulmonary vascular resistance.
Figure 4.
A, Absolute changes in mean PAP and (B) percent decrease in PVR in the normally perfused and poorly perfused groups. See Figure 3 legend for expansion of abbreviations.
In the normally perfused group, arterial oxygen pressure improved (65.9 ± 9.2 mm Hg to 76.4 ± 11.6 mm Hg; P < .001), and nine of 20 patients (45.0%) who had ambulatory oxygen therapy were able to discontinue it. In the poorly perfused group, arterial oxygen pressure showed no significant change (64.8 ± 11.1 mm Hg to 68.3 ± 10.1 mm Hg; P = .616), and only one of six patients (16.7%) discontinued ambulatory oxygen therapy.
There was no significant difference in the incidence of lung injury between the normally and poorly perfused groups (12.2% [59 of 482 sessions] vs 11.7% [14 of 120 sessions]; P = .863).
Predictors of Failure of BPA
Table 4 presents the results of the logistic regression analyses of baseline variables associated with BPA failure. In univariate analysis, small total area of bronchial arteries, PSP, and low baseline Dlco were associated with BPA failure, whereas no baseline hemodynamic variables were risk factors for BPA failure. In forward stepwise multivariate analysis, only PSP was independently associated with BPA failure (adjusted OR, 4.02 [95% CI, 1.17-13.89]; P = .028).
Table 4.
Univariate and Multivariate Logistic Regression Analyses of Predictive Variables of BPA Failure
| Variable | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Baseline characteristics | ||||||
| Age, y | 1.02 | 0.98-1.06 | .415 | … | … | |
| Male | 1.37 | 0.45-4.17 | .580 | … | … | |
| BAA, mm2 | 0.80 | 0.65-0.99 | .045 | … | … | |
| NYHA functional class (I-II vs III-IV) | 0.65 | 0.17-2.52 | .531 | … | … | |
| 6MWD, m | 0.99 | 0.99-1.01 | .482 | … | … | |
| Subpleural perfusion (poorly perfused vs normally perfused) | 5.396 | 1.65-17.62 | .005 | 4.02 | 1.17-13.89 | .028 |
| Dlco, % | 0.95 | 0.90-0.99 | .038 | … | … | |
| Baseline hemodynamic variables | … | … | ||||
| Mean RAP, mm Hg | 1.03 | 0.88-1.19 | .745 | … | … | |
| Systolic PAP, mm Hg | 0.99 | 0.96-1.03 | .629 | … | … | |
| Diastolic PAP, mm Hg | 1.04 | 0.97-1.12 | .266 | … | … | |
| Mean PAP, mm Hg | 1.01 | 0.95-1.06 | .825 | … | … | |
| PAWP, mm Hg | 0.93 | 0.79-1.09 | .372 | … | … | |
| Cardiac index, L/min/m2 | 1.35 | 0.47-3.88 | .578 | … | … | |
| PVR, Wood unit | 0.99 | 0.83-1.18 | .904 | … | … | |
| Svo2, % | 0.92 | 0.84-1.01 | .077 | … | … | |
See Table 1 legend for expansion of abbreviations.
Discussion
In the present study, PSP predicted BPA failure according to prespecified hemodynamic criteria, despite the observation that patients with PSP had baseline hemodynamic variables similar to patients with normal subpleural perfusion. Patients with PSP also had a lower baseline Dlco, suggesting the presence of small pulmonary vessel disease with diffuse distal thrombosis. Thus, PSP is a risk factor for BPA failure. The present study also showed that approximately 15% of patients with nonoperable CTEPH exhibited poor responses to BPA.
Predictive Factors for Failure of BPA
Although the efficacy of BPA in terms of clinical status, hemodynamic variables, and exercise capacity has been previously reported, studies regarding predictors of BPA failure are limited. Tsuji et al22 reported that the duration between onset of symptoms to BPA and diastolic PAP at baseline were predictive factors for poor response to BPA (mean PAP > 30 mm Hg at follow-up). This group also showed that diastolic PAP in patients with PAH was higher than in patients with CTEPH.26 Tsuji et al considered that long-standing PH might lead to secondary microvascular arteriopathy and that higher diastolic PAP reflected more extensive small vessel remodeling. In addition, the diastolic pulmonary gradient (difference between diastolic PAP and mean pulmonary capillary wedge pressure) is associated with pulmonary vascular disease and poor prognosis in patients with left heart disease,27, 28 and it is also independently related to survival in patients with PAH.29 It might thus be proposed that elevated diastolic PAP and diastolic pulmonary gradient reflect microvasculopathy in patients with CTEPH. Moreover, a lower Dlco was associated with poor outcomes of patients with CTEPH and might indicate a pronounced microvasculopathy.30 In the present study, patients in the poorly perfused group had significantly higher diastolic PAP, higher diastolic pulmonary gradient, lower Dlco, lower mixed oxygen saturation, and worse NYHA functional class. It is thus likely that PSP reflects the presence of small pulmonary vessel disease with diffuse distal thrombosis in nonoperable CTEPH.
Interestingly, in our study, no baseline hemodynamic variables were associated with outcomes following BPA. In the aforementioned study, Tsuji et al22 defined poor responders as patients who had mean PAP > 30 mm Hg at follow-up. We defined poor responders as patients with both mean PAP > 30 mm Hg and PVR decrease < 30% following BPA. Therefore, patients with a mean PAP > 30 mm Hg but significantly decreased PVR following BPA were classified as BPA successes. The degree of hemodynamic improvement depends somewhat on the baseline severity. Patients with high baseline mean PAP might be more likely to remain > 30 mm Hg even if mean PAP substantially decreased, and patients with high baseline PVR tend to exhibit larger relative decreases following BPA. Moreover, patients with mild elevations in mean PAP and PVR at baseline have smaller relative changes but might more easily achieve a mean PAP ≤ 30 mm Hg, potentially explaining why baseline hemodynamic variables were not predictors of BPA failure in the present study.
Indications for BPA
Although BPA has had promising results in patients with nonoperable CTEPH, there is no established consensus regarding the indications and contraindications for BPA. Our present study has yielded important insights into predictive factors for BPA failure. However, we do not suggest that PSP is a contraindication for BPA. Figure 3 shows that mean PAP and PVR also improved significantly in the poorly perfused group. The range between the upper and lower quartiles in PVR following BPA was particularly wide in the poorly perfused group, and some patients in the poorly perfused group had successful BPA outcomes. Further studies are needed to identify why some patients with small pulmonary vessel disease still have the potential for improvement. This may depend on the severity of small vessel disease, or the extent of PSP, because some poorly perfused regions may have open arterioles without small vessel disease distal to the completely occluded proximal segment. Nevertheless, assessment of subpleural perfusion in the capillary phase seems to be a reasonably useful tool to predict poor responders to BPA. In patients who exhibit a poor hemodynamic response after several sessions of BPA, discontinuing the BPA procedure and initiation of other medical treatments should be considered.
Distal Disease and Bronchial Arteries in CTEPH
The development of hypertrophied bronchial arteries is a well-known feature in patients with CTEPH.31 They might reflect collaterals between the systemic and pulmonary arterial circulation.31, 32 It has been proposed that pre-existing anastomoses between the bronchial and pulmonary arteries might be opened by the abnormal pressure gradient resulting from a decrease in pulmonary artery pressure distal to an obstructing lesion. Anastomoses are not only observed between bronchial arteries and precapillary pulmonary arterioles but also with postcapillary venules and small veins.12 Collateral anastomoses from the systemic circulation have an important role to maintain perfusion flow and maintain viability of ischemic pulmonary parenchyma downstream of proximal pulmonary artery obstructions.33 Shimizu et al21 reported that the cross-sectional area of bronchial arteries correlated with the central extent of the thromboembolic material from analysis of CT imaging of 59 patients with CTEPH. Their study showed that bronchial artery total area in the proximal type of CTEPH was significantly greater than in the distal type. Simonneau et al10 suggested that distal thrombosis could be diffuse when small pulmonary arterioles distal to more proximal complete obstructions are not maintained open because bronchial arteries and anastomoses fail to develop. The present study showed that in patients with nonoperable CTEPH, bronchial artery total area in the normally perfused group was larger than that of the poorly perfused group (Table 3). Poorly developed bronchial arteries might be involved in the development of diffuse distal thrombosis in patients with CTEPH.
Limitations
The main limitation of this study is its retrospective observational nature. Therefore, the occurrence of some missing values was unavoidable and might have influenced the results in the multivariate regression model. Furthermore, it is undeniable that less experience with the procedure early on during our program might have affected BPA outcomes in the early period.
Conclusions
PSP in the capillary phase of pulmonary angiography, suggesting the presence of small pulmonary vessel disease with diffuse distal thrombosis, is a predictor of BPA failure. In the present study, PSP affected approximately 15% of patients with nonoperable CTEPH who underwent BPA. PSP was also associated with less developed bronchial arteries, suggesting a key role of bronchial-pulmonary anastomoses in maintaining pulmonary capillary bed patency downstream of the more proximal pulmonary artery obstructions in CTEPH.
Acknowledgments
Author contributions: Y. T. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. P. B. and O. P. participated in the collection of data and interpretation of the data, and revised the manuscript. J. W., C. G., and X. J. participated in data collection and revised the manuscript. E. F. and M. H. revised the manuscript. G. S. designed the study and revised the manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: G. S. has relationships with drug companies, including Actelion, Bayer, MSD, GlaxoSmithKline, Biotrial, and Arena; in addition to being investigator in trials involving these companies, his relationships include consultancy service and membership of scientific advisory boards. X. J. has relationships with drug companies, including Actelion, Bayer, GlaxoSmithKline, and MSD; in addition to being investigators in trials involving these companies, other relationships include research grants, speaking fees, consultancy services, and memberships of scientific advisory boards. J. W. has received research grants from the European Respiratory Society, Canadian Thoracic Society, and the Canadian Vascular Network; he has also received consultant fees from Actelion, advisory board activities from Actelion, travel support from Actelion and Bayer, and speaking fees from Actelion, Bayer, and Novartis. M. H. has relationships with drug companies, including Actelion, Bayer, GlaxoSmithKline, and Merck; in addition to being investigator in trials involving these companies, relationships include consultancy service and membership of scientific advisory boards. None declared (Y. T., P. B., C. G., O. P., E. F.).
Footnotes
FUNDING/SUPPORT: The authors have reported to CHEST that no funding was received for this study.
References
- 1.Dartevelle P., Fadel E., Mussot S. Chronic thromboembolic pulmonary hypertension. Eur Respir J. 2004;23(4):637–648. doi: 10.1183/09031936.04.00079704. [DOI] [PubMed] [Google Scholar]
- 2.Hoeper M.M., Mayer E., Simonneau G. Chronic thromboembolic pulmonary hypertension. Circulation. 2006;113(16):2011–2020. doi: 10.1161/CIRCULATIONAHA.105.602565. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M. Pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: pathophysiology. Eur Respir Rev. 2010;19(115):59–63. doi: 10.1183/09059180.00007309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedel M., Stanek V., Widimsky J. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81(2):151–158. doi: 10.1378/chest.81.2.151. [DOI] [PubMed] [Google Scholar]
- 5.Ende-Verhaar Y.M., Cannegieter S.C., Vonk Noordegraaf A. Incidence of chronic thromboembolic pulmonary hypertension after acute pulmonary embolism: a contemporary view of the published literature. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.01792-2016. [DOI] [PubMed] [Google Scholar]
- 6.Simonneau G., Hoeper M.M. Evaluation of the incidence of rare diseases: difficulties and uncertainties, the example of chronic thromboembolic pulmonary hypertension. Eur Respir J. 2017;49(2) doi: 10.1183/13993003.02522-2016. [DOI] [PubMed] [Google Scholar]
- 7.Hoeper M.M., Barbera J.A., Channick R.N. Diagnosis, assessment, and treatment of non-pulmonary arterial hypertension pulmonary hypertension. J Am Coll Cardiol. 2009;54(suppl 1):S85–S96. doi: 10.1016/j.jacc.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Pepke-Zaba J., Delcroix M., Lang I. Chronic thromboembolic pulmonary hypertension (CTEPH): results from an international prospective registry. Circulation. 2011;124(18):1973–1981. doi: 10.1161/CIRCULATIONAHA.110.015008. [DOI] [PubMed] [Google Scholar]
- 9.Kim N.H. Group 4 pulmonary hypertension: chronic thromboembolic pulmonary hypertension: epidemiology, pathophysiology, and treatment. Cardiol Clin. 2016;34(3):435–441. doi: 10.1016/j.ccl.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 10.Simonneau G., Torbicki A., Dorfmuller P. The pathophysiology of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26(143) doi: 10.1183/16000617.0112-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang I.M., Dorfmuller P., Vonk Noordegraaf A. The pathobiology of chronic thromboembolic pulmonary hypertension. Ann Am Thorac Soc. 2016;13(suppl 3):S215–S221. doi: 10.1513/AnnalsATS.201509-620AS. [DOI] [PubMed] [Google Scholar]
- 12.Dorfmuller P., Gunther S., Ghigna M.R. Microvascular disease in chronic thromboembolic pulmonary hypertension: a role for pulmonary veins and systemic vasculature. Eur Respir J. 2014;44(5):1275–1288. doi: 10.1183/09031936.00169113. [DOI] [PubMed] [Google Scholar]
- 13.Tanabe N., Sugiura T., Jujo T. Subpleural perfusion as a predictor for a poor surgical outcome in chronic thromboembolic pulmonary hypertension. Chest. 2012;141(4):929–934. doi: 10.1378/chest.11-0769. [DOI] [PubMed] [Google Scholar]
- 14.Pepke-Zaba J., Jansa P., Kim N.H. Chronic thromboembolic pulmonary hypertension: role of medical therapy. Eur Respir J. 2013;41(4):985–990. doi: 10.1183/09031936.00201612. [DOI] [PubMed] [Google Scholar]
- 15.Feinstein J.A., Goldhaber S.Z., Lock J.E. Balloon pulmonary angioplasty for treatment of chronic thromboembolic pulmonary hypertension. Circulation. 2001;103(1):10–13. doi: 10.1161/01.cir.103.1.10. [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi H., Ogawa A., Munemasa M. Refined balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension. Circ Cardiovasc Interv. 2012;5(6):748–755. doi: 10.1161/CIRCINTERVENTIONS.112.971077. [DOI] [PubMed] [Google Scholar]
- 17.Taniguchi Y., Miyagawa K., Nakayama K. Balloon pulmonary angioplasty: an additional treatment option to improve the prognosis of patients with chronic thromboembolic pulmonary hypertension. EuroIntervention. 2014;10(4):518–525. doi: 10.4244/EIJV10I4A89. [DOI] [PubMed] [Google Scholar]
- 18.Olsson K.M., Wiedenroth C.B., Kamp J.C. Balloon pulmonary angioplasty for inoperable patients with chronic thromboembolic pulmonary hypertension: the initial German experience. Eur Respir J. 2017;49(6) doi: 10.1183/13993003.02409-2016. [DOI] [PubMed] [Google Scholar]
- 19.Matsubara H., Ogawa A. A long way to go after the initial experience with balloon pulmonary angioplasty. Eur Respir J. 2017;49(6) doi: 10.1183/13993003.00718-2017. [DOI] [PubMed] [Google Scholar]
- 20.Galie N., Humbert M., Vachiery J.L. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu H., Tanabe N., Terada J. Dilatation of bronchial arteries correlates with extent of central disease in patients with chronic thromboembolic pulmonary hypertension. Circ J. 2008;72(7):1136–1141. doi: 10.1253/circj.72.1136. [DOI] [PubMed] [Google Scholar]
- 22.Tsuji A., Ogo T., Ueda J. Predictors of residual pulmonary hypertension after balloon pulmonary angioplasty in patients with chronic thromboembolic pulmonary hypertension. Int J Cardiol. 2017;226:118–120. doi: 10.1016/j.ijcard.2016.09.132. [DOI] [PubMed] [Google Scholar]
- 23.Jais X., D'Armini A.M., Jansa P. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52(25):2127–2134. doi: 10.1016/j.jacc.2008.08.059. [DOI] [PubMed] [Google Scholar]
- 24.Ghofrani H.A., D'Armini A.M., Grimminger F. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319–329. doi: 10.1056/NEJMoa1209657. [DOI] [PubMed] [Google Scholar]
- 25.Ghofrani H.A., Simonneau G., D'Armini A.M. Macitentan for the treatment of inoperable chronic thromboembolic pulmonary hypertension (MERIT-1): results from the multicentre, phase 2, randomised, double-blind, placebo-controlled study. Lancet Respir Med. 2017;5(10):785–794. doi: 10.1016/S2213-2600(17)30305-3. [DOI] [PubMed] [Google Scholar]
- 26.Nakayama Y., Nakanishi N., Sugimachi M. Characteristics of pulmonary artery pressure waveform for differential diagnosis of chronic pulmonary thromboembolism and primary pulmonary hypertension. J Am Coll Cardiol. 1997;29(6):1311–1316. doi: 10.1016/s0735-1097(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 27.Naeije R., Vachiery J.L., Yerly P. The transpulmonary pressure gradient for the diagnosis of pulmonary vascular disease. Eur Respir J. 2013;41(1):217–223. doi: 10.1183/09031936.00074312. [DOI] [PubMed] [Google Scholar]
- 28.Gerges C., Gerges M., Lang M.B. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143(3):758–766. doi: 10.1378/chest.12-1653. [DOI] [PubMed] [Google Scholar]
- 29.Mazimba S., Mejia-Lopez E., Black G. Diastolic pulmonary gradient predicts outcomes in group 1 pulmonary hypertension (analysis of the NIH primary pulmonary hypertension registry) Respir Med. 2016;119:81–86. doi: 10.1016/j.rmed.2016.08.024. [DOI] [PubMed] [Google Scholar]
- 30.Suda R., Tanabe N., Ishida K. Prognostic and pathophysiological marker for patients with chronic thromboembolic pulmonary hypertension: usefulness of diffusing capacity for carbon monoxide at diagnosis. Respirology. 2017;22(1):179–186. doi: 10.1111/resp.12883. [DOI] [PubMed] [Google Scholar]
- 31.Endrys J., Hayat N., Cherian G. Comparison of bronchopulmonary collaterals and collateral blood flow in patients with chronic thromboembolic and primary pulmonary hypertension. Heart. 1997;78(2):171–176. doi: 10.1136/hrt.78.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ley S., Kreitner K.F., Morgenstern I. Bronchopulmonary shunts in patients with chronic thromboembolic pulmonary hypertension: evaluation with helical CT and MR imaging. AJR Am J Roentgenol. 2002;179(5):1209–1215. doi: 10.2214/ajr.179.5.1791209. [DOI] [PubMed] [Google Scholar]
- 33.Mitzner W., Wagner E.M. Vascular remodeling in the circulations of the lung. J Appl Physiol (1985) 2004;97(5):1999–2004. doi: 10.1152/japplphysiol.00473.2004. [DOI] [PubMed] [Google Scholar]