Abstract
In order to evaluate the effect of daily consumption of fruit and vegetable juice on the human intestinal microbial community, we compared changes in the gut microbiota and extracellular vesicles in human feces and bowel, and skin symptoms at the baseline and 3 weeks post juice consumption of 22 participants. After 3 weeks of juice consumption, a significant increase in the richness of microbiota (α-diversity, P < 0.05) was observed. It was accompanied by an abundance in Faecalibacterium (bacterial: from 1.62 ± 0.80% to 2.14 ± 0.72% and extra vesicle: 2.49 ± 1.49% to 6.06 ± 3.07%; P < 0.05 in all cases). At the end of the study period, there were reductions in body weight regardless of sex (P < 0.05) and improvements of the symptoms including diarrhea, constipation, fatigue, and skin problems. Eating fruits and vegetables could help modulate the profile of the fecal microbiota and alleviate bowel and skin troubles, and fatigue.
Keywords: Faecalibacterium, Fruit, Vegetable, Extracellular vesicle, Abdominal pain
Introduction
Emerging insights have indicated that the alteration of the genetic composition and metabolic activity of the human gut microbiome may be an important mediator in the pathogenesis of chronic diseases (Turnbaugh et al., 2009). Patients with irritable bowel syndrome (IBS), which was diagnosed by the symptom criteria, such as abdominal pain and/or discomfort, and altered bowel habits, have been reported to have a different composition of microbiota compared to normal individuals (Rajilić-Stojanović et al., 2011). The disrupted microbial architecture has been shown to be associated with clinical manifestation via altered gut-brain axis, visceral hypersensitivity, altered GI motility, epithelial barrier dysfunction, and immune activation (Bhattarai, 2017).
Diet is one of the most important factors affecting the composition of gut microbiota. “Western diet” is rich in animal proteins and fats, and sugars (Cordain et al., 2005), but lacks fruits and vegetables (Grotto and Zied, 2010). Fruits and vegetables are good sources of dietary fiber, phytochemicals, ascorbate, folate, and omega-6 polyunsaturated fatty acids (Orlich et al., 2013). Children fed fiber-rich diets showed a significant richness in the members of Bacteroidetes and a depletion in members of Firmicutes, when compared to those fed a Western diet (De Filippo et al., 2010). A reduction of A. muciniphila and Lactobacillus in those with high-fat diet has been reported (Singh et al., 2017).
Although the link between diet and gut microbiota has been studied extensively, the results have not been consistent, and it is largely unknown whether the daily consumption of just one cup of fruits and vegetable juice could alter the architecture of the fecal microbiome.
Meanwhile, bacteria secrete extracellular vesicles (EVs) into the extracellular milieu (Kim et al., 2013; Zhou et al., 1998). The EVs contain nanoparticles that play an important biological function in intercellular communication by transferring genetic information in the form of DNA and RNA (Kim et al., 2013). Interestingly, Gram-positive bacteria also secrete EVs (Lee et al., 2009). Recent evidence has shown that bacteria-derived EVs could be etiological agents for the development of inflammatory diseases that were once believed to be non-infectious (Kim et al., 2012). To the best of our knowledge, bacteria-derived EVs have not been thoroughly evaluated.
On the basis of this background, using high-throughput molecular approaches, we aimed to investigate the effect of the daily consumption of just one cup of fruit and vegetable juice on the composition of the human fecal bacterial and extravesicular microbiota. Additionally, we investigated the alterations in bowel symptoms, skin trouble, and the physical and mental energy.
Materials and methods
Participants
Subjects with abdominal symptoms related to pain or discomfort, constipation, or diarrhea were recruited by advertisements on online noticeboards (www.naver.com) from May 2016 to August 2016. Most of participants preferred meat-based diet.
Subjects who took medication including antibiotics and probiotics during the previous 6 months, and those with organic diseases or food allergies were excluded. Pregnant and lactating women were also excluded. The methods were conducted in accordance with the Helsinki Declaration of 1983, and informed consent was obtained from all of the subjects. The anonymized met genomic data and questionnaire including details about intestinal symptoms, skin trouble, and physical and mental energy were retrospectively analyzed. This study was approved by the Institutional Review Board of Seoul National University Bundang Hospital (B-1709-420-101).
Study protocol
Symptoms including bowel habits, skin trouble, and both physical and mental energy were collected from the subjects at baseline and at a 3-week time point. Subjects were asked to record all of the food and drink intakes for 3 days prior to baseline. The juice was made by Hurom Co., Ltd. (Kimhae, South Korea) using an H-AA slow juicer. Slow Squeezing Technology uses an auger to squeeze juice from fruits and vegetables (similar to how a person would hand-squeeze an orange), instead of shredding with high-speed blades like other conventional juicers and blenders, to minimize the damage to ingredients and to preserve the natural taste and nutritive content. The ingredients of the juice and the total amount of juice consumed per day are described in Table 1. The juice was delivered individually to subjects every morning for 3 weeks, and the subjects were instructed to maintain their usual dietary pattern during the 3 days before their stool collection in week 3, as far as possible.
Table 1.
Ingredients, composition, and total dose of the juice consumed per day
| Variable | Amount |
|---|---|
| Volume (ml) | 400 |
| Recipe, dry (g) | Kale, 240 |
| Broccoli, 80 | |
| Apple, 240 | |
| Lemon, 5 | |
| Fructose (g) | 16.08 |
| Glucose (g) | 8.44 |
| Sucrose (g) | 2.06 |
| Total sugar (g) | 26.58 |
| Total dietary fiber (g) | 1.24 |
Data and fecal sample collection
Trained interviewers collected general demographic and clinical information, including age, sex, weight, height, and information on health-related behaviors, such as smoking and alcohol consumption, from the participants. At baseline and week 3, fecal samples were collected and delivered in pre-weighed plastic containers for the analyses of the gut microbiota.
Fecal samples were collected by the subjects at home, stored below 0 °C, delivered to the sequencing company (MD healthcare, Seoul, South Korea) within 24 h of collection, and subsequently stored at − 80 °C. After thawing, the feces were evaluated in 2 ways: as total feces and after the isolation of EVs.
EV isolation and DNA extraction from human fecal samples
EVs in human feces were isolated using a differential centrifugation method, as described previously (Kang et al., 2013). Metagenomic analysis was used to evaluate the proportions of bacteria and bacteria-derived EVs in the large intestine. Fecal samples were used to indirectly evaluate the proportions of bacteria and bacteria-derived EVs in the large intestine. Since fecal EVs comprise both host-and bacteria-derived EVs, we amplified the EVs using indigenous 16S ribosomal DNA (16S rDNA), which encodes the 16S ribosomal RNA of bacterial genomic DNA, to exclude host cell-derived EVs. Then, the bacteria and bacteria-derived EVs were assigned to operational taxonomic units (OTUs) using the amplified 16S rDNA.
Stool samples were dissolved in phosphate-buffered saline and centrifuged at 5, 20, and 340g for 5 min each. The supernatant fractions were pelleted once at 10,000 g for 30 min, then filtered through a 0.45-μm syringe filter (Sartorius Stedim Biotech, Goettingen, Germany), followed by filtration through a 0.22-μm syringe filter (Sartorius Stedim Biotech). The filtrates were then subjected to density-gradient centrifugation in a Beckman ultracentrifuge (Beckman Coulter, Fullerton, CA, USA) at 100,000 g for 2 h at 4 °C. The fraction between the 10 and 40% OptiPrep™ solutions (Sigma-Aldrich, St. Louis, MO, USA) was taken, and EVs were prepared by centrifugation at 150 000 g for 2 h at 4 °C using a Beckman ultracentrifuge. The EVs were diluted in phosphate-buffered saline and stored at − 80 °C. The protein concentration of the EVs was assessed by a BCA assay (Thermo Fisher Scientific, Waltham, MA, USA).
Metagenomic analysis using bacteria and bacteria-derived EVs
16S rDNA was amplified with the 16S_V3_F (5′-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGCCTACGGGNGGCWGCAG -3′) and 16S_V4_R (5′-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGACTACHVGGGTATCTAATCC-3′) primers, which are specific for the V3–V4 hypervariable regions of 16S rDNA. The libraries were prepared using PCR products, according to the MiSeq System guide (Illumina, USA) and quantified using a QIAxpert (QIAGEN, Germany). Each amplicon was then quantified, assigned an appropriate equimolar ratio, pooled, and sequenced on a MiSeq (Illumina, USA), according to the manufacturer’s recommendations.
Selection of 16S rDNAs and taxonomic assignments
Sequencing reads of high quality were retained after checking both the score quality (average Phred score > 20) and read lengths (> 300 bp). OTUs were defined using UCLUST and chimeric sequences were eliminated using USEARCH (Edgar, 2010); taxonomic assignment was achieved using QIIME against the 16S rDNA sequence database of GreenGenes 8.15.13 (Caporaso et al., 2010; Lozupone et al., 2006).
Statistical analyses
Data are expressed as the mean (± standard deviation) or as percentages. The basic characteristics, including weight, and the changes in the clustering characteristics, were compared using a paired t test with a Bonferroni correction. All statistical tests were two-tailed, and a P value < 0.05 was considered statistically significant. Statistical analyses were performed using the SPSS 21.0 statistical package (version 21.0, IBM, Armonk, NY).
Results and discussion
Study population
Between May 2016 and August 2016, a total of 24 adults were enrolled in the study. After excluding 2 adults who declined to submit follow-up feces, the results from 22 adults were analyzed. The baseline characteristics of the subjects are shown in Table 2. Among the 22 participants, 7 were male and mean age was 37.0 ± 3.30 years. All of them drank the juice every day for 3 weeks.
Table 2.
Characteristics and symptoms of the 22 participants at baseline and 3 weeks after daily juice intake
| Variables | Baseline | After 3 weeks | ||
|---|---|---|---|---|
| Sex, male (n, [%]) | 7 (31.82) | |||
| Age (years, mean ± SD) | 37.0 ± 3.30 | |||
| Body weight (kg) | ||||
| Total | 68.96 ± 9.15 | 66.89 ± 9.10a | ||
| Men | 76.67 ± 9.44 | 73.78 ± 10.56a | ||
| Women | 63.18 ± 1.60 | 61.73 ± 1.68a | ||
| Symptoms | Improved | Aggravated | Not changed | |
| Fatigue (n, [%]) | 15 (68.18) | 14 (63.63) | 0 | 1 |
| Diarrhea/constipation (n, [%]) | 18 (81.81) | 13 (59.09) | 1 | 4 |
| Skin problems (n, [%]) | 10 (45.45) | 9 (40.90) | 0 | 1 |
| Overall symptoms (n, [%]) | 21 (95.45) | 18 (81.81) | 0 | 3 |
SD standard deviation
aP < 0.05 analyzed by paired t-test
Alpha-diversity of fecal samples
The fecal microbiomes of 22 adults were analyzed by 16S rDNA next-generation sequencing. The species diversity within each microbiome sample (α-diversity) was evaluated by the species-richness metric Chao1 (Fig. 1) (Chao, 1984). Rarefaction curves (Chao1 index) showing the microbial community complexes in 22 samples on day 0 [Fig. 1(A)] and day 21 (3 weeks post juice consumption) [Fig. 1(B)] were obtained. On an average, no statistical difference was detected in the composition of bacteria [Fig. 1(C)], while a significant difference in the Chao1 metric for the EVs was detected at a sampling size of 26,818 OTUs [Fig. 1(D)], that is, a higher 16S rDNA richness in EVs was shown on day 21, when compared to the case for day 0 (P < 0.05). Similar to the present study, children or adults living in rural areas consuming a fiber- and vegetable-rich diet were reported to have more diverse gut microbiota than those residing in the United States consuming a Western diet (De Filippo et al., 2010; Yatsunenko et al., 2012). The loss of microbial diversity has been shown consistently in several diseases including Crohn’s disease, colorectal cancer, and even autism (Ahn et al., 2013; Kang et al., 2013; Sha et al., 2013). Carroll et al., (2012) has reported that the richness of 16S rDNA sequences was significantly decreased in IBS patients, unlike the case for healthy controls. Decreased alpha diversity in IBS has been quite a consistent finding (Bhattarai, 2017).
Fig. 1.
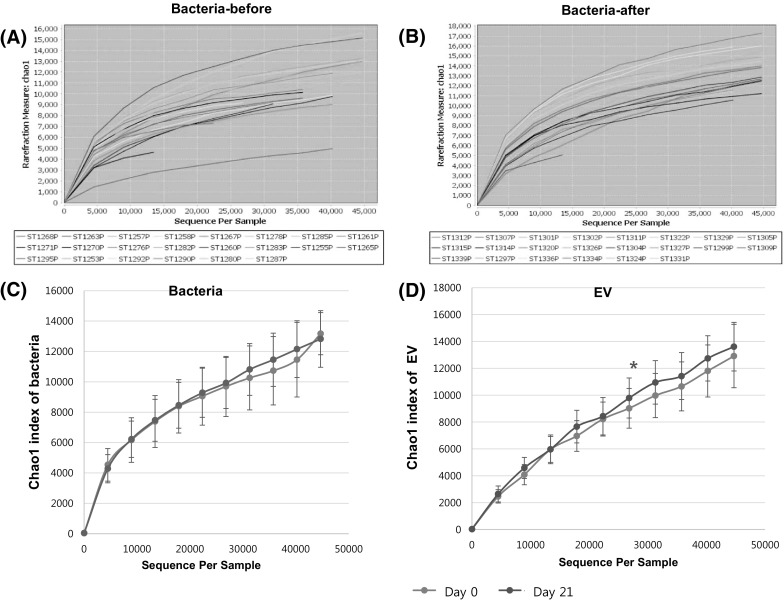
Ecological diversity of the gut microbiome in the subjects. The α-diversities (richness of 16S rDNA) were evaluated based on the rarefied OTU tables. The sampling sizes were 10, 4478, 8946, 13 414, 17 882, 22 350, 26 818, 31 286, 35 754, 40 222, and 44 690 OTUs. (A) Richness of 16S rDNA metric based on the Chao1 method at baseline (left) and week 3 (right). (B) Mean values of α-diversities are illustrated. Significant differences (asterisks) were observed at a sampling size of 26 818 OTUs (P < 0.05, paired t-test). The bars denote standard errors
Composition of fecal bacteria and bacteria-derived EVs at the phylum level
Because diet is the most essential factor that alters the composition and diversity of intestinal bacterial community (Ley et al., 2008; Yatsunenko et al., 2012), the compositions of the fecal bacteria and EVs collected before and after 3 weeks of juice-consumption were compared.
In the fecal sample on day 0, nine bacterial phyla were mostly detected in the gut microbiomes of participants [Fig. 2(A), (C)], including 4 frequently detected phyla (Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria) and 5 minor phyla (Tenericutes, Fusobacteria, Verrucomicrobia, TM7, and Cyanobacteria).
Fig. 2.
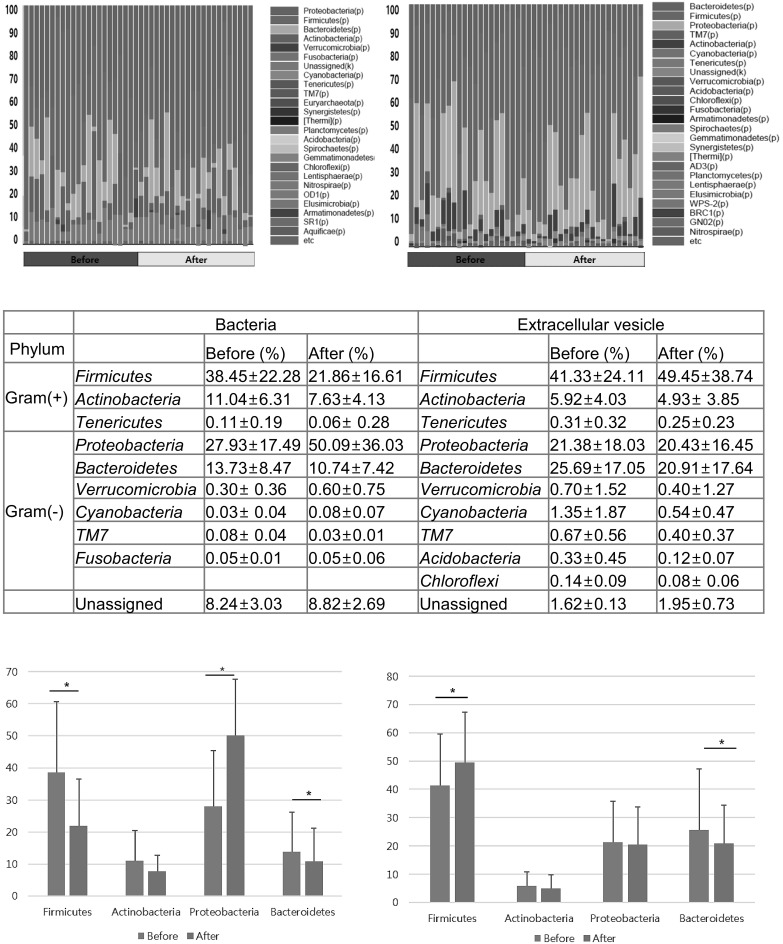
The proportion of fecal bacteria and bacterial EVs at the phylum level. (A) Bacteria, (B) bacteria-derived EVs, (C) proportions of the representative phyla, and compositional change in (D) fecal bacteria and (E) bacterial EVs at the phylum level are listed. The asterisks denote statistical significances
After consumption of the fruit and vegetable juice, the bacterial composition of the feces changed, as reflected by the 16S rDNA sequencing analysis [Fig. 2(B), (C)]. The compositions of Firmicutes, Actinobacteria, and Bacteroidetes decreased as a proportion of total bacteria (mean proportion of change in Firmicutes, Actinobacteria and Bacteroidetes: from 38.45 ± 22.28 to 21.86 ± 16.61%, from 11.04 ± 6.31 to 7.63 ± 4.13%, and from 13.73 ± 8.47 to 10.74 ± 7.42%, respectively; P < 0.05 in all cases) [Fig. 2(D)], but the change in Actinobacteria was not statistically significant. The reduction in the proportion of Firmicutes led to a decrease in the Firmicutes-to-Bacteroidetes (F/B) ratio; however, this did not reach clinical significance (mean ± SD: 15.73 ± 13.58 and 12.67 ± 19.25).
In contrast, the proportion of Proteobacteria, Verrucomicrobia, and TM7 significantly increased (change of mean proportions of above three phyla: from 27.93 ± 17.49 to 50.09 ± 36.03%, from 0.30 ± 0.36 to 0.60 ± 0.75%, and from 0.08 ± 0.04 to 0.03 ± 0.01%, respectively) [Fig. 2(C)] (P < 0.05 in all cases). Compositions of other bacteria including Cyanobacteria, Fusobacteria, and Tenericutes did not change significantly.
Many studies have focused on the impact of high-fat diet on shaping gut microbiota. They have reported that high-calorie diets induced an abundance of Firmicutes and a reduction in Bacteroidetes (Cani et al., 2007; Hildebrandt et al., 2009; Jumpertz et al., 2011). A reduction in the number of Bifidobacterium in the colon has been observed (Cani et al., 2007; Nava et al., 2012). Although the opposite results have been reported in rural areas where meat was rarely consumed, the effect of short-term dietary intervention of vegetable or fruit is largely unknown. Recently, Duque et al. (Duque et al., 2016) observed an increase in Lactobacillus spp. and Bifidobacterium spp. populations after the 14 days of orange juice intake.
With respect to bacteria-derived EVs, a total of 10 bacterial phyla were identified [Fig. 2(B), (C)]. The proportion of Firmicutes’ EVs increased from 41.33 ± 24.11 to 49.45 ± 38.74%, while that of Bacteroidetes’ EVs decreased from 25.69 ± 17.05 to 20.91 ± 17.64% (P < 0.05) [Fig. 2(E)]. This resulted in an increase in the F/B ratio from 4.13 ± 5.99 to 7.67 ± 10.67, but it was not statistically significant. The following EVs showed insignificant changes in the proportion (Actinobacteria, 5.92 ± 4.03 to 4.93 ± 3.85%; Tenericutes, 0.31 ± 0.32 to 0.25 ± 0.23%. Proteobacteria, 21.38 ± 18.03 to 20.43 ± 16.45%; Cyanobacteria, 1.35 ± 1.87 to 0.54 ± 0.47%; Verrucomicrobia, 0.70 ± 1.52 to 0.40 ± 1.27%; TM7, 0.67 ± 0.56 to 0.40 ± 0.37%; and Acidobacteria, 0.33 ± 0.45 to 0.12 ± 0.07%) [Fig. 2(C)].
Composition of fecal bacteria and bacteria-derived EVs at the genus level
At the genus level, it was difficult to assess all of the genera and species due to the vast diversity; thus, we set a cut-off of 1% bacterial occupancy. The bacterial components that significantly increased in participants after the consumption of the juice for 3 weeks, were Faecalibacterium and Pseudomonas (from 1.62 ± 0.80 to 2.14 ± 0.72% and from 1.29 ± 0.96 to 8.50 ± 0.39%, respectively; P < 0.05 in both cases) [Fig. 3(A)–(D)]. Similarly, the proportion of EVs from Faecalibacterium increased (2.49 ± 1.49 to 6.06 ± 3.07%, P < 0.05), while that of bacterial and vesicular Bacteroides decreased (Bacteria: 10.06 ± 4.28 to 8.24 ± 3.72% and EV: 12.28 ± 7.98 to 9.47 ± 5.06; P < 0.05 in all cases) [Fig. 3(D), (E)].
Fig. 3.
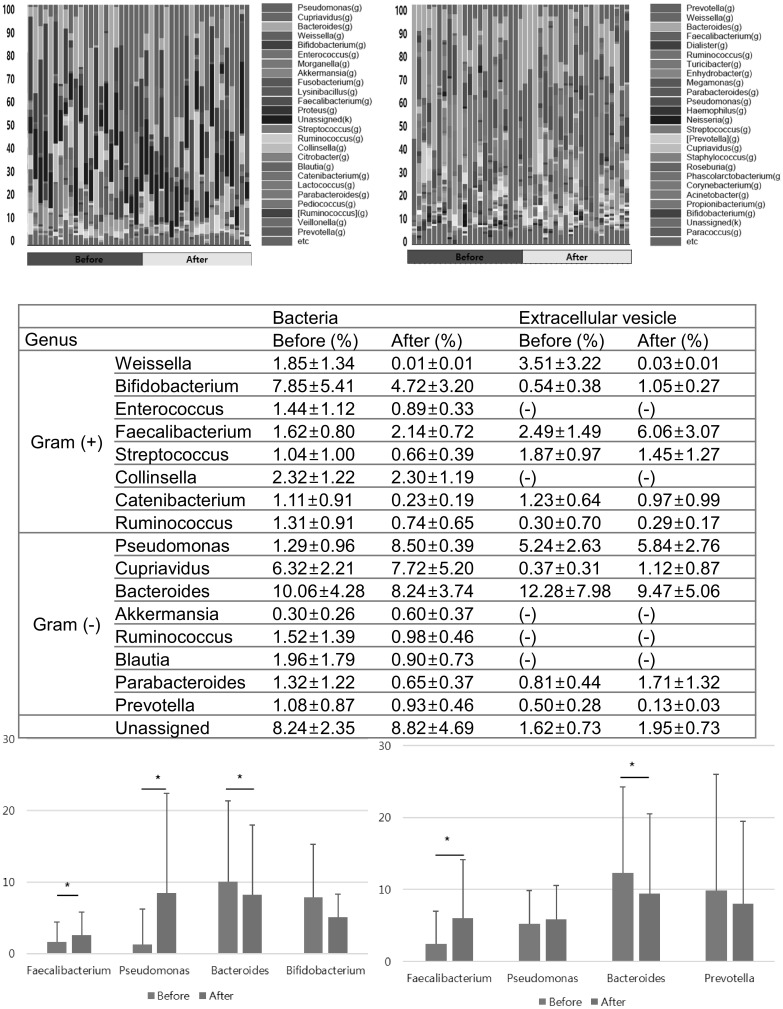
The proportion of fecal bacteria and bacterial EVs at the genus level. (A) Bacteria, (B) bacteria-derived EVs (C) proportions of the representative genera, and compositional change in (D) fecal bacteria and (E) bacterial EVs at the genus level are listed. The asterisks denote statistical significances
Induction of Faecalibacterium is a significant finding in the present study. Faecalibacterium prausnitzii is sole known species among Faecalibacterium and one of the most abundant and important commensal bacteria of the healthy human (Lay et al., 2005). This bacteria produce beneficial short-chain fatty acids via fermentation of dietary fiber. Growing evidence indicates that a reduction in F. prausnitzii is associated with obesity, inflammatory bowel diseases or depression (Fujimoto et al., 2013; Jiang et al., 2015; Newton et al., 2015). Although we did not evaluate microbiota at the species level, this genus could be F. prausnitzii. Further identification study is warranted.
Otherwise, with regards to both bacteria and EVs, proportions of the genera Weissella, Bifidobacterium, Streptococcus, Enterococcus, Collinsella, Ruminococcus, Cupriavidus, Blautia, Parabacteroides, Catenibacterium, and Prevotella changed within a statistically insignificant range [Fig. 3(C)]. The proportion of other genera, including Pseudomonas, remained stable when assessed from EVs.
Changes in demographic characteristics and symptoms
While many studies have focused on the effects of probiotics or different geographical diets on the composition of fecal microbiota, we investigated the effects of a simple consumption of a cup of fruit and vegetable juice on the fecal microbiota, bowel habits, skin symptoms, and physical or mental energy.
After consumption of the juice for 3 weeks, body weights decreased significantly from 68.96 ± 9.15 kg to 66.89 ± 9.10 kg (P = 0.001). This result was shown in among both men and women (Men: 76.67 ± 9.44 to 73.78 ± 10.56 and women: 63.18 ± 1.60 to 61.73 ± 1.68, all P < 0.05) (Table 2).
Of the 22 participants, 21 answered the symptom questionnaires. Specifically, at the baseline, 15 participants had chronic fatigue, 18 had diarrhea or constipation, and 10 had skin problems including atopic dermatitis. After 3 weeks of juice consumption, 18 of 21 (86%) participants reported improvements in their major complaints. Table 2 shows the changes in each symptom.
Subgroup analysis for microbiota based on the symptom change was not performed due to a small sample size. We could not exclude the placebo effect of fruit and vegetable juice because this study is a single-arm study. Nonetheless, because previous studies have consistently reported a low number of members from the Faecalibacterium genus in IBS patients (Carroll et al., 2012; Liu et al., 2017), the findings of the current study suggest a possibility of a close association between the genus Faecalibacterium and the improvement of symptoms. Further studies to evaluate whether the modulation of the profile of fecal microbiota contributes to the weight reduction or symptom improvement are warranted.
Differences from previous literature
A decrease in Bacteroidetes or Bacteroides, known to be positively associated with vegetable-rich diet, was a somewhat unexpected result in the present study. It may indicate that daily drinking one cup of vegetable/fruit juice was not enough to make a large shift in microbiota composition to the signature under long-term veggie-rich diet. Participants were instructed to continue their usual dietary pattern, but unfortunately, we did not collect the food diary.
The difference in Firmicutes’ compositional change between EVs and bacteria is difficult to explain due to a lack of studies that evaluated the microbiota after segregating the 16S rDNA of EVs from whole bacterial genetic information. However, the discrepancy has been often reported (Kang et al., 2013). The microbiota profile assessed from EVs is not likely a simple alternative for the microbiota profile assessed from stool. Recent evidence has indicated that bacteria-derived EVs have key roles in the intercellular communication between the host and commensal microbes, thus reflecting their functional activity (Chu et al., 2016). Given the recent emphasis on EVs as key factors, the increment of Firmicutes’ EV might be a compensation for the decrease in bacterial numbers, abrupt gush out from dying bacteria or a simple result from participants’ excessive consumption of “Western diet” during a study period.
Apart from these hypothesis, the increase in a proportion of Proteobacteria and pseudomonas was unexpected. Although diverse prevalence of asymptotic colonization of Psuedomonas has been reported (Somekh et al., 1996), the increase in a proportion of pseudomonas could be related with a contamination. Further studies regarding the true physiologic role and clinical implications of EVs and identification of increased Proteobacteria and Faecalibacterium at the species level are required. The small sample size of the present study is a limitation.
In conclusion, our study shows that the intake of fruit and vegetable juice for 3 weeks enriched the diversity of the host fecal microbiota and increased the proportion of bacteria of the genus Faecalibacterium and their EVs. Dietary interventions, eating vegetables and fruits might help alleviate bowel symptoms and fatigue. Well-controlled studies, particularly in dietary aspect, with a large sample size and evaluation of the true role of EVs are needed.
Acknowledgements
The anonymized and processed data was provided by Naver corporation and Hurom company via a Korean food forum without cost. This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through the High Value-added Food Technology Development Program, and funded by the Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No. 116017032HD030).
Compliance with ethical standards
Conflicts of interest
The authors declare that they have no conflict of interest.
References
- Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk of colorectal cancer. J Natl Cancer Inst. 2013;105:2907–2911. doi: 10.1093/jnci/djt300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai Yogesh. David A. Muniz Pedrogo, Purna C. Kashyap. Irritable bowel syndrome: a gut microbiota-related disorder? Am J Physiol Gastrointest Liver Physiol. Am J Physiol Gastrointest Liver Physiol. 2017;312:G52–G62. doi: 10.1152/ajpgi.00338.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, Gibson GR, Delzenne NM. Selective increases of Bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–2383. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll IM, Ringel-Kulka T, Siddle JP, Ringel Y. Alterations in composition and diversity of the intestinal microbiota in patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil. 2012;24:521–530. doi: 10.1111/j.1365-2982.2012.01891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao A. Nonparametric estimation of the number of classes in a population. Scand J Stat. 1984;11:265–270. [Google Scholar]
- Chu H, Khosravi A, Kusumawardhani IP, Kwon AH, Vasconcelos AC, Cunha LD, et al. Gene-microbiota interactions contribute to the pathogenesis of inflammatory bowel disease. Science. 2016;352:1116–1120. doi: 10.1126/science.aad9948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O’Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am J Clin Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- C-s Kang, Ban M, Choi E-J, et al. Extracellular vesicles derived from gut microbiota, especially Akkermansia muciniphila, protect the progression of dextran sulfate sodium-induced colitis. PLoS One. 2013;8:e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Filippo Carlotta, Cavalieri Duccio, Di Paola Monica, Ramazzotti Matteo. Jean Baptiste Poullet, Sebastien Massart, Silvia Collini, Giuseppe Pieraccini, and Paolo Lionetti. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;17:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duque ALRF, Monteiro M, Adorno MAT, Sakamoto IK, Sivieri K. An exploratory study on the influence of orange juice on gut microbiota using a dynamic colonic model. Food Res. Int. 2016;84:160–169. doi: 10.1016/j.foodres.2016.03.028. [DOI] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Imaeda H, Takahashi K, et al. Decreased abundance of Faecalibacterium prausnitzii in the gut microbiota of Crohn’s disease. J Gastroenterol Hepatol. 2013;28:613–619. doi: 10.1111/jgh.12073. [DOI] [PubMed] [Google Scholar]
- Grotto D, Zied E. The standard American diet and its relationship to the health status of Americans. Nutr Clin Pract. 2010;25:603–612. doi: 10.1177/0884533610386234. [DOI] [PubMed] [Google Scholar]
- Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen Y-Y, Knight R, Ahima RS, Bushman F, Wu GD, et al. High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology. 2009;137:1716–1724. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015;48:186–194. doi: 10.1016/j.bbi.2015.03.016. [DOI] [PubMed] [Google Scholar]
- Jumpertz R, Duc Son L, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am. J. Clin. Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Chil-sung, Ban Mingi, Choi Eun-Jeong, Moon Hyung-Geun, Jeon Jun-Sung, Kim Dae-Kyum, Park Soo-Kyung, Jeon Seong Gyu, Roh Tae-Young, Myung Seung-Jae, Gho Yong Song, Kim Jae Gyu, Kim Yoon-Keun. Extracellular Vesicles Derived from Gut Microbiota, Especially Akkermansia muciniphila, Protect the Progression of Dextran Sulfate Sodium-Induced Colitis. PLoS ONE. 2013;8(10):e76520. doi: 10.1371/journal.pone.0076520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-W, Park JG, Ilhan ZE, et al. Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One. 2013;8:e68322. doi: 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-R, Hong S-W, Choi E-B, et al. Staphylococcus aureus-derived extracellular vesicles induce neutrophilic pulmonary inflammation via both Th1 and Th17 cell responses. Allergy. 2012;67:1271–1281. doi: 10.1111/all.12001. [DOI] [PubMed] [Google Scholar]
- Kim OY, Hong BS, Park K-S, et al. Immunization with Escherichia coli outer membrane vesicles protects bacteria-induced lethality via Th1 and Th17 cell responses. J Immunol. 2013;190:4092–4102. doi: 10.4049/jimmunol.1200742. [DOI] [PubMed] [Google Scholar]
- Lay C, Sutren M, Rochet V, Saunier K, Dore J, Rigottier-Gois L. Design and validation of 16S rDNA probes to enumerate members of the Clostridium leptum subgroup in human faecal microbiota. Environ Microbiol. 2005;7:933–946. doi: 10.1111/j.1462-2920.2005.00763.x. [DOI] [PubMed] [Google Scholar]
- Lee E-Y, Choi D-Y, Kim D-K, et al. Gram-positive bacteria produce membrane vesicles: Proteomics-based characterization of Staphylococcus aureus-derived membrane vesicles. Proteomics. 2009;9:5425–5436. doi: 10.1002/pmic.200900338. [DOI] [PubMed] [Google Scholar]
- Ley RE, Hamady M, Lozupone C, et al. Evolution of mammals and their gut microbes. Science. 2008;320:1647–1651. doi: 10.1126/science.1155725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HN, Wu H, Chen YZ, Chen YJ, Shen XZ, Liu TT. Altered molecular signature of intestinal microbiota in irritable bowel syndrome patients compared with healthy controls: A systematic review and meta-analysis. Dig Liver Dis. 2017;49:331–337. doi: 10.1016/j.dld.2017.01.142. [DOI] [PubMed] [Google Scholar]
- Lozupone C, Hamady M, Knight R. UniFrac–an online tool for comparing microbial community diversity in a phylogenetic context. BMC Bioinformatics. 2006;7:371. doi: 10.1186/1471-2105-7-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava GM, Carbonero F, Ou J, Benefiel AC, O’Keefe SJ, Gaskins HR. Hydrogenotrophic microbiota distinguish native Africans from African and European Americans. Environ. Microbiol. Rep. 2012;4:307–315. doi: 10.1111/j.1758-2229.2012.00334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RJ, McLellan SL, Dila DK, Vineis JH, Morrison HG, Eren AM, Sogin ML. Sewage Reflects the Microbiomes of Human Populations. MBio. 2015;6(2):e02574–e025714. doi: 10.1128/mBio.02574-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlich MJ, Singh PN, Sabaté J, et al. Vegetarian dietary patterns and mortality in Adventist Health Study 2. JAMA Intern Med. 2013;173:1230–1238. doi: 10.1001/jamainternmed.2013.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajilić-Stojanović M, Biagi E, Heilig HG, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–1801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- Sha S, Xu B, Wang X, et al. The biodiversity and composition of the dominant fecal microbiota in patients with inflammatory bowel disease. Diagn Microbiol Infect Dis. 2013;75:245–251. doi: 10.1016/j.diagmicrobio.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Singh Rasnik K, Chang Hsin-Wen, Yan Di, Lee Kristina M, Ucmak Derya, Wong Kirsten, Abrouk Michael, Farahnik Benjamin, Nakamura Mio. Tian Hao Zhu, Tina Bhutani, and Wilson Liao. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017;15:73. doi: 10.1186/s12967-017-1175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somekh E, Abishai V, Hanani M, Gutman R, Mintz M. The clinical significance of Pseudomonas aeruginosa isolation from stool of neonates. Arch Pediatr Adolesc Med. 1996;150:108–109. doi: 10.1001/archpedi.1996.02170260112021. [DOI] [PubMed] [Google Scholar]
- Turnbaugh P. J., Ridaura V. K., Faith J. J., Rey F. E., Knight R., Gordon J. I. The Effect of Diet on the Human Gut Microbiome: A Metagenomic Analysis in Humanized Gnotobiotic Mice. Science Translational Medicine. 2009;1(6):6ra14–6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatsunenko T, Rey FE, Manary MJ, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Srisatjaluk R, Justus D, Doyle R. On the origin of membrane vesicles in gram-negative bacteria. FEMS Microbiol Lett. 1998;163:223–228. doi: 10.1111/j.1574-6968.1998.tb13049.x. [DOI] [PubMed] [Google Scholar]


