Abstract
Biochar amendments have been used in agriculture to improve soil fertility and enhance crop productivity. A greenhouse experiment was conducted to test the hypothesis that biochar amendment could also enhance the productivity of salt-affected soils. The trial was conducted over two consecutive growing seasons to investigate the effect of biochar amendment (four application rates as: B1 = 0%, B2 = 2%, B3 = 4%, and B4 = 8% by mass of soil) on yield and quality of tomatoes grown in a silt loam soil using non-saline water (I0 = 0.7 dS m−1) and saline water (I1 = 1 dS m−1; I2 = 3 dS m−1) irrigation. Furthermore, the study investigated the mechanism by which biochar addresses the salt stress on plant. The results showed that soil productivity as indicated by the vegetative growth and tomato yield components was adversely and significantly affected by saline water irrigation (P < 0.05). Tomato yield decreased from 689 ± 35.6 to 533 ± 79.0 g per plant as salinity of irrigation water increased from I0 to I2. Then, biochar amendment increased vegetative growth, yield, and quality parameters under saline irrigation water regimes, and ameliorated the salt stresses on crop growth. The highest (8.73 ± 0.15 and 4.10 ± 0.82 g kg−1) and the lowest (8.33 ± 0.08 and 2.42 ± 0.76 g kg−1) values of soil pH and soil organic matter were measured at B4I0 and B1I2 treatments, respectively. Also, the highest rate of biochar amendment combining with non-saline water irrigation (B4I0) produced tomato with the highest plant photosynthetic (17.08 ± 0.19 μmol m−2 s−1) and transpiration rate (8.16 ± 0.18 mmol H2O m−2 s−1). Mechanically, biochar amendment reduced transient sodium ions by adsorption and released mineral nutrients such as potassium, calcium, and magnesium into the soil solution. Therefore, biochar amendments have the potential in ameliorating salt stress and enhancing tomato production.
Introduction
Water shortage represents a serious risk to global food security1–3. The total global water withdrawal for agricultural, domestic and industrial use is expected to increase by 23% from 1995 to 20254. Irrigated agricultural production in a number of the foremost populated areas of the world, such as China and Pakistan are expected to face extreme water crisis in the near future.
Irrigation is hailed as an intervention to enable agricultural production in areas where rainfall is inadequate, but scarcity of fresh water means some farmers are forced to irrigate plants using poor quality water5. This is particularly so where saline water is irrigated and then exacerbating the problem on agricultural fields that suffer the effects of salinization. Currently, about 33% of the global irrigated lands are affected by salinization6. Salinity affects plant growth by influencing plant physiological processes including photosynthesis and transpiration7. Salt stress decreased soil water potential (osmotic stress), causing an ion toxicity and consequently plant death. Decreased osmotic stress reduces water uptake by plants, which usually through closing leaf stomata and decreasing transpiration, and this negatively affects plant growth by decreasing plant photosynthesis8. Therefore, a direct correlation of plant growth with soluble salt concentration and duration of stress has been identified9.
The impact of salt stress and approaches to diminish the negative effects has been a subject of a number of studies. Techniques involved in these studies range from management options to identifying plant characteristics that help mitigate the effects of salinity. For example, Jouyban10 investigated the use of different techniques i.e. scraping, flushing, and leaching to remove excess salt from root zone of plants. Others have targeted on the utilization of various irrigation techniques in decreasing salinity11. Improvement of salt tolerance/resistance is another technique used to address the constraint of soil salinity12. Researchers have employed methods to improve plant salt tolerance, including inoculating seeds with halotolerant plant-growth-promoting rhizobacteria, using plant growth regulators and developing salt resistant cultivars13–15. However, these approaches can be unprofitable, limited by the high costs and labor-consuming requirements of that are involved in order to counter the salinization problems. More recently, applications of organic conditioners have become a more sustainable and popular approach for enhancing crop productivity in salt-affected soils5.
Recent work has elaborated the potentiality of biochar to enhance soil fertility and improve crop productivity16. Many studies conducted in different parts of the world have reported improved soil water holding capacity, nutrient availability to plants, and plant productivity as a result of using biochar amendment1,5,17–19. Schmidt20 reported that a fourfold increase in pumpkin production after soil amended with biochar when mixed with and without cow urine. Kamman21 gave a molecular interpretation about the positive effect of biochar on crop production, where he found that development of acid and basic functional groups and organo-mineral complexes on the biochar-matrix surfaces which responsible for nutrients retention. Joseph22 also reported that organic coating on biochar explained its ability on nutrients retention. However, a few researches had been done to evaluate/investigate the potential of biochar amendment (BA) in reducing the soil soluble salt under saline water (SW) irrigation as well as enhancing fruit quality. For example, Usman5 used conocarpus biochar under SW irrigation to investigate the changes of soil nutrient availability and tomato growth. Additionally, Lashari23 concluded that BA increased the plant growth, biomass, and yield and also increased photosynthesis, nutrient uptake, although under salt stress. The feedstock of biochar is of the main factor determining the effects of BA on plant growth. Therefore, the aims of this study were to investigate 1) the effects of wheat straw biochar soil amendment under SW irrigation on growth as well as physiology, yield, fruit quality of tomato; 2) the capability of BA to alleviate salt stress.
Results
Effects of BA and SW irrigation on selected soil properties
The analysis of variance results indicated that both BA and SWirrigation significantly (P < 0.05) influenced soil EC, SOM and pH as presented in Table 1. The pattern across treatments is greater EC with greater biochar composition, irrespective of irrigation treatment. For example, in 2014, the EC values for the B1 treatment increased in the order 0.20 ± 0.07, 0.85 ± 0.07, and 1.64 ± 0.15 dS m−1 for the I0, I1, and I2 irrigation treatments, respectively, while the corresponding values for the B4 treatment were 0.82 ± 0.07, 1.40 ± 0.21, and 2.25 ± 0.07 dS m−1. Similarly, soil pH and SOM increased significantly (P < 0.05) by increasing BA rate. For instance, in 2014 and 2015, SOM for I0B1 was 2.7 ± 0.08 and 2.71 ± 0.07 g kg−1, and these increased to, respectively 3.67 ± 0.69 and 4.10 ± 0.82 g kg−1 in I0B4 when the soil amended with 8% biochar.
Table 1.
Mean values of soil electrical conductivity (EC), pH and soil organic matter (SOM) content for different biochar amendments and irrigation regimes during the 2014 and 2015 tomato growing seasons.
| Irrigation regime | Biochar treatment | EC (dS m−1) | pH | SOM (g kg−1) | |||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | ||
| I0 | B1 | 0.20cD | 0.57cC | 8.44aB | 8.46aB | 2.70aC | 2.71aD |
| B2 | 0.45cC | 0.75cB | 8.65aA | 8.69aA | 3.30aB | 3.40aC | |
| B3 | 0.72cB | 0.72cB | 8.68aA | 8.71aA | 3.51aA | 3.81aB | |
| B4 | 0.82cA | 0.94cA | 8.70aA | 8.73aA | 3.67aA | 4.10aA | |
| I1 | B1 | 0.85bD | 1.05bD | 8.41bB | 8.42bB | 2.50bC | 2.63bC |
| B2 | 1.18bC | 1.61bC | 8.54bA | 8.55bA | 2.80bC | 3.10bB | |
| B3 | 1.28bB | 1.90bB | 8.56bA | 8.56bA | 3.14bB | 3.34bB | |
| B4 | 1.40bA | 2.10bA | 8.56bA | 8.58bA | 3.44bA | 3.71bA | |
| I2 | B1 | 1.64aD | 1.90aC | 8.31cA | 8.33cB | 2.30cC | 2.42cC |
| B2 | 1.82aC | 1.99aC | 8.40cA | 8.42cA | 2.40cB | 2.70cB | |
| B3 | 2.15aB | 2.40aB | 8.43cA | 8.44cA | 2.45cB | 2.73cB | |
| B4 | 2.25aA | 2.72aA | 8.45cA | 8.46cA | 2.76cA | 2.90cA | |
| Biochar | *** | *** | ns | ns | *** | *** | |
| Salinity | *** | *** | * | * | ** | ** | |
| Interaction | ns | ns | ns | ns | ns | ns | |
Note: In each column, different uppercase and lowercase letters indicate a significant difference among the biochar application rates and irrigation salinity levels, respectively, at P < 0.05 (Least significant difference, LSD); Analysis of variance (ANOVA): ns, not significant; *, **, and ***, denote significance at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively. B1, B2, B3 and B4 represent mixtures of soil with 0%, 2%, 4% and 8% of biochar by mass. I0, I1 and I2 represent irrigation water salinity of tap water, 1 and 3 dS m−1.
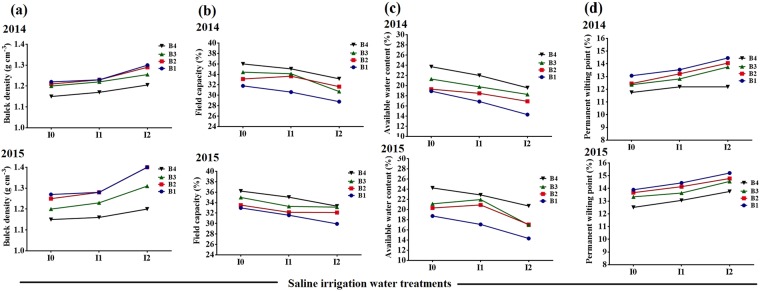
Bulk density (Bd) increased significantly in SW irrigation treatments compared to I0, and decreased with increasing BA rate (Fig. 1). Treatment I2 resulted in 13% and 14% lower Bd under the B4 biochar compared to the respective non-biochar control, for 2014 and 2015 seasons, respectively. The soil field capacity (FC) was also affected significantly by both SW irrigation and BA treatments. It was decreased significantly in SW treatment compared to I0 treatment. However, FC was greater in all BA treatments compared to non-biochar. Figure 1 also shows the soil permanent wilting point (PWP) as influenced by SW irrigation and BA treatments. Soil PWP increased significantly by SW irrigation. However, BA decreased PWP in both I1 and I2 soil compared to their non-biochar control. Available water content (AWC) for plant decreased significantly under I1 and I2 compared to I0, and increased with the increased rate of BA, for all the SW treatments (Fig. 1). Maximum AWC was observed in the combination of B4I0.
Figure 1.
Bulk density, Bd (a); Field capacity, FC (b); Available water content, AWC (c) and Permanent wilting point, PWP (d) for different irrigation water salinity and biochar treatments for season 2014 and 2015. B1, B2, B3 and B4 represent mixtures of soil with 0%, 2%, 4% and 8% of biochar by mass. I0, I1 and I2 represent irrigation water salinity of tap water, 1 and 3 dS m−1.
The effects of BA and SW irrigation on plant growth
The measured plant growth parameters were significantly (P < 0.05) affected by both BA and SW irrigation as shown in Table 2. These growth parameters generally increased significantly with increases in the BA rate and decreased significantly with increases salinity of SW (P < 0.05). This was reflected by greater biomass observed for I0B4 treatments in both growing seasons. However, among all the measured growth parameters, there were no significant interaction effects of BA and SW.
Table 2.
Mean values of various plant growth parameters for different saline water irrigation regimes and biochar amendments during the 2014 and 2015 tomato growing seasons.
| Irrigation regime | Biochar treatment | FAGB (g) | DAGB (g) | FBGB (g) | DBGB (g) | LRWC (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | ||
| I0 | B1 | 240.2aD | 302.9aD | 127.5aD | 190.5aD | 25.40aC | 6.32aB | 92.72aD | 94.00aC | ||
| B2 | 264.3aC | 327.3aC | 146.6aC | 153.8aC | 26.45aC | ND | 6.33aB | ND | 93.80aC | 95.08aB | |
| B3 | 289.7aB | 352.7aB | 156.7aB | 219.7aB | 27.88aB | 7.22aA | 94.12aB | 95.07aB | |||
| B4 | 307.3aA | 383.6aA | 165.7aA | 228.7aA | 29.75aA | 7.58aA | 95.48aA | 96.10aA | |||
| I1 | B1 | 181.6bD | 299.9bD | 125.2bD | 188.2bD | 22.09bD | 6.18bC | 91.52bC | 92.14bC | ||
| B2 | 248.0bC | 331.0bC | 125.5bC | 199.5bC | 25.14bC | ND | 6.88bB | ND | 92.04bB | 92.99bB | |
| B3 | 285.5bB | 348.5bB | 133.8bB | 192.9bB | 25.11bC | 6.71bB | 92.59bB | 94.54bB | |||
| B4 | 300.6bA | 372.3bA | 146.1bA | 209.1bA | 27.65bA | 6.92bA | 94.29bA | 95.24bA | |||
| I2 | B1 | 172.0cD | 251.5cD | 57.49cD | 143.5cD | 20.90cD | 5.70cB | 90.61bC | 90.90cC | ||
| B2 | 181.6cC | 258.0cC | 61.53cC | 147.5cC | 22.80cC | 6.14cB | 91.57bB | 92.47cB | |||
| B3 | 192.3cB | 287.6cA | 70.69cB | 163.7cB | 24.67cA | ND | 6.34cA | ND | 92.02bB | 92.64cA | |
| B4 | 239.9cA | 286.9cB | 67.78cA | 209.6cA | 24.20cA | 6.92cA | 92.89bA | 93.51cA | |||
| Biochar | *** | *** | *** | *** | *** | ND | * | ND | ** | *** | |
| Salinity | ** | *** | ** | *** | *** | ND | ** | ND | ** | *** | |
| Interaction | ns | ns | ns | ns | ns | ND | ns | ND | ns | ns | |
Note: fresh above- ground biomass (FAGB), dry above- ground biomass (DAGB), fresh below- ground biomass (FBGB), dry below- ground biomass (DBGB), and leaf relative water content (LRWC). In each column, different uppercase and lowercase letters indicate a significant difference among the biochar application rates and irrigation salinity levels, respectively, at P < 0.05 (Least significant difference, LSD); Analysis of variance (ANOVA): ns, not significant; *, **, and ***, denote significance at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively. ND, no data. B1, B2, B3 and B4 represent mixtures of soil with 0%, 2%, 4% and 8% of biochar by mass. I0, I1 and I2 represent irrigation water salinity of tap water, 1 and 3 dS m−1.
The Pn and Tr rates were influenced by BA rates and SW irrigation (Fig. 2). Plants irrigated by saline water had significantly lower leaf photosynthetic compared with the control. Within each irrigation treatment, addition of biochar resulted in significant increases in photosynthetic rate. The highest (17.08 ± 0.19 μmol m−2 s−1) and lowest (13.0 ± 0.25 μmol m−2 s−1) Pn rates were observed for treatments I0B4 and I2B1, respectively. Transpiration rate followed a similar trend with I0B4 recording the highest value (8.16 ± 0.18 mmol H2O m−2 s−1) while the lowest one (4.85 ± 0.27 mmol H2O m−2 s−1) was recorded under I2B1. The interaction effects of BA and SW on the Pn and Tr rates were statistically not significant.
Figure 2.
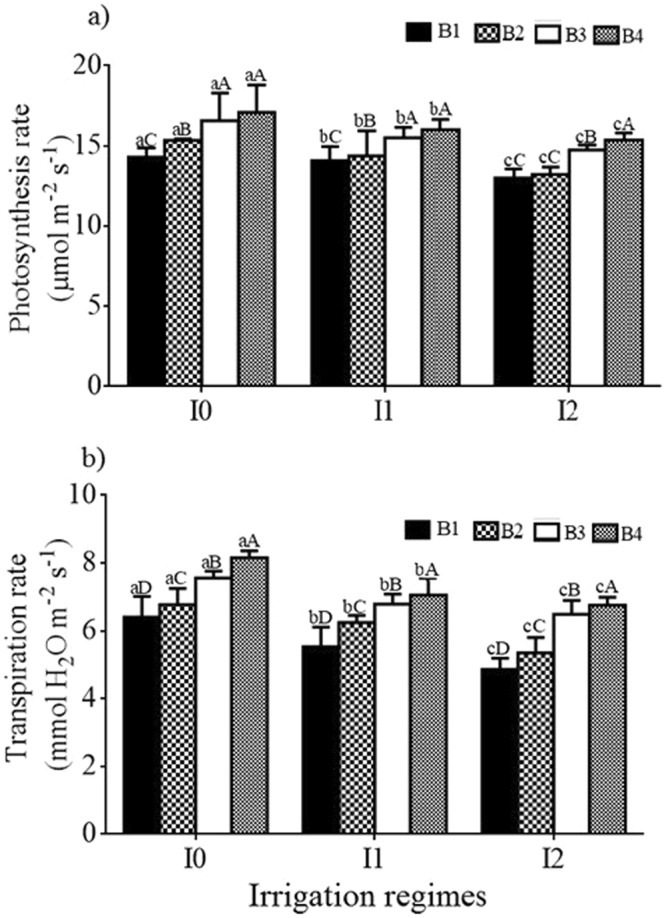
Rates of (a) photosynthesis and (b) transpiration for different irrigation water salinity and biochar treatments for season 2014. Different uppercase and lowercase letters indicate a significant difference between different biochar application rates (for a given irrigation regime) and different salinity levels, respectively, at P < 0.05. B1, B2, B3 and B4 represent mixtures of soil with 0%, 2%, 4% and 8% of biochar by mass. I0, I1 and I2 represent irrigation water salinity of tap water, 1 and 3 dS m−1.
Effects of BA and SW irrigation on tomato yield
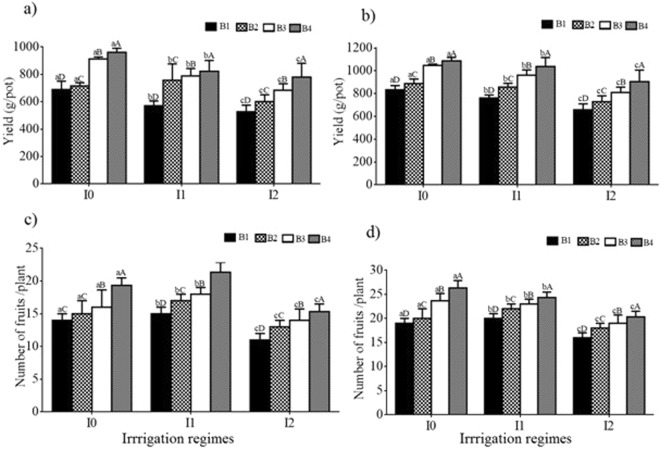
Both BA rate and SW irrigation significantly (P < 0.05) influenced tomato yield and the number of fruits per plant (NFP). In both growing seasons, these two yield parameters significantly increased with decreases in irrigation water salinity and increased with increases in BA rate (Fig. 3). For example, in the 2014 control irrigation treatment (I0), the B4 treatment resulted in an increase of 6 more fruits plant−1 and 272 g greater mass than the B1 treatment. Increased salinity had a negative effect on yield. For the B1 treatment in 2014 for example, the yield decreased from 689 ± 35.6 to 533 ± 79.0 g plant−1 as salinity increased from I0 to I2, which was due to the decreased NFP from 21 to 15. There was no interaction significant (P > 0.05) effect of BA and SW on yield.
Figure 3.
Yield (a,b) and number of fruits per plant (c,d) in 2014 and 2015, respectively, for different irrigation water salinity and biochar treatments. For each year, different uppercase and lowercase letters indicate a significant difference among different biochar application rates (for a given irrigation regime) and salinity levels, respectively, at P < 0.05. B1, B2, B3 and B4 represent mixtures of soil with 0%, 2%, 4% and 8% of biochar by mass. I0, I1 and I2 represent irrigation water salinity of tap water, 1 and 3 dS m−1.
Fruit quality as affected by BA and SW irrigation
Generally, the concentrations of total soluble solid (TSS), soluble sugar content (SS), titratable acidity (TA), vitamin C content (VC), and fruit color index (CI) increased with either BA rate increasing or SW irrigation (Table 3). Overall, there were no significant (P > 0.05) interaction effects of BA and SW irrigation on fruit quality parameters. Significant differences in the TSS content were observed among both BA and SW irrigation treatments. The results elaborated that the I2B4 combination gave the greatest values of TSS and VC while I0B1 had the lowest values for these quality measures. Statistically there were no significant effects on CI value, but numerically increases in SW decreased the CI value while increases in the BA rate got the opposite results. In all cases, the SS content of fruits significantly (P < 0.05) increased with increases in SW and BA in both seasons as displayed in Table 3. Significant (P < 0.05) differences in the TA content were realized among both the BA and SW irrigation treatments, where highest and lowest TA contents values were found in the treatments I2B4 and I0B1, respectively.
Table 3.
Mean values of tomato quality parameters including total soluble solids (TSS), soluble sugar (SS), titratable acids (TA), vitamin C (VC), color index (CI), and sugar/acid content ratio (SS/TA) for different saline water irrigation treatments and biochar amendments during the 2014 and 2015 growing seasons.
| Irrigation regime | Biochar treatment | TSS (Brix %) | VC (mg 100 g−1) | TA (g 100 g−1) | SS (g 100 g−1) | SS/TA | CI | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | 2014 | 2015 | ||
| I0 | B1 | 4.0cC | 5.0cC | 4.5cC | 4.7cD | 0.35cC | 0.33cC | 1.14cC | 1.12cD | 3.3aA | 3.5aA | 1.9aA | 1.96aA |
| B2 | 5.0cB | 5.3cB | 4.7cC | 5.0cC | 0.36cB | 0.37cB | 1.16cB | 1.18cC | 3.2aB | 3.2aB | 1.91aA | 1.97aA | |
| B3 | 5.5cA | 5.5cA | 4.9cB | 5.2cB | 0.39cA | 0.40cA | 1.20cA | 1.20cB | 3.0aC | 3.0aC | 1.93aA | 1.97aA | |
| B4 | 5.5cA | 5.7cA | 5.3cA | 5.5cA | 0.40cA | 0.41cA | 1.20cA | 1.24cA | 3.0aC | 3.0aC | 1.95aA | 1.99aA | |
| I1 | B1 | 5.5bC | 6.0bA | 4.9bC | 5.3bD | 0.37bD | 0.39bD | 1.19bC | 1.28bD | 3.2cA | 3.3cA | 1.86bA | 1.94bA |
| B2 | 5.8bB | 6.0bA | 5.1bB | 5.5bC | 0.41bC | 0.45bC | 1.24bB | 1.29bC | 3.0cB | 2.9cA | 1.88bA | 1.95bA | |
| B3 | 6.2bA | 6.2bA | 5.2bB | 5.7bB | 0.44bB | 0.47bB | 1.31bA | 1.31bB | 3.0cB | 2.8cB | 1.89bA | 1.97bA | |
| B4 | 6.3bA | 6.0bA | 5.6bA | 5.9bA | 0.46bA | 0.481bA | 1.31bA | 1.34bA | 2.9cC | 2.8cB | 1.90bA | 1.98bA | |
| I2 | B1 | 6.7aB | 6.7aB | 5.7aC | 6.1aC | 0.41aC | 0.42aC | 1.33aC | 1.38aC | 3.2bA | 3.3bA | 1.85cA | 1.90cA |
| B2 | 6.7aB | 6.8aB | 6.1aB | 6.2aC | 0.45aB | 0.46aB | 1.38aB | 1.42aB | 3.1bB | 3.1bB | 1.85cA | 1.90cA | |
| B3 | 7.0aA | 7.0aA | 6.4aB | 6.7aB | 0.50aA | 0.51aA | 1.39aB | 1.42aB | 2.8bC | 2.8bC | 1.86cA | 1.91cA | |
| B4 | 7.3aA | 7.0aA | 6.8aA | 6.9aA | 0.50aA | 0.52aA | 1.43aA | 1.46aA | 3.1bB | 2.8cB | 1.88cA | 1.93cA | |
| Biochar | * | ns | ** | ** | *** | *** | * | * | * | *** | ns | ns | |
| Salinity | *** | *** | *** | *** | *** | *** | *** | *** | * | * | ns | ns | |
| Interaction | ns | ns | ns | ns | ns | ns | ns | ns | ns | * | ns | ns | |
Note: In each column, different uppercase and lowercase letters indicate a significant difference among the biochar application rates and irrigation salinity levels, respectively, at P < 0.05 (Least significant difference, LSD); Analysis of variance (ANOVA): ns, not significant; *, **, and ***, denote significance at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively. B1, B2, B3 and B4 represent mixtures of soil with 0%, 2%, 4% and 8% of biochar by mass. I0, I1 and I2 represent irrigation water salinity of tap water, 1 and 3 dS m−1.
Discussion
Effects on soil properties
Results of this study clearly indicated that soil chemical properties (i.e., EC and SOM) are affected by BA and SW irrigation. The significant increase in soil EC with higher rates of BA is consistent with previous findings24, and is due to soluble salts in the biochar entering to the soil solution (Table 4)24–26. The SOM decreased with the SW irrigation for a given rate of BA. This indicated that high salinity probably decreased the biochar decomposition rate, which would also reduce the rate at which nutrients were released27. These impacts of increased salinity, both due to the BA and the SW irrigation, would typically be unfavorable to plant productivity28. However, increasing BA significantly increased SOM which is essential for water retention and nutrients in the soil for plants, thus, alleviating the negative effects of salinity.
Table 4.
Mean values of soil cation contents (g kg−1) for different biochar application rates (B) and irrigation water salinity levels (I).
| Irrigation regime | Biochar treatment | Ca | K | Mg | Na |
|---|---|---|---|---|---|
| I0 | B1 | 0.07dC | 0.01bC | 0.01cB | 0.05eA |
| B2 | 0.35dB | 0.09cB | 0.04bA | 0.04dA | |
| B3 | 0.49eA | 0.23dA | 0.05cA | 0.02fA | |
| I1 | B1 | 0.17cC | 0.02bC | 0.02bB | 0.67dA |
| B2 | 0.37cB | 0.10cB | 0.04bA | 0.23cB | |
| B3 | 0.51eA | 0.25dA | 0.06cA | 0.14eC | |
| I2 | B1 | 0.19cC | 0.03bC | 0.02bC | 1.23cA |
| B2 | 0.39bB | 0.12bB | 0.04bB | 0.53cB | |
| B3 | 0.58dA | 0.30cA | 0.07bA | 0.14eC | |
| Biochar | *** | ** | ** | *** | |
| Salinity | * | ns | * | *** | |
| Interaction | ** | ns | * | ** |
Note: In each column, different uppercase and lowercase letters indicate a significant difference among the biochar application rates and irrigation salinity levels, respectively, at P < 0.05 (Least significant difference, LSD); Analysis of variance (ANOVA): ns, not significant; *, **, and ***, denote significance at P ≤ 0.05, P ≤ 0.01, and P ≤ 0.001, respectively. B1, B2, and B3 represent mixtures of soil with 0%, 2%, and 4% of biochar by mass. I0, I1 and I2 represent irrigation water salinity of tap water, 1 and 3 dS m−1.
The positive effects of BA on soil physical properties were well documented. The results indicated a significant decrease in soil Bd, and an increase in SWC, FC, PWP, and AWC in the biochar-amended soils, even at the low biochar application rate, under both SW and non-SW irrigation treatments (Fig. 1). Moreover, salt stress adversely affected soil productivity, as indicated by the higher Bd and PWP, and lower FC and AWC of the soil under SW irrigation; however, this suppressing effects on soil physical properties tended to decline with BA, especially at the high biochar application rates29. Soil water retention capacity (AWC and FC) is very important property with respect to plant growth30. Laird29 described that the biochar amended soil retained 15% more moisture contents as compared controlled treatment, which was consistent with our results.
Effects on plant growth
Negative impacts of increased salt stress on plant growth were reflected by the responses of measured plant parameters. Both Pn and Tr rates declined due to salt stress, and this was linked to changes in water potential of leaf in previous studies. As described by Kazuhiro31, leaf water potential controls stomatal conductance which affects Tr and Pn, and affects water uptake by plant driven by the potential difference between leaf and soil water19,32. Salt stress caused stomata closure which reduce the CO2/O2 ratio in leaves and inhabit CO2 fixation33. The net effect of BA to soil irrigated with SW on these processes was favorable in this study, since plant Tr rates were significantly higher when tomato grown under I2B4 combination comparing to I0B1. This indicated that BA can be utilized to compensate for the negative effects of salinity on leaf Tr rates, especially at sufficiently high biochar application rate (Fig. 2).
Previous studies illustrated that organic amendments are capable to enhance soil characteristics and plant growth34. This typically, was due to the improvement of soil environment, such as by enhancing macro-nutrient and water availability35,36, which also could increase the plant resistance to salt stresses36–38. This study elaborated that all of the growth parameters were adversely impacted by SW irrigation. This occurs because salts negatively impact plants by both inducing physical drought by osmotic effects that impede water transport in the plants and by ion toxicity39. In contrast, BA significantly increased plant growth and the yield, which implied that biochar, could ameliorate the adverse effects of salt stress on plants. The improved vegetative growth in soils treated with biochar was inline with the findings of Hossain40, who found that BA improved the vegetative growth of cherry tomato.
Effects on fruit quality
Although increasing the salinity in irrigation water decreased tomato yield, it increased significantly fruit quality. In other studies on tomato, similar trends were observed whereby TSS, vitamin C, and acidity increased with increases EC in the soil solution41–44. Increased salinity of irrigation water might cause the plant to regulate metabolic processes that decreased the production of sucrose and organic acids and/or reallocated them to different plant parts, particularly to the fruits, and thus increased the concentration gradient of sucrose from leaves to fruits45.
Similarly, values of the fruit quality parameters increased significantly as BA rate increased. Of particle note is that BA increased the TA content in all the irrigation treatments. These enhancements in fruit quality may be attributed to the BA which affected the root distribution46. Although rooting depth was not measured in this study, the BA increased root biomass. Usman5 obtained similar results, who found that BA increased both TSS and VC slightly under both SW and non-SW irrigation treatments. Moreover, Akhtar44 and Agebna16 reported that BA improved the quality of tomato under deficit irrigation.
Mechanisms by which BA ameliorates salt stress on plants
This study provided evidences that BA can be utilized in salt-affected soils and/or when irrigation water is of low quality. It is probable that BA ameliorated negative impacts of salinity in the tomato plants by three main mechanisms: 1) reducing transient sodium ions by adsorption; 2) releasing mineral nutrients such as potassium, calcium, and magnesium into the soil solution (Table 4); and 3) decreasing osmotic stress by improving the soil AWC18. Novak47 stated that BA has strong absorptive characteristics binding refer to its high porosity, surface area and cation exchange capacity. By adsorbing toxic ions, especially sodium, and/or by releasing more beneficial ions18, BA can therefore reduce the negative effects of salt stress on plants, either by decreasing the exposure of plants to stress agents or by mitigating the stress responses of plants. Soil water content can be increased because that BA increased soil water holding capacity, especially increasing the proportion of larger pores where water is held at lower potentials allowing plants to uptake water more readily (Fig. 1)47. Increasing water availability might also interpret the alleviation of salt stress observed47. Some studies on photosynthetic responses to biochar additions have illustrated the increased water use efficiency at the leaf or whole plant scale36–38.
Conclusions
Saline water irrigation resulted remarkable decrement in tomato growth parameters and physiological processes such as Pn and Tr rates. Soil BA reduced the adverse impacts of salt stress, particularly at higher biochar application rates. The positive effects of BA on tomato growth were due to its capability to adsorb sodium ions and thus reducing its hazards and releasing mineral nutrients such as potassium, calcium, and magnesium into soil solution. Therefore, BA could be used on salt affected soils to improve tomato yield and quality, which would apply to saline-sodic soils elsewhere in the world. In particular, BA has the potential to be widely used combination with SW irrigation in agricultural production to struggle with fresh water crisis.
Materials and Methods
Study site
The experiment was carried out in a greenhouse from March 2014 to August 2015 at the Water-Saving Park of Hohai University (31°57′N, 118°50′E), China. The climate in the area is humid subtropical monsoon with annual mean pan evaporation and precipitation of 900 and 1073 mm, respectively. The mean daily temperature inside the greenhouse was 27.8 °C. More details about this area can be found in Agebna16. The physio-chemical soil properties are displayed in Table 1.
Experimental design
To evaluate the combination effects of soil BA and SW irrigation on tomato growth, the experiment was conducted as a 3 × 4 factorial laid out in a completely randomized design with three replicates. Irrigation (I) water with three different salt concentrations were evaluated; I0 (control; tap water had electrical conductivity (EC) of 0.7 dS m−1), I1 (EC = 1 dS m−1) and I2 (EC = 3 dS m−1). Sodium chloride (NaCl) was mixed with tap water to prepare the irrigation water with a given salinity. Soil BA treatments comprising four different proportions of biochar in biochar-mixtures i.e. 0%, 2%, 4%, and 8% and designated as B1, B2, B3, and B4, respectively, were evaluated. The wheat straw biochar was used in this study, which was pyrolyzed at 350–550 °C. Typically, 30% dry matter of the wheat straw would be converted into biochar48. The initial biochar properties are presented in Table 5.
Table 5.
Basic physical and chemical properties of biochar and the soil in the study area.
| Soil Properties | Soil | Biochar |
|---|---|---|
| pH | 7.7 | 9.9 |
| Electrical Conductivity (dS m−1) | 1.42 | 1.0 |
| Ca (g kg−1) | 0.16 | 0.016 |
| Mg (g kg−1) | 0.07 | 1.3 |
| Cl (g kg−1) | 0.11 | 1.44 |
| HCO3 (g kg−1) | 0.20 | 0.85 |
| total N (g kg−1) | 1.8 | 59 |
| total P (g kg−1) | 0.66 | 144.3 |
| total K (g kg−1) | 0.4 | 115 |
| CEC (cmol kg−1) | 14.94 | 217 |
| Bulk density (g cm−3) | 1.35 | 0.40 |
| Field capacity (%) | 25.8 | — |
| Silt (%) | 30.1 | — |
| Sand (%) | 50.2 | — |
| Clay (%) | 19.7 | — |
Agronomic practices
Tomato (Lycopersiconesculentum Mill, Yazhoufenwang), which is a pink tomato, infinite growth variety, was chosen as the experimental crop. Seeds were sown on March 6 (2014, season 1) and March 10 (2015, season 2) in a nursery, respectively. The Seedlings were transplanted when plants had reached four-leaf stage (April 14 in 2014 and April 19 in 2015, respectively). During the growing period, the weigh method was utilized to verify water losses and then irrigating replace the lost amount of water. The total amounts of water irrigated in each treatment were 147.2 mm and 142.3 mm for 2014 and 2015 growing seasons, respectively. The agricultural applications during the tomato growth periods were the same as Agebna16.
Measurements of soil physio-chemical properties
At the end of each growing seasons (August 12 in 2014 and August 5 in 2015, respectively), soil physio-chemical properties were measured. For each treatment, a disturbed soil sample (∼1 kg) was collected from the upper 20 cm layer, and air-dried. The air-dried samples were packed down to pass through a 1-mm mesh before being extracted using a 5:1 soil: leaching liquor to analyze soil pH, EC and cation contents (K+, Na+, Ca2+, Mg2+)49–54. Cation exchange capacity was determined using the ammonium acetate method52. Soil organic matter (SOM) content was measured using oil bath K2Cr2O7 titration method49,52. Soil total nitrogen (TN) was determined using the method described by Bremneran53, and the total phosphorus (TP) was determined following Olsen and Sommers54. An undisturbed soil core was removed from the surface (0–20 cm) layer at each treatment for soil bulk density (Bd) measurement55. The core sampler had an internal diameter of 5 cm and height of 5 cm56. Field capacity (FC) and soil permanent wilting point (PWP) were determined as described by Michael57. Soil available water content (AWC) was obtained by subtracting values of PWP from FC58.
Measurements of tomato growth and yield
Tomato growth parameters, including fresh above- (FAGB) and below- (FBGB) ground biomass, dry above- (DAGB) and below- (DBGB), were measured at the end of each growing seasons, following the details at Agebna16. Plant leaf relative water content (LRWC) was measured at one day closing to the middle of the growing season (June 13 in 2014 and July 18 in 2015), utilizing the method describe by Smart59. Two mature leaves in plant upper canopy of each treatment were selected to measure plant photosynthesis (Pn) and transpiration (Tr) rates. Four measurements were carried out in the growing season of 2014 (May 23, June 11, July 6 and July 17, respectively), using a portable photosynthesis system (Li-Cor, Lincoln, NE, USA) during the period of 09:00–12:00 h. Mean Pn and Tr rates were calculated from the four measurements.
Tomato fruits were harvested from July 18 to August 12 in 2014 and July 14 to August 5 in 2015. The number of fruits/plant (NFP) was accounted and weighed immediately to determine fruit mass (Yield). Fruits were homogenized using a blender, and chemical composition was quantified to determine fruit quality parameters, including parameters of total soluble solid (TSS, Brix %), titratable acidity (TA, g 100 g−1), vitamin C content (VC, mg 100 g−1 fresh mass, as ascorbic acid), soluble sugar content (SS, g 100 g−1) and fruit color index (CI)60–64.
Statistical analysis
All the treatments were subjected to a two way analysis of variance (ANOVA) using the MSTATC statistical package65,66. The Least Significant Difference (LSD; P ≤ 0.05) was used to determine differences between means.
Acknowledgements
We acknowledge and are grateful for the financial support provided by the National Key Research and Development Program of China (2017YFC0403300-04), by the National Natural Science Foundation of China through grant No. 41471180, by the CAS “Light of West China” Program, by the Fundamental Research Funds for the Central Universities (2018B12214), and by the Qing Lan Project of Jiangsu Province.
Author Contributions
Dongli She and Shuang’en Yu wrote the main manuscript text. Xiaoqin Sun and Agbna H. D. Gamareldawla done the experiment. Elshaikh A. Nazar prepared figures. Wei Hu and Khaembah Edith revised the English.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ahmad A, Wu YY, Xing DK, Qaiser J, Ikram U. Photosynthetic response of two okra cultivars under salt stress and re-watering. Journal of Plant Interactions. 2017;12:67–77. doi: 10.1080/17429145.2017.1385867. [DOI] [Google Scholar]
- 2.Shah T, Singh OP, Mukherji A. Some aspects of South Asia’s groundwater irrigation economy: analyses from a survey in India, Pakistan, Nepal Terai and Bangladesh. Hydrogeology Journal. 2006;14:286–309. doi: 10.1007/s10040-005-0004-1. [DOI] [Google Scholar]
- 3.Hanjra MA, Qureshi ME. Global water crisis and future food security in an era of climate change. Food Policy. 2010;35:365–377. doi: 10.1016/j.foodpol.2010.05.006. [DOI] [Google Scholar]
- 4.Rosegrant MW, Cai XM. Global water demand and supply projections: part 2. Results and prospects to 2025. Water International. 2002;27:170–182. doi: 10.1080/02508060208686990. [DOI] [Google Scholar]
- 5.Usman ARA, et al. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere. 2016;26:27–38. doi: 10.1016/S1002-0160(15)60019-4. [DOI] [Google Scholar]
- 6.Munns R. Genes and salt tolerance: bringing them together. New Phytologist. 2005;167:645–663. doi: 10.1111/j.1469-8137.2005.01487.x. [DOI] [PubMed] [Google Scholar]
- 7.Wang WY, Xiao-Feng Y, Ying J, Bo Q, Yu-Feng X. Effects of salt stress on water content and photosynthetic characteristics in Iris lacteavar. chinensis seedlings Middle-East. Journal of Scientific Research. 2012;12:70–74. [Google Scholar]
- 8.Romero-Aranda R, Soria T, Cuartero J. Tomato plant-water uptake and plant-water relationships under saline growth conditions. Plant Science. 2001;160:265–272. doi: 10.1016/S0168-9452(00)00388-5. [DOI] [PubMed] [Google Scholar]
- 9.Tavakkoli E, Fatehi F, Coventry S, Rengasamy P, McDonald GK. Additive effects of Na+ and Cl− ions on barley growth under salinity stress. Journal of Experimental Botany. 2011;62:2189–2203. doi: 10.1093/jxb/erq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jouyban Z. The effects of salt stress on plant growth. Technical Journal of Engineering and Applied Sciences. 2012;2:7–10. [Google Scholar]
- 11.Belkheiri O, Mulas M. The effects of salt stress on growth, water relations and ion accumulation in two halophyte Atriplex species. Environmental and Experimental Botany. 2013;86:17–28. doi: 10.1016/j.envexpbot.2011.07.001. [DOI] [Google Scholar]
- 12.Riccardi M, Pulvento C, Lavini A, d’Andria R, Jacobsen SE. Growth and ionic content of quinoa under saline irrigation. Journal of agronomy and crop science. 2014;200:246–260. doi: 10.1111/jac.12061. [DOI] [Google Scholar]
- 13.Hanay A, Buyuksanmz F, Kiziloglu FM, Canbolat MV. Reclamation of saline-sodic soils with gypsum and MSW compost. Compost science and utilization. 2004;12:175–179. doi: 10.1080/1065657X.2004.10702177. [DOI] [Google Scholar]
- 14.Ahmad M, Zahir ZA, Asghar HN, Arshad M. The combined application of rhizobial strains and plant growth promoting rhizobacteria improves growth and productivity of mung bean (Vignaradiata L.) under salt-stressed conditions. Annals of microbiology. 2012;62:1321–1330. doi: 10.1007/s13213-011-0380-9. [DOI] [Google Scholar]
- 15.Shrivastava P, Kumar R. Soil salinity: a serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi journal of biological sciences. 2015;22:123–131. doi: 10.1016/j.sjbs.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Agebna GH, et al. Effects of deficit irrigation and biochar addition on the growth, yield, and quality of tomato. Scientia Horticulturae. 2017;222:90–101. doi: 10.1016/j.scienta.2017.05.004. [DOI] [Google Scholar]
- 17.Atkinson CJ, Fitzgerald JD, Hipps NA. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: a review. Plant and Soil. 2010;337:1–18. doi: 10.1007/s11104-010-0464-5. [DOI] [Google Scholar]
- 18.Akhtar SS, Andersen MN, Naveed M, Zahir ZA, Liu F. Interactive effect of biochar and plant growth-promoting bacterial endophytes on ameliorating salinity stress in maize. Functional Plant Biology. 2015;42:770–781. doi: 10.1071/FP15054. [DOI] [PubMed] [Google Scholar]
- 19.Xu CY, et al. Effect of biochar amendment on yield and photosynthesis of peanut on two types of soils. Environmental Science and Pollution Research. 2015;22:6112–6125. doi: 10.1007/s11356-014-3820-9. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt HP, et al. Fourfold increase in pumpkin yield in response to low-dosage root zone application of urine-enhanced biochar to a fertile tropical soil. Agriculture. 2015;5:723–741. doi: 10.3390/agriculture5030723. [DOI] [Google Scholar]
- 21.Kammann CI, et al. Plant growth improvement mediated by nitrate capture in co-composted biochar. Scientific reports. 2015;5:11080. doi: 10.1038/srep11080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Joseph NS, et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nature Communications. 2017;8:1089. doi: 10.1038/s41467-017-01123-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lashari MS, et al. Effects of amendment of biochar –manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from central China Great Plain. Field Crop. Research. 2013;144:113–118. [Google Scholar]
- 24.Wang Y, et al. Effects of biochar on photosynthesis and antioxidative system of Malus hupehensisRehd. Seedlings under replant conditions. Scientia Horticulturae. 2014;175:9–15. doi: 10.1016/j.scienta.2014.05.029. [DOI] [Google Scholar]
- 25.Nguyen BT, Lehmann J. Black carbon decomposition under varying water regimes. Organic Geochemistry. 2009;40:846–853. doi: 10.1016/j.orggeochem.2009.05.004. [DOI] [Google Scholar]
- 26.Ameloot N, et al. Short-term CO2 and N2O emissions and microbial properties of biochar amended sandy loam soils. Soil Biolology and Biochemistry. 2013;57:401–410. doi: 10.1016/j.soilbio.2012.10.025. [DOI] [Google Scholar]
- 27.Wong VNL, Greene RSB, Dalal RC, Murphy BW. Soil carbon dynamics in saline and sodic soils: A review. Soil Use Management. 2010;26:2–11. doi: 10.1111/j.1475-2743.2009.00251.x. [DOI] [Google Scholar]
- 28.Coleman K, et al. Simulating trends in soil organic carbon in long-term experiments using RothC-26.3. Geoderma. 1997;81:29–44. doi: 10.1016/S0016-7061(97)00079-7. [DOI] [Google Scholar]
- 29.Laird DA, et al. Impact of biochar amendments on the quality of a typical Midwestern agricultural soil. Geoderma. 2010;158:443–449. doi: 10.1016/j.geoderma.2010.05.013. [DOI] [Google Scholar]
- 30.Uzoma K, et al. Effect of cow manure biochar on maize productivity under sandy soil condition. Soil use and management. 2011;27:205–212. doi: 10.1111/j.1475-2743.2011.00340.x. [DOI] [Google Scholar]
- 31.Kazuhiro N, Nasir MK, Sho S. Effects of salt accumulation on the leaf water potential and transpiration rate of pot-grown wheat with a controlled saline groundwater table. Soil Science & Plant Nutrition. 2009;55(3):375–384. doi: 10.1111/j.1747-0765.2009.00368.x. [DOI] [Google Scholar]
- 32.Plaut Z, Meinzer FC, Federman E. Leaf development, transpiration and ion uptake and distribution in sugarcane cultivars grown under salinity. Plant Soil. 2000;218:59–69. doi: 10.1023/A:1014996229436. [DOI] [Google Scholar]
- 33.Sharma N, Gupta NK, Gupta S, Hasegawa H. Effect of NaCl salinity on photosynthetic rate, transpiration rate, and oxidative stress tolerance in contrasting wheat genotypes. Photosynthetica. 2005;43:609–613. doi: 10.1007/s11099-005-0095-x. [DOI] [Google Scholar]
- 34.Wong VNL, Dalal RC, Greene RSB. Carbon dynamics of sodic and saline soil following gypsum and organic material additions: A laboratory incubation. Applied Soil Ecology. 2009;41:29–40. doi: 10.1016/j.apsoil.2008.08.006. [DOI] [Google Scholar]
- 35.Demir K, Sahin O, Kadioglu YK, Pilbeam DJ, Gunes A. Essential and non-essential element composition of tomato plants fertilized with poultry manure. Scientia Horticulturae. 2010;127:16–22. doi: 10.1016/j.scienta.2010.08.009. [DOI] [Google Scholar]
- 36.Thomas SC, et al. Biochar mitigates negative effects of salt additions on two herbaceous plant species. Journal of Environmental Management. 2013;129:62–68. doi: 10.1016/j.jenvman.2013.05.057. [DOI] [PubMed] [Google Scholar]
- 37.Alling V, et al. The role of biochar in retaining nutrients in amended tropical soils. Journal of Plant Nutrition and Soil Sciences. 2014;177:671–680. doi: 10.1002/jpln.201400109. [DOI] [Google Scholar]
- 38.Fiaz K, et al. Drought impact on Pb/Cd toxicity remediated by biochar in Brassica campestris. Journal of Plant Nutrition and Soil Sciences. 2014;14:845–854. [Google Scholar]
- 39.Munns R, Tester M. Mechanisms of salinity tolerance. Ann. Rev. Plant Biology. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 40.Hossain MK, Strezov V, Chan KY, Nelson PF. Agronomic properties of wastewater sludge biochar and bioavailability of metals in production of cherry tomato (Lycopersiconesculentum) Chemosphere. 2010;78:1167–1171. doi: 10.1016/j.chemosphere.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell JP, Shennan C, Grattan SR, May DM. Tomato fruit yields and quality under water deficit and salinity. Journal of the American Society for Horticultural Science. 1991;116:215–221. [Google Scholar]
- 42.Malash NM, Flowers TJ, Ragab R. Effect of irrigation methods, management and salinity of irrigation water on tomato yield, soil moisture and salinity distribution. Irrigation Science. 2008;26:313–323. doi: 10.1007/s00271-007-0095-7. [DOI] [Google Scholar]
- 43.Sánchez-Rodríguez E, Moreno DA, Ferreres F, del Mar Rubio-Wilhelmi M, Ruiz JM. Differential responses of five cherry tomato varieties to water stress: changes on phenolic metabolites and related enzymes. Phytochemistry. 2011;72:723–729. doi: 10.1016/j.phytochem.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 44.Akhtar SS, Li G, Andersen MN, Liu F. Biochar enhances yield and quality of tomato under reduced irrigation. Agricultural Water Management. 2014;138:37–44. doi: 10.1016/j.agwat.2014.02.016. [DOI] [Google Scholar]
- 45.Wang F, Kang SZ, Du TS, Li FS, Qiu RJ. Determination of comprehensive quality index for tomato and its response to different irrigation treatments. Agricultural Water Management. 2011;98:1228–1238. doi: 10.1016/j.agwat.2011.03.004. [DOI] [Google Scholar]
- 46.Pérez-López AJ, López-Nicolás JM, Carbonell-Barrachina AA. Effects of organic farming on minerals contents and aroma composition of Clemenules mandarin juice. European Food Research and Technology. 2007;225:255–260. doi: 10.1007/s00217-006-0412-z. [DOI] [Google Scholar]
- 47.Novak JM, et al. Biochars impact on soil-moisture storage in an ultisol and two aridisols. Soil Science. 2012;177:310–320. doi: 10.1097/SS.0b013e31824e5593. [DOI] [Google Scholar]
- 48.Liu Z, et al. Effects of biochar amendment on rapeseed and sweet potato yields and water stable aggregate in upland red soil. Catena. 2014;123:45–51. doi: 10.1016/j.catena.2014.07.005. [DOI] [Google Scholar]
- 49.Nelson DW, et al. Total carbon, organic carbon, and organic matter. Methods of soil analysis. Part3-chemical methods. 1996;1:961–1010. [Google Scholar]
- 50.Gupta, P. K. Soil, plant, water and fertilizer analysis. Agrobios (India), Jodhpur, New Delhi, India. pp. 438 (2000).
- 51.Richards LA. Book Reviews: Diagnosis and improvement of saline and alkali soils. Science. 1954;120:800. [Google Scholar]
- 52.Chapman HD, Kelly WP. The determination of the replaceable bases and the base-exchange capacity of soils. Soil Science. 1930;30(5):391–406. doi: 10.1097/00010694-193011000-00004. [DOI] [Google Scholar]
- 53.Bremner, J. M. & Mulvaney, C. S. Nitrogen-total. In: Methods of soil analysis (Eds Page, A. L., Miller, R. H. & Keeney, D. R.). American Society of soil. Inc., Madison, WI. pp. 595–624 (1982).
- 54.Olsen, S. R. & Sommers, L. E. Phosphorus. In: Methods of Soil Analysis, 2nd ed., part 2 (Eds Page, A. L., Miller, R. H. & Keeney,D. R.). Agronomy No. 9. American Society ofAgronomy, Madison, WI, USA. pp. 403–430 (1982).
- 55.Herath HMSK, Arbestain MC, Hedley M. Effect of biochar on soil physical properties in two contrasting soils: An Alfisol and an Andisol. Geoderma. 2013;209-210:188–197. doi: 10.1016/j.geoderma.2013.06.016. [DOI] [Google Scholar]
- 56.Abel S, et al. Impact of biochar and hydrochar addition on water retention and water repellency of sandy soil. Geoderma. 2013;202:183–191. doi: 10.1016/j.geoderma.2013.03.003. [DOI] [Google Scholar]
- 57.Michael, A. M. Irrigation: Theory and practice. Vikas Publishing House. Pvt. LTD. New Delhi, India (1978).
- 58.Zotarelli, L., Dukes, M. d. & Morgan, K. T. Interpretation of soil moisture content to determine soil field capacity and avoid over-irrigating sandy soils using soil moisture sensors. The Agricultural and Biological Engineering Department, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida, AE460. Chicago (2010).
- 59.Smart RE, Bingham GE. Rapid estimates of relative water content. Plant Physiology. 1974;53:258–260. doi: 10.1104/pp.53.2.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.AOAC. Official Methods of Analysis of AOAC International 16th Edn. Gaithersburg: Ed. AOAC International (1999).
- 61.Patanè C, Tringali S, Sortino O. Effects of deficit irrigation on biomass, yield, water productivity and fruit quality of processing tomato under semi-arid Mediterranean climate conditions. Scientia Horticulturae. 2011;129:590–596. doi: 10.1016/j.scienta.2011.04.030. [DOI] [Google Scholar]
- 62.Chen J, et al. Quantitative response of greenhouse tomato yield and quality to water deficit at different growth stages. Agricultural Water Management. 2013;129:152–162. doi: 10.1016/j.agwat.2013.07.011. [DOI] [Google Scholar]
- 63.AOAC. Association of Official Agriculture Chemists: Official Methods of Analysis. 10th Edn., AOAC., Washington, D.C., USA (1995).
- 64.LópezCamelo AF, Gómez PA. Comparison of color indexes for tomato ripening. Horticultura Brasileira. 2004;22:534–537. doi: 10.1590/S0102-05362004000300006. [DOI] [Google Scholar]
- 65.Fischer, S.D. MSTAT-C statistical package. Michigan State University, USA (1990).
- 66.Cao XC, et al. Assessing water scarcity in agricultural production system based on the generalized water resources and water footprint framework. Science of the Total Environment. 2017;609:587–597. doi: 10.1016/j.scitotenv.2017.07.191. [DOI] [PubMed] [Google Scholar]