Abstract
Duchenne muscular dystrophy (DMD) is a lethal muscle disease caused by dystrophin gene mutation. Conceptually, replacing the mutated gene with a normal one would cure the disease. However, this task has encountered significant challenges due to the enormous size of the gene and the distribution of muscle throughout the body. The former creates a hurdle for viral vector packaging and the latter begs for whole-body therapy. To address these obstacles, investigators have invented the highly abbreviated micro-dystrophin gene and developed body-wide systemic gene transfer with adeno-associated virus (AAV). Numerous microgene configurations and various AAV serotypes have been explored in animal models in many laboratories. Preclinical data suggests that intravascular AAV micro-dystrophin delivery can significantly ameliorate muscle pathology, enhance muscle force, and attenuate dystrophic cardiomyopathy in animals. Against this backdrop, several clinical trials have been initiated to test the safety and tolerability of this promising therapy in DMD patients. While these trials are not powered to reach a conclusion on clinical efficacy, findings will inform the field on the prospects of body-wide DMD therapy with a synthetic micro-dystrophin AAV vector. This review discusses the history, current status, and future directions of systemic AAV micro-dystrophin therapy.
Keywords: Duchenne muscular dystrophy, dystrophin, micro-dystrophin, adeno-associated virus, systemic gene therapy, AAV, DMD, Becker muscular dystrophy, BMD, clinical trial
Adeno-associated virus (AAV)-mediated systemic micro-dystrophin gene therapy has entered clinical trials in Duchenne muscular dystrophy patients. This paper reviews the history of the development of the AAV micro-dystrophin vector, preclinical results in mice and canines, current status of the field, existing problems, and future directions of this revolutionary therapeutic approach.
Main Text
Introduction
Duchenne muscular dystrophy (DMD) is an inherited X-linked recessive muscle-wasting disease caused by mutations in the dystrophin gene.1, 2 DMD affects approximately 1 in every 5,000 newborn boys.3 Patients start to show symptoms of muscle weakness at 2 to 3 years of age.4 Muscle function deteriorates rapidly at ∼7 years of age.5, 6, 7 Most patients lose ambulation at ∼10–12 years of age and die in the second to third decade of their life due to diaphragm failure and/or cardiac complications.4, 8
For a disease caused by mutation(s) in a single gene, a highly appealing therapy is to replace the diseased gene with a normal gene using gene therapy. Luxturna, an adeno-associated virus (AAV) gene therapy drug recently approved by the Food and Drug Administration (FDA) for treating Leber congenital amaurosis (LCA) provides an outstanding proof-of-concept for gene-replacement therapy.9 LCA is a hereditary recessive blindness disease caused by mutations in the retinal pigment epithelium-specific 65-kDa protein (RPE65) gene. AAV delivery of a normal RPE65 gene to the retina partially restores vision in patients.10
Tremendous efforts have been made over the last three decades to develop gene-replacement therapy for DMD. Although conceptually similar to that of AAV RPE65 gene therapy for LCA, DMD gene-replacement therapy has turned out to be a much more challenging task. The first problem is the size of the gene. Unlike the RPE65 gene, the dystrophin gene greatly exceeds the packaging capacity of the AAV vector. The second problem is the distribution of the diseased tissue. Unlike the retina, muscle spreads all over the body. Direct muscle injection offers limited benefits. Furthermore, the heart and the diaphragm, two muscles that are most important for the prognosis, are located deep inside the body and not readily accessible from the surface. Micro-dystrophin genes and systemic AAV delivery are developed to solve these problems.11, 12 Below, I review the progress and current status of preclinical and clinical development of AAV micro-dystrophin gene therapy for DMD. I also discuss unanswered questions and future research directions.
Preclinical Development of Systemic AAV Micro-dystrophin Gene Therapy
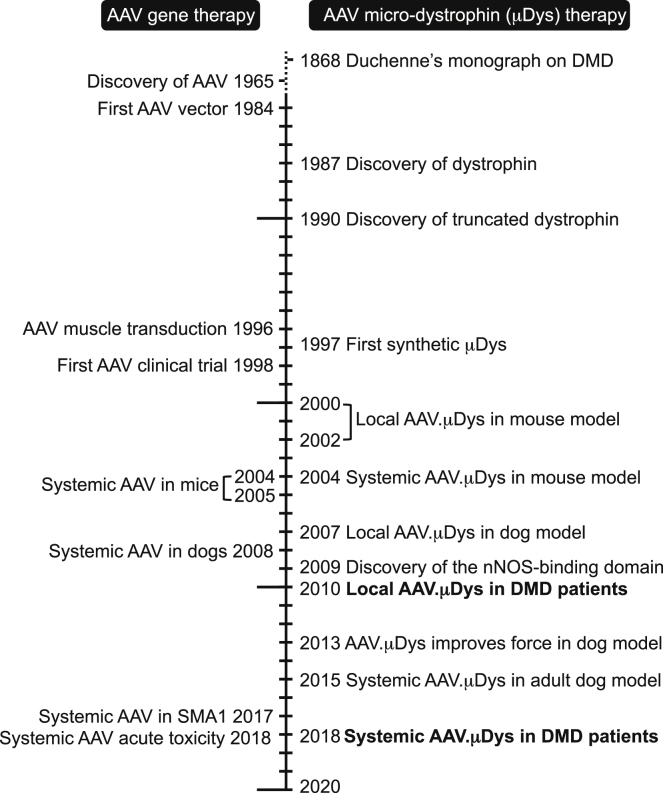
The history of AAV micro-dystrophin gene therapy can be traced back to the growth of two fascinating research disciplines, DMD genetics and AAV biology (Figure 1). Studies on the genetics of DMD not only resulted in the discovery of the dystrophin gene but also provided the rationale for treating DMD with an abbreviated gene. Studies on AAV biology open the door to the development the AAV vector, an enormously powerful viral vector for human gene therapy (Figure 2).13
Figure 1.
Historical Milestones in the Development of Systemic AAV Micro-dystrophin Gene Therapy
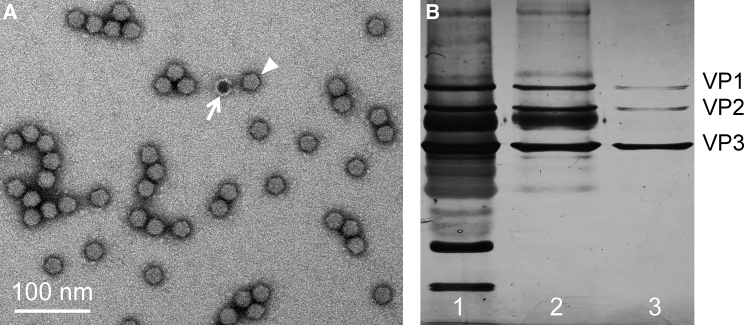
Figure 2.
Adeno-associated Viral Vector
(A) A representative electron microscopic image of the AAV vector. Arrowhead, a fully packaged AAV particle. Arrow, an empty AAV particle. (B) Examination of AAV purity with SDS-PAGE silver staining. Lanes 1, 2, and 3 show a gradual increase of the purity after one, two, and three rounds of purification. The highly pure AAV stock has three viral proteins (VPs) at the ratio of VP1:VP2:VP2 ≈ 1:1:10.
DMD-like symptoms have been described in the literature since 1850.2 The disease got its name following the publication of a clinical monograph in 1968 by French physician Duchenne de Boulogne.14 The gene name was coined in 1987 by Louise Kunkel following the discovery of the gene and its protein product by the Kunkel laboratory.1, 15, 16 The full-length dystrophin gene is 2.6 mb. It encodes 79 exons. The 11.5-kb coding sequence translates into a 427-kD protein. Dystrophin can be divided into four major domains, including the N-terminal domain, rod domain, cysteine-rich domain, and C-terminal domain. The rod domain can be further divided into 24 spectrin-like repeats and four hinges (Figure 3).
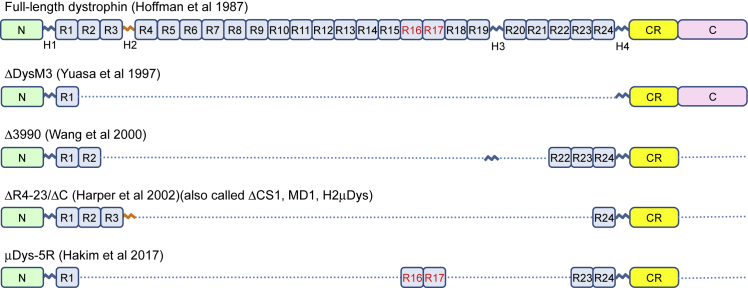
Figure 3.
Full-Length Dystrophin and Representative Micro-dystrophins
Full-length dystrophin contains an N-terminal domain (N), 24 spectrin-like repeats (R1 to R24), four hinges (H1 to H4), a cysteine-rich domain (CR), and a C-terminal domain (CT). ΔDysM3 is the first synthetic micro-dystrophin. Δ3990, ΔR4–23/ΔC and μDys5R are three micro-dystrophins currently in use in clinical trials. H2 is marked in orange to indicate that it compromises micro-dystrophin function in the mouse DMD model (see Banks et al.53 for details). R16 and R17 are marked in red to indicate that they are the nNOS-binding domain (see Lai et al.46, 98 for details).
The availability of the dystrophin coding sequence immediately raised hopes for curing DMD at its genetic root.17 Indeed, dystrophin gene-replacement therapy has been on the very top of the research agenda ever since the discovery of the gene. Because the full-length dystrophin cDNA exceeds the packaging capacity of the first generation adenoviral and retroviral vectors, early proof-of-principle studies used simple intramuscular injection of plasmid DNA, which is an inefficient method for in vivo muscle gene therapy.18 The discovery of the highly functional Δ17–48 mini-dystrophin protein by Kay Davies’s laboratory in 199019 changed the situation. Despite being a half-size protein, it provided excellent muscle protection. One patient was ambulant at age 61, and another was a body builder at age 25.19 The therapeutic potential of mini-dystrophin has since been confirmed in numerous genotype-phenotype correlation studies in human patients (Table 1).20, 21, 22, 23, 24, 25, 26, 27, 28, 29 The 6.2 kb Δ17–48 minigene was evaluated in a series of studies using adenoviral and retroviral vectors and in transgenic mice.30 While encouraging results were achieved in animal models, these viral vectors have not been translated to DMD patients. Adenoviral vectors have strong immunotoxicity, while retroviral vectors are difficult to use for in vivo gene therapy. Notably, these vectors are ineffective for systemic delivery. DMD gene therapy was thus in need of more suitable vector systems.
Table 1.
Dystrophin Large In-Frame Deletion and Clinical Phenotype
| Genotype | % Lost | Level of Expression | Clinical Phenotype | Reference |
|---|---|---|---|---|
| Full-length | 0% | +++ | normal | 16 |
| Δ17–48 | 46% | +++ | BMD | 19 |
| Δ13–47 | 47% | ++ ∼+++ | BMD | 23 |
| Δ10–44 | 48% | ++ | DMD | 143 |
| Δ10–44 | 48% | N/A | BMD | 28 |
| Δ10–44 | 48% | N/A | BMD | 28 |
| Δ13–48 | 49% | N/A | BMD | 27 |
| Δ13–48 | 49% | ++ ∼+++ | BMD | 24 |
| Δ13–48 | 49% | ++ | BMD | 26 |
| Δ4–41 | 50% | + | DMD | 24 |
| Δ4–41 | 50% | ++ | DMD | 145 |
| Δ4–41 | 50% | − | DMD | 26 |
| Δ3–41 | 51% | ++ | DMD | 26 |
| Δ3–41 | 51% | ++ | IMD | 144 |
| Δ3–41 | 51% | + | DMD | 143 |
| Δ3–42 | 52% | + | IMD | 24 |
| Δ11–48 | 52% | N/A | DMD | 142 |
| Δ5–44 | 54% | N/A | DMD | 28 |
| Δ10–53 | 60% | N/A | DMD | 25 |
| Δ10–53 | 60% | +++ | DMD | 141 |
| Δ14–60 | 61% | N/A | DMD | 140 |
| Δ2–50 (Δ2–44)a | 63% | ++ | DMD | 139 |
Abbreviations: BMD, Becker muscular dystrophy; DMD, Duchenne muscular dystrophy; IMD, intermediate muscular dystrophy (clinical phenotype between BMD and DMD); N/A, information not available.
The patient has a Δ2–44 deletion in DNA but a Δ2–50 deletion in mRNA due to alternative splicing.
AAV was discovered as a contaminating particle in an adenovirus stock in 1965 (Figures 1 and 2).31 In 1984, the Nicholas Muzyczka laboratory built the first recombinant AAV virus for gene transfer32. In 1998, the first AAV gene therapy clinical trial was conducted in cystic fibrosis patients.33 AAV has also been tested for muscle gene transfer. In contrast to adenovirus and retrovirus, direct muscle injection of the AAV vector produced high-level and persistent gene transfer in muscle.34, 35 Most importantly, a single intravenous delivery of AAV-6, -8, and -9 resulted in whole-body muscle transduction in rodents and large mammals.36, 37, 38 These desirable features encouraged the development of AAV as a vector for muscle gene therapy.39, 40 However, there is a major hurdle to applying AAV for DMD gene therapy. The maximal packaging capacity of an AAV virus is 5 kb, smaller than the 6.2 kb mini-dystrophin gene.
The problem was addressed with the development of synthetic microgenes that are less than 4 kb. In 1997, Yuasa et al.41 published the 3.7 kb ΔDysM3 gene (Figure 3; Table 2). This microgene encodes the N-terminal domain, hinges 1 and 4, a single spectrin-like repeat, the cysteine-rich domain, and C-terminal domain. Unfortunately, the ΔDysM3 gene did not reduce dystrophic phenotype in the mouse model.42 The first series of protective AAV micro-dystrophin vectors were reported in 2000 and 2002 by the Xiao lab43 and Chamberlain lab,44 respectively (Table 2). The therapeutic potency of these microgenes has been significantly improved with subsequent modifications, in particular, codon optimization by the Dickson lab in 200845 and inclusion of the dystrophin spectrin-like repeats 16 and 17 (R16/17) neuronal nitric oxide synthase (nNOS)-binding domain by the Duan lab in 2009.46 Hitherto, more than 30 different configurations of synthetic microgenes have been published (Table 2).41, 43, 44, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57 Among these, the Δ3990-, ΔR4–23/ΔC- (deletion of repeats 4 to 23 and deletion of the C-terminal domain) and R16/17-containing micro-dystrophin are particularly noteworthy given the extensive safety and efficacy data on these constructs in the murine and canine models. As exemplified in the representative data from two publications (Figure 4),58, 59 a large body of preclinical studies from many laboratories has now provided compelling evidence that AAV delivery of a rationally designed synthetic micro-dystrophin gene can significantly reduce muscle pathology, increase muscle force, enhance cardiac function, and prolong lifespan in animal models.12, 60
Table 2.
Structural and Functional Features of Micro-dystrophins Developed between 1997 and 2017
| Year | Name | Other Name | MW | % Lost | NT | Rod (Hinges) | Rod (Repeats) | CR | CT | Pathology | Force | Comments | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | ΔDysM3 | M3 | 125 | 71% | Yes | 2 (H1, H4) | 2 (R1,a R24) | Yes | Yes | reduced | no improvement | this is different from M3 reported in 2009 | 41 |
| 1998 | ΔDysH1 | 103 | 76% | Yes | 1 (H1) | 0 | Yes | Yes | N/A | N/A | 47 | ||
| 1998 | ΔDysH4 | 103 | 76% | Yes | 1 (H4) | 0 | Yes | Yes | N/A | N/A | 47 | ||
| 1998 | ΔDysAH3 | 138 | 68% | Yes | 2 (H1, H4) | 3 (R1, R2,a R24) | Yes | Yes | N/A | N/A | 47 | ||
| 1998 | ΔDysAX2 | 150 | 65% | Yes | 2 (H1, H4) | 4 (R1, R2, R3,a R24) | Yes | Yes | N/A | N/A | 47 | ||
| 1998 | ΔDysAX11 | AX11 | 150 | 65% | Yes | 2 (H1, H4) | 4 (R1, R2,a R23, R24) | Yes | Yes | reduced | no improvement | 47 | |
| 2000 | Δ3849 | ∼130 | 70% | Yes | 2 (H1, H4) | 5 (R1, R2, R22, R23, R24) | Yes | No | reduced | improved | 43 | ||
| 2000 | Δ3990 | ∼140 | 67% | Yes | 3 (H1, H3, H4) | 5 (R1, R2, R22, R23, R24) | Yes | No | reduced | improved | clinical trial candidate | 43 | |
| 2000 | Δ4173 | ∼140 | 67% | Yes | 2 (H1, H4) | 6 (R1, R2, R3, R22, R23, R24) | Yes | No | reduced | N/A | 43 | ||
| 2002 | ΔR1–R24 | 108 | 75% | Yes | 2 (H1, H4) | 0 | Yes | Yes | no improment | N/A | 44 | ||
| 2002 | ΔR4–R23/ΔCT | ΔCS1, MD1, H2μDys | 138 | 68% | Yes | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | improved | clinical trial candidate | 44 |
| 2002 | ΔR4–R23 | CS1 | 167 | 61% | Yes | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | Yes | reduced | improved | 44 | |
| 2002 | ΔR2–R21 | 165 | 61% | Yes | 2 (H1, H4) | 4 (R1, R22, R23, R24) | Yes | Yes | reduced | improved | 44 | ||
| 2002 | ΔR2–R21+H3 | 169 | 60% | Yes | 3 (H1, H3, H4) | 4 (R1, R22, R23, R24) | Yes | Yes | reduced | improved | 44 | ||
| 2002 | Δ3788 | ΔAB/ΔR3–18/ΔCT | 144 | 66% | Yesa | 3 (H1, H3, H4) | 8 (R1, R2, R19, R20, R21 R22, R23, R24) | Yes | No | reduced | improved | 48 | |
| 2002 | CS1 | 165 | 61% | Yes | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | Yes | reduced | improved | 49 | ||
| 2004 | ΔCS1 | ΔR4–R23/ΔCT, MD1, H2μDys | 138 | 68% | Yes | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | improved | clinical trial candidate | 50 |
| 2007 | ABS1,2μDys | N/A | >60% | Yesa | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | no improment | 51 | ||
| 2007 | ABS1μDys | N/A | >60% | Yesa | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | no improment | 51 | ||
| 2009 | ΔR2–15/ΔR18–23/ΔC | R16–17/ΔC, YL90 | 132 | 69% | Yes | 2 (H1, H4) | 4 (R1, R16, R17, R24) | Yes | No | reduced | improved | 46 | |
| 2009 | ΔR3–15/ΔR18–23/ΔC | YL93 | 144 | 66% | Yes | 2 (H1, H4) | 5 (R1, R2, R16, R17, R24) | Yes | No | reduced | improved | 46 | |
| 2009 | ΔR3–15/ΔR17–23/ΔC | YL113 | 132 | 69% | Yes | 2 (H1, H4) | 4 (R1, R2, R16, R24) | Yes | No | N/A | N/A | 46 | |
| 2009 | M1 | 127 | 73% | Yes | 2 (H1, H4) | 1.5 (R23,a R24) | Yes | Yes | reduced | N/A | 52 | ||
| 2009 | M2 | 125 | 71% | Yes | 2 (H1, H4) | 1.5 (R1, R24a) | Yes | Yes | reduced | improved | no force increase in similar constructs (ΔDysM3, AX11) | 52 | |
| 2009 | M3 | 125 | 71% | Yes | 2 (H1, H4) | 1 (R24) | Yes | Yes | reduced | N/A | this is different from M3 reported in 1997 | 52 | |
| 2009 | M4 | 115 | 70% | Yes | 2 (H1, H4) | 0.5 (R24a) | Yes | Yes | reduced | N/A | 52 | ||
| 2010 | ΔH2-R24/ΔCT | N/A | >60% | Yes | 2 (H1, H4) | 3 (R1, R2, R3) | Yes | No | reduced | N/A | 53 | ||
| 2010 | ΔH2-R23+H3/ΔCT | H3μDys | N/A | >60% | Yes | 3 (H1, H3, H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | improved | H2 is bad | 53 |
| 2010 | ΔR2–R23+R18-H3/ΔCT | N/A | >60% | Yes | 3 (H1, H3, H4) | 4 (R1, R18, R19, R24) | Yes | No | reduced | N/A | 53 | ||
| 2010 | ΔR4–R23/ΔCT/ΔpolyP | N/A | >60% | Yes | 3 (H1, H2,a H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | N/A | polyproline site in H2 is bad | 53 | |
| 2011 | MD1 | ΔR4–R23/ΔCT, ΔCS1, H2μDys | 138 | 68% | Yes | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | improved | clinical trial candidate | 54 |
| 2011 | MD2 | 154 | 64% | Yes | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | Yesa | reduced | improved | 54 | ||
| 2011 | ΔCS2 | 140 | 67% | Yes | 3 (H1, H2, H4) | 4 (R1, R2, R3, R24) | Yes | No | reduced | improved | ΔCS1+Dys2 epitope | 55 | |
| 2012 | R16–17/H3/ΔC | ΔR2–15/ΔR18–19/Δ20–23/ΔC, YL196 | 133 | 69% | Yes | 3 (H1, H3, H4) | 4 (R1, R16, R17, R24) | Yes | No | reduced | improved | 56 | |
| 2017 | mDys5R | ΔR2–15/ΔR18–19/Δ20-22/ΔC, XP42, μDys5 | 147 | 66% | Yes | 2 (H1, H4) | 5 (R1, R16, R17, R23, R24) | Yes | No | reduced | improved | clinical trial candidate | 57 |
MW, moleclar weight (in kD); N/A, information not available; Yes, The domain is present in the construct; No, The domain is absent in the construct.
The domain is truncated.
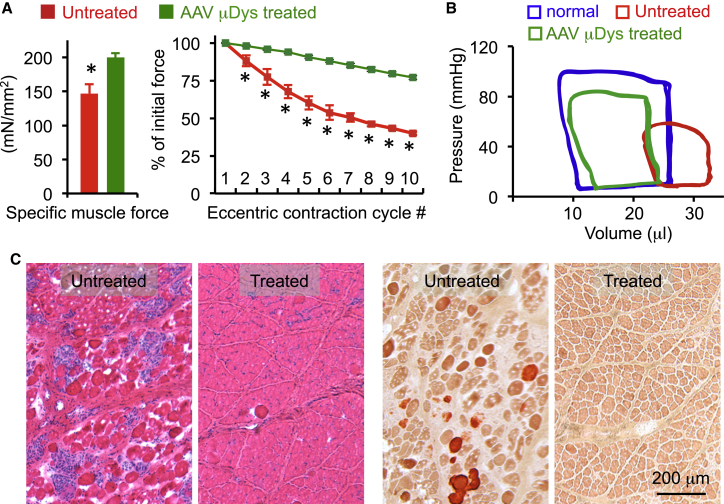
Figure 4.
AAV Micro-dystrophin Gene Therapy Ameliorated Muscle Disease in the Murine and Canine DMD Models
(A) Systemic AAV micro-dystrophin injection improved skeletal muscle function in mdx mice. Treatment significantly improved specific tetanic force and resistance to eccentric contraction-induced force drop in the extensor digitorum longus muscle (see Shin et al.58 for details). Error bar, mean ± SEM. (B) Systemic AAV micro-dystrophin injection improved cardiac hemodynamics in mdx mice (see Bostick et al.59 for details). (C) AAV micro-dystrophin therapy improved histology (left) and reduced pathological muscle calcification (right) in the extensor carp ulnaris muscle in affected dogs (see Shin et al.58 for details).
The next critical achievement in the development of DMD gene therapy is vascular AAV delivery for body-wide therapy.40 This is of utmost importance for DMD in order to significantly change the clinical course of the disease. Treating a single muscle or a group of muscles can only improve the function of the treated muscle. This may improve life quality (for example treating forearm flexors can help wheelchair-bound patients to grasp), but it will not slow down the progression of the disease and will not reduce mortality. Except for the extra-ocular muscle, essentially all body muscles undergo continuous deterioration in DMD. Diaphragm necrosis results in respiratory failure. Myocardial dystrophy leads to heart failure and sudden cardiac death. The breakthrough in systemic gene transfer occurred in 2004 and 2005 when the Chamberlain lab36 and Xiao lab37 showed effective whole-body muscle transduction in rodents with AAV serotype-6 and -8, respectively. In 2008, the Duan lab38 demonstrated that systemic AAV transduction also worked in canines using AAV-9. Currently, systemic delivery has been achieved using a large collection of AAV capsids that are either isolated from nature or engineered in the laboratory.40, 61
In parallel with the development of systemic AAV delivery technology, AAV micro-dystrophin vectors were tested in the mouse and dog DMD models.12, 60, 62 In 2004, the Chamberlain lab36 showed widespread systemic AAV micro-dystrophin transduction in muscles and an improvement of limb muscle function in young mdx mice, a commonly used mouse DMD model. However, young mdx mice are poor models because they do not show dystrophic symptoms.62 To more stringently test systemic AAV therapy in clinically relevant models, experiments were conducted in a number of more severely affected mouse models, such as aged mdx mice, utrophin and dystrophin double knockout mice, and DBA/2J-mdx mice.57, 59, 63, 64, 65, 66 These studies have provided unequivocal evidence for body-wide improvement with systemic AAV micro-dystrophin therapy in the mouse model. Systemic AAV micro-dystrophin therapy in the canine DMD model was first performed in newborn puppies (Table 3).67 Despite widespread micro-dystrophin expression in muscles throughout the body, treated puppies displayed weight loss and clinical signs of inflammatory myopathy.67 In light of the concern on high-dose systemic AAV delivery to a dystrophic large mammal, regional perfusion was proposed as an intermediate step to derisk whole-body administration.40 The technique was tested in dogs, non-human primates, and human patients. Results from these studies revealed several limitations, including (1) uncertainty on consistently delivering AAV to the intended limb, (2) failure to reach the heart and diaphragm, two most critical targets, and (3) safety concerns on applying a tourniquet to a DMD patient to achieve vascular escape of the vector. The first successful systemic AAV micro-dystrophin therapy in young adult dystrophic dogs was published in 2015 (Table 3).68 The authors treated affected dogs at 2 months of age and observed body-wide muscle transduction and amelioration of muscle pathology.68 A recent study further confirmed the safety and durability of systemic AAV micro-dystrophin therapy in juvenile affected dogs (Table 3).69 Importantly, this study demonstrated a dose-dependent improvement in clinical score and dog gait. The safety, durability, histological, and functional amelioration, and the dose response of systemic AAV micro-dystrophin therapy were further confirmed in two additional studies (Table 3).70, 71, 72, 73
Table 3.
Systemic AAV Micro-dystrophin Study in the Canine DMD Model
| References | Injection Age (Months) | Sample Size | AAV Serotype | AAV Dose (× 1014 vg/kg) | Expression Cassette |
Follow-up (Months) | μ-Dystrophin Expression |
Disease Amelioration | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Promoter | Transgene | Bodywide | Persistent | |||||||
| 67 | 0.13 | 3 | AAV-9 | 1.5 | CMV | human m-Dys | 4 | confirmed | confirmed | N/A |
| 68 | 1.8 | 2 | Y731F AAV-9 | 5.0, 6.2 | CMV | canine m-Dys | 4 | confirmed | confirmed | improvement in muscle histology |
| 69 | 2∼2.5 | 8 | AAV-8 | 0.2, 1.0 | Spc5-12 | canine m-Dys | 6.5∼24 | N/A | confirmed | improvement with clinical score and gait in 1.0 × 1014 vg/kg group |
| 71, 72 | 2.5∼3.5 | 5 | AAV-9 | 0.5, 1.0, 3.0 | CK8 | canine m-Dys | 8, 30 | confirmed | confirmed | improvement in muscle histology and force |
| 70 | 3 | 9 | AAV-9 | 0.1, 1.0, 2.0 | CK8 | canine m-Dys | 3 | confirmed | confirmed | dose-dependent improvement in muscle histology and function |
Prelude to Systemic AAV Micro-dystrophin Gene Therapy Trials in DMD Patients
The first AAV micro-dystrophin gene therapy in human patients was initiated on March 28, 2006 by Jerry Mendell and colleagues at the Nationwide Children’s Hospital in Columbus, Ohio USA74 (Figure 5). In this study, a cytomegalovirus (CMV) promoter-driven Δ3990 minigene cassette was packaged in AAV-2.5 and injected to the biceps of six 5- to 11-year-old patients (2 × 1010 vg/kg or 1 × 1011 vg/kg) (Figure 3; Tables 2 and 4).74, 75 Four hours before injection, all patients received methypredinisolone (2 mg/kg). A biopsy was performed at 42 or 90 days after injection. 3∼4 positive myofibers were detected in one low-dose patient at day 42, and one positive myofiber was detected in one high-dose patient at day 42 (Table 4).74 No positive myofiber was detected in the remaining patients. Among these “non-responders,” one showed T cell response to micro-dystrophin, another showed T cell response to revertant fibers (rare dystrophin positive fibers in patient muscle), and a third patient showed T cell response to AAV capsid (Table 4).74, 75 Collectively, the level of micro-dystrophin expression seen in this trial is far from sufficient for therapy. Several explanations have been suggested for the negative outcome in patients, such as immunity to new antigenic epitopes in micro-dystrophin, immunity to preexisting antigenic epitopes in revertant dystrophin, and immunity to viral capsid. The use of the ubiquitous (rather than the muscle-specific) promoter and failure to screen patients for preexisting anti-AAV antibodies may have also contributed to the immune response. Addressing these issues may likely lead to a successful trial in the future. In summary, this first in-patient study has provided critical insights for the design of future human trials, especially, in regards to AAV serotype, expression cassette, immune response, and patient selection.
Figure 5.
First AAV Micro-dystrophin Clinical Trial
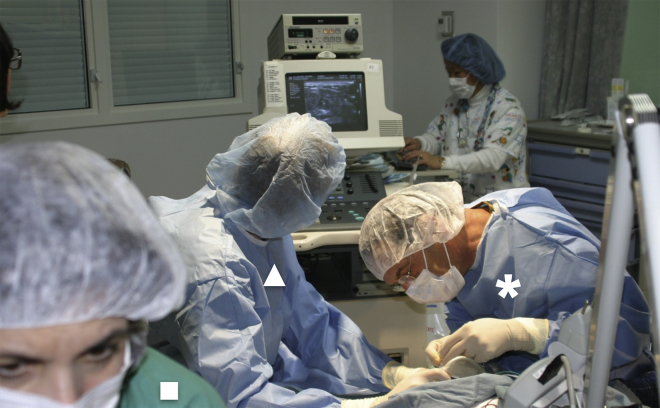
Direct injection of the AAV vector to the biceps of a patient by Dr. Jerry Mendell (asterisk). The injection was assisted by an interventional radiologist (triangle) and a neurologist (square). The radiologist and the neurologist guided and monitored the injection process with ultrasound and electromyography, respectively, to make sure AAV was delivered into viable muscle (see Mendell et al.74 for details).
Table 4.
First AAV Micro-dystrophin Clinical Trial (Local Injection)
| Patient | Dose (vg/kg) | Cortico Steroid | Pre-Nab to AAV | Positive Myofiber | AAV Genome | CTL to m-Dystrophin | CTL to Revertant Fiber | CTL to AAV Capsid |
|---|---|---|---|---|---|---|---|---|
| 1 | 2 × 1010 | yes | + | + | ||||
| 2 | 2 × 1010 | yes | 1:800 | + | ± | |||
| 3 | 2 × 1010 | no | 3∼4 (day 42) | + | ± | |||
| 4 | 1 × 1011 | no | ± | |||||
| 5 | 1 × 1011 | yes | 1:100 | ++ | + | |||
| 6 | 1 × 1011 | yes | 1 (day 42) | ++ |
AAV genome: +, 0.5∼1 copy/nucleus; ++, 1.5∼3 copies/nucleus. Abbreviations: CTL, cytotoxic T cell response; Pre-Nab, preexisting neutralizing antibody.
Favorable results from a series of recently published high-dose systemic AAV therapy clinical trials have instilled enthusiasm for systemic micro-dystrophin therapy.76, 77, 78, 79 The first trial is for treating spinal muscular atrophy type 1 (SMA1). SMA1 is a motor neuron disease caused by mutations in the survival motor neuron 1 (SMN1) gene. Patients die before 2 years of age. Jerry Mendell and colleagues76 delivered a CAG promoter (CMV enhancer-chicken β-actin promoter)-driven SMN1 gene cassette with AAV-9 intravenously to 15 patients (0.9- to 7.9-month-old) at the dose of 6.3 × 1013 vg/kg (three patients) and 2.0 × 1014 vg/kg (12 patients) (trial number NCT02122952). Gene therapy significantly changed the course of the disease and improved motor function. All patients were alive and event-free at 20 months of age. The longest survivor has reached 33 months of age and remained healthy. Two patients (one at low dose and one at high dose) had treatment-related severe adverse events (serum aminotransferase >30 times normal upper limit) and two patients showed non-serious adverse events (serum aminotransferase > normal upper limit but < 10 times). Nonetheless, none of these events was accompanied with abnormal liver function. Further, all events were controlled with prednisolone treatment. The unprecedented clinical success of the SMA1 trial suggests that systemic AAV therapy can be tolerated, is safe, and effective for treating certain neuromuscular diseases.
A second systemic AAV trial for neuromuscular diseases was initiated in August 2017 by Audentes Therapeutics to treat X-linked myotubular myopathy (XLMTM) (trial number NCT03199469). XLMTM is a fatal congenital muscle disorder caused by mutations in the myotubularin (MTM1) gene. Mortality reached 64% for patients ≤18 months and 31% for patients >18 months.80 In this phase 1/2 (phases 1 and 2) open-label randomized trial, patients receive intravenous injection of an AAV-8 vector expressing the human MTM1 gene from the muscle specific desmin promoter. Three doses (four patients for each dose) have been planned, including 1.0 × 1014 vg/kg, 3.0 × 1014 vg/kg, and 5.0 × 1014 vg/kg. Audentes Therapeutics recently released the data from the low-dose cohort.77, 78 All four patients (9 months to 4.1 years) tolerated AAV injection well. Two patients showed asymptomatic elevation in liver enzymes, and one patient had elevated troponin levels. All adverse events responded well to steroid treatment. Motor and respiratory function assay were clearly improved in patients that have received the therapy for 8 weeks or longer.77, 78
Besides neuromuscular diseases, intravenous high-dose systemic AAV delivery has also been used to treat hemophilia A.79 Seven adult hemophilia A patients were treated with 6.0 × 1013 vg/kg of an AAV-5 vector. Treatment resulted in significant and persistent clinical benefits for at least one year. Moderate asymptomatic elevation of transaminase was observed, but there was no clinical sequelae. The only serious adverse event was progression of preexisting arthropathy in one patient.79
In summary, these new results have raised the hope of treating inherited diseases with systemic AAV delivery. However, whether they can be translated to DMD patients remain to be tested due to the differences in disease nature, target tissue, transgene, and patient population.
Clinical Development of Systemic AAV Micro-dystrophin Gene Therapy
Preclinical data in the murine and canine DMD models, as well as promising findings from SMA1, XLMTM, and hemophilia A trials suggest that systemic AAV micro-dystrophin gene therapy may represent a viable approach to treat DMD. It is against this background that three clinical trials were initiated in DMD patients in the USA in December 2017 and one more trial has been planned in Europe (Table 5).81 While all these trials aim to establish the safety and gene transfer efficiency of an AAV micro-dystrophin vector, there are important differences in the details with regards to AAV serotype and dose and methods of AAV production and purification, promoter, micro-dystrophin configuration, patient age, and gene mutation (Table 5). AAV-9 is used in trials by Solid Biosciences and Pfizer, and AAV-rh74 (a serotype very similar to AAV-8) is used in the Mendell trial (Nationwide Children’s Hospital). AAV is produced with a scalable herpesvirus-based system, a scalable transient transfection system using suspension cell culture and the traditional transient transfection system using adherent cell culture in the Solid trial, Pfizer trial, and Mendell trial, respectively.82, 83 A muscle-specific promoter is used in all three trials. Specifically, the Solid trial uses the CK8 promoter,57 the Mendell trial uses the MHCK7 promoter,84 and the Pfizer trial uses a minimized murine muscle creatine kinase (MCK) promoter.85 The Solid trial and Pfizer trial have a dose-escalation design. The Mendell trial has only one dose. Ambulatory patients will be recruited in all trials. In addition, the Solid trial will recruit non-ambulatory patients and the Mendell trial will recruit infant patients (Table 5). The Solid trial and Pfizer trial are open to all patients irrespective of mutation. The Mendell trial only takes patients who have frameshift or nonsense mutation within exons 18–58 (Table 5). The rational for restricting patients to a particular mutation region is not explicitly stated in the trial protocol published in Clinicaltrials.gov (NCT03375164). But it may likely relate to the configuration of the microgene construct proposed in this trial.
Table 5.
Overview of Systemic AAV Micro-dystrophin Clinical Trials
| Solid Biosciences | Nationwide Children’s Hospital | Pfizer | |
|---|---|---|---|
| ClinicalTrials.gov Identifier | NCT03368742 | NCT03375164 | NCT03362502 |
| Trial name | microdystrophin gene transfer study in adolescents and children with DMD (IGNITE DMD) | systemic gene delivery clinical trials for Duchenne muscular dystrophy | a study to evaluate the safety and tolerability of PF-06939926 gene therapy in Duchenne muscular dystrophy |
| Start date (actual) | December 6, 2017 | December 11, 2017 | January 23, 2018 |
| Completion date (estimated) | March, 2021 | January, 2021 | June 7, 2024 |
| Location | University of Florida | Nationwide Children’s Hospital and Washington University | Duke University, UCLA and University of Utah |
| Responsible party | Solid Biosciences | Jerry R. Mendell | Pfizer |
| Study nature | phase 1 and 2, open-label, randomized, controlled | phase 1 and 2, open-label, non-randomized | phase 1b, open-label, non-randomized |
| Drug name | SGT-001 | rAAVrh74.MHCK7.Micro-dystrophin | PF-06939926 |
| AAV-serotype | AAV-9 | AAV-rh74 | AAV-9 |
| Dose | 3 doses (start at 5 × 1013 vg/kg) | 1 dose (2 × 1014 vg/kg) | 2 doses |
| Patient number | 16 | 12 | up to 12 |
| Patient age | 4 to 17 years | 3 months to 7 years | 5 to 12 years |
| Disease stage | both ambulatory and non-ambulatory | ambulatory only | ambulatory only |
| Corticosteroid use | daily for ≥2 years | no prior use for ≤3 years old; >12 weeks use for ≥4 years old | ≥6 month use and ≥3 month daily use |
| Dystrophin gene mutation | any mutation | Frameshift or nonsense mutation within exons 18-58 | any mutation |
| Pre-Nab to AAV | negative | ≤1:400 | negative |
| Primary outcome | safety and micro-dystrophin expression in biopsy | safety | safety and tolerability |
| Secondary outcome | micro-dystrophin expression in biopsy and motor function | micro-dystrophin expression in biopsy |
A major difference among these trials is the configuration of the particular microgene used in the trial (Figure 3). At least three different microgenes have been proposed. These include a five-repeat microgene (Δ3990), a four-repeat microgene (ΔR4–23/ΔC) and a different five-repeat microgene (μDys5R) (Figure 3).43, 44, 57 The common features of these micro-dystrophins are the presence of the N-terminal domain, the cysteine-rich domain, spectrin-like repeats 1 and 24, hinges 1 and 4, as well as the absence of the C-terminal domain (Figure 3). The differences are in the central hinges and R16/17 nNOS-binding domain. Δ3990 micro-dystrophin contains hinge 3 and ΔR4–23/ΔC micro-dystrophin contains hinge 2. μDys5R micro-dystrophin has no central hinge. Only μDys5R micro-dystrophin carries the R16/17 nNOS-binding domain (Figure 3).
It is worth pointing out that the differences in the rod domain of the microgene may have important clinical implications. Of particular interests are hinge 2 and R16/17. Banks et al.53 found that the polyproline site in hinge 2 profoundly influenced the functional capacity of micro-dystrophin in the mouse model. Specifically, it altered the normal structure of the muscle tendentious junction and neuromuscular junction, reduced myofiber size, and resulted in the formation of abnormal ring-shaped myofibers in some muscles.53
Dystrophin accomplishes its biological function through assembly of the dystrophin-associated glycoprotein complex (DGC). A pivotal DGC component in skeletal muscle is nNOS.86, 87 nNOS is involved in a number of muscle activities, including metabolism, regeneration, mitochondrial biogenesis, muscle perfusion, fatigue, and atrophy.88 During contraction, elevated cytosolic calcium activates sarcolemmal nNOS to produce nitric oxide (NO). Diffusion of NO to the surrounding vasculatures counteracts sympathetic vasoconstriction and hence allows sufficient blood perfusion in working muscle (Figure 6). In DMD, nNOS is delocalized from the sarcolemma.86, 87 This has two consequences. First it compromises the ability of muscle to counteract functional ischemia and hence leads to focal ischemic damage (Figure 6).46, 89, 90, 91 In fact, focal ischemic muscle injury is not only the first observable lesions on histological examination in affected dogs and DMD patients (Figure 6),92 but also a characteristic feature that distinguishes DMD from other types of muscular dystrophy.92 Second, mislocalized nNOS elicits nitrosative stress, which directly compromises force production in dystrophic muscle.93, 94 Becker muscular dystrophy (BMD) is a mild form of DMD caused by in-frame deletion of the dystrophin gene. To study the clinical consequence of sarcolemmal nNOS delocalization, Gentil et al.94 performed a genotype-phenotype correlation study in BMD patients. The authors found that patients with sarcolemmal nNOS consistently showed much milder clinical manifestations.94 Collectively, sarcolemmal nNOS plays an essential role in the initiation and progression of muscle disease in DMD. Restoration of nNOS homeostasis should be considered in dystrophin replacement therapy.95
Figure 6.
Sarcolemmal nNOS Delocalization Contributes to DMD Pathogenesis
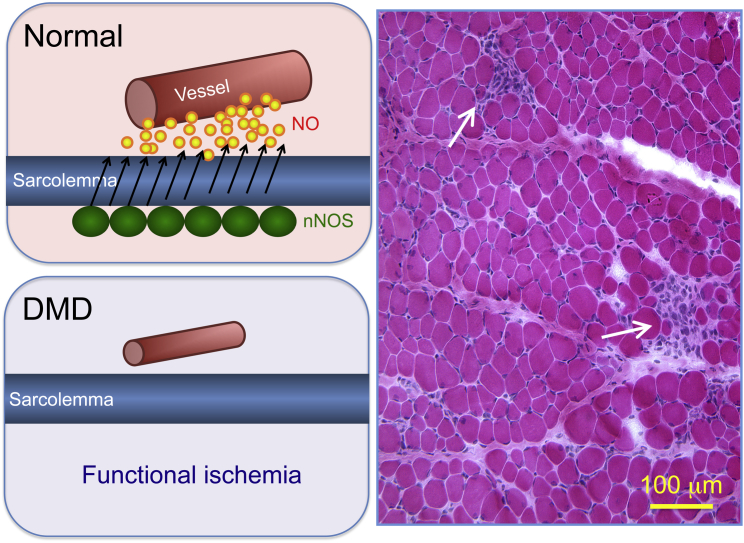
In normal muscle, nNOS is localized at the sarcolemma. This allows immediate diffusion of nitric oxide (NO) to the vasculature and vasodilation in contracting muscle. In DMD, the loss of sarcolemmal nNOS compromises this process and leads to functional ischemia. The H&E-stained image illustrates focal ischemic lesions (arrow) as the first observable histological change in a 3-week-old affected dog. Despite the absence of dystrophin, histologically, the majority of myofibers appeared normal at this age.
The molecular mechanism underlying dystrophin-mediated nNOS membrane localization has been perplexing. Early studies suggest that nNOS is recruited to the sarcolemma by the dystrophin C-terminal domain via interaction with syntrophin.96 Surprisingly, we found that the presence of the dystrophin C-terminal domain and/or membrane-associated syntrophin is not sufficient to anchor nNOS to the sarcolemma.97 Our recent studies suggest that dystrophin spectrin-like repeats 16 and 17 are the nNOS-binding domain.46 Specifically, alpha-helices 2 and 3 from R16 and R17 frame a 10-residue peptide in the α-helix 1 of R17 for direct interaction with the groove region of the nNOS post synaptic density protein, Drosophila disc large tumor suppressor and zonula occludens-1 protein (PDZ) domain.98 R16/17-containing synthetic dystrophin genes successfully restored sarcolemmal nNOS expression in the murine and canine models, effectively enhanced muscle perfusion, prevented functional ischemia, and significantly improved muscle force and exercise capacity.46, 57, 58, 68, 99, 100 In a nutshell, R16/17-containing micro-dystrophin offers much better protection than those without this domain in animal models.
Safety and Immune Response to High-Dose Systemic AAV Administration
As the pace of DMD gene therapy continues to accelerate, it is important to reflect on the lessons learned from patients and animal models. There is no doubt that the most important question is whether systemic AAV micro-dystrophin therapy can be tolerated and whether it is safe in human patients. Systemic AAV delivery has been performed in numerous studies at various dose ranges in mouse models of neuromuscular diseases.40 No safety concern has arisen in these studies. However, toxic responses have been noted when high-dose (≥7.5 × 1013 vg/kg) AAV was delivered intravenously in large mammals. Kornegay et al.67 delivered an AAV-9 vector to three 4-day-old dystrophin null dog puppies at the dose of 1.5 × 1014 vg/kg. This vector expressed a human micro-dystrophin gene from the ubiquitous CMV promoter. One puppy showed persistent lethargy and was euthanized 9 days later (Table 6). Congenital liver steatosis found at necropsy was thought to be the culprit. The remaining two puppies developed muscle atrophy and contracture resembling clinical manifestations of inflammatory myopathy. These two puppies were euthanized at 16 weeks of age (Table 6). Blood chemistries were unremarkable. CD4+ and CD8+ T cells were not observed in micro-dystrophin-positive muscle, suggesting the observed toxicity was likely due to an innate immune response rather than the T cell response.67 Two recent studies from the Wilson laboratory101, 102 further cautioned potential toxicity of high-dose systemic AAV delivery in large-animal models. In one study, Hordeaux et al.102 delivered 7.5 × 1013 vg/kg vectors to two 4-year-old rhesus macaques, one with AAV-9 and the other with an AAV-9 variant called AAV-PHP.B (Table 6). The vector expressed the GFP gene from the ubiquitous CB7 promoter. The subject injected with AAV-PHP.B developed acute liver toxicity and thrombocytopenia on day 3 and was euthanized on day 5 due to diffuse hemorrhage. The AAV-9 injected subject also showed liver enzyme elevation on day 3 (Table 6).102 In another study, Hinderer et al.101 delivered a different AAV-9 variant called AAV-hu68 to three 14-month-old rhesus macaques and three 3- to 30-day-old piglets at the dose of 2 × 1014 vg/kg. The vector expressed the human SMN1 gene from the ubiquitous CB7 promoter. Of three macaques, one developed acute liver failure and was euthanized on day 5 due to disseminated intravascular coagulation (DIC). This animal also had marked elevation of inflammatory cytokines on day 4. The remaining two animals showed liver enzyme elevation and thrombocytopenia on day 5. Histological evidence of sensory neuron toxicity was observed at the scheduled necropsy on day 28. Three piglets did not show liver enzyme elevation, but all showed signs of sensory neuron toxicity within 2 weeks after injection and were euthanized on days 13–14.101 In light of the acute onset of the toxicity and lack of strong evidence supporting a cellular immune response, the authors reasoned that the activation of innate immune response might be responsible for the observed liver toxicity and coagulopathy in nonhuman primates. The cause of sensory neuropathy remained unclear. Collectively, all three papers point to the activation of the innate immune response as an important concern for high-dose systemic AAV gene therapy.103, 104, 105
Table 6.
Side Reactions to High-Dose Systemic AAV Injection in Large Animal Models and Human Patients
| Subject |
AAV |
Side Reaction |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | Disease | Age | n | Type | ×1014vg/kg | Promoter | Transgene | Onset | Presentation | Suspected Cause | Outcome | |
| 67 | dog | DMD | neonatal | 1 | 9 | 1.5 | ubiquitous | hum-μDys | days | lethargy and anorexia | congenital liver disease | euthanized |
| 67 | dog | DMD | neonatal | 2 | 9 | 1.5 | ubiquitous | hum-μDys | weeks | weight loss, muscle atrophy, and contracture | innate immune response and inflammatory response | euthanized |
| 102 | NHP | normal | adult | 1 | PHP.B | 0.75 | ubiquitous | GFP | days | liver enezyme elevation, platelet reduction, hemorrahge | innate immune response and systemic inflammation | euthanized |
| 102 | NHP | normal | adult | 1 | 9 | 0.75 | ubiquitous | GFP | days | liver enezyme elevation | innate immune response | scheduled euthanization |
| 101 | NHP | normal | adolescent | 1 | Hu68 | 2 | ubiquitous | hum-SMN | days | liver failure, DIC | innate immune response and systemic inflammation | euthanized |
| 101 | NHP | normal | adolescent | 2 | Hu68 | 2 | ubiquitous | hum-SMN | days and weeks | liver enezyme elevation, platelet reduction, neural toxicity | innate immune response and systemic inflammation; unknown for neural toxicity | scheduled euthanization |
| 101 | pig | normal | neonatal | 3 | Hu68 | 2 | ubiquitous | hum-SMN | weeks | neural toxicity | unknown | euthanized |
| 76 | human | SMA1 | neonatal | 3 | 9 | 0.67 | ubiquitous | hum-SMN | weeks | liver enzyme elevation (1 patient) | cellular immune response | resolved |
| 76 | human | SMA1 | neonatal | 12 | 9 | 2 | ubiquitous | hum-SMN | weeks | liver enzyme elevation (3 patients) | cellular immune response | resolved |
| 77, 78 | human | XLMTM | neonatal and preschool | 4 | 8 | 1 | muscle-specific | hum-MTM1 | weeks | liver enzyme elevation (1 patient), troponin elevation (1 patient) | N/A | resolved |
| 115, 116 | human | DMD | adolescent | 1 | 9 | 0.5 | muscle-specific | hum-μDys | days | platelet and red blood cell reduction, complement activation and transient renal impairment | innate immune response? | resolved |
Abbreviations: DIC, disseminated intravascular coagulation; DMD, Duchenne muscular dystrophy; Dys, dystrophin gene; Hu68, an AAV-9 variant; MTM1, myotubular myopathy gene; NHP, nonhuman primate; PHP.B, an AAV-9 variant; SMA1, type 1 spinal muscular atrophy; SMN, survival of motor neuron gene; XLMTM, X-linked myotubular myopathy.
The innate immune response is a well-recognized barrier for adenovirus, but not AAV, gene therapy.106, 107 Indeed, AAV vectors are much weaker than adenoviral vectors in activating genes involved in the innate immune response.108, 109 However, recent studies suggest that the innate immune response may likely play an important role in shaping the outcome of AAV gene therapy.103, 105, 110, 111 AAV capsids and the vector genome can be sensed by toll-like-receptor-2 and -9, respectively.112, 113 Zaiss et al.108 found a dose-dependent transient induction of chemokine expression at 1 hr following intravenous injection of AAV-2. Importantly, the level of induction was comparable to that of adenovirus at this time point. Martino et al.114 reported a transient but profound induction of pro-inflammatory cytokines at 2 hr following intravascular injection of self-complementary AAV-2 and -8. The authors also showed that higher doses resulted in significantly stronger induction.114 These results suggest that high-dose systemic AAV delivery faces a unique innate immunity challenge that is absent or marginal in low-dose intravascular AAV administration.
Recently, Solid Biosciences announced the hold of its clinical trial due to suspected unexpected serious adverse reaction.115, 116 Specifically, 5 × 1013 vg/kg of an AAV-9 micro-dystrophin vector was injected intravenously to a non-ambulatory adolescent DMD patient. Several days later, the patient showed platelet count reduction, followed by red blood cell count reduction and transient renal impairment. There was also evidence of complement activation. Nevertheless, there were no signs of bleeding or clotting abnormalities. The liver function was not altered from the baseline either. The patient recovered from the event smoothly, and the trial was resumed.116 The exact reason(s) and/or trigger(s) of this unexpected response are yet to be investigated. However, the rapid onset of the reaction (within days) is reminiscent of the time course observed in nonhuman primates by the Wilson laboratory,101, 102 suggesting innate immune response may have played a role. This notion is further supported by the observation of complement activation in the patient.115 The complement system is a primary component of innate immunity. An in vitro study suggests that AAV capsid not only interacted with various components of the complement system, but also directly activated the complement system in a dose-dependent manner.117 Alternatively, the complement system can also be activated through the classic pathway by the immune complex formed from anti-AAV capsid antibodies.102, 118 Activated complement not only promotes inflammation but also damages cells that are in constant contact with plasma such as red blood cells, platelets, and endothelial cells.118, 119 Injured endothelium, activated platelets, and hemoglobin released from red blood cells can in turn further activate the complement system.120, 121, 122, 123 If unchecked, this malicious feedback cycle may lead to severe thrombocytopenia and anemia, organ injury, bleeding, and death. Together, data from nonhuman primates and a DMD patient indicate that careful monitoring and management of the innate immune response should be included in the protocol in ongoing systemic micro-dystrophin gene therapy trials.
Despite the concern on acute and/or sub-acute toxicity of the innate immune response, it should be pointed out that high-dose systemic AAV delivery remains a viable and highly promising therapy to improve all affected muscles in DMD patients. This positive attitude is backed up by (1) safety and efficacy data from high-dose systemic AAV therapy in SMA1, XLMTM, and hemophilia A patients,76, 77, 78, 79 (2) lack of toxicity in young adult DMD dogs at dose as high as 5 × 1014 vg/kg,68, 69, 70, 71, 72, 73 (3) encouraging preliminary results in DMD boys who have already received systemic AAV micro-dystrophin gene therapy.124, 125, 126 Clearly, a dystrophic body and a high AAV dose are not sufficient to induce systemic toxicity within days after injection in large mammals. A careful comparison of the protocols from different studies should give a hint on the cause(s) and solution(s) to this important safety concern.
In contrast to innate immunity, the adaptive immune response is more frequently discussed in AAV gene therapy.127, 128, 129, 130, 131, 132 T cell response to AAV capsid has been reported in hemophilia B trials that have used a relatively low dose of the AAV vector (<1 × 1013 vg/kg).133, 134, 135 In these cases, the loss of transgene expression is often accompanied by an elevation of transaminase.127, 131 Transient application of high-dose glucocorticosteroids has been shown to effectively control liver enzyme and maintain expression of the therapeutic protein (factor IX).134 Transaminase elevation has also been observed in high-dose systemic AAV gene therapy for SMA1, XLMTM, and hemophilia A.76, 77, 78, 79 However, it is currently unclear whether liver enzyme elevation is caused by T cell immunity to the AAV vector in these studies. Besides viral capsid, the cellular immune response may also target transgene product expressed from the AAV vector. The anti-dystrophin T cell response was highlighted in the local injection trial (Table 4).74 Interestingly, using the enzyme-linked immunospot assay, Flanigan et al.136 revealed a high prevalence of anti-dystrophin T cell immunity in DMD patients (up to 53% in corticosteroid naive patients). The implication of this finding to systemic AAV micro-dystrophin therapy is unclear but should be carefully investigated in the future.
The humoral response is a hurdle for patients with preexisting AAV neutralizing and/or binding antibodies.132, 137 Patients with high titers are usually excluded from the trial (Table 4). For patients that have received AAV therapy, the antibody response from initial exposure creates a barrier for re-administration. Considering the degenerative nature of the disease, it is very likely DMD patients may have to receive repeated therapy. Several strategies are currently under development to overcome the humoral response, such as plasmapheresis, AAV capsid engineering, sheltering capsid with encapsulation, the use of decoy empty capsid, and pharmacological modulation of the B cell and/or T cell activation.129, 132, 137, 138 Each of these methods has its advantages and shortcomings. Ultimately, a combinatorial approach may be required to overcome this important challenge.
Micro-dystrophin Structure Optimization
An equally important question is whether micro-dystrophin can alleviate disease in human patients. The concept of treating DMD with an abbreviated gene originates from observations that patients with large in-frame deletions display a mild clinical course19 (Table 1). However, it should be noted that in these cases, only ≤49% the dystrophin protein is lost.19, 23, 24, 26, 27, 28, 139, 140, 141, 142, 143, 144, 145 In other words, the patients still carry a half-size protein. Patients who have lost ≥50% of dystrophin due to larger in-frame deletions often show the severe DMD clinical manifestation rather than the milder BMD phenotype (Table 1).24, 25, 26, 139, 140, 141, 142, 143, 144, 145 In 1996, Fanin et al.141 proposed the dystrophin length threshold theory. The authors presented a case in which a high level of an ∼160-kD truncated dystrophin protein was detected on immunostaining and western blot using an antibody that recognizes the C-terminal domain (Table 1). Despite the abundant presence of this micro-size dystrophin, the patient displayed a clinical phenotype of DMD instead of BMD. After reviewing a large collection of in-frame deletion patients, they found that smaller dystrophins (deletion larger than 36 exons) were always associated with a severe phenotype. They hypothesized that a dystrophin protein of at least 200 kD might be needed for muscle protection.141 Mouse studies suggest that there may indeed exist a length threshold (∼150 kD instead of 200 kD in mouse muscle). For example, the highly truncated ΔDysM3 and ΔR1–R24 micro-dystrophins cannot reduce dystrophic phenotypes in mice (Table 2).42, 44 It is currently unclear where the line should be drawn for the length threshold in human patients. Nevertheless, the existing AAV micro-dystrophin literature in the murine and canine models suggests that a rationally designed micro-dystrophin can certainly protect mice and dogs. Ongoing trials should offer critical clinical efficacy data regarding the effectiveness of micro-dystrophin in human patients (Table 5).
Besides the length, the configuration of the microgene is also of paramount importance. Among three candidate micro-dystrophins (Figure 3), two contain five repeats and one has four repeats. A side-by-side comparison of the five-repeat microgene versus the four-repeat microgene has yet to be published. Harper et al.44 previously demonstrated that the rod domain functions better when it has an even (rather than odd) number of repeats. This is in agreement with the evolution of spectrin family proteins, which are stemmed from a common four-repeat ancestor.146, 147 However, a counter-argument in support of the five-repeat microgene is that a larger micro-dystrophin would carry more genetic information.
Another complexity is the central hinge. Two micro-dystrophins used in clinical trials have a hinge in the middle, and one has no hinge (Figure 3). The question is whether a central hinge should be included in micro-dystrophin and, if yes, which hinge should be included. So far, a side-by-side comparison has not been performed using AAV micro-dystrophin vectors. However, we may gain some hints from a transgenic study.44 Harper et al.44 compared two transgenic mdx lines: one expressed the ΔR2–R21 microgene (without a central hinge), and the other expressed the ΔR2-R21+H3 microgene (with a central hinge). Although the ΔR2-R21 line expressed less dystrophin, this line outperformed the ΔR2–R21+H3 line on histological and function assays. Specifically, the ΔR2–R21 line showed less degeneration and produced a higher force. This result suggests that in the context of micro-dystrophin, a construct without a central hinge may function better. However, the result may also suggest that hinge 3 negatively influences micro-dystrophin function. A study in patients seems to support this second view. Carsana et al. examined 108 unrelated DMD and BMD patients and found that in-frame deletion of hinge 3 yielded significantly much milder disease.142 They concluded that “when hinge 3 is lost, the protein, though shorter, is able to function better.”142 But it should be pointed out that evidence also exists suggesting inclusion of hinge 3 may lead to better rescue in the context of a larger minigene. Specifically, Harper et al.44 showed that the hinge 3-containg ΔH2-R19 minigene was much more effective in ameliorating muscle pathology and boosting muscle force than a similar minigene (ΔH2–H3 minigene) that is identical to the ΔH2-R19 minigene except without hinge 3. While the jury is still out on whether hinge 3 should be included, results from Banks et al.53 have made it clear that hinge 2 should be excluded because it impairs normal muscle structure.
In above discussions, I touched on the issues related to the length of micro-dystrophin, the number of repeats, and the central hinge. However, it should be kept in mind that dystrophin is not a simple protein; it interacts with many different cellular proteins such as γ-actin (in both skeletal muscle and heart) and α-actin (in heart only), various types of intermediate filaments (e.g., keratin 8 and 19, synemin, and synemin 2), tubulin (microtubule), ankyrin, myospryn, plectin, dystrobrevin, syntrophin, nNOS, dystroglycan, polarity-regulating kinase partitioning-defective 1b, cavin-1, ahnak1, cipher, and crystalline αB. Dystrophin also interacts with the membrane lipid bilayer. It is unlikely that a highly truncated micro-dystrophin will establish all these interactions, especially in light of the fact that the region(s) that bind to some of the above proteins remain to be identified. For the development of next-generation micro-dystrophin, the question is to determine which interactions are absolutely required for dystrophin function and which ones are less essential.
Alone the same line, recent studies have revealed many previously unappreciated or not fully appreciated aspects of dystrophin biology. Of particular interest is the recent discovery of new dystrophin membrane-binding domains.148 It is well established that dystrophin binds to the sarcolemma via its cysteine-rich domain. However, there also exists evidence suggesting that truncated dystrophins (either naturally occurring or synthetic) may directly bind to the sarcolemma in the absence of the cysteine-rich domain. To clarify this issue, we conducted a comprehensive in vivo screening in both mouse and dog models.148 Our data suggest that in addition to the cysteine-rich domain, dystrophin indeed contains additional membrane-binding domains that can independently anchor to the sarcolemma in skeletal muscle and the heart.148 Since sarcolemmal interaction is essential for dystrophin to protect muscle, it is likely that inclusion of more membrane-binding domains may result in better muscle protection. In support, a recent transgenic study found better resistance to eccentric contraction injury for micro-dystrophin with two, rather than one, membrane-binding domains.149
The C-terminal domain (exons 71–79) has a length of 975 bp and translates into 325 amino acid residues (∼36 kD). It contains the α1-and β1-syntrophin binding site and α-dystrobrevin binding site. These binding sites span exons 73 to 75 (450 bp). The C-terminal domain is absent in all candidate microgenes proposed for clinical trials (Figure 3). The decision to remove the C-terminal domain originates from a study by Crawford et al.150 The authors created a transgenic mdx mouse that expressed a C-terminal truncated dystrophin gene (ΔC mice). Muscle histology and force of young adult (≤6-month-old) ΔC mice were identical to that of normal mice, suggesting that the C-terminal domain might be disposable. This notion is further supported by morphological improvement and functional rescue by AAV micro-dystrophin in animal models. While there is no doubt that C-terminal truncated dystrophins could be highly protective in skeletal muscle, a recent clinical study raised the possibility that the C-terminal domain might be critical for the heart. Tandon et al.151 performed a genotype-phenotype correlation study in 274 patients using cardiac magnetic imaging. Interestingly, they found that the presence of the C-terminal domain is linked with a milder cardiac phenotype, suggesting a cardiac protection role of this domain.151 Besides the heart, patient studies suggest that the C-terminal domain may also be important for cognitive function.152, 153 It is worth pointing out that even in skeletal muscle, ΔC dystrophin may become less competent as animals age. For example, in the Crawford et al.150 paper, the percentage of centrally nucleated fibers in ΔC mice was 1% at 4 months of age, and it increased to 10% at 12 months of age. Due to the size restriction of the AAV vector, it is unlikely that a highly functional AAV microgene vector can be generated to carry the entire C-terminal domain. Future studies are needed to identify smaller functional motifs that can be included in the AAV micro-dystrophin vector.54
The basic function of dystrophin is to protect muscle from contraction-induced damage. The heart is the only muscle in the body that contracts constantly. Theoretically, the heart should be the earliest and most severely damaged muscle. Surprisingly, cardiomyopathy becomes apparent only at late stages of the disease.154 This suggests that dystrophin in the heart may cope with mechanical stress using a different mechanism. In support of this, it was recently discovered that cardiac dystrophin interacts with a distinctive set of cellular proteins.155 We also found that modification of the rod domain configuration substantially improved myocardial protection.156, 157 Heart rescue has only been studied in a few microgenes in mdx mice, and most of these studies have used the ΔR4–23/ΔC microgene.55, 59, 63, 65, 158, 159, 160 As more patients survive to the third and fourth decade of their life due to modern medicine, cardiomyopathy is becoming a more prominent issue. Considering the scarce data available on DMD cardiomyopathy gene therapy, there is an urgent need to study AAV micro-dystrophin in the heart, especially to compare the potency of different microgenes and to evaluate efficacy in the heart of affected dogs.161, 162, 163
Improving Micro-dystrophin Therapy with the Optimized Expression Cassette and Engineered AAV Capsids
Another area that has room for improvement is the regulatory elements that are used to drive micro-dystrophin expression. To minimize toxicity and immunogenicity from untoward expression in non-muscle tissues, a muscle-specific promoter is strongly recommended. A number of muscle-specific promoters have been tested for muscle gene therapy such as the CK6, CK8, desmin, MHCK7, miniMCK, myoglobin, and SPc5-12 promoter.164 Among these, the CK8, MHCK7, miniMCK, and SPc5-12 promoter have been proposed for systemic micro-dystrophin therapy in DMD patients.57, 84, 85, 165 Except for the SPc5-12 promoter,165 all other promoters are derived from naturally existing muscle promoters.166 Animal studies suggest that all these promoters can drive muscle-specific expression. However, these studies have also raised issues on fiber type specificity and leaky expression in antigen-presenting cells (APCs).84, 85, 167, 168 Skeletal muscle is composed of at least five different types of myofibers that express type I, IIa, IIb, IIx, and embryonic myosin heavy chains. Cardiac muscle is composed of myofibers that express α and β myosin heavy chains. Fiber type preference has been noted in some promoters in mouse studies.84, 85 Upcoming clinical trials will help to elucidate whether some promoters can drive robust expression in all types of myofibers in DMD patients. Leaky expression in APCs is a major concern for any tissue specific promoter. Unfortunately, some muscle promoters (such as the desmin and SPc5-12 promoter) have been shown to drive expression in APCs.167, 168 A promising solution to this problem is through post-transcriptional silencing using the hematopoietic miRNA-142-3p binding site.167, 169, 170 Alternatively, one may consider the use of small viral genes that interfere with antigen presentation.171 Inclusion of these strategies in future AAV micro-dystrophin vectors may help reduce micro-dystrophin-specific immune reactions. It is worth mentioning that there is still room to increase the specificity and activity of current muscle promoters by molecular engineering.
Based on preclinical studies, it has been suggested that an effective systemic AAV therapy for DMD may require dosing ≥1015 vg particles per patient.11 This creates a significant burden for the production and purification of good manufacturing practice (GMP) grade vectors for trials and future commercialization.172, 173 The high cost associated with AAV production will undoubtedly increase the price tag of the gene therapy drug, and this may further heat the already hotly debated price issue.174 Most importantly, it causes important safety concerns, such as the immune response to the administration of large quantities of viral capsid proteins.175 Since AAV transduction properties are largely dependent on the viral capsids, targeted engineering and/or forced in vivo evolution in patient muscle should provide clues to a cost-effective way to generate novel AAV capsids that are more potent than the ones used in the current trials.40, 61, 176 In this regards, several newly engineered AAV variants (such as AAV-B1 and AAV-6 tyrosine mutant) have shown significantly improved muscle transduction efficiency in rodent studies.177, 178
Level and Duration of Micro-dystrophin Expression
Another important question is the level of expression needed for treatment. The threshold has to be defined in regards to the percentage of dystrophin-positive cells, level of expression in each positive cell, and the total amount of dystrophin in whole muscle. The threshold is likely going to be different for heart and skeletal muscle, for different skeletal muscles, for different aspects of the disease (histology versus force), for different stages of the disease (early versus late), and for different therapeutic goals (halting progression versus reversing the disease). The threshold may also need to be adjusted to meet growth needs. Although marginal level (∼3 to 5%) expression has been shown to improve the outcome in mice and patients,179, 180, 181, 182, 183, 184, 185, 186 a substantial correction may likely require the total muscle dystrophin level to reach 20%–30% of normal and mosaic expression in ∼50% myofibers.187, 188, 189, 190, 191, 192, 193, 194, 195, 196 Based on comprehensive necropsy in canine studies, this may likely be reachable in human patients.67, 68, 71, 72
Last but not least is the durability of the therapy. DMD is a chronic disease and requires continuous dystrophin expression.197 One study in the canine model suggests that intravenous AAV-8 micro-dystrophin injection in the absence of immune suppression can lead to persistent expression for 2 years without a detectable T cell response to either micro-dystrophin or AAV capsid.69 Based on the literature, the chance is likely low to translate such an ideal scenario to human patients. For example, intravascular AAV factor IX therapy in hemophilia B dogs was not associated with cytotoxic T cell responses.198, 199, 200 However, a similar approach in human patients resulted in a significant T cell response to AAV capsid.133 Several studies suggest that the dystrophic microenvironment (leaky membrane and oxidative stress) may promote the cellular immune response and even cause the loss of AAV in dystrophic muscle.201, 202, 203, 204, 205 Re-administration is very likely needed to provide life-long therapy in DMD patients. Several strategies proposed for treating patients with preexisting neutralizing antibodies (such as the use of alternative AAV capsid, pharmacological modulation of the immune system, and plasmapheresis) may also help with re-administration.137, 206, 207, 208, 209 Future studies in the canine DMD model will reveal the suitability of these approaches in dystrophic large mammals.
Conclusion
In summary, research over the last three decades has laid the foundation for systemic AAV micro-dystrophin gene therapy for DMD. Several clinical trials have been initiated to test the safety and tolerability in patients. Results from these trials will shed critical light on this promising therapeutic modality. However, there is still a long way to go. It took ∼10 years from the report of the successful phase I trial to the approval of the AAV drug Luxturna by the FDA.9, 10, 210 Considering the disease complexity, broad distribution of muscle and the need for systemic high-dose AAV administration, it is very likely that the path forward will be much more challenging for eventual regulatory approval and commercialization of AAV micro-dystrophin gene therapy for DMD. Nevertheless, given the rapid development of the entire gene therapy field, clinical success of AAV in multiple diseases and results from the murine and canine DMD models, there are reasons to be cautiously optimistic. It should also be pointed out there is clearly room for improving the current generation of AAV micro-dystrophin therapy. Future studies are needed to improve AAV capsid, maximize micro-dystrophin potency, and minimize immunological risk. Efforts in these directions may also benefit other AAV-based DMD gene therapy approaches such as reframing the mutated dystrophin gene at the RNA levels by U7 small nuclear RNA therapy and at the DNA level by short palindromic repeats (CRISPR)-CRISPR-associated protein 9 (Cas9)-mediated genome editing.
Author Contributions
D.D. is the sole author of the paper. D.D. is responsible for all aspects of the paper.
Conflicts of Interest
The author is a member of the scientific advisory board for Solid Biosciences and an equity holder of Solid Biosciences. The Duan lab has received research support from Solid Biosciences.
Acknowledgments
The author would like to thank Jesse Davidson, Jackson Freel, Eytani Ganot, Mark McDonald, and Javier Romero for their inspiration. AAV micro-dystrophin gene therapy research in the Duan lab is currently supported by the National Institute of Neurological Disorders and Stroke (NS-90634), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR-67985), Solid Biosciences, and the Jackson Freel DMD Research Fund. In the past, the Duan lab has received funding from the Department of Defense (MD130014), Jesse’s Journey: The Foundation for Gene and Cell Therapy, Hope for Javier, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (AR-49419), the National Heart, Lung, and Blood Institute (HL-91883), Muscular Dystrophy Association, and the Parent Project Muscular Dystrophy to develop AAV micro-dystrophin gene therapy. The author thanks Dr. Jerry Mendell for providing the photo in Figure 5, Dr. Xiufang Pan and Yongping Yue for the help in the preparation of Figure 2, and Dr. Kasun Kodippili for the help in the preparation of Figure 6. The author thanks Sean Duan for the critical reading of the manuscript and Duan lab members for the helpful discussion. The author thanks Parent Project Muscular Dystrophy for sponsoring the open access option.
References
- 1.Kunkel L.M. 2004 William Allan award address. cloning of the DMD gene. Am. J. Hum. Genet. 2005;76:205–214. doi: 10.1086/428143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drouin E., Péréon Y. Duchenne or Meryon muscular dystrophy? Mol. Genet. Metab. 2014;113:241–242. doi: 10.1016/j.ymgme.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Mendell J.R., Lloyd-Puryear M. Report of MDA muscle disease symposium on newborn screening for Duchenne muscular dystrophy. Muscle Nerve. 2013;48:21–26. doi: 10.1002/mus.23810. [DOI] [PubMed] [Google Scholar]
- 4.Bushby K., Finkel R., Birnkrant D.J., Case L.E., Clemens P.R., Cripe L., Kaul A., Kinnett K., McDonald C., Pandya S., DMD Care Considerations Working Group Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010;9:77–93. doi: 10.1016/S1474-4422(09)70271-6. [DOI] [PubMed] [Google Scholar]
- 5.Mendell J.R., Province M.A., Moxley R.T., 3rd, Griggs R.C., Brooke M.H., Fenichel G.M., Miller J.P., Kaiser K.K., King W., Robison J. Clinical investigation of Duchenne muscular dystrophy. A methodology for therapeutic trials based on natural history controls. Arch. Neurol. 1987;44:808–811. doi: 10.1001/archneur.1987.00520200012009. [DOI] [PubMed] [Google Scholar]
- 6.Hyde S.A., Steffensen B.F., Fløytrup I., Glent S., Kroksmark A.K., Salling B., Werlauff U., Erlandsen M. Longitudinal data analysis: an application to construction of a natural history profile of Duchenne muscular dystrophy. Neuromuscul. Disord. 2001;11:165–170. doi: 10.1016/s0960-8966(00)00175-9. [DOI] [PubMed] [Google Scholar]
- 7.Mercuri E., Signorovitch J.E., Swallow E., Song J., Ward S.J., DMD Italian Group; Trajectory Analysis Project (cTAP) Categorizing natural history trajectories of ambulatory function measured by the 6-minute walk distance in patients with Duchenne muscular dystrophy. Neuromuscul. Disord. 2016;26:576–583. doi: 10.1016/j.nmd.2016.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koeks Z., Bladen C.L., Salgado D., van Zwet E., Pogoryelova O., McMacken G., Monges S., Foncuberta M.E., Kekou K., Kosma K. Clinical Outcomes in Duchenne Muscular Dystrophy: A Study of 5345 Patients from the TREAT-NMD DMD Global Database. J. Neuromuscul. Dis. 2017;4:293–306. doi: 10.3233/JND-170280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.News FDA approves hereditary blindness gene therapy. Nat. Biotechnol. 2018;36:6. doi: 10.1038/nbt0118-6a. [DOI] [PubMed] [Google Scholar]
- 10.Russell S., Bennett J., Wellman J.A., Chung D.C., Yu Z.F., Tillman A., Wittes J., Pappas J., Elci O., McCague S. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390:849–860. doi: 10.1016/S0140-6736(17)31868-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duan D. Dystrophin Gene Replacement and Gene Repair Therapy for Duchenne Muscular Dystrophy in 2016: An Interview. Hum. Gene Ther. Clin. Dev. 2016;27:9–18. doi: 10.1089/humc.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chamberlain J.R., Chamberlain J.S. Progress toward gene therapy for Duchenne muscular dystrophy. Mol. Ther. 2017;25:1125–1131. doi: 10.1016/j.ymthe.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muzyczka N., Berns K.I. AAV’s Golden Jubilee. Mol. Ther. 2015;23:807–808. doi: 10.1038/mt.2015.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rondot P. G. B. A. Duchenne de Boulogne (1806-1875) J. Neurol. 2005;252:866–867. doi: 10.1007/s00415-005-0874-0. [DOI] [PubMed] [Google Scholar]
- 15.Hoffman E.P., Brown R.H., Jr., Kunkel L.M. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 16.Koenig M., Hoffman E.P., Bertelson C.J., Monaco A.P., Feener C., Kunkel L.M. Complete cloning of the Duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell. 1987;50:509–517. doi: 10.1016/0092-8674(87)90504-6. [DOI] [PubMed] [Google Scholar]
- 17.Kunkel L.M. The Wellcome lecture, 1988. Muscular dystrophy: a time of hope. Proc. R. Soc. Lond. B Biol. Sci. 1989;237:1–9. doi: 10.1098/rspb.1989.0032. [DOI] [PubMed] [Google Scholar]
- 18.Duan D. Myodys, a full-length dystrophin plasmid vector for Duchenne and Becker muscular dystrophy gene therapy. Curr. Opin. Mol. Ther. 2008;10:86–94. [PubMed] [Google Scholar]
- 19.England S.B., Nicholson L.V., Johnson M.A., Forrest S.M., Love D.R., Zubrzycka-Gaarn E.E., Bulman D.E., Harris J.B., Davies K.E. Very mild muscular dystrophy associated with the deletion of 46% of dystrophin. Nature. 1990;343:180–182. doi: 10.1038/343180a0. [DOI] [PubMed] [Google Scholar]
- 20.Love D.R., England S.B., Speer A., Marsden R.F., Bloomfield J.F., Roche A.L., Cross G.S., Mountford R.C., Smith T.J., Davies K.E. Sequences of junction fragments in the deletion-prone region of the dystrophin gene. Genomics. 1991;10:57–67. doi: 10.1016/0888-7543(91)90484-v. [DOI] [PubMed] [Google Scholar]
- 21.Ikeya K., Saito K., Hayashi K., Tanaka H., Hagiwara Y., Yoshida M., Yamauchi A., Fukuyama Y., Ishiguro T., Eguchi C. Molecular genetic and immunological analysis of dystrophin of a young patient with X-linked muscular dystrophy. Am. J. Med. Genet. 1992;43:580–587. doi: 10.1002/ajmg.1320430315. [DOI] [PubMed] [Google Scholar]
- 22.Beggs A.H., Hoffman E.P., Snyder J.R., Arahata K., Specht L., Shapiro F., Angelini C., Sugita H., Kunkel L.M. Exploring the molecular basis for variability among patients with Becker muscular dystrophy: dystrophin gene and protein studies. Am. J. Hum. Genet. 1991;49:54–67. [PMC free article] [PubMed] [Google Scholar]
- 23.Nicholson L.V., Bushby K.M., Johnson M.A., Gardner-Medwin D., Ginjaar I.B. Dystrophin expression in Duchenne patients with “in-frame” gene deletions. Neuropediatrics. 1993;24:93–97. doi: 10.1055/s-2008-1071521. [DOI] [PubMed] [Google Scholar]
- 24.Matsumura K., Burghes A.H., Mora M., Tomé F.M., Morandi L., Cornello F., Leturcq F., Jeanpierre M., Kaplan J.C., Reinert P. Immunohistochemical analysis of dystrophin-associated proteins in Becker/Duchenne muscular dystrophy with huge in-frame deletions in the NH2-terminal and rod domains of dystrophin. J. Clin. Invest. 1994;93:99–105. doi: 10.1172/JCI116989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koenig M., Beggs A.H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C.R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am. J. Hum. Genet. 1989;45:498–506. [PMC free article] [PubMed] [Google Scholar]
- 26.Winnard A.V., Klein C.J., Coovert D.D., Prior T., Papp A., Snyder P., Bulman D.E., Ray P.N., McAndrew P., King W. Characterization of translational frame exception patients in Duchenne/Becker muscular dystrophy. Hum. Mol. Genet. 1993;2:737–744. doi: 10.1093/hmg/2.6.737. [DOI] [PubMed] [Google Scholar]
- 27.Passos-Bueno M.R., Vainzof M., Marie S.K., Zatz M. Half the dystrophin gene is apparently enough for a mild clinical course: confirmation of its potential use for gene therapy. Hum. Mol. Genet. 1994;3:919–922. doi: 10.1093/hmg/3.6.919. [DOI] [PubMed] [Google Scholar]
- 28.Flanigan K.M., Dunn D.M., von Niederhausern A., Soltanzadeh P., Gappmaier E., Howard M.T., Sampson J.B., Mendell J.R., Wall C., King W.M., United Dystrophinopathy Project Consortium Mutational spectrum of DMD mutations in dystrophinopathy patients: application of modern diagnostic techniques to a large cohort. Hum. Mutat. 2009;30:1657–1666. doi: 10.1002/humu.21114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aartsma-Rus A., Van Deutekom J.C., Fokkema I.F., Van Ommen G.J., Den Dunnen J.T. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34:135–144. doi: 10.1002/mus.20586. [DOI] [PubMed] [Google Scholar]
- 30.Chamberlain J.S. Gene therapy of muscular dystrophy. Hum. Mol. Genet. 2002;11:2355–2362. doi: 10.1093/hmg/11.20.2355. [DOI] [PubMed] [Google Scholar]
- 31.Atchison R.W., Casto B.C., Hammon W.M. Adenovirus-associated defective virus particles. Science. 1965;149:754–756. doi: 10.1126/science.149.3685.754. [DOI] [PubMed] [Google Scholar]
- 32.Hermonat P.L., Muzyczka N. Use of adeno-associated virus as a mammalian DNA cloning vector: transduction of neomycin resistance into mammalian tissue culture cells. Proc. Natl. Acad. Sci. USA. 1984;81:6466–6470. doi: 10.1073/pnas.81.20.6466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner J.A., Reynolds T., Moran M.L., Moss R.B., Wine J.J., Flotte T.R., Gardner P. Efficient and persistent gene transfer of AAV-CFTR in maxillary sinus. Lancet. 1998;351:1702–1703. doi: 10.1016/S0140-6736(05)77740-0. [DOI] [PubMed] [Google Scholar]
- 34.Xiao X., Li J., Samulski R.J. Efficient long-term gene transfer into muscle tissue of immunocompetent mice by adeno-associated virus vector. J. Virol. 1996;70:8098–8108. doi: 10.1128/jvi.70.11.8098-8108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kessler P.D., Podsakoff G.M., Chen X., McQuiston S.A., Colosi P.C., Matelis L.A., Kurtzman G.J., Byrne B.J. Gene delivery to skeletal muscle results in sustained expression and systemic delivery of a therapeutic protein. Proc. Natl. Acad. Sci. USA. 1996;93:14082–14087. doi: 10.1073/pnas.93.24.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gregorevic P., Blankinship M.J., Allen J.M., Crawford R.W., Meuse L., Miller D.G., Russell D.W., Chamberlain J.S. Systemic delivery of genes to striated muscles using adeno-associated viral vectors. Nat. Med. 2004;10:828–834. doi: 10.1038/nm1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Zhu T., Qiao C., Zhou L., Wang B., Zhang J., Chen C., Li J., Xiao X. Adeno-associated virus serotype 8 efficiently delivers genes to muscle and heart. Nat. Biotechnol. 2005;23:321–328. doi: 10.1038/nbt1073. [DOI] [PubMed] [Google Scholar]
- 38.Yue Y., Ghosh A., Long C., Bostick B., Smith B.F., Kornegay J.N., Duan D. A single intravenous injection of adeno-associated virus serotype-9 leads to whole body skeletal muscle transduction in dogs. Mol. Ther. 2008;16:1944–1952. doi: 10.1038/mt.2008.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang D., Zhong L., Nahid M.A., Gao G. The potential of adeno-associated viral vectors for gene delivery to muscle tissue. Expert Opin. Drug Deliv. 2014;11:345–364. doi: 10.1517/17425247.2014.871258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Duan D. Systemic delivery of adeno-associated viral vectors. Curr. Opin. Virol. 2016;21:16–25. doi: 10.1016/j.coviro.2016.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuasa K., Ishii A., Miyagoe Y., Takeda S. [Introduction of rod-deleted dystrophin cDNA, delta DysM3, into mdx skeletal muscle using adenovirus vector] Nihon Rinsho. 1997;55:3148–3153. [PubMed] [Google Scholar]
- 42.Takeda S. [Development of new therapy on muscular dystrophy] Rinsho Shinkeigaku. 2001;41:1154–1156. [PubMed] [Google Scholar]
- 43.Wang B., Li J., Xiao X. Adeno-associated virus vector carrying human minidystrophin genes effectively ameliorates muscular dystrophy in mdx mouse model. Proc. Natl. Acad. Sci. USA. 2000;97:13714–13719. doi: 10.1073/pnas.240335297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harper S.Q., Hauser M.A., DelloRusso C., Duan D., Crawford R.W., Phelps S.F., Harper H.A., Robinson A.S., Engelhardt J.F., Brooks S.V., Chamberlain J.S. Modular flexibility of dystrophin: implications for gene therapy of Duchenne muscular dystrophy. Nat. Med. 2002;8:253–261. doi: 10.1038/nm0302-253. [DOI] [PubMed] [Google Scholar]
- 45.Foster H., Sharp P.S., Athanasopoulos T., Trollet C., Graham I.R., Foster K., Wells D.J., Dickson G. Codon and mRNA sequence optimization of microdystrophin transgenes improves expression and physiological outcome in dystrophic mdx mice following AAV2/8 gene transfer. Mol. Ther. 2008;16:1825–1832. doi: 10.1038/mt.2008.186. [DOI] [PubMed] [Google Scholar]
- 46.Lai Y., Thomas G.D., Yue Y., Yang H.T., Li D., Long C., Judge L., Bostick B., Chamberlain J.S., Terjung R.L., Duan D. Dystrophins carrying spectrin-like repeats 16 and 17 anchor nNOS to the sarcolemma and enhance exercise performance in a mouse model of muscular dystrophy. J. Clin. Invest. 2009;119:624–635. doi: 10.1172/JCI36612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yuasa K., Miyagoe Y., Yamamoto K., Nabeshima Y., Dickson G., Takeda S. Effective restoration of dystrophin-associated proteins in vivo by adenovirus-mediated transfer of truncated dystrophin cDNAs. FEBS Lett. 1998;425:329–336. doi: 10.1016/s0014-5793(98)00251-8. [DOI] [PubMed] [Google Scholar]
- 48.Fabb S.A., Wells D.J., Serpente P., Dickson G. Adeno-associated virus vector gene transfer and sarcolemmal expression of a 144 kDa micro-dystrophin effectively restores the dystrophin-associated protein complex and inhibits myofibre degeneration in nude/mdx mice. Hum. Mol. Genet. 2002;11:733–741. doi: 10.1093/hmg/11.7.733. [DOI] [PubMed] [Google Scholar]
- 49.Sakamoto M., Yuasa K., Yoshimura M., Yokota T., Ikemoto T., Suzuki M., Dickson G., Miyagoe-Suzuki Y., Takeda S. Micro-dystrophin cDNA ameliorates dystrophic phenotypes when introduced into mdx mice as a transgene. Biochem. Biophys. Res. Commun. 2002;293:1265–1272. doi: 10.1016/S0006-291X(02)00362-5. [DOI] [PubMed] [Google Scholar]
- 50.Yoshimura M., Sakamoto M., Ikemoto M., Mochizuki Y., Yuasa K., Miyagoe-Suzuki Y., Takeda S. AAV vector-mediated microdystrophin expression in a relatively small percentage of mdx myofibers improved the mdx phenotype. Mol. Ther. 2004;10:821–828. doi: 10.1016/j.ymthe.2004.07.025. [DOI] [PubMed] [Google Scholar]
- 51.Banks G.B., Gregorevic P., Allen J.M., Finn E.E., Chamberlain J.S. Functional capacity of dystrophins carrying deletions in the N-terminal actin-binding domain. Hum. Mol. Genet. 2007;16:2105–2113. doi: 10.1093/hmg/ddm158. [DOI] [PubMed] [Google Scholar]
- 52.Jørgensen L.H., Larochelle N., Orlopp K., Dunant P., Dudley R.W., Stucka R., Thirion C., Walter M.C., Laval S.H., Lochmüller H. Efficient and fast functional screening of microdystrophin constructs in vivo and in vitro for therapy of duchenne muscular dystrophy. Hum. Gene Ther. 2009;20:641–650. doi: 10.1089/hum.2008.162. [DOI] [PubMed] [Google Scholar]
- 53.Banks G.B., Judge L.M., Allen J.M., Chamberlain J.S. The polyproline site in hinge 2 influences the functional capacity of truncated dystrophins. PLoS Genet. 2010;6:e1000958. doi: 10.1371/journal.pgen.1000958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koo T., Malerba A., Athanasopoulos T., Trollet C., Boldrin L., Ferry A., Popplewell L., Foster H., Foster K., Dickson G. Delivery of AAV2/9-microdystrophin genes incorporating helix 1 of the coiled-coil motif in the C-terminal domain of dystrophin improves muscle pathology and restores the level of α1-syntrophin and α-dystrobrevin in skeletal muscles of mdx mice. Hum. Gene Ther. 2011;22:1379–1388. doi: 10.1089/hum.2011.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shin J.-H., Nitahara-Kasahara Y., Hayashita-Kinoh H., Ohshima-Hosoyama S., Kinoshita K., Chiyo T., Okada H., Okada T., Takeda S. Improvement of cardiac fibrosis in dystrophic mice by rAAV9-mediated microdystrophin transduction. Gene Ther. 2011;18:910–919. doi: 10.1038/gt.2011.36. [DOI] [PubMed] [Google Scholar]
- 56.Shin J.-H., Yue Y., Srivastava A., Smith B., Lai Y., Duan D. A simplified immune suppression scheme leads to persistent micro-dystrophin expression in Duchenne muscular dystrophy dogs. Hum. Gene Ther. 2012;23:202–209. doi: 10.1089/hum.2011.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hakim C.H., Wasala N.B., Pan X., Kodippili K., Yue Y., Zhang K., Yao G., Haffner B., Duan S.X., Ramos J. A five-repeat micro-dystrophin gene ameliorated dystrophic phenotype in the severe DBA/2J-mdx model of Duchenne muscular dystrophy. Mol. Ther. Methods Clin. Dev. 2017;6:216–230. doi: 10.1016/j.omtm.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shin J.-H., Pan X., Hakim C.H., Yang H.T., Yue Y., Zhang K., Terjung R.L., Duan D. Microdystrophin ameliorates muscular dystrophy in the canine model of duchenne muscular dystrophy. Mol. Ther. 2013;21:750–757. doi: 10.1038/mt.2012.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bostick B., Shin J.-H., Yue Y., Duan D. AAV-microdystrophin therapy improves cardiac performance in aged female mdx mice. Mol. Ther. 2011;19:1826–1832. doi: 10.1038/mt.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duan D. Duchenne muscular dystrophy gene therapy in the canine model. Hum. Gene Ther. Clin. Dev. 2015;26:57–69. doi: 10.1089/humc.2015.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kotterman M.A., Schaffer D.V. Engineering adeno-associated viruses for clinical gene therapy. Nat. Rev. Genet. 2014;15:445–451. doi: 10.1038/nrg3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McGreevy J.W., Hakim C.H., McIntosh M.A., Duan D. Animal models of Duchenne muscular dystrophy: from basic mechanisms to gene therapy. Dis. Model. Mech. 2015;8:195–213. doi: 10.1242/dmm.018424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregorevic P., Allen J.M., Minami E., Blankinship M.J., Haraguchi M., Meuse L., Finn E., Adams M.E., Froehner S.C., Murry C.E., Chamberlain J.S. rAAV6-microdystrophin preserves muscle function and extends lifespan in severely dystrophic mice. Nat. Med. 2006;12:787–789. doi: 10.1038/nm1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gregorevic P., Blankinship M.J., Allen J.M., Chamberlain J.S. Systemic microdystrophin gene delivery improves skeletal muscle structure and function in old dystrophic mdx mice. Mol. Ther. 2008;16:657–664. doi: 10.1038/mt.2008.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bostick B., Shin J.-H., Yue Y., Wasala N.B., Lai Y., Duan D. AAV micro-dystrophin gene therapy alleviates stress-induced cardiac death but not myocardial fibrosis in >21-m-old mdx mice, an end-stage model of Duchenne muscular dystrophy cardiomyopathy. J. Mol. Cell. Cardiol. 2012;53:217–222. doi: 10.1016/j.yjmcc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang B., Li J., Fu F.H., Xiao X. Systemic human minidystrophin gene transfer improves functions and life span of dystrophin and dystrophin/utrophin-deficient mice. J. Orthop. Res. 2009;27:421–426. doi: 10.1002/jor.20781. [DOI] [PubMed] [Google Scholar]
- 67.Kornegay J.N., Li J., Bogan J.R., Bogan D.J., Chen C., Zheng H., Wang B., Qiao C., Howard J.F., Jr., Xiao X. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol. Ther. 2010;18:1501–1508. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yue Y., Pan X., Hakim C.H., Kodippili K., Zhang K., Shin J.-H., Yang H.T., McDonald T., Duan D. Safe and bodywide muscle transduction in young adult Duchenne muscular dystrophy dogs with adeno-associated virus. Hum. Mol. Genet. 2015;24:5880–5890. doi: 10.1093/hmg/ddv310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Le Guiner C., Servais L., Montus M., Larcher T., Fraysse B., Moullec S., Allais M., François V., Dutilleul M., Malerba A. Long-term microdystrophin gene therapy is effective in a canine model of Duchenne muscular dystrophy. Nat. Commun. 2017;8:16105. doi: 10.1038/ncomms16105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Birch S.M., Lawlor M.W., Guo L.-J., Crudele J.M., Hawkins E.C., Nghiem P.P., Styner M.A., Struharik M.J., Brown K.J., Golebiowski D. A blinded placebo-controlled systemic gene therapy efficacy study in the GRMD model of Duchenne muscular dystrophy. Mol. Ther. 2017;25(Suppl 1):193. [Google Scholar]
- 71.Hakim C.H., Kodippili K., Jenkins G., Yang H.T., Pan X., Lessa T.B., Leach S.B., Emter C., Yue Y., Zhang K. Single systemic AAV micro-dystrophin therapy ameliorates muscular dystrophy in young adult Duchenne muscular dystrophy dogs for up to two years. Mol. Ther. 2017;25(Suppl 1):192–193. [Google Scholar]
- 72.Hakim C.H., Kodippili K., Jenkins G., Yang H.T., Pan X., Lessa T.B., Leach S., Emter C., Yue Y., Zhang K. AAV micro-dystrophin therapy ameliorates muscular dystrophy in young adult Duchenne muscular dystrophy dogs for up to 30 months following injection. Mol. Ther. 2018;26(Suppl 1):5. [Google Scholar]
- 73.Crudele J.M., Birch S.M., Hakim C.H., Golebiowski D., Shanks C., Morris C., Schneider J.S., Hauschka S.D., Duan D., Kornegay J.N., Chamberlain J.S. Assessing anti-dystrophin T-cell responses by ELISPOT following AAV-9 micro-dystrophin gene therapy in dogs. Mol. Ther. 2018;26(Suppl 1):104. [Google Scholar]
- 74.Mendell J.R., Campbell K., Rodino-Klapac L., Sahenk Z., Shilling C., Lewis S., Bowles D., Gray S., Li C., Galloway G. Dystrophin immunity in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2010;363:1429–1437. doi: 10.1056/NEJMoa1000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bowles D.E., McPhee S.W., Li C., Gray S.J., Samulski J.J., Camp A.S., Li J., Wang B., Monahan P.E., Rabinowitz J.E. Phase 1 gene therapy for Duchenne muscular dystrophy using a translational optimized AAV vector. Mol. Ther. 2012;20:443–455. doi: 10.1038/mt.2011.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mendell J.R., Al-Zaidy S., Shell R., Arnold W.D., Rodino-Klapac L.R., Prior T.W., Lowes L., Alfano L., Berry K., Church K. Single-dose gene-replacement therapy for spinal muscular atrophy. N. Engl. J. Med. 2017;377:1713–1722. doi: 10.1056/NEJMoa1706198. [DOI] [PubMed] [Google Scholar]
- 77.Audentes Therapeutics (2018). Audentes Announces Positive Interim Data from First Dose Cohort of ASPIRO, a Phase 1/2 Clinical Trial of AT132 in Patients With X-Linked Myotubular Myopathy. Audentes, http://investors.audentestx.com/phoenix.zhtml?c=254280&p=irol-newsArticle_print&ID=2324833.
- 78.Kuntz N., Shieh P.B., Smith B., Bonnemann C.G., Dowling J.J., Lawlor M.W., Müller-Felber W., Noursalehi M., Rico S., Servais L., Prasad S. ASPIRO phase 1/2 gene therapy trail in X-linked myotubular myopathy (XLMTM): preliminary safety and efficacy findings. Mol. Ther. 2018;26(Suppl 1):4. [Google Scholar]
- 79.Rangarajan S., Walsh L., Lester W., Perry D., Madan B., Laffan M., Yu H., Vettermann C., Pierce G.F., Wong W.Y., Pasi K.J. AAV5-Factor VIII Gene Transfer in Severe Hemophilia A. N. Engl. J. Med. 2017;377:2519–2530. doi: 10.1056/NEJMoa1708483. [DOI] [PubMed] [Google Scholar]
- 80.Beggs A.H., Byrne B.J., De Chastonay S., Haselkorn T., Hughes I., James E.S., Kuntz N.L., Simon J., Swanson L.C., Yang M.L. A multicenter, retrospective medical record review of X-linked myotubular myopathy: The recensus study. Muscle Nerve. 2018;57:550–560. doi: 10.1002/mus.26018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarepta Therapeutics (2017). Sarepta Therapeutics and Genethon Announce a Gene Therapy Research Collaboration for the Treatment of Duchenne Muscular Dystrophy. Sarepta Therapeutics, http://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-and-genethon-announce-gene-therapy-research.
- 82.Clément N., Knop D.R., Byrne B.J. Large-scale adeno-associated viral vector production using a herpesvirus-based system enables manufacturing for clinical studies. Hum. Gene Ther. 2009;20:796–806. doi: 10.1089/hum.2009.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Grieger J.C., Soltys S.M., Samulski R.J. Production of Recombinant Adeno-associated Virus Vectors Using Suspension HEK293 Cells and Continuous Harvest of Vector From the Culture Media for GMP FIX and FLT1 Clinical Vector. Mol. Ther. 2016;24:287–297. doi: 10.1038/mt.2015.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salva M.Z., Himeda C.L., Tai P.W., Nishiuchi E., Gregorevic P., Allen J.M., Finn E.E., Nguyen Q.G., Blankinship M.J., Meuse L. Design of tissue-specific regulatory cassettes for high-level rAAV-mediated expression in skeletal and cardiac muscle. Mol. Ther. 2007;15:320–329. doi: 10.1038/sj.mt.6300027. [DOI] [PubMed] [Google Scholar]
- 85.Wang B., Li J., Fu F.H., Chen C., Zhu X., Zhou L., Jiang X., Xiao X. Construction and analysis of compact muscle-specific promoters for AAV vectors. Gene Ther. 2008;15:1489–1499. doi: 10.1038/gt.2008.104. [DOI] [PubMed] [Google Scholar]
- 86.Brenman J.E., Chao D.S., Xia H., Aldape K., Bredt D.S. Nitric oxide synthase complexed with dystrophin and absent from skeletal muscle sarcolemma in Duchenne muscular dystrophy. Cell. 1995;82:743–752. doi: 10.1016/0092-8674(95)90471-9. [DOI] [PubMed] [Google Scholar]
- 87.Chang W.J., Iannaccone S.T., Lau K.S., Masters B.S., McCabe T.J., McMillan K., Padre R.C., Spencer M.J., Tidball J.G., Stull J.T. Neuronal nitric oxide synthase and dystrophin-deficient muscular dystrophy. Proc. Natl. Acad. Sci. USA. 1996;93:9142–9147. doi: 10.1073/pnas.93.17.9142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Stamler J.S., Meissner G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001;81:209–237. doi: 10.1152/physrev.2001.81.1.209. [DOI] [PubMed] [Google Scholar]
- 89.Thomas G.D., Sander M., Lau K.S., Huang P.L., Stull J.T., Victor R.G. Impaired metabolic modulation of alpha-adrenergic vasoconstriction in dystrophin-deficient skeletal muscle. Proc. Natl. Acad. Sci. USA. 1998;95:15090–15095. doi: 10.1073/pnas.95.25.15090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sander M., Chavoshan B., Harris S.A., Iannaccone S.T., Stull J.T., Thomas G.D., Victor R.G. Functional muscle ischemia in neuronal nitric oxide synthase-deficient skeletal muscle of children with Duchenne muscular dystrophy. Proc. Natl. Acad. Sci. USA. 2000;97:13818–13823. doi: 10.1073/pnas.250379497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Thomas G.D. Functional muscle ischemia in Duchenne and Becker muscular dystrophy. Front. Physiol. 2013;4:381. doi: 10.3389/fphys.2013.00381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mendell J.R., Engel W.K., Derrer E.C. Duchenne muscular dystrophy: functional ischemia reproduces its characteristic lesions. Science. 1971;172:1143–1145. doi: 10.1126/science.172.3988.1143. [DOI] [PubMed] [Google Scholar]
- 93.Li D., Yue Y., Lai Y., Hakim C.H., Duan D. Nitrosative stress elicited by nNOSμ delocalization inhibits muscle force in dystrophin-null mice. J. Pathol. 2011;223:88–98. doi: 10.1002/path.2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gentil C., Leturcq F., Ben Yaou R., Kaplan J.C., Laforet P., Pénisson-Besnier I., Espil-Taris C., Voit T., Garcia L., Piétri-Rouxel F. Variable phenotype of del45-55 Becker patients correlated with nNOSμ mislocalization and RYR1 hypernitrosylation. Hum. Mol. Genet. 2012;21:3449–3460. doi: 10.1093/hmg/dds176. [DOI] [PubMed] [Google Scholar]
- 95.Harper S.Q. Molecular dissection of dystrophin identifies the docking site for nNOS. Proc. Natl. Acad. Sci. USA. 2013;110:387–388. doi: 10.1073/pnas.1220256110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hillier B.J., Christopherson K.S., Prehoda K.E., Bredt D.S., Lim W.A. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–815. [PubMed] [Google Scholar]
- 97.Yue Y., Liu M., Duan D. C-terminal-truncated microdystrophin recruits dystrobrevin and syntrophin to the dystrophin-associated glycoprotein complex and reduces muscular dystrophy in symptomatic utrophin/dystrophin double-knockout mice. Mol. Ther. 2006;14:79–87. doi: 10.1016/j.ymthe.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lai Y., Zhao J., Yue Y., Duan D. α2 and α3 helices of dystrophin R16 and R17 frame a microdomain in the α1 helix of dystrophin R17 for neuronal NOS binding. Proc. Natl. Acad. Sci. USA. 2013;110:525–530. doi: 10.1073/pnas.1211431109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang Y., Duan D. Novel mini-dystrophin gene dual adeno-associated virus vectors restore neuronal nitric oxide synthase expression at the sarcolemma. Hum. Gene Ther. 2012;23:98–103. doi: 10.1089/hum.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y., Yue Y., Li L., Hakim C.H., Zhang K., Thomas G.D., Duan D. Dual AAV therapy ameliorates exercise-induced muscle injury and functional ischemia in murine models of Duchenne muscular dystrophy. Hum. Mol. Genet. 2013;22:3720–3729. doi: 10.1093/hmg/ddt224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hinderer C., Katz N., Buza E.L., Dyer C., Goode T., Bell P., Richman L.K., Wilson J.M. Severe Toxicity in Nonhuman Primates and Piglets Following High-Dose Intravenous Administration of an Adeno-Associated Virus Vector Expressing Human SMN. Hum. Gene Ther. 2018;29:285–298. doi: 10.1089/hum.2018.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hordeaux J., Wang Q., Katz N., Buza E.L., Bell P., Wilson J.M. The Neurotropic Properties of AAV-PHP.B Are Limited to C57BL/6J Mice. Mol. Ther. 2018;26:664–668. doi: 10.1016/j.ymthe.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rogers G.L., Martino A.T., Aslanidi G.V., Jayandharan G.R., Srivastava A., Herzog R.W. Innate immune responses to AAV vectors. Front. Microbiol. 2011;2:194. doi: 10.3389/fmicb.2011.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Shayakhmetov D.M., Di Paolo N.C., Mossman K.L. Recognition of virus infection and innate host responses to viral gene therapy vectors. Mol. Ther. 2010;18:1422–1429. doi: 10.1038/mt.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mingozzi F., High K.A. Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. 2013;122:23–36. doi: 10.1182/blood-2013-01-306647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Raper S.E., Chirmule N., Lee F.S., Wivel N.A., Bagg A., Gao G.P., Wilson J.M., Batshaw M.L. Fatal systemic inflammatory response syndrome in a ornithine transcarbamylase deficient patient following adenoviral gene transfer. Mol. Genet. Metab. 2003;80:148–158. doi: 10.1016/j.ymgme.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 107.Hendrickx R., Stichling N., Koelen J., Kuryk L., Lipiec A., Greber U.F. Innate immunity to adenovirus. Hum. Gene Ther. 2014;25:265–284. doi: 10.1089/hum.2014.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zaiss A.K., Liu Q., Bowen G.P., Wong N.C., Bartlett J.S., Muruve D.A. Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J. Virol. 2002;76:4580–4590. doi: 10.1128/JVI.76.9.4580-4590.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.McCaffrey A.P., Fawcett P., Nakai H., McCaffrey R.L., Ehrhardt A., Pham T.T., Pandey K., Xu H., Feuss S., Storm T.A., Kay M.A. The host response to adenovirus, helper-dependent adenovirus, and adeno-associated virus in mouse liver. Mol. Ther. 2008;16:931–941. doi: 10.1038/mt.2008.37. [DOI] [PubMed] [Google Scholar]
- 110.Shayakhmetov D.M. Virus infection recognition and early innate responses to non-enveloped viral vectors. Viruses. 2010;2:244–261. doi: 10.3390/v2010244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zaiss A.K., Muruve D.A. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15:808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- 112.Hösel M., Broxtermann M., Janicki H., Esser K., Arzberger S., Hartmann P., Gillen S., Kleeff J., Stabenow D., Odenthal M. Toll-like receptor 2-mediated innate immune response in human nonparenchymal liver cells toward adeno-associated viral vectors. Hepatology. 2012;55:287–297. doi: 10.1002/hep.24625. [DOI] [PubMed] [Google Scholar]
- 113.Zhu J., Huang X., Yang Y. The TLR9-MyD88 pathway is critical for adaptive immune responses to adeno-associated virus gene therapy vectors in mice. J. Clin. Invest. 2009;119:2388–2398. doi: 10.1172/JCI37607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Martino A.T., Suzuki M., Markusic D.M., Zolotukhin I., Ryals R.C., Moghimi B., Ertl H.C., Muruve D.A., Lee B., Herzog R.W. The genome of self-complementary adeno-associated viral vectors increases Toll-like receptor 9-dependent innate immune responses in the liver. Blood. 2011;117:6459–6468. doi: 10.1182/blood-2010-10-314518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Solid Biosciences (2018). Solid Biosciences announces clinical hold on SGT-001 phase I/II clinical trial for Duchenne muscualr dystrophy. Solid Biosciences, https://www.solidbio.com/content/solid-biosciences-announces-clinical-hold-sgt-001-phase-iii-clinical-trial-duchenne-muscular.
- 116.Solid Biosciences (2018). Solid Biosciences announces FDA removes clinical hold on SGT-001. Solid Biosciences, https://www.solidbio.com/content/solid-biosciences-announces-fda-removes-clinical-hold-sgt-001.
- 117.Zaiss A.K., Cotter M.J., White L.R., Clark S.A., Wong N.C., Holers V.M., Bartlett J.S., Muruve D.A. Complement is an essential component of the immune response to adeno-associated virus vectors. J. Virol. 2008;82:2727–2740. doi: 10.1128/JVI.01990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Noris M., Remuzzi G. Overview of complement activation and regulation. Semin. Nephrol. 2013;33:479–492. doi: 10.1016/j.semnephrol.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ricklin D., Reis E.S., Lambris J.D. Complement in disease: a defence system turning offensive. Nat. Rev. Nephrol. 2016;12:383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huber-Lang M., Ignatius A., Brenner R.E. Role of Complement on Broken Surfaces After Trauma. Adv. Exp. Med. Biol. 2015;865:43–55. doi: 10.1007/978-3-319-18603-0_3. [DOI] [PubMed] [Google Scholar]
- 121.Del Conde I., Crúz M.A., Zhang H., López J.A., Afshar-Kharghan V. Platelet activation leads to activation and propagation of the complement system. J. Exp. Med. 2005;201:871–879. doi: 10.1084/jem.20041497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wilson W.A., Thomas E.J. Activation of the alternative pathway of human complement by haemoglobin. Clin. Exp. Immunol. 1979;36:140–144. [PMC free article] [PubMed] [Google Scholar]
- 123.Kaca W., Roth R. Activation of complement by human hemoglobin and by mixtures of hemoglobin and bacterial endotoxin. Biochim. Biophys. Acta. 1995;1245:49–56. doi: 10.1016/0304-4165(95)00077-o. [DOI] [PubMed] [Google Scholar]
- 124.Furlong, P. (2018). First Duchenne Patient Dosed in Microdystrophin Gene Therapy! Parent Project Muscular Dystrophy, http://community.parentprojectmd.org/profiles/blogs/first-duchenne-patient-dosed-in-microdystrophin-gene-therapy.
- 125.Furlong, P. (2018). Positive preliminary results from the first three children dosed in phase 1/2A gene therapy micro-dystrophin trial. Parent Project Muscular Dystrophy,(https://www.parentprojectmd.org/positive-preliminary-results-from-the-first-three-children-dosed-in-phase-1-2a-gene-therapy-micro-dystrophin-trial/).
- 126.News & Media (2018). Pfizer doses first patient using investigational mini-dystrophin gene therapy for the treatment of Duchenne muscular dystrophy. Pfizer, http://press.pfizer.com/press-release/pfizer-doses-first-patient-using-investigational-mini-dystrophin-gene-therapy-treatmen.
- 127.Mingozzi F., High K.A. Overcoming the Host Immune Response to Adeno-Associated Virus Gene Delivery Vectors: The Race Between Clearance, Tolerance, Neutralization, and Escape. Annu. Rev. Virol. 2017;4:511–534. doi: 10.1146/annurev-virology-101416-041936. [DOI] [PubMed] [Google Scholar]
- 128.Basner-Tschakarjan E., Bijjiga E., Martino A.T. Pre-clinical assessment of immune responses to adeno-associated virus (AAV) vectors. Front. Immunol. 2014;5:28. doi: 10.3389/fimmu.2014.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Masat E., Pavani G., Mingozzi F. Humoral immunity to AAV vectors in gene therapy: challenges and potential solutions. Discov. Med. 2013;15:379–389. [PubMed] [Google Scholar]
- 130.Mays L.E., Wilson J.M. The complex and evolving story of T cell activation to AAV vector-encoded transgene products. Mol. Ther. 2011;19:16–27. doi: 10.1038/mt.2010.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Vandamme C., Adjali O., Mingozzi F. Unraveling the Complex Story of Immune Responses to AAV Vectors Trial After Trial. Hum. Gene Ther. 2017;28:1061–1074. doi: 10.1089/hum.2017.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Calcedo R., Wilson J.M. Humoral Immune Response to AAV. Front. Immunol. 2013;4:341. doi: 10.3389/fimmu.2013.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Manno C.S., Pierce G.F., Arruda V.R., Glader B., Ragni M., Rasko J.J., Ozelo M.C., Hoots K., Blatt P., Konkle B. Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response. Nat. Med. 2006;12:342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 134.Nathwani A.C., Reiss U.M., Tuddenham E.G., Rosales C., Chowdary P., McIntosh J., Della Peruta M., Lheriteau E., Patel N., Raj D. Long-term safety and efficacy of factor IX gene therapy in hemophilia B. N. Engl. J. Med. 2014;371:1994–2004. doi: 10.1056/NEJMoa1407309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.George L.A., Sullivan S.K., Giermasz A., Rasko J.E.J., Samelson-Jones B.J., Ducore J., Cuker A., Sullivan L.M., Majumdar S., Teitel J. Hemophilia B Gene Therapy with a High-Specific-Activity Factor IX Variant. N. Engl. J. Med. 2017;377:2215–2227. doi: 10.1056/NEJMoa1708538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Flanigan K.M., Campbell K., Viollet L., Wang W., Gomez A.M., Walker C.M., Mendell J.R. Anti-dystrophin T cell responses in Duchenne muscular dystrophy: prevalence and a glucocorticoid treatment effect. Hum. Gene Ther. 2013;24:797–806. doi: 10.1089/hum.2013.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Louis Jeune V., Joergensen J.A., Hajjar R.J., Weber T. Pre-existing anti-adeno-associated virus antibodies as a challenge in AAV gene therapy. Hum. Gene Ther. Methods. 2013;24:59–67. doi: 10.1089/hgtb.2012.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tse L.V., Moller-Tank S., Asokan A. Strategies to circumvent humoral immunity to adeno-associated viral vectors. Expert Opin. Biol. Ther. 2015;15:845–855. doi: 10.1517/14712598.2015.1035645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Arikawa-Hirasawa E., Koga R., Tsukahara T., Nonaka I., Mitsudome A., Goto K., Beggs A.H., Arahata K. A severe muscular dystrophy patient with an internally deleted very short (110 kD) dystrophin: presence of the binding site for dystrophin-associated glycoprotein (DAG) may not be enough for physiological function of dystrophin. Neuromuscul. Disord. 1995;5:429–438. doi: 10.1016/0960-8966(94)00087-p. [DOI] [PubMed] [Google Scholar]
- 140.Den Dunnen J.T., Grootscholten P.M., Bakker E., Blonden L.A., Ginjaar H.B., Wapenaar M.C., van Paassen H.M., van Broeckhoven C., Pearson P.L., van Ommen G.J. Topography of the Duchenne muscular dystrophy (DMD) gene: FIGE and cDNA analysis of 194 cases reveals 115 deletions and 13 duplications. Am. J. Hum. Genet. 1989;45:835–847. [PMC free article] [PubMed] [Google Scholar]
- 141.Fanin M., Freda M.P., Vitiello L., Danieli G.A., Pegoraro E., Angelini C. Duchenne phenotype with in-frame deletion removing major portion of dystrophin rod: threshold effect for deletion size? Muscle Nerve. 1996;19:1154–1160. doi: 10.1002/mus.880190902. [DOI] [PubMed] [Google Scholar]
- 142.Carsana A., Frisso G., Tremolaterra M.R., Lanzillo R., Vitale D.F., Santoro L., Salvatore F. Analysis of dystrophin gene deletions indicates that the hinge III region of the protein correlates with disease severity. Ann. Hum. Genet. 2005;69:253–259. doi: 10.1046/j.1529-8817.2005.00160.x. [DOI] [PubMed] [Google Scholar]
- 143.Nevo Y., Muntoni F., Sewry C., Legum C., Kutai M., Harel S., Dubowitz V. Large in-frame deletions of the rod-shaped domain of the dystrophin gene resulting in severe phenotype. Isr. Med. Assoc. J. 2003;5:94–97. [PubMed] [Google Scholar]
- 144.Takeshima Y., Nishio H., Narita N., Wada H., Ishikawa Y., Ishikawa Y., Minami R., Nakamura H., Matsuo M. Amino-terminal deletion of 53% of dystrophin results in an intermediate Duchenne-Becker muscular dystrophy phenotype. Neurology. 1994;44:1648–1651. doi: 10.1212/wnl.44.9.1648. [DOI] [PubMed] [Google Scholar]
- 145.Vainzof M., Takata R.I., Passos-Bueno M.R., Pavanello R.C., Zatz M. Is the maintainance of the C-terminus domain of dystrophin enough to ensure a milder Becker muscular dystrophy phenotype? Hum. Mol. Genet. 1993;2:39–42. doi: 10.1093/hmg/2.1.39. [DOI] [PubMed] [Google Scholar]
- 146.Pascual J., Castresana J., Saraste M. Evolution of the spectrin repeat. BioEssays. 1997;19:811–817. doi: 10.1002/bies.950190911. [DOI] [PubMed] [Google Scholar]
- 147.Broderick M.J., Winder S.J. Spectrin, alpha-actinin, and dystrophin. Adv. Protein Chem. 2005;70:203–246. doi: 10.1016/S0065-3233(05)70007-3. [DOI] [PubMed] [Google Scholar]
- 148.Zhao J., Kodippili K., Yue Y., Hakim C.H., Wasala L., Pan X., Zhang K., Yang N.N., Duan D., Lai Y. Dystrophin contains multiple independent membrane-binding domains. Hum. Mol. Genet. 2016;25:3647–3653. doi: 10.1093/hmg/ddw210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Nelson D.M., Lindsay A., Judge L.M., Duan D., Chamberlain J.S., Lowe D.A., Ervasti J.M. Variable rescue of microtubule and physiological phenotypes in mdx muscle expressing different miniaturized dystrophins. Hum. Mol. Genet. 2018;27:2090–2100. doi: 10.1093/hmg/ddy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Crawford G.E., Faulkner J.A., Crosbie R.H., Campbell K.P., Froehner S.C., Chamberlain J.S. Assembly of the dystrophin-associated protein complex does not require the dystrophin COOH-terminal domain. J. Cell Biol. 2000;150:1399–1410. doi: 10.1083/jcb.150.6.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Tandon A., Jefferies J.L., Villa C.R., Hor K.N., Wong B.L., Ware S.M., Gao Z., Towbin J.A., Mazur W., Fleck R.J. Dystrophin genotype-cardiac phenotype correlations in Duchenne and Becker muscular dystrophies using cardiac magnetic resonance imaging. Am. J. Cardiol. 2015;115:967–971. doi: 10.1016/j.amjcard.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ricotti V., Mandy W.P., Scoto M., Pane M., Deconinck N., Messina S., Mercuri E., Skuse D.H., Muntoni F. Neurodevelopmental, emotional, and behavioural problems in Duchenne muscular dystrophy in relation to underlying dystrophin gene mutations. Dev. Med. Child Neurol. 2016;58:77–84. doi: 10.1111/dmcn.12922. [DOI] [PubMed] [Google Scholar]
- 153.Daoud F., Candelario-Martínez A., Billard J.M., Avital A., Khelfaoui M., Rozenvald Y., Guegan M., Mornet D., Jaillard D., Nudel U. Role of mental retardation-associated dystrophin-gene product Dp71 in excitatory synapse organization, synaptic plasticity and behavioral functions. PLoS ONE. 2008;4:e6574. doi: 10.1371/journal.pone.0006574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.McNally E.M., Kaltman J.R., Benson D.W., Canter C.E., Cripe L.H., Duan D., Finder J.D., Groh W.J., Hoffman E.P., Judge D.P., Working Group of the National Heart, Lung, and Blood Institute. Parent Project Muscular Dystrophy Contemporary cardiac issues in Duchenne muscular dystrophy. Working Group of the National Heart, Lung, and Blood Institute in collaboration with Parent Project Muscular Dystrophy. Circulation. 2015;131:1590–1598. doi: 10.1161/CIRCULATIONAHA.114.015151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johnson E.K., Zhang L., Adams M.E., Phillips A., Freitas M.A., Froehner S.C., Green-Church K.B., Montanaro F. Proteomic analysis reveals new cardiac-specific dystrophin-associated proteins. PLoS ONE. 2012;7:e43515. doi: 10.1371/journal.pone.0043515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bostick B., Yue Y., Long C., Marschalk N., Fine D.M., Chen J., Duan D. Cardiac expression of a mini-dystrophin that normalizes skeletal muscle force only partially restores heart function in aged Mdx mice. Mol. Ther. 2009;17:253–261. doi: 10.1038/mt.2008.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wasala L., Shin J.-H., Lai Y., Yue Y., Montanaro F., Duan D. Cardiac specific expression of ΔH2-R15 mini-dystrophin normalized all ECG abnormalities and the end-diastolic volume in a 23-m-old mouse model of Duchenne dilated cardiomyopathy. Hum. Gene Ther. 2018;29:737–748. doi: 10.1089/hum.2017.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Yue Y., Li Z., Harper S.Q., Davisson R.L., Chamberlain J.S., Duan D. Microdystrophin gene therapy of cardiomyopathy restores dystrophin-glycoprotein complex and improves sarcolemma integrity in the mdx mouse heart. Circulation. 2003;108:1626–1632. doi: 10.1161/01.CIR.0000089371.11664.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bostick B., Yue Y., Lai Y., Long C., Li D., Duan D. Adeno-associated virus serotype-9 microdystrophin gene therapy ameliorates electrocardiographic abnormalities in mdx mice. Hum. Gene Ther. 2008;19:851–856. doi: 10.1089/hum.2008.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Townsend D., Blankinship M.J., Allen J.M., Gregorevic P., Chamberlain J.S., Metzger J.M. Systemic administration of micro-dystrophin restores cardiac geometry and prevents dobutamine-induced cardiac pump failure. Mol. Ther. 2007;15:1086–1092. doi: 10.1038/sj.mt.6300144. [DOI] [PubMed] [Google Scholar]
- 161.Duan D. Challenges and opportunities in dystrophin-deficient cardiomyopathy gene therapy. Hum. Mol. Genet. 2006;15:R253–R261. doi: 10.1093/hmg/ddl180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lai Y., Duan D. Progress in gene therapy of dystrophic heart disease. Gene Ther. 2012;19:678–685. doi: 10.1038/gt.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Yue Y., Binalsheikh I.M., Leach S.B., Domeier T.L., Duan D. Prospect of gene therapy for cardiomyopathy in hereditary muscular dystrophy. Expert Opin. Orphan Drugs. 2016;4:169–183. doi: 10.1517/21678707.2016.1124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Himeda C.L., Chen X., Hauschka S.D. Design and testing of regulatory cassettes for optimal activity in skeletal and cardiac muscles. Methods Mol. Biol. 2011;709:3–19. doi: 10.1007/978-1-61737-982-6_1. [DOI] [PubMed] [Google Scholar]
- 165.Li X., Eastman E.M., Schwartz R.J., Draghia-Akli R. Synthetic muscle promoters: activities exceeding naturally occurring regulatory sequences. Nat. Biotechnol. 1999;17:241–245. doi: 10.1038/6981. [DOI] [PubMed] [Google Scholar]
- 166.Shield M.A., Haugen H.S., Clegg C.H., Hauschka S.D. E-box sites and a proximal regulatory region of the muscle creatine kinase gene differentially regulate expression in diverse skeletal muscles and cardiac muscle of transgenic mice. Mol. Cell. Biol. 1996;16:5058–5068. doi: 10.1128/mcb.16.9.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Boisgerault F., Gross D.A., Ferrand M., Poupiot J., Darocha S., Richard I., Galy A. Prolonged gene expression in muscle is achieved without active immune tolerance using microrRNA 142.3p-regulated rAAV gene transfer. Hum. Gene Ther. 2013;24:393–405. doi: 10.1089/hum.2012.208. [DOI] [PubMed] [Google Scholar]
- 168.Wang L., Dobrzynski E., Schlachterman A., Cao O., Herzog R.W. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood. 2005;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Brown B.D., Venneri M.A., Zingale A., Sergi Sergi L., Naldini L. Endogenous microRNA regulation suppresses transgene expression in hematopoietic lineages and enables stable gene transfer. Nat. Med. 2006;12:585–591. doi: 10.1038/nm1398. [DOI] [PubMed] [Google Scholar]
- 170.Majowicz A., Maczuga P., Kwikkers K.L., van der Marel S., van Logtenstein R., Petry H., van Deventer S.J., Konstantinova P., Ferreira V. Mir-142-3p target sequences reduce transgene-directed immunogenicity following intramuscular adeno-associated virus 1 vector-mediated gene delivery. J. Gene Med. 2013;15:219–232. doi: 10.1002/jgm.2712. [DOI] [PubMed] [Google Scholar]
- 171.Shao W., Chen X., Samulski R.J., Hirsch M.L., Li C. Inhibition of antigen presentation during AAV gene therapy using virus peptides. Hum. Mol. Genet. 2017;27:601–613. doi: 10.1093/hmg/ddx427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Clément N., Grieger J.C. Manufacturing of recombinant adeno-associated viral vectors for clinical trials. Mol. Ther. Methods Clin. Dev. 2016;3:16002. doi: 10.1038/mtm.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kotin R.M., Snyder R.O. Manufacturing clinical grade recombinant adeno-associated virus using invertebrate cell lines. Hum. Gene Ther. 2017;28:350–360. doi: 10.1089/hum.2017.042. [DOI] [PubMed] [Google Scholar]
- 174.Brennan T.A., Wilson J.M. The special case of gene therapy pricing. Nat. Biotechnol. 2014;32:874–876. doi: 10.1038/nbt.3003. [DOI] [PubMed] [Google Scholar]
- 175.Wilson J.M. Lessons learned from the gene therapy trial for ornithine transcarbamylase deficiency. Mol. Genet. Metab. 2009;96:151–157. doi: 10.1016/j.ymgme.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 176.Nance M.E., Duan D. Perspective on Adeno-Associated Virus Capsid Modification for Duchenne Muscular Dystrophy Gene Therapy. Hum. Gene Ther. 2015;26:786–800. doi: 10.1089/hum.2015.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Qiao C., Zhang W., Yuan Z., Shin J.H., Li J., Jayandharan G.R., Zhong L., Srivastava A., Xiao X., Duan D. Adeno-associated virus serotype 6 capsid tyrosine-to-phenylalanine mutations improve gene transfer to skeletal muscle. Hum. Gene Ther. 2010;21:1343–1348. doi: 10.1089/hum.2010.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Choudhury S.R., Fitzpatrick Z., Harris A.F., Maitland S.A., Ferreira J.S., Zhang Y., Ma S., Sharma R.B., Gray-Edwards H.L., Johnson J.A. In vivo selection yields AAV-B1 capsid for central nervous system and muscle gene therapy. Mol. Ther. 2016;24:1247–1257. doi: 10.1038/mt.2016.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Li D., Yue Y., Duan D. Preservation of muscle force in Mdx3cv mice correlates with low-level expression of a near full-length dystrophin protein. Am. J. Pathol. 2008;172:1332–1341. doi: 10.2353/ajpath.2008.071042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li D., Yue Y., Duan D. Marginal level dystrophin expression improves clinical outcome in a strain of dystrophin/utrophin double knockout mice. PLoS ONE. 2010;5:e15286. doi: 10.1371/journal.pone.0015286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Wasala N.B., Yue Y., Vance J., Duan D. Uniform low-level dystrophin expression in the heart partially preserved cardiac function in an aged mouse model of Duchenne cardiomyopathy. J. Mol. Cell. Cardiol. 2017;102:45–52. doi: 10.1016/j.yjmcc.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.van Putten M., Hulsker M., Young C., Nadarajah V.D., Heemskerk H., van der Weerd L., ’t Hoen P.A., van Ommen G.J., Aartsma-Rus A.M. Low dystrophin levels increase survival and improve muscle pathology and function in dystrophin/utrophin double-knockout mice. FASEB J. 2013;27:2484–2495. doi: 10.1096/fj.12-224170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.van Putten M., van der Pijl E.M., Hulsker M., Verhaart I.E., Nadarajah V.D., van der Weerd L., Aartsma-Rus A. Low dystrophin levels in heart can delay heart failure in mdx mice. J. Mol. Cell. Cardiol. 2014;69:17–23. doi: 10.1016/j.yjmcc.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 184.van Putten M., Hulsker M., Nadarajah V.D., van Heiningen S.H., van Huizen E., van Iterson M., Admiraal P., Messemaker T., den Dunnen J.T., ’t Hoen P.A., Aartsma-Rus A. The effects of low levels of dystrophin on mouse muscle function and pathology. PLoS ONE. 2012;7:e31937. doi: 10.1371/journal.pone.0031937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nicholson L.V., Johnson M.A., Bushby K.M., Gardner-Medwin D. Functional significance of dystrophin positive fibres in Duchenne muscular dystrophy. Arch. Dis. Child. 1993;68:632–636. doi: 10.1136/adc.68.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Waldrop M.A., Gumienny F., El Husayni S., Frank D.E., Weiss R.B., Flanigan K.M. Low-level dystrophin expression attenuating the dystrophinopathy phenotype. Neuromuscul. Disord. 2018;28:116–121. doi: 10.1016/j.nmd.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Phelps S.F., Hauser M.A., Cole N.M., Rafael J.A., Hinkle R.T., Faulkner J.A., Chamberlain J.S. Expression of full-length and truncated dystrophin mini-genes in transgenic mdx mice. Hum. Mol. Genet. 1995;4:1251–1258. doi: 10.1093/hmg/4.8.1251. [DOI] [PubMed] [Google Scholar]
- 188.Wells D.J., Wells K.E., Asante E.A., Turner G., Sunada Y., Campbell K.P., Walsh F.S., Dickson G. Expression of human full-length and minidystrophin in transgenic mdx mice: implications for gene therapy of Duchenne muscular dystrophy. Hum. Mol. Genet. 1995;4:1245–1250. doi: 10.1093/hmg/4.8.1245. [DOI] [PubMed] [Google Scholar]
- 189.Godfrey C., Muses S., McClorey G., Wells K.E., Coursindel T., Terry R.L., Betts C., Hammond S., O’Donovan L., Hildyard J. How much dystrophin is enough: the physiological consequences of different levels of dystrophin in the mdx mouse. Hum. Mol. Genet. 2015;24:4225–4237. doi: 10.1093/hmg/ddv155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Sharp P.S., Bye-a-Jee H., Wells D.J. Physiological characterization of muscle strength with variable levels of dystrophin restoration in mdx mice following local antisense therapy. Mol. Ther. 2011;19:165–171. doi: 10.1038/mt.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Neri M., Torelli S., Brown S., Ugo I., Sabatelli P., Merlini L., Spitali P., Rimessi P., Gualandi F., Sewry C. Dystrophin levels as low as 30% are sufficient to avoid muscular dystrophy in the human. Neuromuscul. Disord. 2007;17:913–918. doi: 10.1016/j.nmd.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 192.Chamberlain J.S. Dystrophin levels required for correction of Duchenne muscular dystrophy. Basic Appl. Myol. 1997;7:251–255. [Google Scholar]
- 193.Hoffman E.P., Arahata K., Minetti C., Bonilla E., Rowland L.P. Dystrophinopathy in isolated cases of myopathy in females. Neurology. 1992;42:967–975. doi: 10.1212/wnl.42.5.967. [DOI] [PubMed] [Google Scholar]
- 194.Arpke R.W., Darabi R., Mader T.L., Zhang Y., Toyama A., Lonetree C.L., Nash N., Lowe D.A., Perlingeiro R.C., Kyba M. A new immuno-, dystrophin-deficient model, the NSG-mdx(4Cv) mouse, provides evidence for functional improvement following allogeneic satellite cell transplantation. Stem Cells. 2013;31:1611–1620. doi: 10.1002/stem.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yue Y., Skimming J.W., Liu M., Strawn T., Duan D. Full-length dystrophin expression in half of the heart cells ameliorates beta-isoproterenol-induced cardiomyopathy in mdx mice. Hum. Mol. Genet. 2004;13:1669–1675. doi: 10.1093/hmg/ddh174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Bostick B., Yue Y., Long C., Duan D. Prevention of dystrophin-deficient cardiomyopathy in twenty-one-month-old carrier mice by mosaic dystrophin expression or complementary dystrophin/utrophin expression. Circ. Res. 2008;102:121–130. doi: 10.1161/CIRCRESAHA.107.162982. [DOI] [PubMed] [Google Scholar]
- 197.Wasala N.B., Lai Y., Shin J.-H., Zhao J., Yue Y., Duan D. Genomic removal of a therapeutic mini-dystrophin gene from adult mice elicits a Duchenne muscular dystrophy-like phenotype. Hum. Mol. Genet. 2016;25:2633–2644. doi: 10.1093/hmg/ddw123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mount J.D., Herzog R.W., Tillson D.M., Goodman S.A., Robinson N., McCleland M.L., Bellinger D., Nichols T.C., Arruda V.R., Lothrop C.D., Jr., High K.A. Sustained phenotypic correction of hemophilia B dogs with a factor IX null mutation by liver-directed gene therapy. Blood. 2002;99:2670–2676. doi: 10.1182/blood.v99.8.2670. [DOI] [PubMed] [Google Scholar]
- 199.Wang L., Nichols T.C., Read M.S., Bellinger D.A., Verma I.M. Sustained expression of therapeutic level of factor IX in hemophilia B dogs by AAV-mediated gene therapy in liver. Mol. Ther. 2000;1:154–158. doi: 10.1006/mthe.2000.0031. [DOI] [PubMed] [Google Scholar]
- 200.Snyder R.O., Miao C., Meuse L., Tubb J., Donahue B.A., Lin H.F., Stafford D.W., Patel S., Thompson A.R., Nichols T. Correction of hemophilia B in canine and murine models using recombinant adeno-associated viral vectors. Nat. Med. 1999;5:64–70. doi: 10.1038/4751. [DOI] [PubMed] [Google Scholar]
- 201.Le Hir M., Goyenvalle A., Peccate C., Précigout G., Davies K.E., Voit T., Garcia L., Lorain S. AAV genome loss from dystrophic mouse muscles during AAV-U7 snRNA-mediated exon-skipping therapy. Mol. Ther. 2013;21:1551–1558. doi: 10.1038/mt.2013.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Peccate C., Mollard A., Le Hir M., Julien L., McClorey G., Jarmin S., Le Heron A., Dickson G., Benkhelifa-Ziyyat S., Piétri-Rouxel F. Antisense pre-treatment increases gene therapy efficacy in dystrophic muscles. Hum. Mol. Genet. 2016;25:3555–3563. doi: 10.1093/hmg/ddw201. [DOI] [PubMed] [Google Scholar]
- 203.Dupont J.B., Tournaire B., Georger C., Marolleau B., Jeanson-Leh L., Ledevin M., Lindenbaum P., Lecomte E., Cogné B., Dubreil L. Short-lived recombinant adeno-associated virus transgene expression in dystrophic muscle is associated with oxidative damage to transgene mRNA. Mol. Ther. Methods Clin. Dev. 2015;2:15010. doi: 10.1038/mtm.2015.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ferrand M., Galy A., Boisgerault F. A dystrophic muscle broadens the contribution and activation of immune cells reacting to rAAV gene transfer. Gene Ther. 2014;21:828–839. doi: 10.1038/gt.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Cordier L., Gao G.P., Hack A.A., McNally E.M., Wilson J.M., Chirmule N., Sweeney H.L. Muscle-specific promoters may be necessary for adeno-associated virus-mediated gene transfer in the treatment of muscular dystrophies. Hum. Gene Ther. 2001;12:205–215. doi: 10.1089/104303401750061267. [DOI] [PubMed] [Google Scholar]
- 206.Velazquez V.M., Meadows A.S., Pineda R.J., Camboni M., McCarty D.M., Fu H. Effective depletion of pre-existing anti-AAV antibodies requires broad immune targeting. Mol. Ther. Methods Clin. Dev. 2017;4:159–168. doi: 10.1016/j.omtm.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chicoine L.G., Montgomery C.L., Bremer W.G., Shontz K.M., Griffin D.A., Heller K.N., Lewis S., Malik V., Grose W.E., Shilling C.J. Plasmapheresis eliminates the negative impact of AAV antibodies on microdystrophin gene expression following vascular delivery. Mol. Ther. 2014;22:338–347. doi: 10.1038/mt.2013.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Corti M., Liberati C., Smith B.K., Lawson L.A., Tuna I.S., Conlon T.J., Coleman K.E., Islam S., Herzog R.W., Fuller D.D. Safety of Intradiaphragmatic Delivery of Adeno-Associated Virus-Mediated Alpha-Glucosidase (rAAV1-CMV-hGAA) Gene Therapy in Children Affected by Pompe Disease. Hum. Gene Ther. Clin. Dev. 2017;28:208–218. doi: 10.1089/humc.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Corti M., Elder M., Falk D., Lawson L., Smith B., Nayak S., Conlon T., Clément N., Erger K., Lavassani E. B-Cell Depletion is Protective Against Anti-AAV Capsid Immune Response: A Human Subject Case Study. Mol. Ther. Methods Clin. Dev. 2014;1:14033. doi: 10.1038/mtm.2014.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Maguire A.M., Simonelli F., Pierce E.A., Pugh E.N., Jr., Mingozzi F., Bennicelli J., Banfi S., Marshall K.A., Testa F., Surace E.M. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N. Engl. J. Med. 2008;358:2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]