Abstract
Introduction:
Sarcoidosis is a chronic granulomatous inflammatory disease that commonly causes lung disease, but can affect other vital organs and tissues. The cause of sarcoidosis is unknown, and current therapies are commonly limited by lack of efficacy, adverse side effects, and excessive cost.
Areas covered:
The manuscript will provide a review of current concepts relating to the pathogenesis of sarcoidosis, and how these disease mechanisms may be leveraged to develop more effective treatments for sarcoidosis. It provides only a brief summary of currently accepted therapy, while focusing more extensively on potential novel therapies.
Expert Opinion:
Current sarcoidosis therapeutic agents primarily target the M1 or pro-inflammatory pathways. Agents that prevent M2 polarization, a regulatory phenotype favoring fibrosis, are attractive treatment alternatives that could potentially prevent fibrosis and associated life threatening complications. Effective treatment of sarcoidosis potentially requires simultaneous modulation both M1/M2 polarization instead of suppressing one pathway over the other to restore immune competent and inactive (M0) macrophages.
Keywords: Sarcoidosis, therapeutic, immunotherapy, macrophage, inflammation, fibrosis, treatment, mechanism
1. Introduction
Sarcoidosis is a rare multisystem granuloma forming disease of unclear etiology. It is likely due to polygenic mechanisms resulting in alteration of the host’s immune response to common environmental exposures. Worldwide prevalence ranges from 1 to 40 per 100,000 with adult onset typically before the 4th decade, and disproportionately afflicts African American and Northern Europeans1. Sarcoidosis can affect almost any organ or tissue, though most commonly affects the lungs, heart, eyes, lymph nodes, and skin. Non-necrotizing granuloma on biopsy supports the diagnosis in the setting of compatible clinical and radiological features2. Clinical presentation varies greatly from self-limited or asymptomatic disease to severe phenotypes experience chronic fibrosing disease of one or more vital organs. The highly variable clinical features and diseases course, together with a lack of understanding of disease mechanisms, explains why sarcoidosis treatment is not well standardized. Furthermore, it is clear from recent studies that current treatment options often fail in terms of quality of life indicators such that the disease burden remains unacceptably high3,4. The objective of this article is to briefly review some of the current sarcoidosis treatments, and further emphasizing how disease mechanisms can be leveraged to guide the development of novel therapies.
2. Disease Mechanisms and Goals of Therapy
The immunopathogenesis of sarcoidosis is complex and remains enigmatic. It is postulated that individuals with certain genetic susceptibility develop uncontrolled cell-mediated immune responses when exposed to as yet unidentified antigens. Granuloma formation is presumably initiated by environmental antigens presented by mononuclear cells and is subsequently propagated through recruitment of circulating immune cells to the site of inflammation5. In sarcoidosis, undifferentiated macrophages (M0 phenotype) are classically activated (M1 phenotype) via Toll-like receptors located on the cell surface or in the endosome compartment, a transition that requires interferon-gamma (INF-γ). M1 macrophages, in turn, present antigens to T cells via major histocompatibility complex (MHC) molecules and promote further granuloma formation by producing tumor necrosis factor alpha (TNF-α) and IL-12, as well as IL-1, IL-16, and IL-23. TNF-α is critical for granuloma formation through the recruitment of naïve T cells and by promoting pro-inflammatory T helper cell (Th1) polarization. Th1 cells undergo oligoclonal expansion and produce INF-γ to further promote M1 activation. Thus, initial phases of granuloma formation are supported by Th1/M1 immune polarization, which is a state of exaggerated TNF-α and INF-γ6–8. There is also evidence supporting the role of Th17 cells to support granulomatous inflammation and subsequent fibrosis by secreting IL-17, and a subset of Th17 cells (Th17.1) contribute by producing significant amounts of INF-γ9,10.
Regulatory T cells (Tregs) are of fundamental importance in sarcoidosis. Self-limited sarcoidosis is characterized by high tissue levels of Tregs, which suppress T cell proliferation and activation by producing anti-inflammatory mediators (IL-10, TGF-β) .. In contrast, patients with chronically active sarcoidosis have fewer Tregs in diseased tissue and paradoxically have higher numbers of peripheral Tregs11. Furthermore, the Tregs of chronically active sarcoidosis patients are functionally less active in terms suppressing pro-inflammatory functions12.
Mechanisms contributing to a transition from acute inflammation (granulomas) to fibrosis are of particular interest, as tissue fibrosis represents an untreatable manifestation with attendant loss of organ function. As opposed to typical TNF-α and INF-γ mediated M1/Th1 polarization during the acute phase of sarcoidosis, the chronic, fibrotic disease state is associated with polarization towards alternatively activated, immune-suppressive M2 macrophages and CD4 T cells of the Th2 phenotype. The M2/Th2 cytokine profile is proximally regulated by IL-4 and IL-13, which induces STAT6 activation to promote the production of IL-10 in favor of INF-γ. Lower levels of INF-γ promote fibroblast matrix production leading to tissue remodeling and fibrosis13,14. In milder cases of sarcoidosis the initial pro-inflammatory M1/Th1 polarization phase presumably does not transition to a sustained M2/Th2 phase. Instead, macrophages and T cell populations are restored to an undifferentiated state through the elimination of differentiated/activated macrophages by programmed cell death (apoptosis)15.
The aforementioned granulomatous response is somehow abnormal in patients with sarcoidosis, persisting in the absence of an identifiable pathogen or foreign body. Chen et al suggest that sarcoidosis is a manifestation of microbial antigens, possibly mycobacterial antigens. Alternatively, an inducible acute-phase reactant and amyloid precursor known serum amyloid A (SAA), was shown to accumulate adjacent to sarcoidosis granulomas and is sensed by TLR2 to induce the release of Th1 cytokines, such as IFN-γ and TNF. IFN-γ, in turn, inhibits SAA clearance. Chen and colleagues hypothesize that SAAcould thereby sustain inflammation in the context of chronic and progressive forms of sarcoidosis16. Notably, TLR2 is capable of promoting both M1 and M2 immune responses, depending the conditions17.
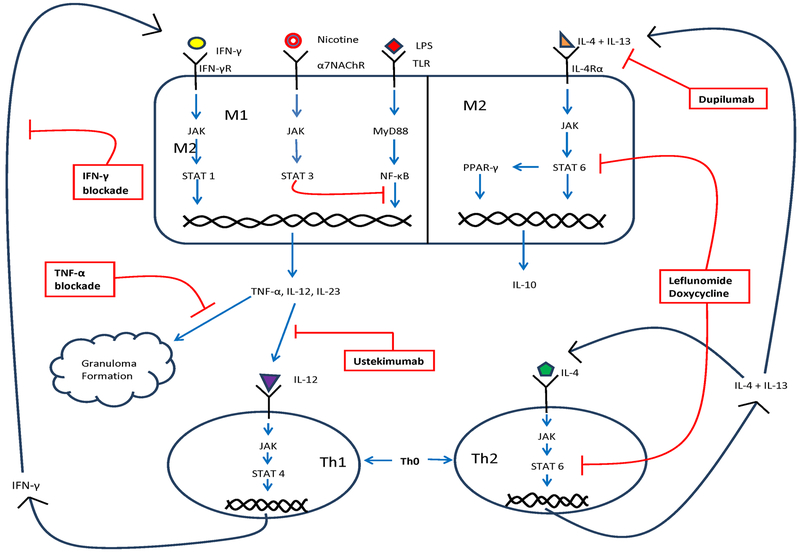
Based upon current knowledge, it follows that the goal of immune therapies for sarcoidosis are to achieve a balance between M1/Th1 (inflammation) and M2/Th2 (favoring fibrosis) while maintaining the vigilance of the immune system for the detection of potential pathogens to avoid infections. Emerging concepts in the field of sarcoidosis includes immune modulating therapies to potentially restore M1/Th1 and M2/Th2 balance, whereas antimicrobial agents are proposed by some experts to promote the clearance of chronic infections that are not readily identified by existing laboratory techniques15,18. Figure 1 provides a schematic of putative disease mechanisms, further discussed below.
Figure 1.
Schematic depicting mechanisms of sarcoidosis granuloma formation and potential therapeutic targets.
3. Current Therapies
Sarcoidosis can affect any organ system, with pulmonary involvement in >90% of cases. While as many as 60% of patients experience spontaneous disease resolution; 10 to 30% develop chronic or progressive disease requiring systemic treatment 19. A subset of the latter will not tolerate the available therapies or will progress despite usual therapies to chronic disability with irreversible tissue fibrosis20. We will begin with a discussion of commonly used immune modulating therapies for which the mechanism of action is understood.
3A. Corticosteroids
Corticosteroids (CS) are considered the first line therapeutic approach due to potent and rapid anti-inflammatory actions. CS have diverse mechanisms of action, including the inhibition of TNF-α, INF-γ, and related (e.g., NF-κB) signaling pathways21. However, the optimal treatment dose and duration of therapy are not standardized due to lack of adequately powered randomized-controlled clinical trials. When used in the short term, CS can dramatically improve disease related symptoms, normalize molecular biomarkers, restore lung function, and x-ray findings22,23. However, their long-term effects/benefits are unclear in that they often fail to slow disease progression, prevent fibrosis, and there is no proof that they improve survival. CS have numerous side effects and these contribute to a decreased perceived quality of life in CS treated patients compared to those with similar disease manifestation who are not treated15. Despite the unfavorable side-effect profile of CS, they are the mostly widely used first line therapy, especially in acute life threatening or symptomatic disease.
3B. Cytolytic Agents: Nucleotide Analogues and Precursor Analogues
Methotrexate (MTX) is commonly used as a second line therapy for sarcoidosis. MTX targets dihydrofolate reductase, a critical enzyme for folic acid metabolism required for purine and thymidine (DNA) synthesis. However, DNA integrity is not influenced significantly at within the lower dose range (10–20 mg/week) used for the treatment of sarcoidosis18. Within this dose range MTX induces the extracellular release of adenosine, a potent regulator of M1 and M2 macrophages, favoring polarization towards the latter. As proof of this concept, in vivo and animal studies of chronic inflammation showed MTX was ineffective in the presence of adenosine antagonists, adenosine deaminase, adenosine receptor antagonists, or the deletion of adenosine receptors24. MTX suppresses TNF-α production via adenosine A2A receptors while inducing IL-4 and IL-13, upstream regulators of M2 polarization. MTX can also polarize M0 to M2 via IL-4 receptor independent pathways20. Thus, the desirable anti-inflammatory actions of MTX are offset by an increased risk of fibrosis21. Other undesirable side effects of MTX, such as hair loss, leukopenia, anemia, relate to the anti-metabolic actions, which are mitigated by folic acid supplementation.
MTX is used clinically either as a “CS-sparing” agent or as the sole agent for patients requiring chronic immune suppression for sarcoidosis22,23. Evidence of clinical efficacy for MTX is extrapolated from a handful of trials, mostly retrospective and conducted in the setting of concomitant CS use, and demonstrating synergy with concomitant use ofMTX and CS. MTX is often used in conjunction with other therapies (e.g., anti-TNF-α) to optimize disease suppression, while exploiting its favorable side effect profile.
Azathioprine (AZA) is a purine analog that can block DNA and RNA synthesis thereby suppressing T- and B-cell proliferation. AZA has been shown to inhibit T-cell/APC (antigen presenting cell) engagement and related T-cell activation and IFN-γ production25,26, such as occurs during early granuloma formation.. Whereas randomized controlled trials assessing AZA efficacy in sarcoidosis are lacking, an open-label clinical trial of 11 patients with steroid-dependent chronic sarcoidosis demonstrated synergy of AZA with CS in terms of less severe symptoms and improved physiological, serological, and radiographic parameters27. Likewise, a retrospective analysis compared 145 patients compared MTX with AZA showing similar benefits, as reflected by CS-sparing effects and improvement of FEV1 and DLCO. However, AZA treatment was more often complicated by infections28.
3C. TNF-alpha inhibitors
For those who fail to respond to or are unable to tolerate corticosteroids, MTX or AZA, anti- TNF-α agents can be an effective alternative. As shown in Figure 1. TNF-α is crucial for the formation and maintenance of granulomas 29. Many of the therapies commonly used for sarcoidosis influence TNF-α production or function.
3C.1. Inhibitors of TNF-α production
Pentoxifylline (POF) is a methylxanthine derivative and a non-selective phosphodiesterase inhibitor. POF modulates inflammation by suppressing cytokine production in macrophages. In vitro studies indicate that POF was comparable to CS as an inhibitor of spontaneous and LPS-induced production of TNF-α, IL-6, −8, and −10 by alveolar macrophages. 30–32. Efficacy of POF was demonstrated in a randomized, double-blind, and placebo-controlled trial of 27 sarcoidosis subjects treated with POF in which fewer sarcoidosis flares and lower corticosteroid dependency were reported33. Another clinical trial enrolled 23 treatment-naive pulmonary sarcoidosis patients who were treated for 6 months with POF, of which eleven had improved DLCO and PaO2 after 6 months to follow up, and seven had stable disease34. This was an observational trial lacking a control group, and many patients were excluded at the time of screening. The inconvenient dosing of the POF (thrice daily) and frequent gastrointestinal side effects limit the routine use of this drug for the treatment of sarcoidosis.
Thalidomide is a suppressor of TNF-α production that has been successful in treating granulomatous diseases, such as leprosy and tuberculosis. Thalidomide accelerates TNF-α mRNA degradation, and has been shown to reduce TNF-α production by alveolar macrophages35,36. However, clinical data on thalidomide for sarcoidosis are not promising. A randomized, double-blind, placebo controlled trial evaluating efficacy for the treatment of cutaneous sarcoidosis reported frequent adverse side effects and lack of efficacy37. Likewise, a prospective open-label of 10 patients with corticosteroid-dependent pulmonary sarcoidosis showed no improvement of spirometry, quality of life, or dyspnea after 24 weeks of thalidomide. Moreover, 90% of patients experienced intolerable side effects38. Another small observational study (19 patients) treated for 24 months with low-dose thalidomide showed improved skin, x-ray, and pulmonary function (lung diffusing capacity); however these benefits were offset by the high frequency of adverse events39. Thalidomide is also prohibitively expensive, and other anti- TNF-α treatments are better tolerated, which explains why thalidomide is rarely used for the treatment of sarcoidosis.
Apremilast is a phosphodiesterase-4 inhibitor that is used for psoriasis that potently suppresses TNF-α production40. There is very limited data supporting the use of Apremilast for the treatment of sarcoidosis. Encouraging results were reported following the treatment of 18 patients with cutaneous sarcoidosis in whom an objective improvement of skin manifestations was documented41, but the effect of treatment in other organ systems has not been established. The high cost of Apremilast currently limits the routine use of this drug for sarcoidosis.
3C.2. Biological TNF-alpha blocking agents
Biological agents show great promise for sarcoidosis treatment given their specificity. Infliximab is a murine chimeric monoclonal antibody against the soluble component of TNF-α to prevent its interaction with TNF-α receptors. Infliximab is administered IV over 2–4 hours, requires several loading doses, followed by regular maintenance dosing at 4–8 week intervals. Infliximab is the most studied biological agent for sarcoidosis treatment, mostly focused on patients who are refractory or intolerant of conventional treatments for sarcoidosis42–46. However, infusion reactions or neutralizing antibody formation are common, necessitating the concomitant use of other immune suppressants.
Other anti-TNF-α agents are less well studied. Adalimumab is a human anti-TNF-α monoclonal antibodyshown to be effective in refractory cases of sarcoidosis47,48. In a retrospective study of 18 patients unable to tolerate Infliximab who were subsequently treated with Adalimumab, there was a reported clinical improvement in 39%, stabilization in 33%, and 28% had worsened disease49. Etanercept, a dimeric fusion protein designed to bind the human TNF receptor extracellular ligand-binding domain, is not shown to be effective for sarcoidosis For instance, of 17 patients with progressive pulmonary sarcoidosis treated with Etanercept, a majority had treatment failure. Others with chronic ocular sarcoidosis (n=9) had no significant improvement with Etanercept when compared to placebo (n=9)50,51. Moreover, a number of case reports indicate that Etanercept paradoxically triggers a sarcoidosis-like illness52. Golimumab, another human monoclonal anti-TNF-α antibody, was carefully studied in a multicenter, randomized, three-arm, double-blind trial compared to placebo and Ustekimumab (a dual IL-12 and IL-23 inhibitor) for the treatment of pulmonary and/or cutaneous sarcoidosis. Unfortunately, neither agent showed significant benefit compared to placebo53.
At present, infliximab and adalimumab are most commonly used for the treatment of refractory forms of sarcoidosis. The high cost of these medications, and the increased risks of infections, and to a lesser degree malignancy and neurological complications, are barriers to their routine use.
4. Novel Therapies
4A. Interferon-gamma pathway inhibitors
The active inflammatory form of sarcoidosis is characterized by an exaggerated production of TNF-α and INF-γ. INF-γ is produced by pro-inflammatory T-cells (Th1), and is maintained through continuous positive feedbacks loop by M1 macrophages leading to sustained M1/Th1 polarization. As such, INF-γ is a potential therapeutic target in sarcoidosis. By partially blocking INF-γ, there is the potential to depolarize M1 back to an inactive (M0) state. In this state, inflammation could be controlled while the innate immune system retains the capacity to resist infections and malignancies. Though never tested in humans, quinolone derivative TAK-603 was shown to block INF-γ production by BAL cells of sarcoidosis patients, and was touted as a possible treatment for refractory sarcoidosis54. Fontolizumab, a humanized monoclonal antibody against INF-γ, has been tested in clinical trials to treat moderate to severe Crohn’s disease. Crohn’s disease is similar to sarcoidosis in that the disease is presumably provoked by environmental antigenic triggers in genetic predisposed individuals forming non-caseating granulomas, a M1/Th1 INF-γ driven process55,56. However, the results of a single randomized, double-blind, placebo-controlled, and multiple-dose trial of 201 patients with moderate to severe Crohn’s Disease receiving anti-INF-γ treatment, was disappointing57,58. Whereas Fontolizumab was safe and well-tolerated, efficacy was lacking. The potential role of this drug as a treatment for sarcoidosis is unclear.
4B. Targeting M2 Polarization
Currently therapeutics primarily target pro-inflammatory pathways59. However, M2 polarization in sarcoidosis promotes fibrosis and associated irreversible tissue damage culminating in the most severe disease manifestations. The same is true of asthma and COPD, wherein treatment-refractory cases are associated with airway remodeling and lung fibrosis60–62. Thus, there is increasing interest in therapeutics designed to prevent M2 polarization in chronic inflammatory diseases, including sarcoidosis. As per Figure 1, M2 polarization is proximally regulated by IL-4 and IL-13 to promote the expression and phosphorylation of STAT-6, a transcription factor that promotes a signaling cascade that includes the PPAR-γ pathway63.
Leflunomide, a promising treatment for sarcoidosis, is a tyrosine kinase inhibitor preventing STAT-6 phosphorylation. Leflunomide also regulates inflammation by suppressing Th17 cells and promoting the function of regulatory T cells (Tregs) 62,64,65. The largest trial of Leflunomide for the treatment of sarcoidosis was a retrospective analysis of 76 patients with pulmonary and extrapulmonary disease. Leflunomide treatment resulted in significant improvement in lung function, as reflected by forced vital capacity (FVC), and was CS sparing66. Additional, prospective studies are needed to validate this encouraging report.
Doxycycline and related drugs (minocycline, tetracycline) inhibit M2 polarization in vitro67, and are used clinically for sarcoidosis. The mechanism of action of this drug class is unclear, but may relate to dose-dependent inhibition of STAT6. In a small related study of twelve patients with cutaneous sarcoidosis treated with minocycline, eight had a complete clinical response and two had a partial response. Three patients experienced a relapse, but remission was subsequently achieved when treated with doxycycline68.
Dupilumab is a new biological agent that modulates M2/Th2 polarization. It is a fully human monoclonal antibody against interleukin-4 receptor alpha. As such, Dupilumab can inhibit both IL-4 and IL-13 signaling, and has been used in moderate to severe asthma and atopic dermatitis with encouraging results69,70. It is reasonable to postulate that Dupilumab could be useful for the prevention of granulomatous inflammation in the context of active sarcoidosis.
4C. Antibacterial Therapy
Chronic stimulation of APCs and T-cells with immunogenic molecules can promote granuloma formation, and this is postulated to be the case in sarcoidosis. The source of disease-causing antigens is unclear; however, microorganisms such as Mycobacterium and Priopionibacterium are of primary interest71. Although viable organisms are not present in sarcoidosis (by definition), molecular testing confirms the presence microorganism DNA at a higher rate in patients with sarcoidosis. Furthermore, Mycobacterium tuberculosis catalase-peroxidase protein is commonly detected in sarcoidosis patients72,73. Could this finding represent insidious infection? To address this question a randomized, placebo controlled trial of 30 patients with cutaneous sarcoidosis investigated the effects concomitant levofloxacin, ethambutol, azithromycin, and rifampin (CLEAR protocol) treatment for 8 weeks, showing significant improvement in the size of cutaneous sarcoidosis lesions74. The CLEAR protocol also showed benefit in a small cohort (n= 15) with chronic pulmonary sarcoidosis, based on significant improvement of FVC, and quality-of-life metrics75. An ongoing NIH-supported multicenter, randomized, double-blind, place-controlled trial is underway to investigate CLEAR for progressive pulmonary sarcoidosis. It is important to note that the antimicrobial components of CLEAR may, like doxycycline, have intrinsic anti-inflammatory effects, such that demonstration of efficacy does not prove a causal link between active infection and sarcoidosis.
4D. Nicotine Therapy
Epidemiological studies suggest that chronic smoking is a protective factor for granulomatous diseases, particularly sarcoidosis76–78. In this regard, nicotine engagement with alpha-7 nicotinic cholinergic receptor (α7nAChR) on macrophages interferes with NF-κB activation and translocation to the nucleus mediated by the JAK-2/STAT3 pathway79. As shown in Figure 1, NF-κB is needed for M1 polarization and pro-inflammatory cytokine production. Nicotine’s effects on CD4 cells includes a reduction of Th17/Treg ratio80. Testing the hypothesis that nicotine may be of benefit in sarcoidosis, a small (n=13) randomized, controlled clinical trial evaluated the effect of nicotine treatment for 12 weeks in conjunction with conventional therapy on surrogate inflammatory endpoints. Compared with conventional therapy alone, transdermal nicotine normalized Toll-like receptors (TLR)-2 and TLR-9 responsiveness and increased the prevalence of specific “pre-activated” Tregs in sarcoidosis patients. Furthermore, no significant tachyphylaxis was observed in this trial as reflected by nicotine receptor expression on circulating immune cells81. Nicotine is an appealing therapeutic option because it is readily available (FDA approved for other indications) and could be readily repurposed for the treatment of sarcoidosis.
Nicotine has many side effects, some of which may be beneficial for patients with active sarcoidosis. Besides symptoms arising from directly involved organs, patients with sarcoidosis have high prevalence of non-specific quality-of-life-altering symptoms such fatigue, cognitive slowing, depression, and anxiety3,4. Nicotine is shown to improve mood, alleviate depression, and increased self-rated vigor in non-sarcoidosis patients82–85. There is an ongoing NIH/NHLBI-support clinical trial to further investigate nicotine’s effects on sarcoidosis treatment which is designed to further evaluate the safety of nicotine and to initially evaluate surrogate biomarkers reflecting pulmonary disease resolution (NCT02265874).
4E. Immunotherapy to Restore Normal Granuloma Function
While most experts believe sarcoidosis to be an exaggerated immune response to common environmental exposures, some posit that sarcoidosis to be an impaired immune response (immune exhaustion) that fails to effectively clear immunogenic antigens. Immune checkpoint inhibitors are a class of molecules that suppress immune responses to antigenic stimulation. Programmed cell death protein 1 (PD-1) and its receptor/ligand (PD-L1) are potent immune checkpoint inhibitors and are molecule of interest for the reversal of immune exhaustion by restoring T cell cytokine responses and proliferation capacity. It has been shown that T cells from patients with active progressive sarcoidosis T cells exhibit increased PD-1 expression and reduced proliferation capacity when compared to self-limited disease phenotypes, and PD-1 blocking antibodies restore T cell proliferative capacity and function86,87. Likewise, cytotoxic T lymphocyte antigen 4 (CTLA-4) is a molecule expressed on activated T cells which inhibits T-cell activation and proliferation by blocking B7 with CD28 co-stimulation. CTLA-4 blockage increases Th17 while impairing Tregs functions, which has important implications for sarcoidosis pathogenesis88,89. CTLA-4 expression therefore might be a potential therapy to promote more effective antigen clearance.
While anti-PD-1 and anti-CTLA-4 therapy do enjoy success in the realm of cancer, no data is yet available for sarcoidosis. However, there are case reports of patients experiencing a flare of preexisting sarcoidosis, and new onset of sarcoid-like reactions while on anti-PD-1 or anti-CTLA-4 therapy for different diseases process90–92. Such reports highlight uncertainties relating to the pathogenesis of sarcoidosis, and further suggests that optimal treatment of sarcoidosis may involve achieving a balance between the extremes of pro-inflammatory (granuloma promoting) and regulatory (pro-fibrotic) pathways so as to restore normal tissue and immune cell function.
4F. B-Cell Therapy
The role of B cells in the pathogenesis of sarcoidosis is unclear, but emerging evidence suggests that they do play a supporting role. Sarcoidosis patients often have evidence B cell hyperactivity, such as hypergammaglobulinemia, autoantibody production, and circulating immune complexes, such as is typically seen in patients with autoimmune disease93. While B cells are normally not detected within granulomas, they are more abundant in surrounding tissues. In the context of severe chronic sarcoidosis these B cells, like T cells, are functionally altered, exhibiting blunted responses to antigenic stimulation, as reflected by reduced expression of activation markers, decreased proliferation, and impaired cell differentiation94. During the early stages of granuloma development it is proposed that M1/Th1 IFN-γ production increases B-cell-activating factor (BAFF), which plays an important role in B-cell maturation and function leading to an exaggerated autoimmune reaction. It has been demonstrated BAFF is elevated in sarcoidosis and may serve as a biomarker of the disease. In vitro studies show that IFN-γ increased BAFF production by monocytes, and was associated with enhanced IL-10 and immunoglobulin production. These B cell populations regressed with effective anti-TNFα treatment, suggesting a supportive role in disease pathogenesis93,95,96.
Rituximab, a humanized anti-CD20 monoclonal antibody used to deplete B cells, has been investigated in sarcoidosis patients failing to respond to other therapies. In contrast to anecdotal success reported in a few case reports, a prospective, open-label trial involving 10 patients with treatment refractory pulmonary sarcoidosis showed no clear clinical benefit, and perhaps increased harm (one death)97. While this particular disease phenotype may not benefit, other disease phenotypes, such as those with high immunoglobulin titers or with autoimmune features, may respond better. Further investigation is needed to determine if a more personalized approach to treating sarcoidosis will improved clinical outcomes.
5. Conclusions
Sarcoidosis is a rare multisystem granulomatous disease of unclear etiology. The clinical presentation varies greatly from one individual to the next, ranging from asymptomatic or self-limited disease compared with others experiencing chronic progression, tissue fibrosis and life altering disease manifestations. Current mainstays of treatment include corticosteroids, cytolytic agents, and TNF-α targeted therapies. At present, treatment decisions are not tailored to specific disease phenotypes or related disease mechanisms. In this regard, it is apparent that some individuals exhibit active inflammation, conforming more to a Th1/M1 phenotype, whereas others present with progressive fibrosis and loss of organ function, presumably reflecting M2 polarization. We speculate that a “one size fits all” approach to treating sarcoidosis is not ideal, and that some patients may benefit from anti-inflammatory treatments targeting Th1/M1 immune responses, whereas others may benefit from treatments that block regulatory/pro-fibrotic Th2/M2 polarization. Ideally, a balance would be struck to restore the immune system to a “resting state” wherein immune surveillance is intact and granulomatous inflammation is suppressed.
6. Expert Opinion
Sarcoidosis was first described in 1869; however, fundamental concepts relating to its pathogenesis remain unclear and optimal treatment protocols are not well standardized due to the lack of well-designed clinical trials. Sarcoidosis is traditionally thought to be M1/Th1 driven process, and current mainstays of treatment for sarcoidosis primarily target the M1 inflammatory pathways. More recently, M2/Th2 polarization is emerging as a concern as relates to disease resolution in the form of chronic fibrosis with life threatening complications. We posit that the ultimate goal of sarcoidosis treatment is to restore a healthy balance of inflammatory and regulatory pathways, with the goal of “resetting” the immune system such that immune competence remains intact to reduce the risks of infection and malignancy. Treatment of sarcoidosis therefore could potentially benefit from simultaneous modulation and fine tuning of M1/Th1 and M2/Th2 pathways rather than targeting one pathway or the other.
Large knowledge gaps currently exist in the field of sarcoidosis due, in part, to the lack of appropriate models to study disease mechanisms and treatment outcomes. In this regard, and as an example of how models could be of benefit, a mathematical model of the granulomatous immune response during sarcoidosis has been recently developed and was validated compared to human disease98. Models such as these could be used to accelerate our understanding of disease mechanisms, to predict clinical responses, and to guide the design of clinical trials involving one or more immunotherapeutic agents while keeping costs down. However, these models do have limitations in that they are built upon existing concepts of disease pathogenesis, for which many unanswered questions remain.
One of the great unknowns of sarcoidosis relates to the early stages of granuloma formation. Experiments performed on isolated cell lines or in human tissues do not represent the complex interactions of diverse immune cells during granuloma-neogenesis. To address this research limitation, we have developed a novel in vitro human granuloma model that is shown to mimic many of the molecular features of human tissue granulomas99. This model will be useful for refining our understanding of mechanisms regulating early granuloma formation, and the model provides a high throughput platform for pre-clinical testing of novel therapies or for more precisely guiding the treatment of individuals with distinct disease phenotypes. Biomarkers associated with early granuloma formation and reflective of distinct disease mechanisms may ultimately serve to guide therapeutic decisions.
As is the case for other rare diseases, international collaboration between sarcoidosis centers will be important to make future advancements. We propose to establish standards of clinical and research practice among a network of “centers of excellence”. Such centers would provide a platform for more rapidly advancing scientific research, including the identification and testing of novel therapeutic targets in well-designed clinical trials. Such centers would reach out to the regional sarcoidosis communities to provide education, access to care, and to research trials. In so doing, future investigations will be inclusive of underserved populations that are often excluded from clinical trials, many of which (e.g., African American women) have the most severe sarcoidosis disease manifestations. In collaboration with the National Institutes of Health, National Heart Lung and Blood Institute, a Workshop on Sarcoidosis was convened in 2015 to “leverage scientific advancements to understand sarcoidosis variability and improve outcomes”100,101. Among the recommendations of the expert panel was to prioritize clinical trials for severe sarcoidosis phenotypes, and to consider novel trial strategies, such as “randomized withdrawal design”102 in order to address the limitation of conducting conventional randomized, controlled clinical trial in cohorts of limited sizes.
With the recent advent of more advanced research tools, and plans to create more standardized and sophisticated clinical research networks in the near future, the field of sarcoidosis is now poised to establish precision therapies for patients with diverse sarcoidosis disease manifestations.
Article Highlights.
Sarcoidosis etiology is unclear at this point, but likely due to polygenic mechanisms and maladapted host’s immune response to common environmental exposures resulting in highly variable clinical phenotypes.
Current therapy primarily targets M1/Th1 inflammatory pathway, but M2/Th2 inhibitory agents could potentially be beneficial in preventing fibrosis and lethal complications.
Sarcoidosis treatment might require restoration of a healthy balance of inflammatory and regulatory pathways by modulating both M1/M2 instead of targeting one over the other.
Lack of reliable research models for sarcoidosis hinders progress towards elucidating disease mechanisms and establishing novel therapies.
Collaboration among sarcoidosis centers with commitment to develop highly standardized and reproducible scientific techniques to accelerate scientific discovery and to promote effective clinical trials.
Acknowledgments
Funding
This paper has received funding in the form of the following grants NIH/NHLBI R34HL123586 (EDC); NIH/NHLBI T32–007946 (VL)
Footnotes
Declaration of Interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose
References
Papers of special note have been highlighted as:
* of interest
** of considerable interest
- 1.Cozier YC. Assessing the worldwide epidemiology of sarcoidosis: challenges and future directions. Eur Respir J 2016;48:1545–8. [DOI] [PubMed] [Google Scholar]
- 2.Judson MA. The clinical features of sarcoidosis: a comprehensive review. Clin Rev Allergy Immunol 2015;49:63–78. [DOI] [PubMed] [Google Scholar]
- 3.Drent M, Lower EE, De Vries J. Sarcoidosis-associated fatigue. Eur Respir J 2012;40:255–63. [DOI] [PubMed] [Google Scholar]
- 4.Goracci A, Fagiolini A, Martinucci M, et al. Quality of life, anxiety and depression in sarcoidosis. Gen Hosp Psychiatry 2008;30:441–5. [DOI] [PubMed] [Google Scholar]
- 5.Iannuzzi MC, Fontana JR. Sarcoidosis clinical presentation, immunopathogenesis, and therapeutics. JAMA 2011;305:391–9.* Provides a consise review of sarcoidosis clinical presentation, immunopathogenesis, and therapeutic agents.
- 6.Zissel G, Muller-Quernheim J. Cellular Players in the Immunopathogenesis of Sarcoidosis. Clin Chest Med 2015;36:549–60.** Provides an in-depth review of immune mechanisms of sarcoidosis pathogenesis; a good reference point for novice to understand sarcoidosis.
- 7.Timmermans WM, van Laar JA, van Hagen PM, van Zelm MC. Immunopathogenesis of granulomas in chronic autoinflammatory diseases. Clin Transl Immunology 2016;5:e118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noor A, Knox KS. Immunopathogenesis of sarcoidosis. Clin Dermatol 2007;25:250–8. [DOI] [PubMed] [Google Scholar]
- 9.Ramstein J, Broos CE, Simpson LJ, et al. IFN-gamma-Producing T-Helper 17.1 Cells Are Increased in Sarcoidosis and Are More Prevalent than T-Helper Type 1 Cells. Am J Respir Crit Care Med 2016;193:1281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortaz E, Rezayat F, Amani D, et al. The roles of T Helper 1, T Helper 17 and Regulatory T Cells in the pathogenesis of sarcoidosis. Iran J Allergy Asthma Immunol 2016;15:334–9. [PubMed] [Google Scholar]
- 11.Miyara M, Amoura Z, Parizot C, et al. The immune paradox of sarcoidosis and regulatory T cells. J Exp Med 2006;203:359–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taflin C, Miyara M, Nochy D, et al. FoxP3+ regulatory T cells suppress early stages of granuloma formation but have little impact on sarcoidosis lesions. Am J Pathol 2009;174:497–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moller M, Aries SP, Dromann D, Mascher B, Braun B, Dalhoff K. Intracellular cytokine repertoire in different T cell subsets from patients with sarcoidosis. Thorax 2001;56:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson KC, Hogarth K, Husain AN, Sperling AI, Niewold TB. The clinical and immunologic features of pulmonary fibrosis in sarcoidosis. Transl Res 2012;160:321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox CE, Donohue JF, Brown CD, Kataria YP, Judson MA. Health-related quality of life of persons with sarcoidosis. Chest 2004;125:997–1004. [DOI] [PubMed] [Google Scholar]
- 16.Chen ES, Song Z, Willett MH, et al. Serum amyloid A regulates granulomatous inflammation in sarcoidosis through Toll-like receptor-2. Am J Respir Crit Care Med 2010;181:360–73.**Compelling argument for Mycobacterial tuberculosis antigens as possible triggers for sarcoidosis propagated by immune-mediated release of endogenous serum amyloid A causing chronic sarcoidosis.
- 17.Herrtwich L, Nanda I, Evangelou K, et al. DNA Damage Signaling Instructs Polyploid Macrophage Fate in Granulomas. Cell 2016;167:1264–80 e18. [DOI] [PubMed] [Google Scholar]
- 18.Montesinos MC, Desai A, Delano D, et al. Adenosine A2A or A3 receptors are required for inhibition of inflammation by methotrexate and its analog MX-68. Arthritis Rheum 2003;48:240–7. [DOI] [PubMed] [Google Scholar]
- 19.Society AT. Statement on sarcoidosis. Am J Respir Crit Care Med 1999;160:736–55. [DOI] [PubMed] [Google Scholar]
- 20.Ferrante CJ, Pinhal-Enfield G, Elson G, et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation 2013;36:921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hasko G, Cronstein B. Regulation of inflammation by adenosine. Front Immunol 2013;4:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isshiki T, Yamaguchi T, Yamada Y, et al. Usefulness of Low-dose Methotrexate Monotherapy for Treating Sarcoidosis. Internal Medicine 2013;52:2727–32. [DOI] [PubMed] [Google Scholar]
- 23.Lower EE, Baughman RP. Prolonged use of methotrexate for sarcoidosis. Arch Intern Med 1995;155:846–51. [PubMed] [Google Scholar]
- 24.Chan ESL, Cronstein BN. Molecular action of methotrexate in inflammatory diseases. Arthritis Res 2002;4:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maltzman JS, Koretzky GA. Azathioprine: old drug, new actions. J Clin Invest 2003;111:1122–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Poppe D, Tiede I, Fritz G, et al. Azathioprine Suppresses Ezrin-Radixin-Moesin-Dependent T Cell-APC Conjugation through Inhibition of Vav Guanosine Exchange Activity on Rac Proteins. The Journal of Immunology 2005;176:640–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller-Quernheim J, Kienast K, Held M, Pfeifer S, Costabel U. Treatment of chronic sarcoidosis with an azathioprine/prednisolone regimen. Eur Respir J 1999;14:1117–22. [DOI] [PubMed] [Google Scholar]
- 28.Vorselaars ADM, Wuyts WA, Vorselaars VMM, et al. Methotrexate vs azathioprine in second-line therapy of sarcoidosis. Chest 2013;144:805–12. [DOI] [PubMed] [Google Scholar]
- 29.Antoniu SA. Targeting the TNF-alpha pathway in sarcoidosis. Expert Opin Ther Targets 2010;14:21–9. [DOI] [PubMed] [Google Scholar]
- 30.Kreth S, Ledderose C, Luchting B, Weis F, Thiel M. Immunomodulatory properties of pentoxifylline are mediated via adenosine-dependent pathways. Shock 2010;34:10–6. [DOI] [PubMed] [Google Scholar]
- 31.Marques LJ, Zheng L, Poulaski N, Guzman J, Costabel U. Pentoxifylline inhibits TNF-alpha production from human alveolar macrophages. Am J Respir Crit Care Med 1999;159:508–11. [DOI] [PubMed] [Google Scholar]
- 32.Tong Z, Dai H, Chen B, Abdoh Z, Guzman J, Costabel U. Inhibition of Cytokine Release From Alveolar Macrophages in Pulmonary Sarcoidosis by Pentoxifylline. Chest 2003;124:1526–32. [DOI] [PubMed] [Google Scholar]
- 33.Park MK, Fontana JR, Babaali H, et al. Steroid-sparing effects of pentoxifylline in pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Disease 2009;26:121–31. [PMC free article] [PubMed] [Google Scholar]
- 34.Zabel P, Entzian P, Dalhoff K, Schlaak M. Pentoxifylline in treatment of sarcoidosis. Am J Respir Crit Care Med 1997;155:1665–9. [DOI] [PubMed] [Google Scholar]
- 35.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med 1993;177:1675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tavares JL, Wangoo A, Dilworth P, Marshall B, Kotecha S, Shaw RJ. Thalidomide reduces tumour necrosis factor-alpha production by human alveolar macrophages. Respir Med 1997;91:31–9. [DOI] [PubMed] [Google Scholar]
- 37.Droitcourt C, Rybojad M, Porcher R, et al. A randomized, investigator-masked, double-blind, placebo-controlled trial on thalidomide in severe cutaneous sarcoidosis. Chest 2014;146:1046–54. [DOI] [PubMed] [Google Scholar]
- 38.Judson MA, Silvestri J, Hartung C, Byars T, Cox CE. The effect of thalidomide on corticosteroid-dependent pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Disease 2006;23:51–7. [DOI] [PubMed] [Google Scholar]
- 39.Fazzi P, Manni E, Cristofani R, et al. Thalidomide for improving cutaneous and pulmonary sarcoidosis in patients resistant or with contraindications to corticosteroids. Biomed Pharmacother 2012;66:300–7. [DOI] [PubMed] [Google Scholar]
- 40.Schafer P Apremilast mechanism of action and application to psoriasis and psoriatic arthritis. Biochem Pharmacol 2012;83:1583–90. [DOI] [PubMed] [Google Scholar]
- 41.Baughman RP, Judson MA, Ingledue R, Craft NL, Elyse EL. Efficacy and safety of apremilast in chronic cutaneous sarcoidosis. Arch Dermatology 2012;148:262–4. [DOI] [PubMed] [Google Scholar]
- 42.Baughman RP, Drent M, Kavuru M, et al. Infliximab therapy in patients with chronic sarcoidosis and pulmonary involvement. Am J Respir Crit Care Med 2006;174:795–802. [DOI] [PubMed] [Google Scholar]
- 43.Baughman RP, Judson MA, Lower EE, et al. Infliximab for chronic cutaneous sarcoidosis: a subset analysis from a double-blind randomized clinical trial. Sarcoidosis Vasc Diffuse Lung Disease 2016;32:289–95. [PubMed] [Google Scholar]
- 44.Baughman RP, Shipley R, Desai S, et al. Changes in chest roentgenogram of sarcoidosis patients during a clinical trial of infliximab therapy: comparison of different methods of evaluation. Chest 2009;136:526–35. [DOI] [PubMed] [Google Scholar]
- 45.Judson MA, Baughman RP, Costabel U, et al. Efficacy of infliximab in extrapulmonary sarcoidosis: results from a randomised trial. Eur Respir J 2008;31:1189–96. [DOI] [PubMed] [Google Scholar]
- 46.Saleh S, Ghodsian S, Yakimova V, Henderson J, Sharma OP. Effectiveness of infliximab in treating selected patients with sarcoidosis. Respir Med 2006;100:2053–9. [DOI] [PubMed] [Google Scholar]
- 47.Erckens RJ, Mostard RL, Wijnen PA, Schouten JS, Drent M. Adalimumab successful in sarcoidosis patients with refractory chronic non-infectious uveitis. Graefes Arch Clin Exp Ophthalmol 2012;250:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Milman N, Graudal N, Loft A, Mortensen J, Larsen J, Baslund B. Effect of the TNF-alpha inhibitor adalimumab in patients with recalcitrant sarcoidosis: a prospective observational study using FDG-PET. Clin Respir J 2012;6:238–47. [DOI] [PubMed] [Google Scholar]
- 49.Crommelin HA, van der Burg LM, Vorselaars AD, et al. Efficacy of adalimumab in sarcoidosis patients who developed intolerance to infliximab. Respir Med 2016;115:72–7. [DOI] [PubMed] [Google Scholar]
- 50.Baughman RP, Lower EE, Bradley DA, Raymond LA, Kaufman A. Etanercept for Refractory Ocular Sarcoidosis. Chest 2005;128:1062–7. [DOI] [PubMed] [Google Scholar]
- 51.Utz JP, Limper AH, Kalra S, et al. Etancercept for the treatment of stage II and III progressive pulmonary sarcoidosis. Chest 2003;124:177–85. [DOI] [PubMed] [Google Scholar]
- 52.Amber KT, Bloom R, Mrowietz U, Hertl M. TNF-alpha: a treatment target or cause of sarcoidosis? J Eur Acad Dermatol Venereol 2015;29:2104–11. [DOI] [PubMed] [Google Scholar]
- 53.Judson MA, Baughman RP, Costabel U, et al. Safety and efficacy of ustekinumab or golimumab in patients with chronic sarcoidosis. Eur Respir J 2014;44:1296–307. [DOI] [PubMed] [Google Scholar]
- 54.Takahashi R, Shijubo R, Shigehara K, Hiraga Y, Abe S, Sato N. T helper 1 inhibitor TAK-603 inhibits IFN-gamma and IL-12 production with no effect on IL-18: an observation in sarcoidosis patients. Sarcoidosis Vasc Diffuse Lung Disease 2004;21:204–11. [PubMed] [Google Scholar]
- 55.Alwan W, Nestle FO. Pathogenesis and treatment of psoriasis: exploiting pathophysiological pathways for precision medicine. Clinical and Experimental Rheumatology 2015;33:S2–S6. [PubMed] [Google Scholar]
- 56.Baumgart DC, Sandborn WJ. Crohn’s disease. The Lancet 2012;380:1590–605. [DOI] [PubMed] [Google Scholar]
- 57.Reinisch W, de Villiers W, Bene L, et al. Fontolizumab in moderate to severe Crohn’s disease: a phase 2, randomized, double-blind, placebo-controlled, multiple-dose study. Inflamm Bowel Dis 2010;16:233–42.*A review of current sarcoidosis therapeutics and their clinical data--great resource for busy clinical physicians.
- 58.Harden JL, Johnson-Huang LM, Chamian MF, et al. Humanized anti-IFN-gamma (HuZAF) in the treatment of psoriasis. J Allergy Clin Immunol 2015;135:553–6. [DOI] [PubMed] [Google Scholar]
- 59.Sah BP, Goyal S, Iannuzzi MC. Novel pharmacotherapy of sarcoidosis. Pharmacol Ther 2016;157:1–9. [DOI] [PubMed] [Google Scholar]
- 60.Jiang Z, Zhu L. Update on the role of alternatively activated macrophages in asthma. J Asthma Allergy 2016;9:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu YC, Zou XB, Chai YF, Yao YM. Macrophage polarization in inflammatory diseases. Int J Biol Sci 2014;10:520–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 2004;25:677–86. [DOI] [PubMed] [Google Scholar]
- 63.Jiang H, Harris MB, Rothman P. IL-4/IL-13 signaling beyond JAK/STAT. Journal of Allergy and Clinical Immunology 2000;105:1063–70. [DOI] [PubMed] [Google Scholar]
- 64.Zeyda M, Poglitsch M, Geyeregger R, et al. Disruption of the interaction of T cells with antigen-presenting cells by the active leflunomide metabolite teriflunomide: involvement of impaired integrin activation and immunologic synapse formation. Arthritis Rheum 2005;52:2730–9. [DOI] [PubMed] [Google Scholar]
- 65.Siemasko K, Chong ASF, Jack HM, Gong H, Williams JW, Finnegan A. Inhibition of JAK3 and STAT6 tyrosine phosphorylation by the immunosuppressive drug leflunomide leads to a block in IgG1 production. Journal of Immunology 1998;160:1581–8. [PubMed] [Google Scholar]
- 66.Sahoo DH, Bandyopadhyay D, Xu M, et al. Effectiveness and safety of leflunomide for pulmonary and extrapulmonary sarcoidosis. Eur Respir J 2011;38:1145–50. [DOI] [PubMed] [Google Scholar]
- 67.He L, Marneros AG. Doxycycline inhibits polarization of macrophages to the proangiogenic M2-type and subsequent neovascularization. J Biol Chem 2014;289:8019–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bachelez H, Senet P, Cadranel J, Kaoukhov A, Dubertret L. The use of tetracyclines for the treatment of sarcoidosis. Arch Dermatology 2001;137:69–73. [DOI] [PubMed] [Google Scholar]
- 69.Simpson EL, Bieber T, Guttman-Yassky E, et al. Two Phase 3 Trials of Dupilumab versus Placebo in Atopic Dermatitis. N Engl J Med 2016;375:2335–48. [DOI] [PubMed] [Google Scholar]
- 70.Wenzel S, Ford L, Pearlman D, et al. Dupilumab in persistent asthma with elevated eosinophil levels. N Engl J Med 2013;368:2455–66. [DOI] [PubMed] [Google Scholar]
- 71.Eishi Y, Suga M, Ishige I, et al. Quantitative Analysis of Mycobacterial and Propionibacterial DNA in Lymph Nodes of Japanese and European Patients with Sarcoidosis. Journal of Clinical Microbiology 2002;40:198–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song Z, Marzilli L, Greenlee BM, et al. Mycobacterial catalase-peroxidase is a tissue antigen and target of the adaptive immune response in systemic sarcoidosis. J Exp Med 2005;201:755–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rotsinger JE, Celada LJ, Polosukhin VV, Atkinson JB, Drake WP. Molecular Analysis of Sarcoidosis Granulomas Reveals Antimicrobial Targets. Am J Respir Cell Mol Biol 2016;55:128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Drake WP, Oswald-Richter K, Richmond BW, et al. Oral antimycobacterial therapy in chronic cutaneous sarcoidosis: a randomized, single-masked, placebo-controlled study. JAMA Dermatol 2013;149:1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Drake WP, Richmond BW, Oswald-Richter K, et al. Effects of broad-spectrum antimycobacterial therapy on chronic pulmonary sarcoidosis. Sarcoidosis Vasc Diffuse Lung Disease 2014;30:201–11. [PMC free article] [PubMed] [Google Scholar]
- 76.Ungprasert P, Crowson CS, Matteson EL. Smoking, obesity and risk of sarcoidosis: A population-based nested case-control study. Respir Med 2016;120:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newman LS, Rose CS, Bresnitz EA, et al. A case control etiologic study of sarcoidosis: environmental and occupational risk factors. Am J Respir Crit Care Med 2004;170:1324–30. [DOI] [PubMed] [Google Scholar]
- 78.Valeyre D, Soler P, Clerici C, et al. Smoking and pulmonary sarcoidosis: effect of cigarette smoking on prevalence, clinical manifestations, alveolitis, and evolution of the disease. Thorax 1988;43:516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sugano N, Shimida K, Ito K, Murai S. Nicotine inhibits the prodiction of inflammatory mediators in U937 cells through modulation of nuclear factor-kB activation. Biochemal and Biophysical Research Communications 1998;252:25–8. [DOI] [PubMed] [Google Scholar]
- 80.Zheng YS, Wu ZS, Zhang LY, et al. Nicotine ameliorates experimental severe acute pancreatitis via enhancing immunoregulation of CD4+ CD25+ regulatory T cells. Pancreas 2015;44:500–6. [DOI] [PubMed] [Google Scholar]
- 81.Julian MW, Shao G, Schlesinger LS, et al. Nicotine treatment improves Toll-like receptor 2 and Toll-like receptor 9 responsiveness in active pulmonary sarcoidosis. Chest 2013;143:461–70. [DOI] [PubMed] [Google Scholar]
- 82.Salin-Pascual RJ, Rosas M, Jimenez-Genchi A, Rivera-Meza BL, Delgado-Parra V. Antidepressant effect of transdermal nicotine patches in nonsmoking patients with major depression. J Clin Psychiatry 1996;57:387–9. [PubMed] [Google Scholar]
- 83.Warburton DM, Mancuso G. Evaluation of the information processing and mood effects of a transdermal nicotine patch. Psychopharmacology 1998;135:305–10. [DOI] [PubMed] [Google Scholar]
- 84.Le Houezec J, Halliday R, Benowitz NL, Callaway E, Naylor H, Herzig K. A low dose of subcutaneous nicotine improves information processing in non-smokers. Psychopharmacology 1994;114:628–34. [DOI] [PubMed] [Google Scholar]
- 85.Gentry MV, Hammersley JJ, Hale CR, Nuwer PK, Meliska CJ. Nicotine patches improve mood and response speed in a lexical decision task. Addictive Behaviors 2000;25:549–57. [DOI] [PubMed] [Google Scholar]
- 86.Braun NA, Celada LJ, Herazo-Maya JD, et al. Blockade of the programmed death-1 pathway restores sarcoidosis CD4(+) T-cell proliferative capacity. Am J Respir Crit Care Med 2014;190:560–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Celada LJ, Rotsinger JE, Young A, et al. Programmed Death-1 Inhibition of Phosphatidylinositol 3-Kinase/AKT/Mechanistic Target of Rapamycin Signaling Impairs Sarcoidosis CD4(+) T Cell Proliferation. Am J Respir Cell Mol Biol 2017;56:74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Broos CE, van Nimwegen M, Kleinjan A, et al. Impaired survival of regulatory T cells in pulmonary sarcoidosis. Respir Res 2015;16:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Broos CE, Veen JC, Hoogsteden HC. Decreased cytotoxic T-Lymphocyte antigen 4 expression on regulatory T cells and Th17 cells in sarcoidosis: double trouble? American Journal of Respiratory and Critical Care Medicine 2015;192:763–5. [DOI] [PubMed] [Google Scholar]
- 90.Lomax AJ, McGuire HM, McNeil C, et al. Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: Case series and immunophenotypic analysis. Int J Rheum Dis 2017;20:1277–85. [DOI] [PubMed] [Google Scholar]
- 91.Reddy SB, Possick JD, Kluger HM, Galan A, Han D. Sarcoidosis Following Anti-PD-1 and Anti-CTLA-4 Therapy for Metastatic Melanoma. J Immunother 2017;40:307–11. [DOI] [PubMed] [Google Scholar]
- 92.Cousin S, Italiano A. Pulmonary sarcoidosis or post-immunotherapy granulomatous reaction induced by the anti-PD-1 monoclonal antibody pembrolizumab: the termilogy is not the key point. Ann Oncol 2016;27:1974–5. [DOI] [PubMed] [Google Scholar]
- 93.Kamphuis LS, van Zelm MC, Lam KH, et al. Perigranuloma localization and abnormal maturation of B cells: emerging key players in sarcoidosis? Am J Respir Crit Care Med 2013;187:406–16. [DOI] [PubMed] [Google Scholar]
- 94.Lee NS, Barber L, Akula SM, Sigounas G, Kataria YP, Arce S. Disturbed homeostasis and multiple signaling defects in the peripheral blood B-cell compartment of patients with severe chronic sarcoidosis. Clin Vaccine Immunol 2011;18:1306–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saussine A, Tazi A, Feuillet S, et al. Active chronic sarcoidosis is characterized by increased transitional blood B cells, increased IL-10-producing regulatory B cells and high BAFF levels. PLoS One 2012;7:e43588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ueda-Hayakawa I, Tanimura H, Osawa M, et al. Elevated serum BAFF levels in patients with sarcoidosis: association with disease activity. Rheumatology (Oxford) 2013;52:1658–66. [DOI] [PubMed] [Google Scholar]
- 97.Sweiss NJ, Lower EE, Mirsaedi M, et al. Rituximan in the treatment of refractory pulmonary sarcoidosis Eur Respir J 2014;43:1525–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hao W, Crouser ED, Friedman A. Mathematical model of sarcoidosis. Proc Natl Acad Sci U S A 2014;111:16065–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Crouser ED, White P, Caceres EG, et al. A Novel In Vitro Human Granuloma Model of Sarcoidosis and Latent Tuberculosis Infection. Am J Respir Cell Mol Biol 2017;57:487–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Maier LA, Crouser ED, Martin WJ, Eu J. Executive summary of the NHLBI workshop report: leveraging current scientific advances to better undertsand sarcoidosis variability and improve outcome. Ann Am Thorac Soc 2017. October 19. doi: 10.1513/AnnalsATS.201707-563OT. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Crouser ED, Fingerlin TE, Yang IV, Maier LA, Nana-Sinkam P, Collman RG, Kaminsky N. Application of ‘Omics’ and Systems Biology to Sarcoidosis Research. Ann Am Thorac Soc 2017. October 20 doi: 10.1513/AnnalsATS.201707-567OT. [Epub ahead of press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Abrahamyan L, Feldman BM, Tomlinson G, et al. Alternative designs for clinical trials in rare diseases. Am J Med Genet C Semin Med Genet 2016;172:313–31. [DOI] [PubMed] [Google Scholar]