Abstract
In this paper we report the differentiating properties of platelet-rich plasma releasates (PRPr) on human chondrocytes within elastomeric polycaprolactone triol–citrate (PCLT–CA) porous scaffold. Human-derived chondrocyte cellular content of glycosaminoglycans (GAGs) and total collagen were determined after seeding into PCLT–CA scaffold enriched with PRPr cells. Immunostaining and real time PCR was applied to evaluate the expression levels of chondrogenic and extracellular gene markers. Seeding of chondrocytes into PCLT–CA scaffold enriched with PRPr showed significant increase in total collagen and GAGs production compared with chondrocytes grown within control scaffold without PRPr cells. The mRNA levels of collagen II and SOX9 increased significantly while the upregulation in Cartilage Oligomeric Matrix Protein (COMP) expression was statistically insignificant. We also report the reduction of the expression levels of collagen I and III in chondrocytes as a consequence of proximity to PRPr cells within the scaffold. Interestingly, the pre-loading of PRPr caused an increase of expression levels of following extracellular matrix (ECM) proteins: fibronectin, laminin and integrin β over the period of 3 days. Overall, our results introduce the PCLT–CA elastomeric scaffold as a new system for cartilage tissue engineering. The method of PRPr cells loading prior to chondrocyte culture could be considered as a potential environment for cartilage tissue engineering as the differentiation and ECM formation is enhanced significantly.
Keywords: Cartilage tissue engineering, PCLT–CA scaffolds, PRPr cells, Human chondrocytes, ECM formation
Introduction
Cartilage healing is a slow and sensitive process that often leads to a permanent cartilage defect. Cartilage repair is commonly suppressed by factors such as avascular condition of articular cartilage, limited chondrocytes in mature and aged tissue and inability of chondrocytes to migrate to the damaged site. In most cases, the fibro-cartilage would replace the damaged cartilage, which does not provide sustained repair and desirable mechanical support for affected joints [1]. In addition, chondrocytes are the sole source of extracellular matrix (ECM) synthesis within cartilage tissue. The number of chondrocytes reduces progressively with age through chondrocytes apoptosis, which also indicates the absence of the renewal of chondrocyte population, resulting in a turnover failure of cartilage ECM [2]. Despite the fact that chondrocytes apoptosis is essential during normal skeletal growth, the chondrocyte-derived apoptotic bodies deliver degradative properties that promote ECM degradation and calcification [3]. In order to achieve cartilage repair by principles of tissue engineering, an appropriate scaffold should provide initial 3D support for chondrocyte cells which are considered to be extremely sensitive to mechanical properties of scaffolding material. The cartilage tissue is a natural, highly hydrated, shock absorbent material that possess substantial elasticity necessary to provide a smooth joint movement over the lifetime. Due to their elasticity and hydrophilicity, elastomeric polyesters seem to be materials of choice for cartilage tissue engineering scaffolds [4].
In recent years, autologous articular chondrocytes have been isolated from small biopsies, expanded in vitro and used to repair articular cartilage damage through either direct injection into cartilage defect or applying engineered implantable grafts [5]. Though such techniques have shown considerable outcome through extensive in vitro and in vivo studies, many limitations still need to be addressed such as low yield of chondrocytes that are obtained from biopsies and the low capacity of flask-expended chondrocytes to re-differentiate towards cartilage-forming cells [5]. Consequently, chondrogenic conditional medium has been used to maintain chondrogenic phenotype as well as induction of chondrocytes proliferation. However, such medium has not been considered to scale up due to the high cost of growth factors and their side effects [6]. Possibly the key strategy for cartilage repair is to utilize scaffolds with sustained and controlled release of growth factors. The applied scaffolds should express dual function: 1) temporary mechanical support as artificial ECM; and 2) biochemical cues that would stimulate tissue growth within 3D porous network. The widely accepted approach in tissue engineering is to load biodegradable scaffolds with growth factors and several important issues must be considered such as: loading capacity, load distribution and long term stability of growth factor molecules [7]. It is obvious that the whole process would require careful material design in order to tune the protein release to closely match the release kinetics found in biological systems.
Platelets rich plasma releasates (PRPr) are natural reservoir of growth factors which represent an alternative approach for inducing successful tissue repair in cost- and time-effective manner [8, 9]. PRPr are derived from the non-coagulated blood and contain approximately five times more platelets than the baseline of whole blood platelets account [10]. Therefore, the activated PRP is able to release high levels of the essential growth factors which stimulate cells proliferation and ECM formation [11]. In this paper we report elastomeric polymer scaffolds, loaded with PRPr cells as a tissue engineering platform for cartilage repair. We took advantage of the fact the release of growth factors from PRPr cells does not directly rely on the supporting material (scaffold) and the growth factor release originates from biological system (PRPr cells), not from pre-designed growth factor loading into biodegradable polymer matrix.
Citric acid (CA)-based elastomers are one of the prominent biodegradable materials for porous scaffolds fabrication aimed for variety of tissue types, such as: blood vessels, kidney tissue, bone and cartilage [12]. In particular, in vitro study from Kang et al. described polyoctanediol citrate (POC) scaffold that have potential for cartilage tissue reconstruction. Cell compatibility evaluations of the POC scaffold confirmed that chondrocytes were able to attach to the pore walls within the scaffold structure, maintain their round morphology, and form a cartilaginous tissue during 28 days in culture [4]. Although POC (and other similar polymer systems) have shown good physical characteristics for cartilage repair, the growth factor release is necessary in order to closely mimic natural environment for chondrocyte cells. In our previous work we have reported the synthesis and characterization of polyester elastomer based on polycondensation between polycaprolactone triol (PCLT) and CA [13]. This material can be processed into porous scaffolds with highly controlled pore size and distribution. Here we report the concept of loading elastomeric PCLT–CA scaffolds with PRPr cells prior to seeding with human chondrocytes in order to provide a natural source of growth factor molecules for optimal cartilage repair. The results demonstrate a strong potential of our multifunctional elastomeric scaffolds for future applications in clinical cartilage repair.
Materials and methods
Polycaprolactone triol-citrate (PCLT-CA) synthesis and scaffold preparation
Polycaprolactone triol (PCLT, 300) and citric acid (CA) were purchased from Sigma, and used as received. Dulbecco’s modified Eagle’s Medium (DMEM) and fetal bovine serum (FBS) were purchased from Gibco-Invitrogen, USA.
The polymer was synthesised by previously reported method [13]. In brief, equimolar amounts of citric acid (CA) and (PCLT; Mn = 300 gmol−1) were placed in round bottom flask and heated to 160–165 °C until CA crystals melted. The reaction mixture was further stirred at 140–145 °C for 1 h under a constant stream of nitrogen. Thus formed polycaprolactone triol–citrate (PCLT–CA) pre-polymer was processed into scaffolds by solvent-casting/particulate leaching method by using sieved NaCl particles (200 µm) and EtOH as a solvent. Polymer solution was mixed with NaCl (9:1 polymer-to-salt ratio) and the polymer/salt slurry was moulded into PTFE casts to be cured at 80 °C for 7 days. After curing, scaffolds were soaked in water for 2 days in order to leach out the salt. After salt leaching scaffolds were freeze dried for 24 hours prior to loading with PRPr cells and subsequent seeding with chondrocytes.
Preparation of platelet rich plasma releasates and scaffold preparation
Ethical approval was granted by the Medical Ethics Committee, Faculty of Medicine University of Malaya (DFOP 1213/0079 (L)). Preparation of platelet rich plasma releasates was performed as previously described [14]. In brief, Blood samples (10 ml) were collected in EDTA-tube from 5 healthy volunteers and centrifuged at 400 g for 15 min and the supernatant that contained platelet rich plasma (PRP) was collected in a new tube. Then PRP was incubated in 25 mM CaCl2 at room temperature for 1 hour in order to activate the platelets. The activated PRP was then centrifuged at 2500 rpm for 15 min to collect soluble platelet rich clot releasate (PRCr) that was used in experiments. Prior to cell culture experiments, scaffold samples (5 mm in diameter, 2 mm in thickness; Fig. 1) were soaked overnight at 4 °C into DMEM medium containing 5, 10, 15 and 20% of isolated human PRPr and then freeze-dried for 24 h.
Fig. 1.
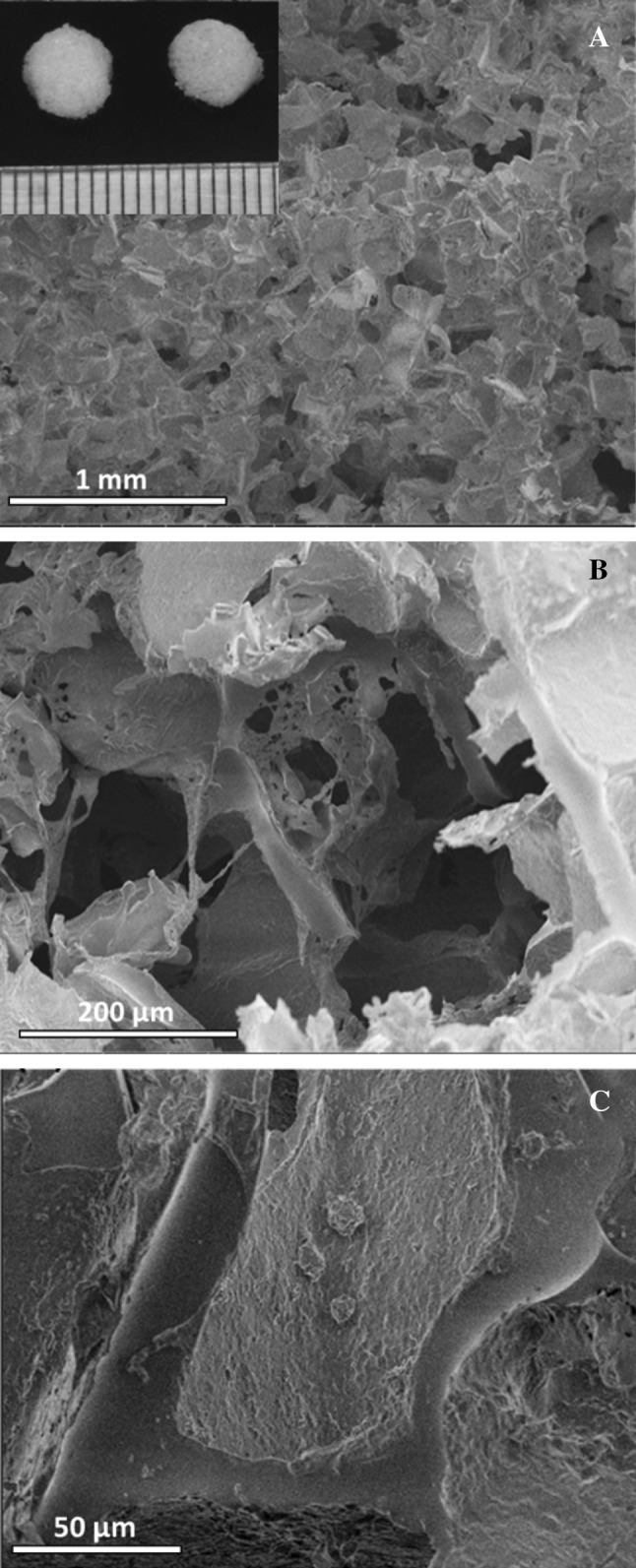
SEM images of PCLT–CA scaffolds. Polycaprolactone triol–citrate (PCLT–CA) polymer was processed into scaffolds by solvent-casting/particulate leaching method by using sieved NaCl particles and EtOH as a solvent. A, B PCLT–CA scaffolds were cut in uniform dimensions as presented in for SEM surface morphology observation, prior and after the seeding with chondrocyte cells. C Chondrocytes showed rounded shape of in PCLT–CA scaffold which is similar to the cell shape in native cartilage
Human chondrocyte culture
Human chondrocytes (Lonza, USA) were cultured in growth medium consistent of Dulbecco’s modified Eagle’s Medium (DMEM; Gibco-Invitrogen, USA) supplemented with 10% fetal bovine serum (FBS; Gibco-Invitrogen, USA) or 2% FBS in maintenance medium. Cells were incubated at standard conditions (humidified atmosphere, 5% CO2, 37 °C) for enough expansion. Then, the cell suspension with pre-determined concentration of cells (5 × 105 cells/scaffold) was dropped into the scaffold and left for 1 h in the incubator (37 °C and 5% CO2) for initial cell attachment. Scaffolds were then soaked in medium and left in incubator for further proliferation. Medium was replenished every 2 days during the culture.
Cell proliferation assay
Chondrocyte viability was determined by standard MTT assay method. Human chondrocytes were seeded at 105/scaffold in triplicates at optimal conditions (5% CO2, 37 °C in humidified incubator) and scaffolds were placed in 96 well plates for further incubation. Following 24, 48 and 72 h of incubation with increasing concentrations of PRPr (5, 10, 15 and 20%), a 10 μl solution of freshly prepared 5 mg/ml MTT in PBS was added to each well and allowed to incubate for an additional 2 to 4 hours. Next, the media was aspirated and DMSO was added at 100 μl/well. The plates were swirled gently to facilitate formazan crystal solubilization. By using a microplate reader (Tecan Infinite M200 Pro), the absorbance was measured at 570 nm. The percent of cell viability was calculated as follows: 100 − (absorbance PRP-treated cells/absorbance untreated cells) × 100.
Total collagen content
Cellular production of total collagen was measured as described previously [14]. In brief, human chondrocytes were seeded into T25 flask (5 × 105 cells/ flask, n = 6) or into scaffolds (5 × 105 cells/scaffold, n = 6) and grown in DMEM medium supplemented with 10% FBS as controls or scaffold enriched with 10% PRPr for 10 and 20 days. Total soluble collagen kit was used (QuickZyme BioSciences, Leiden- Netherlands) in all experiments. Briefly, the cells were trypsinised in both culture systems, washed with PBS and equal number of cells was used for this assay. The PBS was replaced by 0.5 M acetic acid (250 μl/well of 24-well-plate) and incubated overnight at 4 °C on a rotating platform. The cellular extracts were centrifuged for 10 minutes and the supernatant was tested in the assay in 1-fold to 10-fold dilutions made in PBS. Measurements were performed at a wavelength of 540 nm in triplicates using Tecan Infinite M200 spectrophotometer (Tecan Group Ltd., Switzerland). A standard curve for calculating collagen concentration was obtained using a manufacturer- supplied acid soluble type I collagen calibration standard solution.
Indirect Immunostaining
The chondrocyte cells that were initially seeded at density of 5 × 105 cells/scaffold into PCLT–CA elastomeric scaffolds enriched with 10% PRPr or 10% FBS as control to evaluate the expression levels of collagen II. After 10 days, the cells were trypsinised and reseeded on cover slides fixed in 6-well plates for 24 h. Next, the cells were washed three times with PBS and fixed with ice-cold methanol for 15 min at −20 °C. After the washing steps, the cells were incubated with a coating buffer for 1 h at room temperature. A mouse antibody specific to collage II (abcam, USA, cat. # ab3092) was added and the cells were incubated overnight at 4 °C. After incubation, cells were washed three times with PBS and further incubated for 30 min in anti-mouse IgG conjugated with horse radish peroxidase (HRP; abcam, USA, cat. # ab97046). After incubation with the substrate, the cells were washed with PBS and examined under light microscopy.
Real time PCR analysis
Real time PCR was applied to study the expression levels of chondrogenic (collagen II, Cartilage Oligomeric Matrix Protein COMP and SOX9) and extracellular gene markers (Collagen I, III, Fibronectin, Laminin and Integrin Beta1) in reference to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression levels. Briefly, equal number of human chondrocytes were seeded in scaffold with or without PRPr. Cells were harvested after 72 hours and total RNA was extracted using Trizol method (Invitrogen, USA), purified by RNA purification kit (Promega, USA) and quantified by nanospectrophotometer. The amount of 1 µg of pure RNA was used for gene expression analysis. Reverse-transcription was performed using cDNA synthesis kit (Invitrogen, USA) to prepare first strand cDNA. Real time PCR was performed using SYBR Green PCR kit (Applied Biosystems). The PCR program consisted of an initial step of 10 sec at 95 °C, followed by 40 cycles of denaturing at 95 °C, annealing at 50 °C for 5 seconds and extension at 60 °C for 31 sec. The relative level of genes expression was quantified using GAPDH gene as an endogenous control.
Scanning electron microscopy (SEM)
Human chondrocytes were cultured for 5 days into PCLT–CA scaffolds (1 × 105/scaffold) previously enriched with PRPr cells and then fixed with 3% glutaraldehyde in PBS for 24 h at 4 °C. After thorough washing with PBS, scaffold samples were dehydrated sequentially in 50%, 70%, 95% and 100% ethanol. Then the fixed samples were freeze dried, sputter coated with gold and observed with SEM (HitachiS-530).
Statistical analysis
Statistical analysis was performed on all collected data using GraphPad Prism version 5.01 (GraphPad Software, San Diego, CA). P values of < 0.05 were considered significant.All the results are presented with error bars, expressed as ± standard deviation.
Results
Scaffold morphology, chondrocyte attachment and viability
PCLT–CA scaffolds were cut in uniform dimensions as presented in Fig. 1 for SEM surface morphology observation, prior and after the seeding with chondrocyte cells. The results confirmed optimal pore distribution and successful control over the pore size within the scaffold (Fig. 1, A and B). Apart from mechanical compatibility, the cells attached and expended within the scaffold as shown in SEM results (Fig. 1, C). In addition, chondrocytes showed rounded shape of in PCLT-CA scaffold which is similar to the cell shape in native cartilage as presented in Fig. 1.
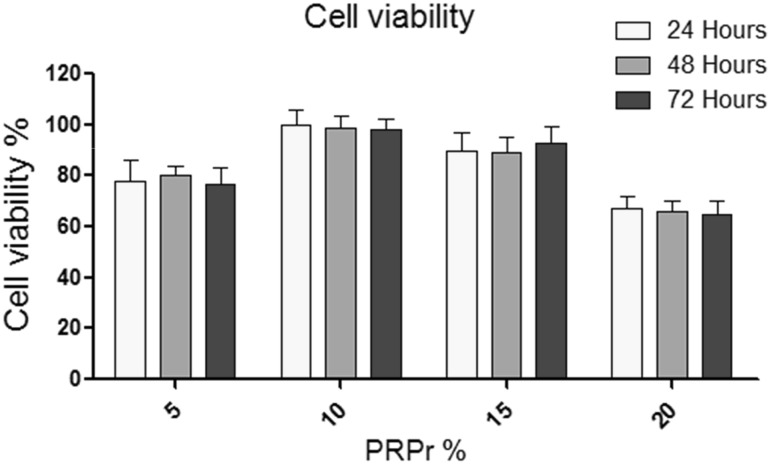
MTT assay was used to determine in vitro chondrocytes viability in the scaffolds within the periods of 24, 48 and 72 h and results are presented in Fig. 2. The results were compared for different concentration of loaded PRPr cells (5–20%) in order to determine the optimal PRPr concentration for chondrocyte seeding and subsequent proliferation. Results from Fig. 2 indicate the highest cell viability was observed when the cells were seeded in PCLT–CA scaffolds loaded with 10% of PRPr cells. This particular scaffold (named as “PCLT–CA/PRPr” further in text) was used in all other experiments that revealed more detailed information about cartilaginous ECM formation within the scaffold porous network.
Fig. 2.
Chondrocyte viability after the treatment with increased concentrations of PRPr. Human chondrocytes were seeded at 105/scaffold in triplicates at optimal conditions and scaffolds were placed in 96 well plates for further incubation. Following 24, 48 and 72 h of incubation with increased concentrations of PRPr (5, 10, 15 and 20%), cell viability was measured by MTT assay. The highest cell viability was observed when the cells were seeded in PCLT–CA scaffolds loaded with 10% of PRPr cells. The data were collected from three independent experiments. The statistical analysis (t-test) showed significant differences in cell viability between 10% PRPr and 5% PRPr (p < 0.05) and between 10% PRPr and 20% PRPr (p < 0.01), while the difference between 10% PRPr and 15% PRPr was insignificant (p > 0.05)
Time dependent effects on total collagen and glycosaminoglycan (GAG) content
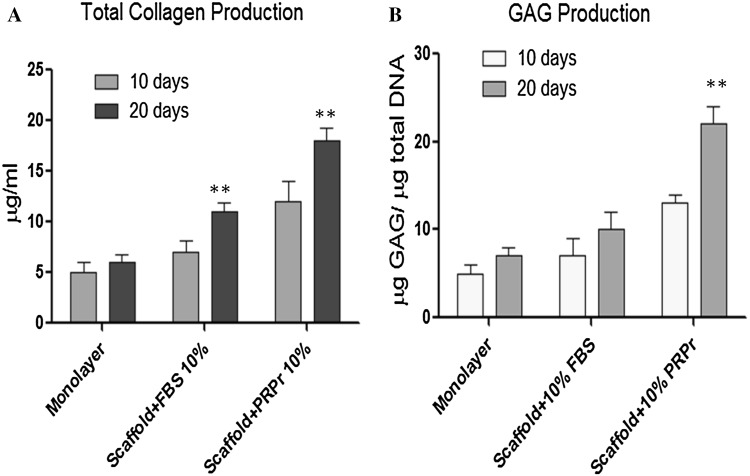
Human chondrocytes were cultured in PCLT–CA/PRPr scaffold in order to determine the effect of PRPr on cartilage ECM formation, including total collagen and GAGs production. All the results obtained for PCLT–CA/PRPr scaffolds were compared to two controls: 1) cell monolayer on polystyrene plate; and 2) FBS conditioned PCLT–CA scaffold without PRPr cells. Cell seeding into PCLT–CA/PRPr scaffold resulted in increased total collagen and GAGs production significantly (p < 0.01) with increasing incubation time from 10 days to 20 days (Fig. 3A and B). The production levels of total collagen increased 1.7-fold and 1.6-fold due to the presence of PRPr compared to FBS treated scaffolds after 10 and 20 h incubation respectively. In addition, the production levels of total collagen were higher 2.4-fold and 3-fold in PCLT–CA/PRPr scaffold after 10 and 20 h incubation respectively, when compared to chondrocyte monolayer (Fig. 3). Furthermore, seeded chondrocytes produced high levels of GAGs (1.9-fold and 2.2-fold) after 10 and 20 h incubation respectively (Fig. 3B). To characterise the expression level of collagen type II (the abundant collagen type in cartilage ECM), the cells were trypsinised from scaffolds and reseeded on cover slip. The expression levels of collagen II were detected by antibody specific to collagen II that was visualised by secondary antibody conjugated with HRP (Fig. 4). The results showed higher expression levels of intracellular collagen II in chondrocytes seeded into PCLT–CA/PRPr scaffold compared with pure PCLT–CA scaffold conditioned with 10% FBS prior to chondrocyte seeding. Obviously, growth factor molecules released from PRPr cells had an impact on collagen II extraction from proliferated chrondocytes after prolonged period of time (10 days).
Fig. 3.
Total collagen A and GAGs B produced by human chondrocytes seeded on PRPr loaded PCLT–CA porous scaffolds in comparison to controls (chondrocyte monolayer and scaffold treated with 10% FBS). Human chondrocytes were seeded into T25 flask (5 × 105 cells/ flask, n = 6) or into scaffolds (5 × 105 cells/scaffold, n = 6) and grown in DMEM medium supplemented with 10% FBS as controls or scaffold enriched with 10% PRPr for 10 and 20 days. The production levels of total collagen increased 1.7-fold and 1.6-fold due to the presence of PRPr compared to FBS treated scaffolds after 10 and 20 h incubation respectively. Seeded chondrocytes produced high levels of GAGs (1.9-fold and 2.2-fold) due to the presence of PRPr compared to FBS treated scaffolds after 10 and 20 h incubation respectively. The data were collected from three independent experiments.**t-test p < 0.01
Fig. 4.
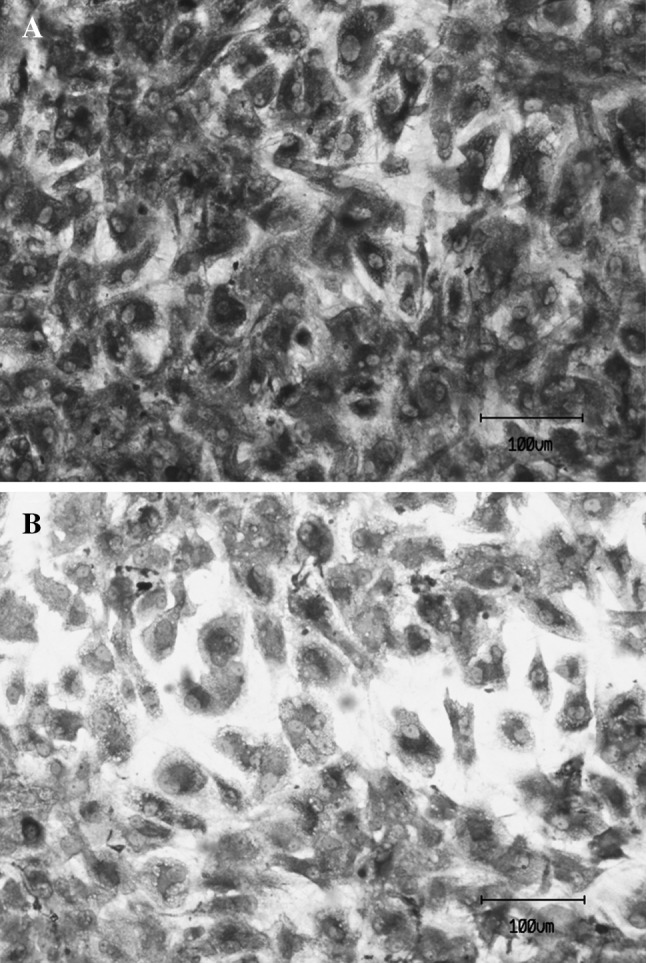
Indirect immunostaining of collagen II of human chondrocytes after 10 day period within PCLT–CA scaffolds. The cells that were seeded at density of 5 × 105 cells/scaffold into PCLT–CA scaffolds enriched with 10% PRPr or 10% FBS as control. After 10 days, the cells were trypsinised and reseeded on cover slides. Collagen II was detected using mouse antibody specific to collage II followed by anti-mouse IgG conjugated with horse radish peroxidase. The expression level of collagen II was higher due to the treatment with PRPr A compared with FBS-treated cells B
Gene expression analysis
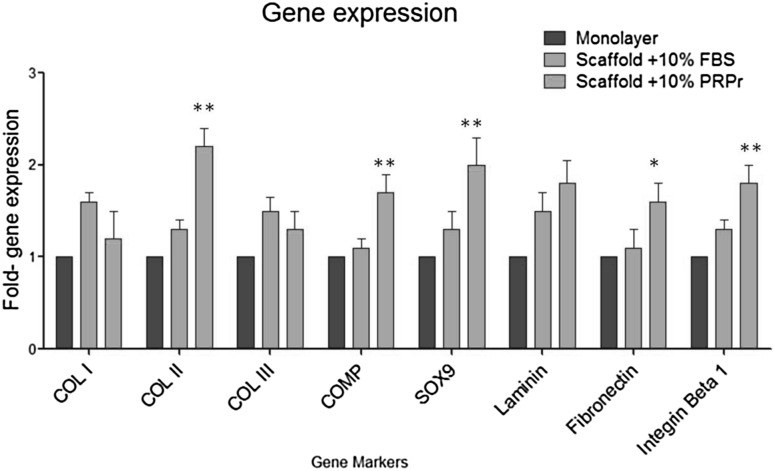
Gene expression of chondrogenic markers were normalized to the GAPDH expression level and was used to determine the maintenance of chondrocyte phenotype and expression of ECM genes in both cultural environments: PCLT–CA/PRPr scaffolds and controls (Fig. 5). The gene markers are characterized into transcription factor SOX9, Cartilage Oligomeric Matrix Protein (COMP), collagen network (collagen types I, II and III) and receptor and its ligand (integrin β1 and the fibronectin and laminin). In PCLT/PRPr scaffolds the expression level of COMP, SOX9, collagen type II, fibronectin and integrin β1 increased significantly when compared to controls. COMP, SOX9 and collagen type II showed considerable upregulation, while, the differences in the expression levels of collagen types I, III and lamanin were insignificant (p > 0.05; Fig. 5). Collagen type II gene expression level approximately increased 2-fold compared with collagen type I and III. The upregulation in the gene expression of fibronectin and integrin β1 were obviously significant (p < 0.05) in the presence of PRPr within the scaffold structure (compared with controls). Overall, PRPr cells loading had proven to be an effective method for cartilage ECM formation by seeded chondrocytes in vitro.
Fig. 5.
The expression levels of chondrogenic and extracellular gene markers. Equal number of human chondrocytes were seeded in scaffold in presence of PRPr or FBS and monolayer seeded- cells were used as control. Cells were harvested after 72 hours and total RNA was extracted using Trizol method and the amount of 1 µg of pure RNA was used for gene expression analysis. Reverse-transcription was performed using cDNA synthesis kit to prepare first strand cDNA. Real time PCR was performed using SYBR Green PCR kit. scaffolds the expression level of COMP, Sox9, collagen type II, fibronectin and integrin β1 increased significantly when compared to controls. While, the differences in the expression levels of collagen types I, III and laminin were insignificant. The data were collected from three independent experiments. t-test * p < 0.05, ** p < 0.01
Discussion
As previously mentioned in introduction, elastomeric scaffolds are expected to provide optimal microenvironment for chondrocyte cell growth. PCLT-CA (when reacted in PCLT:CA = 1:1 molar ratio) have shown a hydrophilic surface (water-in-air contact angle of ~60°) and Young’s modulus of ~0.3 MPa with recovery rate of ~70% after tensile strain [13]; these values are very close to ones published previously for CA-based elastomeric scaffolds that had shown excellent performance with chondrocytes in comparison to most common materials for cartilage tissue engineering (alginate, agarose and PLGA) [4].
Previous studies indicated that chondrocytes expansion in flasks tend to form fibroblastic-like morphology due to dedifferentiation phenomenon [15–17]. Furthermore, the 2D culture environment leads to a considerable reduction in ECM formation, upregulation of collagen type I gene expression and down regulation of collagen type II gene expression as well as a changed in chondrocyte cell surface antigen [3, 15–17]. Our results showed significant increase in total collagen and GAGs production in PCLT–CA scaffold, most likely as a result of 3D environment and the presence of PRPr cells, which in turn deliver necessary growth factors for tissue formation. The high production of total collagen and GAGs showed that PRPr further enhanced the chondrogenic environment imposed by the scaffold 3D porous network. In addition, the results of immunostaining and gene expression showed a prominent upregulation in collagen type II production, suggesting that chondrocytes in the new environment retained their chondrogenic properties and did not undergo dedifferentiation.
The maintenance of the rounded chondrocyte phenotype depicted in 3D culture is in agreement with the results of several studies which had proved that the 3D environment favours the maintenance of chondrocyte phenotype [18–20]. Furthermore, the expression level of chondrogenic markers (COPM and SOX9) was enhanced, which is an encouraging result for in vitro cartilage ECM formation. It should be noted that COMP plays its structural role in assembly and stabilization of ECM by interacting with collagen fibrils and matrix components such as fibronectin via carboxyterminal globular domain [21]. SOX9 is an early marker which mediates the activation of COMP by binding itself to the positive regulatory regions with the association of transcription factor Sox5 and Sox6. In this study, the considerable upregulation of SOX9 was noted, which in turn may lead to increase the expression levels of COMP. This expectation is supported by the finding that explains how chondrogenic medium (COMP gene expression) increased gradually with SOX9 within the first week of incubation [22]. It has been known that fibronectin promotes cell-cell interaction thereby defines the development of a chondrogenic differentiation mediated by cell microenvironment [23]. The expression pattern of integrin subunits varies during chondrogenesis where the expression of integrin β1 is substantial in mature chondrocytes [24]. Integrin β1, the heterodimeric transmembrane glycoprotein, modulates the interactions between ECM proteins and chondrocytes thus regulating the cell-matrix adhesion and inter cellular interaction [25]. Our study showed that the expression levels of integrin β1, an essential protein for chondrocytes adherence to ECM, were upregulated in the chondrocytes proliferated within PCLT–CA scaffold enriched with PRPr (Fig. 5).
In our previous work we have demonstrated that the PCLT–CA scaffolds (loaded with PRPr cells) support and enhance proliferation of skin fibroblasts [14]. Unlike skin or all other vascular tissues, cartilage tissue engineering requires different approach where the formed ECM by seeded cells does not generate vascular network. For that reason, chondrocyte cells are quite unique and each proposed strategy for cartilage repair should be thoroughly tested with tissue-specific cells. Cartilage tissue is highly hydrated where the water in ECM can reach up to 80% [26]. On the other hand, the material should be able to support initial cell attachment and provide appropriate micro-mechanical environment. If the scaffold-cell composite is used for in vivo engineering after implantation, the implanted tissue constructs are a subject to repetitive shear forces induced by joint movements and compression under the body weight. Since these forces are vital for chondrocyte cells and maintenance of healthy cartilage metabolism, the mechanical integrity of the scaffolding material should be conducive to physical strain in multiple directions. In particular, CA-based elastomers have demonstrated flexibility, hydrophilic surface and substantial swelling in water, with mechanical properties that are proven to be beneficial to chondrocyte cells [4].
Apart from their elastic mechanical properties for engineering of soft tissues, CA-based polyester materials could be formed into hard composites for orthopedic implants [27]. The solid material phase can be introduced in various filler forms such as “bioglasses” or nanoparticles [28, 29]. This opportunity was explored previously for production of multi-functional scaffolds that promote chondrocyte proliferation while at the same time deplete the bacteria in their environment by controlled release of zinc oxide (ZnO) nanoparticles from biodegradable polymer matrix [28]. The problem of bacterial biofilm formation upon surgical intervention must be considered when designing a potential material for scaffold fabrication. Currently we are exploring the possibility of PRPr cells loading into PCLT–CA/ZnO nanocomposite scaffolds that would combine both antibacterial effect of ZnO nanoparticles release and generation of growth factors for proliferation of chondrocytes within the scaffold volume. PCLT–CA material offers many other possibilities as the material itself is produced from non-toxic components with high degree of control over physico-chemical properties. The strategy described in this paper provide a fundamental aspect that could be explored further for development of tissue engineering devices used in clinical practice.
In conclusion, platelet rich plasma cells were loaded into elastomeric porous scaffolds in order to achieve the natural growth factor delivery for chondrocyte cells. The proliferation rate and extracellular matrix formation was significantly enhanced by this approach in comparison to controls. Scaffolds were originally produced in polyesterification reaction between polycaprolactone triol and citric acid which are both considered to be nontoxic and biocompatible. These highly elastic and hydrophilic polyester scaffolds provide biomimetic microenvironment for human-derived tissue-specific chondrocyte cells. In addition, growth factor release from plasma cells, loaded prior to chrondocyte cell seeding into the scaffolds, showed a universal strategy to produce multifunctional tissue engineering scaffolds that would provide both optimal physical support for cells and enhancement of cellular proliferation.
Acknowledgement
This project was funded by University of Malaya-Malaysia (RG377-15AFR).
Conflict of interest
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Ethical statement
This study was approved by the Medical Ethics Committee, Faculty of Medicine University of Malaya (DFOP 1213/0079 (L)).
Contributor Information
Hussin A. Rothan, Email: rothan@um.edu.my
Suhaeb A. Mahmod, Email: suhaeb@um.edu.my
Ivan Djordjevic, Email: ivandjordjevich@hotmail.com.
Mojtaba Golpich, Email: m_golpich@yahoo.com.
Rohana Yusof, Email: rohana@um.edu.my.
Simmrat Snigh, Email: simmratsingh@um.edu.my.
References
- 1.Snyder TN, Madhavan K, Intrator M, Dregalla RC, Park D. A fibrin/hyaluronic acid hydrogel for the delivery of mesenchymal stem cells and potential for articular cartilage repair. J Biol Eng. 2014;8:10. doi: 10.1186/1754-1611-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sandell LJ, Aigner T. Articular cartilage and changes in arthritis. An introduction: Cell biology of osteoarthritis. Arthritis Res. 2001;3:107–113. doi: 10.1186/ar148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobasheri A, Kalamegam G, Musumeci G, Batt ME. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas. 2014;78:188–198. doi: 10.1016/j.maturitas.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Kang Y, Yang J, Khan S, Anissian L, Ameer GA. A new biodegradable polyester elastomer for cartilage tissue engineering. J Biomed Mater Res Part A. 2006;77A:331–339. doi: 10.1002/jbm.a.30607. [DOI] [PubMed] [Google Scholar]
- 5.Hildner F, Albrecht C, Gabriel C, Redl H, Van Griensven M. State of the art and future perspectives of articular cartilage regeneration: a focus on adipose-derived stem cells and platelet-derived products. J Tissue Eng Regen Med. 2011;5:e36–e51. doi: 10.1002/term.386. [DOI] [PubMed] [Google Scholar]
- 6.Hao T, Wen N, Cao J-K, Wang H-B, Lü S-H, Liu T, et al. The support of matrix accumulation and the promotion of sheep articular cartilage defects repair in vivo by chitosan hydrogels. Osteoarthr Cartil. 2010;18:257–265. doi: 10.1016/j.joca.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Whitaker MJ, Quirk RA, Howdle SM, Shakesheff KM. Growth factor release from tissue engineering scaffolds. J Pharm Pharmacol. 2001;53:1427–1437. doi: 10.1211/0022357011777963. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Gomez L, Alvarez-Lorenzo C, Concheiro A, Silva M, Dominguez F, Sheikh FA, et al. Biodegradable electrospun nanofibers coated with platelet-rich plasma for cell adhesion and proliferation. Mater Sci Eng C. 2014;40:180–188. doi: 10.1016/j.msec.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shimojo AA, Perez AG, Galdames SE, Brissac IC, Santana MH, et al. Stabilization of porous chitosan improves the performance of its association with platelet-rich plasma as a composite scaffold. Mater Sci Eng C. 2016;60:538–546. doi: 10.1016/j.msec.2015.11.080. [DOI] [PubMed] [Google Scholar]
- 10.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA, et al. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–72. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 11.Demidova-Rice TN, Hamblin MR, Herman IM. Acute and impaired wound healing: pathophysiology and current methods for drug delivery, part 2: role of growth factors in normal and pathological wound healing: therapeutic potential and methods of delivery. Adv Skin Wound Care. 2012;25(8):349–370. doi: 10.1097/01.ASW.0000418541.31366.a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran RT, Yang J, Ameer GA. Citrate-based biomaterials and their applications in regenerative engineering. Annu Rev Mater Res. 2015;45:277–310. doi: 10.1146/annurev-matsci-070214-020815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shirazi HS, Forooshani PK, Pingguan-Murphy B, Djordjevic I. Processing and characterization of elastomeric polycaprolactone triol–citrate coatings for biomedical applications. Prog Org Coat. 2014;77:821–829. doi: 10.1016/j.porgcoat.2014.01.011. [DOI] [Google Scholar]
- 14.Rothan HA, Djordjevic I, Bahrani H, Paydar M, Ibrahim F, Abd Rahmanh N, et al. Three-dimensional culture environment increases the efficacy of platelet rich plasma releasate in prompting skin fibroblast differentiation and extracellular matrix formation. Int J Med Sci. 2014;11:1029–1038. doi: 10.7150/ijms.8895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caron MM, Emans PJ, Coolsen MM, Voss L, Surtel DA, Cremers A, et al. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr Cartil. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 16.Lin L, Shen Q, Xue T, Duan X, Fu X, Yu C. Sonic hedgehog improves redifferentiation of dedifferentiated chondrocytes for articular cartilage repair. PLoS One. 2014;9:e88550. doi: 10.1371/journal.pone.0088550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu F, L Xu, Wang Q, Ye Z, Zhou Y, Tan WS. 3D dynamic culture of rabbit articular chondrocytes encapsulated in alginate gel beads using spinner flasks for cartilage tissue regeneration. BioMed Res Int. 2014;2014:539789. doi: 10.1155/2014/539789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernardini G, Chellini F, Frediani B, Spreafico A, Santucci A. Human platelet releasates combined with polyglycolic acid scaffold promote chondrocyte differentiation and phenotypic maintenance. J Biosci. 2015;40:61–69. doi: 10.1007/s12038-014-9492-2. [DOI] [PubMed] [Google Scholar]
- 19.Gigout A, Jolicoeur M, Buschmann MD. Low calcium levels in serum-free media maintain chondrocyte phenotype in monolayer culture and reduce chondrocyte aggregation in suspension culture. Osteoarthr Cartil. 2005;13:1012–1024. doi: 10.1016/j.joca.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 20.Yates KE, Allemann F, Glowacki J. Phenotypic analysis of bovine chondrocytes cultured in 3D collagen sponges: effect of serum substitutes. Cell Tissue Bank. 2005;6:45–54. doi: 10.1007/s10561-005-5810-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tseng S, Reddi AH, Di Cesare PE. Cartilage oligomeric matrix protein (COMP): A biomarker of arthritis. Biomark Insights. 2009;4:33–44. doi: 10.4137/bmi.s645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamid AA, Idrus RB, Saim AB, Sathappan S, Chua KH. Characterization of human adipose-derived stem cells and expression of chondrogenic genes during induction of cartilage differentiation. Clinics. 2012;67:099–106. doi: 10.6061/clinics/2012(02)03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tavella S, Bellese G, Castagnola P, Martin I, Piccini D, Doliana R, Colombatti A, Cancedda R, Tacchetti C, et al. Regulated expression of fibronectin, laminin and related integrin receptors during the early chondrocyte differentiation. J Cell Sci. 1997;110:2261–2270. doi: 10.1242/jcs.110.18.2261. [DOI] [PubMed] [Google Scholar]
- 24.Fukumoto T, Sanyal A, Fitzsimmons JS, O'Driscoll SW. Expression of β1 integrins during periosteal chondrogenesis. Osteoarthr Cartil. 2002;10:135–144. doi: 10.1053/joca.2001.0490. [DOI] [PubMed] [Google Scholar]
- 25.Knudson W, Loeser RF. CD44 and integrin matrix receptors participate in cartilage homeostasis. Cell Mol Life Sci. 2002;59:36–44. doi: 10.1007/s00018-002-8403-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sophia Fox AJ, Bedi A, Rodeo SA. The basic science of articular cartilage: structure, composition, and function. Sports Health A Multidiscip Approach. 2009;1:461–468. doi: 10.1177/1941738109350438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu Hongjin, Yang J, Kodali P, Koh J, Ameer GA. A citric acid-based hydroxyapatite composite for orthopedic implants. Biomaterials. 2006;27(34):5845–5854. doi: 10.1016/j.biomaterials.2006.07.042. [DOI] [PubMed] [Google Scholar]
- 28.Mirza EH, Pan-Pan C, Ibrahim W, Bin WMA, Djordjevic I, Pingguan-Murphy B. Chondroprotective effect of zinc oxide nanoparticles in conjunction with hypoxia on bovine cartilage-matrix synthesis. J Biomed Mater Res A. 2015;103:3554–3563. doi: 10.1002/jbm.a.35495. [DOI] [PubMed] [Google Scholar]
- 29.Zeimaran E, Pourshahrestani S, Pingguan-Murphy B, Kadri NA, Rothan HA, Yusof R, et al. Fabrication and characterization of poly(octanediol citrate)/gallium-containing bioglass microcomposite scaffolds. J Mater Sci. 2015;50:2189–2201. doi: 10.1007/s10853-014-8782-2. [DOI] [Google Scholar]