Abstract
Mesenchymal stem cells (MSCs), which are multipotent and have self-renewal ability, support the regeneration of damaged normal tissue. A number of external stimuli promote migration of MSCs into peripheral blood and support their participation in wound healing. In an attempt to harness the potential beneficial effects of such external stimuli, we exposed human MSCs (hMSCs) to one such stimulus—low-dose ionizing radiation (LDIR)—and examined their biological properties. To this end, we evaluated differences in proliferation, cell cycle, DNA damage, expression of surface markers (CD29, CD34, CD90, and CD105), and differentiation potential of hMSCs before and after irradiation with γ-rays generated using a 137CS irradiator. At doses less than 50 mGy, LDIR had no significant effect on the viability or apoptosis of hMSCs. Interestingly, 10 mGy of LDIR increased hMSC viability by 8% (p < 0.001) compared with non-irradiated hMSCs. At doses less than 50 mGy, LDIR did not induce DNA damage, including DNA strand breaks, or cause cellular senescence or cell-cycle arrest. Surface marker expression and in vitro differentiation potential of hMSCs were maintained after two exposures to LDIR at 10 mGy per dose. In conclusion, a two-dose exposure to LDIR at 10 mGy per dose not only facilitates proliferation of hMSCs, it also maintains the stem cell characteristics of hMSCs without affecting their viability. These results provide evidence for the potential of LDIR as an external stimulus for in vitro expansion of hMSCs and application in tissue engineering and regenerative medicine.
Electronic supplementary material
The online version of this article (doi:10.1007/s13770-017-0045-2) contains supplementary material, which is available to authorized users.
Keywords: Low dose ionizing radiation, Human mesenchymal stem cells, Proliferation, DNA damages, Differentiation
Introduction
Mesenchymal stem cells (MSCs) are a type of bone marrow stem cell that possesses self-renewal ability and the capacity to differentiate into various mesenchymal lineages [1]. MSCs have been applied to repair bone fractures, cartilage defects, ischemic heart disease, tendon damage, and skin wounds [2–5].
MSCs must be expanded in vitro to clinically meaningful cell numbers in order to be used for tissue engineering applications. However, expanded MSCs at later passages tend to lose their stemness potential, proliferation and differentiation ability [6, 7]. Various modifications of human MSCs (hMSCs) culture methods, such as inclusion of growth factors or small molecules and exposure to external stimuli, have been reported to enhance proliferation as well as differentiation during subsequence passages in culture [8, 9]. Among external cellular stimuli that have been studied for tissue engineering applications are cyclic tensile, compression, ultrasound, and hypoxic conditions [10–13].
Interestingly, several studies have reported that exposure of cells to low-dose ionizing radiation (LDIR) not only provides protection against radiation-induced damage, but also stimulates cellular processes such as proliferation, protein synthesis, and immune responses—a concept referred to as radiation hormesis (or homeostasis) [14]. These effects of LDIR have been reported for various cell types, including germ cells, immune cells, and hematopoietic progenitor cells [15–18]. Cai et al. [15, 16] investigated the effects of LDIR with X-rays at doses up to 200 mGy (milligrays) in lymphocytes and spermatocyte. Li et al. [17] reported that LDIR with X–rays at a dose of 75 mGy stimulates proliferation of bone marrow hematopoietic progenitor cells and induces their mobilization into peripheral blood. Guo et al. [18] reported that repeated exposure to LDIR with X-rays at a dose of 75 mGy accelerates skin wound healing in a rat model of diabetes. Other studies have reported radiation responses of hMSCs, examining changes in cellular morphology, apoptosis, cell cycle, cellular senescence, and differentiation [19–22]. Liang et al. [19] studied proliferation of rat MSCs (rMSCs) stimulated with LDIR at doses up to 100 mGy through activation of the mitogen-activated protein kinase (MAPK)/extracellular signal regulated kinase (ERK) pathway. Kurpinski et al. [20] compared the biological properties of hMSCs after LDIR with X-rays or 56Fe ions at doses up to 1 Gy. Nicolay et al. [21] defined the stem cell characteristics of hMSCs after exposure to up to 10 Gy, and Alessio et al. [22] reported cellular senescence of hMSCs after exposure to X-rays at doses up to 2 Gy. However, to date, ionizing radiation (IR) has not been used for external cellular stimulation of hMSCs in tissue engineering applications. Moreover, the effects of LDIR on the biological properties and stem cell characteristics of hMSCs at doses less than 50 mGy have remained largely unknown.
In this study, we assessed LDIR as an external hMSC stimulus for tissue engineering applications. Specifically, we evaluated the effects of LDIR with γ-rays on the biological properties and characteristics of hMSCs, focusing on doses less than 50 mGy. hMSCs were exposed two or three times to LDIR at doses less than 50 mGy; 3 Gy was used as a negative control. Proliferation and viability of irradiated and control hMSCs were evaluated by counting viable cells. LDIR-induced DNA damage of hMSCs was examined by measuring apoptosis and cell cycle changes using flow cytometry and immunofluorescence, respectively. We also characterized the maintenance of hMSC stemness, phenotypic surface markers, and in vitro differentiation potential after exposure to LDIR.
Materials and methods
γ-Irradiation of hMSCs
hMSCs (passage 2) were purchased from Lonza (Switzerland, Lot number: 000394413) and maintained according to the supplier’s protocol. Briefly, hMSCs were seeded on 100 mm culture dishes at a density of 3.0 × 105 cells per dish in 10 ml mesenchymal stem cell growth medium (MSCGM, MSCGM™ BulletKit™, LONZA, Switzerland) at 37°C in a humidified 5% CO2 incubator. The medium was changed every 2–3 days until the cultures reached ~70–80% confluence. hMSCs at passage 5 to 6 were used in this study. The cell density and type of culture plate used are indicated for each experimental method.
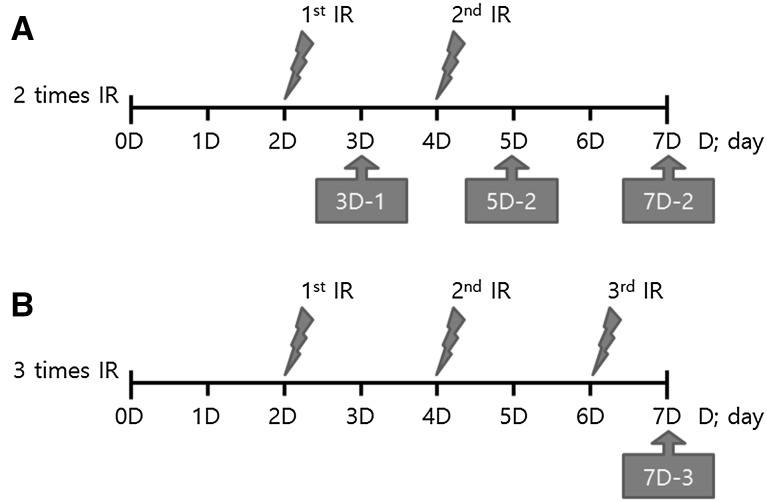
After culturing for 2 days, hMSCs were exposed to γ-ray irradiation at experimental doses of 10 and 50 mGy, and a control dose of 3 Gy, using a 137CS irradiator. LDIR doses less than 50 mGy were administered using a MDI-KIRAMS 137 (KIRAMS, Korea), and the higher control dose of 3 Gy was administered using a BioBeam 8000 (Gamma-Service Medical GmbH, Germany). After culturing for an additional 2 days, hMSCs were exposed to two or three doses of γ-ray irradiation. The control group was processed under the same conditions without γ–ray irradiation. Changes in γ-ray–irradiated hMSCs were evaluated at multiple times, as indicated in Fig. 1.
Fig. 1.
Time schedules of LDIR γ-ray irradiation of hMSCs. A hMSCs irradiated one or two times; B hMSCs irradiated three times
Proliferation and viability of irradiated hMSCs
Cell proliferation and viability were determined directly by counting viable cells. hMSCs were plated on 48-well culture plates at a density of 5.0 × 103 cells/well in 200 µl of MSCGM. After irradiating hMSCs according to the indicated irradiation schedule, viable cells were counted by first trypsinizing hMSCs using 0.05% trypsin–EDTA (GIBCO, USA) and then staining with trypan blue (WelGene, Korea) to distinguish live and dead cells. The proliferation and viability of irradiated hMSCs was determined by counting the number of hMSCs using a hemocytometer.
Apoptosis of irradiated hMSCs
hMSCs were seeded in a 6-well culture plate at a density of 5.5 × 104 cells/well in 2 ml of MSCGM, and irradiated according to the irradiation schedule. On culture day 7, hMSCs were trypsinized and evaluated for apoptotic status using a terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay (APO-BrdU™ TUNEL Assay Kit, Invitrogen, USA), according the manufacturer’s protocol. Briefly, hMSCs at a density of 1.0 × 106 cells/well were incubated for 30 min with 100 µl of Alexa Fluor 488-conjugated anti-BrdU antibody (1:20, APO-BrdU™ TUNEL Assay Kit, Invitrogen, USA), and nuclei were stained using 500 µl of propidium iodide (PI, APO-BrdU™ TUNEL Assay Kit, Invitrogen, USA). The percentage of apoptotic cells was analyzed using fluorescence-activated cell sorting (FACS, FACSCalibeur™, BD science, USA).
Morphology of irradiated hMSCs
hMSCs were seeded in a 24-well culture plate at a density of 5.0 × 103 cells/well in 0.5 ml of MSCGM, and irradiated according to the irradiation schedule. Images of hMSCs for morphological assessments were collected on days 3, 5, and 7 using an IX-70 microscope (Olympus, Japan) equipped with a DFC 280 camera (Leica, German) and Leica Application Suite version 4.2 software (LAS v4.2 software, Leica, German).
Immunofluorescence analysis of γ-H2AX and measurement of nucleus size
The DNA-damage response of irradiated hMSCs was determined by immunofluorescence analysis of γ-H2AX (histone 2A) foci. hMSCs were seeded on 12 mm coverslip in a 24-well culture plate at a density of 5.0 × 103 cells/well in 0.5 ml of MSCGM, and irradiated according to the irradiation schedule. On day 7, irradiated hMSCs were fixed by incubating with 4.0% paraformaldehyde (PFA, Sigma-Aldrich, USA) in phosphate-buffered saline (PBS) and then permeabilized by incubating with 0.1% (v/v) Triton X-100 (USB, USA) in PBS. hMSCs were blocked by incubating with 5% bovine serum albumin (BSA, USB, USA) and then incubated with an anti-phospho-histone H2A.X (Ser139) antibody (1:200, Merck Millipore, USA) at 4°C, overnight. After washing three times with PBS, hMSCs were incubated with goat anti-mouse IgG H&L (Alexa Fluor 488, 1:200, Abcam, UK) at room temperature for 1 h. hMSCs were then mounted with 4′,6-diamidino-2-phenylindole (DAPI)-containing mounting medium (VECTASHIELD with DAPI, Vector Laboratories, USA) and imaged using an LSM 710 confocal microscope (Carl Zeiss, Germany). The number of γ-H2AX foci was manually counted in collected images, and the sizes of nuclei were measured with ImageJ software (National Institutes of Health, USA).
Cell cycle of irradiated hMSCs
hMSCs were plated in a 6-well culture plate at a density of 5.5 × 104 cells/well in 2 ml of MSCGM, and irradiated according to the irradiation schedule. On day 7, hMSCs were trypsinized and suspended at a density of 1.0 × 106 cells per 5.0 mL of PBS. hMSCs were fixed for 30 min with 5.0 ml of a 75% ethanol solution. After washing three times with PBS, fixed hMSCs were incubated for 30 min with 0.5 mL of PI (Sigma, USA) at 37°C. The cell cycle in each group was then analyzed by FACS.
CD marker expression in irradiated hMSCs
hMSCs were plated in a 48-well culture plate at a density of 5.5 × 103 cells/well in 2 ml of MSCGM, and irradiated according to the irradiation schedule. hMSCs were trypsinized with 0.05% EDTA-trypsin and adjusted to a density of 1.0 × 106 cells/ml of PBS. Suspended hMSCs were blocked by incubating for 30 min with 2.0% BSA, USB, USA). After washing three times with PBS, hMSCs were incubated for 1 h with antibodies (200 μl each) against the positive hMSC surface markers, CD29 (1:100, mouse anti-human CD29:FITC, Serotec, UK), CD44 (1:100, mouse anti-human CD44:FITC, Serotec, UK), CD90 (1:100, mouse anti-hum9an CD90:FITC, Serotec, UK) and CD105 (1:100, mouse anti-human CD105:FITC, Serotec, UK), and negative hMSC surface markers, CD34 (1:100, monoclonal mouse anti-human CD34 Class 3, Clone BIRMA-K3, Dako, Denmark) and CD45. After staining hMSCs nuclei using 500 µl of PI according to the manufacturer’s instructions, expression of each CD marker was analyzed using FACS.
In vitro differentiation of LDIR-exposed hMSCs
Osteogenic, adipogenic, and chondrogenic differentiation potentials of LDIR-exposed hMSCs compared with those of non–irradiated hMSCs were examined using commercial in vitro differentiation assays (LONZA, Switzerland). hMSCs were plated on 100-mm culture dishes at a density of 3.0 × 105 cells per 10 ml of MSCGM. hMSCs were irradiated at a dose of 10 or 50 mGy on days 2 and 4 of culturing (7D-2), then trypsinized on day 7 and seeded at an appropriate density. Osteogenic, adipogenic, and chondrogenic differentiation was confirmed, according to the manufacturer’s instructions.
For osteogenic differentiation, LDIR-exposed hMSCs were seeded in 6-well plates at a density of 2.0 × 104 cells per well in 2 ml of osteogenic induction medium (hMSC differentiation BulletKit™-osteogenic, LONZA, Switzerland), and cultured at 37°C in a humidified 5% CO2 incubator. After culturing for 28 days, calcium deposits in hMSC cultures were stained with alizarin red S (Sigma-Aldrich, USA).
For adipogenic differentiation, LDIR-exposed hMSCs were seeded in 6-well plates at a density of 2.0 × 105 cells/well in 2 ml of MSCGM. The medium was changed every 2–3 days until the cultures reached confluence. At 100% confluence, three cycles of induction/maintenance were conducted to differentiate hMSCs into adipogenesis. Each cycle consisted of feeding hMSCs with adipogenic induction medium (hMSC differentiation BulletKit™-adipogenic, LONZA, Switzerland) and culturing for 3 days, followed by 2 days of culture in adipogenic maintenance medium (hMSC differentiation BulletKit™-adipogenic, LONZA, Switzerland) at 37°C in a 5% CO2 incubator. The medium was changed every 3 days. After culturing for 15 days, lipid droplets were stained with oil red O (Sigma-Aldrich, USA).
For chondrogenic differentiation, LDIR-exposed hMSCs were pellet-cultured at a density of 1.0 × 106 cells per pellet in 1 ml of chondrogenic induction medium (hMSC differentiation BulletKit™-chondrogenic, LONZA, Switzerland) at 37°C in a 5% CO2 incubator. After culturing for 6 weeks, chondrogenic differentiated pellets were formalin fixed, paraffin embedded, thin-sectioned, and stained with safranin O (Sigma-Aldrich, USA).
Statistical analysis
Data are presented as mean ± standard deviation (SD). The significance of differences between the control and irradiated groups was determined using ANOVA’s t-test. A P–value <0.05 was considered statistically significant. Individual P-values for specific comparisons are indicated in figure legends.
Results
Proliferation and viability of irradiated hMSCs
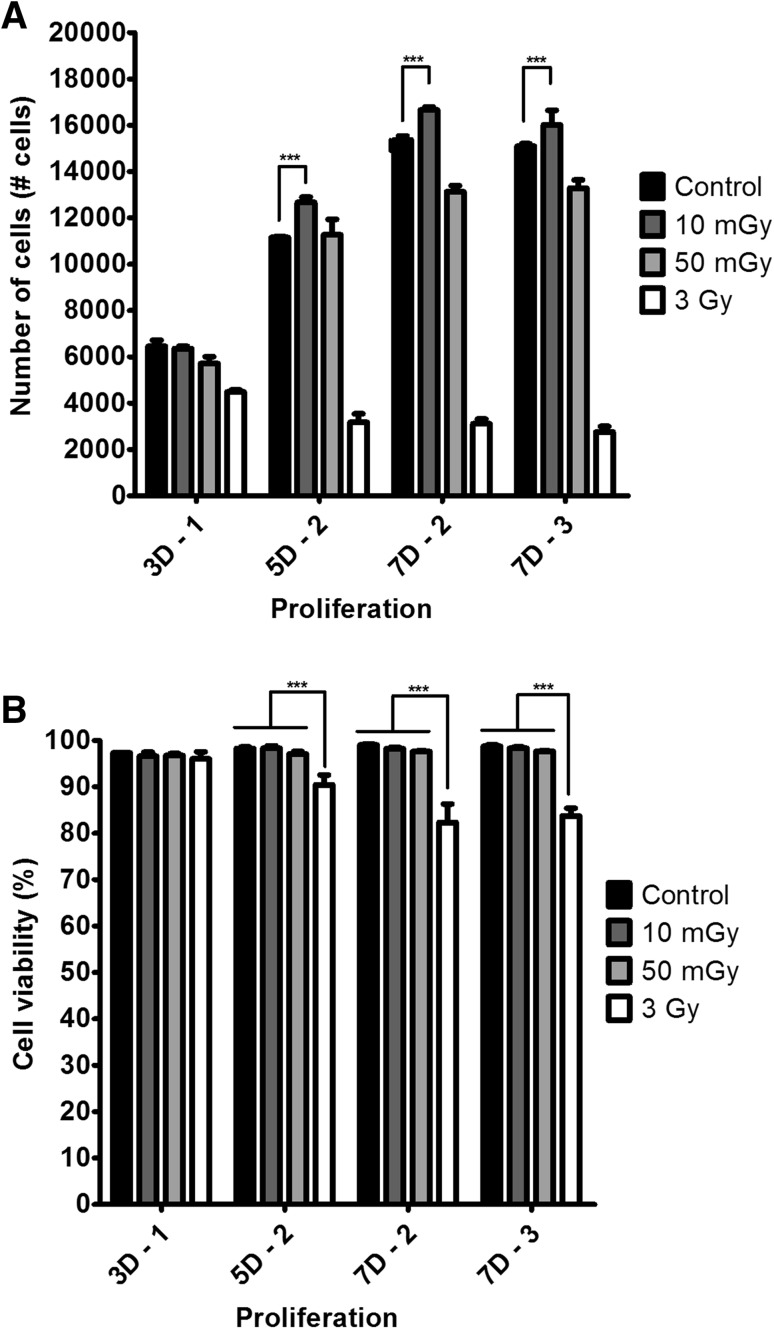
To evaluate the effects of LDIR on the proliferation and viability of hMSCs, we irradiated hMSCs at a dose of 10 or 50 mGy for 7 days, and then counted the number of hMSCs. As shown in Fig. 2A, two-dose 5-day (5D–2), two-dose 7-day (7D–2), and three-dose 7-day (7D–3) irradiation of hMSCs at 10 mGy per dose increased the proliferation of hMSCs by 13.60% ± 2.04%, 8.36% ± 0.88% and 6.11% ± 4.17%, respectively, compared with non-irradiated hMSCs. The viability of LDIR-exposed hMSCs was greater than 95% on day 7. By contrast, the viability of hMSCs exposed to 3-Gy irradiation was significantly lower than that of LDIR-exposed hMSCs (Fig. 2B).
Fig. 2.
At dose 10 mGy LDIR enhanced proliferations of hMSCs and no effect on viabilities of hMSCs compared with control. Proliferations A and viabilities B of two or three times at dose 10 mGy or 50 mGy LDIR irradiated hMSCs (n = 4). (*p < 0.05, ** p < 0.01, and *** p < 0.001)
Apoptosis of irradiated hMSCs
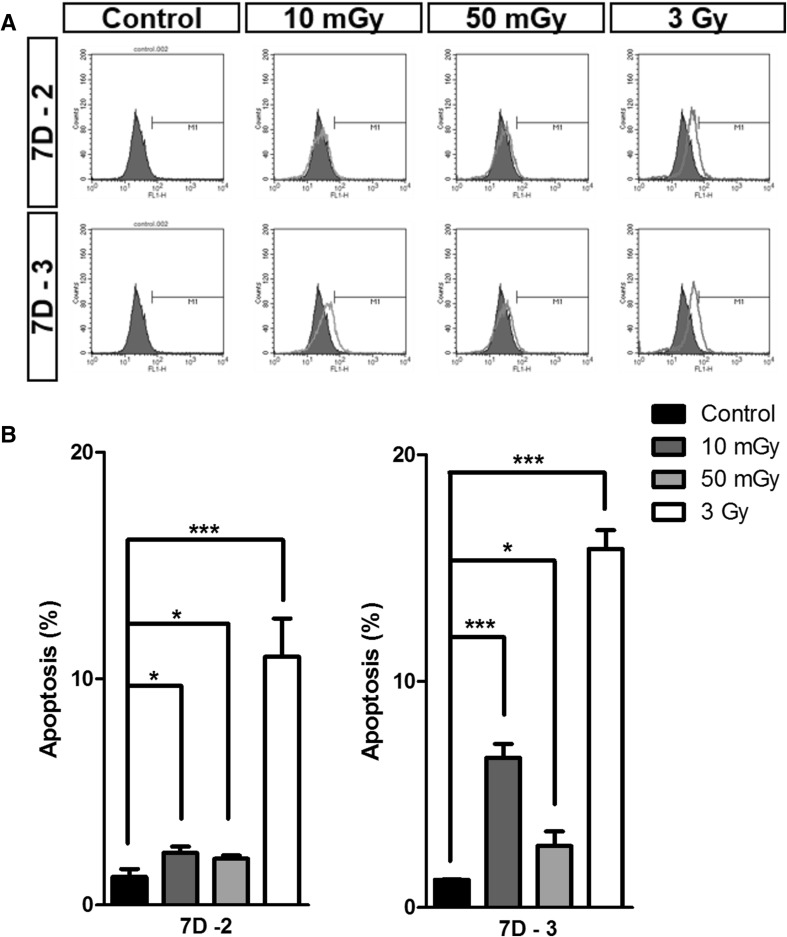
To evaluate cellular damage after irradiation, we compared apoptosis in LDIR-exposed hMSCs with that in 3-Gy–irradiated hMSCs using TUNEL assays (Fig. 3A, B). The frequency of apoptotic cells in the 7D-2 group exposed to 10 mGy, 50 mGy and 3 Gy irradiation was 2.31% ± 0.14%, 2.04% ± 0.32% and 10.97% ± 0.94%, respectively, compared with 1.24% ± 0.03% in controls (0 Gy). In the 7D-3 group, the corresponding values were 6.61% ± 0.62%, 2.72% ± 0.63%, and 15.84% ± 0.83%, respectively, compared with 1.23% ± 0.02% in controls (0 Gy). These latter data indicate that, although apoptosis in LDIR-exposed hMSCs was significantly lower than that in 3-Gy–irradiated hMSCs, more than three doses of LDIR might lead to hMSC apoptosis.
Fig. 3.
LDIR induced apoptosis were less than 10% at day 7. A LDIR induced apoptosis of hMSCS were measured by TUNEL assay. B The graphs presented LDIR induced apoptosis at day 7 two times or three times irradiated hMSCs (n = 3). (*p < 0.05, ** p < 0.01, and *** p < 0.001)
Cellular senescence and DNA-damage responses of irradiated hMSCs
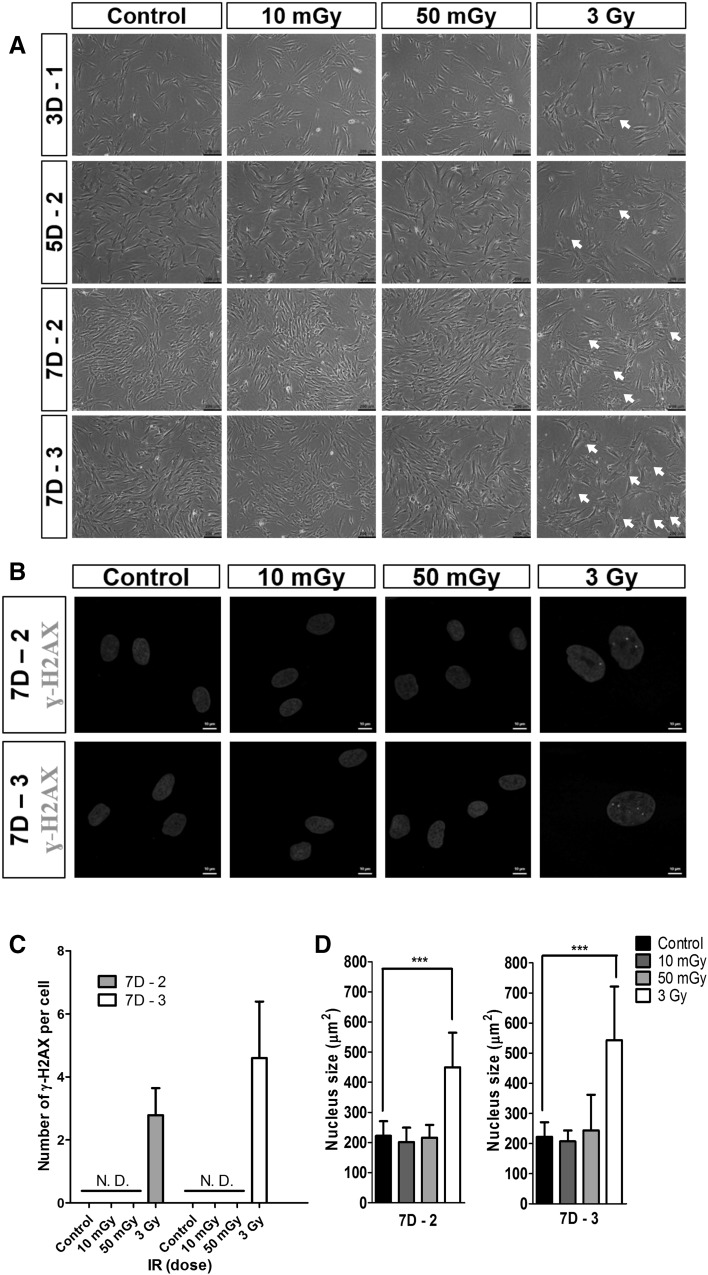
To determine how LDIR affects cellular senescence and DNA-damage responses in hMSCs, we compared dose effects on morphological changes of hMSCs. As shown in Fig. 4A, hMSCs exposed to 10 or 50 mGy LDIR maintained their normal spindle-shaped fibroblast morphology, showing no change in morphology compared with the non-irradiated control group. They also showed a time-dependent increase in their cell density. However, hMSCs irradiated with a dose of 3 Gy showed enlarged or flattened, senescent morphologies on day 3 (Fig. 4A, arrow). This senescent behavior of hMSCs increased gradually with culture time and number of irradiation doses. hMSCs irradiated with a dose of 3 Gy also showed lower cell densities than LDIR-exposed hMSCs.
Fig. 4.
LDIR did not induce DNA damage response in hMSCs. A Morphologies after two or three times LDIR irradiated hMSCs. LDIR irradiated hMSCs increased cell density. 3 Gy irradiated hMSCs showed senescence morphology (white arrow). B Expressions of γ-H2AX foci. C Numbers of γ-H2AX foci per two or three times 3 Gy irradiated hMSCs. D Sizes of nuclei of LDIR irradiated hMSCs. More than twenty nuclei in Fig. 4B were analyzed by ImageJ. (*** p < 0.001)
We evaluated LDIR effects on DNA damage and nuclei size. To assess the DNA-damage effects of LDIR, we evaluated expression of γ-H2AX foci, a marker of radiation-induced DNA strand breaks, using immunofluorescence and a quantitative assay. As shown in Fig. 4 (B and C), no γ-H2AX foci were detected in nuclei of LDIR-exposed hMSCs or non-irradiated hMSCs, indicating that the low dose used in these experiments did not induce DNA strand breaks. By contrast, clear evidence of DNA damage was detected following exposure to 3 Gy IR; at this dose, the average numbers of γ-H2AX foci in hMSCs exposed twice or three times to a dose of 3 Gy were 2.78 ± 0.86 and 4.60 ± 1.79 per cell, respectively. To evaluate the relationship between DNA damage and changes in nuclear size after exposure to IR, we measured the sizes of nuclei in at least 20 hMSC nuclei using image J (Fig. 4B, D). We found no significant differences in DNA-damage and nuclear size between LDIR-exposed hMSCs and non-irradiated controls. Interestingly, nucleus size in 3-Gy–irradiated hMSCs was almost 2-fold greater than that of LDIR-exposed hMSCs. The sizes of nuclei in irradiated hMSCs were increased after IR irradiation in a dose-dependent manner, especially at higher doses (>3 Gy).
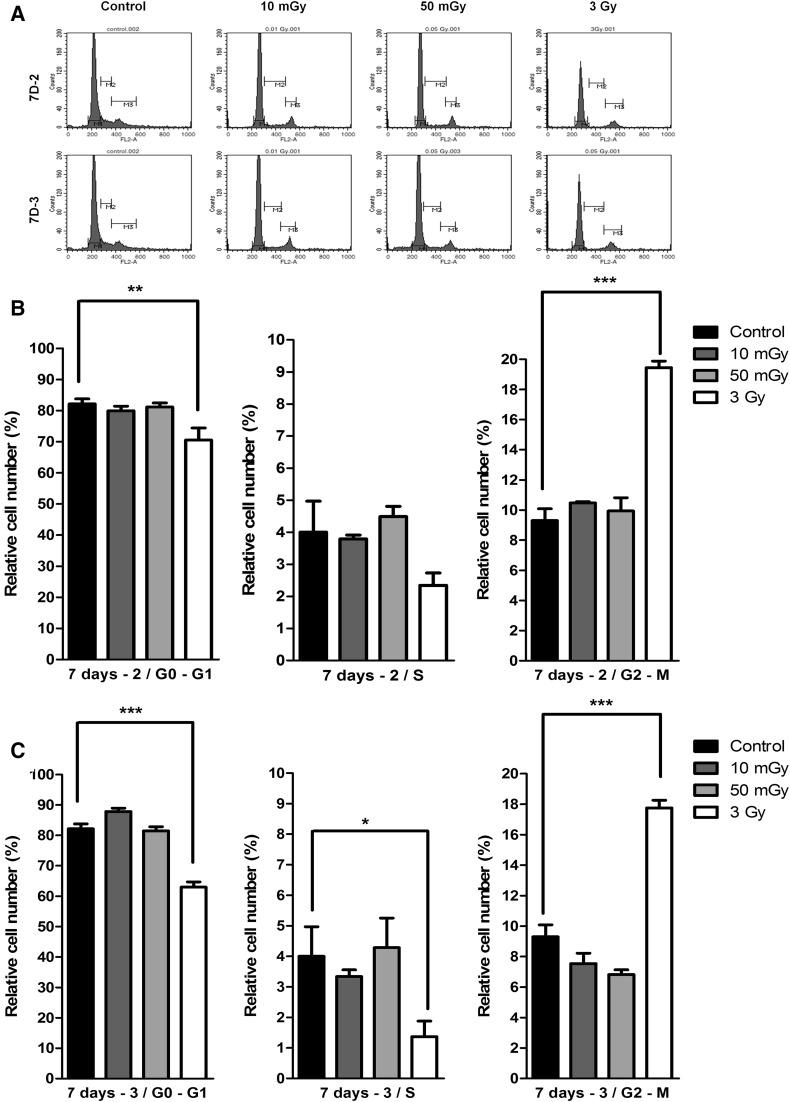
Cell cycle of irradiated hMSCs
We evaluated the effect of LDIR on the cell cycle of hMSCs using flow cytometry (Fig. 5). Quantitative analyses of G0/G1, S, and G2/M phase populations revealed by flow cytometry showed that 3-Gy–irradiated hMSCs exhibited a decrease in G0/G1 and S phase populations and an increase in the G2/M phase population (Fig. 5B). However, hMSCs exposed to two-dose LDIR at 10 mGy per dose showed no difference in the G2/M phase population compared with non-irradiated controls (Fig. 5C).
Fig. 5.
Cell cycle arrests were not occurred by LDIR to hMSCs. Whereas 3 Gy irradiated hMSCs showed G2-arrest. A Cell cycles were measured by flow cytometry. The graphs presented G0/G1, S, and G2/M phase at day 7 two times irradiated hMSCs B and three times irradiated hMSCs C (n = 3). (*p < 0.05, ** p < 0.01, and *** p < 0.001)
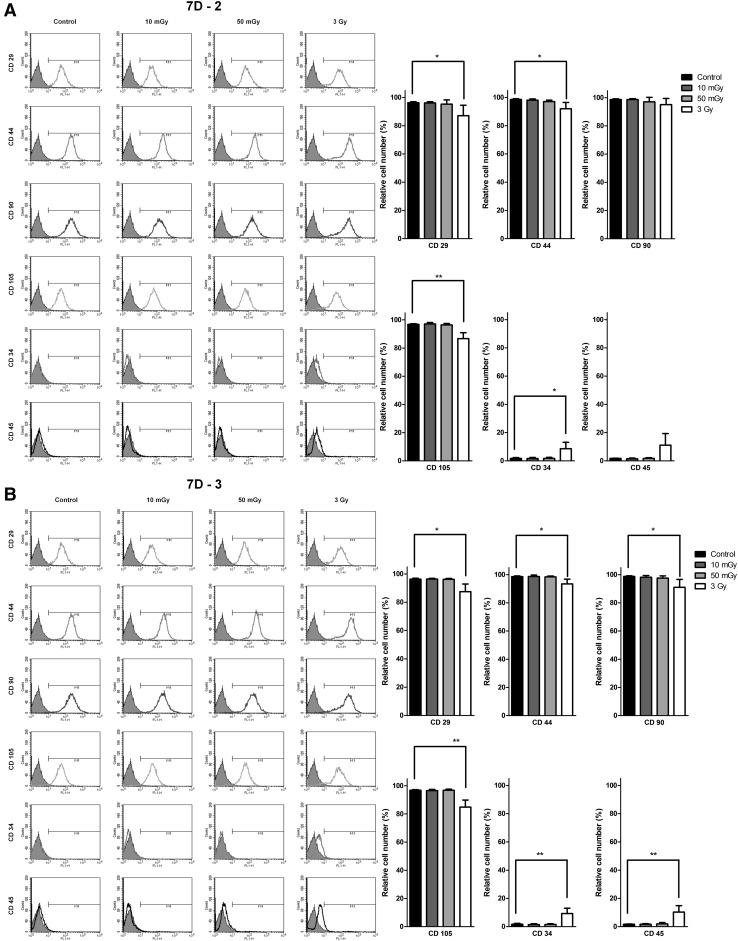
Stemness of irradiated hMSCs
To further determine whether LDIR affects the stemness of hMSCs, we examined the expression of surface CD markers after two-dose LDIR at 10 mGy per dose. To this end, we compared surface expression of positive markers (CD29, CD44, CD90, and CD105) and negative markers (CD34 and CD45) of hMSCs by FACS before and after γ-irradiation (Fig. 6). Quantitative analyses of FACS data showed no difference in the expression of positive CD markers between LDIR-exposed hMSCs and non-irradiated controls. In contrast, hMSCs irradiated at a dose of 3 Gy showed reduced expression of positive hMSC CD markers.
Fig. 6.
Phenotypic characteristics and differentiation potentials of hMSCs were maintained after LDIR. LDIR irradiated hMSCs were analyzed with flow cytometry. The graphs showed that the means and S.D. for hMSCs CD marker after two times LDIR A or three times LDIR B at day 7 (n = 4). (*p < 0.05, ** p < 0.01, and *** p < 0.001)
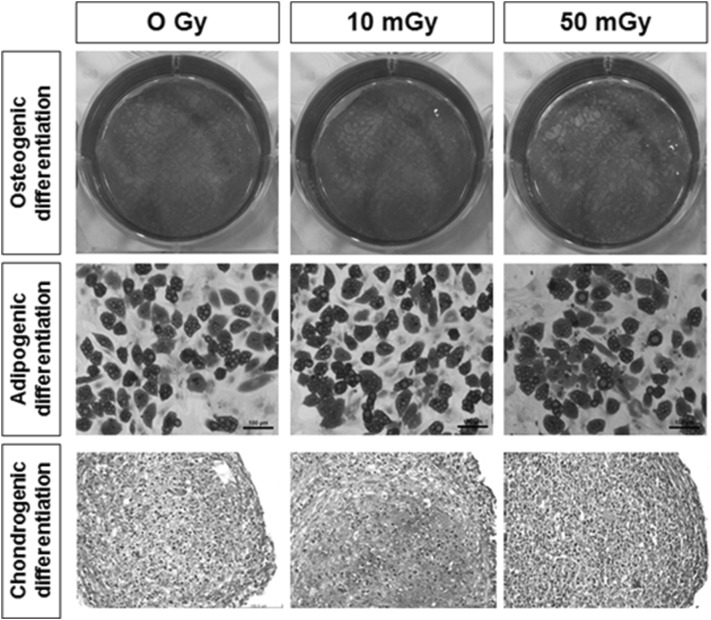
To examine the effects of LDIR (10 and 50 mGy) on the stemness of hMSCs, we evaluated changes in the differentiation potential of hMSCs before and after two-dose LDIR at 10 mGy per dose. As shown in Fig. 7, intact hMSCs and LDIR-exposed hMSCs were induced to differentiate into osteogenic-, adipogenic-, and chondrogenic-lineage cells. LDIR-exposed hMSCs were cultured in osteogenic or adipogenic induction media for 28 and 15 days, respectively. To confirm osteoblast differentiation, we stained LDIR-exposed hMSCs with alizarin red S to evaluated calcium deposition around cells. Adipogenic differentiation was determined by assessing lipid droplet formation, using oil red O staining. For chondrogenic differentiation, LDIR-exposed hMSCs were pellet-cultured and maintained for 6 weeks in chondrogenic induction media, after which chondrocyte-differentiated pellets were treated with safranin O to stain glycosaminoglycans. These analyses revealed no significant differences in the in vitro differentiation potential between LDIR-exposed hMSCs and non-irradiated controls.
Fig. 7.
Osteogenic, adipogenic and chondrogenic differentiation potentials of LDIR irradiated hMSCs. Osteogenic differentiation was confirmed by alizarin red S staining. Adipogenic differentiation was stained with oil red O staining. And pellet cultured hMSCs were stained by safranin O staining to confirm Chondrogenic differentiation
Discussion
Several studies have reported that exposure of cells to LDIR not only provides protection against radiation-induced damage, but also stimulates cellular processes such as proliferation, protein synthesis, and immune responses [14]. For example, Liang et al. studied that LDIR at doses up to 100 mGy could stimulate the proliferation of rMSCs [19]. To date, IR has not been used for external cellular stimulation of hMSCs in tissue engineering applications. The effects of LDIR less than 50 mGy on biological properties and stem cell characteristics of hMSCs remain largely unknown.
In this study, we examined the possibility of using LDIR as an external stimulator of hMSCs for tissue engineering applications. hMSCs were exposed two or three times to LDIR at doses less than 50 mGy; 3 Gy was used as a negative control. Proliferation and viability of irradiated and control hMSCs were evaluated by counting viable cells. Interestingly, 10 mGy of LDIR increased hMSC viability by 8% (p < 0.001) compared with non-irradiated hMSCs (Fig. 2). The observation on proliferation of hMSCs were consistent with those of rMSCs [19]. LDIR less than 50 mGy had no significant effect on the viability or apoptosis of hMSCs (Fig. 3). These data indicate that two-dose LDIR at 10 mGy per dose enhanced the proliferation of hMSCs and maintained their viability.
Before examining the effects of LDIR on the stemness of hMSCs, we compared dose effects on cellular senescence and DNA-damage responses in hMSCs. It is well known that cellular senescence, characterized by the indicated cellular morphological changes and elevated expression of senescence-associated β-galactosidase, is commonly caused by IR-induced DNA damage and oxidative stress. Multiple mechanisms, including telomere shortening, strong mitotic signals, activation of tumor-suppressor genes and DNA damage, can trigger cellular senescence [23]. Thus, IR-induced cellular senescence might affect the stemness characteristics of hMSCs.
Thus, we evaluated its effects on DNA damage and nuclei size, using a quantitative assay, and expression of γ-H2AX foci, as a marker of radiation-induced DNA strand breaks, respectively. As shown in Fig. 4 (B and C), no γ-H2AX foci were detected in nuclei of LDIR-exposed hMSCs or non-irradiated hMSCs, indicating that the low dose used in these experiments did not induce DNA strand breaks. By contrast, clear evidence of DNA damage was detected following exposure to 3 Gy IR.
Besides, at 3 Gy, irradiated hMSCs exhibited enlarged or flattened morphologies, consistent with a DNA damage-induced senescence-like morphology [22–24]. However, LDIR-exposed hMSCs showed no evidence of a senescence-like morphology that would be indicative of DNA damage (Fig. 4). It has been reported that cellular senescence, a DNA damage response that occurs after exposure to IR, accelerates the increase in the size of cancer cell nuclei [25]. The sizes of nuclei in irradiated hMSCs were increased after IR irradiation in a dose-dependent manner, especially at higher doses (>3 Gy). The DNA damage-associated increase in the size of nuclei in 3-Gy–irradiated hMSCs might account for the senescence morphologies of hMSCs. In contrast, LDIR-exposed hMSCs showed no evidence of induction of not only cellular senescence or DNA damage, but also G2/M cell cycle arrest in hMSCs.
Finally, we characterized the maintenance of hMSC stemness, phenotypic surface markers, and in vitro differentiation potential after exposure to LDIR. hMSCs irradiated at a dose of 3 Gy showed reduced expression of positive hMSC CD markers (Fig. 6). Because cellular senescence is associated with changes in the stemness characteristics of hMSCs, these results are consistent with the effects of 3 Gy IR on senescence, noted above, as well as the absence of a cellular senescence effect of LDIR at doses of 10 or 50 mGy. Then, we evaluated changes in the differentiation potential of hMSCs before and after two-dose LDIR at 10 mGy per dose. LDIR-exposed hMSCs were induced to differentiate into osteogenic-, adipogenic-, and chondrogenic-lineage cells. To confirm osteoblast differentiation, we stained LDIR-exposed hMSCs with alizarin red S to evaluated calcium deposition around cells, a marker of osteogenic differentiation. Adipogenic differentiation was determined by assessing lipid droplet formation, a marker of adipogenic differentiation, using oil red O staining. For chondrogenic differentiation, pellet-cultured LDIR-exposed hMSCs were treated with safranin O to stain glycosaminoglycans, markers of chondrogenic differentiation. These in vitro differentiation analyses revealed no significant differences in the differentiation potential between LDIR-exposed hMSCs and non-irradiated controls (Fig. 7).
Clinical applications of hMSCs rely on the ability to acquire sufficient numbers of cells. To date, a number of physical stimuli, including tensile, compression, ultrasound and hypoxic conditions, have been used to enhance proliferation and differentiation of hMSCs for tissue engineering applications [10–13]. In addition, exposure of cells to LDIR imparts a radiation hormesis effect, providing not only protection from IR-induced damage, but also stimulating processes such as cell proliferation, protein synthesis, and immune responses. For example, Hong et al. [26] reported that low-dose γ-radiation blocks IL-1β–induced cartilage disorders by inhibiting catenin signaling. We also showed that two-dose LDIR at 10 mGy per dose enhanced the proliferation of hMSCs, but did not affect cellular senescence or DNA damage in hMSCs. LDIR-exposed hMSCs maintained their fibroblastic morphology and nuclear size compared with non-irradiated controls, and did not form γ-H2AX foci. hMSCs exposed to this LDIR regimen also maintained their stemness, as reflected in their phenotypic characteristics and in vitro differentiation potential.
In summary, we evaluated the possibility of using LDIR as an external biological stimulus for hMSCs. Two-dose LDIR at 10 mGy per dose increased the proliferation of hMSCs while maintaining their stem cell characteristics and in vitro differentiation potential. Importantly, LDIR at doses less than 50 mGy did not affect cellular properties, promote cellular senescence, or cause DNA damage responses in hMSCs. Thus, LDIR stimulation at doses below 50 mGy using γ-rays could be a promising external-stimulation approach for achieving in vitro expansion of hMSCs for tissue engineering and regenerative medicine applications. This hormesis effect of LDIR might also be extended to hMSCs in vivo as a therapeutic strategy for repairing normal tissue damaged during radiotherapy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This research was supported by the National R&D Program through the Korea Institute of Radiological and Medical Sciences funded by the Ministry of Science, ICT & Future Planning (1711021779) and the Technology Innovation Program (10053595, Development of functionalized hydrogel scaffold based on medical grade biomaterials with 30% or less of molecular weight reduction) funded by the Ministry of Trade, Industry and Energy (MOTIE, Korea).
Conflict of interest
The authors have no conflicts of interest.
Ethical statement
There are no animal experiments carried out for this article.
References
- 1.Ohishi M, Schipani E. Bone marrow mesenchymal stem cells. J Cell Biochem. 2010;109:277. doi: 10.1002/jcb.22399. [DOI] [PubMed] [Google Scholar]
- 2.Bajada S, Mazakova I, Richardson JB, et al. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2:169. doi: 10.1002/term.83. [DOI] [PubMed] [Google Scholar]
- 3.Park J-B, Lee K, Lee W, et al. Establishment of the chronic bone defect model in experimental model mandible and evaluation of the efficacy of the mesenchymal stem cells in enhancing bone regeneration. Tissue Eng Regen Med. 2013;10:18. doi: 10.1007/s13770-013-0368-6. [DOI] [Google Scholar]
- 4.Shetty AA, Kim SJ, Shetty V, et al. Autologous bone-marrow mesenchymal cell induced chondrogenesis: single-stage arthroscopic cartilage repair. Tissue Eng Regen Med. 2014;11:247. doi: 10.1007/s13770-014-0061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hong HS, Son Y. Substance-p-mobilized mesenchymal stem cells accelerate skin wound healing. Tissue Eng Regen Med. 2014;11:483. doi: 10.1007/s13770-014-0062-3. [DOI] [Google Scholar]
- 6.Xiong Y, Yang S, Liao W, et al. Autonomic dysreflexia during cystolitholapaxy in patients with spinal cord injury. Minerva Urol Nefrol. 2015;67:85. [PubMed] [Google Scholar]
- 7.Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Transl Med. 2014;3:643. doi: 10.5966/sctm.2013-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eom YW, Oh JE, Lee JI, et al. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2014;445:16. doi: 10.1016/j.bbrc.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 9.Al-Habib M, Yu Z, Huang GT. Small molecules affect human dental pulp stem cell properties via multiple signaling pathways. Stem Cells Dev. 2013;22:2402. doi: 10.1089/scd.2012.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Charoenpanich A, Wall ME, Tucker CJ, et al. Cyclic tensile strain enhances osteogenesis and angiogenesis in mesenchymal stem cells from osteoporotic donors. Tissue Eng Part A. 2014;20:67. doi: 10.1089/ten.tea.2013.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pelaez D, Huang CY, Cheung HS. Cyclic compression maintains viability and induces chondrogenesis of human mesenchymal stem cells in fibrin gel scaffolds. Stem Cells Dev. 2009;18:93. doi: 10.1089/scd.2008.0030. [DOI] [PubMed] [Google Scholar]
- 12.Wang Y, Peng W, Liu X, et al. Study of bilineage differentiation of human-bone-marrow-derived mesenchymal stem cells in oxidized sodium alginate/N-succinyl chitosan hydrogels and synergistic effects of RGD modification and low-intensity pulsed ultrasound. Acta Biomater. 2014;10:2518. doi: 10.1016/j.actbio.2013.12.052. [DOI] [PubMed] [Google Scholar]
- 13.Fotia C, Massa A, Boriani F, et al. Hypoxia enhances proliferation and stemness of human adipose-derived mesenchymal stem cells. Cytotechnology. 2015;67:1073. doi: 10.1007/s10616-014-9731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luckey TD. Radiation hormesis: the good, the bad, and the ugly. Dose Response. 2006;4:169. doi: 10.2203/dose-response.06-102.Luckey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai L, Liu SZ. Induction of cytogenetic adaptive response of somatic and germ cells in vivo and in vitro by low-dose X-irradiation. Int J Radiat Biol. 1990;58:187. doi: 10.1080/09553009014551541. [DOI] [PubMed] [Google Scholar]
- 16.Cai L, Jiang J, Wang B, et al. Induction of an adaptive response to dominant lethality and to chromosome damage of mouse germ cells by low dose radiation. Mutat Res. 1993;303:157. doi: 10.1016/0165-7992(93)90017-P. [DOI] [PubMed] [Google Scholar]
- 17.Li W, Wang G, Cui J, et al. Low-dose radiation (LDR) induces hematopoietic hormesis: LDR-induced mobilization of hematopoietic progenitor cells into peripheral blood circulation. Exp Hematol. 2004;32:1088. doi: 10.1016/j.exphem.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 18.Guo WY, Wang GJ, Wang P, et al. Acceleration of diabetic wound healing by low-dose radiation is associated with peripheral mobilization of bone marrow stem cells. Radiat Res. 2010;174:467. doi: 10.1667/RR1980.1. [DOI] [PubMed] [Google Scholar]
- 19.Liang X, So YH, Cui J, et al. The low-dose ionizing radiation stimulates cell proliferation via activation of the MAPK/ERK pathway in rat cultured mesenchymal stem cells. J Radiat Res. 2011;52:380. doi: 10.1269/jrr.10121. [DOI] [PubMed] [Google Scholar]
- 20.Kurpinski K, Jang DJ, Bhattacharya S, et al. Differential effects of x-rays and high-energy 56Fe ions on human mesenchymal stem cells. Int J Radiat Oncol Biol Phys. 2009;73:869. doi: 10.1016/j.ijrobp.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Nicolay NH, Sommer E, Lopez R, et al. Mesenchymal stem cells retain their defining stem cell characteristics after exposure to ionizing radiation. Int J Radiat Oncol Biol Phys. 2013;87:1171. doi: 10.1016/j.ijrobp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 22.Alessio N, Del Gaudio S, Capasso S, et al. Low dose radiation induced senescence of human mesenchymal stromal cells and impaired the autophagy process. Oncotarget. 2015;6:8155. doi: 10.18632/oncotarget.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim KS, Kim JE, Choi KJ, et al. Characterization of DNA damage-induced cellular senescence by ionizing radiation in endothelial cells. Int J Radiat Biol. 2014;90:71. doi: 10.3109/09553002.2014.859763. [DOI] [PubMed] [Google Scholar]
- 24.Ma J, Shi M, Li J, et al. Senescence-unrelated impediment of osteogenesis from Flk1 + bone marrow mesenchymal stem cells induced by total body irradiation and its contribution to long-term bone and hematopoietic injury. Haematologica. 2007;92:889. doi: 10.3324/haematol.11106. [DOI] [PubMed] [Google Scholar]
- 25.Menendez JA, Cufi S, Oliveras-Ferraros C, et al. Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer. Aging (Albany NY) 2011;3:1063. doi: 10.18632/aging.100407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hong EH, Song JY, Lee SJ, et al. Low-dose gamma-radiation inhibits IL-1beta-induced dedifferentiation and inflammation of articular chondrocytes via blockage of catenin signaling. IUBMB Life. 2014;66:128. doi: 10.1002/iub.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.