Abstract
Adverse drug reactions, also called side effects, range from mild to fatal clinical events and significantly affect the quality of care. Among other causes, side effects occur when drugs bind to proteins other than their intended target. As experimentally testing drug specificity against the entire proteome is out of reach, we investigate the application of chemogenomics approaches. We formulate the study of drug specificity as a problem of predicting interactions between drugs and proteins at the proteome scale. We build several benchmark datasets, and propose NN-MT, a multi-task Support Vector Machine (SVM) algorithm that is trained on a limited number of data points, in order to solve the computational issues or proteome-wide SVM for chemogenomics. We compare NN-MT to different state-of-the-art methods, and show that its prediction performances are similar or better, at an efficient calculation cost. Compared to its competitors, the proposed method is particularly efficient to predict (protein, ligand) interactions in the difficult double-orphan case, i.e. when no interactions are previously known for the protein nor for the ligand. The NN-MT algorithm appears to be a good default method providing state-of-the-art or better performances, in a wide range of prediction scenario that are considered in the present study: proteome-wide prediction, protein family prediction, test (protein, ligand) pairs dissimilar to pairs in the train set, and orphan cases.
1 Introduction
1.1 Drug specificity
The current paradigm in rationalized drug design is to identify a small molecular compound that binds to a protein involved in the development of a disease in order to alter disease progression. Once a hit ligand has been identified, often by combining in silico and in vitro approaches, this molecule needs to be optimized in order to meet the ADME (Absorption, Distribution, Metabolism, Elimination), toxicity, and industrial synthesis requirements. Finally, pre-clinical and clinical assays are organized to obtain agreement from the regulatory agencies. When successful, this process often lasts more than ten years, and recent estimates set the cost of drug development in US$2.5 billion in 2013 [1].
This complex, long, and costly process is often interrupted because of adverse drug reactions (ADR, also called side effects) that appear at various stages of drug development, or even after the drug has reached the market. In the USA, ADRs have been estimated to have an annual direct hospital cost of US$1.56 billion [2]. A meta-analysis estimated that, in hospitalized patients, the incidence of severe ADR was 1.9% − 2.3%, while the incidence of fatal ADR was 0.13% − 0.26%. For the year 1994, this means that 2216000 patients hospitalized in the US suffered from a serious ADR, and approximately 106000 died [3]. Finally, a recent review [4] found that between 1950 and 2014, 462 medicinal products were withdrawn from the market in at least one country due to ADR. Of these 462 withdrawn drugs, 114 were associated with deaths.
Side effects frequently occur when drugs lack specificity, which means that they bind to proteins other than their intended target [5]. In that case, the molecular mechanisms at the source of the therapeutic effect and of the unwanted side effects are of similar nature: they both involve interactions between the drug and a protein. However, the complete study of drug specificity at early stages of drug development is experimentally out of reach, since it would require the evaluation of potential interactions between the hit molecule and the entire human proteome. Therefore, there is a strong incentive to develop in silico methods that predict specificity. The goal is to reduce the number of experiments to be performed, identify drug candidates that should be dropped because of their lack of specificity, protect patients from deleterious ADRs, and reduce the expense of time and money for the pharmaceutical industry.
1.2 Protein-ligand interactions prediction
The study of a drug’s specificity mainly boils down to predicting its protein targets in the space of the human proteome, or at least at the scale of “druggable” human proteins, i.e. proteins that present pockets into which drugs can bind. The approaches that have been developed to predict interactions between a protein and a small molecule can be separated into three categories.
First, ligand-based approaches such as Quantitative Structure Activity Relationship (QSAR) (refer to [6] for a recent review on QSAR) build a model function that predicts the affinity of any molecule for a given target, based on the affinities of known ligands for this target. They are efficient to study the affinity of molecules against a given protein target, but they are not suitable to study the specificity of a molecule against a large panel of proteins. This would indeed require, for each of the considered proteins, that the binding affinities of multiple ligands were available.
The second category is docking (refer to [7] for a recent review on docking), also called target-based approaches. Docking is a molecular modeling method that predicts the affinity of a ligand for a protein based on the estimated interaction energy between the two partners. However, it relies on the 3D structure of the proteins, which strongly limits its application on a large scale.
Finally, chemogenomic approaches [8] can be viewed as an attempt to fill a large binary interaction matrix where rows are molecules and columns are proteins, partially filled with the known protein-ligand interaction data available in public databases such as the PubChem database at NCBI [9]. In this context, drug specificity prediction is formulated as a classification problem, where the goal is to distinguish protein-ligand pairs that bind from those that do not: the aim is to predict “interacting” or “not interacting” labels for all pairs, but not to predict the strength of the interaction, which would correspond to a regression problem. Chemogenomics mainly belong to supervised machine learning (ML) methods, which learn mathematical models from available data, and use these models to make predictions on unknown data.
Various chemogenomics methods have been proposed in the last decade [10–24]. They all rely on the assumption that “similar” proteins are expected to bind “similar” ligands. They differ by (i) the descriptors used to encode proteins and ligands, (ii) how similarities between these objects are measured, (iii) the ML algorithm that is used to learn the model and make the predictions.
Predecessors of our approach include Support Vector Machine (SVM) [11], kernel Ridge Linear Regression (kernelRLS) [10, 12, 18–20], and matrix factorization (MF) [22–24].
MF approaches decompose the interaction matrix that lives in the (protein, molecule) space into the product of matrices of lower rank, living in the two latent spaces of proteins and of molecules. The most recent and efficient MF based approach by [24] consider more specifically Logistic Matrix Factorization [25]. They display good performances and are also computationally efficient. [24] also generalized their approach to orphan molecules and proteins by computing latent representations of orphan molecules and proteins as a weighted sum of the latent representation of their neighbors.
BLM make prediction for a (protein, molecule) pair based first on the prediction of target proteins for the considered molecule, and then on the prediction of ligand molecules for the considered protein. The predictor used is the kernelRLS. This gives two independent predictions for each putative drug-target interaction, which are combined into a final prediction.
Finally, kernel methods using the Kronecker product of the molecule and protein space (presented in the next section) can handle orphan cases, but are more computationally expensive. Among them, although the KronRLS method (a Kernel Regularized Least Square classifier) succeeded to dramatically reduce the computational complexity of its exact solution when used on the Kronecker product of the molecule and protein space, and is hence applicable to large scale chemogenomics studies, SVM-based methods are still computationally inappropriate at such scale. The present study propose a SVM-based approach and aims at addressing this issue.
In most cases, previous studies have been implemented to predict interactions of molecules with proteins belonging to the same family, such as kinases or GPCRs [10, 18–24, 26, 27]. A few studies have been devoted to larger scales in the protein space, such as [17] which however does not focus on settings relevant to the prediction of drug specificity. Some rely on the 3D structure of the binding pocket [28, 29], which limits the number of proteins that can be considered, others on coarse protein descriptors based on the presence of structural or functional domains [30]. In the present paper, we propose a computationally efficient approach to study the applicability of these ML techniques to the entire druggable proteome.
1.3 Single-task and multi-task algorithms
In the context of the present paper, a Single-Task method consists in predicting protein targets for a given molecule m. In this setting, the specificity of m is studied by learning a model function fm(p) that predicts whether molecule m interacts with protein p, based on known protein targets for m. This means that a new model function is learned for each molecule. We refer to this setting as ligand-based ST. Conversely, a single-task method could learn a model function fp(m) that predicts whether protein p interacts with molecule m, based on all ligands known for protein p. We call this the target-based ST setting.
In contrast, Multi-Task methods predict whether p and m interact by training a model based on all known interactions, including those involving neither m nor p. In other words, the task of predicting ligands for protein p is solved not only based on the data available for this task (i.e. known ligands for this protein), but also based on the data available for the other tasks (i.e. known ligands any other protein). The main issue is to define how the data available in all tasks can be used to make the predictions made for a given task.
More generally, the main idea behind multi-task learning is that, when solving several related tasks for which the data available for each task are scarce, a multi-task framework defines how to share information across tasks, which can improve the performance of the final prediction models that can be built.
In our case, the tasks are predictions of ligands for proteins whose sequences can be compared, which explains how the tasks are related. Such approaches are of particular interest when few interactions are known for a given ligand or protein, as is often the case when looking for secondary targets at the size of the human proteome. Even within the multi-task framework, orphan proteins (for which no ligand is known) and proteins for which the only known ligands are very dissimilar to the tested molecule, are those for which predictions are the most difficult. Therefore, our study will focus more particularly on these cases.
Various multi-task methods have been proposed in a number of applications that do not refer to bioinformatics [31–34]. Among them, multi-tasks methods have been proposed to solve the problem using kernel with Support Vector Methods (SVM, their basic principles are recalled in Supplementary Materials). Such multi-task kernel methods have widely been used in bioinformatics, including for chemogenomics applications [8]. Our contributions in the present paper belong to this category of methods that we briefly review in the next section.
1.4 Kernel methods for chemogenomics
In this study, we formalize and solve the problem of drug-target interaction prediction with Support Vector Machines (SVM), an algorithm for learning a classification or a regression rule from labeled examples [35].
Intuitively, SVMs seeks to find the optimal hyperplane separating two classes of data points. In addition to the choice of kernels, SVMs requires an important regularization parameter classically called C. This parameter controls the trade-off between maximizing the margin (i.e. the distance separating the hyperplane and the classes distributions) and classification errors on the training points. A deeper intuition on how SVM works and how this regularization parameters plays its role is given in Supplementary Materials S1. Additionally, as recalled in Supplementary Materials S1, although SVMs can be solved from vector representations of the data, they can also be solved using the “kernel trick”, based only on the definition of a kernel function K which gives the similarity value K(x, x′) between all pairs of data points x and x′, without needing an explicit representation of the data. Many kernels have been proposed for molecules and for proteins, and an overview of such kernels is presented in the Material and Methods section.
In chemogenomics, our goal can be viewed as finding the optimal hyperplane that separates the pairs (m, p) of molecules and proteins that interact from those that do not interact. This classification task can be solved using an SVM with a kernel Kpair defined on (ligand, protein) pairs. Given N example pairs, solving the SVM in the space of (m, p) pairs using the Kpair kernel corresponds to finding the optimal αi coefficients such that (see Supplementary Materials S1):
| (1a) |
| (1b) |
| (1c) |
A general method to build a kernel on such pairs is to use the Kronecker product of the molecule and protein kernels [36]. Given a molecule kernel Kmolecule and a protein kernel Kprotein, the Kronecker kernel Kpair is defined by:
| (2) |
Thus, the Kronecker kernel Kpair captures interactions between features of the molecule and features of the protein that govern their interactions (see Supplementary Materials S2 for an explicit definition of the Kronecker product of two matrices). If Kmolecule is a n × n matrix and Kprotein is a p × p matrix, their Kronecker product Kpair has size np #x00D7; np. In the context of chemogenomics, this can correspond to a very large size, leading to intractable computations. However, one interesting property of the Kronecker kernel is that calculating its values on a data set of (m, p) pairs does not require storing this entire matrix since it is sufficient to store Kmolecule(m, m′) and Kprotein(p, p′).
Therefore, solving the SVM (Eq 1) only requires calculation of the Kmolecule and Kprotein kernels according to Eq 2.
Once the αi coefficients have been determined, the ability of a given (m, p) pair to interact is predicted based on:
| (3) |
This equation illustrates why the use of such a kernel can be viewed as a multi-task method. Indeed, in a single-task approach where one task corresponds to the prediction of ligands for a given protein p, the ability of molecule m to bind protein p would be estimated by:
| (4) |
where the mi molecules are ligand and non-ligand molecules known for protein p.
In the multi-task setting, f((m, p)) evaluates the ability of m to bind to protein p using mi molecules that are all ligand or non-ligand molecules known for all proteins pi. However, the contribution of the labels yi of ligands for pi proteins that are different from p to calculate f((m, p)) is weighted by Kprotein(p, pi). In other words, the more similar two tasks (i.e. the corresponding proteins) are, the more known instances for one of the task will be taken into account to make predictions for the other task.
Such kernel-based multi-task approaches have been successfully applied to biological problems, including the prediction of protein-ligand interactions [8, 11, 37, 38].
1.5 Contribution
The goal of this paper is to investigate the application of multi-task Support Vector Machines (SVM) to the prediction of drug specificity, by predicting interactions between the drug of interest and the entire druggable proteome. To this end, we evaluate our multi-task SVMs based methods and state-of-the-art approaches in several key scenario that explore the impact of the similarity between the query (protein, molecule) pair and the training data on the prediction performance, a point that is rarely discussed in the literature. We also explore their applicability to orphan settings, a situation often encountered large scale studies, and where single-task methods are not applicable.
We first discuss how to generate negative training examples, and we optimize protein and molecule kernels in the spaces of drug-like molecules and druggable proteins. Our observations lead us to propose the NN-MT algorithm, a multi-task SVM for chemogenomics that is trained on a limited number of data points: for a query (protein, molecule) pair (p*, m*), the training data is composed of (1) all intra-task (protein, ligand) pairs defined by pairs (p, m) with either p = p* or m = m*; (2) a limited number of extra-task (protein, ligand) pairs, defined by pairs (p, m) with p ≠ p* and m ≠ m*, chosen based on the similarity of p and m to p* and m*, respectively; and (3) randomly picked negative examples (about ten times more than positive training pairs).
While the applicability of multi-task approaches can be limited in practice by computational times, our approach only requires training on a dataset of size similar to those used by single-task methods. We evaluate the performance on various assembled datasets in which the protein and/or the ligand are orphan.
Finally, compare the NN-MT algorithm to state-of-the-art approaches in drug-target interaction prediction [18–24]. We used and updated the PyDTI package [24], adding an implementation of NN-MT together with key cross-validation schemes and a DrugBank-based dataset built in the present study. In addition to all other experiments performed in the present study, this benchmark study concludes that NN-MT is a good default method providing state-of-the-art or better performances, in a wide range of prediction scenario that can be encountered in real-life studies: proteome-wide prediction, protein family prediction, test (protein, ligand) pairs dissimilar to pairs in the train set, and orphan cases.
All codes and all the datasets that were built are available at https://github.com/bplaye/efficient_MultiTask_SVM_for_chemogenomics/. The updated PyDTI package is available at https://github.com/bplaye/PyDTI/.
2 Materials and methods
2.1 Protein kernels
We used sequence-based kernels since they are suitable for proteome-wide approaches, unlike kernels relying on the 3D structure of the proteins or on binding pocket descriptions. Numerous studies have already been devoted defining descriptors of proteins based on amino-acid sequence [39–43]. We considered three sequence-based kernels: the Profile kernel [43], the SWkernel, and the LAkernel.
The Profile kernel uses as protein descriptors the set of all possible subsequences of amino acids of a fixed length k, and considers their position-dependent mutation probability. This kernel is available at http://cbio.mskcc.org/leslielab/software/string_kernels.html.
We also used two kernels that rely on local alignment scores. The first one is directly based on the Smith-Waterman (SW) alignment score between two proteins [44] and is called the SWkernel in the present paper. SW scores were calculated with the EMBOSS Water tool available at http://www.ebi.ac.uk/Tools/psa/emboss_water/. We built a kernel based on the SW score matrix by subtracting its most negative eigenvalue from all diagonal values. We also used the Local Alignment kernel (LAkernel) [42] which mimics the behavior of the SW score. However, while the SW score only keeps the contribution of the best local alignment between two sequences to quantify their similarity, the LAkernel sums up the contributions of all possible local alignments, which proved to be efficient for detecting remote homology [42]. This kernel is available at http://members.cbio.mines-paristech.fr/~jvert/software/.
Kernel hyperparameters values
The Profile kernel has two hyperparameters: the size k of the amino acid subsequences that are searched and compared, and the threshold t used to define the probabilistic mutation neighborhoods. We considered k ∈ {4, 5, 6, 7} and t ∈ {6, 7.5, 9, 10.5}. The SWkernel also has two hyperparameters: the penalties for opening a gap (o) and for extending a gap (e). We considered o ∈ {1, 10, 50, 100} and e ∈ {0.01, 0.1, 0.5, 1, 10}. The LAkernel has three hyperparameters: the penalties for opening (o) and extending (e) a gap, and the β parameter which controls the importance of the contribution of non-optimal local alignments in the final score. We considered o ∈ {1, 20, 50, 100}, e ∈ {0.01, 0.1, 1, 10}, and β ∈ {0.01, 0.5, 0.05, 0.1, 1}. All kernels hyperparameters were optimized by cross-validation (see Section 2.3).
In the last part of the study, we also considered kernels on proteins based on their family hierarchy. Indeed, the most important classes of drug targets have been organized into hierarchies established on the sequence and the function of the proteins within these families (GPCR [45], kinases [46] and ion channels [47]). As in [11], the hierarchy kernel is built based on the number of common ancestors shared by two proteins in the hierarchy. More precisely, Khierarchy(t, t′) = 〈ϕ(t), ϕ(t′)〉, where ϕ(t) is a binary vector for which each entry corresponds to a node in the hierarchy and is set to 1 if the corresponding node is part of t’s hierarchy and 0 otherwise.
All protein kernels were centered and normalized.
2.2 Small molecule kernels
Many descriptors have been proposed for molecules, based on physico-chemical and structural properties [48–51]. To measure the similarity between molecules, we considered two state-of-the-art kernels based on molecular graphs that represent the 2D structure of the molecules, with atoms as vertices and covalent bonds as edges. Both kernels compute similarities between molecules via the comparison of linear fragments found in their molecular graphs. They are available at http://chemcpp.sourceforge.net/.
The first one, called the Marginalized kernel [50], calculates the similarity between two molecules based on the infinite sets of random walks over their molecular graphs.
The second kernel, called the Tanimoto kernel, uses a description of molecules by vectors whose elements count the number of fragments of a given length. The similarity between molecules is based on the Tanimoto metric [48].
Kernel hyperparameters values
The Marginalized kernel has two hyperparameters: the stopping probability (while building a path) q in {0.01, 0.05, 0.1, 0.5}, and the Morgan Index (MI) in {2, 3, 4}. For both kernels, hyperparameters were selected by cross-validation (see Section 2.3). The Tanimoto kernel has one hyperparameter: the length d of the paths, which we considered in {2, 4, 6, 8, 10, 12, 14}. All molecule kernels were centered and normalized.
2.3 Evaluation of prediction performance
Prediction performance is commonly evaluated with a cross-validation (CV) scheme [52]: 1) the dataset is randomly split into K folds 2) the model is run K times, each run using the union of (K-1) folds as the training set, and measuring the performance on the remaining fold. Prediction performance are averaged over all folds. When hyperparameters had to be selected, we used a nested cross validation (Nested-CV) scheme [53]. It consists in a (K-1) folds cross validation (inner-CV) nested in a K folds cross validation (outer-CV). At each step of the outer-CV, the inner-CV is repeated for all considered values of the hyperparameters. The values leading to the best prediction performance are retained as optimal. We used K = 5, a classical value in CV.
We also considered leave-one-out cross-validation (LOO-CV), for which the number of folds is the number of available points in the dataset. The LOO-CV scheme is particularly useful when the number of samples is small. It was used in the present paper when the size of the considered dataset was too small to perform 5-fold-CV.
We estimated prediction performance using two scores that are classically employed to judge the quality of a classifier in case of drug-target interaction prediction. The first one is the area under the Receiver Operating Characteristic curve [54] (ROC-AUC). The ROC curve plots true positive rate as a function of false positive rate, for all possible thresholds on the prediction score. Intuitively, the ROC-AUC score of a classifier represents the probability that if a positive and a negative interaction are each picked at random from the dataset, the positive one will have a higher positive score than the negative one. The second one is the area under the Precision-Recall curve [55] (AUPR). It indicates how far the prediction scores of true positive interactions are from false positive interactions, on average. Although we used both the ROC-AUC and AUPR scores, since negative interactions are actually unknown interactions in protein-ligand interaction datasets, the AUPR is considered a more significant quality measure of the prediction method than the ROC-AUC. Indeed, it emphasizes the recovery of the positive samples and penalizes the presence of false positive examples among the best ranked points.
We used the Python library scikit-learn [56] to implement our SVM-based machine learning algorithms and we used the PyDTI package [24] for the other recent algorithms.
2.4 Datasets
Many publicly available databases such as KEGG Drug [57], DrugBank [58], or ChEMBL [59] can be used to build a learning dataset of protein-ligand interactions. We chose to build all the datasets used in the present study from the DrugBank database v4.3, because it contains FDA-approved drugs, or drug candidate molecules. This allowed optimize and test our models on drug-like molecules, on which they intend be applied. In addition, we assumed that the list of human proteins appearing as targets for molecules of DrugBank can represent a relevant “druggable” human proteome on which we could train models that predicting the specificity of drug-like molecules.
We built a first learning dataset called S, based on Version 4.3 of the DrugBank [58]. We selected all molecules targeting at least one human protein, and having a molecular weight between 100 and 600 g.mol−1, a range in which most small molecule marketed drugs are found [60]. This leads to a dataset composed of 3980 molecules targeting 1821 proteins, and including 9536 protein-ligand interactions that correspond to the positive training pairs. All other protein-ligand pairs are unlabeled because no interactions were recorded for them in the database. Most of these pairs are expected not to interact, but a small number of them are in fact missing interactions. However, we considered that all unlabeled pairs as negative examples, allowing the predictor to re-classify some of these pairs as positive examples.
We built several other datasets using exactly the same training pairs as those in S, but 5-folded in various ways. Datasets S1, S2, S3, and S4 are folded so as to correspond to random, orphan protein, orphan ligand, and double orphan prediction situations. The construction of these four datasets is detailed in Section 3.2, where they are used. Datasets , , , and are also folded to mimic the same situations, but with the additional constraint that proteins and ligands were clustered based on their similarities, and each fold contains only one cluster of proteins and of ligands. The goal is to test the performance of the method in situations similar to those of S1, S2, S3, and S4, but with the added difficulty that the test set (one fold) and the train set (the 4 other folds) contain pairs that have low similarities. This setting is relevant when considering proteome-wide predictions: many of the proteins to consider may not have close neighbors among the proteins for which the most information (i.e. ligands) are known. The construction of these four datasets is detailed in Section 3.3, where they are used.
We also built a dataset called S0 by keeping only molecules and proteins in S which are involved at least in two interactions, in order to compare the prediction performance of the proposed methods with those of ligand-based and target-based approaches. Indeed, these two single-task approaches require at least two data points, one used as train, and one as test. Consequently, when a LOO-CV scheme is used, no ligand and no protein are orphans in S0. S0 contains 5908 positive interactions and was used in Sections 3.4 and 3.5. In addition, we randomly generated four sets of 5908 negative interactions involving proteins and ligands found in the positive interactions, while ensuring that each protein and each ligand are present in the same number of positive and negative interactions. Then, we assessed performance by computing the mean and standard deviation of the AUPR scores over test sets including the positive interactions set and one of the negative interactions sets.
Finally, we built three protein family datasets by extracting from S0 all protein-ligand interactions involving respectively only G-Protein Coupled Receptors (GPCR set), ion channels (IC set), and kinases (Kinases set). These datasets were used to evaluate performance of our method within a family of proteins, and compare it to those of single-task approaches. They were extracted from S0 (and not from the larger dataset S) since again, these comparisons used the LOO-CV scheme, which requires at least two data points per protein and per molecule.
Table 1 gives some statistics about the datasets, including the distribution of targets per drug and the distribution of ligands per protein.
Table 1. Dataset statistics.
| S | S0 | GPCR | IC | Kinases | |
|---|---|---|---|---|---|
| number of interactions | 9536 | 5908 | 1735 | 1603 | 847 |
| number of proteins | 1821 | 788 | 85 | 140 | 143 |
| number of molecules | 3980 | 1180 | 482 | 295 | 577 |
| number of targets per drug (mean/median) | 5.2/2 | 7.5/3 | 20.3/6 | 5.9/3 | 11.5/4 |
| number of targets per drug (min—max) | 1 − 136 | 2 − 82 | 1 − 86 | 1 − 67 | 1 − 136 |
| number of ligands per protein (mean/median) | 2.4/1 | 5.0/3 | 3.6/3 | 5.4/3 | 1.5/7 |
| number of ligands per protein (min—max) | 1 − 70 | 2 − 48 | 1 − 31 | 1 − 26 | 1 − 18 |
3 Results
3.1 Kernel selection and parametrization
The goal of this section is to choose a protein kernel and a molecule kernel that we will use throughout the remainder of this study. We assumed that kernels optimized on a large dataset of interactions between drug-like molecules and druggable human proteins such as dataset S would be good default kernels for the prediction of drug candidates specificity. Therefore, we optimized kernels on dataset S (the largest dataset built in the present study), and used the best-performing couple of kernels in the remainder of the paper.
The set of (protein, ligand) pairs in S were randomly 5-folded, and we performed a nested 5-fold-CV experiment in order to evaluate the six possible kernel combinations and their best hyperparameters.
Table 2 gives the best prediction performance for the six combinations of protein and molecule kernels, together with the corresponding hyperparameters. All protein kernels gave the best AUPR when coupled to the Tanimoto kernel. The Marginalized kernel obtained good performance only when coupled to the Profile kernel. Overall, the Profile kernel (k = 5, t = 7.5) associated to the Tanimoto kernel (d = 8) gave the best performance. Therefore, in what follows, we only consider these two kernels, and call MT the Multi-Task SVM that uses their Kronecker product.
Table 2. Best nested 5-fold-CV AUPR for each kernel combination, together with optimal hyperparameters.
| Kernels | Tanimoto | Marginalized |
|---|---|---|
| LAkernel | AUPR = 0.938±0.001 | AUPR = 0.930±0.001 |
| Tanimoto: d = 14 | Marginalized: q = 0.1, MI = 4 | |
| LAkernel: o = 20, e = 1, β = 1 | LAkernel: o = 20, e = 1, β = 1 | |
| SWkernel | AUPR = 0.878±0.002 | AUPR = 0.868±0.002 |
| Tanimoto: d = 6 | Marginalized: q = 0.1, MI = 2 | |
| SWkernel: o = 100, e = 0.01 | SWkernel: o = 50, e = 10 | |
| Profile | AUPR = 0.941 ± 0.001 | AUPR = 0.935±0.001 |
| Tanimoto: d = 8 | Marginalized: q = 0.1, MI = 2 | |
| Profile: k = 5, t = 7.5 | Profile: k = 4, t = 6 |
We also considered one-class SVM using the same kernels [61]. However, the performance of one-class SVM were clearly lower than those of KronSVM. The AUPR scores of one-class SVM were in the range of 0.6 for all considered kernels when those of KronSVM were in the range of 0.9. Therefore, we did not further consider one-class SVM.
It is worth noting that the SW-kernel gave the worst performance, although it is used in many studies [10, 15, 18, 20]. Overall, the good performance of the six multi-task methods observed on S is consistent with previously reported results [17, 20]. However, S was built from the DrugBank, which is mostly fueled by application-specific screens of either related proteins or related small molecules. Therefore, (protein, ligand) pairs of the test sets will usually have close pairs in the train set (i.e. pairs involving the same or similar proteins and ligands), which will facilitate the prediction. The performance in real-case prediction of drug specificity is expected to be lower than that obtained on S, since at the proteome scale, some of the test (protein, ligand) pairs will be far from all pairs of the train set. This will be particularly true in the case of new drugs and therapeutic targets, as already pointed by [26].
The question of interest is now to which extent the proposed MT method is effective to make predictions on more challenging situations that are relevant in the context of drug specificity prediction. Therefore, in the following, we study the evolution of MT’s performance in more realistic settings where the protein, the molecule, or both, are orphan, or where the tested (protein, ligand) pair has low similarity with the pairs belonging to the train set. Before pursuing, we bring to the reader’s attention that, for all experiments that follow, the numerical values of the performance measures and their standard deviations can be found in the Supporting Information.
3.2 Performance of multi-task approaches in orphan situations
The goal of this section is to evaluate the performance of MT in cases where the queried (protein, molecule) pairs contain proteins and/or molecules that are not in the training set, as proposed by [26]. For that purpose, all the pairs of dataset S were used and 5-folded as follows in order to create four cross-validation data sets:
S1: randomly and balanced in positive and negative pairs;
S2 (corresponding to the “orphan ligand” case): (protein, molecule) pairs in one fold only contain molecules that are absent from all other folds; prediction on each test set (each fold) is performed using train sets (the four other folds) in which no the ligands of the test set are absent.
S3 (corresponding to the “orphan protein” case): (protein, molecule) pairs in one fold only contain proteins that are absent from all other folds; prediction on each test set is performed using train sets in which no the proteins of the test set are absent.
S4 (corresponding to the “double orphan” case): (protein, molecule) pairs in one fold only contain proteins and molecules that are both absent from all other folds. Prediction on each test set is performed using train sets in which no the proteins and the ligands of the test set are absent. The folds of S4 were built by intersecting those of S2 and S3 and S4. Thus, S4 contains 25 folds and not 5.
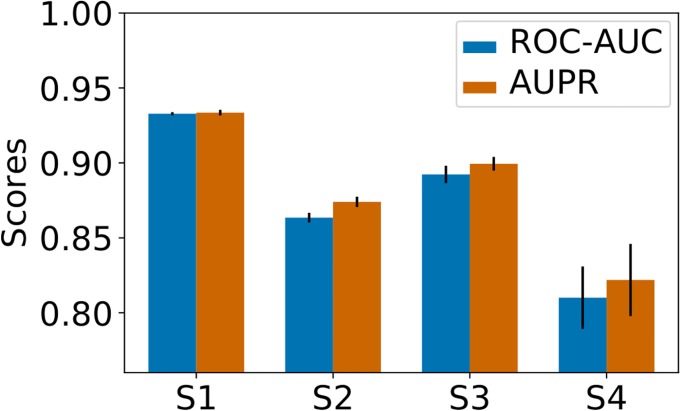
Fig 1 shows the nested-CV AUC and AUPR scores obtained by the MT method on the S1-S4 datasets. As expected, the best scores are obtained for S1, and the worst for S4, since in S4, no pairs of the train set contain the protein or the ligand of the tested pair to guide the predictions. The loss of performance between the random and the double orphan settings is about 0.12 both in AUC and AUPR. However, the performance on the S4 dataset remains well above those of a random predictor. These results confirm that MT chemogenomics can make predictions for (protein, ligands) pairs made of unseen proteins and unseen ligands, even in datasets containing very diverse types of proteins. This confirms previous observations made on less diverse datasets [26]. It is important to point that single-task approaches would not be able to provide any prediction on the S4 dataset.
Fig 1. Nested 5-fold-CV performance of the MT method on the S1 − S4 datasets.
Numerical values can be found in Supporting Information S1 Table.
The scores obtained in the S2 and S3 datasets are intermediate between those observed on S1 and S4. This was to be expected, as in these datasets, the algorithm can rely on training pairs containing either the same proteins (S2) or the same ligands (S3) as the test set. The AUC and AUPR scores are both slightly better for S3 than for S2, which suggests that predicting ligand for new protein targets is easier than predicting targets for new compounds, as already noticed in [26]. We also observed similar behaviors when replacing the SVM with a kernel ridge regression (see Supporting Information S1 Fig) and hence did not further consider this algorithm.
Overall, our results suggest that the performance of MT is driven by known (protein, molecule) pairs that are similar to the query pair, in the sense that they share either their protein or their molecule. In the next section, we will evaluate how the actual similarity between query and train pairs influences the prediction performance of this multi-task algorithm.
3.3 Impact of the similarity of the training examples to the test set
To evaluate the impact on performance of the dissimilarity between training and test pairs, we re-folded the pairs of S following the “clustered cross-validation” approach [62]. More precisely, we clustered proteins (resp. ligands) into 5 clusters by hierarchical clustering [63]. We then built four cross-validation datasets, , generated based on folds similarly as S1 − S4, but with the added constraint that all pairs in a given fold are made of proteins from a single protein cluster and ligands from a single ligand cluster. Therefore, test pairs are more dissimilar from train pairs than in the S1 − S4 datasets, which makes the problem more difficult.
Overall, all the pairs of dataset S were 5-folded as follows in order to create four cross-validation data sets:
: randomly and balanced in positive and negative pairs, each fold containing proteins and ligands belonging to the same cluster;
(corresponding to the “orphan ligand” case): (protein, molecule) pairs in one fold only contain molecules that are absent from all other folds; prediction on each test set (each fold) is performed using train sets (the four other folds) in which no the ligands of the test set are absent, with the additional constraint that each fold contains proteins and ligands belonging to the same cluster.
(corresponding to the “orphan protein” case): (protein, molecule) pairs in one fold only contain proteins that are absent from all other folds; prediction on each test set is performed using train sets in which no the proteins of the test set are absent, with the additional constraint that each fold contains proteins and ligands belonging to the same cluster.
(corresponding to the “double orphan” case): (protein, molecule) pairs in one fold only contain proteins and molecules that are both absent from all other folds. Prediction on each test set is performed using train sets in which no the proteins and the ligands of the test set are absent, with the additional constraint that each fold contains proteins and ligands belonging to the same cluster. The folds of S4 were built by intersecting those of S2 and S3 and S4. Thus, S4 contains 25 folds and not 5.
We used the same kernels as for the MT method. Fig 2 shows the prediction performance of MT on these new cross-validation folds. For all the datasets, we observed a strong decrease in prediction scores with respect to those obtained on the corresponding S1 − S4 datasets. This suggests that good performance on a query pair (p*, m*) is driven by the presence in the training set of pairs made both of proteins similar to p* and of molecules similar to m*, even if the query pair (p*, m*) is a double orphan, as in S4. Our results also suggest that it is more important to train on pairs similar to the double orphan query pair (p*, m*), as in S4, than on data containing, for example, p* itself, but paired only with molecules quite dissimilar to m*, as in .
Fig 2. Nested 5-fold-CV performance of the MT method on the datasets.
Numerical values can be found in Supporting Information S2 Table.
In the most difficult case of , the performance is even lower than that of a random predictor, which would display an AUC of 0.5. This is somewhat intriguing. One explanation could be that, when the data points are randomly dispatched in the folds used to build the train and test sets, this value is of 0.5 can be considered as a baseline. In the case of S’4, the folds are built using clustered protein-ligand pairs, so that the data distribution in the test set (one of the clusters/folds) may be different from the data distribution in the train set (the remaining samples). This explains why a machine-learning algorithm like MT is unable to learn a model that is relevant for the test set. In other words, the baseline performance expected when systematically learning on differently distributed data might be worse than that observed by a random predictor.
These results suggest that pairs in the training set that are very dissimilar to the query pair do not help making more accurate predictions. In other words, although the kernels used in multi-task approaches modulates how information available in one task is shared for training other tasks (the further the tasks are, the less information is shared), using information from distant tasks seems to degrade performance. This insight is interesting since the MT algorithm requires calculating the Kronecker kernel on all (protein, molecule) pairs, which is computationally demanding. Therefore, the next two sections evaluate whether removing distant pairs from the training set can improve computational efficiency without degrading performance.
3.4 Multi-task approaches on reduced training sets
Based on the insight that MT prediction is driven by training examples that are close to the query data, we propose to build training tests of reduced sizes by removing training examples distant from the query. The goal of this section is also to compare the prediction performance of the MT method trained on these reduced data sets to that of the simpler and faster single-task method, since there would be no point in using the more complex MT method if a single-task method performs better. Because this study is motivated by ligand specificity prediction, we chose to focus on comparisons with the ligand-based ST method rather than target-based ST.
In what follows, n+ (resp. n−) will refer to the number of positive (resp. negative) examples in the train set.
In all the following experiments, we used the LOO-CV scheme because intra-task and extra-task pairs can only be defined for each pair separately, which prevents from using K-fold-CV schemes. In addition, in single-task approaches, the size of the training set was relatively small in most cases (see datasets statistics in Section 2), which does not allow to fold the data. We checked that the LOO-CV scheme did not trigger a bias, as sometimes observed [26] (see Supporting Information S2 Fig and S3 Table).
Because prediction of a given (protein, ligand) interaction can only be made by single-task if the pair partners are present in at least another pair of the train set, in the following experiments, we used the S0 dataset in which all ligands and all proteins are involved in at least two known interactions, as explained in Section 2.4.
3.4.1 Training on intra-task positive examples
The goal of this section is to compare the prediction performance of the MT method trained on a reduced data set (of size similar to that employed in single-task methods) to those of single-task methods. Since ligand-based ST can only use intra-task positive examples, the only positive training pairs we use for the MT method are the intra-task pairs as well. Note that MT still gets more training examples than ligand-based ST, since pairs formed with the query protein and a different ligand are also included. By reducing the training set size, the computational times required by the MT method are now similar to those of the single-task method. In the following, we call MT-intra this variant of MT. For each test ligand, we build the negative training examples by randomly selecting a number n− of proteins that do not interact with the ligand in S0. We vary n− from 1 to 100 × n+.
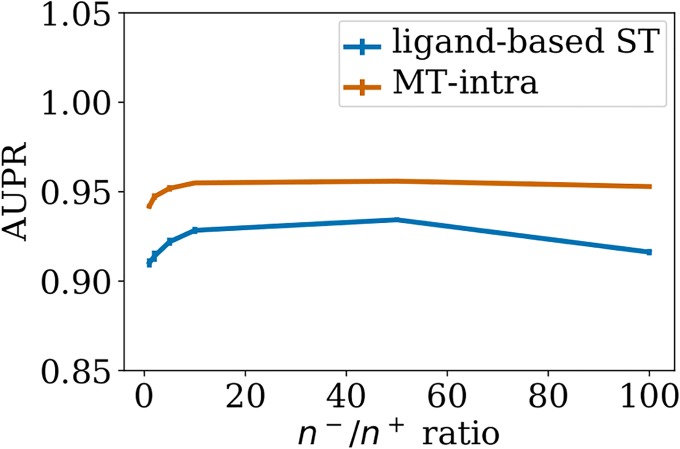
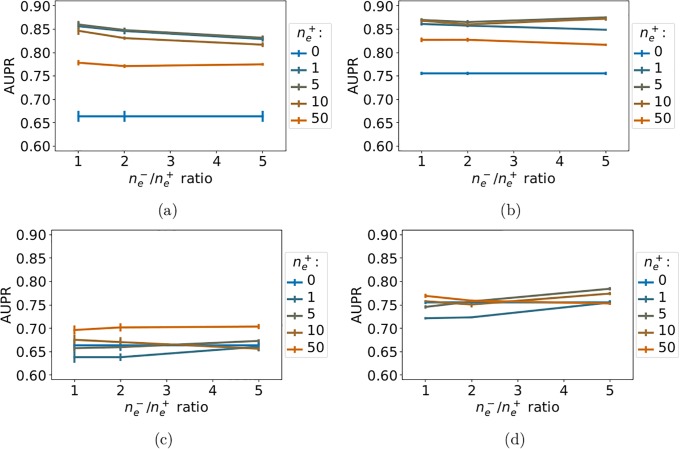
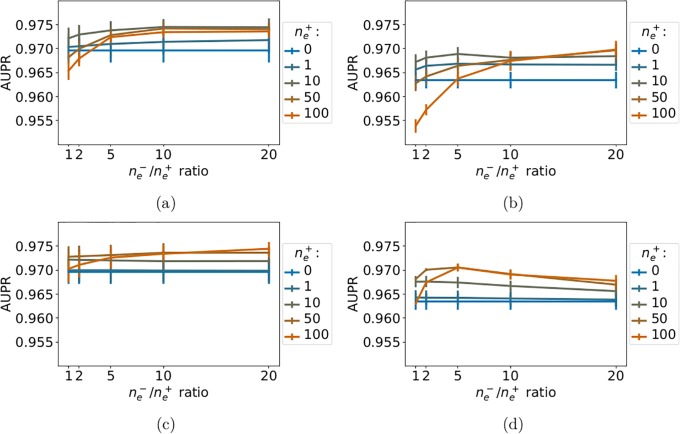
Fig 3 shows the LOO-CV AUPR of MT-intra and ligand-based ST on S0, for increasing values of the n−/n+ ratio. For both methods, the AUPR score increases with the number of negative pairs in the train set, before decreasing for large numbers of negative pairs. A good trade-off for both computational and predictive performance seems to be in the range of 10 times more negative points than positive points. We therefore set n−/n+ to 10 for the remaining experiments of this section.
Fig 3. Performance of single-task and MT-intra as a function of the n−/n+ ratio.
Numerical values can be found in Supporting Information S4 Table.
The AUPR scores of MT-intra outperform those of the ligand-based ST method. Interestingly, the performance of the MT-intra with a n−/n+ ratio of 10 is close to 0.96 which outperforms the AUPR score of 0.93 obtained with MT (see Section 3.2). This indicates that including in the train set pairs displaying low similarity to the tested pair degrades both the computational time and the quality of the prediction of MT.
3.4.2 Adding similar extra-task positive examples
The results from Section 3.3 suggest to explore the performance of the MT-intra method when trained on various datasets including extra-task pairs close to the tested pair, in addition to the intra-task pairs. Therefore, we built train sets made of:
the train set of MT-intra
closest extra-task positive pairs with respect to the tested pair ().
closest extra-task negative pairs with respect to the tested pair, so that the ratio varies from 1 to 10.
We call NN-MT (for Nearest Neighbor MT) the resulting variant of MT. We also considered a similar approach in which the extra-task pairs were chosen at random rather than according to their similarity to the test pair. We refer to this method as RN-MT (for Random Neighbor MT).
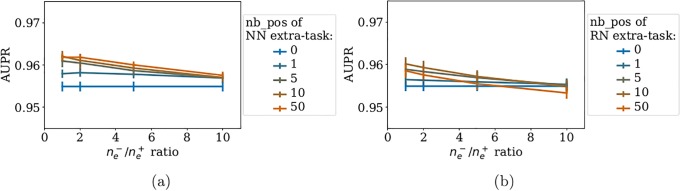
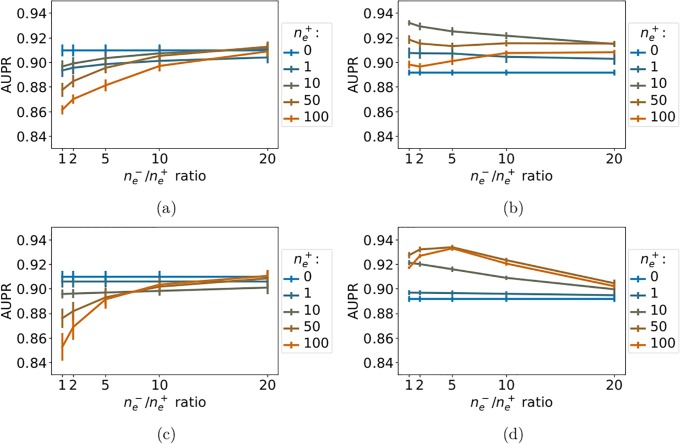
We report the LOO-CV performance of NN-MT and RN-MT on Fig 4. Fig 4(a) shows that, while adding to the train set 0 to 50 nearest neighbor extra-task positive pairs with respect to the tested pair, the prediction performance of NN-MT slightly and monotonously increases. Fig 4(b) shows that the performance of RN-MT also slightly increases (although not monotonously) when random extra-task pairs are added. However, its best performance remains under that of NN-MT. Finally, using a high ratio did not improve the performance. This is an interesting observation, since limiting the size of the train set is computationally favorable.
Fig 4. AUPR as a function of the ratio for increasing numbers random extra-task points in the train set.
(a): NN-MT. (b): RN-MT. The blue horizontal line corresponds to MT-intra (which is trained only on intra-task pairs). Numerical values can be found in Supporting Information S5 and S6 Tables.
Taken together, these results show that NN-MT outperforms not only MT-intra, but also the more computationally demanding MT method trained in the LOO-CV setting in Section 3.2. AUPR scores for (protein, ligand) pairs involving non-orphan ligands and non-orphan proteins are expected to be very high (around 0.96).
However, predicting the specificity of a given ligand requires the ability to make predictions for proteins that are far from the known targets. In these cases, the high prediction scores obtained in this section might not hold. Therefore, in the next section, we study the performance of NN-MT when the test pairs are far from the train set.
3.5 Impact of the distance of the intra-task examples to the query pair
The goal of this section is to evaluate the performance of the MT-intra and NN-MT proposed methods, and to compare them to those of the ligand-based ST method, when the similarity between the test pair and the training data varies.
3.5.1 Training on dissimilar intra-task positive examples
We first evaluated the performance of ligand-based ST and MT-intra when the similarity between the test pair and the training data varies. To do so, we computed the percentiles of the molecules (respectively proteins) similarity distribution in S0.
For each test pair (p*, m*), the training set only included the positive intra-task pairs (p, m) such that Kprotein(p, p*) and Kmolecule(m, m*) is lower than a percentile-based threshold θ. We then added n− random intra-task negative pairs. We did not apply a similarity constraint to negative pairs, since, unlike the positive pairs, they are available in large numbers and at all distances from the tested pairs.
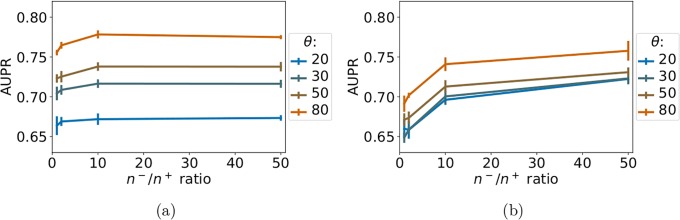
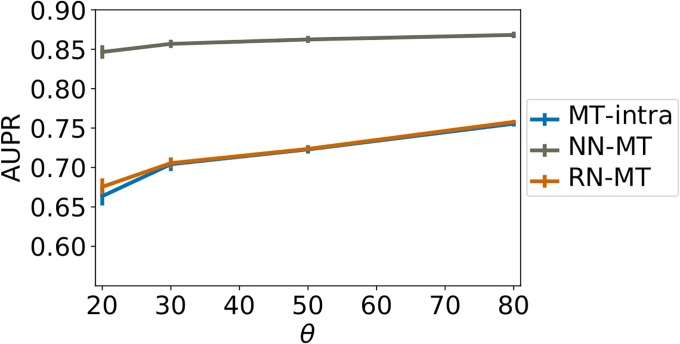
Fig 5 reports the LOO-CV AUPR scores of ligand-based ST and MT-intra for varying values of θ (20th, 30th, 50th, and 80th percentiles) and of the n−/n+ ratio (from 1 to 50).
Fig 5. AUPR scores as a function of the n−/n+ ratio, for percentile-based threshold θ ranging from 20% to 80%.
(a): MT-intra method. (b): ligand-based ST method. Numerical values can be found in Supporting Information, respectively S7 and S8 Tables.
Fig 5(a) shows that, as expected, the performance of MT-intra increases when the similarity of the tested pair to the train set increases from the 20th to the 80th percentiles (AUPR of 0.67 to 0.77). However, the performance is still much lower than when the closest pairs are allowed in the training set (AUPR of 0.96, see Section 3.4.1). Fig 5(a) also suggests that a n−/n+ ratio of 10 is again an appropriate choice, as when all intra-task positive example are used (see Section 3.4.1). We therefore set n−/n+ to this value for the remaining experiments of this section.
Fig 5(b) shows that ligand-based ST behaves similarly to MT-intra: the AUPR score increases from 0.70 to 0.75 for ligand-based ST when threshold θ increases from the 20th to the 80th percentiles. These values again remain much under the AUPR score of 0.93 observed when all intra-task pairs are used. Although modest, the performance of MT-intra remains above those of ligand-based ST for all tested thresholds of similarity (except θ = 20) between the tested pair and pairs of the train set.
3.5.2 Adding similar extra-task positive examples
We then explored to which extent adding extra-task (protein, ligand) pairs to the training set of the MT-intra method improves the prediction scores.
Applying the same percentile-based similarity constraint to the intra-task positive pairs, we compared the performance of NN-MT and RN-MT when respectively adding nearest neighbors or random extra-task positive to the training set. We did not apply a similarity constraint to the extra-task pairs, since the principle underlying multi-task methods is precisely to learn from extra-task data, which is particularly critical when the intra-task pairs of the train set are scarce or far from the tested pair, as illustrated by the poor performance of ligand-based ST in the previous section. A number of nearest neighbors (respectively random) negative extra-task pairs were also added for NN-MT (respectively RN-MT).
Fig 6(a) and 6(b) report the LOO-CV AUPR of NN-MT, as a function of (ratio of negative over positive extra-task pairs) and for a number of extra-task positive pairs varying from 0 to 50, respectively for percentile similarity constraints θ of 20 and 80. The blue horizontal line (for ) corresponds to the performance of the MT-intra methods. Fig 6(a) and 6(b) show that adding extra-task pairs to the train set dramatically improves performance. The AUPR score reaches values above 0.85, independently of θ, suggesting that when no close intra-task pairs are available, performance is driven mainly by extra-task training pairs, confirming our observations in Section 3.3. Moreover, when the number of extra-task pairs increases, the performance of NN-MT increases, then tends towards that of RN-MT, and then degrades at larger values of because too many dissimilar extra-task pairs are included in the training set. This implies that only a limited number of the closest extra-task pairs is required to reach optimal performance. Adding the same number of negative extra-task pairs ( = 1) provides the best AUPR, which again limits the size of the required training set. Unsurprisingly, the best AUPR in the absence of the closest intra-task pairs (around 0.87 for θ = 0.80) is still lower than when all available intra-task pairs are used (AUPR = 0.93, see Section 3.4). Note that, although the performance of MT-intra can be biased when considering similarity thresholds of 20th and 80th percentile, because the corresponding sizes of the train sets might be different, this is not the case for the NN-MT method because the prediction is driven by extra-task pairs.
Fig 6. AUPR score of NN-MT and RN-MT as a function of the ratio, for a number of extra-task positive pairs varying from 0 to 50, and for percentile-based similarity threshold θ of 20 and 80 applied to the intra-task positive pairs.
(a): NN-MT, θ = 0.20. (b): NN-MT, θ = 0.80. (c): RN-MT, θ = 0.20. (d): RN-MT, θ = 0.80. Numerical values can be found in Supporting Information, respectively S9–S12 Tables.
On the contrary, Fig 6(c) and 6(d) show that the performance does not improve when the extra-task training pairs are chosen at random, and therefore, are on average further from the test pair. It might even degrade when the number of extra-task pairs becomes large. Finally, Fig 7 shows that, for all similarity thresholds, the performance of the MT-intra and RN-MT methods are similar, and far beyond that of the NN-MT method.
Fig 7. AUPR score as a function of percentile-based similarity θ, for n−/n+ = 10, a number of extra-task positive pairs = 10 and a ratio of = 1 for extra-task pairs.
Numerical values can be found in Supporting Information S13 Table.
In conclusion, in settings where (protein, ligand) pairs similar to the test pair are available, our results suggest the best prediction performance are obtained using the NN-MT method trained with 10 times more negative intra-task pairs than positive ones, 1 to 5 extra-task nearest neighbor positive pairs, and the same number of extra-task negative pairs. The computational time will be reasonably comparable to that of ligand-based ST, and performance should be high enough (AUPR above 0.85) to guide experimental evaluations for drug specificity prediction.
3.5.3 Adding dissimilar extra-task positive examples
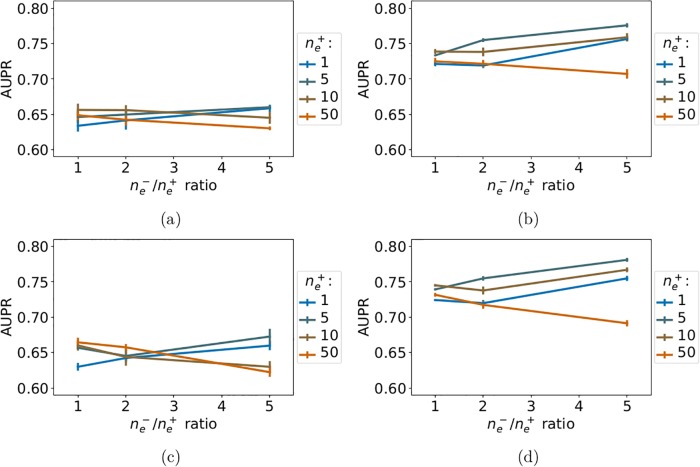
While we previously argued that the point of multi-task approaches is to leverage similar extra-task data to improve prediction performance, ligand specificity studies can require the prediction of interactions between proteins and ligands for which very little similar extra-task data is available. We therefore repeated the experiments from the previous section, but this time applying the percentile-based similarity constraint to both intra-task and extra-task positive pairs of the train set. We report the corresponding LOO-CV AUPR on Fig 8.
Fig 8. AUPR scores of the NN-MT and RN-MT multi-task methods as a function of the ratio, for a number of extra-task positive pairs varying from 1 to 50.
(a): NN-MT, θ = 0.20. (b): NN-MT, θ = 0.80. (c): RN-MT, θ = 0.20. (d): RN-MT, θ = 0.80. The two methods are trained with intra-task and extra-task examples that are both dissimilar to the tested pair (percentile-based similarity thresholds θ of 20 and 80). Exact values can be found in Supporting Information respectively in S14–S17 Tables.
We observe that the performance of NN-MT remains overall low (best AUPR score of 0.75 for θ = 0.80). Adding dissimilar extra-task positive pairs fails to improve the scores obtained when only intra-task positive pairs are included in the training set. Hence, if neither close intra-task nor close extra-task positive pairs are available, no method can provide performance good enough for the purpose of drug sensitivity prediction. These interactions would have to be experimentally tested if they are critical in the context of a drug’s development program. These observations were expected given that adding random extra-task training pairs, possibly far from the tested pair, did not improve performance of the MT-intra method (see Section 3.5.2).
Taken together, our results show that the proposed NN-MT method is the most appropriate for predicting the specificity of a molecule. Indeed, it outperforms all its comparison partners independently of the number of known (protein, ligand) interacting pairs involving the same or similar ligands or proteins as the query pair. In addition, it requires much fewer training pairs than the classical MT approach, and its computational time is therefore close to that of a single-task method. Finally, in the most challenging setting where no similar intra-task nor extra-task training data is available, it performs significantly better than random, in a context where ligand-based ST could not make any prediction.
The results we have presented so far address the issue of using kernel methods with SVM in the context of proteome-wide specificity prediction, at a tractable computational cost thanks to the choice of a reduced learning dataset, without loss in prediction performance. Reducing datasets to the most informative data points is also the underlying idea of active-learning methods [64]. In the latter, the goal is to guide step by step which data points are needed (i.e. have to be observed and labeled) to best improve the prediction performance. These methods are used when acquiring data is the limiting factor. By essence, their goal is also to reduce the size of the data sets that are used.
However, another key issue corresponds to study the specificity of a molecule within a family of related proteins. Indeed, when a new drug candidate is identified against a given therapeutic target, proteins belonging to the same family are important off-target candidates. This corresponds to the setting where similar training pairs are available, since proteins of the same family are similar in terms of sequence.
In the next section, we therefore assess whether the proposed NN-MT method, initially dedicated and tuned in proteome-wide prediction problems, also provides good performance for molecule specificity prediction within a family of proteins.
3.6 Specificity prediction within families of proteins
We considered three families of proteins because they gather a wide range of therapeutic targets, and have also been considered in other chemogenomics studies, thus providing reference prediction scores: G-Protein Coupled Receptors (GPCRs), ion channels (IC), and kinases. All the (protein, molecule) pairs involving GPCRs, ICs, or kinases that were present in the dataset S described in Section 2.4 were used to build the three corresponding family datasets.
We compared the performance of the MT-intra method (trained using only positive pairs involving the protein or the ligand of the tested pair) to those of the NN-MT and RN-MT methods, in order to evaluate the interest of the multi-task approach in family studies. We considered two versions: one in which the Profile protein kernel is used, as in the above sections, and another in which a family-based hierarchy kernel is used (Section 2.1), because a sequence-based kernel may not be optimal to study the specificity of the molecule within a family of proteins [11, 27]. The corresponding methods are called MT-intra-family, NN-MT-family, and RN-MT-family.
As in the above section, each (protein, ligand) test pair is considered in turn in a LOO-CV scheme. We used a learning dataset containing: all positive intra-task positive pairs, ten times more random negative intra-task pairs (this value was found adequate in previous sections), a varying number of positive extra-task pairs (nearest neighbors for NN-MT or NN-MT-family, random for RN-MT or RN-MT-family), and a number of negative extra-task pairs so that the ratio of varies from 1 to 20.
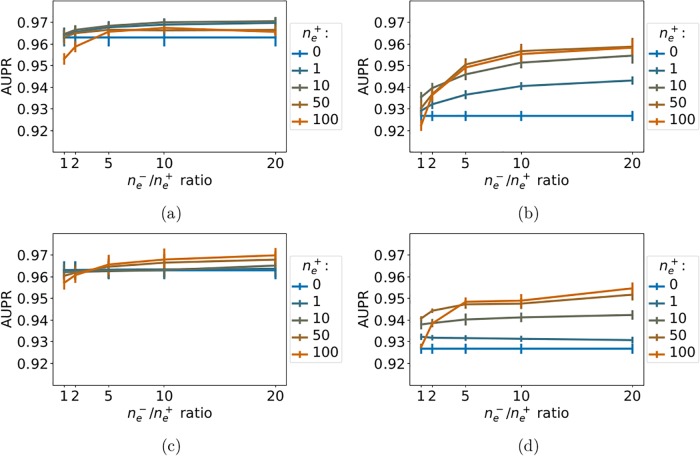
3.6.1 G-Protein Coupled Receptor family
Fig 9 shows that all methods perform very well, with AUPR scores above 0.95. Including extra-task positive pairs in the train set improves the AUPR score, even when added randomly. This indicates that, contrary to studies in larger scales in the protein space, in family studies, extra-task pairs are always close to the tested pair because they belong to the same family. However, the performance reached when adding positive nearest-neighbor extra-task pairs remains above those reached when adding positive random extra-task pairs, as observed in the larger scale studies presented above. Overall, adding 10 to 50 extra-task positive pairs to the train set, and around 10 times more random negative extra-task pairs leads to the best performance.
Fig 9. AUPR score of the considered multi-task methods on the GPCR family as a function of the ratio, for a varying number of extra-task positive pairs.
(a): NN-MT-family (family hierarchy kernel). (b): NN-MT (sequence kernel). (c): RN-MT-family (family hierarchy kernel). (d): RN-MT (sequence kernel). The blue horizontal line corresponds to the MT-intra method trained only on intra-task pairs. Numerical values can be found in Supporting Information, respectively S18–S21 Tables.
The best AUPR scores of the NN-MT and the NN-MT-family methods are close (0.96 and 0.97). Although the best scores of the NN-MT-family method are slightly above those of NN-MT, one should note that the family GPCR kernel is based on a GPCR hierarchy that was established using known GPCR ligands. Therefore, the results obtained by the NN-MT-family might be biased, which is not the case for those obtained by the NN-MT.
3.6.2 Ion Channel family
The conclusions obtained above in the GPCR family also hold in the IC family, as shown in Fig 10. Again, all methods perform very well, reaching AUPR scores above 0.97. As for the GPCR family, adding 10 to 50 extra-task positive pairs to the train set, and around 10 times more random negative extra-task pairs leads to the best performance.
Fig 10. AUPR of the multi-task methods on the IC family.
(a): NN-MT-family (family hierarchy kernel). (b): NN-MT (sequence kernel). (c): RN-MT-family (family hierarchy kernel). (d): RN-MT (sequence kernel). The blue horizontal line corresponds to the MT-intra method trained only on intra-task pairs. Numerical values can be found in Supporting Information, respectively S22–S25 Tables.
3.6.3 Kinase family
In the kinase family, the results are somewhat different from those obtained on IC and GPCRs. The NN-MT and RN-MT methods both outperform the MT-intra method that is trained using only intra-task pairs, as shown in Fig 11(b) and 11(d). Again, 10 to 50 extra-task pairs, with a ratio in the range of 1 to 5 leads to the best results, with AUPR scores in the range of 0.93. Unexpectedly, the NN-MT-family and RN-MT-family methods, which both use the kinase family hierarchy kernel, tend to perform not as well when extra-task pairs are added to the training set, than when only the intra-task pairs are used, as shown in Fig 11(a) and 11(c). In addition, their best AUPR scores reaches 0.90, which is lower than those of the NN-MT and RN-MT methods which are in the range of 0.93. These observations may reflect the fact that the kinase family gathers proteins that are relatively more diverse than GPCRs and IC. For example, one can distinguish Tyrosine kinases and Serine/Threonine kinases, or globular protein kinases and receptor protein kinases. This diversity is illustrated by the organization of the kinome in some 50 distinct sub-families [46]. In this context, the sequence kernel that was optimized in proteome-wide studies might better capture the degree of similarity between two kinases than the hierarchy kernel does.
Fig 11. AUPR score of the multi-task methods within the kinase family.
(a): NN-MT-family (family hierarchy kernel). (b): NN-MT (sequence kernel). (c): RN-MT-family (family hierarchy kernel). (d): RN-MT (sequence kernel). The blue horizontal line corresponds to the MT-intra method trained only on intra-task pairs. Numerical values can be found in Supporting Information, respectively S26–S29 Tables.
Overall, the above results on the IC, GPCR and kinase families indicate that the proposed NN-MT method leads to the best results when the train set includes all positive intra-task pairs, 10 times more random negative intra-task pairs, a small number of nearest neighbors positive extra-task pairs (in the range of 10) and around 5 times more random negative extra-task pairs. These conditions are very similar to those leading to the best prediction scores when ligand specificity is studied on large scale in the protein space. Even if the performance of NN-MT on family datasets is better than those reached by other methods on similar datasets [11, 20], they remain in the same order of magnitude.
4 Discussion: Comparison to other methods
As mentioned in Section 1.2, a few methods have been proposed to predict interactions between proteins and ligands. We compared the prediction performances of the proposed NN-MT method to those of two state-of-the art methods: a recent Matrix Factorization method called Neighborhood Regularized Logistic Matrix Factorization (NRLMF) [24], and the Kronecker (kernel) Regularized Least Square regression method KronRLS (a kernel-based method, as NN-MT) [18, 19].
The KronRLS and NRLMF methods were published based on their prediction performance on four protein family datasets, Nuclear Receptors (NR), GPCR, Ion channels (IC), and Enzymes (E) that contained respectively 90, 636, 1476 and 2926 interactions [10].
The KronRLS method uses a kernel Kmolecule for molecules that is defined by:
| (5) |
where KSIMCOMP is a structure similarity kernel [65], and where KGIP,m is a Gaussian kernel that compares the interaction profiles of molecules against the proteins of the dataset [18]. For proteins, the kernel Kprotein is defined by:
| (6) |
where Ksequence is a protein sequence similarity kernel also based on the Smith-Waterman score [44], and KGIP,p is a Gaussian kernel that compares the interaction profile of proteins against the molecules of the dataset.
A specific feature of the NRLMF method is that it integrates a neighborhood regularized method which allows to take into account only the K nearest neighbors to predict a given (protein, ligand) interaction (in practice, the authors used K = 5).
We performed benchmark experiments on these family datasets using the PyDTI package. This package initially contained the KronRLS, a variant of it called KronRLS-WNN, and the NRLMF methods, and the kernel matrices Kprotein and Kmolecule calculated for the four family datasets. In all experiments, we compare the intrinsic performances of the algorithms: the similarity measures used are the same for all methods. More precisely, the three methods used the kernels available in PyDTI: the structure-based KSIMCOMP for molecules, and a kernel Ksequence based on the Smith-Waterman score. In addition, KronRLS also used the KGIP,m kernel, leading to the Kmolecule kernel for molecules defined in Eq 5, and the KGIP,p kernel, and to the Kprotein kernel for proteins defined in Eq 6. The two other methods do not use the KGIP kernels because they do not take into account information about interaction profiles.
We also performed benchmark experiments on a dataset gathering more diverse protein and ligands. To this end, we used the DrugBank-based dataset S0 described in Section 2.4) containing 5 908 interactions. Because KronRLS and NRLMF could not make predictions on S0 at a manageable computational cost in the LOO-CV scheme, we randomly sampled 2 000 of the 5 908 interactions of this data set to create a smaller test data set called S0,2000. We still used all of S0 (minus the test example) for training.
We calculated the Tanimoto and Profile kernels optimized in the present study (see Section 3.1), and these matrices were uploaded in PyDTI so that the three considered methods could used them. In addition, since KronRLS also use the KGIP,m and the KGIP,p kernels, we described all molecules and proteins in S0 by their interaction profile. We calculated the KGIP,m and KGIP,p kernels on S0 and uploaded these kernels in PyDTI. Only KronRLS used these additional kernels. All cross-validation experiments were performed building test sets from S0,2000 and using all remaining data points in S0 for training.
Table 3 presents the performance of the three considered methods on the protein family datasets.
Table 3. AUPR scores and standard deviations in 10-fold-CV, test sets balanced in positive and randomly chosen negative samples.
| Method / Dataset | NR | GPCR | IC | E |
|---|---|---|---|---|
| KronRLS | 0.75 ± 0.14 | 0.9 ± 0.03 | 0.96 ± 0.01 | 0.96 ± 0.01 |
| NN-MT | 0.89 ± 0.09 | 0.95 ± 0.02 | 0.97 ± 0.01 | 0.97 ± 0.01 |
| NRLMF | 0.96 ± 0.04 | 0.96 ± 0.02 | 0.98 ± 0.01 | 0.98 ± 0.0 |
Globally, the performance of all methods are high and close, with AUPR scores above 0.9 in most of the cases. On average, the NRLMF and NN-MT methods are on par and lead to the best results. These results are consistent with those reported in [24].
Table 4 confirms the tendencies observed in Table 3. Although the performances are slightly lower on this more diverse S0,2000 dataset than on the family datasets, they remain high, with NRLMF and NN-MT keeping the best AUPR scores.
Table 4. AUPR scores and standard deviations in 10-fold-CV, test sets balanced in positive and randomly chosen negative samples.
| Method / Dataset | S0,2000 |
|---|---|
| KronRLS | 0.91 ± 0.02 |
| NN-MT | 0.95 ± 0.01 |
| NRLMF | 0.96 ± 0.01 |
As discussed in Sections 3.1 and 3.2, various prediction methods lead to such high performances because, in the protein family or S0,2000 datasets, predictions are averaged over test pairs in which the protein and/or the molecule might be orphan, or not. These averaged results hide less favorable situations, typically double orphan samples. Because these cases are common and important when predicting specificity of a new drug candidate at the proteome scale, we would like to stress that comparing methods in orphan cases is a more stringent and relevant test. In such cases, the performance are expected to be more modest and the methods might not rank in the same order.
Therefore, we ran the three methods using a LOO-CV scheme on double orphan (protein, molecule) pairs on the same datasets. We considered the LOO-CV scheme as it is particularly convenient to test double orphan drug-target interactions. In these experiments, for each tested (p, m) pair, interactions involving the considered protein or the molecule are ignored in the train set. The LOO-CV schemes were balanced in positive and randomly chosen negative pairs.
In order to better explore the performance of the considered methods in this double-orphan setting, we used two versions of the two kernel-based methods, initially introduced for KronRLS. More precisely, in [19], the authors proposed an approach called WNN (weighted nearest neighbor) that, for each orphan molecule m (resp. protein), an interaction profile is computed by summing the weighted profiles of non orphan molecules in the dataset. The weighting depends on the similarity between the orphan molecule and all other non orphan molecules. This predicted profile is used in the training to predict labels to all (protein, m) pairs of the dataset. Thus, in the first version of KronRLS [18], all the labels of (protein, m) pairs involving the orphan molecule m were set to 0. Based on this WNN procedure some of these non interactions might be re-qualified as true interaction before training the predictor. In other words, the WNN algorithm can be viewed as a mean to de-orphanize molecules or proteins in order to help the predictions on such cases. In the following, we will call KronRLS-WNN the KronRLS method ran with the WNN algorithm. Using the PyDTI package, we also considered a version of NN-MT in which the WNN algorithm is the implemented, and call it NN-MT-WNN in the following.
Table 5 presents the results of the double-orphan benchmark on the family datasets. Surprisingly, in these double-orphan experiments, the NRLMF method has very modest results and does not perform as well as the other methods. The results of NN-MT remain well above the random performance of 0.5, but not the KronRLS method. The WNN algorithm dramatically improves the performance of KronRLS, and to a lesser extent those of NN-MT, and overall, the NN-MT-WNN algorithm leads to the best performance in most cases.
Table 5. AUPR scores and standard deviations on double orphan LOO-CV, balanced number of positive and randomly chosen negative test samples.
| Method / Dataset | NR | GPCR | IC | E |
|---|---|---|---|---|
| KronRLS-WNN | 0.78 ± 0.03 | 0.83 ± 0.01 | 0.78 ± 0.01 | 0.84 ± 0.0 |
| KronRLS | 0.55 ± 0.01 | 0.62 ± 0.01 | 0.64 ± 0.0 | 0.56 ± 0.0 |
| NRLMF | 0.19 ± 0.03 | 0.15 ± 0.0 | 0.26 ± 0.01 | 0.23 ± 0.0 |
| NN-MT | 0.72 ± 0.04 | 0.76 ± 0.01 | 0.72 ± 0.01 | 0.66 ± 0.0 |
| NN-MT-WNN | 0.77 ± 0.05 | 0.85 ± 0.0 | 0.79 ± 0.01 | 0.84 ± 0.0 |
Table 6 presents the results of the double-orphan benchmark S0,2000 dataset. We did not run the NRLMF method in this experiment, because it was computationally too intensive in this LOO-CV, and because it already gave very poor results on the easier family dataset. Moreover we shortened the train set of KronRLS and KronRLS-WNN methods by considering only the thousand molecules (resp. proteins) closest to the molecule (resp. protein) of the test sample. Thus, the computation time was reduced to some hours instead of months which made those methods computationally reasonable.
Table 6. AUPR scores and standard deviations on double orphan LOO-CV, balanced number of positive and randomly chosen negative test samples.
| Method / Dataset | S0,2000 |
|---|---|
| NRLMF | None |
| KronRLS-WNN | 75.6 ± 0.43 |
| KronRLS | 0.4979 ± 0.0071 |
| NN-MT | 0.60 ± 0.01 |
| NN-MT-WNN | 0.85 ± 0.01 |
Overall, the scores are lower on this dataset than on the family datasets because S0,2000 is a more diverse dataset on which predictions are more difficult, in general, than on the family datasets. However, the same tendency is observed: NN-MT performs better than KronRLS, and when the WNN algorithm is used, NN-MT-WNN performs better than KronRLS-WNN.
Overall, the results of these benchmarks show that the NN-MT method present state-of-the-art or better results on the protein family datasets and the more diverse DrugBank-based dataset. In the general case, it appears to be a good default method in terms of performance, number of parameters and computational efficiency, which are important issues for non expert users.
In the specific double-orphan case, only the two kernel-based methods NN-MT and KronRLS lead to performance well above those of a random predictor. The WNN algorithm, proposed in [19] improves the performance of KronRLS and of NN-MT, but resulting NN-MT-WNN method lead to the best performance.
Finally, it is interesting to compare the computational complexities of the methods as a function of the number of hyper-parameters that they contain. Indeed, these hyper-parameters need to be optimized by cross-validation, leading to heavy computational issues in the case of the large-scale datasets used in proteome-wide chemogenomics. As can be seen in the PyDTI package, NRLMF has 5 regularization parameters, KronRLS has 2 hyper-parameters (decay parameter T and the weight parameter used to combine kernels; the regularization parameter and the bandwidth of the GIP kernel are fixed), and NN-MT has 1 hyper-parameter (regularization parameter C for SVM). In practice, the optimization of NRLMF in the LOO-CV scheme was out of reach, requiring several days of calculation while the other methods required hours, which represents a limitation of this method. This could explain in part the very low performances displayed by NRLMF in the double-orphan experiment. In addition, the double-orphan case was not considered in [24], and therefore, we do not have any performance reference for this case. In this setting, latent vectors describing proteins and ligands are both estimated from non-orphan neighbors, and interaction prediction may fail in this situation, and contribute to the poor performance observed. However, we did cross-validate NRLMF parameters in the double-orphan setting in the case of the family NR dataset (the smallest dataset used in this section). This allowed a modest increase in AUPR score from 0.14 to 0.19 (reported in Table 5). Therefore, even if the NRLMF method had been optimized on the other datasets, we do not expect that this would have changed the overall conclusion that this method is not suitable for handling orphan cases.
5 Conclusion
The present study tackles prediction of ligand specificity on large scale in the space of proteins. More precisely, our goal was to propose a method to explore the specificity of molecules with state-of-the-art or better performance over a wide range of prediction situations: at the proteome or protein family scales, on average or in specific situations such as tested pairs far from the train set, or such as orphan proteins and ligands. In other words, the aim was to propose a robust default method, applicable to many types of studies, thus avoiding development of ad hoc complex and specific methods to non expert users. We chose to formulated it as a problem of predicting (protein, ligand) interactions within a multi-task framework based on SVM and Kronecker products of kernels on proteins and molecules. Within the kernel-based SVM methods tested in the Results section, we showed that the NN-MT method fulfills these requirements. In particular, NN-MT outperforms both the multi-task MT method and the corresponding single-task kernel-based methods, while it also keeps a computational cost close to that of single-task approaches. The NN-MT algorithm fulfills these requirements, leading to the best prediction performance for the three tested settings which cover most of the prediction situations that would be encountered in real-case studies.
To summarize the main characteristics of the proposed NN-MT method (detailed in Sections 3.1, 3.4 and 3.5), we suggest to predict each (protein, ligand) interaction using the Profile kernel for proteins (with subsequences length k of 5 and threshold equaled to 7.5) and the Tanimoto kernel for molecules (with length of path 8), with a train set including:
all positive intra-task pairs (i.e. all known interactions involving the protein or the ligand of the test pair), and around ten times more randomly chosen intra-task negative pairs.
a small number of the closest positive extra-task pairs (i.e. a number similar to that of intra-task positive pairs), and a similar number of randomly chosen negatives extra-tasks pairs.
This should provide good default parameters to use the NN-MT method in a straightforward manner for users that are not familiar with machine learning approaches.
We used the DrugBank database to build several datasets that illustrate various prediction contexts and that we made available online to the community for future benchmarking studies. We also updated the PyDTI package [24] with an implementation of NN-MT together with several cross-validation schemes and our DrugBank-based dataset. This allowed us to compare the NN-MT method to recent approaches developed on drug target interaction prediction [18–24]. In the context of wide-scale prediction of molecule specificity, the DrugBank-based dataset is more relevant that the family datasets that have been widely used. Indeed, it contains a set of proteins that can be viewed as a relevant druggable proteome to train and test computational models for drug specificity prediction.
The benchmark study comparing NN-MT to the matrix factorization NRLMF and the kernel-based KronRLS algorithms on family and DrugBank-based datasets showed that, all methods displayed high performances, NRLMF and NN-MT leading to the best results. However, on the more demanding double-orphan tests performed on the same datasets, NRLMF performed much poorer than the kernel-based NN-MT and KronRLS algorithms. In this orphan case, the WNN algorithm makes it possible not only to significantly improve the performance of KronRLS, but also that of NN-MT, the NN-MT-WNN algorithm leading to the best results.
We formalized (protein, ligand) interaction prediction as a classification problem because they can be solved at a reasonable computational cost on large datasets. Whenever the purpose would be to predict the relative affinities of molecules for a set of proteins, the predicted scores can be used to rank all interactions. However, this question could be also solved using a regression algorithm when predicting the affinity between pair of molecule and protein [26]. Note that in such an approach, the affinity of all (protein, ligand) pairs in the training data is required, which is rarely available on large scale.
Although the protein-ligand interaction process takes place in the 3D space, we chose to encode the two partners based on features that do not require 3D information. Indeed, the bound conformation of the ligand, and the 3D structure of the protein binding pocket is unknown in most cases, which prevents predictions on large scale. However, we are aware that a method using 3D information to encode the interaction can be of interest on more restricted datasets (i.e. not covering the druggable human proteome) as those available from the PDB database [28, 29].
Since the prediction performance strongly depends on the distance between the predicted interactions and the train set, it could be relevant to apply the multi-kernel learning (MKL) framework [66]. Indeed, different feature spaces will lead to different metrics which could modulate the distance between the test and train sets. This idea was explored in [67], but in this work the MKL approach employed L2 regularization between kernels, which did not lead to the improvements that could be expected from an L1 (i.e. sparsity-inducing) regularization term.
To conclude, this study suggests that including only similar samples to the tested sample forces the algorithm to adjust the hyperplane to segregate positive and negative sample distributions in a better way. In the future, it might be relevant to consider a weighted-sample SVM in order to integrate such behavior in a more continuous way, by decreasing the error importance on train samples depending on their distance to the tested sample.
Supporting information
(A) Scores of MT Kernel Ridge regression on datasets S1/2/3 with a nested 5-fold-CV scheme. (B) Scores of MT Kernel Ridge regression on S1 depending on the CV scheme.
(TIFF)
Overall, all CV schemes provide high prediction performance on this dataset, in the range of 0.93-0.94 in AUC and AUPR. The nested 5-fold-CV leads to performance very close to those of 5-fold-CV, showing that on the S1 dataset, 5-fold-CV did not suffer from overestimation of the performance due to data over-fitting. LOO-CV leads to slightly better results, although very close to those of the other CV schemes. In general, the LOO-CV scheme is expected to provide better results because the model is trained on more data points than in 5-fold-CV. Again, this problem seems to be limited here, since the performance of LOO-CV does not differ much from that of nested 5-fold-CV.
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(PDF)
(PDF)
Data Availability
Code is available at https://github.com/bplaye/efficient_MultiTask_SVM_for_chemogenomics. All data are available at http://members.cbio.mines-paristech.fr/~bplaye/efficient_MT_chemo.tar.gz.
Funding Statement
This work is founded by the French ministry of Industry (https://www.economie.gouv.fr/) as founding our institution (http://www.mines-paristech.eu/). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. Journal of health economics. 2016;47:20–33. 10.1016/j.jhealeco.2016.01.012 [DOI] [PubMed] [Google Scholar]
- 2. Miguel A, Azevedo LF, Araújo M, Pereira AC. Frequency of adverse drug reactions in hospitalized patients: a systematic review and meta-analysis. Pharmacoepidemiology and drug safety. 2012;21(11):1139–1154. 10.1002/pds.3309 [DOI] [PubMed] [Google Scholar]
- 3. Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. Jama. 1998;279(15):1200–1205. 10.1001/jama.279.15.1200 [DOI] [PubMed] [Google Scholar]
- 4. Onakpoya IJ, Heneghan CJ, Aronson JK. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: a systematic review of the world literature. BMC medicine. 2016;14(1):10 10.1186/s12916-016-0553-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Scheiber J, Chen B, Milik M, Sukuru SCK, Bender A, Mikhailov D, et al. Gaining insight into off-target mediated effects of drug candidates with a comprehensive systems chemical biology analysis. Journal of chemical information and modeling. 2009;49(2):308–317. 10.1021/ci800344p [DOI] [PubMed] [Google Scholar]
- 6. Martinez-Lopez Y, Caballero Y, J Barigye S, Marrero-Ponce Y, Millan-Cabrera R, Madera J, et al. State of the Art Review and Report of New Tool for Drug Discovery. Current topics in medicinal chemistry. 2017;17(26):2957–2976. 10.2174/1568026617666170821123856 [DOI] [PubMed] [Google Scholar]
- 7. Xu X, Huang M, Zou X. Docking-based inverse virtual screening: methods, applications, and challenges. Biophysics reports. 2018; p. 1–16. 10.1007/s41048-017-0045-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vert JP, Jacob L. Machine learning for in silico virtual screening and chemical genomics: new strategies. Combinatorial chemistry & high throughput screening. 2008;11(8):677–685. 10.2174/138620708785739899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bolton EE, Wang Y, Thiessen PA, Bryant SH. PubChem: integrated platform of small molecules and biological activities. Annual reports in computational chemistry. 2008;4:217–241. 10.1016/S1574-1400(08)00012-1 [DOI] [Google Scholar]
- 10. Yamanishi Y, Araki M, Gutteridge A, Honda W, Kanehisa M. Prediction of drug–target interaction networks from the integration of chemical and genomic spaces. Bioinformatics. 2008;24(13):i232–i240. 10.1093/bioinformatics/btn162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jacob L, Vert JP. Protein-ligand interaction prediction: an improved chemogenomics approach. Bioinformatics. 2008;24(19):2149–2156. 10.1093/bioinformatics/btn409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bleakley K, Yamanishi Y. Supervised prediction of drug–target interactions using bipartite local models. Bioinformatics. 2009;25(18):2397–2403. 10.1093/bioinformatics/btp433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yamanishi Y, Kotera M, Kanehisa M, Goto S. Drug-target interaction prediction from chemical, genomic and pharmacological data in an integrated framework. Bioinformatics. 2010;26(12):i246–i254. 10.1093/bioinformatics/btq176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hizukuri Y, Sawada R, Yamanishi Y. Predicting target proteins for drug candidate compounds based on drug-induced gene expression data in a chemical structure-independent manner. BMC medical genomics. 2015;8(1):1 10.1186/s12920-015-0158-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Takarabe M, Kotera M, Nishimura Y, Goto S, Yamanishi Y. Drug target prediction using adverse event report systems: a pharmacogenomic approach. Bioinformatics. 2012;28(18):i611–i618. 10.1093/bioinformatics/bts413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yamanishi Y. Inferring Chemogenomic Features from Drug-Target Interaction Networks. Molecular Informatics. 2013;32(11-12):991–999. 10.1002/minf.201300079 [DOI] [PubMed] [Google Scholar]
- 17. Yuan Q, Gao J, Wu D, Zhang S, Mamitsuka H, Zhu S. DrugE-Rank: improving drug–target interaction prediction of new candidate drugs or targets by ensemble learning to rank. Bioinformatics. 2016;32(12):i18–i27. 10.1093/bioinformatics/btw244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. van Laarhoven T, Nabuurs SB, Marchiori E. Gaussian interaction profile kernels for predicting drug–target interaction. Bioinformatics. 2011;27(21):3036–3043. 10.1093/bioinformatics/btr500 [DOI] [PubMed] [Google Scholar]
- 19. van Laarhoven T, Marchiori E. Predicting drug-target interactions for new drug compounds using a weighted nearest neighbor profile. PloS one. 2013;8(6):e66952 10.1371/journal.pone.0066952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mei JP, Kwoh CK, Yang P, Li XL, Zheng J. Drug–target interaction prediction by learning from local information and neighbors. Bioinformatics. 2013;29(2):238–245. 10.1093/bioinformatics/bts670 [DOI] [PubMed] [Google Scholar]
- 21. Xia Z, Wu LY, Zhou X, Wong ST. Semi-supervised drug-protein interaction prediction from heterogeneous biological spaces. BMC systems biology. 2010;4(Suppl 2):S6 10.1186/1752-0509-4-S2-S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng X, Ding H, Mamitsuka H, Zhu S. Collaborative matrix factorization with multiple similarities for predicting drug-target interactions. In: Proceedings of the 19th ACM SIGKDD international conference on Knowledge discovery and data mining. ACM; 2013. p. 1025–1033.
- 23. Gönen M. Predicting drug–target interactions from chemical and genomic kernels using Bayesian matrix factorization. Bioinformatics. 2012;28(18):2304–2310. 10.1093/bioinformatics/bts360 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Wu M, Miao C, Zhao P, Li XL. Neighborhood regularized logistic matrix factorization for drug-target interaction prediction. PLoS computational biology. 2016;12(2):e1004760 10.1371/journal.pcbi.1004760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Johnson CC. Logistic matrix factorization for implicit feedback data. Advances in Neural Information Processing Systems. 2014;27. [Google Scholar]
- 26. Pahikkala T, Airola A, Pietilä S, Shakyawar S, Szwajda A, Tang J, et al. Toward more realistic drug–target interaction predictions. Briefings in bioinformatics. 2014; p. bbu010. 10.1093/bib/bbu010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jacob L, Hoffmann B, Stoven V, Vert JP. Virtual screening of GPCRs: an in silico chemogenomics approach. BMC bioinformatics. 2008;9(1):363 10.1186/1471-2105-9-363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paul N, Kellenberger E, Bret G, Müller P, Rognan D. Recovering the true targets of specific ligands by virtual screening of the protein data bank. Proteins: Structure, Function, and Bioinformatics. 2004;54(4):671–680. 10.1002/prot.10625 [DOI] [PubMed] [Google Scholar]
- 29. Kellenberger E, Foata N, Rognan D. Ranking targets in structure-based virtual screening of three-dimensional protein libraries: methods and problems. Journal of chemical information and modeling. 2008;48(5):1014–1025. 10.1021/ci800023x [DOI] [PubMed] [Google Scholar]
- 30. Hue M, Riffle M, Vert JP, Noble WS. Large-scale prediction of protein-protein interactions from structures. BMC bioinformatics. 2010;11(1):144 10.1186/1471-2105-11-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caruana R. Multitask learning. In: Learning to learn. Springer; 1998. p. 95–133.
- 32. Bakker B, Heskes T. Task clustering and gating for bayesian multitask learning. Journal of Machine Learning Research. 2003;4(May):83–99. [Google Scholar]
- 33. Arora N, Allenby GM, Ginter JL. A hierarchical Bayes model of primary and secondary demand. Marketing Science. 1998;17(1):29–44. 10.1287/mksc.17.1.29 [DOI] [Google Scholar]
- 34. Allenby GM, Rossi PE. Marketing models of consumer heterogeneity. Journal of econometrics. 1998;89(1-2):57–78. 10.1016/S0304-4076(98)00055-4 [DOI] [Google Scholar]
- 35. Cortes C, Vapnik V. Support-vector networks. Machine learning. 1995;20(3):273–297. 10.1007/BF00994018 [DOI] [Google Scholar]
- 36. Erhan D, L’Heureux PJ, Yue SY, Bengio Y. Collaborative filtering on a family of biological targets. Journal of chemical information and modeling. 2006;46(2):626–635. 10.1021/ci050367t [DOI] [PubMed] [Google Scholar]
- 37. Faulon JL, Misra M, Martin S, Sale K, Sapra R. Genome scale enzyme–metabolite and drug–target interaction predictions using the signature molecular descriptor. Bioinformatics. 2008;24(2):225–233. 10.1093/bioinformatics/btm580 [DOI] [PubMed] [Google Scholar]
- 38. Napolitano F, Zhao Y, Moreira VM, Tagliaferri R, Kere J, D’Amato M, et al. Drug repositioning: a machine-learning approach through data integration. J Cheminformatics. 2013;5:30 10.1186/1758-2946-5-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Jaakkola T, Diekhans M, Haussler D. A discriminative framework for detecting remote protein homologies. Journal of computational biology. 2000;7(1-2):95–114. 10.1089/10665270050081405 [DOI] [PubMed] [Google Scholar]
- 40.Leslie CS, Eskin E, Noble WS. The spectrum kernel: A string kernel for SVM protein classification. In: Pacific symposium on biocomputing. vol. 7; 2002. p. 566–575. [PubMed]
- 41.Eskin E, Weston J, Noble WS, Leslie CS. Mismatch string kernels for SVM protein classification. In: Advances in neural information processing systems; 2002. p. 1417–1424.
- 42. Saigo H, Vert JP, Ueda N, Akutsu T. Protein homology detection using string alignment kernels. Bioinformatics. 2004;20(11):1682–1689. 10.1093/bioinformatics/bth141 [DOI] [PubMed] [Google Scholar]
- 43. Kuang R, Ie E, Wang K, Wang K, Siddiqi M, Freund Y, et al. Profile-based string kernels for remote homology detection and motif extraction. Journal of bioinformatics and computational biology. 2005;3(03):527–550. 10.1142/S021972000500120X [DOI] [PubMed] [Google Scholar]
- 44. Smith TF, Waterman MS. Identification of common molecular subsequences. Journal of molecular biology. 1981;147(1):195–197. 10.1016/0022-2836(77)90031-6 [DOI] [PubMed] [Google Scholar]
- 45. Okuno Y, Tamon A, Yabuuchi H, Niijima S, Minowa Y, Tonomura K, et al. GLIDA: GPCR—ligand database for chemical genomics drug discovery—database and tools update. Nucleic acids research. 2007;36(suppl_1):D907–D912. 10.1093/nar/gkm948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S. The protein kinase complement of the human genome. Science. 2002;298(5600):1912–1934. 10.1126/science.1075762 [DOI] [PubMed] [Google Scholar]
- 47. Kanehisa M, Araki M, Goto S, Hattori M, Hirakawa M, Itoh M, et al. KEGG for linking genomes to life and the environment. Nucleic acids research. 2007;36(suppl_1):D480–D484. 10.1093/nar/gkm882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Swamidass SJ, Chen J, Bruand J, Phung P, Ralaivola L, Baldi P. Kernels for small molecules and the prediction of mutagenicity, toxicity and anti-cancer activity. Bioinformatics. 2005;21(suppl 1):i359–i368. 10.1093/bioinformatics/bti1055 [DOI] [PubMed] [Google Scholar]
- 49. Kashima H, Tsuda K, Inokuchi A. Marginalized kernels between labeled graphs. In: ICML; vol. 3; 2003. p. 321–328. [Google Scholar]
- 50. Mahé P, Ueda N, Akutsu T, Perret JL, Vert JP. Graph kernels for molecular structure-activity relationship analysis with support vector machines. Journal of chemical information and modeling. 2005;45(4):939–951. 10.1021/ci050039t [DOI] [PubMed] [Google Scholar]
- 51. Azencott CA, Ksikes A, Swamidass SJ, Chen JH, Ralaivola L, Baldi P. One-to four-dimensional kernels for virtual screening and the prediction of physical, chemical, and biological properties. Journal of chemical information and modeling. 2007;47(3):965–974. 10.1021/ci600397p [DOI] [PubMed] [Google Scholar]
- 52. Golub GH, Heath M, Wahba G. Generalized cross-validation as a method for choosing a good ridge parameter. Technometrics. 1979;21(2):215–223. 10.1080/00401706.1979.10489751 [DOI] [Google Scholar]
- 53. Friedman J, Hastie T, Tibshirani R. The elements of statistical learning. vol. 1 Springer series in statistics; New York; 2001. [Google Scholar]
- 54. Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36. [DOI] [PubMed] [Google Scholar]
- 55. Raghavan V, Bollmann P, Jung GS. A critical investigation of recall and precision as measures of retrieval system performance. ACM Transactions on Information Systems (TOIS). 1989;7(3):205–229. 10.1145/65943.65945 [DOI] [Google Scholar]
- 56. Pedregosa F, Varoquaux G, Gramfort A, Michel V, Thirion B, Grisel O, et al. Scikit-learn: Machine Learning in Python. Journal of Machine Learning Research. 2011;12:2825–2830. [Google Scholar]
- 57.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic acids research. 2011; p. gkr988. [DOI] [PMC free article] [PubMed]
- 58. Law V, Knox C, Djoumbou Y, Jewison T, Guo AC, Liu Y, et al. DrugBank 4.0: shedding new light on drug metabolism. Nucleic acids research. 2013;42(D1):D1091–D1097. 10.1093/nar/gkt1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gaulton A, Bellis LJ, Bento AP, Chambers J, Davies M, Hersey A, et al. ChEMBL: a large-scale bioactivity database for drug discovery. Nucleic acids research. 2012;40(D1):D1100–D1107. 10.1093/nar/gkr777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Advanced drug delivery reviews. 1997;23(1-3):3–25. 10.1016/S0169-409X(96)00423-1 [DOI] [PubMed] [Google Scholar]
- 61. Schölkopf B, Platt JC, Shawe-Taylor J, Smola AJ, Williamson RC. Estimating the support of a high-dimensional distribution. Neural computation. 2001;13(7):1443–1471. 10.1162/089976601750264965 [DOI] [PubMed] [Google Scholar]
- 62. Kramer C, Gedeck P. Leave-cluster-out cross-validation is appropriate for scoring functions derived from diverse protein data sets. Journal of chemical information and modeling. 2010;50(11):1961–1969. 10.1021/ci100264e [DOI] [PubMed] [Google Scholar]
- 63. Johnson SC. Hierarchical clustering schemes. Psychometrika. 1967;32(3):241–254. 10.1007/BF02289588 [DOI] [PubMed] [Google Scholar]
- 64. Warmuth MK, Liao J, Rätsch G, Mathieson M, Putta S, Lemmen C. Active learning with support vector machines in the drug discovery process. Journal of chemical information and computer sciences. 2003;43(2):667–673. 10.1021/ci025620t [DOI] [PubMed] [Google Scholar]
- 65. Hattori M, Okuno Y, Goto S, Kanehisa M. Development of a chemical structure comparison method for integrated analysis of chemical and genomic information in the metabolic pathways. Journal of the American Chemical Society. 2003;125(39):11853–11865. 10.1021/ja036030u [DOI] [PubMed] [Google Scholar]
- 66. Rakotomamonjy A, Bach FR, Canu S, Grandvalet Y. SimpleMKL. Journal of Machine Learning Research. 2008;9(Nov):2491–2521. [Google Scholar]
- 67. Nascimento AC, Prudêncio RB, Costa IG. A multiple kernel learning algorithm for drug-target interaction prediction. BMC bioinformatics. 2016;17(1):1 10.1186/s12859-016-0890-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Scores of MT Kernel Ridge regression on datasets S1/2/3 with a nested 5-fold-CV scheme. (B) Scores of MT Kernel Ridge regression on S1 depending on the CV scheme.
(TIFF)
Overall, all CV schemes provide high prediction performance on this dataset, in the range of 0.93-0.94 in AUC and AUPR. The nested 5-fold-CV leads to performance very close to those of 5-fold-CV, showing that on the S1 dataset, 5-fold-CV did not suffer from overestimation of the performance due to data over-fitting. LOO-CV leads to slightly better results, although very close to those of the other CV schemes. In general, the LOO-CV scheme is expected to provide better results because the model is trained on more data points than in 5-fold-CV. Again, this problem seems to be limited here, since the performance of LOO-CV does not differ much from that of nested 5-fold-CV.
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(TIFF)
(PDF)
(PDF)
Data Availability Statement
Code is available at https://github.com/bplaye/efficient_MultiTask_SVM_for_chemogenomics. All data are available at http://members.cbio.mines-paristech.fr/~bplaye/efficient_MT_chemo.tar.gz.