Abstract
India is considered the diabetes capital of the world, and a significant proportion of patients undergoing cataract surgery are diabetic. Considering this, we reviewed the principles and guidelines of managing cataract in patients with diabetes. The preoperative, intraoperative, and postoperative factors are of paramount importance in the management of diabetic cataract patients. Particularly, the early recognition and treatment of diabetic retinopathy or maculopathy before cataract surgery influence the final visual outcome and play a major role in perioperative decision-making. Better understanding of various factors responsible for favorable outcome of cataract surgery in diabetic patients may guide us in better overalll management of these patients and optimizing the results.
Keywords: Cataract, diabetes mellitus, diabetic retinopathy, treatment outcome, vitrectomy
Worldwide, more than 285 million people are affected by diabetes mellitus. This number is expected to increase almost twofold to 439 million by 2030 according to the International Diabetes Federation.[1,2] In India, approximately 69 million people are affected by diabetes mellitus as of 2015.[3] Diabetic patients are 2–5 times more likely to develop cataract and it tends to happen more so at an earlier age.[3] On the other hand, diabetes-induced microangiopathy leads to diabetic retinopathy (DR), nephropathy, and neuropathy. Recently, retinal neurodegeneration has been described in diabetes and has been suggested to precede or occur concurrently and aggravate retinal vasculopathy.[4,5,6] Diabetic retinal neurodegeneration clinically manifests as reduction in the retinal nerve fiber layer, ganglion cell layer, as well as Muller cells,[7,8,9,10] color vision loss, and spatial frequency changes on electrophysiology.[11,12] In addition to the retinal changes, diabetes leads to structural and morphological alterations in other parts of the eye including the cornea, tear film, and crystalline lens, which lead to changes in the optical quality of the diabetic eye.[13]
Globally, cataract remains the leading cause of blindness, affecting approximately 18 million people.[1,2] Cataract is considered a major cause of visual impairment in diabetic patients as the incidence and progression of cataract are higher in patients with diabetes mellitus.[14] Several clinical studies have shown that cataracts occur more frequently and at an earlier age in diabetics.[15] Overall, it is estimated that up to 20% of all cataract surgeries are performed in diabetic patients.[16]
Some studies have reported that cataract surgery when performed in diabetic patients may lead to relatively rapid progression of DR, precipitate vitreous hemorrhage, induce iris neovascularization, and ultimately lead to decrease or loss of vision.[17] Uneventful cataract surgery results in increased inflammatory cytokines in the eye.[18,19] Patel et al. reported that 1 day after uneventful phacoemulsification and intraocular lens (IOL) implantation, there were significant increases in vascular endothelial growth factor (VEGF), hepatocyte growth factor, interleukin-1 (IL-1), and pigment epithelium derived factor concentrations and that these increases took up to 1 month to decline to preoperative levels.[18] These cytokines may induce clinical or subclinical worsening of DR and maculopathy.[18] Although there have been a number of studies on the progression of DR status and factors influencing such progression after cataract surgery, further studies are still required, especially in the longer term.
Previously, monitoring changes in DR, especially maculopathy, were based on clinical examination including biomicroscopy and fundus fluorescein angiography.
The recent introduction of optical coherence tomography (OCT), especially spectral-domain models, into clinical practice has improved the noninvasive monitoring of retinal thickness changes in DR including postcataract surgery.[20]
This article reviews the development and progression of cataract, as well as cataract surgery and its outcomes in diabetic patients with emphasis on the relationship between cataract surgery and DR progression.
Methods
To ensure the accuracy of this review, this literature review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses statement. A comprehensive search of the literature was conducted for papers using the online biomedical search engines PubMed, EMBASE, and the Cochrane library. The following terms were used for the searches: diabetes AND (cataract) AND (timing OR pathophysiology OR phacoemulsification OR complications OR visual outcome OR visual prognosis OR retinopathy progression OR diabetic macular edema). Two authors identified the articles, and all relevant articles were included in this review. Limits for our literature search filters included papers published in English between 1987 and June 2017, including human studies published as randomized controlled trials and nonrandomized comparative studies (systematic reviews, cohort or retrospective studies, or case–control series).
Results
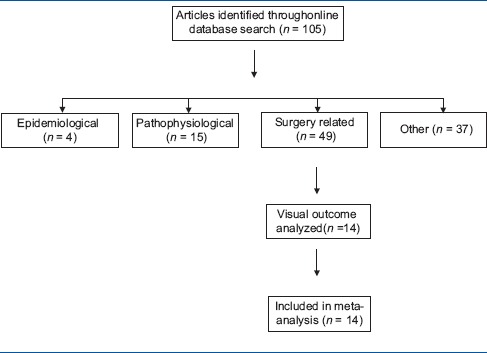
Of 105 studies included in this review article, 14 studies reported the visual outcome as an endpoint after the cataract surgery. The meta-analysis[21] was carried out using poor visual outcome as an outcome variable. Table 1 shows the flowchart for the literature search and study selection.
Table 1.
Literature search and study selection for meta-analysis
Meta-analysis
The results are presented in Table 2. It is clear that there is a significant heterogeneity being reported in the literature in terms of poor visual outcome, following the cataract surgery (Q-Statistic - 386.2768, P < 0.001). Fig. 1 shows the forest plot that includes the proportions with 95% confidence interval found in the studies included in the meta-analysis. Fig. 2 shows the funnel plot[22] as a graphical tool for detecting bias in the meta-analysis. Since the funnel plot is asymmetric, there is a clear evidence of publication bias. Meta-analysis was carried out using MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium; http://www.medcalc.org; 2018).
Table 2.
Meta-analysis for proportions of poor visual outcome
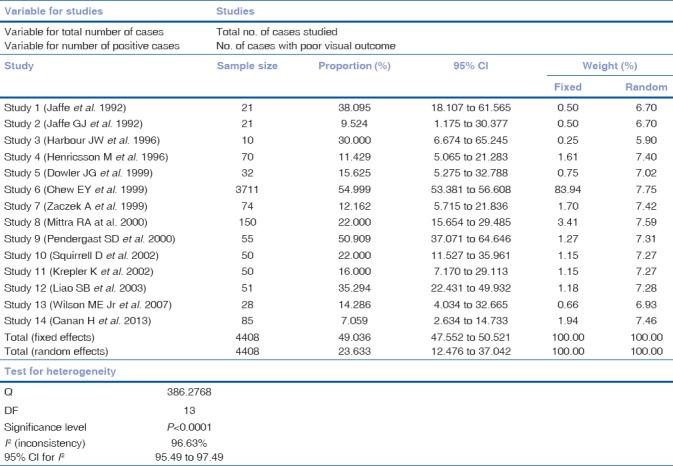
Figure 1.
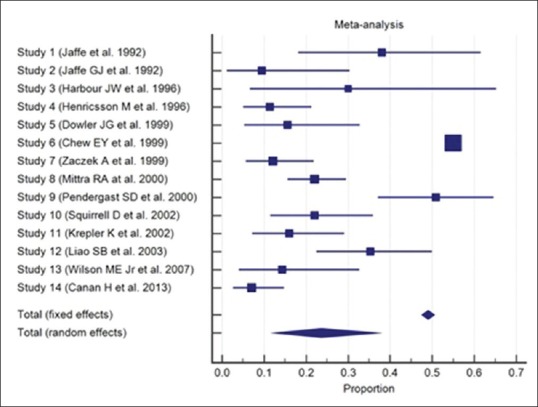
Forest plot showing the proportions with 95% confidence interval found in the studies included in the meta-analysis
Figure 2.
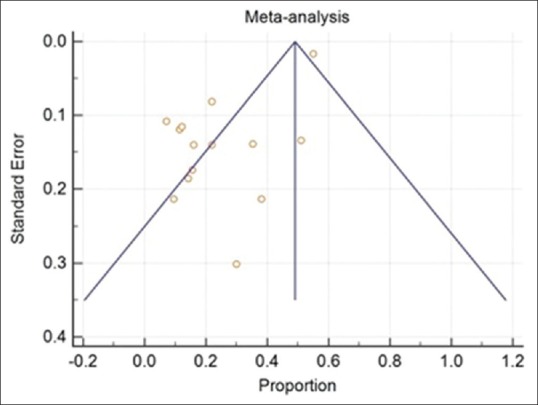
A funnel plot for detecting the publication bias in the meta-analysis
Discussion
Pathogenesis of diabetic cataract
Various types of lens changes occur in diabetic patients. Snowflake cataract is a type of cataract seen very commonly in Type 1 diabetics. However, the most frequent type of cataract seen in diabetics is senile type.[23] Various studies have been carried out to study the type of cataract associated with diabetes. Posterior subcapsular cataract has been shown to be significantly associated with diabetes.[24] In fact, increased levels of glycated hemoglobin was shown by some authors to be associated with increased risk of nuclear and cortical cataract.[25] In further analysis, it had shown that diabetic patients were prone to develop cortical cataract and it was associated with duration of diabetes.[26]
Various mechanisms have been proposed for the pathogenesis of cataract in diabetes mellitus. In the lens, sorbitol is produced faster than it is converted to fructose by the enzyme sorbitol dehydrogenase, a process that is increased in diabetics compared to nondiabetics.[27] The increased accumulation of sorbitol creates a hyperosmotic effect that results in an infusion of fluid to countervail the osmotic gradient. Hyperglycemia per se may also contribute significantly to the osmotic retention of fluid in the lens fibers with resultant osmotic stress and is aggravated by increased intracellular production of cytokines/growth factors and oxidative stress.[28,29,30] The role of osmotic stress is particularly important for the rapid cataract formation in young patients with Type 1 diabetes mellitus[31,32] due to the extensive swelling of cortical lens fibers.[32]
Accumulation of sorbitol and advanced glycation endproducts lead to the generation of superoxide radicals and hydrogen peroxide (H2O2). Normally, antioxidant enzymes help in degradation of superoxide radicals into H2O2 and oxygen. However, lens antioxidant enzymes such as superoxide dismutase and catalase are compromised in diabetes, thus leading to oxidative stress which contributes to cataract formation.[33,34,35]
Pathophysiological changes in crystalline lens with fluctuations in blood glucose levels
Hyperglycemia causes a myopic shift and is the major cause of transient refractive changes in diabetic patients. With intensive medical therapy, a considerable number of patients tend to become more hyperopic as compared to hyperglycemic state. The refractive changes observed during periods of unstable blood sugar are thought to be related to both morphologic and functional changes in the crystalline lens.[36] In addition, changes in corneal topographic parameters during glycemic fluctuations are a potential source of error in keratorefractive and cataract surgery assessments.[37] The basement membrane of the lens (or lens capsule) is known to be thicker in people with diabetes, leading to refractive changes. Saxena et al.[38] found a twofold higher incidence of cortical cataracts in patients with diabetes mellitus over 5 years and postulated the same mechanism.
Timing of cataract surgery
The growing tendency toward earlier cataract surgery in patients with diabetes has contributed to improved visual outcomes.[39] Visual outcomes are likely to be worse in patients in whom surgery is deferred until late when it is not possible to identify or adequately treat DME before cataract surgery. Where cataract surgery is undertaken before lens opacities obscure adequate macular assessment preventing the recognition of retinal thickening, the risk of DME is decreased and the visual outcome may be improved considerably.[40]
Preoperative considerations
Preoperative counseling is crucial. Before surgery, patients should have good glycemic control and no evidence of ocular or periocular infection. Some organisms in the conjunctiva are more prevalent in diabetic patients such as Staphylococcus aureus, Enterococci, certain Streptococci, and Klebsiella species compared to nondiabetics.[41] Hence, the need for thorough asepsis cannot be overemphasized in these patients.[41] A thorough and comprehensive ophthalmologic examination including assessment of visual acuity (VA), best-corrected VA, relative afferent pupillary defect, slit-lamp biomicroscopy to look for corneal health and neovascularization of iris, tonometry, and dilated fundoscopy and gonioscopy (in patients with high-risk PDR with particular attention to new vessels), is mandatory. Ancillary diagnostic evaluations such as fluorescein angiography, OCT, and B-scan ultrasonography may be helpful in selected cases.
Patients with preexisting untreated PDR are more likely to progress and develop vitreous hemorrhage and other retinal complications rapidly after cataract surgery. Therefore, PRP is recommended preoperatively. When the lens opacity precludes preoperative PRP, it could be administered as indirect PRP immediately following the cataract procedure or planned and performed within a week after cataract surgery.[42] Alternatively, combined cataract surgery with vitrectomy and endolaser photocoagulation may be undertaken, especially for cases with dense vitreous hemorrhage or for eyes with posterior pole tractional retinal detachment (TRD). Some studies have shown that uncomplicated phacoemulsification modern surgery does not lead to progression of DR and that the progression of DR after cataract surgery is mainly related to poor glycemic control.[40,43,44] Thus, data on this particular topic are conflicting and large studies in the future may lead to a clearer picture.
ME should be adequately treated before surgery as preexisting maculopathy may be aggravated postoperatively with associated poor visual outcome.[43] Treatment of the maculopathy may be with laser photocoagulation or pharmacotherapy with intravitreal injections of anti-VEGF agents or steroids.[45,46]
It is well known that increased retinal vascular permeability factors such as IL-6 and VEGF play a major role in diabetic ME (DME).[47] In patients with preexisting DME or prior treatment for the same, cataract surgery can increase the risk of progression or re-development of ME to 20%–50%.[48,49] Thus, perioperative intravitreal steroids and anti-VEGF agents are a good option in these cases. It has been shown that intraoperative intravitreal ranibizumab with cataract surgery is more effective than preoperative or postoperative injection for DME although an ongoing treatment is needed in these patients.[50] Steroids, on the other hand, have shown to be effective for persistent or refractory DME.[50] Dexamethasone has potentially less risk of intraocular pressure (IOP) elevation and cataract formation compared to fluocinolone acetonide and triamcinolone acetate.[51] Furthermore, the intravitreal dexamethasone implants and fluocinolone implants require less frequent injections.[51] Studies on intravitreal dexamethasone implant have shown a significant improvement in clinically significant ME (CSME) over 3 years.[52] In fact, it has also shown to also slow the progression of DR. Furthermore, a recent study showed that patients who were treated with preoperative fluocinolone acetonide 0.2 μg implant and requiring subsequent cataract surgery had good visual outcome.[53]
Recently, few studies have suggested that preoperative use of nonsteroidal anti-inflammatory drugs such as diclofenac and nepafenac may reduce the chances of postoperative ME in patients with DR.[54,55]
Patients with neovascularization of the iris (NVI) need prompt treatment to induce regression before cataract surgery. Conventionally, this was done with PRP. When neovascular glaucoma (NVG) develops, medical therapy alone is usually not effective. Topical beta-adrenergic antagonists, alpha-2-adrenergic agonists, carbonic anhydrase inhibitors, cycloplegics, and corticosteroids may be useful in reducing IOP and decreasing inflammation. Eyes with NVG have demonstrated dramatic short-term regression of neovascularization and IOP reduction with intravitreal injection of anti-VEGF agents.[56,57] Once NVI regresses, phacoemulsification should be considered with or without vitrectomy at the earliest to enable treatment of the posterior-segment pathology. Alternatively, combining phacoemulsification with endoscopic diode laser cyclophotocoagulation is an option for eyes where the anterior-chamber angles are closed with mature (fibrosed) new vessels.[58] Combining trabeculectomy with phacoemulsification may also be planned in eyes with coexistent NVG and cataract when NVI has regressed. However, the visual outcomes following phacoemulsification in eyes with NVG are usually poor.
Cataract surgery (intraoperative considerations)
Phacoemulsification with IOL implantation yields better visual results and less inflammation as compared to extracapsular cataract surgery.[59,60] Anterior capsular phimosis is more common in diabetic eyes.[61] The capsulorhexis size should, therefore, be larger than normal but smaller than IOL optic diameter to prevent anterior IOL displacement and posterior capsular opacification (PCO).[62] A large diameter optic (i.e., 6.0 mm or larger) also facilitates diagnosis and treatment of peripheral retinal pathology postoperatively.[38] Longer duration and complicated cataract surgery are associated with a greater risk of progression of retinopathy and subsequent visual compromise.[63] In diabetic patients, there is high likelihood of poor pupillary dilatation due to damage to pupillary parasympathetic supply.[64] Thus, iris hooks, malyugin ring, or other iris expanders should be considered for intraoperative use in these patients. In addition, complications such as intraoperative hyphema due to the presence of rubeosis iridis need to be kept in mind. Some studies have shown that preoperative intravitreal anti-VEGF agents may decrease the chances of bleeding from iris neovascularization.[65] Cetinkaya et al.[66] showed that photic retinopathy during cataract surgery was more prevalent in diabetic patients than nondiabetics. The mere presence of diabetes does not increase the risk of intraoperative complications such as posterior capsular rupture, zonular dehiscence, and vitreous loss.[60] However, the diabetic eye is more prone to keratoepitheliopathy, including corneal epithelial defects/abrasions, which may heal slowly.[67] The impaired corneal wound healing has multiple etiologies including neurogenic (subbasal nerve abnormalities) and impaired corneal stem cell and epithelial cell division.[68] Studies have also shown greater predisposition to corneal endothelial cell loss in people with diabetes compared to nondiabetics.[68,69,70,71] Hence, routine specular microscopy is recommended for all people with diabetes, and greater care should be taken with regard to endothelial protection when operating on diabetic patients.
Intraocular lens choice
Large diameter IOLs are preferred to facilitate visualization and treatment of the peripheral retina in DR.[59] Diabetic patients seem to develop more severe PCO than nondiabetics.[72] The level of phosphorus in the serum and aqueous humor of diabetic patients, particularly those with PDR, is significantly higher than normal individuals and may lead to opacification of hydrophilic acrylic IOLs.[73,74] Hydrophobic acrylic IOLs are associated with slightly more anterior-chamber flare in the early postoperative period. However, PCO develops less frequently, and hydrophobic acrylic lenses have the lowest propensity for silicone oil adhesion and should be the IOL of choice in diabetic patients when anticipating vitreoretinal surgery.[75]
Rodríguez-Galietero et al.[76] evaluated blue-light-filtering IOLs and reported that such lenses did not cause chromatic discrimination defects and even improved color vision in the blue-yellow chromatic axis. The use of multifocal and accommodative IOLs in people with diabetes remains controversial. Multifocal IOLs, especially, may make postoperative laser treatment (focal/grid) and fundus visualization during vitrectomy difficult, considering the optics of multifocal IOLs.[77] In addition, reduced contrast sensitivity due to multifocal IOLs can get aggravated and be a cause of visual dissatisfaction in patients with preexisting maculopathy.[77] Iris claw lenses, both anterior and posterior claw, should be avoided in patients with diabetes who are at an increased risk for iris neovascularization. In addition, ovalization of the pupil and poor mydriasis after iris–claw IOL make posterior-segment evaluation difficult. In addition, the theoretical risk of cystoid ME is higher when iris–claw IOLs are used, especially in diabetics.
Visual prognosis following cataract surgery
Recent studies on cataract surgery in diabetics tend to report a lower incidence of complications and better visual outcomes[78,79] due to better preoperative management of retinopathy, evolutions in operative techniques, better glycemic and hypertensive control, and better surgical technique of phacoemulsification. Diabetic patients with little or no retinopathy enjoy good visual prognosis similar to that in individuals without diabetes.[80] The presence of DME and poor preoperative VA (reflecting diabetic maculopathy, ischemia, and traction) has been recognized as risk factors for poor postoperative VA following cataract surgery.[60,81]
Mozaffarieh et al.[81] evaluated patients with more advanced DR and showed no functional improvement in VA. Pollack et al.[82] reported VA better than 20/40 in 31% of patients, noted CME as the main cause of poor visual outcome following cataract extraction in patients with diabetes, and proposed that “cataract extraction should not be recommended for eyes with DR until VA has deteriorated to 20/100–20/200.” This view was later endorsed by Schatz et al.,[83] who reported in their study that only 9% of eyes achieved postoperative VA better than 20/40. Overall, visual recovery after uncomplicated phacoemulsification and IOL implantation is governed by the macular perfusion status, presence of ME, and stage of DR.[84]
Indicators of poor visual outcomes following cataract surgery
According to the Early Treatment of Diabetic Retinopathy Study (ETDRS), the presence of CSME at the time of cataract surgery is significantly associated with poor VA and a predictor of final VA worse than 20/200 following uncomplicated phacoemulsification.[85] The severity of DR at the time of cataract surgery is also a significant determinant of postoperative VA; more severe retinopathy seems to be associated with an increased prevalence of macular ischemia[39,60] or edema[20,60,86] and a reduced tendency for spontaneous resolution of postoperative ME with associated poor postoperative VA. Treatment-naïve PDR before cataract surgery may progress to vitreous hemorrhage and TRD following phacoemulsification, thus threatening good visual outcome.[87]
Cataract surgery and intravitreal injection
Intravitreal steroids may be considered during cataract surgery in the eyes with DME without epiretinal membrane or tractional component, particularly if the patient has not been treated previously.[88,89] Intravitreal injections of bevacizumab (Avastin, Roche) have been employed for the treatment of neovascular and exudative ocular diseases since 2005. Since then, several small studies have evaluated the effect of intravitreal bevacizumab on neovascular complications of diabetes.[56,57,90,91] Coexistent center-involving DME at the time of cataract surgery warrants combined phacoemulsification and anti-VEGF injections as treatment for the DME simultaneously. Repeat intravitreal anti-VEGF injections can be planned based on the DRCR.net retreatment criteria.[92]
Combined cataract surgery and vitrectomy
Diabetic patients undergoing vitrectomy often have coexisting cataracts. Furthermore, lens opacities often progress following vitrectomy.[93] Phacoemulsification may be combined with pars plana vitrectomy in the presence of coexistent cataract and nonclearing vitreous hemorrhage, macular TRD, combined mechanism retinal detachment, and persistent DME not responding to intravitreal anti-VEGF agents and/or steroids. Several studies have suggested that the vitreoretinal interface is a contributing factor in the development of persistent DME following laser photocoagulation and have demonstrated significant anatomic and visual improvement with combined surgery when indicated.[39,94,95] Internal limiting membrane (ILM) peeling in the eyes with persistent DME has been shown to be beneficial.[96] However, it is also known that the ILM in diabetics is more difficult to peel due to increased adhesions.
Careful patient selection and combining the two procedures can offer more rapid visual rehabilitation, avoid a second operation, and simplify surgical interventions in patients who are likely to require multiple procedures.[97,98,99] Patients with severe traction and ischemia and those with active rubeosis are less suitable candidates. Younger patients with little preoperative lens opacity may be better managed without lens extraction.[98] Table 3 shows the results of some major studies on outcomes of combined phacoemulsification and vitrectomy surgery.[97,100,101,102] Table 4 shows the compilation of various complication rates with the combined surgery.[97,100,101,102,103]
Table 3.
Summary table of visual outcomes after combined phacoemulsification and vitrectomy in diabetic patients in some major studies
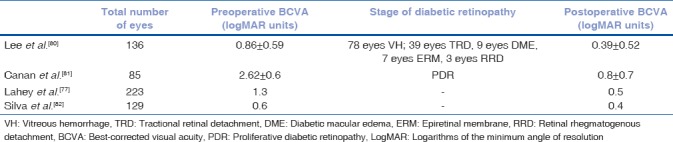
Table 4.
Summary table showing complication rates in combined phacoemulsification and vitrectomy surgery in some major studies

Effect of cataract surgery on retinopathy
Some studies have demonstrated DR progression after phacoemulsification surgery, while others reported no significant change.[99,104] Squirrell et al.[43] have shown an increased risk of DR progression following cataract surgery in patients with elevated hemoglobin A1c. In a retrospective study, Krepler et al.[105] found that DR was associated with male sex, disease duration, and poor glycemic control.
To differentiate the effect of cataract surgery from the natural course of the disease, Dowler et al.[40] designed a prospective study which showed that uncomplicated phacoemulsification cataract surgery does not accelerate progression of DR as smaller incision size and shorter surgical time in phacoemulsification decrease inflammation and may induce less breakdown of the blood–ocular barrier. Similarly, Shah and Chen in a recent review reported that recent studies do not support the generalized conclusion that phacoemulsification causes progression of retinopathy and ME in all diabetic patients.[44] The ETDRS study, however, suggested a trend toward increased retinopathy progression and worsening VA in eyes undergoing cataract surgery compared to unoperated fellow eyes in people with diabetes.[39] Denniston et al. have recently reported significant postoperative progression of center-involving DME which was associated with the preoperative grade of DR in their large UK cohort.[20]
Effect of cataract surgery on macular edema
Altered concentrations of angiogenic factors after cataract surgery may aggravate maculopathy. Following uneventful cataract surgery, OCT imaging showed increased retinal thickness in diabetic eyes without retinopathy in comparison to eyes from non-diabetics.[106] Recently, report 2 from the UK DR electronic medical record users group published real-world data for the impact of cataract surgery on DME.[20] They studied 4850 eyes without any DME before cataract surgery and followed them up for 2 years using the electronic records. VA and “treatment requiring DME” were available on this large cohort. The authors found that the risk of “treatment-requiring DME” increased sharply after surgery and peaked in the 3–6 months’ period (with annualized rates of 5.2%, 6.8%, 5.6%, and 4.0% for the 0–3, 3–6, 6–9, and 9–12 months’ postoperative time periods, respectively). The risk of ME was associated with preoperative grade of retinopathy; the risk of DME in the 1st year postoperatively was 1.0% (no DR preoperatively), 5.4% (mild non-PDR [NPDR]), 10.0% (moderate NPDR), 13.1% (severe NPDR), and 4.9% (PDR). This large real-world study proves that the risk of treatment requiring DME increases sharply in the 1st year after cataract surgery and that those with moderate and severe NPDR are most at risk of such progression.
Postoperative laser photocoagulation for DME is now less performed than previously. In the current era of anti-VEGF therapy, the role of focal and grid laser photocoagulation has diminished generally as the treatment of DME and especially for DME following cataract surgery.[86] Pollack et al. evaluated the natural course of DME after cataract surgery and found that only a minority of patients who developed ME required focal laser photocoagulation.[42] Dowler et al.[40] suggested that early laser treatment of all cases of postoperative DME was unnecessary because many cases of edema resolve spontaneously if it arose after surgery but not when present before surgery.
Differentiating diabetic macular edema from pseudophakic macular edema in diabetic patients
It is important to differentiate DME from pseudophakic cystoid ME (PCME), especially because people with diabetes are more prone to develop PCME.[107] The pathogenesis, treatment, natural course, and outcomes are very different in these two entities: (1) presence of underlying DR, exudates, and ME point toward DME (2) no or minimal DR and absence of exudates in the posterior pole hint toward PCME. When in doubt, fundus fluorescein angiography should be performed which reveals optic disc staining (“hot disc”) and typical petalloid pooling of dye in PCME, whereas the disc is usually normal and leaking micro-aneurysms and/or capillary plexus are identified as the causes of ME in DME.
PCME is managed predominantly with topical steroids and nonsteroidal anti-inflammatory drugs including bromfenac (Yellox, Bausch, and Lomb) and nepafenac (Nevanac, Alcon) and rarely requires steroids in the form of posterior subtenon's or intravitreal injections, whereas DME is managed mainly with intravitreal injections of anti-VEGF agents or steroids with or without laser photocoagulation.
Postoperative consideration
All patients diagnosed with NPDR should undergo detailed retinal examination within 3 months before cataract extraction. Patients with diabetes, especially those with proliferative retinopathy or those with inadequate view of the retina before cataract extraction, should be evaluated closely after surgery for monitoring retinal status.[17]
Diabetic patients are prone to corneal epithelial defects and persistent erosions due to impaired corneal innervation; these occur more frequently with increasing patient age and duration of diabetes.[108] Eyes of diabetic patients showed more severe corneal endothelial cell damage following cataract surgery and delayed recovery of corneal edema as described previously.[70,109] Other anterior-segment complications such as severe iritis, posterior synechiae, pupillary block, and pigmented precipitates on the IOL are more frequently observed in diabetic patients.[110] The incidence of NVI which is the most dreaded anterior segment complication in diabetic patients following cataract surgery has been reduced with modern cataract surgery which is less traumatic than previous techniques. In addition, PRP and intravitreal injections of anti-VEGF agents have been reported to control NVI albeit for short periods.[57,90,91,92] Diabetic patients may have increased risk of postoperative endophthalmitis which may be associated with a poor visual prognosis.[111]
Conclusion
The number of people with diabetes mellitus is increasing exponentially. People with diabetes have not always shared the favorable outcomes after cataract surgery as their nondiabetic counterparts. Diabetic patients with visually significant cataracts pose unique challenges during surgery and postoperative recovery, which vary according to the severity of the DR. However, with careful pretreatment of the DR and minimally invasive surgical techniques, these patients do very well and recover excellent vision just like other cataract patients without diabetes. Special attention to systemic and ocular conditions is needed.
Modern surgical and pharmacologic therapies may allow for safer and more effective surgery in diabetic individuals. This emphasizes the importance of patient education before surgery.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We acknowledge Dr. Shrikant B. Kelkar for his guidance. We would also like to acknowledge the assistance of Dr. Sabyasachi Sengupta from Sengupta's Research Academy and Dr. Neha Patel for content editing of this manuscript. We would also like to acknowledge Dr Mehmood G. Sayyad, Consultant Bio-Statistician, NIO, Pune, for statistical analysis.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. [Last accessed on 2006 Sep 07]. Available from: http://www.who.int/blindness/causes .
- 3.International Diabetes Federation (IDF). Diabetes Atlas. 7th Edition, International Diabetes Federation, Brussels, Belgium. 2015. [Last accessed on 2015 Nov 06]. http://www.diabetesatlas.org .
- 4.Fletcher EL, Phipps JA, Ward MM, Puthussery T, Wilkinson-Berka JL. Neuronal and glial cell abnormality as predictors of progression of diabetic retinopathy. Curr Pharm Des. 2007;13:2699–712. doi: 10.2174/138161207781662920. [DOI] [PubMed] [Google Scholar]
- 5.Simó R, Hernández C European Consortium for the Early Treatment of Diabetic Retinopathy (EUROCONDOR) Neurodegeneration is an early event in diabetic retinopathy: Therapeutic implications. Br J Ophthalmol. 2012;96:1285–90. doi: 10.1136/bjophthalmol-2012-302005. [DOI] [PubMed] [Google Scholar]
- 6.Adams AJ, Bearse MA., Jr Retinal neuropathy precedes vasculopathy in diabetes: A function-based opportunity for early treatment intervention? Clin Exp Optom. 2012;95:256–65. doi: 10.1111/j.1444-0938.2012.00733.x. [DOI] [PubMed] [Google Scholar]
- 7.Mizutani M, Gerhardinger C, Lorenzi M. Müller cell changes in human diabetic retinopathy. Diabetes. 1998;47:445–9. doi: 10.2337/diabetes.47.3.445. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk HW, Kok PH, Garvin M, Sonka M, Devries JH, Michels RP, et al. Selective loss of inner retinal layer thickness in type 1 diabetic patients with minimal diabetic retinopathy. Invest Ophthalmol Vis Sci. 2009;50:3404–9. doi: 10.1167/iovs.08-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Dijk HW, Verbraak FD, Kok PH, Garvin MK, Sonka M, Lee K, et al. Decreased retinal ganglion cell layer thickness in patients with type 1 diabetes. Invest Ophthalmol Vis Sci. 2010;51:3660–5. doi: 10.1167/iovs.09-5041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Dijk HW, Verbraak FD, Stehouwer M, Kok PH, Garvin MK, Sonka M, et al. Association of visual function and ganglion cell layer thickness in patients with diabetes mellitus type 1 and no or minimal diabetic retinopathy. Vision Res. 2011;51:224–8. doi: 10.1016/j.visres.2010.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Leo MA, Falsini B, Caputo S, Ghirlanda G, Porciatti V, Greco AV, et al. Spatial frequency-selective losses with pattern electroretinogram in type 1 (insulin-dependent) diabetic patients without retinopathy. Diabetologia. 1990;33:726–30. doi: 10.1007/BF00400342. [DOI] [PubMed] [Google Scholar]
- 12.Di Leo MA, Caputo S, Falsini B, Porciatti V, Greco AV, Ghirlanda G, et al. Presence and further development of retinal dysfunction after 3-year follow up in IDDM patients without angiographically documented vasculopathy. Diabetologia. 1994;37:911–6. doi: 10.1007/BF00400947. [DOI] [PubMed] [Google Scholar]
- 13.Calvo-Maroto AM, Perez-Cambrodí RJ, Albarán-Diego C, Pons A, Cerviño A. Optical quality of the diabetic eye: A review. Eye (Lond) 2014;28:1271–80. doi: 10.1038/eye.2014.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harding JJ, Egerton M, van Heyningen R, Harding RS. Diabetes, glaucoma, sex, and cataract: Analysis of combined data from two case control studies. Br J Ophthalmol. 1993;77:2–6. doi: 10.1136/bjo.77.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benson WE. Cataract surgery and diabetic retinopathy. Curr Opin Ophthalmol. 1992;3:396–400. doi: 10.1097/00055735-199206000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton AM, Ulbig MW, Polkinghorne P, Sharma S. Management of Diabetic Retinopathy. Can Med Assoc J. 1997;157:192. [Google Scholar]
- 17.Jaffe GJ, Burton TC, Kuhn E, Prescott A, Hartz A. Progression of nonproliferative diabetic retinopathy and visual outcome after extracapsular cataract extraction and intraocular lens implantation. Am J Ophthalmol. 1992;114:448–56. doi: 10.1016/s0002-9394(14)71857-4. [DOI] [PubMed] [Google Scholar]
- 18.Patel JI, Hykin PG, Cree IA. Diabetic cataract removal: Postoperative progression of maculopathy – Growth factor and clinical analysis. Br J Ophthalmol. 2006;90:697–701. doi: 10.1136/bjo.2005.087403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong N, Xu B, Wang B, Chu L, Tang X. Aqueous cytokines as predictors of macular edema in patients with diabetes following uncomplicated phacoemulsification cataract surgery. Biomed Res Int 2015. 2015:126984. doi: 10.1155/2015/126984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Denniston AK, Chakravarthy U, Zhu H, Lee AY, Crabb DP, Tufail A, et al. The UK diabetic retinopathy electronic medical record (UK DR EMR) users group, report 2: Real-world data for the impact of cataract surgery on diabetic macular oedema. Br J Ophthalmol. 2017;101:1673–8. doi: 10.1136/bjophthalmol-2016-309838. [DOI] [PubMed] [Google Scholar]
- 21.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. Introduction to Meta-Analysis. Chichester, UK: Wiley; 2009. [Google Scholar]
- 22.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollreisz A, Schmidt-Erfurth U. Diabetic cataract-pathogenesis, epidemiology and treatment. J Ophthalmol 2010. 2010 doi: 10.1155/2010/608751. 608751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rowe NG, Mitchell PG, Cumming RG, Wans JJ. Diabetes, fasting blood glucose and age-related cataract: The Blue Mountains Eye Study. Ophthalmic Epidemiol. 2000;7:103–14. [PubMed] [Google Scholar]
- 25.Klein BE, Klein R, Lee KE. Diabetes, cardiovascular disease, selected cardiovascular disease risk factors, and the 5-year incidence of age-related cataract and progression of lens opacities: The Beaver Dam Eye Study. Am J Ophthalmol. 1998;126:782–90. doi: 10.1016/s0002-9394(98)00280-3. [DOI] [PubMed] [Google Scholar]
- 26.Klein BE, Klein R, Wang Q, Moss SE. Older-onset diabetes and lens opacities. The beaver dam eye study. Ophthalmic Epidemiol. 1995;2:49–55. doi: 10.3109/09286589509071451. [DOI] [PubMed] [Google Scholar]
- 27.Kador PF, Wyman M, Oates PJ. Aldose reductase, ocular diabetic complications and the development of topical Kinostat(®) Prog Retin Eye Res. 2016;54:1–29. doi: 10.1016/j.preteyeres.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 28.Obrosova IG, Chung SS, Kador PF. Diabetic cataracts: Mechanisms and management. Diabetes Metab Res Rev. 2010;26:172–80. doi: 10.1002/dmrr.1075. [DOI] [PubMed] [Google Scholar]
- 29.Zhang P, Xing K, Randazzo J, Blessing K, Lou MF, Kador PF, et al. Osmotic stress, not aldose reductase activity, directly induces growth factors and MAPK signaling changes during sugar cataract formation. Exp Eye Res. 2012;101:36–43. doi: 10.1016/j.exer.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hashim Z, Zarina S. Osmotic stress induced oxidative damage: Possible mechanism of cataract formation in diabetes. J Diabetes Complications. 2012;26:275–9. doi: 10.1016/j.jdiacomp.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Wilson ME, Jr, Levin AV, Trivedi RH, Kruger SJ, Elliott LA, Ainsworth JR, et al. Cataract associated with type-1 diabetes mellitus in the pediatric population. J AAPOS. 2007;11:162–5. doi: 10.1016/j.jaapos.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Datiles MB, 3rd, Kador PF. Type I diabetic cataract. Arch Ophthalmol. 1999;117:284–5. doi: 10.1001/archopht.117.2.284. [DOI] [PubMed] [Google Scholar]
- 33.Behndig A, Karlsson K, Reaume AG, Sentman ML, Marklund SL. In vitro photochemical cataract in mice lacking copper-zinc superoxide dismutase. Free Radic Biol Med. 2001;31:738–44. doi: 10.1016/s0891-5849(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 34.Olofsson EM, Marklund SL, Karlsson K, Brännström T, Behndig A. In vitro glucose-induced cataract in copper-zinc superoxide dismutase null mice. Exp Eye Res. 2005;81:639–46. doi: 10.1016/j.exer.2005.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Olofsson EM, Marklund SL, Behndig A. Enhanced diabetes-induced cataract in copper-zinc superoxide dismutase-null mice. Invest Ophthalmol Vis Sci. 2009;50:2913–8. doi: 10.1167/iovs.09-3510. [DOI] [PubMed] [Google Scholar]
- 36.Saito Y, Ohmi G, Kinoshita S, Nakamura Y, Ogawa K, Harino S, et al. Transient hyperopia with lens swelling at initial therapy in diabetes. Br J Ophthalmol. 1993;77:145–8. doi: 10.1136/bjo.77.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonmez B, Bozkurt B, Atmaca A, Irkec M, Orhan M, Aslan U, et al. Effect of glycemic control on refractive changes in diabetic patients with hyperglycemia. Cornea. 2005;24:531–7. doi: 10.1097/01.ico.0000151545.00489.12. [DOI] [PubMed] [Google Scholar]
- 38.Saxena S, Mitchell P, Rochtchina E. Five-year incidence of cataract in older persons with diabetes and pre-diabetes. Ophthalmic Epidemiol. 2004;11:271–7. doi: 10.1080/09286580490510733. [DOI] [PubMed] [Google Scholar]
- 39.Chew EY, Benson WE, Remaley NA, Lindley AA, Burton TC, Csaky K, et al. Results after lens extraction in patients with diabetic retinopathy: Early treatment diabetic retinopathy study report number 25. Arch Ophthalmol. 1999;117:1600–6. doi: 10.1001/archopht.117.12.1600. [DOI] [PubMed] [Google Scholar]
- 40.Dowler JG, Sehmi KS, Hykin PG, Hamilton AM. The natural history of macular edema after cataract surgery in diabetes. Ophthalmology. 1999;106:663–8. doi: 10.1016/S0161-6420(99)90148-3. [DOI] [PubMed] [Google Scholar]
- 41.Fernández-Rubio ME, Rebolledo-Lara L, Martinez-García M, Alarcón-Tomás M, Cortés-Valdés C. The conjunctival bacterial pattern of diabetics undergoing cataract surgery. Eye (Lond) 2010;24:825–34. doi: 10.1038/eye.2009.218. [DOI] [PubMed] [Google Scholar]
- 42.Pollack A, Dotan S, Oliver M. Course of diabetic retinopathy following cataract surgery. Br J Ophthalmol. 1991;75:2–8. doi: 10.1136/bjo.75.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Squirrell D, Bhola R, Bush J, Winder S, Talbot JF. A prospective, case controlled study of the natural history of diabetic retinopathy and maculopathy after uncomplicated phacoemulsification cataract surgery in patients with type 2 diabetes. Br J Ophthalmol. 2002;86:565–71. doi: 10.1136/bjo.86.5.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shah AS, Chen SH. Cataract surgery and diabetes. Curr Opin Ophthalmol. 2010;21:4–9. doi: 10.1097/ICU.0b013e328333e9c1. [DOI] [PubMed] [Google Scholar]
- 45.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early treatment diabetic retinopathy study report number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology. 1987;94:761–74. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 46.Diabetic Retinopathy Clinical Research Network. Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117:1064–77.e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meyer CH. Current treatment approaches in diabetic macular edema. Ophthalmologica. 2007;221:118–31. doi: 10.1159/000098257. [DOI] [PubMed] [Google Scholar]
- 48.Diabetic Retinopathy Clinical Research Network Authors/Writing Committee. Baker CW, Almukhtar T, Bressler NM, Glassman AR, Grover S, et al. Macular edema after cataract surgery in eyes without preoperative central-involved diabetic macular edema. JAMA Ophthalmol. 2013;131:870–9. doi: 10.1001/jamaophthalmol.2013.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen XY, Song WJ, Cai HY, Zhao L. Macular edema after cataract surgery in diabetic eyes evaluated by optical coherence tomography. Int J Ophthalmol. 2016;9:81–5. doi: 10.18240/ijo.2016.01.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yumuşak E, Örnek K. Comparison of perioperative ranibizumab injections for diabetic macular edema in patients undergoing cataract surgery. J Ophthalmol 2016. 2016 doi: 10.1155/2016/7945619. 7945619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dugel PU, Bandello F, Loewenstein A. Dexamethasone intravitreal implant in the treatment of diabetic macular edema. Clin Ophthalmol. 2015;9:1321–35. doi: 10.2147/OPTH.S79948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sadda S, Danis RP, Li XY, Cui H, Hashad Y, Whitcup SM. Anatomic effects of dexamethasone intravitreal implant in diabetic macular edema: Pooled analysis of findings from two randomized phase 3 studies. 32nd Annual Meeting of the American Society of Retina Specialists. San Diego; 9-13 August. 2014 [Google Scholar]
- 53.Yang Y, Bailey C, Holz FG, Eter N, Weber M, Baker C, et al. Long-term outcomes of phakic patients with diabetic macular oedema treated with intravitreal fluocinolone acetonide (FAc) implants. Eye (Lond) 2015;29:1173–80. doi: 10.1038/eye.2015.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medić A, Jukić T, Matas A, Vukojević K, Sapunar A, Znaor L, et al. Effect of preoperative topical diclofenac on intraocular interleukin-12 concentration and macular edema after cataract surgery in patients with diabetic retinopathy: A randomized controlled trial. Croat Med J. 2017;58:49–55. doi: 10.3325/cmj.2017.58.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yüksel B, Karti Ö, Kusbeci T. Topical nepafenac for prevention of post-cataract surgery macular edema in diabetic patients: Patient selection and perspectives. Clin Ophthalmol. 2017;11:2183–90. doi: 10.2147/OPTH.S132810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yazdani S, Hendi K, Pakravan M. Intravitreal bevacizumab (Avastin) injection for neovascular glaucoma. J Glaucoma. 2007;16:437–9. doi: 10.1097/IJG.0b013e3180457c47. [DOI] [PubMed] [Google Scholar]
- 57.Chilov MN, Grigg JR, Playfair TJ. Bevacizumab (Avastin) for the treatment of neovascular glaucoma. Clin Exp Ophthalmol. 2007;35:494–6. doi: 10.1111/j.1442-9071.2007.01521.x. [DOI] [PubMed] [Google Scholar]
- 58.Kahook MY, Lathrop KL, Noecker RJ. One-site versus two-site endoscopic cyclophotocoagulation. J Glaucoma. 2007;16:527–30. doi: 10.1097/IJG.0b013e3180575215. [DOI] [PubMed] [Google Scholar]
- 59.Apple DJ, Solomon KD, Tetz MR, Assia EI, Holland EY, Legler UF, et al. Posterior capsule opacification. Surv Ophthalmol. 1992;37:73–116. doi: 10.1016/0039-6257(92)90073-3. [DOI] [PubMed] [Google Scholar]
- 60.Sadiq SA, Sleep T, Amoaku WM. The visual results and changes in retinopathy in diabetic patients following cataract surgery. Eur J Ophthalmol. 1999;9:14–20. doi: 10.1177/112067219900900103. [DOI] [PubMed] [Google Scholar]
- 61.Kato S, Oshika T, Numaga J, Hayashi Y, Oshiro M, Yuguchi T, et al. Anterior capsular contraction after cataract surgery in eyes of diabetic patients. Br J Ophthalmol. 2001;85:21–3. doi: 10.1136/bjo.85.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takamura Y, Tomomatsu T, Yokota S, Matsumura T, Takihara Y, Inatani M, et al. Large capsulorhexis with implantation of a 7.0 mm optic intraocular lens during cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2014;40:1850–6. doi: 10.1016/j.jcrs.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 63.Mittra RA, Borrillo JL, Dev S, Mieler WF, Koenig SB. Retinopathy progression and visual outcomes after phacoemulsification in patients with diabetes mellitus. Arch Ophthalmol. 2000;118:912–7. [PubMed] [Google Scholar]
- 64.Cahill M, Eustace P, de Jesus V. Pupillary autonomic denervation with increasing duration of diabetes mellitus. Br J Ophthalmol. 2001;85:1225–30. doi: 10.1136/bjo.85.10.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jardeleza MS, Miller JW. Review of anti-VEGF therapy in proliferative diabetic retinopathy. Semin Ophthalmol. 2009;24:87–92. doi: 10.1080/08820530902800330. [DOI] [PubMed] [Google Scholar]
- 66.Cetinkaya A, Yilmaz G, Akova YA. Photic retinopathy after cataract surgery in diabetic patients. Retina. 2006;26:1021–8. doi: 10.1097/01.iae.0000254895.78766.af. [DOI] [PubMed] [Google Scholar]
- 67.Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T, et al. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea. 2001;20:798–801. doi: 10.1097/00003226-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 68.Shih KC, Lam KS, Tong L. A systematic review on the impact of diabetes mellitus on the ocular surface. Nutr Diabetes. 2017;7:e251. doi: 10.1038/nutd.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Morikubo S, Takamura Y, Kubo E, Tsuzuki S, Akagi Y. Corneal changes after small-incision cataract surgery in patients with diabetes mellitus. Arch Ophthalmol. 2004;122:966–9. doi: 10.1001/archopht.122.7.966. [DOI] [PubMed] [Google Scholar]
- 70.Hugod M, Storr-Paulsen A, Norregaard JC, Nicolini J, Larsen AB, Thulesen J, et al. Corneal endothelial cell changes associated with cataract surgery in patients with type 2 diabetes mellitus. Cornea. 2011;30:749–53. doi: 10.1097/ICO.0b013e31820142d9. [DOI] [PubMed] [Google Scholar]
- 71.Yang R, Sha X, Zeng M, Tan Y, Zheng Y, Fan F, et al. The influence of phacoemulsification on corneal endothelial cells at varying blood glucose levels. Eye Sci. 2011;26:91–5. doi: 10.3969/j.issn.1000-4432.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Ebihara Y, Kato S, Oshika T, Yoshizaki M, Sugita G. Posterior capsule opacification after cataract surgery in patients with diabetes mellitus. J Cataract Refract Surg. 2006;32:1184–7. doi: 10.1016/j.jcrs.2006.01.100. [DOI] [PubMed] [Google Scholar]
- 73.Kim CJ, Choi SK. Analysis of aqueous humor calcium and phosphate from cataract eyes with and without diabetes mellitus. Korean J Ophthalmol. 2007;21:90–4. doi: 10.3341/kjo.2007.21.2.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee DH, Seo Y, Joo CK. Progressive opacification of hydrophilic acrylic intraocular lenses in diabetic patients. J Cataract Refract Surg. 2002;28:1271–5. doi: 10.1016/s0886-3350(02)01245-2. [DOI] [PubMed] [Google Scholar]
- 75.Eaton AM, Jaffe GJ, McCuen BW, 2nd, Mincey GJ. Condensation on the posterior surface of silicone intraocular lenses during fluid-air exchange. Ophthalmology. 1995;102:733–6. doi: 10.1016/s0161-6420(95)30961-x. [DOI] [PubMed] [Google Scholar]
- 76.Rodríguez-Galietero A, Montés-Micó R, Muñoz G, Albarrán-Diego C. Blue-light filtering intraocular lens in patients with diabetes: Contrast sensitivity and chromatic discrimination. J Cataract Refract Surg. 2005;31:2088–92. doi: 10.1016/j.jcrs.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 77.Braga-Mele R, Chang D, Dewey S, Foster G, Henderson BA, Hill W, et al. Multifocal intraocular lenses: Relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40:313–22. doi: 10.1016/j.jcrs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 78.Henricsson M, Heijl A, Janzon L. Diabetic retinopathy before and after cataract surgery. Br J Ophthalmol. 1996;80:789–93. doi: 10.1136/bjo.80.9.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antcliff RJ, Poulson A, Flanagan DW. Phacoemulsification in diabetics. Eye (Lond) 1996;10(Pt 6):737–41. doi: 10.1038/eye.1996.171. [DOI] [PubMed] [Google Scholar]
- 80.Zaczek A, Olivestedt G, Zetterström C. Visual outcome after phacoemulsification and IOL implantation in diabetic patients. Br J Ophthalmol. 1999;83:1036–41. doi: 10.1136/bjo.83.9.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mozaffarieh M, Heinzl H, Sacu S, Wedrich A. Clinical outcomes of phacoemulsification cataract surgery in diabetes patients: Visual function (VF-14), visual acuity and patient satisfaction. Acta Ophthalmol Scand. 2005;83:176–83. doi: 10.1111/j.1600-0420.2005.00407.x. [DOI] [PubMed] [Google Scholar]
- 82.Pollack A, Leiba H, Bukelman A, Oliver M. Cystoid macular oedema following cataract extraction in patients with diabetes. Br J Ophthalmol. 1992;76:221–4. doi: 10.1136/bjo.76.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Schatz H, Atienza D, McDonald HR, Johnson RN. Severe diabetic retinopathy after cataract surgery. Am J Ophthalmol. 1994;117:314–21. doi: 10.1016/s0002-9394(14)73138-1. [DOI] [PubMed] [Google Scholar]
- 84.Kim SJ, Equi R, Bressler NM. Analysis of macular edema after cataract surgery in patients with diabetes using optical coherence tomography. Ophthalmology. 2007;114:881–9. doi: 10.1016/j.ophtha.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 85.Harbour JW, Smiddy WE, Flynn HW, Jr, Rubsamen PE. Vitrectomy for diabetic macular edema associated with a thickened and taut posterior hyaloid membrane. Am J Ophthalmol. 1996;121:405–13. doi: 10.1016/s0002-9394(14)70437-4. [DOI] [PubMed] [Google Scholar]
- 86.Egan C, Zhu H, Lee A, Sim D, Mitry D, Bailey C, et al. The United Kingdom diabetic retinopathy electronic medical record users group, report 1: Baseline characteristics and visual acuity outcomes in eyes treated with intravitreal injections of ranibizumab for diabetic macular oedema. Br J Ophthalmol. 2017;101:75–80. doi: 10.1136/bjophthalmol-2016-309313. [DOI] [PubMed] [Google Scholar]
- 87.Liao SB, Ku WC. Progression of diabetic retinopathy after phacoemulsification in diabetic patients: A three-year analysis. Chang Gung Med J. 2003;26:829–34. [PubMed] [Google Scholar]
- 88.Massin P, Audren F, Haouchine B, Erginay A, Bergmann JF, Benosman R, et al. Intravitreal triamcinolone acetonide for diabetic diffuse macular edema: Preliminary results of a prospective controlled trial. Ophthalmology. 2004;111:218–24. doi: 10.1016/j.ophtha.2003.05.037. [DOI] [PubMed] [Google Scholar]
- 89.Murtha T, Cavallerano J. The management of diabetic eye disease in the setting of cataract surgery. Curr Opin Ophthalmol. 2007;18:13–8. doi: 10.1097/ICU.0b013e32801129fc. [DOI] [PubMed] [Google Scholar]
- 90.Lynch SS, Cheng CM. Bevacizumab for neovascular ocular diseases. Ann Pharmacother. 2007;41:614–25. doi: 10.1345/aph.1H316. [DOI] [PubMed] [Google Scholar]
- 91.Avery RL, Pearlman J, Pieramici DJ, Rabena MD, Castellarin AA, Nasir MA, et al. Intravitreal bevacizumab (Avastin) in the treatment of proliferative diabetic retinopathy. Ophthalmology. 2006;113:1695.e1–15. doi: 10.1016/j.ophtha.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 92.Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, Bressler SB, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: Two-year results from a comparative effectiveness randomized clinical trial. Ophthalmology. 2016;123:1351–9. doi: 10.1016/j.ophtha.2016.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kataria AS, Thompson JT. Cataract formation and progression in patients less than 50 years of age after vitrectomy. Ophthalmol Retina. 2017;1:149–53. doi: 10.1016/j.oret.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 94.Pendergast SD, Hassan TS, Williams GA, Cox MS, Margherio RR, Ferrone PJ, et al. Vitrectomy for diffuse diabetic macular edema associated with a taut premacular posterior hyaloid. Am J Ophthalmol. 2000;130:178–86. doi: 10.1016/s0002-9394(00)00472-4. [DOI] [PubMed] [Google Scholar]
- 95.Lewis H. The role of vitrectomy in the treatment of diabetic macular edema. Am J Ophthalmol. 2001;131:123–5. doi: 10.1016/s0002-9394(00)00660-7. [DOI] [PubMed] [Google Scholar]
- 96.Talcott KE, Eliott D. Cystoid Macular Edema. Cham: Springer International Publishing; 2017. Surgical management of diabetic macular edema; pp. 163–76. [Google Scholar]
- 97.Lahey JM, Francis RR, Kearney JJ. Combining phacoemulsification with pars plana vitrectomy in patients with proliferative diabetic retinopathy: A series of 223 cases. Ophthalmology. 2003;110:1335–9. doi: 10.1016/S0161-6420(03)00454-8. [DOI] [PubMed] [Google Scholar]
- 98.Scharwey K, Pavlovic S, Jacobi KW. Combined clear corneal phacoemulsification, vitreoretinal surgery, and intraocular lens implantation. J Cataract Refract Surg. 1999;25:693–8. doi: 10.1016/s0886-3350(99)00022-x. [DOI] [PubMed] [Google Scholar]
- 99.Amino K, Tanihara H. Vitrectomy combined with phacoemulsification and intraocular lens implantation for diabetic macular edema. Jpn J Ophthalmol. 2002;46:455–9. doi: 10.1016/s0021-5155(02)00526-9. [DOI] [PubMed] [Google Scholar]
- 100.Lee DY, Jeong HS, Sohn HJ, Nam DH. Combined 23-gauge sutureless vitrectomy and clear corneal phacoemulsification in patients with proliferative diabetic retinopathy. Retina. 2011;31:1753–8. doi: 10.1097/IAE.0b013e31820d4057. [DOI] [PubMed] [Google Scholar]
- 101.Canan H, Sizmaz S, Altan-Yaycioğlu R. Surgical results of combined pars plana vitrectomy and phacoemulsification for vitreous hemorrhage in PDR. Clin Ophthalmol. 2013;7:1597–601. doi: 10.2147/OPTH.S47780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Silva PS, Diala PA, Hamam RN, Arrigg PG, Shah ST, Murtha TL, et al. Visual outcomes from pars plana vitrectomy versus combined pars plana vitrectomy, phacoemulsification, and intraocular lens implantation in patients with diabetes. Retina. 2014;34:1960–8. doi: 10.1097/IAE.0000000000000171. [DOI] [PubMed] [Google Scholar]
- 103.Honjo M, Ogura Y. Surgical results of pars plana vitrectomy combined with phacoemulsification and intraocular lens implantation for complications of proliferative diabetic retinopathy. Ophthalmic Surg Lasers. 1998;29:99–105. [PubMed] [Google Scholar]
- 104.Wagner T, Knaflic D, Rauber M, Mester U. Influence of cataract surgery on the diabetic eye: A prospective study. Ger J Ophthalmol. 1996;5:79–83. [PubMed] [Google Scholar]
- 105.Krepler K, Biowski R, Schrey S, Jandrasits K, Wedrich A. Cataract surgery in patients with diabetic retinopathy: Visual outcome, progression of diabetic retinopathy, and incidence of diabetic macular oedema. Graefes Arch Clin Exp Ophthalmol. 2002;240:735–8. doi: 10.1007/s00417-002-0530-7. [DOI] [PubMed] [Google Scholar]
- 106.Jurecka T, Bátková Z, Ventruba J, Synek S. Macular edema after cataract surgery in diabetic patients without retinopathy. Cesk Slov Oftalmol. 2007;63:274–84. [PubMed] [Google Scholar]
- 107.Hayashi K, Igarashi C, Hirata A, Hayashi H. Changes in diabetic macular oedema after phacoemulsification surgery. Eye (Lond) 2009;23:389–96. doi: 10.1038/sj.eye.6703022. [DOI] [PubMed] [Google Scholar]
- 108.Wylegała E, Moćko L, Woyna-Orlewicz A, Teper S, Orzechowska-Wylegała B. Diabetic complications within ocular surface. Pol Merkur Lekarski. 2006;21:495–7. [PubMed] [Google Scholar]
- 109.Lee JS, Lee JE, Choi HY, Oum BS, Cho BM. Corneal endothelial cell change after phacoemulsification relative to the severity of diabetic retinopathy. J Cataract Refract Surg. 2005;31:742–9. doi: 10.1016/j.jcrs.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 110.Krupsky S, Zalish M, Oliver M, Pollack A. Anterior segment complications in diabetic patients following extracapsular cataract extraction and posterior chamber intraocular lens implantation. Ophthalmic Surg. 1991;22:526–30. [PubMed] [Google Scholar]
- 111.Doft HH. The endophthalmitis vitrectomy study. In: Kertes PI, Conway MD, editors. Clinical Trials in Ophthalmology: A Summary and Practice Guide. Philadelphia: Lippincott, Williams & Wilkins; 1998. pp. 97–111. [Google Scholar]


