Abstract
Nineteen years have passed since our previous review in this journal in 1999 regarding estrogen receptors. At that time, we described the current assessments of the physiological activities of estrogen and estrogen receptors. Since that time there has been an explosion of progress in our understanding of details of estrogen receptor–mediated processes from the molecular and cellular level to the whole organism. In this review we discuss the basic understanding of estrogen signaling and then elaborate on the progress and current understanding of estrogen receptor actions that have developed using new models and continuing clinical studies.
Essential Points
Since our previous review of estrogen receptors 19 years ago, understanding of biological and molecular details driving estrogen response has greatly progressed
More comprehensive description of the structural details of the estrogen receptor protein complex illustrates important aspects of its functions
Continued development of cell culture and animal models has enabled closer study of both molecular and biological details of estrogen signaling
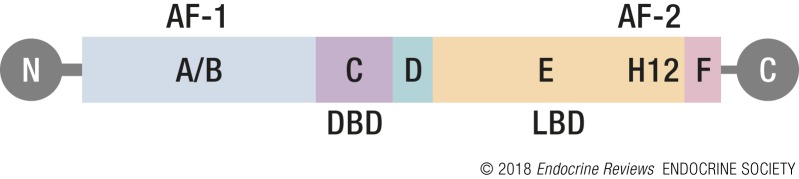
Estrogen (E2) is a steroid hormone synthesized by mammalian ovaries and secreted into the blood (1) and is also synthesized peripherally in cells expressing the enzyme aromatase. Its lipophilic nature allows it to pass freely through cell membranes, accessing cells within many tissues. What allows E2 to specifically target certain cells is its transducer, the estrogen receptor (ER). In mammals there are two ERs, ERα and ERβ, both members of the nuclear receptor superfamily of hormone-inducible transcription factors (2–4). As the name indicates, nuclear receptors work in the nucleus of cells and are receptors for various specific hormones. The easiest way to illustrate how ERs function is to describe their protein domain structure (Fig. 1). All nuclear receptor family members share a general multidomain structure, with each domain directing the mechanistic interactions and functions necessary for hormone response. The ERs have six domains, A through F (3–5). The two key functions of high-affinity and high-specificity binding to (1) its hormonal activator E2 and (2) its target gene DNA motif, the estrogen-responsive element (ERE), are located in the ligand-binding domain (LBD) and the DNA-binding domain (DBD), respectively. Each of these domains, along with the four other domains, encodes structural features critical to their activity, which is described in more detail below.
Figure 1.
Obligatory domain structure schematic. Drawing showing the six ERα domains, A to F, oriented from the amino (N) to carboxyl (C) terminus. The domains in which each of several key functions is located are indicated: AF-1 and AF-2 mediate transcriptional activity. The DBD interacts with ERE DNA motifs, and the LBD binds E2. Helix 12 (H12) interacts with transcriptional activators and repressors.
Update on Estrogen Receptors: The Basics
A/B amino terminal domain
The amino terminal, or N-terminal, A/B domain is the largest domain and includes the transcriptional activation function (AF)-1, which interacts with transcriptional coregulators and can maximize rates of RNA transcription in a cell- and promoter-dependent manner. Several phosphorylation sites located in the A/B domain are important for ER activity. Although this domain has been difficult to study, owing to its structural fluctuations, this flexibility is thought to confer its function as to being amenable for a variety of modifications and interactions. The A/B domain of the ER contains intrinsically disordered regions, which lend flexibility to the response functions of the ER via interactions with other ER domains, inducing A/B domain structure that then allosterically controls ER interactions with ligands, DNA, and other transcriptional modulators (6, 7). Recent cryo-electron microscopy studies of the ER revealed positioning of the A/B domain nearer the LBD than previously thought, thereby facilitating interactions with transcriptional coactivators (8).
C domain/DBD
The DBD contains two zinc finger structures, each formed by zinc cation chelation with four cysteine residues. Amino acids at the base of the more amino terminal zinc finger are called the proximal box, or P box, and are critical for recognizing the specific DNA sequence of the ERE motif (GGTCAnnnTGACC) (9). Accordingly, this finger is sometimes referred to as the recognition helix. Amino acids at the base of the second zinc finger are termed the distal box, or D box, and are important in recognizing the spacing between the arms of the ERE palindrome. Additionally, the D box also contributes to ER dimer formation. From the end of the second zinc finger and extending into the D domain is a stretch of amino acids termed the C-terminal extension that also confers DNA binding activity (7).
D domain/hinge region
The D domain, also called the hinge region, contains part of the C-terminal extension of the DBD, as well as sequences important for nuclear localization of the ER protein. Hinge region sumoylation or p300-mediated acetylation has been shown to impact transcriptional activity (10–12). Additionally, the D domain contains an intrinsically disordered region that, similar to that of the A/B domain, offers a mode of allosteric regulation of ER interactions and activity (13).
E domain/LBD
The LBD is folded into a complex pocket in which 11 α-helical structures (termed helices 1 and 3 through 12) create a site of high-affinity interaction with E2 ligands (dissociation rate constant of 0.1 nM) (13). Binding of the estrogen ligand repositions helix 12, which is part of a second transcriptional activation function, AF-2. AF-2 then interacts with mediators of chromatin accessibility and RNA transcription rates. The E domain also encodes sequences in helix 11 important for ER dimerization and nuclear localization.
F domain
The carboxyl-terminal F domain is a feature unique to ERs and is not observed in other nuclear receptor family members. It has roles in ER intramolecular interaction and ER protein stability. Its functionality and contribution to ER activity is not well understood because few studies have focused on this domain. A recent study has shown that species differences in responsiveness to tamoxifen appear to reside in the altered sequence homology of the F domain (14).
Structure of ER
The full-length ER has proven difficult to study structurally, in part because its size and the presence of disordered regions make x-ray crystallography impossible, as the full-length ER is not amenable to forming crystals (15). Cocrystals of the ERαDBD and ERE DNA sequence were successfully produced and revealed several aspects regarding the DBD–ERE interaction (16). Similarly, crystals of the ERα LBD together with E2 or other ERα-binding substances have informed understanding of these interactions (17). Most revealing, however, was the derivation of a three-dimensional structure of ERα in a complex with ERE DNA and two transcriptional coactivators (p300 and SRC3). The complex structure was obtained using cryo-electron microscopy, together with structural knowledge from DBD/DNA crystallography and use of antibodies to localize the positions of p300 and ERα (8).
Basic Mechanisms of ER-Mediated Response
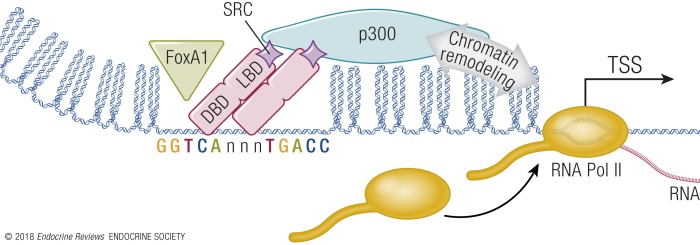
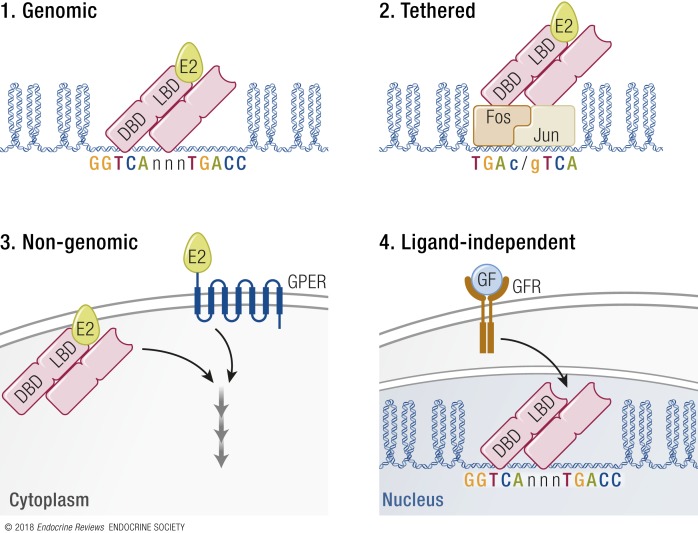
In general, the basics of estrogen response can be inferred from the preceding description of the ER domains. Pioneer factors, such as FOXA1, provide accessibility by binding and partially opening chromatin to facilitate ER–ERE interaction at appropriate sites in the cell (18). ER DBD interacts with ERE motifs in these accessible regions of chromatin, and the LBD binds E2, initiating conformational structural shifts in the ER protein. This allows interaction between the E2/ER and transcriptional coactivators, including those with chromatin remodeling activities. This leads to a further increase in chromatin accessibility and engagement of RNA polymerase II complexes and ultimately altered rates of transcription of responding genes (Fig. 2). This core understanding of ER’s function has been expanded to incorporate variations to the mechanisms, which are classified as “genomic,” “tethered,” “nongenomic,” and “ligand-independent” mechanisms (Fig. 3). The mechanism described above is the genomic mechanism, which occurs via direct interactions between dimers of ER and ERE DNA motifs. The tethered mode of response is called such, as it involves indirect DNA interactions “tethering” ER proteins to a transcriptional response motif in DNA, such as the AP-1 DNA motif, via binding between ER and other transcription factors, such as AP-1 Fos/Jun dimers. This mechanism has been primarily described utilizing in vitro systems (19, 20). Studies in mouse models utilizing mutant forms of the ER suggest that on its own, tethering does not mediate many primary ER physiological or transcriptional responses (21, 22). Nongenomic signaling involves interaction between E2 and cellular membrane-associated receptors, either the ER itself or possibly via a G protein–coupled receptor called G protein–coupled ER1 (GPER) (23, 24). The membrane interactions trigger rapid signaling responses (not involving transcriptional components), including activation of intracellular signaling exemplified by AKT and MAPK pathways. This mechanism seems to have been shown to play its greatest documented role in peripheral E2 cellular responses, such as those seen in endothelial cells, some neural regions, and pancreatic tissues. Studies using mouse models indicate that in cells with strong genomic ER signaling, nongenomic signaling on its own is not effective in mediating responses. Other effects, including cardiovascular responses and male reproductive functions, are mediated by nongenomic signals (25–28). Ligand-independent responses are ER-mediated effects seen after activating other pathways, such as IGF1 receptor–mediated signaling, that results in ER-mediated transcriptional responses independent of estrogenic steroid ligands. Recent work indicates that IGF1 stimulation can actually lead to recruitment of ERα to chromatin (29).
Figure 2.
Basic mechanisms of ER-mediated transcriptional regulation. Access to target genes is controlled, in part, by chromatin state. Pioneering factors, such as FOXA1 (green triangle), provide areas of more open chromatin, facilitating access of ER (pink) to ERE DNA motifs. Interaction with E2 leads to recruitment of steroid receptor coactivator (SRC) molecules to ER and interaction with p300. Chromatin remodeling activity of p300, especially histone acetyl transferase activity, facilitates RNA Pol II assembly at the TSS, leading to increased RNA transcription of ER target genes.
Figure 3.
Variations in the basic mechanism of E2 response. Four different E2 response mechanisms have been described. (1) The genomic mechanism involves interaction between ER and ERE DNA motifs. (2) The tethered mechanism involves indirect interaction between ER and other transcriptional regulators, such as the AP-1 DNA motif that binds the FOS/JUN dimer. Thus, ER is “tethered” to the DNA via, in this example, FOS/JUN binding to its AP-1 DNA motif. (3) Nongenomic signaling is so-called because it initiates a signal from extracellular E2 that leads to rapid signal cascades in the cytoplasm, and thus the response does not involve interaction with genomic features. The responses are mediated by membrane-associated ER, or by GPER, a G protein–coupled receptor. (4) Ligand-independent signaling involves transduction of extracellular growth factor (GF) activation of cell membrane GF receptor (GFR), which initiates signaling cascades, such as MAPK. The signal is received by the ER, activating its transcriptional modulation of target genes, despite lacking E2 ligand.
Using Model Systems to Discern Mechanistic Details of ERs
Two general types of biological systems are currently used to study E2 responses. First, numerous in vitro cell culture–based models are employed. These are derived from cell lines as well as from primary cells cultured after isolation from tissues. Many mechanistic details of ER-mediated signaling have been worked out using in vitro cell culture models. These include endogenous ER-expressing cells, such as MCF7 breast cancer cells, or cells without endogenous ER expression, which are then transfected to introduce full-length ER protein, or ER with deletion or mutations in the domain or function of interest. These systems are useful because they are comprised of a single cell type and are amenable to several different types of manipulations. Knockdown of molecules of interest using small interfering RNA allows evaluation of their importance in molecular and biological responses. Introduction of expression plasmids into cultured cells using transfection is another way in which molecular details can be evaluated. Similarly, reporter gene plasmids can be transfected into the cells and used to understand the molecular mechanistic actions in addition to the DNA sequences needed to drive ERα-mediated responses. Additionally, reporter gene plasmids analyzed with either endogenous ER or cotransfected mutant ER forms have been used for screening the activities of numerous chemicals and substances, including pharmaceuticals, exogenous chemicals, natural products (phytoestrogens), and endocrine-disrupting chemicals.
To understand biological roles, studies have been extended to genetically engineered mice and rats. These include models in which ERα or ERβ have been eliminated (“knocked out”), or generation of a mutated form of ERα by way of “knock-in” (KI) technology, and “conditional” genetic deletion of ERα from specific cells or tissues while leaving endogenous ERα intact in others. Table 1 (21, 22, 24, 26–28, 30–55) lists mouse models currently reported and briefly summarizes observations. Observing the effects of these deletions and mutations informs our understanding of the biological roles of the ERs and impacts of mechanistic details on in vivo E2 responses in actual tissues. Additionally, mouse models engineered to express ER-driven fluorescent tags (56, 57) promise to substantially inform understanding of ER-mediated processes during development and in peripheral tissues by facilitating tracking of cells expressing ERs.
Table 1.
Summary of Mouse Models
| Model | Names | Description | Findings/Conclusions | References |
|---|---|---|---|---|
| ERα-null mouse | ERαKO, αERKO, Ex3αERKO | Global deletion of ERα | Both sexes infertile | (30–33) |
| Males: disrupted mating behaviors; loss of spermatogenesis due to impaired efferent duct function | ||||
| Females: disrupted mating behavior, lack of ovulation, hypoplastic nonfunctional uterus | ||||
| ERα-null rat | Esr1-null | Global deletion of ERα | Both sexes infertile | (34) |
| Females: lack of ovulation, hypoplastic nonfunctional uterus | ||||
| ERβ-null mouse | βERKO, Ex3βERKO | Global deletion of ERβ | Males: normal | (31, 35–37) |
| Females: reduced ovulation | ||||
| ERβ-null rat | Esr2ΔE3 | Global deletion of ERβ | Males: normal | (38) |
| Females: reduced ovulation | ||||
| ERβ-DBD rat | Esr2ΔE4 | In-frame disruption of DNA binding | Males: normal | (38) |
| Females: reduced ovulation | ||||
| Uterine epithelial ERα cKO | Wnt7aCre;Esr1f/f | Deletion of ERα in the epithelial cells of the female reproductive tract | Impaired oviductal function, detrimental to early embryonic development and transport. Impaired uterine response, lack of implantation | (39, 40) |
| Uterine stromal ERα cKO | Amhr2Cre;Esr1f/f | Deletion of ERα in the antimesometrial stromal cells of the female reproductive tract | Reduced female fertility. Retained uterine response in epithelial cells adjacent to ERα-positive stromal cells | (41) |
| Uterine ERα cKO | PgrCre;Esr1f/f | Deletion of ERα in uterine cells | Development of ovarian tumors. Hypoplastic nonfunctional uterus | (40) |
| DBD mutant | NERKI | Mutation of DBD | NERKI/WT females infertile due to ovarian and uterine issues | (42) |
| GPER-null | GPER KO | Deletion of GPER | Normal reproductive system; nonreproductive phenotypes, including bone, cardiovascular, and insulin/glucose | (24, 43–46) |
| DBD mutant | KIKO,ERαAA/− | DBD mutation on ERα-null background | Males and females infertile. Aberrant uterine transcriptional response due to DNA-binding preference change form ERE to HRE, the motif that normally binds PGR | (21, 47, 48) |
| DBD mutant | EAAE | Mutation of DBD | Males and females infertile. No uterine transcriptional response to E2 | (21, 22) |
| AF-1 mutant | ERα AF-1° | Deletion of AF-1 | Males and females infertile. Blunted uterine response to E2 | (49) |
| AF-2 mutant | ERα AF-2° | Deletion of AF-2 | Males and females infertile. Insensitive to E2 | (50) |
| AF-2 mutant | AF2ERKI | Mutation of AF-2 | Males and females infertile. No uterine response to E2, partial uterine response to ICI 182 or 780 or tamoxifen | (51) |
| Extranuclear ERα signaling | ENERKI, ERαG525L | Mutation of LBD | Males and females infertile. No uterine response to E2 | (52) |
| Membrane localization mutant | C451AERα, NOER | Palmitoylation site mutant | Results differ: C451AERα, normal uterine E2 response; NOER, no uterine E2 response in one study, uterine growth in later study. Males and females infertile | (26–28, 53) |
| Membrane-only ERα | MOER | ERα LBD fused to multiple palmitoylation sites | Males and females infertile, no uterine E2 response | (54) |
| Nuclear localization mutant | H2NES | Mutations in the nuclear localization sequence and addition of a nuclear exclusion sequence | Males and females infertile | (55) |
cKO, conditional knockout; PGR, progesterone receptor.
ER-null or ER mutated models
Prior to the development of gene targeting, biological models of the E2 response relied on ovariectomized rodents that were then treated with E2 or test substances to characterize responses. These studies highlighted the central role of E2 signaling in certain target tissues, including the female reproductive tract, especially the uterus, and the mammary gland. Additionally, impacts were reported in the hypothalamic–pituitary–gonadal (HPG) axis. Subtler but notable effects were also observed in bone, adipose, brain (behavior), immune, pulmonary, and cardiovascular vascular systems. The development of genetic tools to manipulate components that mediate E2 signaling allowed further advancement by providing models in which individual components could be isolated and tested. Because E2’s effects could be mediated by three different receptors (ERα, ERβ, or GPER), studies specifically targeting each have informed our understanding of their relative roles. Of the three ERs, deletion of ERα leads to the greatest disruption in biological outcomes, including sterility of both sexes. Evaluation of various responses and phenotypes indicates that ERα is critical to many E2-initiated processes. Since the first report of ERα deletion in 1993 (30), dozens, if not hundreds, of studies have used this model system to confirm and evaluate ERα’s impact on E2 activity. The effects leading to female sterility include lack of E2-mediated regulation of the HPG axis, which results in an altered endocrine environment (elevated E2, testosterone, and LH) and lack of ovulation (58). Additionally, the uterine tissue is hypoplastic and lacks any response to E2, and thus no embryo implantation occurs (59). Furthermore, although initial studies indicated an E-independent decidual response (60), it was later shown that the first ERα-null mouse model used [αER knockout (KO)] had expression of a truncated variant form of the ERα in its uterus that likely mediated the decidual response. Use of later models with more complete ERα disruption lacked decidual response (40). An ERα-null rat model has also been reported, with similar female reproductive defects (34). Overall, these findings in ERα-null females demonstrated the essential roles of ERα-mediated activity in HPG and uterine function. Additionally, other notable phenotypes observed include lack of mammary duct outgrowth (61, 62) and obesity with insulin insensitivity (63, 64). The metabolic phenotype was recapitulated using a mouse with muscle cell selective ERα deletion, indicating the importance of muscle ERα in preventing obesity and insulin resistance (65). Some peripheral effects of ERα are also disrupted, including the inability of E2 to protect from cardiovascular vasculature injury, as measured using carotid artery re-endothelialization (25), endothelial vasodilation (66), atherosclerosis (67), pulmonary hyperresponsiveness (68), sensitivity to developing lupus (69), and pancreatic β-cell viability and functionality related to insulin resistance and energy metabolism (70, 71). Additionally, cortical bone density is decreased and cancellous bone density is increased in ERα-null females (72); however, these findings are thought to be impacted by the altered endocrine milieu of the ERα KO (73).
ERα role in the male
The observation of male sterility in ERα-null mice was totally unexpected, as E2 signaling was thought to be a female hormone primarily involved in female reproduction. The loss of male fertility was shown to be a result of age-dependent progressive accumulation of testicular fluid, leading to impaired sperm production due to efferent duct and epididymal defects [recently reviewed in (74)]. Molecular analysis revealed the ERα-dependent expression of Slc9a3, which encodes a sodium–hydrogen exchanger, in the efferent duct epithelial cells (74–76). Loss of Slc9a3 expression leads to the inability of the efferent ducts to reabsorb fluid, which causes fluid accumulation in the seminiferous tubules, resulting in testicular enlargement, compression of the tubules, disrupted spermatogenesis, loss of male germ cells, and finally testicular atrophy. Additionally, ERα-null males have altered mating behaviors consisting of reduced intromissions and no ejaculations, but normal mounting behavior (77). In male mice lacking both ERα and ERβ, none of these mating behaviors is present, as male mounting behavior is now absent (78). Such a compromising effect on mating behavior when both ERα and ERβ are lost indicates either a compensatory mechanism of the ERs for each other in the same cells or neurons, or the potential that the neurologic paths regulated by ERα and ERβ may converge to mediate male mating response. Additionally, the same overall impact on male fertility was reported in ERα-null rats (34). Mutations that disrupt ERα activity in humans are quite rare. Decreased sperm counts due to disruptions in spermatogenesis and sperm viability, cardiovascular endothelial dysfunction, obesity, and insulin resistance were reported in the first male patient described to have E2 insensitivity (see below). The patient harbored a mutation resulting in a premature stop codon in exon 2 and did not express any ERα protein (79). These observations indicate the surprising findings and importance of ERα action for male fertility and reproduction.
Clinical characteristics of ER dysfunction
There have been reports of both male and female patients with E2 insensitivity/resistance, including a familial case with three affected siblings (one male, two females) (79–81). All show lack of postpubertal epiphyseal closure and exhibit low bone density. The females who express mutant forms of ERα present clinically with delayed puberty and lack of breast development and have low bone density, cystic ovaries, and thin endometrium (80, 81). In all cases of ER insensitivity/resistance, the patients’ origins were from consanguineous relatives resulting in homozygous offspring. Observations in these patients also highlight the clinical importance of ERα in human health.
Mutations in ERα frequently occur in breast cancers and greatly impact effectiveness of treatments. The occurrence of mutations contributes to metastasis and resistance to tamoxifen therapy. This important topic is more thoroughly reviewed elsewhere (82, 83).
ERβ-null models
Deletion of ERβ in mice or rats, in contrast to the profound impacts seen from ERα deletion, leads to subtler issues. Several different lines of mice (31, 35, 36) and rat models (38) have been generated. ERβ-null males appear normal and are fertile, whereas ERβ-null females are principally infertile, but occasionally delivered a small litter (4, 38, 84). ERβ-null females become pregnant less frequently and have smaller sized litters than do their wild-type (WT) littermates. This is primarily due to decreased ovulation efficiency, which is influenced by the occurrence of suboptimal LH surges (84), and is consistent with the high level of selective expression of ERβ in ovarian granulosa cells. Thus, ERβ is primarily required for regulating folliculogenesis, optimal ovulatory efficiency, and ovarian function (58, 85).
Deletion of both ERα and ERβ results in many phenotypes principally similar to those from loss of the ERα, again emphasizing the more important role of ERα in mediating estrogenic responses. However, in the females, a highly novel phenotype was observed (86). On certain strain backgrounds, evidence in double ER KO of trans-differentiation of granulosa cells to Sertoli calls, with the follicle acquiring tubule-like appearance, highlights a role of ERβ in maintaining granulosa cell differentiation in the absence of ERα (86). Interestingly, no detectable ERα expression is normally found in granulosa cells; however, ERα transcript is detectable in ERβ-null granulosa cells (87). ERα expression does not, however, rescue the granulosa cell phenotype or hormone responsiveness, emphasizing the selective action of these two ER proteins in certain tissues and cell types.
There are four different GPER-null mouse lines that have been developed; none exhibits reproductive phenotypes, and they are fully fertile [reviewed in (24)]. In addition to using GPER KO models, researchers have used overlapping and selective agonists and antagonists to ER and GPER. E2 is an ER and GPER agonist, whereas the ER antagonist ICI/fulvestrant acts as a GPER agonist. Selective ER agonists such as tamoxifen and raloxifene work as agonists for GPER. Furthermore, a GPER-specific agonist, G1, and antagonist, G15, have been developed. Studies using GEPR-null mice or GPER ligands have illustrated GPER’s roles in nonreproductive systems [recently reviewed in detail (24, 88)]. For example, obesity and glucose intolerance occur in aged GPER-null males, and studies indicate roles for GPER in cardiovascular responses to E2, as well as in E2-induced calcium transport in kidneys.
Models to study details of ER-mediated responses
Knowing that ERα mediates many of the E2 responses, much effort has been focused on dissecting the importance of the various cellular mechanistic activities of ERα in each of the observed consequences of ERα deletion. This has been accomplished via replacement, or KI, of mutated forms of ERα. These include mutations that selectively disrupt DNA binding, interactions with transcriptional co-regulators, or subcellular localization.
Mutations that disrupt ERα DNA binding activity were made to address the relative contribution of nongenomic and tethered regulatory mechanisms (Fig. 3) in E2 biological responses. Two mouse models were created using similar strategies. The first model, named “nonclassical ER KI” (NERKI), introduced two point mutations (E207A/G208A) in the base of the first zinc finger (42); this mutation has been shown in vitro to eliminate ERα DNA binding activity (89). Female mice heterozygous for this mutation are infertile due to ovarian dysfunction and uterine hyperplasia. Because heterozygotes could not be bred to produce homozygous KI mice, to create a mouse model in which the DBD-mutated ERα was the only functional ERα, male NERKI heterozygous mice (WT/KI) were bred with female mice that were heterozygous for the ERα-null allele (WT/KO) to obtain offspring that contain one KI and one KO ERα allele (KIKO; also call ERαAA) (47). The second model mutated four amino acids (Y201E, K210A, K214A, and R215E) in the first zinc finger and between the two zinc fingers of the DBD (22). Heterozygous males and females for this “EAAE” mutation were fertile and could be bred to produce EAAE homozygous mice. Differences in the phenotypes of KIKO and EAAE mice were observed, although overall both exhibited phenotypes that were similar to ERα-null mice, highlighting the importance of DNA binding activity in ERα biological function. Phenotypic comparisons also illustrated that for the major estrogenic endocrine physiological responses that tethering gene regulation was not a primary activity and likely contributed in a complementary manner to the DNA response actions. The KIKO model developed a normal-sized uterus, in contrast to the hypoplastic uterus seen in the EAAE and ERα-null females. This difference was shown to be the result of a gain-of-function interaction between KIKO ERα and hormone response element (HRE) DNA motifs, which normally bind progesterone receptor (21). Therefore, the KIKO mouse receptor had an aberrant unexpected DNA binding activity, and interpretation of regulatory mechanisms for tethering need to take into account the HRE-mediated action of this mutant receptor.
Mutations that impact the AF-1 and AF-2 transcription activation functions of the ERα were made to determine the relative contributions of these two regions of the receptor to E2 responses. Deletion of the AF-1 (ERα AF-1°) or deletion or mutation of AF-2 (ERα AF-2°, Af2ERKI) causes male and female infertility (49–51, 90, 91). E2 responses are blunted in the AF-1° mouse and absent in the AF-2 mutated mice.
To address the membrane-associated ER activity, a mutation in the palmitoylation residue was created by two different researchers, with differing outcomes (26, 27). The C451AERα retains genomic signaling responses and normal uterine growth response to E2. In contrast, the NOER, which is exactly the same mutation, exhibits an ERα-null–like uterine phenotype and lacks response to E2. A later study using the NOER mice indicated a uterine growth response (53). The different observations resulting from these two versions of the same model appear to result from residual membrane ERα observed in the C451AERα, whereas the NOER has complete loss of membrane ERα signaling (26–28). Males exhibit an ERα-null phenotype as well (28).
Recently, to address the impact of nuclear localization, a mouse model was made with mutations that exclude ERα from the nucleus, called the H2NES. Both males and females exhibit phenotypes very similar to the ERα-null models (55). Another model, the MOER, restricts ERα to the membrane, and similarly exhibits ERα-null phenotypes (54).
A common observation with many of these mouse models is an infertile, estrogen-insensitive phenotype. Overall, these findings using different ERα mutations emphasize that the DNA binding and AF functions are key for mediating most biological responses, and also emphasize that, on their own, nongenomic mechanisms cannot modulate the primary endocrine response to E2.
Mouse uterine model
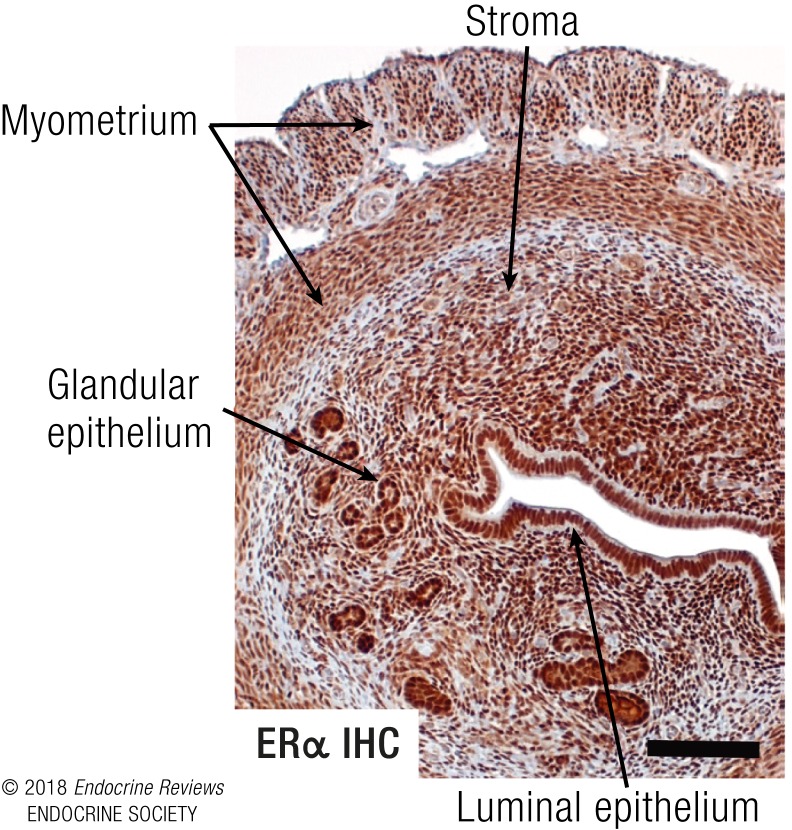
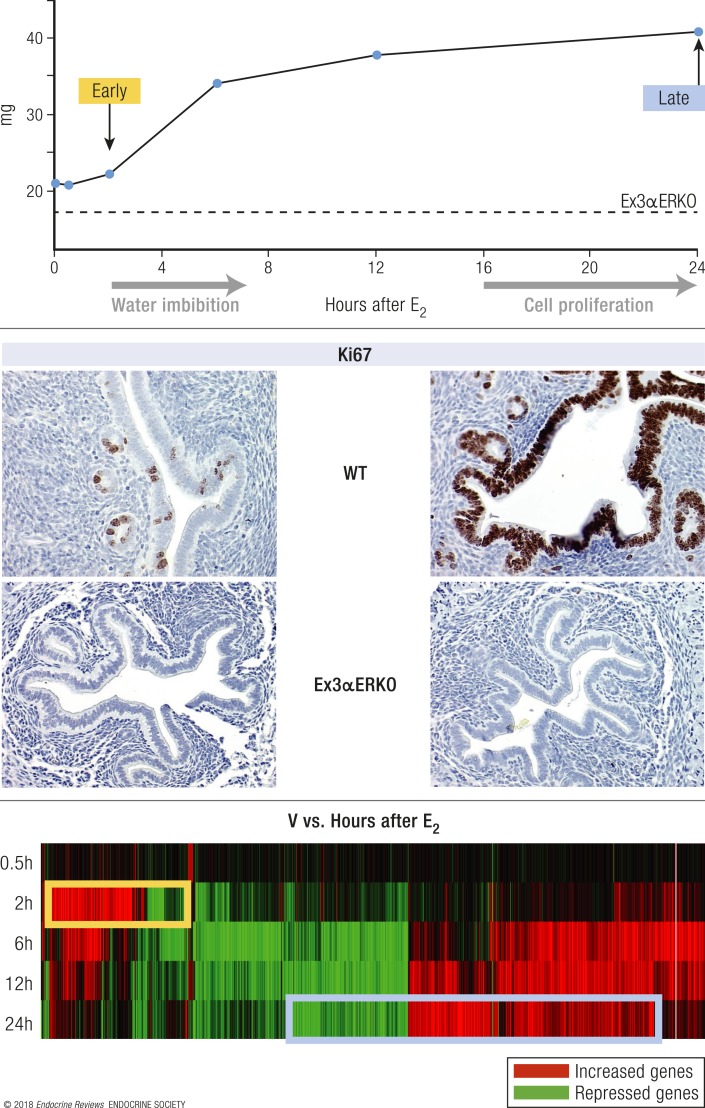
KO and KI models can also allow study of molecular mechanisms within selective tissues. Additionally, endogenous gene regulation by E2 now can be evaluated as a spectrum of responses rather than relying on exogenously engineered reporter genes. Much of the progress in understanding mechanisms and biology of the E2 response in the last 15 years has resulted from the ability to comprehensively evaluate transcriptional responses with microarray and RNA sequencing analysis and to explore the chromatin and cistromic landscape for ER interactions using chromatin immunoprecipitation sequencing (ChIP-seq). The ovariectomized mouse uterus has been an especially useful experimental system in exploring molecular details of E2 biological response. It is highly responsive to E2 due to the abundance of ERα in uterine cells (Fig. 4). Biological endpoints observed following E2 injection include increased tissue weight due initially to increased vascular permeability, plasma transudation, and fluid uptake culminating in epithelial cell growth beginning ∼18 to 24 hours after E2 treatment (summarized in Fig. 5). This temporal pattern of response is bimodal, as it involves an initial early phase, followed by a late phase. These responses require ERα, as they are not observed in ERα-null mice.
Figure 4.
Mouse uterus as ERα-mediated E2 response model. Cross-section of a mouse uterus stained by immunohistochemistry (IHC) with an antibody to ERα illustrating plentiful ERα in all cells, including the luminal and glandular epithelial cells, stromal cells, and myometrial cells. Photo taken with ×10 objective, scale bar = 100 μM.
Figure 5.
Biphasic uterine response to E2. (Top) Schematic of uterine weight response of WT (solid line) and Ex3αERKO (ERα-null) mice. Administering a single dose of E2 to an ovariectomized female mouse results in an early uterine weight increase, caused by water imbibition, and a later increase due to epithelial cell proliferation. Ex3αERKO mice lack any E2 response. (Middle) Ki67 proliferative marker in samples from WT and Ex3αERKO mice treated for 24 h with vehicle (V, left) or E2 (right). Photos taken with ×20 objective. (Bottom) Heat map of differentially expressed (vs V) transcripts in uterus samples from WT mice treated for 0.5, 2, 6, 12, or 24 h with E2.
Evaluation of Transcriptional Responses
Microarray, or RNA sequencing, can be used to evaluate the transcriptional responses of endogenous uterine genes that underlie the biphasic response, and to explore pathways that are impacted by E2 signaling. A principal question regarding the bimodal uterine response was whether the two phases reflected differential gene responses or the stimulation of the same gene more than once. Microarray studies revealed that distinct early and late responding clusters that mirror the early (fluid uptake) and late (epithelial cell proliferation) biological responses were different. These are clearly seen in the 2-hour and 24-hour samples (Fig. 5) (92). Responses at intermediate time points overlap with those seen at 2 hours or 24 hours; therefore, most of our studies sample at these two times to obtain a “snapshot” of uterine response. Co-treatment with the ER antagonist fulvestrant prevented transcriptional response, and no transcriptional responses to E2 treatment were seen in ERα-null samples, indicating that all of the mouse uterine transcriptional responses to E2 require ERα. Transcriptional evaluation of the E2 response of mouse models in which mutated ERα has been knocked in indicated that DBD mutation (EAAE mouse) completely impairs the E2 transcriptional response, whereas transcriptional responses remain in KIKO uterine tissue (21, 48). Mutation of AF-2 (AF2ERKI mouse) prevents transcriptional response to E2 (51). As a result of the structural changes caused by the AF-2 mutation, the estrogen antagonists fulvestrant and tamoxifen behave as agonists rather than antagonists of the AF2ERKI ERα. The agonist activity of this AF-2 mutant was shown to occur through the AF-1 function of the ERα (93). Consequently, these ligands can be used as agonists to study and probe the selective AF-1–mediated transcriptional responses in the uterus of the AF2ERKI mouse. Selective deletion of ERα with Wnt7aCre resulted in loss of uterine epithelial cell ERα, which impacted the early transcriptional responses (2-hour) less than the late (24-hour) responses (39, 94). The growth response also reflects this, as the initial growth (24 hours) is comparable to WT, but the epithelial ERα KO growth is not maintained and after several days is blunted compared with WT and shows indications of apoptosis. Thus, the early transcripts seem to drive initial uterine proliferative growth and seem to be primarily dependent on stromal ERα, whereas further response is driven by the late transcriptional responses and requires epithelial ERα (94).
Next-Generation Sequencing: Chromatin Landscape of ERα-Driven Transcriptional Complexes
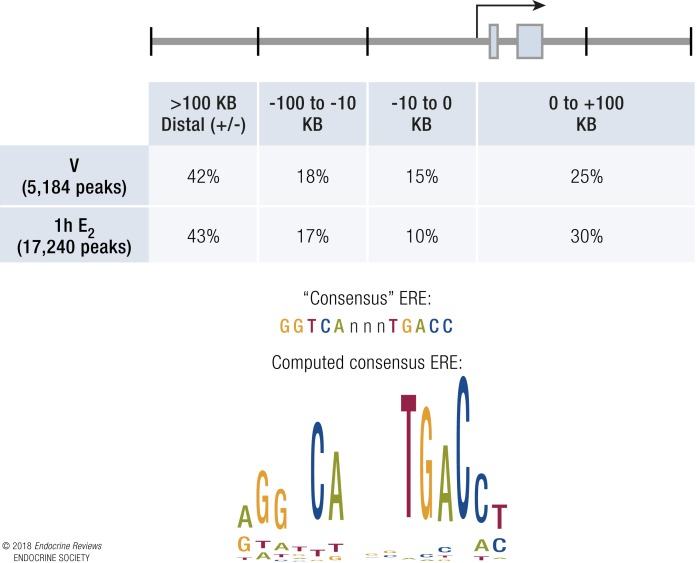
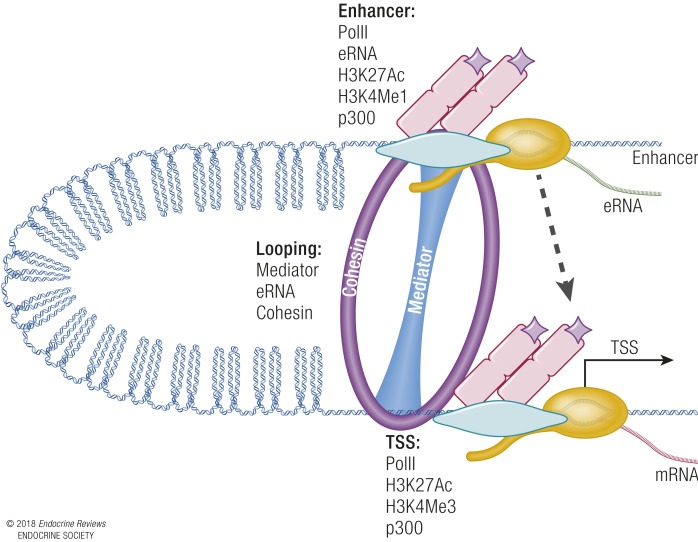
The transcriptional responses described above engage chromatin modifiers to enable access for ERα and regulation involving transcriptional mediators. Identifying the sites of ER interactions with DNA/chromatin enables an understanding of the responses and precise mechanism for establishing comparative gene profiles in certain tissues. Combining immunoprecipitation of chromatin-interacting factors that are crosslinked to DNA (chromatin immunoprecipitation or ChIP) with comprehensive next-generation sequencing of enriched DNA (ChIP-seq) enables mapping of sites of interaction within cells or tissues. Using these ChIP-seq methods, ERα binding sites were initially mapped in MCF-7 breast cancer cells and have now also been mapped in mouse and other animal tissues (95, 96). In general, mapping the ERα “cistrome” has revealed that, although there are ERα sites of interaction proximal to transcription start sites (TSSs), most sites are distal from TSSs, often >100 kb away from regulated transcripts. In our studies using ovariectomized mouse samples, we showed that ERα in the absence of hormone is prerecruited at some sites prior to E2 treatment, and that E2 induces an increase in the number of ERα binding sites and also increases the amount of ERα bound to prebound sites (Fig. 6) (97). ChIP-seq studies have also confirmed the preferential binding of ERα to the GGTCAnnnTGACC palindrome, although one to two base substitutions are accommodated (97, 98). Distal ERα binding has made it difficult to mechanistically assign binding events to transcriptional regulation of genes. Often, other mediators of transcription, including RNA polymerase II (PolII) transcription factors, as well as activating histone modifications, are also enriched at these distal regions, called enhancers. Analysis of prebound ERα sites in a study done in MCF-7 cells indicated that ERα was prerecruited to “mother” sites that contained consensus motif EREs, and that E2 exposure resulted in further ERα recruitment at “daughter” sites (99). These secondary sites orchestrate formation of super-enhancers. Super-enhancers are regions with especially high enrichment of transcriptional enhancer-associated characteristics (99–102) containing groups of coordinately regulated enhancers. Super-enhancers are thought to determine the cell-specific regulatory program in different tissues, and their dysregulation is a key contributor to carcinogenesis. Interaction between distal ERα binding regions and TSSs occur via looping of chromatin. The looping mechanisms involve production of small RNA transcripts called enhancer RNAs from distal regions and assembly of CCTF and cohesin complexes, which facilitate enhancer–TSS interactions (Fig. 7) (103). Activation of enhancers occurs via recruitment of p300 and its associated acetyltransferase activity, and this is then communicated to target gene promoters, leading to increased transcription. Mechanisms of ERα-mediated transcriptional repression are not as well characterized, but we know from gene profiling that repressed genes are part of the normal physiological response. However, recruitment of polycomb complexes to enhancers are known to inhibit interaction with p300/CBP, thus preventing transcriptional progression (104). Hence, interaction with polycomb may be a way in which ER and associated transcriptional machinery could decrease transcriptional rates.
Figure 6.
ERα ChIP-seq indicates sites of interaction in mouse uterus. (Top) Schematic and table show positions of ERα-binding peaks relative to a generic gene model. Both pre-bound (V) and E2-induced (1 h E2) peaks are primarily localized distal from genes. (Bottom) Consensus ERE DNA motif and motif computed from actual ERα binding in uterine tissue.
Figure 7.
Model of looping to facilitate interaction between enhancers and promoters/TSSs. RNA PolII, enhancer RNA (eRNA) transcription, acetylation of histone H3 lysine 27 (H3K27Ac), monomethylation of histone H3 lysine 4 (H3K4Me1), and p300 are found at enhancers. TSSs have PolII, H3K27Ac, trimethylation of histone H3 lysine 4 (H3K4Me3), and p300. Cohesin/mediator form a looping structure that facilitates interaction between the enhancer and TSSs (dashed arrow).
Where We Are and What Is on the Horizon
The key findings in recent years have increased understanding of details of ER-mediated biological responses. Continued development of methods and technologies have enlarged the body of knowledge regarding molecular mechanisms and biological functions of ERs. Questions remain regarding specific details driving tissue responses.
How are tissue cell-specific responses “set” and when?
How do specific ER-binding enhancers regulate transcription of targeted distal transcripts?
How do the different cellular modes of ER signaling interact and contribute to the complete hormone response?
What role does ligand-independent signaling play in biological responsiveness?
What leads to ER-mediated repression of certain E2 responsive genes?
Developing novel approaches promises to provide means by which to study these and other details of ER molecular mechanisms and roles in physiological systems. One emerging approach is use of CRISPR-Cas9 as a means to rapidly target and study candidate molecules or DNA enhancer regions in cells or in animal models. Additionally, novel epigenetic analysis tools are rapidly being developed and driving further discovery of cellular development and gene regulation. Considering how much more is understood about estrogen response than was known in 1999, undoubtedly advancements and efforts during the next decades will enable researchers to develop a better understanding and fill in further important details, which will have application to disease conditions and therapeutic enhancements.
Acknowledgments
Financial Support: This work was supported in part by the Intramural Research Program of the National Institutes of Health through National Institute of Environmental Health Sciences 1ZIAES070065 to K.S.K.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations
- AF
activation function
- ChIP-seq
chromatin immunoprecipitation sequencing
- DBD
DNA-binding domain
- ER
estrogen receptor
- ERE
estrogen-responsive element
- GPER
G protein–coupled estrogen receptor 1
- HPG
hypothalamic–pituitary–gonadal
- HRE
hormone response element
- KI
knock-in
- KIKO
one knock-in and one knockout ERα allele
- LBD
ligand-binding domain
- NERKI
nonclassical ER knock-in
- PolII
RNA polymerase II
- TSS
transcription start site
- WT
wild-type
References
- 1. Cui J, Shen Y, Li R. Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends Mol Med. 2013;19(3):197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Aranda A, Pascual A. Nuclear hormone receptors and gene expression. Physiol Rev. 2001;81(3):1269–1304. [DOI] [PubMed] [Google Scholar]
- 3. Gibson DA, Saunders PT. Estrogen dependent signaling in reproductive tissues—a role for estrogen receptors and estrogen related receptors. Mol Cell Endocrinol. 2012;348(2):361–372. [DOI] [PubMed] [Google Scholar]
- 4. Hewitt SC, Winuthayanon W, Korach KS. What’s new in estrogen receptor action in the female reproductive tract. J Mol Endocrinol. 2016;56(2):R55–R71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McEwan IJ. The nuclear receptor superfamily at thirty. Methods Mol Biol. 2016;1443:3–9. [DOI] [PubMed] [Google Scholar]
- 6. Hilser VJ, Thompson EB. Structural dynamics, intrinsic disorder, and allostery in nuclear receptors as transcription factors. J Biol Chem. 2011;286(46):39675–39682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aagaard MM, Siersbæk R, Mandrup S. Molecular basis for gene-specific transactivation by nuclear receptors. Biochim Biophys Acta. 2011;1812(8):824–835. [DOI] [PubMed] [Google Scholar]
- 8. Yi P, Wang Z, Feng Q, Pintilie GD, Foulds CE, Lanz RB, Ludtke SJ, Schmid MF, Chiu W, O’Malley BW. Structure of a biologically active estrogen receptor-coactivator complex on DNA. Mol Cell. 2015;57(6):1047–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Helsen C, Kerkhofs S, Clinckemalie L, Spans L, Laurent M, Boonen S, Vanderschueren D, Claessens F. Structural basis for nuclear hormone receptor DNA binding. Mol Cell Endocrinol. 2012;348(2):411–417. [DOI] [PubMed] [Google Scholar]
- 10. Sentis S, Le Romancer M, Bianchin C, Rostan MC, Corbo L. Sumoylation of the estrogen receptor α hinge region regulates its transcriptional activity. Mol Endocrinol. 2005;19(11):2671–2684. [DOI] [PubMed] [Google Scholar]
- 11. Wang C, Fu M, Angeletti RH, Siconolfi-Baez L, Reutens AT, Albanese C, Lisanti MP, Katzenellenbogen BS, Kato S, Hopp T, Fuqua SAW, Lopez GN, Kushner PJ, Pestell RG. Direct acetylation of the estrogen receptor α hinge region by p300 regulates transactivation and hormone sensitivity. J Biol Chem. 2001;276(21):18375–18383. [DOI] [PubMed] [Google Scholar]
- 12. Le Romancer M, Poulard C, Cohen P, Sentis S, Renoir JM, Corbo L. Cracking the estrogen receptor’s posttranslational code in breast tumors. Endocr Rev. 2011;32(5):597–622. [DOI] [PubMed] [Google Scholar]
- 13. Kumar R, McEwan IJ. Allosteric modulators of steroid hormone receptors: structural dynamics and gene regulation. Endocr Rev. 2012;33(2):271–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Arao Y, Korach KS. The F domain of estrogen receptor α is involved in species-specific, tamoxifen-mediated transactivation. J Biol Chem. 2018;293(22):8495–8507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. White JT, Motlagh HN, Li J, Thompson EB, Hilser VJ. Allosteric regulation and intrinsic disorder in nuclear hormone receptors In: McEwan IJ, Kumar R, eds. Nuclear Receptors: From Structure to the Clinic. Cham, Switzerland: Springer International Publishing; 2015:73–91. [Google Scholar]
- 16. Schwabe JW, Chapman L, Finch JT, Rhodes D. The crystal structure of the estrogen receptor DNA-binding domain bound to DNA: how receptors discriminate between their response elements. Cell. 1993;75(3):567–578. [DOI] [PubMed] [Google Scholar]
- 17. Brzozowski AM, Pike AC, Dauter Z, Hubbard RE, Bonn T, Engström O, Ohman L, Greene GL, Gustafsson JA, Carlquist M. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature. 1997;389(6652):753–758. [DOI] [PubMed] [Google Scholar]
- 18. Hah N, Kraus WL. Hormone-regulated transcriptomes: lessons learned from estrogen signaling pathways in breast cancer cells. Mol Cell Endocrinol. 2014;382(1):652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kushner PJ, Webb P, Uht RM, Liu MM, Price RH. Estrogen receptor action through target genes with classical and alternative response elements. Pure Appl Chem. 2003;75(11–12):1757–1769. [Google Scholar]
- 20. Safe S, Kim K. Non-classical genomic estrogen receptor (ER)/specificity protein and ER/activating protein-1 signaling pathways [published correction appears in J Mol Endocrinol. 2009;42(4):359]. J Mol Endocrinol. 2008;41(5):263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hewitt SC, Li L, Grimm SA, Winuthayanon W, Hamilton KJ, Pockette B, Rubel CA, Pedersen LC, Fargo D, Lanz RB, DeMayo FJ, Schütz G, Korach KS. Novel DNA motif binding activity observed in vivo with an estrogen receptor α mutant mouse. Mol Endocrinol. 2014;28(6):899–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ahlbory-Dieker DL, Stride BD, Leder G, Schkoldow J, Trölenberg S, Seidel H, Otto C, Sommer A, Parker MG, Schütz G, Wintermantel TM. DNA binding by estrogen receptor-alpha is essential for the transcriptional response to estrogen in the liver and the uterus. Mol Endocrinol. 2009;23(10):1544–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levin ER. Extranuclear steroid receptors are essential for steroid hormone actions. Annu Rev Med. 2015;66:271–280. [DOI] [PubMed] [Google Scholar]
- 24. Prossnitz ER, Hathaway HJ. What have we learned about GPER function in physiology and disease from knockout mice? J Steroid Biochem Mol Biol. 2015;153:114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chambliss KL, Wu Q, Oltmann S, Konaniah ES, Umetani M, Korach KS, Thomas GD, Mineo C, Yuhanna IS, Kim SH, Madak-Erdogan Z, Maggi A, Dineen SP, Roland CL, Hui DY, Brekken RA, Katzenellenbogen JA, Katzenellenbogen BS, Shaul PW. Non-nuclear estrogen receptor α signaling promotes cardiovascular protection but not uterine or breast cancer growth in mice. J Clin Invest. 2010;120(7):2319–2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Adlanmerini M, Solinhac R, Abot A, Fabre A, Raymond-Letron I, Guihot AL, Boudou F, Sautier L, Vessières E, Kim SH, Lière P, Fontaine C, Krust A, Chambon P, Katzenellenbogen JA, Gourdy P, Shaul PW, Henrion D, Arnal JF, Lenfant F. Mutation of the palmitoylation site of estrogen receptor α in vivo reveals tissue-specific roles for membrane versus nuclear actions. Proc Natl Acad Sci USA. 2014;111(2):E283–E290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pedram A, Razandi M, Lewis M, Hammes S, Levin ER. Membrane-localized estrogen receptor α is required for normal organ development and function. Dev Cell. 2014;29(4):482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nanjappa MK, Hess RA, Medrano TI, Locker SH, Levin ER, Cooke PS. Membrane-localized estrogen receptor 1 is required for normal male reproductive development and function in mice. Endocrinology. 2016;157(7):2909–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hewitt SC, Winuthayanon W, Lierz SL, Hamilton KJ, Donoghue LJ, Ramsey JT, Grimm SA, Arao Y, Korach KS. Role of ERα in mediating female uterine transcriptional responses to IGF1. Endocrinology. 2017;158(8):2427–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162–11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dupont S, Krust A, Gansmuller A, Dierich A, Chambon P, Mark M. Effect of single and compound knockouts of estrogen receptors α (ERα) and β (ERβ) on mouse reproductive phenotypes. Development. 2000;127(19):4277–4291. [DOI] [PubMed] [Google Scholar]
- 32. Hewitt SC, Kissling GE, Fieselman KE, Jayes FL, Gerrish KE, Korach KS. Biological and biochemical consequences of global deletion of exon 3 from the ERα gene. FASEB J. 2010;24(12):4660–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Antonson P, Omoto Y, Humire P, Gustafsson JA. Generation of ERα-floxed and knockout mice using the Cre/LoxP system. Biochem Biophys Res Commun. 2012;424(4):710–716. [DOI] [PubMed] [Google Scholar]
- 34. Rumi MA, Dhakal P, Kubota K, Chakraborty D, Lei T, Larson MA, Wolfe MW, Roby KF, Vivian JL, Soares MJ. Generation of Esr1-knockout rats using zinc finger nuclease-mediated genome editing. Endocrinology. 2014;155(5):1991–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, Sar M, Korach KS, Gustafsson JA, Smithies O. Generation and reproductive phenotypes of mice lacking estrogen receptor β. Proc Natl Acad Sci USA. 1998;95(26):15677–15682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Antal MC, Krust A, Chambon P, Mark M. Sterility and absence of histopathological defects in nonreproductive organs of a mouse ERβ-null mutant. Proc Natl Acad Sci USA. 2008;105(7):2433–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wada-Hiraike O, Hiraike H, Okinaga H, Imamov O, Barros RP, Morani A, Omoto Y, Warner M, Gustafsson JA. Role of estrogen receptor β in uterine stroma and epithelium: insights from estrogen receptor β−/− mice. Proc Natl Acad Sci USA. 2006;103(48):18350–18355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rumi MAK, Singh P, Roby KF, Zhao X, Iqbal K, Ratri A, Lei T, Cui W, Borosha S, Dhakal P, Kubota K, Chakraborty D, Vivian JL, Wolfe MW, Soares MJ. Defining the role of estrogen receptor β in the regulation of female fertility. Endocrinology. 2017;158(7):2330–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Winuthayanon W, Hewitt SC, Orvis GD, Behringer RR, Korach KS. Uterine epithelial estrogen receptor α is dispensable for proliferation but essential for complete biological and biochemical responses. Proc Natl Acad Sci USA. 2010;107(45):19272–19277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Pawar S, Laws MJ, Bagchi IC, Bagchi MK. Uterine epithelial estrogen receptor-α controls decidualization via a paracrine mechanism. Mol Endocrinol. 2015;29(9):1362–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Winuthayanon W, Lierz SL, Delarosa KC, Sampels SR, Donoghue LJ, Hewitt SC, Korach KS. Juxtacrine activity of estrogen receptor α in uterine stromal cells is necessary for estrogen-induced epithelial cell proliferation. Sci Rep. 2017;7(1):8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, Jameson JL. An estrogen receptor (ER)α deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16(10):2188–2201. [DOI] [PubMed] [Google Scholar]
- 43. Wang C, Dehghani B, Magrisso IJ, Rick EA, Bonhomme E, Cody DB, Elenich LA, Subramanian S, Murphy SJ, Kelly MJ, Rosenbaum JS, Vandenbark AA, Offner H. GPR30 contributes to estrogen-induced thymic atrophy. Mol Endocrinol. 2008;22(3):636–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mårtensson UE, Salehi SA, Windahl S, Gomez MF, Swärd K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grände PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, Leeb-Lundberg LM. Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology. 2009;150(2):687–698. [DOI] [PubMed] [Google Scholar]
- 45. Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 does not mediate estrogenic responses in reproductive organs in mice. Biol Reprod. 2009;80(1):34–41. [DOI] [PubMed] [Google Scholar]
- 46. Isensee J, Meoli L, Zazzu V, Nabzdyk C, Witt H, Soewarto D, Effertz K, Fuchs H, Gailus-Durner V, Busch D, Adler T, de Angelis MH, Irgang M, Otto C, Noppinger PR. Expression pattern of G protein-coupled receptor 30 in LacZ reporter mice. Endocrinology. 2009;150(4):1722–1730. [DOI] [PubMed] [Google Scholar]
- 47. O’Brien JE, Peterson TJ, Tong MH, Lee EJ, Pfaff LE, Hewitt SC, Korach KS, Weiss J, Jameson JL. Estrogen-induced proliferation of uterine epithelial cells is independent of estrogen receptor α binding to classical estrogen response elements. J Biol Chem. 2006;281(36):26683–26692. [DOI] [PubMed] [Google Scholar]
- 48. Hewitt SC, O’Brien JE, Jameson JL, Kissling GE, Korach KS. Selective disruption of ERα DNA-binding activity alters uterine responsiveness to estradiol. Mol Endocrinol. 2009;23(12):2111–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Abot A, Fontaine C, Raymond-Letron I, Flouriot G, Adlanmerini M, Buscato M, Otto C, Bergès H, Laurell H, Gourdy P, Lenfant F, Arnal JF. The AF-1 activation function of estrogen receptor α is necessary and sufficient for uterine epithelial cell proliferation in vivo. Endocrinology. 2013;154(6):2222–2233. [DOI] [PubMed] [Google Scholar]
- 50. Billon-Galés A, Krust A, Fontaine C, Abot A, Flouriot G, Toutain C, Berges H, Gadeau AP, Lenfant F, Gourdy P, Chambon P, Arnal JF. Activation function 2 (AF2) of estrogen receptor-α is required for the atheroprotective action of estradiol but not to accelerate endothelial healing. Proc Natl Acad Sci USA. 2011;108(32):13311–13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Arao Y, Hamilton KJ, Ray MK, Scott G, Mishina Y, Korach KS. Estrogen receptor α AF-2 mutation results in antagonist reversal and reveals tissue selective function of estrogen receptor modulators. Proc Natl Acad Sci USA. 2011;108(36):14986–14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, Sugg SL, Temple KA, Wondisford FE, Korach KS, Woodruff TK, Greene GL. An estrogen receptor-α knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149(6):2970–2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gustafsson KL, Farman H, Henning P, Lionikaite V, Movérare-Skrtic S, Wu J, Ryberg H, Koskela A, Gustafsson JA, Tuukkanen J, Levin ER, Ohlsson C, Lagerquist MK. The role of membrane ERα signaling in bone and other major estrogen responsive tissues. Sci Rep. 2016;6(1):29473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pedram A, Razandi M, Kim JK, O’Mahony F, Lee EYHP, Luderer U, Levin ER. Developmental phenotype of a membrane only estrogen receptor alpha (MOER) mouse. J Biol Chem. 2009;284(6):3488–3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Stefkovich ML, Arao Y, Hamilton KJ, Korach KS. Experimental models for evaluating non-genomic estrogen signaling. Steroids. 2018;133:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Park CJ, Chen G, Koo Y, Lin PP, Cacioppo JA, Prohaska H, Ko CJ. Generation and characterization of an estrogen receptor α-iCre knock-in mouse. Genesis. 2017;55(12):e23084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cacioppo JA, Koo Y, Lin PC, Osmulski SA, Ko CD, Ko C. Generation of an estrogen receptor β-iCre knock-in mouse. Genesis. 2016;54(1):38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Couse JF, Yates MM, Walker VR, Korach KS. Characterization of the hypothalamic–pituitary–gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17(6):1039–1053. [DOI] [PubMed] [Google Scholar]
- 59. Couse JF, Curtis SW, Washburn TF, Lindzey J, Golding TS, Lubahn DB, Smithies O, Korach KS. Analysis of transcription and estrogen insensitivity in the female mouse after targeted disruption of the estrogen receptor gene. Mol Endocrinol. 1995;9(11):1441–1454. [DOI] [PubMed] [Google Scholar]
- 60. Curtis SW, Clark J, Myers P, Korach KS. Disruption of estrogen signaling does not prevent progesterone action in the estrogen receptor α knockout mouse uterus. Proc Natl Acad Sci USA. 1999;96(7):3646–3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hamilton KJ, Arao Y, Korach KS. Estrogen hormone physiology: reproductive findings from estrogen receptor mutant mice. Reprod Biol. 2014;14(1):3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hamilton KJ, Hewitt SC, Arao Y, Korach KS. Estrogen hormone biology. Curr Top Dev Biol. 2017;125:109–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Heine PA, Lubahn DB, Cooke PS. Role of estrogen receptor alpha (ER alpha) in white adipose tissue (WAT) deposition in mice. Biol Reprod. 1999;60:244. [Google Scholar]
- 64. Bryzgalova G, Gao H, Ahren B, Zierath JR, Galuska D, Steiler TL, Dahlman-Wright K, Nilsson S, Gustafsson JA, Efendic S, Khan A. Evidence that oestrogen receptor-α plays an important role in the regulation of glucose homeostasis in mice: insulin sensitivity in the liver. Diabetologia. 2006;49(3):588–597. [DOI] [PubMed] [Google Scholar]
- 65. Ribas V, Drew BG, Le JA, Soleymani T, Daraei P, Sitz D, Mohammad L, Henstridge DC, Febbraio MA, Hewitt SC, Korach KS, Bensinger SJ, Hevener AL. Myeloid-specific estrogen receptor α deficiency impairs metabolic homeostasis and accelerates atherosclerotic lesion development [published correction appears in Proc Natl Acad Sci USA. 2012;109(2):645]. Proc Natl Acad Sci USA. 2011;108(39):16457–16462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rubanyi GM, Freay AD, Kauser K, Sukovich D, Burton G, Lubahn DB, Couse JF, Curtis SW, Korach KS. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J Clin Invest. 1997;99(10):2429–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hodgin JB, Krege JH, Reddick RL, Korach KS, Smithies O, Maeda N. Estrogen receptor α is a major mediator of 17β-estradiol’s atheroprotective effects on lesion size in Apoe−/− mice. J Clin Invest. 2001;107(3):333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Carey MA, Card JW, Voltz JW, Germolec DR, Korach KS, Zeldin DC. The impact of sex and sex hormones on lung physiology and disease: lessons from animal studies. Am J Physiol Lung Cell Mol Physiol. 2007;293(2):L272–L278. [DOI] [PubMed] [Google Scholar]
- 69. Svenson JL, EuDaly J, Ruiz P, Korach KS, Gilkeson GS. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clin Immunol. 2008;128(2):259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, Mauvais-Jarvis F. Estrogens protect pancreatic β-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proc Natl Acad Sci USA. 2006;103(24):9232–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ribas V, Drew BG, Zhou Z, Phun J, Kalajian NY, Soleymani T, Daraei P, Widjaja K, Wanagat J, de Aguiar Vallim TQ, Fluitt AH, Bensinger S, Le T, Radu C, Whitelegge JP, Beaven SW, Tontonoz P, Lusis AJ, Parks BW, Vergnes L, Reue K, Singh H, Bopassa JC, Toro L, Stefani E, Watt MJ, Schenk S, Akerstrom T, Kelly M, Pedersen BK, Hewitt SC, Korach KS, Hevener AL. Skeletal muscle action of estrogen receptor α is critical for the maintenance of mitochondrial function and metabolic homeostasis in females. Sci Transl Med. 2016;8(334):334ra54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sims NA, Dupont S, Krust A, Clement-Lacroix P, Minet D, Resche-Rigon M, Gaillard-Kelly M, Baron R. Deletion of estrogen receptors reveals a regulatory role for estrogen receptors-β in bone remodeling in females but not in males. Bone. 2002;30(1):18–25. [DOI] [PubMed] [Google Scholar]
- 73. Khosla S. Estrogen, selective estrogen receptor modulators and now mechanism-specific ligands of the estrogen or androgen receptor? Trends Pharmacol Sci. 2003;24(6):261–263. [DOI] [PubMed] [Google Scholar]
- 74. Cooke PS, Nanjappa MK, Ko C, Prins GS, Hess RA. Estrogens in male physiology. Physiol Rev. 2017;97(3):995–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, Verkman A, Lubahn D, Fisher JS, Katzenellenbogen BS, Hess RA. Estrogen action and male fertility: roles of the sodium/hydrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc Natl Acad Sci USA. 2001;98(24):14132–14137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hess RA, Bunick D, Lee KH, Bahr J, Taylor JA, Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997;390(6659):509–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ogawa S, Lubahn DB, Korach KS, Pfaff DW. Behavioral effects of estrogen receptor gene disruption in male mice. Proc Natl Acad Sci USA. 1997;94(4):1476–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ogawa S, Chester AE, Hewitt SC, Walker VR, Gustafsson JA, Smithies O, Korach KS, Pfaff DW. Abolition of male sexual behaviors in mice lacking estrogen receptors α and β (αβERKO). Proc Natl Acad Sci USA. 2000;97(26):14737–14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, Williams TC, Lubahn DB, Korach KS. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N Engl J Med. 1994;331(16):1056–1061. [DOI] [PubMed] [Google Scholar]
- 80. Bernard V, Kherra S, Francou B, Fagart J, Viengchareun S, Guéchot J, Ladjouze A, Guiochon-Mantel A, Korach KS, Binart N, Lombès M, Christin-Maitre S. Familial multiplicity of estrogen insensitivity associated with a loss-of-function ESR1 mutation. J Clin Endocrinol Metab. 2017;102(1):93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Quaynor SD, Stradtman EW Jr, Kim HG, Shen Y, Chorich LP, Schreihofer DA, Layman LC. Delayed puberty and estrogen resistance in a woman with estrogen receptor α variant. N Engl J Med. 2013;369(2):164–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Jeselsohn R, Buchwalter G, De Angelis C, Brown M, Schiff R. ESR1 mutations—a mechanism for acquired endocrine resistance in breast cancer. Nat Rev Clin Oncol. 2015;12(10):573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Pejerrey SM, Dustin D, Kim J-A, Gu G, Rechoum Y, Fuqua SAW. The impact of ESR1 mutations on the treatment of metastatic breast cancer. Hormones and Cancer. 2018;9(4):215–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Jayes FL, Burns KA, Rodriguez KF, Kissling GE, Korach KS. The naturally occurring luteinizing hormone surge is diminished in mice lacking estrogen receptor β in the ovary. Biol Reprod. 2014;90(2):24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Deroo BJ, Rodriguez KF, Couse JF, Hamilton KJ, Collins JB, Grissom SF, Korach KS. Estrogen receptor β is required for optimal cAMP production in mouse granulosa cells. Mol Endocrinol. 2009;23(7):955–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Couse JF, Hewitt SC, Bunch DO, Sar M, Walker VR, Davis BJ, Korach KS. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science. 1999;286(5448):2328–2331. [DOI] [PubMed] [Google Scholar]
- 87. Binder AK, Rodriguez KF, Hamilton KJ, Stockton PS, Reed CE, Korach KS. The absence of ER-β results in altered gene expression in ovarian granulosa cells isolated from in vivo preovulatory follicles. Endocrinology. 2013;154(6):2174–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Barton M. Not lost in translation: Emerging clinical importance of the G protein-coupled estrogen receptor GPER. Steroids. 2016;111:37–45. [DOI] [PubMed] [Google Scholar]
- 89. Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276(17):13615–13621. [DOI] [PubMed] [Google Scholar]
- 90. Arao Y, Hamilton KJ, Goulding EH, Janardhan KS, Eddy EM, Korach KS. Transactivating function (AF) 2–mediated AF-1 activity of estrogen receptor α is crucial to maintain male reproductive tract function. Proc Natl Acad Sci USA. 2012;109(51):21140–21145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Billon-Galés A, Fontaine C, Filipe C, Douin-Echinard V, Fouque MJ, Flouriot G, Gourdy P, Lenfant F, Laurell H, Krust A, Chambon P, Arnal JF. The transactivating function 1 of estrogen receptor α is dispensable for the vasculoprotective actions of 17β-estradiol. Proc Natl Acad Sci USA. 2009;106(6):2053–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hewitt SC, Deroo BJ, Hansen K, Collins J, Grissom S, Afshari CA, Korach KS. Estrogen receptor-dependent genomic responses in the uterus mirror the biphasic physiological response to estrogen. Mol Endocrinol. 2003;17(10):2070–2083. [DOI] [PubMed] [Google Scholar]
- 93. Arao Y, Coons LA, Zuercher WJ, Korach KS. Transactivation function-2 of estrogen receptor α contains transactivation function-1-regulating element. J Biol Chem. 2015;290(28):17611–17627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Winuthayanon W, Hewitt SC, Korach KS. Uterine epithelial cell estrogen receptor α-dependent and -independent genomic profiles that underlie estrogen responses in mice. Biol Reprod. 2014;91(5):110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Gilfillan S, Fiorito E, Hurtado A. Functional genomic methods to study estrogen receptor activity. J Mammary Gland Biol Neoplasia. 2012;17(2):147–153. [DOI] [PubMed] [Google Scholar]
- 96. Cheung E, Kraus WL. Genomic analyses of hormone signaling and gene regulation. Annu Rev Physiol. 2010;72(1):191–218. [DOI] [PubMed] [Google Scholar]
- 97. Hewitt SC, Li L, Grimm SA, Chen Y, Liu L, Li Y, Bushel PR, Fargo D, Korach KS. Research resource: whole-genome estrogen receptor α binding in mouse uterine tissue revealed by ChIP-seq. Mol Endocrinol. 2012;26(5):887–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Coons LA, Hewitt SC, Burkholder AB, McDonnell DP, Korach KS. DNA sequence constraints define functionally active steroid nuclear receptor binding sites in chromatin. Endocrinology. 2017;158(10):3212–3234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bojcsuk D, Nagy G, Balint BL. Inducible super-enhancers are organized based on canonical signal-specific transcription factor binding elements. Nucleic Acids Res. 2017;45(7):3693–3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sengupta S, George RE. Super-enhancer-driven transcriptional dependencies in cancer. Trends Cancer. 2017;3(4):269–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pott S, Lieb JD. What are super-enhancers? Nat Genet. 2015;47(1):8–12. [DOI] [PubMed] [Google Scholar]
- 102. Kim T-K, Shiekhattar R. Architectural and functional commonalities between enhancers and promoters. Cell. 2015;162(5):948–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Wang J, Meng X, Chen H, Yuan C, Li X, Zhou Y, Chen M. Exploring the mechanisms of genome-wide long-range interactions: interpreting chromosome organization. Brief Funct Genomics. 2016;15(5):385–395. [DOI] [PubMed] [Google Scholar]
- 104. Piunti A, Shilatifard A. Epigenetic balance of gene expression by Polycomb and COMPASS families. Science. 2016;352(6290):aad9780. [DOI] [PubMed] [Google Scholar]