The structure of bile acids was first described in 1932, and it is well established that bile acids are important in the physiologic and pathophysiologic processes of fat absorption, cholesterol secretion, and cholesterol gallstone formation. The primary human bile acids cholic acid (CA) and chenodeoxycholic acid (CDCA) are synthesized in the liver, secreted into the intestine and undergo bacterial biotransformation to generate secondary bile acids including deoxycholic acid (DCA) and lithocholic acid (LCA). The importance of the microbiome in bile acid metabolism has been well recognized for sixty years (1, 2), preceding the more recent appreciation of the role of the microbiome in a host of other gastrointestinal and non-gastrointestinal disease processes. More recently, bile acid signaling pathways by the nuclear receptor farnesoid X receptor (FXR) and the Takeda G protein couple receptor 5 (TGR5, also termed G protein-coupled bile acid receptor-1, Gpbar-1) have been identified and well-characterized as mediators of insulin-resistance, obesity, lipid metabolism and systemic metabolic processes. Other nuclear receptors including SXR (steroid and xenobiotic receptor, and its rodent homolog PXR), vitamin D receptor (VDR), and constitutive androstane receptor (CAR) have also been identified to be important for bile acid signaling, albeit responding to a more restricted set of bile acid species. In fact, several drugs targeting bile acid pathways are currently in clinical trials for the treatment of fatty liver and other metabolic disorders.
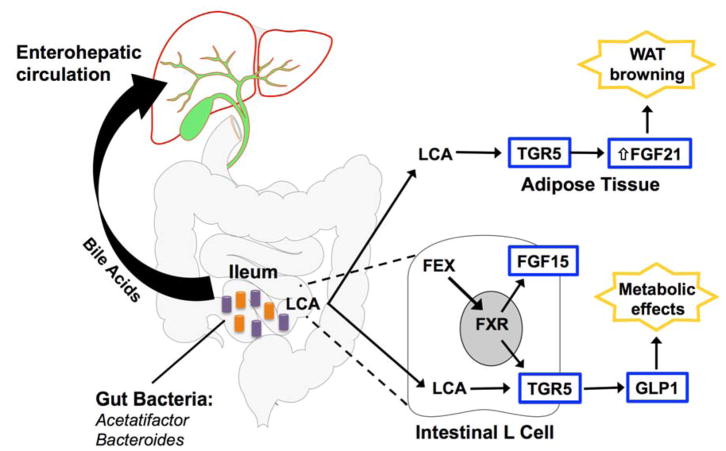
Experimental studies have sought to better define the effects of intestinal FXR signaling on features of the metabolic syndrome. However, seemingly contrasting evidence demonstrates improvement in metabolic parameters with both intestinal-specific FXR activation and inhibition. The reason for these seemingly paradoxical findings of FXR activation and inhibition have been unclear. In this issue of Hepatology, Pathak et al. addresses this controversy by demonstrating that the gut microbiota has a critical role in regulating FXR-driven metabolic effects through LCA-mediated activation of TGR5, with the resultant production of glucagon-like peptide-1 (GLP-1) by intestinal L cells (Figure 1) (3). They demonstrate that the synthesis of LCA by intestinal Acetatifactor and Bacteroides may help explain the seemingly paradoxical metabolic effects of FXR signaling. In this study, while the FXR agonist fexaramine (FEX) acts only in the intestine and is not absorbed, FEX treatment regulates hepatic metabolism and improves lipid profiles, insulin sensitivity, and white adipose tissue (WAT) browning in wild-type mice. The intestinal-specific signaling effects of FEX treatment were confirmed by the lack of changes in the expression of FXR-responsive genes in the liver. FEX treatment increases taurolithocholic acid (TLCA) concentrations approximately 1,000-fold in gallbladder bile, but did not affect the concentration of the primary bile acid CA (suggesting that the effects of FEX treatment are largely independent of primary hepatic synthesis). In the intestine of FEX treated mice, Acetatifactor and Bacteroides, which are known to produce LCA from CDCA and ursodeoxycholic acid (UDCA), are increased in abundance. Intestinal antibiotic treatment altered bile acid composition and significantly reduced LCA production, preventing the increased GLP-1 secretion, and reversing the improvements of glucose tolerance and WAT browning. Overall, these findings support a role for microbiota-mediated FXR regulation of obesity and insulin resistance, acting through LCA production with the resultant activation of TGR5/GLP-1 signaling in the intestinal L cells.
Figure 1. Mechanism of intestine-specific FXR activation.
Fexaramine (FEX) activates intestinal FXR, causing alterations in the gut microbiota and production of LCA by Acetatifactor and Bacteroides. LCA activates TGR5, which induces GLP-1 production from intestinal L cells and improves insulin sensitivity. TGR5 activation and increased FGF21 production then mediate WAT browning (see online Supplemental Figure S7 from Pathak et al).
Microbiota-mediated changes in bile acid composition have previously been shown to regulate host metabolism via the bile acid receptor FXR. Seemingly contrasting evidence exists, however, as to whether FXR signaling promotes or prevents insulin resistance and obesity-related metabolic disease (4, 5). Previous studies on the role of microbiota-mediated FXR antagonism have demonstrated improvement in metabolic diseases. In mice, decreased levels of intestinal Lactobacillus result in an increase of the FXR bile acid antagonist tauro-β-muricholic acid, conferring a resistance to the high-fat diet-induced metabolic phenotype (6). The administration of the intestinal FXR antagonist glycine-β-muricholic acid to mice also improves metabolic disease, and is associated with a decreased ratio of Firmicutes to Bacteroides (7). The importance of the microbiome on the metabolic syndrome is further supported by the demonstration that FXR-null mice are protected from high-fat diet induced obesity, and transfer of their microbiota into germ free wild-type mice results in less obesity and improved glucose tolerance (8).
Interestingly, the study by Pathak et al. demonstrates that antibiotic-treated mice receiving FEX have reduced WAT browning and GLP-1 production by intestinal L cells. These mice also have decreased hepatic gene expression of liver FXR targets such as CYP7A1), likely secondary to an increase in intestinal fibroblast growth factor 15 (FGF15) production. However, FGF21 levels did not significantly change in antibiotic treated mice. Taken together, these data support a key role for TGR5 regulation of GLP-1 signaling, as well as FGF21 pathways, regulating the metabolic effects seen with FEX treatment. In addition, these findings suggest that the gut microbiota, acting via changes of bile acid composition, may alter FEX efficacy and limit the hepatic effects of FXR agonist signaling. Difference of intestinal microbiota may thus help explain the differing findings of previous studies examining the metabolic phenotypes that occur in intestine-specific FXR-null mice and in mice treated with FXR agonists and antagonists (6, 7). Intestinal FXR signaling in the setting of the different gut microbiota may occur due to the specific murine phenotypes, as well as the housing environment at different institutions. Additional studies evaluating the effects of FEX treatment on FXR-null and TGR5-null mice would help confirm that GLP-1 induction via TGR5 is a primary pathway controlling these metabolic outcomes.
Many recent advances in our understanding of the role of the intestinal microbiome on hepatic and systemic metabolism have been achieved using high-throughput exploratory techniques including rRNA sequencing, metagenomics, metaproteomics and metabolomics (9). The current paper, however, highlights the role of the microbiota on metabolism using a hypothesis-driven investigation focusing on the gut microbiota and LCA production. The interaction of the gut microbiome and bile acids demonstrated by Pathak et al. further supports the importance of bacterial flora-bile acid interactions that was first appreciated over 6 decades ago. Although LCA is a toxic bile acid, the pharmacologic targeting of TGR5 agonists (10) and TGR5/GLP-1 signaling may offer new targets for pharmacologic therapies to treat obesity, insulin-resistance and hepatic steatosis. While the interplay between gut microbiota and bile acid metabolism has been known for over 60 years, this study highlights the importance of further investigating this interaction in the regulation of host physiology and disease.
Abbreviations
- CA
cholic acid
- CDCA
chenodeoxycholic acid
- DCA
deoxycholic acid
- LCA
lithocholic acid
- FXR
farnesoid X receptor
- TGR5
Takeda G protein couple receptor 5
- Gpbar-1
G protein-coupled bile acid receptor-1
- SXR
steroid and xenobiotic receptor
- PXR
pregnane X receptor
- VDR
vitamin D receptor
- CAR
constitutive androstane receptor
- GLP-1
glucagon-like peptide-1
- FEX
fexaramine
- WAT
white adipose tissue
- TLCA
taurolithocholic acid
- UDCA
ursodeoxycholic acid
- CYP7A1
cholesterol 7-alpha-hydroxylase
- FGF15
fibroblast growth factor 15
- FGF21
fibroblast growth factor 21
- rRNA
ribosomal ribonucleic acid
References
- 1.Norman A, Sjovall J. On the transformation and enterohepatic circulation of cholic acid in the rat: bile acids and steroids 68. J Biol Chem. 1958;233:872–885. [PubMed] [Google Scholar]
- 2.Hofmann AF, Hagey LR. Key discoveries in bile acid chemistry and biology and their clinical applications: history of the last eight decades. J Lipid Res. 2014;55:1553–1595. doi: 10.1194/jlr.R049437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pathak P, Xie C, Nichols RG, Ferrell JM, Boehme S, Krausz KW, Patterson AD, et al. Intestine farnesoid X receptor agonist and the gut microbiota activate G-protein bile acid receptor-1 signaling to improve metabolism. Hepatology. 2018 doi: 10.1002/hep.29857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat Med. 2015;21:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jiang C, Xie C, Li F, Zhang L, Nichols RG, Krausz KW, Cai J, et al. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J Clin Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez FJ, Jiang C, Patterson AD. An Intestinal Microbiota-Farnesoid X Receptor Axis Modulates Metabolic Disease. Gastroenterology. 2016;151:845–859. doi: 10.1053/j.gastro.2016.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang L, Xie C, Nichols RG, Chan SH, Jiang C, Hao R, Smith PB, et al. Farnesoid X Receptor Signaling Shapes the Gut Microbiota and Controls Hepatic Lipid Metabolism. mSystems. 2016:1. doi: 10.1128/mSystems.00070-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parseus A, Sommer N, Sommer F, Caesar R, Molinaro A, Stahlman M, Greiner TU, et al. Microbiota-induced obesity requires farnesoid X receptor. Gut. 2017;66:429–437. doi: 10.1136/gutjnl-2015-310283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez KB, Leone V, Chang EB. Microbial metabolites in health and disease: Navigating the unknown in search of function. J Biol Chem. 2017;292:8553–8559. doi: 10.1074/jbc.R116.752899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pathak P, Liu H, Boehme S, Xie C, Krausz KW, Gonzalez F, Chiang JYL. Farnesoid X receptor induces Takeda G-protein receptor 5 cross-talk to regulate bile acid synthesis and hepatic metabolism. J Biol Chem. 2017;292:11055–11069. doi: 10.1074/jbc.M117.784322. [DOI] [PMC free article] [PubMed] [Google Scholar]