Abstract
The recent report of the existence of meningeal lymphatic vessels (MLVs) in human and nonhuman primates used both histology and magnetic resonance imaging (MRI). Many questions about the physiology and function of these lymphatic vessels remain unanswered. Through the combination of appropriately positioned saturation bands and time-of-flight angiography sequences, MRI can resolve direction of flow within vessels without the use of exogenous contrast agent. Six healthy volunteers underwent high-resolution MRI of the MLVs running alongside the superior sagittal sinus to determine the direction of the lymphatic flow. In all subjects, the lymphatic flow was posterior to anterior, countercurrent to the direction of venous flow in the superior sagittal sinus and alongside the superior sagittal sinus. This flow strongly supports that a large proportion of the CNS lymphatic flow in humans is directed to the cribriform plate. The countercurrent direction of flow in the MLVs relative to venous flow in the superior sagittal sinus has implications for modeling flow of fluid and solutes across the various compartments of the CNS. A hypothetical compartmental model incorporating countercurrent flow is presented here.
Keywords: meningeal lymphatic vessels, flow, human, countercurrent
Introduction
The central nervous system (CNS) has an incompletely understood lymphatic system that is quite different from the systemic lymphatic system. The CNS lymphatics is made more complex by the interaction of the glial-associated lymphatic compartment (glymphatics) with the meningeal lymphatic vessels (MLVs) (1). In addition, the brain lymphatic system is made even more unique by its relationship with the cerebrospinal fluid (CSF) compartment with its own circulatory pathways and dynamic pressures. Research supports that nerve sheaths, adventitia of blood vessels, and the cribriform plate are significant routes of CSF drainage into the lymphatic system (2).
Although the CNS lymphatic system has been extensively studied in animal models such as rodents, a 2017 report of the existence of MLVs in humans was groundbreaking for this area of clinical investigation into normal and pathophysiology. This study's utilization of noninvasive MRI also opened the door for development of the wide variety of MRI sequences for shedding new light on the anatomy and function of the CNS lymphatics (3).
To understand the functioning of a vascular compartment, the direction of flow must be known. A common technique used in MRI for imaging of arteries is time-of-flight (TOF) angiography. The key principle for this technique is that blood flowing from outside the image section to inside the image section will have greater signal intensity than the relatively saturated protons in the imaged section and thus appear “brighter” (4). Of course, radiologists already know the direction of blood flow in the arteries from their knowledge of anatomy, so directionality of flow does not need to be additionally tested for in the clinical setting. To ascertain the direction of flow, TOF sequences can be modified by the addition of saturation bands placed parallel on either side of the image section. The protons in the fluid flowing into the image section from the direction on the same side of the saturation band will be saturated before entering the image section and therefore will have low signal intensity and not appear “brighter.” Vice versa, protons in fluid flowing into the image section from the opposite side of the saturation band will not be saturated before entering the image section and thus still will appear “brighter.” In this study, we take advantage of these MRI techniques to determine the direction of flow in the MLVs running alongside the superior sagittal sinus (SSS) in human subjects.
Methods and Materials
This study was approved by the institutional review board. Informed and signed consent was obtained. MRI safety questionnaires were completed and reviewed before all volunteer subjects entered the MRI scanner room.
Six healthy volunteers (women, 2; men, 4; age range, 30–56 years) underwent MRI scanning (3 T MRI unit; Skyra, Siemens Healthcare, Erlangen, Germany; body coil was used for radiofrequency transmission; and 16-channel shoulder phased array coil was used for reception). Compared with a standard head coil, the small curved half-shell design of this high density shoulder coil contoured to the vertex of the cranium quite closely and improved the signal-to-noise ratio (SNR) in the region of the vertex of the head. The improved signal-to-noise ratio was helpful for the high-resolution demands of the following MRI techniques:
Coronal SPACE FLAIR (Sampling Perfection with Application optimized Contrasts using varying flip angle Evolutions): acquisition matrix, 222 × 222, 1.2 mm thick sections, 176s; field of view (FOV), 120 mm; voxel dimensions, 0.54 mm × 0.54 mm × 1.2 mm; repetition time (TR)/apparent echo time (TE)/inversion time = 7000/100/2100 milliseconds; echo train length, 264; fat saturation, weak; flip angle evolution optimized for T2 constrast (T2 var), Caipirinha iPAT method; acquisition time, 6 minutes 24 seconds.
Axial SPACE T2 (Sampling Perfection with Application optimized Contrasts using varying flip angle Evolutions): acquisition matrix, 192 × 192, sections, 72; FOV phase, 77 mm; FOV reads, 64 mm; voxel dimensions, 0.4 × 0.33 × 0.4 mm; TR/TE, 3500/697 milliseconds; echo train length, 180; bandwidth, 280 Hz/pixel; averages, 1.7 parallel image factor of 2, fat saturation, constant flip angle; acquisition time, 5 minutes 31 seconds.
- Coronal gradient echo inflow-sensitive sequence for TOF angiography: matrix, 160 × 160; section, 1; section thickness, 1.5 mm; FOV, 50 mm; voxel dimension, 0.31 × 0.31 × 1.5 mm; TR/TE, 30/4.49 milliseconds; bandwidth, 320 Hz/pixel; flip angle, 10° to reduce slow flow saturation effects; signal averages, 10. By using the axial and coronal SPACE sequences, the angle of the coronal section was adjusted to be perpendicular to the course of the SSS and adjacent MLVs in order to maximize sensitivity for inflow of fluid into the imaging plane. This sequence was acquired 3 times, each time with differing placements of the saturation bands parallel to the image section as follows:
- saturation bands anterior and posterior (negative control);
- saturation band anterior only; and
- saturation band posterior only.
Imaging was supervised and reviewed by a board-certified radiologist.
Results
All subjects completed all sequences of the MRI study and reported no significant side effects.
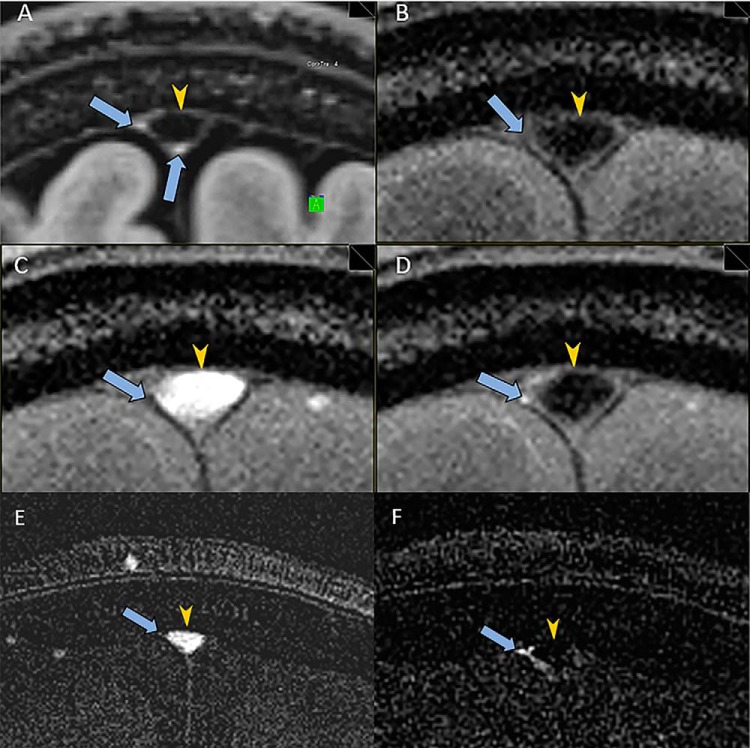
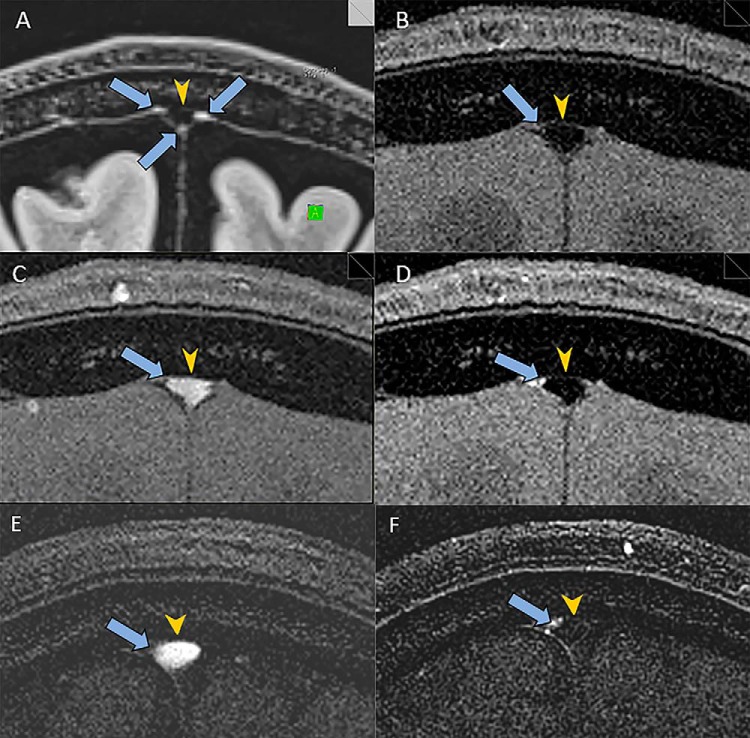
The MLVs running alongside the SSS were visualized in all 6 subjects (Figures 1A and 2A). A coronal section slightly anterior to the vertex was found to provide good visualization of the MLVs most consistently. The appearance of the MLV was similar to that previously reported with MLV visualized at the corners of the SSS (3). The cross-sectional dimension of the visualized lymphatic vessels was difficult to measure but was ∼0.5 mm, the resolution of the high-resolution sequences. This estimate is in line with that observed by MRI and by histology in the previous work by Absinta et al. (3).
Figure 1.
Utilization of magnetic resonance imaging (MRI) to determine the direction of flow in meningeal lymphatic vessels (MLVs) of a 56-year-old woman. High-resolution coronal SPACE FLAIR image shows MLVs (arrows) adjacent to the superior sagittal sinus (SSS) (arrowhead) (A). Coronal time-of-flight (TOF) sequence with saturation bands, both anterior and posterior to the image section, shows the expected low signal in the SSS (arrowhead) and no signal in the region of the MLVs (arrows) (B). Coronal TOF sequence with a saturation band posterior to the image section shows bright signal in the SSS (arrowhead) (C). The MLVs are not visualized (arrows). Coronal TOF sequence with a saturation band anterior to the image section shows the expected low signal in the SSS (arrowhead) (D). MLVs show increased signal consistent (arrows) with the rostral flow of lymphatic fluid posterior to anterior, countercurrent to the venous flow in the SSS. Difference image generated by subtracting coronal TOF sequence with a saturation band posterior (C) from coronal TOF sequence with saturation bands both anterior and posterior (B) shows the expected flow-related enhancement in the SSS only (arrowhead) and not in the MLVs (arrow) (E). Difference image generated by subtracting coronal TOF sequence with a saturation band anterior (D) from coronal TOF sequence with saturation bands both anterior and posterior (B) shows more clearly the flow-related enhancement in the MLV (arrow) and lack of enhancement in the SSS (arrowhead) (F).
Figure 2.
Utilization of MRI to determine the direction of flow in MLVs of a 45-year-old man. High-resolution coronal SPACE FLAIR image shows MLVs (arrows) adjacent to the SSS (arrowhead) (A). Coronal TOF sequence with saturation bands both anterior and posterior to the image section shows the expected low signal in the SSS (arrowhead) and no signal in the region of the MLVs (arrows) (B). Coronal TOF sequence with a saturation band posterior to the image section shows bright signal in the SSS (arrowhead). The MLV are not visualized (arrows) (C). Coronal TOF sequence with a saturation band anterior to the image section shows the expected low signal in the SSS (arrowhead) (D). MLVs show increased signal consistent (arrows) with rostral flow of the lymphatic fluid posterior to anterior, countercurrent to the venous flow in the SSS. Difference image generated by subtracting coronal TOF sequence with a saturation band posterior (C) from coronal TOF sequence with saturation bands both anterior and posterior (B) shows the expected flow-related enhancement in the SSS only (arrowhead) and not in the MLV (arrow) (E). Difference image generated by subtracting coronal TOF sequence with a saturation band anterior (D) from coronal TOF sequence with saturation bands both anterior and posterior (B) shows more clearly the flow-related enhancement in the MLV (arrow) and lack of enhancement in the SSS (arrowhead) (F).
In all subjects, the coronal TOF sequence with saturation bands both anterior and posterior to the image section showed the expected low signal in the SSS and lack of visualization of the MLVs (Figures 1B and 2B). This negative control was important to demonstrate that the saturation bands effectively nullified the signal from flow into the image section.
In all subjects, the coronal TOF sequence with a saturation band posterior to the image section showed the expected bright signal in the SSS form the influx of unsaturated venous blood flowing anterior to posterior (Figures 1C and 2C). The MLVs were not visualized, suggesting that the flow was in a direction opposite to the venous flow; however, very slow lymphatic flow might not bring in the adequate flux of unsaturated protons and thus could also produce a similar finding.
In all subjects, coronal TOF sequence with a saturation band anterior to the image section showed the expected low signal in the SSS (Figures 1D and 2D) owing to the saturation of the venous blood flowing anterior to posterior through the saturation band before entering the image section. Crucially, the MLV in all subjects showed definitively increased signal consistent with flow of lymphatic fluid posterior to anterior, opposite or countercurrent to the venous flow in the SSS (Figures 1D and 2D). The MLV did not all show the same level of increased signal. The greatest signal increase is expected from flow perfectly perpendicular to the image section; thus, the different levels of signal increase may be because of the different obliquities of the vessels in relation to the image section. Also, different flow velocities may contribute to the different levels of signal increase, as a slower flow could result in less enhancement.
Discussion
This study shows that lymphatic flow in the MLV runs countercurrent to venous flow in the SSS in the human brain. This result has implications for how we model the interactions among the various compartments of the CNS, such as the meningeal lymphatics, glymphatics, meninges, meningeal spaces, arteries, veins, perivascular spaces/adventitia, perineural spaces/nerve sheaths, brain parenchyma, and cerebrospinal fluid (1, 2, 5, 6). Layer upon that interactions outside of the CNS such as with the cribriform plate, nasal mucosa, systemic lymphatic system, and trafficking of cytokines and immune cells (1). Animal studies were principally used to develop these models, so imaging of the human CNS lymphatic system may shed new light on extrapolating these models to humans. For example, human models would have to compensate for the greater metabolic needs and waste concomitant with our larger brains.
The flow of these relatively large MLVs posteriorly to anteriorly (rostral) alongside the SSS strongly supports that a large proportion of the CNS lymphatic flow is directed to the cribriform plate. Animal data have shown that protein and T lymphocytes in the brain migrate from the CNS along the olfactory nerves, to beneath the nasal mucosa and into the cervical lymph nodes (7, 8). Our data support that in humans, this pathway is also a major route of lymphatic flow and trafficking of immune cells.
The countercurrent direction of flow of fluid in the MLVs relative to venous flow in the SSS may necessitate a rethinking of the interactions between the meningeal lymphatics, venous structures, and the glymphatics, which are thought to drain fluid along veins in the same direction as venous flow (1, 9, 10). While initially the flow, both concurrent and countercurrent to venous flow, may seem counterintuitive or impossible, the cellular-based (astrocytic) glymphatic system is very different from the meningeal lymphatics, which constitute a vasculature. Indeed, the perivascular spaces of cerebral vessels have been referred to as pseudolymphatics (1).
Countercurrent flow of the MLV in relation to the venous flow in the SSS provides to the complex compartmental model a new hypothetical component, which allows solutes, waste products, and pathologic proteins to be cleared from the CNS more rapidly through exchange to the dural venous sinuses that more promptly drain back into the systemic venous system (Figure 3). Fluid may also follow the solutes in this alternate clearance path. This more efficient path for the transfer of fluid and other substances may also act as an effective sump system and helps drive the hydraulic forces that drive the flow of fluid and solutes through the chain of compartments in the CNS. Perhaps even antigens and immune cells can traverse from the MLV countercurrent into the SSS and thus bypass the rostral pathway and its connection to the immunogenic, extracranial lymphatic system. In this way, the CNS glymphatic/lymphatic system may more functionally resemble the drainage of the systemic lymphatic system into the venous system, although anatomically opposite (possibly mandated by the unique systems in the CNS that the MLV must collaborate with). Countercurrent flow may be necessary to accomplish this more efficiently, as the MLVs do no directly connect to the venous system like the thoracic duct of the systemic lymphatic system. The mechanism of this hypothetical route of clearance would presumably require a novel interaction between lymphovascular and venovascular endothelium and adventitia.
Figure 3.
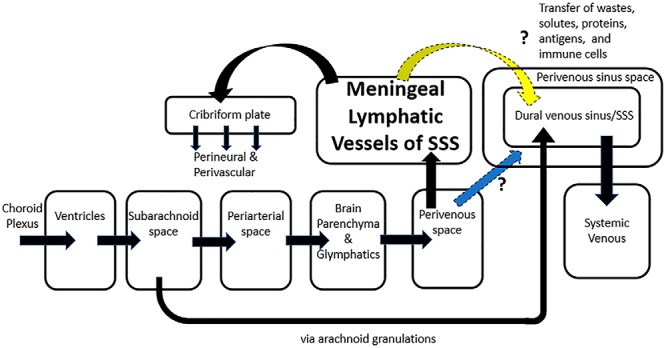
Hypothetical compartmental model incorporating countercurrent flow. Countercurrent flow of the MLV in relation to the venous flow in the SSS provides a new component to the compartmental model (yellow arrow), which hypothesizes the advantage of an additional path that solutes, waste products, pathologic proteins, antigens, and immune cells could be cleared from the CNS more rapidly through exchange from the MLV to the dural venous sinus. An alternative mechanism is that countercurrent, caudal flow of fluid is in the perivenous sinus space of the SSS (blue arrow) akin to the flow of fluid in the perivenous space from glymphatic drainage. Significant advantages potentially exist when perivenous flow has options of flow rostrally in the MLV and/or caudally along the perivenous sinus space in the direction of venous blood flow, as emphasized in this figure by arrows pointing in opposite directions for flow to the cribriform plate versus in the direction of flow of the dural venous sinus/SSS.
An alternate hypothesis using countercurrent flow is the potential advantage of flow in the perivenous sinus space (Figure 3) akin to the flow of fluid in the perivenous space from glymphatic drainage (1). This hypothesis may be more appealing in the context of current models, as the mechanism is an extension of the perivenous flow path. Perivenous flow would then have both options, to flow either rostrally in the MLV or caudally in the perivenous sinus following venous blood flow. Because the MLVs reside adjacent or in the perivenous sinus space, they are in an ideal location to potentially transfer substances with that compartment in an effective countercurrent exchange. The corollary is that the flow to the cribriform plate is either exclusively or almost exclusively from the MLV.
This clearance of substances from the MLVs both to the cribriform plate and countercurrent with the SSS (either directly into the venous flow or perivenous sinus space) may provide an important redundancy in case of damage or reduced flow to one path. In rodents, data support that sleeping in the lateral position is best for elimination of waste from the brain and least efficient with the head elevated (5, 11). This rodent model supports that the rate of waste clearance from the brain is affected by gravity. Because neither the venous sinuses nor their MLVs have valves, the bidirectionality of clearance (anteriorly/rostrally to the cribriform plate and posteriorly/caudally to the confluence of the sinuses) may be important to ensure clearance regardless of the head position. Therefore, this system of redundancy may be more developed in humans with our upright posture, and by inference, the clearance of fluids and substances along the spinal nerve sheaths may play a greater role in humans and primates than in quadruped mammals. A long-lived species like humans would perhaps require greater need for redundant systems that could handle clearing brain waste regardless of the head position, for example, sleeping in the supine position with rostral flow going against gravity. Notably, the MRI was acquired with the subject in supine position so that the fluid in the MLV alongside the SSS can continue to flow against gravity; further experiments are required to determine the extent of the effect of gravity on the flow rate.
Perhaps, a countercurrent pathway also became more developed in humans than in other mammals, as our brains are much larger in relation to our cribriform plate and other anatomy for the olfactory system. The shape of our skulls also became more convex to accommodate our larger brains, resulting in greater gravitational effects on drainage compared with the flatter skulls of other mammals.
For assessing the prospects of CNS lymphatics as a therapeutic target (12), the further confirmation in humans that the nasal mucosa is an important interface between the MLVs of the CNS and systemic lymphatic system has a myriad of potential implications such as immune modulation or enhanced clearance of wastes and pathologic proteins. Interesting questions arise such as potential effects on the CNS immune system of nasal delivery of drugs like the common nasal steroids or the effect of trauma or radiation to the brain and particularly the sinonasal region.
Interestingly, the results of this study may provide further insights into the physiological and immunological bases for the effectiveness of certain meditative and health practices as well as aspects of traditional Chinese medicine (13). For example, the cribriform plate co-locates with yintang, an acupuncture point used to treat neurological conditions (14). The emphasis on breathing in many health practices may connect consciously controlled respiration to the dynamic pulsatility of the CSF (15) and thus drive lymphatic flow in the CNS to clear wastes and pathologic proteins. Vascular pulsatility has also been shown to be critical for driving the paravascular influx of CSF through the brain parenchyma (16), and therefore, purposeful control of the cardiovascular system may also help to drive lymphatic flow.
Future MRI studies in humans can further develop the compartmental model, for example, by determining direction of flow in other MLVs, flow rates, and if flow is altered in various pathologic states or normal aging. Further optimization of the technique by varying TOF parameters may allow for visualization of smaller MLVs and thus build a whole brain map of directionality of flow. Dynamic studies with MRI could also help to elucidate the relationships among the glymphatics/lymphatics and the respiration and cardiovascular systems.
Acknowledgments
PHK would like to thank Dr. Marlys Witte, Dr. Shaojun Lu, and John Alton for the many thoughtful discussions related to this topic over the years, the volunteer subjects for giving their valuable time to this research, and Dr. Gregory Woodhead for his helpful input on the manuscript.
Disclosures: No disclosures to report.
Conflict of Interest: The authors have no conflict of interest to declare.
Footnotes
- MLVs
- Meningeal lymphatic vessels
- MRI
- magnetic resonance imaging
- CNS
- central nervous system
- CSF
- cerebrospinal fluid
- TOF
- time-of-flight
- SSS
- superior sagittal sinus
- FOV
- field of view
- TR
- repetition time
- TE
- echo time
References
- 1. Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210–3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–316. [DOI] [PubMed] [Google Scholar]
- 3. Absinta M, Ha S-K, Nair G, Sati P, Luciano NJ, Palisoc M, Louveau A, Zaghloul KA, Pittaluga S, Kipnis J, Reich DS. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. Elife. 2017;6:pii:e29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morita S, Masukawa A, Suzuki K, Hirata M, Kojima S, Ueno E. Unenhanced MR angiography: techniques and clinical applications in patients with chronic kidney disease. Radiographics. 2011;31:E13–E33. [DOI] [PubMed] [Google Scholar]
- 5. Benveniste H, Nedergaard M. Glymphatic System. Neuroscience in the 21st Century. 2nd ed Eds. Pfaff DW, Volkow ND. New York, NY: Springer; 2016:1945–1962. [Google Scholar]
- 6. Plog BA, Nedergaard M. The glymphatic system in central nervous system health and disease: past, present, and future. Annu Rev Pathol. 2018;13:379–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathology. 1992;2:269–276. [DOI] [PubMed] [Google Scholar]
- 8. Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797–801. [DOI] [PubMed] [Google Scholar]
- 9. Cserr HF, Cooper DN, Milhorat TH. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res. 1977;25:461–473. [DOI] [PubMed] [Google Scholar]
- 10. Cserr HF, Ostrach LH. Bulk flow of interstitial fluid after intracranial injection of blue dextran 2000. Exp Neurol. 1974;45:50–60. [DOI] [PubMed] [Google Scholar]
- 11. Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, Logan J, Nedergaard M, Benveniste H. The effect of body posture on brain glymphatic transport. J Neurosci. 2015;35:11034–11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sun BL, Wang LH, Yang T, Sun JY, Mao LL, Yang MF, Yuan H, Colvin RA, Yang XY. Lymphatic drainage system of the brain: A novel target for intervention of neurological diseases. 2018;163–164:118–143. [DOI] [PubMed] [Google Scholar]
- 13. Alton J, Rich T, Potter M, Hughes J, Eckman M. Autonomic Intelligence: Pathway to the Pulsatile Self and Sustainable Health. Crozet, VA: Pulsatile International Books; 2016;116–118. [Google Scholar]
- 14. Gliedt JA, Daniels CJ, Wuollet A. Narrative review of perioperative acupuncture for clinicians. J Acupunct Meridian Stud. 2015;8:264–269. [DOI] [PubMed] [Google Scholar]
- 15. Dreha-Kulaczewski S, Joseph AA, Merboldt K-D, Ludwig H-C, Gärtner J, Frahm J. Inspiration is the major regulator of human CSF flow. J Neurosci. 2015;35:2485–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, Deane R, Nedergaard M. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–18199. [DOI] [PMC free article] [PubMed] [Google Scholar]