Abstract
Background
The majority of patients with Parkinson's disease (PD) have handwriting abnormalities. Micrographia (abnormally small letter size) is the most commonly reported and easily detectable handwriting abnormality in patients with PD. However, micrographia is perhaps the tip of the iceberg representing the handwriting abnormalities in PD. Digitizing tablet technology, which has evolved over the last 2 decades, has made it possible to study the pressure and kinematic features of handwriting. This has resulted in a surge of studies investigating graphomotor impairment in patients with PD.
Methods
The objectives of this study were to review the evolution of the kinematic analysis of handwriting in PD and to provide an overview of handwriting abnormalities observed in PD along with future directions for research in this field. Articles for review were searched from the PubMed and SCOPUS databases.
Results
Digitizing tablet technologies have resulted in a shift of focus from the analysis of only letter size to the analysis of several kinematic features of handwriting. Studies based on the kinematic analysis of handwriting have revealed that patients with PD may have abnormalities in velocity, fluency, and acceleration in addition to micrographia. The recognition of abnormalities in several kinematic parameters of handwriting has given rise to the term PD dysgraphia. In addition, certain kinematic properties potentially may be helpful in distinguishing PD from other parkinsonian disorders.
Conclusion
The journey from micrographia to PD dysgraphia is indeed a paradigm shift. Further research is warranted to gain better insight into the graphomotor impairments in PD and their clinical implications.
Keywords: dysgraphia, handwriting, micrographia, Parkinson's disease
Parkinson's disease (PD) is a progressive neurodegenerative disorder characterized by cardinal motor symptoms, such as tremor at rest, bradykinesia, rigidity, and postural instability.1 The diagnosis of clinically probable PD in the early stages relies primarily on clinical assessment by a neurologist.2 When motor symptoms affect the dominant hand, patients may report worsening of handwriting as 1 of the initial symptoms.3, 4, 5 The presence of any of the cardinal motor symptoms may substantially hamper the quality of handwriting in patients with PD. Handwriting is a complex process requiring cognitive, perceptual, and fine motor abilities. Although not formally included in the diagnostic criteria of PD, handwriting impairment remains one of the initial signs that prompt the patients to consult a physician. McLennan et al. reported that micrographia (abnormally small letter size) may be observed in approximately 5% of patients with PD before the onset of motor symptoms, and 30% of those patients later report further worsening of handwriting.3
Alterations in the kinematics of handwriting are among the recently proposed biomarkers of PD. Recent research has suggested the potential of handwriting analysis for both early diagnosis and assessment of disease progression.6, 7, 8, 9 Assessing the improvement of motor symptoms in response to dopaminergic medications is crucial for formulating the differential diagnoses and monitoring the progress of the disease. Although an improvement in motor symptoms with dopaminergic medication is 1 of the hallmarks of PD, the significance of an improvement in fine motor activities with such medication is not clear. However, recent advances in digitizing tablet technology have been revolutionary in studying several components of handwriting. These technologies enable the precise study of velocity and fluency of handwriting in addition to quantification of the size of the letters, which is not possible to study objectively using the paper‐and‐pen method. Objective study of several parameters related to handwriting may provide more insights into the involvement of fine motor performance in PD.
Several studies have utilized digitizing tablet technology to explore the kinematics of handwriting in PD. The objectives of this review are: (1) to provide an overview of the methods and technology used today in handwriting analysis research, (2) to review studies focused on analyses of handwriting in patients with PD using digitizing tablet technology, (3) to discuss the characteristics of handwriting studied and justify the shift of focus from the term micrographia to “PD dysgraphia,” and (4) to explore additional developments and new perspectives on graphomotor impairment in PD.
Methodology
We searched for published literature in the PubMed and Scopus databases that focused on handwriting kinematic analysis using digitizing tablets in PD. A broad search strategy was applied by using several key words and combinations (see Table S1). Many studies that were not relevant to the current article were excluded after screening the titles, abstracts, or full texts of articles obtained from the 2 databases. Studies were considered for review if: (1) they were either original or review articles, (2) the full text was available in English, and (3) digitizing tablet technology was used to study handwriting size, pressure, or kinematics in patients with PD. The details of the steps of database search are provided in a flow chart in Figure S1.
We aimed to incorporate a wide variety of studies related to handwriting analysis in patients with PD, ranging from reviews on micrographia to kinematic handwriting analyses for diagnostic and therapeutic purposes, and re‐learning of handwriting. Because of the heterogeneity of the articles selected, we organized the current review into 3 broad sections: the first discusses the advent of digitizing tablets and handwriting analysis software systems, the second examines the spectrum of handwriting abnormalities in PD, and the third explores the new areas of research in PD for which handwriting analysis has been employed.
Handwriting Analysis: Journey from Paper‐and‐Pen to Digitizing Tablets
Ever since difficulties with handwriting were first observed by James Parkinson in patients with the “shaking palsy,” described as “the hand failing to answer with exactness to the dictates of the will,” handwriting has been subject to examination using the pen‐and‐paper method. McLennan et al.,3 in 1 of the earliest published articles studying micrographia, collected handwriting samples from selected patients who had significant, observable micrographia. The selected patients were required to submit handwriting samples from the periods before the onset of PD and during disease progression. The most common sources reportedly were serial signatures from cancelled checks. McLennan et al. remarked that the “nature of data did not lend itself readily to quantification,” a drawback that now can be overcome. Sandyk and Iacono published a case study of a patient with PD and included hand‐drawn samples by the patient before and after treatment with magnetic fields.10 A classic example of micrographia recorded using the paper‐and‐pen method is provided in Figure S2 (authors’ personal collection).
Even after the development of digitizing tablets, paper‐and‐pen methods have been used. Ondo and Satija, instructed patients to write a simple sentence on paper with their eyes open and closed to study the effects of visual feedback on micrographia in both the on‐drug (on) and off‐drug (off) states. Balas et al. also included handwritten samples before and after stereotactic surgery in patients with PD.11
Introduction of the Digitizing Tablets
Researchers today primarily rely on digitizing tablet technology to study handwriting in patients with PD. The collection of handwriting samples using digitizing tablets is easy and noninvasive. A tablet connected to a computer is placed in front of the individual performing the task, who is seated comfortably, and the task is performed with a stylus on the tablet.
Possible limitations of using digitizing tablets to study handwriting include differences in virtually frictionless surface of the tablet. This is overcome by keeping a piece of paper on top of the surface of the tablet to simulate the physical conditions attained while writing on paper. Another drawback could be the difference in the nature of the stylus used to write compared with the pen. However, recently developed technologies are able to amply mimic the “feel” of writing with a pen. Digital inking pens, which incorporate sensors, are also in the process of being tested for research. Such pens record pen movement when written on any piece of paper and are more affordable, because the tablet is no longer required. However, the sensorized digital inking pens are limited in spatial and temporal resolution and do not have the ability to detect pen pressure. Hence, currently, digitizing tablets are inarguably the best tools for handwriting research.
Software Used for Handwriting Analysis
In addition to the x/y‐coordinates of the pen position while writing, the pressure from the pen acting orthogonal to the digitizing tablet surface can also recorded. Digitizing tablets used today for research are dominated by WACOM tablets (Wacom Technology Corporation, Portland, OR). The WACOM Intuos Pro tablets, which offer the highest spatial and temporal resolutions among its competitors, have been widely used in handwriting movement analysis. Digitizers initially had sampling rates of 100 Hz; however, today, a sampling rate of 133 to 200 Hz helps in capturing the signal components with higher frequencies, thus enabling the analysis of a wider spectrum.
Various software systems are then used to calculate several kinematic features of handwriting. The first known software used was CSWin, which was developed by Marquardt and May in Germany. Other programs include MEDDRAW (by a joint project between the University of Kent, UK and the University of Rouen, France). It was developed with the aim of producing a computer‐based assessment of hand‐drawing tasks to assist in the diagnosis and assessment of several neuropsychological conditions and to ensure the adoption of effective rehabilitation techniques. Pullman12 extracted the coordinates of hand‐drawn spirals collected through a digitizing tablet and calculated various spiral indices, which included first‐order smoothness, first‐order zero crossing, tightness of the spiral, and degree of severity.
Teulings and Stelmach of NeuroScript developed the MovAlyzeR, a widely used handwriting analysis software to visualize the data and calculate of various kinematic qualities of handwriting (NeuroScript Software, Tempe, AZ). The most recent version of the MovAlyzeR allows the building of norm databases, including subject‐specific averages and overall averages. MovAlyzeR has been used in the fields of neurology, kinesiology, forensic, and psychiatry.13, 14, 15, 16, 17
At the University of Haifa in Israel, Rosenblum and Weiss have developed the Computerized Penmanship Evaluation Tool, previously known as the Penmanship Objective Evaluation Tool using MATLAB (MathWorks, Natick, MA). Notable research has been conducted using the Computerized Penmanship Evaluation Tool in PD, Alzheimer's disease, and developmental coordination disorder.7, 18, 19
Spectrum of Handwriting Abnormalities in PD
Micrographia
Studies have highlighted the potential of micrographia as a presymptomatic neurobehavioral biomarker of PD.3, 20, 21 Micrographia may be defined as “an obvious reduction in size of the letters of the writer in comparison to the calligraphy before the development of the organic lesion effecting the change” (Wilson, 1925).22 A reduction in letter size is fairly simple to detect with paper‐and‐pen tools. Although several studies have reported the presence of micrographia in PD,21, 23, 24, 25, 26 the exact prevalence of micrographia in PD is not clear and may range from 9% to 60% of patients with PD.27, 28, 29
Micrographia may be classified into 2 categories: (1) consistent micrographia (an overall reduction in letter size compared with that before the onset of PD) and (2) progressive micrographia (decreasing letter size as writing progresses).30, 31 Both types of micrographia are presumed to have different neural correlates. Wu et al.30 used functional magnetic resonance imaging to study neural correlates of micrographia. Those authors reported that consistent micrographia is related to dysfunction of the basal ganglia circuits, possibly because of dopamine depletion; whereas progressive micrographia, which is a sequential movement, may be caused by disconnections between the anterior supplementary motor area, the rostral cingulate motor area, and the cerebellum. The 2 categories of micrographia also have differential responses to dopaminergic medications. Although dopaminergic medication may result in a significant improvement in consistent micrographia by restoring the function of the cortical‐basal ganglia‐thalamocortical motor circuit, there is no significant improvement in progressive micrographia. A potential explanation for this is that dopaminergic drugs have a negligible effect on the connectivity of the anterior supplementary motor area, the rostral cingulate motor area, and the cerebellum, which may be altered in progressive micrographia.30 However, the major disadvantage of the study, as acknowledged by the authors, is the coexistence of consistent and progressive micrographia in varying degrees.
Handwriting requires both finger and wrist movements.32 A straight line at an angle 90 degrees from the baseline is a purely vertical stroke, and a straight line at an angle of 0 or 180 degrees from the baseline is a purely horizontal stroke. Ma et al. examined Chinese handwriting in a cohort of patients with PD and reported the presence of micrographia only while patients were writing in the horizontal direction. Because extension of the wrist is required when writing in a horizontal direction, those authors suggested that wrist extension stiffness may have a role in progressive micrographia.26, 33 Progressive micrographia was caused by a reduction in the size of the horizontal stroke, rather than the vertical stroke. Smits et al. also reported similar observations, indicating that the width of the letter “e” in the “elel” task was significantly smaller in patients who had PD compared with controls.34 Hence a shortening of the width of letters in patients with PD, or “horizontal micrographia,” merits further investigation. It has been postulated that horizontal micrographia may also have subtypes: consistent and progressive. This is a new concept and has not yet been investigated. Future studies need to address the subtypes of horizontal micrographia specific to PD. Horizontal micrographia, like vertical micrographia, may also provide additional information in differentiating idiopathic PD from other parkinsonian syndromes. Figure 1 (authors’ personal collection) illustrates consistent horizontal micrographia in a patient with PD who improved with levodopa (l‐dopa) (vertical size was also reduced in the above sample; however, the change in horizontal size in response to l‐dopa was more prominent).
Figure 1.
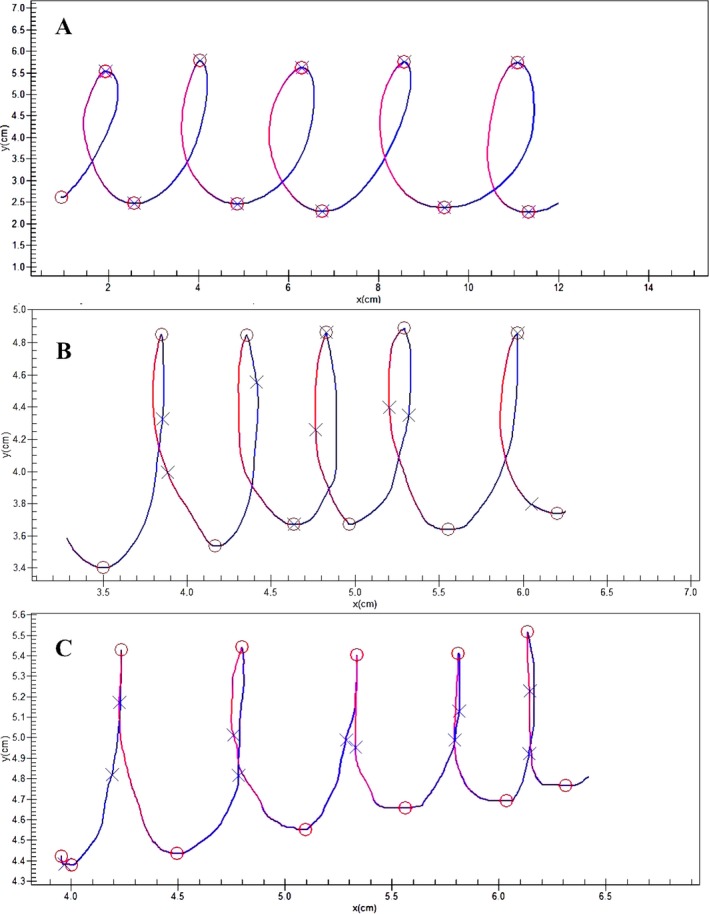
A: Handwriting sample from an age‐matched, healthy control. B:) Handwriting sample from a patient during best on‐drug state showing visible improvement of horizontal micrographia. C:) Handwriting sample from a patient with Parkinson's disease during drug‐off state showing consistent, horizontal micrographia (from the authors’ unpublished collection).
Several intrinsic and extrinsic factors have been associated with (vertical) micrographia in PD. These include disease severity, cognitive impairment, PD phenotype, increased cognitive demands/dual tasking, and the presence of visual feedback. In a study of 43 patients with PD, Wagle Shukla et al.27 reported a significant correlation between micrographia with measures of PD stage and severity of motor symptoms. They also reported that a subgroup of 12 patients who had severe micrographia (defined as the demonstration of a reduction in writing surface area >50% on a writing task) showed even higher correlations with the aforementioned measures of disease severity and cognitive impairment.
Characteristics of handwriting may vary across PD phenotypes. Bajaj et al., in a comparison of the frequency of micrographia between 2 PD phenotypes (tremor‐dominant and akinetic‐rigid), reported a higher prevalence of micrographia in the akinetic‐rigid phenotype.35 In the same study, micrographia reportedly to had 90% sensitivity and 55% specificity for distinguishing the akinetic‐rigid phenotype from the tremor‐dominant phenotype.
Teulings et al. used visual distortion to study the dependence of patients with PD on visual feedback while writing. Using display digitizers, the trace of the pen was visible underneath the pen tip as the participant wrote on the screen of the digitizer (i.e., visual feedback was provided). In that study, although participants performed the writing task at the required size, the visual feedback of the writing provided was either enlarged in size (to 140%) or diminished in size (to 70%).25 The authors observed that young, healthy controls adapted to the size of the letters appropriately, according to the visual distortion; whereas the older controls did not make much use of visual feedback. Conversely, patients with PD amplified the distortion.25 This suggests that patients with PD constantly rely on the visual trace feedback while writing. The importance of visual feedback was further reinforced in a study by Ondo and Satija. Those authors reported that, upon the removal of visual feedback (by asking participants to close their eyes), there was significant improvement in micrographia in patients with PD who were in the drug‐off state. However, the same result could not be replicated in patients during the best drug‐on state.36 Results from that study suggest a possible role of dopaminergic medications in reducing dependency on visual feedback.
Van Gemmert et al. reported that patients with PD significantly reduced their stroke size when the processing demands were increased (when a longer second phrase was required to be planned and written while executing the first word).26 Broeder et al. reported that, although no significant difference in size was observed between patients with PD and controls when they performed single tasks, the addition of another cognitive task (dual‐task condition), in which participants were asked to count the number of high‐pitched and low‐pitched tones, resulted in a significant reduction in writing amplitude.37 These results highlight the effect of dual tasks on writing size. However, additional variables, such as movement time and normalized jerk of writing, may also be affected by dual tasking.26
Velocity and Acceleration
Velocity and acceleration are considered to be the main kinematic features of handwriting. Velocity is defined as the rate of change of position with time, whereas acceleration is the rate of change of velocity with time. Absolute velocity and absolute acceleration take the velocities and accelerations of both x and y axes into consideration and thus are the preferred measures to assess velocity and acceleration.
Jerk
Jerk is the third derivative of position with respect to time. Like velocity and acceleration, jerk can be calculated for all 3 axes. Hence absolute or normalized jerk is commonly used. Although jerk has been regarded as a kinematic feature, it can also be a measure of fluency, because the value of jerk is sensitive to subtle changes of acceleration that affect the smoothness of writing.
Fluency
Fluency can be defined as the ease with which the task of writing is performed. Several measures, such as “number of peak velocity/acceleration points,” “submovement analysis,” and “ratio of deceleration phase” (RDP), have been proposed to assess fluency. The number of peak velocity/acceleration points has also been defined in several studies as the number of inversions in velocity (NIV) and acceleration (NIA). The number of local maxima in the velocity or acceleration profiles are calculated. Ideally, the velocity profile of a fluent, curved upstroke would grow to a local maximum and then decay to a local minimum (Fig. 2A). The following downstroke continues from the local minima, proceeds to another local maximum, and drops again. The process repeats itself. The ideal number of maxima for a single upstroke is 1; and, the greater the number of extrema, the more dysfluent the writing.
Figure 2.
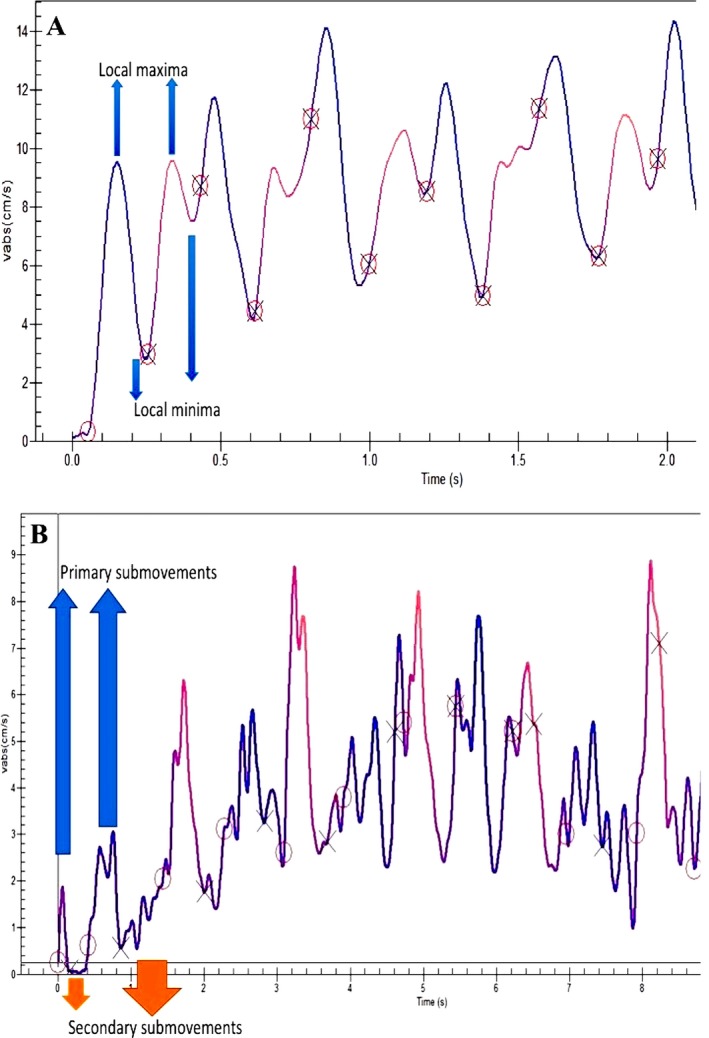
A: Absolute velocity‐versus‐time graph from a healthy control. Local maxima and local minima are located. B: Absolute velocity‐versus‐time graph from a patient demonstrating primary and secondary submovements. In the graph, (O) indicates the start of the primary submovement, and (X) indicates the start of the secondary submovement (from the authors’ unpublished collection).
Submovement analysis is a technique for studying fluency and was first described in with MovAlyzeR (NeuroScript LLC, Tempe, AZ). Submovement analysis, according to the MovAlyzeR, parses each stroke in to 3 segments: the primary and secondary submovements and the total stroke (= the sum of the primary and secondary submovements).
Primary submovement
The initial ballistic segment of a stroke that is under feed‐forward control is the primary submovement and is considered from movement onset to the first negative‐to‐positive point (or second zero‐crossing) of the acceleration profile after peak velocity. All movements have a primary submovement.
Secondary submovement
The corrective, or homing‐in, segment of a movement (or stroke) that is under feed‐back control is the secondary submovement, which begins at the end of the primary submovement and ends at movement offset (Fig. 2B). The frequency, relative size, and relative duration of the secondary submovement to the total submovement is an indicator of fluency of the writing. The greater the frequency of the secondary submovement, the less fluent the writing.
The RDP is another measure of fluency recently proposed by Yu et al38 and is calculated as ([(total movement duration − duration till peak velocity of the movement)/total movement duration] × 100%). The greater the ratio above 50%, the less efficient the movement control.
The Concept of PD Dysgraphia
Micrographia has been regarded as the cardinal handwriting abnormality in PD. However, summarizing the studies conducted over the last 2 decades, Letanneux et al. concluded that handwriting abnormality in PD is not limited to micrographia, and the spectrum of abnormalities includes several dynamic and kinematic features of duration, velocity, and fluency.39 In fact, these variables are more effective than micrographia in differentiating between controls and patients with PD and between drug‐off and best‐on states in patients with PD.9, 39, 40, 41, 42, 43, 44, 45 Although letter size remains sensitive in discriminating healthy controls from patients with PD, it may not distinguish patients with PD in the drug‐off state from the best drug‐on state, because letter size improves in only 50% of patients in response to medications. However, other kinematic factors usually improve and reportedly are very sensitive to medications.39 Figure 3 illustrates the improvement of change in the absolute velocity of handwriting during the drug‐on state in a patient with PD. In another example, Poluha et al. observed that, although l‐dopa significantly improved the duration of the upstroke of handwriting, it had little effect on the upstroke size.42 Siebner et al. reported the positive effects of high‐frequency substantia nigra stimulation on the speed and smoothness of handwriting along with an increase in the mean vertical stroke length.45 However, most reports fail to state clearly whether the micrographia being studied in response to medication is progressive, consistent, or both. Because progressive micrographia may not improve with medication and consistent micrographia may, in addition to their coincident nature,30 it seems possible that the total effect of medication on micrographia is limited compared with kinematic variables. Researchers need to clearly state whether progressive, consistent, or both are being considered.
Figure 3.
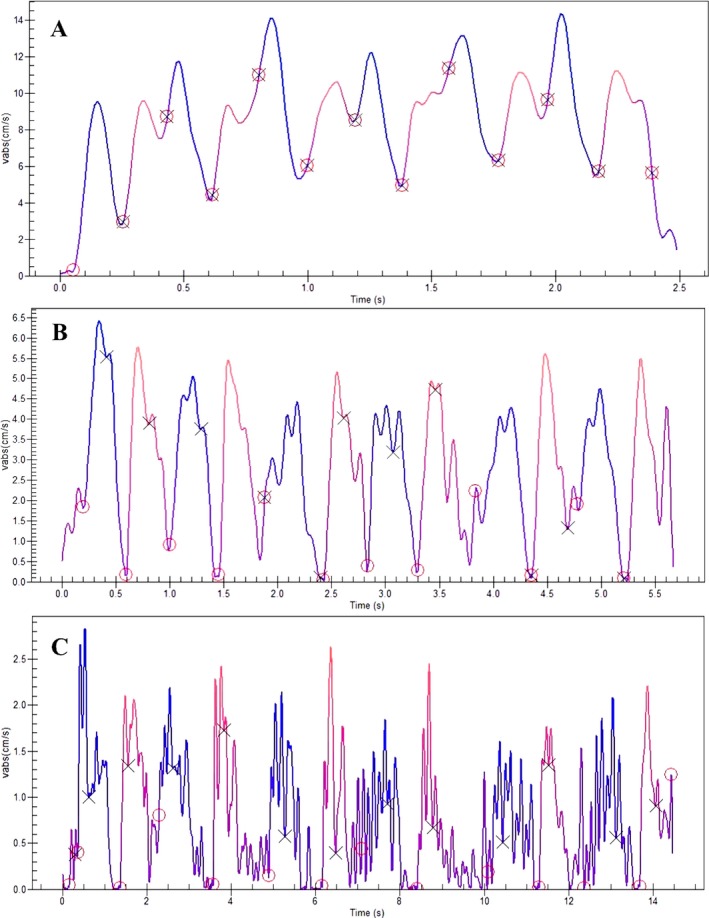
A: Absolute velocity‐versus‐time graph from a healthy control (corresponding to the handwriting sample in Fig. 1A) clearly shows fewer peaks and higher absolute velocity compared with patients who had Parkinson's disease (PD) during both drug‐off and drug‐on states. B: Absolute velocity‐versus‐time graph from a patient with PD during best drug‐on state (corresponding to the handwriting sample in Fig. 1B) shows a significant reduction in the number of peaks in velocity, suggesting a visible improvement in handwriting fluency. C: Absolute velocity‐versus‐time graph from a patient with PD during drug‐off state (corresponding to the handwriting sample in Fig. 1C) shows several peaks at various points, indicating a larger value of the number of inversions of velocity, hence suggesting dysfluent movement (from the authors’ unpublished collection).
Considering the importance of several parameters of handwriting in addition to the size of the letters, Letanneux et al. proposed using the term PD dysgraphia, encompassing 4 variables (duration, velocity, and fluency in addition to size), to study graphomotor impairment in PD.39 The term “PD dysgraphia” is used to convey the meaning, “the inability to perform fluent graphomotor movements,” and should be clearly differentiated from the common use of the term in developmental disorders, in which motor and nonmotor aspects are not clearly distinguished from “agraphia,” which is related to aphasia—difficulties in language processing caused by injury to specific brain areas. Figure 4 depicts the spectrum of handwriting abnormalities in patients with PD.
Figure 4.
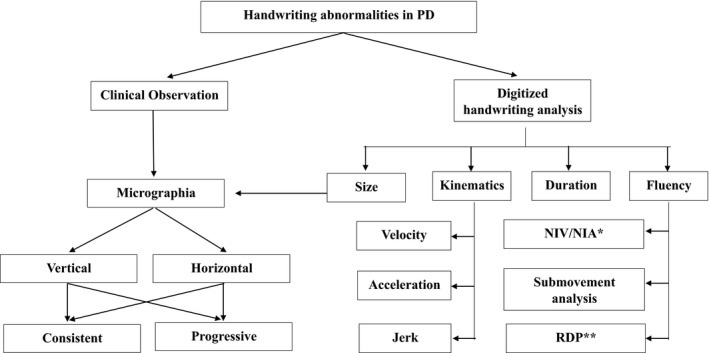
Schematic presentation of a spectrum of handwriting abnormalities in patients with Parkinson's disease (PD). Note that certain parameters of handwriting, such as velocity and acceleration, are dependent on others, such as amplitude and size, respectively. Therefore, although the figure depicts the spectrum of handwriting abnormalities in PD, they may not be independent, as indicated in the figure. NIV indicates number of inversions of velocity; NIA, inversions of acceleration; RDP, ratio of deceleration phase.
Newly Proposed Additional Parameters of Handwriting
In‐air movements remain an interesting field of investigation, because the pen must be lifted from the surface of the paper and placed again at another position to continue the writing task. With slowness in initiating movements, it can be assumed that the in‐air time in patients with PD would be significantly longer than that in controls. Drotar et al.46, 47, 48 and Rosenblum et al.7 have emphasized the importance of in‐air movements and pressure in the handwriting of patients with PD. In addition to requiring more performance time and writing in a smaller size, patients with PD also reportedly applied significantly less pressure on the writing surface than controls. Interestingly, the difference between groups in stroke duration in the air was greater than the difference in stroke duration on paper. Drotar et al. confirmed this by carefully distinguishing between on‐surface movement, in‐air movement, and pressure and examined their relative contributions in distinguishing patients with PD from healthy controls. To classify samples as PD or controls, Drotar et al. used a supervised machine‐learning algorithm support vector machine with a nonlinear radial basis function kernel.46 Using basic kinematics and pressure features, an accuracy of approximately 90% may be achieved.47 However, the question remains whether the air‐stroke can be studied the same way as the on‐surface stroke. It seems probable that the kinematic features of the in‐air stroke, such as velocity, acceleration, and jerk, would differ in patients with PD compared with healthy controls.
Certainly, the additional parameters of kinematics, such as duration (both on paper and in air), pressure, and fluency, need to be regularly included in future studies investigating graphomotor impairment in PD. Table 1 summarizes major studies based on kinematic analysis of variables that contribute to dysgraphia in PD.
Table 1.
Summary of studies on additional modalities of handwriting contributing to Parkinson's disease dysgraphia
| Reference | Participants | Handwriting task | Comparisons | Modalities | Conclusion |
|---|---|---|---|---|---|
| Poluha et al., 199842 | PD, n = 10 | Sequence “le” and “hell” | On vs. off | Upstroke duration; upstroke size | There was a significant reduction in upstroke duration during on state, whereas upstroke size did not change significantly |
| Tucha et al., 20069 | PD, n = 27; HC, n = 27 | Letter combination “ll”; off vs. on | PD vs. HC; off vs. on | Distance of writing trace, movement time, fluency, (NIV/NIA), maximum velocity, and acceleration | Compared with the HC group, patients who had PD had shorter writing trace, longer movement time, less fluency, and lower maximum velocity and acceleration; these parameters (except distance of writing trace) improved significantly with medication |
| Lange et al., 200643 | PD, n = 12; HC, n = 12 | “helles,” “grelles” | PD vs. HC | Movement distance, movement time, fluency (NIV/NIA) maximum velocity, and acceleration | Compared with the HC group, patients who had PD had shorter movement distance, longer movement time, less fluency, and lower maximum velocity and acceleration; these parameters (except movement distance) improved significantly with medication |
| Broderick et al. 200963 | PD, n = 20; EHC, n = 16; YHC, n = 16 | “^” shape with vertex angle of 40 degrees | EHC vs. PD; YHC vs. PD | Mean acceleration, peak acceleration, mean velocity, normalized jerk | In both age groups, compared with HCs, patients who had PD had significantly lower mean acceleration, peak acceleration, and mean velocity and higher normalized jerk |
| Rosenblum et al., 20137 | PD, n = 20; HC, n = 20 | Full name, address | PD vs. HC | Mean stroke duration (on paper and in air), stroke width, velocity, and pressure | Compared with the HC group, patients who had PD had significantly longer mean stroke duration, both on paper and in air (more pronounced in air); the PD group also had shorter width, lesser velocity, and applied less pressure on paper while writing |
| Yu et al., 201638 | PD, n = 12; ET, n = 13; HC, n = 21 | (1) Crosses, 50 mm2; (2) 20‐mm‐tall cursive “ill”; (3) single circle with 20‐mm diameter; (4) 10 overlapping circles | Mean velocity, progressive vertical and horizontal size | Patients who had PD had lower velocity compared with HCs D in most tasks; the PD group had a significant reduction in progressive vertical size, and the ET group had an increase in progressive horizontal size |
Abbreviations: PD: Parkinson's disease; on, on medication; off, off medication, HC, healthy controls; NIV, number of inversions in velocity; NIA, number of inversions in acceleration; EHC, elderly healthy controls; YHC, young health controls; ET, essential tremor.
There was only 1 trial9 in which investigators attempted to study the parameters of handwriting that were more significant in discriminating patients from controls (controls vs on state, controls vs off, on vs off). For the letter combination “ll,” parameters indicating fluency (the number of inversions of velocity and acceleration) were reported, with a higher effect size for all 3 group comparisons. However, for the analysis of individual ascending and descending strokes, the movement time was reported with the highest effect size for all comparisons followed by parameters of velocity. Distance of the writing trace (size) had a comparatively low effect size.
Clinical Implications
The clinical features of PD may overlap with several other diseases, such as essential tremor (ET) and atypical parkinsonian disorders like progressive supranuclear palsy (PSP), multiple system atrophy, and early stage corticobasal degeneration. Hence kinematic analysis of handwriting possibly may be useful in differentiating the aforementioned disorders. Yu et al. reported significant differences in handwriting kinematics between patients with PD and ET.38 Patients who had PD displayed a significant progressive decrease of vertical size of cursive “l”‐loops and a marginally significant progressive decrease in horizontal size (P = 0.077) compared with patients who had ET. In contrast, patients in the ET group had significantly increased horizontal size of the cursive loops as the writing progressed. The authors also observed an increase in vertical size of cursive loops in patients with ET that tended toward significance (P = 0.06). An additional variable, RDP, was studied in the 3 groups. The authors reported that patients with PD had significantly larger RDP than those with ET and controls.38
Ling et al. reported that micrographia was more common in patients with PSP (75%) than in those with idiopathic PD (15%).49 The presence of progressive micrographia can help to differentiate idiopathic PD from Parkinson‐plus disorders. Progressive micrographia is more characteristic of PD, whereas a lack of decrement is more commonly observed in Parkinson‐plus syndromes.28, 31, 49 This information may prove to be crucial in differentiating PD from atypical parkinsonism during early stages of the disease.
Digitized handwriting analysis permits quantification of the improvement in performance in response to medication, which is essential in a clinical setting. Poluha et al.42 used digitized handwriting analysis to study the handwriting changes along the l‐dopa cycle in order to determine the ideal time and dosage of the drug. This has immense potential in clinical practice and needs to be further investigated.
In addition to the relatively small sample size, the other important limitation of most studies is the lack of consideration given to motor practice and verbal intelligence, because these variables reportedly have a significant effect on handwriting.50 It is also important to take these variables into consideration, along with age, sex, and education level, when recruiting healthy controls for comparison. Although no studies, to our knowledge, have studied the effect of PD laterality on handwriting, it also may be important to take this into consideration. Another limitation arises from the fact that, in several studies, the type of pen used (inking or non‐inking) was not specified. Having discussed the effects of visual feedback on handwriting, the type of pen used may provide relevant information to interpret findings.
Recent Developments
Freezing of the upper limb
Although freezing of gait (FOG) is a debilitating symptom of PD, freezing of the upper limb (FOUL) or “upper limb motor block”51 has rarely been the subject of scientific investigation. FOUL is characterized by a significant reduction of amplitude and erratic frequency followed by inefficient movement, rather than a complete termination of movement.52, 53 FOUL appears to be correlated with FOG.52, 54 Nackaerts et al. confirmed that making small, bimanual movements may induce FOUL. They also reported that FOUL episodes may be triggered during the gradual scaling down of writing size.54 Because FOUL may result in significant worsening of handwriting, it would be interesting to explore whether patients can overcome FOUL by using certain visual or auditory cues, which usually help patients with FOG. Further studies are warranted to explore the association of FOG and FOUL. FOUL can affect the kinematic variables of handwriting, because the erratic movement that follows the freezing would most likely result in a higher value for jerk and inconsistencies in acceleration.
Rehabilitation
Graphomotor impairment in PD can only be alleviated in part by conventional treatment strategies. Thus, re‐learning and rehabilitation of graphomotor skills in patients with PD have recently become an area of active research. Doyon et al.55, 56 described 2 types of motor learning: (1) motor sequence learning (MSL) and (2) motor adaptation (MA). MSL is the process of learning and performance of the movement as a well‐articulated behavior through practice. The striatum, along with the motor cortices, plays a crucial role in MSL. The corticocerebellar systems are also activated during the initial learning process, but their activity decreases as performance approaches automatization.56 MA is the process of adaptation of learned movements to environmental influences. It is well recognized that the cerebellar system plays a decisive role in MA.56
Cueing is the use of external stimuli to aid the commencement and maintenance of movement.57 Although the effects of cueing on gait have been studied extensively, its effects on writing remain elusive.58 Among the different types of cueing, auditory and verbal cues were found to benefit the patients, whereas the effect of visual cues are still debatable.58 Nackaerts et al. explored the effects of visual cueing in 15 patients with PD and 15 controls by providing target lines at amplitudes of 0.6 cm and 1.0 cm as the participants performed sequential loops. The results indicated that, although the cues at 1.0‐cm amplitude showed improvements in size, variability, and speed in both groups, the cues at 0.6‐cm amplitude seemed to result in deterioration of writing performance (i.e., decreased size of letters in both groups and reduced speed of writing in the controls).59 The authors suggested that, in a neurorehabilitatory setting, the size of the fine motor task should be taken into consideration before visual cues are provided. For tasks requiring sizes of greater than 1.0 cm, visual cues may be beneficial; whereas, for tasks requiring smaller amplitudes, visual cues may result in further deterioration of handwriting.
Doyon et al. emphasized the importance of the corticostriatal system in the encoding and retention of learned movements in MSL.56 Therefore, a dysfunction of the striatal system would be associated with difficulty in learning and retaining learned movements. However, Nackaerts et al. reported that intensive amplitude training with the aid of visual target zones can result in consolidation of writing skills (including automatization, transfer, and retention) and improvement of micrographia.58 Those authors proposed that intensive training of writing skills should be used as a part of the neurorehabilitation for PD.
As described above, several studies have reported an association of FOG with handwriting abnormalities, possibly as a result of FOUL. Hence, in addition to providing appropriate gait and balance training, patients may also be advised to undergo handwriting training. However, the pattern of training should be different for patients with and without FOG. In a study comparing motor learning in PD patients with and without FOG, Heremans et al. reported that, although significant short‐term improvements in amplitude and reduced variability were observed in patients who had PD with and without FOG, retention effects on writing amplitude was observed only in those without FOG.60 This reinforces the requirement of alternative methods of handwriting training to benefit patients who have PD with FOG. Heremans et al. also reported that the withdrawal of cues resulted in a significant deterioration of handwriting performance.
Unexplored areas
Areas that still remain elusive include the study of motor memory in patients with PD. Considering the function of the basal ganglia in motor learning, it can be postulated that patients with PD would have trouble in learning new fine motor tasks. The fluency of performance on the handwriting tasks after a new fine motor sequence has been taught can be assessed, and the time taken to master the new sequence can be determined. The effects of consolidation with sleep can also be tested. These may have implications for the methodology used in the relearning of handwriting. A major complaint of patients with PD is their inability to sign legibly. For all practical purposes, further research on handwriting rehabilitation should focus on improving the signature.
The direct effects of visual and auditory cueing can also be studied using digitizing tablets. Recent evidence suggests the positive effects of auditory cueing and mixed effects of visual feedback on handwriting performance, as discussed above. Fluency of a handwriting task with and without the provision of feedback can be helpful in determining the extent to which the task is useful. Further research can also study correlations between handwriting parameters, the Hoehn and Yahr scale, and subsections of the Unified Parkinson's Disease Rating Scale to establish the use of handwriting analysis as a tool in clinical assessment.
Digitized handwriting analysis can also be used to objectively study various cognitive deficits, such as attention61 and visuospatial deficits.62 By manipulating the dictation of the instructions (oral or written), motor memory deficits can also be subject to study. Because patients with PD may present with cognitive symptoms, investigation into this area is novel and may open up possibilities for future research on such nonmotor symptoms of PD.
Conclusions and Perspectives
Research in the field of handwriting in PD has undergone tremendous transformation since the introduction of kinematic analysis by application of digitizing tablets. Micrographia, which has been the characteristic handwriting abnormality in PD, is now considered as a part of the “PD dysgraphia” spectrum. Because studies based on kinematic analyses of handwriting have reported specific abnormalities in different PD phenotypes and in several PD mimics, such as ET and PSP, these may be used as clinical markers during the early stages of disease. Because PD is sensitive to medications, handwriting may also be a reliable marker of disease progression. More studies are warranted in patients with other atypical parkinsonism, because the graphomotor impairment in disorders such as multiple system atrophy and corticobasal degeneration have not been systematically studied. Future research should also focus on the concept of FOUL considering its association with FOG in patients with PD. Rehabilitation and relearning of graphomotor skills remain subjects of active investigation, and further studies are required to gain better insight into the natural course of graphomotor impairment in patients with PD.
Author Roles
1. Research Project: A. Conception, B. Organization, C. Execution; 2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique; 3. Manuscript Preparation: A. Writing the First Draft, B. Review and Critique.
M.T.: 1A, 1B, 3A, 3B
A.L.: 1A, 1B, 3A, 3B
P.K.P.: 1A, 1B, 3A, 3B
Disclosures
Ethical Compliance Statement: We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this work is consistent with those guidelines.
Funding Sources and Conflicts of Interest: The authors report no sources of funding and no conflicts of interest.
Financial Disclosures for the previous 12 months: The authors report no sources of funding and no conflicts of interest.
Supporting information
Table S1. Results of PubMed and Scopus Database Search with Various Keywords and Combinations
Figure S1. Schematic flow of search strategy adopted for papers screened using the keywords.
Figure S2. A case of coexistent, consistent, and progressive micrographia collected using paper and pen (from the authors’ unpublished collection).
Relevant disclosures and conflicts of interest are listed at the end of this article.
Supporting information may be found in the online version of this article.
References
- 1. Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79:368–376. [DOI] [PubMed] [Google Scholar]
- 2. Gaenslen A, Berg D. Early diagnosis of Parkinson's disease. Int Rev Neurobiol 2010;90(C):81–92. 10.1016/S0074-7742(10)90006-8. [DOI] [PubMed] [Google Scholar]
- 3. McLennan JE, Nakano K, Tyler HR, Schwab RS. Micrographia in Parkinson's disease. J Neurol Sci 1972;15:141–152. [DOI] [PubMed] [Google Scholar]
- 4. Becker G, Muller A, Braune S, et al. Early diagnosis of Parkinson's disease. J Neurol 2002;249(Suppl 3):III40–III48. [DOI] [PubMed] [Google Scholar]
- 5. Ponsen MM, Daffertshofer A, Wolters EC, Beek PJ, Berendse HW. Impairment of complex upper limb motor function in de novo Parkinson's disease. Park Relat Disord 2008;14:199–204. [DOI] [PubMed] [Google Scholar]
- 6. Phillips JG, Stelmach GE, Teasdale N. What can indices of handwriting quality tell us about Parkinsonian handwriting? Hum Mov Sci 1991;10(2–3):301–314. [Google Scholar]
- 7. Rosenblum S, Samuel M, Zlotnik S, Erikh I, Schlesinger I. Handwriting as an objective tool for Parkinson's disease diagnosis. J Neurol 2013;260:2357–2361. [DOI] [PubMed] [Google Scholar]
- 8. Saunders‐Pullman R, Derby C, Stanley K, et al. Validity of spiral analysis in early Parkinson's disease. Mov Disord 2008;23:531–537. [DOI] [PubMed] [Google Scholar]
- 9. Tucha O, Mecklinger L, Thome J, et al. Kinematic analysis of dopaminergic effects on skilled handwriting movements in Parkinson's disease. J Neural Transm 2006;113:609–623. [DOI] [PubMed] [Google Scholar]
- 10. Sandyk R, Iacono RP. Reversal of micrographia in Parkinson's disease by application of picoTesla range magnetic fields. Int J Neurosci 1994;77(1–2):77–84. [DOI] [PubMed] [Google Scholar]
- 11. Balas I, Llumiguano C, Doczi TP. Ablative stereotactic surgery improves manual performance time in Parkinson's disease. Park Relat Disord 2006;12:223–227. [DOI] [PubMed] [Google Scholar]
- 12. Pullman SL. Spiral analysis: a new technique for measuring tremor with a digitizing tablet. Mov Disord 1998;13(Suppl 3):85–89. [DOI] [PubMed] [Google Scholar]
- 13. Pan Z, Van Gemmert AW. Peripheral neuropathy reduces asymmetries in inter‐limb transfer in a visuo‐motor task. Laterality 2016;21:255–266. 10.1080/1357650X.2015.1134563. [DOI] [PubMed] [Google Scholar]
- 14. Pantelyat A, Freedman R, Pawlowski S, Kesari A, Duda J, Morley J. Quantitative assessment of bradykinesia in Parkinson's disease (PD) [abstract]. Neurology 2013;80(7 Suppl):P04.192. [Google Scholar]
- 15. Grunich K, Garcia‐Hoyos V, Stinear C, Ackerley S, Tiemensma J, Broadbent E. Kinematic measures of brain drawings are associated with illness perceptions in people with stroke. Int Psychogeriatr 2016;28:1637–1642. [DOI] [PubMed] [Google Scholar]
- 16. Dean DJ, Orr JM, Newberry RE, Mittal VA. Motor behavior reflects reduced hemispheric asymmetry in the psychosis risk period. Schizophr Res 2016;170:137–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mohammed L, Found B, Caligiuri M, Rogers D. Dynamic characteristics of signatures: effects of writer style on genuine and simulated signatures. J Forensic Sci 2015;60:89–94. [DOI] [PubMed] [Google Scholar]
- 18. Rosenblum S. Do motor ability and handwriting kinematic measures predict organizational ability among children with developmental coordination disorders? Hum Mov Sci 2015;43:201–215. [DOI] [PubMed] [Google Scholar]
- 19. Werner P, Rosenblum S, Bar‐On G, Heinik J, Korczyn A. Handwriting process variables discriminating mild Alzheimer's disease and mild cognitive impairment. J Gerontol B Psychol Sci Soc Sci 2006;61:P228–P236. [DOI] [PubMed] [Google Scholar]
- 20. Sharma S, Moon CS, Khogali A, et al. Biomarkers in Parkinson's disease (recent update). Neurochem Int 2013;63:201–229. [DOI] [PubMed] [Google Scholar]
- 21. Contreras‐Vidal JL, Teulings HL, Stelmach GE. Micrographia in Parkinson's disease. NeuroReport 1995;6:2089–2092. [DOI] [PubMed] [Google Scholar]
- 22. Wilson SAK. Disorders of motility and muscle tone with special reference to the corpus striatum. Lancet 1925;2:1–10. [Google Scholar]
- 23. Margolin DI, Wing AM. Agraphia and micrographia: clinical manifestations of motor programming and performance disorders. Acta Psychol (Amst) 1983;54(1–3):263–283. [DOI] [PubMed] [Google Scholar]
- 24. Van Gemmert AW, Teulings HL, Contreras‐Vidal JL, Stelmach GE. Parkinson's disease and the control of size and speed in handwriting. Neuropsychologia 1999;37:685–694. [DOI] [PubMed] [Google Scholar]
- 25. Teulings HL, Contreras‐Vidal JL, Stelmach GE, Adler CH. Adaptation of handwriting size under distorted visual feedback in patients with Parkinson's disease and elderly and young controls. J Neurol Neurosurg Psychiatry 2002;72:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van Gemmert AW, Teulings HL, Stelmach GE. Parkinsonian patients reduce their stroke size with increased processing demands. Brain Cogn 2001;47:504–512. [DOI] [PubMed] [Google Scholar]
- 27. Wagle Shukla A, Ounpraseuth S, Okun MS, Gray V, Schwankhaus J, Metzer WS. Micrographia and related deficits in Parkinson's disease: a cross‐sectional study [serial online]. BMJ Open 2012;2:e000628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim EJ, Lee BH, Park KC, Lee WY, Na DL. Micrographia on free writing versus copying tasks in idiopathic Parkinson's disease. Park Relat Disord 2005;11:57–63. [DOI] [PubMed] [Google Scholar]
- 29. Ishihara LS, Khaw KT, Luben R, Bingham S, Welch A, Day N, Brayne C. Self‐reported parkinsonian symptoms in the EPIC‐Norfolk cohort [serial online]. BMC Neurol 2005;5:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wu T, Zhang J, Hallett M, Feng T, Hou Y, Chan P. Neural correlates underlying Micrographia in Parkinson's disease. Brain 2016;139:144–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Inzelberg R, Plotnik M, Harpaz NK, Flash T. Micrographia, much beyond the writer's hand. Park Relat Disord 2016;26:1–9. [DOI] [PubMed] [Google Scholar]
- 32. Linderman M, Lebedev MA, Erlichman JS. Recognition of handwriting from electromyography [serial online]. PLoS ONE 2009;4e0006791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ma HI, Hwang WJ, Chang SH, Wang TY. Progressive micrographia shown in horizontal, but not vertical, writing in Parkinson's disease. Behav Neurol 2013;27:169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smits EJ, Tolonen AJ, Cluitmans L, et al. Standardized handwriting to assess bradykinesia, micrographia and tremor in Parkinson's disease [serial online]. PLoS ONE 2014;9:e0097614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bajaj NPS, Wang L, Gontu V, Grosset DG, Bain PG. Accuracy of subjective and objective handwriting assessment for differentiating Parkinson's disease from tremulous subjects without evidence of dopaminergic deficits (SWEDDs): an FP‐CIT‐validated study. J Neurol 2012;259:2335–2340. [DOI] [PubMed] [Google Scholar]
- 36. Ondo WG, Satija P. Withdrawal of visual feedback improves micrographia in Parkinson's disease. Mov Disord 2007;22:2130–2131. [DOI] [PubMed] [Google Scholar]
- 37. Broeder S, Nackaerts E, Nieuwboer A, Smits‐Engelsman BCM, Swinnen SP, Heremans E. The effects of dual tasking on handwriting in patients with Parkinson's disease. Neuroscience 2014;263:193–202. [DOI] [PubMed] [Google Scholar]
- 38. Yu NY, Van Gemmert AWA, Chang SH. Characterization of graphomotor functions in individuals with Parkinson's disease and essential tremor. Behav Res Methods 2016;35:795–806. [DOI] [PubMed] [Google Scholar]
- 39. Letanneux A, Danna J, Velay JL, Viallet F, Pinto S. From micrographia to Parkinson's disease dysgraphia. Mov Disord 2014;29:1467–1475. [DOI] [PubMed] [Google Scholar]
- 40. Eichhorn TE, Gasser T, Mai N, Marquardt C, Arnold G, Schwarz J, Oertel WH. Computational analysis of open loop handwriting movements in Parkinson's disease: a rapid method to detect dopamimetic effects. Mov Disord 1996;11:289–297. [DOI] [PubMed] [Google Scholar]
- 41. Contreras‐Vidal JL, Poluha P, Teulings HL, Stelmach GE. Neural dynamics of short and medium‐term motor control effects of levodopa therapy in Parkinson's disease. Artif Intell Med 1998;13(1–2):57–79. [DOI] [PubMed] [Google Scholar]
- 42. Poluha PC, Teulings HL, Brookshire RH. Handwriting and speech changes across the levodopa cycle in Parkinson's disease. Acta Psychol (Amst) 1998;100(1–2):71–84. [DOI] [PubMed] [Google Scholar]
- 43. Lange KW, Mecklinger L, Walitza S, Becker G, Gerlach M, Naumann M, Tucha O. Brain dopamine and kinematics of graphomotor functions. Hum Mov Sci 2006;25(4–5):492–509. [DOI] [PubMed] [Google Scholar]
- 44. Caligiuri MP, Teulings HL, Filoteo JV, Song D, Lohr JB. Quantitative measurement of handwriting in the assessment of drug‐induced parkinsonism. Hum Mov Sci 2006;25(4–5):510–522. [DOI] [PubMed] [Google Scholar]
- 45. Siebner HR, Ceballos‐Baumann A, Standhardt T, Auer C, Conrad B, Alesch T. Changes in handwriting resulting from bilateral high‐frequency stimulation of the subthalamic nucleus in Parkinson's disease. Mov Disord 1999;14:964–971. [DOI] [PubMed] [Google Scholar]
- 46. Drotar P, Mekyska J, Rektorova I, Masarova L, Smekal Z, Faundez‐Zanuy M. Analysis of in‐air movement in handwriting: a novel marker for Parkinson's disease. Comput Methods Programs Biomed 2014;117:405–411. [DOI] [PubMed] [Google Scholar]
- 47. Drotar P, Mekyska J, Rektorova I, Masarova L, Smekal Z, Faundez‐Zanuy M. Evaluation of handwriting kinematics and pressure for differential diagnosis of Parkinson's disease. Artif Intell Med 2016;67:39–46. [DOI] [PubMed] [Google Scholar]
- 48. Drotar P, Mekyska J, Rektorova I, Masarova L, Smekal Z, Faundez‐Zanuy M. Decision support framework for Parkinson's disease based on novel handwriting markers. IEEE Trans Neural Syst Rehabil Eng 2015;23:508–516. [DOI] [PubMed] [Google Scholar]
- 49. Ling H, Massey LA, Lees AJ, Brown P, Day BL. Hypokinesia without decrement distinguishes progressive supranuclear palsy from Parkinson's disease. Brain 2012;135:1141–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mergl R, Tigges P, Schroter A, Moller HJ, Hegerl U. Digitized analysis of handwriting and drawing movements in healthy subjects: methods, results and perspectives. J Neurosci Methods 1999;90:157–169. [DOI] [PubMed] [Google Scholar]
- 51. Brown MJ, Almeida QJ, Rahimi F. The dopaminergic system in upper limb motor blocks (ULMB) investigated during bimanual coordination in parkinsonnulls disease (PD). J Neurol 2015;262:41–53. [DOI] [PubMed] [Google Scholar]
- 52. Nieuwboer A, Vercruysse S, Feys P, Levin O, Spildooren J, Swinnen S. Upper limb movement interruptions are correlated to freezing of gait in Parkinson's disease. Eur J Neurosci 2009;29:1422–1430. [DOI] [PubMed] [Google Scholar]
- 53. Ziv I, Avraham M, Dabby R, Zoldan J, Djaldetti R, Melamed E. Early‐occurrence of manual motor blocks in Parkinson's disease: a quantitative assessment. Acta Neurol Scand 1999;99:106–111. [DOI] [PubMed] [Google Scholar]
- 54. Heremans E, Nackaerts E, Vervoort G, et al. Amplitude manipulation evokes upper limb freezing during handwriting in patients with Parkinson's disease with freezing of gait. PLoS ONE 2015;10:e0142874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Doyon J. Motor sequence learning and movement disorders. Curr Opin Neurol 2008;21:478–483. [DOI] [PubMed] [Google Scholar]
- 56. Doyon J, Bellec P, Amsel R, et al. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav Brain Res 2009;199:61–75. [DOI] [PubMed] [Google Scholar]
- 57. Nieuwboer A, Kwakkel G, Rochester L, et al. Cueing training in the home improves gait‐related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry 2007;78:134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nackaerts E, Heremans E, Vervoort G, et al. Relearning of writing skills in Parkinson's disease after intensive amplitude training. Mov Disord 2016;31:1209–1216. [DOI] [PubMed] [Google Scholar]
- 59. Nackaerts E, Nieuwboer A, Broeder S, Smits‐Engelsman BC, Swinnen SP, Vandenberghe W, Heremans E. Opposite effects of visual cueing during writing‐like movements of different amplitudes in Parkinson's disease. Neurorehabil Neural Repair 2016;30:431–439. [DOI] [PubMed] [Google Scholar]
- 60. Heremans E, Nackaerts E, Vervoort G, Broeder S, Swinnen SP, Nieuwboer A. Impaired retention of motor learning of writing skills in patients with Parkinson's disease with freezing of gait [serial online]. PLoS ONE 2016;11:e0148933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Grace N, Enticott PG, Johnson BP, Rinehart NJ. Do handwriting difficulties correlate with core symptomology, motor proficiency and attentional behaviours? J Autism Dev Disord 2017;47:1006–1017. [DOI] [PubMed] [Google Scholar]
- 62. Giammarco E, Di Sano S, Aureli T, Cerratti P, Fano‐Illic G, Pietrangelo T. Psychological and physiological processes in figure‐tracing abilities measured using a tablet computer: a study with 7 and 9 years old children [serial online]. Front Psychol 2016;7:1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Broderick et al. Hypometria and bradykinesia during drawing movements in individuals with Parkinson's disease. Exp Brain Res. 2009;197(3):223–233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Results of PubMed and Scopus Database Search with Various Keywords and Combinations
Figure S1. Schematic flow of search strategy adopted for papers screened using the keywords.
Figure S2. A case of coexistent, consistent, and progressive micrographia collected using paper and pen (from the authors’ unpublished collection).


