Abstract
The search for genes of complex traits is aided by the availability of multiple quantitative phenotypes collected in geographically isolated populations. Here we provide rationale for a large‐scale study of gene‐environment interactions influencing brain and behavior and cardiovascular and metabolic health in adolescence, namely the Saguenay Youth Study (SYS). The SYS is a retrospective study of long‐term consequences of prenatal exposure to maternal cigarette smoking (PEMCS) in which multiple quantitative phenotypes are acquired over five sessions (telephone interview, home, hospital, laboratory, and school). To facilitate the search for genes that modify an individual's response to an in utero environment (i.e. PEMCS), the study is family‐based (adolescent sibships) and is carried out in a relatively geographically isolated population of the Saguenay Lac‐Saint‐Jean (SLSJ) region in Quebec, Canada. DNA is acquired in both biological parents and in adolescent siblings. A genome‐wide scan will be carried out with sib‐pair linkage analyses, and fine mapping of identified loci will be done with family‐based association analyses. Adolescent sibships (12–18 years of age; two or more siblings per family) are recruited in high schools throughout the SLSJ region; only children of French‐Canadian origin are included. Based on a telephone interview, potential participants are classified as exposed or nonexposed prenatally to maternal cigarette smoking; the two groups are matched for the level of maternal education and the attended school. A total of 500 adolescent participants in each group will be recruited and phenotyped. The following types of datasets are collected in all adolescent participants: (1) magnetic resonance images of brain, abdominal fat, and kidneys, (2) standardized and computer‐based neuropsychological tests, (3) hospital‐based cardiovascular, body‐composition and metabolic assessments, and (4) questionnaire‐derived measures (e.g. life habits such as eating and physical activity; drug, alcohol use and delinquency; psychiatric symptoms; personality; home and school environment; academic and vocational attitudes). Parents complete a medical questionnaire, home‐environment questionnaire, a handedness questionnaire, and a questionnaire about their current alcohol and drug use, depression, anxiety, and current and past antisocial behavior. To date, we have fully phenotyped a total of 408 adolescent participants. Here we provide the description of the SYS and, using the initial sample, we present information on ascertainment, demographics of the exposed and nonexposed adolescents and their parents, and the initial MRI‐based assessment of familiality in the brain size and the volumes of grey and white matter. Hum Brain Mapp 2007. © 2007 Wiley‐Liss, Inc.
Keywords: genetics, isolated population, mri, siblings, heritability, familiarity, morphometry, cognition, cardiovascular, obesity
BACKGROUND
Genetics of Complex Traits
There are two main approaches used to establish a link between a complex genetic trait (phenotype) and genotype: case‐control and family‐based studies. The case‐control approach allows investigators to reveal a trait‐genotype association in a population of unrelated individuals based on cooccurrence of a specific gene variant (allele) and a phenotypic value of a trait of interest. Several studies have used this strategy to describe associations between different quantitative measures of brain anatomy and allelic variations of candidate genes in healthy human subjects. These include associations between polymorphisms in the promotor of the 5‐HT transporter gene and the grey‐matter volume in the subgenual cingulate cortex [Pezawas et al., 2005; n = 114], polymorphisms in monoamine oxidase A gene and amygdala volume [Meyer‐Lindenberg et al., 2006; n = 97], and polymorphism in brain derived neurotrophic factor gene and hippocampal volume [Bueller et al., 2006; n = 36]. Such case‐control studies, when compared with family‐based investigations, have certain limitations. First, they do not allow carrying out a linkage analysis and, as such, they are less suitable for a search for genes influencing a trait of interest throughout the entire genome. This is despite the fact that massive efforts are currently being made to develop new strategies that would allow investigators to perform a genome‐wide search in case‐control cohorts as well [International HapMap Consortium, 2005]. Second, case‐control studies do not permit distinguishing between allelic identity “by state” and “by descent” (i.e. to define whether an apparently same allele comes from the mother or the father) and, therefore, are associated with reduced fidelity when defining the genotype. Third, case‐control studies are more sensitive to biases associated with population admixture and, hence, more prone to spurious results. The latter limitation (population admixture) can also be reduced by studying individuals from a geographically isolated population. Such populations offer several advantages in genetic studies. First, genetic heterogeneity in such populations is lower compared with ethnically mixed populations; this reduces the number of genes influencing a particular complex trait. Second, geographically isolated populations usually have good genealogical records that can improve further the fidelity of defining the genotype. Third, geographically isolated populations typically live in a more uniform environment (e.g. diet, parenting style) that reduces occurrence of confounding factors such as phenocopies. To our knowledge, there are only two large‐scale MR‐based studies carried out in geographically isolated populations: (1) the Saguenay Youth Study (SYS) described here; and (2) the AGES‐Reykjavik study carried out in Iceland in a population cohort of 67 to 93‐year‐old individuals [Sigurdsson et al., 2006]. The Sagunay Youth Study is carried out in the Saguenay Lac‐Saint‐Jean (SLSJ) region of Quebec, Canada in a population‐based cohort of high‐school students (age 12–18 years). It is designed to investigate the interaction between the genetic background of the individual and his/her prenatal exposure to maternal cigarette smoking (PEMCS). Therefore, we will now provide background information on the known effects of this particular in utero environment on brain and body.
Prevalence of Maternal Cigarette Smoking During Pregnancy
The prevalence of cigarette smoking in pregnant women varies widely in different countries [Ebrahim et al., 2000; Kendrick and Merritt, 1996]. In the United States, for example, the average prevalence was 16.3% in 1984 and decreased to 11.8% in 1994 [Ebrahim et al., 2000]. In Canada, 25% of women age 15 years and above are current smokers [Health Canada, 1995b]. In Quebec, we found that 25% of expectant mothers smoked cigarettes throughout pregnancy [Japel et al., 2000]. It is important to note that the prevalence rates of cigarette smoking in general, and of cigarette smoking during pregnancy in particular, are not uniform across different groups of women. The strongest predictor of smoking both before and during pregnancy is a woman's socioeconomic status (SES); for example, a prospective study of 589 preadolescent children found that 52% of their low‐SES mothers smoked cigarettes during pregnancy [Cornelius et al., 2000]. Tobacco smoking is particularly frequent in young mothers; in Canada, 40–50% of teen mothers reported that they smoked during their pregnancy [Paquette and Morrisson, 1999]. Similar relationships were observed in the SLSJ region; we found that prevalence of tobacco use is negatively correlated with the level of schooling and with household income [Institute de la statistique du Quebec, 2001].
Cigarette Smoking, the Fetus and the Fetal–Placental Unit
The impact of maternal smoking on the developing fetus is complex. First of all, tobacco smoke may affect the fetus in several ways [Lambers and Clarke, 1996]: (a) inhaled nicotine induces vasoconstriction of the uteroplacental vasculature, leading to uteroplacental underperfusion and, in turn, decreased flow of nutrients and oxygen to the fetus, (b) increased levels of carboxyhemoglobin reduce tissue oxygenation of the fetus, (c) nicotine suppresses the mother's appetite, leading to reduced energy intake by the mother and hence reduced energy supply to the fetus and (d) nicotine causes alterations in the cellular growth and activity of the central and peripheral nervous systems [Slotkin, 1998]. Second, tobacco smoking is frequently associated with epiphenomena, such as risky behaviors, coabuse of other substances, poor prenatal care, and low SES [Slotkin, 1998], which themselves may exert adverse effects on the developing fetus. Finally, interindividual variability in genetic background is likely to modify the response of the fetus to tobacco smoke, i.e. gene–environment interactions [Pausova et al., 1999]. Cigarette smoking during pregnancy has been associated with a number of adverse outcomes related to the child's mental and cardiovascular and metabolic health.
PEMCS and Behavior
In human subjects, there is growing evidence that PEMCS is associated with cognitive sequelae and increased incidence of psychiatric disorders in childhood and adolescence. In a prospective cohort study, Fried [1995] observed systematic differences between children born to “heavy smokers” (>20 cigarettes/day) compared with nonsmoking mothers, in several cognitive domains, including processing of auditory stimuli, attention, and language comprehension. Other [Lassen and Oei, 1998; Obel et al., 1998; Olds et al., 1994] but not all [MacArthur et al., 2001] investigators have observed similar effects. Several studies revealed an increased incidence of externalizing disorders in general [Breslau and Chilcoat, 2000], and attention‐deficit hyperactivity [Milberger et al., 1998] and conduct [Wakschlag et al., 1997; Weissman et al., 2000] disorders in particular. Some studies also observed an association between PEMCS and criminal behavior in adulthood [Brennan et al., 1999; Räsänen et al., 1999], and higher rates of aggression in children and adults [Orlebeke et al., 1999; Räsänen et al., 1999]. Furthermore, PEMCS may increase the probability of experimenting with cigarette smoking in childhood [Cornelius et al., 2000] and of developing cigarette‐smoking addiction in adolescence [Kandel and Udry, 1999; Weissman et al., 2000].
PEMCS and the Brain
The effect of PEMCS on the human brain is largely unknown. A recent study demonstrated subtle differences in the expression of nicotinic and muscarinic acetylcholine receptors in the brains of 5 to 12‐week‐old fetuses exposed and nonexposed to maternal cigarette smoking [Falk et al., 2005]. Several lines of indirect evidence suggest detrimental effects of PEMCS on brain growth and development. Kallen [2000] analyzed the Swedish Medical Birth Registry (1983–1996: 1,362,169 infants) and found significant negative correlation between PEMCS and head circumference at birth. Maternal cigarette smoking was found to increase the relative risk of periventricular‐intraventricular hemorrhage in premature babies [Bada et al., 1990].
PEMCS and the Offspring's Metabolic and Cardiovascular Health
Despite being related to intrauterine growth retardation [Kramer, 2000], PEMCS is associated with obesity in later life. The British National Child Development Study reported that PEMCS significantly increases the risk of obesity beginning in childhood and adolescence [Power and Jefferis, 2002; Toschke et al., 2003; von Kries et al., 2002; Wideroe et al., 2003], and diabetes mellitus in adulthood [Montgomery and Ekbom, 2002]. In prospective cohort studies, the impact of PEMCS on obesity increased with age and remained robust even after adjustment for known confounding factors, such as SES, infant feeding, current diet, and physical activity [Power and Jefferis, 2002; Wideroe et al., 2003].
PEMCS may also increase the susceptibility to hypertension later in life. Significant associations between PEMCS and increased blood pressure in children of different age categories, including neonates and adolescents, have been reported [Beratis et al., 1996; Blake et al., 2000; Morley et al., 1995; O'Sullivan et al., 1996; Williams et al., 1999]. In experimental animals, prenatal exposure to nicotine has been related to a decreased kidney size and increased blood pressure in adolescence [Pausova et al., 2003].
DESIGN
To evaluate long‐term consequences of PEMCS in adolescence and to assess how such an adverse intrauterine environment interacts with the individual's genetic background, the design of the SYS has the following features: (1) a family‐based (sib‐ship) design where only children with one or more siblings and with both biological parents are included; (2) a cross‐sectional design with participants' age ranging from 12 to 18 years, and an equal proportion of exposed (n = 500) and nonexposed (n = 500) adolescents of both sexes (1:1 male‐to‐female ratio); (3) a retrospective‐cohort design where in utero exposure is assessed retrospectively, while the acquisition of the majority of phenotypes in the offspring is carried out in a prospective fashion; (4) quantitative assessment of brain and behavior and cardiovascular and metabolic phenotypes; and (5) acquisition of DNA samples from the adolescent participants and their biological parents.
SETTING AND PARTICIPANTS
The sib‐ships are being recruited from a relatively geographically isolated population with a known founder effect [Grompe et al., 1994] living in the SLSJ region of Quebec, Canada.
History of the SLSJ Population
The initial settlement of the SLSJ region occurred between 1838 and 1911. From a total of 28,656 settlers who moved there during that time, 75% originated from the neighboring Charlevoix region [Gradie et al., 1988]. The settling of the Charlevoix region itself started in 1675 when 599 founders of mostly French descent moved to this region from the Quebec City area. Because of relatively high birth‐rates and low immigration into the SLSJ region, today the population represents one of the largest population isolates in North America, with almost 300,000 inhabitants. As a consequence of the SLSJ population history, the prevalence of several recessive disorders is higher in the SLSJ region than in other populations [De Braekeleer et al., 1991], and a limited allelic diversity exist among patients with these disorders [De Braekeleer et al., 1998; Grompe et al., 1994]. These studies indicate that genetic heterogeneity is reduced in the SLSJ population. Moreover, extensive computerized genealogical records exist in the BALSAC population register maintained at the Inter‐university Institute for Population Research. The register contains ascending genealogies going back as far as the 16th century; they have been constructed on the basis of parish records of baptisms, marriages, and deaths of ∼1,500,000 individuals [Roy et al., 1991].
The SLSJ Population at Present
According to the 2001 census, the region has a population of 278,279 inhabitants. In 2001, there were 19,114 French‐speaking students in 24 secondary schools (20 public and 4 private). A large part of the SLSJ population (60%) is living in the Chicoutimi‐Jonquière urban area. There are four school boards in the region, two in Saguenay and two in the Lac Saint‐Jan region. According to the 2001 census, there were 80,325 families living in the region. More than half (51,660) of all families have children living at home: 46.5% families have one child; 38.1% two children; 15.4% three or more children.
Selection Criteria
The following selection criteria are used for the exposed subjects: (1) Age 12–18 years; (2) One or more siblings in the same age group; (3) Maternal and paternal grand‐parents of French–Canadian ancestry; and (4) Positive history of maternal cigarette smoking (>1 cigarette/day in the 2nd trimester of pregnancy). The main exclusion criteria are: (1) Positive history of alcohol abuse during pregnancy; (2) Positive medical history for meningitis, malignancy, and heart disease requiring heart surgery; (3) Severe mental illness (e.g. autism, schizophrenia) or mental retardation (IQ < 70); and (4) MRI contraindications. The nonexposed subjects are matched to the exposed ones based on the level of maternal education and the school attended. In the case of mothers of nonexposed offspring, we require negative history of maternal cigarette smoking during pregnancy and during the 12‐month period preceding the pregnancy. The full list of exclusion‐inclusion criteria is presented in Supplementary Table I.
Recruitment
Subjects are recruited through regional high schools; the number of students in each school varies between 600 and 1,900. Following a briefing of the teachers, the team visits individual classrooms and presents the project. Before the visit, we mail a letter to all parents of students attending the visited school; the letter contains an information brochure, a letter from the principal, a consent form for a telephone interview and a self‐addressed and stamped response card. We ask the parents to mail the response card back to the team and indicate whether or not they are interested in participating in the project, how many children aged 12–18 years they have and to provide the home phone number for a follow‐up call by a research nurse. During the follow‐up call to the interested families, the nurse verifies basic eligibility for the project (number of children and their age), solicits consent to a telephone interview and, if agreed, proceeds with the interview. Given the nature of the interview (see later), the respondent should be the children's biological mother whenever possible. The telephone interview is carried out on a laptop computer using software written for the project, providing the nurse with the script and allowing her to record all responses on line; the software navigates the nurse through the interview by skipping “not‐applicable” questions. The interview covers the following areas: demographics of the parents (French Canadian origin, age, level of education), pregnancy (smoking, drinking, drug use, medical complications), and medical history of the children and parents. If the nurse deems the family eligible, she sets up an appointment for a home visit; this can be done immediately only for the exposed subjects, whereas nonexposed subjects are called back once selected by matching to the exposed subjects from the same school. The home visit that concludes the recruitment phase begins with the signing of the consent (parents) and assent (adolescents) forms.
MEASUREMENTS
Data collection begins with the telephone interview, continues with a home visit, neuropsychological testing during a laboratory visit, a hospital visit for the cardiovascular and metabolic phenotyping and magnetic resonance imaging (MRI), and it concludes with a school visit; hospital visits always take place on Saturdays. In this section, we will describe the various instruments by types of data sets rather than by place/time of their collection (Supplementary Table II).
Questionnaires
Demographics and measures of SES: Supplementary Table IIIA provides a summary of datasets acquired in these domains, together with the instruments used for their collection. Note that a large number of SES measures, as well as stressful life events, are recorded for the following four periods of each child's life: birth, 3 years and 10 years of age and present. Medical and psychiatric history: Supplementary Table IIIB summarizes measures collected in this domain. In addition to the medical history of each child from conception to present (pregnancy, delivery, breastfeeding, concussions, etc), we also collect data on the parental mental health (depression and anxiety) and, in the adolescents, evaluate the likelihood of psychiatric diagnosis with DISC Predictive Scales. Cigarettes, alcohol, and drugs: Supplementary Table IIIC shows that several instruments are used to collect information about the use of cigarettes, alcohol, and illegal substances by the adolescent, as well as his/her mother and father. Maternal reports of the use of these substances during pregnancy are checked against medical records. In utero exposure to second‐hand cigarette smoke is also documented. As seen in Supplementary Table IIID, anti‐social behavior is documented extensively in both adolescents (self‐report and parental report) and in their parents (maternal and paternal self‐report). Lifestyle measures provide information about the adolescents' engagement in physical activities, their food habits, sleep pattern, sexuality, extracurricular activities, attitudes towards school, as well as their academic/vocational aspirations (Supplementary Table IIIE). A number of self‐attributes and personality traits (Supplementary Table IIIE) are evaluated using two instruments, namely the 5C (Competence, Connection, Character, Confidence, and Caring/Compassion) questionnaire and the NEO‐Personality Inventory. In addition, we employ two body‐esteem and body‐image instruments [Aleong et al., 2007], and a peer‐pressure questionnaire.
Neuropsychological Assessment
This assessment (see Supplementary Table II: Section 3) consists of two types of tests: (1) standardized psychological instruments and (2) domain‐specific tests. Total testing time is 6 h, divided into two 3‐h blocks separated by a lunch break. To characterize the two groups of subjects and collect clinically relevant information, we administer several standardized tests including the WISC‐III (except Mazes), Woodcock‐Johnson Achievement subtests (reading comprehension and arithmetic and a spelling test) and Children's Memory Scale (Dot Locations and Stories). We have also included an extensive battery of domain‐specific tests based on neuropsychological and cognitive‐neuroscience research. The following domains are assessed: (1) executive functions, including working memory (self‐ordered pointing, [Petrides and Milner, 1982]), word fluency (phonetic and semantic), and resistance to interference (Stroop test [Stroop, 1935]), (2) phonological skills and frequency‐modulation (FM) auditory threshold [Talcott et al., 2000], delayed auditory feedback, phonological learning, (3) fine motor skills (Grooved Pegboard, Simple tapping) and motor coordination (bimanual coordination [Stephan et al., 1999]), (4) number sense [Galistel and Gelman, 2000] and (5) emotion and motivation (Pollak faces [Pollak and Kistler, 2002], repeated failure test, and card playing task [Newman et al., 1987]).
Magnetic Resonance Imaging—Acquisition
MRI data are collected on a Phillips 1.0‐T superconducting magnet; the session lasts between 45 and 60 min. (1) Structural MRI of the brain: High‐resolution anatomical MR images are acquired using the following three sequences: T1‐weighted images (T1W; 3D RF‐spoiled gradient echo scan with 140–160 slices, 1‐mm isotropic resolution, TR = 25 ms, TE = 5 ms, flip angle = 30°), T2‐weighted images (T2W; 2D multi‐slice fast spin echo scan with 70–80 2‐mm contiguous slices with a 1 mm in‐plane resolution, TR = 3,300 ms, TE effective = 11 ms) and proton‐density weighted images (PDW; same as for T2 scan, but with TE effective = 105 ms). Magnetization‐transfer ratio: Magnetization transfer (MT) imaging [Wolff and Balaban, 1989] is a quantitative MRI technique that provides information on the macromolecular content and structure of tissue; it has been shown to reveal subtle white matter abnormalities that are undetected by conventional MRI, in diseases such as multiple sclerosis [Dousset et al., 1992; Gass et al., 1994; Pike et al., 2000]. In the context of this study, MT data should provide a more sensitive and accurate measure of myelination as compared with white matter (WM) density maps obtained via the methods discussed below. MT data are acquired using a dual acquisition (3D RF‐spoiled gradient echo scan with ∼50 slices of 3 mm thickness and 1 mm in‐plane resolution, TR = 41 ms, TE = 7.9 ms, flip angle = 20°) with and without an MT saturation pulse (Gaussian RF pulse of 7.68 ms duration, 1.5 kHz off resonance, and 500° effective pulse angle). Magnetization transfer ratio (MTR) images are calculated as the percent signal change between the two acquisitions [Pike, 1996]. (2) MRI of abdominal fat: Imaging of adipose tissue using MRI is relatively straightforward, as T1 relaxation time of adipose tissue is much shorter than that of most other tissues [Snijder et al., 2002]. Thus, in the present project, the MR images of abdominal fat are acquired with a heavily T1‐weighted, single breath‐hold, multi‐slice (10 axial slices of 10‐mm thickness) spin‐echo (TR/TE = 200 ms/20 ms) measurement that is centered on the L4–L5 region (Fig. 3). (3) Kidney MRI: The kidneys can be well visualized on T1‐weighted MRI acquisitions, providing that respiratory motion can be controlled for. Therefore, anatomical MR images of the kidneys are obtained with a multi‐slice T1‐weighted gradient recalled echo acquisition (3D Gradient Echo, TR/TE = 4 ms/1.9 ms; flip angle = 10°), collected within a single breath‐hold. Approximately, 27 coronal slices of 3‐mm thickness and 3‐mm in‐plane resolution are acquired.
Figure 3.
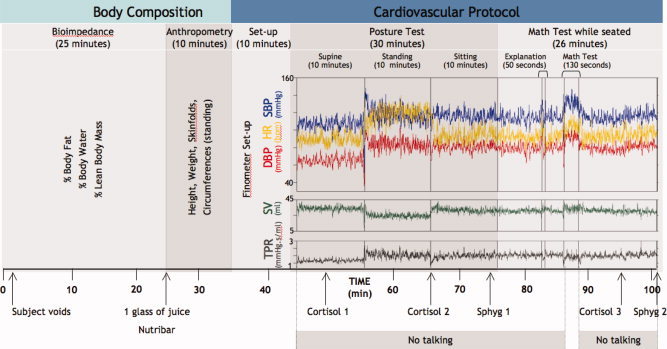
Cardiovascular and body‐composition protocol. Subjects undergo a 101‐min protocol that includes body composition measurements (bioimpedance and anthropometry), and continuous beat‐to‐beat monitoring of blood pressure and other cardiovascular parameters (see later) using a Finometer™. The cardiovascular component involves two main challenges: a posture test and a math stress‐test. SBP, systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SV, stroke volume; TPR, total peripheral resistance; Sphyg 1 and 2, additional measurements of blood pressure using sphygmomanometer; Cortisol 1, 2, and 3, samples of saliva obtained for cortisol measurements.
Magnetic Resonance Imaging—Analysis
Computational Analysis of the Brain MR images is used to assess differences in brain structure between exposed and nonexposed adolescents, as well as to identify more general effects of age and sex, structure‐function relationships, and genetic/familial influences on brain structure. A typical image‐processing “pipeline” (Fig. 1) begins with the linear registration of a T1W image from the acquisition (“native”) space to standardized stereotaxic space, namely that of the average MNI‐305 atlas [Evans et al., 1993], aligned with Talairach and Tournoux space [Talairach and Tournoux, 1988]. We use as a registration target the average brain (SYS333) computed from our adolescent population (n = 333), and aligned with the average MNI‐305 atlas [Guimond et al., 2000]. Note that there is a negligible difference in the overall brain size between adolescent brains and those of young adults (23.4 ± 4.1 years) who constitute the MNI‐305 atlas. The next step involves brain‐tissue classification into grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF); an automatic classification is achieved by combining information from different types of MR images, namely T1W, T2W, and PDW acquisitions [Cocosco et al., 2003; Zijdenbos et al., 2002]. The tissue‐classification step yields three sets of binary 3D images (i.e. GM, WM, and CSF). Each of the binary images can be smoothed to generate probabilistic “density” images. These maps are used in voxel‐wise analyses of age‐ or group‐related differences in GM or WM density [see Ashburner and Friston, 2000 for methodological overview]. Another important processing step is a nonlinear registration of the subject's image to a template brain; local differences between the subject and the template are captured in a deformation field. The template brain contains information about anatomical boundaries; this anatomical information can be “projected” onto each subject's brain using the corresponding deformation field and fine‐tuned by combining it with the tissue‐classification map [Collins et al., 1994, 1995]. In this way, the pipeline provides automatic estimates of regional volumes. Another voxel‐wise approach has been developed to quantify individual differences in the 3D anatomy of the cerebral cortex. These include estimates of the cortical thickness [Fischl and Dale, 2000; Lerch and Evans, 2005; MacDonald et al., 2000] and the quantification of individual differences in the position, depth, and length of the cerebral sulci [Le Goualher et al., 1999; Mangin et al., 2004].
Figure 1.
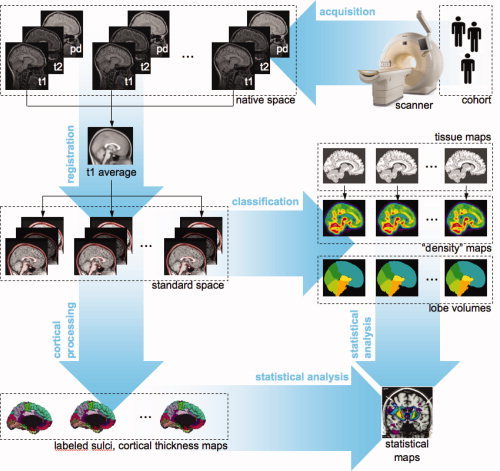
Acquisition and analysis of brain magnetic resonance images. T1W, T2W, PDW, T1‐weighted, T2‐weighted, and proton‐density weighted images, respectively.
Computational Analysis of Fat and Kidneys
Fat is well contrasted on T1W images and therefore amenable to semi‐automated or automated segmentation (Fig. 2). To this end, we developed a histogram‐based classification algorithm where the combination of local intensity measures and geometrical constraints enabled the segmentation and volumetric measurements of subcutaneous and intra‐abdominal (visceral) adipose tissues. We are also developing deformable‐model based techniques for the automated segmentation of the kidneys to provide an overall volume of each kidney, and semi‐automated classification approaches to partition the kidney into the cortex and medulla to calculate cortex/medulla ratio.
Figure 2.
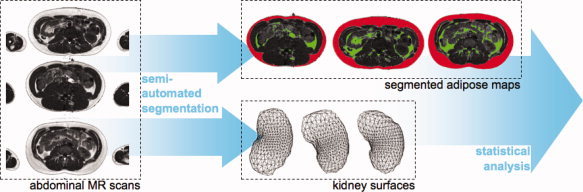
Acquisition and analysis of abdominal magnetic resonance images.
Blood Pressure and Heart Rate Measurements at Rest and in Response to Postural and Mental Challenges
The cardiovascular protocol is carried out in a hospital setting and lasts 56 min (Fig. 3). It includes posture and mental‐stress tests [McAdoo et al., 1990]. During the cardiovascular protocol, blood pressure and heart rate are measured (1) with an automated blood pressure monitor (Omron) at two time points and (2) with the Finometer (Finapres Medical Systems, Arnhem, Netherlands) continuously, i.e. beat‐by‐beat, throughout the protocol (Fig. 3). Using Beatscope software (Finapres Medical Systems, Arnhem, Netherlands), the Finometer provides the following parameters at each beat: upstroke, systolic, diastolic, and mean blood pressure, heart rate, inter‐beat interval, stroke volume, cardiac output, ventricular ejection time, peripheral resistance, aortic impedance, and aortic compliance. During the entire protocol, respiration rate is recorded with a sensor (Model 1132 Pneumotrace II Respiration Transducer, UFI, Morro Bay, CA, USA). In addition, salivary cortisol is measured in response to mental stress and posture changes [Fig. 3, Roy et al., 2001].
Assessments of the Quantity and Distribution of Body Fat, Energy Intake, and Energy Expenditure
The quantity and distribution of body fat is measured with anthropometry, bioelectrical impedance (Xitron Technologies, San Diego, CA, USA) and MRI (described above). Energy intake and expenditure are assessed with a 24‐h food‐recall interview, and food‐frequency and physical activity questionnaires. The 24‐h food recall, conducted by a trained nutritionist, is employed to collect quantitative and qualitative information on foods and drinks that the subject has consumed during the past 24 h. The recall is complemented by food‐frequency and physical activity questionnaires used by Santé Québec in its survey of Quebec children aged 6–16 years.
Biochemical Analyses of a Fasting Blood Sample
In all subjects, a fasting blood sample is drawn between 8 a.m. and 9 a.m. This sample is used to evaluate (1) glucose and lipid metabolism (glucose, insulin, total cholesterol, LDL‐cholesterol, HDL‐cholesterol, triglycerides, free fatty acids, glycerol, and apolipoproteins A, B, C, and E), (2) pro‐inflammatory and pro‐thrombotic states (C‐reactive protein and PAI‐I), (3) the hypothalamic‐pituitary‐adrenal axis and sympatho‐adrenal system (cortisol, epinephrine, and norepinephrine), (4) sexual maturation (total and bio‐available testosterone, estradiol, and leptin) and (5) current cigarette smoking (cotinine). The sample is also used for DNA extraction.
WEB‐BASED PROJECT MANAGEMENT SYSTEM
To manage smooth communication across the various project sites, we have implemented a number of web‐based tools. The entire procedure manual is available on a password‐protected web site. The manual contains a detailed version of the study flowchart (Supplementary Table II) and electronic versions (Word, PDF) of all instruments; a total of 107 individual documents are available in this electronic manual.
The key element for the management of recruitment, screening and scheduling is the field module. This is a web‐based system, developed with MySQL/PHP programming tools; it allows a research nurse to track a subject through the different stages of the study, from recruitment to study completion. When the team receives the response card from an interested family, the family name, and the telephone number are entered into the field module, and the family is assigned automatically an identification code (FAM9999). To ensure confidentiality, nominal information is restricted to local access (nurse) and is not accessible through the web. In order to proceed with the screening of families, this information is transferred electronically to the telephone interview software installed on stand‐alone personnel computers employed by research nurses. The software guides the nurse through a structured interview with the parent; the generic text is tailored to each particular family by inserting automatically family‐specific text (e.g. children's names) and skipping irrelevant questions based on the recorded answers. The first data‐collection step in the interview identifies all biological children of the mother and their biological father. At this point, a six‐digit identification code is assigned automatically to each family member; this code is combined with the family identification number assigned in the field module (see above). This combination of the family and subject identification numbers (e.g. FAM9999_888888) is carried together throughout the study. Information recorded during the telephone interview, together with the family and subject identification codes, is transferred electronically back to the field module. At this point, all nominal information is stripped away and denominalized data are released to the web‐based part of the field module that is accessible to all members of the research team. Tracking of data and data entry is an integral component of the system; this is described later.
DATA ENTRY, DATA TRANSFER, AND QUALITY CONTROL
The project database is a relational database developed with MySQL/PHP programming tools. Each participant (i.e. biological mother, biological father, and all their biological children) is identified using a unique six‐digit code assigned automatically by the telephone‐interview software (see earlier). This combination of the family and subject identification (ID) number (e.g. FAM9999_888888) is entered on all paper forms (e.g. questionnaires and testing forms) and in the subject field of the various computer‐based instruments (e.g. Finometer, MRI, neuropsychological tests). Given the variety of instruments, there are several modes of data entry (see Supplementary Table II). Web‐based manual data entry is employed for the majority of standardized neuropsychological tests and some other data‐entry forms. Individual data‐entry modules are available on a password‐protected data‐entry web site. Graphical user interface (GUI) is designed (PHP/Java script) to mirror the actual layout of a given form; this facilitates data entry and reduces possible errors due to spatial incompatibilities between the GUI and the hardcopy. Each data‐entry form begins with the subject identification; the operator is requested to enter both subject and family ID and the computer validates whether the combination of the two numbers (e.g. FAM9999 and 888888) is correct. This system minimizes the possibility of entering data under an incorrect ID number (e.g. FAM9999_898888). In addition to data entry, this module allows investigators to view and export (in text format) all entered data. Most of the questionnaires are entered using the Remark Office Optical Mark Recognition (OMR) software (http://www.PrincipiaProducts.com; Paoli, Pennsylvania, USA). The software works in conjunction with an image scanner to collect the data (Remark Office OMR, Version 5.5 for Windows, User's Guide, May 2002). The output forms generated by the Remark Office OMR software are uploaded to the project database, raw data stored in questionnaire‐specific tables and relevant scales calculated and stored.
Magnetic Resonance Images are stored at the MR console in DICOM (Digital Imaging and Communication in Medicine; http://medical.nema.org) format (12 bits) and copied onto a compact disk. Using a secure file transfer protocol, DICOM images are then transferred into a dedicated MR database and converted into MINC (Medical Imaging NetCDF; http://www.bic.mni.mcgill.ca/software/minc/) format. All other electronic files collected using various computer programs (e.g. computer‐based neuropsychological tests) or specialized hardware (e.g. Finometer, Bioimpedance) are stored in their proprietary format, copied onto a compact disk and uploaded to the project database using again a secure file transfer protocol.
STATISTICAL ANALYSES
Here we highlight only the main principles that will guide statistical analyses of the entire dataset. These analyses will rely on the Generalized Estimating Equations (GEE) extension of the multiple linear regressions for clustered data [Liang and Zeger, 1986]. This methodology will allow us to account for: (1) quantitative (continuous) measurement scales of primary and intermediary variables; (2) potential imbalances between exposed and non‐exposed subjects in the distribution of characteristics related to the variables, such as duration of breast feeding and second‐hand smoke; and (3) a likely correlation between the variables within sib‐pairs. Point (1) calls for statistical analyses designed for continuous, normally distributed data, such as linear regression analysis. Point (2) requires multivariable linear regression methods that will adjust the estimated PEMCS effect for potential confounding factors. Point (3) requires the GEE extension of the conventional multiple linear regression to account for the dependence of observations within sib‐pairs. In GEE, we will assume the exchangeable covariance structure of the residuals, with just two observations per cluster, i.e. a pair of siblings [Jennrich and Schluchter, 1986].
The sibship‐based design of the SYS allows us to evaluate the degree of “familiality” of the various phenotypes; familiality is defined here as the proportion of variance attributable to the combination of shared family environment and shared genes. Familiality estimates were derived from intraclass correlations (ICC) computed with the FCOR program (version 5.3.0, S.A.G.E. package, Department of Epidemiology and Biostatistics, Case Western Reserve University, Cleveland, Ohio; http://darwin.epbi.cwru.edu/sage/). Within‐trait and cross‐trait estimates of familiality were computed; the former reflects the proportion of variance of a single trait determined by familial factors shared between siblings (e.g. brother–brother), whereas the latter indicates the proportion of variance between two traits determined by familial factors shared between siblings.
FINDINGS
As of October 2006, we have collected full data‐sets in 408 adolescents and 195 sets of their biological parents. In this section, we provide information on ascertainment, demographics, PEMCS, and the initial data on the degree of familiarity of brain‐MRI phenotypes.
Ascertainment
Between November 2003 and October 2006, the research team has approached a total of 5,033 students. An overall response rate (i.e. number of returned response cards) was 19% and varied between 8 and 36% across schools. Of the number of returned response cards, 64% of families agreed to being contacted by the research nurse. Based on the telephone interview (n = 768), 37% families were excluded for a variety of reasons: cigarette smoking 12 months before but not during pregnancy (n = 104), one of the two adolescents not interested (n = 73), child not of eligible age (n = 75), MR contraindications (braces: n = 59), other than French Canadian origin (n = 46), medical reasons (n = 42 [most common: heart abnormalities: 12, epilepsy: 6, diabetes: 4]), one parent not available (n = 30), twins (n = 28), premature birth (n = 14), and placental detachment/rupture (n = 5). In the total of 423 eligible families, there were 229 (26%) exposed and 657 (73%) nonexposed adolescents eligible for the study. Note that the ratio of the exposed and nonexposed adolescents is similar to the population average of maternal cigarette smoking during pregnancy established in this region previously [Japel et al., 2000]. From the pool of all eligible families, we have matched (maternal education, school attended) 199 exposed and 209 nonexposed adolescents belonging to a total of 198 families. These subjects and families have completed the entire protocol and are considered below.
Prenatal Exposure to Maternal Cigarette Smoking
The exposure status and the number of cigarettes smoked during each pregnancy rely on information provided by the mother during the telephone interview. For all subjects born in the two main hospitals in the region (Chicoutimi [159 adolescents] and Jonquiere [140 adolescents]), we have verified the mother's report by extracting relevant antenatal information from the medical chart completed during pregnancy (1st trimester [73%], 2nd trimester [7%], and 3rd trimester [20%]). We were able to obtain relevant information in a total of 260 adolescents (87%), with an equal distribution across the exposed and nonexposed adolescents. To assess the overall agreement between the exposure statuses ascertained antenatally from the medical records and by the maternal report during the telephone interview, we calculated Kappa statistics (range: −1 to +1) and found that, with a value of 0.69 ± 0.04, it indicates a “good” strength of agreement [Landis and Koch, 1977; good agreement: ≫0.6 to ≤0.8].
By definition, mothers of exposed adolescents smoked during pregnancy whereas the mothers of nonexposed subjects did not (Table IA). As a group, the mothers reported smoking an average of 11 ± 6.7 cigarettes/day during the 2nd trimester (range: 1–42; quartiles: 1–6, 7–10, 11–15, ≫15 cigarettes/day). A significant number of mothers of nonexposed adolescents never smoked in their life (51%). On the other hand, many more mothers of exposed (vs. nonexposed) adolescents smoke at present (ξ 2 = 52.2, P < 0.0001). When smoking, the number of cigarettes smoked per day at present is higher in the mothers of exposed versus mothers of nonexposed adolescents (F(3,61) = 5.6, P = 0.002, Effect size defined as Cohen's d = 1.23) and, in the mothers of exposed subjects, it is similar to the number of cigarettes smoked during pregnancy (r 2 = 0.47, P < 0.0001).
Table I.
Results
| Nonexposed | Exposed | Mixed | |
|---|---|---|---|
| A. Maternal cigarette smoking | |||
| Mother: Cig smoking during pregnancy | 0 (209) | 11 ± 6.7 (199) | N/A |
| [No cig/day in 2nd trimester; M ± SD (n)] | |||
| Mother: Cig smoking during breastfeeding [No cig/day; M ± SD (n)] | 0 | 7.5 ± 7.5 (65) | N/A |
| Mother: Age of onset for cig smoking [years; M ± SD (n)] | 12.4 ± 5.2 (35) | 14.3 ± 2.6 (59) | 15.4 ± 2.9 (14) |
| Mother: Negative life long history of cig smoking (No of mothers) | 45 (88) | 0 (82) | 0 (23) |
| Mother: Cig smoking at present (No of mothers) | 8 (88) | 48 (82) | 5 (23) |
| Mother: Cig smoking at present [No cig/day; M ± SD (n)] | 7 ± 4.2 (8) | 16.8 ± 7.98 (48) | 12.4 ± 3.6 (5) |
| B. Demographics | |||
| Ado: Age (M ± SD) | 14.5 ± 1.87 (209) | 14.7 ± 1.86 (199) | N/A |
| Ado: Sex (F:M) | 103:106 | 107:92 | N/A |
| Female Ado: Menstruation (Percent menstruating) | 84% (83/99) | 83% (87/105) | N/A |
| Female Ado: Menarche [M ± SD; years; (n)] | 11.8 ± 1.4 (83) | 12.1 ± 1.4 (87) | N/A |
| Puberty [M ± SD; Tanner stage; (n)] | 3.75 ± 0.9 (191) | 3.86 ± 0.85 (173) | N/A |
| Sibship Size [M ± SD (n)] | 2.2 ± 0.45 (89) | 2.1 ± 0.39 (82) | 2.2 ± 0.4 (24) |
| Mother: Age [M ± SD (n)] | 42.01 ± 3.65 (89) | 41.6 ± 3.6 (82) | 41.7 ± 3.8 (24) |
| Father: Age [M± SD (n)] | 44.8 ± 3.8 (88) | 43.96 ± 3.3 (82) | 44.4 ± 4.5 (24) |
| A07f: Mother: Edu [M ± SD (n)] | 3.3 ± 0.9 (78) | 3.3 ± 0.8 (67) | 3.7 ± 0.9 (20) |
| A07f: Father: Edu [M ± SD (n)] | 3.6 ± 0.9 (74) | 3.3 ± 0.8 (66) | 3.5 ± 0.96 (19) |
| B09f: Mother: Edu [M ± SD (n)] | 4.7 ± 1.5 (82) | 4.5 ± 1.45 (71) | 5.5 ± 1.4 (19) |
| B09f: Father: Edu [M ± SD (n)] | 5.3 ± 1.7 (77) | 4.7 ± 1.6 (66) | 5.1 ± 1.4 (19) |
| Mother: Employment [M ± SD (n)] | 2.5 ± 1.8 (86) | 2.7 ± 1.9 (81) | 2.3 ± 2.1 (24) |
| Father: Employment [M ± SD (n)] | 1.6 ± 1.6 (83) | 1.7 ± 1.6 (75) | 1.3 ± 1.3 (23) |
| Household income [in thousands CAD, M ± SD (n)] | 54.9 ± 21.4 (87) | 51.7 ± 25.4 (81) | 59.2 ± 18.5 (24) |
| Perceived economic situation [M ± SD (n)] | 1.9 ± 0.6 (84) | 1.9 ± 0.6 (75) | 1.7 ± 0.5 (23) |
| Number of children in household [M ± SD (n)] | 2.46 ± 0.7 (84) | 2.3 ± 0.6 (75) | 2.4 ± 0.8 (23) |
| Number of persons in household [M ± SD (n)] | 4.57 ± 0.9 (84) | 4.3 ± 0.8 (74) | 4.5 ± 1.03 (23) |
| C. Pregnancy, birth, and breastfeeding | |||
| Number of pregnancies [M ± SD (n)] | 3.3 ± 1.2 (89) | 3.2 ± 1.4 (81) | 3.58 ± 1.5 (24) |
| Number of spontaneous abortion and stillbirths [M ± SD (n)] | 0.48 ± 0.8 (89) | 0.54 ± 0.9 (81) | 0.79 ± 1.14 (24) |
| Pregnancy duration [weeks; M ± SD (n)] | 39.1 ± 1.6 (206) | 39.2 ± 1.5 (198) | N/A |
| Pregnancy and birth complications [M ± SD (n)] | 0.28 ± 0.5 (208) | 0.26 ± 0.5 (195) | N/A |
| Birth weight (g; M ± SEM) | 3530 ± 468 (205) | 3239 ± 486 (195) | N/A |
| Breastfeeding: Frequency of yes (total n) | 116 (206) | 61 (195) | N/A |
| Breastfeeding: Total duration [age in weeks; M ± SD (n)] | 18.6 ± 16.3 (116) | 15.2 ± 12.3 (61) | N/A |
| Breastfeeding: First other milk [age in weeks; M ± SD (n)] | 13.8 ± 12.5 (116) | 11.3 ± 8.7 (61) | N/A |
| Breastfeeding: First other liquid [age in weeks; M ± SD (n)] | 12.6 ± 9.3 (208) | 10.45 ± 8.6 (193) | N/A |
| Breastfeeding: First solids [age in weeks; M ± SD (n)] | 14.02 ± 9.7 (208) | 12.2 ± 8.5 (191) | N/A |
| D. Medical History, psychiatric history and antisocial behavior | |||
| Child's medical history: Positive answers [M ± SD (n)] | 0.09 ± 0.3 (208) | 0.1 ± 0.32 (195) | N/A |
| Ado: History of head trauma (Frequency) | 27/200 | 19/191 | N/A |
| Ado: Psychiatric symptoms (Sum of DPS symptoms) | 15.5 ± 11.5 (201) | 18.8 ± 12.8 (188) | N/A |
| Family's Medical History, Mother's side: Positive answers [M ± SD (n)] | 3.7 ± 2.01 (88) | 3.2 ± 2.2 (81) | 3.04 ± 2.2 (24) |
| Family's Medical History, Father's side: Positive answers [M ± SD (n)] | 3.98 ± 2.3 (88) | 3.3 ± 2.2 (81) | 3.5 ± 2.00 (24) |
| Mother's depression [M ± SD (n)] | 16.25 ± 4.0 (84) | 17.1 ± 4.0 (77) | 17.04 ± 5.4 (23) |
| Mother's anxiety [M ± SD (n)] | 19.3 ± 5.98 (84) | 19.1 ± 4.9 (77) | 18.3 ± 4.1 (23) |
| Father's depression [M ± SD (n)] | 16.5 ± 4.1 (82) | 15.8 ± 4.3 (73) | 16.7 ± 5.6 (23) |
| Father's anxiety [M ± SD (n)] | 18.1 ± 5.9 (82) | 17.3 ± 4.96 (73) | 17.7 ± 5.4 (23) |
| Mother's antisocial behavior in adolescence [M ± SD (n)] | 0.13 ± 0.4 (84) | 0.36 ± 0.6 (77) | 0.17 ± 0.4 (23) |
| Mother's antisocial behavior in aduthood [M ± SD (n)] | 0.05 ± 0.26 (84) | 0.05 ± 0.28 (77) | 0.04 ± 0.21 (23) |
| Father's antisocial behavior in adolescence [M ± SD (n)] | 0.39 ± 0.86 (82) | 0.7 ± 0.9 (73) | 0.4 ± 0.9 (23) |
| Father's antisocial behavior in adulthood [M ± SD (n)] | 0.16 ± 0.46 (82) | 0.24 ± 0.6 (73) | 0.13 ± 0.46 (23) |
| Ado's physical aggression [M ± SD (n)] | 3.34 ± 0.9 (200) | 3.3 ± 0.9 (181) | N/A |
| Ado's indirect aggression [M ± SD (n)] | 6.3 ± 1.6 (200) | 6.3 ± 1.5 (181) | N/A |
| Ado's fighting [M ± SD (n)] | 5.2 ± 0.8 (200) | 5.2 ± 0.8 (181) | N/A |
| Ado's stealing [M ± SD (n)] | 4.6 ± 1.4 (200) | 4.7 ± 1.4 (181) | N/A |
| Ado's vandalism [M ± SD (n)] | 2.1 ± 0.4 (200) | 2.2 ± 0.6 (181) | N/A |
M, Mean; SD, Standard deviation; F, Female; M, Male; CAD, Canadian dollars; Exposed family = all children exposed to maternal cigarette smoking during pregnancy; Non‐exposed family = no children exposed; Mixed family = some children exposed, some non‐exposed.
Bold indicates statistical significant differences between the groups (see results for details).
Demographics
The exposed and nonexposed groups do not differ in their mean age and sex distribution (Table IB). All subjects are of French–Canadian origin and live within the region (98,709 km2). The two groups do not differ in the different measures of SES, including the level of maternal education (used for matching nonexposed adolescents to the exposed ones), level of employment of both parents and household income (Table IB).
Pregnancy, Birth Weight, and Breastfeeding
Duration of pregnancy did not differ between the two groups; but note that pregnancy shorter than 35 weeks was exclusionary (Table IC). As expected, birth weight of the exposed offspring was lower compared with the nonexposed offspring by 291 g, or 8.5% (Table IC; P < 0.0001; d = 0.6). When comparing the difference between exposed and nonexposed subjects, the effect of exposure was similar in males (d = 0.57) and females (d = 0.61). In the exposed males, we observed a small but significant dose effect in that the number of cigarettes smoked during the 2nd trimester of pregnancy correlated negatively with the birth weight (r 2 = 0.05, F(1,90) = 4.6, P = 0.04). This was not the case in the exposed female subjects. Exposed adolescents were less likely to have been breastfed (ξ 2 = 25.7, P < 0.0001); when breastfed though, there were no group differences in the total duration of breastfeeding (Table IC).
Medical History, Psychiatric Symptoms, and Antisocial Behavior
The two groups were comparable in the overall frequency of positive answers to various questions related to the children's medical history (Table ID). As for the mental health of the parents at the time of the home visit, we found no significant group differences on the scales of depression and anxiety reported by the mothers and fathers in the Mental Health and Antisocial Behavior questionnaire. We have observed no differences in the current antisocial behavior (Table ID) of the mothers and fathers. But we found a slightly higher score on the scale of antisocial behavior during adolescence in mothers of exposed versus nonexposed adolescents (Table ID; F(3,188) = 2.95, P = 0.03, d = 0.38).
Familiality of Brain Size and Volumes of Grey and White Matter
Using a fully automatic processing pipeline (see earlier), we have derived the following measures: brain size (based on the combination of X, Y, and Z scaling factors derived from the nine‐parameter linear registration of each brain to the SYS333 template aligned with the MNI305 atlas), and the volumes of GM and WM constituting each of the four lobes (frontal, parietal, temporal, and occipital) in the left and right hemispheres. The initial analysis included 337 adolescents who passed our quality control of the registration (linear for brain size, nonlinear for volumes) and tissue classification (GM, WM, CSF) steps of the processing pipeline. This sample gives rise to a total of 171 sibships consisting of 38 brother–brother, 75 brother–sister, and 58 sister–sister pairs. Given the significant effect of age on GM and WM volumes, intra‐class correlations were computed using age‐adjusted residuals.
Table II lists ICC computed for brain size, as well as for the relative (i.e. brain‐size corrected) volumes of GM and WM constituting each of the four lobes in the left and right hemispheres; GM and WM volumes were age‐adjusted using linear regressions carried out separately for male and female adolescents. Pooled across the different types of siblings (171 pairs), familiarity indexed by ICC varied between 0.141 (occipital WM volume) and 0.337 (temporal GM volume). Overall, familiarity appeared somewhat higher for GM than WM but comparable for the left and right hemispheres. Familiality (ICC) values were highest in brother–brother sibpairs (0.454–0.543 for GM), as compared with sister–sister (0.221–0.399 for GM) and brother–sister (0.324–0.429 for GM) sibpairs.
Table II.
Familiality (intraclass correlation coefficients)
| Lobe, Tissue | Brother–Brother (n = 38) | Sister–Sister (n = 58) | Brother–Sister (n = 75) | Siblings (n = 171) |
|---|---|---|---|---|
| Left hemisphere | ||||
| Frontal, Grey | 0.45 | 0.37 | 0.39 | 0.33 |
| Frontal, White | 0.44 | 0.38 | 0.29 | 0.25 |
| Parietal, Grey | 0.47 | 0.40 | 0.37 | 0.34 |
| Parietal, White | 0.30 | 0.38 | 0.34 | 0.23 |
| Temporal, Grey | 0.50 | 0.30 | 0.43 | 0.33 |
| Temporal, White | 0.16 | 0.32 | 0.33 | 0.21 |
| Occipital, Grey | 0.47 | 0.28 | 0.32 | 0.31 |
| Occipital, White | 0.37 | 0.09 | 0.21 | 0.15 |
| Right hemisphere | ||||
| Frontal, Grey | 0.46 | 0.36 | 0.36 | 0.30 |
| Frontal, White | 0.41 | 0.39 | 0.30 | 0.24 |
| Parietal, Grey | 0.50 | 0.34 | 0.35 | 0.31 |
| Parietal, White | 0.31 | 0.35 | 0.33 | 0.23 |
| Temporal, Grey | 0.54 | 0.30 | 0.42 | 0.33 |
| Temporal, White | 0.23 | 0.33 | 0.30 | 0.20 |
| Occipital, Grey | 0.49 | 0.22 | 0.34 | 0.32 |
| Occipital, White | 0.30 | 0.19 | 0.13 | 0.14 |
| Brain size | 0.42 | 0.33 | 0.35 | 0.27 |
Figure 4 illustrates cross‐trait familiarity in brother–brother and sister–sister pairs. In the former group of pairs, for example, GM volumes in the temporal lobe show relatively high cross‐trait correlations across the two hemispheres. In sister–sister pairs, such a pattern is present for the frontal and parietal volumes.
Figure 4.
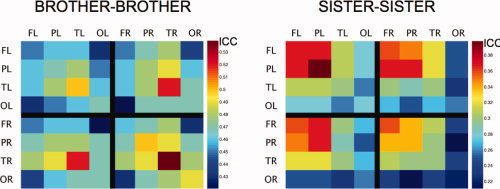
Familiality of brain‐size corrected grey‐matter volumes. Estimates of intra‐trait (e.g. FL vs. FL) and cross‐trait (e.g. FL–PL) familiality indexed by intra‐class correlation coefficients computed for brother‐brother (left; 38 pairs) and sister‐sister (right; 58 pairs) pairs. F, P, T, and O denote, respectively, Frontal, Parietal, Temporal, and Occipital lobes. L and R denote the Left and Right hemispheres.
DISCUSSION
The main purpose of this paper is the description of the SYS. Nonetheless, the few initial findings mentioned here are largely consistent with the existing literature. For example, birth weight of offspring born to mothers who smoked during pregnancy, as compared with the nonexposed control group, is lower by 8.5% (291 g). Note, however, that we have not adjusted our data using any of the possible confounding variables and corrected for within‐sibling similarity; this will be done in the upcoming detailed reports of our findings. One of the possible limitations of the SYS is the retrospective nature of the questionnaire‐based assessments of perinatal events, most importantly the exposure to maternal cigarette smoking during pregnancy. But as concluded by Rice et al. [2006b] in their recent paper on the reliability of maternal reports of various perinatal events, “mothers can provide accurate reports in comparison to information from medical records.” In their study, which compared medical records with questionnaire‐based maternal reports obtained 4–9 years later, Kappa statistics varied between “very good” for smoking during pregnancy (κ = 0.81) and “poor” for alcohol during pregnancy (κ = 0.17). For the birth weight, the correlation between the two time‐points was excellent (r = 0.99). In our study, Kappa statistics for smoking during pregnancy was comparable to the Rice et al. findings (κ = 0.69).
The sibship‐based design of the SYS allows us to examine familiarity of the range of phenotypes collected in our sample, from questionnaire‐based assessment of personality, through cognitive performance, to brain structure. Although we cannot distinguish between the effect of shared environment and genes, respectively, and calculate true heritability of a given phenotype, familiarity values are informative in different respects. First of all, we can compare familiarity of different phenotypes, such as the volumes of grey and white matter, both within and across different types of siblings (brother–brother vs. sister–sister); in the initial sample, familiarity appears higher for grey than white matter especially for male adolescents. This may reflect continuing maturation of white matter in boys, compared with girls, during this period of human development. Wallace et al. [2006] provides comparable data for monozygotic and dizygotic twins in the age range between 5 and 18 years; the number of same‐sex pairs varied between 52 male MZ pairs and 15 female DZ pairs. Overall, values of ICC in the DZ twins (male and female pairs combined) varied between 0.29 (parietal GM) and 0.65 (temporal WM). After splitting the groups into “younger” and “older” subgroups, Wallace et al. observed lower ICC values in older versus younger DZ twins for GM volumes and vice versa for WM volumes, with no changes for the MZ twins. If we consider sex‐differences in the stage of sexual maturation, that is female adolescents being ahead of male adolescents, this pattern is consistent with our observation of sex differences in familiarity: lower ICC in sister–sister versus brother–brother pairs for GM and vice versa for WM. Note, however, that the above pattern of differences in ICC is not statistically significant and may not be stable given relatively low number of pairs (38 brother–brother and 58 sister–sister pairs). As the study progresses, the number of available pairs will increase thus providing more statistical power and reliability for the estimates of familiarity. Finally, the access to a wide range of quantitative phenotypes both within the brain and behavior domain and across the brain and body (i.e. cardiovascular and metabolic phenotypes) will provide unique opportunities for estimating cross‐trait familiarity. This approach is illustrated using cross‐lobe familiarity estimates for brother–brother and sister–sister datasets (Fig. 4).
Inclusion of the genetic component in the SYS is motivated conceptually by the emerging view in which the expression of a given phenotype is determined by a combination of environmental and genetic factors [Caspi et al., 2003; Pausova et al., 1999; Rice et al., 2006b; Thapar et al., 2005; Wang et al., 2002]. Identifying genes that modulate individuals' response to PEMCS should also provide the initial pointers vis‐à‐vis possible molecular pathways, and hence possible mechanisms, mediating such effects. The family‐based design involving sibships (full phenotyping and genotyping) and their parents (partial phenotyping and genotyping) will allow us to carry out genome‐wide linkage and association analyses, and identify regions of the genome not necessarily predicted by previous knowledge. At the same time, candidate‐gene studies can also be used to test specific hypotheses. The reduced genetic heterogeneity of a geographically isolated population should increase the power of these analyses, as the number of genes involved in determining individual complex traits is expected to be lower in this population than in multiethnic populations (e.g. Montreal metropolitan area). The overall advantage of the genetic approach is the control of yet another variable—the genome—that may explain some of the variance in the studied phenotypes. Disadvantages of this approach, other than its additional cost, include organizational demands associated with the siting of the study in a specific geographical location and a selective recruitment (siblings only).
The full phenotype is being acquired in adolescents 12–18 years of age. Adolescence is a highly dynamic period of human development during which the children's brains and bodies adopt adult roles. It is also a time when adolescents begin to make their own choices affecting their health: sexual behavior, eating habits, physical activity, consumption of alcohol, cigarette smoking, and the use of recreational drugs, to name but a few. What are the factors determining such choices? The relatively comprehensive design of the SYS and its large sample size (n = 1,000) should provide a suitable platform for predicting variance in a particular phenotype, such as blood pressure, cardiovascular reactivity, and school performance, using multiple independent variables capturing the individual's environment and life habits, his/her genetic background and the state of other organs (e.g. fat metabolism, brain morphology). The structure and function of the adolescent's organs are less likely to be affected by a disease process that requires treatment, a situation quite common in adults; this minimizes possible confounding effects of medication. Furthermore, recent epidemiological studies suggest that processes underlying the development of common disorders, such as the metabolic syndrome, may be emerging as early as in adolescence [Weiss et al., 2004]. Thus, adolescence may be a critical period during which meaningful prevention strategies are still possible.
The inter‐disciplinary design of the SYS requires input from many disparate fields, such as medicine, computer science, genetics and statistics, physics, psychology, and sociology. Although the different conceptual background of investigators working in the respective fields often hampers the initial communication and the setting of common goals, the richness of the various approaches and tools benefits the program at the end. To succeed, individual investigators must be willing to adapt their approaches to fit the overall design. For example, questionnaires must be designed in a way that maximizes collection of quantitative data based on an individual (e.g. a composite index of academic achievement) rather than a group (e.g. frequency of individuals with a particular grade in math). Or, MR acquisition protocols must be robust to allow for a consistent collection of images over a long period of time (e.g. 5 years) at the expense, perhaps, of fine‐tuning the acquisition as new sequences are developed. As the data become available, the biggest challenge is faced by colleagues working at the interface between data processing (bioinformatics, computer science, and statistics) and their biological interpretation (genetics, psychology, sociology, and medicine). In our view, this challenge can be best met by having a core group of investigators working in the same physical location for an extended period of time.
Overall, we hope that the above description of the SYS will be helpful to colleagues interested in similar studies of environmental and genetic factors influencing human health.
Supporting information
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .
Exclusion and inclusion criteria
Instruments
Questionnaires
Acknowledgements
The authors thank the following individuals for their contributions in designing the protocol, acquiring, and analyzing the data: MR technicians (Sylvie Masson, Suzanne Castonguay, Julien Grandisson, Marie‐Josée Morin), cardio nurses (Jessica Blackburn, Mélanie Gagné, Jeannine Landry, Lisa Pageau, Réjean Savard, Jacynthe Tremblay), psychometricians (Chantale Belleau, Mélanie Drolet, Catherine Harvey, Stéphane Jean, Mélanie Tremblay Hélène Simard), ÉCOBES team (Julie Auclair, Marie‐Ève Blackburn, Marie‐Ève Bouchard, Annie Houde, Catherine Lavoie), nutritionists (Caroline Benoit and Henriette Langlais), laboratory technicians (Denise Morin and Nadio Mior), Dr. Michel Bérubé, Julie Bérubé, Celine Bourdon, Rosanne Aleong, Dr. Jennifer Barrett, Candice Cartier, Valerie Legge, Helena Jelicic, and Dale Einarson.
Contributor Information
Zdenka Pausova, Email: zdenka.pausova@nottingham.ac.uk.
Tomás˘ Paus, Email: tomas.paus@nottingham.ac.uk.
REFERENCES
- Aleong R,Duchesne S,Paus T ( 2007): Assessment of Adolescent Body Perception: Development and Characterization of a Novel Tool for Morphing Images of Adolescent Bodies. Behavior Research Methods (in press). [DOI] [PubMed] [Google Scholar]
- Ashburner J,Friston KJ ( 2000): Voxel‐based morphometry—The methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- Bada HS,Korones SB,Perry EH,Arheart KL,Pourcyrous M,Runyan JW III,Anderson GD,Magill HL,Fitch CW,Somes GW ( 1990): Frequent handling in the neonatal intensive care unit and intraventricular hemorrhage. J Pediatr 117(1, Part 1): 126–131. [DOI] [PubMed] [Google Scholar]
- Behar L,Stringfield S ( 1974): A behavior rating scale for the preschool child. Dev Psychol 10: 601–640. [Google Scholar]
- Belanger PW,Rocher G ( 1972): Le projet de recherche : Étude des aspirations scolaires et des orientations professionnelles des étudiants (ASOPE). L'orientation professionnelle 8: 114–127. [Google Scholar]
- Benson PL,Leffert N,Scales PC,Blyth DA ( 1998): Beyond the “village” rhetoric: Creating healthy communities fro children and adolescents. Appl Dev Sci 2: 138–159. [Google Scholar]
- Beratis NG,Panagoulias D,Varvarigou A ( 1996): Increased blood pressure in neonates and infants whose mothers smoked during pregnancy. J Pediatr 128: 806–812. [DOI] [PubMed] [Google Scholar]
- Blake KV,Gurrin LC,Evans SF,Beilin LJ,Landau LI,Stanley FJ,Newnham JP ( 2000): Maternal cigarette smoking during pregnancy, low birth weight and subsequent blood pressure in early childhood. Early Hum Dev 57: 137–147. [DOI] [PubMed] [Google Scholar]
- Brennan PA,Grekin ER,Mednick SA ( 1999): Maternal smoking during pregnancy and adult male criminal outcomes. Arch Gen Psychiatry 56: 215–219. [DOI] [PubMed] [Google Scholar]
- Breslau N,Chilcoat HD ( 2000): Psychiatric sequelae of low birth weight at 11 years of age. Biol Psychiatry 47: 1005–1011. [DOI] [PubMed] [Google Scholar]
- Buchanan CM Holmbeck GN ( 1998): Measuring beliefs about adolescent personality and behavior. J Youth Adolesc 27: 607–627. [Google Scholar]
- Bueller JA,Aftab M,Sen S,Gomez‐Hassan D,Burmeister M,Zubieta JK ( 2006): BDNF Val66Met allele is associated with reduced hippocampal volume in healthy subjects. Biol Psychiatry 59: 812–815. [DOI] [PubMed] [Google Scholar]
- Cohen J ( 1988): Statistical power analysis for the behavioral sciences. Hillsdale, NJ: Erlbaum Associates. [Google Scholar]
- Caspi A,Sugden K,Moffitt TE,Taylor A,Craig IW,Harrington H,McClay J,Mill J,Martin J,Braithwaite A,Poulton R ( 2003): Influence of life stress on depression: Moderation by a polymorphism in the 5‐HTT gene. Science 301: 386–389. [DOI] [PubMed] [Google Scholar]
- Cocosco CA,Zijdenbos AP,Evans AC ( 2003): A fully automatic and robust MRI tissue classification method. Med Image Anal 7: 513–527. [DOI] [PubMed] [Google Scholar]
- Collins DL,Neelin P,Peters TM,Evans AC ( 1994): Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr 18: 192–205. [PubMed] [Google Scholar]
- Collins DL,Holmes CJ,Peters TM,Evans AC ( 1995): Automatic 3D model‐based neuroanatomical segmentation. Hum Brain Mapp 3: 190–208. [Google Scholar]
- Cornelius MD,Leech SL,Goldschmidt L,Day NL ( 2000): Prenatal tobacco exposure: Is it a risk factor for early tobacco experimentation? Nicotine Tob Res 2: 45–52. [DOI] [PubMed] [Google Scholar]
- De Braekeleer M ( 1991): Hereditary disorders in Saguenay‐Lac‐St‐Jean (Quebec, Canada). Hum Hered 41: 141–146. [DOI] [PubMed] [Google Scholar]
- De Braekeleer M,Mari C,Verlingue C,Allard C,Leblanc JP,Simard F,Aubin G,Ferec C ( 1998): Complete identification of cystic fibrosis transmembrane conductance regulator mutations in the CF population of Saguenay‐Lac‐Saint‐Jean (Quebec, Canada). Clin Genet 53: 44–46. [DOI] [PubMed] [Google Scholar]
- Dousset V,Grossman RI,Ramer KN, et al. ( 1992): Experimental allergic encephalomyelitis and multiple sclerosis: Lesion characterization with magnetization transfer imaging. Radiology 182: 483–491. [DOI] [PubMed] [Google Scholar]
- Ebrahim SH,Decoufle P,Palakathodi AS ( 2000): Combined tobacco and alcohol use by pregnant and reproductive‐aged women in the United States. Obstet Gynecol 96: 767–771. [DOI] [PubMed] [Google Scholar]
- Eisenberg N,Fabes RA,Murphy BC,Karbon M,Smith M,Maszk P ( 1996): The relations of children's dispositional empathy‐related responding to their emotionality. Dev Psychol 32: 195–209. [DOI] [PubMed] [Google Scholar]
- Enquête nationale sur la santé de la population (National Population Health Survey) Available at http://www.statcan.ca/english/concepts/nphs/nphs1.htm; http://www.phac-aspc.gc.ca/dca-dea/7-18yrs-ans/hbschealth_e.html.
- Evans AC,Collins DL,Mills SR,Brown ED,Kelly RL,Peters TM ( 1993): 3D statistical neuroanatomical models from 305 MRI volumes. In: Proceedings of theIEEE Nuclear Science Symposium and Medical Imaging Conference, San Francisco. pp 1813–1817.
- Falk L,Nordberg A,Seiger A,Kjaeldgaard A,Hellstrom‐Lindahl E ( 2005): Smoking during early pregnancy affects the expression pattern of both nicotinic and muscarinic acetylcholine receptors in human first trimester brainstem and cerebellum. Neuroscience 132: 389–397. [DOI] [PubMed] [Google Scholar]
- Fischl B,Dale AM ( 2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97: 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund AM,Bates PB ( 2002): Life‐management strategies of selection, optimization and compensation: Measurement by self‐report and construct validity. J Pers Soc Psychol 82: 642–662. [PubMed] [Google Scholar]
- Fried PA ( 1995): Prenatal exposure to marihuana and tobacco during infancy, early and middle childhood: Effects and an attempt at synthesis. Arch Toxicol Suppl 17: 233–260. [DOI] [PubMed] [Google Scholar]
- Galland O ( 1994): Âge et valeurs. Dans H. Riffault (dir.). Les valeurs des Français. Paris: PUF. 332 p.
- Gallistel CR,Gelman II ( 2000): Non‐verbal numerical cognition: From reals to integers. Trends Cogn Sci 4: 59–65. [DOI] [PubMed] [Google Scholar]
- Gass A,Barker GJ,Kidd D,Thorpe JW,MacManus D,Brennan A,Tofts PS,Thompson AJ,McDonald WI,Miller DH ( 1994): Correlation of magnetization transfer ratio with clinical disability in multiple sclerosis. Ann Neurol 36: 62–67. [DOI] [PubMed] [Google Scholar]
- Gradie MI,Jorde LB,Bouchard G ( 1988): Genetic structure of the Saguenay, 1852–1911: evidence from migration and isonymy matrices. Am J Phys Anthropol 77: 321–333. [DOI] [PubMed] [Google Scholar]
- Grompe M,St. Louis M,Demers S,Al‐Dhalimy M,Leclerc B,Tanguay R ( 1994): A single mutation in the fumaryl acetoacetate hydrolase gene in French Canadians with hereditary tyrosinemia type I. N Engl J Med 331: 353–357. [DOI] [PubMed] [Google Scholar]
- Guimond J,Meunier J,Thirion J‐P ( 2000): Average Brain Models: A Convergence Study. Comput Vis Image Understand 77: 192–210. [Google Scholar]
- Harter S ( 1983): Supplementary description of the self‐perception profile for children: Revision of the perceived competence scale for children (unpublished manuscript). Denver, CO: University of Denver. [Google Scholar]
- Health Canada ( 1995a): Fact Sheet 5. Selected TDRS Priority Groups. Survey on Smoking in Canada, Cycle 4. Ottawa, June 1995.
- Health Canada ( 1995b): Fact Sheet 4. Smoking behaviour of women—November 1994. Survey on Smoking in Canada, Cycle 3. Ottawa, August 1995.
- Institut de la statistique du Québec ( 2001):L'enquête sociale et de santé 1998. Division Santé Québec, Collection la santé et le bien‐être. Sainte‐Foy: Publications du Québec. p 642.
- International HapMap Consortium ( 2005): A haplotype map of the human genome. Nature 437: 1299–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janosz M,Lacroix M,et Rondeau N ( 1999): A decisionnal balance measure for assessing school disengagement in adolescence. Communication présenté au Biennal meeting of the society for research in Child Development (SRCD), Alburqueque, NM.
- Japel C,Tremblay RE,McDuff P ( 2000): Parent's Health and Social Adjustment, Part I – Lifestyle Habits and Health Status” in Longitudinal Study of Child Development in Québec (ÉLDEQ 1998‐2002), Québec, Institut de la statistique du Québec, Vol. 1, No. 9. [Google Scholar]
- Jennrich R,Schluchter M ( 1986): Unbalanced repeated‐measures models with structured covariance matrices. Biometrics 42: 805–820. [PubMed] [Google Scholar]
- Kallen K ( 2000): Maternal smoking during pregnancy and infant head circumference at birth. Early Hum Dev 58: 197–204. [DOI] [PubMed] [Google Scholar]
- Kandel DB,Udry JR ( 1999): Prenatal effects of maternal smoking on daughters' smoking: Nicotine or testosterone exposure? Am J Public Health 89: 1377–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick JS,Merritt RK ( 1996): Women and smoking: An update for the 1990s. Am J Obstet Gynecol 175: 528–535. [DOI] [PubMed] [Google Scholar]
- Kramer MS ( 2000): Invited commentary: Association between restricted fetal growth and adult chronic disease: Is it causal? Is it important? Am J Epidemiol 152: 605–608. [DOI] [PubMed] [Google Scholar]
- Le Blanc M ( 1996): MASPAQ: Mesures de l'adaptation sociale et personnelle pour les adolescents québécois. Manuel et guide d'utilisation 3rd éd. Montréal,École de psychoéducation, Groupe de recherche sur les adolescents en difficulté, Université de Montréal. Logiciel MASPAQ (Windows et MacIntosh).
- LeBlanc M,Tremblay RE ( 1988): A study of factors associated with the stability of hidden delinquency. Int J Adolesc Youth 1: 269–292. [Google Scholar]
- Lambers DS,Clark KE ( 1996): The maternal and fetal physiologic effects of nicotine. Semin Perinatol 20; 115–126. [DOI] [PubMed] [Google Scholar]
- Landis JR,Koch GG ( 1977): The measurement of observer agreement for categorical data. Biometrics 33: 159–174. [PubMed] [Google Scholar]
- Larose S,Boivin M ( 1998): Attachment to parents, social support expectations, and socioemotional adjustment during the high school—College transition. J Res Adolesc 8: 1–27. [Google Scholar]
- Lassen K,Oei TP ( 1998): Effects of maternal cigarette smoking during pregnancy on long‐term physical and cognitive parameters of child development. Addict Behav 23: 635–653. [DOI] [PubMed] [Google Scholar]
- Leffert N,Benson PL,Scales PC,Sharma AR,Drake DR,Blyth DA ( 1998): Developmental assets: Measurement and prediction of risk behaviors among adolescents. Appl Dev Sci 2: 209–230. [Google Scholar]
- Le Goualher G,Procyk E,Collins DL,Venugopal R,Barillot C,Evans AC ( 1999): Automated extraction and variability analysis of sulcal neuroanatomy. IEEE Trans Med Imaging 18: 206–217. [DOI] [PubMed] [Google Scholar]
- Lerch J,Evans AC ( 2005): Cortical thickness analysis examined through power analysis and a population simulation. Neuroimage 24: 163–173. [DOI] [PubMed] [Google Scholar]
- Lerner RM,Lerner JV,Almerigi J,Theokas C,Phelps E,Gestsdottir S,Naudeau S,Jelicic H,Alberts A,Ma L,Smith L,Bobek D,Simpson I,Christiansen E,von Eye A ( 2005): Positive youth development, participation in community youth development programs and contributions of fifth grade adolescents: Findings from the first wave of the 4‐h study of positive youth development (special issue). J Early Adolesc 25(1). [Google Scholar]
- Liang K,Zeger S ( 1986): Longitudinal data analysis using generalized linear models. Biometrics 73: 13–22. [Google Scholar]
- Loiselle J ( 2000): Enquête québécoise sur le tabagisme chez les élèves du secondaire (Rep. No. 1). Québec, QC, Canada: Institut de la Statistique du Québec. [Google Scholar]
- Lucas CP,Zhang H,Fisher PW,Shaffer D,Regier DA,Narrow WE,Bourdon K,Dulcan MK,Canino G,Rubio‐Stipec M,Lahey BB,Friman P ( 2001): The DISC predictive scales (DPS): efficiently screening for diagnoses. J Am Acad Child Adolesc Psychiatry 40: 443–449. [DOI] [PubMed] [Google Scholar]
- MacArthur C,Knox EG,Lancashire RJ ( 2001): Effects at age nine of maternal smoking in pregnancy: Experimental and observational findings. BJOG 108: 67–73. [DOI] [PubMed] [Google Scholar]
- MacDonald D,Kabani N,Avis D,Evans AC ( 2000): Automated extraction of inner and outer surfaces of cerebral cortex from MRI. Neuroimage 11: 564–574. [DOI] [PubMed] [Google Scholar]
- Malo J,Tremblay RE ( 1995): Rates of precocity of sexual intercourse among adolescents of disadvantaged sections of the population. Contracept Fertil Sex 23: 545–551. [PubMed] [Google Scholar]
- Mangin JF,Riviere D,Cachia A,Duchesnay E,Cointepas Y,Papadopoulos‐Orfanos D,Scifo P,Ochiai T,Brunelle F,Regis J ( 2004): A framework to study the cortical folding patterns. Neuroimage 23 ( Suppl 1): S129–S138. [DOI] [PubMed] [Google Scholar]
- McAdoo WG,Weinberger MH,Miller JZ,Fineberg NS,Grim CE ( 1990): Race and gender influence hemodynamic responses to psychological and physical stimuli. J Hypertens 8: 961–967. [DOI] [PubMed] [Google Scholar]
- Meyer‐Lindenberg A,Buckholtz JW,Kolachana B,R Hariri A,Pezawas L,Blasi G,Wabnitz A,Honea R,Verchinski B,Callicott JH,Egan M,Mattay V,Weinberger DR ( 2006): Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci USA 103: 6269–6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milberger S,Biederman J,Faraone SV,Jones J ( 1998): Further evidence of an association between maternal smoking during pregnancy and attention deficit hyperactivity disorder: Findings from a high‐risk sample of siblings. J Clin Child Psychol 27: 352–358. [DOI] [PubMed] [Google Scholar]
- Montgomery SM,Ekbom A ( 2002): Smoking during pregnancy and diabetes mellitus in a British longitudinal birth cohort. Br Med J 324: 26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley R,Payne CL,Lucas A ( 1995): Maternal smoking and blood pressure in 7.5 to 8 year old offspring. Arch Dis Child 72: 120–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JP,Patterson CM,Kosson DS ( 1987): Response preservation in psychopaths. J Abnorm Psychol 96: 145–148. [DOI] [PubMed] [Google Scholar]
- Ntamakiliro L,Monnard I,Gurtner J‐L ( 2000): Mesure de la motivation scolaire des adolescents: Construction et validation de trois échelles complémentaires. L'orientation scolaire et professionnelle 29: 673–693. [Google Scholar]
- Obel C,Henriksen TB,Hedegaard M,Secher NJ,Ostergaard J ( 1998): Smoking during pregnancy and babbling abilities of the 8‐month‐old infant. Paediatr Perinat Epidemiol 12: 37–48. [PubMed] [Google Scholar]
- Offord DR,Boyle MH,Szatmari P,Rae‐Grant NI,Links PS,Cadman DT,Byles JA,Crawford JW,Blum HM,Byrne C,Thomas H,Woodward CA ( 1987): Ontario child health study. II. Six‐month prevalence of disorder and rates of service utilization. Arch Gen Psychiatry 44: 832–836. [DOI] [PubMed] [Google Scholar]
- Olds DL,Henderson CR Jr,Tatelbaum R ( 1994): Intellectual impairment in children of women who smoke cigarettes during pregnancy. Pediatrics 93: 221–227. [PubMed] [Google Scholar]
- Orlebeke JF,Knol DL,Verhulst FC ( 1999): Child behavior problems increased by maternal smoking during pregnancy. Arch Environ Health 54: 15–19. [DOI] [PubMed] [Google Scholar]
- O'Sullivan MJ,Kearney PJ,Crowley MJ ( 1996): The influence of some perinatal variables on neonatal blood pressure. Acta Paediatr 85: 849–853. [DOI] [PubMed] [Google Scholar]
- Paquette D,Morrisson D ( 1999):Un profil descriptif de 100 mères adolescentes: étude préliminaire dans le cadre du projet la Mère veille [A descriptive profile of 100 adolescent mothers : Preliminary study]. Montréal: Institut de recherche pour le développement social des jeunes [Research Institute on Youth Social Development].
- Pausova Z,Paus T,Sedova L,Berube J ( 2003): Prenatal exposure to nicotine modifies kidney weight and blood pressure in genetically susceptible rats: A case of gene‐environment interaction. Kidney Int 64: 829–835. [DOI] [PubMed] [Google Scholar]
- Pausova Z,Tremblay J,Hamet P ( 1999): Gene‐environment interactions in hypertension. Curr Hypertens Rep 1: 42–50. [DOI] [PubMed] [Google Scholar]
- Petersen AC,Crockett L,Richards M,Boxer A ( 1988): A self‐report measure of pubertal status: Reliability, validity, and initial norms. J Youth Adolesc 17: 117–133. [DOI] [PubMed] [Google Scholar]
- Petrides M,Milner B ( 1982): Deficits on subject‐ordered tasks after frontal‐ and temporal‐lobe lesions in man. Neuropsychologia 20: 249–262. [DOI] [PubMed] [Google Scholar]
- Pezawas L,Meyer‐Lindenberg A,Drabant EM,Verchinski BA,Munoz KE,Kolachana BS,Egan MF,Mattay VS,Hariri AR,Weinberger DR ( 2005): 5‐HTTLPR polymorphism impacts human cingulate‐amygdala interactions: A genetic susceptibility mechanism for depression. Nat Neurosci 8; 828–834. [DOI] [PubMed] [Google Scholar]
- Pike GB ( 1996): Pulsed magnetization transfer contrast in gradient echo imaging: A two‐pool analytic description of signal response. Magn Reson Med 36: 95–103. [DOI] [PubMed] [Google Scholar]
- Pike GB,De Stefano N,Narayanan S,Worsley KJ,Pelletier D,Francis GS,Antel JP,Arnold DL ( 2000): Multiple sclerosis: Magnetization transfer MR imaging of white matter before lesion appearance on T2‐weighted images. Radiology 215: 824–830. [DOI] [PubMed] [Google Scholar]
- Pollak SD,Kistler DJ ( 2002): Early experience is associated with the development of categorical representations for facial expressions of emotion. Proc Natl Acad Sci USA 99: 9072–9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power C,Jefferis B ( 2002): Fetal environment and subsequent obesity: A study of maternal smoking. Int J Epidemiol 31: 413–419. [PubMed] [Google Scholar]
- Radloff LS ( 1977): The CES‐D scale: A self‐report depression scale for research in general population. Appl Psychol Meas 1: 385–401. [Google Scholar]
- Räsänen P,Hakko H,Isohanni M,Hodgins S,Järvelin MR,Tiihonen J ( 1999): Maternal smoking during pregnancy and risk of criminal behavior among adult male offspring in the Northern Finland 1966 Birth Cohort. Am J Psychiatry 156: 857–862. [DOI] [PubMed] [Google Scholar]
- Rice F,Harold GT,Thapar A ( 2006a): The effect of birth‐weight with genetic susceptibility on depressive symptoms in childhood and adolescence. Eur Child Adolesc Psychiatry 15: 383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F,Lewis A,Harold G,van den Bree M,Boivin J,Hay DF,Owen MJ,Thapar A ( 2006b): Agreement between maternal report and antenatal records for a range of pre and peri‐natal factors: The influence of maternal and child characteristics. Early Hum Dev. [DOI] [PubMed] [Google Scholar]
- Robins LN,Helzer JE,Croughan J,Ratcliff KS ( 1981): National Institute of Mental Health Diagnostic Interview Schedule. Its history, characteristics, and validity. Arch Gen Psychiatry 38: 381–389. [DOI] [PubMed] [Google Scholar]
- Rosenberg M,Schooler C,Schoenbach C,Rosenberg F ( 1995): Global self‐esteem and specific self‐esteem: Different concepts, different outcomes. Am Sociol Rev 60: 141–156. [Google Scholar]
- Rosenthal D,Gurbey R,Moore S ( 1981): From trust to intimacy: A new inventory for examining Erikson's stages of psychosocial development. J Youth Adolesc 10; 525–535. [DOI] [PubMed] [Google Scholar]
- Roy MP,Kirschbaum C,Steptoe A ( 2001): Psychological, cardiovascular, and metabolic correlates of individual differences in cortisol stress recovery in young men. Psychoneuroendocrinology 26: 375–391. [DOI] [PubMed] [Google Scholar]
- Roy R,Bouchard G,Declos M ( 1991): La premiere generation de Saguenayens In: Bouchard G,De Braekeleer M, editors. Histoire d'un genome. Quebec: Presses de l'Universite du Quebec; pp 164–186. [Google Scholar]
- Rutter M ( 1967): A children's behaviour questionnaire for completion by teachers: Preliminary findings. J Child Psychol Psychiatry 8: 1–11. [DOI] [PubMed] [Google Scholar]
- Schludermann E,Schludermann S ( 1970): Replicability of factors in children's reports of parent behavior (CRPBI). J Psychol 76: 239–249. [Google Scholar]
- Séguin JR,Freeston MH,Tarabulsy GM,Zoccolillo M,Tremblay RE,Carbonneau R ( 2000): Développement des comportements anxieux au préscolaire: De nouvelles mesures et influences familiales. Montréal,Canada, 2 juin: Presented at the annual meeting of Québec's Mental Health Network.
- Selzer ML ( 1971): Michigan alcoholism screening test—Quest for a new diagnostic instrument. Am J Psychiatry 127: 1653. [DOI] [PubMed] [Google Scholar]
- Sigurdsson G,Aspelund T,Chang M,Jonsdottir B,Sigurdsson S,Eiriksdottir G,Gudmundsson A,Harris TB,Gudnason V,Lang TF ( 2006): Increasing sex difference in bone strength in old age: The Age, Gene/Environment Susceptibility‐Reykjavik study (AGES‐REYKJAVIK). Bone 39: 644–651. [DOI] [PubMed] [Google Scholar]
- Slotkin TA ( 1998): Fetal nicotine or cocaine exposure: Which one is worse? J Pharmacol Exp Ther 85: 931–945. [PubMed] [Google Scholar]
- Small SA,Kerns D ( 1993): Unwanted sexual activity among peers during early and miiddle adolescence: Incidence and risk factors. J Marriage Fam 55: 941–952. [Google Scholar]
- Small SA,Rodgers KB ( 1995): Teen Assesment Project (TAP) Survey Question Bank. Madison, WI: University of Wisconsin‐Madison. [Google Scholar]
- Snijder M B,Visser M,Dekker JM,Seidell JC,Fuerst T,Tylavsky F,Cauley J,Lang T,Nevitt M,Harris TB ( 2002): The prediction of visceral fat by dual‐energy X‐ray absorptiometry in the elderly: A comparison with computed tomography and antropometry. Int J Obes 26: 984–993. [DOI] [PubMed] [Google Scholar]
- Steinberg L ( 2002): Resistance to peer pressure (RPI) scale. Instrument developed for the MacArthur Juvenile Adjudicative Competence Study (unpublished measure available from the author). Philadelphia, PA: Department of Psychology, Temple University. [Google Scholar]
- Stephan KM,Binkofski F,Halsband U,Dohle C,Wunderlich G,Schnitzler A, et al. ( 1999): The role of ventral medial wall motor areas in bimanual co‐ordination. A combined lesion and activation study. Brain 122: 351. [DOI] [PubMed] [Google Scholar]
- Stroop JR ( 1935): Studies of interference in serial verbal reactions. J Exp Psychol 18: 643–662. [Google Scholar]
- Talcott JB,Witton C,McLean MF,Hansen PC,Rees A,Green GG,Stein JF ( 2000): Dynamic sensory sensitivity and children's word decoding skills. Proc Natl Acad Sci USA 97: 2952–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J,Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme Medical Publishers. [Google Scholar]
- Thapar A,Langley K,Fowler T,Rice F,Turic D,Whittinger N,Aggleton J,Van den Bree M,Owen M,O'Donovan M ( 2005): Catechol O‐methyltransferase gene variant and birth weight predict early‐onset antisocial behavior in children with attention‐deficit/hyperactivity disorder. Arch Gen Psychiatry 62: 1275–1278. [DOI] [PubMed] [Google Scholar]
- Theokas C,Almerigi JB,Lerner RM,Dowling EM,Benson PL,Scales PC,von Eye A ( 2005): Conceptualizing and modeling individual and ecological asset components of thriving in early adolescence. J Early Adolesc 25: 113–143. [Google Scholar]
- Toschke AM,Montgomery SM,Pfeiffer U,von Kries R ( 2003): Early intrauterine exposure to tobacco‐inhaled products and obesity. Am J Epidemiol 158: 1068–1074. [DOI] [PubMed] [Google Scholar]
- von Kries R,Toschke AM,Koletzko B,Slikker W Jr ( 2002): Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol 156: 954–961. [DOI] [PubMed] [Google Scholar]
- Vaillancourt T,Brendgen M,Boivin M,Tremblay RE ( 2003): A longitudinal confirmatory factor analysis of indirect and physical aggression: Evidence of two factors over time? Child Dev 74: 1628–1638. [DOI] [PubMed] [Google Scholar]
- Wang X,Zuckerman B,Pearson C,Kaufman G,Chen C,Wang G,Niu T,Wise PH,Bauchner H,Xu X ( 2002): Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA 287: 195–202. [DOI] [PubMed] [Google Scholar]
- Weir K,Duveen G ( 1981): Further development and validation of the prosocial behaviour questionnaire for use by teachers. J Child Psychol Psychiatry 22: 357–374. [DOI] [PubMed] [Google Scholar]
- Wakschlag LS,Lahey BB,Loeber R,Green SM,Gordon RA,Leventhal BL ( 1997): Maternal smoking during pregnancy and the risk of conduct disorder in boys. Arch Gen Psychiatry 54: 670–676. [DOI] [PubMed] [Google Scholar]
- Wallace GL,Eric Schmitt J,Lenroot R,Viding E,Ordaz S,Rosenthal MA,Molloy EA,Clasen LS,Kendler KS,Neale MC,Giedd JN ( 2006): A pediatric twin study of brain morphometry. J Child Psychol Psychiatry 47: 987–993. [DOI] [PubMed] [Google Scholar]
- Weiss R,Dziura J,Burgert T,Tamborlane WV,Taksali SE,Yeckel CW,Allen K,Lopes M,Savoye M,Morrison J,Sherwin RS,Caprio S ( 2004): Obesity and the metabolic syndrome in children and adolescents. N Engl J Med 350: 2362–2374. [DOI] [PubMed] [Google Scholar]
- Weissman MM,Wickramaratne PJ,Kandel DB ( 2000): Maternal smoking during pregnancy and psychopathology in offspring followed to adulthood. J Am Acad Child Adolesc Psychiatry 38: 892–899. [DOI] [PubMed] [Google Scholar]
- Wideroe M,Vik T,Jacobsen G,Bakketeig LS ( 2003): Does maternal cigarette smoking during pregnancy cause childhood overweight? Paediatr Perinat Epidemiol 17: 171–179. [DOI] [PubMed] [Google Scholar]
- Williams S,Poulton R ( 1999): Twins and maternal smoking: Ordeals for the fetal origins hypothesis? A cohort study. Br Med J 318: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff SD,Balaban RS ( 1989): Magnetization transfer contrast (MTC) and tissue water proton relaxation in vivo. Magn Reson Med 10: 135–144. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP,Forghani R,Evans AC ( 2002): Automatic 'pipeline' analysis of 3D MRI data for clinical trials: Application to multiple sclerosis IEEE Trans Med Imaging 21: 1280–1291. [DOI] [PubMed] [Google Scholar]
- Zoccolillo M,Price RK,Ji T,Hwu H‐G ( 1999): Antisocial personality disorder: Comparisons of prevalence, symptoms, and correlates in four countries In: Cohen P, Slomkowski C, Robins LLL, editors. Historical and Geographic Effects on Psychopathology. Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/1065-9471/suppmat .
Exclusion and inclusion criteria
Instruments
Questionnaires


