Abstract
Allergen immunotherapy (AIT) is a safe, effective treatment for allergic rhinoconjunctivitis and allergic asthma. However, AIT's clinical effect is still contested—primarily due to heterogeneity in clinical trial designs, study populations, therapeutic formulations, and efficacy criteria. After discussing current concepts and unmet needs, an international panel of experts made several recommendations: (i) explore and validate definitions for (clinical) responders in AIT trials; (ii) use of well‐documented, standardized provocation tests prior to inclusion of subjects with relevant diseases in AIT trials; (iii) monitoring neo‐sensitizations and occurrence of new allergy in extended AIT trials, and exclusion of polyallergic participants; (iv) validation of allergen exposure chambers with regard to natural exposure; (v) in studies of seasonal allergies, focus on peak exposure but also consider organizing two parallel, geographically distinct but otherwise identical trials; (vi) discuss adaptive trial designs with the regulatory authorities; (vii) use e‐health and m‐health technologies to capture more information on individual exposure to allergens; (viii) initiate research on potential psychological, biochemical, immune, neural, and even genomic markers of the placebo response; (ix) identify trial designs and primary endpoints that will give children with allergies easier, faster access to AIT formulations; and (x) promote and apply standardized methods for reporting systemic and local adverse events. The latest technologies and trial designs may provide novel, ethical ways of reducing bias and heterogeneity in AIT clinical trials. There is scope for physicians, patient organizations, companies, and regulators to improve clinical trials in AIT and, ultimately, to provide patients with better treatments.
Keywords: allergen immunotherapy, allergic asthma, allergic rhinoconjunctivitis, clinical development, trial design
Abbreviations
- AEC
allergen exposure chamber
- AIT
allergen immunotherapy
- AR
allergic rhinoconjunctivitis
- CPT
conjunctival provocation test
- CSMS
combined symptom and medication score
- DBPC
double‐blind, placebo‐controlled
- NPT
nasal provocation test
- PIP
pediatric investigation plan
- QoL
quality of life
- RCT
randomized clinical trials
- SCIT
subcutaneous immunotherapy
- SLIT
sublingual immunotherapy
Highlights.
An international panel of experts in allergen immunotherapy (AIT) discussed current concepts and unmet needs in clinical trial methodology in AIT.
Ten domains on recommendations for improvement of future study designs were outlined.
Following these recommendations will help to provide novel, ethical ways of reducing bias and heterogeneity in AIT clinical trials.
1. INTRODUCTION
A large body of evidence from meta‐analyses and systematic reviews of double‐blind, placebo‐controlled, randomized clinical trials (DBPC RCTs) shows that allergen immunotherapy (AIT, whether delivered subcutaneously [SCIT] or sublingually [SLIT]) is a safe, effective treatment for allergic rhinoconjunctivitis (AR) and allergic asthma.1, 2, 3, 4, 5, 6
As such, AIT is the only causal treatment option for allergic patients, as it directly targets the pro‐inflammatory immune response and thus has disease‐modifying properties.7, 8, 9 Accordingly, AIT has the potential to decrease the neo‐sensitization rate (ie, the development of sensitizations to secondary allergens)10 and has been shown to reduce the risk of developing allergic asthma in AR patients11, 12, 13. Accordingly, many medical societies and expert groups have recommended the use of AIT in selected individuals; this mainly covers patients with moderate‐to‐severe AR who either (i) do not gain sufficient relief from symptomatic medications or (ii) do obtain sufficient relief from symptomatic medications but consider that AIT may counter the severity of their AR symptoms and prevent progression to asthma.2, 3, 4, 14, 15, 16, 17 Despite these observations, levels of AIT acceptance (both by patients and physicians) are rather modest, as only a minority of eligible patients receive this treatment option.18, 19
However, there is a high degree of heterogeneity in the clinical trial designs, study populations, therapeutic formulations, and efficacy criteria used in clinical trials on AIT; these include the source of the allergen tested (pollen, house dust mite, animal dander, etc.), the kind of allergen preparation (native allergens vs chemically modified allergens), the administration route (SCIT vs SLIT), and other factors (Figure 1). Recently, the European Academy of Allergy and Clinical Immunology (EAACI) published a systematic review and meta‐analysis of published clinical trials in AIT and strongly emphasized the need for more thorough standardization in designing future trials.1 In response to this need, the EAACI also published a position paper on clinical endpoints in AIT trials and notably proposed a harmonized, standardized definition of the combined symptom and medication score (CSMS) for use as a primary endpoint in future pivotal AIT trials.20 Furthermore, several regulatory authorities, medical societies, and expert groups have issued recommendations on clinical trial design, reporting, and interpretation in the field of allergic disease in general and AIT in particular.21, 22, 23, 24, 25, 26, 27, 28 These recommendations are valuable but tend to emphasize current good practice, rather than the introduction of truly novel approaches. Hence, an international panel of experts in clinical practice and in the clinical development of AIT products met to discuss current standards and important unmet needs in the conception and design of clinical trials in AIT. The present report highlights the challenges and recommendations identified by the group in ten domains (Table 1).
Figure 1.
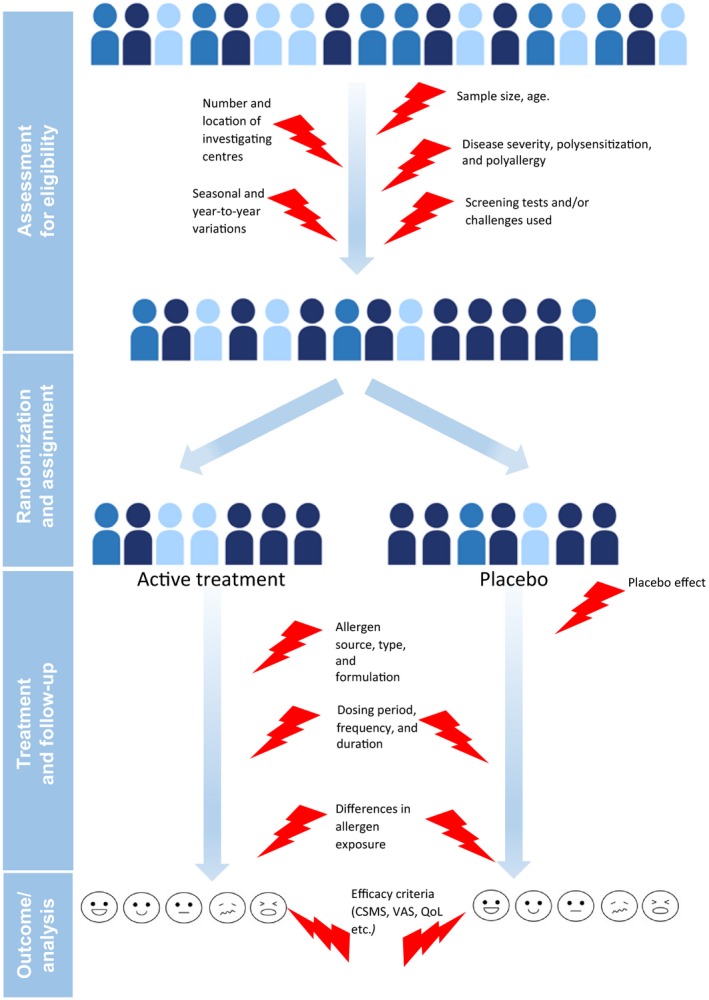
Sources of heterogeneity in double‐blind, placebo‐controlled (DBPC) randomized clinical trials (RCTs) of allergen immunotherapy (AIT) formulations
Table 1.
Domains identified and recommendations made by the expert group
| Domain | Summary of recommendations | |
|---|---|---|
| (i) | Clinical definitions of the response to AIT | Further research should address the important question of (clinically defined) responders in AIT trials |
| (ii) | Inclusion of allergic patients with relevant disease(s) in AIT trials | Trial participants should be systematically screened at least once for target and confounding allergens with an objective, standardized nasal or conjunctival provocation test or a provocation test in an allergen exposure chamber |
| (iii) | Exclusion of polyallergic patients (with clinically relevant, overlapping allergen exposures) in AIT trials | Polysensitized participants can be included in trials, but polyallergic participants with clinical manifestation of symptoms caused by overlapping allergen exposures should preferably be excluded. In multiyear trials, yearly neo‐sensitization assays and allergen challenge tests should be performed in all participants |
| (iv) | AEC facilities in AIT trials | The clinical validation of allergen exposure chambers as an adjunct to or proxy for exposure in the field should be further addressed |
| (v) | Allergen exposure—differences in regional and seasonal exposure | For seasonal allergens, peak pollen periods should be primarily investigated. The organisation of two simultaneous, geographically distinct but otherwise identical trials with identical protocols should be considered. All participants in a Phase III trial on seasonal or perennial allergies should be recruited and evaluated during a single season |
| (vi) | Adaptive trial designs | The introduction of high‐quality, ethical, adaptive trials should be discussed with regulatory bodies. Treatment‐free or placebo‐only baseline periods should not be required, for both ethical and analytical reasons |
| (vii) | Patient‐to‐patient differences in treatment adherence and allergen exposure | With appropriate ethical and privacy safeguards, the use of “e‐health” and “m‐health” technologies is recommended for capturing more information (on an individual patient basis) as a proxy for allergen exposure |
| (viii) | The placebo effect in AIT | Possible psychological, biochemical, immune, neural and even genomic markers of the placebo response by mining data on patients in active treatment and placebo groups should be identified |
| (ix) | Ethical and technical aspects of DBPC RCTs, especially in pediatric populations | New modes for AIT trials in the pediatric population should be identified and implemented – notably to seek to avoid 3 y of placebo treatment and 2 y of post‐treatment (blinded) follow‐up in pediatric trials. Primary endpoints other than a combined symptom and medication score should be considered and further explored in pediatric trials |
| (x) | The importance of safety reporting | World Allergy Organization guidelines for reporting systemic and local adverse events should be applied |
2. METHODOLOGY
The present expert consensus was achieved through a multistep in‐person and electronic process. In an initial in‐person meeting in June 2017, the expert panel explored the issues and the existing guidelines related to clinical trial design, reporting, and interpretation in the field of AIT. The first draft of the consensus was then circulated for comments by the lead author. The suggested revisions were discussed in a second (and final) in‐person meeting in November 2017. This led to the production of a second revision, which was approved by all panel members.
3. RESULTS OF THE DISCUSSION: AN EXPERT CONSENSUS
3.1. Domain (i): clinical definitions of the response to AIT
Recommendation 1: further research should address the important question of [clinically defined] responders in AIT trials.
The definitions of clinically relevant responses to AIT are essential for understanding the therapeutic properties of this disease‐modifying modality. However, there are limited data on responder analyses of DBPC RCTs of AIT. By applying a responder operating characteristic curve analysis, a multicenter trial of SCIT in birch‐allergic adult patients demonstrated that the ideal cutoff for the improvement in a symptom‐medication score in the active group (vs the placebo group) was 30%; based on this definition, 64% of the study participants in the active group and only 32% of the study participants in the placebo group were defined as “AIT responders”.29 Responder analyses should be further investigated in future DBPC RCTs in AIT. The recently published EAACI guideline on AIT in allergic rhinoconjunctivitis stated that the identification of responders (eg, using further stratification approaches) would be useful.2
3.2. Domain (ii): inclusion of allergic patients with relevant disease(s) in AIT trials
Recommendation 2: Trial participants should be systematically screened at least once for target and confounding allergens with an objective, standardized nasal or conjunctival provocation test or a provocation test in an allergen exposure chamber. The difference between (silent) allergen sensitization and the patient symptoms of allergen‐induced AR is critical. Maximization of the active treatment vs placebo difference in efficacy requires intense patient exposure (ie, high allergen levels) and a strong patient response (ie, signs and symptoms when exposed). Sublingual immunotherapy regimens for treating seasonal allergies (such as grass pollen allergy) are typically initiated 2‐4 months before the start of the pollen season.30 In this context, patients are included out of season and thus are asymptomatic at inclusion, with the expectation that they will develop moderate‐to‐severe symptoms once allergen exposure starts. Many trials recruit patients with a history of symptoms (ie, retrospective scoring, which has a number of methodological limitations) or with biomarkers of IgE‐linked sensitization (high absolute and/or relative allergen‐specific IgE serum levels), but this does not guarantee the future occurrence of symptoms. Hence, we suggest that in clinical trials on AIT, well‐defined allergen challenges should be performed on inclusion and then (in extended trials for several years) annually whenever possible. The key to success will be the implementation of a standardized operating procedure by trained, dedicated personnel.31 With the objective of further (internationally) standardizing and harmonizing allergen challenge methods for future trials in AIT, the EAACI recently published a position paper on the standardization of nasal allergen challenges32 and a guideline on conjunctival allergen provocation tests in daily practice.33 However, the type of challenge must be chosen to match the study population's profile; for example, we consider that children are less likely to cooperate during CPTs than during NPTs. Whenever possible, an allergic reaction during a challenge (eg, redness of the conjunctiva in a CPT) should be documented objectively and/or digitally in a format that is compatible with (semi)automated processing (ie, digital photography).34, 35
3.3. Domain (iii): exclusion of polyallergic patients (with clinically relevant, overlapping allergen exposures) in AIT trials
Recommendation 3: Polysensitized participants can be included in trials, but polyallergic participants with clinical manifestation of symptoms caused by overlapping allergen exposures should preferably be excluded. In multiyear trials, yearly neo‐sensitization assays and allergen challenge tests should be performed in all participants.
Most patients consulting a specialist physician for allergic disease will be polysensitized; hence, AIT trials should reflect this by including polysensitized patients. However, polyallergic patients with clinically relevant, overlapping allergen exposures should not be included. In multiyear RCTs, neo‐sensitization (using conventional specific IgE and/or multiplex assays) and the possible occurrence of new allergies (using NPT/CPTs) should be yearly monitored in all participants.
3.4. Domain (iv): AEC facilities in AIT trials
Recommendation 4: The clinical validation of allergen exposure chambers as an adjunct to or proxy for exposure in the field should be further addressed. At present, AECs are not considered for pivotal Phase III studies by regulatory bodies21—mainly because the relationship between allergen exposure in the field and allergen exposure in an AEC has not been validated. Hence, there is a strong need for collaboration between industry, chamber operators, and regulators on field‐AEC correlation studies.36 By analogy with CPTs and NPTs, we particularly encourage AEC vendors/operators to publish data on titrated challenges, that is, the exposure of participants to different levels of allergen for defined periods of time during a single AEC session or during several consecutive sessions in a short space of time.
3.5. Domain (v): allergen exposure—differences in regional and seasonal exposure
Recommendation 5a: For seasonal allergens, peak pollen periods should be primarily investigated. The most accurate assessments of efficacy and safety require the best‐defined disease signal. In pivotal Phase III trials, regulatory authorities should allow a primary efficacy criterion focused on the “peak pollen period” (PPP, as defined in the recent EAACI position paper),37 rather than the pollen season as a whole—the objective being to more closely reflect the patient's unmet needs and clinical demands.
Recommendation 5b: The organisation of two simultaneous, geographically distinct but otherwise identical trials with identical protocols should be considered. The organization of two simultaneous, geographically distinct but otherwise identical trials (rather than a single, geographically dispersed trial that will potentially be weakened by a low‐pollen season or other geographically variable factors) should be considered.
Recommendation 5c: All participants in a Phase III trial on seasonal or perennial allergies should be recruited and evaluated during a single season. Recruitment and evaluation in different seasons are likely to increase heterogeneity and bias.38
3.6. Domain (vi): adaptive trial designs
Recommendation 6a: The introduction of high‐quality, ethical, adaptive trials should be discussed with regulatory bodies. Currently, DBPC RCTs are the gold standard for demonstrating efficacy and safety and thus obtaining a marketing authorization.21 In some disease areas, however, the European Medicines Agency appears to be relatively open to adaptations such as sample size reassessment, population enrichment, and the dropping of treatment arms.39 The potential for the use of adaptive trial designs in AIT should be investigated. Again, upstream, well‐grounded dialogue with the regulatory authorities will be essential.
Recommendation 6b: Treatment‐free or placebo‐only baseline periods should not be required, for both ethical and analytical reasons. A baseline period may provide valuable information on the patients’ disease severity under natural exposure. However, ethical factors and variability in environmental exposure and compliance mean that a year‐long or season‐long “run‐in” or “baseline” period (ie, treatment with symptomatic medications only, no treatments, or placebo only) with symptom scoring should not be considered as a mandatory solution. A baseline period may serve solely to either assess the clinical relationship with exposure or acquire some baseline measurements through which efficacy can be compared with post‐treatment data. In the second case, efficacy must be assessed by directly comparing the placebo and active treatment groups.
3.7. Domain (vii): patient‐to‐patient differences in treatment adherence and allergen exposure
Recommendation 7: With appropriate ethical and privacy safeguards, the use of “e‐health” and “m‐health” technologies is recommended for capturing more information (on an individual patient basis) as a proxy for allergen exposure. As mentioned above, the best possible disease signal is preferable when seeking to establish the true treatment effect of an AIT formulation in field‐based trials. Individual (wearable) allergen traps (for pollen or house dust mite allergens, for example) can be used to estimate patient exposure but are not practical in everyday life. We suggest that with appropriate ethical and privacy safeguards for informed, volunteer participants, the use of modern IT (primarily the geolocalization of smartphones) could be used to estimate the time spent outdoors, indoors or in public transport, etc., and might serve as a proxy for allergen exposure. These technologies provide relevant information on efficacy and safety under real‐life conditions, and this tracking might flag up relative differences, that is, between‐center or active vs placebo differences in patient mobility patterns. At the very least, the use of a patient diary and/or treatment reminder applications on smartphones (predominantly developed for patients with asthma)40 might reduce the number of missing data, promote participant engagement, and increase the level of adherence during a trial.41, 42, 43
3.8. Domain (viii): the placebo effect in AIT
Recommendation 8: Possible psychological, biochemical, immune, neural and even genomic markers of the placebo response by mining data on patients in active treatment and placebo groups should be identified. The placebo effect in AIT is common and relevant.44 Patients randomized to placebo have even reported up to a 60% decrease in their symptoms.45, 46 With a view to distinguishing between placebo responders and nonresponders, we encourage research on possible psychological, biochemical, immunological, neural, and even genomic markers of the placebo response.47, 48, 49 Most of the known predictors of the placebo response are psychological constructs related to goal‐seeking, self‐esteem, locus of control, optimism, expectation bias, body consciousness, and baseline symptom severity.47 Manufacturers of AIT products possess large bodies of (partially unpublished) data on patients in active treatment and placebo groups. These datasets could be mined to identify (probably complex) correlations between biological parameters (immunoglobulin levels, basophil activation, dendritic cell and T‐cell markers, epigenetic markers, proteomic profiles, etc.), symptom scores, medication scores, quality of life (QoL) scores, and other patient‐reported outcomes in active treatment vs placebo groups. The same holds true for the determination of AIT responders vs nonresponders. Here, we strongly encourage AIT product manufacturers to concentrate on biomarkers of high vs low responses (allergen‐specific IgE, IgG4, regulatory T‐cell activity, and basophil reactivity, for example8) in the placebo arm and not only in the active treatment arm. The patients’ perception of the treatment arm to which they have been allocated may also provide some insight into the possible placebo effect.
3.9. Domain (ix): ethical and technical aspects of DBPC RCTs, especially in pediatric populations
Recommendation 9a: New modes for AIT trials in the pediatric population should be identified and implemented – notably to seek to avoid 3 years of placebo treatment and 2 years of post‐treatment (blinded) follow‐up in pediatric trials. The current regulatory guidelines21, 50 have triggered discussion of critical ethical aspects in pediatric trial designs51, 52. In countries regulated by the European Medicines Agency, an applicant for the marketing authorization of an AIT product must submit a pediatric investigation plan (PIP) for assessment by the Agency's Pediatric Committee.53 The lack of an approved PIP will prohibit marketing authorization, even at the national level. Therefore, dialogue with regulatory authorities should be emphasized with regard to selecting robust but practical primary endpoints, decreasing the length of (or omitting) placebo treatment for pediatric patients, and thus giving children easier, faster access to AIT products that have been proven effective in adults. For ethical reasons, we consider that the 5‐year DBPC RCT for long‐term efficacy in adults with AR should not be mandatory in a PIP for an AIT product. Such a lengthy trial will deprive children in the placebo group of symptom relief and (perhaps just as importantly) a potentially disease‐modifying treatment during a critical period in their development. Indeed, a growing body of evidence demonstrates that AIT can counter neo‐sensitization and the progression of allergic respiratory disease.11, 12, 13, 54, 55, 56, 57 Hence, there may be a window of opportunity for AIT in early childhood. High‐quality RCTs of AIT products are required in pediatric populations, but more effort should be devoted to developing and validating controlled trials in which the control group receives some form of active treatment (eg, a head‐to‐head, noninferiority study comparing the investigational formulation with a high‐quality, registered comparator), rather than a placebo. Furthermore, waiting for 5‐year efficacy data from adult studies prior to starting a pediatric program unnecessarily delays market access to an effective AIT formulation for use in children. This policy will inevitably result in a gap in the availability of AIT products between adult and pediatric patients.
Recommendation 9b: Primary endpoints other than a CSMS should be considered and further explored in pediatric trials. Although the CSMS has not yet been psychometrically validated, it is still the best primary endpoint in adults for AR.20 However, there is some room for (i) improvement in the CSMS (eg, by changing the weighting between the symptom score and the medication score) in adults and (ii) the exploration of other systems (a visual analog scale, a disease control score, QoL, etc.), particularly in studies of children and adolescents and in asthma trials58, 59. Scoring a CSMS poses a number of problems in pediatric trials. Firstly, the amount of rescue medication consumed may not necessarily accurately reflect the severity of the child's symptoms. On one hand, parents may adopt a contrasting “give no rescue at all or give rescue every day” strategy. On the other hand, children may ask for medication (as a comforter) when symptoms are not severe or, conversely, may not ask for medication even when symptoms are severe (but are not fully perceived). All these issues should be discussed with the regulatory authorities, with a view to choosing statistically robust, clinically relevant outcomes.
3.10. Domain (x): the importance of safety reporting
Recommendation 10: World Allergy Organization (WAO) guidelines for reporting systemic and local adverse events should be applied. The WAO criteria are harmonized and standardized for safety reporting in both SCIT and SLIT.60, 61 More generally, reports of DBPC RCTs should follow the CONSORT guidelines.62, 63, 64
4. CONCLUSIONS
Evidence from meta‐analyses and systematic reviews demonstrate that AIT is a safe and effective treatment for AR and allergic asthma. Even more important, AIT is the only causal treatment option for allergic patients directly targeting the allergic immune reaction, thus bearing disease‐modifying properties. Despite these observations, levels of AIT acceptance are rather modest, as only a minority of eligible patients receive this treatment option. This limited acceptance may in part be accentuated by rigid regulatory requirements that prevent more specific investigations of the patients’ unmet “real‐world” needs and do not sufficiently consider the vast heterogeneity in patient‐related and environmental factors. We strongly believe that addressing these difficulties—by implementing new methodological approaches such as use of biomarkers, knowledge about placebo effects, e‐health technologies, and trial designs—may provide novel, ethical ways of reducing bias and heterogeneity in AIT clinical trials. In turn, these changes would allow the broader, more effective use of AIT in patients with allergic respiratory diseases.
CONFLICTS OF INTEREST
Dr. Pfaar reports personal fees from LETI Pharma/Laboratorios LETI, during the conduct of the project; grants and personal fees from ALK‐Abelló, grants and personal fees from Allergopharma, grants and personal fees from Stallergenes Greer, grants and personal fees from HAL Allergy Holding B.V./HAL Allergie GmbH, grants and personal fees from Bencard Allergie GmbH/Allergy Therapeutics, grants and personal fees from Lofarma, grants from Biomay, grants from Nuvo, grants from Circassia, grants and personal fees from Biotech Tools S.A., grants and personal fees from Laboratorios LETI/LETI Pharma, personal fees from Novartis Pharma, personal fees from MEDA Pharma, grants and personal fees from Anergis S.A., personal fees from Mobile Chamber Experts (a GA2LEN Partner), personal fees from Pohl‐Boskamp, personal fees from Indoor Biotechnologies, grants from Glaxo Smith Kline, all outside the submitted work. M. Alvaro reports personal fees from LETI Pharma/Laboratorios LETI for the conduct of the present project, and consulting fees, honoraria for lectures, and/or research funding from ALK, Allergopharma, Stallergenes, Thermofisher, and Novartis and Uriach outside the present project. V. Cardona reports personal fees from LETI Pharma/Laboratorios LETI for the conduct of the present project, and consulting fees, honoraria for lectures, and/or research funding from ALK, Allergopharma, Allergy Therapeutics, Circassia, HAL, Thermofisher, and Stallergenes outside the present project. E. Hamelmann reports personal fees from LETI Pharma/Laboratorios LETI for the conduct of the present project, and consulting fees, honoraria for lectures from AllergoPharma, ALK, Bencard, Boehringer Ingelheim, HAL Allergy, Novartis, Nutricia, Stallergenes, and research grants from Deutsche Forschungsgemeinschaft (DFG) and Bundesministerium für Bildung und Forschung (BMBF) outside the present project. R. Mösges reports personal fees from LETI Pharma/Laboratorios LETI for the conduct of the present project, and reports personal fees from ALK; grants from ASIT biotech, LETI Pharma, Optima, BitopAG, Hulka, and Ursapharm; personal fees from allergopharma, Allergy Therapeutics, Friulchem, Hexal, Servier, Klosterfrau, Bayer, FAES, GSK, MSD, Johnson&Johnson, Meda, Stada, UCB, and Nuvo; and grants and personal fees from Bencard, Stallergenes; grants, personal fees, and nonfinancial support from Lofarma; nonfinancial support from Roxall, Atmos, Bionorica, Otonomy, and Ferrero; and personal fees and nonfinancial support from Novartis. J. Kleine‐Tebbe reports personal fees from LETI Pharma/Laboratorios LETI for the conduct of the present project. Outside the present project, he also reports consultancy fees from Merck (USA) and Circassia; and is on the advisory boards of ALK, Novartis, Leti, and Bencard; has received institutional grants from Circassia, Leti, and Stallergenes Greer; has received payment for lectures from Allergopharma, Allergy Therapeutics, ALK‐Abelló, Bencard, HAL Allergy, Leti, Lofarma, Novartis, and Stallergenes Greer.
AUTHOR CONTRIBUTIONS
Oliver Pfaar, Montserrat Alvaro, Victòria Cardona, Eckard Hamelmann, Ralph Mösges, and Jörg Kleine‐Tebbe all contributed substantially in drafting the article and revising it critically before submission. All authors have given their final approval of the submitted article.
ACKNOWLEDGMENTS
The authors would like to thank LETI Pharma/Laboratorios LETI Germany/Spain for organizing the workshops. Lastly, the authors acknowledge medical writing and editorial support for the preparation of this review from David Fraser (Biotech Communication SARL, Ploudalmézeau, France), which was funded by LETI Pharma/Laboratorios LETI Germany/Spain. This sponsor also paid for open access of the article (OnlineOpen publication).
Pfaar O, Alvaro M, Cardona V, Hamelmann E, Mösges R, Kleine‐Tebbe J. Clinical trials in allergen immunotherapy: Current concepts and future needs. Allergy. 2018;73:1775–1783. 10.1111/all.13429
REFERENCES
- 1. Dhami S, Nurmatov U, Arasi S, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta‐analysis. Allergy. 2017;72:1597‐1631. [DOI] [PubMed] [Google Scholar]
- 2. Roberts G, Pfaar O, Akdis CA, et al. EAACI guidelines on allergen immunotherapy: allergic rhinoconjunctivitis. Allergy. 2018;73:765‐798. [DOI] [PubMed] [Google Scholar]
- 3. Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556‐568. [DOI] [PubMed] [Google Scholar]
- 4. Pfaar O, Bachert C, Bufe A, et al. Guideline on allergen‐specific immunotherapy in IgE‐mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto‐ Rhino‐Laryngology, Head and Neck Surgery (DGHNO‐KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV‐HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin SY, Erekosima N, Suarez‐Cuervo C, et al. Allergen‐Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review. AHRQ Comparative Effectiveness Reviews. Rockville (MD). 2013. [PubMed]
- 6. Normansell R, Kew KM, Bridgman AL. Sublingual immunotherapy for asthma. Cochrane Database Syst Rev. 2015:CD011293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jutel M, Agache I, Bonini S, et al. International consensus on allergen immunotherapy II: mechanisms, standardization, and pharmacoeconomics. J Allergy Clin Immunol. 2016;137:358‐368. [DOI] [PubMed] [Google Scholar]
- 8. Shamji MH, Kappen JH, Akdis M, et al. Biomarkers for monitoring clinical efficacy of allergen immunotherapy for allergic rhinoconjunctivitis and allergic asthma: an EAACI Position Paper. Allergy. 2017;72:1156‐1173. [DOI] [PubMed] [Google Scholar]
- 9. Pfaar O, Bonini S, Cardona V, et al. Perspectives in allergen immunotherapy: 2017 and beyond. Allergy. 2018; Suppl 104:5‐23. [DOI] [PubMed] [Google Scholar]
- 10. Kristiansen M, Dhami S, Netuveli G, et al. Allergen immunotherapy for the prevention of allergy: a systematic review and meta‐analysis. Pediatr Allergy Immunol. 2017;28:18‐29. [DOI] [PubMed] [Google Scholar]
- 11. Schmitt J, Schwarz K, Stadler E, Wüstenberg EG. Allergy immunotherapy for allergic rhinitis effectively prevents asthma: results from a large retrospective cohort study. J Allergy Clin Immunol. 2015;136:1511‐1516. [DOI] [PubMed] [Google Scholar]
- 12. Zielen S, Devillier P, Heinrich J, Richter H, Wahn U. Sublingual immunotherapy provides long‐term relief in allergic rhinitis and reduces the risk of asthma: a retrospective, real‐world database analysis. Allergy. 2018;73:165‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Valovirta E, Petersen TH, Piotrowska T, et al. Results from the 5‐year SQ grass sublingual immunotherapy tablet asthma prevention (GAP) trial in children with grass pollen allergy. J Allergy Clin Immunol. 2018;141:529‐538. [DOI] [PubMed] [Google Scholar]
- 14. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines‐2016 revision. J Allergy Clin Immunol. 2017;140:950‐958. [DOI] [PubMed] [Google Scholar]
- 15. Brozek JL, Bousquet J, Baena‐Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466‐476. [DOI] [PubMed] [Google Scholar]
- 16. Wallace DV, Dykewicz MS, Bernstein DI, et al. The diagnosis and management of rhinitis: an updated practice parameter. J Allergy Clin Immunol. 2008;122(2 suppl):S1‐S84. [DOI] [PubMed] [Google Scholar]
- 17. Canonica GW, Cox L, Pawankar R, et al. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014;7:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Biermann J, Merk HF, Wehrmann W, Klimek L, Wasem J. Allergic disorders of the respiratory tract – findings from a large patient sample in the German statutory health insurance system. Allergo J. 2013;22:366‐373. [Google Scholar]
- 19. Hankin CS, Cox L, Bronstone A, Wang Z. Allergy immunotherapy: reduced health care costs in adults and children with allergic rhinitis. J Allergy Clin Immunol. 2013;131:1084‐1091. [DOI] [PubMed] [Google Scholar]
- 20. Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854‐867. [DOI] [PubMed] [Google Scholar]
- 21. European Medicines Agency (EMA) . Guideline on the clinical development of products for specific immunotherapy for the treatment of allergic diseases [Internet]. 2008. London, UK: European Medicines Agency; http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003605.pdf. Accessed January 22, 2018. [Google Scholar]
- 22. Akerlund A, Andersson M, Leflein J, Lildholdt T, Mygind N. Clinical trial design, nasal allergen challenge models, and considerations of relevance to pediatrics, nasal polyposis, and different classes of medication. J Allergy Clin Immunol. 2005;115(3 suppl 1):S460‐S482. [DOI] [PubMed] [Google Scholar]
- 23. Bousquet J, Schünemann HJ, Bousquet PJ, et al. How to design and evaluate randomized controlled trials in immunotherapy for allergic rhinitis: an ARIA‐GA(2)LEN statement. Allergy. 2011;66:765‐774. [DOI] [PubMed] [Google Scholar]
- 24. Canonica GW, Baena‐Cagnani CE, Bousquet J, et al. Recommendations for standardization of clinical trials with Allergen Specific Immunotherapy for respiratory allergy. A statement of a World Allergy Organization (WAO) taskforce. Allergy. 2007;62:317‐324. [DOI] [PubMed] [Google Scholar]
- 25. Casale TB, Canonica GW, Bousquet J, et al. Recommendations for appropriate sublingual immunotherapy clinical trials. J Allergy Clin Immunol. 2009;124:665‐670. [DOI] [PubMed] [Google Scholar]
- 26. Gödicke V, Hundt F. Registration trials for specific immunotherapy in Europe: advanced guidance from the new European Medical Agency guideline. Allergy. 2010;65:1499‐1505. [DOI] [PubMed] [Google Scholar]
- 27. Passalacqua G. Recommendations for appropriate sublingual immunotherapy clinical trials. World Allergy Organ J. 2014;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pfaar O, Kleine‐Tebbe J, Hörmann K, Klimek L. Allergen‐specific immunotherapy: which outcome measures are useful in monitoring clinical trials? Immunol Allergy Clin North Am. 2011;31:289‐309. [DOI] [PubMed] [Google Scholar]
- 29. Pfaar O, Robinson DS, Sager A, Emuzyte R. Immunotherapy with depigmented‐polymerized mixed tree pollen extract: a clinical trial and responder analysis. Allergy. 2010;65:1614‐1621. [DOI] [PubMed] [Google Scholar]
- 30. Demoly P, Calderon MA, Casale TB, Malling HJ, Wahn U. The value of pre‐ and co‐seasonal sublingual immunotherapy in pollen‐induced allergic rhinoconjunctivitis. Clin Transl Allergy. 2015;5:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Riechelmann H, Bachert C, Goldschmidt O, et al. Application of the nasal provocation test on diseases of the upper airways. Position paper of the German Society for Allergology and Clinical Immunology (ENT Section) in cooperation with the Working Team for Clinical Immunology. Laryngorhinootologie. 2003;82:183‐188. [DOI] [PubMed] [Google Scholar]
- 32. Augé J, Vent J, Agache I, et al. Position paper on the standardization of nasal allergen challenges. Allergy. 2018;73:1597‐1608. [DOI] [PubMed] [Google Scholar]
- 33. Fauquert JL, Jedrzejczak‐Czechowicz M, Rondon C, et al. Conjunctival allergen provocation test: guidelines for daily practice. Allergy. 2017;72:43‐54. [DOI] [PubMed] [Google Scholar]
- 34. Astvatsatourov A, Reydelet Y, Mösges R. Photodocumentation of allergic severity under conjunctival provocation. Stud Health Technol Inform. 2015;213:11‐14. [PubMed] [Google Scholar]
- 35. Gloistein C, Astvatsatourov A, Allekotte S, Mosges R. Digitally analyzed conjunctival redness: does repeated conjunctival provocation intrinsically cause local desensitization of the eye? Int Arch Allergy Immunol. 2015;168:277‐284. [DOI] [PubMed] [Google Scholar]
- 36. Pfaar O, Calderon MA, Andrews CP, et al. Allergen exposure chambers: harmonizing current concepts and projecting the needs for the future ‐ an EAACI Position Paper. Allergy. 2017;72:1035‐1042. [DOI] [PubMed] [Google Scholar]
- 37. Pfaar O, Bastl K, Berger U, et al. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen‐induced rhinoconjunctivitis ‐ an EAACI position paper. Allergy. 2017;72:713‐722. [DOI] [PubMed] [Google Scholar]
- 38. Durham SR, Nelson HS, Nolte H, et al. Magnitude of efficacy measurements in grass allergy immunotherapy trials is highly dependent on pollen exposure. Allergy. 2014;69:617‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Elsäβer A, Regnstrom J, Vetter T, et al. Adaptive clinical trial designs for European marketing authorization: a survey of scientific advice letters from the European Medicines Agency. Trials. 2014;15:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Marcano Belisario JS, Huckvale K, Greenfield G, Car J, Gunn LH. Smartphone and tablet self management apps for asthma. Cochrane Database Syst Rev. 2013;CD010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bianchi A, Tsilochristou O, Gabrielli F, Tripodi S, Matricardi PM. The smartphone: a novel diagnostic tool in pollen allergy? J Investig Allergol Clin Immunol. 2016;26:204‐207. [DOI] [PubMed] [Google Scholar]
- 42. Demoly P, Passalacqua G, Pfaar O, Sastre J, Wahn U. Patient engagement and patient support programs in allergy immunotherapy: a call to action for improving long‐term adherence. Allergy Asthma Clin Immunol. 2016;12:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bousquet J, Bewick M, Arnavielhe S, et al. Work productivity in rhinitis using cell phones: the MASK pilot study. Allergy. 2017;72:1475‐1484. [DOI] [PubMed] [Google Scholar]
- 44. Wedi B, Wieczorek D, Kapp A. Placebo effect in clinical trials with allergen‐specific immunotherapy with inhalant allergens. Hautarzt. 2017;68:297‐306. [DOI] [PubMed] [Google Scholar]
- 45. Demoly P, Emminger W, Rehm D, Backer V, Tommerup L, Kleine‐Tebbe J. Effective treatment of house dust mite‐induced allergic rhinitis with 2 doses of the SQ HDM SLIT‐tablet: results from a randomized, double‐blind, placebo‐controlled phase III trial. J Allergy Clin Immunol. 2016;137:444‐451. [DOI] [PubMed] [Google Scholar]
- 46. Circassia Announces Top‐Line Results from Cat Allergy Phase III Study [Internet]. 2016. Oxford, UK: Circassia Pharmaceuticals plc; http://www.circassia.com/media/press-releases/circassia-announces-top-line-results-from-cat-allergy-phase-iii-study [press release]. Accessed January 22, 2018. [Google Scholar]
- 47. Horing B, Weimer K, Muth ER, Enck P. Prediction of placebo responses: a systematic review of the literature. Front Psychol. 2014;5:1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benedetti F, Carlino E, Pollo A. How placebos change the patient's brain. Neuropsychopharmacology. 2011;36:339‐354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hall KT, Loscalzo J, Kaptchuk TJ. Genetics and the placebo effect: the placebome. Trends Mol Med. 2015;21:285‐294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. European Medicines Authority (EMA) . EMA/PDCO Standard Paediatric Investigation Plan for Allergen Products for Specific Immunotherapy. Human Medicines Development and Evaluation; 2015. http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/11/WC500015814.pdf. Accessed on January 22, 2018.
- 51. Rose K, Kopp MV. Pediatric investigation plans for specific immunotherapy: questionable contributions to childhood health. Pediatr Allergy Immunol. 2015;26:695‐701. [DOI] [PubMed] [Google Scholar]
- 52. Valovirta E, Berstad AK, de Blic J, et al. Design and recruitment for the GAP trial, investigating the preventive effect on asthma development of an SQ‐standardized grass allergy immunotherapy tablet in children with grass pollen‐induced allergic rhinoconjunctivitis. Clin Ther. 2011;33:1537‐1546. [DOI] [PubMed] [Google Scholar]
- 53. Bonertz A, Roberts GC, Hoefnagel M, et al. Challenges in the implementation of EAACI guidelines on allergen immunotherapy: a global perspective on the regulation of allergen products. Allergy. 2018;73:64‐76. [DOI] [PubMed] [Google Scholar]
- 54. Marogna M, Tomassetti D, Bernasconi A, et al. Preventive effects of sublingual immunotherapy in childhood: an open randomized controlled study. Ann Allergy Asthma Immunol. 2008;101:206‐211. [DOI] [PubMed] [Google Scholar]
- 55. Niggemann B, Jacobsen L, Dreborg S, et al. Five‐year follow‐up on the PAT study: specific immunotherapy and long‐term prevention of asthma in children. Allergy. 2006;61:855‐859. [DOI] [PubMed] [Google Scholar]
- 56. Novembre E, Galli E, Landi F, et al. Coseasonal sublingual immunotherapy reduces the development of asthma in children with allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2004;114:851‐857. [DOI] [PubMed] [Google Scholar]
- 57. Shaker M. New insights into the allergic march. Curr Opin Pediatr. 2014;26:516‐520. [DOI] [PubMed] [Google Scholar]
- 58. Klimek L, Bergmann KC, Biedermann T, et al. Visual analogue scales (VAS): measuring instruments for the documentation of symptoms and therapy monitoring in cases of allergic rhinitis in everyday health care: position Paper of the German Society of Allergology (AeDA) and the German Society of Allergy and Clinical Immunology (DGAKI), ENT Section, in collaboration with the working group on Clinical Immunology, Allergology and Environmental Medicine of the German Society of Otorhinolaryngology, Head and Neck Surgery (DGHNOKHC). Allergo J Int. 2017;26:16‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bousquet PJ, Combescure C, Klossek JM, Daures JP, Bousquet J. Change in visual analog scale score in a pragmatic randomized cluster trial of allergic rhinitis. J Allergy Clin Immunol. 2009;123:1349‐1354. [DOI] [PubMed] [Google Scholar]
- 60. Cox L, Larenas‐Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. J Allergy Clin Immunol. 2010;125:569‐574. [DOI] [PubMed] [Google Scholar]
- 61. Passalacqua G, Baena‐Cagnani CE, Bousquet J, et al. Grading local side effects of sublingual immunotherapy for respiratory allergy: speaking the same language. J Allergy Clin Immunol. 2013;132:93‐98. [DOI] [PubMed] [Google Scholar]
- 62. Begg C, Cho M, Eastwood S, et al. Improving the quality of reporting of randomized controlled trials. The CONSORT statement. JAMA. 1996;276:637‐639. [DOI] [PubMed] [Google Scholar]
- 63. Bousquet PJ, Calderon MA, Demoly P, et al. The Consolidated Standards of Reporting Trials (CONSORT) Statement applied to allergen‐specific immunotherapy with inhalant allergens: a Global Allergy and Asthma European Network (GA(2)LEN) article. J Allergy Clin Immunol. 2011;127:49‐56. [DOI] [PubMed] [Google Scholar]
- 64. Bousquet PJ, Brozek J, Bachert C, et al. The CONSORT statement checklist in allergen‐specific immunotherapy: a GA2LEN paper. Allergy. 2009;64:1737‐1745. [DOI] [PubMed] [Google Scholar]


