Summary
For patients with chronic myeloid leukaemia (CML), treatment guidelines recommend monitoring response to treatment with tyrosine kinase inhibitors (TKIs) by testing the BCR‐ABL1 fusion gene transcript level using reverse transcriptase quantitative polymerase chain reaction. Despite recent efforts to standardise protocols for BCR‐ABL1 testing, some variability remains among laboratories in the UK regarding the techniques used and the approach to reporting results. This increases the risk of misinterpretation of results by both clinicians and patients. An expert panel met to discuss current issues surrounding BCR‐ABL1 testing in the UK and to develop guidance for laboratories, with emphasis on the optimal approach to reporting laboratory results. Topics included the minimum required information to include in the laboratory report, units of measurement, test sensitivity and BCR‐ABL1 transcript variants. To aid communication between laboratories and clinics, standard forms were generated that could be used by (i) clinics when submitting samples to laboratories, and (ii) laboratories when reporting results to clinics. Standardising the way in which BCR‐ABL1 test results are reported from laboratories to clinics should help to improve communication, interpretation of results and patient care.
Keywords: chronic myeloid leukaemia, BCR‐ABL1, laboratory assay, laboratory report, United Kingdom
Molecular testing for the fusion gene BCR‐ABL1 is the most sensitive routine test for monitoring response to therapy in patients with chronic myeloid leukaemia (CML) (Foroni et al, 2011). The technique utilises reverse transcriptase quantitative polymerase chain reaction (RT‐qPCR) to estimate the amount of BCR‐ABL1 mRNA relative to an internal reference gene (typically ABL1, GUSB, or BCR) (Cross et al, 2015). Results are expressed on the International Scale (IS) as a percentage relative to the standardised baseline used in the pivotal IRIS (International Randomized Study of Interferon and STI571) trial, which evaluated the tyrosine kinase inhibitor (TKI) imatinib in patients with CML (Hughes et al, 2003, 2006; Cross et al, 2015). BCR‐ABL1 testing is used to define molecular response (MR) to TKIs, and a major molecular response (MMR) is defined as a 3‐log reduction from the standardised baseline (MR3 or 0·1% BCR‐ABL IS) (Baccarani et al, 2013). Beyond MMR, deep molecular responses (DMRs) of MR4, MR4·5, and MR5 are defined as ≤0·01%, ≤0·0032%, and ≤0·001% BCR‐ABL IS, respectively (Table 1) (Cross et al, 2015).
Table 1.
Molecular response in patients with CML: BCR‐ABL1 transcript levels according to the International Scale (Baccarani et al, 2014; Cross et al, 2015)
| BCR‐ABL IS, % | Log reduction from standardised baseline | MR category | Minimum number of ABL1 transcripts |
|---|---|---|---|
| 100 | 0 | – | – |
| ≤0·1 | 3 | MR3 (MMR) | >10 000 |
| ≤0·01 | 4 | MR4 | 10 000–31 999 |
| ≤0·0032 | 4·5 | MR4·5 | 32 000–99 999 |
| ≤0·001 | 5 | MR5 | ≥100 000 |
CML, chronic myeloid leukaemia; MMR, major molecular response; MR, molecular response.
As described in the current European LeukaemiaNet (ELN) CML recommendations (Baccarani et al, 2013), regular ongoing BCR‐ABL1 testing provides essential information required to make timely important treatment decisions, such as whether to continue current TKI, or switch to a different TKI or alternative therapy. More recently, the National Comprehensive Cancer Network (NCCN) and European Society of Medical Oncology (ESMO) guidelines have been updated to include recommendations on stopping TKI treatment in patients who have achieved a sustained DMR on TKI treatment, initiating a period of treatment‐free remission (TFR) (Hochhaus et al, 2017; NCCN, 2017). The feasibility of TFR following achievement of DMR has been demonstrated in numerous clinical studies (reviewed in Saussele et al, 2016; Rea & Cayuela, 2017). However, across these studies, approximately 50% of patients had molecular recurrence (loss of MMR) during discontinuation and required TKI re‐initiation. Patients who re‐initiated treatment remained sensitive to TKI treatment, and re‐achieved DMR in the majority of cases. Molecular recurrence generally occurred within 6 months following discontinuation, although more recent studies show later molecular recurrence continues to occur (Campiotti et al, 2017). Thus, while all CML patients on TKI treatment require ongoing regular BCR‐ABL1 monitoring, patients entering TFR require increased frequency of monitoring of BCR‐ABL1 levels (Hochhaus et al, 2017; NCCN, 2017). This will likely increase laboratory workload and highlights the need for fast and reliable BCR‐ABL1 test results that are effectively communicated between the laboratory and clinician.
To ensure accurate BCR‐ABL1 testing, laboratories should participate in standardisation and external quality assessment programmes, establish conversion factors or use calibrated kits for reporting on the IS, determine the variability of their assay at high and low levels of disease (Branford & Hughes, 2006; Branford et al, 2008), and validate that their assay is capable of detecting MR4·5 in most patient samples. To assist accurate interpretation of BCR‐ABL1 results in the clinic, reports should be easily interpretable and use standardised definitions of MR (Cross et al, 2015). Despite efforts to standardise procedures (Foroni et al, 2011; Cross et al, 2015), some variability remains among laboratories in the UK regarding technique and reporting results of BCR‐ABL1 testing (Foroni et al, 2011), which underscores the need for further standardisation of protocols.
In June 2017, an expert panel met in London to discuss potential alignment on BCR‐ABL1 reporting in the UK. The purpose of the meeting was to develop guidance to support the accurate communication of BCR‐ABL1 molecular monitoring results from laboratories to clinics, to enable optimal management of patients with CML. Topics for discussion included laboratory requirements for accurate BCR‐ABL1 reporting (such as use of standardised definitions to present results of BCR‐ABL1IS, MR4·5, transcript type, etc.), frequency of testing, the minimum clinical information that a laboratory needs in order to provide results and accurate response interpretation, the minimum information that should be included in the laboratory report and additional laboratory considerations for molecular monitoring requirements during TFR.
Laboratory requirements for providing an optimal report
According to the ELN CML recommendations, molecular testing to determine BCR‐ABL1 transcript level is recommended for patients with CML treated with TKIs to establish the level of response and to monitor changes over time (Baccarani et al, 2013). In addition, it may be useful to quantify BCR‐ABL1 levels prior to starting therapy to determine the velocity of response at 3 months, which can help identify patients at risk of treatment failure (Branford et al, 2014; Hanfstein et al, 2014).
Depending on local circumstances, patients can be monitored using molecular tests, such as RT‐qPCR, cytogenetic tests, such as G‐band analysis or fluorescence in situ hybridization (FISH), or both (Baccarani et al, 2013). When using molecular tests, it is recommended to use standardised sensitive assays capable of detecting MR4·5 on the IS, because these allow for accurate response monitoring during TKI treatment and during TFR. Furthermore, IS results are necessary for comparing patient results with the ELN recommendations and data from clinical trials.
Before initiating therapy, the BCR‐ABL1 transcript variant type should be determined in all patients so that molecular testing can target the correct subtype and false‐negative results can be excluded (Foroni et al, 2011). Identification of the individual transcript type is also important as this may correlate with clinical outcome (Claudiani et al, 2017). Standard BCR‐ABL1 testing and reporting in IS units can only be applied reliably in patients with typical transcript variants (e13a2 and/or e14a2), which account for 97–98% of CML patients (Foroni et al, 2011). For patients with atypical variants, bespoke assays that target the correct variant can be used to monitor general trends in disease levels on treatment. This may be used to inform clinical management, but the results cannot be expressed on the IS. Given that these patients with atypical variants are so rare, we consider that these bespoke monitoring assays should be carried out by specialised laboratories or ideally a single central laboratory. However, it is essential that all laboratories should be able to detect atypical variants in patients before treatment, in order to provide faster and comprehensive in‐house testing results.
For patients achieving TFR, molecular monitoring is a critical part of care to identify a potential loss of MR3, necessitating restarting of TKI treatment. From the laboratory perspective, TFR presents many challenges: more frequent monitoring is required and the need for rapid results with a 2‐week turnaround will probably increase laboratory workload. Patients entering TFR have very low or undetectable BCR‐ABL1 levels, and laboratories monitoring these patients must ensure that they are capable of detecting MR4·5, using regular external or internal validation, and reporting on the IS to ensure DMR can be accurately monitored prior to and during TKI discontinuation. Due to the requirement for standardised results reported on the IS prior to and during TFR, treatment discontinuation is currently only recommended in patients with typical transcripts and where IS results are available (NCCN, 2017). In addition, regular monitoring of patients in long‐term TFR will require careful coordination between the laboratory and haematologist/oncologist to ensure that reintroduction of treatment in the case of molecular recurrence can be started promptly.
BCR‐ABL1 kinase domain point mutations reflect disease evolution and may be used to inform subsequent therapy (Soverini et al, 2011). Therefore, mutational analysis is recommended in case of disease progression and treatment failure, and for patients in the ELN ‘Warning’ response category (Baccarani et al, 2013). According to the ELN recommendations (Baccarani et al, 2013), mutational analysis should be performed using Sanger sequencing until the clinical relevance of mutations detected with more sensitive techniques has become clear.
Frequency of monitoring
International treatment guidelines are generally consistent with regard to the frequency of BCR‐ABL1 testing when monitoring response to TKIs. The ELN recommends testing every 3 months until BCR‐ABL1 ≤ 0·1%IS (MMR) is achieved and then every 3–6 months thereafter (Baccarani et al, 2013). In the American NCCN guidelines, testing is recommended at diagnosis, every 3 months after starting treatment until BCR‐ABL1 0·1–1%IS is achieved, then every 3 months for 2 years, and every 3–6 months thereafter (NCCN 2017).
The ELN CML recommendations include response categories (Optimal, Warning and Failure) and monitoring frequency requirements for patients receiving TKIs as first‐line (Table 2) or second‐line treatment in the case of failure to first line imatinib (Baccarani et al, 2013). If a patient falls in the ‘Failure’ category, they should initiate a different treatment (e.g. an alternative TKI or allogeneic stem cell transplant) in order to decrease the risk of disease progression and mortality. In addition, cytogenetic analysis of marrow cell metaphases, RT‐qPCR and, when appropriate, mutational analysis should be performed. In some cases, repeat testing on the same sample, if possible, may be required. If a test result is significantly different from the previous result, the test should be repeated within the laboratory before being reported. If the result remains significantly different, the clinician should be notified and arrangements should be made for the patient to return for repeat sampling. It should be noted that repeat sampling can cause anxiety in patients; efforts to minimise distress regarding repeat testing should be considered.
Table 2.
ELN response criteria and recommended monitoring frequency in first‐line treatment of CMLa (Baccarani et al, 2013)
| Time since start of TKI treatment | ELN response category | |||||
|---|---|---|---|---|---|---|
| Optimal | Warning | Failure | ||||
| Response criteria | Monitoring | Response criteria | Monitoring | Response criteria | Monitoring | |
| Baseline | NA | CBA, Qualitative PCR | High risk or CCA/Ph+, major route | CBA, Qualitative PCR | NA | CBA, Qualitative PCR |
| 3 months | BCR‐ABL1 ≤10% and/or Ph+ ≤35% | RT‐qPCR every 3 months until MMR, then every 3–6 months and/or CBA at 3, 6 and 12 months until CCyR, then FISH | BCR‐ABL1 >10% and/or Ph+ 36–95% | Molecular/cytogenetic tests to be performed more frequently (up to monthly)b | No CHR and/or Ph+ >95% | RT‐qPCR, mutational analysis, and CBA should be performed. Immunophenotyping in blastic phase. |
| 6 months | BCR‐ABL1 <1% and/or Ph+ 0 | BCR‐ABL1 1–10% and/or Ph+ 1–35% | BCR‐ABL1 >10% and/or Ph+ >35% | |||
| 12 months | BCR‐ABL1 ≤0·1% | BCR‐ABL1 >0·1–1% | BCR‐ABL1 >1% and/or Ph+ >0 | |||
| ≥12 months | BCR‐ABL1 ≤0·1% |
CCA/Ph−
(−7, or 7q−) |
Loss of CHR, Loss of CCyR, confirmed loss of MMRc | |||
CBA, chromosome banding analysis of marrow cell metaphases; CCA/Ph+, clonal chromosome abnormalities in Philadelphia chromosome‐positive cells; CCA/Ph−, clonal chromosome abnormalities in Philadelphia chromosome‐negative cells; CCyR, complete cytogenetic response; CHR, complete haematological response; CML, chronic myeloid leukaemia; ELN, European LeukaemiaNet; FISH, fluorescence in situ hybridisation; MMR, major molecular response; NA, not applicable; PCR, polymerase chain reaction; Ph, Philadelphia chromosome; RT‐qPCR, reverse transcriptase quantitative polymerase chain reaction.
The definitions are the same for patients in chronic phase, accelerated phase and blast phase and also apply to second‐line treatment, when first‐line treatment was changed for intolerance.
CBA recommended in case of myelodysplasia or CCA/Ph‐ with chromosome 7 involvement.
In two consecutive tests of which one is with a BCR‐ABL1 transcript level of ≥1%.
How to report: clinician to laboratory
Good communication among members of the multidisciplinary team is essential for supporting good communication between the clinician and patient, thus ensuring more effective disease management (Fig 1). This includes submitting sufficient clinical information with blood samples, and providing a comprehensive yet practical laboratory report. Currently, samples are often submitted for BCR‐ABL1 testing without sufficient clinical information about the patient, leading to difficulty in providing an interpretative laboratory report (Claustres et al, 2014). Although providing brief clinical details with a BCR‐ABL1 test request can be challenging within the context of a busy clinic, this information is important to ensure good laboratory–clinician communication. To reduce workload, some laboratories have developed CML‐specific online forms that clinicians can fill out when submitting samples, and this approach is encouraged. Ideally, the following clinical information should be submitted to the laboratory: TKI therapy and any recent known treatment interruptions (e.g. pregnancy, TFR, intolerance) and possible issues with treatment adherence. For patients who are being transferred between hospitals, BCR‐ABL1 transcript type and, in cases where resistance has been encountered, TK domain mutation status should be reported as well. Sample forms are shown in Figs 2 and 3. Various aspects of the clinician's report are discussed in more detail below.
Figure 1.
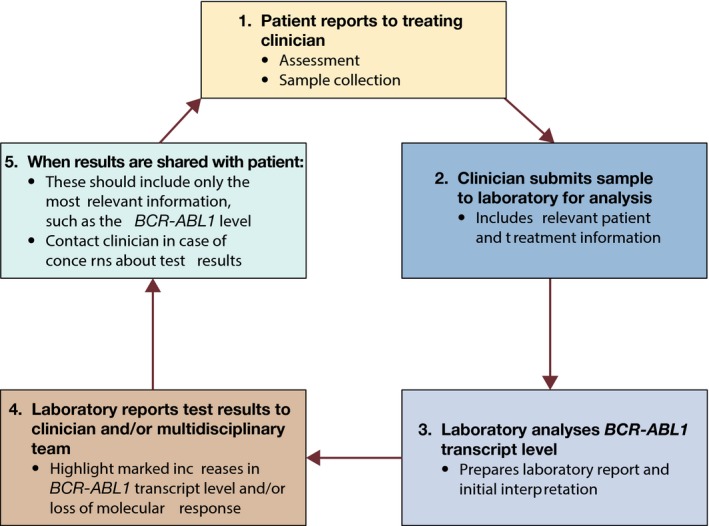
Patterns of communication among the CML healthcare team regarding BCR‐ABL1 testing. CML, chronic myeloid leukaemia. *Multidisciplinary team: including pharmacy.
Figure 2.

Information to accompany samples submitted for BCR‐ABL1 testing. NHS, National Health Service; TKI, tyrosine kinase inhibitor.
Figure 3.
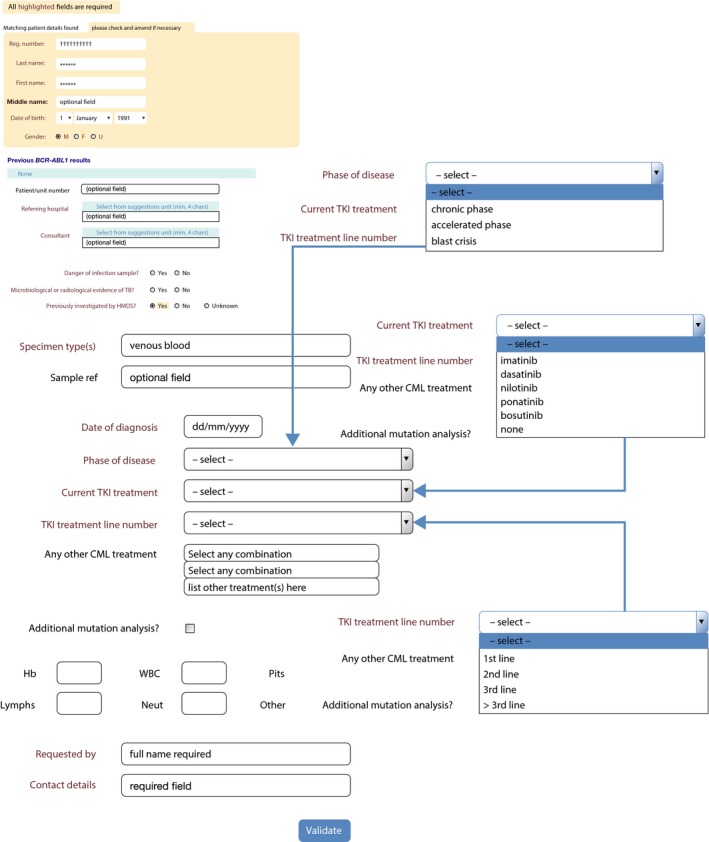
Screenshot of an online request form for BCR‐ABL1 testing. CML, chronic myeloid leukaemia; F, female; Hb, haemoglobin; HMDS, Haematological Malignancy Diagnostic Service; Lymphs, lymphocytes; M, male; Neut, neutrophils; Plts, platelets; TB, tuberculosis; TKI, tyrosine kinase inhibitor; U, unknown; WBC, white blood cells.
Clinical details
Accurate clinical details are essential in order to offer appropriate clinical guidance for patients receiving TKI therapy. Bespoke online request forms offer an attractive means to achieve this. Linked to a departmental laboratory information management system, patient demographics can be populated using the National Health Service (NHS) number following a patient's initial registration on the system, usually at diagnosis. Additional disease and treatment information can then be added using a simple drop‐down menu each time the patient attends the clinic for molecular monitoring. Phase of disease, line of therapy and current TKI usage can all be captured on the form, making informed clinical interpretation possible. Dose escalation, modification, and cessation of TKI can also be documented in a similar manner on the request form. If clinical details are not available, laboratory reports should clearly state that interpretation of the results according to ELN recommendations is not possible.
Therapy
Samples submitted for BCR‐ABL1 testing should include the line of therapy of TKI treatment, which is essential to provide ELN response category as part of the report, and should be included in the laboratory report whenever possible. Changes in treatment can influence the interpretation of results, and the laboratory should be informed of any significant changes to treatment, including switching to a different TKI, treatment interruptions or discontinuation.
Timing of sample in relation to start of TKI therapy
If available, including the sample time point (e.g. 3 months after starting TKI) is essential for the interpretation of results. If this information is not provided with the sample, it may be found in electronic regional prescribing systems, if available.
Expert panel opinion.
Ideally, when submitting samples for BCR‐ABL1 testing, clinicians should provide the following information: line of therapy, start date for current TKI, and any recent treatment interruptions (e.g. pregnancy, TFR)
Laboratories should be informed of whether the patient is in TFR and date of treatment cessation as this can affect the frequency of monitoring
How to report: laboratory to clinician
The following minimum required information should be included in the molecular genetics laboratory report: patient and physician information, test performed, test result and broad interpretation to help guide the final interpretation by the referring clinician, and any relevant supplemental information (Scheuner et al, 2012; Claustres et al, 2014). A sample laboratory report is shown in Fig 4, and examples of laboratory reports illustrating various clinical scenarios are shown in Appendix S1 (Figures S1 –S4). Various aspects of the laboratory report are discussed in more detail below.
Figure 4.
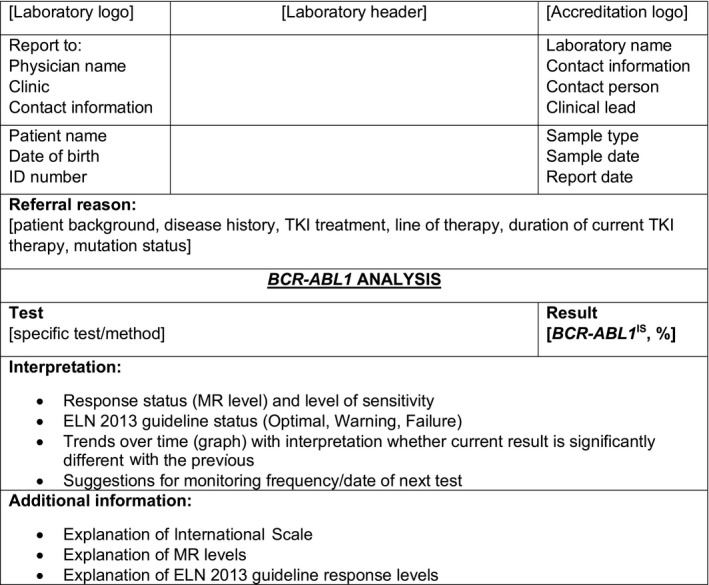
Standard laboratory report for BCR‐ABL1 testing. CML, chronic myeloid leukaemia; ELN, European LeukaemiaNet; ID, identification; IS, International Scale; MR, molecular response; TKI, tyrosine kinase inhibitor.
BCR‐ABL1 transcript variants
As discussed above, the BCR‐ABL1 transcript variant type should be established at the time of diagnosis to determine the most appropriate method for monitoring changes in BCR‐ABL1 transcript level (Foroni et al, 2011). Variant type has important implications, not only for testing protocols but also for treatment decisions. For example, stopping nilotinib in patients who have achieved sustained DMR is currently only recommended for patients with confirmed typical variants (i.e. e13a2 and e14a2) (http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000798/WC500034394.pdf; Hochhaus et al, 2017; NCCN 2017). Therefore, the variant type should be included in the laboratory report and atypical variants should be highlighted.
Cumulative timeline of BCR‐ABL1 transcript level
A list or graph describing previous test results is strongly recommended to present results over time to allow the clinician to easily interpret the current result in the context of previous results. Ideally a graph should include some indication of the limit of detection of the assay and ELN response category. This interpretation would mandate the date of TKI initiation and line of therapy to be stated. Examples of such graphs are given in Appendix S1 (Figures S1–S4).
TK domain mutations
Ideally, a timeline graph should be generated indicating the time points at which mutation analysis was carried out, the type of mutation(s) present (using Human Genome Variation Society [HGVS] nomenclature; see http://varnomen.hgvs.org) and the time point at which each mutation was first detected, as well as the sensitivity of mutation detection, the level of the mutation, and a brief summary of whether it is likely to be sensitive or resistant to other TKIs.
Units
All laboratories should report results using the IS (Hughes et al, 2006). Unconverted results should only be included during a transition phase to IS, as routine reporting of unconverted results can lead to misinterpretation of ELN response by clinicians. At this time, not all laboratories in the UK report BCR‐ABL1 results using the IS, and some laboratories are currently transitioning to the IS system. If units other than IS are used, the laboratory report should state clearly that the results are not reported in IS. Transitioning requires good communication with clinicians and patients. It is essential that patients are adequately counselled about the change to IS, so that they are not unduly alarmed by a marked change in their test results.
Reference gene
The laboratory report should mention which reference gene (ABL1, GUSB or BCR) was used.
Test sensitivity
For patients with undetectable BCR‐ABL1 levels, it is important to state the level at which BCR‐ABL1 is undetectable (e.g. MR4 vs. MR4·5). In the laboratory report, placing the result in context by including standard levels of response (MMR, MR4, MR4·5) may aid clinicians. Efforts to establish confidence intervals are under way at individual laboratories, but there is no consensus on how to report this information at this time. Nevertheless, testing laboratories need to understand their measurement uncertainty at high and low BCR‐ABL1 levels (Branford & Hughes, 2006; Branford et al, 2008).
Technique used
Technical details, such as the level of sensitivity and methodology used, should also be included in the laboratory report.
Response status (MMR, MR4, MR4·5)
Laboratory reports should include both the actual BCR‐ABL1 result (e.g. 0·08%) and the corresponding response status (e.g. MMR) to aid in interpretation.
Response status according to ELN CML recommendations
The current ELN recommendations include three response categories (Optimal, Warning and Failure) (Baccarani et al, 2013). In some cases, laboratories may not have access to sufficient clinical information to determine response status according to the ELN recommendations. If the information is available, interpretation of results according to the current ELN recommendations could be a useful addition to the laboratory report. As a minimum, a reference to the ELN recommendations for clinical interpretation should be provided on the report.
Suggestions for frequency of testing
Frequency of monitoring should be as per current ELN recommendations (see Table 2). The laboratory should promptly notify the clinician and/or other members of the multidisciplinary team when there is a significant increase in BCR‐ABL1 level and/or when a change in monitoring frequency is required. What constitutes a significant change needs to be defined locally on the basis of the level of disease and the uncertainty of measurement of the assay used, but in general a 1‐log increase or loss of MMR in a patient with previous stable MMR would be considered as a significant change. Any change reported as potentially significant should be confirmed before making any alterations to management, and the laboratory report should contain appropriate caveats plus a request for an urgent repeat sample. A laboratory may suggest a change in testing frequency, but it should be noted that the suggestion may be incorrect if the laboratory is provided with incomplete or inaccurate clinical information (see below).
Date of next test
Providing or suggesting a date for the next test may be useful but is considered optional, because the laboratory may not have sufficient clinical information to determine the date of the next test. If a patient misses a visit, it may be useful for laboratories to have standard procedures in place to alert the multidisciplinary team so that the patient can be contacted. While this is not normally the laboratory's responsibility, this could help ensure that patients are followed appropriately.
Expert panel opinion.
The laboratory report should ideally include the following:
Transcript variant type
Line of therapy
-
Results reported in IS only
-
☐
If units other than IS are currently used, it should be clearly stated in the laboratory report that results are not reported in IS
-
☐
Unconverted results should only be included during a transition phase to IS
-
☐
Reference gene used (ABL1, GUSB, or BCR)
Technical details, such as the level of sensitivity
-
Both the actual BCR‐ABL1 result (e.g. 0·08%) and the corresponding response status (e.g. MMR)
-
☐
Laboratory results should be interpreted in the context of prior results, response status, and clinical circumstances
-
☐
Results should be interpreted according to the current ELN recommendations
-
☐
-
The laboratory should promptly notify the clinician and/or multidisciplinary team when there is a marked change in BCR‐ABL1 level and/or when a change in monitoring frequency is required. What constitutes a ‘marked’ change needs to be defined locally on the basis of the level of disease and the measured variation of the essay used, but in general a 1‐log increase or loss of MMR would be considered as a marked change
-
☐
Changes to monitoring frequency should be finalised after the laboratory has consulted with the treating haematologist/oncologist; this is usually determined by the clinician rather than the laboratory
-
☐
If available, the laboratory report could also include the following:
Mutation status and the time at which the mutation was first detected (including details of TKI sensitivity)
Timing of the test in relation to the start of TKI (e.g. 3 months after start of TKI)
A list or graph describing previous BCR‐ABL1 test results is strongly recommended
Suggesting a date for the next test is considered optional
How to report: patient‐directed communication
Increasingly, patients have access to laboratory results, and complex or poorly worded reports can lead to unnecessary alarm and confusion. The UK government has made a commitment that patient clinical records will be digitalised and accessible to patients and healthcare providers in real time by 2020.
If reports are being sent to the patient, these should contain the most important information only, such as the BCR‐ABL1 level and whether the level has increased, decreased or remained stable since the last test, as shown in Fig 5. Their current test result should be contextualised by including their two previous BCR‐ABL1 testing results. It would also be important to indicate to patients if the sample had been a technical failure or not, if a result cannot be given. A comment could be added to the report that the patient should contact their clinician if they have a concern about their test results.
Figure 5.
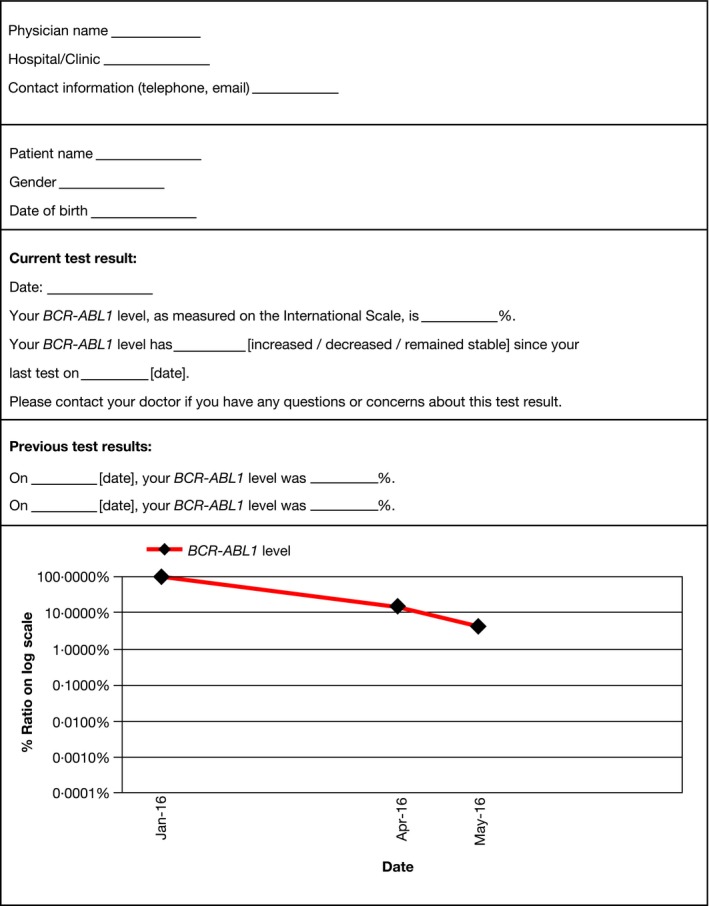
Standard patient‐directed report of BCR‐ABL1 testing results.
Expert panel opinion.
If reports are sent to the patient, these should include only the most relevant information, such as the BCR‐ABL1 level and whether the level has significantly increased or decreased, contextualised by including at least 2 previous test results
Concluding remarks
The remarkable improvements in outcomes observed in patients with CML in recent years have occurred in tandem with advances in molecular monitoring of the disease. However, considerable variability persists among laboratories in the UK regarding testing methods and reporting results for BCR‐ABL1 transcript levels. This consensus report provides a framework for developing a more standardised approach to presenting BCR‐ABL1 results. This will hopefully encourage greater uniformity across laboratories in the UK and support the accurate translation of results from laboratory to clinic, which is essential for the delivery of optimal CML patient care and disease management.
Supporting information
Fig S1. Example laboratory report for treatment response: optimal.
Fig S2. Example laboratory report for treatment response: warning.
Fig S3. Example laboratory report for treatment response: failure.
Fig S4. Example laboratory report for treatment switch and ABL kinase domain mutation.
Appendix S1. Examples of laboratory reports.
Acknowledgements
The authors are members of a publication steering committee organised and funded by Novartis Pharmaceuticals Ltd UK. Additional authors were invited by the steering committee, and all authors fulfil the criteria as author as per the International Committee of Medical Journal Editors (http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html). NCPC, HEW, PASE, JH, MC and AJM participated in the consensus meeting. NCPC developed the concept for this article. All authors reviewed and commented on the drafts and approved the final manuscript. Financial support for medical editorial assistance was organised and funded by Novartis Pharmaceuticals Ltd UK. The authors take full responsibility for the final wording of the manuscript but would like to thank Nicky Dekker, MD, PhD, of Excerpta Medica BV for medical editorial assistance. The authors would like to thank the following persons and parties for their critical review of the content of this manuscript: Mark Catherwood (Belfast Health and Social Care Trust), Polly Talley (Leeds Teaching Hospitals NHS Trust), and the UK NCRI Haematological Oncology Clinical Studies Group – CML Subgroup.
References
- Baccarani, M. , Deininger, M.W. , Rosti, G. , Hochhaus, A. , Soverini, S. , Apperley, J.F. , Cervantes, F. , Clark, R.E. , Cortes, J.E. , Guilhot, F. , Hjorth‐Hansen, H. , Hughes, T.P. , Kantarjian, H.M. , Kim, D.‐W. , Larson, R.A. , Lipton, J.H. , Mahon, F.‐X. , Martinelli, G. , Mayer, J. , Müller, M.C. , Niederwieser, D. , Pane, F. , Radich, J.P. , Rousselot, P. , Saglio, G. , Saußele, S. , Schiffer, C. , Silver, R. , Simonsson, B. , Steegmann, J.‐L. , Goldman, J.M. & Hehlmann, R. (2013) European LeukemiaNet recommendations for the management of chronic myeloid leukemia. Blood, 122, 872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccarani, M. , Soverini, S. & Benedittis, C.D. (2014) Molecular monitoring and mutations in chronic myeloid leukemia: how to get the most out of your tyrosine kinase inhibitor. American Society of Clinical Oncology Educational Book, 2014, 167–175. [DOI] [PubMed] [Google Scholar]
- Branford, S. & Hughes, T. (2006) Diagnosis and monitoring of chronic myeloid leukemia by qualitative and quantitative RT‐PCR. Methods in Molecular Medicine, 125, 69–92. [DOI] [PubMed] [Google Scholar]
- Branford, S. , Fletcher, L. , Cross, N.C. , Müller, M.C. , Hochhaus, A. , Kim, D.W. , Radich, J.P. , Saglio, G. , Pane, F. , Kamel‐Reid, S. , Wang, Y.L. , Press, R.D. , Lynch, K. , Rudzki, Z. , Goldman, J.M. & Hughes, T. (2008) Desirable performance characteristics for BCR‐ABL measurement on an international reporting scale to allow consistent interpretation of individual patient response and comparison of response rates between clinical trials. Blood, 112, 3330–3338. [DOI] [PubMed] [Google Scholar]
- Branford, S. , Yeung, D.T. , Parker, W.T. , Roberts, N.D. , Purins, L. , Braley, J.A. , Altamura, H.K. , Yeoman, A.L. , Georgievski, J. , Jamison, B.A. , Phillis, S. , Donaldson, Z. , Leong, M. , Fletcher, L. , Seymour, J.F. , Grigg, A.P. , Ross, D.M. & Hughes, T.P. (2014) Prognosis for patients with CML and >10% BCR‐ABL1 after 3 months of imatinib depends on the rate of BCR‐ABL1 decline. Blood, 124, 511–518. [DOI] [PubMed] [Google Scholar]
- Campiotti, L. , Suter, M.B. , Guasti, L. , Piazza, R. , Gambacorti‐Passerini, C. , Grandi, A.M. & Squizzato, A. (2017) Imatinib discontinuation in chronic myeloid leukaemia patients with undetectable BCR‐ABL transcript level: a systematic review and a meta‐analysis. European Journal of Cancer, 77, 48–56. [DOI] [PubMed] [Google Scholar]
- Claudiani, S. , Apperley, J.F. , Gale, R.P. , Clark, R. , Szydio, R. , Deplano, S. , Palanicawandar, R. , Khorashad, J. , Foroni, L. & Milojkovic, D. (2017) E14a2 BCR‐ABL1 transcript is associated with a higher rate of treatment‐free remission in individuals with chronic myeloid leukemia after stopping tyrosine kinase inhibitor therapy. Haematologica, 102, e297–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claustres, M. , Kožich, V. , Dequeker, E. , Fowler, B. , Hehir‐Kwa, J.Y. , Miller, K. , Oosterwijk, C. , Peterlin, B. , van Ravenswaaij‐Arts, C. , Zimmermann, U. , Zuffardi, O. , Hastings, R.J. & Barton, D.E. ; on behalf of the ESHG Quality committee . (2014) Recommendations for reporting results of diagnostic genetic testing (biochemical, cytogenetic and molecular genetic). European Journal of Human Genetics, 22, 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross, N.C.P. , White, H.E. , Colomer, D. , Ehrencrona, H. , Foroni, L. , Gottardi, E. , Lange, T. , Lion, T. , Machova Polakova, K. , Dulucq, S. , Martinelli, G. , Oppliger Leibundgut, E. , Pallisgaard, N. , Barbany, G. , Sacha, T. , Talmaci, R. , Izzo, B. , Saglio, G. , Pane, F. , Müller, M.C. & Hochhaus, A. (2015) Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia, 29, 999–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroni, L. , Wilson, G. , Gerrard, G. , Mason, J. , Grimwade, D. , White, H.E. , Gonzalez de Castro, D. , Austin, S. , Awan, A. , Burt, E. , Clench, T. , Farruggia, J. , Hancock, J. , Irvine, A.E. , Kizilors, A. , Langabeer, S. , Milner, B.J. , Nickless, G. , Schuh, A. , Sproul, A. , Wang, L. , Wickham, C. & Cross, N.C.P. (2011) Guidelines for the measurement of BCR‐ABL1 transcripts in chronic myeloid leukaemia. British Journal of Haematology, 153, 179–190. [DOI] [PubMed] [Google Scholar]
- Hanfstein, B. , Shlyakhto, V. , Lauseker, M. , Hehlmann, R. , Saussele, S. , Dietz, C. , Erben, P. , Fabarius, A. , Proetel, U. , Schnittger, S. , Krause, S.W. , Schubert, J. , Einsele, H. , Hänel, M. , Dengler, J. , Falge, C. , Kanz, L. , Neubauer, A. , Kneba, M. , Stegelmann, F. , Pfreundschuh, M. , Waller, C.F. , Spiekermann, K. , Baerlocher, G.M. , Pfirrmann, M. , Hasford, J. , Hofmann, W.K. , Hochhaus, A. & Müller, M.C. ; SAKK and the German CML Study Group . (2014) Velocity of early BCR‐ABL transcript elimination as an optimized predictor of outcome in chronic myeloid leukemia (CML) patients in chronic phase on treatment with imatinib. Leukemia, 28, 1988–1992. [DOI] [PubMed] [Google Scholar]
- Hochhaus, A. , Saussele, S. , Rosti, G. , Mahon, F‐X. , Janssen, J.J.W.M. , Hjorth‐Hansen, H. , Richter, J. & Buske, C. (2017) Chronic myeloid leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 28, iv41–iv51. [DOI] [PubMed] [Google Scholar]
- Hughes, T.P. , Kaeda, J. , Branford, S. , Rudzki, Z. , Hochhaus, A. , Hensley, M.L. , Gathmann, I. , Bolton, A.E. , Van Hoomissen, I.C. , Goldman, J.M. & Radich, J.P. ; for the International Randomised Study of Interferon versus STI571 (IRIS) Study Group . (2003) Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. New England Journal of Medicine, 349, 1423–1432. [DOI] [PubMed] [Google Scholar]
- Hughes, T. , Deininger, M. , Hochhaus, A. , Branford, S. , Radich, J. , Kaeda, J. , Baccarani, M. , Cortes, J. , Cross, N.C.P. , Druker, B.J. , Gabert, J. , Grimwade, D. , Hehlmann, R. , Kamel‐Reid, S. , Lipton, J.H. , Longtine, J. , Martinelli, G. , Saglio, G. , Soverini, S. , Stock, W. & Goldman, J.M. (2006) Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR‐ABL transcripts and kinase domain mutations and for expressing results. Blood, 108, 28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCCN (National Comprehensive Cancer Network). (2017) NCCN Clinical Practice Guidelines in Oncology: Chronic Myeloid Leukemia. Version 1.2018. Available at: http://www.NCCN.org.
- Rea, D. & Cayuela, J.M. (2017) Treatment‐free remission in patients with chronic myeloid leukemia. International Journal of Hematology, 106, 1–10. 10.1007/s12185-017-2295-0 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Saussele, S. , Richter, J. , Hochhaus, A. & Mahon, F.‐X. (2016) The concept of treatment‐free remission in chronic myeloid leukemia. Leukemia, 30, 1638–1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner, M.T. , Hilborne, L. , Brown, J. & Lubin, I.M. (2012) A report template for molecular genetic tests designed to improve communication between the clinician and laboratory. Genetic Testing and Molecular Diagnosis, 16, 761–769. [DOI] [PubMed] [Google Scholar]
- Soverini, S. , Hochhaus, A. , Nicolini, F.E. , Gruber, F. , Lange, T. , Saglio, G. , Pane, F. , Müller, M.C. , Ernst, T. , Rosti, G. , Porkka, K. , Baccarani, M. , Cross, N.C.P. & Martinelli, G. (2011) BCR‐ABL kinase domain mutation analysis in chronic myeloid leukemia patients treated with tyrosine kinase inhibitors: recommendations from an expert panel on behalf of European LeukemiaNet. Blood, 118, 1208–1215. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Example laboratory report for treatment response: optimal.
Fig S2. Example laboratory report for treatment response: warning.
Fig S3. Example laboratory report for treatment response: failure.
Fig S4. Example laboratory report for treatment switch and ABL kinase domain mutation.
Appendix S1. Examples of laboratory reports.


