Abstract
Background
Immunotherapy with peptide hydrolysates from Lolium perenne (LPP) is an alternative treatment for seasonal allergic rhinitis with or without asthma. The aim of this study was to assess the clinical efficacy and safety of a cumulative dose of 170 μg LPP administered subcutaneously over 3 weeks.
Methods
In a randomized, double‐blind, placebo‐controlled trial, 554 adults with grass pollen rhinoconjunctivitis were randomized (1:2 ratio) to receive 8 subcutaneous injections of placebo or 170 μg LPP administered in increasing doses in 4 visits over 3 weeks. The primary outcome was the combined symptom and medication score (CSMS) measured over the peak pollen season. Reactivity to conjunctival provocation test (CPT) and quality of life (QOL) was assessed as secondary endpoints.
Results
The mean reduction in CSMS in the LPP vs placebo group was −15.5% (P = .041) during the peak period and −17.9% (P = .029) over the entire pollen season. LPP‐treated group had a reduced reactivity to CPT (P < .001) and, during the pollen season, a lower rhinoconjunctivitis QOL global score (P = .005) compared with placebo group. Mostly mild and WAO grade 1 early systemic reaction (ESR) were observed ≤30 minutes in 10.5% of LPP‐treated patients, whereas 3 patients with a medical history of asthma (<1%) experienced a serious ESR that resolved with rescue medication.
Conclusion
Lolium perenne pollen peptides administered over 3 weeks before the grass pollen season significantly reduced seasonal symptoms and was generally safe and well‐tolerated.
Keywords: clinical trial, grass pollen, Lolium perenne, peptide immunotherapy, subcutaneous immunotherapy
Abbreviations
- (E)SR
(early) systemic reaction
- AIT
allergen immunotherapy
- CPT
conjunctival provocation test
- CSMS
combined clinical symptom/medication score
- EAACI
European Academy of Allergy and Clinical Immunology
- ESS
Eye symptoms score
- LPP
Lolium perenne pollen peptides
- NSS
nose symptom score
- QOL
quality of life
- RMS
rescue medication score
- RTSS
rhinoconjunctivitis total symptom score
- SCIT
subcutaneous immunotherapy
- WAO
World Allergy Organization
1. INTRODUCTION
Allergic rhinitis with and without asthma affects at least 400 million people worldwide, and the current estimated prevalence is up to 40%. Pharmacotherapy with antihistamines and nasal corticosteroids can provide symptomatic relief in most but not all patients.1, 2 Allergen immunotherapy (AIT), administered either subcutaneously or sublingually, provides long‐term relief of allergy symptoms and is recommended for patients whose seasonal allergies are not effectively controlled by pharmacotherapy.3, 4, 5, 6 However, patient adherence to AIT is low, and therefore, efficiency is suboptimal because current immunotherapy regime requires frequent treatment over several years.7, 8, 9
Adjuvant‐free allergen peptide hydrolysates ranging 1‐10 kDa from Lolium perenne (LPP) for immunotherapy use have been shown to have limited IgE binding, basophil, and mast cell reactivity and hence are considered as a safe alternative that can be administered at higher doses and for a shorter period to improve treatment adherence.10, 11 Moreover, in a dose‐response, double‐blind, placebo‐controlled study where 198 adults with grass pollen‐induced allergic rhinitis received either placebo, 70, 170, or 370 μg, a cumulative dose of 170 μg of LPP over 3 weeks was the optimal dose in terms of surrogate efficacy and benefit/risk balance.12 In this study, a dose‐dependent allergen‐specific IgG4 and blocking antibodies were induced.
We sought to investigate the clinical efficacy and safety of a cumulative dose of 170 μg LPP administered subcutaneously over 3 weeks. Here, we present the results of a multicenter, randomized, double‐blind, placebo‐controlled trial with grass pollen‐allergic patients treated in 4 visits over 3 consecutive weeks with LPP. Clinical efficacy was primarily assessed using the combined clinical symptom and medication score (CSMS) as recommended by the European Academy of Allergy and Clinical Immunology (EAACI).13 In addition, patient QOL reactivity and CPT reactivity to grass pollen allergen were assessed, and adverse events were documented.
2. METHODS
2.1. Study design and ethics
This was a randomized, double‐blind, placebo‐controlled, international and multicenter trial conducted in 57 sites in six countries in Europe (Belgium, Czech Republic, Germany, France, Italy, and Spain) between January and September, 2016 (ClinicTrials.gov no. NCT02560948; EudraCT no. 2015‐002105‐11). The primary objective was to demonstrate the clinical efficacy of a cumulative dose of 170 μg LPP over the peak pollen season as measured using the CSMS. The study was reviewed and approved by local regulatory authorities and independent ethics committees in each country and was conducted in accordance with the Declaration of Helsinki in its revised edition (64th World Association General Assembly, Fortaleza, Brazil, October 2013) and International Conference on Harmonization Good Clinical Practice. All patients provided written informed consent.
2.2. Patients
Between January 14, 2016 and March 26, 2016, 554 adults were included according to inclusion criteria below and randomized (182 to placebo and 372 to LPP). Follow‐up of the last patient was completed on September 15, 2016. Nine patients withdrew before the treatment phase and did not receive study product, 28 withdrew during the treatment phase, and 5 withdrew after the treatment phase, so that among those who completed the study, ITT efficacy analysis included 171 patients in the placebo group and 339 patients in the LPP group (Figure 1). Adults aged 18‐64 years and allergic to grass pollen were enrolled if they had a medical history of moderate‐to‐severe seasonal allergic rhinoconjunctivitis during at least the two previous seasons as defined by the Allergic Rhinitis and its Impact on Asthma guidelines.14 Patients also had a positive skin prick test (wheal diameter ≥ 3 mm, mean of orthogonal diameters) to grass pollen mix extract, specific IgE against grass pollen allergens > 0.7 kU/L, a positive CPT to grass pollen allergen (≤10 000 standardized quality units; see Data S1) and have received anti‐allergic medications for at least two consecutive grass pollen seasons. Patients with confirmed diagnosis of grass pollen‐induced controlled asthma according to the 2014 Global Initiative for Asthma guidelines were included in the study as well.15
Figure 1.
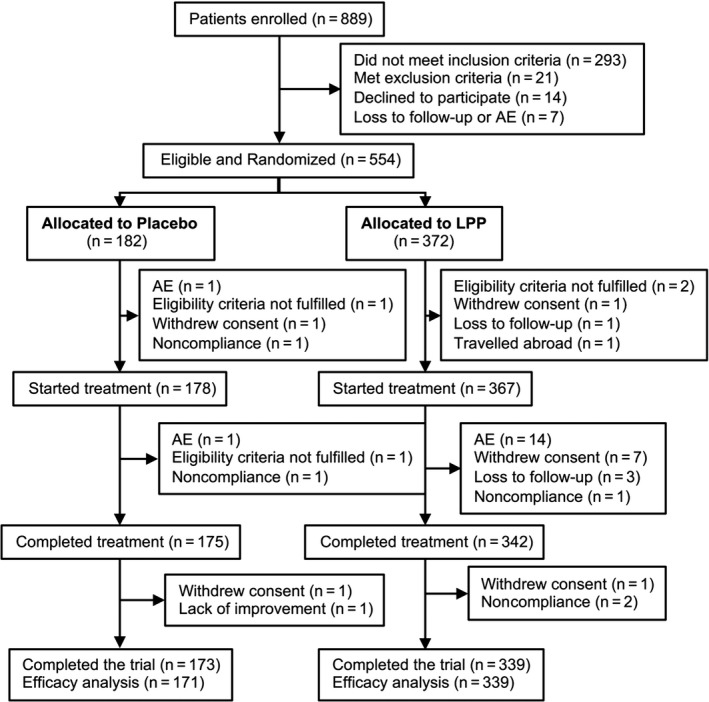
Patient disposition. A total of 554 patients were included and randomized. Of the 182 patients randomized to placebo, 178 started treatment, of which 3 discontinued treatment early, 2 discontinued before the end of the study, and 173 completed the trial. Of the 372 patients randomized to LPP, 367 started treatment, of which 22 discontinued treatment early, 6 discontinued before the end of the study, and 339 completed the trial. ITT population consisted of 171 placebos and 339 LPP‐treated patients. AE, adverse event; LPP, Lolium perenne pollen peptides
Patients were excluded if they had received immunotherapy with grass pollen allergens within the preceding 5 years, were currently receiving immunotherapy of any kind, had a history of anaphylaxis, were hypersensitive to the excipients of the investigational product, had a forced expiratory volume in 1 second <80% of the predicted value or a peak expiratory flow <70%, were symptomatic to other inhaled allergens present during the grass pollen season or to perennial inhaled allergens (house dust mites, cat, dog) to which they were regularly exposed, or had a contraindication for epinephrine. Patients with a history of significant renal disease, chronic hepatic disease, malignant disease, and severe autoimmune disease were excluded.
2.3. LPP and placebo
Lolium perenne pollen peptides is an adjuvant‐free mixture of peptides (1‐10 kDa) resulting from the enzymatic hydrolysis of L. perenne purified proteins as described in Shamji et al.11 LPP was supplied in ready‐to‐use vials containing 1.5 mL of 100 μg/mL grass pollen peptides in sterile aqueous‐buffered solution (pH 7.4). The placebo was provided in identical ready‐to‐use vials containing 1.5 mL of sterile aqueous‐buffered solution. The vials were numbered with computer‐generated randomization codes.
2.4. Study design
Eligible patients were randomized 2:1 to LPP or placebo. Treatments were assigned using a central allocation system. Each treatment consisted of eight subcutaneous injections at 4 visits over 3 consecutive weeks between January and April, 2016. At each treatment visit, the patient received a first injection in one arm, followed 30 minutes later by a second injection in the opposite arm. Doses were increased incrementally starting with 2 × 5 μg at 1st visit, 2 × 10 μg at 2nd visit, 2 × 20 μg at 3rd visit and 2 × 50 μg of LPP at the last treatment visit, (Figure S1). The resulting cumulative dose was 170 μg of LPP. After the two injections, patients remained under physician supervision for another 30 minutes. Local reactions and SRs occurring within 48 hours following each injection were reported. SRs were graded according to the World Allergy Organization (WAO) scale from 1 to 4.16 Patients were discontinued if they had a serious SR (grade 3 or 4) at any visit or had a local wheal reaction >8 cm in diameter within 30 minutes after injection or a confirmed grade 2 SR during the first treatment visit. In case of a local wheal of 5‐8 cm in diameter (within 30 minutes) or a grade 1 SR, the same dose was to be repeated at the next treatment visit. Patients who had a local wheal >8 cm (within 30 minutes) or a grade 2 SR during one of the last three treatment visits were de‐escalated to the dose injected at the previous visit for the next visit. In case, a dose adjustment was necessary, up to two additional treatment visits were allowed to reach the cumulative dose of 170 μg. All patients were provided with fexofenadine (180 mg) as rescue medication to relieve any local reaction. Follow‐up visits occurred before (March/April), during (June), and after the grass pollen season (August). During the pollen season, all patients were provided with desloratadine 5‐mg tablets, levocabastine 0.5 mg/mL eye drops, and fluticasone propionate 50 μg/dose nasal spray. Upon request, patients were provided with methylprednisolone 16‐mg tablets. Asthmatic patients were also provided with budesonide 160 μg/formoterol fumarate dihydrate 4.5 μg.
2.5. Endpoints
The primary endpoint was the daily CSMS during the peak pollen period, which was calculated as the daily rhinoconjunctivitis total symptom score (RTSS)/6+ rescue medication score (RMS) over the peak of the grass pollen season, as described previously.13 Secondary efficacy endpoints included individual symptom (NSS, ESS) and medication scores over the peak and entire pollen season, and number of well days. A well day was defined as a day with a RTSS ≤ 2 and no rescue medication needed (RMS = 0). Change in CPT reactivity was assessed before and after treatment and was scored from 0 (unreactive) to 4 (highest) as described previously17, 18 (see Data S1 for details). Quality of life was evaluated using the Rhinoconjunctivitis Quality of Life Questionnaire (RQLQ)19 and Nocturnal Rhinoconjunctivitis Quality of life questionnaire (NRQLQ)20 at a visit prior to the pollen season (V6) and at a visit during the pollen season (V7). Safety endpoints included local reactions and SRs graded according to the WAO scale,16 and unsolicited treatment‐emergent adverse events (TEAEs) and serious adverse events coded using MedDRA version 19.0 (MedDRA MSSO, McLean, VA, USA).
2.6. Definition of pollen season and the pollen peak
Start and end dates of the grass pollen season and of the grass pollen peak period were determined for each region or country based on official pollen count data. The pollen season start was defined as the first of 5 consecutive days with a pollen count ≥10 grains/m3 of air and the last day of the pollen season as the first of 5 consecutive days with a pollen count <10 grains/m3 of air. The grass pollen peak period was defined as the 14‐day period with the highest pollen counts.
2.7. Study size estimate
Using a 2:1 randomization ratio and assuming a mean daily CSMS of 1.55 in the placebo group with a standard deviation of 1.0, a sample size of 165 patients in the placebo group and 330 patients in the LPP group is needed to detect a statistically significant (P < .05) difference in mean daily CSMS of 20% between the 2 groups with a power of 90%.13 To account for a 10% and a 15% dropout rate during the treatment phase and the pollen season, respectively, 654 randomized patients were targeted for corticosteroids.
2.8. Statistical analysis
Statistical analyses were performed using SAS® version 9.4 (SAS Institute, Cary, NC, USA). The primary analysis was the intention‐to‐treat (ITT) using the efficacy assessments from all patients who received at least one dose of study treatment and had at least one record of the primary efficacy measure on at least 1 day during the observation period. The primary efficacy assessment was the reduction in CSMS over the peak pollen season for LPP vs placebo, with missing data replaced as recommended by the Committee for Medicinal Products for Human Use Guideline on Missing Data in Confirmatory Clinical Trials (see Data S1).21 Baseline demographics and allergy characteristics were analyzed in all randomized patients. CSMS, its related subscores, well days, and QOL scores were compared by Wilcoxon rank sum test as they were not normally distributed. Improvement in CPT reactivity was compared by Cochran‐Mantel Haenszel chi‐squared test, and change in CPT score from baseline was compared by Mann‐Whitney test. A P‐value below .05 was considered to indicate statistical significance.
3. RESULTS
Five hundred and fifty‐four patients were randomized and 512 (92%) completed treatment (Figure 1). Demographics and baseline disease characteristics were similar between the placebo and LPP groups (Table S1). The most common cosensitization in both groups was birch pollen, followed by house dust mites, cat epithelia, and dog epithelia. Approximately one‐quarter (24.1%) of patients were asthmatic. The full cumulative dose was reached by 329 patients (89.4%) in the LPP and 171 patients (96.6%) in the placebo group. Most of these received the full treatment in the planned eight injections (312/329 [94.8%] for LPP and 168/171 [98.3%] for placebo). Seventeen (5.2%) patients in the LPP group and three (1.7%) in the placebo group reached the full treatment in more than eight injections. Eight additional patients in the LPP group (0.6%) received the maximum of 12 injections but did not reach the targeted cumulative dose of 170 μg. The remaining patients discontinued for various reasons, as detailed in Figure 1.
3.1. Clinical efficacy outcomes
The mean daily CSMS during the peak pollen period was significantly lower in the LPP group than in the placebo group (the primary efficacy assessment), indicating better control of symptoms in the LPP group than in the placebo group (Table 1). The treatment effect corresponded to a relative mean difference of 15.5% in favor of LPP (P = .041). Similarly, mean daily RTSS, NSS, ESS were also significantly lower in the LPP group compared with placebo (Table 1), but not for RMS. Patients in the LPP group had more number of well days (+23.0%, P = .044) during the peak pollen period than patients in the placebo group.
Table 1.
Efficacy assessments
| Periods | Measures | Treatments | N | Mean ± SD | Absolute differences | Relative differences (%) | P‐values |
|---|---|---|---|---|---|---|---|
| Pollen peak | CSMS (primary endpoint) | Placebo | 136 | 1.475 ± 1.049 | −0.228 | −15.5 | .041 |
| LPP | 264 | 1.247 ± 0.972 | |||||
| RTSS | Placebo | 150 | 4.498 ± 3.513 | −0.833 | −18.5 | .013 | |
| LPP | 284 | 3.665 ± 3.169 | |||||
| RMS | Placebo | 108 | 0.698 ± 0.620 | −0.104 | −14.9 | .152 | |
| LPP | 222 | 0.594 ± 0.595 | |||||
| Nose symptom score | Placebo | 156 | 3.318 ± 2.502 | −0.614 | −18.5 | .007 | |
| LPP | 289 | 2.704 ± 2.335 | |||||
| Eye symptom score | Placebo | 150 | 1.222 ± 1.215 | −0.248 | −20.3 | .046 | |
| LPP | 287 | 0.974 ± 1.093 | |||||
| Well days | Placebo | 102 | 33.193 ± 37.063 | 7.638 | 23.0 | .044 | |
| LPP | 208 | 40.831 ± 36.131 | |||||
| Entire season | CSMS | Placebo | 95 | 1.189 ± 0.856 | −0.213 | −17.9 | .029 |
| LPP | 201 | 0.976 ± 0.810 | |||||
| RTSS | Placebo | 86 | 3.168 ± 2.423 | −0.495 | −15.6 | .073 | |
| LPP | 186 | 2.673 ± 2.171 | |||||
| RMS | Placebo | 64 | 0.577 ± 0.577 | −0.125 | −21.7 | .127 | |
| LPP | 144 | 0.452 ± 0.486 | |||||
| Nose symptom score | Placebo | 98 | 2.340 ± 1.661 | −0.285 | −12.2 | .097 | |
| LPP | 196 | 2.055 ± 1.656 | |||||
| Eye symptom score | Placebo | 94 | 0.892 ± 0.947 | −0.194 | −21.8 | .115 | |
| LPP | 202 | 0.698 ± 0.751 | |||||
| Well days | Placebo | 46 | 42.188 ± 34.968 | 10.530 | 24.9 | .082 | |
| LPP | 113 | 52.718 ± 33.019 |
Values are shown according to the treatment planned. P‐values were calculated by Wilcoxon rank sum test.
CSMS, combined symptom and medication score; LPP, Lolium perenne peptides; RMS, rescue medication score; RTSS, rhinoconjunctivitis total symptom score; SD, standard deviation.
When assessed over the entire pollen season, the difference in CSMS was 17.9% in favor of LPP (P = .029; Figure S1, Table 1). Similarly, RTSS, RMS, ESS, NSS, and number of well days were all better in the LPP group, although differences were not significant.
Reactivity to CPT before and after treatment was assessed as a secondary endpoint, as surrogate of the clinical effect anticipated. CPT reactivity decreased in significantly more patients in the LPP group (177/295 [60.0%]) than in the placebo group (56/149 [37.6%]) (P < .0001) (Figure 2). The mean change in CPT score was also significantly larger in the LPP group than in the placebo group (−0.8 ± 0.8 vs −0.4 ± 0.9, P < .001; Table S2). In a post hoc analysis, the relationship between CPT reactivity at baseline and the CSMS during the peak pollen period and entire pollen season was assessed. Both the mean CSMS in the placebo group and the difference in mean CSMS between the placebo and LPP group, increased when the CPT reactivity at baseline was higher (Table S3). When looking at the most CPT reactive patients, that is, those with a baseline CPT score of 3 or 4 (representing 57.9% the patients, Table S3), the mean relative difference of CSMS was −19.8% (P = .051) over the peak period and −24.4% (P = .047) over the entire pollen season (Figure 3A‐B and Table S4).
Figure 2.
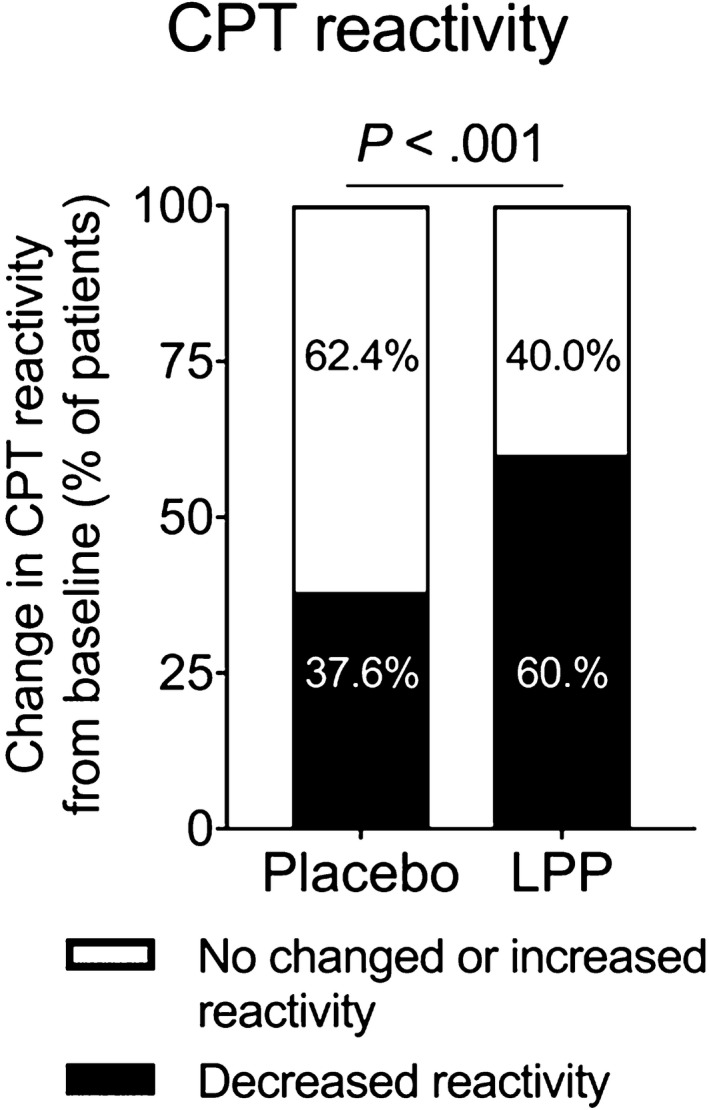
Conjunctival provocation test (CPT) reactivity. A CPT with grass pollen extract was performed at baseline and after completion of treatment (visit 6). Shown are the proportions of patients receiving placebo (n = 56/149) and LPP (n = 177/295) with a decrease in CPT score from baseline, and those without. Results are for the ITT population. Proportions were compared by chi‐squared test. LPP, Lolium perenne pollen peptides
Figure 3.
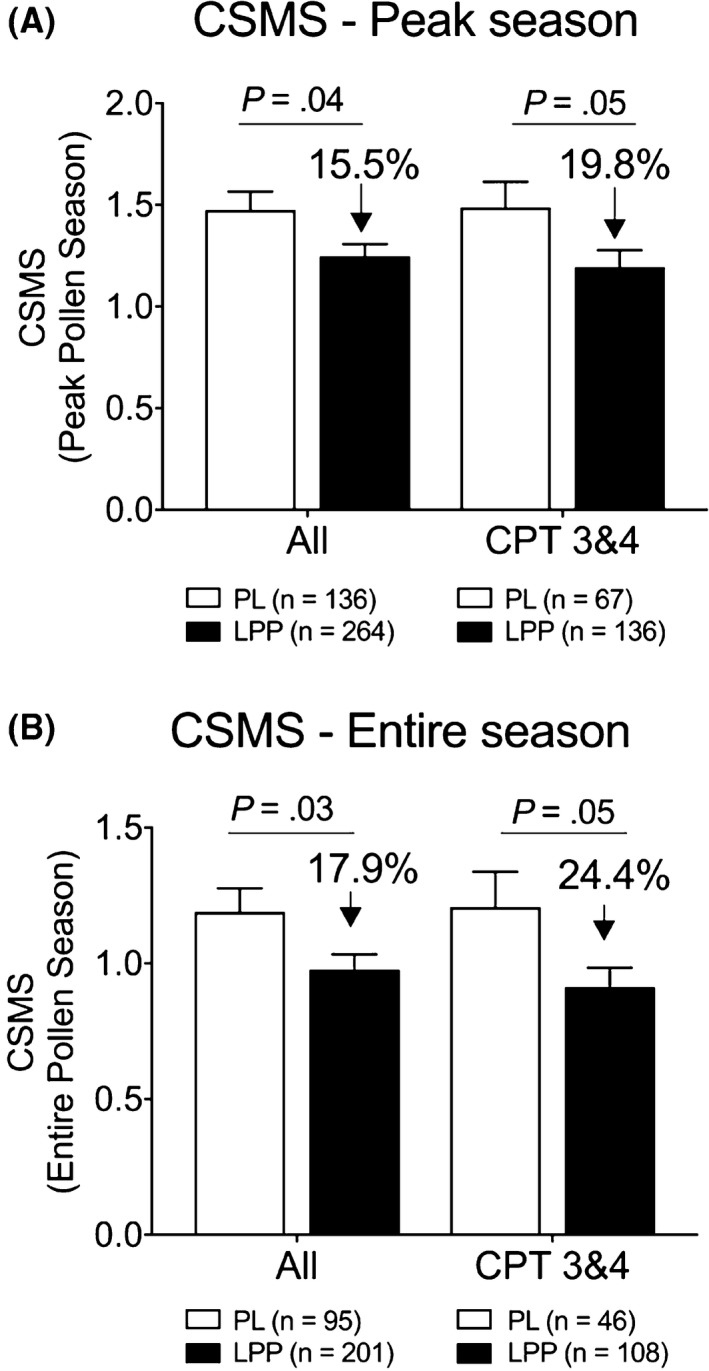
Impact of baseline conjunctival provocation test (CPT) reactivity on CSMSs. Comparison of the mean daily combined symptom and medication score (CSMS) during the peak pollen period (A) and during the entire pollen season (B) in the placebo and Lolium perenne pollen peptides (LPP) groups for the whole population and for the subpopulation of patients with a CPT score of 3 and 4 at baseline. Data are presented as mean ± SEM
3.2. Quality of life assessments
Whereas overall RQLQ and NRQLQ scores were similar in both groups prior to the pollen season, patients in the LPP group reported a better rhinoconjunctivitis‐related QOL during the pollen season than those in the placebo group (Table 2). A significant treatment effect was observed at V7 (−17.1%; P = .005) with the overall RQLQ assessment. Similarly, overall NRQLQ score was significantly lower in the LPP group at the visit performed during the grass pollen season. Also in most RQLQ and NRQLQ subdomains, mean scores were significantly lower in the LPP group compared with placebo. In a post hoc analysis, the treatment effect on QOL in patients with highest CPT reactivity at baseline was found to reach 29.2% for RQLQ and 20.7% for NRQLQ (Table S5).
Table 2.
Quality of Life assessments during the pollen season
| Measures | Treatments | N | Mean ± SD | Relative differences (%) | P‐values |
|---|---|---|---|---|---|
| Overall RQLQ Scores | Placebo | 170 | 1.407 ± 1.104 | −17.1 | .005 |
| LPP | 332 | 1.167 ± 1.063 | |||
| Activity limitations | Placebo | 170 | 1.831 ± 1.451 | −12.3 | .093 |
| LPP | 336 | 1.606 ± 1.381 | |||
| Sleep problems | Placebo | 170 | 0.914 ± 1.260 | −23.1 | .066 |
| LPP | 336 | 0.702 ± 1.096 | |||
| Non‐nose/eye symptoms | Placebo | 170 | 1.240 ± 1.259 | −20.9 | .011 |
| LPP | 335 | 0.981 ± 1.188 | |||
| Practical problem | Placebo | 170 | 2.220 ± 1.594 | −13.8 | .025 |
| LPP | 335 | 1.914 ± 1.557 | |||
| Nose symptoms | Placebo | 170 | 1.875 ± 1.342 | −15.3 | .015 |
| LPP | 335 | 1.588 ± 1.276 | |||
| Eye symptoms | Placebo | 170 | 1.224 ± 1.220 | −12.0 | .122 |
| LPP | 332 | 1.076 ± 1.166 | |||
| Emotional function | Placebo | 170 | 0.860 ± 1.133 | −21.2 | .018 |
| LPP | 333 | 0.687 ± 1.086 | |||
| Overall NRQLQ Scores | Placebo | 170 | 0.944 ± 0.907 | −16.5 | .006 |
| LPP | 338 | 0.789 ± 0.910 | |||
| Sleep problems | Placebo | 171 | 0.754 ± 1.077 | −24.3 | .014 |
| LPP | 338 | 0.571 ± 0.961 | |||
| Sleep time problems | Placebo | 170 | 0.774 ± 0.927 | −17.3 | .028 |
| LPP | 339 | 0.640 ± 0.888 | |||
| Symptoms on waking in the morning | Placebo | 171 | 1.069 ± 1.123 | −17.2 | .009 |
| LPP | 338 | 0.885 ± 1.121 | |||
| Practical problems | Placebo | 171 | 1.310 ± 1.004 | −8.0 | .025 |
| LPP | 339 | 1.205 ± 1.246 |
Values are shown according to the treatment planned based on observed cases. P‐values were calculated by Wilcoxon rank sum test.
LPP, Lolium perenne peptides, SD, standard deviation.
3.3. Safety outcomes
Early systemic reactions (ESR, ie, within 30 minutes of treatment) were reported in 10.1% of patients (37/368) in the LPP group and in 2.3% (4/177) in the placebo group (Table 3). All but two were WAO grade 1 or 2. Three ESRs were reported as serious in 3 patients with a history of asthma belonging to the LPP group: one grade 2, one grade 3, and one grade 4 ESR (WAO). These were resolved with rescue medication but resulted in withdrawal from the trial. Only the grade 4 ESR required the administration of epinephrine. Seventeen participants who had dose adjustments due to ESRs eventually reached the full target dose. Systemic reactions occurring later than 30 minutes were less severe (Table 3), and none was serious: Only 4 (1.1%) patients experienced a WAO grade 2 reaction (compared to 3.5% within 30 minutes), and no grade 3 and 4 WAO systemic reaction occurred. Local reactions at the injection site were reported for 28.5% (105 of 368) of patients in the LPP group and 2.8% (5 of 177) in the placebo group. Most were mild (99 for LPP, 7 for placebo). The remainder were moderate (12 for LPP, 1 for placebo). In all cases, the event resolved without medical assistance, and none was considered as serious. No wheal diameter exceeded 5 cm, 30 minutes after injection, and so no dose adjustment was required per protocol. Nineteen patients (5.2%) discontinued the study treatment due to a TEAE considered as related to LPP (some of which continued in the study), the main cause being a SR.
Table 3.
Solicited systemic reactions
| WAO grade | Placebo | LPP | ||
|---|---|---|---|---|
| N = 177 | N = 368 | |||
| Events | Patient, n (%) | Events | Patient, n (%) | |
| Occurrence within 30 min of injection | ||||
| Any | 5 | 4 (2.3) | 49 | 37 (10.1) |
| Grade 1 | 5 | 4 (2.3) | 34 | 25 (6.8 |
| Grade 2 | 0 | 0 (0.0 | 13a | 13 (3.5) |
| Grade 3 | 0 | 0 (0.0) | 1b | 1 (0.3) |
| Grade 4 | 0 | 0 (0.0) | 1c | 1 (0.3) |
| Occurrence later than 30 min after injection | ||||
| Any | 4 | 4 (2.3) | 48 | 41 (11.1) |
| Grade 1 | 3 | 3 (1.7) | 44 | 37 (10.1) |
| Grade 2 | 1 | 1 (0.6) | 4 | 4 (1.1) |
Results are shown according to the treatment received. Reactions were graded according to the WAO scale.14
LPP, Lolium perenne peptides; WAO, World Allergy Organization.
Included one serious event of bronchospasm with dyspnea and wheezing, followed by generalized urticaria.
Included one serious event of conjunctival injection, throat clearing, and urticaria, followed by abdominal cramps, retrosternal pain, dyspnea, nausea, and extension of the urticaria.
Included one serious event of tightness of the chest, followed by dizziness and decreased blood pressure.
4. DISCUSSION
For the first time, we report the clinical efficacy and safety of a 3‐week treatment administered subcutaneously over 4 visits prior the grass pollen season with an adjuvant‐free Lollium perenne peptides (LPP) ranging 1‐10 kDa. CSMS was reduced in LPP‐treated group compared with placebo. Furthermore, LPP treatment was well tolerated as the majority of the patients reached accumulative dose of 170 μg.
The primary outcome measure was the assessment of the daily CSMS during the peak pollen season. In this study, we showed a significantly lower mean CSMS over the peak pollen season of 15% for LPP vs placebo. The low magnitude in the reduction may have been due to a relatively mild grass pollen allergy season, as indicated by relatively low symptom scores (mean RTSS <4.5 out of a maximum of 18 during peak and <3.2 over the entire season) and low rescue medication use in the placebo arm: The mean RMS in the placebo group during both the pollen peak (0.698) and the full pollen season (0.577) indicated that, on average, only 2 of 3 patients used any rescue medications daily. It is well known that the magnitude of efficacy measurements in grass allergy immunotherapy trials is highly dependent on the natural pollen exposure.22, 23 Consistent with these findings, post hoc analysis showed that the difference in CSMS during the pollen peak was about 20% when limited to patients with more severe grass pollen sensitivity at baseline (representing 57.9% of the whole study population) and 24.4% over the entire pollen season.
Short‐course immunotherapy approach with whole allergen extract has been used in grass pollen‐induced allergic rhinitis. One study showed a 26.6% decrease in median combined score during the first year and a 48.4% decrease the second year in grass pollen‐allergic adults who received weekly subcutaneous of alum‐adsorbed six‐grass pollen extract just before each pollen season.24 A decrease of 12.7% during the entire grass pollen season and 13.6% during the pollen peak was reported in patients who received four preseason subcutaneous injections of monophosphoryl lipid A‐adjuvanted tyrosine‐adsorbed 13‐grass pollen extract.25 A decrease of 15.6% the first year and 33% the second year was reported in patients who each year received five preseason subcutaneous injections of alum‐adsorbed depigmented grass pollen extract.26 Although comparison of results of different studies has to be performed with caution because scores, statistical methods, study designs, pollen seasons, and regions may be different, the 15.5%‐24.4% improvement in CSMS observed after LPP treatment appeared to be within the range reported for other short‐term grass pollen immunotherapies with adjuvanted products.
The effect of LPP appeared to be more pronounced on the changes in allergic symptoms (mean RTSS, NSS, and ESS were significantly lower with LPP than with placebo), than on rescue medication. In agreement with this, patients receiving LPP also had more well days, a lower impairment of quality of life in season (diurnal and nocturnal) and a greater decrease in grass pollen CPT scores than patients receiving placebo.
Injections with whole pollen antigens can cause severe systemic reactions, so injections must be performed under medical supervision where resuscitation equipment is available, and patients need to be monitored for 30‐60 minutes after injections.27 At the same doses, LPP are less likely than the whole grass pollen allergen protein to cross‐link high‐affinity IgE receptors and cause allergic reactions, although LPP is administered at higher dose to allow for the short‐course treatment regimen.11 Three patients (<1%) treated with LPP had grades 2‐4 serious systemic hypersensitivity reactions, resulting in discontinuation from the study. However, all occurred within 30 minutes of injection and resolved with rescue medication. In each of the three cases, the patient had a history of asthma, a known risk factor for early SRs to SCIT.28, 29 By comparison, Frew et al30 reported that alum‐adsorbed grass pollen extract (Phleum pratense) induced ESRs in 32.5% of patients and EAACI grade 3 ESRs in 4.4% of patients with grass pollen allergy. In this study, ESRs were reported in 10.5% of the patients and WAO grade ≥3 ESR in 0.6%. Although a different grading system was used than the current study, the data of this study suggest that LPP was overall well tolerated, in agreement with the concept of peptide immunotherapy.10 Importantly, 89% reached the full dose of 170 μg LPP in the planned eight injections (four treatment visits of two injections) and another 5% reached the full dose after a dose adjustment.
A limitation of this study was an unexpectedly high number of screening failures, which was mainly due to the exclusion of patients in whom birch pollen allergy was dominant. Despite this, the population was large enough to detect clinically and statistically significant improvements in allergic rhinitis symptoms as well as relevant safety data. The modest CSMS treatment effect observed may also be due to the inclusion of patients with limited allergy severity, as suggested by the increased treatment effect observed in patients with highest CPT reactivity at baseline. Another apparent limitation is the number of missing data, especially outside the peak pollen period, which is due to noncompliance of patients with the requirement of scoring symptoms and medication on a daily basis for several weeks, even in the absence of symptoms. Considering that all efficacy data show similar results, including the QOL scores for which only a limited number of data were available, these missing data are not considered to impact significantly on efficacy data and conclusions.
In conclusion, this study showed that a short‐course treatment with LPP over 3 weeks just before the pollen season was effective and limited CSMS in patients with seasonal allergic rhinitis with and without asthma. LPP offers the possibility of a much shorter treatment course and therefore better compliance and efficiency than standard SCIT using conventional whole allergen extracts. LPP treatment was safe and well tolerated. However, as with conventional SCIT, patients should be monitored for adverse reactions, and dose adjustment should be made if necessary.
CONFLICTS OF INTEREST
RM received research grants via the Institute of Medical Statistics, Informatics and Epidemiology from ASIT biotech. CB received research grants via Ghent University Hospital from ASIT biotech. MHS and SRD received research grants via Imperial College London from ASIT biotech. MS received grants and personal fees from ALK‐Abelló, grants from Regeneron and Merck, personal fees from Allergopharma. SRD received grants and personal fees from ALK‐Abelló, grants from Regeneron and Merck, personal fees from Biomay, UCB, Boehringer Ingelheim, Allergy Therapeutics, Pneumo Update GmbH and Anergis. OP reports grants and personal fees from ASIT biotech. for the conduct of the study; grants and personal fees from ALK‐Abelló, Allergopharma, Stallergenes Greer, HAL Allergy Holding B.V./HAL Allergie GmbH, Bencard Allergie GmbH/Allergy Therapeutics, Lofarma, Laboratorios LETI/LETI Pharma, Anergis S.A, grants from Biomay, Nuvo, Circassia, personal fees from Novartis Pharma, MEDA Pharma Sanofi US Services, Mobile Chamber Experts (a GA2LEN Partner), Pohl‐Boskamp. PP received honoraria for participation as investigators in this study. MAC received personal feels from ALK, Merck, Stallergenes Greer, Hal Allergy, Allergopharma. SP, NW, MAB and TL are employees of ASIT biotech. LH and RVF are consultants for ASIT biotech and received fees from ASIT biotech for their contribution.
AUTHOR CONTRIBUTIONS
RM, CB, MHS, SP, and TL conceptualized and delineated research hypotheses. SP, MAB, and LH coordinated the clinical study. CB, OP, and PP performed the study as investigator. RM, CB, MHS, SP, MAC, LH, NW, TL, MC, OP, and RVF participated in the discussions of data analysis and interpretation and contributed to manuscript preparation. The manuscript was finalized by MHS, RM, CB, LH, NW, and SP with the assistance of all authors.
Supporting information
ACKNOWLEDGMENTS
We thank all investigators for their continuation to this study, M. Calderon, P. Demoly, and W. Lehmacher for their work as members of the data safety monitoring board. Medical writing was provided by Dr. Phillip Leventhal (4Clinics, Paris, France). We also thank Denys Research Consultants, Business & Decision Life Sciences, Institute of Medical Statistics, Informatics and Epidemiology (University Hospital of Cologne), and Keyrus Biopharma for clinical trial management.
Mösges R, Bachert C, Panzner P, et al. Short course of grass allergen peptides immunotherapy over 3 weeks reduces seasonal symptoms in allergic rhinoconjunctivitis with/without asthma: A randomized, multicenter, double‐blind, placebo‐controlled trial. Allergy. 2018;73:1842–1850. 10.1111/all.13433
Funding information
This research was funded via institutional grants by ASIT biotech.sa.
REFERENCES
- 1. Meltzer EO, Blaiss MS, Derebery MJ, et al. Burden of allergic rhinitis: results from the Pediatric Allergies in America survey. J Allergy Clin Immunol. 2009;124(3 suppl):S43‐S70. [DOI] [PubMed] [Google Scholar]
- 2. Salo PM, Calatroni A, Gergen PJ, et al. Allergy‐related outcomes in relation to serum IgE: results from the National Health and Nutrition Examination Survey 2005‐2006. J Allergy Clin Immunol. 2011;127:1226‐1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: allergic rhinitis. Otolaryngol Head Neck Surg. 2015;152:S1‐S43. [DOI] [PubMed] [Google Scholar]
- 4. Didier A, Malling HJ, Worm M, Horak F, Sussman GL. Prolonged efficacy of the 300IR 5‐grass pollen tablet up to 2 years after treatment cessation, as measured by a recommended daily combined score. Clin Transl Allergy. 2015;5:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jutel M, Agache I, Bonini S, et al. International consensus on allergy immunotherapy. J Allergy Clin Immunol. 2015;136:556‐568. [DOI] [PubMed] [Google Scholar]
- 6. Pfaar O, Bachert C, Bufe A, Buhl R, Ebner C, et al. Guideline on allergen‐specific immunotherapy in IgE‐mediated allergic diseases: S2k Guideline of the German Society for Allergology and Clinical Immunology (DGAKI), the Society for Pediatric Allergy and Environmental Medicine (GPA), the Medical Association of German Allergologists (AeDA), the Austrian Society for Allergy and Immunology (OGAI), the Swiss Society for Allergy and Immunology (SGAI), the German Society of Dermatology (DDG), the German Society of Oto‐ Rhino‐Laryngology, Head and Neck Surgery (DGHNO‐KHC), the German Society of Pediatrics and Adolescent Medicine (DGKJ), the Society for Pediatric Pneumology (GPP), the German Respiratory Society (DGP), the German Association of ENT Surgeons (BV‐HNO), the Professional Federation of Paediatricians and Youth Doctors (BVKJ), the Federal Association of Pulmonologists (BDP) and the German Dermatologists Association (BVDD). Allergo J Int. 2014;23:282‐319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Musa F, Al‐Ahmad M, Arifhodzic N, Al‐Herz W. Compliance with allergen immunotherapy and factors affecting compliance among patients with respiratory allergies. Hum Vaccin Immunother. 2017;13:514‐517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reisacher WR, Visaya JM. Patient adherence to allergy immunotherapy. Curr Opin Otolaryngol Head Neck Surg. 2013;21:256‐262. [DOI] [PubMed] [Google Scholar]
- 9. Kiel MA, Röder E, van Wijk RG, Al MJ, Hop WCJ, Rutten‐van Mölken MP. Real‐life compliance and persistence among users of subcutaneous and sublingual allergen immunotherapy. J Allergy Clin Immunol. 2013;132:353‐360. [DOI] [PubMed] [Google Scholar]
- 10. Moldaver D, Larche M. Immunotherapy with peptides. Allergy. 2011;66:784‐791. [DOI] [PubMed] [Google Scholar]
- 11. Shamji MH, Ceuppens J, Bachert C, et al. Lolium perenne peptides for the treatment of grass pollen allergy: a randomized double blind placebo‐controlled clinical trial. J Allergy Clin Immunol. 2018;141:448‐451. [DOI] [PubMed] [Google Scholar]
- 12. Mösges R, Kasche EM, Raskopf E, et al. A randomized, double‐blind, placebo‐controlled, dose‐finding trial with Lolium perenne peptide immunotherapy. Lolium perenne peptides: significant efficacy in 3 weeks. Allergy. 2018;73:896‐904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pfaar O, Demoly P, Gerth van Wijk R, et al. Recommendations for the standardization of clinical outcomes used in allergen immunotherapy trials for allergic rhinoconjunctivitis: an EAACI Position Paper. Allergy. 2014;69:854‐867. [DOI] [PubMed] [Google Scholar]
- 14. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol. 2001;108:S147‐S334. [DOI] [PubMed] [Google Scholar]
- 15. Global Initiative for Asthma . Global strategy for asthma management and prevention. 2014. http://bcrt.ca/wp-content/uploads/2014/05/GINA_Report_2014.pdf. Accessed 10th of June 2017.
- 16. Cox L, Larenas‐Linnemann D, Lockey RF, Passalacqua G. Speaking the same language: the world allergy organization subcutaneous immunotherapy systemic reaction grading system. J Allergy Clin Immunol. 2010;125:569‐574. [DOI] [PubMed] [Google Scholar]
- 17. Riechelmann H, Epple B, Gropper G. Comparison of conjunctival and nasal provocation test in allergic rhinitis to house dust mite. Int Arch Allergy Immunol. 2003;130:51‐59. [DOI] [PubMed] [Google Scholar]
- 18. Dogan S, Astvatsatourov A, Deserno TM, et al. Objectifying the conjunctival provocation test: photography‐based rating and digital analysis. Int Arch Allergy Immunol. 2014;163:59‐68. [DOI] [PubMed] [Google Scholar]
- 19. Juniper EF, Thompson AK, Ferrie PJ, Roberts JN. Validation of the standardized version of the Rhinoconjunctivitis Quality of life Questionnaire. J Allergy Clin Immunol. 1999;104:364‐369. [DOI] [PubMed] [Google Scholar]
- 20. Juniper EF, Rohrbaugh T, Meltzer EO. A questionnaire to measure quality of life in adults with nocturnal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2003;111:484‐490. [DOI] [PubMed] [Google Scholar]
- 21. European Medicines Agency Committee for Medicinal Products for Human Use . Guideline on Missing Data in Confirmatory Clinical Trials. EMA/CPMP/EWP/1776/99 Rev. 1. 2010. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/09/WC500096793.pdf. Accessed 10th of June 2017.
- 22. Pfaar O, Bastl K, Berger U, Buters J, Calderon MA, et al. Defining pollen exposure times for clinical trials of allergen immunotherapy for pollen‐induced rhinoconjunctivitis — an EAACI position paper. Allergy. 2017;72:713‐722. [DOI] [PubMed] [Google Scholar]
- 23. Durham SR, Nelson HS, Nolte H, Bernstein DI, Creticos PS, et al. Magnitude of efficacy measurements in grass allergy immunotherapy trials is highly dependent on pollen exposure. Allergy. 2014;69:617‐623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Corrigan CJ, Kettner J, Doemer C, Cromwell O, Narkus A. Efficacy and safety of preseasonal‐specific immunotherapy with an aluminium‐adsorbed six‐grass pollen allergoid. Allergy. 2005;60:801‐807. [DOI] [PubMed] [Google Scholar]
- 25. DuBuske LM, Frew AJ, Horak F, et al. Ultrashort‐specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc. 2011;32:239‐247. [DOI] [PubMed] [Google Scholar]
- 26. Pfaar O, Urry Z, Robinson DS, et al. A randomized placebo‐controlled trial of rush preseasonal depigmented polymerized grass pollen immunotherapy. Allergy. 2012;67:272‐279. [DOI] [PubMed] [Google Scholar]
- 27. Alvarez‐Cuesta E, Bousquet J, Canonica GM, Durham SR, Maling HJ, Valovirta E, EAACI Immunotherapy Task force . Standards for practical allergen‐specific immunotherapy. Allergy. 2006;61:1‐20. [DOI] [PubMed] [Google Scholar]
- 28. Iglesias‐Cadarso A, Hernandez‐Weigand P. Risk factors for systemic reactions to allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2011;11:579‐585. [DOI] [PubMed] [Google Scholar]
- 29. Dhami S, Nurmatov U, Arasi S, Khan T, Asaria M, et al. Allergen immunotherapy for allergic rhinoconjunctivitis: a systematic review and meta‐analysis. Allergy. 2017;72:1597‐1631. [DOI] [PubMed] [Google Scholar]
- 30. Frew AJ, Powell RJ, Corrigan CJ, Durham SR, UK Immunotherapy Study Group . Efficacy and safety of specific immunotherapy with SQ allergen extract in treatment‐resistant seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006;117:319‐325. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


