Abstract
The sodium bicarbonate cotransporter (NBCe2, aka NBC4) was originally isolated from the human testis and heart (Pushkin et al. IUBMB Life 50:13–19, 2000). Subsequently, NBCe2 was found in diverse locations where it plays a role in regulating sodium and bicarbonate transport, influencing intra-cellular, extracellular, interstitial, and ultimately plasma pH (Boron et al. J Exp Biol. 212:1697–1706, 2009; Parker and Boron, Physiol Rev. 93:803–959, 2013; Romero et al. Mol Asp Med. 34:159–182, 2013). NBCe2 is located in human and rodent renal-collecting duct and proximal tubule. While much is known about the two electrogenic sodium bicarbonate cotransporters, NBCe1 and NBCe2, in the regulation of sodium homeostasis and pH balance in the rodent kidney, little is known about their roles in human renal physiology. NBCe2 is located in the proximal tubule Golgi apparatus under basal conditions and then disperses throughout the cell, but particularly into the apical membrane microvilli, during various maneuvers that increase intracellular sodium. This review will summarize our current understanding of the distribution and function of NBCe2 in the human kidney and how genetic variants of its gene, SLC4A5, contribute to salt sensitivity of blood pressure.
Keywords: Renal sodium, Sodium bicarbonate cotransporter, pH homeostasis, NBCe2, Salt sensitivity of blood pressure
Renal Bicarbonate Transport and NBCe2 (SLC4A5), NBCe1 (SLC4A4), and the Cl−/HCO3− Exchanger (SLC26A6)
Various transporters are involved with bicarbonate transport since reabsorbing bicarbonate filtered by the kidney is necessary to maintain acid-base and pH balance in the body. Approximately 4320 meq/day of HCO3−(24 meq/L × 180 L/day) are filtered by the glomeruli. Much (~90 %) of this bicarbonate is reabsorbed by the renal proximal tubule (RPT) and the remainder in the thick ascending limb and collecting duct. The reabsorption process is thought to occur via the combination of H+ ions secreted into the tubular lumen by the sodium hydrogen exchanger (NHE3) and the vacuolar H+ATPase [1], and reabsorption of the filtered bicarbonate by a series of events initiated by carbonic anhydrase type 4 (CA IV) (Fig. 1). CA IV dissociates carbonic acid (H2CO3) into H2O and CO2, which is membrane permeable, diffuses into the cell. Bicarbonate can also be reabsorbed from or secreted into the tubule lumen, the latter occurring if there is an excess generated in the renal tubule, through members of the solute carrier protein family (SLC4). Therefore, we will briefly review this transporter family and what is known about its location in the kidney.
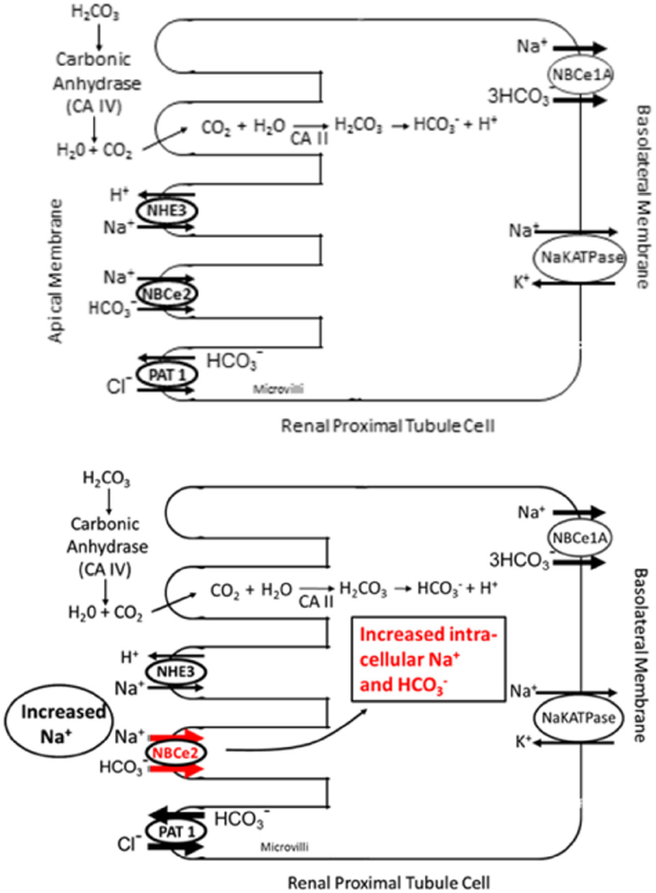
Fig. 1.
Model of a human renal proximal tubule cell (RPTC). Top figure—under basal conditions the principal avenue for bicarbonate (HCO3−) uptake from the lumen into cytosol is via the breakdown of carbonic acid (H2CO3) to water and carbon dioxide (CO2) by luminal carbonic anhydrase type IV (CA IV), followed by the diffusion of CO2 into the cytosol via the membranes of the microvilli. CA II then converts water and CO2 back into carbonic acid where it spontaneously breaks down into bicarbonate and hydrogen ion, with the latter secreted into the lumen via the sodium hydrogen exchanger type 3 (NHE3). Two to three bicarbonate ions can then be reabsorbed into the blood stream along with each sodium ion via NBCe1-A at the basolateral membrane. Bottom figure—increasing intracellular sodium concentration caused by high extracellular sodium concentration or monensin increases NBCe2 mRNA and protein expressions and activity that become persistent in the presence of single nucleotide polymorphisms of NBCe2, while only marginally attenuating the protein expression and activity of NBCe1-A in RPTCs. This results in a net increase in sodium transport into the basolateral space. PAT1 activity increases because of an increase in intracellular bicarbonate. NHE3 activity also increases because the increase in luminal NBCe2 activity increases intracellular H+ following the conversion of intracellularly transported HCO3− to H2CO3 and its dissociation to H+ and HCO3− resulting in a further increase in sodium reabsorption
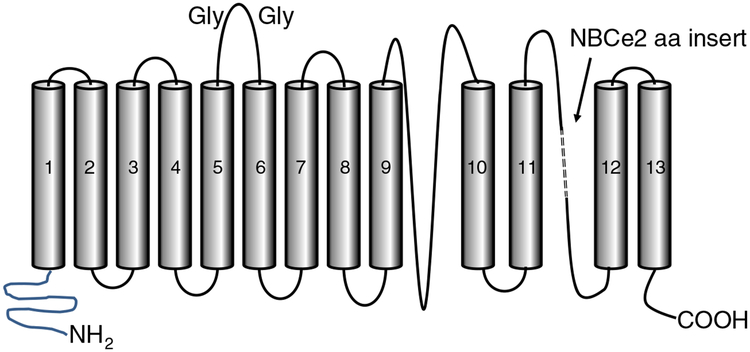
There are 10 members of the bicarbonate transporter family (encoded by the SLC4 gene family), many of which are found in the kidney [2••, 3••]. This review is focused on the electrogenic members of the SLC4 family, extensively studied by Boron and Boulpaep, and discovered in the renal proximal tubule (RPT) [4], as well as NBCe2 (SLC4A5) originally found in the liver, testis, and spleen by Pushkin [5], which mediate cotransport of 2–3 bicarbonate ions along with each sodium ion. NBCe2 is found predominantly at the apical side of the proximal tubule cell (vide infra), while NBCe1, originally found in the salamander and rodent [4, 6], is found exclusively at the basolateral membrane [3••, 7•, 8]. Although there are six published splice variants of NBCe2, only two (NBCe2-A and NBCe2-C) are expressed with all the transmembrane domains intact and thus may be the only functional members of the NBCe2 variant family [9]. NBCe2-A contains a unique 16 amino acid insert almost at the end of the carboxy terminal tail between transmembrane segments 11 and 12 when compared with NBCe2-C (Fig. 2). NBCe2-C is the only NBCe2 variant to have electrogenic activity, and thus, the 16 amino acid insert probably prevents NBCe2-A from expressing electrogenic activity. In order to visualize both isoforms by immunoblotting or immunohistochemistry, an amino terminal tail-directed antibody should be selected.
Fig. 2.
Generic model of NBCe2 showing its three domain structure, carboxy and amino terminal tails, and glycosylation (Gly) sites along the extracellular domain between transmembrane domains 5 and 6. NBCe2 exists in two isoforms (NBCe2-A and NBCe2-C). NBCe2-A differs from NBCe2-C mainly by the presence of an 18 amino acid (aa) insert in the connector between transmembrane domains 11 and 12 (adapted from reference [9])
NBCe1 is transcribed under the control of two distinct promoters making four possible messenger RNAs. However, only three isoforms have been identified as follows: NBCe1-A, NBCe1-B, andNBCe1-C [9]. Of these three transcripts, only NBCe1-A is expressed in the kidney where it is located in the basolateral membrane of the S1 and S2 segments of the proximal tubule [8]. By contrast, NBCe2 is found in the luminal membrane of all segments of the RPT (vide infra).
Recently, we demonstrated that under basal conditions, the human RPT expresses low levels of NBCe2 which can be enhanced by increasing the intracellular sodium concentration, either by increasing the extracellular concentration of sodium or adding monensin, an ionophore, into the incubating media. Presumably, this results in an increase in sodium bicarbonate cotransport [10•]. The increase in NBCe2 activity in RPT cells (RPTC) is transient in those carrying wild-type SLC4A5 and persistent in those carrying rs7571842 SLC4A5 [11••]. Others have determined that approximately 75 % of bicarbonate secretion into the intestinal lumen in the sea bass is due to the activity of carbonic anhydrase, while another 10–20 % is 4,4’-diisothiocyanatostilbene-2,2’-disulfonate sensitive. NBCe2 and NBCe1 are inhibited by 4,4’-diisothiocyanatostilbene-2,2’-disulfonate [2••, 3••]. This suggests that a sodium bicarbonate cotransporter, such as NBCe2, may be involved with this activity [12]. Thus, there is some precedent for a sodium bicarbonate cotransport beyond the well-accepted carbonic anhydrase pathway in the regulation of bicarbonate transport [1, 2••, 3••, 4–6, 7•, 9].
Human SLC4 proteins come from 10 different genes that encode sodium-dependent transporters and sodium- independent exchangers, including bicarbonate [1, 2••, 3••, 4–6, 7•, 8, 9, 13]. They can be ubiquitously or discretely expressed with specific functions. In the renal proximal tubule, it appears that bicarbonate and chloride secretion into the tubule lumen via the apical membrane is also mediated by SLC26A6 encoding the putative anion transporter 1 (PAT1) (aka CFEX) [14]. When studied in RPTCs from the spontaneously hypertensive rat (SHR) and its normotensive control, the Wistar-Kyoto rat (WKY), PAT1 activity was found to be increased in the SHR [15–17]. However, there are other SLC26−encoded proteins in the renal proximal tubule that may contribute to bicarbonate transport [14–20]. The Cl−/HCO3− exchanger activity in the kidney appears to be the sum of the activities of SLC26A4, SLC26A6, and SLC26A9 in WKY and SHR RPTCs; all these transporters are overexpressed in the SHR [15]. Simão et al. [15] make a compelling argument that these transporters may be an adaptive process to the sustained increase in sodium and bicarbonate transport in the RPTCs in the SHR. However, the increased transport could also be a result of gain-of-function single nucleotide polymorphisms (SNPs) in SLC4A5 [11••] (vide infra).
NBCe1 and NBCe2 may regulate renal sodium and bicarbonate transport to varying degrees depending on the salt balance of the individual. The activities of other sodium-dependent transporters and sodium-independent exchangers in the SLC4 and SLC26 families affect the activities of NBCe1 and NBCe2. These transporters and exchangers, in turn, affect the activity of proteins involved in sodium and hydrogen transport, such as NHE3, Na+/K+−ATPase, and the H+−ATPase in the renal proximal tubule. Since a full review of all the sodium, bicarbonate, and hydrogen transporters is beyond the scope of this review, we suggest the following review articles [1, 2••, 3••, 7•, 9, 18–21].
The Role of the Kidneys in the Regulation of Blood Pressure
Seminal studies on blood pressure regulation were performed by Guyton et al. [22] who hypothesized that ultimately the kidney contributes to the most critical regulation of intra- arterial pressure aided by interactions with the nervous, cardiovascular, and endocrine systems, among others. The gastrointestinal tract also contributes to the regulation of blood pressure [23–26]. Within the kidney, the angiotensin type 1 receptor (AT1R) is considered one of the dominant regulators of blood pressure homeostasis [27]. However, the D1−like dopamine receptors (D1 receptor (D1R) and D5 receptor (D5R)) and angiotensin type 2 receptor (AT2R) exert important counter regulatory roles when excess salt and volume need to be excreted [28]. There is no evidence that NBCe2 and NBCe1 are regulated directly by anti-natriuretic (AT1R) or natriuretic renal receptors (D1R, D5R, and AT2R) since adding their agonists in RPTCs were ineffective in changing the expression ofNBCe1 orNBCe2 (unpublished data from our laboratory). We speculate that intracellular sodium may be the primary stimulus in the regulation of NBCe1 and NBCe2.
NBCe2 Localization in the Kidney
NBCe2 protein is minimally expressed in the human and rodent RPT under basal conditions; this may have limited the detection of its protein expression by immunohistochemistry and its functional activity, even though its mRNA was detectable [29••]. Well-characterized antibodies against NBCe2 have been lacking. In addition, the commercially available antibodies were ambiguously labeled due to changes in nomenclature for NBCe2 (aka NBC4) which further hindered progress in this area of research. Newer NBCe2 antibodies have made it recently possible to determine the location of NBCe2 in the human kidney [10•]. We validated a commercial NBCe2 antibody by preadsorption of NBCe2 immunore-activity with the immunizing peptide. We then used this antibody to study NBCe2 expression in empty vector- and NBCe2-shRNA-treated cells, by western blot and immunofluorescence microscopy of RPTCs and HEK293 cells expressing an epitope-tagged NBCe2 lentiviral expression construct. NBCe2 immunofluorescence was alsofound in the cortical collecting duct [10•], but not in the distal convoluted tubule in fresh and frozen renal tissue sections; the latter finding in agreement with a previous report [30].
NBCe2 Subcellular Location and Translocation
Understanding the subcellular localization of NBCe2 is important in determining whether or not NBCe2 contributes to the transport of bicarbonate from the tubular (luminal) fluid into the RPTC. We studied NBCe2 expression in connecting tubule and cortical collecting duct (identified by L1-CAM) and RPT (identified by CD13 and Lotus tetragonolobus agglutinin) in fresh human renal slices [10•]. NBCe2 staining in the renal cortical collecting duct is consistent with earlier reports of its expression in principal cells [30]. We found that an increase in intracellular sodium caused by increasing the NaCl concentration in the incubation medium from 120 to 170 mmol/L or exposure to the ionophore, monensin (1–10 μimol/L), was the stimulus that increased NBCe2 expression and activity, i.e., increase bicarbonate transport across the luminal membrane [10•]. Under basal conditions, in the RPTC, NBCe2 is concentrated in the Golgi bodies with some diffuse staining throughout the cell. Increasing intracellular sodium causes the recruitment of NBCe2 to intracellular punctate structures subjacent to the apical membrane of the RPT. Electron microscopy demonstrated migration of NBCe2 from the sub-apical compartment to the microvilli following the increase in intracellular sodium. Total internal reflection fluorescence microscopy demonstrated vesicle-like structures at the apical membrane in polarized RPTCs. This location is similar to that shown for NHE3 in RPTs of rats after the induction of hypertension where NHE3-mediated sodium transport is still functional, albeit at a lower level, even after its movement in the microvilli or in the inter-microvilliary cleft. [31] We also confirmed apical RPT localization of NBCe2 by western blot studies of apical membranes isolated by two different methods: CD-13 immunoprecipitation and magnesium precipitation [10•].
NBCe1 Versus NBCe2
In the kidney, NBCe1 (particularly NBCe1-A) has been well characterized to provide electrogenic transport of sodium and bicarbonate across the basolateral membrane of the RPT in rodents [1, 2••, 3••, 9, 32] and humans [33]. NBCe1, encoded by SLC4A4 [1, 2••, 3••, 9], is located in the human RPT [10•, 30, 33], medullary thick ascending limb (mTAL), and collecting duct [30]. NBCe1, by transporting bicarbonate from inside the RPT across the basolateral membrane, promotes H+ ion secretion into the tubular lumen which can then combine with bicarbonate that was exchanged with chloride via PAT1. The resultant carbonic acid is acted upon by CA IV to continue the process of bicarbonate reabsorption. The renal tubular reabsorption of bicarbonate helps to maintain normal plasma bicarbonate and pH [1, 2••, 3••, 4, 13, 18, 19]. Germline deletion of SLC4A4 (NBCe1) [33] or SLC4A5 (NBCe2) [34] in mice causes metabolic acidosis and hypertension in the case of SLC4A5. The latter occurs because of an increase in sodium reabsorption in the distal nephron [35].
The electrogenic sodium bicarbonate cotransporter activities of NBCe1 and NBCe2 are considered indistinguishable [2••, 3••]. In humans, the relationship of these two transporters is better understood, in that RPTC NBCe1 appears to be located in the basolateral membrane [36], whereas NBCe2 is located in the apical membrane [10•]. Our immunofluorescence studies were performed in various model systems to ensure the validity of our interpretation of the results. We measured NBCe2 protein in the apical membranes and NBCe1 in the basolateral membranes of RPT of flash-frozen human kidney, primary cultures of RPTCs exfoliated into human urine, primary cultures of RPTCs isolated from surgical-discard fresh human kidneys, and immortalized human RPTCs from cell lines isolated from seven different individuals. We localized NBCe2 expression to the RPT subapical membrane in flash-frozen human kidney tissue, as well as in fresh renal cortical tissue. Higher expression of NBCe2 and lower expression of NBCe1 were found when intracellular sodium was increased (vide supra). We also demonstrated that bicarbonate-dependent pH recovery in RPTCs was due, in part, to NBCe2 at the apical membrane [10•]. These data support our hypothesis that NBCe2 expression is increased and recruited to the RPTC apical membrane microvilli by an increase in intracellular sodium.
Dysregulation of NBCe2 Activity
Essential hypertension is likely caused by genetic variants in key blood pressure-regulating pathways, instigated or exacerbated by environmental factors. The nephron segments responsible for the bulk of sodium retention in human polygenic/essential hypertension are the renal proximal tubule (RPT) and the medullary thick ascending limb of Henle (mTAL) [37–40]. However, renal distal tubular mechanisms also contribute to the increased sodium retention in hypertension [39, 40], especially in monogenic forms of hypertension [39–41]. NBCe2 is located in these nephron segments [10•, 30]. The gene SLC4A5, which encodes NBCe2 [2••, 3••, 9, 19], has been significantly associated with high blood pressure and/or salt sensitivity [42••, 43••, 44••, 45••, 46, 47]. The increased activity ofNBCe2 [44••, 45••, 46, 47] at the luminal membrane [43••], and decreased activity of NBCe1, at the basolateral membrane [48], in hypertension do not conflict with the increase in RPT sodium transport in the genetic hypertension [37–40]. The increased sodium reabsorption in the renal proximal tubule of young SHRs is a consequence of the high activity of the main mechanisms of sodium transport in this nephron segment, NHE3, and Na+/K+−ATPase [49–51]; SLC26A6 activity is also increased in the SHR [15–17]. The increased activity of these exchangers and pump [37–40, 49–53], with the increased activity of NBCe2 in the RPT [43••] and the decreased activity of NBCe1 [48] result in a decrease in overall HCO3− reabsorption. Low plasma HCO3− and high anion gap are associated with hypertension [54–57]. Mice with germline deletion of SLC4A5 on SV129/C57 background were reported to be acidotic with elevated blood pressure that was thought to be due to increased distal tubule bicarbonate transport via other bicarbonate sodium transporters, e.g., SLC26A4 and SLC4A7 [34, 35]. Increasing bicarbonate consumption in wild-type SV129/C57 mice elevated their blood pressure to the levels seen in SLC4A5 knockout mice [34]. Mice with germline deletion of SLC4A5 on mostly C57BL/6 background have normal blood pressure on a normal diet but an acid diet caused hypertension that was due to increased epithelial sodium channel-mediated sodium reabsorption [35, 58].
Human essential hypertension is also caused by increased renal reabsorption of electrolytes, including bicarbonate and sodium [59, 60]. Salt sensitivity has been estimated to be present in 51 % of hypertensive and 26 % of normotensive subjects [60]. Salt sensitivity of blood pressure, even in the absence of hypertension, is similar to hypertension in that they both lead to significant increases in morbidity and mortality due to stroke, blindness, heart attack, and renal failure [61]. We, and others, examined the relationship between SNPs in NBCe2 and salt sensitivity. At the University of Virginia (UVA), we examined the genetic associations with blood pressure in 185 subjects of European ancestry ages 18–70 years and body mass index (BMI) of 18–30 [43••]. In a collaborative study, the genetic associations with blood pressure traits were performed on specimens from the HyperPATH Cohort with subjects with mild hypertension studied from four international centers (Brigham and Women’s Hospital, University of Utah Medical Center, Vanderbilt University, and Hospital Broussais (Paris, France)) [43••, 62]. We tested the hypothesis that SNPs in SLC4A5 are associated with salt sensitivity (≥7-mmHg increase in mean arterial pressure during a randomized transition between high- and low-sodium diets) in 185 whites consuming an isocaloric constant diet starting with either 7 days of low (10 mmol Na+/day) or 7 days of high sodium (300 mmol Na+/day) intake and then switching to the other diet. Three variants were associated with salt sensitivity, two in SLC4A5 (P<0.001) and one in GRK4 (P =0.020). Of these, two SNPs in SLC4A5 (rs7571842 and rs10177833) demonstrated highly significant results and large effect sizes, using logistic regression. These two SNPs had P values of 1.0×10−4 and 3.1×10−4 with odds ratios of 0.221 and 0.221 in unadjusted regression models, respectively, with the G allele at both sites conferring protection. The association of these SNPs with salt sensitivity was replicated in the HyperPATH Cohort at Harvard with a meta-analysis demonstrating significant associations of both SNPs with salt sensitivity (rs7571842 (P = 1.2×10−5); rs1017783 (P =1.1× 10−4)) [43••]. Our results [43••] are consistent with the association ofrs1017783 and increased blood pressure in African-Americans, Mexican-Americans, Euro-Americans, and Taiwanese [42••, 44••, 45••, 46, 47, 63••, 64]. Another SLC4A5 SNP (rs 10022637) (P =2.07×10−6) was found to be associated with salt sensitivity in a large cohort of Han Chinese; SLC4A5 rs1017783 was not genotyped [65].
Some investigators believe that despite the strong association between the SLC4A5 locus and salt sensitivity in the absence of hypertension, NBCe2 may not contribute to the phenotypes of salt sensitivity or hypertension since it has only a minor role to play (if at all) in renal sodium and bicarbonate transport, under conditions of “normal” sodium intake. However, there are two compelling reasons to support the notion that SLC4A5 contributes, at least in part, to the sodium retention in hypertension. Increased sodium transport is involved in genetic hypertension [37–41, 43••, 59, 66••]. Although SLC4A5 may not be a major contributor to sodium balance, relative to other sodium transporters, it may be an important player under conditions of high salt intake. Thus, even a decrease of only 0.1 % in sodium excretion over a period of time can lead to hypertension. For illustrative purposes, an average individual excretes 1 % of filtered sodium (~250 mmol/day). A reduction in sodium excretion of only 0.1 % leads to sodium retention of 25 mmol/day or 125 mmol in 5 days provided that there is no corresponding natriuresis. A short-term (5 days) change in sodium diet in normotensive and hypertensive human subjects from low to high and vice versa can also lead to a directional change in plasma sodium of about 3 mmol [67, 68]. One-month reduction of sodium intake from ~ 170 to 100 mmol/day has also been reported to be associated with a 0.4-mmol decrease in plasma sodium [68]. Fortunately, extrarenal regulatory mechanisms [69] participate in the maintenance of sodium homeostasis and pressure-natriuresis mechanism, in addition to the increased production and action of natriuretic hormones/factors and decreased production and action of anti-natriuretic hormones/factors help to eliminate most of the ingested sodium [25]. However, we have demonstrated that SLC4A5 mRNA, as well as its protein prod-uctNBCe2, is increased by sodium intake [10•]. Individuals with polymorphisms in SLC4A5 would have a further increase in NBCe2 expression and therefore an increase in sodium and bicarbonate cotransport in their RPT. Thus, we have demonstrated a functional link between SLC4A5 polymorphisms and renal sodium transport that could make contributions to salt sensitivity [11••]. As previously mentioned, studies from various investigators including ourselves have demonstrated a genetic link between NBCe2 polymorphisms and hypertension [11••, 42••, 43••, 44••, 45••, 46, 47, 63••, 64, 65]. However, these polymorphisms are not in the coding region for SLC4A5 (NBCe2). We have further clarified the mechanism by which SLC4A5 rs10177833 may lead to an increase in expression and activity of NBCe2. HNF4A is a transcriptional regulator present in the RPT that plays a key regulatory role in a large number of pathways [70–72]. We found that SLC4A5 rs10177833 causes an increase in HNF4A binding to the SLA4A5 gene resulting in an increase in NBCe2 mRNA, NBCe2 protein expression, and increased NBCe2-mediated bicarbonate and sodium transport under conditions of elevated intracellular sodium [11••] (Fig. 3).
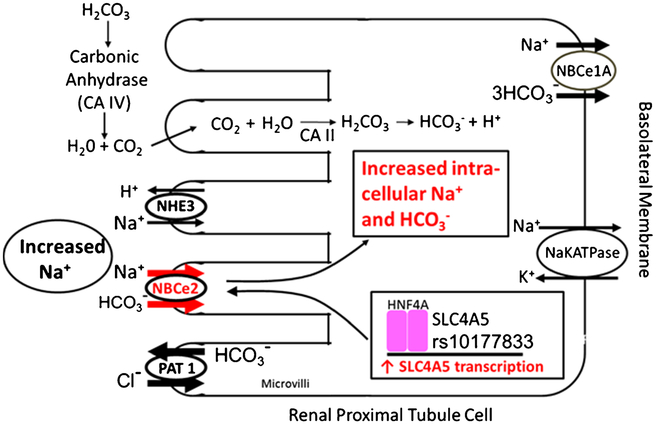
Fig. 3.
The mechanism causing the increase in sodium transport in RPTCs carrying the SLC4A5 rs10177833 is shown on the same model depicted in Fig. 1. These RPTCs have an increase in HNF4A binding to the SLC4A5 gene resulting in an increase in NBCe2 mRNA, as well as an increase in NBCe2 protein and activity under high salt conditions. The resulting increase in intracellular sodium and bicarbonate is associated with an increase in PAT1 activity and slight reduction in NBCe1 activity
Summary and Conclusion
Ion transporters and exchangers mediate the balance of influx and outflux of ions through the cell membrane in all tissues. The normal balance of pH and sodium is critical to the maintenance of life in the short-term and health in the long-term. Since pH is so critical to immediate cell health, nature seems to have endowed cells throughout the body with a rich variety of bicarbonate cotransporters and hydrogen pump and channel. We have studied NBCe2 and NBCe1 regulation of renal bicarbonate transport and how they work in concert to maintain sodium balance. A consequence of a hyperactive NBCe2, as a result of SNPs in the SLC4A5 gene, may be that in a subset of salt-sensitive individuals, NBCe2 polymorphisms, e.g., SLC4A5 rs1017783, lead to an increase in renal sodium bicarbonate reabsorption, which apparently is only partially compensated by a partial reduction in NBCe1 and increase in PAT1 activities. We speculate that blocking the increased synthesis and/or activity of NBCe2 may be a novel approach to mitigate the increased renal sodium reabsorption in some salt-sensitive individuals.
Footnotes
Compliance with Ethical Standards
Conflict of Interest Drs. Felder, Jose, Xu, and Gildea declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
This article is part of the Topical Collection on Hypertension and the Kidney
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.Wagner CA, Finberg KE, Breton S, Marshansky V, Brown D, Geibel JP. Renal vacuolar h+ −atpase. Physiol Rev. 2004;84: 1263–314. [DOI] [PubMed] [Google Scholar]
- 2.••.Parker MD, Boron WF. The divergence, actions, roles, and relatives of sodium-coupled bicarbonate transporters. Physiol Rev. 2013;93: 803–959.This paper is the most comprehensive review of sodium-coupled bicarbonate transporters.
- 3.••.Romero MF, Chen AP, Parker MD, Boron WF. The slc4 family of bicarbonate (hco3−) transporters. Mol Asp Med. 2013;34:159–82.This paper is also a good comprehensive review of bicarbonate transporters.
- 4.Boron WF, Boulpaep EL. Intracellular pH regulation in the renal proximal tubule of the salamander. Basolateral hco3- transport. J Gen Physiol. 1983;81:53–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pushkin A, Abuladze N, Newman D, Lee I, Xu G, Kurtz I. Two c-terminal variants ofnbc4, a new member of the sodium bicarbonate cotransporter family: cloning, characterization, and localization. IUBMB Life. 2000;50:13–9. [DOI] [PubMed] [Google Scholar]
- 6.Schmitt BM, Biemesderfer D, Romero MF, Boulpaep EL, Boron WF. Im m unolocalization of the electrogenic na + −hco −3 cotransporter in mammalian and amphibian kidney. Am J Physiol. 1999;276:F27–38. [DOI] [PubMed] [Google Scholar]
- 7.•.Skelton LA, Boron WF, Zhou Y. Acid-base transport by the renal proximal tubule. J Nephrol. 2010;23 Suppl 16:S4–18.This paper is a good summary of acid-base transport.
- 8.Maunsbach AB, Vorum H, Kwon TH, Nielsen S, Simonsen B, Choi I, et al. Immunoelectron microscopic localization of the electrogenic na/hco(3) cotransporter in rat and ambystoma kidney. J Am Soc Nephrol. 2000;11:2179–89. [DOI] [PubMed] [Google Scholar]
- 9.Boron WF, Chen L, Parker MD. Modular structure of sodium-coupled bicarbonate transporters. J Exp Biol. 2009;212:1697–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.•.Gildea JJ, Xu P, Carlson JM, Gaglione RT, Bigler Wang D, Kemp BA, et al. The sodium-bicarbonate cotransporter nbce2 (slc4a5) expressed in human renal proximal tubules shows increased apical expression under high-salt conditions. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1447–59.This paper from our group demonstrates the presence of NBCe2 on the apical membrane of the human renal proximal tubule.
- 11.••.Gildea JJ, Xu P, Carlson J, Gaglione R, Wang DB, Kemp B, Reyes C, McGrath H, Carey R, Jose PA, Felder RA. Role of human renal proximal tubule sodium bicarbonate cotransporter nbce2 (slc4a5) in salt sensitivity of blood pressure. American Heart Council for High Blood Pressure Research, 2015. Poster Abstract 242 http://my.americanheart.org/idc/groups/ahamahpublic/@wcm/@sop/@scon/documents/downloadable/ucm_477293.pdf:PA242.This abstract is the first demonstration of a link between polymorphisms in SLC4A5 and aberrant renal sodium regulation.
- 12.Faggio C, Torre A, Lando G, Sabatino G, Trischitta F. Carbonate precipitates and bicarbonate secretion in the intestine of sea bass, Dicentrarchus labrax. J Comp Physiol B. 2011;181:517–25. [DOI] [PubMed] [Google Scholar]
- 13.Choi I, Aalkjaer C, Boulpaep EL, Boron WF. An electroneutral sodium/bicarbonate cotransporter nbcn1 and associated sodium channel. Nature. 2000;405:571–5. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Wang T, Petrovic S, Tuo B, Riederer B, Barone S, et al. Renal and intestinal transport defects in slc26a6-null mice. Am J Physiol Cell Physiol. 2005;288:C957–65. [DOI] [PubMed] [Google Scholar]
- 15.Simao S, Gomes P, Pinho MJ, Soares-da-Silva P. Identification of slc26a transporters involved in the cl−/hco3− exchange in proximal tubular cells from wky and shr. Life Sci. 2013;93:435–40. [DOI] [PubMed] [Google Scholar]
- 16.Simao S, Gomes P, Jose PA, Soares-da-Silva P. Increased responsiveness to jnk1/2 mediates the enhanced h2o2-induced stimulation of cl-/hco3- exchanger activity in immortalized renal proximal tubular epithelial cells from the shr. Biochem Pharmacol. 2010;80:913–9. [DOI] [PubMed] [Google Scholar]
- 17.Simao S, Pedrosa R, Hopfer U, Mount DB, Jose PA, Soares-da-Silva P. Short-term regulation of the cl-/hco3(−) exchanger in imm ortalized shr proxim al tubular epithelial cells. Biochem Pharmacol. 2008;75:2224–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuo JL, LiXC. Proximal nephron. Compr Physiol. 2013;3:1079–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aalkjaer C, Boedtkjer E, Choi I, Lee S. Cation-coupled bicarbonate transporters. Compr Physiol. 2014;4:1605–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohana E, Yang D, Shcheynikov N, Muallem S. Diverse transport modes by the solute carrier 26 family of anion transporters. J Physiol. 2009;587:2179–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer LG, Schnermann J. Integrated control of Na transport along the nephron. Clin J Am Soc Nephrol. 2015;10:676–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guyton A, Hall J, Coleman T. The dominant role of the kidneys in long-term arterial pressure regulation in normal and hypertensive states. New York: Raven; 1995. [Google Scholar]
- 23.Banday AA, Lokhandwala MF. Novel gastro-renal axis and sodium regulation during hypertension. Hypertension. 2013;62:834–5. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y, Asico LD, Zheng S, Villar VA, He D, Zhou L, et al. Gastrin and D1 dopamine receptor interact to induce natriuresis and diuresis. Hypertension. 2013;62:927–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jose PA, Yang Z, Zeng C, Felder RA. The importance of the gastrorenal axis in the control of body sodium homeostasis. Exp Physiol. 2016;101:465–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spencer AG, Labonte ED, Rosenbaum DP, Plato CF, Carreras CW, Leadbetter MR, et al. Intestinal inhibition of the Na+/H+ exchanger 3 prevents cardiorenal damage in rats and inhibits Na+ uptake in humans. Sci Transl Med. 2014;6, 227ra236. [DOI] [PubMed] [Google Scholar]
- 27.Coffman TM, Crowley SD. Kidney in hypertension: guyton redux. Hypertension. 2008;51:811–6. [DOI] [PubMed] [Google Scholar]
- 28.Jose PA, Eisner GM, Felder RA. Regulation of blood pressure by dopamine receptors. Nephron Physiol. 2003;95:p19–27. [DOI] [PubMed] [Google Scholar]
- 29.••.Xu J, Wang Z, Barone S, Petrovic M, Amlal H, Conforti L, et al. Expression of the Na+;HCO-3 cotransporter nbc4 in rat kidney and characterization of a novel NBC4 variant. Am J Physiol Renal Physiol. 2003;284:F41–50.This paper is the first demonstration of SLC4A5 mRNA in the kidney.
- 30.Damkier HH, Nielsen S, Praetorius J. Molecular expression of SLC4-derived Na+ -dependent anion transporters in selected human tissues. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2136–46. [DOI] [PubMed] [Google Scholar]
- 31.Yang L, Leong PK, Chen JO, Patel N, Ham m-Alvarez SF, McDonough AA. Acute hypertension provokes internalization of proximal tubule NHE3 without inhibition of transport activity. Am J Physiol Renal Physiol. 2002;282:F730–40. [DOI] [PubMed] [Google Scholar]
- 32.Burnham CE, Flagella M, Wang Z, Amlal H, Shull GE, Soleimani M. Cloning, renal distribution, and regulation of the rat Na+-HCO3- cotransporter. Am JPhysiol. 1998;274:F1119–26. [DOI] [PubMed] [Google Scholar]
- 33.Kurtz I, Zhu Q. Proximal renal tubular acidosis mediated by mutations in NBCe1-A: unraveling the transporter’s structure-functional properties. Front Physiol. 2013;4:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gröger N, Vitzthum H, Frohlich H, Kruger M, Ehmke H, Braun T, et al. Targeted mutation of SLC4A5 induces arterial hypertension and renal metabolic acidosis. Hum Mol Genet. 2012;21:1025–36. [DOI] [PubMed] [Google Scholar]
- 35.Wen D, Sansom SC. Physiological role of NBCe2 in the regulation of electrolyte transport in the distal nephron. Am J Physiol Renal Physiol. 2015;309:F489–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamada H, Yamazaki S, Moriyama N, Hara C, Horita S, Enomoto Y, et al. Localization of NBC-1 variants in human kidney and renal cell carcinoma. Biochem Biophys Res Commun. 2003;310:1213–8. [DOI] [PubMed] [Google Scholar]
- 37.Aviv A, Hollenberg NK, Weder A. Urinary potassium excretion and sodium sensitivity in blacks (response: reinterpreting sodium- potassium data in salt sensitivity hypertension: a prospective debate). Hypertension. 2005;43:707–13. [DOI] [PubMed] [Google Scholar]
- 38.Doris PA. Renal proximal tubule sodium transport and genetic mechanisms of essential hypertension. J Hypertens. 2000;18:509–19. [DOI] [PubMed] [Google Scholar]
- 39.Ortiz PA, Garvin JL. Intrarenal transport and vasoactive substances in hypertension. Hypertension. 2001;38:621–4. [DOI] [PubMed] [Google Scholar]
- 40.Khalil RA. Dietary salt and hypertension: new molecular targets add more spice. Am J Physiol Regul Integr Comp Physiol. 2006;290:R509–13. [DOI] [PubMed] [Google Scholar]
- 41.Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell. 2001;104:545–56. [DOI] [PubMed] [Google Scholar]
- 42.••.Barkley RA, Chakravarti A, Cooper RS, Ellison RC, Hunt SC, Province MA, et al. Positional identification of hypertension susceptibility genes on chromosome 2. Hypertension. 2004;43:477–82.This paper demonstrates a genetic link between chromosome 2, the locationi of SLC4A5, and hypertension.
- 43.••.Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, et al. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension. 2012;60:1359–66.This paper from our group dem onstrates a strong association between two SNPs in SLC4A5 and salt sensitivity of blood pressure.
- 44.••.Hunt SC, Xin Y, Wu LL, Cawthon RM, Coon H, Hasstedt SJ, et al. Sodium bicarbonate cotransporter polymorphisms are associated with baseline and 10-year follow-up blood pressures. Hypertension. 2006;47:532–6.This paper corroborates the findings of Carey et al in reference 43.
- 45.••.Stutz AM, Teran-Garcia M, Rao DC, Rice T, Bouchard C, Rankinen T. Functional identification of the promoter of SLC4a5, a gene associated with cardiovascular and metabolic phenotypes in the heritage family study. Eur J Hum Genet. 2009;17:1481–9.This paper corroborates the work of Carey et al in reference 43 and the work of Hunt in reference 44.
- 46.Taylor JY, Maddox R, Wu CY. Genetic and environmental risks for high blood pressure among African American mothers and daughters. Biol Res Nurs. 2009;11:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Padmanabhan S, Menni C, Lee WK, Laing S, Brambilla P, Sega R, et al. The effects of sex and method of blood pressure measurement on genetic associations with blood pressure in the PAMELA study. J Hypertens. 2010;28:465–77. [DOI] [PubMed] [Google Scholar]
- 48.Pedrosa R, Gongalves N, Hopfer U, Jose PA, Soares-da-Silva P. Activity and regulation of Na+−HCO3- cotransporter in immortalized spontaneously hypertensive rat and Wistar-Kyoto rat proximal tubular epithelial cells. Hypertension. 2007;49:1186–93. [DOI] [PubMed] [Google Scholar]
- 49.Beach RE, DuBose TD. Adrenergic regulation of (Na+-K+)- ATPase activity in proximal tubules of spontaneously hypertensive rats. Kidney Int. 1990;38:402–8. [DOI] [PubMed] [Google Scholar]
- 50.Hayashi M, Yoshida T, Monkawa T, Yamaji Y, Sato S, Saruta T. Na+/H+-exchanger 3 activity and its gene in the spontaneously hypertensive rat kidney. J Hypertens. 1997;15:43–8. [PubMed] [Google Scholar]
- 51.Xu J, Li XX, Albrecht FE, Hopfer U, Carey RM, Jose PA. Dopamine(1) receptor, G(salpha), and Na(+)-H(+) exchanger interactions in the kidney in hypertension. Hypertension. 2000;36:395–9. [DOI] [PubMed] [Google Scholar]
- 52.Yao LP, Li XX, Yu PY, Xu J, Asico LD, Jose PA. Dopamine D1 receptor and protein kinase C isoforms in spontaneously hypertensiverats. Hypertension. 1998;32:1049–53. [DOI] [PubMed] [Google Scholar]
- 53.Ehret GB, Munroe PB, Rice KM, Bochud M, Johnson AD, Chasman DI, et al. Genetic variants in novel pathways influence blood pressure and cardiovascular disease risk. Nature. 2011;478:103–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Forman JP, Rifas-Shiman SL, Taylor EN, Lane K, Gillman MW. Association between the serum anion gap and blood pressure among patients at Harvard Vanguard Medical Associates. J Hum Hypertens. 2008;22:122–5. [DOI] [PubMed] [Google Scholar]
- 55.Farwell WR, Taylor EN. Serum anion gap, bicarbonate and biomarkers of inflammation in healthy individuals in a national survey CMAJ. 2010;182:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abramowitz MK, Hostetter TH, Melamed ML. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int. 2012;81:1033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharma AM, Cetto C, Schorr U, Spies KP, Distler A. Renal acid- base excretion in normotensive salt-sensitive humans. Hypertension. 1993;22:884–90. [DOI] [PubMed] [Google Scholar]
- 58.Wen D, Yuan Y, Warner PC, Wang B, Cornelius RJ, Wang-France J, et al. Increased epithelial sodium channel activity contributes to hypertension caused by Na+-HCO3- cotransporter electrogenic 2 deficiency. Hypertension. 2015;66:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Elijovich F, Weinberger MH, Anderson CAM, Appel LJ, Bursztyn M, Cook NR, Dart RA, Newton-Cheh CH, Sacks FM, Laffer CL, American Heart Association Professional and Public Education Committee of the Council on Hypertension; Council on Functional Genomics and Translational Biology; and Stroke Council. Salt sensitivity of blood pressure: a scientific statement from the American Heart Association. Hypertension. 2016;68:e7–e46. [DOI] [PubMed] [Google Scholar]
- 60.Weinberger MH, Miller JZ, Luft FC, Grim CE, Fineberg NS. Definitions and characteristics of sodium sensitivity and blood pressure resistance. Hypertension. 1986;8:II127–34. [DOI] [PubMed] [Google Scholar]
- 61.Weinberger MH, Fineberg NS, Fineberg SE, Weinberger M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension. 2001;37:429–32. [DOI] [PubMed] [Google Scholar]
- 62.Pojoga LH, Underwood PC, Goodarzi MO, Williams JS, Adler GK, Jeunemaitre X, et al. Variants of the caveolin-1 gene: a translational investigation linking insulin resistance and hypertension. J Clin Endocrinol Metab. 2011;96:E1288–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.•.Taylor JY, Wu CY, Darling D, Sun YV, Kardia SL, Jackson JS. Gene-environment effects of SLC4a5 and skin color on blood pressure among african american women. Ethn Dis. 2012;22:155–61.This paper links polym orphism s of SLC4A5 with blood presssure regulation.
- 64.Lynn KS, Li LL, Lin YJ, Wang CH, Sheng SH, Lin JH, et al. A neural network model for constructing endophenotypes of common complex diseases: an application to male young-onset hypertension microarray data. Bioinformatics. 2009;25:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guo L, Liu F, Chen S, Yang X, Huang J, He J, et al. Common variants in the Na(+)-coupled bicarbonate transporter genes and salt sensitivity of blood pressure: The gensalt study. J Hum Hypertens. 2016;30:543–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.••.Oh YS, Appel LJ, Galis ZS, Hafler DA, He J, Hernandez AL, et al. National heart, lung, and blood institute working group report on salt in human health and sickness: building on the current scientific evidence. Hypertension. 2016;68:281–8.This review covers the importance of sodium reduction in the human diet.
- 67.de Wardener HE, He FJ, MacGregor GA. Plasma sodium and hypertension. Kidney Int. 2004;66:2454–66. [DOI] [PubMed] [Google Scholar]
- 68.Stocker SD, Monahan KD, Browning KN. Neurogenic and sym pathoexcitatory actions of nacl in hypertension. Curr Hypertens Rep. 2013;15:538–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Titze J Sodium balance is not just a renal affair. Curr Opin Nephrol Hypertens. 2014;23:101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thiagarajan RD, Georgas KM, Rumballe BA, Lesieur E, Chiu HS, Taylor D, et al. Identification of anchor genes during kidney development defines ontological relationships, molecular subcompartments and regulatory pathways. PLoS One. 2011;6, e17286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gallegos TF, Martovetsky G, Kouznetsova V, Bush KT, Nigam SK. Organic anion and cation slc22 “drug” transporter (oat1, oat3, and oct1) regulation during development and maturation of the kidney proximal tubule. PLoS One. 2012;7, e40796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hahn-Windgassen A, Van Gilst MR. The Caenorhabditis elegans HNF4alpha homolog, NHR-31, mediates excretory tube growth and function through coordinate regulation of the vacuolar ATPase. PLoS Genet. 2009;5, e1000553. [DOI] [PMC free article] [PubMed] [Google Scholar]