Abstract
Background and Aims:
Smokers can regulate their nicotine intake by altering the number of cigarettes smoked per day (CPD) and their smoking intensity. The current study aimed to compare the utility of self-reported CPD, total nicotine equivalents (TNE), and urinary cotinine to estimate nicotine intake during pregnancy.
Design:
Longitudinal smoking behavior and biomarker data was collected at early pregnancy, late pregnancy, and at postpartum as part of a smoking cessation trial to examine voucher-based incentives for decreasing smoking.
Setting:
Obstetric practices in Burlington, Vermont, United States.
Participants:
A subset of participants (n=47) from the parent trial, recruited between December 2006 and June 2012, who provided a urine sample at each assessment during early pregnancy, late pregnancy, and postpartum.
Measurements:
Smoking was assessed using self-reported CPD, TNE, TNE/CPD, and urinary cotinine.
Findings:
Pregnant smokers reported smoking 10.4 CPD at early pregnancy, 7.2 CPD at late pregnancy (a 31% reduction at late pregnancy, P=0.001), and 8.6 at postpartum (a 19% increase from late pregnancy, P=0.08). TNE exposure was 41% (P=0.07) and 48% (P=0.03) lower at early and late pregnancy, respectively, compared with postpartum. TNE/CPD was on average 167% higher at late pregnancy compared to early pregnancy (P=0.01) and remained high at postpartum where it was 111% higher compared to early pregnancy (P=0.007). Uriniary cotinine underestimated nicotine intake by 55% during early pregnancy and by 65% during late pregnancy compared with postpartum (Pinteraction<0.001); the underestimation was greater in slower (Pinteraction<0.001) versus faster (Pinteraction=0.04) nicotine metabolizers.
Conclusions:
Neither cigarettes smoked per day (CPD) nor cotinine provides an accurate estimates of nicotine exposure during pregnancy. CPD substantially underestimates nicotine intake due to under-reporting and/or higher intensity of smoking while cotinine markedly underestimates nicotine intake due to accelerated nicotine (and cotinine) metabolism during pregnancy.
Keywords: Smoking, Pregnancy, Biomarkers, Nicotine, Cotinine, Total nicotine equivalents
INTRODUCTION
Maternal cigarette smoking is the leading preventable cause of poor pregnancy outcomes (1), yet approximately 14% of pregnant women are regular cigarette smokers (2). On average only 40% of women quit smoking during pregnancy with more than 90% relapsing within one year (3). Compared to pregnant women who continue to smoke during pregnancy, those who quit have more favorable smoking behaviors (e.g. smoking fewer cigarettes per day, reporting higher motivation for abstinence and less dependency) (4).
A dose-effect relationship exists between nicotine intake and smoking-related outcomes of pregnancy (5–8). Smokers can regulate their nicotine intake to maintain relatively constant circulating nicotine levels throughout the day by altering the number of cigarettes they smoke per day (CPD) and their smoking intensity, including puff frequency, volume, and duration. Nicotine intake is most commonly quantified by self-reported CPD (9–11). Despite the simplicity of collecting CPD data, CPD remains a crude measure of nicotine intake as it does not take into account smoking intensity which can alter nicotine intake per cigarette. The best biochemical biomarker of nicotine intake is determined by the molar sum of nicotine and metabolites in urine and is referred to as total nicotine equivalents (TNE) (12, 13). Following smoking in twelve non-pregnant smokers, approximately 88% of the systemic nicotine dose was accounted for by urinary TNE (13). A second widely used biochemical measure of nicotine intake is nicotine’s primary metabolite, cotinine (14, 15). Cotinine has a relatively long half-life (~16 hours), suggesting cotinine can be detected for a few days after smoking cessation (14, 15).
Nicotine is primarily metabolized by hepatic CYP2A6, and nicotine’s major metabolite, cotinine, is exclusively metabolized by CYP2A6 to trans-3’-hydroxycotinine (3HC) (16–18). Due to the long half-life of cotinine and the 3HC formation dependency, the ratio of nicotine’s metabolites 3HC/cotinine, referred to as nicotine metabolic ratio (NMR), functions as a surrogate measure of the rate of nicotine metabolism (19–21). The rate of nicotine metabolism is highly correlated with CYP2A6 enzymatic activity and has been identified as an important factor influencing nicotine intake (22, 23). CYP2A6 activity is influenced by a variety of genetic (i.e. polymorphisms) and non-genetic (i.e. diet, medications, sex) factors (24). Individuals who metabolize nicotine faster (who have higher CYP2A6 activity and NMR) smoke more to maintain similar levels of nicotine in the body compared to slower nicotine metabolizers (who have lower CYP2A6 activity and NMR) (22, 23, 25). The rate of nicotine metabolism is accelerated during pregnancy as evidenced by faster nicotine clearance (26) and a lower proportion of nicotine excreted unchanged (as a fraction of TNE) (27). Estrogen levels begin to rise after conception and are approximately five-fold and twenty-fold higher at early and late pregnancy, respectively, compared to pre-pregnancy levels (27). Elevated estrogen during pregnancy is thought to induce CYP2A6 and UGT2B10 enzymatic activity, leading to accelerated nicotine metabolism by increasing the rate of nicotine and cotinine C-oxidation and N-glucuronidation, respectively; the increase in nicotine metabolism rate is even greater at late compared to early pregnancy stages (27). Consistent with elevated CYP2A6 activity during pregnancy, pregnant smokers have higher NMR compared to non-pregnant women (28, 29), suggesting they may increase their smoking to maintain similar nicotine levels and may need to be treated with higher doses of NRT.
Elevated rates of nicotine metabolism may further influence the ability of biomarkers to accurately estimate nicotine intake during pregnancy. TNE is not substantially impacted by variation in the rate of nicotine and cotinine metabolism as the parent substrates and resulting metabolite levels are assessed together (30, 31). Unlike TNE, there is individual variability in the quantitative relationship between steady state cotinine concentrations and intake of nicotine. This is because people convert different percentages of nicotine to cotinine (range 50–90%), and metabolize cotinine at different rates (clearance range 20–75 ml/min) (13, 16). For example, individuals with higher CYP2A6 activity have lower ratios of cotinine formation to cotinine removal (i.e. relatively lower cotinine levels for a given nicotine intake) compared to individuals with lower CYP2A6 activity (32). Similarly, pregnant smokers who have higher CYP2A6 and UGT2B10 activity may have relatively lower cotinine levels for the same intake of nicotine. Thus, cotinine is a weaker biomarker than TNE, but it is unclear by how much cotinine underestimates nicotine intake in pregnant smokers and how this may be affected at different stages of pregnancy.
The objective of this study was to investigate the utility of biomarkers to accurately estimate nicotine intake during pregnancy. We aimed to 1) compare self-reported CPD, TNE, TNE/CPD (i.e. nicotine intake per self-reported CPD), and urinary cotinine during early pregnancy, late pregnancy, and postpartum. 2) We then aimed to measure the extent to which cotinine underestimates nicotine intake at each pregnancy stage and 3) among those with slower versus faster nicotine metabolism.
METHODS
Trial Design
Current smoking pregnant women (n=118) were recruited from obstetric practices in Burlington, VT to participate in a smoking cessation trial to examine the efficiency of voucher-based incentives for decreasing smoking during pregnancy (33). The inclusion criteria for the parent trial were self-reported smoking in the past 7 days, urinary cotinine > 80 ng/ml, and gestational age ≤ 25 weeks. The exclusion criteria for the parent trial included self-reported use of prescribed opioid, psychomotor stimulant, or antipsychotic medications. The recruitment period for the parent trial was December 2006 to June 2012. The follow-up rate for the 6 months postpartum assessment was 71% for the incentive arm and 69% for the non-incentive arm. Women who terminated the pregnancy or had a fetal demise post-randomization were withdrawn from the parent trial. A subset of participants (n=47; n=27 from incentive arm and n=20 from non-incentive arm) met the inclusion criteria for the current analysis which included providing a spot urine sample at all three trial assessments conducted during early pregnancy (estimate gestational age (EGA) 12.5 ± 4.5 weeks), late pregnancy (EGA 28.9 ± 2.0 weeks), and at 6 months postpartum (24.7 ± 1.2 weeks since birth). The final sample of 47 provided 89% power to detect differences in TNE between stages of pregnancy (Effect size ~0.39; G*Power 3.1.9.3, Dusseldorf, DE) (34). No participants were taking any forms of NRT. Demographic characteristics for the subset of participants included in the current analysis (n=47) are presented in Table-1. The University of Toronto and University of Vermont Institutional Review Boards approved this study and all participants provided written informed consent.
Table-1.
Participant Characteristics
| % or mean ± standard deviation | Total sample (n=47) |
|---|---|
| Age at intakea (yrs) | 24.5 ± 0.8 |
| Education (yrs) | 12.3 ± 0.2 |
| % Caucasian | 89% |
| % Married | 26% |
| Age of smoking initiation | 15.2 ± 0.5 |
| Minnesota Nicotine Withdrawal Scale Scores at intakea | 1.5 ± 0.1 |
| Time to first cigarette | |
| < 5 min | 19% |
| 6 to 30 min | 21% |
| 31 to 59 min | 19% |
| 1 to 2 hr | 26% |
| ≥ 2 hr | 15% |
| % Living with other smoker(s) | 76% |
| % with none or few friends/family who smoke | 24% |
| % with no smoking allowed at home | 57% |
| % attempted to quit before pregnancy | 77% |
Intake refers to when subjects were enrolled in the study during early pregnancy (estimated gestational age mean 12.5 ± standard deviation 4.5 weeks).
Analytical procedures
Spot urine samples (5 ml) were collected, frozen, and sent to University of Toronto for nicotine metabolite assessment. TNE was analyzed in urine using liquid chromatography tandem mass spectrometry (LC-MS/MS) as described previously (35). Urinary creatinine concentrations were determined using a colorimetric assay (Creatinine Assay Kit MAK080) from Sigma (St. Louis, MO) with a SynergyMX Analyzer (BioTek, Winooski, VT, USA) and were adjusted for the effect of pregnancy on creatinine clearance (i.e. scaled creatinine) as described previously (27, 36).
Measures
We assessed nicotine intake at each pregnancy stage with the following variables: CPD and urinary biomarkers of nicotine consisting of TNE, free cotinine, and total cotinine. CPD was assessed by self-report. TNE was quantified as the molar sum of nicotine and nine metabolites (free cotinine, free 3HC, nicotine glucuronide, cotinine glucuronide, 3HC glucuronide, nicotine N-oxide, cotinine N-oxide, nornicotine, and norcotinine). Total cotinine was quantified as the molar sum of free cotinine and cotinine glucuronide. Nicotine intake per self-reported CPD was assessed by the ratio of TNE per CPD.
The NMR was determined as the ratio of 3HC+3HC glucuronide/free cotinine in urine. Nicotine metabolism status was determined by a median split on the NMR measured at postpartum (37), and participants were categorized into a slower (NMR ≤ 4.6, n=24) versus faster strata (NMR > 4.6, n=23) (Figure-S3).
Data Analysis
Urinary TNE, free cotinine, and total cotinine were corrected for creatinine using scaled creatinine concentrations (27, 36). All biomarkers were measured three times, at early and late pregnancy and at 6 months postpartum. Additionally, a retrospective report of pre-pregnancy CPD was collected at the time of entry into the trial during early pregnancy. General linear repeated measures analysis with Bonferroni correction was used to compare changes in nicotine intake longitudinally across the four (for CPD) and the three (for all other biomarkers) time points. As participants in the incentive arm may be more motivated to under-report their smoking quantity compared to those in the non-incentive arm, the relationship between TNE and CPD was tested separately in subjects from these two arms. No significant difference in nicotine intake per cigarette between the two groups was observed (Figure-S1). Furthermore, more dependent smokers (i.e. those with higher FTND scores) may be more prone to adopting compensatory smoking styles during pregnancy. As such, the relationship between TNE and CPD was tested in those who reported smoking within first hour of waking (i.e. time to first cigarette < 60 minutes; a component of FTND) compared to those smoking their first cigarette later in the day. No significant difference in nicotine intake per cigarette between the two groups was observed (Figure-S2). Spearman’s correlation was used to evaluate the relationship between 1) free cotinine and TNE and 2) total cotinine and TNE. Linear regression was used to calculate the slopes (± standard error of the slopes) for the relationship between TNE, free cotinine, and total cotinine. All analyses were conducted with GraphPad Prism (v5.0; La Jolla, CA) and SPSS (v24.0; IBM).
RESULTS
Changes in self-reported CPD during pregnancy
Pregnant smokers reported smoking 19.6 CPD prior to pregnancy, 10.4 CPD at early pregnancy (a 47% reduction, P<0.001), 7.2 CPD at late pregnancy (further 31% reduction, P=0.001), and 8.6 at postpartum (a 19% increase, P=0.08) (Table-2, Figure-1 A).
Table-2.
Smoking Biomarkers
| Mean ± standard deviation | Total sample (n=47) | Main effect of timea |
|---|---|---|
| Cigarettes per day (CPD) | ||
| Prior to pregnancyb | 19.6 ± 9.0 | |
| Early pregnancy | 10.4 ± 7.2 | |
| Late pregnancy | 7.2 ± 6.1 | <0.001 |
| Postpartum | 8.6 ± 5.6 | |
| Urinary total nicotine equivalentsc | ||
| Early pregnancy | 75 ± 72 | |
| Late pregnancy | 67 ± 71 | 0.05 |
| Postpartum | 128 ± 181 | |
| Total nicotine equivalents/CPD | ||
| Early pregnancy | 9 ± 9 | |
| Late pregnancy | 24 ± 37 | 0.008 |
| Postpartum | 19 ± 22 | |
| Urinary free cotininec | ||
| Early pregnancy | 7 ± 1 | |
| Late pregnancy | 5 ± 5 | 0.008 |
| Postpartum | 18 ± 43 | |
| Urinary total cotininec | ||
| Early pregnancy | 24 ± 29 | |
| Late pregnancy | 22 ± 25 | 0.20 |
| Postpartum | 39 ± 67 | |
| Nicotine metabolite ratiod | ||
| Early pregnancy | 8.1 ± 8.0 | |
| Late pregnancy | 10.4 ± 8.0 | <0.001 |
| Postpartum | 5.2 ± 3.5 |
P-values are for the main effect of time and are derived from repeated measures general linear modelling corrected for age of smoking initiation, age at intake, ethnicity, years of education, and MNWS scores. Statistical testing was done on non log-transformed values with the exception of the NMR. However, when tested on log-transformed values, the P-values did not differ substantially from those reported here on non-logged data.
Intake refers to when subjects were enrolled in the study during early pregnancy (estimated gestational age mean 12.5 ± standard deviation 4.5 weeks). Prior to pregnancy refers to retrospective assessment of the number of cigarettes smoked before enrollment in the study when subjects were not pregnant.
Urinary total nicotine equivalents, free cotinine, and total cotinine (nmol/mg creatinine) were corrected for creatinine using creatinine values that were adjusted for the effect of pregnancy. Same data uncorrected for creatinine, or corrected for creatinine using creatinine values un-adjusted for the effect of pregnancy on creatinine clearance are presented in supplementary material Table-S1.
Statistical testing was done on log-transformed values.
Figure-1.
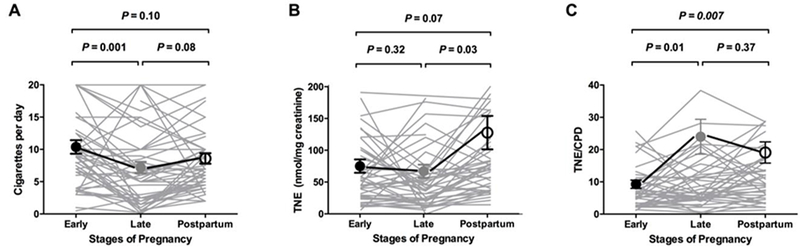
Nicotine intake measured by CPD (A), TNE (B), and smoking intensity estimated by TNE/CPD (C) during pregnancy and in postpartum.
Individual longitudinal data are presented, in addition to mean ± SD. Statistical testing was done on all n=47 subjects at all three stages, but one outlier from early pregnancy and two from postpartum were removed from the illustrations in A and B, respectively. P-values are for between-stage comparisons and are derived from repeated measures general linear modelling (Bonferroni correction).
Changes in TNE during pregnancy
TNE was on average 11% lower at late pregnancy compared to early pregnancy (P=0.32), suggesting a modest reduction in intake between these two pregnancy stages. TNE exposure was 41% (P=0.07) and 48% (P=0.03) lower at early and late pregnancy, respectively, compared to postpartum (Table-2, Figure-1B), suggesting a relatively large increase in intake between pregnancy and postpartum.
Changes in nicotine intake per cigarette during pregnancy
Despite reporting smoking fewer CPD, nicotine intake per self-reported CPD was on average 167% (2.67-fold) higher (as measured by the ratio of TNE per CPD for each subject which was then averaged across all 47 subjects) at late pregnancy compared to early pregnancy (P=0.01). Nicotine intake per self-reported CPD remained relatively high at postpartum where it was 111% (2.1-fold) higher compared to early pregnancy (P=0.007) (Table-2, Figure-1C).
Changes in urinary cotinine during pregnancy
The quantitative relationship between nicotine intake (i.e. TNE exposure) with free cotinine is presented in Figure-2A. Free cotinine was highly correlated with TNE at all three pregnancy stages (Spearman rho = 0.77–0.84, all P-values < 0.0001). Independent regression lines between urinary TNE and free cotinine were constructed for each stage of pregnancy (n=47 for each stage). The slope of the regression lines in early and late pregnancy was significantly lower than the slope in postpartum (slope: 0.09 and 0.07 in early and late pregnancy, respectively, versus 0.20 in postpartum, P<0.001), with a statistically significant interaction between pregnancy stage and free cotinine levels in the linear regression analysis with TNE as the dependent variable (Pinteraction<0.001; Table-3). These observations indicate that free cotinine predicts nicotine intake differently at different stages of pregnancy. As illustrated in Figure-2A, a 200 nmol/mg creatinine TNE exposure was suggestive of an 18 and 14 nmol/mg creatinine free cotinine level at early and late pregnancy, whereas the same TNE exposure was indicative of 40 nmol/mg creatinine cotinine level at postpartum. This reflects, on average, 55% and 65% underestimation of nicotine intake by free cotinine at early and late pregnancy compared to postpartum.
Figure-2.
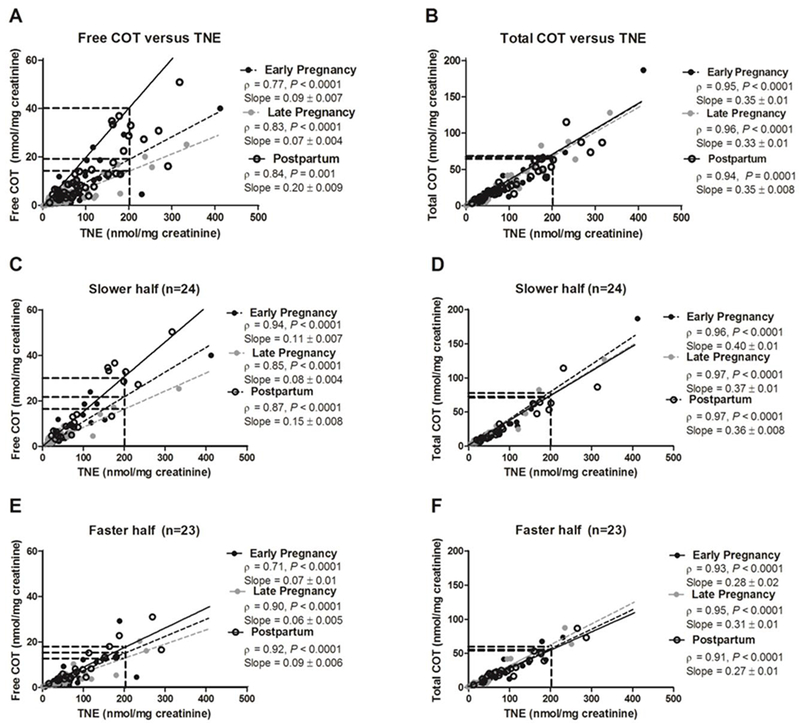
(Left) Free cotinine’s ability to predict tobacco exposure was different between stages of pregnancy (A) and by nicotine metabolism status (C & E). (Right) Total cotinine’s (i.e cotinine + cotinine glucuronide) ability to predict tobacco exposure was not different between stages of pregnancy (B) or by nicotine metabolism status (D & F). The slope between urinary TNE and urinary total cotinine was not different between stages of pregnancy (trend lines derived from linear regression modelling are overlapping). The same data analysis, uncorrected for creatinine (free and total cotinine in ng/ml and TNE in nmol/L), can be found in supplementary material Figure-S5.
Nicotine metabolism status was determined by a median split on the nicotine metabolite ratio at 6 months postpartum, an approximation of baseline NMR during non-pregnancy. The numbers after the slopes are standard error.
Table-3.
Regression analyses of the predictive ability of cotinine on total nicotine equivalents exposure during pregnancy
| Y=TNE | |||||
| All Subjects (n=47) | r2=0.87 P<0.0001 | B | β | 95% CI | P |
| Free cotinine (Increasing, per nmol/mg Cre) | 10.6 | 2.3 | 8.4 to 12.9 | <0.001 | |
| Pregnancy stage | 19.7 | 0.13 | 8.8 to 30.5 | <0.001 | |
| Interaction term | −2.2 | −1.4 | −2.9 to −1.4 | <0.001 | |
| Y=TNE | |||||
| Slower half (n=24) | r2=0.94 P<0.0001 | B | β | 95% CI | P |
| Free cotinine (Increasing, per nmol/mg Cre) | 11.5 | 2.6 | 9.1 to 13.9 | <0.001 | |
| Pregnancy stage | 22.1 | 0.11 | 8.8 to 35.4 | 0.002 | |
| Interaction term | −2.5 | −1.7 | −3.3 to −1.7 | <0.001 | |
| Y=TNE | |||||
| Faster half (n=23) | r2=0.65 P<0.0001 | B | β | 95% CI | P |
| Free cotinine (Increasing, per nmol/mg Cre) | 6.4 | 0.61 | 2.3 to 10.4 | 0.002 | |
| Pregnancy stage | −5.6 | −0.07 | −23.0 to 11.8 | 0.521 | |
| Interaction term | 0.98 | 0.24 | 0.11 to 2.7 | 0.043 | |
| Y=TNE | |||||
| All Subjects (n=47) | r2=0.96 P<0.0001 | B | β | 95% CI | P |
| Total cotinine (Increasing, per nmol/mg Cre) | 2.2 | 0.83 | 1.9 to 2.6 | <0.001 | |
| Pregnancy stage | 1.6 | 0.01 | −4.3 to 7.5 | 0.599 | |
| Interaction term | 0.15 | 0.16 | −0.24 to 0.27 | 0.180 | |
| Y=TNE | |||||
| Slower half (n=24) | r2=0.98 P<0.0001 | B | β | 95% CI | P |
| Total cotinine (Increasing, per nmol/mg Cre) | 2.0 | 0.76 | 1.67 to 2.3 | <0.001 | |
| Pregnancy stage | −0.51 | −0.003 | −7.5 to 6.5 | 0.884 | |
| Interaction term | 0.22 | 0.14 | −0.10 to 0.34 | 0.100 | |
| Y=TNE | |||||
| Faster half (n=23) | r2=0.89 P<0.0001 | B | β | 95% CI | P |
| Total cotinine (Increasing, per nmol/mg Cre) | 2.6 | 0.78 | 1.8 to 3.3 | <0.001 | |
| Pregnancy stage | −2.1 | −0.003 | −12.6 to 8.4 | 0.691 | |
| Interaction term | 0.26 | 0.17 | −0.08 to 0.60 | 0.131 | |
Changes in urinary cotinine by nicotine metabolism status during pregnancy
Cotinine levels, as a measure of intake, may be differentially affected in individuals with slower versus faster NMR. A difference in regression line slopes was observed between pregnancy stages in the slower NMR strata (n=24) (slope: 0.11 and 0.08 in early and late pregnancy, respectively, versus 0.15 in postpartum, Pinteraction<0.001; Table-3 and Figure-2 C). A 200 nmol/mg creatinine TNE exposure was suggestive of a 22 and 16 nmol/mg creatinine free cotinine level at early and late pregnancy, whereas the same TNE exposure was indicative of 30 nmol/mg creatinine free cotinine at postpartum. In the slower NMR strata, this reflects, on average, 27% and 47% underestimation of nicotine intake by free cotinine compared to when TNE is used at early and late pregnancy compared to postpartum.
A similar, albeit smaller, difference in regression line slopes was observed between the stages of pregnancy in the faster NMR strata (slope: 0.07 and 0.06 in early and late pregnancy, respectively, versus 0.09 in postpartum, Pinteraction= 0.04; Table-3 and Figure-2 E). A 200 nmol/mg creatinine TNE exposure was indicative of a 14 and 12 nmol/mg creatinine free cotinine level at early and late pregnancy, whereas the same TNE exposure was indicative of 18 nmol/mg creatinine free cotinine at postpartum. In the faster NMR strata, this reflects, on average, 22% and 33% underestimation of nicotine intake by free cotinine compared to when TNE is used at early and late pregnancy compared to postpartum.
Compared to free cotinine, total cotinine may be a better biomarker for measuring nicotine intake in part because it takes into account variation in removal of cotinine via glucuronidation, which is increased in pregnancy via induction of UGT2B10 (27).The quantitative relationship between TNE and total cotinine is presented in Figure-2B. Total cotinine was highly correlated with TNE at all three pregnancy stages (Spearman rho = 0.94–0.96, all P<0.0001). Similar to free cotinine, independent regression lines between urinary TNE and total cotinine were constructed for each stage of pregnancy. The slopes of the regression lines were not different between early pregnancy, late pregnancy, and postpartum (slope: 0.35 and 0.33 in early and late pregnancy, respectively, versus 0.35 in postpartum), suggesting total cotinine predicts nicotine intake similarly at different stages of pregnancy (Table-3 and Figure-2B). Likewise, there was no statistically significant difference in total cotinine estimates of dose between stages of pregnancy in the slower or faster NMR strata (Table-3; Figure-2D and 2F).
DISCUSSION
We found self-reported CPD was 21% higher at early pregnancy and 16% lower at late pregnancy compared to postpartum. TNE exposure was 41% and 48% lower at early and late pregnancy, respectively, compared to postpartum. This suggests 1) under-reporting of CPD and/or 2) an increase in nicotine intake per self-reported CPD at late pregnancy which also remained relatively high at postpartum potentially due to more intense smoking. We further found free cotinine underestimated nicotine intake by 55% as early as 12 weeks gestation during early pregnancy and by 65% by 29 weeks gestation during late pregnancy compared to postpartum. Differential effects of the stages of pregnancy on the quantitative relationship between cotinine and TNE were larger in phenotypically slower compared to faster nicotine metabolizers. In the absence of TNE data, we found total cotinine (free cotinine plus cotinine glucuronide) provided better estimates of nicotine intake than free cotinine reducing the effects of stages of pregnancy on the accuracy of the estimations.
The self-reported CPD at each pregnancy stage closely matched those reported across many previous studies (9–11, 38–40), suggesting, in general, pregnant smokers report substantially reducing their CPD at early pregnancy and making further smaller reductions at late pregnancy. To our knowledge, no studies have measured nicotine intake by TNE from early pregnancy, throughout gestation, and into postpartum. Despite lower self-reported CPD, we found the TNE was relatively comparable at early and late pregnancy and substantially higher at postpartum, suggesting intake of nicotine may be much higher than suggested by CPD. Mean TNE levels measured for non-pregnant female smokers of similar ethnicity were 37.5 nmol/L in 99 non-pregnant female smokers (average of 20 CPD) (41). In contrast, TNE levels measured at early pregnancy, late pregnancy, and postpartum in this study were 68.9, 55.8, and 82.9 nmol/L (converted to the same unit of concentration). This suggests pregnant smokers may under-report their CPD, for example due to social pressures, which is frequently observed in studies of pregnancy (42–45); under-reporting of CPD may also be observed during postpartum, for example due to social expectation around smoking with a new born. This also suggests, in addition to under-reporting of CPD, pregnant smokers may smoke each cigarette much more intensely during pregnancy and after birth. In both cases, CPD appears to substantially underestimate nicotine intake. As such, studies that estimate fetal exposure using CPD may severely underestimate the magnitude of toxic exposure (46–48).
Our observation of the nicotine intake remaining high into the postpartum period is novel and suggests learning to smoke more intensely, and adaptation to sensory effects associated with more intense smoking, during pregnancy may lead to continuation of this smoking behavior well into the postpartum period. Further, it suggests pre-pregnancy and postpartum are distinct periods in terms of smoking behaviors and that CPD may be an even poorer biomarker of nicotine intake in postpartum compared to non-pregnant female smokers. Of note, a previous study comparing puffing behavior between pregnant and non-pregnant subjects found no difference in puff frequency, volume, and duration, suggesting smoking topography was not influenced by faster nicotine metabolism during pregnancy (49). Their study points to underreporting of CPD being a reason for the disconnect in CPD versus TNE levels we observed, as well as potential increases in nicotine intake per self-reported CPD (TNE/CPD), for example due to smoking a greater portion of each cigarette. While these alternatives could be tested empirically, both indicate that CPD substantially underestimates nicotine intake relative to TNE during both pregnancy and in postpartum.
Following birth, it is unknown when nicotine metabolism rates return to pre-pregnancy levels. In this same dataset, we found that the profile of nicotine and metabolites at postpartum was similar to those reported for non-pregnant populations (27). Thus, while this postpartum time point likely differs from pre-pregnancy in terms of smoking behaviours, it appears to be a good estimate for baseline metabolic differences and for biomarker assessment (i.e. cotinine). Cotinine’s underestimation of nicotine intake during pregnancy suggests that studies estimating fetal exposure using cotinine may substantially underestimate the magnitude of nicotine and toxin exposure. Furthermore, studies that use cotinine to verify smoking abstinence may inaccurately classify pregnant smokers as abstinent. For example, the cotinine cut-off of 15 ng/ml in plasma widely used to distinguish smokers from non-smokers in clinical trials is approximately equivalent to cotinine of 60 ng/mg creatinine in urine (50). Cotinine at 60 ng/mg creatinine represents a postpartum TNE exposure of 300 nmol/mg creatinine (see Figure-2). However, during pregnancy a TNE exposure of 300 nmol/mg creatinine is equivalent to urinary cotinine of 27 and 21 ng/mg creatinine at early and late pregnancy, respectively; well below the urinary cotinine cut off of 60 ng/mg creatinine. Together, this suggests that while actively smoking based on their TNE exposure, urinary cotinine levels would have classified these pregnant smokers as non-smokers at both early and late pregnancy due to more rapid metabolism of cotinine at these times. We found total cotinine, compared to free cotinine, was a better measure of nicotine intake during pregnancy since the quantitative relationship between total cotinine and TNE exposure at early and late pregnancy was more closely related to the relationship observed at postpartum.
A limitation of our study is that TNE data was not collected prior to pregnancy limiting our ability to biochemically verify changes in nicotine intake that occur upon learning of pregnancy (i.e. pre-pregnancy to early pregnancy). A second potential limitation is that subjects were recruited as part of a larger smoking cessation study which may have altered their smoking, thus limiting the generalizability of our findings. Moreover, subjects were not asked about use of alternative nicotine products (e.g. e-cigarettes) which could have influenced their nicotine intake also.
In conclusion, the intake of nicotine was higher during pregnancy and in postpartum than is suggested by self-reported CPD. Cotinine underestimated nicotine intake by as much as 55% as early as 12 weeks gestation with the underestimation increasing as pregnancy progresses. Lastly, the quantitative relationship between cotinine and TNE was more substantially affected in subjects who are slower compared to faster nicotine metabolizers. These findings provide insights into changes in smoking behaviors and utility of self-reported and biochemical biomarkers of nicotine intake during pregnancy.
Supplementary Material
ACKNOWLEDGEMENTS
This research was undertaken, in part, thanks to funding from the Canada Research Chairs program (Dr. Tyndale, the Canada Research Chair in Pharmacogenomics). We acknowledge the support of CIHR grant (FDN-154294), the Campbell Family Mental Health Research Institute of CAMH, the CAMH Foundation, the Canadian Foundation for Innovation (#20289 and #16014) to R. F. Tyndale and the Ontario Ministry of Research and Innovation, and National Institute on Drug Abuse award (R01DA014028), National Institute of Child Health and Human Development award (R01HD075669), National Institute on Drug Abuse and Food and Drug Administration Tobacco Centers of Regulatory Science award (P50DA036114), and National Institute of General Medical Sciences Center of Biomedical Research Excellence award (P20GM103644) to S. T. Higgins.
R. F. Tyndale has served as a paid consultant to Apotex and Quinn Emmanuel and received unrestricted research funding from Pfizer as part of the Global Research Awards for Nicotine Dependence (GRAND), an independently reviewed competitive grants program.
Footnotes
Conflicts of interest: All other authors declare no conflict of interest.
REFERENCES
- 1.Ebrahim SH, Floyd RL, Merritt RK 2nd, Decoufle P, Holtzman D. Trends in pregnancy-related smoking rates in the United States, 1987–1996. Jama. 2000;283(3):361–6. [DOI] [PubMed] [Google Scholar]
- 2.Kurti AN, Redner R, Lopez AA, Keith DR, Villanti AC, Stanton CA, et al. Tobacco and nicotine delivery product use in a national sample of pregnant women. Prev Med. 2017;5(17):30277–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tong VT, Jones JR, Dietz PM, D’Angelo D, Bombard JM. Trends in smoking before, during, and after pregnancy - Pregnancy Risk Assessment Monitoring System (PRAMS), United States, 31 sites, 2000–2005. Morbidity and mortality weekly report Surveillance summaries (Washington, DC : 2002). 2009;58(4):1–29. [PubMed] [Google Scholar]
- 4.Kia F, Tosun N, Carlson S, Allen S. Examining characteristics associated with quitting smoking during pregnancy and relapse postpartum. Addictive behaviors. 2018;78:114–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kallen K The impact of maternal smoking during pregnancy on delivery outcome. Eur J Public Health. 2001;11(3):329–33. [DOI] [PubMed] [Google Scholar]
- 6.Murphy DJ, Dunney C, Mullally A, Adnan N, Deane R. Population-based study of smoking behaviour throughout pregnancy and adverse perinatal outcomes. Int J Environ Res Public Health. 2013;10(9):3855–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rogers JM. Tobacco and pregnancy. Reprod Toxicol. 2009;28(2):152–60. [DOI] [PubMed] [Google Scholar]
- 8.Salihu HM, Wilson RE. Epidemiology of prenatal smoking and perinatal outcomes. Early Hum Dev. 2007;83(11):713–20. [DOI] [PubMed] [Google Scholar]
- 9.Coleman T, Cooper S, Thornton JG, Grainge MJ, Watts K, Britton J, et al. A randomized trial of nicotine-replacement therapy patches in pregnancy. N Engl J Med. 2012;366(9):808–18. [DOI] [PubMed] [Google Scholar]
- 10.Heil SH, Higgins ST, Bernstein IM, Solomon LJ, Rogers RE, Thomas CS, et al. Effects of voucher-based incentives on abstinence from cigarette smoking and fetal growth among pregnant women. Addiction. 2008;103(6):1009–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins ST, Heil SH, Solomon LJ, Bernstein IM, Lussier JP, Abel RL, et al. A pilot study on voucher-based incentives to promote abstinence from cigarette smoking during pregnancy and postpartum. Nicotine Tob Res. 2004;6(6):1015–20. [DOI] [PubMed] [Google Scholar]
- 12.Scherer G, Engl J, Urban M, Gilch G, Janket D, Riedel K. Relationship between machine-derived smoke yields and biomarkers in cigarette smokers in Germany. Regulatory Toxicology and Pharmacology. 2007;47(2):171–83. [DOI] [PubMed] [Google Scholar]
- 13.Benowitz NL, Jacob P, Fong I, Gupta S. Nicotine metabolic profile in man: comparison of cigarette smoking and transdermal nicotine. Journal of Pharmacology and Experimental Therapeutics. 1994;268(1):296. [PubMed] [Google Scholar]
- 14.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev. 1996;18(2):188–204. [DOI] [PubMed] [Google Scholar]
- 15.Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–59. [DOI] [PubMed] [Google Scholar]
- 16.Benowitz NL, Jacob P 3rd. Metabolism of nicotine to cotinine studied by a dual stable isotope method. Clin Pharmacol Ther. 1994;56(5):483–93. [DOI] [PubMed] [Google Scholar]
- 17.Messina ES, Tyndale RF, Sellers EM. A major role for CYP2A6 in nicotine C-oxidation by human liver microsomes. J Pharmacol Exp Ther. 1997;282(3):1608–14. [PubMed] [Google Scholar]
- 18.Nakajima M, Yamamoto T, Nunoya K, Yokoi T, Nagashima K, Inoue K, et al. Characterization of CYP2A6 involved in 3’-hydroxylation of cotinine in human liver microsomes. J Pharmacol Exp Ther. 1996;277(2):1010–5. [PubMed] [Google Scholar]
- 19.Benowitz NL, Kuyt F, Jacob P 3rd, Jones RT, Osman AL. Cotinine disposition and effects. Clin Pharmacol Ther. 1983;34(5):604–11. [DOI] [PubMed] [Google Scholar]
- 20.Benowitz NL, Jacob P 3rd. Trans-3’-hydroxycotinine: disposition kinetics, effects and plasma levels during cigarette smoking. Br J Clin Pharmacol. 2001;51(1):53–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dempsey D, Tutka P, Jacob P 3rd, Allen F, Schoedel K, Tyndale RF, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. [DOI] [PubMed] [Google Scholar]
- 22.Fujieda M, Yamazaki H, Saito T, Kiyotani K, Gyamfi MA, Sakurai M, et al. Evaluation of CYP2A6 genetic polymorphisms as determinants of smoking behavior and tobacco-related lung cancer risk in male Japanese smokers. Carcinogenesis. 2004;25(12):2451–8. [DOI] [PubMed] [Google Scholar]
- 23.Rao Y, Hoffmann E, Zia M, Bodin L, Zeman M, Sellers EM, et al. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 2000;58(4):747–55. [DOI] [PubMed] [Google Scholar]
- 24.Hukkanen J, Jacob P 3rd, Benowitz NL. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005;57(1):79–115. [DOI] [PubMed] [Google Scholar]
- 25.Wassenaar CA, Dong Q, Wei Q, Amos CI, Spitz MR, Tyndale RF. Relationship Between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 Variation and Smoking Behaviors and Lung Cancer Risk. Journal of the National Cancer Institute. 2011;103(17):1342–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dempsey D, Jacob P, Benowitz NL. Accelerated Metabolism of Nicotine and Cotinine in Pregnant Smokers. Journal of Pharmacology and Experimental Therapeutics. 2002;301(2):594–8. [DOI] [PubMed] [Google Scholar]
- 27.Taghavi T, Arger CA, Heil SH, Higgins ST, Tyndale RF. Longitudinal Influence of Pregnancy on Nicotine Metabolic Pathways. J Pharmacol Exp Ther. 2017;20(117):245126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bowker K, Lewis S, Coleman T, Cooper S. Changes in the rate of nicotine metabolism across pregnancy: a longitudinal study. Addiction. 2015;110(11):1827–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arger CA, Taghavi T, Heil SH, Higgins ST, Tyndale RF. Pregnancy-Induced Increases in the Nicotine Metabolite Ratio: Examining Changes During Antepartum and Postpartum. Nicotine & Tobacco Research; Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benowitz NL, Dains KM, Dempsey D, Yu L, Jacob P 3rd . Estimation of nicotine dose after low-level exposure using plasma and urine nicotine metabolites. Cancer Epidemiol Biomarkers Prev. 2010;19(5):1160–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J, Liang Q, Mendes P, Sarkar M. Is 24h nicotine equivalents a surrogate for smoke exposure based on its relationship with other biomarkers of exposure? Biomarkers. 2011;16(2):144–54. [DOI] [PubMed] [Google Scholar]
- 32.Zhu AZ, Renner CC, Hatsukami DK, Swan GE, Lerman C, Benowitz NL, et al. The ability of plasma cotinine to predict nicotine and carcinogen exposure is altered by differences in CYP2A6: the influence of genetics, race, and sex. Cancer Epidemiol Biomarkers Prev. 2013;22(4):708–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Higgins ST, Washio Y, Lopez AA, Heil SH, Solomon LJ, Lynch ME, et al. Examining two different schedules of financial incentives for smoking cessation among pregnant women. Prev Med. 2014;68:51–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods. 2009;41(4):1149–60. [DOI] [PubMed] [Google Scholar]
- 35.Taghavi T, Novalen M, Lerman C, George TP, Tyndale RF. A comparison of direct and indirect analytical approaches to measuring total nicotine equivalents in urine. Cancer Epidemiol Biomarkers Prev. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davison JM, Noble MC. Serial changes in 24 hour creatinine clearance during normal menstrual cycles and the first trimester of pregnancy. Br J Obstet Gynaecol. 1981;88(1):10–7. [DOI] [PubMed] [Google Scholar]
- 37.Chenoweth MJ, Novalen M, Hawk LW Jr., Schnoll RA, George TP, Cinciripini PM, et al. Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev. 2014;23(9):1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rigotti NA, Park ER, Regan S, Chang Y, Perry K, Loudin B, et al. Efficacy of telephone counseling for pregnant smokers: a randomized controlled trial. Obstet Gynecol. 2006;108(1):83–92. [DOI] [PubMed] [Google Scholar]
- 39.Ussher M, Lewis S, Aveyard P, Manyonda I, West R, Lewis B, et al. The London Exercise And Pregnant smokers (LEAP) trial: a randomised controlled trial of physical activity for smoking cessation in pregnancy with an economic evaluation. Health Technol Assess. 2015;19(84):1–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heil SH, Herrmann ES, Badger GJ, Solomon LJ, Bernstein IM, Higgins ST. Examining the timing of changes in cigarette smoking upon learning of pregnancy. Prev Med. 2014;68:58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Derby KS, Cuthrell K, Caberto C, Carmella SG, Franke AA, Hecht SS, et al. Nicotine metabolism in three ethnic/racial groups with different risks of lung cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(12):3526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.England LJ, Grauman A Fau - Qian C, Qian C Fau - Wilkins DG, Wilkins Dg Fau - Schisterman EF, Schisterman Ef Fau - Yu KF, Yu Kf Fau - Levine RJ, et al. Misclassification of maternal smoking status and its effects on an epidemiologic study of pregnancy outcomes. (1462–2203 (Print)). [DOI] [PubMed]
- 43.Kvalvik LG, Nilsen Rm Fau - Skjaerven R, Skjaerven R Fau - Vollset SE, Vollset Se Fau - Midttun O, Midttun O Fau - Ueland PM, Ueland Pm Fau - Haug K, et al. Self-reported smoking status and plasma cotinine concentrations among pregnant women in the Norwegian Mother and Child Cohort Study. (1530–0447 (Electronic)). [DOI] [PMC free article] [PubMed]
- 44.Lindqvist R, Lendahls L Fau - Tollbom O, Tollbom O Fau - Aberg H, Aberg H Fau - Hakansson A, Hakansson A. Smoking during pregnancy: comparison of self-reports and cotinine levels in 496 women. (0001–6349 (Print)). [DOI] [PubMed]
- 45.Shipton D, Tappin Dm Fau - Vadiveloo T, Vadiveloo T Fau - Crossley JA, Crossley Ja Fau - Aitken DA, Aitken Da Fau - Chalmers J, Chalmers J. Reliability of self reported smoking status by pregnant women for estimating smoking prevalence: a retrospective, cross sectional study. (1756–1833 (Electronic)). [DOI] [PMC free article] [PubMed]
- 46.Poets CF, Schlaud M, Kleemann WJ, Rudolph A, Diekmann U, Sens B. Sudden infant death and maternal cigarette smoking: results from the Lower Saxony Perinatal Working Group. European journal of pediatrics. 1995;154(4):326–9. [DOI] [PubMed] [Google Scholar]
- 47.Ellard GA, Johnstone FD, Prescott RJ, Ji-Xian W, Jian-Hua M. Smoking during pregnancy: the dose dependence of birthweight deficits. Br J Obstet Gynaecol. 1996;103(8):806–13. [DOI] [PubMed] [Google Scholar]
- 48.Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: findings from a large population-based study. Am J Obstet Gynecol. 2005;192(6):1856–62; discussion 62–3. [DOI] [PubMed] [Google Scholar]
- 49.Bergeria CL, Heil SH, Bunn JY, Sigmon SC, Higgins ST. Comparing Smoking Topography and Subjective Measures of Usual Brand Cigarettes Between Pregnant and Non-Pregnant Smokers. Nicotine Tob Res. 2017;27(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benowitz NL, Dains KM, Dempsey D, Herrera B, Yu L, Jacob P 3rd. Urine nicotine metabolite concentrations in relation to plasma cotinine during low-level nicotine exposure. Nicotine Tob Res. 2009;11(8):954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


