Abstract
AIM
To investigate the effects of VSL#3 on tumor formation, and fecal and intestinal mucosal microbiota in azoxymethane/dextran sulfate sodium (AOM/DSS) induced mice model.
METHODS
C57BL/6 mice were administered AOM/DSS to develop the ulcerative colitis (UC) carcinogenesis model. Mice were treated with 5-ASA (75 mg/kg/d), VSL#3 (1.5 × 109 CFU/d), or 5-ASA combined with VSL#3 by gavage from the day of AOM injection for three months (five days/week). The tumor load was compared in each group, and tumor necrosis factor (TNF-α) and interleukin (IL)-6 levels were evaluated in colon tissue. The stool and intestinal mucosa samples were collected to analyze the differences in the intestinal microbiota by 16s rDNA sequencing method.
RESULTS
VSL#3 significantly reduced the tumor load in AOM/DSS-induced mice model and decreased the level of TNF-α and IL-6 in colon tissue. The model group had a lower level of Lactobacillus and higher level of Oscillibacter and Lachnoclostridium in fecal microbiota than the control group. After the intervention with 5-ASA and VSL#3, Bacillus and Lactococcus were increased, while Lachnoclostridium and Oscillibacter were reduced. 5-ASA combined with VSL#3 increased the Lactobacillus and decreased the Oscillibacter. The intestinal mucosal microbiota analysis showed a lower level of Bifidobacterium and Ruminococcaceae_UCG-014 and higher level of Alloprevotella in the model group as compared to the control group. After supplementation with VSL#3, Bifidobacterium was increased. 5-ASA combined with VSL#3 increased the level of both Lachnoclostridium and Bifidobacterium.
CONCLUSION
VSL#3 can prevent UC-associated carcinogenesis in mice, reduce the colonic mucosal inflammation levels, and rebalance the fecal and mucosal intestinal microbiota.
Keywords: Tumor necrosis factor-α, VSL#3, Ulcerative colitis carcinogenesis, Interleukin-6, Intestinal microbiota
Core tip: Microbiota and chronic inflammation play an important role in the process of ulcerative colitis (UC)-associated carcinogenesis. Our study found VSL#3 could effectively prevent UC-associated carcinogenesis in azoxymethane/dextran sulfate sodium induced mice and decrease the level of tumor necrosis factor-α and IL-6 in colon tissue. The intestinal microbiota dysbiosis exists in UC-associated carcinogenesis. Supplementary VSL#3 is beneficial for rebalancing the fecal and mucosal intestinal microbiota. Based on the data presented here, VSL#3 may be a potential therapeutic agent for UC-associated carcinogenesis prevention.
INTRODUCTION
Recently, the incidence of ulcerative colitis (UC) has shown an upward trend, leading to increased clinical attention on UC-associated carcinogenesis. A recent meta-analysis encompassing eight population-based cohort studies reported a 1.6% prevalence of colorectal cancer (CRC) in patients with UC, and the rate of CRC was 2.4-fold higher than that in the general population[1]. Moreover, the existing treatment for UC is not satisfactory for the prevention of carcinogenesis, involving several risks and side effects with long-term usage. Thus, finding new treatment regimens are essential.
Although the etiology of UC is yet to be elucidated, several studies have indicated that the host intestinal microbiota triggers an immune response that is requisite for the onset of the disease[2]. Microbiota also plays a major role in promoting UC-associated carcinogenesis. It downregulates the host immune response, improves the epithelial barrier function, and increases the mucus production[3]. Previous studies demonstrated that in the sterile intestinal environment, i.e., the lack of intestinal microbiota, a significant reduction in carcinogenic mutations and intestinal tumor formation was observed[4]. Chronic inflammation plays a crucial role in UC-associated tumorigenesis via cellular DNA damage, telomere shortening, and senescence[5]. Previous studies demonstrated that probiotics exert a superior therapeutic effect on inflammation and UC[6]. VSL#3 is a mixture of Lactobacillus casei, Lactobacillus plantarum, Lactobacillus acidophilus, Lactobacillus delbrueckii subsp. bulgaricus, Bifidobacterium longum, Bifidobacterium breve, Bifidobacterium infantis, and Streptococcus salivarius[7]. It proved to be beneficial in the treatment of UC, including remission and relief of the relapse in mild to moderate disease[8-10]. Thus, we speculated that probiotic treatment or adjuvant treatment of UC could prevent carcinogenesis. One study demonstrated that VSL#3 can inhibit UC-associated carcinogenesis in a mouse model[11]. However, the mechanism underlying the VSL#3 treatment of UC carcinogenesis is yet to be elucidated.
Therefore, in the present study, VSL#3 was selected to investigate the effect of prevention on UC-associated carcinogenesis and the differences between fecal and mucosal microbiota were analyzed to gain a theoretical insight for the prevention of UC-associated carcinogenesis.
MATERIALS AND METHODS
Animals
Eight week old C57BL/6 male mice were purchased from the Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China), housed under 12 h light/dark cycle conditions (temperature 22 ± 1 °C, humidity 40%-60%) in the National Cancer Center/Cancer Hospital animal facilities, and fed a standard diet for the duration of the study. All animal experiments were conducted in accordance with the recommendations of the Animal Care Ethics and Use Committee of Peking Union Medical College Hospital and approved by the same Committee (XHDW-2015-0032).
Development of UC-associated carcinogenesis model and in vivo treatment
All mice (n = 90) were initially housed together (5 animals/cage) for adaption one week before randomization into five experimental groups: control (no induction of UC-associated carcinogenesis, n = 10), model (no treatment) (n = 20), 5-ASA treatment (n = 20), VSL#3 treatment (n = 20), and 5-ASA + VSL#3 treatment (n = 20). In order to establish the UC-associated carcinogenesis model, mice were injected with 12.5 mg/kg body weight (BW) Azoxymethane (AOM) intraperitoneally, and after one week, 2.5% dextran sulfate sodium (DSS) (Mpbio, Solon, OH, United States) was added to their drinking water for five days, followed by ten weeks and two days of regular drinking water. This modeling method was based on a method described previously with some changes[12]. The three treatment groups, including 5-ASA, VSL#3, and 5-ASA + VSL#3 were gavaged 5-ASA (75 mg/kg BW, QD, Ferring Pharmaceuticals Ltd, solubilized in drinking water), VSL#3 (1.5 × 109 CFU/mice, QD, Sigma-Tau Pharmaceuticals Ltd, solubilized in drinking water), and 5-ASA + VSL#3 (75 mg/kg BW + 1.5 × 109 CFU/mice, QD) from the day of AOM injection. The control and model group were not subjected to gavage (Figure 1).
Figure 1.
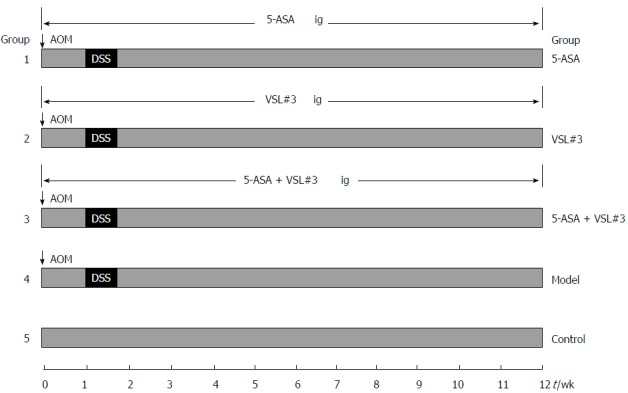
Experimental protocol for ulcerative colitis-associated carcinogenesis model and treatment.
Specimen collection
The mice were sacrificed by the 12th week via transcardiac perfusion, and colon tissues were removed. The colons were slit longitudinally along the main axis and washed with 0.9% saline. The long and short diameter of each tumor was measured using sliding calipers, and the total tumor load of each colon was calculated (sum of the product of long and short diameter of each tumor). Subsequently, the whole colon was divided into four sections. The section near the anus washed with 0.9% saline to remove the non-adherent bacteria were flash-frozen in liquid nitrogen and stored at -80 °C for subsequent microbiota analysis. The remaining sections were used for enzyme-linked immunosorbent assays (ELISA) and histopathological examinations. A stool sample was collected just before AOM injection and sacrifice. A total of six mice were randomly selected from each group, and their stool and intestinal mucosa samples were sent to Allwegene (Beijing, China) for analyzing the differences in intestinal microbiota by 16S rDNA sequencing method.
Fecal DNA extraction and pyrosequencing
Microbial genomic DNA was isolated using a QIAamp DNA Micro Kit according to the manufacturer’s instructions. The final quantity and quality of the DNA were assessed at 260 nm and 280 nm using an ultraviolet spectrophotometer and stored at -20 °C before further analysis. The V3-V4 hypervariable regions of the 16S rDNA gene were subjected to high-throughput sequencing by Allwegene using the Illumina Miseq PE300 sequencing platform (Illumina Inc., CA, United States).
ELISA for tumor necrosis factor-α and interleukin-6 in colon mucosa
The levels of tumor necrosis factor (TNF)-α and interleukin (IL)-6 in the colon mucosa were measured using commercial mouse TNF-α and IL-6 ELISA Kits (eBioscience, United States), according to the manufacturer’s protocols. The absorbance was measured at 450 nm. The results were expressed as pg/mg tissue. A total of eight mice were selected randomly from each group for ELISA.
Statistical analysis
Data are presented as mean ± SE. All statistical analyses were performed using GraphPad Prism Software Version 6.0 (GraphPad Software Inc., La Jolla, CA, United States). Statistical differences between experimental variants were assessed by two-tailed independent t-test, and data from more than two groups were analyzed by one-way ANOVA. Anosim and metastats analysis were used for microbiota analysis. P < 0.05 was considered statistically significant.
RESULTS
General health of mice in each group
As shown in Figure 2, compared to the control mice, the body weight loss was significantly higher in mice treated with azoxymethane/dextran sulfate sodium (AOM/DSS) after day 10 of DSS administration, which was accompanied by colitis symptoms, such as loose and bloody stool and dim body hair, fatigue, and less movement. These symptoms were alleviated when the mice received ordinary drinking water. In week 9, some mice treated with AOM/DSS presented bloody stool again, as well as, anal prolapse in week 10. However, no apparent weight loss was observed in the control mice, and no significant differences were detected among the five groups at the end of week 12.
Figure 2.
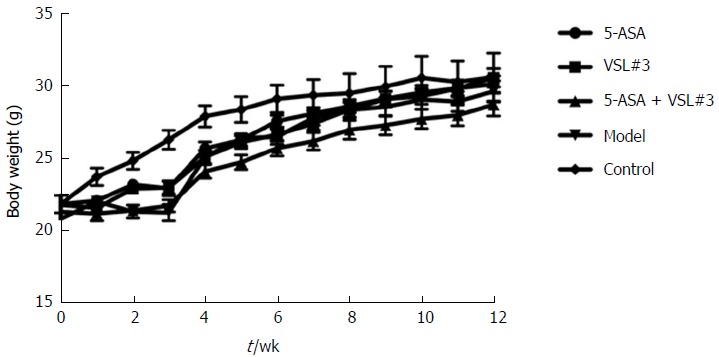
Body weight in each group.
Establishment of UC-associated carcinogenesis mice model
The mice were sacrificed by week 12, and the colorectal tumors were observed in the model and treatment groups (5-ASA, VSL#3, and 5-ASA + VSL#3). Strikingly, the tumor was primarily localized in the distal two-thirds of the colon. Anal tumor fusion and ring growth at the end of the rectum were observed in mice with anal prolapse (Figure 3). The pathological analysis showed mucosal carcinoma or high-grade intraepithelial neoplasia in mice treated with AOM/DSS. They were manifested with colonic gland structure disorder, large nuclei, deep staining, and nucleoplasmic ratio imbalance (Figure 4).
Figure 3.
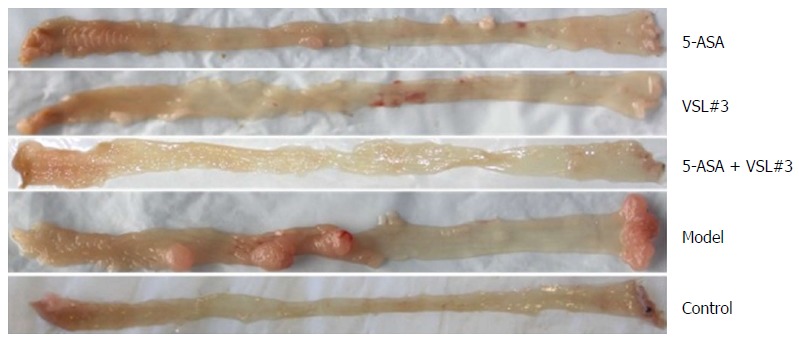
Representative image of colonic tumor in each group that was examined under naked eye.
Figure 4.
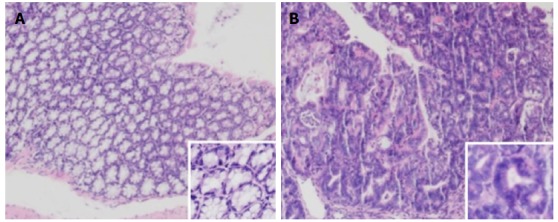
Representative image of hematoxylin-eosin staining of colon tissue examined under a microscope (40 × and 100 ×). A: Control group, the colonic mucosa glands were normal in the control group, the structure was regular, and the opening was good; B: Model group, the colonic gland structure presented disorder, large nuclei, deep staining, and nucleoplasmic ratio imbalance.
Effects of VSL#3 on UC-associated carcinogenesis
Treatment with AOM and DSS led to 100% (19/19, one mouse died during the experiment due to fighting) incidence of colonic neoplasms in the model group with the mean tumor load of 0.97 ± 0.19 cm. 5-ASA and VSL#3 administration significantly reduced both the tumor formation rate and the tumor load (Table 1 and Figure 5). Furthermore, no colonic tumor was detected in the control group.
Table 1.
Tumor formation rate and tumor load in each group
| Group | n | Tumor formation rate (%) | Tumor load (cm) | P value (vs model group) |
| 5-ASA | 20 | 65.0 (13/20) | 0.43 ± 0.14 | 0.0269 |
| VSL#3 | 20 | 65.0 (13/20) | 0.25 ± 0.07 | 0.0009 |
| 5-ASA + VSL#3 | 19 | 63.2 (12/19) | 0.46 ± 0.11 | 0.0261 |
| Model | 19 | 100.0 (19/19) | 0.97 ± 0.19 | - |
| Control | 10 | 0 | 0 | - |
Figure 5.
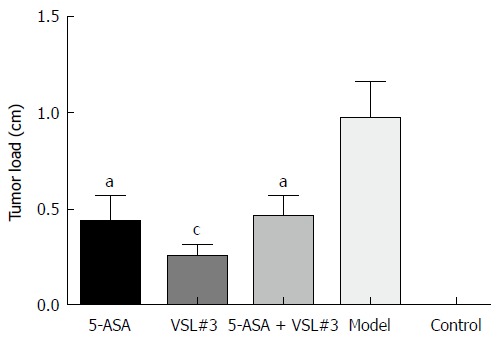
Tumor load in each group. aP < 0.05, bP < 0.01, cP < 0.001.
Colonic TNF-α and IL-6 level comparison
As illustrated in Figure 6 and Tables 2 and 3, the levels of colonic tissue TNF-α and IL-6 in the model group were significantly higher than that in the control group. The increased levels of these inflammatory factors induced by AOM/DSS were attenuated by 5-ASA and VSL#3 treatment.
Figure 6.
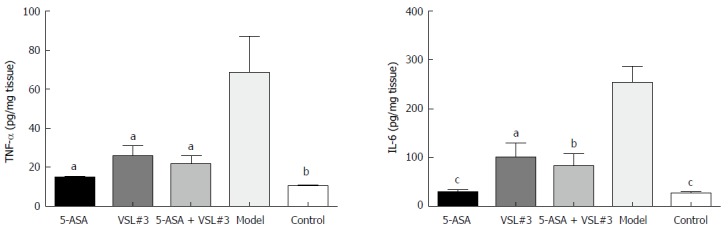
Colonic tumor necrosis factor-α and interleukin-6 levels in different groups. aP < 0.05, bP < 0.01, cP < 0.001. TNF-α: Tumor necrosis factor-α; IL-6: Interleukin-6.
Table 2.
Level of tumor necrosis factor-α in colon tissue in each group
| Group | n | TNF-α (pg/mg tissue) | P value (vs model group) |
| 5-ASA | 8 | 14.66 ± 0.72 | < 0.05 |
| VSL#3 | 8 | 25.89 ± 5.25 | < 0.05 |
| 5-ASA + VSL#3 | 8 | 21.33 ± 4.55 | < 0.05 |
| Model | 8 | 68.38 ± 18.73 | - |
| Control | 8 | 10.49 ± 0.30 | < 0.01 |
TNF-α: Tumor necrosis factor.
Table 3.
Level of interleukin-6 in colon tissue in each group
| Group | n | IL-6 (pg/mg tissue) | P value (vs model group) |
| 5-ASA | 8 | 28.19 ± 6.80 | < 0.0001 |
| VSL#3 | 8 | 99.71 ± 31.14 | < 0.0500 |
| 5-ASA+VSL#3 | 8 | 81.43 ± 26.98 | < 0.0100 |
| Model | 8 | 254.20 ± 32.49 | - |
| Control | 8 | 25.47 ± 5.50 | < 0.0001 |
IL-6: Interleukin 6.
VSL#3 treatment alters the composition of fecal microbiota in AOM/DSS treated mice
In order to characterize the diversity of fecal-associated community in UC-associated carcinogenesis, we used Chao 1 and the observed species indexes, as well as the Shannon and Simpson indexes. No significant difference was detected in the diversity and composition of fecal microbiota in each group at the beginning of the experiment. After the 12-wk experiment, although no statistically significant difference was detected in the diversity among groups, the microbiota composition was altered considerably. The change in the composition of fecal microbiota induced by AOM/DSS administration was characterized by a decrease in Lactobacillus coupled with an increase in Oscillibacter and Lachnoclostridium as indicated by metastats analysis (P < 0.05). Both 5-ASA and VSL#3 supplementation was associated with a significant increase in Bacillus and Lactococcus and a decrease in Oscillibacter and Lachnoclostridium as compared to the model group (P < 0.05). 5-ASA combined with VSL#3 increased the level of Lactobacillus and decreased that of Oscillibacter (P < 0.05) (Table 4).
Table 4.
Comparison of fecal microbiota (abundance)
| Genus | Control (%) | Model (%) | 5-ASA (%) | VSL#3 (%) | 5-ASA + VSL#3 (%) |
| Lactobacillus | 4.77 | 3.261 | 2.65 | 4.06 | 9.864 |
| Oscillibacter | 0.64 | 1.301 | 0.542 | 0.523 | 0.794 |
| Lachnoclostridium | 0.45 | 1.191 | 0.482 | 0.393 | 1.08 |
| Bacillus | 1.01 | 0.98 | 24.002 | 23.343 | 0.66 |
| Lactococcus | 2.30 | 2.29 | 8.582 | 7.863 | 1.59 |
P < 0.05 between the model and control groups;
P < 0.05 between the model and 5-ASA groups;
P < 0.05 between the model and VSL#3 groups;
P < 0.05 between the model and 5-ASA + VSL#3 groups.
VSL#3 treatment alters the composition of mucosal microbiota in AOM/DSS treated mice.
For the mucosal microbiota, no difference was observed in the community diversity among the groups after the 12-wk experiment. However, the distinct shift in the microbiota composition was observed by PCA and Anosim analysis (R > 0, P < 0.05). Further investigation into the discrete bacterial taxa revealed that Ruminococcaceae UCG-014 and Bifidobacterium decreased, while Alloprevotella increased in the model group compared to the control group. After supplementation with VSL#3, Bifidobacterium was increased. Although 5-ASA alone did not alter the mucosal microbiota, the combination with VSL#3 increased Lachnoclostridium and Bifidobacterium in the mucosa (Table 5).
Table 5.
Comparison of mucosal microbiota (abundance)
| Genus | Control (%) | Model (%) | 5-ASA (%) | VSL#3 (%) | 5-ASA + VSL#3 (%) |
| Alloprevotella | 0.26 | 1.571 | 1.16 | 0.95 | 1.22 |
| Ruminococcaceae_UCG-014 | 6.63 | 1.491 | 1.64 | 1.50 | 1.15 |
| Bifidobacterium | 3.45 | 0.241 | 0.19 | 3.342 | 1.903 |
| Lachnoclostridium | 0.24 | 0.40 | 2.05 | 0.25 | 2.033 |
P < 0.05 between the model and the control groups;
P < 0.05 between the model and the VSL#3 groups;
P < 0.05 between the model and the 5-ASA + VSL#3 groups.
DISCUSSION
The current study found that the rate of tumor formation and tumor load decreased after VSL#3 treatment compared to the model group, while the levels of TNF-α and IL-6 in the colon tissue in the model group were significantly higher than the control group. After the 12 wk treatment of VSL#3, the increase in TNF-α and IL-6 caused by AOM/DSS declined significantly. These findings were consistent with that of previous studies[11,13,14]. The major risk of long-term chronic inflammation is tumor occurrence[2]. Thus, we speculated that VSL#3 could prevent UC carcinogenesis by inhibiting the inflammatory response.
Herein, we found differences between the fecal and mucosal microbiota. In the case of fecal microbiota, the model group mice possessed less Lactobacillus and more Oscillibacter and Lachnoclostridium as compared to the control group. Previous studies have shown that Lactobacillus bulgaricus can reduce colitis[15], and Lactobacillus rhamnosus can effectively maintain UC remission[16]. Oscillibacter and Lachnoclostridium are newly discovered genera with respect to digestive diseases. In the case of mucosal microbiota, the level of the genus UCG-014 of Ruminococcaceae and Bifidobacterium decreased, while that of Alloprevotella increased in the model group as compared to the control group. Some genus of Ruminococcaceae can consume hydrogen to produce acetate, which is subsequently used by Roseburia to produce butyrate that is not only the main source of energy for intestinal epithelial cells but can also inhibit the signaling pathway of proinflammatory cytokines[17]. Bifidobacterium can produce bacteriocin and organic acids against pathogens on intestinal mucosal invasion[18]. It regulates the intestinal mucosal immunity and prevents the colonization of pathogens. The role of Alloprevotella is not yet clarified as it is not reported frequently in the digestive disease. Therefore, we hypothesize that dysbiosis occurs during UC-associated carcinogenesis, which reduces the beneficial types and increases the detrimental types.
Previous studies have shown that supplementation of probiotics can balance the intestinal microbiota of UC patients[6], which led us to speculate that supplementation of probiotics can also balance the intestinal microbiota of UC-associated carcinogenesis. The current study demonstrated that Bacillus and Lactococcus were increased, while Oscillibacter and Lachnoclostridium were decreased in the feces following VSL#3 treatment as compared to the model group. Some species of Bacillus and Lactococcus are widely used as probiotics. For example, Bacillus subtilis can significantly reduce DSS-induced colonic mucosal injury and inflammatory factors in mice and improve the levels of short-chain fatty acids[19]. Lactococcus lactis exerts a protective effect on DSS-induced colitis model mice[20].
Furthermore, Bifidobacterium increased in the mucosa after VSL#3 supplementation, thereby suggesting that VSL#3 supplementation, following the onset of AOM/DSS-induced colitis, promotes a healthy gastrointestinal bacterial community. Interestingly, VSL#3 is composed of eight strains, including one Streptococcus, three Bifidobacterium, and four Lactobacillus. However, none of the above strains increased significantly in the fecal intestinal microbiota after three-month gavage, suggesting that the positive effect of probiotics on the intestinal microbiota of the host is by regulating the proportion of beneficial and harmful bacteria.
For the differences between fecal and mucosal microbiota, we make the following explanation. There are three kinds of Bifidobacterium in VSL#3, and Bifidobacterium increased in mucosal microbiota but not in fecal. This phenomenon indicated that Bifidobacterium is easily colonized in the mucosa. Conversely, Bacillus and Lactococcus increased in fecal microbiota after VSL#3 intervention but not in the mucosa, indicating that Bacillus and Lactococcus can colonize easily in the feces. Strikingly, the four types of Lactobacillus in VSL#3 did not increase either in the fecal or mucosal microbiota, thereby suggesting that the intestinal environment of UC-associated carcinogenesis is not optimal for the growth of Lactobacillus. Only in the 5-ASA + VSL#3 group, the increase in Lactobacillus was observed in feces, which might be attributed to the low luminal pH. However, these hypotheses necessitate further studies for substantiation.
5-ASA is the first-line treatment for mild-to-moderate UC, and studies have found that 5-ASA ≥ 1.2 g/d could reduce the risk of carcinogenesis in patients with mild-to-moderate UC[21]. Thus, considering the clinical significance, we designed the 5-ASA monotherapy group and the 5-ASA + VSL#3 group. Interestingly, the change in the fecal microbiota in the 5-ASA group was similar to that in the VSL#3 monotherapy group. The potential mechanisms regulating the microbiota by 5-ASA are as follows: (1) Change in the colonic luminal pH: 5-ASA is released in the colon and translated into acetylsalicylic acid, which in turn, can decrease the luminal pH[22]. Low luminal pH is optimal for the growth of Bifidobacteria and Lactobacilli[23]; (2) improvement in the anoxia environment: 5-ASA can inhibit the production of chemotactic eicosanoids and cyclooxygenase 2 (COX2), which induces anoxia and can inactivate the oxygen-derived free radicals, improving the anoxia situation, which might affect the composition of intestinal microbiota[22]; and (3) 5-ASA can downregulate the expression of genes that are involved in bacterial metabolism, invasiveness, and antibiotic/stress resistance[24].
Nevertheless, the present study has some limitations. Herein, we only observed the phenomenon of gut microbiota changes while the specific role of flora is yet to be explored. Our future in vitro studies would focus on the underlying mechanisms.
In conclusion, the current study demonstrated that VSL#3 prevented UC-associated carcinogenesis in the AOM/DSS-induced mice model and decreased the level of TNF-α and IL-6 in colon tissue. The intestinal microbiota dysbiosis was exhibited in UC-associated carcinogenesis mice. Supplementary VSL#3 was beneficial for a balanced fecal and mucosal microbiota in UC-associated carcinogenesis mice. Taken together, VSL#3 may serve as a potential therapeutic agent for the prevention of UC-associated carcinogenesis. Ongoing studies in our group are focused on the underlying mechanisms.
ARTICLE HIGHLIGHTS
Research background
Recently, an upward trend has been observed in the incidence of ulcerative colitis (UC) leading to increased clinical attention on UC-associated carcinogenesis.
Research motivation
Existing treatment for UC in the prevention of carcinogenesis involves several risks and side effects with long-term usage. Finding new treatment regimens are essential.
Research objectives
To investigate the effects of VSL#3 on tumor formation, and fecal and intestinal mucosal microbiota in the azoxymethane/dextran sulfate sodium (AOM/DSS) induced mice model.
Research methods
C57BL/6 mice were administered AOM/DSS to develop the UC-associated carcinogenesis model. The treatment group was gavaged with 5-ASA (75 mg/kg/d), VSL#3 (1.5 × 109 CFU/d), and 5-ASA + VSL#3 from the day of AOM injection for three months (five days/week). The tumor load was compared in each group, and tumor necrosis factor (TNF-α) and interleukin (IL)-6 levels evaluated in colon tissue. The stool and intestinal mucosa samples were collected to analyze the differences in the intestinal microbiota by 16s rDNA sequencing.
Research results
VSL#3 significantly reduced the tumor load in the AOM/DSS-induced mice model, and decreased the level of TNF-α and IL-6 in colon tissue. The model group had a lower level of Lactobacillus and higher level of Oscillibacter and Lachnoclostridium in fecal microbiota than the control group (UC-associated carcinogenesis not induced). Bacillus and Lactococcus were increased after the intervention with 5-ASA and VSL#3, while Lachnoclostridium and Oscillibacter were reduced. 5-ASA + VSL#3 increased the Lactobacillus and decreased the Oscillibacter. The intestinal mucosal microbiota analysis showed a lower level of Bifidobacterium and Ruminococcaceae_UCG-014 and higher level of Alloprevotella in the model group compared to the control group. Bifidobacterium was increased after supplementation with VSL#3. 5-ASA + VSL#3 increased the level of both Lachnoclostridium and Bifidobacterium.
Research conclusions
In mice, VSL#3 can prevent UC-associated carcinogenesis, reduce the colonic mucosal inflammation levels, and is beneficial for rebalancing the fecal and mucosal intestinal microbiota.
Research perspectives
VSL#3 may be a potential therapeutic agent for UC-associated carcinogenesis prevention based on the data presented here.
ACKNOWLEDGMENTS
We thank the staff at the National Cancer Center/Cancer Hospital of the Chinese Academy of Medical Sciences and Peking Union Medical College.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Institutional animal care and use committee statement: All animal experiments were conducted in accordance with the recommendations of the Animal Care Ethics and Use Committee of Peking Union Medical College Hospital and approved by the same Committee (XHDW-2015-0032).
Conflict-of-interest statement: To the best of our knowledge, no conflict of interest exists.
Data sharing statement: No additional data are available.
ARRIVE guidelines statement: The ARRIVE Guidelines have been adopted.
Peer-review started: May 27, 2018
First decision: July 6, 2018
Article in press: August 24, 2018
P- Reviewer: Gardlik R, Suzuki H S- Editor: Wang XJ L- Editor: Filipodia E- Editor: Huang Y
Contributor Information
Chun-Sai-Er Wang, Department of Gastroenterology, PUMC Hospital, CAMS and PUMC, Beijing 100730, China.
Wen-Bin Li, Department of Gastroenterology, PUMC Hospital, CAMS and PUMC, Beijing 100730, China.
Hong-Ying Wang, National Cancer Center/Cancer Hospital, CAMS and PUMC, Beijing 100021, China.
Yi-Ming Ma, National Cancer Center/Cancer Hospital, CAMS and PUMC, Beijing 100021, China.
Xin-Hua Zhao, National Cancer Center/Cancer Hospital, CAMS and PUMC, Beijing 100021, China.
Hong Yang, Department of Gastroenterology, PUMC Hospital, CAMS and PUMC, Beijing 100730, China.
Jia-Ming Qian, Department of Gastroenterology, PUMC Hospital, CAMS and PUMC, Beijing 100730, China.
Jing-Nan Li, Department of Gastroenterology, PUMC Hospital, CAMS and PUMC, Beijing 100730, China. lijn2008@126.com.
References
- 1.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clin Gastroenterol Hepatol. 2012;10:639–645. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Uronis JM, Mühlbauer M, Herfarth HH, Rubinas TC, Jones GS, Jobin C. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS One. 2009;4:e6026. doi: 10.1371/journal.pone.0006026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Derikx LA, Dieleman LA, Hoentjen F. Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol. 2016;30:55–71. doi: 10.1016/j.bpg.2016.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Rakoff-Nahoum S, Medzhitov R. Role of toll-like receptors in tissue repair and tumorigenesis. Biochemistry (Mosc) 2008;73:555–561. doi: 10.1134/s0006297908050088. [DOI] [PubMed] [Google Scholar]
- 5.Risques RA, Lai LA, Himmetoglu C, Ebaee A, Li L, Feng Z, Bronner MP, Al-Lahham B, Kowdley KV, Lindor KD, et al. Ulcerative colitis-associated colorectal cancer arises in a field of short telomeres, senescence, and inflammation. Cancer Res. 2011;71:1669–1679. doi: 10.1158/0008-5472.CAN-10-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verna EC, Lucak S. Use of probiotics in gastrointestinal disorders: what to recommend? Therap Adv Gastroenterol. 2010;3:307–319. doi: 10.1177/1756283X10373814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang J, Sha SM, Wu KC. Role of the intestinal microbiota and fecal transplantation in inflammatory bowel diseases. J Dig Dis. 2014;15:641–646. doi: 10.1111/1751-2980.12211. [DOI] [PubMed] [Google Scholar]
- 8.Shen J, Zuo ZX, Mao AP. Effect of probiotics on inducing remission and maintaining therapy in ulcerative colitis, Crohn’s disease, and pouchitis: meta-analysis of randomized controlled trials. Inflamm Bowel Dis. 2014;20:21–35. doi: 10.1097/01.MIB.0000437495.30052.be. [DOI] [PubMed] [Google Scholar]
- 9.Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol. 2009;7:1202–1209, 1209.e1. doi: 10.1016/j.cgh.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 10.Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, et al. Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol. 2010;105:2218–2227. doi: 10.1038/ajg.2010.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talero E, Bolivar S, Ávila-Román J, Alcaide A, Fiorucci S, Motilva V. Inhibition of chronic ulcerative colitis-associated adenocarcinoma development in mice by VSL#3. Inflamm Bowel Dis. 2015;21:1027–1037. doi: 10.1097/MIB.0000000000000346. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka T, Kohno H, Suzuki R, Yamada Y, Sugie S, Mori H. A novel inflammation-related mouse colon carcinogenesis model induced by azoxymethane and dextran sodium sulfate. Cancer Sci. 2003;94:965–973. doi: 10.1111/j.1349-7006.2003.tb01386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassaganya-Riera J, Viladomiu M, Pedragosa M, De Simone C, Hontecillas R. Immunoregulatory mechanisms underlying prevention of colitis-associated colorectal cancer by probiotic bacteria. PLoS One. 2012;7:e34676. doi: 10.1371/journal.pone.0034676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Takamura T, Harama D, Fukumoto S, Nakamura Y, Shimokawa N, Ishimaru K, Ikegami S, Makino S, Kitamura M, Nakao A. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol Cell Biol. 2011;89:817–822. doi: 10.1038/icb.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zocco MA, dal Verme LZ, Cremonini F, Piscaglia AC, Nista EC, Candelli M, Novi M, Rigante D, Cazzato IA, Ojetti V, et al. Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment Pharmacol Ther. 2006;23:1567–1574. doi: 10.1111/j.1365-2036.2006.02927.x. [DOI] [PubMed] [Google Scholar]
- 17.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 2012;13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhiwei L, Xuequn W, tiantian L. The clinical significance of intestinal flora changes in patients with ulcerative colitis. Chin J Gastroenterol Hepatol. 2016;25:554–556. [Google Scholar]
- 19.Zhang HL, Li WS, Xu DN, Zheng WW, Liu Y, Chen J, Qiu ZB, Dorfman RG, Zhang J, Liu J. Mucosa-reparing and microbiota-balancing therapeutic effect of Bacillus subtilis alleviates dextrate sulfate sodium-induced ulcerative colitis in mice. Exp Ther Med. 2016;12:2554–2562. doi: 10.3892/etm.2016.3686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berlec A, Perše M, Ravnikar M, Lunder M, Erman A, Cerar A, Štrukelj B. Dextran sulphate sodium colitis in C57BL/6J mice is alleviated by Lactococcus lactis and worsened by the neutralization of Tumor necrosis Factor α. Int Immunopharmacol. 2017;43:219–226. doi: 10.1016/j.intimp.2016.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Zhao LN, Li JY, Yu T, Chen GC, Yuan YH, Chen QK. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: an updated meta-analysis. PLoS One. 2014;9:e94208. doi: 10.1371/journal.pone.0094208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue L, Huang Z, Zhou X, Chen W. The possible effects of mesalazine on the intestinal microbiota. Aliment Pharmacol Ther. 2012;36:813–814. doi: 10.1111/apt.12034. [DOI] [PubMed] [Google Scholar]
- 23.Kerr BJ, Weber TE, Ziemer CJ, Spence C, Cotta MA, Whitehead TR. Effect of dietary inorganic sulfur level on growth performance, fecal composition, and measures of inflammation and sulfate-reducing bacteria in the intestine of growing pigs. J Anim Sci. 2011;89:426–437. doi: 10.2527/jas.2010-3228. [DOI] [PubMed] [Google Scholar]
- 24.Kaufman J, Griffiths TA, Surette MG, Ness S, Rioux KP. Effects of mesalamine (5-aminosalicylic acid) on bacterial gene expression. Inflamm Bowel Dis. 2009;15:985–996. doi: 10.1002/ibd.20876. [DOI] [PubMed] [Google Scholar]


