Abstract
OX40 and its ligand are members of the TNF/TNF receptor superfamily, which includes various molecules influencing cellular signaling and function of both tumor and immune cells. The ability of OX40 to promote proliferation and differentiation of activated T cells fueled present attempts to modulate this immune checkpoint to reinforce antitumor immunity. While we recently found evidence for the involvement of OX40 in pathophysiology of acute myeloid leukemia including natural killer (NK) cell immunosurveillance, less is known on its role in acute lymphoblastic leukemia (ALL). In the present study, OX40 expression on ALL cells was significantly associated with positivity for the adverse risk factor BCR-ABL. In line, signaling via OX40 increased metabolic activity of primary ALL cells and resulted in release of cytokines involved in disease pathophysiology. Furthermore, interaction of ALL-expressed OX40 with its cognate ligand on NK cells stimulated ALL cell lysis. The data presented thus not only identify the yet unknown involvement of OX40/OX40L in ALL pathophysiology and NK cell immunosurveillance but also point to the necessity to thoroughly consider the consequences of modulating the OX40/OX40L molecule system beyond its effects on T cells when developing OX40-targeting approaches for cancer immunotherapy.
Introduction
Immune checkpoint therapy has become a pillar of cancer treatment [1], [2]. The first three approved checkpoint antibodies ipilimumab, nivolumab, and pembrolizumab represent a novel strategy for the treatment of a multitude of malignancies by targeting inhibitory pathways that prevent effective antitumor T cell responses [3], [4], [5], [6], [7]. Therapeutic concepts for checkpoint modulation utilizing antibodies providing an agonistic signal via activating receptors on T cells are less advanced and presently under investigation. One of the targets is OX40 (CD134), a member of the tumor necrosis factor receptor (TNFR) superfamily [8], [9], [10]. This costimulatory molecule is upregulated on effector T cells after activation and supports differentiation, proliferation, and long-term survival. In addition, it mediates inhibition of the suppressive activity of regulatory T cells [11], which contribute to evasion of tumor cells from T cell immunity. In line, the frequency of tumor-infiltrating OX40-positive T cells has been reported to correlate with patient survival [12], [13]. Application of OX40 agonists, alone or in combination with other checkpoint modulators, stimulated the cytotoxic activity of T cells and caused tumor regression in preclinical models [14], [15], [16], [17], [18], [19]. First evidence from early clinical trials also indicates that OX40 stimulation could be effective in cancer patients [20]. A multitude of clinical trials targeting OX40 as monotherapy or in combination with vaccination, radiotherapy, checkpoint blockade, chemotherapy, or targeted therapy are currently ongoing (for review, see [10]). Notably, OX40 was also found to be expressed by T cell–derived leukemic cells and in acute myeloid leukemia (AML). Its counterpart OX40 ligand (OX40L) is upregulated on natural killer (NK) cells following activation and stimulates their reactivity via reverse signaling into the ligand-bearing cells, while forward signaling into AML cells stimulated cellular functions of the leukemic cells [21], [22]. So far, less is known regarding the OX40/OX40L system in acute lymphoblastic leukemia (ALL) of B cell lineage and its functional role in ALL cells. Here we report that primary ALL cells and cell lines partially express OX40 and that OX40 surface expression is significantly associated with BCR-ABL status, which constitutes a powerful predictor of treatment outcome and prognosis in ALL. We further show that OX40 stimulation promotes metabolic activity of ALL cells and results in release of cytokines like tumor necrosis factor (TNF), interleukin-6 (IL-6), and IL-8 that influence growth and survival of the malignant cells. In line with the stimulatory role of OX40L in NK cells, we further demonstrate that disruption of OX40/OX40L interaction impairs NK cell reactivity against OX40-positive ALL cell lines and provide data on the poor prognostic relevance of OX40 expression.
Material and Methods
ALL Cell Lines
The human ALL cell lines JURKAT, NALM-16, REH, SD-1, SUP-B15, and TOM-1 were obtained from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Cell lines were cultured in RPMI-1640 medium (Biochrom, Berlin, Germany) supplemented with 1% penicillin/streptomycin (Lonza, Basel, Switzerland) and 10% fetal calf serum (FCS, Biochrom) (JURKAT, NALM-16, SD-1, and TOM-1) or 20% FCS (REH). SUP-B15 cells were cultured in IMDM medium (GIBCO, Carlsbad, CA) with 1% penicillin/streptomycin (Lonza), 1% L-glutamine (Lonza), 1% nonessential amino acids (Lonza), 1% sodium pyruvate (Sigma Aldrich, St. Louis, MO), and 10% FCS. Cells were kept in a humidified atmosphere at 37°C and 5% CO2.
Mycoplasma contamination was excluded by routine testing of cell lines every 3 months. Cell lines were authenticated by single nucleotide profiling.
Patients
Peripheral blood samples of ALL patients were obtained after written informed consent at the University of Tübingen. Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll/Paque (Biochrom) density gradient centrifugation and viably stored in liquid nitrogen until analysis. This study was approved by the institutional review board to be in accordance with the ethical standards and the Declaration of Helsinki. Diagnosis of precursor B cell and T cell ALL was confirmed by morphologic analysis, immunophenotyping, and genetic features.
Reagents
For flow cytometry, the following unconjugated monoclonal antibodies were used: OX40 BerAct35, OX40L ANC10G1 (cat. no. 355-820 and 400-820, both Ancell Corporation, Bayport, MN), and mouse IgG1 isotype control (cat. no. 557273, BD Biosciences, Heidelberg, Germany). Secondary PE goat anti-mouse was from Dako (cat. no. R0480, BIOZOL, Eching, Germany). Fluorescence conjugates were from BD Biosciences (PE-Cy5 mouse anti-human CD3, cat. no. 555334; APC mouse anti-human CD5, cat. no. 555355; APC mouse anti-human CD34, cat. no. 555824; FITC mouse anti-human CD19, cat. no. 555412; FITC mouse anti-human CD34, cat. no. 555821; FITC mouse anti-human CD56, cat. no. 345811), and BioLegend (San Diego, CA) (PE-Cy7 mouse anti-human CD10, cat. no. 312213; FITC mouse anti-human CD7, cat. no. 343104; FITC mouse anti-human CD15, cat. no. 301904) or eBioscience (ThermoFisher Scientific, Waltham, MA) (APC-eFlour780 mouse anti-human CD19, cat. no. 47-0199-42). 7-Aminoactinomycin D (7-AAD, cat. no. 559925) was from BD Biosciences. For stimulation of OX40 on ALL cells, the agonistic antibody M-OX17 was used [22]. To disrupt OX40/OX40L interactions in cytotoxicity assays, F(ab')2 fragments of the blocking OX40 antibody M-OX2 were applied [23]. Mouse F(ab')2 IgG1 (cat. no. 0115-14, SouthernBiotech, Birmingham, AL) served as control. Recombinant human IL-2 for NK cell generation was obtained from ImmunoTools (Friesoythe, Germany).
Flow Cytometry
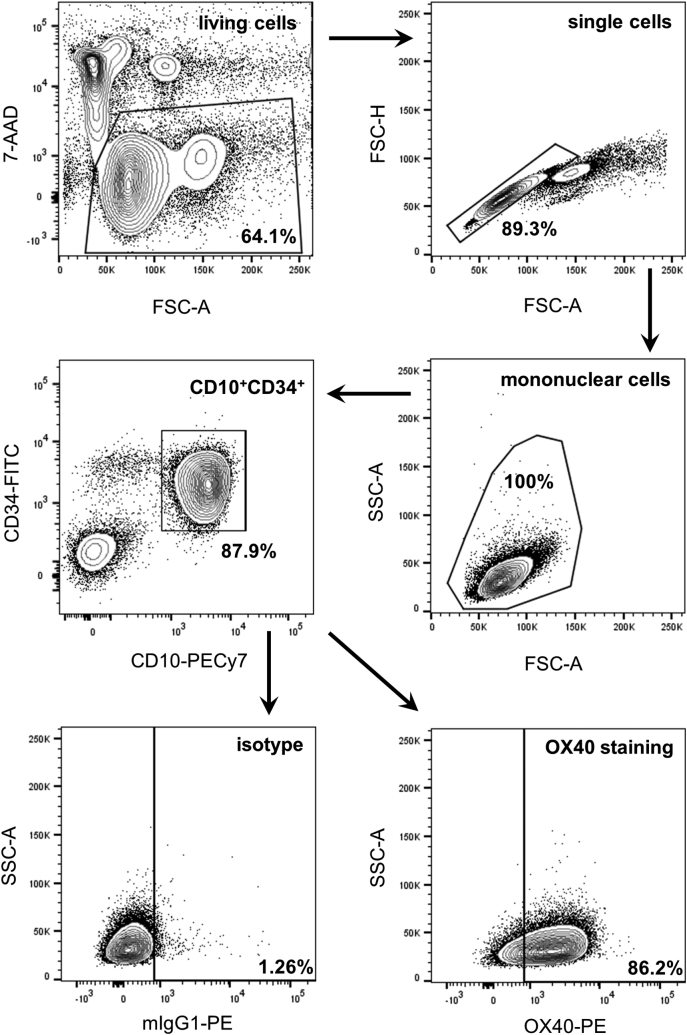
Cells were stained with the respective unconjugated antibody or isotype control (10 μg/ml) followed by PE goat anti-mouse conjugate as secondary reagent (dilution 1:100). Leukemia cells within PBMC of ALL patients were selected according to their prespecified immunophenotype by counterstaining with CD10, CD15, CD19, or CD34 for precursor B cell ALL and with CD5 and CD7 for precursor T cell ALL (Supplementary Figure 1). Analysis was performed using a BD FACSCanto II (BD Biosciences). Dead cells were excluded from analysis by 7-AAD staining (1:200). Specific fluorescence indices (SFIs) were calculated by dividing median fluorescences obtained with specific monoclonal antibodies by median fluorescences obtained with isotype control. Expression was considered positive in case of SFI ≥2.0. Data analysis was performed using FlowJo software (FlowJo LCC, Ashland, OR).
Supplementary Figure 1.
Representative gating strategy for ALL samples.
Note that different leukemic antigens were used for selection of ALL cells by flow cytometry.
PCR
OX40 primers were 5′-TGTAACCTCAGAAGTGGGAGTG-3′ and 5′-GGTCCCTGTCCTCACAGATTG-3′. GAPDH primers were 5′-AGCCACATCGCTCAGACAC-3′ and 5′-GCCCAATACGACCAAATCC-3′. Reverse transcriptase–polymerase chain reaction (RT-PCR) was performed as described previously [24]. PCR products were visualized via gel electrophoresis (1.5% agarose gel).
For quantitative PCR, total RNA was isolated using the High Pure RNA Isolation Kit (Roche, Mannheim, Germany) and transcribed into cDNA using qScript XLT cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD) according to the manufacturer's instructions. Amplification of OX40 cDNA was performed using PerfeCTa SYBR Green FastMix (Quanta Biosciences) on a LightCycler 480 (Roche) instrument. Primer assays (QuantiTect Primer Assay, Qiagen, Hilden, Germany) for OX40 and GAPDH RNA were used according to the manufacturer's instructions. Relative mRNA expression was calculated by the ΔΔ cycle-threshold (Ct) method. BCR-ABL status was quantified with the ipsogen mbcr BCR-ABL1 mbcr CE Kit (Qiagen) according to the manufacturer’s instructions.
Preparation of NK Cells
Polyclonal NK cells were generated by culturing plastic nonadherent PBMC with irradiated K562-mb15-41BBL feeder cells provided from St. Jude Children´s Research Hospital as described previously [25], [26], [27]. Functional experiments were performed when purity of NK cells (CD56+CD3−) was above 80% as determined by flow cytometry.
Determination of Cytokine Levels and Metabolic Activity
For analysis of cytokine production, ALL cells were seeded at a concentration of 2 × 106 cells/ml followed by incubation at 37°C in a humidified atmosphere. Supernatants were collected and stored at −80°C until analysis for cytokine production by two-site sandwich ELISA using commercially available OptEIA kits from BD Pharmingen according to manufacturer’s instructions.
Effects of OX40 stimulation on metabolic activity were determined using the CellTiter-Glo Assay (Promega, Madison, WI). Therefore, ALL cells were seeded in triplicates at a concentration of 5 × 105 cells/ml in 100 μl culture medium followed by incubation at 37°C in a humidified atmosphere. Luminescence was analyzed according to manufacturer’s instructions.
Cytotoxicity Assays
Cytotoxicity of polyclonal NK cells against ALL cell lines was analyzed by BATDA Europium assays after 2 hours as described previously [28]. Percentage of lysis was calculated as follows: 100 × [(experimental release) – (spontaneous release)]/[(maximum release) – (spontaneous release)].
Statistical Analysis
For statistical analysis, GraphPad Prism 7 software (GraphPad, La Jolla, CA) was used. Mean or median values and standard deviation (SD) are shown. The 95% confidence level was used, and P values were calculated with a two-tailed unpaired Mann-Whitney test for not normally distributed data. Survival analyses were performed using SPSS version 24 (IBM, Ehningen, Germany). Overall survival (OS) was calculated using the Kaplan-Meier estimate and defined as the time from diagnosis to death from any cause. If no event occurred, data were censored, and the time from diagnosis or relapse until last patient contact was considered. Patient characteristics were analyzed using Mann-Whitney test, Kruskal-Wallis, or Spearman Rho tests.
Results
OX40 Is Expressed in BCR-ABL–Positive ALL Cell Lines
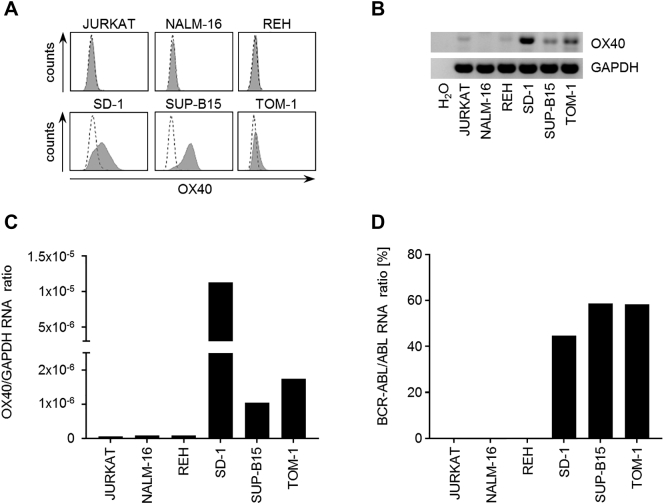
OX40 is expressed in different solid tumors and hematological malignancies and was found in leukemic cells of myeloid and T cell origin [22], [29], but less is known regarding its expression in precursor B cell ALL. As a first step, we analyzed expression of OX40 in different ALL cell lines. Flow cytometry revealed a variable extent of OX40 cell surface expression. Interestingly, only ALL cell lines carrying a minor breakpoint BCR-ABL fusion gene like SD-1, SUP-B15, and TOM-1 displayed an elevated OX40 expression, whereas the BCR-ABL–negative precursor B cell ALL cell lines NALM-16 and REH as well as the precursor T cell ALL cell line JURKAT showed no relevant surface expression (Figure 1A). Corresponding results were obtained when OX40 mRNA levels were investigated by PCR analysis (Figure 1B). While quantitative PCR revealed higher OX40 mRNA levels in surface-positive compared to surface-negative ALL cell lines, the presence of mRNA in the latter indicates that OX40 expression may also be regulated posttranscriptionally. Interestingly, surface expression did not directly correlate with OX40 mRNA amount (Figure 1C) or BCR-ABL quantity (Figure 1D).
Figure 1.
mRNA and protein expression of OX40 in ALL cell lines.
(A) OX40 surface expression of six different ALL cell lines was investigated by flow cytometry using the monoclonal OX40 antibody BerAct35 (shaded peaks) and mouse IgG1 isotype serving as control (open peaks). Representative results of at least three independent experiments with similar results are shown.
(B) OX40 mRNA expression of six different ALL cell lines as determined by RT-PCR with GAPDH and H2O serving as controls. PCR products were visualized by agarose gel electrophoresis.
(C) Quantitative RT-PCR analysis of OX40 mRNA relative to GAPDH expression in six different ALL cell lines.
(D) For quantitative detection of BCR-ABL mbcr e1a2 transcripts relative to ABL control gene expression ALL cell lines were analyzed by quantitative RT-PCR.
OX40 Is Expressed in Primary ALL Cells
Next, primary ALL cells from a total of 44 patients with proven precursor B and T cell ALL were analyzed for OX40 expression. Leukemic cells were classified at the time of diagnosis using the immunological classification proposed by the European Group for the Immunological Characterization of Leukemias [30]. Detailed patient characteristics and individual OX40 expression levels are listed in Table 1.
Table 1.
Clinical Characteristics and OX40 Surface Expression
| BCR-ABL | ALL type | Risk | Age | Sex | WBC [G/l] | Hb [g/dl] | Plt [G/l] | Karyotype | PB % | BM % | Relapse | SFI OX40 | % OX40+ cells |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | pro B | HR | 63 | m | 39.97 | 10.1 | 125 | 46,XY | 96 | 96 | - | 1.3 | 3.6 |
| − | pro B | HR | 36 | f | 51.99 | 12.4 | 247 | 48,XX,+Xor+7,t(4;11)(q21:q23),+der(4)t(4;11)(q21;q23),+22/46,XX | 79 | 80 | - | 1.0 | 1.1 |
| − | common | HR | 21 | m | 28.27 | 11.7 | 280 | NA | 92 | ND | - | 1.1 | 0.7 |
| − | common | HR | 31 | f | 14.23 | 5.4 | 80 | NA | 68 | 79 | - | 2.9 | 60.3 |
| − | common | HR | 66 | m | 300.95 | 13.3 | 121 | NA | 88 | ND | - | 1.0 | 0.8 |
| − | common | HR | 39 | m | 33.50 | 13.3 | 65 | 47,XY,+21 | 61 | 88 | - | 2.9 | 21.7 |
| − | common | SR | 20 | f | 11.50 | 7.1 | 33 | 46,XX | 60 | 91 | - | 1.4 | 1.7 |
| − | common | SR | 19 | m | 4.99 | 5.8 | 110 | 46,XY,ish del(9)(p21p21)/46;XY | 47 | 59 | - | 1.3 | 0.8 |
| − | common | SR | 61 | m | 7.87 | 8.5 | 21 | 46,XY | 54 | 44 | - | 1.4 | 1.5 |
| − | common | SR | 20 | m | 44.53 | 10.0 | 153 | NA | 88 | ND | - | 7.5 | 86.2 |
| − | common | SR | 61 | f | 1.80 | 9.1 | 7 | NA | 59 | 98 | - | 1.2 | 4.8 |
| − | common | HR | 36 | m | 33.59 | 5.0 | 11 | 46,XY | 92 | 95 | - | 1.6 | 3.5 |
| − | common | HR | 24 | m | 38.63 | 7.3 | 76 | NA | 99 | 100 | - | 1.1 | 1.4 |
| − | common | SR | 33 | m | 13.50 | 13.0 | 136 | 46,XY | 50 | 100 | - | 1.0 | 2.1 |
| − | pre B | HR | 61 | f | 123.81 | 8.0 | 40 | NA | 98 | 97 | - | 1.3 | 1.4 |
| − | pre B | HR | 22 | m | 74.14 | 13.2 | 13 | 46,XY | 95 | 97 | - | 4.6 | 27.9 |
| − | pre B | SR | 41 | m | 11.67 | 9.1 | 36 | 46,XY | 80 | 50 | - | 1.6 | 22.3 |
| − | pre B | SR | 45 | m | 10.55 | 9.4 | 21 | 46,XY | 66 | 98 | - | 1.0 | 0.9 |
| − | pre B | SR | 24 | f | 29.22 | 10.3 | 33 | 46,XX | 86 | 97 | - | 1.2 | 0.9 |
| − | cortical | SR | 20 | f | 54.08 | 13.4 | 288 | 46,XX | 24 | 70 | - | 1.8 | 25.0 |
| − | cortical | SR | 20 | m | 98.62 | 11.3 | 76 | NA | 63 | 65 | relapse | 1.0 | 11.2 |
| − | cortical | SR | 45 | m | 224.80 | 8.2 | 46 | 46,XY | 95 | 94 | - | 1.4 | 2.1 |
| − | cortical | SR | 69 | m | 37.71 | 10.7 | 119 | 46,XY | 70 | 72 | - | 1.1 | 1.4 |
| − | cortical | SR | 29 | m | 355.70 | 10.5 | 81 | 46,XY,ish del(1)(p32p32)(TAL1,SIL−,3’ of TAL1+),del(9)(p21p21)(p16−)/46, XY | 82 | 64 | relapse | 1.1 | 6.2 |
| − | cortical | SR | 32 | m | 93.31 | 12.5 | 57 | 46,XY | 93 | 95 | - | 2.3 | 8.8 |
| − | γ/δ | HR | 38 | m | 244.20 | 15.0 | 204 | 46,XY | 84 | 93 | - | 12.1 | 48.7 |
| + | pro B | VHR | 74 | f | 20.86 | 9.0 | 22 | 46,XX,t(9;22)(q34;q11)/45,idem,−21/46,XX | 72 | 97 | - | 2.3 | 38.0 |
| + | common | VHR | 64 | m | 30.01 | 13.6 | 141 | 46,XY,t(9;22)(q34;p11)/46,XY | 57 | ND | - | 3.6 | 48.6 |
| + | common | VHR | 81 | f | 114.17 | 8.8 | 35 | 46,XX | 97 | ND | - | 50.7 | 87.8 |
| + | common | VHR | 38 | m | 13.81 | 4.8 | 30 | 46,XY,t(9;22)(q34;q11)/46,XY | 65 | 97 | - | 5.7 | 48.0 |
| + | common | VHR | 45 | m | 10.34 | 9.3 | 64 | 46,XY,t(9;22)(q34;q11)/45,idem,−20/46,XY | 59 | 94 | - | 7.1 | 84.6 |
| + | common | VHR | 25 | m | 4.69 | 4.8 | 15 | 46,XY | 60 | 81 | - | 2.4 | 21.0 |
| + | common | VHR | 61 | f | 172.76 | 7.7 | 50 | 46,XX,?t(9;22)(q34;q11) | 80 | ND | - | 14.2 | 45.7 |
| + | common | VHR | 21 | m | 463.01 | 11.6 | 31 | NA | 87 | 93 | - | 1.3 | 1.4 |
| + | common | VHR | 49 | f | 56.81 | 10.3 | 48 | 46,XX | 92 | 94 | - | 10.8 | 22.3 |
| + | common | VHR | 32 | f | 19.37 | 7.2 | 170 | NA | 56 | 98 | - | 2.5 | 12.4 |
| + | common | VHR | 31 | m | 57.83 | 13.4 | 270 | 46,XY,t(9;22)(q34;q11)/46,XY | 18 | 37 | - | 2.0 | 16.7 |
| + | common | VHR | 50 | f | 364.80 | 8.3 | 30 | 48,XX,t(9;22)(q34;q11),+der(22)t(9;22)(q34;q11),+C,?inc | 97 | ND | - | 2.3 | 10.4 |
| + | common | VHR | 68 | m | 29.00 | 13.1 | 28 | 45,XY,−7,t(9;22)(q34;q11,2),del(11)(p10),+2~3mar,inc/46,XY | 79 | 83 | - | 6.0 | 37.9 |
| + | common | VHR | 23 | m | 41.39 | 12.9 | 124 | NA | 64 | ND | relapse | 1.8 | 38.1 |
| + | common | VHR | 76 | m | 68.75 | 9.6 | 20 | 47,XY,t(2;16)(p11;p11),+der(8)t(8;8)(p23;q23),der(8)t(8;8)(p23;q23),t(9;22)(q34;q11)/47,XY,t(2;16)(p11;p11),+der(8)t(8;8)(p23;q23),der(8)t(8;8)(p23;q23),t(9;22)(q34;q11)/47,XY,t(2;16)(p11;p11),+der(8)t(8;8)(p23;q23),der(8)t(8;8)(p23;q23),t(9;22)(q34;q11)[8];/48,XY,+X,t(2;16)(p11;p11)der(8)t(8;8)(p23;q23),t(9;22)(q34;q11)+der(22)t(9;22)(q34;q11)/46,XY | 82 | ND | - | 75.5 | 71.7 |
| + | pre B | VHR | 54 | f | 7.35 | 7.2 | 111 | 46,XX,t(9;22)(q34;q11)/46,XX | 28 | ND | - | 4.2 | 28.7 |
| + | pre B | VHR | 31 | f | 11.72 | 10.6 | 111 | NA | ND | 94 | - | 3.8 | 34.1 |
| + | pre B | VHR | 61 | f | 187.40 | 13.3 | 204 | 46,XX,t{4;9)(q35;q12-13)?c,t{9;22)(q34;q11) | 80 | 66 | - | 5.1 | 46.3 |
Abbreviations: BCR, breakpoint cluster region; ABL, Abelson murine leukemia viral oncogene homolog 1; −, negative; +, positive; SR, standard risk; HR, high risk; VHR, very high risk; f, female; m, male; WBC, white blood count; G/l, Giga per liter; Hb, hemoglobin; g/dl, gram per deciliter; Plt, platelets; PB, peripheral blood blasts among nucleated cells; BM, bone marrow blasts; NA, not available; ND, not determined; SFI, specific fluorescence index.
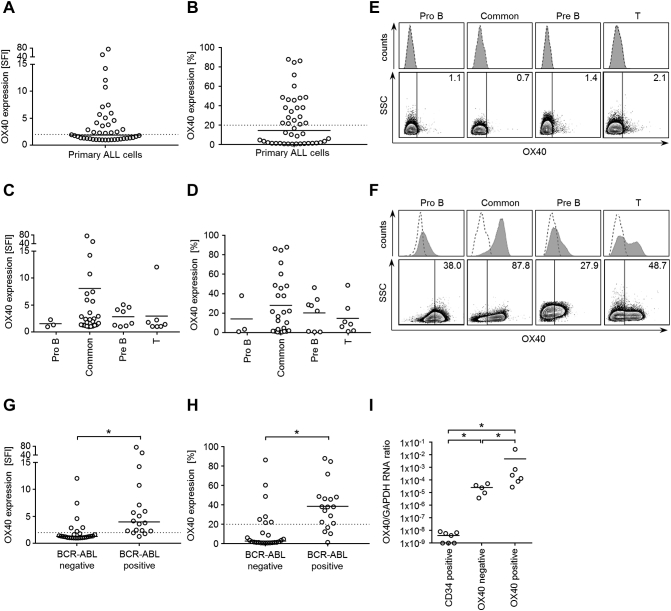
Leukemic cells within mononuclear cells of patients were identified using different surface marker combinations based on immunophenotyping results at initial diagnosis or relapse. Substantial surface expression of OX40 (SFI ≥2.0) was detected in 48% of cases (n=21) with a median expression of 1.9 (SFI range, 1.0-75.5) (Figure 2A).
Figure 2.
Primary ALL cells express OX40 on mRNA and protein level.
(A and B) Immunophenotypic OX40 expression on ALL patient samples using the monoclonal OX40 antibody BerAct35 or the respective isotype control followed by secondary anti-mouse PE after selection with the predetermined immunophenotype. Combined SFI levels (A) and percentage of OX40-positive cells (B) are shown. Patients with SFI levels ≥2.0 or at least 20% OX40-expressing cells were considered positive (cutoff values are indicated as dotted lines). Horizontal bars represent median of results.
(C and D) OX40 expression [SFI levels (C); % positive cells (D)] according to ALL subtype. Horizontal bars represent mean.
(E and F) PBMC from ALL patients were analyzed by flow cytometry using the monoclonal OX40 antibody BerAct35 (shaded peaks) and isotype control (open peaks) followed by anti-mouse PE. Exemplary histograms (upper panels) and dot plots (lower panels) of OX40-negative (E) and OX40-positive (F) ALL cells of different subtypes are shown.
(G and H) Combined SFI levels (G) and combined results showing % OX40 positivity (H) are depicted for BCR-ABL–negative and –positive ALL cells. Horizontal bars represent median of results in each group.
(I) Quantitative RT-PCR analysis of OX40 mRNA expression of CD34-enriched bone marrow cells from seven different healthy donors and from five different OX40 surface-negative and six different-positive ALL patients. GAPDH served as control. Horizontal bars represent the mean of the results in each group.
*Statistically significant differences, P<.05.
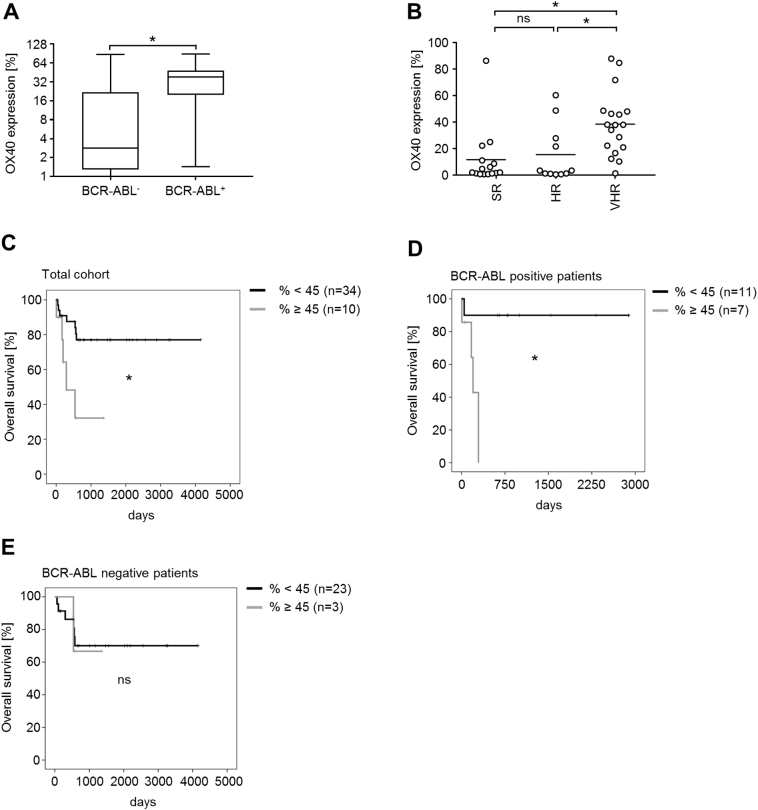
When analyzing the percentage of OX40-positive cells among leukemic cells of each sample, 61% of patients (n=27) showed at least 5% OX40-positive cells with a median expression of 14.6% (range, 0.7-87.8). In 48% of samples (n=21), an OX40 expression above 20% could be detected (Figure 2B). When analyzing different ALL subtypes, no statistically significant differences were observed (Figure 2, C-F). However, significantly differing OX40 expression levels were detected when primary ALL samples were stratified according to BCR-ABL status with positivity being significantly associated with higher OX40 expression, both with regard to SFI levels (P<.0001, Mann-Whitney test) (Figure 2G) and percentage of positive cells (P<.0001, Mann-Whitney test) (Figure 2H).
When OX40 expression in ALL was studied on mRNA level, OX40 amplicons were observed in all analyzed surface-positive but also in surface-negative ALL samples (Figure 2I), which, like the results obtained with cell lines, points to posttranscriptional regulation of OX40 expression. Quantification of OX40 mRNA levels in ALL samples and healthy CD34-positive bone marrow (BM) cells showed significantly higher mRNA levels in surface-positive compared to surface-negative samples (P=.02, Mann-Whitney test) or healthy CD34-positive BM cells (P=.001, Mann-Whitney test).
OX40 Stimulation Promotes Metabolic Activity and Induces Cytokine Release of Primary ALL Cells
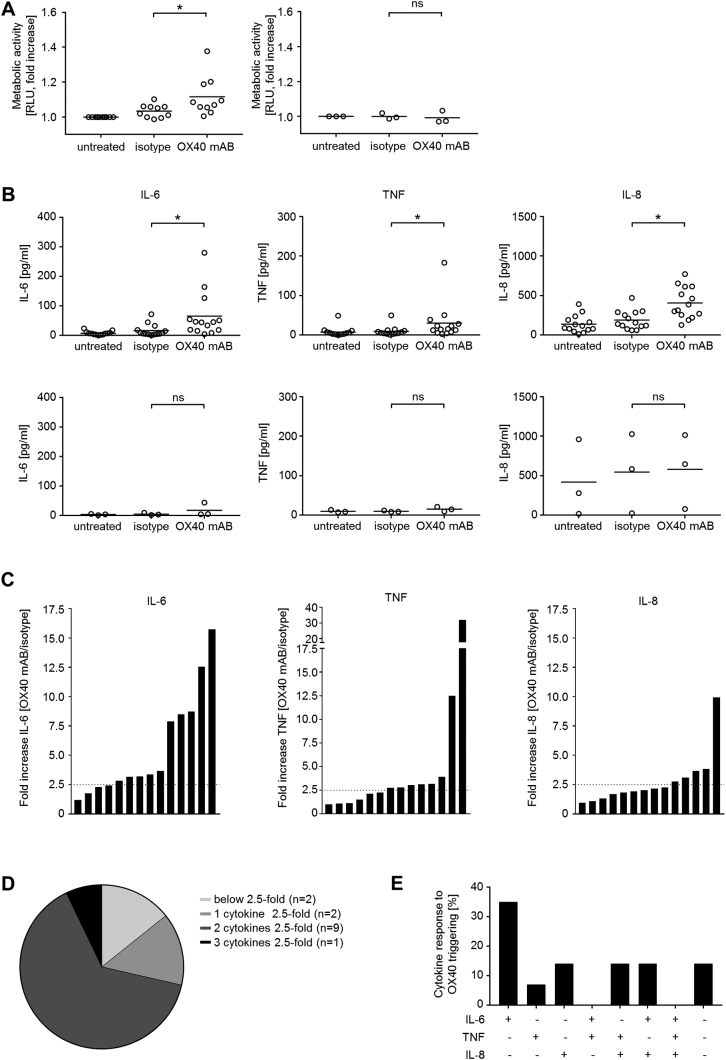
In order to examine if OX40 plays a functional role in ALL cells, an agonistic antibody recently described to stimulate OX40 was employed [22]. It induced a significant increase of metabolic activity as an indicator of cell proliferation and viability in OX40-positive primary ALL samples (n=10, P=.04, Mann-Whitney test), while metabolic activity remained unaltered in OX40 surface-negative samples (n=3, P=.75) (Figure 3A). Notably, interindividual differences concerning metabolic activity of ALL cells upon OX40 stimulation were detected, but no association of OX40 expression and change in metabolic activity was observed (Supplementary Figure 2).
Figure 3.
OX40 signaling induces stimulation of metabolic activity and cytokine release in ALL cells.
PBMC of ALL patients were either cultured alone, on immobilized M-OX17 monoclonal antibody or human IgG isotype control (10 μg/ml).
(A) Metabolic activity of ALL cells was determined by CellTiter Glo assay after 24 hours. Results of OX40-positive (n=10) and OX40-negative samples (n=3) are shown. For normalization, results obtained with untreated ALL cells were set to 1. Horizontal bars represent mean.
(B) Cytokine levels of IL-6 (after 24 hours), TNF, and IL-8 (both after 6 hours) in culture supernatants were determined by ELISA. Results obtained with OX40-positive (n=14, upper panel) and OX40-negative ALL samples (n=3, lower panel) are shown. Horizontal bars represent mean.
(C) Fold increase (OX40/isotype) in cytokine production (IL-6, TNF, and IL-8) of ALL cells after OX40 stimulation. Each patient is shown as individual bar. Dotted lines represent a 2.5-fold increase in cytokine production.
(D) Quantification of cytokine release by ALL cells after OX40 stimulation. Numbers of patients showing fold increase <2.5 (light gray), ≥2.5-fold increase for one cytokine (gray), two cytokines (dark grey) or three cytokines (black) are displayed.
(E) Analysis of results obtained with 14 OX40-positive patient samples with regard to release of specific cytokine combinations. Positive response (+) was defined as ≥2.5-fold increase of each individual cytokine upon OX40 signaling. The percentage of samples responding with the indicated cytokine pattern is depicted.
*Statistically significant differences, P<.05. ns, not significant.
Supplementary Figure 2.
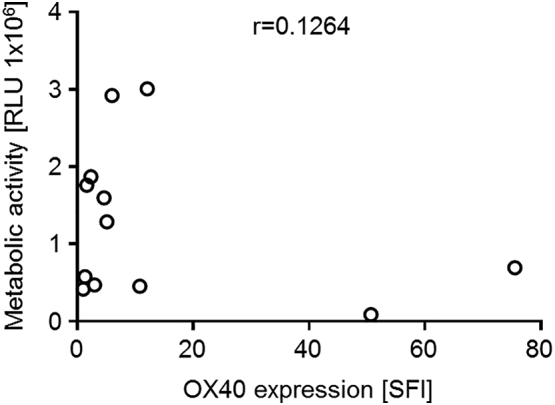
Basal metabolic activity of ALL cells does not correlate with OX40 expression intensity. PBMC of ALL patients were cultured for 24 hours before metabolic activity was measured using CellTiter-Glo assay. Metabolic activity and corresponding OX40 surface expression levels (SFI) are shown for individual patient samples. Correlation coefficient r was calculated using GraphPad Prism 7 software.
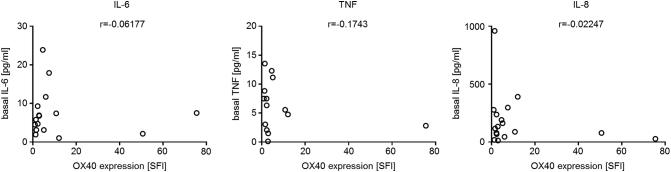
Antibody-mediated stimulation of OX40 also led to a significant induction of IL-6, TNF, and IL-8 production (P=.003, P=.009, and P=.002; Mann-Whitney test) by OX40 surface-positive ALL cells (n=14), while no significant effect was observed in OX40 surface-negative ALL samples (IL-6, P=.4; TNF, P=.2; IL-8, P>.99; Mann-Whitney test) (Figure 3B). Again, substantial interindividual differences regarding cytokine release of ALL cells upon OX40 signaling were detected (Figure 3C). Notably, no association of OX40 surface expression and basal release of the cytokines was observed (Supplementary Figure 3). In the majority of investigated cases, OX40 stimulation led to an at least 2.5-fold increase in secretion of cytokines, whereas only in two cases could this not be detected in OX40 surface-positive ALL samples (Figure 3, D and E).
Supplementary Figure 3.
Basal cytokine release of ALL cells does not correlate with OX40 expression intensity. PBMC of ALL patients were cultured for 24 (IL-6) or 6 hours (TNF and IL-8) before cytokine levels in culture supernatant were determined by ELISA. Release of IL-6 (left), TNF (middle), or IL-8 (right) and corresponding OX40 surface expression levels (SFI) are shown for individual patient samples. Correlation coefficient r was calculated using GraphPad Prism 7 software.
OX40/OX40L Interaction Enhances NK Cell Cytotoxicity Against ALL Cells
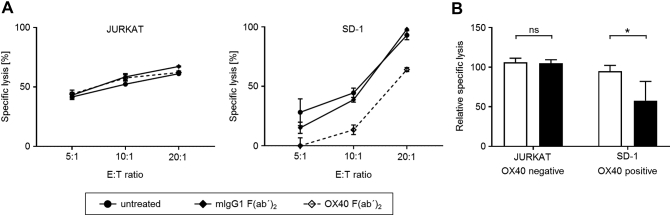
Recently, interaction between OX40 on AML cells and OX40L expressed by NK cells was reported to enhance NK cytotoxicity. Blockade of OX40/OX40L interaction was to shown to impair polyclonal NK cell reactivity against leukemic cells [22]. In order to investigate and confirm such consequences of OX40/OX40L interaction with ALL cells, the OX40-negative cell line JURKAT and the OX40-positive cell line SD-1 were employed in cocultures with OX40L-expressing polyclonal NK cells using nonstimulatory OX40 F(ab’)2-fragments [23] to disrupt OX40/OX40L interaction. Blockade of OX40/OX40L signaling significantly (P=.008, Mann-Whitney test) decreased lysis of OX40 surface-positive ALL cell lines, while no effect on lysis was detected employing surface-negative ALL cell lines (P=.97, Mann-Whitney test) (Figure 4, A and B).
Figure 4.
OX40/OX40L interaction increases NK cell cytotoxicity in response to ALL cells.
(A and B) OX40L-positive polyclonal NK cells were cultured for 2 hours with OX40-negative JURKAT (left) or OX40-positive SD-1 ALL cells (right) in the absence or presence of blocking OX40 F(ab')2 or isotype control (2 μg/ml each). NK cell reactivity was evaluated by time-resolved fluorometric assays. (A) Exemplary results representing means of triplicates are shown. (B) Combined data of five independent experiments for each ALL cell line at an E:T ratio of 10:1 (control: white bars, OX40 blockade: black bars). Mean values and SD are depicted.
*Statistically significant differences, P<.05.
OX40 Expression Is Associated with Clinical Characteristics in ALL
Next, we set out to study whether OX40 expression was associated with clinical characteristics in our patient cohort. The median age at time of diagnosis was 38 years (range, 19-81), 30% of patients were 55 years or older, and 64% were male. Median white blood cell (WBC) count was 38.17 G/l (range, 1.80-463.01) (Table 1).
When we grouped ALL cases into subtypes, we did not observe significant association with OX40 expression (see Figure 2, C and D), but OX40 expression was significantly associated with BCR-ABL status (Figure 5A). Whereas BCR-ABL–negative ALL cells had a median OX40 expression of 1.3 which is below the threshold we defined for surface positivity (range, 1.0-12.1), median OX40 expression was 4.0 (range, 1.3-75.5) in BCR-ABL–positive cases. Furthermore, OX40 expression was significantly associated with very high risk (VHR) status in ALL, while no association was found for high-risk (HR) or standard-risk (SR) patients [%: SR vs. VHR, P=.005; HR vs. VHR, P=.03; SR vs. HR, P>.99; SFI: SR vs. VHR, P=.02; HR vs. VHR; P=.03; SR vs. HR: P>.99 (data not shown); both Kruskal-Wallis test] (Figure 5B). Higher OX40 levels defined as surface expression ≥45% were also associated with significantly shorter overall survival [P=.007 (%), log-rank Mantel-Cox] (Figure 5C). Interestingly, when subgrouped according to BCR-ABL status, higher OX40 expression in BCR-ABL–positive patients also resulted in significantly shorter overall survival (P=.009, log-rank Mantel-Cox) (Figure 5D). This effect could not be observed in the BCR-ABL–negative subgroup, where only three patients showed an OX40 value of ≥45% (P=.843, log-rank Mantel-Cox). Higher OX40 levels defined as a cutoff value of SFI ≥5 were also associated with significantly shorter overall survival in the total cohort (P=.02, log-rank Mantel-Cox, data not shown). No association was identified with other genetic abnormalities, disease etiology, clinical parameters such as WBC count, or treatment response.
Figure 5.
OX40 positivity is associated with high-risk features and poor survival of ALL patients.
(A) Association of OX40 expression showing % positive cells and BCR-ABL status of ALL patients. Horizontal bars indicate median expression for BCR-ABL–negative and BCR-ABL–positive patients; whiskers indicate maximal and minimal expression levels.
(B) Patients were divided into subgroups according to risk stratification. Association of OX40 expression (% positive cells) and disease risk is depicted.
(C) Correlation of OX40 expression (% positive cells) with overall survival in days was calculated using predictive cutoff values for OX40 expression. Patients were divided into two groups. Black curve: OX40 expression above cutoff value (% ≥45), gray curve: OX40 expression below cutoff value (% <45).
(D and E) Correlations of OX40 expression (% positive cells) and overall survival of patients with BCR-ABL–positive ALL (D) and BCR-ABL–negative ALL (E) were calculated as described in (C).
*Statistically significant differences, P<.05. ns, not significant.
Discussion
During the last decades, OX40 has gained increasing attention due to its function as a potent positive regulatory surface molecule on T cells that is able to reestablish T cell antitumor reactivity [10]. Activation of this stimulatory immune checkpoint leads to sustained T cell proliferation and survival, rendering OX40 a popular therapeutic target for immune-based elimination of tumor cells. The ability of OX40 to potently stimulate both CD4+ and CD8+ T cells makes it a suitable candidate for cancer immunotherapy. Various OX40 targeting approaches were developed in the last two decades [31], and several strategies are being evaluated in clinical trials [10].
In contrast to its use in chimeric antigen receptor T cells, where the costimulatory signaling domain of OX40 is frequently incorporated into chimeric antigen receptors to specifically enhance function of transfected T cells, systemic application of monoclonal antibodies targeting OX40 might also affect other OX40-bearing cells of the immune system or nonimmune cells.
The consequences of OX40 expression on malignant cells and its potential role in pathophysiology and prognosis have even less been taken into account and only investigated in a few studies to our knowledge so far.
Imura et al. demonstrated that 15 out of 17 adult T cell leukemia cases, a peripheral T cell neoplasm associated with infection by the human T-lymphotropic virus type I, expressed significant levels of OX40. While OX40 mRNA was detected by RT-PCR in all tested patient samples, OX40 protein expression could be identified by Western blot in only two out of five adult T cell leukemia cases. The precursor T ALL cell line JURKAT that served as control was negative in both analyses [32].
Immunophenotypic analyses of different lymphoid malignancies by Koubek et al. showed relevant OX40 surface expression on malignant cells from patients with precursor T cell but not B cell ALL. Six of seven analyzed precursor T cell ALL cases expressed OX40 with a median of 8% (range, 4-31), whereas all eight analyzed precursor B cell ALL cases exhibited only very low levels of OX40 not exceeding 4% (median 1; range, 1-4) [33]. Furthermore, in this study, one of two T cell non-Hodgkin lymphoma (NHL) cases expressed OX40 >5%. B cell chronic lymphocytic leukemia, B NHL, prolymphocytic leukemia with B cell markers, and plasmocytoma cells expressed very low levels of OX40 which did not exceed 3%.
In our study, flow cytometry analyses of OX40 surface expression in a cohort of 44 precursor B and T cell ALL patients revealed that 61% exhibited at least 5% OX40-positive cells with a median expression of 14.6% (range, 0.7-87.8) and in 48%, an OX40 expression above 20% could be detected.
In line with previous studies, five of seven analyzed precursor T cell ALL samples expressed substantial amounts of OX40 in the majority of cases (median 8.8%, range 1.4-48.7). In contrast to earlier reports, in our cohort, OX40 surface expression was also observed for precursor B cell ALL with a median expression of 21.0% (range, 0.7-87.8). The observed discrepancy may be due to the higher number of analyzed cases, the heterogeneity among primary samples, and the use of different OX40 antibodies in our analyses.
Interestingly, also ALL samples without relevant surface expression showed OX40 mRNA expression. One explanation for this observation could be the contamination with OX40-expressing healthy cells that may have influenced the respective PCR results, but also regulatory or mutational blockade of surface expression by posttranscriptional or posttranslational mechanisms. This may comprise, as reported for many other TNF/TNFR members, cell surface shedding and release in soluble form, which is supported by reports on the presence of soluble OX40 in sera of patients with malignant and autoimmune diseases [34], [35], [36], [37].
To our knowledge, expression of OX40 in the context of hematological malignancies has further up to now only been reported for AML [22]. Our group recently reported OX40 expression on the surface of primary AML samples in 54% of cases and could show that OX40 stimulation led to enhanced proliferation and release of proleukemic cytokines. When we performed functional analyses using well-characterized monoclonal antibodies with defined specificity and agonistic property, we could show that OX40 signaling results in release of cytokines that act as autocrine/paracrine growth and survival factors also in ALL. Furthermore, like in T cells, OX40 signaling enhanced viability/metabolic activity in a substantial proportion of ALL cases. It seems thus possible that OX40 confers a survival benefit for leukemic cells, e.g., upon interaction with OX40L bearing immune or bystander cells. This is in line with increasing evidence regarding the important role of the immune and stromal microenvironment in malignancies [38].
Regarding prognostic impact of OX40 expression, Xie et al. comparatively analyzed immunohistochemistry and RNA sequencing data to assess the biologic relevance of OX40 in hepatocellular carcinoma and could detect higher OX40 expression in malignant cells in comparison to adjacent healthy liver tissue. OX40 expression was associated with elevated serum alpha-fetoprotein level, vascular invasion, and shorter survival [39].
When assessing the biological relevance of OX40 expression in ALL, we grouped ALL subtypes according to risk group. Risk stratification is based on clinical and biological prognostic factors like ALL-subtype, high WBC count at diagnosis, or late achievement of complete remission [40]. Patients with translocation t(9;22) or the corresponding molecular aberration BCR-ABL are defined as a separate group because they are treated with tyrosine kinase inhibitors as causal therapy.
Evaluation of these prognostic factors is crucial at initial diagnosis for the development of a suitable treatment strategy governing intensity of treatment. With our study, we identify OX40 as a negative prognostic marker and show its association with BCR-ABL status. Additionally, we demonstrated that disruption of OX40/OX40L interaction between ALL cells and polyclonal NK cells resulted in diminished lysis rates, implying that OX40-targeting approaches should be considered carefully.
Moreover, our findings support our above-mentioned line of argument regarding potential unexpected consequences of a therapeutic application of agonistic OX40 monoclonal antibodies, and it is noteworthy that other investigators reported OX40 expression (without analyzing functionality) on cancer cells of various origins beyond ALL [41]. Another layer of complexity when applying OX40 monoclonal antibodies is added by the issue of whether and how antibody binding to OX40 affects interaction with cells that express its cognate ligand. This is of particular relevance because OX40/OX40L interaction can lead to transduction of bidirectional signals, e.g., into the receptor and the ligand-bearing cell, a characteristic feature of many ligands of the TNF family [42], [43]. Besides healthy tissues like endothelial cells, antigen-presenting cells including B cells, and monocytes/dendritic cells express OX40L. Various cellular functions of these cells are affected by OX40L "reverse signaling" [43], [44], [45], [46], which may also occur upon their interaction with OX40-expressing ALL cells.
Thus, additional work is warranted to fully unravel the complex role of the OX40/OX40L molecule system, which in turn may help to fully exploit the potential of OX40 stimulation for cancer immunotherapy.
The following are the supplementary data related to this article.
Author Contributions
K. R. and I. H. designed and performed the experiments, analyzed and interpreted data, and wrote the manuscript. M. R. collected patient samples and provided clinical data. G. B. performed bioinformatic analyses. M. H. and G. J. provided critical reagents. T. N. contributed to the study design, performed experiments, and provided important advice. H. S. contributed to the study design, critically revised the manuscript, and supervised the study. D. D. collected clinical samples and provided patient data, contributed to the study design, wrote the manuscript as lead author, and supervised the study. All authors critically reviewed the manuscript and approved the final version.
Conflict of Interest Disclosure
The authors declare no competing financial interests.
Note on Previous Publication
Data of this manuscript have in part been orally presented at the Annual Meeting of the German, Austrian and Swiss Societies for Hematology and Oncology, DGHO, 2017 September 29-October 03, Stuttgart, Germany.
Acknowledgements
We thank Melanie Kraft and Didem Ünal for excellent technical assistance and Frank Grünebach for providing important advice. Flow cytometry sample acquisition was done on shared instruments of the Flow Cytometry Core Facility Tübingen.
Footnotes
Funding: This work was supported by grants of Deutsche Krebshilfe, Germany (70112914, 111828), DFG, Germany (SA1360/7-3, NU341/1-1) and Wilhelm Sander Stiftung, Germany (2007.115.3).
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56–61. doi: 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 3.Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol. 2015;33(17):1974–1982. doi: 10.1200/JCO.2014.59.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robert C, Thomas L, Bondarenko I, O'Day S, Weber J, Garbe C. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 6.Larkin J, Lao CD, Urba WJ, McDermott DF, Horak C, Jiang J. Efficacy and safety of nivolumab in patients with BRAF V600 mutant and BRAF wild-type advanced melanoma: a pooled analysis of 4 clinical trials. JAMA Oncol. 2015;1(4):433–440. doi: 10.1001/jamaoncol.2015.1184. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Ribas A, Wolchok JD, Hodi FS, Hamid O, Kefford R. Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet. 2014;384(9948):1109–1117. doi: 10.1016/S0140-6736(14)60958-2. [DOI] [PubMed] [Google Scholar]
- 8.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes—a molecule related to nerve growth factor receptor. EMBO J. 1990;9(4):1063–1068. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4(6):420–431. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 10.Buchan SL, Rogel A, Al-Shamkhani A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood. 2018;131(1):39–48. doi: 10.1182/blood-2017-07-741025. [DOI] [PubMed] [Google Scholar]
- 11.Croft M. Control of immunity by the TNFR-related molecule OX40 (CD134) Annu Rev Immunol. 2010;28:57–78. doi: 10.1146/annurev-immunol-030409-101243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134) Am J Surg. 2002;183(5):512–518. doi: 10.1016/s0002-9610(02)00831-0. [DOI] [PubMed] [Google Scholar]
- 13.Ladanyi A, Somlai B, Gilde K, Fejos Z, Gaudi I, Timar J. T-cell activation marker expression on tumor-infiltrating lymphocytes as prognostic factor in cutaneous malignant melanoma. Clin Cancer Res. 2004;10(2):521–530. doi: 10.1158/1078-0432.ccr-1161-03. [DOI] [PubMed] [Google Scholar]
- 14.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205(4):825–839. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ali SA, Ahmad M, Lynam J, McLean CS, Entwisle C, Loudon P. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine. 2004;22(27-28):3585–3594. doi: 10.1016/j.vaccine.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 16.Lee SJ, Myers L, Muralimohan G, Dai J, Qiao Y, Li Z. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173(5):3002–3012. doi: 10.4049/jimmunol.173.5.3002. [DOI] [PubMed] [Google Scholar]
- 17.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68(13):5206–5215. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 18.Gough MJ, Crittenden MR, Sarff M, Pang P, Seung SK, Vetto JT. Adjuvant therapy with agonistic antibodies to CD134 (OX40) increases local control after surgical or radiation therapy of cancer in mice. J Immunother. 2010;33(8):798–809. doi: 10.1097/CJI.0b013e3181ee7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linch SN, McNamara MJ, Redmond WL. OX40 agonists and combination immunotherapy: putting the pedal to the metal. Front Oncol. 2015;5:34. doi: 10.3389/fonc.2015.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73(24):7189–7198. doi: 10.1158/0008-5472.CAN-12-4174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173(6):3716–3724. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 22.Nuebling T, Schumacher CE, Hofmann M, Hagelstein I, Schmiedel BJ, Maurer S. The immune checkpoint modulator OX40 and its ligand OX40L in NK-cell immunosurveillance and acute myeloid leukemia. Cancer Immunol Res. 2018;6(2):209–221. doi: 10.1158/2326-6066.CIR-17-0212. [DOI] [PubMed] [Google Scholar]
- 23.Jung G, Freimann U, Von Marschall Z, Reisfeld RA, Wilmanns W. Target cell-induced T cell activation with bi- and trispecific antibody fragments. Eur J Immunol. 1991;21(10):2431–2435. doi: 10.1002/eji.1830211020. [DOI] [PubMed] [Google Scholar]
- 24.Schmiedel BJ, Arelin V, Gruenebach F, Krusch M, Schmidt SM, Salih HR. Azacytidine impairs NK cell reactivity while decitabine augments NK cell responsiveness toward stimulation. Int J Cancer. 2011;128(12):2911–2922. doi: 10.1002/ijc.25635. [DOI] [PubMed] [Google Scholar]
- 25.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res. 2009;69(9):4010–4017. doi: 10.1158/0008-5472.CAN-08-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baltz KM, Krusch M, Bringmann A, Brossart P, Mayer F, Kloss M. Cancer immunoediting by GITR (glucocorticoid-induced TNF-related protein) ligand in humans: NK cell/tumor cell interactions. FASEB J. 2007;21(10):2442–2454. doi: 10.1096/fj.06-7724com. [DOI] [PubMed] [Google Scholar]
- 27.Kropp KN, Maurer S, Rothfelder K, Schmied BJ, Clar KL, Schmidt M. The novel deubiquitinase inhibitor b-AP15 induces direct and NK cell-mediated antitumor effects in human mantle cell lymphoma. Cancer Immunol Immunother. 2018;67(6):935–947. doi: 10.1007/s00262-018-2151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koerner SP, Andre MC, Leibold JS, Kousis PC, Kubler A, Pal M. An Fc-optimized CD133 antibody for induction of NK cell reactivity against myeloid leukemia. Leukemia. 2017;31(2):459–469. doi: 10.1038/leu.2016.194. [DOI] [PubMed] [Google Scholar]
- 29.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183(5):2185–2195. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9(10):1783–1786. [PubMed] [Google Scholar]
- 31.Croft M, So T, Duan W, Soroosh P. The significance of OX40 and OX40L to T-cell biology and immune disease. Immunol Rev. 2009;229(1):173–191. doi: 10.1111/j.1600-065X.2009.00766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Imura A, Hori T, Imada K, Kawamata S, Tanaka Y, Imamura S. OX40 expressed on fresh leukemic cells from adult T-cell leukemia patients mediates cell adhesion to vascular endothelial cells: implication for the possible involvement of OX40 in leukemic cell infiltration. Blood. 1997;89(8):2951–2958. [PubMed] [Google Scholar]
- 33.Koubek K, Stary J, Kumberova A, Klamova H, Filipec M. Occurrence of cytokine receptors on different lymphoid leukaemic cells. Eur J Haematol. 1999;63(1):1–10. doi: 10.1111/j.1600-0609.1999.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 34.Lum L, Wong BR, Josien R, Becherer JD, Erdjument-Bromage H, Schlondorff J. Evidence for a role of a tumor necrosis factor-alpha (TNF-alpha)–converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem. 1999;274(19):13613–13618. doi: 10.1074/jbc.274.19.13613. [DOI] [PubMed] [Google Scholar]
- 35.Schmiedel BJ, Scheible CA, Nuebling T, Kopp HG, Wirths S, Azuma M. RANKL expression, function, and therapeutic targeting in multiple myeloma and chronic lymphocytic leukemia. Cancer Res. 2013;73(2):683–694. doi: 10.1158/0008-5472.CAN-12-2280. [DOI] [PubMed] [Google Scholar]
- 36.Taylor L, Schwarz H. Identification of a soluble OX40 isoform: development of a specific and quantitative immunoassay. J Immunol Methods. 2001;255(1-2):67–72. doi: 10.1016/s0022-1759(01)00424-0. [DOI] [PubMed] [Google Scholar]
- 37.Komura K, Yoshizaki A, Kodera M, Iwata Y, Ogawa F, Shimizu K. Increased serum soluble OX40 in patients with systemic sclerosis. J Rheumatol. 2008;35(12):2359–2362. [PubMed] [Google Scholar]
- 38.Doron B, Handu M, Kurre P. Concise review: adaptation of the bone marrow stroma in hematopoietic malignancies: current concepts and models. Stem Cells. 2018;36(3):304–312. doi: 10.1002/stem.2761. [DOI] [PubMed] [Google Scholar]
- 39.Xie K, Xu L, Wu H, Liao H, Luo L, Liao M. OX40 expression in hepatocellular carcinoma is associated with a distinct immune microenvironment, specific mutation signature, and poor prognosis. Oncoimmunology. 2018;7(4) doi: 10.1080/2162402X.2017.1404214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532–543. doi: 10.1200/JCO.2010.30.1382. [DOI] [PubMed] [Google Scholar]
- 41.Xie F, Wang Q, Chen Y, Gu Y, Shi Q, Ge Y. Characterization and application of two novel monoclonal antibodies against human OX40: costimulation of T cells and expression on tumor as well as normal gland tissues. Tissue Antigens. 2006;67(4):307–317. doi: 10.1111/j.1399-0039.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 42.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 43.Eissner G, Kolch W, Scheurich P. Ligands working as receptors: reverse signaling by members of the TNF superfamily enhance the plasticity of the immune system. Cytokine Growth Factor Rev. 2004;15(5):353–366. doi: 10.1016/j.cytogfr.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 44.Stuber E, Neurath M, Calderhead D, Fell HF, Strober W. Cross-linking of Ox40 ligand, a member of the Tnf/Ngf cytokine family, induces proliferation and differentiation in murine splenic B-cells. Immunity. 1995;2(5):507–521. doi: 10.1016/1074-7613(95)90031-4. [DOI] [PubMed] [Google Scholar]
- 45.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159(8):3838–3848. [PubMed] [Google Scholar]
- 46.Kotani A, Hori T, Matsumura Y, Uchiyama T. Signaling of gp34 (OX40 ligand) induces vascular endothelial cells to produce a CC chemokine RANTES/CCL5. Immunol Lett. 2002;84(1):1–7. doi: 10.1016/s0165-2478(02)00082-2. [DOI] [PubMed] [Google Scholar]