SUMMARY.
Phosphorylated histone 3 (PH3) and cleaved caspase 3 (CCASP3) were used to detect proliferating and apoptotic cells, respectively, in the jejunums of female sibling poults, with and without enteritis and depressed growth, from hatch to day 35. Poults that developed enteritis and depressed growth (SIB flock) were raised on a commercial farm in eastern North Carolina, whereas poults with normal growth and no enteritis (TAU flock) were raised in the Teaching Animal Unit at North Carolina State University College of Veterinary Medicine. Beginning on day 5 through day 35 and at processing, TAU poults were significantly heavier than SIB poults. Jejunal weights, relative jejunal weights, and jejunal densities were greater in SIB poults from day 10 through 35. Jejunal efficiency (body weight /jejunal length) was higher in TAU poults at day 5 and days 10 through 35. Mucosal thickness was greater in SIB poults between days 7 and 21 but greater in TAU poults at days 28 and 35. From day 7 to 35, villus-to-crypt ratios were higher for TAU poults and lower for SIB poults because hyperplastic crypts formed a greater percentage of the mucosa in SIB poults. By day 7, PH3- and CCASP3-positive cells were increased in SIB poults, showing that mucosal changes resulted from combined crypt epithelial hyperplasia and increased apoptosis of villous enterocytes. Findings in this study confirm that enteritis, in the absence of clinical signs, and depressed growth in turkey poults begins by day 7, can be identified microscopically, persists for at least 35 days, is associated with lower processing weights, and has a profound negative effect on turkey growth.
Keywords: turkey, poult enteritis, immunohistochemistry, histomorphometry, gross morphometry, crypt hyperplasia, growth, gut health
Enteritis in poults is described in various syndromes, including poult enteritis complex (5), stunting syndrome (2), poult malabsorption syndrome (37), light turkey syndrome (30), transmissible enteritis (1), poult enteritis mortality syndrome (4,22), spiking mortality (6), enteric disease in poults (12), turkey enteritis (27), and big bird–little bird syndrome (47). Collectively, these are all lumped together under the global term of poult enteritis complex (25). Estimates of annual losses of $300 million to $400 million were based on growth depression of 10%–15% in affected poults (7).
Viruses isolated from poults with enteritis include turkey astrovirus (3,34,35,36,42), coronavirus (10,21,23), enterovirus-like virus (25), parvovirus (49) picornavirus (12), reovirus (27), rotavirus (9,23), small round virus (41), torovirus (39), and others (11). Viruses associated with poult enteritis also have been demonstrated by electron microscopy (48). Bacteria, notably enteropathogenic Escherichia coli, are associated with some of these syndromes in the poult enteritis complex (24). The multifactorial pathogenesis of poult enteritis with emphasis on viruses as initiators and the role of bacteria and/or protozoa including cryptosporidia and coccidia as well as management and nutrition are reviewed (33).
Comparison of the gut virome in commercially raised poults (termed SIB for this study) with the virome in poults from the same breeder flocks, hatched in the same hatchery, but raised in comparative isolation in the Teaching Animal Unit (TAU) at the North Carolina College of Veterinary Medicine showed differences in several enteric picornavirus sequences (12). The availability of similar paired SIB-TAU poults provided an opportunity to repeat earlier studies (unpublished results) using body weight and gross morphometry to show differences between poults raised in these two different environments. Availability of immunohistochemistry and histomorphometry used in the study of enteric disease in horses and porcine models provided additional tools for evaluation of enteric disease in turkey poults (17,18,19,20).
Using gross morphology, histomorphology, and immunohistochemistry, crypt hyperplasia with increased numbers of proliferating and apoptotic cells in the jejunums from SIB poults occurring by day 7 and persisting through day 35 is described.
MATERIALS AND METHODS
Poults and flock management.
Sibling female poults from the same breeder flock were hatched together and divided into two flocks. One flock (n = 2000) was placed in the Teaching Animal Unit (TAU) at North Carolina State University, College of Veterinary Medicine, in Raleigh, North Carolina. The sibling (SIB) flock (n = 8000) was placed in one section of a brooder house on a commercial farm in eastern North Carolina. Poults in both flocks received the same formulated feed from the same mill and were provided free access to water. SIB flocks were raised using commercial turkey production methods, whereas TAU poults were raised in relative isolation, intensively managed with lower stocking density, and used as a teaching flock. Protocols for animal care were approved by the Institutional Animal Care and Use Committee of North Carolina State University.
Sample collection.
Samples were obtained from 6 poults on the day of hatch and from 6 poults in each flock on days 3, 5, 7, 10, 12, 14, 21, 28, and 35 (n = 114) to determine body weights and jejunal weights and lengths and to collect jejunal segments for histomorphometry and immunohistochemistry. Preprocessing body weights were obtained on days 72 and 73 for the TAU and SIB flocks, respectively. On sampling days, approximately 60 poults in each flock were caught in a catch pen and weighed individually. Until 6 poults were obtained, every third poult was selected for sampling when it was weighed and euthanized by cervical dislocation. The jejunum (small intestine between the hepatoduodenal ligament and attachment of ileocecal ligaments) was removed, weighed (g), and measured (cm). Jejunal density (jejunal weight/jejunal length), relative jejunal weight (jejunal weight/body weight × 100), and jejunal efficiency (body weight/jejunal length) were calculated. A 2–3 cm section of jejunum proximal to Meckel’s diverticulum was opened longitudinally, stapled to cardboard, and placed into 10% neutral buffered formalin. After 24 hr fixation, jejunal sections were transferred to 70% ethanol and stored at 4° C until processed for histomorphology and immunohistochemistry.
Histomorphometry.
Paraffin embedded tissue was sectioned at ~5 μm and stained with hematoxylin and eosin. Villous height and crypt depth were measured (CellSens™, ver. 1.11, Olympus Corp., www.olympus-sis.com) for 8–10 paired regions. Inappropriately angled or poorly fixed samples were excluded. Mucosal thickness and villus-to-crypt ratio (V:C) were calculated.
Immunohistochemistry.
Jejunal sections (~5 μm) were mounted on positively charged glass slides, deparaffinized, and rehydrated. Heat-induced epitope retrieval was done by placing slides into citrate Target Retrieval Solution™ (DakoCytomation, Glostrup, Denmark) for 30 sec at 119° C followed by 90° C for 10 sec in a Pascal pressure chamber (DakoCytomation). Slides were cooled to room temperature and moved to a DakoAutostainerPlus™ (DakoCytomation) after which they were incubated in a peptide-blocking agent, Background Buster™ (Innovex Biosciences, Richmond, CA), for 30 min. Primary antibodies were applied to tissue sections diluted in Common Antibody Diluent™ (BioGenex, Fremont, CA). For staining of phosphorylated histone 3 (PH3), α-phospho-histone H3 (PH3, rabbit, EMD Millipore, Darmstadt, Germany) was diluted 1:500. Cleaved caspase 3 (CCASP3) was detected using α-cleaved caspase 3 (CCASP3, rabbit, Cell Signaling Technology, Inc., Danvers, MA) diluted 1:400. Primary antibody incubation was done for 30 minutes at room temperature. Subsequently, slides were incubated for 30 min in a 1:1 dilution of ImmPRESS™ (peroxidase) polymer anti-rabbit IgG reagent (Vector Laboratories, Burlingame, CA) followed by 30 min in ImmPact DAB peroxidase (HPR) substrate solution™ (Vector Laboratories). To identify nonspecific binding, Rabbit Super Sensitive Negative Control™ (BioGenex) was applied to tissue sections.
PH3-positive enterocytes were counted in 100 crypts. For CCASP3, the number of positive enterocytes in 30 crypt-villus pairs was determined. If a full set of 100 crypts or 30 crypt-villus pairs could not be evaluated, that sample was eliminated from the study. Except for samples obtained on the day- of hatch and day 3, which were not counted, at least 4 jejunal sections were analyzed for each sampling.
Statistical analyses.
The Kruskal-Wallis test (JMP™, Pro 13.0.0, SAS Institute Inc., Cary, NC) was used to determine significance for all data except preprocessing body weights at 72 and 73 days, which were analyzed using a t-test to compare means because of their normal distribution. Significance was set at p ≤ 0.05.
RESULTS
Gross morphometry.
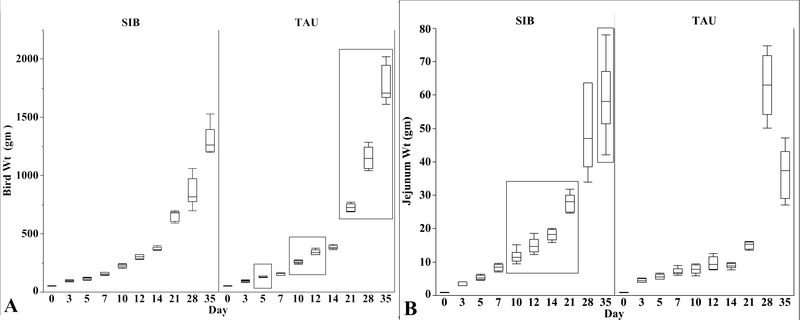
Beginning on day 5 through day 35, sampled TAU poults were significantly heavier than sampled SIB poults. At day 35, sampled SIB poults weighed 29.2% less than sampled TAU poults (1707 g vs. 1208 g) (Fig. 1A). Prior to processing, weights of turkeys in the TAU flock were still significantly greater than those in the SIB flock (6398 g vs. 5426 g; 15.2%).
Fig. 1.
Poult (A) and jejunal (B) weights for sampled SIB and TAU poults from day 0 to 35. Box plots show minimum, first quartile, median, third quartile, and maximum values. Significantly different values (p ≤ 0.05) are enclosed in rectangles. (A) TAU poults at 5, 10, 12, and day 21 to 35 were significantly heavier than SIB poults. (B) Except for day 28, jejunal weights of SIB poults were significantly heavier than TAU poults between days 10 and 35. Differences in poult and jejunal weights were greatest at day 35.
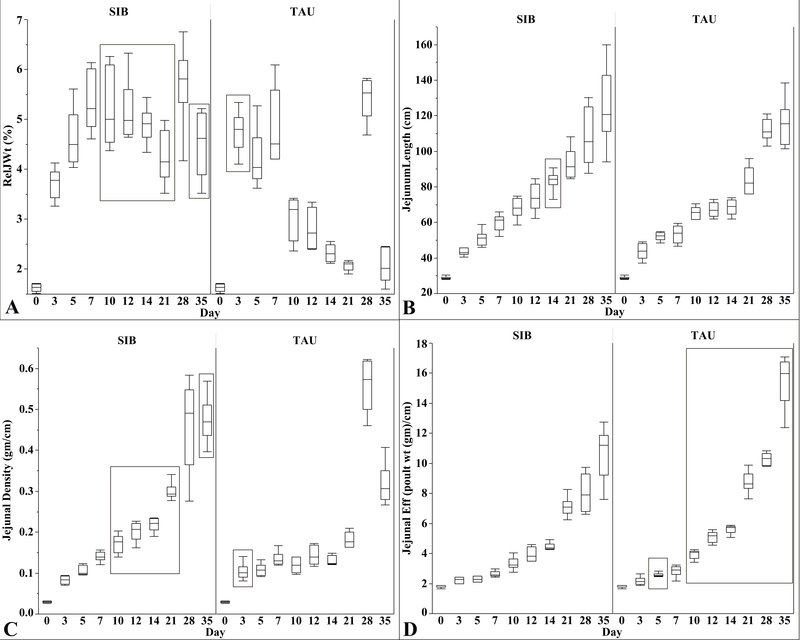
Jejunal weights were similar in poults from both flocks until day 10. Median jejunal weight increased 1.1 g in TAU poults from 6.8 g at day 7 to 7.9 g at day 10, whereas median jejunal weights in SIB poults increased 2.9 g from a median of 8.6 g at day 7 to 11.5 g at day 10. Jejunal weights remained significantly heavier in SIB poults compared to TAU poults between days 10, 14, 21, and 35, except for day 28 (Fig. 1B). Relative jejunal weight was higher in TAU poults at day 3 (4.8% compared to 3.8%), but higher in SIB poults at days 10, 14, 21, and 35 (Fig. 2A). Relative median weight of the jejunum in TAU poults at day 35 was 2.02% compared to 4.62% in SIB poults.
Fig. 2.
Relative jejunal weight (A), jejunal length (B), jejunal density (C), and jejunal efficiency (D) for sampled SIB and TAU poults from day 0 to 35. Box plots show minimum, first quartile, median, third quartile, and maximum values. Significantly different values (p ≤ 0.05) are enclosed in rectangles. (A) Relative jejunal weight was greater in TAU poults at day 3 and greater in SIB poults at days 10 to 35, except for day 28 when the relative jejunal weight was high in TAU poults. (B) Jejunal length was similar in both SIB and TAU poults except for day 14 when the jejunum of SIB poults was significantly longer. (C) At day 3, jejunal density was greater in TAU poults. Jejunal density was significantly greater in SIB poults at days 10 to 35, except for day 28. Median values at day 28 were similar for both SIB and TAU poults. (D) Jejunal efficiency was significantly greater in TAU poults at day 5 and days 10 to 35.
Jejunal length was similar in both TAU and SIB poults except for day 14 when the median length in SIB poults was 84 cm compared to 69 cm for TAU poults (Fig. 2B). Jejunal density was higher in TAU poults at day 3 but higher in SIB poults at days 10, 14, 21, and 35 (Fig. 2C). Median jejunum density for SIB poults at day 35 was 0.467 g/cm compared to 0.306 g/cm for TAU poults. Day 28 values were not statistically different, although median jejunum density in TAU poults was higher (0.572 g/cm) compared to SIB poults (0.491g/cm).
Jejunal efficiency was higher in TAU poults at days 10, 14, 21, 28, and 35 (Fig. 2D). At day 35, 1 g of jejunum supported 16 g of body weight in TAU poults compared to 11.2 g of body weight in SIB poults.
Gross enteric lesions observed in the SIB flock were watery intestinal contents in 1 of 6 poults each at days 3 and 5, congested mesenteric blood vessels in 1 of 6 poults at day 7, and mild catarrhal enteritis in 3 of 6 poults at day 10. Gross lesions of enteritis were not observed in TAU poults. Clinical signs of enteritis were not observed in either TAU or SIB poults.
Histomorphometry.
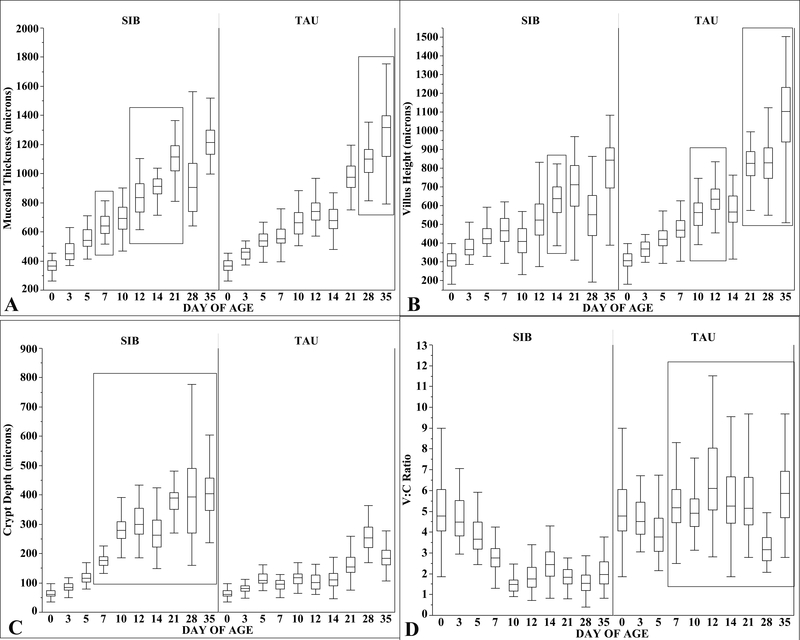
Mucosal thickness was greater in SIB poults at days 7, 12, 14, and 21 but lower at days 28 and 35 compared to TAU poults (Fig. 3A).
Fig. 3.
Median mucosal thickness (A), villous height (B), crypt depth (C), and V:C (D) in sampled SIB and TAU poults from day 0 to 35. Box plots show minimum, first quartile, median, third quartile, and maximum values. Significantly different values (p < 0.05) are enclosed in rectangles. (A) Compared to TAU poults, mucosal thickness was significantly greater in SIB poults at days 7 and 12 to 21, but significantly lower at days 28 and 35. (B) Villous height was greater in SIB poults at day 14, but greater in TAU poults at days 10, 12, and 21 to 35. (C) Crypt depth was greater in SIB poults at days 7 to 35. (D) V:Cs were greater in TAU poults at days 7 to 35.
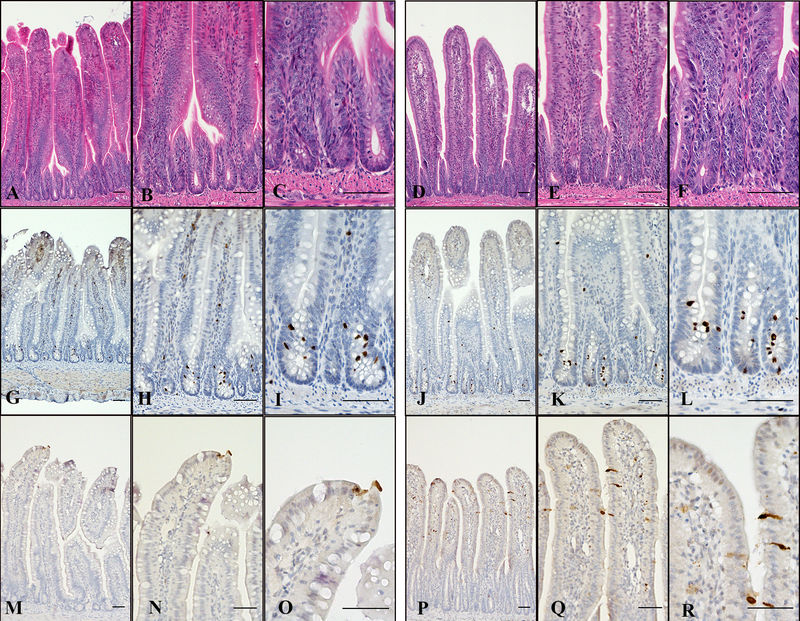
Villous height increased from 367 μm at day 3 to 845 μm (478 μm increase) at day 35 in SIB poults and from 369 μm at day 3 to 1105 μm (736 μm increase) at day 35 in TAU poults. Villous height was greater in TAU poults at days 10, 12, 21, 28, and 35 with SIB poults higher only at day 14 (Fig. 3B). Median crypt depth at day 3 was 84.7 μm in SIB poults and 81 μm in TAU poults. Crypt depth was significantly higher in SIB poults at days 7 through 35 with the median for SIB poults at day 35 being 404 μm compared to 184 μm in TAU poults (Fig. 3C). V:Cs were higher in TAU poults from day 7 through 35 (Fig. 3D). Sections of H&E–stained jejunums from 7-day-old TAU and SIB poults show relationships of crypts to villi at successively higher magnifications (Fig. 4A–F). Increased crypt depth due to proliferating cells is shown in a 7-day-old SIB poult (Fig. 4F). Crypt hyperplasia was characterized by disruption of normal crypt architecture, bulging of epithelial cells into the lumen, crowding of enlarged epithelial cells, karyomegaly of crypt epithelial cells, prominent nucleoli, and increased mitotic figures.
Fig. 4.
Day 7 TAU and SIB poults, jejunums. (A–C, TAU poult, H&E). (A) Normal villi, crypts, and portion of muscularis; (B) relationship of crypts to base of villi; (C) organized arrangement of crypt enterocytes with occasional mitotic figures. (D–F, SIB poult, H&E, compare with A–C). (D) Mild to moderate crypt hyperplasia; (E) early crypt hyperplasia with dysplasia of crypt enterocytes; (F) hyperplasia and dysplasia of crypt enterocytes. (G–I, TAU poult, PH3). (G) PH3-positive cells in the crypts, brown staining at the tips of villi is not specific; (H) cells positive for PH3 expression in the crypts; (I) higher magnification showing PH3-positive cells in crypts. (J–L, SIB poult, PH3, compare with G–I). (J) PH3-positive cells in the crypts; (K) PH3-positive cells in the crypts; (L) higher magnification showing PH3-positive cells in crypts. (M–O, TAU poult, CCASP3). (M) Relatively few positive cells at villous tips; (N) CCASP3-positive cell at the villus tip; (O) higher magnification showing CCASP3-positive cell at the villous tip. Compare with the number of CCASP3-positive cells in the SIB poult. (P–R, SIB poult, CCASP3, compare with M–O). (P) CCASP3-positive cells in upper third of villi; (Q) CCASP3-positive cells along upper third of villus; (R) higher magnification showing detail of CCASP3-positive cells. Bars, low magnification = 50 μm, two higher magnifications both = 5 μm.
No other histologic lesions were found.
Immunohistochemistry.
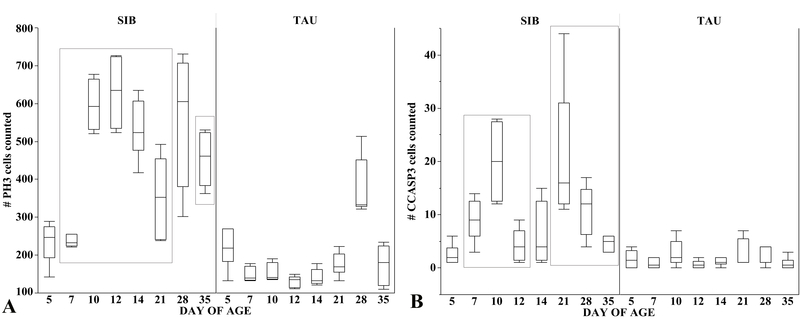
Proliferating PH3-positive cells occurred in the crypts (Fig. 4G–L) while CCASP3-positive enterocytes undergoing apoptosis were located on tips and upper parts of villi (Fig. 4M–R). SIB poults had more proliferating PH3-positive cells than TAU poults from day 7 through days 21 and 35 (Fig. 5A). CCASP3-positive cells were increased in SIB poults compared to TAU poults at days 7 through 12 and days 21 through 35 (Fig. 5B).
Fig. 5.
Numbers of immunopositive cells expressing PH3 (A) and CCASP3 (B) in SIB and TAU poults. Box plots show minimum, first quartile, median, third quartile, and maximum values. Significantly different values (p < 0.05) are enclosed in rectangles. (A) The number of PH3-positive cells was significantly higher in SIB poults from day 7 through 35, except for day 28 when the number of PH3-positive cells was also high in TAU poults. (B) Compared to TAU poults, SIB poults had greater numbers of CCASP3-positive cells from day 7 to 35, except for day 14. TAU poults had consistently low numbers of CCASP3-positive cells at all sampling times.
DISCUSSION
Crypt hyperplasia and increased apoptosis were identified by immunohistochemistry in SIB poults as early as day 7 and was the first evidence of intestinal disease in the poults. Histomorphometry correlated with immunohistochemistry, which showed decreased jejunal V:Cs at day 7. Crypt hyperplasia, apoptosis, and decreased V:C in SIB poults persisted until at least day 35. Gross morphometry, while useful for assessing enteric health, did not show differences between SIB and TAU poults until day 10. Discovery of early and persistent jejunal lesions is important for designing and implementing management and treatment interventions to reduce economic losses from enteritis in poults. The jejunum was chosen because this region has a major role in the absorption of nutrients (14).
SIB poults met several criteria for poult enteritis including reduction in body weight, increased jejunal weight, relative jejunal weight, and jejunal density, reduced jejunal efficiency, and decreased V:C (7,33). It was not an objective of this study to investigate the possible causes of these jejunal lesions, but confirmation of differences in the jejunums and time course of these changes between SIB and TAU flocks supports this TAU-SIB model as an important one for the study of enteric disease in turkeys. A study using a previous SIB-TAU model documented differences in the enteric picornavirus virome of SIB poults (11), but those procedures were not applied in this study.
In this study, dysregulation of the relationship between crypt and villous epithelial cells occurred in SIB poults with enteritis. Proliferation of crypt epithelial cells normally correlates with the number of enterocytes that move up the sides of villi and are eventually extruded at villous tips. Increasing crypt cells coupled with decreasing enterocyte loss results in increased villous length. In contrast to TAU poults, this relationship was not maintained in SIB poults with enteritis. Marked hyperplasia of crypt epithelium was not matched by increased length of villi, which resulted in villi remaining short or even decreasing in length (villous atrophy). Physical changes in villi resulted in decreased villous area and decreased ability of affected poults to absorb nutrients. These changes are not etiology specific. It is also possible that enterocytes being lost prematurely as indicated by increased apoptosis of enterocytes were also functionally deficient in addition to being decreased numerically. Decreased mucosal absorption is indicated by the lower jejunal efficiencies in SIB poults compared to TAU poults. Increases in jejunal length and weight in SIB poults resulted from an attempt to compensate for lost nutrient absorption and inflammation respectively. Decreased growth rate paralleled changes in mucosal morphology. Lower body weights of SIB turkeys just prior to processing indicate the functionally impaired nutrient uptake when the birds were younger remained incompletely compensated to market. Some compensatory growth occurred as evidenced by the improvement in relative body weight differences between the TAU and SIB poults at day 35 (29.2%) and preprocessing (15.2%). However, as the birds grew, absolute weight differences continued to increase until there was an average difference of 972 g (2.14 lb) at preprocessing. At a current average value of US$0.98 (USDA Turkey Market News Report 64:40; October 6, 2017), losses from weight alone would approximate US$2.12/bird.
Differences between TAU and SIB poults are consistent throughout the 35-day experimental period except for day 28. Jejunal weight, relative jejunal weight, and jejunal density were increased in TAU poults at day 28 and were not significantly different from values in SIB poults at the same age. Reasons for this are unknown, as there were no clinical signs of enteric or other disease in the TAU poults at any time interval. Jejunal efficiency remained higher in TAU than SIB poults at day 28. V:Cs were lower in TAU poults but still significantly higher than SIB poults. PH3-positive cells were increased in TAU poults, suggesting some stimulus for cell proliferation, but CCASP3-positive cells were not increased. The impact at day 28 in TAU poults did not affect poults at day 35 as no lesions were identified by gross morphometry, histomorphometry, or immunohistochemistry in the TAU poults. It is possible the TAU flock experienced mild transient enteritis at this time, but the cause is unknown.
In chickens, crypts are rudimentary at hatch but are well organized by day 2 with a peak in number of crypts per villi by day 3 (16). Villous height in poults measured immediately post-hatch through day 11 showed a significant increase in villous height. Crypt depth increased, but not significantly after day 4 (26). Crypts begin to form at hatch and are recognizable within several days post-hatch (45). We were able to measure crypt depth and calculate V:Cs from hatch until day 35 when the study was concluded. TAU and SIB poults did not differ significantly in V:Cs until day 7 when crypts became hyperplastic and occupied a greater percentage of the mucosa.
Growth and development of the small intestine in chicks and poults peak within days 6–10 post-hatch. Enterocyte proliferation occurs throughout villi in the pre-hatch and immediate post-hatch period but rapidly shifts to localized proliferation within the crypts (28). A continuing increase in the difference between villous height and crypt depth occurs between days 1 and 3 post-hatch (28). Gross morphometry, histomorphometry, and measurement of mucosal enzyme activities in poults from hatch through day 12 showed intestinal weight increased to a peak at day 6, villous length increased rapidly, and crypt depth increased but at a slower rate than villous height (46). In our findings, jejunal weight increased from day 3 to 35, but, except for the 28-day value, relative jejunal weights progressively declined. A marked rise in jejunal efficiency occurred after day 10 in TAU poults. The V:C in TAU poults from day 0 through 35 remained relatively constant.
We do not know if TAU poults had access to feed after placement before SIB poults. Early access to feed has a positive effect on increasing body weight and increasing relative intestinal weight by day 2 post-hatch (31). Intestinal development is slower when access to first feed is delayed (44). The lack of significant differences in body weight, jejunal weight, and jejunal efficiency between TAU and SIB poults at day 3 suggests no early access to feed effects. TAU poults did have significantly higher jejunal density and relative jejunal weight at day 3 than SIB poults; thus an effect of early access to feed as a factor influencing our results cannot be ruled out.
Expression of PH3 and CCASP3 using immunohistochemistry is reported in avian tissues including PH3 expression in turkey (38) and chicken erythrocyte nuclei (43), PH3 expression in a study on male meiosis in finches (13), PH3 expression in the brain of Bobwhite quail (8), PH3 and CCASP3 expression in an investigation of the cell cycle in the proliferative zone of chick embryo retinas (32), CCASP3 for detection of apoptotic cells in reovirus infected chick embryo fibroblasts (40), Mycoplasma synoviae infection of chicken chondrocytes (15), and apoptosis in the early development of the bursa of Fabricius (29). In this study, PH3-positive cells in crypts correlated with hyperplasia of crypt enterocytes. Apoptotic cells as detected by CCASP3-positive staining were comparable to patterns observed in a porcine model (20).
Comparison of jejunal changes using multiple techniques as described in this study clearly show SIB poults have lesions detectable by day 7 that persist through day 35. A significant loss of body weight amounting to approximately 15% was associated with early enteritis in the SIB poults and persistence intestinal lesions. Immunohistochemistry and histomorphometry detected lesions by day 7 compared to detection at day 10 by gross morphometry. All methods detected jejunal lesions in the absence of clinical signs of enteritis. These observations are critical in guiding efforts to control enteric disease in poults. Interventions undertaken by the time clinical signs appear or growth depression is noted are likely to be too late. Based on this study’s findings, measures to prevent enteritis in young poults must be done in ovo or in the first few days of life to avert the millions of dollars in industry losses associated with decreased body weight.
ACKNOWLEDGMENTS
Support for this project was provided by a gift from Zoetis, LLC, Florham Park, NJ.
Abbreviations:
- CCASP3
cleaved caspase 3
- H&E
hematoxylin and eosin
- PH3
phosphorylated histone 3
- SIB
commercial poults
- TAU
Teaching Animal Unit
- V:C
villus-to-crypt ratio
REFERENCES
- 1.Adams NR, Ball RA, Annis CL, and Hofstad MS. Ultrastructural changes in the intestines of turkey poults and embryos affected with transmissible enteritis. J. Comp. Pathol. 82:187–192. 1972. [DOI] [PubMed] [Google Scholar]
- 2.Akbar A, and Reynolds DL. Stunting syndrome in turkey poults: isolation and identification of the etiologic agent. Avian Dis. 41:870–881. 1997. [PubMed] [Google Scholar]
- 3.Azad S, Mor SK, Naresh J, Devi P, Sobhy NM, Nhungoc Ti L, and Goyal SM. Detection and molecular characterization of astroviruses in turkeys. Arch. Virol. 161:939–946. 2016. [DOI] [PubMed] [Google Scholar]
- 4.Barnes HJ Prevention, control and treatment of poult enteritis-mortality syndrome. Poult. Dig. 56:16–18. 1997. [Google Scholar]
- 5.Barnes HJ PEC: what is it and what is the economic significance? World Poult. 18:14–16. 2002. [Google Scholar]
- 6.Barnes HJ, Guy JS, Brown TP, and Edens FW. Poult enteritis-mortality syndrome (“spiking mortality of turkeys”) and related disorders—an update and overview. Proc. Annual Meeting United States Animal Health Assoc 100:564–575. 1996. [Google Scholar]
- 7.Barnes HJ, Guy JS, and Vaillancourt JP. Poult enteritis complex. Rev. Sci. Tech. Off. Int. Épizoot. 19:565–588. 2000. [DOI] [PubMed] [Google Scholar]
- 8.Charvet CJ, and Striedter GF. Developmental species differences in brain cell cycle rates between northern bobwhite quail (Colinus virginianus) and parakeets (Melopsittacus undulatus): implications for mosaic brain evolution. Brain Behav. Evol 72:295–306. 2008. [DOI] [PubMed] [Google Scholar]
- 9.Day JM Rotavirus infections In: Diseases of poultry, 13th ed. Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, and Nair V, eds. Wiley-Blackwell, Ames, IA: pp. 730–746. 2013. [Google Scholar]
- 10.Day JM, Gonder E, Jennings S, Rives D, Robbins K, Tilley B, and Wooming B. Investigating turkey enteric coronavirus circulating in the Southeastern United States and Arkansas during 2012 and 2013. Avian Dis. 58:313–317. 2014. [DOI] [PubMed] [Google Scholar]
- 11.Day JM, and Zsak L. Recent progress in the characterization of avian enteric viruses. Avian Dis. 57:573–580. 2013. [DOI] [PubMed] [Google Scholar]
- 12.Day JM, and Zsak L. Investigating turkey enteric picornavirus and its association with enteric disease in poults. Avian Dis. 59:138–142. 2015. [DOI] [PubMed] [Google Scholar]
- 13.del Priore L, and Pigozzi MI. Histone modifications related to chromosome silencing and elimination during male meiosis in Bengalese finch. Chromosoma 123:293–302. 2014. [DOI] [PubMed] [Google Scholar]
- 14.Denbow DM Gastrointestinal anatomy and physiology In: Stukie’s avian physiology (electronic resource), 6th ed. Scanes CG, ed. Elsevier/Academic Press, London: pp. 356–361. 2015. [Google Scholar]
- 15.Dusanic D, Bencina D, Oven I, Cizelj I, Bencina M, and Narat M. Mycoplasma synoviae induces upregulation of apoptotic genes, secretion of nitric oxide and appearance of an apoptotic phenotype in infected chicken chondrocytes. Vet. Res 43:7 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geyra A, Uni Z, and Sklan D. Enterocyte dynamics and mucosal development in the posthatch chick. Poult. Sci 80:776–782. 2001. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez LM, Kinnin LA, and Blikslager AT. Characterization of discrete equine intestinal epithelial cell lineages. Am. J. Vet. Res 76:358–366. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gonzalez LM, Moeser AJ, and Blikslager AT. Animal models of ischemia-reperfusion-induced intestinal injury: progress and promise for translational research. Am. J. Physiol. Gastrointest. Liver Physiol 308:G63–75. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez LM, Moeser AJ, and Blikslager AT. Porcine models of digestive disease: the future of large animal translational research. Transl. Res 166:12–27. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez LM, Williamson I, Piedrahita JA, Blikslager AT, and Magness ST. Cell lineage identification and stem cell culture in a porcine model for the study of intestinal epithelial regeneration. PLoS One 8:e66465 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guy JS Turkey Cornavirus enteritis. In: Diseases of poultry, 13th ed. Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL and Nair V, eds. Wiley-Blackwell, Ames, IA: pp. 722–730. 2013. [Google Scholar]
- 22.Guy JS, and Barnes HJ. Poult enteritis and mortality syndrome (“spiking mortality”): an acute, transmissible disease of unknown etiology. Zootec. Int 20:42–47. 1997. [Google Scholar]
- 23.Guy JS, Barnes J, Smith LG, and Breslin J. Antigenic characterization of a turkey coronavirus identified in poult enteritis- and mortality syndrome-affected turkeys. Avian Dis. 41:583–590. 1997. [PubMed] [Google Scholar]
- 24.Guy JS, Smith LG, Breslin JJ, Vaillancourt JP, and Barnes HJ. High mortality and growth depression experimentally produced in young turkeys by dual infection with enteropathogenic Escherichia coli and turkey coronavirus. Avian Dis. 44:105–113. 2000. [PubMed] [Google Scholar]
- 25.Haychow C Avian enterovirus-like virus infections In: Diseases of poultry, 13th ed. Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL and Nair V, eds. Wiley-Blackwell, Ames, IA: pp. 751–758. 2013. [Google Scholar]
- 26.Hutsko SL, Meizlisch K, Wick M, and Lilburn MS. Early intestinal development and mucin transcription in the young poult with probiotic and mannan oligosaccharide prebiotic supplementation. Poult. Sci 95:1173–1178. 2016. [DOI] [PubMed] [Google Scholar]
- 27.Jindal N, Mor SK, and Goyal SM. Enteric viruses in turkey enteritis. Virusdisease 25:173–185. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lilburn MS, and Loeffler S. Early intestinal growth and development in poultry. Poult. Sci 94:1569–1576. 2015. [DOI] [PubMed] [Google Scholar]
- 29.Makino K, Omachi R, Suzuki H, Tomobe K, Kawashima T, Nakajima T, and Kawashima-Ohya Y. Apoptosis occurs during early development of the bursa of Fabricius in chicken embryos. Biol. Pharm. Bull. 37:1982–1985. 2014. [DOI] [PubMed] [Google Scholar]
- 30.Mor SK, Sharafeldin TA, Abin M, Kromm M, Porter RE, Goyal SM, and Patnayak DP. The occurrence of enteric viruses in Light Turkey Syndrome. Avian Pathol. 42:497–501. 2013. [DOI] [PubMed] [Google Scholar]
- 31.Noy Y, Geyra A, and Sklan D. The effect of early feeding on growth and small intestinal development in the posthatch poult. Poult. Sci 80:912–919. 2001. [DOI] [PubMed] [Google Scholar]
- 32.Ornelas IM, Silva TM, Fragel-Madeira L, and Ventura AL. Inhibition of PI3K/Akt pathway impairs G2/M transition of cell cycle in late developing progenitors of the avian embryo retina. PLoS One 8:e53517 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pantin-Jackwood MJ Multicausal enteric diseases In: Diseases of poultry, 13th ed. Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL and Nair V, eds. Wiley-Blackwell, Ames, IA: pp. 2428–2433. 2013. [Google Scholar]
- 34.Pantin-Jackwood MJ, Spackman E, and Day JM. Pathogenesis of type 2 turkey astroviruses with variant capsid genes in 2-day-old specific pathogen free poults. Avian Pathol. 37:193–201. 2008. [DOI] [PubMed] [Google Scholar]
- 35.Pantin-Jackwood MJ, Spackman E, Day JM, and Rives D. Periodic monitoring of commercial turkeys for enteric viruses indicates continuous presence of astrovirus and rotavirus on the farms. Avian Dis. 51:674–680. 2007. [DOI] [PubMed] [Google Scholar]
- 36.Pantin-Jackwood MJ, Strother KO, Mundt E, Zsak L, Day JM, and Spackman E. Molecular characterization of avian astroviruses. Arch. Virol 156:235–244. 2011. [DOI] [PubMed] [Google Scholar]
- 37.Perry RW, Rowland GN, and Glisson JR. Poult malabsorption syndrome. I. Malabsorption in poult enteritis. Avian Dis. 35:685–693. 1991. [PubMed] [Google Scholar]
- 38.Polioudaki H, Markaki Y, Kourmouli N, Dialynas G, Theodoropoulos PA, Singh PB, and Georgatos SD. Mitotic phosphorylation of histone H3 at threonine 3. FEBS Lett. 560:39–44. 2004. [DOI] [PubMed] [Google Scholar]
- 39.Reynolds DR, and Ali A. Turkey torovirus infection In: Diseases of poultry, 12th ed. Saif YM, Fadley AM, Glisson JR, McDougald LR, Nolan LK and Swayne DE, eds. Wiley-Blackwell, Ames, IA: pp. 361–365. 2008. [Google Scholar]
- 40.Rodriguez-Grille J, Busch LK, Martinez-Costas J, and Benavente J. Avian reovirus-triggered apoptosis enhances both virus spread and the processing of the viral nonstructural muNS protein. Virology 462–463:49–59. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saif YM, Saif EJ, Hofacre CL, Hayhow C, Swayne DE, and Dearth RN. A small round virus associated with enteritis in turkey poults. Avian Dis. 34:762–764. 1990. [PubMed] [Google Scholar]
- 42.Schultz-Cherry SL Astrovirus infections In: Diseases of poultry, 13th ed. Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, and Nair V, eds. Wiley-Blackwell, Ames, IA: pp. 746–751. 2013. [Google Scholar]
- 43.Sun JM, Chen HY, Espino PS, and Davie JR. Phosphorylated serine 28 of histone H3 is associated with destabilized nucleosomes in transcribed chromatin. Nucleic Acids Res. 35:6640–6647. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uni Z Functional development of the small intestine in domestic birds: cellular and molecular aspects. Poult. Avian Biol. Rev 10:167–179. 1999. [Google Scholar]
- 45.Uni Z Early development of small intestinal function In: Avian gut function in health and disease. Perry GC, ed. CABI, Wallingford, UK: 2006. [Google Scholar]
- 46.Uni Z, Noy Y, and Sklan D. Posthatch development of small intestinal function in the poult. Poult. Sci 78:215–222. 1999. [DOI] [PubMed] [Google Scholar]
- 47.Waibel PE, Pomeroy BS, Hansen MH, Bruin TK, and Newman JA. Attempt to reproduce turkey ‘big bird-little bird syndome’ in battery brooders at the university. In: University of Minnesota, Agricultural Experiment Station. pp. 29–34. 1978. [Google Scholar]
- 48.Woolcock PR, and Shivaprasad HL. Electron microscopic identification of viruses associated with poult enteritis in turkeys grown in California 1993–2003. Avian Dis. 52:209–213. 2008. [DOI] [PubMed] [Google Scholar]
- 49.Zsak L Enteric parvovirus infections of chickens and turkeys In: Diseases of poultry, 13th ed. Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL and Nair V, eds. Wiley-Blackwell, Ames, IA: pp. 758–766. 2013. [Google Scholar]