ABSTRACT
Streptococcus suis (S. suis) causes meningitis, arthritis and endocarditis in piglets. The aim of this study was to characterize the IgM degrading enzyme of S. suis (IdeSsuis) and to investigate the role of IgM cleavage in evasion of the classical complement pathway and pathogenesis. Targeted mutagenesis of a cysteine in the putative active center of IdeSsuis abrogated IgM cleavage completely. In contrast to wt rIdeSsuis, point mutated rIdeSsuis_C195S did not reduce complement-mediated hemolysis indicating that complement inhibition by rIdeSsuis depends on the IgM proteolytic activity. A S. suis mutant expressing IdeSsuis_C195S did not reduce IgM labeling, whereas the wt and complemented mutant showed less IgM F(ab’)2 and IgM Fc antigen on the surface. IgM cleavage increased survival of S. suis in porcine blood ex vivo and mediated complement evasion as demonstrated by blood survival and C3 deposition assays including the comparative addition of rIdeSsuis and rIdeSsuis_C195S. However, experimental infection of piglets disclosed no significant differences in virulence between S. suis wt and isogenic mutants without IgM cleavage activity. This work revealed for the first time in vivo labeling of S. suis with IgM in the cerebrospinal fluid of piglets with meningitis. In conclusion, this study classifies IdeSsuis as a cysteine protease and emphasizes the role of IgM cleavage for bacterial survival in porcine blood and complement evasion though IgM cleavage is not crucial for the pathogenesis of serotype 2 meningitis.
KEYWORDS: Streptococcus suis, IdeSsuis, IgM, opsonization, cysteine protease, IdeS-family protease
Introduction
S. suis disease is one of the main reasons for high economic losses in pig husbandry worldwide [1]. S. suis colonizes the mucosal surfaces of healthy pigs but can lead to invasive disease, mainly in growing piglets of approximately five to ten weeks of age [2,3]. These piglets might develop suppurative meningitis, arthritis, endocarditis, serositis and septicemia upon S. suis infection [1]. S. suis is also an emerging zoonotic agent especially in Southeast Asia [4].
The species S. suis is very heterogeneous. It has been divided into 35 serotypes and more than 700 sequence types (ST) [5]. Serotype 2 is worldwide the most frequently isolated from diseased pigs and humans [4]. However, other serotypes such as serotypes 9, 7, 3 and 1/2 can cause invasive disease as well. The distribution of serotypes depends on the geographical region [4]. However, special adaption to its main host, the pig, is not well understood. A myriad of virulence-associated factors involved in immune evasion have been described for S suis [6,7]. Virulence factors involved specifically in complement evasion on the other hand are scarce. Proven or potential candidates are the polysaccharide capsule [8], factor H binding proteins FhB and Fhbp [9] and lastly the IgM protease IdeSsuis [10]. IdeSsuis, expressed universally among S. suis serotypes, is a highly specific protease, with porcine IgM as its sole substrate [11]. IdeSsuis shows homology to other streptococcal immunoglobulin degrading enzymes such as IdeS of S. pyogenes, IdeZ of S. equi subsp. zooepidemicus, IdeE of S. equi subsp. equi and IdeP of S. phocae subsp. phocae [12]. IdeSsuis, however, remains the only specific IgM protease and, in contrast to the above mentioned IgG proteases, possesses a long C-terminal domain not involved in protease activity [11].
IgM has important effector functions in immunity. First of all, IgM represents not only the main class of natural antibodies [13], but is also the first antibody being produced in response to antigenic stimulation [14]. Secondly, IgM is the main activator of the classical complement cascade. It is an estimated 1000 times more potent in activating complement than IgG [15]. Activation of complement leads to opsonophagocytosis, direct killing of bacteria via the terminal membrane attack complex (MAC), induction of inflammation and recruitment of immune cells [16]. Recently, the complement system, traditionally regarded as part of the innate immune system, has been linked to adaptive immunity, especially T-and B-lymphocytes [16]. Both T-and B-lymphocytes possess the IgM receptor FcμR [15] and IgM can enhance the induction of memory B cells in mice [17]. Lastly, monomeric IgM is the only B-cell receptor (BCR) of naïve porcine B-cells since pigs do not express IgD as a BCR [18]. The possession of an IgM protease can be pivotal for pathogens early in infection and in young animals when IgM titers are highest. Previous studies of our group showed, that expression of IdeSsuis reduced IgM-mediated C3 deposition on the bacterial surface and has a positive effect on survival of S. suis in porcine blood in the presence of specific IgM titers [10]. The aim of this study was to investigate if IdeSsuis is a cysteine protease and to specifically test if the IgM cleavage activity of IdeSsuis is involved in complement evasion and virulence.
Results
Point mutation of cysteine 195 to serine in the putative active center of rIdeSsuis leads to abrogation of IgM cleavage activity
Sequence homologies to known streptococcal cysteine proteases and a protease inhibitor profile [11] suggested that the IgM protease IdeSsuis is a cysteine protease. In order to proof this hypothesis and further elucidate the role of IgM cleavage by IdeSsuis in complement evasion and virulence, a point mutation was introduced into the putative active center of rIdeSsuis. The cysteine at position 195 was replaced by a serine, the rest of the gene remaining intact. The point mutation was also introduced into the truncated construct rIdeSsuis_homologue lacking the large C-terminal domain. Expression and purification of full length protein was verified for the different recombinant IdeSsuis constructs by Coomassie staining (Fig. S1). Analyzing the IgM cleaving activity of cysteine to serine point mutated recombinant rIdeSsuis_C195S and rIdeSsuis_homologue_C195S by Western Blot analysis revealed that both recombinant constructs are unable to cleave porcine IgM (Figure 1). Hence, the presence of a cysteine at position 195 is crucial for IgM cleavage. This finding indicates that IdeSsuis is indeed a cysteine protease, in accordance with its homology to the IdeS-family of streptococcal immunoglobulin degrading enzymes.
Figure 1.
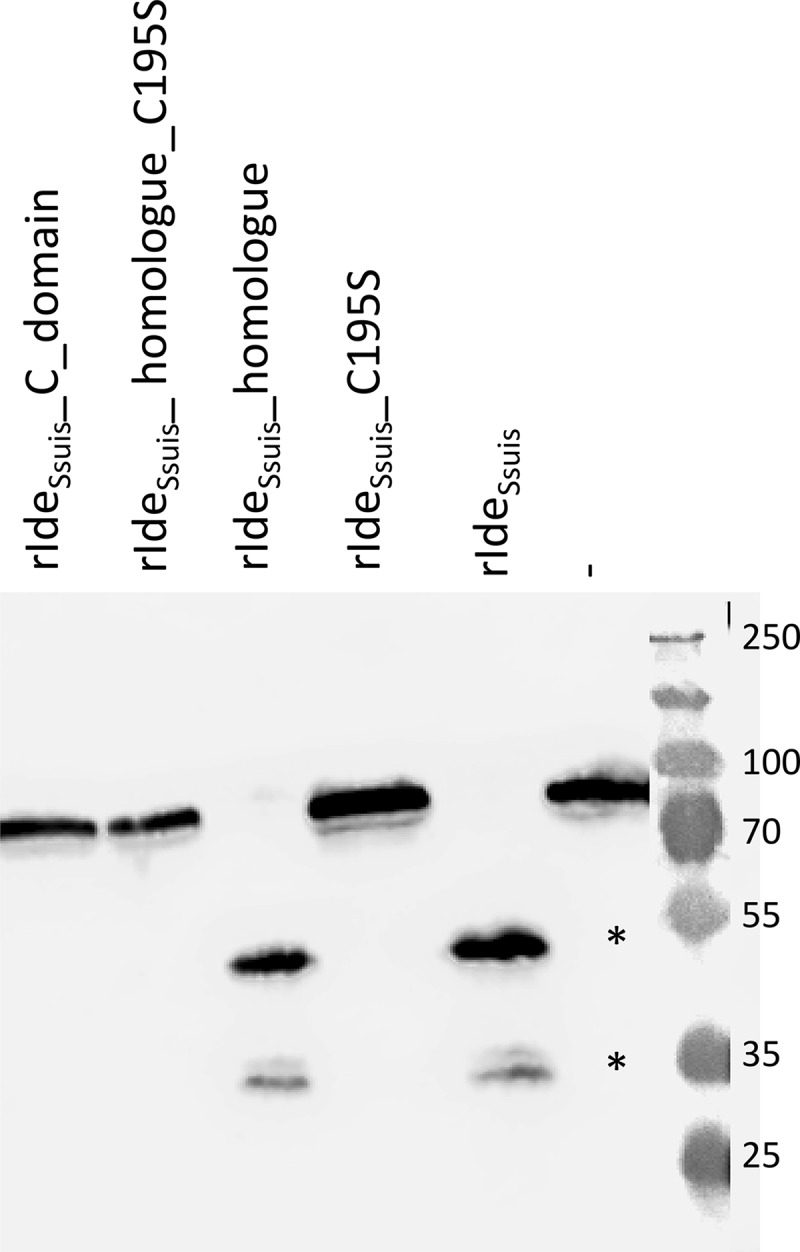
Point mutation of the cysteine 195 within the putative catalytic center of rIdeSsuis leads to loss of IgM cleavage activity. Porcine serum was incubated with 5 µg/ml of the indicated rIdeSsuis constructs, followed by anti-pig IgM Western blot analysis with a polyclonal anti-IgM antibody. Serum incubated with phosphate buffered saline served as negative control (-). A 10% percent polyacrylamide gel was used for SDS-PAGE under reducing conditions. Marker bands in kDa are shown on the right-hand side. IgM cleavage products are indicated by asterisks.
Reduction of complement-mediated hemolysis depends on IgM cleavage
To investigate the role of IgM cleavage in inhibition of the classical complement pathway, a hemolysis assay was conducted. It could be shown that 5 µg/ml rIdeSsuis suffices to reduce complement-mediated hemolysis by over 90% in the presence of high anti-erythrocyte IgM titers (Figure 2(a)) as elicited by prime vaccination of a pig with sheep erythrocytes. Hemolysis could not be reduced in the presence of high anti-erythrocyte IgG titers as shown by hemolysis assays conducted with a porcine anti-erythrocyte serum drawn fourteen days after prime booster immunization (Fig. S2). Hemolysis caused by the classical complement pathway was abrogated only by rIdeSsuis and rIdeSsuis_homologue, not by rIdeSsuis_C195S, rIdeSsuis_homologue_C195S or rIdeSsuis_C_domain (Figure 2(b)). Thus, the IgM cleaving activity of rIdeSsuis alone must be responsible for abrogation of complement activation in the presence of high IgM titers. An impact of the C-terminal domain of IdeSsuis on complement activity could not be demonstrated since hemolysis levels remained unaffected by addition of rIdeSsuis_C-domain (Figure 2(b)). Hemolysis assays in the presence of high anti-erythrocyte IgG titers revealed that none of the tested rIdeSsuis constructs, even those with IgM protease activity, were able to reduce hemolysis (Fig. S2). In concordance with the results obtained by the hemolysis assay, IgM labeling of sheep erythrocytes was significantly reduced by rIdeSsuis and rIdeSsuis_homologue, but not by the point mutated constructs rIdeSsuis_C195S and rIdeSsuis_homologue_C195S as shown by flow cytometry (Figure 2(c)).
Figure 2.
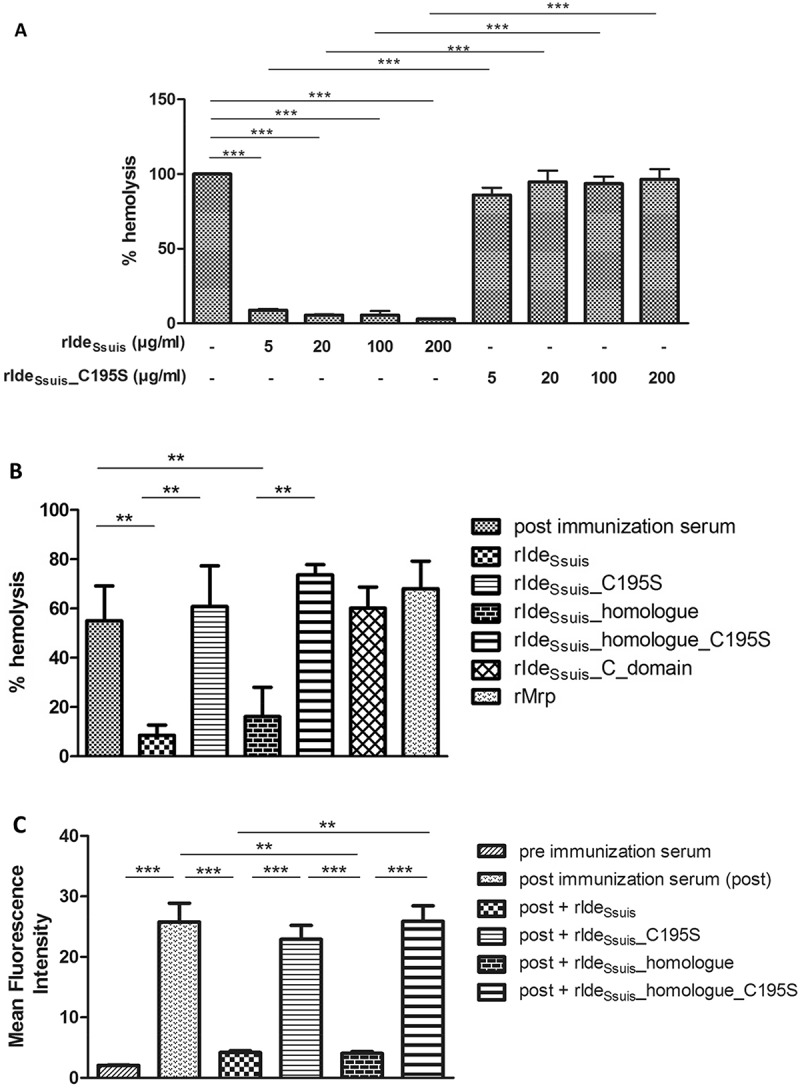
In the presence of high S. suis specific IgM titers, complement-mediated hemolysis and labeling of sheep erythrocytes with IgM are significantly reduced by rIdeSsuis constructs with IgM cleaving activity, but not by rIdeSsuis constructs lacking IgM cleavage activity due to the C195S point mutation. Hemolysis assays were performed by addition of purified sheep erythrocytes to pig anti-sheep erythrocyte serum which had been pretreated with either different concentrations of rIdeSsuis and rIdeSsuis_C195S (a) or different recombinant IdeSsuis constructs (b). The assays were performed with porcine serum drawn seven days after immunization with sheep erythrocytes (n = 4). Bars and error bars represent mean and standard deviation and significant differences are indicated. (a) Hemolysis induced by water was defined as one hundred percent and is represented by the first bar. (b) rIdeSsuis wt, rIdeSsuis_C195S, rIdeSsuis_homologue, rIdeSsuis_homologue_C195S, rIdeSsuis_C_domain, rMrp were compared regarding their ability to reduce complement mediated hemolysis at 18 µg/ml. Recombinant Mrp served as a control protein and was purified the same way as rIdeSsuis constructs. Post immunization serum without either rIdeSsuis construct served as negative control. (c) Flow cytometric analysis of IgM labeled sheep erythrocytes was performed after addition of purified sheep erythrocytes to a heat inactivated porcine anti-sheep erythrocyte serum drawn seven days after immunization and pretreated with 18 µg/ml of the indicated rIdeSsuis constructs. Serum drawn prior to immunization with sheep erythrocytes served as negative control. Serum drawn seven days post immunization served as positive control. Bars and error bars show mean values and standard deviations (n = 6). Significant differences are indicated by asterisks. Probabilities were considered as follows p < 0.05 *, p < 0.01 **, p < 0.001 ***.
Addition of rIdeSsuis and rIdeSsuis_homologue, but not rIdeSsuis_C195S and rIdeSsuis_homologue_C195S promotes survival of S. suis 10∆ideSsuis in porcine blood ex vivo
Previous work showed that the deletion mutant 10∆ideSsuis is attenuated in survival in porcine blood with high specific anti-S. suis IgM titers ex vivo [10]. To investigate if the attenuation is due to the inability of the mutant to cleave porcine IgM, blood survival assays with external complementation of the mutant with functional rIdeSsuis and non-functional rIdeSsuis_C195S were performed. In these assays, S. suis 10∆ideSsuis could survive significantly better in porcine blood of growing piglets if rIdeSsuis was externally added (Figure 3(a)). Survival was increased in a concentration depend manner starting at a rIdeSsuis concentration of 2 µg/ml. Addition of 2 µg/ml rIdeSsuis led to a survival factor of the deletion mutant that was almost twice as high as that of the wt. Addition of 20 µg/ml rIdeSsuis increased the survival factor of the deletion mutant to levels almost nine times as high as the survival factor of the wt. Importantly, there was no increase in bacterial survival by addition of rIdeSsuis_C195S, even at a concentration of 20 µg/ml (Figure 3(a)). In the plasma of samples where rIdeSsuis had been added to 10∆ideSsuis, IgM cleavage products were detectable by anti-IgM Western blot analysis (Figure 3(b)). Cleavage products started to appear at 2 µg rIdeSsuis/ml. IgM cleavage products were not detectable for blood samples incubated with S. suis wt or 10∆ideSsuis supplemented with rIdeSsuis_C195S. A further independent blood survival assay was conducted with the addition of 20 µg/ml rIdeSsuis_homologue and rIdeSsuis_homologue_C195S to S. suis 10∆ideSsuis. This assay confirmed that survival of 10∆ideSsuis can only be increased by addition of rIdeSsuis constructs with IgM cleavage activity (Figure 3(c)). In conclusion, survival of S. suis 10∆ideSsuis in porcine blood is restricted by IgM under the chosen experimental conditions. Furthermore, IgM cleavage through addition of rIdeSsuis or rIdeSsuis_homologue is responsible for the significant increase in survival of the S. suis 10∆ideSsuis mutant.
Figure 3.
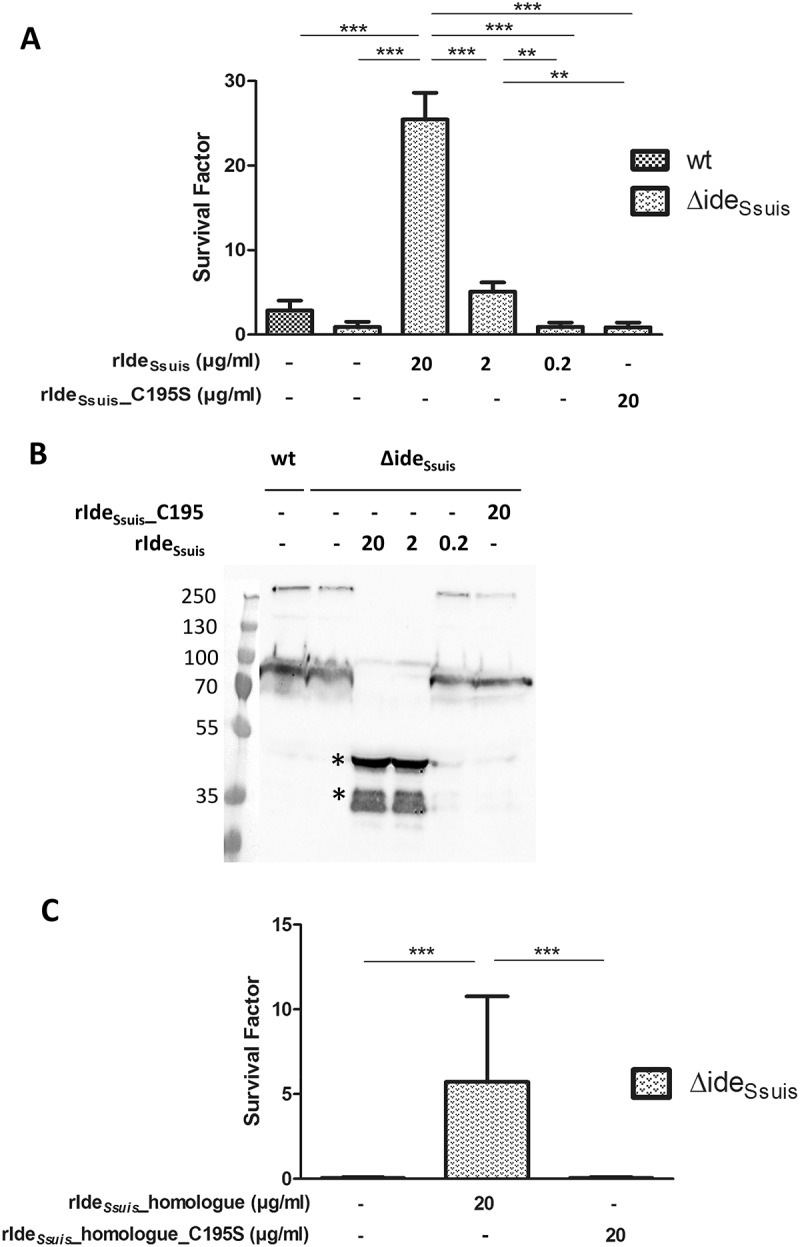
IgM proteolysis by rIdeSsuis and rIdeSsuis_homologue promotes survival of S. suis 10∆ideSsuis in porcine blood. (a) Blood survival assay in whole porcine blood of growing piglets with high specific anti-S. suis IgM antibody titers. S. suis 10∆ideSsuis was supplemented with 0.2, 2, 20 µg rIdeSsuis or 20 µg rIdeSsuis_C195S (n = 8) per ml blood. The survival factor of S. suis strain 10 and the isogenic deletion mutant 10∆ideSsuis in porcine blood with the addition of rIdeSsuis and rIdeSsuis_C195S after a two-hour incubation period at 37°C is depicted. Bars and error bars represent means and standard deviations. Significant differences are indicated. Probabilities were considered as follows p < 0.05 *, p < 0.01 **, p < 0.001 ***. (b) Anti-IgM Western blot analysis of plasma after the blood survival assay shown in Figure 4(a). IgM cleavage products were detectable in plasma after a blood survival assay including 2 and 20 µg rIdeSsuis and as faint bands in association with incomplete cleavage also for 0.2 µg rIdeSsuis. Marker bands in kDa are shown on the left-hand side. Asterisks indicate IgM cleavage products for the first positive lane. (c) Blood survival assay of S. suis 10∆ideSsuis in whole porcine blood of growing piglets (n = 6). S. suis 10∆ideSsuis was supplemented with 20 µg rIdeSsuis_homologue or rIdeSsuis_homologue_C195S per ml blood (n = 6). Survival factors were calculated and are depicted analogously to Fig. (A).
Reduction of bacterial labeling with IgM depends on the IgM cleaving activity of IdeSsuis
Two complemented S. suis mutants were generated to investigate the role of IgM cleavage by IdeSsuis in the pathogenesis of S. suis diseases further. Firstly, 10∆ideSsuis was chromosomally complemented to render 10∆ideSsuis∇ideSsuis_ EcoRI expressing wt IdeSsuis. A silent mutation (EcoRI site) was introduced in this strain for further discrimination of wt and complemented mutant. Secondly, the cysteine to serine point mutation of IdeSsuis was implemented in the chromosome of S. suis itself to render ∆ideSsuis∇ideSsuis_C195S. Site-directed mutagenesis was confirmed using sequencing and Southern blot analysis including confirmation of the silent mutation leading to the new EcoRI site in 10∆ideSsuis∇ideSsuis_ EcoRI (Fig. S4 and S5). Anti-IgM Western blot analysis showed that the complemented strain 10∆ideSsuis∇ideSsuis_EcoRI possesses full IgM cleavage activity whereas 10∆ideSsuis∇ideSsuis_C195S is unable to cleave porcine IgM (Figure 4(a)) even though it expresses a stable IdeSsuis antigen (Fig. S3).
Figure 4.
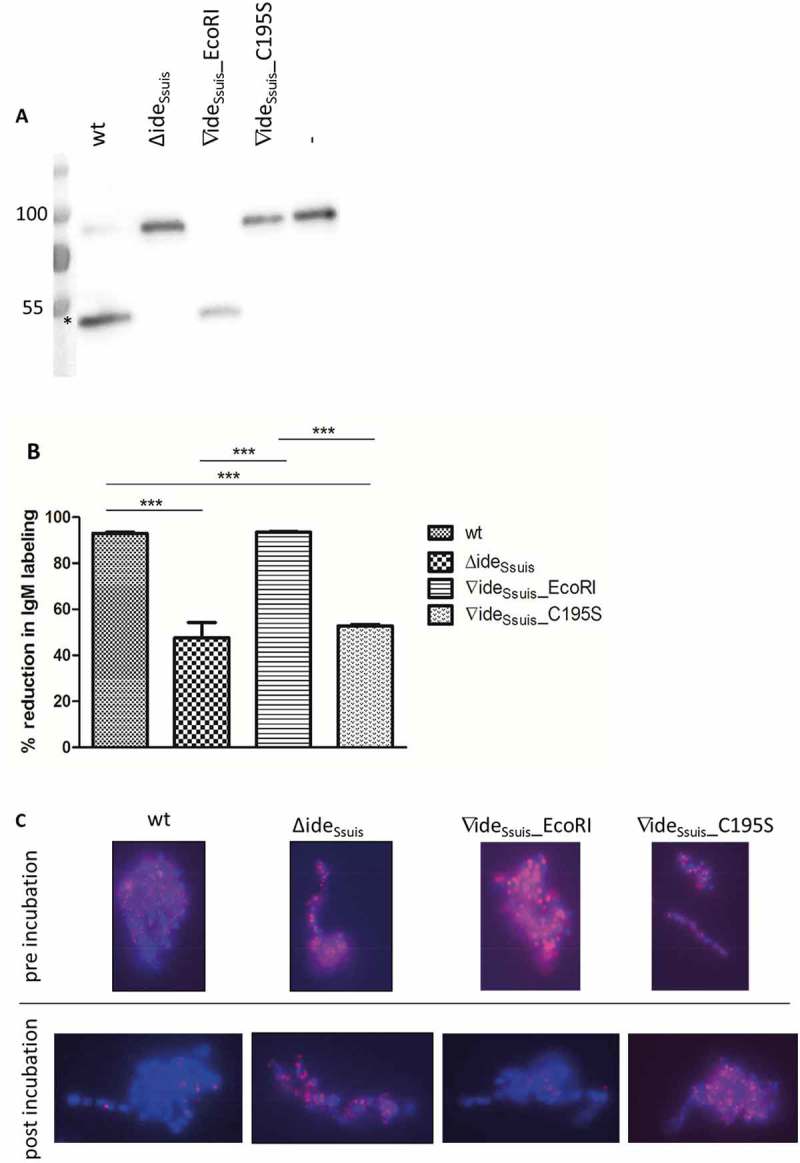
S. suis strain 10 (wt) and the complemented mutant 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI) cleave porcine IgM (a) and reduce bacterial IgM labeling by over 90% (B,C), in contrast to 10∆ideSsuis (∆ideSsuis) and the point mutated mutant 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S). (a) Twenty-four-fold concentrated supernatants of the respective strains were incubated with 1:100 diluted porcine serum, followed by anti-porcine IgM Western Blot analysis under reducing conditions. Incubation of serum with phosphate-buffered saline was used as negative control (-). An 8% separating gel was used for gel electrophoresis and a polyclonal anti-IgM antibody for detection of IgM. Marker bands in kDa are shown on the left-hand side. Asterisks indicate IgM cleavage products on the left side of the first positive lane. (b, c) The indicated S. suis strains were grown to an OD600 of 0.8, incubated in anti-S. suis serotype 2 hyperimmune serum for 0.5 hours at 4°C and then for four hours at 37°C. IgM labeling of the bacterial surface was analyzed before and after incubation at 37°C by flow cytometry (n = 6) (b) and fluorescent microscopy (c) using a monoclonal anti-IgM antibody and a phycoerythrin (PE)-labeled secondary antibody. The % reduction in IgM labeling was calculated by subtracting the percental amount of IgM positive bacteria after a four-hour incubation period at 37°C from an initially one hundred percent positive population before incubation at 37°C. (c) DAPI (4ʹ,6 diamidino-2-phenylindole) dye in blue was used to stain DNA. IgM, labeled by the monoclonal anti IgM antibody and the PE-labeled secondary antibody appears in pink. Bars and error bars indicate mean and standard deviation. Significant differences are indicated by asterisks. Probabilities were considered as follows p < 0.05 *, p < 0.01 **, p < 0.001 ***.
Opsonization induced by antibodies binding to the bacterial surface is an important immune defense mechanism. Previous work showed that the expression of IdeSsuis leads to reduction of surface bound IgM [11]. We hypothesized that point mutation of the cysteine 195 and consequently loss of IgM cleavage activity would lead to the inability of IdeSsuis to reduce the amount of surface-bound IgM. S. suis wt, 10∆ideSsuis and the complemented mutants 10∆ideSsuis∇ideSsuis_EcoRI and 10∆ideSsuis∇ideSsuis_C195S were therefore analyzed regarding their ability to reduce labeling of bacteria with IgM. Flow cytometry (Figure 4(b)) and immunofluorescence microscopy (Figure 4(c)) showed that S. suis wt and 10∆ideSsuis∇ideSsuis_EcoRI reduced surface-bound IgM by over 90% in contrast to 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S. Reduction by the wt and complemented mutant was significantly higher than that by the mutants 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S. None of the latter possessed IgM cleaving activity. We hypothesized that upon IgM cleavage, Fc fragments would become detached from the bacterial surface, whereas F(ab’)2 fragments might remain attached to it as IdeSsuis cleaves the heavy chain of IgM at the N-terminus of the C3 domain [9]. We therefore analyzed bacterial labeling with IgM using IgM-F(ab’)2 and IgM-Fc specific antibodies, that were tested in Western Blot analysis to recognize the respective parts of porcine IgM only (Fig. S6). Flow cytometry with these specific antibodies revealed that both the percentage of F(ab’)2 positive and Fc positive cells within the S. suis population were reduced by IdeSsuis activity (Figure 5(a,c)). Analogously, the geometric mean fluorescence intensity (MFI) of both the F(ab’)2 and the Fc signal was reduced by IdeSsuis activity (Figure 5(b,d)). The reduction of the IgM-F(ab’)2 signal was generally greater than that of the IgM Fc signal. This was true also for the percentage of the F(ab’)2 and Fc positive cells. Hence it can be concluded that IdeSsuis-mediated IgM cleavage leads not only to detachment of IgM Fc fragments form the bacterial surface but of IgM F(ab’)2 fragments as well.
Figure 5.
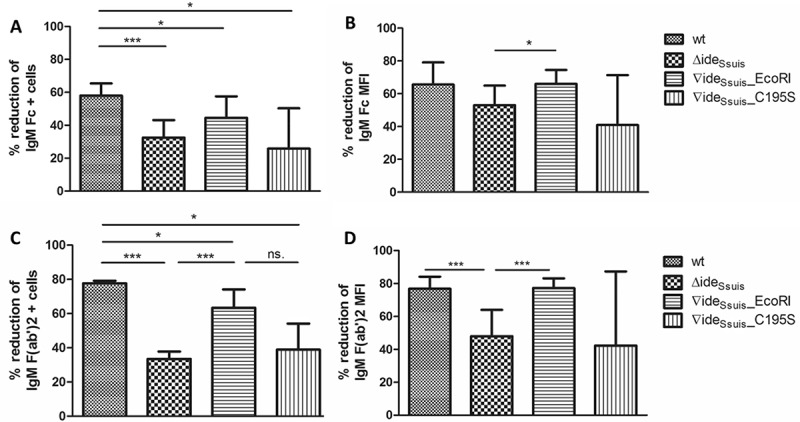
IgM cleavage by IdeSsuis reduces surface bound F(ab‘)2 and Fc antigen of porcine IgM. S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis), 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI), 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S) were incubated in a porcine anti-S. suis serotype 2 hyperimmune serum for 0.5 hours at 4°C and then for four hours at 37°C. Bacteria were stained with IgM F(ab‘)2 and IgM Fc specific antibodies and measured by flow cytometry (n = 7) before and after incubation at 37°C. (a) Reduction of the percentage of IgM Fc positive bacteria. (b) Reduction of the geometric mean fluorescence intensity (MFI) of the IgM Fc signal. (c) Reduction of the percentage of IgM F(ab‘)2 positive bacteria. (d) Reduction of the geometric mean fluorescence intensity (MFI) of the IgM F(ab‘)2 signal. The reduction in IgM labeling was calculated by subtracting the percental amount or MFI of IgM positive bacteria after a four-hour incubation period at 37°C from an initially one hundred percent positive population before incubation at 37°C. Bars and error bars indicate mean and standard deviation. Asterisks indicate significant differences. Probabilities were considered as follows p < 0.05 *, p < 0.01 **, p < 0.001 ***.
IgM cleavage by rIdeSsuis reduces deposition of the complement component C3 on the bacterial surface
Differences in surface-bound IgM in the investigated S. suis strains led us to hypothesize that the loss of IgM cleavage activity of expressed IdeSsuis leads to increased activation of the classical complement pathway and thus increased labeling of bacteria with C3b antigen. Using a serum depleted of IgG antibodies, it was shown that 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S were indeed significantly more labeled with C3 than S. suis wt (Figure 6). Surprisingly, also the complemented mutant 10∆ideSsuis∇ideSsuis_EcoRI was significantly more labeled with C3 than the wt (∇ideSsuis_EcoRI: 28.94% C3+ cells; SD: 2.6 vs. wt: 12.18% C3+ cells; SD: 1.6). When rIdeSsuis was added to 10∆ideSsuis, C3 labeling was reduced by 91% (Figure 6). A significant reduction in the percentage of C3 positive cells also occurred when rIdeSsuis_C195S was added to the deletion mutant, though with 28.5% it was much less pronounced. In fact, 10∆ideSsuis supplemented with rIdeSsuis_C195S was significantly more C3 labeled than 10∆ideSsuis supplemented with functional rIdeSsuis (17.76% C3+ cells; SD: 0.6 vs. 2.22% C3+ cells, SD: 0.3). Furthermore, addition of rIdeSsuis to 10∆ideSsuis led to a significant reduction in C3 labeling even when compared to the level of C3 labeling of the wt (wt: 12.18% C3+ cells; SD: 1.6 vs. 10∆ideSsuis + rIdeSsuis: 2.22% C3+ cells; SD: 0.3). Thence it can be concluded that activation of the classical complement pathway and subsequent C3 labeling of S. suis is reduced by the IgM cleaving activity of rIdeSsuis.
Figure 6.
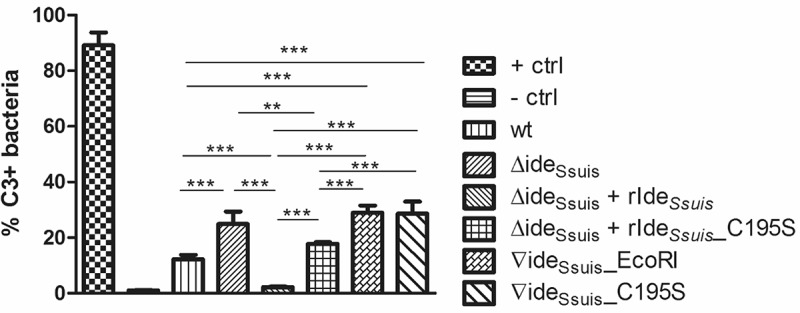
IgM cleavage activity by rIdeSsuis leads to reduction of C3 antigen on the bacterial surface. S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis), 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI), 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S) were incubated in a 1:2 diluted IgG depleted porcine anti-S. suis serotype 2 hyperimmune serum. After incubation at 37°C, bacteria were stained with a FITC-labeled rabbit anti-human C3c antibody and measured by flow cytometry. S. suis strain 10 incubated in undiluted serum served as positive control (+ ctrl). S. suis strain 10 incubated with heat-inactivated serum served as negative control (- ctrl). Bars and error bars represent mean and standard deviation. Significant differences are indicated by asterisks. Probabilities were considered as follows p < 0.05 *, p < 0.01 **, p < 0.001 ***.
S. suis survival in porcine blood is restricted by active complement
The ability to survive in blood is an important feature for any pathogen causing bacteremia and systemic diseases after dissemination in the blood. We hypothesized that loss of IgM cleavage would lead to increased killing of S. suis in porcine blood of growing piglets with high titers of S. suis specific IgM. Blood survival assays revealed significantly higher survival factors (SF) for S. suis wt than for 10∆ideSsuis in the blood of eight-week-old piglets with high anti-S. suis IgM (71.4 ELISA units) and high anti-S. suis IgG (77.6 ELISA units) antibody titers (Figure 7(a)). S. suis wt exhibited also higher survival factors in porcine blood than 10∆ideSsuis∇ideSsuis_C195S and 10∆ideSsuis∇ideSsuis_EcoRI, although the differences were not significant (Figure 7(a)). Nevertheless, S. suis mutants without IgM cleavage activity (10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S) had lower survival factors than S. suis wt and 10∆ideSsuis∇ideSsuis_EcoRI (wt SF: 2.2, SD: 1.7; 10∆ideSsuis∇ideSsuis_C195S SF: 0.7, SD: 0.7; 10∆ideSsuis∇ideSsuis_EcoRI SF: 0.9; SD: 1.2; 10∆ideSsuis SF: 0.5, SD: 0.48).
Figure 7.
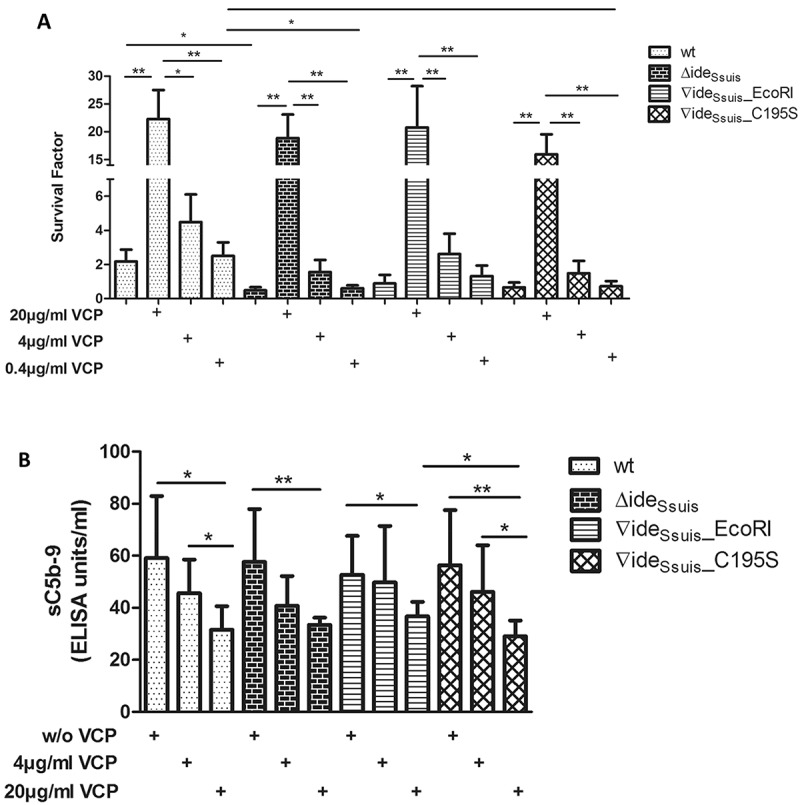
Survival of S. suis serotype 2 in porcine blood is restricted by active complement. (a) Survival of S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis) and the two complemented strains 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI) and 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S) was analyzed in a whole blood survival assay with the addition of the complement inhibitor vaccinia virus complement control protein (VCP). Porcine whole blood from growing piglets (n = 6) with high anti-S. suis IgM titers was pre-incubated with 0.4, 4, 20 µg VCP/ml for five minutes at 37°C. 4 × 106 CFU/ml of the indicated S. suis strains were then added and the survival factor determined by plate counting after two hours at 37°C. (b) Soluble C5b-9 was determined in porcine plasma after the blood survival assay by sC5b-9 ELISA. A concentration dependent reduction in sC5b-9 levels was detectable after addition of VCP to porcine whole blood (Pearson r values: wt: −0,9487; 10∆ideSsuis: −0,8485; 10∆ideSsuis∇ideSsuis_EcoRI: −0,9999; 10∆ideSsuis∇ideSsuis_C195S: 0,9821). Bars and error bars indicate mean and standard deviation. Significant differences are indicated by asterisks. Probabilities were considered as follows p < 0.05 *, p < 0.01 **, p < 0.001 ***.
To investigate the role of complement in survival of S. suis and mutants in porcine blood, the C3 convertase inhibitor vaccinia virus complement control protein (VCP) was added to the blood prior to the addition of bacteria. VCP inhibits both the classical and the alternative C3 convertase and has been shown to inhibit the complement cascade in porcine blood [19]. VCP was used at concentrations where it is known to inhibit complement significantly but not completely [19]. The addition of VCP led to a concentration dependent increase of survival of all strains in porcine blood (Figure 7(a)). Only at concentrations of 20 µg VCP/ml blood the survival of S. suis increased significantly above levels without complement inhibitor. Addition of 4 µg/ml VCP led to a doubling of the survival factor of the wt, a triplication of the survival factor of 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_EcoRI and a 2.4-fold increase of the survival factor of 10∆ideSsuis∇ideSsuis_C195S, but differences to the samples without VCP were not significant. Furthermore, at 4 µg/ml VCP the survival factors of S. suis wt and 10∆ideSsuis∇ideSsuis_EcoRI were higher than those of 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S although differences were not significant (wt vs. 10∆ideSsuis p = 0.109, wt vs. 10∆ideSsuis ∇ideSsuis_ C195S p = 0.101, 10∆ideSsuis ∇ideSsuis_EcoRI vs. 10∆ideSsuis p = 0.943, 10∆ideSsuis ∇ideSsuis_EcoRI vs. 10∆ideSsuis ∇ideSsuis_C195S p = 0.798). Interestingly, addition of 4 µg/ml VCP to 10∆ideSsuis led to an increased survival of the mutant approximating S. suis wt levels (SF wt: 2.2, SD: 1.712; SF 10∆ideSsuis + 4 µg VCP: 1.6, SD: 1.749). As a read-out parameter for complement activation in this assay, soluble C5b-9 (sC5b-9) was measured after the described blood survival assay. The sC5b-9 ELISA revealed a concentration dependent decrease in sC5b-9 levels in samples with VCP (Figure 7(b)). Significant differences in sC5b-9 levels between S. suis strains were not detected. In conclusion, survival of S. suis in porcine blood is restricted by active complement.
S. suis wt strain 10 shows higher survival factors than the mutant strains 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S in opsonophagocytosis assays
As tissue infected with S. suis is mainly infiltrated by neutrophils, opsonophagocytosis assays were conducted to specifically investigate the role of IgM cleavage in evasion of killing by these immune cells. Opsonophagocytosis assays did indeed reveal that the survival factor of S. suis strain 10 (SF mean: 3.06, SD: 1.36) was significantly higher than that of 10∆ideSsuis (SF mean: 1.27, SD: 0.85) and 10∆ideSsuis ∇ideSsuis C195S (mean: 1.36, SD: 0.59) (Fig. S7). However, the survival factor of S. suis wt was also significantly higher than that of 10∆ideSsuis ∇ideSsuis_EcoRI (SF mean: 1.6, SD: 0.81).
The complemented S. suis 10∆ideSsuis∇ideSsuis_EcoRI mutant expresses a thinner and less dense polysaccharide capsule
Blood survival, opsonophagocytosis and C3 deposition assays revealed phenotypic differences between the complemented mutant 10∆ideSsuis∇ideSsuis_EcoRI and the wt. Despite full IgM cleavage activity, the complemented mutant showed lower survival factors in blood survival and opsonophagocytosis assays and was significantly more C3 labeled than the wt. We conducted comparative transmission electron microscopy of S. suis strain 10, 10∆ideSsuis, 10∆ideSsuis∇ideSsuis_EcoRI and 10∆ideSsuis∇ideSsuis_C195S to verify intact capsule expression in these strains. However, electron microscopy indicated that the morphology of the capsule of 10∆ideSsuis∇ideSsuis_EcoRI differed from that of the three other investigated strains (Figure 8). The capsule of this complemented strain was less dense and less compact. Additionally, measurements of the capsule thickness revealed that the capsule of 10∆ideSsuis∇ideSsuis_EcoRI was significantly thinner than that of the wt (mean capsule thickness of 10∆ideSsuis∇ideSsuis_EcoRI: 40.2 nm, SD: 4.0 vs. mean. capsule thickness of the wt: 60.7 nm, SD: 5.3). Differences in capsule expression between S. suis strain 10, 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S were not recorded. PCR analysis of 16 kb of the cps2 locus of S. suis strain 10 and 10∆ideSsuis ∇ideSsuis_EcoRI was conducted to investigate if larger deletions or insertions in cps genes were detectable in the complemented mutant. However, the cps amplification products did not differ in size between S. suis strain 10 and 10∆ideSsuis ∇ideSsuis_EcoRI (Fig. S8). In conclusion, the attenuated phenotype of 10∆ideSsuis∇ideSsuis_EcoRI might be due to an aberrant capsule morphology, but the reason for this phenotypic alteration is not known.
Figure 8.
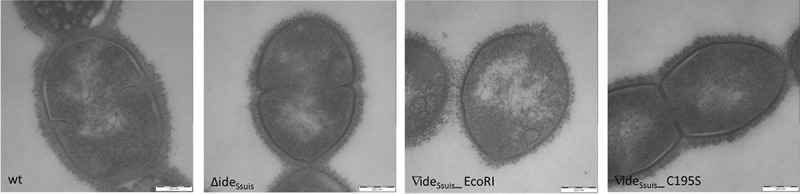
Transmission electron microscopy reveals a different capsule morphology for S. suis 10∆ideSsuis∇ideSsuis_ EcoRI. The expression and thickness of the capsule of S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis), 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S) and ∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI) was investigated by transmission electron microscopy using lysine-ruthenium red staining. The morphology of the capsule of the complemented S. suis mutant ∇ideSsuis_EcoRI differs from that of the other strains in that it appears less dense and significantly thinner.
IgM cleavage by IdeSsuis is not crucial for virulence of S. suis serotype 2 in growing piglets
Former results in our laboratory suggested an attenuation of 10∆ideSsuis in prime-vaccinated growing piglets [20]. Data on bacterial survival in porcine blood generated in this study also hinted at a potential attenuation of S. suis mutants without IgM cleavage activity. An animal infection experiment was consequently conducted to investigate the role of IgM cleavage by IdeSsuis in the pathogenesis of S. suis serotype 2 infection by comparing the mutant 10∆ideSsuis∇ideSsuis_C195S to the wt. Immunological screening of the piglets prior to infection revealed moderate to high anti-S. suis IgM (mean of 52.2 ELISA units over all infection groups) and IgG (mean of 129.6 ELISA units over all infection groups) titers and very low anti-IdeSsuis antibody titers (mean of 1.5 ELISA units over all infection groups) (Fig S.9). Antibody titers did not differ significantly between the infection groups. Sixty-seven percent of piglets infected with either the wt, 10∆ideSsuis∇ideSsuis_EcoRI or 10∆ideSsuis∇ideSsuis_C195S survived the experiment versus 44.4% of piglets infected with 10∆ideSsuis (Figure 9(a)). In the wt, 10∆ideSsuis, 10∆ideSsuis∇ideSsuis_C195S and 10∆ideSsuis∇ideSsuis_EcoRI infection groups four, three, five and six out of nine animals each showed no clinical signs of disease, respectively (Figure 9(b)). Pathological screenings revealed that five of nine 10∆ideSsuis, three of nine 10∆ideSsuis∇ideSsuis_C195S and three of nine wt infected animals had moderate or severe fibrinous-suppurative meningitis (Table 1). Thus, meningitis was found as often or even more often in piglets infected with strains not cleaving IgM. The pathohistological scores for suppurative and fibrinous inflammations of predefined tissues were as follows (ranging from 0 to 5): wt infected group ω = 1.78, 10∆ideSsuis infection group ω = 2.78, 10∆ideSsuis∇ideSsuis_EcoRI group ω = 1.22, 10∆ideSsuis∇ideSsuis_C195S ω = 1.89 (Table 1). The respective infection strains were re-isolated from both the brain and the cerebrospinal fluid (CSF) of all piglets with signs of central nervous system disorder (Table 2). Additionally, the infection strains could be reisolated from various inner organs, namely spleen, liver, lung, endocard and peritoneum (Table 2). EcoRI restriction enzyme digest of ideSsuis-PCR products of reisolates from the wt, the 10∆ideSsuis∇ideSsuis_C195S and the 10∆ideSsuis∇ideSsuis_EcoRI infection groups demonstrated that the complemented strain had kept both the ideSsuis gene and the EcoRI cleavage site within since it was clearly distinguishable from the wt and 10∆ideSsuis∇ideSsuis_C195S by a 799 bp EcoRI cleavage product. Sequencing of the ideSsuis gene of all reisolates of the 10∆ideSsuis∇ideSsuis_C195S infection group confirmed that the C195S mutation was still present. In conclusion, IgM cleavage of S. suis serotype 2 is not crucial for causing meningitis in growing piglets.
Figure 9.
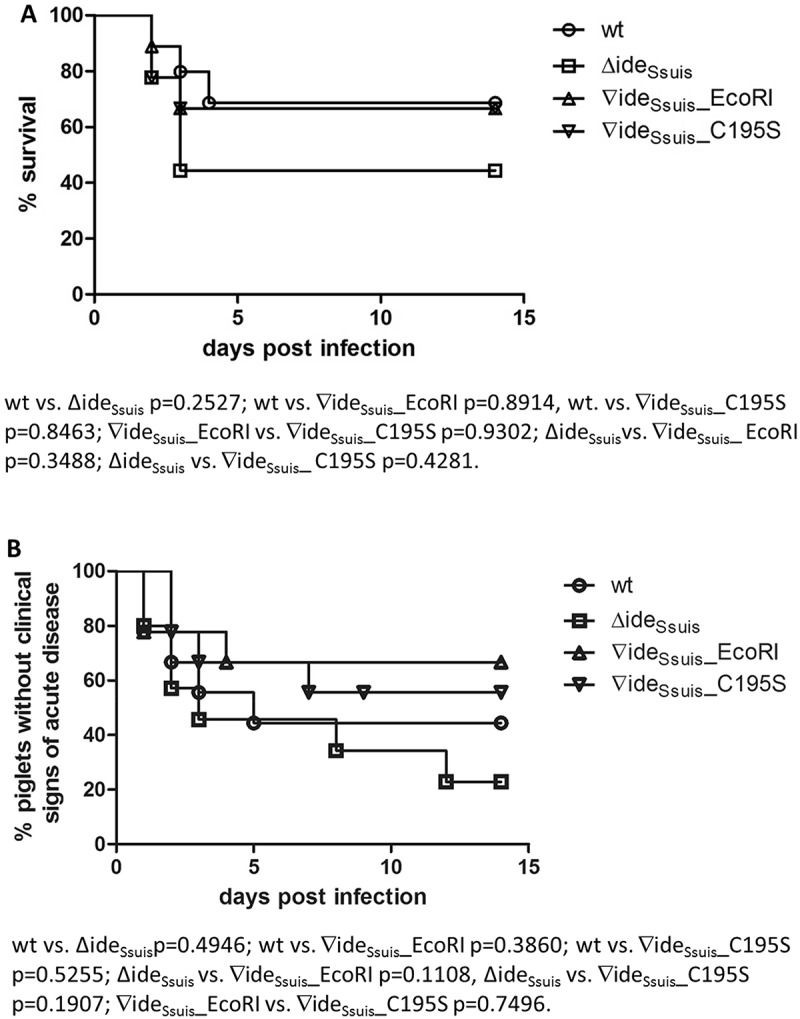
Mortality (a) and morbidity (b) of growing piglets experimentally infected with the indicated S. suis strains. Eight-week old piglets were infected with S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis), 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI), 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S). Morbidity was defined as an inner body temperature equal to or greater than 40.2°C and/or typical clinical signs of S. suis disease such as acute lameness or convulsions. Statistical analysis of the Kaplan-Meier diagrams was performed using the log-rank test. P-values are shown below the diagrams.
Table 1.
Histopathological scoring of fibrinosuppurative lesions of growing piglets challenged with the indicated S. suis strains.
| brain |
serosae |
joint |
spleen, liver |
lung |
heart |
||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| meningitis, chorioiditis |
pleuritis, peritonitis |
synovialitis |
splenitis, hepatitis |
pneumonia |
endocarditis |
||||||||||||||||||
| infection straina | piglets w/o lesions | piglets with lesions ≥ 3 locations |
5b | 3c | 1d | 4b | 2c | 1d | 4b | 2c | 1d | 4b | 2c | 1d | 4b | 2c | 1d | 4b | 2c | 1d | ωe | ||
| wt | 4/9 | 0/9 | 3/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 2/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 1.89 | ||
| ∆ideSsuis | 4/9 | 0/9 | 5/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 3/9 | 0/9 | 1/9 | 0/9 | 0/9 | 0/9 | 0/9 | 2.78 | ||
| ∇ideSsuis_EcoRI | 6/9 | 0/9 | 1/9 | 2/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 1/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 1.22 | ||
| ∇ideSsuis_C195S | 5/9 | 0/9 | 3/9 | 0/9 | 0/9 | 0/9 | 0/9 | 1/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 2/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 0/9 | 1.78 | ||
aInfection strains were S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis), 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI), 10∇ideSsuis ∇ideSsuis_C195S (∇ideSsuis_C195S).
bScores of 4 or 5 were assigned to moderate to severe diffuse or multifocal fibrinosuppurative inflammations
cScores of 3 or 2 were assigned to mild focal fibrinosuppurative inflammations
dA score of 1 was assigned to individual single perivascular neutrophils
eω = ∑scoremax/nanimals
Table 2.
Reisolation of the infection strains from piglets infected with the indicated S.suis strains.
| infection straina | no. of piglets with an isolate of the infection strain in ≥ 1 inner organb | no. of piglets with indicated site of infection straina isolation/total number of piglets |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| tonsils | lungc | serosad | spleen | liver | brain, CSFe | joint fluidf | endocard | blood | ||
| wt | 3/9 | 3/9 | 0/9 | 0/9 | 3/9 | 2/9 | 3/9 | 0/9 | 0/9 | 0/9 |
| ∆ideSsuis | 5/9 | 3/9 | 1/9 | 0/9 | 5/9 | 4/9 | 5/9 | 0/9 | 2/9 | 1/9 |
| ∇ideSsuis_EcoRI | 3/9 | 4/9 | 1/9 | 1/9 | 1/9 | 2/9 | 3/9 | 0/9 | 0/9 | 1/9 |
| ∇ideSsuis_C195S | 3/9 | 3/9 | 1/9 | 0/9 | 3/9 | 3/9 | 3/9 | 0/9 | 1/9 | 0/9 |
aInfection strains were S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis), 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI), 10∇ideSsuis ∇ideSsuis_C195S (∇ideSsuis_C195S).
bIsolates exclusively from the tonsils were not considered
cThe left cranial lobe was investigated
dPleural, peritoneal or pericardial cavity
eCerebrospinal fluid
fAspirates of both left and right carpal and tarsal joints were investigated in each animal
Investigation of putative IgM cleavage in vivo
The CSF of one piglet with meningitis per infection group was investigated for detection of IgM cleavage products by anti-IgM Western Blot analysis. The chosen piglets all had a high intracerebrospinal S. suis burden (3–5 x 107 CFU/ml) but varying intracerebrospinal anti-S. suis IgM titers (< 0.1–7.7 ELISA units/ml). IgM cleavage products were not detected in the CSF of these experimentally infected piglets with acute meningitis (Figure 10, left). Addition of rIdeSsuis to the CSF of the same piglets, however, gave rise to IgM cleavage products (Figure 10, right). S. suis in the CSF of the same animals was analyzed via flow cytometry for detection of IgM on the bacterial surface using anti IgM F(ab’)2 and anti IgM Fc specific antibodies. S. suis in the CSF of experimentally infected piglets was labeled with porcine IgM regardless of the infection strain (Figure 11). However, differences between the individual animals existed regarding the percentage of IgM F(ab’)2 and IgM Fc positive bacteria. In summary, bacteria labeled with uncleaved IgM, as well as bacteria without IgM on the bacterial surface could be detected for all strains. However, IgM labeling seemed to depend on the intracerebrospinal IgM concentration rather than the S. suis strain’s ability to cleave IgM. Animals with intracerebrospinal IgM concentrations greater than 5.6 ELISA units/ml had a higher percentage of IgM F(ab’)2 positive and IgM Fc positive cells than those with lower IgM titers. In conclusion, flow cytometric analysis indicated that IgM might still be bound to the bacterial surface of S. suis wt in the CSF of piglets with acute meningitis, at least in the case of intracerebrospinal IgM concentrations above five ELISA units.
Figure 10.

IgM Western blot analysis of cerebrospinal fluid (CSF) of experimentally infected piglets with acute meningitis and high intracerebrospinal S. suis burden reveals only intact IgM. Piglets were intranasally infected with the indicated strains and the CSF of one pig with meningitis of each infection group was analyzed for the presence of IgM cleavage products by anti-IgM Western Blot analysis under non-reducing conditions with a monoclonal anti-IgM antibody. Protein concentrations in the CSF were adjusted before loading the 6% polyacrylamide gel. As a control (lanes 7–10), CSF of the same piglets was incubated with 5 µg/ml rIdeSsuis. Asterisks indicate IgM cleavage products for the last positive lane. Marker bands (in kDa) are shown on the left-hand side.
Figure 11.
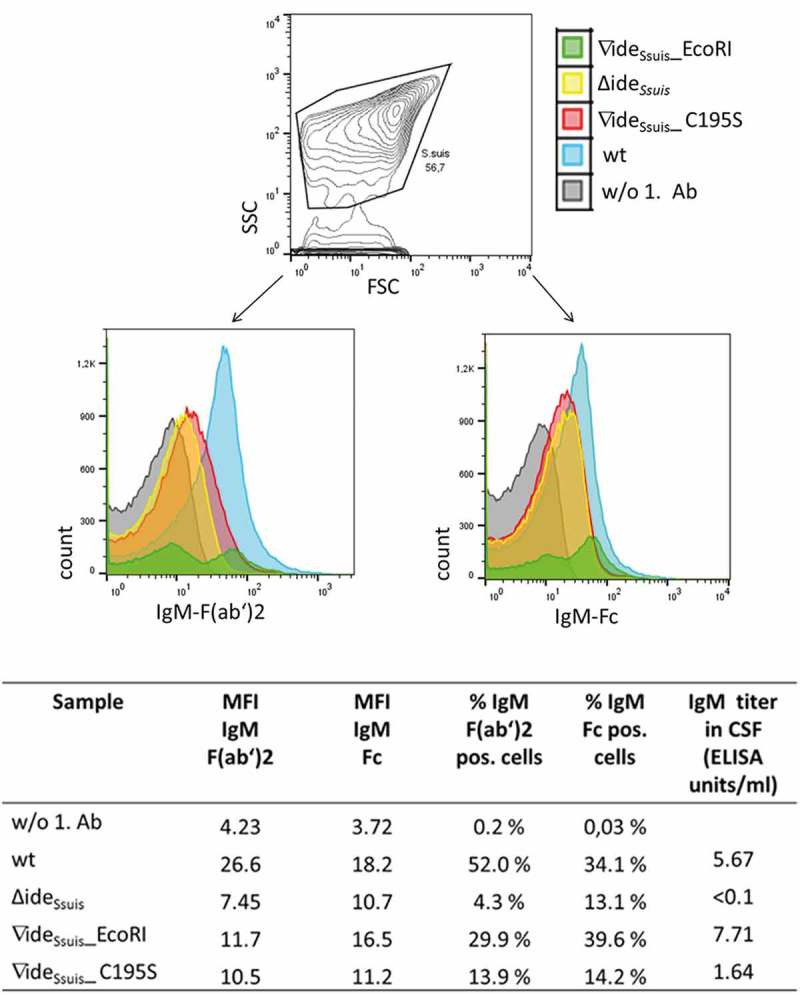
S. suis in the CSF of experimentally infected piglets is labeled with porcine IgM. Eight-week old piglets were intranasally infected with S. suis strain 10 (wt), 10∆ideSsuis (∆ideSsuis), 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI) and 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S), respectively. The CSF of one pig with meningitis of each infection group was centrifuged and the intracerebrospinal S. suis population stained with anti-IgM F(ab’)2 and anti-IgM Fc specific primary antibodies and phycoerythrin (PE) and fluorescein (FITC) labeled secondary antibodies. Bacteria were analyzed for the presence of IgM on their surface via flow cytometry. S. suis stained without the first antibodies served as negative control (w/o 1. Ab). The upper panel shows the gating strategy used to define the S. suis population. The lower panels shows the S. suis population in the CSF of infected piglets as overlay histograms of the F(ab’)2 signals (left hand side) and the Fc signals (right hand side). The table beneath the figure shows the geometric mean fluorescence intensity (MFI), the percentage of IgM F(ab’)2 and IgM Fc positive bacteria and the IgM titers of the four representative piglets.
Discussion
Previous research indicated, that the IgM protease IdeSsuis of S. suis is involved in complement evasion [10]. However, as conclusions were based on the comparison of the wt to the mutant 10∆ideSsuis only, it remained to be shown that the identified phenotypes depend on the lack of the IgM cleavage activity. Noteworthy, IdeSsuis is a very large protein compared to other immunoglobulin proteases and the C-terminal 674 amino acids of the protein are not necessary for cleavage of IgM [11]. This suggests that IdeSsuis might carry out an additional function, which might also be related to complement evasion. Thus, we designed experiments to specifically investigate the role of IgM cleavage, which is a unique phenotype of this bacterial pathogen. To this end, a single point mutation (cysteine to serine) was introduced in the putative active center of the IgM cleaving domain of rIdeSsuis and the isogenic mutant 10∆ideSsuis was chromosomally complemented to express wt IdeSsuis (10∆ideSsuis∇ideSsuisEcoRI), as well as point mutated IdeSsuis (10∆ideSsuis∇ideSsuis_C195S). As strain 10∆ideSsuis∇ideSsuis_C195S expresses stable, point mutated IdeSsuis lacking the IgM cleavage activity, this strain is a state-of-art tool to reveal phenotypes determined by this specific protease activity. Comparative investigations with this strain demonstrated that IdeSsuis-mediated modulation of IgM labeling is due to the IgM cleaving activity alone. Loss of function experiments and comparative phenotypic analysis adding rIdeSsuis and rIdeSsuis_C195S indicated further that IdeSsuis-mediated modulation of C3 deposition is also mainly due to the IgM cleavage activity. However, as addition of rIdeSsuis_C195S also reduced C3 deposition on the bacterial surface significantly, it is not unlikely that the large C-terminus carries out a further function related to complement evasion.
Homology to known cysteine proteases and an inhibitor profile [11] suggested that IdeSsuis is a cysteine protease. The inability of cysteine to serine point mutated rIdeSsuis to cleave porcine IgM, as shown in this study, confirms IdeSsuis as a cysteine protease. Thus, IdeSsuis is a true member of the IdeS-family of cysteine proteases annotated under the name of C66 in the MEROPS peptidase database (https://www.ebi.ac.uk/merops/cgi-bin/famsum?family=C66).
Since IgM is the most important activator of the classical complement cascade, we investigated the impact of IgM cleavage on the complement system. Mechanisms of complement evasion are multiple in pathogenic bacteria, viruses and yeast. Streptococcus pyogenes for example has been shown to use the C5a and C3 peptidase ScpA [21], M protein mediated C4BP binding [22] and the C5b-C9 inhibitory protein SIC [23] to circumvent complement. Gram negative Borrelia burgdorferi inhibits complement via multiple FH binding proteins [24] and enterohemorrhagic Escherichia coli can cleave C1-INH and thus enhance its complement inhibitory function [25]. For S. suis complement evasion has not been intensively studied and so far only the sialic acid containing capsule of certain serotypes and factor H binding molecules have been described as complement evasion factors [26,27]. However, complement plays an important role in immunity to S. suis disease. By using the complement inhibitor VCP we show here that survival of S. suis in porcine blood is very much restricted by active complement. Accordingly, C3 knockout mice are known to be highly susceptible to disseminated S. suis infection [27].
Hemolysis caused by a porcine anti-erythrocyte immune serum with high IgM titers is inhibited by addition of rIdeSsuis, suggesting that IdeSsuis is involved in a novel complement evasion mechanism [11]. In this study, we show that this inhibition depends entirely on the presence of a cysteine at position 195 in the putative active center of IdeSsuis. Since C195S point mutated rIdeSsuis did not influence complement-mediated hemolysis, the IgM cleaving activity of IdeSsuis alone must be responsible for reduction of complement mediated hemolysis. In accordance, IgM labeling of sheep erythrocytes was only significantly reduced by functional rIdeSsuis but not by rIdeSsuis_C195S.
IgM labeling of S. suis can be a prerequisite for subsequent surface-associated complement activation. We therefore analyzed IgM labeling of S. suis wt and mutants deficient in IgM cleavage and could show that labeling of S. suis with IgM is significantly reduced by IdeSsuis activity and that this reduction depends on the presence of a cysteine in the active center of IdeSsuis. Interestingly, not only the Fc fragments of porcine IgM detach from the bacterial surface upon IdeSsuis-mediated IgM cleavage, but the F(ab’)2 fragments disengage also. This phenomenon contrasts with labeling of bacteria with IgG antibodies upon cleavage by IdeS. Björk et al. showed that IgG Fab fragments remain attached to the bacterial surface upon IgG cleavage [28]. We propose that the detachment of IgM F(ab’)2 fragments is due to the lower affinity of IgM binding [29]. Disruption of the pentameric IgM structure could lead to weaker binding of IgM F(ab’)2 fragments to the bacterial surface and lead to subsequent detachment. If the release of IgM F(ab’)2 and Fc fragments modulates host responses remains to be determined. For IgG Fc fragments a priming effect on neutrophils has been reported [30]. Since neutrophils do not possess Fcµ or Fcα/µ receptors it remains questionable if they are pre-activated by IgM Fc cleavage products also.
Previous experiments with the specific classical complement pathway inhibitor EGTA MgCl2 and unencapsulated S. suis showed that C3 deposition on S. suis depends on the classical complement pathway [10]. The importance of the classical complement pathway in immunity to streptococcal infection was demonstrated by Brown et al. who identified it as the dominant pathway required for innate immunity to Streptococcus pneumoniae in mice [31]. We could show in this study, that the reduction of surface bound C3 through IdeSsuis expression of S. suis wt depends mainly on its IgM cleavage activity. The increased C3 deposition of the complemented mutant 10∆ideSsuis∇ideSsuisEcoRI is, to our minds, related to a defective capsule architecture of the mutant which was detected by transmission electron microscopy. Noteworthy, C3 deposition is also significantly enhanced on the surface of an isogenic unencapsulated mutant of S. suis serotype 2 strain 10 [27]. We are still confident to conclude that the reduced C3 deposition on the surface of the wt is influenced by IgM cleavage since strains 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S showed a significant increase in C3 labeling in comparison to the wt and 10∆ideSsuis showed significantly less C3 deposition after addition of rIdeSsuis than after addition of rIdeSsuis_C195S. It was recently pointed out by Kerr et al. that C3 is not only important during systemic disease but also at the site of infection during the initial steps of infection with pneumococci [32]. Additionally, it was shown that resistance to C3b opsonization is important for persistence of pneumococci in the host nasopharynx [31–33] and that complement hinders pneumococci from invasiveness by containing them to mucous membranes [34]. Since S. suis asymptomatically colonizes the nasopharynx of pigs, reduced C3b deposition mediated by preceding IgM cleavage could also be important for colonization of the porcine nasopharynx by S. suis and eventual spreading. Reduced opsonization with complement nonetheless remains very important for survival in blood which is a prerequisite for S. suis to reach sites of infection, especially the cerebrospinal fluid and the meninges. Multiple virulence mechanisms must play in concert to enable S. suis to circumvent killing during bacteremia. Both mutants deficient in IgM proteolysis, 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S, are attenuated in whole blood survival assays. Importantly, attenuation of 10∆ideSsuis in porcine blood is abolished by addition of rIdeSsuis but not rIdeSsuis_C195S. We thus conclude that survival of S. suis in porcine blood with specific IgM titers is influenced by the ability to cleave IgM and that survival is very much restricted by IgM. In this study, we could also demonstrate that the two S. suis mutants 10∆ideSsuis and 10∆ideSsuis∇ideSsuis_C195S survive less well in opsonophagocytosis assays with purified neutrophils than the wt. This distinguishes IdeSsuis from IdeS of S. pyogenes because the IgG cleaving activity of IdeS does not impact killing by neutrophils as shown by Okumura et al [35].
Animal experiments conducted by Xiao et al. [36] and our group [10] already indicated that IdeSsuis is not an essential virulence factor, despite ex vivo data suggesting a role of IgM cleavage by IdeSsuis in pathogenicity. Animal experimental infection as conducted in this study confirmed that IdeSsuis activity is not crucial for induction of severe pathologies, in particular meningitis, and associated mortality in growing piglets. Analogously, IgM-labeled S. suis bacteria were detected in the CSF of piglets from all infection groups. Differences in IgM labeling between the individual animals could possibly be due to varying stages of disease progression and inflammation and thus different amounts of IgM in the CSF at the time of CSF acquisition. Different peak patterns of the fluorescence intensity for IgM-labeled S. suis wt and 10∆ideSsuis∇ideSsuis_EcoRI in the CSF of experimentally infected piglets could not be explained by differing IgM titers or bacterial load within the CSF. Disparate peak patterns might be due to as jet unknown host factors or a divergent behavior of the complemented ideSsuis mutant in the CSF under in vivo conditions, particularly considering its aberrant capsule morphology. However, none of our conclusions on the impact of IgM cleavage are depended on finding an explanation for the two peaks of the complemented S. suis mutant since it expresses stable IdeSsuis and is able to cleave IgM. We conclude that IdeSsuis activity does not suffice to reduce labeling of S. suis with IgM in the CSF, especially in a highly inflammatory setting such as acute meningitis. This would also explain why we could not detect significant differences in the number of animals with meningitis among infection groups. However, to our knowledge, this is the first detection of IgM-labeled streptococci in the CSF of experimentally infected animals. IdeSsuis activity might not be enough to reduce labeling of S. suis with IgM completely in an inflammatory setting but IgM cleavage could have an important role for S. suis’ ability to survive in the nasopharynx since IgM concentrations are lower in nasal secretion and saliva than in serum [37] or inflamed tissue. Our findings that IdeSsuis-mediated IgM cleavage does not influence virulence significantly, resemble results published for IdeS of S. pyogenes which was also shown unessential for virulence of a highly invasive Group A streptococcus strain [35]. Okumura et al. proposed that the reason for this is the highly virulent strain background and that immunoglobulin cleavage might be important for less virulent strains [35]. The same might be true for IdeSsuis since all investigations were carried out with a highly virulent serotype 2 strain and derived mutants.
Former studies on IdeSsuis related virulence were not specifically designed to reveal the role of IgM proteolysis in pathogenesis and a complemented mutant was not included. The experimental design of this study, including a mutant differing from the wt in one amino acid only, allowed to investigate the role of IdeSsuis-mediated IgM cleavage in virulence more thoroughly. Accordingly, we can exclude that the phenotypic comparison is influenced by functions of the large C-terminus of IdeSsuis, which are not yet known.
Despite the finding that IdeSsuis is not an essential virulence factor, the fact that this protease is conserved among S. suis serotypes [11] points to an evolutionary important function. IdeSsuis might not be essential for induction of disease in growing piglets but it might play a role in colonization of mucous membranes. It has recently been shown that human secretory IgM, actively secreted to mucous membranes, can activate complement and offer protection at mucosal surfaces [38]. Therefore, cleavage of IgM by IdeSsuis might be crucial for S. suis to circumvent activation of complement on mucous membranes and thus facilitate colonization. Furthermore, since monomeric IgM is the only virgin B-cell receptor in swine, pigs lacking IgD [39], IgM cleavage might inhibit B-cell activation and thus have far reaching consequences for cell mediated immunity. It has been shown that FcµR, expressed on B-lymphocytes, is an uptake receptor for IgM labeled pathogens [40]. It is therefore reasonable to assume that cleavage of IgM bound to the bacterial surface might hinder uptake of IgM labeled bacteria by B-lymphocytes and thus encumber their antigen presenting ability. Further B-lymphocyte functions such as antibody production and memory cell formation could also potentially be inhibited or at least modulated by IdeSsuis-mediated IgM cleavage. Complement inhibition through IgM cleavage could for example reduce C3d mediated B-cell co-stimulation via CD21. Further studies are needed to investigate this question, in particular the importance of IdeSsuis in colonization as well as in disease progression and immune modulation.
In summary, in this study, the IgM cleaving activity of IdeSsuis of S. suis was investigated regarding complement modulation, survival in porcine blood and virulence. Whereas the IgM cleaving activity of IdeSsuis was shown to be important for reduction of complement-mediated hemolysis, labeling of sheep erythrocytes with IgM and opsonization of S. suis with IgM and complement, an impact of IgM cleavage in induction of disease, especially meningitis, could not be demonstrated.
We conclude, that IdeSsuis of S. suis is a cysteine protease and its IgM cleaving activity is important for bacterial survival in porcine blood and evasion of the classical complement pathway, but not for virulence in growing piglets. Including numerous in vitro assays as well as an in vivo model of infection, this study provides extensive insight into the role of IgM cleavage in S. suis pathogenesis.
Materials and methods
Bacterial strains and growth conditions
S. suis strain 10 is a virulent serotype 2 strain that has been used by different groups in studies on pathogenesis [11,41,42]. All other strains used in this study were derived from strain 10 by targeted mutagenesis. The isogenic mutant 10∆ideSsuis (∆ideSsuis) carries an in-frame deletion of the complete ideSsuis gene except the signal sequence [11]. The other mutants, 10∆ideSsuis∇ideSsuis_EcoRI (∇ideSsuis_EcoRI) and 10∆ideSsuis∇ideSsuis_C195S (∇ideSsuis_C195S) were constructed in this study. S. suis was grown in Todd-Hewitt broth (THB) (Becton Dickinson, catalogue #249,240) and on Columbia agar plates with 6% sheep blood (Oxoid, catalogue #PB5039A) at 37°C. Escherichia coli was grown in Luria-Bertani (LB) medium (Carl Roth, catalogue #X968.2) at 37°C under constant shaking. The following antibiotics were added to the medium if needed: 100 µg/ml ampicillin for E. coli carrying a pET45b-derived plasmid, 8 µg/ml and 3.5 µ/ml chloramphenicol for E. coli and S. suis carrying a pSET5s-derived plasmid, respectively.
DNA techniques, primers and sequencing
Standard DNA manipulations were performed as described [43]. Chromosomal DNA of S. suis strain 10 served as template for PCR. Primers were designed based on the ideSsuis sequence of S. suis P1/7 (Gene ID: 8153996) which is identical to the ideSsuis sequence of strain 10 [11]. Primer sequences are listed in Table S1. PCR was conducted using Phusion polymerase (New England Biolabs, catalogue #M0530L) and sequencing was performed by Seqlab (Göttingen, Germany).
Targeted mutagenesis of pETideSsuis
The plasmid pETideSsuis [11] was point mutated using QuikChange Lightning Multi Site Directed Mutagenesis Kit (Agilent Technologies, catalogue #210513). Plasmid DNA served as template for the mutagenesis primer PrimerC184S. This primer created a single base exchange in ideSsuis at the position 195 resulting in the replacement of a cysteine by a serine in the final protein. Following thermal cycling, a DpnI digest was conducted and the product electro transformed into competent E. coli BL21. A similar procedure was carried out to generate rIdeSsuis_homologue_C195S using QuikChange Lightning Site Directed Mutagenesis Kit (Agilent Technologies, catalogue #210518). More precisely, pETideSsuis_homologue, a plasmid encoding the His-tagged N-terminal domain of IdeSsuis (aa 36 to 432), sufficient for IgM proteolysis [11], was used as template DNA for PCR with the primers IdeSsuis_C195S_for and IdeSsuis_C195S_rev. The final constructs pETideSsuis_C195S and pETideSsuis_homologue_C195S were sequenced to verify the original sequences and the codon encoding the C195S point mutation.
Expression and purification of recombinant his-tagged proteins
E. coli bacteria harboring the respective plasmids were incubated in LB-broth with ampicillin until an OD600 of 0.5. Once the OD was reached, isopropyl-β-D-thiogalactopyranoside (IPTG) (VWR, catalogue #AC121) at a final concentration of 1mM was added and the cultures were then incubated at room temperature (RT) for 2.5h under constant shaking. Following centrifugation, E. coli were subjected to a lysozyme digest (1 mg/ml lysozyme 4°C 30 min) followed by ultrasound treatment (Brandson sonifier, 5mm tip, 35% amplitude, 1min 45sec total with 10 sec pulse on, 20sec pulse off). Recombinant proteins were then purified under native conditions via Ni-TED columns as recommended by the manufacturer (Macherey-Nagel, Protino Ni-TED, catalogue #745120.25) and dialyzed against phosphate buffered saline using dialysis membranes with a molecular weight cut off of 6000–8000Da (Carl Roth, ZelluTrans,catalogue #E660.1) and frozen in liquid nitrogen. Recombinant muramidase-released protein (rMrp) was purified as previously described [44]. His-tagged proteins were subjected to SDS-polyacrylamide gel electrophoresis and stained by Coomassie (expedeon, InstantBlue, catalogue #SKU: ISB1L).
SDS-PAGE and Western blot analysis
SDS-PAGE was performed with 10 or 8% separating gels under reducing conditions or 6% separating gels under non-reducing conditions. Stacking gels were uniformly 4%. Following gel electrophoresis proteins were blotted onto nitrocellulose membranes (Carl Roth, catalogue #HP40.1) and blocked with 5% skim milk powder in Tris-buffered saline with 0.05% Tween 20 (TBST). Incubation with primary and secondary antibodies was performed in TBST with 1% skim milk powder. Antibody dilutions are specified in Table S2. Protein detection was performed using chemiluminescent substrates SuperSignal West Pico (Thermo Fisher, catalogue #34,079) or Quantum (Biozym, catalogue #541013).
Analysis of IgM cleavage activity of recombinant IdeSsuis
Determination of IgM cleavage was performed as described previously [11]. Briefly 5 µg/ml recombinant protein was added to a 1:100 dilution of porcine serum. The whole was incubated at 37°C for 2.5h on a rotator, followed by anti IgM Western blot analysis under reducing conditions.
Complement hemolysis assay
Recombinant proteins (rIdeSsuis, rIdeSsuis_C195S, rIdeSsuis_homologue, rIdeSsuis_homologue_C195S, rIdeSsuis_C-domain, rMrp) were incubated in a concentration of 18 µg/ml with a 1:100 dilution of porcine anti-sheep erythrocyte (αEry) serum drawn either 7 or 14 days post immunization with sheep erythrocytes [10] for 1.5 h at 37°C on a rotator in a total volume of 0.25ml as described previously [10]. For investigating complement inhibition in hemolysis assays, rIdeSsuis and rIdeSsuis_C195S were added to the sera prior to the hemolysis assay to reach final concentrations of 5, 20, 100, 200 µg/ml. Sheep erythrocytes were purified as described [10] and adjusted to a 2% suspension with sodium chloride. Erythrocytes and pre-incubated sera were then mixed at a ratio of 1:1 (100 µl each) in a 96-well plate (V-bottom) and incubated for 30 min at 37°C on an orbital shaker. Plates were then centrifuged (5 min 1000g RT) and the OD405 of the supernatant was measured.
Detection of IgM and IgG on the surface of ovine erythrocytes
The detection of antibodies on ovine erythrocytes was conducted as described previously [10]. Briefly, αEry serum (both 7d and 14d post immunization) was incubated at 56°C for 30min to inactivate complement. Recombinant proteins were then added (18µg/ml) and the whole incubated for 1.5h at 37°C on a rotator. Subsequently, a 2% sheep erythrocyte suspension was added at a ratio of 1:1 (50 µl each) and the mixture incubated at 4°C for 45min on a rotator. Erythrocytes were then centrifuged, blocked with 5% goat serum for 1h at 4°C and subsequently washed with phosphate buffered saline with a pH of 7.4 (PBS). A mouse anti-pig IgM antibody (Serotec catalogue #MCA637) at a dilution of 1:225 or a goat anti-pig IgG antibody (Serotec, catalogue #AHP865P) at a dilution of 1:10,000 was then added and the samples incubated for 1h at 4°C. Cells were washed twice and incubated for 1h at 4°C in the dark with the following secondary antibodies: phycoerythrin (PE)-labeled goat anti-mouse IgG (BioLegend, catalogue #405307) at a dilution of 1:500 for detection of porcine IgM and Alexa Fluor 488-labeled chicken anti-goat IgG (Life Technologies, catalogue #A-21467) at a dilution of 1:800 for detection of porcine IgG. Samples were then washed twice and fixated with 0.375% formaldehyde and measured by flow cytometry (BD FACSCalibur).
Complementation and targeted mutagenesis of S. suis 10∆ideSsuis
S. suis 10∆ideSsuis was chromosomally complemented using the thermosensitive suicide vector pSET5s [45] to render the two mutants S. suis 10∆ideSsuis∇ideSsuis_C195S and 10∆ideSsuis∇ideSsuis_EcoRI. The former mutant contains a point mutation in the ideSsuis gene leading to a serine instead of a cysteine at the position 195 of the final protein. The later mutant contains a silent mutation at the position 1793 of ideSsuis resulting in a new EcoRI cleavage site enabling distinction of the complemented strain from the wt. Chromosomal DNA of S. suis strain 10 served as template in a PCR with the primers preProIdeSsuisPstI, postEndIdeSsuisBamHI to introduce the cleavage sites for the restriction enzymes BamHI and PstI. The PCR product, as well as the pSET5s vector were then digested with the two indicated enzymes and afterwards ligated to render pSET5sideSsuis. To introduce the C195S encoding mutation in ideSsuis, pSET5sideSsuis plasmid DNA was used in a PCR with the mutagenesis primers IdeSsuis_C195S_for, IdeSsuis_C195S_rev resulting in pSET5sideSsuis_C195S. To introduce the EcoRI cleavage site, primers EcoRI1793ideSsf, EcoRI1793ideSsr were used to create pSET5sideSsuis_EcoRI. PCR products were DpnI digested and transformed into competent E. coli CC118. Sequencing of the whole ideSsuis reading frame confirmed the presence of both point mutations at the correct sites. The plasmids pSET5sideSsuis_C195S and pSET5sideSsuis_EcoRI were then transformed into competent S. suis 10∆ideSsuis (25µF, 2.5kV 200Ω, Genpulser, Biorad). Temperature shifts, performed as described previously [46], ensured allelic exchange and later excision of the vector from S. suis.
Genotypic analysis of S. suis mutants
To verify the correct chromosomal complementation, mutants were sequenced, tested in restriction enzyme digest with EcoRI and analyzed by non-radioactive Southern blot. For Southern blotting, chromosomal DNA was digested with HincII, blotted onto a positively charged nylon membrane (Biorad, catalogue #162–0165) and subsequently hybridized with two different biotinylated probes. Between hybridizations with the different probes, the membrane was stripped with 0.2 N NaOH, 1% sodium dodecyl sulfate (SDS). The first probe, 450 bp in size, binding a 685bp fragment within ideSsuis was constructed using the primers Sonde450bpfor and Sonde450bprev. The second probe, 412bp in size, binding within the pSET5s backbone was generated with the primer pair pSET5sSondefor and pSET5sSonderev. After hybridization, the membrane was incubated with peroxidase-labeled streptavidin (Biolegend, catalogue #405210) and signals detected by chemiluminescence (blots see Fig. S5). PCR analysis with the primers pSET5spreMCSfor and postMCSpSET5s was conducted to verify complete excision of the pSET5s plasmid from the S. suis chromosome.
Phenotypic analysis of S. suis mutants for IdeSsuis expression and IgM cleavage
Expression of IdeSsuis was confirmed in bacterial supernatants by anti-IdeSsuis Western blot analysis as described [11]. Furthermore, the mutants’ ability to cleave porcine IgM was tested by incubation of concentrated culture supernatants with porcine serum, followed by anti-IgM Western blotting. Concentration of supernatants was performed as described [11]. Briefly, 50 ml cultures of S. suis strain 10, 10∆ideSsuis, 10∆ideSsuis ∇ideSsuis_EcoRI, ∆ideSsuis∇ideSsuis_C195S were grown to an OD600 of 0.8, centrifuged and the supernatants concentrated 24-fold using 30 kDa cut off centrifugal filters (Merck Milipore, catalogue #UFC903024). One hundred microliter of the 24-fold concentrated supernatants was incubated with a 1:100 dilution of porcine serum, followed by anti-IgM Western blotting.
Transmission electron microscopy
The expression and thickness of the capsule of S. suis strain 10, 10∆ideSsuis, 10∆ideSsuis∇ideSsuis_C195S and 10∆ideSsuis∇ideSsuis_EcoRI was investigated by transmission electron microscopy using lysine-ruthenium red (LRR) staining as described previously [47]. The capsule thickness of S. suis strain 10 and 10∆ideSsuis∇ideSsuis_EcoRI was measured 25 times. Measurements were only conducted of bacteria which capsule had been cut in a 90° angle.
Generation of porcine IgM (Fab’)2 and IgM Fc specific antibodies
To generate an antibody specifically against porcine IgM F(ab’)2 fragments, porcine IgM was purified from serum, digested with rIdeSsuis and F(ab’)2 fragments, purified as described below, were used as antigen for immunization of a rabbit. IgM-Fc specific antibodies were purified from a commercial polyclonal anti-pig IgM antibody (biomol, catalogue #A100-117A). All chromatographic purification procedures were performed with the ÄCTA prime plus chromatography system (GE Healthcare, Little Chalfont, United Kingdom). In detail, the gamma globulin fraction of porcine serum was twice precipitated with 40% ammonium sulfate, followed by dialysis against phosphate buffered saline with 300 kDa cut off membranes (Spectra/Por Float A-Lyzer G2, Spectrum Labs, catalogue #G235072) for five days. The dialysate was then affinity chromatographed over protein G columns (HiTrap protein G column, GE Healthcare via VWR, catalogue #29–0485-81) to exclude porcine IgG. The flow through from protein G columns was purified by thiophilic adsorption chromatography (HiTrap IgM purification columns, GE Healthcare via VWR, catalogue #17–5110-01). The eluate from thiophilic adsorption chromatography was purified further via size exclusion chromatography (HiLoad 16/600 Superdex 200pg, GE Healthcare via VWR, catalogue #28–9893-35). The eluates corresponding in size to pentameric IgM were pooled and concentrated via 10 kDa cut off centrifugal filters (Sartorius, catalogue #VS2001) to render a total volume of 2 ml. Purified IgM was next subjected to a digest with rIdeSsuis (20 µg rIdeSsuis/mg IgM) for 2.5h at 37°C. F(ab’)2 fragments were purified from the digest via size exclusion chromatography. The eluates corresponding to 130kDa F(ab’)2 fragments were pooled and subsequently purified via affinity chromatography with a Ni-sepharose column (HisTrap HP, GE Healthcare via VWR, catalogue #29–0510-21) to exclude remaining rIdeSsuis that had been used to digest IgM. The flow through of this chromatography was then used as antigen for immunization of a rabbit (BioGenes GmbH). The rabbit was immunized three times with 250–500 µg antigen being used for priming and 100 µg antigen for the two booster immunizations. Final bleeding serum was precipitated with 40% ammonium sulfate, the precipitate was then purified by affinity chromatography with Sepharose columns covalently coupled with porcine IgG to exclude those antibodies cross reacting with the light chain of porcine IgG. The flow through was purified further by affinity chromatography with Sepharose columns covalently coupled with porcine IgM-F(ab’)2 fragments generated also by rIdeSsuis digest. The eluate from this chromatography was analyzed for its specificity for porcine IgM-F(ab’)2 portion in Western blot analysis as described above (Fig. S6). Western blot analysis confirmed that the antibody does not cross-react with IdeSsuis and rMrp (results not shown). For purification of anti-IgM Fc specific antibodies, a commercial polyclonal goat anti-pig IgM antibody was purified by affinity chromatography with Sepharose columns coupled with IgM-F(ab’)2. The flow through from this procedure was then applied to Sepharose columns coupled with intact pentameric porcine IgM. The eluate from this chromatography was also verified in Western blot analysis to recognize only IgM Fc fragments (Fig. S6).
Generation of a porcine hyperimmune serum against S. suis serotype 2 and depletion of IgG from this serum
A pig was prime-booster-booster vaccinated with a S. suis bacterin based on strain 10, essentially as described previously for a bacterin vaccination trial [48] except that two booster immunizations were conducted. The generation of hyperimmune sera in piglets is approved by the Landesdirektion Sachsen (permit no. N01/16). This porcine hyperimmune serum was depleted from all IgG using affinity chromatography. The hyperimmune serum was purified via a Protein G sepharose column (GE Healthcare via VWR, catalogue #17–0405-01) using the chromatography system ÄCTA prime plus (GE Healthcare, Little Chalfont, United Kingdom). To assure that no IgG was remaining in the flow through, it was send over the Protein G column again. The IgG free flow through was concentrated using 10 kDa centrifugal filters (Sartorius, catalogue #VS2001).
Detection of IgM on the surface of S. suis
For detection of IgM on the bacterial surface S. suis strain 10, 10∆ideSsuis, 10∆ideSsuis ∇ideSsuis_EcoRI and ∆ideSsuis∇ideSsuis_C195S were grown to an OD600 of 0.8, followed by incubation at 4°C with the anti-S. suis hyperimmune serum against S. suis serotype 2 for 0.5 h. Hereafter, bacteria were incubated in THB at 37°C for 4h. In the first staining protocol, bacteria were labeled with a mouse anti-pig IgM primary antibody (Serotec, catalogue #MCA 637) and a PE-labeled goat anti-mouse IgG secondary antibody (BioLegend, catalogue #405307) as described by Seele et al. [11]. Presence of IgM was then detected on the surface of S. suis using flow cytometry (BD, Fortessa) and immunofluorescent microscopy. The percent reduction of IgM labeling was calculated as follows: The amount of IgM labeled bacteria after opsonization with serum and before incubation at 37°C was set at 100%. The reduction in IgM labeling was then calculated by subtracting the % of IgM positive bacteria after incubation at 37°C from the initial 100%. Staining was also performed with antibodies directed specifically against the F(ab’)2- and Fc-regions of porcine IgM. Prior to this staining, opsonized bacteria were blocked with 1:100 diluted donkey serum (Dianova, catalogue # 017–000-121) at 4°C for 0.5h. Staining with primary and secondary antibodies was performed for 0.5 h at 4°C each. Rabbit anti-pig IgM-F(ab’)2 and goat anti-pig IgM-Fc primary antibodies were both used at a dilution of 1:100. As secondary antibodies, 1:250 dilutedPE-labeled F(ab’)2 donkey anti-goat IgG (Thermo Fisher Scientific, catalogue #31860) and 1:200 diluted fluorescein isothiocyanate (FITC)- labeled donkey anti-rabbit IgG (Dianova, catalogue #DAB-87179) antibodies were used. Detection of IgM labeled S. suis was again performed by flow cytometry (BD FACSCalibur). The % reduction in the IgM F(ab’)2 and IgM Fc signal was calculated as described above. To detect IgM on the surface of S. suis in the CSF of experimentally infected piglets, CSF of piglets with high intracerebrospinal S. suis burden was centrifuged and stained with anti-IgM F(ab’)2 and anti IgM Fc specific antibodies. Cerebrospinal fluid was frozen within five minutes after acquisition and stored at −80°C until analysis. Flow cytometry (BD, FACSCalibur) was used to detect IgM F(ab’)2 and IgM Fc labeling on S. suis. To gate S. suis in the CSF correctly, preliminary tests were conducted with S. suis wt incubated in the CSF of healthy but S. suis infected piglets or in the CSF of the same piglets supplemented with serum with moderate IgM levels (50 ELISA units) or IgG levels (29.1 ELISA units), respectively. The frequencies of parent of IgM F(ab’)2 and IgM Fc-positive S. suis were determined via FlowJoTM_V10 software.
C3 deposition on the surface of S. suis
Complement deposition on the streptococcal surface was assessed basically as described [10], with the differences of the use of an IgG depleted porcine anti-S. suis strain 10 serum as source of IgM and complement. Briefly, S. suis strain 10, 10∆ideSsuis, 10∆ideSsuis ∇ideSsuis_EcoRI, ∆ideSsuis∇ideSsuis_C195S were grown to an OD600 of 0.8. Then, 75µl of the respective cultures were centrifuged and incubated for 1h at 37°C on a rotator with 150 µl of a 1:2 dilution of an anti-S. suis strain 10 hyperimmune serum depleted of all IgG. Staining of C3 labeled bacteria was conducted with 250µl of a 1:150 diluted FITC-labeled cross-reactive rabbit anti-human C3c antibody (Dako, catalogue #F020102-2) for 1h at 4°C under constant rotation. As positive control, undiluted serum was used, as negative control, serum that had been heat inactivated at 56°C for 30 min was used. For samples with the addition of recombinant protein, 5µg of rIdeSsuis or rIdeSsuis_C195S were added to 10∆ideSsuis prior to incubation with serum. Samples were measured using BD, FACSFortessa and analyzed using FlowJoTM_V10 software.
Detection of anti-S. suis IgM, anti-S. suis IgG and anti-IdeSsuis antibodies
Anti-S. suis strain 10 IgM, anti-S. suis strain 10 IgG and anti-IdeSsuis antibody titers were determined by ELISA as described by Seele et al. [20]. Antibody titers below 10 ELISA units were considered as low, ten to seventy ELISA units were considered as moderate and ELISA units greater than 70 were considered as high.
Blood survival assays
Survival of S. suis wt and mutants in porcine blood was analyzed using whole heparinized blood from eight-week old piglets from a commercial pig farm (infected with various S. suis serotypes). The withdrawal of blood was approved under the permit number N19/14 by the responsible authorities of the state of Saxony, Germany (Landesdirektion Sachsen). Assays with the addition of rIdeSsuis_homologue and rIdeSsuis_homologue_C195S were conducted with blood from eleven-week old piglets known to be free of S. suis sly+ mrp+ epf+ cps2, cps7 and cps9 that served as placebo animals in an infection experiment approved by the responsible authorities of the state of Saxony, Germany under the permit number TVV 37–17. Survival factors were determined by dividing the number of CFUs after a 2 h incubation period at 37°C by the number of CFUs at t = 0 (CFU were determined by plating of serial dilutions). As indicated, 0.5 ml porcine whole blood was mixed with 0.1, 1, 10 µg rIdeSsuis or 10 µg rIdeSsuis_C195S, rIdeSsuis_homologue, rIdeSsuis_homologue_C195S. Then 1.5 x 106 CFU S. suis wt or 10∆ideSsuis were added. Bacteria were added from glycerol stocks prepared at the late exponential growth phase. The mixture was incubated for 2 h at 37°C on a rotator. For the detection of IgM cleavage products after the 2 h incubation period, samples were centrifuged and the supernatant analyzed by anti-pig IgM Western blot analysis under reducing conditions as described above.
For survival assays with the complement inhibitor vaccinia virus complement control protein (VCP, GeneBalance, Inc., catalogue #GB-VCP250), 10, 2, 0.2 µg of VCP were added to 0.5 ml whole porcine blood and incubated at 37°C for 5 min on a rotator. S. suis wt, 10∆ideSsuis, 10∆ideSsuis∇ideSsuis_EcoRI, 10∆ideSsuis∇ideSsuis_C195S were then added at 4 × 106 CFU/ml and the whole incubated at 37°C for 2h. VCP was used at concentrations where it is known to inhibit complement significantly but not completely [19].
Opsonophagocytosis assay
For opsonophagocytosis assays with purified porcine neutrophils, 1.5 x 105 CFU S. suis strain 10, 10∆ideSsuis, 10∆ideSsuis ∇ideSsuis_EcoRI, ∆ideSsuis∇ideSsuis_C195S were incubated with 100 µl of porcine serum for 0.5h at 37°C on a rotator. Bacteria were added from glycerol stocks prepared at the late exponential growth phase. The chosen experimental serum was drawn from a piglet with meningitis five days after experimental infection with S. suis strain 10 within a different animal experiment approved under the permit number TVV 11/16 by the ethics committee of the Landesdirektion Sachsen. This serum has moderate levels of specific anti-S. suis IgM (20.4 ELISA units/ml) and relatively low anti-S. suis IgG (35.8 ELISA units/ml) as confirmed by anti-S. suis IgM and IgG ELISA. Serum of colostrum-deprived piglets (CDS) served as positive control, anti-S. suis hyperimmune serum as negative control. Porcine neutrophils were separated as described by Seele et al. 2015 using ficoll density centrifugation [10]. 5 × 106 purified porcine neutrophils were added to the pre-incubated serum containing the above mentioned 1.5 x 105 S. suis corresponding to a multiplicity of infection (MOI) of 0.03. Samples were then incubated for 2h at 37°C on a rotator and the survival factor determined as described for the whole blood survival assays above.
sC5b-9 ELISA
Porcine sC5b-9 was measured by ELISA as described by Messias-Reason et al. [49]. with minor modifications, using a monoclonal antibody against a neoepitope of C5b-9 [50]. Briefly, ELISA plates were coated with a cross-reactive monoclonal anti-C5b-9 antibody, plasma samples were applied at a dilution of 1:3 and detection was performed with a rabbit anti-human cross-reactive C5 IgG primary antibody followed by a peroxidase-conjugated goat anti-rabbit IgG antibody. Zymosan-activated normal porcine serum (set as 1000 ELISA units) was used as standard.
Animal experimental infection
Thirty-six specific-pathogen free German Landrace piglets known to be free of sly+ mrp+ epf+ cps2, cps7 and cps9 S. suis were divided into four infection groups with nine animals per group. The animals in the respective groups were intranasally challenged with 1.5 x 109 CFU S. suis strain 10, 10∆ideSsuis, 10∆ideSsuis ∇ideSsuis_EcoRI, 10∆ideSsuis∇ideSsuis_C195S at an age of 8 weeks after predisposition with 1% acetic acid as described [46]. Bacterial infection cultures were grown to mid-exponential growth phase. The animal experiment was approved by the Committee on Animal Experiments of the Lower Saxonian State Office for Consumer Protection and Food Safety under the permit number 33.12–42,502-04–16/2305A. Handling and treatment of animals was in strict accordance with the principles of the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes as well as the German Animal Protection Law. Animals were clinically monitored including measurement of the inner body temperature, assessment of movements and feed intake every eight hours (piglets were only fed at these time points). Piglets were regarded morbid if the inner body temperature was equal to or greater than 40.2°C. Piglets were euthanized for animal welfare reasons when reaching predefined endpoints such as convulsions or the inability to stand indicating severe meningitis or acute polyarthritis, respectively. Furthermore, piglets were euthanized if high fever (≥ 40.5°C), apathy and anorexia persisted over 36 h. Surviving piglets were euthanized two weeks after infection. Necropsies were performed by a standardized protocol and samples for histopathological as well as bacteriological investigations were taken as described previously [46]. Isolated S. suis strains were screened in a MP-PCR for detection of the cps1, cps2, cps7, cps9, mrp, epf, sly, arcA and gdh [51]. All isolates from the 10∆ideSsuis infection group were tested in an ideSsuis PCR with the primer pair 800vorEcoRI, 800nachEcoRI to confirm the absence of the ideSsuis gene. All isolates from the CSF, the tonsils and chosen inner organs of the 10∆ideSsuis∇ideSsuis _C195S infection group were subjected to an ideSsuis PCR with the primer pair IdeSsuis_con_fo, IdeSsuis_con_rev, followed by sequencing to confirm the presence of the C195S point mutation. All isolates from the 10∆ideSsuis∇ideSsuis EcoRI infection group as well as all CSF isolates from the wt and 10∆ideSsuis∇ideSsuis _C195S infection groups were tested in a PCR with the primer pair 800vorEcoRI, 800nachEcoRI. The resulting 1619 bp PCR products weres cut with EcoRI and separated by 1% agarose gel electrophoresis to discriminate between S. suis wt and the complemented strain 10∆ideSsuis∇ideSsuis _ EcoRI.
Statistical analysis
All experiments were repeated at least three times. Data was analyzed for normal distribution by the Kolmogorov-Smirnov normality test. In case of normal distribution, student’s unpaired, two-tailed t-test was conducted. If data was not normally distributed, the nonparametric two tailed Mann–Whitney test was applied. Kaplan–Meier diagrams representing mortality and morbidity were analyzed with the log-rank test. Concentration dependency was analyzed by calculating the Person correlation coefficient. A confidence interval of 95% was chosen for all analyzes. Figures of all experiments represent the mean and standard deviation. Outliers were calculated by Graphpad QuickCalcs (https://www.graphpad.com/quickcalcs/grubbs1/) and excluded from analysis. Probabilities were considered as follows p < 0.05 *, p < 0.01 **, p < 0.001 ***. Flow cytometric data was analyzed using FlowJoTM _V10 or Flowing Software version 2.5.1.
Funding Statement
This work was supported by the Deutsche Forschungsgemeinschaft [DFG BA 4730/3-1].
Abbreviations
- αEry
porcine anti-sheep erythrocyte serum
- BCR
B-cell receptor
- CDS
colostrum-deprived piglet serum
- CFU
Colony forming units
- CSF
cerebrospinal fluid
- FITC
fluorescein isothiocyanate
- IdeE
Immunoglobulin G degrading enzyme of Streptococcus equi subsp. equi
- IdeP
Immunoglobulin G degrading enzyme of Streptococcus phocae
- IdeS
Immunoglobulin G degrading enzyme of Streptococcus pyogenes
- IdeSsuis
Immunoglobulin M degrading enzyme of Streptococcus suis
- IdeZ
Immunoglobulin G degrading enzyme of Streptococcus equi subsp. zooepidemicus
- LB-broth
Luria Bertani Broth
- MFI
geometric mean fluorescence intensity
- MRP
Muramidase-released protein
- OPA
Opsonophagocytosis assay
- PBS
Phosphate buffered saline
- PCR
Polymerase chain reaction
- PE
phycoerythrin
- POD
horseradish peroxidase
- RT
Room temperature
- SD
Standard deviation
- SF
survival factor
- S. suis
Streptococcus suis
- ST
Streptococcus suis sequence type
- TBST
Tris buffered saline Tween20
- THB
Todd-Hewitt Broth
- VCP
vaccinia virus complement control protein
- wt
wild type
Acknowledgments
We thank H. Smith (DLO-Lelystad, Netherlands) for S. suis strain 10. Daisuke Takamatsu (National Institute of Animal Health, Japan) kindly provided the plasmid pSET5s. Ulrich von Pawel-Rammingen (Department of Molecular Biology, Umeå University, Umeå, Sweden) is acknowledged for providing the plasmid pET45bideSsuis_C195S. Renate Rutz (Institute for Immunology, University of Heidelberg, Heidelberg, Germany) is acknowledged for the execution of the sC5b-9 ELISA. Flow cytometry was performed at the core unit for flow cytometry, CUDZ, of the Veterinary Faculty of the University of Leipzig, Germany. This study was financially supported by the German Research Foundation (DFG BA 4730/3-1).
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental material
Supplemental material can be accessed here
References
- [1].Staats JJ, Feder I, Okwumabua O, et al. Streptococcus suis: past and present. Vet Res Commun. 1997;21:381–407. [DOI] [PubMed] [Google Scholar]
- [2].Baele M, Chiers K, Devriese LA, et al. The Gram-positive tonsillar and nasal flora of piglets before and after weaning. J Appl Microbiol. 2001;91:997–1003. [DOI] [PubMed] [Google Scholar]
- [3].Segura M, Calzas C, Grenier D, et al. Initial steps of the pathogenesis of the infection caused by Streptococcus suis: fighting against nonspecific defenses. FEBS Lett. 2016;590:3772–3799. [DOI] [PubMed] [Google Scholar]
- [4].Goyette-Desjardins G, Auger J-P, Xu J, et al. Streptococcus suis, an important pig pathogen and emerging zoonotic agent—an update on the worldwide distribution based on serotyping and sequence typing. Emerg Microbes Infect. 2014;3:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Okura M, Osaki M, Nomoto R, et al. Current taxonomical situation of Streptococcus suis. Pathogens. 2016;5:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Fittipaldi N, Segura M, Grenier D, et al. Virulence factors involved in the pathogenesis of the infection caused by the swine pathogen and zoonotic agent Streptococcus suis. Futur Microbiol. 2012;7:259–279. [DOI] [PubMed] [Google Scholar]
- [7].Segura M, Fittipaldi N, Calzas C, et al. Critical Streptococcus suis virulence factors: are they all really critical? Trends Microbiol. 2017;25:585–599. [DOI] [PubMed] [Google Scholar]
- [8].Lecours M-P, Gottschalk M, Houde M, et al. Critical role for Streptococcus suis cell wall modifications and suilysin in resistance to complement-dependent killing by dendritic cells. J Infect Dis. 2011;204:919–929. [DOI] [PubMed] [Google Scholar]
- [9].Roy D, Grenier D, Segura M, et al. Recruitment of factor H to the Streptococcus suis Cell surface is multifactorial. Pathogens. 2016;5:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Seele J, Beineke A, Hillermann LM, et al. The immunoglobulin M-degrading enzyme of Streptococcus suis, IdeSsuis, is involved in complement evasion. Vet Res. 2015;46:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Seele J, Singpiel A, Spoerry C, et al. Identification of a novel host-specific IgM Protease in Streptococcus suis. J Bacteriol. 2013;195:930–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Rungelrath V, Wohlsein JC, Siebert U, et al. Identification of a novel host-specific IgG protease in Streptococcus phocae subsp. phocae. Vet Microbiol. 2017;201:42–48. [DOI] [PubMed] [Google Scholar]
- [13].Ochsenbein AF, Zinkernagel RM.. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;5699:624–630. [DOI] [PubMed] [Google Scholar]
- [14].Tizard IR. Veterinary immunology. 9th ed. St. Louis (MO): Elsevier Health Sciences; 2013. [Google Scholar]
- [15].Klimovich VB. IgM and its receptors: structural and functional aspects. Biochemistry (Mosc). 2011;76:534–549. [DOI] [PubMed] [Google Scholar]
- [16].Bennett KM, Rooijakkers SHM, Gorham RD. Let’s tie the knot: marriage of complement and adaptive immunity in pathogen evasion, for better or worse. Front Microbiol. 2017;8:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Heyman B, Wigzell H. Specific IgM enhances and IgG inhibits the induction of immunological memory in mice. Scand J Immunol. 1985;21:255–266. [DOI] [PubMed] [Google Scholar]
- [18].Sun J, Butler JE. Sequence analysis of pig switch mu, C mu, and C mu m. Immunogenetics. 1997;46:452–460. [DOI] [PubMed] [Google Scholar]
- [19].Thorgersen EB, Pharo A, Haverson K, et al. Inhibition of complement and CD14 attenuates the Escherichia coli-induced inflammatory response in porcine whole blood. Infect Immun. 2009;77:725–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Seele J, Hillermann LM, Beineke A, et al. The immunoglobulin M-degrading enzyme of Streptococcus suis, IdeSsuis, is a highly protective antigen against serotype 2. Vaccine. 2015;33:2207–2212. [DOI] [PubMed] [Google Scholar]
- [21].Lynskey NN, Reglinski M, Calay D, et al. Multi-functional mechanisms of immune evasion by the streptococcal complement inhibitor C5a peptidase. PLoS Pathog. 2017;13:1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Buffalo CZ, Bahn-Suh AJ, Hirakis SP, et al. Conserved patterns hidden within group A Streptococcus M protein hypervariability recognize human C4b-binding protein. Nat Microbiol. 2016;1:16155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Frick I-M, Rasmussen M, Schmidtchen A, et al. SIC, a secreted protein of Streptococcus pyogenes that inactivates antibacterial peptides. J Biol Chem. 2003;278(19):16561–16566. [DOI] [PubMed] [Google Scholar]
- [24].Kraiczy P, Hellwage J, Skerka C, et al. Immune evasion of Borrelia burgdorferi: mapping of a complement-inhibitor factor H-binding site of BbCRASP-3, a novel member of the Erp protein family. Eur J Immunol. 2003;33:697–707. [DOI] [PubMed] [Google Scholar]
- [25].Lathem WW, Bergsbaken T, Welch RA. Potentiation of C1 esterase inhibitor by StcE, a metalloprotease secreted by Escherichia coli O157:H7. J Exp Med. 2004;199:1077–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Li Q, Ma C, Fu Y, et al. H specifically capture novel Factor H-binding proteins of Streptococcus suis and contribute to the virulence of the bacteria. Microbiol Res. 2017;196:17–25. [DOI] [PubMed] [Google Scholar]
- [27].Seitz M, Beineke A, Singpiel A, et al. Role of Capsule and Suilysin in Mucosal Infection of Complement-Deficient Mice with Streptococcus suis. Infect Immun. 2014;82:2460–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Nordenfelt P, Waldemarson S, Linder A, et al. Antibody orientation at bacterial surfaces is related to invasive infection. J Exp Med. 2012;209:2367–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mäkelä O, Rouslahti E, Seppälä IJT. Affinity of IgM and IgG antibodies. Immunochemistry. 1970;7:917–932. [DOI] [PubMed] [Google Scholar]
- [30].Söderberg JJ, von Pawel-Rammingen U. The streptococcal protease IdeS modulates bacterial IgGFc binding and generates 1/2Fc fragments with the ability to prime polymorphonuclear leucocytes. Mol Immunol. 2008;45:3347–3353. [DOI] [PubMed] [Google Scholar]
- [31].Brown JS, Hussell T, Gilliland SM, et al. The classical pathway is the dominant complement pathway required for innate immunity to Streptococcus pneumoniae infection in mice. Proc Natl Acad Sci USA. 2002;99:16969–16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kerr AR, Paterson GK, Riboldi-Tunnicliffe A, et al. Innate immune defense against pneumococcal pneumonia requires pulmonary complement component C3. Infect Immun. 2005;73:4245–4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Paterson GK, Mitchell TJ. Innate immunity and the pneumococcus. Microbiology. 2006;152:285–293. [DOI] [PubMed] [Google Scholar]
- [34].Bogaert D, Thompson CM, Trzcinski K, et al. The role of complement in innate and adaptive immunity to pneumococcal colonization and sepsis in a murine model. Vaccine. 2010;28:681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Okumura CYM, Anderson EL, Döhrmann S, et al. IgG protease Mac/IdeS is not essential for phagocyte resistance or mouse virulence of M1T1 group A Streptococcus. MBio. 2013;4:e00499–13 – e00499–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Xiao G, Wu Z, Zhang S, et al. Mac Protein is not an Essential Virulence Factor for the Virulent Reference Strain Streptococcus suis P1/7. Curr Microbiol. 2017;74(1):90–96. [DOI] [PubMed] [Google Scholar]
- [37].Plebani A, Mira E, Mevio E, et al. IgM and IgD concentrations in the serum and secretions of children with selective IgA deficiency. Clin Exp Immunol. 1983;53:689–696. [PMC free article] [PubMed] [Google Scholar]
- [38].Michaelsen TE, Emilsen S, Sandin RH, et al. Human secretory IgM antibodies activate human complement and offer protection at mucosal surface. Scand J Immunol. 2017;85:43–50. [DOI] [PubMed] [Google Scholar]
- [39].Butler JE, Sun J, Navarro P. The swine Ig heavy chain locus has a single JH and no identifiable IgD. Int Immunol. 1996;8:1897–1904. [DOI] [PubMed] [Google Scholar]
- [40].Shima H, Takatsu H, Fukuda S, et al. Identification of TOSO/FAIM3 as an Fc receptor for IgM. Int Immunol. 2010;22:149–156. [DOI] [PubMed] [Google Scholar]
- [41].Smith HE, Damman M, van der Velde J, et al. Identification and characterization of the cps locus of Streptococcus suis serotype 2: the capsule protects against phagocytosis and is an important virulence factor. Infect Immun. 1999;67:1750–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].de Buhr N, Reuner F, Neumann A, et al. Neutrophil extracellular trap formation in the Streptococcus suis-infected cerebrospinal fluid compartment. Cell Microbiol. 2017;19:1–16. [DOI] [PubMed] [Google Scholar]
- [43].Green MR, Sambrook J. Molecular cloning: a laboratory manual. 4th ed. Long Island (NY): Cold Spring Harbor Lab. Press; 2012. [Google Scholar]
- [44].Beineke A, Bennecke K, Neis C, et al. Comparative evaluation of virulence and pathology of Streptococcus suis serotypes 2 and 9 in experimentally infected growers. Vet Microbiol. 2008;128:423–430. [DOI] [PubMed] [Google Scholar]
- [45].Takamatsu D, Osaki M, Sekizaki T. Thermosensitive suicide vectors for gene replacement in Streptococcus suis. Plasmid. 2001;46:140–148. [DOI] [PubMed] [Google Scholar]
- [46].Baums CG, Kaim U, Fulde M, et al. Identification of a novel virulence determinant with serum opacification activity in Streptococcus suis. Infect Immun. 2006;74:6154–6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hammerschmidt S, Wolff S, Hocke A, et al. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect Immun. 2005;73:4653–4667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Baums CG, Kock C, Beineke A, et al. Streptococcus suis bacterin and subunit vaccine immunogenicities and protective efficacies against serotypes 2 and 9. Clin Vaccine Immunol. 2009;16:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Messias-Reason IJ, Hayashi SY, Nisihara RM, et al. Complement activation in infective endocarditis: correlation with extracardiac manifestations and prognosis. Clin Exp Immunol. 2002;127:310–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Mollnes TE, Lea T, Frøland SS, et al. Quantification of the terminal complement complex in human plasma by an enzyme-linked immunosorbent assay based on monoclonal antibodies against a neoantigen of the complex. Scand J Immunol. 1985;22:197–202. [DOI] [PubMed] [Google Scholar]
- [51].Silva LMG, Baums CG, Rehm T, et al. Virulence-associated gene profiling of Streptococcus suis isolates by PCR. Vet Microbiol. 2006;115:117–127. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


