Abstract
In this study, we address one of the major critiques for TIL therapy - the time needed for proper expansion of a suitable product. We postulated that TCR activation in the first phase of expansion combined with an agonistic stimulation of CD137/4-1BB and IL-2 would favor preferential expansion of CD8+ TIL. Indeed, this novel 3-signal approach for optimal T-cell activation resulted in faster and more consistent expansion of CD8+CD3+ TIL. This new method allowed for successful expansion of TIL from cutaneous and uveal melanoma tumors in 100% of the cultures in less than three weeks. Finally, providing the three signals attributed to optimal T-cell activation led to expansion of TIL capable of recognizing their tumor counterpart in cutaneous and uveal melanoma.
This new methodology for the initial phase of TIL expansion brings a new opportunity for translation of TIL therapy in challenging malignancies such as uveal melanoma.
INTRODUCTION
Clinical success using tumor-infiltrating lymphocytes (TIL) in adoptive cell therapy (ACT) has been widely reported in previous phase II studies1–4. Although this success has predominantly been reported in metastatic cutaneous melanoma, given its pioneering status, TIL therapy was later transposed in other malignancies such as colon cancer, HPV-related cervical cancer and uveal melanoma5–7. The methodology for sufficiently expanding numerous TIL is primarily dependent on an initial stage of in vitro expansion of TIL from tumor fragments (referred to as the “pre-REP” process), followed by a large scale expansion and infusion using the rapid expansion protocol (REP). The strategy for the expansion of TIL in the pre-REP process has been solely based on providing a strong signal for T-cell activation through exogenous addition of high-dose IL-2, without direct activation of the TCR. Following culturing in high dose IL-2, TIL expansion occurs within 3 to 5 weeks. Although the techniques used for the expansion of TIL in the pre-REP process are very consistent for metastatic cutaneous melanoma, the final yield of TIL and the time required to attain those numbers are highly variable. It is noteworthy that this variability is not only contingent on the level of infiltration of TIL in the tumor fragments. This is clearly demonstrated in uveal melanoma where the ability to successfully culture TIL in the pre-REP phase is low despite the degree of TIL infiltration in the original tumor fragments comparable to the degree of infiltration in cutaneous melanoma8.
Consequently, we aimed to overcome three major challenges of pre-REP TIL manufacturing: patient’s accessibility to therapy, time required to expand TIL and expertise required to culture TIL given its variability without compromising the degree of T-cell differentiation or tumor reactivity. We first hypothesized that the type of activation given by high dose IL-2, which would be considered a “third signal” for T-cell activation, might not be sufficient to expand TIL in malignancies like uveal melanoma where the level of T-cell infiltration does not correlate with successful pre-REP expansion of TIL. Thus, we decided to optimize the generation of the pre-REP product by combining the 3 signals required for an optimal T-cell activation: translating in the addition of OKT3 antibody (anti-CD3) for TCR stimulation (signal 1) and the use of an agonistic antibody to CD137/4-1BB (a-4-1BB, Urelumab) (signal 2 for co-stimulation), while keeping high dose IL-2 for the 3rd signal. The choice of an agonistic stimulation of 4-1BB over other co-stimulatory molecules was based on our extensive experience in multiple cancer types and the previous demonstration that addition of this agonistic antibody during the REP phase preserved melanoma TIL from over differentiation and protects against activation-induced cell death9–12.
Altogether, this work describes how targeting the 3 signals combined for T-cell activation significantly improved the success of TIL culturing. Additionally, we show greatly reduced variability in time and yield of TIL for the pre-REP process while preserving the capacity for tumor recognition. It is our hope that the standardization of growth conditions will improve the eligibility of patients to TIL ACT for challenging cancer types such as uveal melanoma or when tissue size is a limiting factor. Finally, the development of a reliable and standard TIL manufacturing methodology will facilitate expansion of TIL therapy to additional treatment centers and broaden the pool of treatment eligible patients.
MATERIALS AND METHODS
Patient selection
All melanoma TIL lines were derived from tumor tissue obtained from patients with metastatic melanoma (or primary tumor in some uveal cases) who provided written informed consent for a TIL ACT clinical trial (Institutional review board (IRB)-approved protocol# 2004-0069, NCT00338377) at the University of Texas MD Anderson Cancer Center (MDACC). Male and female patients over the age of 12 with stage IV melanoma, stage III in-transit disease, recurrent regional nodal disease or uveal melanoma were eligible for enrollment. Refer to clinical trial NCT00338377 on www.clinicaltrials.gov for specific exclusion criteria. For this study, samples from cutaneous (n= 16) and uveal (n=12) melanoma patients were assessed.
Reagents
A fully human and purified IgG4 monoclonal antibody against human CD137 (Urelumab, 663513; Lot 6A20377) was kindly provided by Bristol Myers Squibb (BMS, New York, NY) through a Materials Transfer Agreement. Human recombinant IL-2 (Proleukin™) was generously provided by Prometheus Therapeutics and Diagnostics (San Diego, CA). GMP-grade soluble anti-CD3 antibody (OKT3 clone) was obtained from Miltenyi Biotec (Bergisch Gladbach, Germany).
Isolation and expansion of TIL from human metastatic melanoma tumors (clinic vs new)
Regarding the clinic TIL line generation, the tumor samples were cut into 1–3 mm3 fragments and placed in complete TIL culture media (TIL-CM) and 6000 IU/mL IL-2. TIL were cultured in 24-well plates for a period averaging between 3 to 5 weeks. An average of 20 fragments were put in culture in 24 well plates (1 fragment per well)2, 13. For 3-signal expansion, five 1–3 mm3 fragments were put in culture in a G-Rex 10 flask (Wilson Wolf Manufacturing, New Brighton, MN) in 20 mL of TIL-CM with 30 ng/mL of OKT3, 10 µg/mL of agonist 4-1BB antibody and 6000IU/mL IL-2. Four to five days after initiating the culture, 20 mL of TIL-CM containing 6000IU/mL IL-2 were added for a total of 40 mL. Half of the media was replaced with new TIL-CM containing 6000IU/mL IL-2 every 3 to 4 days until the cells completely covered the bottom of the flask. The cell suspension was filtered with a 40 µm cell strainer (Corning, Tewksbury, MA) before performing flow analysis and cryopreservation for later testing. The 4-1BB condition was carried in the same manner but without addition of OKT3. TIL lines were terminated after 3 weeks of culture in absence of TIL growth. All samples described are from the same tumor tissue tested across expansion methods as indicated by the four digit de-identified patient ID in each figure.
Flow Cytometric Analysis of TIL
Expanded TIL were stained in 100 µL of FACS Wash Buffer (Dulbecco’s Phosphate Buffered Saline 1× with 1% Bovine Serum Albumin) for 30 min using fluorochrome-conjugated monoclonal antibodies for CD3 (FITC), CD16 (PE), γδTCR (APC), CD56 (PE-Cy7), CD4 (PerCP Cy5.5), CD8 (PB), BLTA (PE), CD28 (PE-Cy7), TIM3 (APC) (all BD Bioscience, San Jose, CA), PD-1 (PerCP-Cy5.5) (Biolegend, San Diego, CA) and AQUA live/dead dye (Invitrogen, Carlsbad, CA). Stained cells were acquired using the BD FACScanto II and analyzed using FlowJo software v 10.1 (Tree star, Ashland, OR).
IFNγ ELISPOT assay
IFNγ ELISPOT (Enzyme-linked immunospot) assay was performed as described by Harao, Forget et al.10. Briefly, MultiScreen-HA 96-well filter ELISPOT plates (Millipore, Billerica, MA) were coated with 5 µg/mL anti-IFNγ mAb (Mabtech, Cincinnati, OH) in pH 9.5 carbonate buffer (Sigma-Aldrich, St.Louis, MO) overnight at 4°C and blocked with 2% BSA of PBS (Sigma). OKT3 + a-4-1BB expanded TIL fragments were thawed and rested overnight in TIL-CM with 6000 IU/mL of human IL-2 in 37°C. The following day, cells were washed twice and rested in TIL-CM without IL-2 for 6 hours at 37°C. Before setting the co-culture experiment, 1 × 105 cells/well (or indicated numbers of cells/well, for #3111: 5 × 104, for #3116: 2 × 104) of autologous tumor were incubated in the presence of 80 µg/mL of mouse IgG2a kappa isotype control Ab (eBioscience, San Diego, CA) or anti-Human HLA-ABC mAb (clone W6/32, eBioscience) for 3 hours as previously described14. 1 × 105 cells/well of rested TIL were co-cultured with autologous tumor lines in triplicates for overnight. The next day, 1 µg/mL of biotin-labeled anti-IFN-γ mAb (Mabtech) was added and incubated for 1 h at 37°C. After treatment with a 1:5000 dilution of extravidin-alkaline phosphatase (Sigma) for 1.5 h at room temperature, plates were developed with filtered 5-bromo-4-chloro-3-indolyl-phosphate in conjunction with nitro blue tetrazolium (BCIP/NBT substrate; Sigma). Spots were counted on an ImmunoSpot ELISPOT reader (CTL Immunospot Reader, software version 6.0.0.0, Cleveland, OH). TIL only with media condition is a negative control (TIL alone). Cells treated with PMA and ionomycin (Sigma) were used as positive controls in this experiment. The culture was considered reactive to the tumor line when the isotype control was twice that of the MHC class I blocked condition.
Statistical Analysis
GraphPah prism version 7 (GraphPad Software, La Jolla, CA) was used for graphing and statistical analysis. Mean ± SD were calculated for each condition and statistical analysis for comparison of culture duration was performed using an unpaired Student’s t test with Welch’s correction. The clinical expansion conditions were used as a control metric for determination of statistical significance unless otherwise stated in the figure.
RESULTS
Retrospective success of TIL expansion at the pre-REP stage over 11 years at MDACC
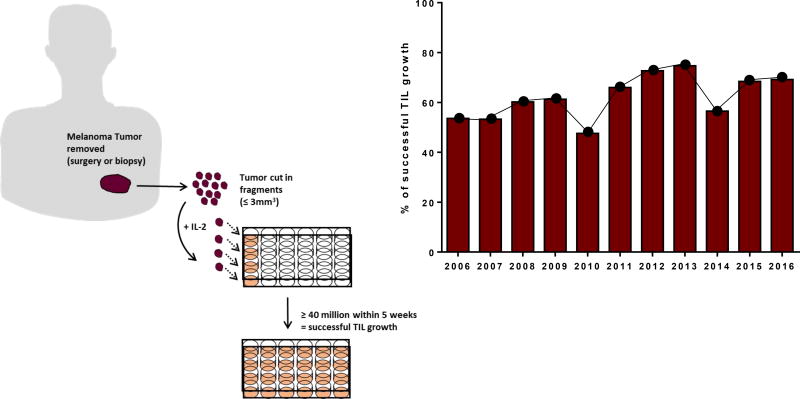
Under the clinical trial at MDACC for the treatment of metastatic melanoma, we have accrued over 900 patients in the 11 years of this protocol’s existence. Since we opted for a “banking” model instead of an “intent to treat only” model, the MDACC TIL lab has accumulated an impressive bank of cryopreserved TIL. Successful TIL culture is defined as the ability to grow at least 40× 106 cells by 5 weeks. The culturing success rate throughout 11 years averages at 62%, with 68% for the last 5 years (n=1135, depicted in Figure 1). Pre-REP TIL are obtained by cutting fragments of less than 5mm3 and culturing them in 24-well plates with high doses of IL-2 for a maximum of 5 weeks before cryopreservation (Figure 1, Schematic). There is no discrimination in tissue accrual based on quality or size.
Figure 1. Diagram of melanoma-derived TIL cultures and yearly success rate.
On the left side: Small fragments (1 to 3 mm3) are excised from melanoma tumors and individually put in culture in 24 well-plates. Subsequent sub-culturing of at least 40 million TIL cells in a maximal timeframe of 5 weeks is considered a successfully grown pre-REP TIL culture. On the right side: Percentage of successfully grown pre-REP TIL cultures according to the method presented on the left side.
Simultaneous engagement of the TCR and CD137/4-1BB molecule shortens the duration and improves the success rate of TIL expansion in both cutaneous and uveal melanoma
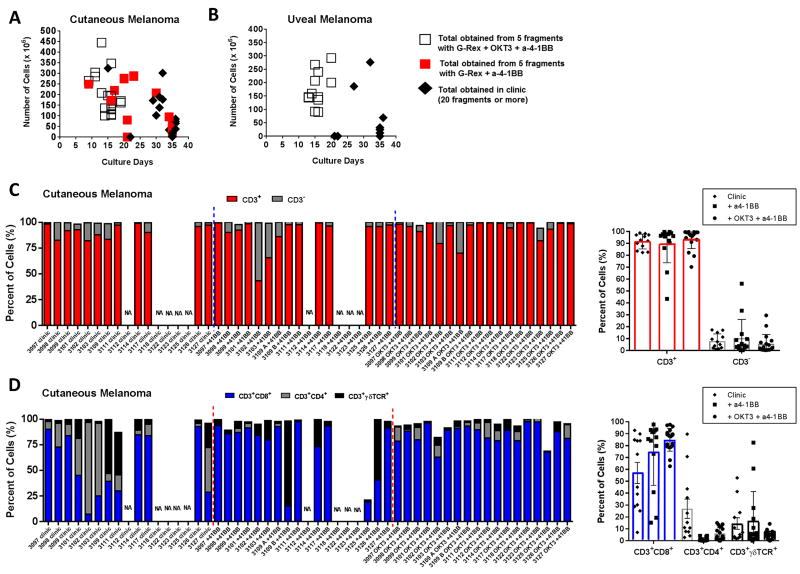
The labor and expertise required to care for the pre-REP stage including the high variability in the number of TIL obtained and the duration of the culture have warranted research efforts to streamline and standardize this procedure. We and others have recently published for multiple solid cancers, including cutaneous melanoma, that the use of an agonistic antibody directed at CD137/4-1BB (Urelumab) expressed on the surface of CD8+ TIL could improve the growth rate and reduce the time of propagation of pre-REP TIL9, 10, 15. Although the introduction of 4-1BB stimulation presents a great advantage, it does not take the expertise and decision-making factor out of the equation throughout the culturing process. Based on our past experiences on the use of different devices for TIL expansion and their beneficial impact on metabolism we designed a new approach to expand pre-REP TIL. This new approach also took into account data showing that some cultures (mainly from uveal melanoma) grow poorly in IL-2 alone but expand well after a TCR activation, as well as our knowledge of the benefit of a CD137/4-1BB co-stimulation of TIL while stimulating the TCR during the REP8, 9, 16. This novel method, which utilizes the 3 signals required for an optimal T-cell activation, incorporates the use of both an agonistic 4-1BB and OKT3 antibodies (anti-CD3) with high dose IL-2 in a small G-Rex10 device. We optimized this new method by using parallel TIL cultures from consented patients. We compared the yield of TIL obtained and duration of culture between our current clinical expansion in 24-well plates with high dose IL-2 and the two other test conditions which were the use of the agonistic 4-1BB antibody alone with IL-2 or the use of the 3 signals (antibodies to 4-1BB and CD3 with IL-2). As demonstrated in Figure 2A, utilizing all 3 signals for T-cell activation in the tumor fragments yields high numbers of TIL in a shorter period of time with an average culture duration of 14.7 ± 2.6 days (white squares, p<0.0001 as compared to control clinical expansion). By comparison, the culture period for the other two growth conditions, while significantly different, was more comparable with an average culture duration of 30.9 ± 6.1 days for the TIL grown for clinical growth (black diamonds) and 24.3 ± 7.8 days for a-4-1BB alone (red squares, p=0.0119). The TIL grown via the clinical lines in Figure 2A constitutes a good representation of our yearly overall success for melanoma in general (between 60 and 70%). It should be noted that the TIL grown from the clinical cultures arise from an average of 20 fragments in 24 well-plates; in contrast to the new method and the agonistic 4-1BB alone where only 5 fragments are used in the G-Rex flask. All data points for all conditions for a given patient came from TIL generated with the same source tissue to ensure maximal standardization. Overall, the addition of agonistic 4-1BB and OKT3 antibodies greatly improved the yield of TIL and halved the culture time required to produce an adequate number of TIL.
Figure 2. Addition of agonistic 4-1BB and OKT3 antibodies increases the success rate of pre-REP TIL cultures, reduces the time-frame for culturing, and greatly favors the growth of CD3+CD8+ TIL cells.
(A) Graph depicting the numbers of TIL vs time of culturing before freeze for cutaneous melanoma-derived TIL following 3 types of expansions: addition of anti-4-1BB (a-4-1BB) with OKT3 and IL-2 (5 tumor fragments in a small G-Rex flaks, white squares n=18), addition of anti-4-1BB only and IL-2 (5 tumor fragments in a small G-Rex flaks, red squares n=18) and TIL expanded if clinical lab with IL-2 only (average of 20 tumor fragments in 24wp, black diamond n=17). The TIL lines #3109 A and B were derived from two different anatomical sites (left and right flank). The TIL from these 2 sites were pooled in the clinic culture (3109), but kept separated on the research side (3109 A and B). (B) Graph depicting the numbers of TIL vs time of culturing before freeze for uveal melanoma-derived TIL following 2 types of expansions: addition of anti-4-1BB with OKT3 and IL-2 (white squares n=12) and TIL expanded if clinic with IL-2 only (black diamond n=12). (C) Percentage of CD3+/CD3−for each pre-REP TIL line (indicated by the four digit ID) grown in clinic, with anti-4-1-BB alone or in combination with OKT3 (left graph). Percent of CD3+/CD3− in samples segregated by group (blue dashed line) and averages for each conditions (right graph). (D) Percentage of CD8+, CD4+ and γδTCR+ cells for each pre-REP TIL line grown in clinic, with anti-4-1-BB alone or in combination with OKT3 (left graph). Percent of CD8+, CD4+ and γδTCR+ cells in samples segregated by group (red dashed line) and averages for each conditions (right graph).
Uveal melanoma is notoriously difficult to expand enough TIL to allow for treatment. Contrary to the notion that uveal melanoma tumors are devoid of an immune infiltrate, our colleagues have recently demonstrated that uveal melanoma tumors contain a comparable immune infiltrate compared to cutaneous melanoma. However, the overall numbers obtained after culturing in high-dose IL-2 remain modest in comparison to cutaneous melanoma8. As demonstrated in Figure 2B, an agonistic stimulation of 4-1BB combined with the use of OKT3 in the tumor fragments allowed uveal melanoma-derived TIL to proliferate to very high numbers in half the normal culture time (average culture duration of 15.8 ± 2.3 days). The latter is greatly contrasting to the samples grown with only IL-2 in the clinical lab, from which only three cultures were considered successful according to our clinical standard from 20 fragments (average culture duration of 29.7 ± 6.5 days, p<0.0001, Figure 2B).
Utilizing the 3 signals preferentially expands the lymphocyte population, mainly the CD8+ TIL
As previously mentioned, addition of the agonistic 4-1BB antibody accelerates the culture process and success rate. However it also has the potential to stimulate the growth of other tumor-infiltrating immune cells such as NK cells and γδ T cells due to their surface expression of CD137/4-1BB17. In a quest to verify if this observation was transposable to our work, we assessed the general phenotype of the pre-REP product obtained from the 3 different methods of expansion. As depicted in Figure 2C (left graph), TIL lines such as TIL#3102 and #3103 expanded more CD3− (grey bar), most likely NK cells, when the propagation was executed with anti-4-1BB alone. On the other hand, the combination with OKT3 gave rise to a more uniform CD3+ T-cell product (Figure 2C, left graph). While specific examples of CD3− cell growth occurred with agonistic 4-1BB expanded cultures, CD3+ T-cell expansion was generally similar across the growth conditions (average 91.5% clinic, 90% a-4-1BB, and 94% OKT3 + a-4-1BB, Figure 2C, right graph). We observed a similar result regarding γδ T cells when we characterized the CD3+ T-cell populations. As shown in Figure 2D, while γδ T-cell expansion was favored in the presence agonistic 4-1BB alone in some cases, CD8+ TIL were predominantly expanded from the tumor fragments when the 3-signal method was used for pre-REP TIL expansion (mean 84.9 ± 9.6% versus 74.7 ± 28.1%, p=0.2). Although this new expansion method resulted in a high degree of TIL proliferation in a short time frame, we observed a high expression of CD28 and B-and-T lymphocyte attenuator (BTLA) and a low expression of PD-1 and TIM3 indicating that the TIL were not overly differentiated (Supplementary Figure 1). Our group has previously reported that expression of BTLA (and CD28) on CD8+ TIL is a signature of less-differentiated TIL, which correlates with clinical response to TIL therapy2, 18.
TCR-dependent proliferation expands tumor-specific TIL in both cutaneous and uveal melanoma
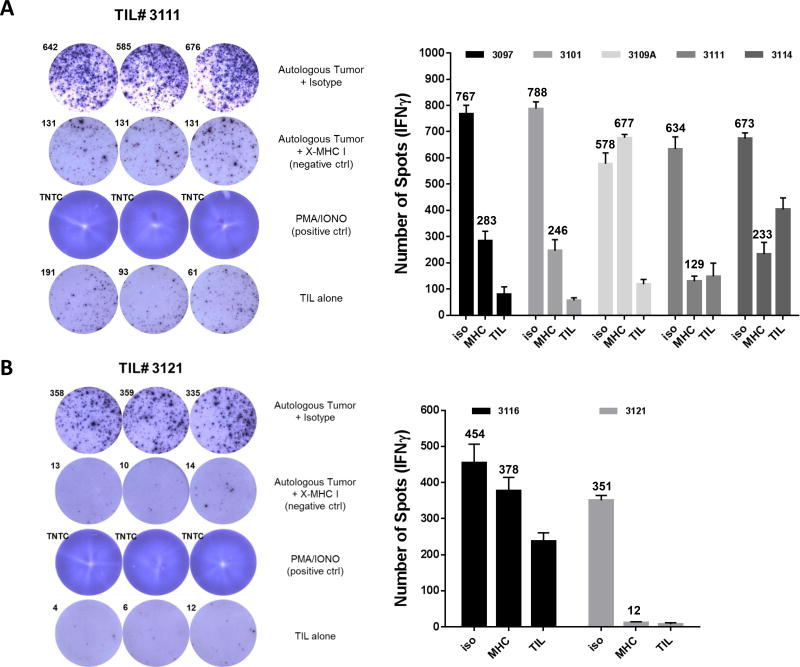
T cells proliferate and accumulate at sites that are rich for the antigen they recognize. Success of TIL therapy relies on a diverse repertoire of tumor-antigen specific T cells infiltrating the tumor, and their capacity to recognize various antigens presented by the tumor. Since this new method considerably changes the way to expand TIL at the pre-REP level, we wanted to verify if the short-term expanded TIL could recognize their autologous tumor line. As demonstrated in Figure 3A, 4 out of the 5 cutaneous TIL lines tested displayed tumor recognition based on secretion of IFNγ detected by ELISPOT. This recognition was MHC class I dependent with the exception of the TIL line #3109 which contained 30% NK cells, providing a potential explanation for the presence of IFNγ independently of the availability of the MHC-I complex (Figures 2C and 3A). Globally, tumor recognition is not lost upon expansion using this new method.
Figure 3. Pre-REP TIL expended using the 3-signal model are capable of autologous tumor recognition.
(A, B) IFNγ ELISPOT showing autologous tumor recognition for 5 pre-REP cutaneous TIL lines (A) and 2 pre-REP uveal TIL lines (B) cultured with addition of agonistic 4-1-BB and OKT3 antibodies. Addition of MHC-I blocking antibody and TIL alone were used as negative controls. Results are presented as number of spots per 1× 105 TIL. The numbers indicate the average number of spots per condition. Positive reactivity was defined as twice the number of spots as compared to the MHC class I blocked control.
Tumor cell lines derived from a uveal tumor are always very challenging to generate. We were able to successfully derive one autologous tumor cell line for TIL #3121 with confirmed MHC class I expression and mainly devoid of CD90+ fibroblasts (Supplementary Figure 2) which was positively recognized by its autologous pre-REP TIL line expanded with OKT3 and a-4-1BB (Figure 3B). Unfortunately, the clinical sample did not proliferate in high dose IL-2 and tumor-reactivity could not be tested. We also tested the uveal pre-REP TIL line #3116 against its autologous tumor that had unfortunately entered in vitro senescence and we could not see any tumor recognition for this line.
Thus, providing the 3 signals attributed to T-cell activation lead to expansion of TIL capable of recognizing their tumor counterpart in cutaneous and uveal melanoma.
DISCUSSION
TIL ACT for metastatic melanoma has demonstrated high clinical response rates in multiple phase II studies, however the ability to reach widespread application will rely on the standardization and efficiency of TIL expansion methodology. The duration of TIL manufacturing remains a hurdle to overcome in order to translate this therapy to more aggressive tumor types. With this work, we have addressed the variability derived from culturing TIL with IL-2 alone by the combination of IL-2, agonistic CD137/4-1BB (Urelumab) and OKT3 antibodies. This combination resulted in a standard production of TIL in terms of success rate and production time. The production time was consistent at approximately 2 weeks and required minimal manipulation which greatly diminished the need for any decision-making by the operator thus abrogated the requirement for expertise in TIL culturing.
One major aspect that needs to be highlighted about this method is that the numbers of TIL obtained from only 5 fragments of less than 3mm3 were equivalent or higher to those obtained from 20 fragments with the standard IL-2 method. Typically, TIL centers set-up variable numbers of fragments in their initial pre-REP culture using IL-2 alone but it usually stands between 20 and 48 tumor fragments2, 15. The fact that we easily achieve 100 × 106 TIL from 5 tumor fragments within 2 weeks opens the possibly of expanding TIL successfully from samples as small as a core needle biopsy. One could also contemplate using more TIL fragments with the 3-signal expansion method and treat a patient using only the pre-REP TIL product, thereby omitting the 2 week REP process.
Remarkably, this new approach resulted in 12 out of 12 successful expansion of pre-REP TIL derived from uveal melanoma. In comparison, only 3 cultures from a uveal tumor were successfully expanded in the clinical lab using IL-2 alone. From our past experience, the success of generating TIL from uveal melanoma has been poor compared to cutaneous melanoma8. Thus, this method not only reduced variability but more importantly allowed to reliably grow TIL from tumor tissue where IL-2 alone was not successful. This enables the possibility of expanding the use of TIL therapy to more patients, including uveal melanoma, where TIL therapy has already shown preliminary success7. This advance may help position TIL therapy as a sensible alternative in malignancies facing limited success with the in vivo T-cell activation through the use of checkpoint inhibitors such as uveal melanoma, pancreas adenocarcinoma, and triple-negative breast cancer19–21.
The general profile of TIL was shifted in favor of CD3+CD8+ cells when co-stimulated with agonistic CD137/4-1BB and OKT3 antibodies. NK cells can also be stimulated through targeting the 4-1BB axis, which is what was observed when 4-1BB was targeted alone. However, in combination with OKT3, the cultures retained a high percentage of CD3+ T cells through TCR stimulation, avoiding any unwanted stimulatory effects of 4-1BB alone on other cell types.
The very last aspect that we wanted to assess is tumor recognition. Even though our clinical TIL study never correlated tumor recognition with clinical response, it was still critical for us to demonstrate that such a short and strong expansion would not impair the capacity of the pre-REP TIL to recognize an autologous tumor. We were able to demonstrate the latter in both cutaneous and uveal melanoma.
Altogether, utilizing T-cell activation signals 1, 2 and 3 greatly improved the culture success rate and absolute expansion numbers and, more importantly, consistently shortened the duration of pre-REP TIL culture for both cutaneous and uveal melanoma. This study broadens the horizon for TIL therapy in cancers where the amount of tissue available for culture is minimal, as well as for rapidly progressing cancer types where the window of treatment is limited and where the time for TIL production becomes of paramount importance.
Supplementary Material
Acknowledgments
The authors would like to thank Bristol Myers Squibb for their generous contribution with the agonistic anti-4-1BB antibody (BMS-663513) as well as Prometheus for kindly providing the IL-2 used in this study and the clinical work. Additionally, we would like to thank all the additional clinical and research staff in the Melanoma Medical Oncology Department as well as the Regulatory Compliance Unit, part of the Center for Cancer Immunotherapy Research and finally the Stem Cell Transplantation Lab at MDACC that contributed to this study.
This work was supported by NIH P30-CA16672 to Chantale Bernatchez and Dr. Elizabeth J. Shpall as well as The University of Texas MD Anderson Cancer Institutional provost funds.
Footnotes
CONFLICT OF INTEREST/ FINANCIAL DISCLOSURES
The authors have no conflict of interest to declare.
References
- 1.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. Journal of Clinical Oncology. 2005;23(10):2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radvanyi LG, Bernatchez C, Zhang M, et al. Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2012;18(24):6758–70. doi: 10.1158/1078-0432.CCR-12-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besser MJ, Shapira-Frommer R, Treves AJ, et al. Clinical responses in a phase II study using adoptive transfer of short-term cultured tumor infiltration lymphocytes in metastatic melanoma patients. Clin Cancer Res. 2010;16(9):2646–55. doi: 10.1158/1078-0432.CCR-10-0041. [DOI] [PubMed] [Google Scholar]
- 4.Andersen R, Donia M, Ellebaek E, et al. Long-lasting complete responses in patients with metastatic melanoma after adoptive cell therapy with tumor-infiltrating lymphocytes and an attenuated IL-2 regimen. Clin Cancer Res. 2016 doi: 10.1158/1078-0432.CCR-15-1879. [DOI] [PubMed] [Google Scholar]
- 5.Tran E, Turcotte S, Gros A, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–5. doi: 10.1126/science.1251102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevanovic S, Draper LM, Langhan MM, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus-targeted tumor-infiltrating T cells. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2015;33(14):1543–50. doi: 10.1200/JCO.2014.58.9093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chandran SS, Somerville RPT, Yang JC, et al. Treatment of metastatic uveal melanoma with adoptive transfer of tumour-infiltrating lymphocytes: a single-centre, two-stage, single-arm, phase 2 study. Lancet Oncol. 2017;18(6):792–802. doi: 10.1016/S1470-2045(17)30251-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qin Y, Petaccia de Macedo M, Reuben A, et al. Parallel profiling of immune infiltrate subsets in uveal melanoma versus cutaneous melanoma unveils similarities and differences: A pilot study. Oncoimmunology. 2017;6(6):e1321187. doi: 10.1080/2162402X.2017.1321187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chacon JA, Sarnaik AA, Chen JQ, et al. Manipulating the tumor microenvironment ex vivo for enhanced expansion of tumor-infiltrating lymphocytes for adoptive cell therapy. Clin Cancer Res. 2015;21(3):611–21. doi: 10.1158/1078-0432.CCR-14-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harao M, Forget MA, Roszik J, et al. 4-1BB-Enhanced Expansion of CD8+ TIL from Triple-Negative Breast Cancer Unveils Mutation-Specific CD8+ T Cells. Cancer Immunol Res. 2017;5(6):439–445. doi: 10.1158/2326-6066.CIR-16-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chacon JA, Wu RC, Sukhumalchandra P, et al. Co-stimulation through 4-1BB/CD137 improves the expansion and function of CD8(+) melanoma tumor-infiltrating lymphocytes for adoptive T-cell therapy. PLoS One. 2013;8(4):e60031. doi: 10.1371/journal.pone.0060031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernandez-Chacon JA, Li Y, Wu RC, et al. Costimulation through the CD137/4-1BB pathway protects human melanoma tumor-infiltrating lymphocytes from activation-induced cell death and enhances antitumor effector function. J Immunother. 2011;34(3):236–50. doi: 10.1097/CJI.0b013e318209e7ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Forget MA, Malu S, Liu H, et al. Activation and propagation of tumor-infiltrating lymphocytes on clinical-grade designer artificial antigen-presenting cells for adoptive immunotherapy of melanoma. J Immunother. 2014;37(9):448–60. doi: 10.1097/CJI.0000000000000056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doucet JD, Forget MA, Grange C, et al. Endogenously expressed matrix protein M1 and nucleoprotein of influenza A are efficiently presented by class I and class II major histocompatibility complexes. J Gen Virol. 2011;92(Pt 5):1162–71. doi: 10.1099/vir.0.029777-0. [DOI] [PubMed] [Google Scholar]
- 15.Hall M, Liu H, Malafa M, et al. Expansion of tumor-infiltrating lymphocytes (TIL) from human pancreatic tumors. J Immunother Cancer. 2016;4:61. doi: 10.1186/s40425-016-0164-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forget MA, Haymaker C, Dennison JB, et al. The beneficial effects of a gas-permeable flask for expansion of Tumor-Infiltrating lymphocytes as reflected in their mitochondrial function and respiration capacity. Oncoimmunology. 2016;5(2):e1057386. doi: 10.1080/2162402X.2015.1057386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakellariou-Thompson D, Forget MA, Creasy C, et al. 4-1BB Agonist Focuses CD8(+) Tumor-Infiltrating T-Cell Growth into a Distinct Repertoire Capable of Tumor Recognition in Pancreatic Cancer. Clinical Cancer Research. 2017;23(23):7263–7275. doi: 10.1158/1078-0432.Ccr-17-0831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haymaker CL, Wu RC, Ritthipichai K, et al. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. Oncoimmunology. 2015;4(8):e1014246. doi: 10.1080/2162402X.2015.1014246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maio M, Danielli R, Chiarion-Sileni V, et al. Efficacy and safety of ipilimumab in patients with pre-treated, uveal melanoma. Ann Oncol. 2013;24(11):2911–5. doi: 10.1093/annonc/mdt376. [DOI] [PubMed] [Google Scholar]
- 20.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–7. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Algazi AP, Tsai KK, Shoushtari AN, et al. Clinical outcomes in metastatic uveal melanoma treated with PD-1 and PD-L1 antibodies. Cancer. 2016;122(21):3344–3353. doi: 10.1002/cncr.30258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.