Abstract
Berberine is a traditional medicine that has multiple medicinal and agricultural applications. However, little is known about whether berberine can be a bioactive molecule toward carbohydrate-active enzymes, which play numerous vital roles in the life process. In this study, berberine and its analogs were discovered to be competitive inhibitors of glycoside hydrolase family 20 β-N-acetyl-d-hexosaminidase (GH20 Hex) and GH18 chitinase from both humans and the insect pest Ostrinia furnacalis. Berberine and its analog SYSU-1 inhibit insect GH20 Hex from O. furnacalis (OfHex1), with Ki values of 12 and 8.5 μm, respectively. Co-crystallization of berberine and its analog SYSU-1 in complex with OfHex1 revealed that the positively charged conjugate plane of berberine forms π–π stacking interactions with Trp490, which are vital to its inhibitory activity. Moreover, the 1,3-dioxole group of berberine binds an unexplored pocket formed by Trp322, Trp483, and Val484, which also contributes to its inhibitory activity. Berberine was also found to be an inhibitor of human GH20 Hex (HsHexB), human GH18 chitinase (HsCht and acidic mammalian chitinase), and insect GH18 chitinase (OfChtI). Besides GH18 and GH20 enzymes, berberine was shown to weakly inhibit human GH84 O-GlcNAcase (HsOGA) and Saccharomyces cerevisiae GH63 α-glucosidase I (ScGluI). By analyzing the published crystal structures, berberine was revealed to bind with its targets in an identical mechanism, namely via π–π stacking and electrostatic interactions with the aromatic and acidic residues in the binding pockets. This paper reports new molecular targets of berberine and may provide a berberine-based scaffold for developing multitarget drugs.
Keywords: glycoside hydrolase, chitinase, inhibitor, protein crystallization, human, insect, β-N-acetyl-D-hexosaminidase, berberine
Introduction
Berberine is an isoquinoline quaternary alkaloid widely distributed in the root, stem, and bark of plants from the Berberis and Coptis families, such as Berberis aristata, Berberis aquifolium, Berberis vulgaris, Coptis chinesis, Coptis japonica, and Coptis rhizome (1–3). Berberine has been used for more than 3,000 years in Ayurvedic, Chinese, and Middle-Eastern folk medicine for its antimicrobial, antiprotozoal, antidiarrheal, and antitrachomatic activities (1–3). With the development of modern biomedicine, berberine has been revealed to have a very wide range of pharmacological properties, including anticancer, antidiabetic, antidepressant, antihyperlipidemic, and antihypertensive activities (4–8). Moreover, berberine has also been revealed to have potential applications in agriculture for its antifungal, insecticidal, and herbicidal activities (9–11). Corresponding to its multispectrum activities, several molecular targets of berberine, such as glycogen synthase kinase, calmodulin kinase, matrix metalloprotease, acetylcholinesterase, butyrylcholinesterase, monoamine oxidase, DNA topoisomerase, cyclin, and transcriptional factor p53 (12–18), have been discovered.
Glycoside hydrolase family 20 β-N-acetyl-d-hexosaminidase (GH20 Hex)3 catalyzes the removal of N-acetyl-d-glucosamine (GlcNAc) or N-acetyl-d-galactosamine (GalNAc) from various glycans, glycolipids, and glycoproteins (19, 20). Insect Hex has been proven to be vital for the survival of agricultural pests (21–26). Human Hex is also important for health. Dysfunction of human Hex results in lysosomal storage diseases and osteoarthritis (27, 28). Glycoside hydrolase family 18 (GH18) chitinase not only catalyzes chitin degradation in bacteria, fungi, and insects but also plays different roles in other organisms (29). For example, human chitinases (HsCht and AMCase) have been reported to be involved in asthma (30) and other immunological disorders (31–33). Chitinases from parasites causing nematodosis (34) and malaria (35) are also important for the development and pathogenesis of these organisms. In view of the abovementioned roles of GH20 Hexs and chitinases, inhibitors targeting these enzymes are potential therapeutic agents and agrochemicals (36–39). Glycoside hydrolase family 84 O-GlcNAcase (HsOGA) removes O-linked GlcNAc (O-GlcNAc) from nucleocytoplasmic proteins that are involved in transcriptional regulation and stress response (40). Glycoside hydrolase family 63 α-glucosidase I (GluI) is a key member of the eukaryotic N-glycosylation-processing pathway. Inhibition of GluI activity decreases infectivity of several enveloped viruses, including hepatitis B and C (41).
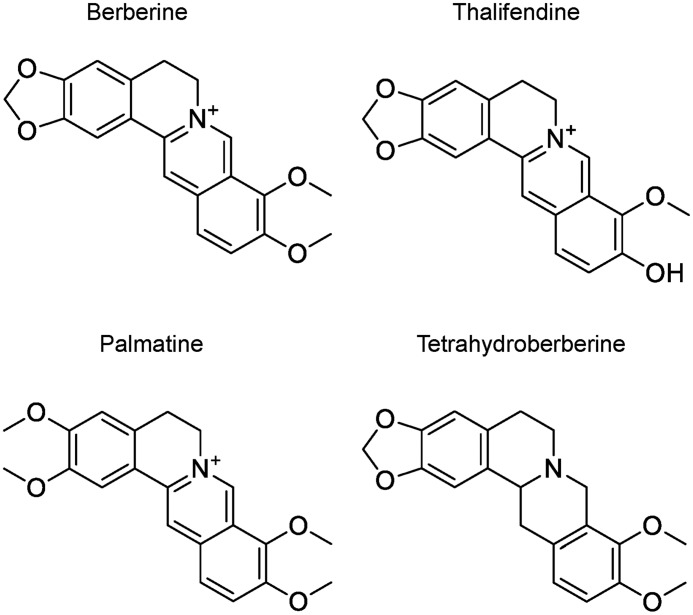
In the previous work, we noticed that compounds with a large conjugated plane were highly potent inhibitors of GH20 Hex (21, 42) and GH18 chitinase (39). Berberine is a typical compound with a large conjugated plane. Here, we report that berberine and its analogs (Fig. 1) act as inhibitors of GH20, GH18, GH84, and GH63 enzymes. The inhibition mechanism of berberine for these enzymes was revealed by crystallography and molecular docking. By comparison with published structures, berberine was revealed to have a similar inhibition mechanism for these structurally and functionally diverse proteins. This work provides the first report of berberine targeting glycoside hydrolases.
Figure 1.
Structure of berberine and its analogs.
Results
Inhibition of GH20, GH18, GH84, GH63, and GH13 enzymes by berberine and its analogs
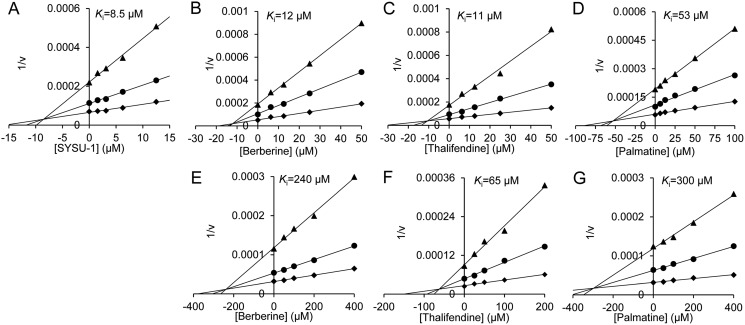
SYSU-1 is a berberine derivative originally reported as a telomeric G-quadruplex DNA-stabilizing ligand (43). In our preliminary screening, SYSU-1 was found to display an inhibition rate of 58.7% against OfHex1, an insect GH20 member, at a concentration of 10 μm. In this study, the Ki value of SYSU-1 for OfHex1 was determined to be 8.5 μm (Fig. 2). Then, the inhibitory activities of berberine itself against OfHex1 as well as HsHexB, a human GH20 member, were studied. Inhibition kinetics demonstrated that berberine inhibits both OfHex1 and HsHexB in a competitive mode, but the Ki value of berberine for HsHexB was 20-fold higher than that of berberine against OfHex1 (Fig. 2 and Table 1). Berberine analogs, including thalifendine and palmatine, were also found to be inhibitors of OfHex1 and HsHexB, and they all showed ∼5-fold higher Ki values for HsHexB than for OfHex1 (Fig. 2 and Table 1). However, tetrahydroberberine did not inhibit OfHex1 and HsHexB at the concentration of 100 μm (its solubility limit in 2% DMSO).
Figure 2.
Inhibition kinetics of berberine and its analogs toward GH20 Hexs. A, inhibition kinetics of SYSU-1 toward OfHex1; B and E, inhibition kinetics of berberine toward OfHex1 and HsHexB; C and F, inhibition kinetics of thalifendine toward OfHex1 and HsHexB; and D and G, inhibition kinetics of palmatine toward OfHex1 and HsHexB.
Table 1.
Ki values of berberine and its analogs against GH20 and GH18 enzymes
| Compound |
Ki (μm) |
|||||||
|---|---|---|---|---|---|---|---|---|
| GH20 |
GH18 |
GH84, HsOGA | GH63, ScGluI | GH13, PPA | ||||
| OfHex1 | HsHexB | OfChtI | HsCht | AMCase | ||||
| Berberine | 12 | 240 | 23 | 19 | 65 | 118 | 130 | NIa |
| Thalifendine | 11 | 65 | 15 | 15 | 55 | 72 | 74 | NIa |
| Palmatine | 53 | 300 | 38 | 15 | 70 | 194 | 600 | NIa |
| Tetrahydroberberine | NIb | NIb | NIb | NIb | NIb | NIb | NIb | NIb |
a NI indicates not inhibited at a concentration of 400 μm.
b NI indicates not inhibited at a concentration of 100 μm.
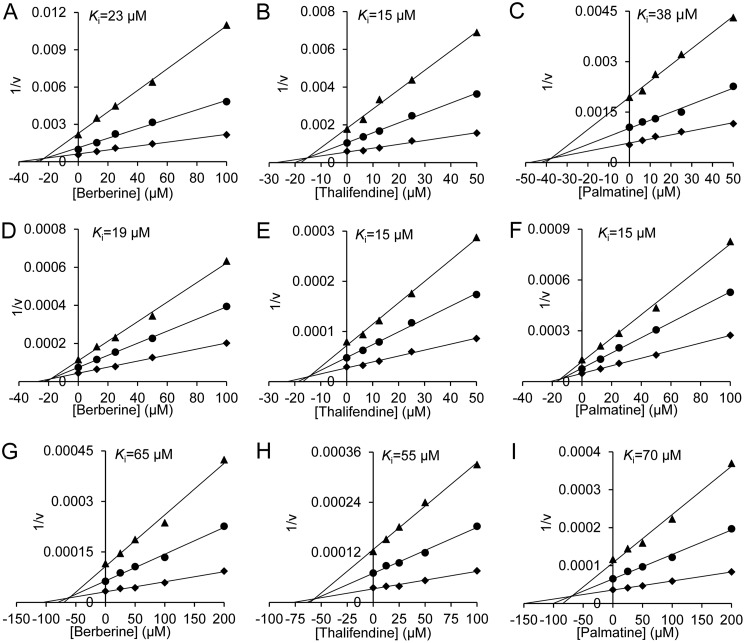
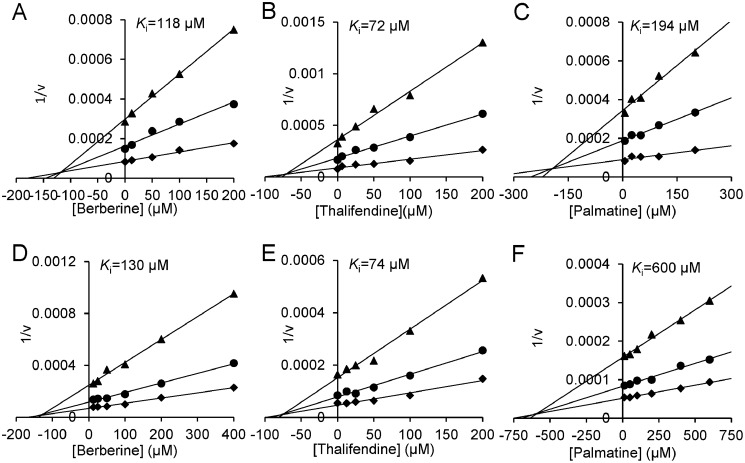
To evaluate whether berberine can act as a scaffold for developing an inhibitor for a broad spectrum of glycosyl hydrolases, the inhibitory activities of berberine and its analogs toward GH18, GH84, GH63, and GH13 enzymes were assayed, and the Ki values were determined. Berberine, thalifendine, and palmatine showed inhibitory activities against GH18, GH84, and GH63 enzymes in a competitive mode, but it did not inhibit GH13 porcine pancreatic α-amylase (PPA) even at a concentration of 400 μm. Tetrahydroberberine did not inhibit all these enzymes at the concentration of 100 μm (Figs. 3 and 4 and Table 1). In addition, berberine and its analogs showed moderate selectivity between two human chitinases. They had 3–5-fold higher Ki values for AMCase than for HsCht.
Figure 3.
Inhibition kinetics of berberine and its analogs toward GH18 chitinases. A, D, and G, inhibition kinetics of berberine toward OfChtI, HsCht, and AMCase; B, E, and H, inhibition kinetics of thalifendine toward OfChtI, HsCht, and AMCase; and C, F, and I, inhibition kinetics of palmatine toward OfChtI, HsCht, and AMCase.
Figure 4.
Inhibition kinetics of berberine and its analogs toward GH84 and GH63 enzymes. A and D, inhibition kinetics of berberine toward HsOGA and ScGluI; B and E, inhibition kinetics of thalifendine toward HsOGA and ScGluI; and C and F, inhibition kinetics of palmatine toward HsOGA and ScGluI.
Crystal structure of OfHex1 in complex with berberine
To reveal the inhibition mechanism of berberine against OfHex1, the complexed structure of OfHex1 and berberine was prepared by soaking and was resolved to a solution of 2.4 Å. The statistics of data collection and structure refinement are shown in Table 2. The coordinates of the OfHex1–berberine complex and OfHex1–SYSU-1 complex have been deposited in the Protein Data Bank under accession numbers 5Y0V and 5Y1B.
Table 2.
Details of data collection and structure refinement
| OfHex1–berberine | OfHex1–SYSU-1 | |
|---|---|---|
| Space group | P3221 | P3221 |
| Unit-cell parameters | ||
| a (Å) | 107.821 | 107.983 |
| b (Å) | 107.821 | 107.983 |
| c (Å) | 175.098 | 175.529 |
| Wavelength (Å) | 0.97775 | 0.97853 |
| Temperature (K) | 100 | 100 |
| Resolution (Å) | 32.79–2.423 (2.51–2.423) | 19.46–2.207 (2.286–2.207) |
| Unique reflections | 41,233 (2260) | 57,623 (3959) |
| Observed reflections | 82,312 (4513) | 115,092 (7911) |
| Rmerge | 0.129 (0.4719) | 0.09773 (0.3229) |
| Average multiplicity | 2.0 (2.0) | 2.0 (2.0) |
| 〈I/σ(I)〉 | 21.55 (8.73) | 24.01 (9.84) |
| Completeness (%) | 90.62 (50.28) | 95.63 (66.59) |
| R/Rfree | 0.1787/0.2026 | 0.1713/0.1918 |
| Protein atoms | 4615 | 4615 |
| Water molecules | 230 | 517 |
| Other atoms | 67 | 65 |
| Root mean square deviation from ideal | ||
| Bond lengths (Å) | 0.003 | 0.003 |
| Bond angles (°) | 0.570 | 0.610 |
| Wilson B factor (Å2) | 35.06 | 28.21 |
| Average B factor (Å2) | 40.95 | 33.06 |
| Protein atoms | 40.37 | 31.77 |
| Water molecules | 41.41 | 40.54 |
| Ramachandran plot (%) | ||
| Favored | 96.49 | 97.54 |
| Allowed | 3.51 | 2.28 |
| Outliers | 0.00 | 0.18 |
| PDB code | 5Y0V | 5Y1B |
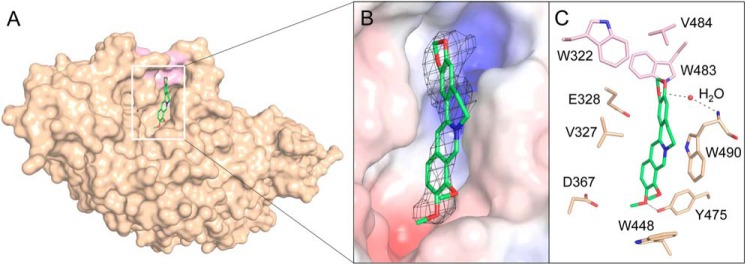
The electron-density map supports the location of berberine in the active pocket of OfHex1 (Fig. 5A). Berberine binds OfHex1 across the −1 and +1 subsites mainly via a π–π stacking interaction with Trp490 and van der Waals interactions with the surrounding residues (Fig. 5, B and C). The positive charge of berberine can be neutralized by the negative electrostatic potential in the active pocket (Fig. 5B). Trp490 appears to be important for berberine's binding because it forms a π–π stacking interaction with the berberine ring and a water-mediated hydrogen bond with the O1 of berberine. The mutant OfHex1–W490A was not inhibited by berberine at 100 μm (data not shown). Moreover, a hydrophobic recess composed of Trp322, Trp483, and Val484 also contributed to the binding of berberine by accommodating its 1,3-dioxole group (Fig. 5). Palmatine without the 1,3-dioxole group showed a Ki value for OfHex1 that was more than 4-fold higher than that of berberine (Table 1). Notably, this hydrophobic recess has not been occupied by other OfHex1 inhibitors, such as N,N,N-trimethyl-d-glucosaminyl-chitotriomycin (TMG-chitotriomycin) (44), N-acetylglucosaminono-1,5-lactone O-(phenylcarbamoyl)-oxime (PUGNAc) (45), 3aR,5R,6S,7R,7aR)-5-(hydroxymethyl)-2-methyl-5,6,7,7a-tetrahydro-3aH-pyrano[3,2-d]thiazole-6,7-diol (NAG-thiazoline) (46), 2-(2-(((5-methyl-1,3,4-thiadiazol-2-yl)methyl)amino)ethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (Q1), and 6-(dimethylamino)-2-(2-(((5-methyl-1,3,4-thiadiazol-2-yl)methyl)amino)ethyl)-1H-benzo[de]isoquinoline-1,3(2H)-dione (Q2) (42).
Figure 5.
Crystal structures of OfHex1 in complex with berberine. A, surface representations of OfHex1 complexed with berberine. B, binding mode of berberine in the active pocket of OfHex1. Electrostatic potential between −6 kT/e and 6 kT/e was shown as a colored gradient from red (acidic) to blue (basic). The 2Fo − Fc electron-density map around the ligand is contoured at the 1.0 σ level. C, amino acid residues involved in the binding of berberine in the active pocket of OfHex1. The hydrophobic recess accommodating the 1,3-dioxole group of berberine is shown in pink. The hydrogen bonds are shown as dashed black lines.
Crystal structure of OfHex1 in complex with SYSU-1
The complexed structure of OfHex1 and SYSU-1 was also prepared by soaking and was resolved to a solution of 2.2 Å. The statistics of data collection and structure refinement are shown in Table 2. The coordinates and the OfHex1-SYSU-1 complex have been deposited into the Protein Data Bank under accession number 5Y1B.
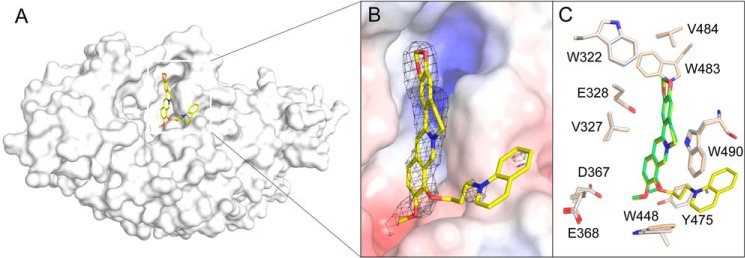
The electron-density map of the berberine ring of SYSU-1 is clear and supports the binding of SYSU-1 in the active pocket of OfHex1 (Fig. 6A). Superimposition of the complex structures of OfHex1–SYSU-1 and OfHex1–berberine revealed that the binding mode of the berberine moiety of SYSU-1 in OfHex1 is identical to that of berberine (Fig. 6B). Most of the residues in the active pockets that interacted with the ligand were in the same conformation except Trp448, which was rotated ∼10° outward from the center of the active pocket. The electron density for the 1-butylquinolin-1-ium group of SYSU-1 is not clear, indicating disorder in this region. Nevertheless, the 1-butylquinolin-1-ium group as well as the linker region may enhance the binding affinity of SYSU-1 via van der Waals interactions or π–π stacking interactions with the surrounding aromatic residues, such as Trp448 and Tyr471 (Fig. 6B).
Figure 6.
Crystal structures of OfHex1 in complex with SYSU-1. A, surface representations of OfHex1 complexed with SYSU-1. B, binding mode of SYSU-1 in the active pocket of OfHex1. Electrostatic potential between −6 kT/e and 6 kT/e was shown as a colored gradient from red (acidic) to blue (basic). The 2Fo − Fc electron-density map around the ligand is contoured at the 1.0 σ level. C, superimposition of the berberine-complexed and SYSU-1–complexed OfHex1. Residues of the berberine-complexed and SYSU-1–complexed OfHex1 are shown in wheat and white, respectively.
Modeled structures of other GH20, GH18, GH84, GH63, and GH13 enzymes in complex with berberine
The binding mode of berberine to HsHexB was studied by the molecular docking of berberine to the crystal structure of HsHexB (47). As shown in Figs. 6B and 7A, berberine could be only partially inserted into the active pocket and formed a π–π stacking interaction with Trp489. Compared with berberine binding to OfHex1, berberine bound to HsHexB is more solvent-exposed, which may weaken the hydrophobic interaction with the active-site residues. Moreover, the positive charge of berberine may be repulsed by the positive electrostatic potential in the active pocket (Fig. 7A).
Figure 7.
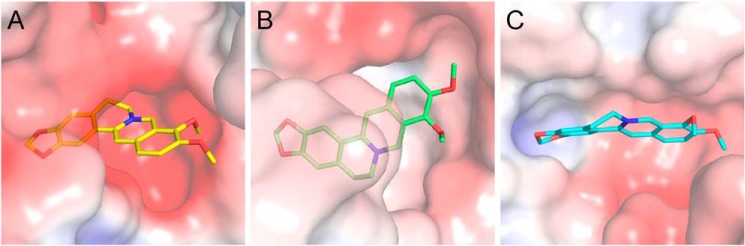
Modeled structures of berberine in complex with GH20 and GH18 enzymes. Electrostatic potential between −6 kT/e and 6 kT/e was shown as a colored gradient from red (acidic) to blue (basic). A, binding mode of berberine in the active pocket of HsHexB. B, locations of the key residues for berberine binding in HsHexB. C, binding modes of berberine in the active pocket of HsCht. D, binding modes of berberine in the active pocket of OfChtI. E, binding modes of berberine in the active pocket of AMCase. F, superimposition of the binding modes of berberine with three chitinases. Residues of the HsCht and its berberine are shown in green. Residues of the OfChtI and its berberine are shown in yellow-orange. Residues of the AMCase and its berberine are shown in cyan.
The binding modes of berberine to HsCht, OfChtI, and AMCase were studied by molecular docking (Fig. 7, C–F). The results demonstrated that berberine was placed into an identical position in the substrate-binding clefts of these chitinases by forming π–π stacking interactions with a conserved tryptophan residue (Fig. 7F). Although the electrostatic potentials in the active pockets of these chitinases are from negative to neutral (Fig. 7, C–E), the positive charge of berberine might be neutralized by a conserved aspartate residue (Fig. 7F).
The binding modes of berberine to HsOGA, ScGluI, and PPA were also studied by molecular docking (Fig. 8). As the electrostatic potentials in the active pockets of HsOGA and ScGluI are negative, the positive charge of berberine might be neutralized by the surrounding negatively charged residues (Fig. 8, A and B). As for PPA, although berberine could be docked into the wide active pocket of PPA, it could not form any π–π stacking interactions or electrostatic interactions with PPA (Fig. 8C).
Figure 8.
Modeled structures of berberine in complex with GH84, GH63, and GH13 enzymes. Electrostatic potential between −6 kT/e and 6 kT/e was shown as a colored gradient from red (acidic) to blue (basic). A, binding mode of berberine in the active pocket of HsOGA. B, binding modes of berberine in the active pocket of ScGluI. C, binding modes of berberine in the active pocket of PPA.
In vivo activity of berberine and SYSU-1
To test their bioactivity, the artificial diet that contained berberine and SYSU-1, respectively, was used to feed 4th-instar day 1 Ostrinia furnacalis larvae. Compared with the control group, compounds fed larvae grew slowly, and some of them died after 6 days (Fig. 9).
Figure 9.

In vivo activity of berberine and SYSU-1. A, larvae before exposed to the compounds. B, larvae of DMSO-fed group 6 days later. C, larvae of berberine-fed group 6 days later. D, larvae of SYSU-1-fed group 6 days later.
Discussion
In this study, berberine was discovered to be a competitive inhibitor of GH20 and GH18 enzymes. Similar to the amylase inhibitor montobretin A (48), berberine binds with the target enzymes by a noncanonical mode that is not the binding mode of the transition state analog inhibitor or a substrate.
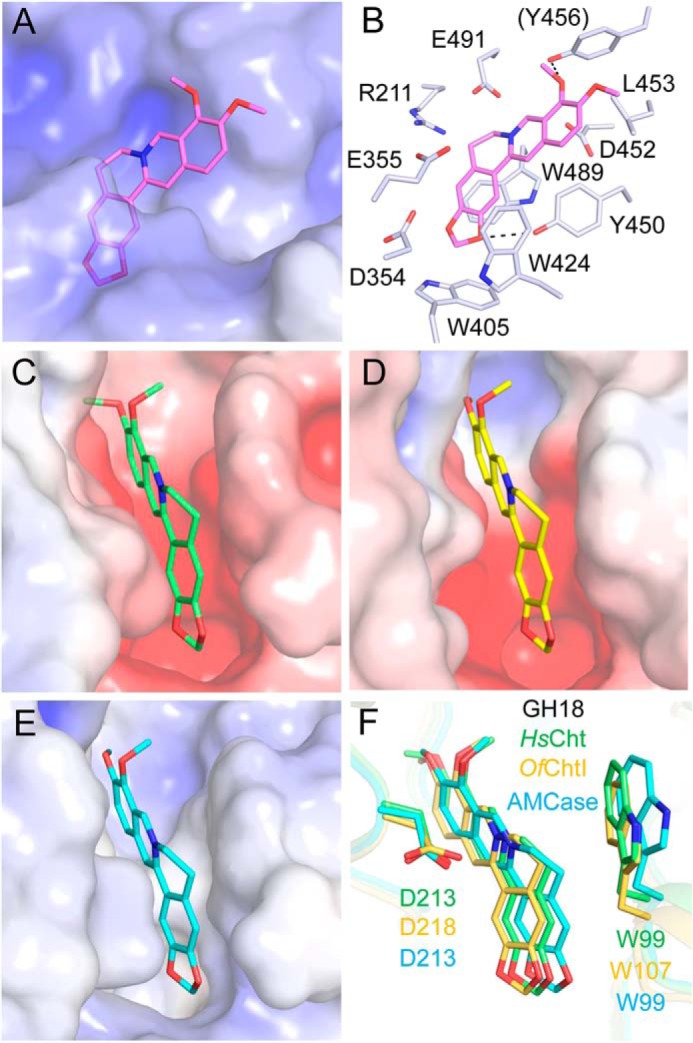
As revealed by X-ray crystallography as well as molecular docking, berberine inhibits these enzymes via an identical mechanism. As a positively charged conjugate plane, berberine usually binds in a narrow pocket with negative electrostatic potential and forms π–π stacking interactions with a conserved tryptophan residue (Trp490 in OfHex1, Trp489 in HsHexB, Trp99 in HsCht, Trp99 in AMCase, and Trp107 in OfChtI) (Figs. 5B and 7, A and B) and electrostatic interactions with a conserved negatively charged residue (Glu328 in OfHex1, Glu491 in HsHexB, Asp213 in HsCht, Asp213 in AMCase, Asp218 in OfChtI, Asp175 in HsOGA, and Glu771 in ScGluI) (Figs. 5B, 7, A and B, and 8). To determine whether berberine binds other known target proteins by the same mechanism, the reported complexed structures of berberine with other protein targets were analyzed. These protein targets included the multidrug binding protein QacR from Staphylococcus aureus (49), the multidrug resistance regulator BmrR from Bacillus subtilis (50), and RamR from Salmonella typhimurium (51). Although the structures and functions of these proteins vary greatly, we observed that berberine binds these proteins in a similar mode to that observed with GH20 and GH18 enzymes (Fig. 10). The conjugate plane of berberine formed π–π stacking interactions with aromatic residues (Trp61, Tyr93, Tyr123 in QacR; Phe224, Tyr229, and Tyr268 in BmrR; Phe155 in RamR). Moreover, the positive charge of berberine could be neutralized by the surrounding negatively charged residues (Glu57 and Glu58 in QacR; Glu253 in BmrR; and Asp152 in RamR).
Figure 10.
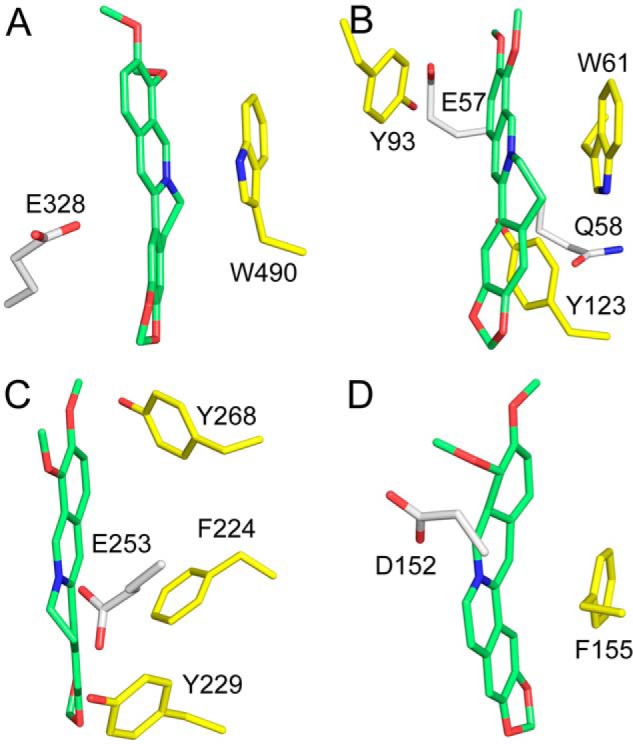
Aromatic residues and negatively charged residues involved in the binding of berberine to OfHex1 (PDB code 5Y0V) (A), QacR (PDB code 3BTI) (B), BmrR (PDB code 3D6Y) (C), and RamR (PDB code 3VW2) (D). Berberine is shown in green. Aromatic and negatively charged residues are shown in yellow and white, respectively.
The above analysis indicated that the positively charged conjugate plane is important for the binding activities of berberine with target proteins. To find the molecular basis for the selectivity of berberine and its analogs for GH20, GH18, GH84, GH63, and GH13 enzymes, the conformational flexibility and electrostatic potential of these compounds were calculated. As shown in Fig. 11, berberine, thalifendine, and palmatine, which are active against the tested enzymes, have a rigid conjugate plane with a positive charge. By contrast, tetrahydroberberine, which is inactive toward the tested enzymes, has a flexible and neutral structure (less than 3% of tetrahydroberberine is positively charged at pH 6.5 because its pKa is predicted to be 4.9). These results demonstrated that the positively charged conjugate plane is the core pharmacophore of berberine and should be retained in the further design of berberine-based inhibitors. Additionally, berberine is a good starting point to pursue better affinity or specificity because it can be readily modified at the C8, C13, and O9 sites (3).
Figure 11.
Analysis of the structural characteristics of berberine and its analogs. The energy-minimized structures of berberine and its analogs were generated with MM2 on ChemBio3D (PerkinElmer Life Sciences). The electrostatic potential surfaces for berberine and its analogs were generated with DelPhi on Accelrys Discovery Studio 2016 (Dassault Systèmes). Red and cyan represent the electronegative and electropositive potentials, respectively, and green represents a potential halfway point between the two extremes. The pKa of tetrahydroberberine was predicted by Marvin Beans (ChemAxon).
Conclusion
In this study, we discovered berberine and its analogs to be inhibitors of GH20, GH18, GH84, and GH63 glycoside hydrolases. By steady inhibition kinetics, X-ray crystallography, and molecular docking, we revealed berberine and its analogs interacted with these enzymes through π–π stacking or electrostatic interactions. This work not only expands the molecular target library of berberine but also provides a scaffold for developing inhibitors of carbohydrate hydrolyases.
Experimental procedures
Materials
4-Methylumbelliferyl-β-d-GlcNAc (MU-β-GlcNAc), 4-methylumbelliferyl-β-d-N,N′-diacetylchitobiose (MU-β-(GlcNAc)2), 4-methylumbelliferyl α-d-glucopyranoside (MU–α-glucose), Saccharomyces cerevisiae α-glucosidase I (ScGluI), PPA, amylase activity assay kit, berberine, and palmatine were purchased from Sigma (Shanghai, China). The compound SYSU-1 was synthesized by Ma et al. (43) and was kindly provided by Associate Prof. Min Li (Sun Yat-Sen University, China). Thalifendine and tetrahydroberberine were kindly provided by Prof. Xuhong Qian (East China University of Science and Technology, China). The yeast strain Pichia pastoris GS115 and the expression vectors pPIC9 and pPIC9K were purchased from Invitrogen (Beijing, China). The chromatographic columns for protein purification were purchased from GE Healthcare. The BCA protein assay kit was purchased from TaKaRa (Dalian, China).
Enzyme preparation
OfHex1 and the mutant OfHex1–W490A were expressed in P. pastoris GS115 and purified as described previously with some modifications (52). Briefly, the positive clones were cultured in BMMY broth at 30 °C for 72 h, and methanol (1% of the total volume) was added every 12 h. WT and mutant OfHex1 were purified from the culture supernatant by ammonium sulfate precipitation (65% saturation), followed by affinity chromatography on a HisTrapTM crude column (5 ml).
HsHexB was also expressed in P. pastoris GS115. The selected region of the gene encoding HsHexB (GenBankTM accession number NM_000521.3) was synthesized, and a C-terminal His6 tag was introduced. The DNA fragment was ligated into pPIC9K, and the expression plasmid pPIC9K–HsHexB was transformed into P. pastoris GS115 by electroporation. The cells expressing HsHexB were grown in 200 ml of BMGY medium at 30 °C for 24 h and then collected and resuspended in 1 liter of fresh BMMY medium. Methanol was added to a final concentration of 1% (v/v) at 24-h intervals as an inducer. After incubation for an additional 72 h, the supernatant was harvested via centrifugation. HsHexB was purified using immobilized metal ion affinity chromatography with a HisTrapTM crude column (5 ml).
The catalytic domains of OfChtI from O. furnacalis, human HsCht, and human AMCase were expressed in P. pastoris GS115 and purified as described previously (39, 53, 54). HsOGA was expressed in Escherichia coli and purified as described previously (40). All of the purified proteins were desalted using a HiTrap desalting column (5 ml) with 20 mm bis-tris at pH 6.5 and quantitated using a commercial BCA protein assay kit. The purities of the target proteins were analyzed by SDS-PAGE followed by Coomassie Brilliant Blue R-250 staining.
Inhibitory activity assay
The activities of GH20 Hex and GH84 HsOGA were determined using MU-β-GlcNAc as a substrate. The reaction mixtures used for inhibitor screening consisted of 100 μl of 0.4 nm enzyme, 10 μm MU-β-GlcNAc, 10 μm inhibitors, and 2% DMSO in the buffer (20 mm sodium phosphate, pH 6.5, for OfHex1; 20 mm sodium citrate, pH 4.5, for HsHexB). The reaction in the absence of inhibitors was used as a positive control. After incubating at 30 °C for OfHex1 and 37 °C for HsHexB and HsOGA for an appropriate time, 0.5 m sodium carbonate was added to the reaction mixture, and the fluorescence produced by the released MU was quantified using a Varioskan Flash microplate reader (ThermoFisher Scientific) at excitation and emission wavelengths of 360 and 450 nm, respectively. Experiments were performed in triplicate. For Ki value determination, three substrate concentrations (10, 20, and 40 μm) and varied inhibitor concentrations were used. The Ki values and types of inhibition were determined by linear fitting of the data in Dixon plots.
The activity of GH18 chitinase was determined using MU-β-(GlcNAc)2 as a substrate. The reaction mixtures used for inhibitor screening consisted of 100 μl of 0.4 nm enzyme, 10 μm MU-β-(GlcNAc)2, 10 μm inhibitors, and 2% DMSO in the buffer (20 mm sodium phosphate, pH 6.5, for OfChtI and HsCht; 20 mm sodium citrate, pH 5.2, for AMCase). The reaction in the absence of inhibitors was used as a positive control. After incubating at 30 °C for OfChtI and 37 °C for HsCht and AMCase for an appropriate time, 0.5 m sodium carbonate was added to the reaction mixture, and the fluorescence produced by the released MU was quantified as described above. Experiments were performed in triplicate. For Ki value determination, three substrate concentrations (1, 2, and 4 μm for OfChtI and 5, 10, and 20 μm for HsCht and AMCase) and varied inhibitor concentrations were used. The Ki values and types of inhibition were also determined by linear fitting of the data in Dixon plots
The activity of GH63 ScGluI was determined using MU–α-glucose as a substrate. The reaction mixtures used for inhibitor screening consisted of 100 μl of 0.4 nm enzyme, 10 μm MU–α-glucose, 10 μm inhibitors, and 2% DMSO in the buffer (20 mm sodium phosphate, pH 6.5). The reaction in the absence of inhibitors was used as a positive control. After incubating at 30 °C for an appropriate time, 0.5 m sodium carbonate was added to the reaction mixture, and the fluorescence produced by the released MU was quantified as described above. Experiments were performed in triplicate. For Ki value determination, three substrate concentrations (10, 20, and 40 μm) and varied inhibitor concentrations were used. The Ki values and types of inhibition were also determined by linear fitting of the data in Dixon plots. The activity of GH13 PPA was determined by the amylase activity assay kit according to the manufacturer's instruction.
Protein crystallization and structure determination
Crystallization experiments were performed by the hanging drop–vapor diffusion method at 4 °C. OfHex1 was desalted in 20 mm bis-tris with 20 mm NaCl, pH 6.5, and concentrated to 15.0 mg/ml by ultracentrifugation. The reservoir solution used for crystallization consisted of 100 mm HEPES, pH 6.6–7.5, 100 mm MgCl2, and 26–35% PEG400. Berberine and SYSU-1 were dissolved in the mother liquor with 5% DMSO at 1 mm and soaked into the crystals 1 h before they were transferred to a glycerol solution and flash-cooled in liquid nitrogen.
Diffraction data were collected at the National Center for Protein Science, Shanghai (BL19U1, Pilatus3–6M detector), and processed using HKL2000 (55). The structures of berberine- and SYSU-1-complexed OfHex1 were solved by molecular replacement with PHASER (56) using the structure of unliganded OfHex1 (PDB code 3NSM) as the search model. PHENIX (57) was used for structure refinement. The molecular models were manually built and extended using Coot (58). The stereochemistry of the models was checked by PROCHECK (59). The coordinates of berberine- and SYSU-1-complexed OfHex1 have been deposited under accession codes 5Y0V and 5Y1B. All structural figures were generated using PyMOL (DeLano Scientific LLC, San Carlos, CA). The electrostatic surfaces were calculated using APBS and PDB 2PQR (60–62).
Molecular docking
The PRODRG2 server was used to generate and optimize the initial structure of the compound before docking (63). The molecular docking methodology, performed using AutoDock4.2 software (64, 65), consisted of two steps. First, the protein–ligand complex was obtained by rigid docking and then by flexible docking via setting the active pocket outside ligand-binding residues as flexible. Polar hydrogen atoms and Gasteiger charges were added using AutoDockTools. The center of the grid box was placed at the center of the active pocket of HsHexB (PDB code 1O7A), HsCht (PDB code 1HKK), OfChtI (PDB code 3WQW), AMCase (PDB code 2YBT), HsOGA (PDB code 5UN9; β-subunit), ScGluI (PDB code 4J5T), and PPA (PDB code 1DHK), and the dimensions of the active site box were set at 50 × 50 × 50 Å, 70 × 70 × 60 Å, 70 × 70 × 70 Å, 60 × 80 × 60 Å, 50 × 50× 60 Å, 90 × 46 × 54 Å, and 50 × 80 × 50 Å. All maps were calculated with a 0.375 Å spacing between the grid points. Docking calculations were carried out using the Lamarckian genetic algorithm, and all parameters were the same for each docking. A population of random individuals (population size: 150), a maximum number of 25,000,000 energy evaluations, and a maximum number of generations of 27,000 were used.
In vivo activity of berberine and SYSU-1
O. furnacalis larvae were fed an artificial diet and reared at 26 ± 1 °C under a 16:8 light/dark photoperiod and 70% relative humidity. Day 1 4th-instar larvae were selected for the feeding experiment. In the experimental groups, an artificial diet containing 0.5 mm compounds (dissolved in DMSO) was used. In the control groups, an artificial diet containing DMSO was used. Each group contained 10 individual larvae and was continuously fed for 7 days. Mortality and developmental defects were recorded every day.
Author contributions
Y. D. and T. D. data curation; Y. D. and Y. Z. software; Y. D., T. L., Y. Z., and T. D. formal analysis; Y. D. and Q. Y. methodology; T. L. and Q. Y. conceptualization; T. L. writing-original draft; T. L. and Q. Y. writing-review and editing; Q. Y. resources; Q. Y. validation; Q. Y. project administration.
Acknowledgments
We thank the staff of BL19U1 beamline at National Center for Protein Sciences Shanghai and Shanghai Synchrotron Radiation Facility, Shanghai, People's Republic of China, for assistance during data collection. We thank Prof. Xuhong Qian (East China University of Science and Technology, Shanghai, China) for providing thalifendine and tetrahydroberberine. We also thank Dr. Min Li (Sun Yat-Sen University, Guangzhou, China) for providing SYSU-1.
This work was supported by the Program for National Natural Science Funds for Distinguished Young Scholar (Grant 31425021) and the National Natural Science Foundation of China (Grants 31830076 and 31871959). The authors declare that they have no conflicts of interest with the contents of this article.
The atomic coordinates and structure factors (codes 5Y0V and 5Y1B) have been deposited in the Protein Data Bank (http://wwpdb.org/).
- GH20 Hex
- glycoside hydrolase family 20 β-N-acetyl-d-hexosaminidase
- AMCase
- acidic mammalian chitinase
- GH18
- glycoside hydrolase family 18
- GH84
- glycoside hydrolase family 84
- GH63
- glycoside hydrolase family 63
- GH13
- glycoside hydrolase family 13
- GlcNAc
- N-acetyl-d-glucosamine
- Hex
- HsCht, human chitotriosidase
- HsHexB
- human β-N-acetyl-d-hexosaminidase B
- MU-β-GlcNAc
- 4-methylumbelliferyl-β-N-acetyl-d-glucosamine
- MU–α-glucose
- 4-methylumbelliferyl α-d-glucopyranoside
- OfChtI
- group I chitinase from O. furnacalis
- OfHex1
- group I β-N-acetyl-d-hexosaminidase from O. furnacalis
- HsOGA
- O-GlcNAcase from human
- ScGluI
- α-glucosidase I from S. cerevisiae
- PPA
- porcine pancreatic α-amylase
- bis-tris
- 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol.
References
- 1. Koehn F. E., and Carter G. T. (2005) The evolving role of natural products in drug discovery. Nat. Rev. Drug Discov. 4, 206–220 10.1038/nrd1657 [DOI] [PubMed] [Google Scholar]
- 2. Vuddanda P. R., Chakraborty S., and Singh S. (2010) Berberine: a potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 19, 1297–1307 10.1517/13543784.2010.517745 [DOI] [PubMed] [Google Scholar]
- 3. Singh I. P., and Mahajan S. (2013) Berberine and its derivatives: a patent review (2009–2012). Expert Opin. Ther. Pat. 23, 215–231 10.1517/13543776.2013.746314 [DOI] [PubMed] [Google Scholar]
- 4. Sun Y., Xun K., Wang Y., and Chen X. (2009) A systematic review of the anticancer properties of berberine, a natural product from Chinese herbs. Anticancer Drugs 20, 757–769 10.1097/CAD.0b013e328330d95b [DOI] [PubMed] [Google Scholar]
- 5. Zhao H. L., Sui Y., Qiao C. F., Yip K. Y., Leung R. K., Tsui S. K., Lee H. M., Wong H. K., Zhu X., Siu J. J., He L., Guan J., Liu L. Z., Xu H. X., Tong P. C., and Chan J. C. (2012) Sustained antidiabetic effects of a berberine-containing Chinese herbal medicine through regulation of hepatic gene expression. Diabetes 61, 933–943 10.2337/db11-1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Peng W. H., Lo K. L., Lee Y. H., Hung T. H., and Lin Y. C. (2007) Berberine produces antidepressant-like effects in the forced swim test and in the tail suspension test in mice. Life Sci. 81, 933–938 10.1016/j.lfs.2007.08.003 [DOI] [PubMed] [Google Scholar]
- 7. Derosa G., Maffioli P., and Cicero A. F. (2012) Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin. Biol. Ther. 12, 1113–1124 10.1517/14712598.2012.704014 [DOI] [PubMed] [Google Scholar]
- 8. Fatehi-Hassanabad Z., Jafarzadeh M., Tarhini A., and Fatehi M. (2005) The antihypertensive and vasodilator effects of aqueous extract from Berberis vulgaris fruit on hypertensive rats. Phytother. Res. 19, 222–225 10.1002/ptr.1661 [DOI] [PubMed] [Google Scholar]
- 9. da Silva A. R., de Andrade Neto J. B., da Silva C. R., Campos Rde S., Costa Silva R. A., Freitas D. D., do Nascimento F. B., de Andrade L. N., Sampaio L. S., Grangeiro T. B., Magalhães H. I., Cavalcanti B. C., de Moraes M. O., and Nobre Júnior H. V. (2016) Berberine antifungal activity in fluconazole-resistant pathogenic yeasts: action mechanism evaluated by flow cytometry and biofilm growth inhibition in Candida spp. Antimicrob. Agents Chemother. 60, 3551–3557 10.1128/AAC.01846-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Miyazawa M., Fujioka J., and Ishikawa Y. (2002) Insecticidal compounds from Phellodendron amurense active against Drosophila melanogaster. J. Sci. Food Agric. 82, 830–833 10.1002/jsfa.1112 [DOI] [Google Scholar]
- 11. Iwasa K., Moriyasu M., and Nader B. (2000) Fungicidal and herbicidal activities of berberine related alkaloids. Biosci. Biotechnol. Biochem. 64, 1998–2000 10.1271/bbb.64.1998 [DOI] [PubMed] [Google Scholar]
- 12. Yu G., Li Y., Tian Q., Liu R., Wang Q., Wang J. Z., and Wang X. (2011) Berberine attenuates calyculin A-induced cytotoxicity and Tau hyperphosphorylation in HEK293 cells. J. Alzheimers Dis. 24, 525–535 10.3233/JAD-2011-101779 [DOI] [PubMed] [Google Scholar]
- 13. Ma C., Tang K., Liu Q., Zhu R., and Cao Z. (2013) Calmodulin as a potential target by which berberine induces cell cycle arrest in human hepatoma Bel7402 cells. Chem. Biol. Drug Des. 81, 775–783 10.1111/cbdd.12124 [DOI] [PubMed] [Google Scholar]
- 14. Peng P. L., Hsieh Y. S., Wang C. J., Hsu J. L., and Chou F. P. (2006) Inhibitory effect of berberine on the invasion of human lung cancer cells via decreased productions of urokinase-plasminogen activator and matrix metalloproteinase-2. Toxicol. Appl. Pharmacol. 214, 8–15 10.1016/j.taap.2005.11.010 [DOI] [PubMed] [Google Scholar]
- 15. Jung H. A., Min B. S., Yokozawa T., Lee J. H., Kim Y. S., and Choi J. S. (2009) Anti-Alzheimer and antioxidant activities of Coptidis rhizoma alkaloids. Biol. Pharm. Bull. 32, 1433–1438 10.1248/bpb.32.1433 [DOI] [PubMed] [Google Scholar]
- 16. Kong L. D., Cheng C. H., and Tan R. X. (2001) Monoamine oxidase inhibitors from rhizoma of Coptis chinensis. Planta Med. 67, 74–76 10.1055/s-2001-10874 [DOI] [PubMed] [Google Scholar]
- 17. Qin Y., Pang J. Y., Chen W. H., Zhao Z. Z., Liu L., and Jiang Z. H. (2007) Inhibition of DNA topoisomerase I by natural and synthetic mono- and dimeric protoberberine alkaloids. Chem. Biodivers. 4, 481–487 [DOI] [PubMed] [Google Scholar]
- 18. Chai Y. S., Hu J., Lei F., Wang Y. G., Yuan Z. Y., Lu X., Wang X. P., Du F., Zhang D., Xing D. M., and Du L. J. (2013) Effect of berberine on cell cycle arrest and cell survival during cerebral ischemia and reperfusion and correlations with p53/cyclin D1 and PI3K/Akt. Eur. J. Pharmacol. 708, 44–55 10.1016/j.ejphar.2013.02.041 [DOI] [PubMed] [Google Scholar]
- 19. Slámová K., Bojarová P., Petrásková L., and Kren V. (2010) β-N-Acetylhexosaminidase: what's in a name? Biotechnol. Adv. 28, 682–693 10.1016/j.biotechadv.2010.04.004 [DOI] [PubMed] [Google Scholar]
- 20. Liu T., Duan Y., and Yang Q. (2018) Revisiting glycoside hydrolase family 20 β-N-acetyl-d-hexosaminidases: crystal structures, physiological substrates and specific inhibitors. Biotechnol. Adv. 36, 1127–1138 10.1016/j.biotechadv.2018.03.013 [DOI] [PubMed] [Google Scholar]
- 21. Liu T., Zhang H., Liu F., Wu Q., Shen X., and Yang Q. (2011) Structural determinants of an insect β-N-acetyl-d-hexosaminidase specialized as a chitinolytic enzyme. J. Biol. Chem. 286, 4049–4058 10.1074/jbc.M110.184796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hogenkamp D. G., Arakane Y., Kramer K. J., Muthukrishnan S., and Beeman R. W. (2008) Characterization and expression of the β-N-acetylhexosaminidase gene family of Tribolium castaneum. Insect Biochem. Mol. Biol. 38, 478–489 10.1016/j.ibmb.2007.08.002 [DOI] [PubMed] [Google Scholar]
- 23. Rong S., Li D. Q., Zhang X. Y., Li S., Zhu K. Y., Guo Y. P., Ma E. B., and Zhang J. Z. (2013) RNA interference to reveal roles of β-N-acetylglucosaminidase gene during molting process in Locusta migratoria. Insect Sci. 20, 109–119 10.1111/j.1744-7917.2012.01573.x [DOI] [PubMed] [Google Scholar]
- 24. Xi Y., Pan P. L., and Zhang C. X. (2015) The β-N-acetylhexosaminidase gene family in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 24, 601–610 10.1111/imb.12187 [DOI] [PubMed] [Google Scholar]
- 25. Zhang H., Zhao K., and Fan D. (2016) Molecular cloning and RNA interference analysis of β-N-acetylglucosaminidase in Mamestra brassicae L. J. Asia-Pacif. Entomol. 19, 721–728 10.1016/j.aspen.2016.06.012 [DOI] [Google Scholar]
- 26. Chen X., Xu K., Yan X., Chen C., Cao Y., Wang Y., Li C., and Yang W. (2018) Characterization of a β-N-acetylglucosaminidase gene and its involvement in the development of Lasioderma serricorne (Fabricius). J. Stored Prod. Res. 77, 156–165 10.1016/j.jspr.2018.04.012 [DOI] [Google Scholar]
- 27. Mahuran D. J. (1999) Biochemical consequences of mutations causing the GM2 gangliosidoses. Biochim. Biophys. Acta 1455, 105–138 10.1016/S0925-4439(99)00074-5 [DOI] [PubMed] [Google Scholar]
- 28. Liu J., Shikhman A. R., Lotz M. K., and Wong C. (2001) Hexosaminidase inhibitors as new drug candidates for the therapy of osteoarthritis. Chem. Biol. 8, 701–711 10.1016/S1074-5521(01)00045-X [DOI] [PubMed] [Google Scholar]
- 29. Adrangi S., and Faramarzi M. A. (2013) From bacteria to human: a journey into the world of chitinases. Biotechnol. Adv. 31, 1786–1795 10.1016/j.biotechadv.2013.09.012 [DOI] [PubMed] [Google Scholar]
- 30. Zhu Z., Zheng T., Homer R. J., Kim Y. K., Chen N. Y., Cohn L., Hamid Q., and Elias J. A. (2004) Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 304, 1678–1682 10.1126/science.1095336 [DOI] [PubMed] [Google Scholar]
- 31. Wiesner D. L., Specht C. A., Lee C. K., Smith K. D., Mukaremera L., Lee S. T., Lee C. G., Elias J. A., Nielsen J. N., Boulware D. R., Bohjanen P. R., Jenkins M. K., Levitz S. M., and Nielsen K. (2015) Chitin recognition via chitotriosidase promotes pathologic type-2 helper T cell responses to cryptococcal infection. PLoS Pathog. 11, e1004701 10.1371/journal.ppat.1004701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Van Dyken S. J., Liang H. E., Naikawadi R. P., Woodruff P. G., Wolters P. J., Erle D. J., and Locksley R. M. (2017) Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell 169, 497–509.e13 10.1016/j.cell.2017.03.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim L. K., Morita R., Kobayashi Y., Eisenbarth S. C., Lee C. G., Elias J., Eynon E. E., and Flavell R. A. (2015) AMCase is a crucial regulator of type 2 immune responses to inhaled house dust mites. Proc. Natl. Acad. Sci. U.S.A. 112, E2891–E2899 10.1073/pnas.1507393112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu Y., Egerton G., Underwood A. P., Sakuda S., and Bianco A. E. (2001) Expression and secretion of a larval-specific chitinase (family 18 glycosyl hydrolase) by the infective stages of the parasitic nematode, Onchocerca volvulus. J. Biol. Chem. 276, 42557–42564 10.1074/jbc.M103479200 [DOI] [PubMed] [Google Scholar]
- 35. Vinetz J. M., Dave S. K., Specht C. A., Brameld K. A., Xu B., Hayward R., and Fidock D. A. (1999) The chitinase PfCHT1 from the human malaria parasite Plasmodium falciparum lacks proenzyme and chitin-binding domains and displays unique substrate preferences. Proc. Natl. Acad. Sci. U.S.A. 96, 14061–14066 10.1073/pnas.96.24.14061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Horsch M., Mayer C., Sennhauser U., and Rast D. M. (1997) β-N-Acetylhexosaminidase: a target for the design of antifungal agents. Pharmacol. Ther. 76, 187–218 10.1016/S0163-7258(97)00110-1 [DOI] [PubMed] [Google Scholar]
- 37. Qian X., Lee P. W., and Cao S. (2010) China: forward to the green pesticides via a basic research program. J. Agric. Food Chem. 58, 2613–2623 10.1021/jf904098w [DOI] [PubMed] [Google Scholar]
- 38. Andersen O. A., Dixon M. J., Eggleston I. M., and van Aalten D. M. (2005) Natural product family 18 chitinase inhibitors. Nat. Prod. Rep. 22, 563–579 10.1039/b416660b [DOI] [PubMed] [Google Scholar]
- 39. Jiang X., Kumar A., Liu T., Zhang K. Y., and Yang Q. (2016) A novel scaffold for developing specific or broad-spectrum chitinase inhibitors. J. Chem. Inf. Model. 56, 2413–2420 10.1021/acs.jcim.6b00615 [DOI] [PubMed] [Google Scholar]
- 40. Li B., Li H., Lu L., and Jiang J. (2017) Structures of human O-GlcNAcase and its complexes reveal a new substrate recognition mode. Nat. Struct. Mol. Biol. 24, 362–369 10.1038/nsmb.3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barker M. K., and Rose D. R. (2013) Specificity of processing α-glucosidase I is guided by the substrate conformation: crystallographic and in silico studies. J. Biol. Chem. 288, 13563–13574 10.1074/jbc.M113.460436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Liu T., Guo P., Zhou Y., Wang J., Chen L., Yang H., Qian X., and Yang Q. (2014) A crystal structure-guided rational design switching non-carbohydrate inhibitors' specificity between two β-GlcNAcase homologs. Sci. Rep. 4, 6188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ma Y., Ou T. M., Tan J. H., Hou J. Q., Huang S. L., Gu L. Q., and Huang Z. S. (2009) Synthesis and evaluation of 9-O-substituted berberine derivatives containing aza-aromatic terminal group as highly selective telomeric G-quadruplex stabilizing ligands. Bioorg. Med. Chem. Lett. 19, 3414–3417 10.1016/j.bmcl.2009.05.030 [DOI] [PubMed] [Google Scholar]
- 44. Yang Y., Liu T., Yang Y., Wu Q., Yang Q., and Yu B. (2011) Synthesis, evaluation, and mechanism of N,N,N-trimethyl-d-glucosamine-(1→4)-chitooligosaccharides as selective inhibitors of glycosyl hydrolase family 20 β-N-acetyl-d-hexosaminidases. ChemBioChem 12, 457–467 10.1002/cbic.201000561 [DOI] [PubMed] [Google Scholar]
- 45. Liu T., Zhang H., Liu F., Chen L., Shen X., and Yang Q. (2011) Active-pocket size differentiating insectile from bacterial chitinolytic β-N-acetyl-d-hexosaminidases. Biochem. J. 438, 467–474 10.1042/BJ20110390 [DOI] [PubMed] [Google Scholar]
- 46. Liu T., Xia M., Zhang H., Zhou H., Wang J., Shen X., and Yang Q. (2015) Exploring NAG-thiazoline and its derivatives as inhibitors of chitinolytic β-acetylglucosaminidases. FEBS Lett. 589, 110–116 10.1016/j.febslet.2014.11.032 [DOI] [PubMed] [Google Scholar]
- 47. Maier T., Strater N., Schuette C. G., Klingenstein R., Sandhoff K., and Saenger W. (2003) The X-ray crystal structure of human β-hexosaminidase B provides new insights into Sandhoff disease. J. Mol. Biol. 328, 669–681 10.1016/S0022-2836(03)00311-5 [DOI] [PubMed] [Google Scholar]
- 48. Williams L. K., Zhang X., Caner S., Tysoe C., Nguyen N. T., Wicki J., Williams D. E., Coleman J., McNeill J. H., Yuen V., Andersen R. J., Withers S. G., and Brayer G. D. (2015) The amylase inhibitor montbretin A reveals a new glycosidase inhibition motif. Nat. Chem. Biol. 11, 691–696 10.1038/nchembio.1865 [DOI] [PubMed] [Google Scholar]
- 49. Peters K. M., Schuman J. T., Skurray R. A., Brown M. H., Brennan R. G., and Schumacher M. A. (2008) QacR-cation recognition is mediated by a redundancy of residues capable of charge neutralization. Biochemistry 47, 8122–8129 10.1021/bi8008246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Newberry K. J., Huffman J. L., Miller M. C., Vazquez-Laslop N., Neyfakh A. A., and Brennan R. G. (2008) Structures of BmrR-drug complexes reveal a rigid multidrug binding pocket and transcription activation through tyrosine expulsion. J. Biol. Chem. 283, 26795–26804 10.1074/jbc.M804191200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yamasaki S., Nikaido E., Nakashima R., Sakurai K., Fujiwara D., Fujii I., and Nishino K. (2013) The crystal structure of multidrug-resistance regulator RamR with multiple drugs. Nat. Commun. 4, 2078 10.1038/ncomms3078 [DOI] [PubMed] [Google Scholar]
- 52. Liu T., Liu F., Yang Q., and Yang J. (2009) Expression, purification and characterization of the chitinolytic β-N-acetyl-d-hexosaminidase from the insect Ostrinia furnacalis. Protein Expr. Purif. 68, 99–103 10.1016/j.pep.2009.06.004 [DOI] [PubMed] [Google Scholar]
- 53. Chen L., Zhou Y., Qu M., Zhao Y., and Yang Q. (2014) Fully deacetylated chitooligosaccharides act as efficient glycoside hydrolase family 18 chitinase inhibitors. J. Biol. Chem. 289, 17932–17940 10.1074/jbc.M114.564534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sutherland T. E., Andersen O. A., Betou M., Eggleston I. M., Maizels R. M., van Aalten D., and Allen J. E. (2011) Analyzing airway inflammation with chemical biology: dissection of acidic mammalian chitinase function with a selective drug-like inhibitor. Chem. Biol. 18, 569–579 10.1016/j.chembiol.2011.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Otwinowski Z., and Minor W. (1997) Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 276, 307–326 10.1016/S0076-6879(97)76066-X [DOI] [PubMed] [Google Scholar]
- 56. McCoy A. J. (2007) Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. D Biol. Crystallogr. 63, 32–41 10.1107/S0907444906045975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Adams P. D., Afonine P. V., Bunkóczi G., Chen V. B., Davis I. W., Echols N., Headd J. J., Hung L. W., Kapral G. J., Grosse-Kunstleve R. W., McCoy A. J., Moriarty N. W., Oeffner R., Read R. J., Richardson D. C., et al. (2010) PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 10.1107/S0907444909052925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Emsley P., Lohkamp B., Scott W. G., and Cowtan K. (2010) Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 10.1107/S0907444910007493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Laskowski R. A., Macarthur M. W., Moss D. S., and Thornton J. M. (1993) Procheck–a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26, 283–291 10.1107/S0021889892009944 [DOI] [Google Scholar]
- 60. Baker N. A., Sept D., Joseph S., Holst M. J., and McCammon J. A. (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc. Natl. Acad. Sci. U.S.A. 98, 10037–10041 10.1073/pnas.181342398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dolinsky T. J., Nielsen J. E., McCammon J. A., and Baker N. A. (2004) PDB2PQR: an automated pipeline for the setup of Poisson-Boltzmann electrostatics calculations. Nucleic Acids Res. 32, W665–W667 10.1093/nar/gkh381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Unni S., Huang Y., Hanson R. M., Tobias M., Krishnan S., Li W. W., Nielsen J. E., and Baker N. A. (2011) Web servers and services for electrostatics calculations with APBS and PDB2PQR. J. Comput. Chem. 32, 1488–1491 10.1002/jcc.21720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schüttelkopf A. W., and van Aalten D. M. (2004) PRODRG: a tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. D Biol. Crystallogr. 60, 1355–1363 10.1107/S0907444904011679 [DOI] [PubMed] [Google Scholar]
- 64. Morris G. M., Goodsell D. S., Halliday R. S., Huey R., Hart W. E., Belew R. K., and Olson A. J. (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 19, 1639–1662 10.1002/(SICI)1096-987X(19981115)19:14%3C1639::AID-JCC10%3E3.0.CO%3B2-B [DOI] [Google Scholar]
- 65. Morris G. M., Huey R., Lindstrom W., Sanner M. F., Belew R. K., Goodsell D. S., and Olson A. J. (2009) AutoDock4 and AutoDockTools4: automated docking with selective receptor flexibility. J. Comput. Chem. 30, 2785–2791 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]