Abstract
Aims
Lisinopril is an angiotensin‐converting‐enzyme inhibitor that is largely administered for off‐label uses. This study aims to provide a comprehensive review of off‐label uses of lisinopril to aid physicians to make evidence‐based decisions.
Methods
The following bibliographic databases were searched from inception up to 30 March 2017: PubMed, EMBASE, the Cochrane Library, Cochrane Central Register of Controlled Trials, Scopus, Ovid and Proquest. This systematic review sought all randomized trials conducted on adult individuals comparing lisinopril on its off‐label uses with alternative drugs or placebos and reported direct or alternative clinical outcomes. Risk of bias assessment by using the Cochrane Collaboration risk‐of‐bias tool and quality evaluation took place.
Results
Included studies demonstrated significant positive effects of lisinopril on proteinuric kidney disease; however, lisinopril caused a slight reduction of glomerular filtration rate (GFR) especially for patients with GFR < 90 ml min–1. Lisinopril offered better outcomes in comparison to other standard treatments of diabetic nephropathy. Other studies showed positive effects of lisinopril for migraine, prevention of diabetes, myocardial fibrosis, mitral valve regurgitation, cardiomyopathy in patients with Duchenne muscular dystrophy, oligospermia and infertility, and diabetic retinopathy. Conversely, the studies reported that lisinopril was ineffective for five other off‐label uses.
Conclusions
The identified studies showed that lisinopril was highly effective for proteinuric kidney disease with a minor but inconsiderable decrease in GFR. Positive effects of lisinopril were demonstrated in seven other off‐label uses; however, lisinopril cannot be recommended as the first choice for these until further clinical trials confirm these positive effects.
Keywords: ACE inhibitor, diabetes, diabetic nephropathy, left ventricular hypertrophy, nephropathy
What is Already Known about this Subject
Off‐label administration of some drugs, including lisinopril has a remarkable prevalence among physicians. However, there is still a need for a comprehensive review to aid physicians to base their decision on the strong evidence and make safe and positive decisions
What this Study Adds
We searched the literature and found related studies for each of lisinopril's off‐label uses, assessed the quality of them, illustrated the eligibility of prescription, and summarized the supporting evidence
Introduction
Hundreds of new drugs emerge each year. Before they can be widely used, they need to be approved by regulatory organizations such as the US Food and Drug Administration (FDA), European Medicines Agency and the Therapeutic Goods Administration. The FDA was initially established to regulate the production of food, drugs and cosmetics in the USA but nowadays it also supervises international drug products 1. In addition to approved FDA uses of drugs, there are a variety of unapproved uses that are called off‐label. The off‐label administration is not the same as the unlicensed use of a drug or substance. While the safety of these drugs has been previously confirmed by the FDA, their efficiency has been established only for their approved uses. The efficiency for their off‐label uses is yet to be confirmed by taking the complicated procedure of approval. Therefore, clinicians may consider the off‐label uses for these drugs if they find them useful for their patients. Off‐label administration of drugs has a remarkable prevalence among physicians 2. Recent North American surveys show that 11% 3 to 21% 4 of the administrations of commonly used drugs were off‐label; among these, up to 80.0% lacked strong scientific evidence of efficacy 3. It has been shown that the prevalence of off‐label prescriptions was higher in some special groups; almost 80% of the children discharged from a paediatric hospitals in the USA received at least one off‐label drug 5. Although these off‐label uses are not approved, it does not necessarily mean that their prescription is unsafe, as some off‐label uses of drugs are recommended by prominent guidelines (e.g. use of tricyclic antidepressants for neuropathic pain or spironolactone for hirsutism 6, 7, 8). That said, clinicians and guideline developers require research syntheses and critical evaluations of available information to assess the extent and quality of the evidence before supporting a drug's off‐label uses.
Lisinopril is an angiotensin‐converting‐enzyme (ACE) inhibitor that is largely being used off‐label 9. Lisinopril was initially approved by the FDA as an oral tablet for the treatment of hypertension in adults and children aged >6 years, adjunct therapy for heart failure and treatment of acute myocardial infarction (Prinivil and Zestril) 10, 11 and in combination with hydrochlorothiazide for the treatment of hypertension (Zestoretic) 12. Lately, an oral solution form (Qbrelis), has been approved for the same indications 13. Lisinopril has been identified as one of the top 10 drugs that require further evidence for its off‐label uses: data from the USA, July 2005–June 2007 showed that 2 374 000 of 2 601 000 records of administrations in its off‐label uses had inadequate supporting evidence 9. Through this paper, we aim to comprehensively review the off‐label uses of lisinopril.
Methods
This study was conducted as a systematic review of randomized clinical trials (RCTs) conducted on patients aged ≥18 years who received lisinopril (on its off‐label uses) as compared to other alternative drugs or placebos and reported direct or alternative clinical outcomes.
Data sources and searches
An expert librarian defined individualized search strategies for the following bibliographic databases, from inception up to 30 March 2017: PubMed, EMBASE, the Cochrane Library, Cochrane Central Register of Controlled Trials, Scopus, Ovid and Proquest. A primary search (Table 1) was conducted by a librarian for any possible off‐label administrations of lisinopril using any available research design. Two reviewers (S.R.S.E., N.P.) independently screened the titles and abstracts to identify all off‐label uses for lisinopril. A comprehensive search was then performed based on the keywords identified in our primary search. The search terms and strategies are available in our electronic supplementary material. A librarian expert on grey literature (sources produced by organizations which are not controlled by commercial publishers) searched regulatory sites, clinical trial registries, conference proceedings and grant‐funded and federally‐funded research sites. We also contacted experts and then reviewed bibliographies and files supplied by the manufacturers' website.
Table 1.
Search terms and strategy for our primary search
| (((((((Lizinopril [Title/Abstract]) OR Lisinopril [Title/Abstract]) OR Lysinopril [Title/Abstract]) OR “Lisinopril”[Mesh])) NOT ((((“heart failure”[Title/Abstract]) OR “Heart Failure”[Mesh]) OR “Cardiac Failure”[Title/Abstract]) OR “myocardial failure”[Title/Abstract])) NOT ((((“High Blood Pressures”[Title/Abstract]) OR “High Blood Pressure”[Title/Abstract]) OR “Hypertension”[Mesh]) OR hypertension [Title/Abstract])) NOT (((((((((“Myocardial Infarcts”[Title/Abstract]) OR “Myocardial Infarct”[Title/Abstract]) OR “Heart Attacks”[Title/Abstract]) OR “Heart Attack”[Title/Abstract]) OR “Cardiovascular Strokes”[Title/Abstract]) OR “Cardiovascular Stroke”[Title/Abstract]) OR “Myocardial Infarction”[Mesh]) OR “myocardial infarction”[Title/Abstract]) OR “myocardial infarctions”[Title/Abstract]) |
Inclusion criteria and study selection
We included RCTs on adult patients aged ≥18 years who received an off‐label use of lisinopril as compared to control [i.e. other alternative drugs, usual care (e.g. β‐blockers, calcium channel blockers, other ACE inhibitors, diuretics) or placebos]. These RCTs should have reported direct or alternative clinical outcomes. Full texts of English‐language studies and references of studies in non‐English languages published from inception until November 2017 were reviewed. We excluded the following studies: nonrandomized clinical trials or observational studies, studies published only as abstracts, studies on transplanted patients (due to possible confounding effects of drugs used in these patients 14), studies based on lisinopril for approved uses, studies of lisinopril combined with or adjunct to other drugs (or combination/adjunct arm of the multiarm studies), studies based on the negative effects of lisinopril or effects of dose adjustments, studies whose outcome measures were not relevant to efficacy, effectiveness or safety (e.g. drug half‐life).
Risk of bias assessment
Two authors (S.R.S.E., N.P.) independently assessed the quality of RCTs using the Cochrane Collaboration Risk‐of‐Bias tool 15. Six domains (i.e. sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and other issues) were evaluated based on what was reported in each study. A judgement (such as high risk, low risk or unclear risk of bias) relating to the risk of bias for that particular domain was assigned to these domains. Other coauthors were consulted in case of absent agreement between the two reviewers. We also took a very conservative approach when evaluating the risk of other bias.
Data extraction
Two reviewers (S.R.S.E., N.P.) independently abstracted study characteristics (study design, population, lisinopril dosing and administration, outcomes assessed, etc.) by using specific forms. We contacted field experts to determine the most important variables. We extracted only the baseline and the final values of variables (or changes if the actual number were not reported). If the outcomes were reported only by means of figures and plots, we used Get Data Graph Digitizer (version 2.24) to extract the numbers. Other coauthors were consulted in case the two reviewers failed to agree.
Results
Our primary search identified 24 off‐label uses for lisinopril (Table 2). Through our complementary search, 9164 articles were found after removing duplicates, all of which were screened by title and abstract. Finally, full papers of 259 studies were assessed for eligibility, 231 studies were excluded [studies on the combination of lisinopril with other drugs (n = 166), adverse effects of lisinopril (n = 16) and approved uses (n = 49)] and the full texts of 28 articles were critically reviewed (Figure 1). Our final pool of 28 papers presented support for 12 off‐label uses. The characteristics of these studies are described in Table 3. Assessments of the risk of bias of the studies are described in Figure 2. An 89% agreement was seen between the authors (S.R.S.E., N.P.) in the evaluation of risk of bias; discrepancies were resolved by discussion and consulting other authors. Most of the studies provided appropriate details about blinding of participants and personnel; however, few trials presented clear details of the blinding of outcome assessment. The most frequent biases were other bias and selective reporting, as well as ambiguity about random sequence generation – the most prevalent problems of included studies. The most common biases presented as other bias were: a potential source of bias related to the specific study design used and lack of a complete elucidation of supporting organization and funding source.
Table 2.
Off‐label uses of lisinopril
| Supported by RCTs | Not supported by RCTs |
|---|---|
|
|
RCT: randomized clinical trial
Figure 1.
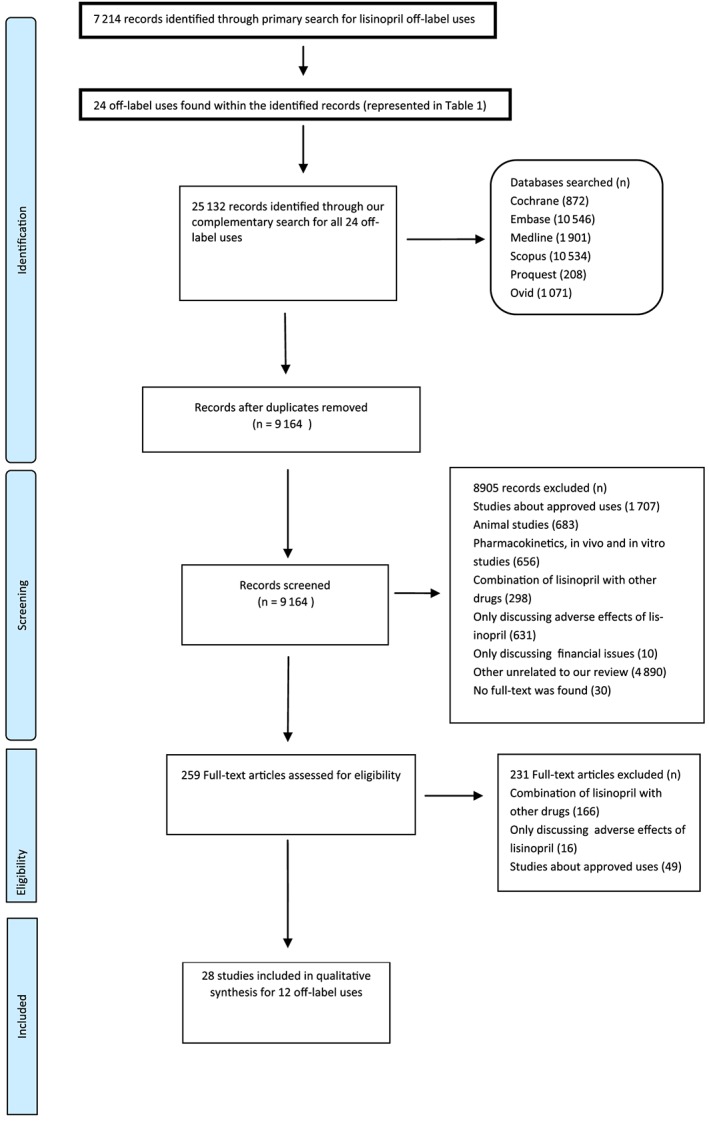
PRISMA flow diagram summarizing retrieved, included, and excluded randomized controlled trials
Table 3.
Characteristics of studies on lisinopril for off‐label uses
| Off‐label | Author (reference) | Comparison | Duration of study (months) | Dosing (mg day–1) | Mean age (years; if NA lisinopril group) | Final comparison group number | Final lisinopril group number | Total number |
|---|---|---|---|---|---|---|---|---|
| Proteinuric kidney disease | Juarez et al. 20 | ARB (irbesartan) | 32 | 40 or 10 | 68.7 ± 6.8 | 28 | 35 | 133 |
| Menne et al. 18 | ARB (valsartan) | 7.5 | 40 or 10 | 59.7 ± 9.5 | 42 | 47 | 133 | |
| Laverman et al. 17 | ARB (losartan) | 3 | 40 or 10 | 51 | 9 | 9 | 10 | |
| Poulsen et al. 25 | Placebo | 24 | 20 | 29.3 ± 8.6 | 10 | 11 | 22 | |
| Mogensen et al. 23 | ARB (candesartan) | 6 | 20 | 60 | 66 | 64 | 199 | |
| Tarnow et al. 27 | CCB (nisoldipine) | 48 | 20 or 10 | 35 ± 6 | 24 | 24 | 52 | |
| Crepaldi et al. 21 | CCB (nifedipine) and placebo | 36 | 2.5 or 10 or 20 | 38 ± 11 | 60 | 32 | 137 | |
| Janssen et al. 16 | CCB (amlodipine) | 4 | 5 or 10 | 47 ± 3.2 | 9 | 11 | 21 | |
| Mitchell et al. 22 | ACE inhibitor (fosinopril) | 5.5 | 20 or 40 | 67 | 14 | 13 | 94 | |
| Chaturvedi et al. 28 | Placebo | 24 | 20 or 10 | 265 | 265 | 530 | ||
| Rossing et al. 26 | CCB (nisoldipine) | 12 | 20 or 10 | 35 ± 7 | 35 | 34 | 71 | |
| Nielsen et al. 24 | β‐blocker (atenolol) | 42 | 20 or 10 | 61 ± 8 | 16 | 16 | 43 | |
| Ranieri. et al. 19 | CCB (amlodipine) | 12 | 20 | 51 ± 3 | 18 | 18 | 36 |
| Off‐label | Author (reference) | Amount of proteinuria | BP range | Type of diabetes | Diabetes | BP at baseline: mean of lisinopril group SP/DP | Underlying disease of patients with proteinuria |
|---|---|---|---|---|---|---|---|
| Proteinuric kidney disease | Juarez et al. 20 | Overt proteinuria | <180/95 | 2 | • | 153 ± 18/80 ± 11 | Diabetic nephropathic patient and stage 2 or 3 chronic kidney disease |
| Menne et al. 18 | Microalbuminuria | 85 < DP < 115 | 2 | 𝚯 | 153 ± 14.3/90.6 ± 8.3 | Essential hypertensive patient | |
| Laverman et al. 17 | Overt proteinuria | 80 < DP < 110 | ‐ | ο | 137/80 | Nondiabetic renal patients with median proteinuria | |
| Poulsen et al. 25 | Microalbuminuria | <160/90 | 1 | • | 128 ± 10.5 | Diabetic nephropathic patient | |
| Mogensen et al. 23 | Microalbuminuria | 90 < DP < 110 | 2 | • | 162.6/85.7 | Hypertensive patients with diabetic nephropathy | |
| Tarnow et al. 27 | ‐ | ‐ | 1 | • | ‐ | Hypertensive patients with diabetic nephropathy | |
| Crepaldi et al. 21 | Microalbuminuria | 115 < SP < 140, 75 < DP < 90 | 1 | • | 126 ± 8/82 ± 5 | Diabetic nephropathic patient | |
| Janssen et al. 16 | Overt proteinuria | DP < 100 with antihypertensive treatment or < 115 without medication | ‐ | ο | 163 ± 7/101 ± 3 |
Membranous glomerulopathy, chronic pyelonephritis, membranoproliferative, glomerular nephropathy, IgA nephropathy, hypertensive glomerulosclerosis, adult polycystic kidney disease, focal glomerulosclerosis, hereditary nephritis (Alport syndrome) |
|
| Mitchell et al. 22 | Microalbuminuria | 95 < DP < 110 | 2 | 𝚯 | 156 ± 3/85 ± 2 | Arteriolar nephrosclerosis or diabetic nephropathy | |
| Chaturvedi et al. 28 | Microalbuminuria | 75 < DP < 90, SP < 155 | 1 | • | 122/79 | Diabetic nephropathic patient | |
| Rossing et al. 26 | Overt proteinuria | 90 < DP < 105 | 1 | • | 155/86 | Diabetic nephropathic patient | |
| Nielsen et al. 24 | Overt proteinuria | ‐ | 2 | • | 162/85 ± 2 | Diabetic nephropathic patient | |
| Ranieri et al. 19 | Microalbuminuria | 95 < DP < 115 | ‐ | 𝚯 | 169.6 ± 15.3/104.2 ± 3.3 | Essential hypertensive patient |
| Off‐label | Author (reference) | Comparison (dose mg day–1) | Duration of study | Dose of lisinopril (mg day–1) | Mean age of total sample (years; if NA lisinopril group) | Final comparison group number | Final lisinopril group number | Total number enrolled |
|---|---|---|---|---|---|---|---|---|
| Atrial fibrillation | Van Den Berg et al. 30 | Placebo | 12 m | 5–10 | 68 ± 6 | 14 | 11 | 30 |
| Haywood et al. 31 | Chlorthalidone (12.5, 25), Amlodipine (2.5, 5, 10), doxazosin (2, 4, 8) | 6 y | 10–20‐40 | >55 | Chlorthalidone (11 695) Amlodipine (6935) doxazosin (6392) | 6702 | 39 056 | |
| CM in patients with DMD | Allen et al. 32 | Losartan (25) | 1 y | 5 | median of 12.5 y (range, 10 to 21 y) | 8 | 7 | 23 |
| Diabetic retinopathy | Mehlsen et al. 36 | Amlodipine (5) | 49 d | 10 | 27.9 ± 0.8 | 25 | 25 | 25 |
| Chaturvedi et al. 37 | Placebo | 2 y | 10–20 | 34 ± 9 | 168 | 156 | 530 | |
| Myocardial fibrosis | Brilla et al. 34 | Hydrochlorothiazide (25) | 6 m | 5–20 | 57 ± 2 | 14 | 11 | 35 |
| Kooij et al. 40 | Placebo | 8 m | 10 | 54.6 ± 12.3 | 36 | 36 | 40 | |
| Inflammatory cystoid macular oedema | Christian et al. 34 | Hydrochlorothiazide (25) | 6 m | 5–20 | 57 ± 2 | 14 | 11 | 35 |
| Left ventricular hypertrophy | Ernst et al. 33. | Chlorthalidone (12.5, 25), Amlodipine (2.5, 5, 10) | 4 y | 10–20‐40 | >55 | Chlorthalidone (12 102) Amlodipine (7151) | 7121 | 26 376 |
| Migraine | Schrader et al. 41 | Placebo | 8 m | 10 | 41 ± 9 | 47 | 47 | 60 |
| Mitral valve regurgitation | Wong et al. 35 | Placebo | 1 y | 5–10‐20 | 53.2 ± 2.4 | 11 | 12 | 23 |
| Oligospermia and infertility | Mbah et al. 42 | Placebo | 5 y | 2.5 | 30.86 ± 8.8 | 14 | 14 | 33 |
| Prevention of diabetes | ALLHAT Group 39 | Chlorthalidone (12.5, 25), Amlodipine (2.5, 5, 10) | 5 y | 10–20‐40 | >55 | Chlorthalidone (15 255) Amlodipine (9048) | 9054 | 42 418 |
| Fogari and Roberto 38 | Losartan (50) | 16 w | 20 | 55 ± 2 | 25 | 25 | 25 | |
| Prevention of pneumonia | Lee et al. 43 | Placebo | 26 w | 2.5 | 83.4 ± 6.8 | 38 | 33 | 93 |
| Off‐label | Author (reference) | Most important inclusion criteria |
|---|---|---|
| Atrial fibrillation | Van Den Berg et al. 30 | Mild to moderate stable CHF and chronic AF |
| Haywood et al. 31 | CVD risk factor | |
| CM in patients with DMD | Allen et al. 32 | Duchenne muscular dystrophy, EF <55% |
| Diabetic retinopathy | Mehlsen et al. 36 | Diabetes (type1) |
| Chaturvedi et al. 37 | Diabetes (type1) | |
| Myocardial fibrosis | Brilla et al. 34 | Symptomatic patients (dyspnoea or angina pectoris), LVH, LV diastolic dysfunction |
| Kooij et al. 40 | Inactive or chronic low‐grade activity of uveitis, inflammatory cystoid macular oedema | |
| Inflammatory cystoid macular oedema | Christian et al. 34 | Symptomatic patients (dyspnoea or angina pectoris), LVH, LV diastolic dysfunction |
| Left ventricular hypertrophy | Ernst et al. 33. | CVD risk factor |
| Migraine | Schrader et al. 41 | Migraine for more than a year |
| Mitral valve regurgitation | Wong et al. 35 | at least moderate, isolated, organic MR and an EF > 60% |
| Oligospermia and infertility | Mbah et al. 42 | being on treatment for oligospermia for at least 2 years |
| Prevention of diabetes | ALLHAT Group 39 | CVD risk factor |
| Fogari and Roberto 38 | Nondiabetic, nonobese | |
| Prevention of pneumonia | Lee et al. 43 | Tube‐fed patients |
BP, blood pressure; SP, systolic blood pressure; DP, diastolic blood pressure; NA, Not available; •, diabetic patients; ο, nondiabetic patients; 𝚯, both diabetic and nondiabetic or no data provided;
M, month; y, year; d, day; CM, cardiomyopathy; DMD, Duchenne muscular dystrophy; CVD, cardiovascular disease; CHF, congestive heart failure; AF, atrial fibrillation; EF = ejection fraction; LVH, left ventricular hypertrophy; MR, mitral regurgitation
Figure 2.
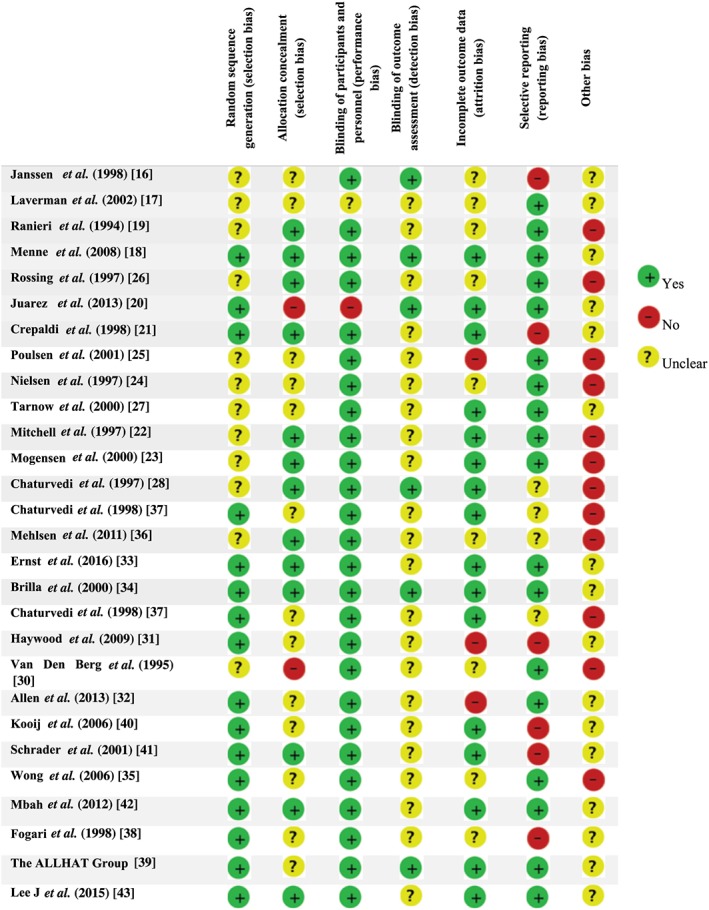
Risk of bias assessment for studies of Lisinopril's off‐label uses
Proteinuric kidney disease
Impact of lisinopril on proteinuria and glomerular filtration rate
We found thirteen studies (Table 4) evaluating lisinopril among patients with proteinuria 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28. These studies were conducted on diabetic nephropathy patients 20, 21, 22, 23, 24, 25, 26, 27, 28, on patients with proteinuria due to essential hypertension 17, 18, 19, and on patients with other underlying conditions (e.g. membranous glomerulopathy, chronic pyelonephritis, membranoproliferative, glomerular nephropathy, IgA nephropathy, hypertensive glomerulosclerosis, adult polycystic kidney disease, focal glomerulosclerosis, and hereditary nephritis) 16. Overall, these studies except one 20, reported significant effects of lisinopril on proteinuria. Most studies showed that lisinopril resulted in statistically, yet not clinically, significant reduction in glomerular filtration rate (GFR), but not if patients had estimated GFR > 90 ml min–1 19, 21. For example, Ranieri et al. demonstrated that lisinopril significantly increased GFR (116.94 ± 11.11 ml min–1 to 127.27 ± 18.89 ml min–1; P < 0.01) 19. This could possibly be due to more efficient renal compensatory mechanisms in higher levels of GFR 29.
Table 4.
Outcomes of comparison group within included studies
| Off‐label | Author (reference) | Main outcome | Baseline vs. final outcome in comparison group | |
|---|---|---|---|---|
| Proteinuric kidney disease | Juarez et al. 20 | Geometric mean (95%CI) of UPCR (g g–1) | 1.33 (0.83–2.18) to 1.01 (0.69–1.80; P = NS) | |
| Mean and SD of GFR (ml min–1 1.73 m–2) | Baseline = 46 ± 16 and decreased with a median rate of 3.3 (95%CI, 1.8–4.7) | |||
| Menne et al. 18 | Mean UACR (mg mmol–1)‐ adjusted ratio | 9.1 to 4.5 | ||
| Laverman et al. 17 | Mean (95%CI) of total proteinuria (g 24‐h–1) | 4.5 (3.5, 6.4) to 2.2 (1.2, 4.8; P < 0.05) | ||
| Mean (95%CI) of creatinine clearance (ml/min) | 80 (66, 96) to 73 (59, 89) | |||
| Poulsen et al. 25 | Geometric mean ± tolerance factor E‐UAE (μg min–1) | 150.1 ± 3.7 to 213.6 ± 6.9 | ||
| Mogensen et al. 23 | Adjusted mean reduction in UACR [Geometric mean (tolerance factor)] | 24 (0 to 43) [Baseline = 5.9 (1.1)] (P = 0.05) | ||
| Tarnow et al. 27 | Mean (95%CI) albuminuria mg 24‐h–1 | Baseline = 1033 (760–1406) and increased by 12% (–10 to 40; P = NS) | ||
| Mean and SE of GFR (ml min–1 1.73‐m–2) | Baseline = 85 ± 6 and declined 0.5 ± 0.1 ml. min–1. month–1 (P < 0.001) | |||
| Crepaldi et al. 21 | Mean and SD of AER (μg/min) | 70 (28–174) to 58 (2–198; P < 0.03)≈ | 88 (21–187) to 119 (4–176)* | |
| Mean and SD of creatinine clearance (ml min–1 1.73‐m–2) | 105 ± 14 to 101 ± 15≈ | 110 ± 15 to 105 ± 15* | ||
| Mean and SD of urinary albumin concentration (g dl–1) | 4.4 ± 0.3 to 4.2 ± 0.4≈ | 4.4 ± 0.2 to 4.4 ± 0.3* | ||
| Mean (95%CI) reduction of risk of progression to macroalbuminuria | 41.4% (22.1–64.3%; P = 0.05)≈ | |||
| regression rate from macroalbuminuria to normoalbuminuria | 0%≈ | 4%* | ||
| Janssen et al. 16 | Mean ± SEM of GFR (ml min–1) | 45 ± 8 to 45 ± 8 (P = NS) | ||
| Mean ± SEM of FF (%) | 23 ± 2 to 25 ± 2 (P = NS) | |||
| Mean ± SEM of UPCR (g mmol–1) | 0.56 to 0.69 (P = NS) | |||
| Mitchell et al. 22 | Mean ± SEM (range) of GFR (ml min–1 1.73‐m–2) | 56 ± 5 (23–65) to 53 ± 7 (13–108; P = NS) | ||
| Mean ± SEM (range) of RPF rate (ml min–1 1.73‐m–2) | 282 ± 27 (130–456) to 319 ± 38 (160–594; P = NS) | |||
| Mean ± SEM (range) of FF (%) | 21 ± 2 (11–33) to 19 ± 3 (9–46) | |||
| Chaturvedi et al. 28 | Geometric mean (IQR) of AER (μg min–1) | 8 (4.7–14) to 9.4 | ||
| Rossing et al. 26 | Means ± antilog SE of urinary IgG (mg 24‐h–1h) | 71 ± 1.19 to 81 ± 1.19 (P = NS) | ||
| Means ± antilog SE of fractional clearance of albumin (× 10–6) | 215 ± 1.23 to 297 ± 1.29 (P < 0.05) | |||
| Means ± antilog SE of GFR (ml min–1 1.73‐m–2) | 85 ± 6 to 81 | |||
| albuminuria (mg 24‐h–1) | 1087 ± 1.2 to 1271 | |||
| Nielsen et al. 24 | Means ± antilog SE of GFR (ml min–1 1.73‐m–2) | 74 ± 8 and – 0.60 ± 0.11 decreased during the study | ||
| Geometric mean ± antilog SE of Albuminuria (mg 24‐h–1) | Baseline = 1578 ± –1.2 and reduced 15% (–13 to 34%; P = NS) | |||
| means ± SE of Fractional albumin clearance (×106) | 553 ± 1.0 and increased 6% (–31 to 31%) | |||
| Ranieri et al. 19 | Mean ± SD of GFR (ml min–1) | 133.06 ± 15.75 to 120.17 ± 11.84 (P = NS)† ‡ | 115.5 ± 1 to 116.94 ± 11.11 (P = NS)ϕ | |
| Mean ± SD of FF (%) | 18.39 ± 2.17 to 19.78 ± 2.44 (P = NS)† ‡ | 20.06 ± 3.81 to 19.72 ± 2.07 (P = NS)ϕ | ||
| Mean ± SD of UAE (mg 24‐h–1) | 44.7 ± 9.5 to 69.3 ± 6.4(P < 0.01)† ‡ | 76.6 ± 5.6 to 71.7 ± 7.5 (P = NS)ϕ | ||
| *placebo, ≈Nifedipine, ϕbaseline to first phase with amlodipine, ‡baseline to first phase of lisinopril, †end of first phase with lisinopril to second phase of amlodipine, ∥end of first phase with amlodipine to second phase of lisinopril | ||||
| Off‐label | Author (reference) | Main outcome | Baseline vs. final outcome in lisinopril group | P‐value lisinopril vs. comparison | |
|---|---|---|---|---|---|
| Proteinuric kidney disease | Juarez et al. 20 | Geometric mean (95%CI) of UPCR (g g–1) | 0.92 (0.52–1.66) to 0.68 (0.38–1.20; P = NS) | ||
| Mean and SD of GFR (ml min–1 1.73‐m–2) | 48 ± 14 and decreased with a median rate of 3.8 (95%CI, 1.8–6.3) | ||||
| Menne et al. 18 | Mean UACR (mg mmol–1)‐ adjusted ratio | 9.6 to 5.7 adjusted ratio: 76%, CI: 48–118% | 0.213 | ||
| Laverman et al. 17 | Mean (95%CI) of total proteinuria (g 24‐h–1) | 4.5 (3.5–6.4) to 1.4 (0.5–2.9; P < 0.05) | <0.05 | ||
| Mean (95%CI) of creatinine clearance (ml/min) | 80 (66–96) to 72 (52–92) | ||||
| Poulsen et al. 25 | Geometric mean ± tolerance factor E‐UAE (μg min–1) | 96.8 ± 1.8 to 48.3 ± 3.1 | 0.04 | ||
| Mogensen et al. 23 | Adjusted mean reduction in UACR [Geometric mean (tolerance factor)] | 39 (20 to 54) [Baseline = 6.6 (1.1)] (<0.001) | |||
| Tarnow et al. 27 | Mean (95%CI) albuminuria mg 24‐h–1 | Baseline = 1554 (980–2465) and reduced by 52% (95%CI 14–73) | <0.001 | ||
| Mean and SE of GFR (ml min–1 1.73‐m–2) | Baseline = 85 ± 5 and declined 0.5 ± 0.1 ml min–1 month–1 (P < 0.001) | NS | |||
| Crepaldi et al. 21 | Mean and SD of AER (μg/min) | 54 (20–128) to 29 (5–173; P < 0.003) | |||
| Mean and SD of creatinine clearance (ml min–1 1.73‐m–2) | 113 ± 16 to 109 ± 19 | ||||
| Mean and SD of Urinary albumin concentration (g/dl) | 4.4 ± 0.4 to 4.3 ± 0.3 | ||||
| Mean (95%CI) reduction of risk of progression to macroalbuminuria | 49.1% (26.8–63.4%; P < 0.03) | ||||
| Regression rate from macroalbuminuria to normoalbuminuria | 15% | < 0.001 | |||
| Janssen et al. 16 | Mean ± SEM of GFR (ml/min) | 55 ± 11 to 50 ± 10 (P < 0.01) | |||
| Mean ± SEM of FF (%) | 24 ± 2 to 21 ± 2 (P < 0.001) | ||||
| Mean ± SEM of UPCR (g/mmol) | 0.39 ± 0.17 to 0.26 ± 0.11 (P < 0.05) | <0.05 | |||
| Mitchell et al. 22 | Mean ± SEM (range) of GFR (ml min–1 1.73‐m–2) | 46 ± 6 (18–78) to 42 ± 6 (18–83; P = NS) | NS | ||
| Mean ± SEM (range) of RPF rate (ml min–1 1.73‐m–2) | 262 ± 37 (122–570) to 247 ± 42 (62–523; P = NS) | NS | |||
| Mean ± SEM (range) of FF (%) | 19 ± 2 (9–37) to 21 ± 4 (8–65; P = NS) | NS | |||
| Chaturvedi et al. 28 | Geometric mean (IQR) of AER (μg min–1) | 8 (4.4–14.8) to 7.3 | 0.03 | ||
| Rossing et al. 26 | Means ± antilog SE of Urinary IgG (mg 24‐h–1) | 101 ± 1.29 to 64 ± 1.38 (P < 0.05) | <0.05 | ||
| Means ± antilog SE of Fractional clearance of albumin (× 10–6) | 380 ± 1.29 to 213 ± 1.35 (P < 0.05) | <0.05 | |||
| Means ± antilog SE of GFR (ml min–1 1.73‐m–2) | 85 ± 5 to 73.5 | <0.05 | |||
| albuminuria (mg 24‐h–1) | 1513 ± 1.3 to 790 | 0.001 | |||
| Nielsen et al. 24 | Means ± antilog SE of GFR (ml min–1 1.73‐m–2) | 75 ± 6 and –0.67 ± 0.10 decreased during the study | 0.63 | ||
| Geometric mean ± antilog SE of Albuminuria (mg 24‐h–1) | Baseline = 963 ± 1.2 and reduced 55% (29–72%; P < 0.05) | 0.01 | |||
| means ± SE of Fractional albumin clearance (×106) | (323 ± – 1.2 and reduced 52% (8–75%) | 0.03 | |||
| Ranieri et al. 19 | Mean ± SD of GFR (ml min–1) | 116.94 ± 11.11 to 127.27 ± 18.89 (P < 0.01)∥ | 119.61 ± 16.01 to 133.06 ± 15.7 (P < 0.01)‡ | ||
| Mean ± SD of FF (%) | 19.72 ± 2.07 to 18.22 ± 2.3 (P < 0.01)∥ | 20.06 ± 2.01 to 18.39 ± 2.17 (P < 0.01)‡ | |||
| Mean ± SD of UAE (mg 24‐h−1) | 71.7 ± 7.5 to 54.3 ± 3 (P < 0.01)∥ | 77.1 ± 8.1 to 44.7 ± 9.5 (P < 0.01)‡ | <0.01 | ||
| *placebo, ≈Nifedipine, ϕbaseline to first phase with amlodipine, ‡baseline to first phase of lisinopril, †end of first phase with lisinopril to second phase of amlodipine, ∥end of first phase with amlodipine to second phase of lisinopril | |||||
| Off‐label use | Author (Ref) | Main outcome | Final outcome vs. baseline in comparison group | ||
|---|---|---|---|---|---|
| Atrial fibrillation | Van Den Berg et al. 30 | Mean ± SD of heart rate (beats min–1) | 83 ± 14 to 81 + 13 (P = NS) | ||
|
Median number of isolated premature ventricular beats h–1 (range) |
23 (5–340) to 109 (22–372) | ||||
| Haywood et al. 31 | New AF per 1000 | 16.3† | 22.4‡ | 20.9¥ | |
| Odds ratio for univariable logistic model | 1.346‡ | 1.073‡ | 1.000¥ | ||
| Odds ratio for multivariable logistic model | 1.326‡ | 1.083‡ | 1.000¥ | ||
| CM in patients with DMD | Allen et al. 32 | Mean EF (%) | 48.4 to 55.2 (P = 0.03) | ||
| Diabetic retinopathy | Mehlsen et al. 36 | Mean (95%CI) of diameter change of retinal artery after exercise + flicker (%) | 1.8 (0.1–3.5) to 2.0 (0.3–3.6) | ||
| Mean (95%CI) of diameter change of retinal vein after exercise + flicker (%) | 4.4 (2.6–6.1) to 5.3 (3.5–7.0) | ||||
| Chaturvedi et al. 37 | Incidence of retinopathy: odds ratio | 15/62 | |||
| Regression of retinopathy | 28/117 | ||||
| Myocardial fibrosis | Brilla et al. 34 | Myocardial hydroxyproline concentration (μg mg–1) | 9.5 ± 0.5 to 10.4 ± 0.6 (P = NS) | ||
| Collagen volume fraction (%) | 6.4 ± 0.8 to 6.5 ± 0.8 (P = NS) | ||||
| Inflammatory cystoid macular oedema | Kooij et al. 40 | Number (%) of patients with improvement of cystoid macular oedema | 9 of 40 (23%) | ||
| Left ventricular hypertrophy | Christian et al. 34 | LV mass index (g m–2.7) | 176 ± 17 to 145 ± 11 (P = 0.08) | ||
| Mean ± SD of Cornell voltage overall (μV) | 1451 ± 620 to 1427 ± 630 (P = 0.0045)‡ | 1440 ± 627 to 1434 ± 636 (P = 0.3438)¥ | |||
| Ernst et al. 33. | Prevalence (%) of LVH by Cornell voltage | 482/7151 (6.74) to 311/4753 (6.54)‡ | 783/12102 (6.47) to 488/7947 (6.14)¥ | ||
| Individuals experienced regression of their LVH (%) | 50.21 | 52.96 | |||
| Migraine | Schrader et al. 41 | Mean ± SD of h with headache | 162 ± 142 | ||
| Mean ± SD of days with migraine | 18.5 ± 10 | ||||
| Mitral valve regurgitation | Wong et al. 35 | Mean ± SD of change in mitral regurgitant fraction (%) | +3.7% ± 3.2 | ||
| Mean ± SD of change of MR jet to LA area ratio | –10.9% ± 2.4 | ||||
| Mean ± SD of LA dimension (mm) measured by M‐mode techniques | 45.4 ± 8 to 46.7 ± 8 (P = NS) | ||||
| Oligospermia and infertility | Mbah et al. 42 | Mean ± SD (95%CI) sperm cell count (106 ml–1) | 17.1 (12.0–2.2) to 12.8 (8.2–19.0; P ≤ 0.02)† | 7.43 ± 3.97 to 7.0 (4.2–10.1; P = NS)ϕ | |
| Mean ± SD (95%CI) sperm cells with good motility (%) | 41.1 (32.1–50.6) to 15.3 (12.2–17.7; P ≤ 0.008)† | 22.12 ± 4.4 to 22.2 (18.6–25.7; P = NS)ϕ | |||
| Mean ± SD (95%CI) sperm cells with abnormal morphology (%) | 15.3 (11.6–19.8) to 28.2 (22.5–33.9; P ≤ 0.03)† | 44.12 ± 2.6 to 44.1 (39.1–50.2; P = NS)ϕ | |||
| Prevention of diabetes | ALLHAT Group 39 | Number (%) of patients with FBS ≥ 126 mg dl–1 among baseline‐nondiabetics | 154 (9.8)‡ | 302 (11.6)¥ | |
| Fogari and Roberto 38 | Glucose infusion rate (mg min–1 kg–1) | 5.79 ± 0.33 to 6.21 ± 0.41 (P = NS) | |||
| Total glucose requirement (g) | 31.9 ± 3.4 to 33.8 ± 3.3 (P = NS) | ||||
| Prevention of pneumonia | Lee et al. 43 | Unadjusted OR (95%CI) of Pneumonia or death | |||
| The incidences of pneumonia (%) | 47.4 | ||||
| ¥Chlorthalidone, ‡Amlodipine, †Doxazosin, ϕbaseline to the first phase with placebo, ‡baseline to the first phase of lisinopril, †end of the first phase with lisinopril to the second phase of placebo, ∥end of the first phase with placebo to the second phase of lisinopril | |||||
| Off‐label use | Author (reference) | Main outcome | Final outcome vs. baseline in lisinopril group (P value) | P‐value lisinopril vs. comparison | |
|---|---|---|---|---|---|
| Atrial fibrillation | Van Den Berg et al. 30 | mean ± SD of heart rate (beats/min) | 92 ± 9 to 88 + 9 (P = NS) | ||
|
Median number of isolated premature ventricular beats/h (range) |
34 (2–228) to 27 (4–335) | 0.040 | |||
| Haywood et al. 31 | New AF per 1000 | 20.6 | |||
| Odds ratio for univariable logistic model | 0.987 | 0.90¥ | |||
| Odds ratio for multivariable logistic model | 0.939 | 0.59¥ | |||
| CM in patients with DMD | Allen et al. 32 | Mean EF (%) | 47.5 to 54.6 (P = 0.02) | NS | |
| Diabetic retinopathy | Mehlsen et al. 36 | Mean (95%CI) of diameter change of retinal artery after exercise + flicker (%) | 1.8 (0.1–3.5) to 1.0 (1.4–3.4) | 0.43 | |
| Mean (95%CI) of diameter change of retinal vein after exercise + flicker (%) | 4.4 (2.6–6.1) to 2.3 (0.2–4.8) | 0.02 | |||
| Chaturvedi et al. 37 | Incidence of retinopathy ‐Odds ratio | 13/72 OR = 0.69 (95%CI 0.30–0.59) | 0.4 | ||
| Regression of retinopathy | 33/103 OR = 1.48 (95%CI 0.82–2.68) | 0.2 | |||
| Myocardial fibrosis | Brilla et al. 34 | Myocardial hydroxyproline concentration (μg mg–1) | 9.9 ± 0.3 to 8.3 ± 0.4 (P = 0.001) | <0.00001 | |
| Collagen volume fraction (%) | 6.9 ± 0.6 to 6.3 ± 0.6 (P < 0.01) | <0.05 | |||
| Inflammatory cystoid macular oedema | Kooij et al. 40 | Number (%) of patients with improvement of cystoid macular oedema | 10 of 40 (25) | 0.79 | |
| Left ventricular hypertrophy | Christian et al. 34 | LV mass index lisinopril (g m–2.7) | 170 ± 16 to177 ± 15 (P = NS) | ||
| Mean ± SD of Cornell voltage overall (μV) | 1425 ± 615 to 1435 ± 637 (P = 0.24) | 0.9433¥ | |||
| Ernst et al. 33. | Prevalence (%) of LVH by Cornell voltage | 473/7123 (6.64) to 292/4494 (6.50) | NS¥ | ||
| Individuals experienced regression of their LVH (%) | 49.27 | NS¥ ‡ | |||
| Migraine | Schrader et al. 41 | Mean ± SD of hours with headache | 129 ± 125 | <0.05 | |
| Mean ± SD of days with migraine | 14.5 ± 11 | <0.05 | |||
| Mitral valve regurgitation | Wong et al. 35 | Mean ± SD of change in mitral regurgitant fraction (%) | –6.4% ± 3.5 | <0.05 | |
| Mean ± SD of change of MR jet to LA area ratio | –15.7% ± 3.7 | NS | |||
| Mean ± SD of LA dimension (mm) measured by M‐mode techniques | 44.3 ± 7 to 44.1 ± 8 (P = NS) | NS | |||
| Oligospermia and infertility | Mbah et al. 42 | Mean ± SD (95%CI) sperm cell count (106 ml–1) | 7.0 (4.2–10.1) to 13.9 (8.7–17.5; P < 0.004)∥ | 5.29 ± 2.6 to 17.1 (12.0–22.2; P < 0.03)‡ | |
| Mean ± SD (95%CI) sperm cells with good motility (%) | 22.2 (18.6–25.7) to 30.3 (26.0–35.1; P ≤ 0.005)∥ | 17.33 ± 3.2 to 41.1 (32.1–50.6; P < 0.05)‡ | |||
| Mean ± SD (95%CI) Sperm cells with abnormal morphology (%) | 44.1 (39.1–50.2) to 11.8 (6.0–17.2; P ≤ 0.03)∥ | 42.91 ± 5.1 to 15.3 (11.6–19.8; P ≤ 0.04)‡ | |||
| Prevention of diabetes | ALLHAT Group 39 | Number (%) of patients with FBS ≥ 126 mg dl–1 among baseline‐nondiabetics | 119 (8.1) | <0.001¥ | |
| Fogari and Roberto 38 | Glucose infusion rate (mg min–1 kg–1) | 5.74 ± 0.31 to 7.21 ± 0.39 (P = 0.05) | 0.05 | ||
| Total glucose requirement (g) | 32.5 ± 3.2 to 39.8 ± 3.6 | ||||
| Prevention of pneumonia | Lee et al. 43 | Unadjusted OR (95%CI) of Pneumonia or death | 1.80 (0.69, 4.72; P = 0.232) | ||
| The incidences of pneumonia (%) | 57.6 | 0.390 | |||
¥Chlorthalidone, ‡Amlodipine, †Doxazosin, ϕbaseline to the first phase with placebo, ‡baseline to the first phase of lisinopril, †end of the first phase with lisinopril to the second phase of placebo, ∥end of the first phase with placebo to the second phase of lisinopril
AER, albumin exertion rate; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; CCB, calcium channel blocker; CI, confidence interval; CM, cardiomyopathy; DMD, Duchenne muscular dystrophy; ERPF, effective renal plasma flow; EF, ejection fraction; FF, filtration fraction; GFR, glomerular filtration rate; RPF, renal plasma flow; LA, left atrium; LAV, left atrium volume; LVH, left ventricular hypertrophy; MR, Mitral valve regurgitation; NS, non‐significant; SD, standard deviation; SEM, Standard Error of the Mean; UAE, urine albumin exertion; UACR, urine albumin: creatinine ratio; UPCR, urine protein: creatinine ratio
Lisinopril was compared with different drugs or placebos regarding their effects on proteinuria and GFR within included studies:
Lisinopril vs. placebo: Studies in normotensive patients with diabetes 21, 28 highlighted a significant regression rate from macroalbuminuria to microalbuminuria with administration of lisinopril compared to a placebo (15% in lisinopril vs. 4% in placebo, P < 0.001) and a significant reduction of the risk of progression to macroalbuminuria [mean risk reduction 49.1%; 95% confidence interval (CI): 26.8–63.4, P < 0.03]. The decrease in the albumin exertion rate was also significantly more in lisinopril than in the placebo [geometric mean (interquartile range) of the albumin exertion rate 8 (4.7–14) to 9.4 μg min–1 in placebo vs. 8 (4.4–14.8) to 7.3 μg min–1 in lisinopril, P = 0.03]. Another study in nondiabetic normotensive patients showed that lisinopril significantly reduced exercise‐urine albumin exertion when compared to a placebo [geometric mean (tolerance factor) 150.1 (3.7) to 213.6 (6.9) μg min–1 in placebo vs. 96.8 (1.8) to 48.3 (3.1) in lisinopril, P = 0.04] 25 (Tables 3 and 4).
Lisinopril vs. angiotensin receptor blockers: Lisinopril in comparison to angiotensin receptor blockers (ARBs; e.g. irbesartan, valsartan, losartan, candesartan) 17, 18, 20, 23 showed different results. Its effects on proteinuria (or albuminuria) and GFR were similar to irbesartan and valsartan [mean rate of reduction of GFR per year; 3.8 ml min–1 (95%CI 1.8–6.3) in lisinopril vs. 3.3 ml min–1 1.73 m–2 (1.8–4.7) in irbesartan) but they were significantly superior when compared to losartan and candesartan [adjusted geometric mean reduction of urine albumin to creatinine ratio 39 (95%CI 20–54) in lisinopril vs. 24 (0–43) in candesartan] (Tables 3 and 4).
Lisinopril vs. calcium channel blockers: Studies on both diabetic and nondiabetic, hypertensive and normotensive patients (Table 3) demonstrated that although calcium channel blockers (CCBs; e.g. nisoldipine, amlodipine and nifedipine) 16, 19, 21, 26, 27 conversely increased albuminuria, lisinopril significantly reduced it (Table 4). Tarnow et al. showed that nisoldipine increased albuminuria by 12% (95%CI –10 to 40) but lisinopril decreased albuminuria by 52% (14–73) 27. Similarly, Ranieri et al. demonstrated that amlodipine significantly increased albuminuria (44.7 ± 9.5 to 69.3 ± 6.4 (mg 24‐h−1); P < 0.01) but lisinopril significantly decreased albuminuria (71.7 ± 7.5 to 54.3 ± 3; P < 0.01) 26. There were controversies about the effect of lisinopril on GFR and creatinine clearance in comparison with CCBs; however, those studies with greater sample sizes and longer duration showed similar effects (Tables 3 and 4).
Lisinopril vs. β‐blockers: Nielsen et al. 24 conducted a study on diabetic patients with diabetic nephropathy comparing lisinopril with atenolol. They highlighted the fact that lisinopril significantly decreased albuminuria better than atenolol [geometric mean reduction 55% (95%CI 29–72) in lisinopril vs. 15% (–13 to 34) in atenolol, P < 0.01] 24. The effects of these drugs on GFR were similar (decrease in means ± antilog standard error of GFR: 0.67 ± 0.10 ml min–1 for lisinopril vs. 0.60 ± 0.11 ml min–1 for atenolol, P = 0.63; Tables 3 and 4).
Lisinopril vs. other ACE inhibitors: One single study compared lisinopril with another ACE inhibitor fosinopril. The result demonstrated a similar nonsignificant reduction in GFR (mean ± standard error of the mean of GFR 46 ± 6 to 42 ± 6 ml min–1 in lisinopril vs. 56 ± 5 to 53 ± 7 ml min–1 in fosinopril, P > 0.05) 22 (Tables 3 and 4).
Atrial fibrillation
Nonsignificant results were reported for using lisinopril for atrial fibrillation (AF). Van Den Berg et al. 30 assessed the effects of daily lisinopril in patients with congestive heart failure and chronic AF. Monitoring the heart rates for 6 weeks showed that reduction of the mean heart rate was not significant in the lisinopril group. However, a minor reduction of the median number of isolated premature ventricular beats h–1 was demonstrated in the lisinopril group [34 (range = 2–228) to 27 (4–335) beats h–1, P (lisinopril vs. placebo) = 0.04]. Additionally, the effect of lisinopril on the maintenance of sinus rhythm after an electrical cardioversion was 71% in the lisinopril group and 36% in the placebo group (P > 0.05). Haywood et al. evaluated the incidence of new AF cases in patients who received lisinopril, amlodipine, doxazosin, or chlorthalidone. The results of this study showed that treatment with lisinopril as well as other antihypertensive drugs did not affect AF incidence when compared with usual care (odds ratio 0.987, P = 0.9 in the univariable logistic model) 31 (Tables 3 and 4).
Cardiomyopathy in patients with Duchenne muscular dystrophy
A multicenter double‐blind prospective study compared the efficacy and safety of lisinopril versus losartan in the treatment of cardiomyopathy (CM) on 22 Duchenne muscular dystrophy (DMD) patients with newly diagnosed CM. Although the ejection fraction after 1 year was significantly improved in each treatment group, the difference was not statistically significant 32 (Tables 3 and 4).
Left ventricular hypertrophy
The effect of lisinopril on left ventricular hypertrophy (LVH) was assessed using two different diagnostic tools. An American study randomized 26 376 subjects into three groups: lisinopril, amlodipine and chlorthalidone, and used electrocardiography to examine LVH and its prevalence (defined as Cornell voltage >2200 μV in women and > 2800 μV in men). After 4 years, the mean Cornell voltage was increased in the lisinopril group; however, LVH prevalence was not changed 33. Another study recruited 35 hypertensive patients with LVH to be treated with either lisinopril or hydrochlorothiazide and evaluated the outcome using echocardiography. Results of this study demonstrated no significant treatment effect of lisinopril on the left ventricular mass index after a 6‐month follow‐up 34 (Tables 3 and 4).
Mitral valve regurgitation
Wong et al. 35 conducted an RCT on 23 patients with mitral regurgitation. A significant reduction in mitral regurgitation fraction occurred during the 1‐year follow‐up with lisinopril. Both maximum and minimum left atrium volumes were reduced in the lisinopril group (88 ± 33 ml reduced to 75 ± 23 ml as compared to 46 ± 20 ml reduced to 38 ± 16 ml for placebos; P < 0.01; Tables 3 and 4).
Myocardial fibrosis
A double‐blind RCT compared the effect of lisinopril and hydrochlorothiazide on patients with primary hypertension, LVH and LV diastolic dysfunction investigating myocardial fibrosis. After 6 months of treatment, myocardial fibrosis significantly regressed with lisinopril 34 (Tables 3 and 4).
Diabetic retinopathy
Mehlsen et al. 36 recruited 25 normotensive diabetic patients with mild retinopathy and randomized them to receive lisinopril, placebo or amlodipine. To show the perfusion disturbances that occur in retinopathy, the diameter response of retinal arterioles during an acute increase in the blood pressure induced by isometric exercise, during flicker stimulation and stimulus conditions simultaneously were studied before and through the treatment period. The results revealed that lisinopril did not significantly change the diameter response of retinal vessels (P = 0.11). However, the study of Chaturvedi et al. 37 assessed the effect of lisinopril on retinopathy in patients with insulin‐dependent diabetes mellitus. Retinopathy was evaluated by retinal photographs. They showed that retinopathy progression was halved in the lisinopril group compared to the placebo group (OR 0.50, P = 0.02) and progression to proliferative retinopathy was also reduced significantly (OR 0.18, P = 0.03; Tables 3 and 4).
Prevention of diabetes
Previous studies using lisinopril raised new hopes for the prevention of diabetes. Fogari et al. 38 compared the effects of the lisinopril and losartan on insulin sensitivity. They involved 25 nondiabetic patients with mild to moderate hypertension and randomized them to receive lisinopril or losartan for 12 weeks. They found that the glucose infusion rate – used as an indicator of insulin sensitivity – was significantly increased by lisinopril but not by losartan (P value for lisinopril vs. losartan <0.05). Also, total glucose requirement (defined as the total amount of exogenous glucose required to maintain a steady‐state blood glucose level in response to a defined increase in plasma insulin concentration) was increased by lisinopril, whereas losartan did not significantly modify it. Another study 39 compared chlorthalidone with lisinopril, doxazosin and amlodipine in a large sample size. This study, after a mean 5 years of follow‐up, demonstrated a significant reduction in the risk of developing diabetes in the lisinopril group vs. chlorthalidone (P < 0.001; Tables 3 and 4).
Inflammatory cystoid macular oedema
Kooij et al. 40 designed a randomized control trial to analyse the effect of lisinopril on inflammatory cystoid macular oedema and visual acuity. They included 40 patients with inflammatory cystoid macular oedema then randomized them to receive lisinopril or placebo. Lisinopril had no effect on cystoid macular oedema, visual acuity, papillary leakage, retinal vasculitis or choroidal leakage 40 (Tables 3 and 4).
Migraine
One RCT randomized 60 patients with migraine to receive lisinopril or placebo for a treatment period of 12 weeks. The lisinopril group had a 20% reduction of hours suffering with a headache, a 21% reduction of days with migraine, and a 20% reduction of the headache severity index as compared with the placebo group 41. Side effects were not significantly different compared to placebo (P = 0.7; Tables 3 and 4).
Oligospermia and infertility
In a crossover RCT, 33 men with idiopathic oligospermia were randomized to receive either lisinopril or placebo. Lisinopril was found to cause a normalization of seminal parameters in 53.6% of the participants. Although the mean ejaculate volume was unchanged, the total sperm cell count and the percentage of motile sperm cells increased, whereas the percentage of sperm cells with abnormal morphology decreased 42 (Tables 3 and 4).
Prevention of pneumonia
One RCT randomized 93 patients with dysphagia from cerebrovascular diseases, who were on tube‐feeding, to receive lisinopril or placebos for 26 weeks. No difference in the incidence of pneumonia or fatal pneumonia was noted between these groups 43 (Tables 3 and 4).
Discussion
In furtherance of conducting a reliable and comprehensive review of off‐label uses of lisinopril for physicians, an extensive search in seven prominent databases was executed and 28 RCTs were acquired. Twenty‐four off‐label uses for lisinopril were identified in our review, but 12 lacked supporting RCT. The overall risk of bias across the studies was moderate. Other bias, selective reporting and inadequate details on random sequence generation were the most prevalent problems of the included studies. We were not able to perform meta‐analysis due to: the heterogeneity of inclusion criteria for patients; dosage of lisinopril, placebos or drugs being compared; variables reported within different RCTs; and the differences in the duration of administration and follow‐ups. However, this heterogeneity somehow helped our review to assess the effects of lisinopril examined in different aspects: for example, the effect of lisinopril on left ventricular hypertrophy was assessed using both electrocardiography and echocardiography in two separate studies.
Among the remaining 12 off‐label uses with RCT support (Table 2), proteinuric kidney disease was the most prominent use. The renoprotective effect of ACE/ARBs have been demonstrated in meta‐analyses 44, 45 but the specific effect of lisinopril was not designated. The result of our systematic review showed a clear benefit from lisinopril for proteinuria and albuminuria as defined by the albumin excretion rate, total proteinuria, total albuminuria, urine protein/creatinine ratio and urine albumin/creatinine ratio. This positive effect was noticed in a variety of underlying diseases such as diabetic nephropathy, essential hypertension, membranous glomerulopathy, chronic pyelonephritis, membranoproliferative, glomerular nephropathy, IgA nephropathy, hypertensive glomerulosclerosis, adult polycystic kidney disease, focal glomerulosclerosis and hereditary nephritis. Lisinopril is approved for hypertension. Treatment of proteinuria in patients with hypertension can be considered as an off‐label use since it has not been specified in any of the FDA approval letters. That said, lisinopril has some positive effects on proteinuria, which are beyond its antihypertensive effects as not all antihypertensive drug can improve proteinuria (e.g. calcium channel blockers) 16, 19. The included studies were primarily aiming at patients with proteinuria, although some of them were conducted on hypertensive or diabetic patients (see Table 3). It should be noted that no included studies determined the proportion of patients with hypertension.
A small yet statistically significant decrease in GFR and creatinine clearance was demonstrated in patients using lisinopril; however, increase in serum creatinine of up to 30% (up to about 23% reduction in GFR) is generally considered not to be clinically significant and is a function, and not an adverse effect, of its mechanism of action in diminishing angiotensin II medicated efferent glomerular arteriolar constriction, thus reducing glomerular filtration pressure 46. This slight reduction of GFR was not seen in those studies which were conducted on patients with GFR > 90 ml min–1. Furthermore, lisinopril, in comparison to other standard treatments of diabetic nephropathy including CCB, ARBs and β‐blockers 47, demonstrated better outcomes; however, its effects on decreasing GFR and creatinine clearance were similar to the others.
Among other off‐label uses of lisinopril, the included RCTs highlight the positive effects of lisinopril for migraine, prevention of diabetes, myocardial fibrosis and mitral valve regurgitation, CM in patients with DMD, and oligospermia and infertility. However, lisinopril appeared to be ineffective for atrial fibrillation, LVH, inflammatory macular oedema and prevention of pneumonia. Moreover, the results of two studies into the effects of lisinopril on retinopathy were contradictory: different methods were used in these studies 36, 37; however, Chaturvedi et al. using retinal photographs (96% sensitivity and 89% specificity 48), larger sample size and longer duration of follow‐up, reported more reliable positive results for lisinopril. Moreover, regarding the effect of lisinopril on LVH, one of the studies had a very short follow up of only 6 months 34. As it is not expected to see the effect of this drug on LVH in such a short period, the result of this study may not clearly represent lisinopril's efficacy for LVH. The ALLHAT (Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial) study with 4 years of follow up could put forward more reliable evidence that lisinopril cannot be considered very effective for LVH 33. Some other off‐label uses (mitral valve regurgitation, CM in patients with DMD, inflammatory cystoid macular oedema and oligospermia and infertility) were investigated with RCTs of small sample sizes, making their results less trustworthy.
One of the studies compared lisinopril with chlorthalidone in reducing the of risk of diabetes and concluded a significant superior effect of lisinopril 39. Although, chlorthalidone can slightly increase blood glucose, it cannot make a patient diabetic as it cannot induce an fasting blood sugar level of ≥126 mg dl–1 (7 mmol l–1). Moreover, the other study comparing lisinopril with losartan confirmed the positive effect of lisinopril for diabetes prevention 38. Therefore, it is more likely that lisinopril can reduce the risk of diabetes.
While the exact mechanism of lisinopril for its off‐label uses are yet to be fully determined, some possible mechanisms have been suggested. It is stated that lisinopril can alter sympathetic activity, inhibit free radical activity, increase prostacyclin synthesis, and block the degradation of bradykinin, encephalin and substance P, resulting in improvement of migraine 41. Moreover, activated angiotensin has been shown to stimulate fibrosis in both renal and cardiac tissues and similar findings have been demonstrated for hepatic fibrosis through pharmacological inhibition and genetic knockdown of angiotensin I, evidenced by reduced collagen deposition, accumulation of myofibroblasts, inflammation and procollagen α2(I) gene expression. Therefore, lisinopril, by inhibiting angiotensin II formation, can reduce this fibrogenesis effect 49. ACE‐inhibitors can modulate the actions of sex hormones, cytokines, growth factors and leptins, thereby improving oligospermia and infertility 42. Studies show that ACE is produced locally by vascular endothelial cells, which may have direct detrimental effects on retinal flow and vascular structure (independent of changes in systemic blood pressure). Therefore, lisinopril, by inhibiting ACE, may play a role in prevention and improvement of diabetic retinopathy 37.
Previous reviews discussing the effects of the class of ACE inhibitors including lisinopril reported findings similar to ours for some of the off‐label uses 49, 50, 51, 52, 53, 54. ACE inhibitors are reported to have significant effects on diabetic retinopathy and might even perform better than ARBs 50. We only found one RCT discussing the positive effect of lisinopril on myocardial fibrosis but previous reviews showed therapeutic effects of lisinopril on hepatic fibrosis 49 and fibrosis in the course of chronic pancreatitis 51. Although we found unsuccessful results about the effect of lisinopril on atrial fibrillation, previous reviews on ACE inhibitors demonstrated significant positive effects; however, they also mentioned that the follow‐up and sample size of the studies were not large enough to recognize AF episodes properly 52, 55. ACE inhibitors were recommended as the second‐ or third‐line migraine preventative. They were not considered as first‐line due to the insufficiency of evidence 53. Also, similar to our findings, ACE inhibitors were shown to reduce the incidence of new‐onset diabetes 54.
Conclusion
Among 24 off‐label uses obtained in our search, proteinuric kidney disease constituted the largest number of studies. We found lisinopril highly effective for proteinuria from a wide range of underlying pathologies, although it resulted in a minor and inconsiderable decrease in GFR in those patients with GFR < 90 ml min–1. Lisinopril, compared to other standard treatments of diabetic nephropathy including ARBs, CCBs and β‐blockers, achieved better outcomes. Our review showed that a few studies reported the effect of lisinopril in other off‐label uses. These studies showed the positive effects of lisinopril for migraine, prevention of diabetes, myocardial fibrosis, mitral valve regurgitation, CM in patients with DMD, oligospermia and infertility, and diabetic retinopathy. Still, we do not recommend considering lisinopril as the first choice for these off‐label uses until more RCTs confirm these positive effects. Conversely, the studies reported that lisinopril was ineffective for atrial fibrillation, LVH, inflammatory macular oedema and prevention of pneumonia. While we did not find any RCTs for some of the off‐label uses of lisinopril as mentioned in Table 2, we are monitoring the development of proper RCTs for future updates to this review.
Competing Interests
There are no competing interests to declare.
This study was funded by a grant from the Iranian Evidence‐Based Medicine Center of Excellence, Tabriz University of Medical Sciences, Iran. We would like to thank Dr Prakeshkumar S. Shah and Dr Fereshteh Ansari for comments that greatly improved the manuscript and Mr. Hossein Hosseinifard for statistical consultations.
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Contributors
S.‐R.S.‐E., conceived of and designed the study, performed the research, analysed the data, and wrote the paper. N.P., conceived of and designed the study, performed the research, analysed the data, and wrote the paper. N.V., performed the research and analysed the data. H.B., designed the study and wrote the paper. M.G., analysed the data and wrote the paper. S.T., analysed the data and wrote the paper. A.A., designed the study, performed the research, analysed the data, and wrote the paper.
Sadat‐Ebrahimi, S.‐R. , Parnianfard, N. , Vahed, N. , Babaei, H. , Ghojazadeh, M. , Tang, S. , and Azarpazhooh, A. (2018) An evidence‐based systematic review of the off‐label uses of lisinopril. Br J Clin Pharmacol, 84: 2502–2521. 10.1111/bcp.13705.
References
- 1. Frankel D . Office of Technology Assessment. Drug labeling in developing countries. OTA‐H‐464, Washington, 1993. [DOI] [PubMed]
- 2. Wittich CM, Burkle CM, Lanier WL, editors. Ten common questions (and their answers) about off‐label drug use. Mayo Clin Proc; 2012: Elsevier. [DOI] [PMC free article] [PubMed]
- 3. Eguale T, Buckeridge DL, Winslade NE, Benedetti A, Hanley JA, Tamblyn R. Drug, patient, and physician characteristics associated with off‐label prescribing in primary care. Arch Intern Med 2012; 172: 781–788. [DOI] [PubMed] [Google Scholar]
- 4. Radley DC, Finkelstein SN, Stafford RS. Off‐label prescribing among office‐based physicians. Arch Intern Med 2006; 166: 1021–1026. [DOI] [PubMed] [Google Scholar]
- 5. Shah SS, Hall M, Goodman DM, Feuer P, Sharma V, Fargason C, et al Off‐label drug use in hospitalized children. Arch Pediatr Adolesc Med 2007; 161: 282–290. [DOI] [PubMed] [Google Scholar]
- 6. Loriaux DL. An approach to the patient with hirsutism. J Clin Endocrinol Metabol 2012; 97: 2957–2968. [DOI] [PubMed] [Google Scholar]
- 7. Dworkin RH, O'Connor AB, Audette J, Baron R, Gourlay GK, Haanpää ML, et al., editors. Recommendations for the pharmacological management of neuropathic pain: an overview and literature update. Mayo Clin Proc; 2010: Elsevier. [DOI] [PMC free article] [PubMed]
- 8. Stafford RS. Regulating off‐label drug use—rethinking the role of the FDA. New Engl J Med 2008; 358: 1427–1429. [DOI] [PubMed] [Google Scholar]
- 9. Walton SM, Schumock GT, Lee KV, Alexander GC, Meltzer D, Stafford RS. Prioritizing future research on off‐label prescribing: results of a quantitative evaluation. Pharmacotherapy 2008; 28: 1443–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Approval letter for Prinivil: Food and Drug Administration of USA. Available at http://www.accessdata.fda.gov/drugsatfda%E2%80%93docs/appletter/2008/019558s051,20s052ltr.pdf (last accessed 20 December 2016).
- 11. Approval letter for Zestril: Food and Drug Administration of USA. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/pre96/19777-S002_ZESTRIL_APPROV.PDF (last accessed 20 December 2016).
- 12. Prescribing information of Zestoretic: AstraZeneca pharmaceuticals LP, Wilmington. DE 19850. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/019888s051lbl.pdf (updated May 2012; last accessed 20 December 2016).
- 13. Approval letter for Qbrelis: Food and Drug Administration of USA. Available at http://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/208401Orig1s000Approv.pdf (last accessed 2 December 2016).
- 14. Paoletti E, Cassottana P, Amidone M, Gherzi M, Rolla D, Cannella G. ACE inhibitors and persistent left ventricular hypertrophy after renal transplantation: a randomized clinical trial. Am J Kidney Dis 2007; 50: 133–142. [DOI] [PubMed] [Google Scholar]
- 15. Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions 5.1. 0. The Cochrane Collaboration 2011; 33–49. [Google Scholar]
- 16. Janssen JJWM, Gans ROB, Van Der Meulen J, Pijpers R, Ter Wee PM. Comparison between the effects of amlodipine and lisinopril on proteinuria in nondiabetic renal failure a double‐blind, randomized prospective study. Am J Hypertens 1998; 11: 1074–1079. [DOI] [PubMed] [Google Scholar]
- 17. Laverman GD, Navis G, Henning RH, De Jong PE, De Zeeuw D. Dual renin‐angiotensin system blockade at optimal doses for proteinuria. Kidney Int 2002; 62: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 18. Menne J, Farsang C, Deak L, Klebs S, Meier M, Handrock R, et al Valsartan in combination with lisinopril versus the respective high dose monotherapies in hypertensive patients with microalbuminuria: the VALERIA trial. J Hypertens 2008; 26: 1860–1867. [DOI] [PubMed] [Google Scholar]
- 19. Ranieri G, Andriani A, Lamontanara G, Cesaris RD. Effects of lisinopril and amlodipine on microalbuminuria and renal function in patients with hypertension. Clin Pharmacol Ther 1994; 56: 323–330. [DOI] [PubMed] [Google Scholar]
- 20. Fernandez Juarez G, Luno J, Barrio V, de Vinuesa SG, Praga M, Goicoechea M, et al Effect of dual blockade of the renin‐angiotensin system on the progression of type 2 diabetic nephropathy: a randomized trial. Am J Kidney Dis 2013; 61: 211–218. [DOI] [PubMed] [Google Scholar]
- 21. Crepaldi G, Carta Q, Deferrari G, Mangili R, Navalesi R, Santeusanio F, et al Effects of lisinopril and nifedipine on the progression to overt albuminuria in IDDM patients with incipient nephropathy and normal blood pressure. Diabetes Care 1998; 21: 104–110. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell HC, Smith RD, Cutler RE, Sica D, Videen J, Thompsen‐Bell S, et al Racial differences in the renal response to blood pressure lowering during chronic angiotensin‐converting enzyme inhibition: a prospective double‐blind randomized comparison of fosinopril and lisinopril in older hypertensive patients with chronic renal insufficiency. Am J Kidney Dis 1997; 29: 897–906. [DOI] [PubMed] [Google Scholar]
- 23. Mogensen CE, Neldam S, Tikkanen I, Oren S, Viskoper R, Watts RW, et al Randomised controlled trial of dual blockade of renin‐angiotensin system in patients with hypertension, microalbuminuria, and non‐insulin dependent diabetes: the candesartan and lisinopril microalbuminuria (CALM) study. BMJ (Clin Res Ed) 2000; 321: 1440–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nielsen FS, Rossing P, Gall M‐A, Skott P, Smidt UM, Parving HH. Long‐term effect of Lisinopril and atenolol on kidney function in hypertensive NIDDM subjects with diabetic nephropathy. Diabetes 1997; 46: 1182–1188. [DOI] [PubMed] [Google Scholar]
- 25. Poulsen PL, Ebbehøj E, Mogensen CE. Lisinopril reduces albuminuria during exercise in low grade microalbuminuric type 1 diabetic patients: a double blind randomized study. J Intern Med 2001; 249: 433–440. [DOI] [PubMed] [Google Scholar]
- 26. Rossing P, Tarnow L, Boelskifte S, Jensen BR, Nielsen FS, Parving H‐H. Differences between Nisoldipine and Lisinopril on glomerular filtration rates and albuminuria in hypertensive IDDM patients with diabetic nephropathy during the first year of treatment. Diabetes 1997; 46: 481–487. [DOI] [PubMed] [Google Scholar]
- 27. Tarnow L, Rossing P, Jensen C, Hansen BV, Parving HH. Long‐term renoprotective effect of nisoldipine and lisinopril in type 1 diabetic patients with diabetic nephropathy. Diabetes Care 2000; 23: 1725–1730. [DOI] [PubMed] [Google Scholar]
- 28. Chaturvedi N, Group ES. Randomised placebo‐controlled trial of lisinopril in normotensive patients with insulin‐dependent diabetes and normoalbuminuria or microalbuminuria. Lancet 1997; 349: 1787–1792. [PubMed] [Google Scholar]
- 29. Schnaper HW. Remnant nephron physiology and the progression of chronic kidney disease. Pediatr Nephrol (Berlin, Germany) 2014; 29: 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Den Berg MP, Cruns HJ, Van Veldhuisen DJ, Griep N, De Kam PJ, Lie K. Effects of lisinopril in patients with heart failure and chronic atrial fibrillation. J Card Fail 1995; 1: 355–363. [DOI] [PubMed] [Google Scholar]
- 31. Haywood LJ, Ford CE, Crow RS, Davis BR, Massie BM, Einhorn PT, et al Atrial fibrillation at baseline and during follow‐up in ALLHAT (antihypertensive and lipid‐lowering treatment to prevent heart attack trial). J Am Coll Cardiol 2009; 54: 2023–2031. [DOI] [PubMed] [Google Scholar]
- 32. Allen HD, Flanigan KM, Thrush PT, Dvorchik I, Yin H, Canter C, et al A randomized, double‐blind trial of Lisinopril and Losartan for the treatment of cardiomyopathy in Duchenne muscular dystrophy. PLoS Curr 2013; 5: ecurrents.md.2cc69a1dae4be7dfe2bcb420024ea865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ernst ME, Davis BR, Soliman EZ, Prineas RJ, Okin PM, Ghosh A, et al Electrocardiographic measures of left ventricular hypertrophy in the antihypertensive and lipid‐lowering treatment to prevent heart attack trial. J Am Soc Hypertens 2016; 10: 9308.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Brilla CG, Funck RC, Rupp H. Lisinopril‐mediated regression of myocardial fibrosis in patients with hypertensive heart disease. Circulation 2000; 102: 1388–1393. [DOI] [PubMed] [Google Scholar]
- 35. Wong GC, Marcotte F, Rudski LG. Impact of chronic lisinopril therapy on left atrial volume versus dimension in chronic organic mitral regurgitation. Can J Cardiol 2006; 22: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mehlsen J, Jeppesen P, Erlandsen M, Poulsen PL, Bek T. Lack of effect of short‐term treatment with amlodipine and lisinopril on retinal autoregulation in normotensive patients with type 1 diabetes and mild diabetic retinopathy. Acta Ophthalmol 2011; 89: 764–768. [DOI] [PubMed] [Google Scholar]
- 37. Chaturvedi N, Sjolie A‐K, Stephenson JM, Abrahamian H, Keipes M, Castellarin A, et al Effect of lisinopril on progression of retinopathy in normotensive people with type 1 diabetes. The Lancet 1998; 351: 28–31. [DOI] [PubMed] [Google Scholar]
- 38. Fogari R, Zoppi A, Corradi L, Lazzari P, Mugellini A, Lusardi P. Comparative effects of lisinopril and losartan on insulin sensitivity in the treatment of non diabetic hypertensive patients. Br J Clin Pharmacol 1998; 46: 467–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs. diuretic. The antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). ACC Curr J Rev 2002; 12: 37. [Google Scholar]
- 40. Kooij B, Fijnheer R, Boer J, Dam‐van Loon N, Bartelink I, Roest M, et al A randomized, masked, cross‐over trial of lisinopril for inflammatory macular edema. Am J Ophthalmol 2006; 141: 646–651. [DOI] [PubMed] [Google Scholar]
- 41. Schrader H, Stovner LJ, Helde G, Sand T, Bovim G. Prophylactic treatment of migraine with angiotensin converting enzyme inhibitor (lisinopril): randomised, placebo controlled, crossover study. BMJ (Clin Res Ed) 2001; 322: 19–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mbah AU, Ndukwu GO, Ghasi SI, Shu EN, Ozoemena FN, Mbah JO, et al Low‐dose lisinopril in normotensive men with idiopathic oligospermia and infertility: a 5‐year randomized, controlled, crossover pilot study. Clin Pharmacol Ther 2012; 91: 582–589. [DOI] [PubMed] [Google Scholar]
- 43. Lee JS, Chui PY, Ma HM, Auyeung TW, Kng C, Law T, et al Does low dose angiotensin converting enzyme inhibitor prevent pneumonia in older people with neurologic dysphagia – a randomized placebo‐controlled trial. J Am Med Dir Assoc 2015; 16: 702–707. [DOI] [PubMed] [Google Scholar]
- 44. Vejakama P, Thakkinstian A, Lertrattananon D, Ingsathit A, Ngarmukos C, Attia J. Reno‐protective effects of renin‐angiotensin system blockade in type 2 diabetic patients: a systematic review and network meta‐analysis. Diabetologia 2012; 55: 566–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Strippoli GF, Craig M, Deeks JJ, Schena FP, Craig JC. Effects of angiotensin converting enzyme inhibitors and angiotensin II receptor antagonists on mortality and renal outcomes in diabetic nephropathy: systematic review. BMJ (Clin Res Ed) 2004; 329: 828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bakris GL, Weir MR. Angiotensin‐converting enzyme inhibitor–associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med 2000; 160: 685–693. [DOI] [PubMed] [Google Scholar]
- 47. American Diabetes Association . Standards of medical Care in Diabetes Guidline. 2017.
- 48. Olson JA, Strachan FM, Hipwell JH, Goatman KA, McHardy KC, Forrester JV, et al A comparative evaluation of digital imaging, retinal photography and optometrist examination in screening for diabetic retinopathy. Diabet Med 2003; 20: 528–534. [DOI] [PubMed] [Google Scholar]
- 49. Munshi MK, Uddin MN, Glaser SS. The role of the renin‐angiotensin system in liver fibrosis. Exp Biol Med 2011; 236: 557–566. [DOI] [PubMed] [Google Scholar]
- 50. Wang B, Wang F, Zhang Y, Zhao SH, Zhao WJ, Yan SL, et al Effects of RAS inhibitors on diabetic retinopathy: a systematic review and meta‐analysis. Lancet Diabetes Endocrinol 2015; 3: 263–274. [DOI] [PubMed] [Google Scholar]
- 51. Madro A, Slomka M, Celinski K. Can we expect progress in the treatment of fibrosis in the course of chronic pancreatitis? Adv Med Sci 2011; 56: 132–137. [DOI] [PubMed] [Google Scholar]
- 52. Disertori M, Barlera S, Staszewsky L, Latini R, Quintarelli S, Franzosi MG. Systematic review and meta‐analysis: renin‐angiotensin system inhibitors in the prevention of atrial fibrillation recurrences. An unfulfilled hope. Cardiovasc Drugs Ther 2012; 26: 47–54. [DOI] [PubMed] [Google Scholar]
- 53. Halker RB, Starling AJ, Vargas BB, Schwedt TJ. ACE and ARB agents in the prophylactic therapy of migraine—how effective are they? Curr Treat Options Neurol 2016; 18: 1–9. [DOI] [PubMed] [Google Scholar]
- 54. Alkhenizan AH, Alswes MA. The role of renin blockers in the prevention of diabetes. Saudi Med J 2007; 28: 91–95. [PubMed] [Google Scholar]
- 55. Southern regional meeting, New Orleans, February 18‐20, 2016[Miscellaneous]:. J Invest Med 2016; 64: 488–634. [Google Scholar]
- 56. Wilson TG, Iyengar AJ, Winlaw DS, Weintraub RG, Wheaton GR, Gentles TL, et al Use of ACE inhibitors in Fontan: rational or irrational? Int J Cardiol 2016; 210: 95–99. [DOI] [PubMed] [Google Scholar]
- 57. Van de Ven L, Van Leeuwen J, Smit A. The influence of chronic treatment with betablockade and angiotensin converting enzyme inhibition on the peripheral blood flow in hypertensive patients with and without concomitant intermittent claudication. A comparative cross‐over trial. VASA Zeitschrift fur Gefasskrankheiten 1993; 23: 357–362. [PubMed] [Google Scholar]
- 58. Buchler T, Krejci M, Svobodnik A, Adam Z, Minarik J, Bacovsky J, et al Outcome of patients with multiple myeloma and hypertension treated with angiotensin‐I‐converting enzyme inhibitors during high‐dose chemotherapy. Hematol J 2005; 5: 559–564. [DOI] [PubMed] [Google Scholar]
- 59. Brozovich FV, Morganroth J, Gottlieb NB, Gottlieb RS. Effect of angiotensin converting enzyme inhibition on the incidence of restenosis after percutaneous transluminal coronary angioplasty. Cathet Cardiovasc Diagn 1991; 23: 263–267. [DOI] [PubMed] [Google Scholar]
- 60. Hackam DG, Thiruchelvam D, Redelmeier DA. Angiotensin‐converting enzyme inhibitors and aortic rupture: a population‐based case‐control study. Lancet 2006; 368: 659–665. [DOI] [PubMed] [Google Scholar]
- 61. Chen FA, Chien CC, Chen YW, Wu YT, Lin CC. Angiotensin converting‐enzyme inhibitors, angiotensin receptor blockers, and calcium channel blockers are associated with prolonged vascular access patency in uremic patients undergoing hemodialysis. PLoS One 2017; 11: e0166362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. London RD, Dikman SH, Spiera H. Recovery of renal function in undifferentiated connective tissue disease after treatment with angiotensin‐converting enzyme inhibitors. Am J Kidney Dis 1991; 18: 716–719. [DOI] [PubMed] [Google Scholar]
- 63. Onder G, Pahor M, Gambassi G, Federici A, Savo A, Carbonin P, et al Association between ACE inhibitors use and headache caused by nitrates among hypertensive patients: results from the Italian group of pharmacoepidemiology in the elderly (GIFA). Cephalalgia 2003; 23: 901–906. [DOI] [PubMed] [Google Scholar]
- 64. Ecder T, Edelstein CL, Fick‐Brosnahan GM, Johnson AM, Chapman AB, Gabow PA, et al Diuretics versus angiotensin‐converting enzyme inhibitors in autosomal dominant polycystic kidney disease. Am J Nephrol 2001; 21: 98–103. [DOI] [PubMed] [Google Scholar]
- 65. Hacihanefioglu B, Somunkiran A, Mahmutoglu I, Sercelik A, Toptani S, Kervancioglu E. Effect of hypertension therapy with the angiotensin‐converting enzyme inhibitor lisinopril on hyperandrogenism in women with polycystic ovary syndrome. Fertil Steril 2002; 77: 526–528. [DOI] [PubMed] [Google Scholar]
- 66. Reja A, Tesfaye S, Harris ND, Ward JD. Is ACE inhibition with lisinopril helpful in diabetic neuropathy? Diabet Med 1995; 12: 307–309. [DOI] [PubMed] [Google Scholar]
- 67. Solfrizzi V, Scafato E, Frisardi V, Seripa D, Logroscino G, Kehoe PG, et al Angiotensin‐converting enzyme inhibitors and incidence of mild cognitive impairment. The Italian longitudinal study on aging. Age 2013; 35: 441–453. [DOI] [PMC free article] [PubMed] [Google Scholar]


