Abstract
The therapeutic application of cannabis is attracting substantial public and clinical interest. The cannabis plant has been described as a veritable ‘treasure trove’, producing more than 100 different cannabinoids, although the focus to date has been on the psychoactive molecule delta‐9‐tetraydrocannabinol (THC) and cannabidiol (CBD). Other numerous secondary metabolites of cannabis, the terpenes, some of which share the common intermediary geranyl diphosphate (GPP) with the cannabinoids, are hypothesized to contribute synergistically to their therapeutic benefits, an attribute that has been described as the ‘entourage effect’. The effective delivery of such a complex multicomponent pharmaceutical relies upon the stable genetic background and standardized growth of the plant material, particularly if the raw botanical product in the form of the dried pistillate inflorescence (flos) is the source. Following supercritical CO2 extraction of the inflorescence (and possibly bracts), the secondary metabolites can be blended to provide a specific ratio of major cannabinoids (THC : CBD) or individual cannabinoids can be isolated, purified and supplied as the pharmaceutical. Intensive breeding strategies will provide novel cultivars of cannabis possessing elevated levels of specific cannabinoids or other secondary metabolites.
Keywords: cannabinoids, cannabis, flos, flower, terpenoids
Cannabis, a single species?
The earliest physical evidence of Cannabis possessing an elevated level of http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=2424 (THC ), believed to have been cultivated in Eurasia for its psychoactive or pharmacological properties, was unearthed in an excavation of a 2700‐year‐old grave of a Caucasoid shaman 1. There is ongoing debate as to whether the Cannabis genus is made up of one highly variable species (Cannabis sativa L.), two or three species based upon morphological, geographical, ecotypic or chemotypic differences 2, 3, 4, 5. Four evolutionarily distinct ‘groups’ can be recognized, and this more flexible taxonomic terminology [derived from the International Code of Nomenclature for Cultivated Plants (ICNCP) 6] provides a relatively simple and suitable means of labelling domesticated forms of a genus such as Cannabis 5 (Figure 1). Although a construct of strict multiple taxonomic divisions is not supported by empirical evidence of any genetic or physiological barriers impeding cross‐fertilization and subsequent gene flow between accessions or varieties, a single‐nucleotide polymorphism (SNP) analysis of 81 ‘marijuana’ and 43 hemp samples revealed that hemp and ‘marijuana’ lines can in fact be significantly differentiated at a genome‐wide level and not exclusively upon variation in major alleles associated with THC content 7.
Figure 1.
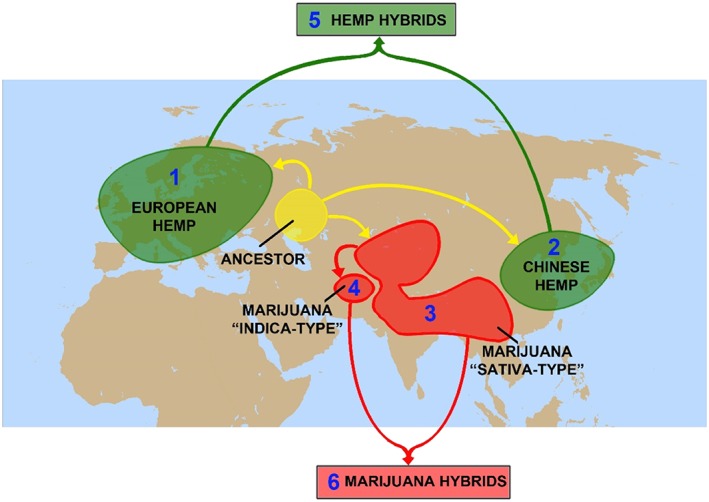
Geographical distribution of the four major domesticated groups 1, 2, 3, 4 of Cannabis sativa, with the centre of origin and ancestral genotype illustrated to be in Central Asia. Significant hybridization, predominantly during the last century, has given rise to two additional groups, hemp and marijuana hybrids (reproduced from 5; ©Government of Canada)
Extraction of the pharmacological cornucopia
Plant secondary metabolites including cannabinoids and terpenoids, so called as they are not critical for plant growth, development and reproduction, are synthesized and stored predominantly in glandular trichomes, hair‐like epidermal protrusions densely concentrated in the bracts and flowers of Cannabis plants. Various strategies have been pursued to extract and deliver the pharmacological agents from Cannabis. The use of chemical solvents such as petroleum ether, ethanol or naptha are likely to leave unwanted residues, whereas extractants such as olive or coconut oil provide a more organic alternative 8.
Cannabinoids and terpenoids contained in the concentrated extract often referred to as ‘oil’ are generally delivered as a medicinal tincture for treatment, although food prepared with the ‘oil’ presents another mode of delivery. The replacement of organic solvents with supercritical CO2 (liquid CO2 under very high pressure) is the method of cannabinoid extraction used to produce the pharmaceutical Sativex®, administered as an oral mucosal spray and licensed in more than 27 countries as a formulation delivering a consistent concentration and at a one‐to‐one ratio of THC : cannabidiol (http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=4150) 9, 10. Pharmaceutical industry and licensing bodies demand a reliable and robust product, more easily achieved by extraction of secondary metabolites from the botanical material and subsequent formulation by blending 10. Cannador® is another such product delivering THC : CBD within a narrow concentration range and at a two‐to‐one ratio, in the form of an orally administered capsule 11. Bedrocan BV, however, the sole supplier of medicinal cannabis to the Dutch government, provides dried, unfertilized female flowers, ‘flos’, as the pharmaceutical product. Heating of the ‘flos’ in a proprietary device at a specific temperature for a defined length of time volatilizes and decarboxylates the cannabinoids, making them available for inhalation. The highly regulated methods of preparation and delivery of Sativex®, Cannador® and Bedrocan materials are also likely to deliver terpenoids as an ancillary and adjunct medicinal product. An ‘entourage effect’, whereby the whole is greater than the sum of the parts, has been hypothesized, in that greater medicinal efficacy results from the delivery of a combination of the cannabinoids and terpenoids 12. However, double‐blind clinical trials have not been conducted on the combination of cannabinoids and terpenes, so evidence remains anecdotal.
The extraction and purification of the single naturally occurring trans isomer of THC is available as Dronabinol, the international nonproprietary name (INN), whereas a synthetic version, Marinol® (Solvay Pharmaceuticals, Brussels, Belgium), is also available, with both pharmaceuticals delivered as a capsule. http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=9071 (Valeant Pharmaceuticals International, Costa Mesa, CA, USA), a synthetic version of THC marketed as binding to the cannabinoid type 1 receptor, is available in a number of countries under the trade name Cesamet® 13. A purified extract of CBD, to be marketed as Epidiolex® (GW Pharmaceuticals, Cambridge, UK), is the subject of a submission to the US Food and Drug Administration.
The medicinal focus to date has been directed at two principal cannabinoids, THC and CBD, although 100 or more are reportedly present in Cannabis 14, 15, 16 and have been described as belonging to 11 different classes, namely: (−)‐delta‐9‐trans‐tetrahydrocannabinol (Δ9‐THC), (−)‐delta‐8‐trans‐tetrahydrocannabinol (Δ8‐THC), cannabigerol (CBG), cannabichromene (CBC), CBD, cannabinodiol (CBND), cannabielsoin (CBE), cannabicyclol (CBL), cannabinol (CBN), cannabitriol (CBT) and miscellaneous‐type cannabinoids (Figure 2). Many of these may only be present in low concentrations, at least in the Cannabis accessions characterized to date, or some may in fact be an artefact of storage, extraction or analysis.
Figure 2.
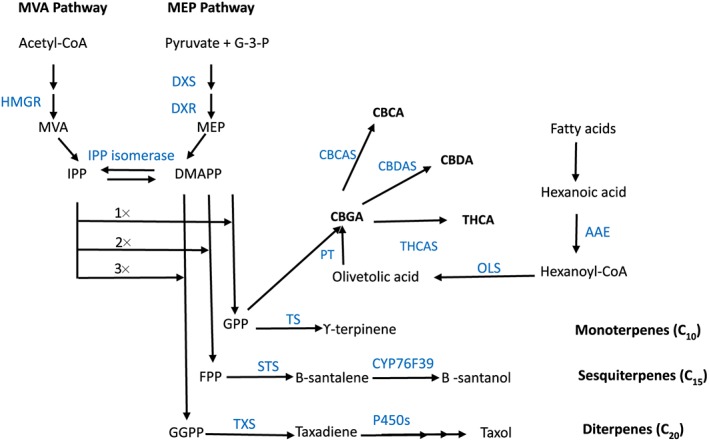
Schematic depiction of cannabinoid and exemplar mono‐, sesqui‐, and diterpenoid biosynthesis. The isoprenoid and prenyl precursors for cannabigerolic acid (CBGA), are provided by the hexanoate and 2‐C‐methyl‐D‐erythritol 4‐phosphate (MEP) pathways, respectively. Geranyl diphosphate (GPP), is a key intermediate metabolite and building block for both cannabinoid and terpenoid biosynthesis. The seven‐step mevalonate (MVA) pathway converts pyruvate and glyceraldehyde‐3‐phosphate (G‐3‐P) into isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). Key catalytic enzymes controlling flux through this pathway include the first two steps, 1‐deoxy‐D‐xylulose 5‐phosphate synthase (DXS) and 1‐deoxy‐D‐xylulose 5‐phosphate reductase (DXR). In the six‐step MEP pathway, three units of acetyl coenzyme A (CoA) are converted to IPP, which is isomerized with DMAPP by IPP isomerase. The enzyme catalysing the synthesis of MEV, 3‐hydroxy‐3‐methylglutaryl‐CoA reductase (HMGR), is considered to control flux through this pathway. The number of consecutive condensations of the five‐carbon monomer isopentenyl diphosphate (IPP) to its isomer, dimethylallyl diphosphate (DMAPP) is indicated by 1x, 2x, 3x. Longer‐chain isoprenoids, GPP, farnesyl diphosphate (FPP) and geranyl geranyl diphosphate (GGPP), are the products of IPP and DMAPP condensation catalysed by GPP synthase, FPP synthase and GGPP synthase, respectively. GPP, FPP and geranyl‐geranyl diphosphate (GGPP) are the precursors for mono‐, sequi‐, and di‐terpines, respectively. The final steps catalysing the synthesis of major active cannabinoids, cannabichromenic acid (CBCA), cannabidiolic acid (CBDA) and Δ9‐tetrahydrocannabinolic acid (THCA), are oxidocyclases, CBCA synthase (CBCAS), CBDA synthase (CBDAS) and THCA synthase (THCAS). Components of Figure 2 are derived from 29. AAE, acyl‐activating enzyme; CBD: cannabidiol; CYP76F39, α/β‐santalene monooxygenase; GPP synthase small subunit; OLS, olivetol synthase; P450: haemoprotein cytochrome P450; PT, prenyltransferase; STS, santalene synthase; TS, gamma‐terpinene synthase; TXS, taxadiene synthase
Creation of novel Cannabis varieties
The introduction of novel traits into commercial cultivars of Cannabis, or other agricultural crops, is reliant upon forward or reverse genetic strategies. Untapped natural genetic diversity harboured in wild accessions and landraces (geographically adapted lines which have not been intensively selected by humans) can be introgressed into elite varieties and represents a forward genetics approach. The reverse genetics approach invokes the use of ionizing radiation or chemical agents to introduce random mutagenic lesions in DNA, thereby creating phenotypic changes. Directed anthropogenic selection and breeding of Cannabis has favoured traits associated with industrial hemp fibre, hemp seed and illicit drug uses. A focused breeding programme, undertaken by GW Pharmaceuticals, UK, has created a series of chemotypes with elevated levels of key cannabinoids including CBD, CBG, CBC as well as the propyl cannabinoids Δ9‐tetrahydrocannabivarin (THCV), Cannabidivarin (CBDV), Cannabigerovarin (CBGV) and Cannabichromevarin (CBCV) 16.
Information about the fragmented global Cannabis germplasm collections is limited, with three major European and a Chinese collection described in published peer‐reviewed literature 17. With a primary focus on the two major cannabinoids, THC and CBD, publicly available information on the range of other cannabinoids and terpenoids in these collections is lacking. Although the sophistication and accuracy of instrumentation required for the measurement of secondary plant metabolites has advanced considerably in recent years, high throughput analysis has been impeded by the lack of purified standards, for the broad array of cannabinoids at least. A recent report has described the positive identification of eight cannabinoids and 36 terpenoids in a single gas chromatographic run 18. A robust, validated method aiming to establish the benchmark for cannabinoid and terpenoid measurement in the USA describes a single‐sample procedure suitable for the extraction and subsequent analysis of cannabinoid and terpenoids by high‐performance liquid chromatography–diode array detector (DAD) and GC‐flame ionization detector (FID), respectively 19.
A mutagenesis strategy employing ethyl methanesulfonate (EMS) has been successfully utilized to modify the seed oil profile of industrial hemp 20, indicating the possible success of a reverse genetics strategy to manipulate the cannabinoid and terpenoid profiles of Cannabis. However, as C. sativa is predominantly a dioecious species, although a small number of hemp accessions or varieties are monoecious, the path towards the generation of a successful commercial variety can be somewhat protracted, as it relies upon the crossing of female lines harbouring superior traits with elite male siblings.
A draft of the complete genome sequence, 534 Mb in size, has been reported for the elevated THC cultivar ‘Purple Kush’. Transcriptome sequences derived from Purple Kush and the hemp cultivar Finola (low THC) exhibit clear expression differences in genes encoding key proteins involved in cannabinoid and precursor biosynthesis 21. Particularly notable was the elevated transcript abundance of the enzyme catalysing Δ9‐tetrahydrocannabinolic acid (THCA) production in all stages of female flower development, THCA synthase (THCAS), in Purple Kush. Finola, characteristically possessing elevated levels of CBD, exhibited elevated transcript levels of cannabidiolic acid (CBDA) synthase and few THCAS transcripts. Although the correlation between synthase transcript and cannabinoid product is not always close 22, 23, the THC : CBD cannabinoid ratio is inherited in accordance with Mendelian principles 24. However, rather than THCA synthase and CBDA synthase being allelic variants of the same locus, it has been proposed that they are linked loci 21, 25.
Impact of growth environment upon secondary metabolism
The overarching and paramount feature of the botanical raw material that constitutes the medicinal Cannabis drug itself, in the form of flos, or from which the standardized multicomponent drug is extracted, is uniformity of the cannabinoid, terpenoid and flavonoid profile. The fundamental driver of the secondary metabolite profile and uniformity is plant genetic makeup, although the growth environment also plays a significant role. The scientific literature addressing the environmental impact upon secondary metabolism and, in particular, the cannabinoids THC and CBD in Cannabis is, unsurprisingly, very limited. The strong likelihood of fungal contamination of the plant or the harvestable inflorescence largely eliminates the possibility of outdoor cultivation if the dried pistillate flower is the principal pharmaceutical product. Hence, glasshouse or indoor cultivation are the preferred options and provide the opportunity for controlling light, temperature and humidity conditions. Furthermore, the ingress of pests and diseases can be controlled by restricting access to the growth facility. However, field‐grown Cannabis may be a suitable source of pharmaceutical cannabinoids if they are extracted using high‐pressure CO2, for example, and good agricultural and manufacturing practices are both observed 26.
Uniformity of plant growth and consistency of cannabinoid and terpenoid profiles are best achieved by vegetatively propagating select cultivars, rather than germinating seed 10. Once established and grown under long‐day conditions to generate a substantial vegetative plant body, flowering is initiated by reducing the day length. The yield of botanical raw material produced per unit area was reported to be linearly proportional to the average irradiance level of the growing environment 10. However, the partitioning of carbohydrate towards primary or secondary metabolites is more likely to be dependent upon the sum total of light energy falling upon the leaf canopy over a defined period of time, rather than the energy level expressed as irradiance per unit area per unit time. Although light is a key factor, nutrient composition and a host of other manipulable environmental factors will influence secondary metabolite concentration and profile in a cultivar‐specific manner.
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY 27.
Competing Interests
The author states explicitly that there are no conflicts of interest in connection with this article. The author is an affiliate of The Australian Centre for Cannabinoid Clinical and Research Excellence, 2018‐2023.
Grof, C. P. L. (2018) Cannabis, from plant to pill. Br J Clin Pharmacol, 84: 2463–2467. 10.1111/bcp.13618.
Footnotes
The major cannabinoids are predominantly found as the acid form in plants, although they are often described in their neutral form, hence ‘cannabidiol’ rather than ‘cannabidiolic acid’.
References
- 1. Russo EB, Jiang HE, Li X, Sutton A, Carboni A, Del Bianco F, et al Phytochemical and genetic analyses of ancient cannabis from Central Asia. J Exp Bot 2008; 59: 4171–4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schultes RE, Klein WM, Plowman T, Lockwood TE. Cannabis: an example of taxonomic neglect. Bot Mus Leafl Harv Univ 1974; 23: 337–367. [Google Scholar]
- 3. Small E, Cronquist A. A practical and natural taxonomy for cannabis. Taxon 1976; 25: 405–435. [Google Scholar]
- 4. Clarke RC, Merlin MD. Cannabis: Evolution and Ethnobotany. Los Angeles: University of California Press, 2013. [Google Scholar]
- 5. Small E. Evolution and classification of Cannabis sativa (marijuana, hemp) in relation to human utilization. Bot Rev 2015; 81: 189–294. [Google Scholar]
- 6. Brickell CD, Alexander C, Cubey JJ, David JC, Hoffman MHA, Leslie AC, et al International code of nomenclature for cultivated plants [online]. International Society for Horticultural Science. 2016. Available from: https://www.ishs.org/sites/default/files/static/ScriptaHorticulturae_18.pdf (last accessed 27 February 2018).
- 7. Sawler J, Stout JM, Gardner KM, Hudson D, Vidmar J, Butler L, et al The genetic structure of marijuana and hemp. PLoS One 2015; 10: e0133292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Romano LL, Hazekamp A. Cannabis oil: chemical evaluation of an upcoming cannabis‐based medicine. Cannabinoids 2013; (1): 1–11. [Google Scholar]
- 9. Russo EB. Current therapeutic cannabis controversies and clinical trial design issues. Front Pharmacol 2016; 7: 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potter DJ. A review of the cultivation and processing of cannabis (Cannabis sativa L.) for production of prescription medicines in the UK. Drug Test Anal 2014; 6: 31–38. [DOI] [PubMed] [Google Scholar]
- 11. Russo E, Guy GW. A tale of two cannabinoids: the therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med Hypotheses 2005; 66: 234–246. [DOI] [PubMed] [Google Scholar]
- 12. Russo EB. Taming THC: potential cannabis synergy and phytocannabinoid‐terpenoid entourage effects. Br J Pharmacol 2011; 163: 1344–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kowal MA, Hazekamp A, Grotenhermen F. Review on clinical studies with cannabis and cannabinoids 2010–2014. Cannabinoids 2016; 11: 1–18. [Google Scholar]
- 14. Brenneisen R. Chemistry and analysis of phytocannabinoids and other Cannabis constituents. Forensic Sci Med Marijuana Cannabinoids 2007; 7: 17–49. [Google Scholar]
- 15. Mehmedic Z, Chandra S, Slade D, Denham H, Foster S, Patel AS, et al Potency trends of Δ9‐THC and other cannabinoids in confiscated cannabis preparations from 1993 to 2008. J Forensic Sci 2010; 55: 1209–1217. [DOI] [PubMed] [Google Scholar]
- 16. Pertwee RG. Handbook of Cannabis. Oxford: Oxford University Press, 2014. 10.1093/acprof:oso/9780199662685.001.0001 [DOI] [Google Scholar]
- 17. Welling MT, Shapter T, Rose TJ, Liu L, Stanger R, King GJ. A belated green revolution for Cannabis: virtual genetic resources to fast‐track cultivar development. Front Plant Sci 2016; 7: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hazekamp A, Tejkalová K, Papadimitriou S. Cannabis: from cultivar to chemovar II – a metabolomics approach to Cannabis classification. Cannabis Cannabinoid Res 2016; (1): 202–215. [Google Scholar]
- 19. Giese MW, Lewis MA, Giese L, Smith KM. Development and validation of a reliable and robust method for the analysis of cannabinoids and terpenes in cannabis. J AOAC Int 2015; 98: 1503–1522. [DOI] [PubMed] [Google Scholar]
- 20. Bielecka M, Kaminski F, Adams I, Poulson H, Sloan R, Li Y, et al Targeted mutation of Δ12 and Δ15 desaturase genes in hemp produce major alterations in seed fatty acid composition including a high oleic hemp oil. Plant Biotechnol J 2014; 12: 613–623. [DOI] [PubMed] [Google Scholar]
- 21. Bakel V, Van Bakel H, Stout JM, Cote AG, Tallon CM, Sharpe AG, et al The draft genome and transcriptome of Cannabis sativa . Genome Biol 2011; 12: R102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Onofri C, De Meijer EPM, Mandolino G. Sequence heterogeneity of cannabidiolic‐ and tetrahydrocannabinolic acid‐synthase in Cannabis sativa L. and its relationship with chemical phenotype. Phytochemistry 2015; 116: 57–68. [DOI] [PubMed] [Google Scholar]
- 23. Cascini F, Passerotti S, Boschi I. Analysis of THCA synthase gene expression in cannabis: a preliminary study by real‐time quantitative PCR. Forensic Sci Int 2013; 231: 208–212. [DOI] [PubMed] [Google Scholar]
- 24. De Meijer EPM, Bagatta M, Carboni A, Crucitti P, Moliterni VMC, Ranalli P, et al The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003; 163: 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weiblen GD, Wenger JP, Craft KJ, ElSohly MA, Mehmedic Z, Treiber EL, et al Gene duplication and divergence affecting drug content in Cannabis sativa . New Phytol 2015; 208: 1241–1250. [DOI] [PubMed] [Google Scholar]
- 26. Chandra S, Lata H, ElSohly MA, Walker LA, Potter D. Cannabis cultivation: methodological issues for obtaining medical‐grade product. Epilepsy Behav 2017; 70: 302–312. [DOI] [PubMed] [Google Scholar]
- 27. Harding SD, Sharman JL, Faccenda E, Southan C, Pawson AJ, Ireland S, et al The IUPHAR/BPS guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acid Res 2018; 46: D1091–D1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alexander SPH, Christopoulos A, Davenport AP, Kelly E, Marrion NV, Peters JA, et al The Concise Guide to PHARMACOLOGY 2017/18: G protein‐coupled receptors. Br J Pharmacol 2017; 174: S17–S129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ikram NKBK, Zhan X, Pan X‐W, King BC, Simonsen HT. Stable heterologous expression of biologically active terpenoids in green plant cells. Front Plant Sci 2015; 6: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]


