Abstract
Altered brain development is a common feature of the neurological sequelae of complex congenital heart disease (CHD). These alterations include abnormalities in brain size and growth that begin prenatally and persist postnatally. However, the longitudinal trajectory of changes in brain volume from the prenatal to postnatal environment have not been investigated. We aimed to evaluate the trajectory of brain growth in a cohort of patients with complex CHD (n = 16) and healthy controls (n = 15) to test the hypothesis that patients with complex CHD would have smaller total brain volume (TBV) prenatally, which would become increasingly prominent by three months of age. Participants underwent fetal magnetic resonance imaging (MRI) at a mean of 32 weeks gestation, a preoperative/neonatal MRI shortly after birth, a postoperative MRI (CHD only), and a 3-month MRI to evaluate the trajectory of brain growth. Three-dimensional volumetric analysis was applied to the MRI data to measure TBV, as well as tissue-specific volumes of the cortical gray matter (CGM), white matter (WM), subcortical (deep nuclear) gray matter (SCGM), cerebellum, and cerebrospinal fluid (CSF). A random coefficients model was used to investigate longitudinal changes in TBV and demonstrated an altered trajectory of brain growth in the CHD population. The estimated slope for TBV from fetal to 3-month MRI was 11.5 cm3 per week for CHD infants compared to 16.7 cm3 per week for controls (p = 0.0002). Brain growth followed a similar trajectory for the CGM (p < 0.0001), SCGM (p = 0.002), and cerebellum (p = 0.005). There was no difference in growth of the WM (p = 0.30) or CSF (p = 0.085). Brain injury was associated with reduced TBV at 3-month MRI (p = 0.02). After removing infants with brain injury from the model, an altered trajectory of brain growth persisted in CHD infants (p = 0.006). These findings extend the existing literature by demonstrating longitudinal impairments in brain development in the CHD population and emphasize the global nature of disrupted brain growth from the prenatal environment through early infancy.
Keywords: Brain volume, Magnetic resonance imaging, Congenital heart disease, Fetal
Highlights
-
•
Pre- to postnatal trajectory of brain growth is altered in congenital heart disease.
-
•
These alterations resulted in a 20% reduction in total brain volume.
-
•
Cortical gray matter, subcortical gray matter, and cerebellum are affected.
1. Introduction
The key pathways to adverse neurological outcomes in infants with congenital heart disease (CHD) continue to be investigated in order to frame approaches for neuroprotection. Such insights require an understanding of the nature, timing, and neurobiological underpinnings of not only brain injury, but also alterations in brain development. To date, cohort studies have identified that alterations in brain development begin prenatally and include impairments in brain volume (Limperopoulos et al., 2010; Sun et al., 2015). These volumetric deficits exist before cardiac surgery, persist postoperatively, and are even present into adolescence and adulthood (Cordina et al., 2014; Rollins et al., 2017; von Rhein et al., 2015; 2014; Heye et al., 2018). Importantly, reductions in tissue- and region-specific brain volumes have been associated with adverse neurodevelopmental outcomes in cognitive, motor, language, and executive function domains (Rollins et al., 2017; von Rhein et al., 2014; Heye et al., 2018; Latal et al., 2016).
Despite evidence of reduced brain volume in CHD patients across multiple stages of childhood development, there are limited data investigating longitudinal brain growth within a single cohort. This type of analysis may provide information regarding the timing of total and tissue-specific alterations in brain volume, which may lend insight to the underlying pathophysiologic processes and/or critical period(s) for neurologic risk. Our laboratory has previously performed repeated two-dimensional (2D) MRI measures of brain size in infants with CHD and identified preoperative deficits across multiple regions, which persisted at three months of age (Ortinau et al., 2012a; 2012b). While these biometric methods can be readily applied at the bedside, they are limited to 2D global brain region measurements, as opposed to three-dimensional (3D) volumetric methods, which can also generate specific tissue-type measures (Gholipour et al., 2011). Furthermore, 2D biometry has variable correlation with 3D volumetry, depending on whether global or tissue-specific measures are being evaluated (Kyriakopoulou et al., 2017; Nguyen The Tich et al., 2009). Thus, application of 3D volumetric methods provide more detailed tissue assessments that may be more relevant for untangling the neurobiological processes of altered brain development in CHD.
The only studies to date that have investigated longitudinal 3D brain volumes in infants with CHD have focused on postnatal comparisons. These data have demonstrated diminished total brain growth perioperatively, over two weeks, in infants with hypoplastic left heart syndrome (HLHS) compared to infants with transposition of the great arteries (TGA) (Peyvandi et al., 2018a). MRI at approximately one year of age demonstrated smaller total brain volume (TBV) in children with single ventricle physiology or TGA when compared to controls. At three years of age, these deficits only persisted in those children with single ventricle physiology (Ibuki et al., 2012). While fetal imaging in CHD has clearly suggested a disruption in brain development prenatally, no data have evaluated the progression of brain volume from the fetal to postnatal environment. Additionally, the interplay between brain development and brain injury pre- and post-natally has yet to be fully defined.
This study was a pilot investigation that aimed to extend the existing literature by determining the trajectory of brain growth in patients with complex CHD, beginning during pregnancy and continuing through the perioperative period and into early infancy. We hypothesized that the CHD population would have smaller TBV than the control population, that the volume difference would exist prenatally, and that volume reduction would become more prominent by three months of age. We also hypothesized that brain injury would be associated with smaller TBV by three months of age.
2. Materials and methods
2.1. Patient population
Pregnant women with a known diagnosis of fetal complex CHD were recruited from the Fetal Care Center at Barnes Jewish Hospital/St. Louis Children's Hospital from 2012 to 2015. Specific lesions targeted for recruitment included HLHS, dextro-transposition of the great arteries (d-TGA), pulmonary atresia (PA), tetralogy of Fallot (TOF), double outlet right ventricle (DORV), truncus arteriosus, and complex single ventricle physiology. The control population included pregnant women cared for in the Obstetrics Clinic at Barnes Jewish Hospital who had an otherwise healthy pregnancy. These women were approximately matched to the CHD population on fetal gestational age (GA) at MRI, fetal sex, and maternal race. Exclusion criteria included fetal diagnosis of a genetic syndrome or chromosomal abnormality known to affect clinical outcome, congenital anomalies (outside of CHD for the study population), suspected or proven congenital infection, or multiple gestation pregnancy. The local Institutional Review Board approved all aspects of the study. Adult participants provided informed, written consent for prenatal study evaluations and data collection. Both parents provided informed, written consent for infant postnatal study evaluations and data collection.
2.2. Demographic and clinical variables
Demographic and clinical variables were collected after informed consent was obtained. Demographic variables included maternal age, maternal and paternal race, and infant sex. Clinical variables included pregnancy, delivery, and hospitalization characteristics. Pregnancy data included co-morbid conditions and new pregnancy diagnoses. Delivery characteristics included mode and indication for delivery, Apgar scores, and delivery complications. GA at birth and anthropometric measures at birth and at each MRI were also collected. Hospitalization variables for CHD subjects included medical variables related to the CHD diagnosis (i.e., need for preoperative prostaglandins or atrial septostomy), cardiac surgical data, extracorporeal life support, length of hospital stay, and survival.
2.3. Magnetic resonance imaging
Brain MRI was performed at four time points for the CHD population and at three time points for the control population. These included: 1) a fetal brain MRI performed during the second or third trimester of pregnancy for both groups, 2) a neonatal brain MRI that occurred preoperatively for CHD subjects and within the first week of life for control subjects, 3) a postoperative MRI for CHD subjects only, and 4) a 3-month MRI for all subjects. The preoperative and postoperative MRIs were used to evaluate for perioperative brain injury in the CHD population. Two raters experienced in neonatal neuroimaging (J.S. and C.S.) who were blinded to the subject's group and clinical history reviewed each MRI and a consensus was formed for presence of brain injury and qualitative abnormalities in brain development. Brain injury was defined as white matter injury, intraventricular hemorrhage, hemorrhagic or ischemic infarct, or other hemorrhage (i.e., cerebellar hemorrhage). A standardized scoring system was applied to evaluate the severity of white matter injury (minimal, moderate, or severe) and to calculate an overall brain injury severity score (0–3) (Dimitropoulos et al., 2013; McQuillen et al., 2007). Qualitative abnormalities in brain development included increased extra-axial space, open Sylvian Fissure, and delayed myelination patterns.
2.3.1. Fetal magnetic resonance imaging acquisition
Pregnant women underwent fetal MRI on a 1.5 Tesla Magnetom Avanto (Siemens Healthcare, Erlangen, Germany) without sedation. The acquisition parameters included a T2 half-fourier acquisition single-shot turbo spin-echo (HASTE) sequence acquired in the axial, coronal, and sagittal planes with a field of view (FOV) of 320 millimeters (mm), repetition time (TR) of 1450 milliseconds (ms), echo time (TE) of 140 ms, flip angle of 180°, and slice thickness of 3.0 mm. To address the possibility of fetal motion and improve the success of volumetric reconstruction, multiple acquisitions were acquired in each plane (Kuklisova-Murgasova et al., 2012; Gholipour et al., 2010). The MR scanner and acquisition protocol utilized were identical for both the CHD and control groups.
2.3.2. Postnatal magnetic resonance imaging acquisition
Postnatal imaging (preoperative, postoperative, and 3-month MRIs) was performed on a 3-Tesla Magnetom Trio (Siemens Healthcare, Erlangen, Germany) using the same scanner and protocol for the CHD and control groups. Infants underwent MRI without sedation. If clinically appropriate, they were fed prior to the MRI and were prepared and wrapped using a Med-Vac fixation device (Mathur et al., 2008). Acquisition parameters for volumetric analysis included a T1 magnetization prepared rapid gradient echo (MP-RAGE) sequence with a FOV of 144 × 192 mm, TR of 1550 ms, TE of 3.05 ms, inversion time (TI) of 1100 ms, flip angle of 15°, and voxel resolution of 1.0 × 1.0 × 1.3 mm3. A T2 fast spin echo sequence was also acquired with a FOV of 144 × 192 mm, TR of 7000–8210 ms, TE of 161 ms, flip angle of 110–120°, and voxel resolution of 1.0 × 1.0 × 1.0 mm3.
2.3.3. Post-processing methods
For the fetal MRI data, an isotropic, high-resolution volume reconstruction was generated at 0.75 cubic mm from the multiple scan slice acquisitions outlined above. This methodology incorporates an inter-slice motion correction that has been shown to be accurate and robust for fetal imaging (Kuklisova-Murgasova et al., 2012; Gholipour et al., 2010; Kainz et al., 2015). A spatiotemporal fetal brain MRI atlas was then used to segment key tissue-types within the fetal brain (Gholipour et al., 2017). To maintain consistency from fetal to postnatal post-processing analyses, labels from the automated fetal segmentation were combined using ITK-SNAP tools (Yushkevich et al., 2006) to generate final segmentations of the cortical gray matter (CGM), white matter (WM), subcortical (deep nuclear) gray matter (SCGM), cerebellum, and cerebrospinal fluid (CSF). The SCGM included the basal ganglia, thalamus, hippocampus, and amygdala. For the postnatal MR data, automated segmentations were generated using the Advanced Normalization Toolkit (ANTS) (Avants et al., 2011) to provide segmentations of the same tissue-types as above. All fetal and postnatal segmentations were then manually modified in ITK-SNAP by staff blinded to clinical status. Segmentations were modified using the T2-weighted images, initially in the coronal plane, from posterior to anterior regions of the brain. Additional modifications were subsequently performed in the axial and sagittal planes while referencing the coronal segmentation. The majority of the manual modifications adjusted overestimations of the cerebellum, corrected misclassified hyperintense WM regions, and delineated the WM region between the basal ganglia and insular cortex. Final segmentations were reviewed by a single rater (D.A.) blinded to group to ensure the accuracy of segmentation results and consistent data quality. Fig. 1 demonstrates examples of the final segmentations. Using the manually modified segmentation, measurements were calculated for each tissue-type. The CGM, WM, SCGM, and cerebellar volumes were then combined to determine TBV.
Fig. 1.
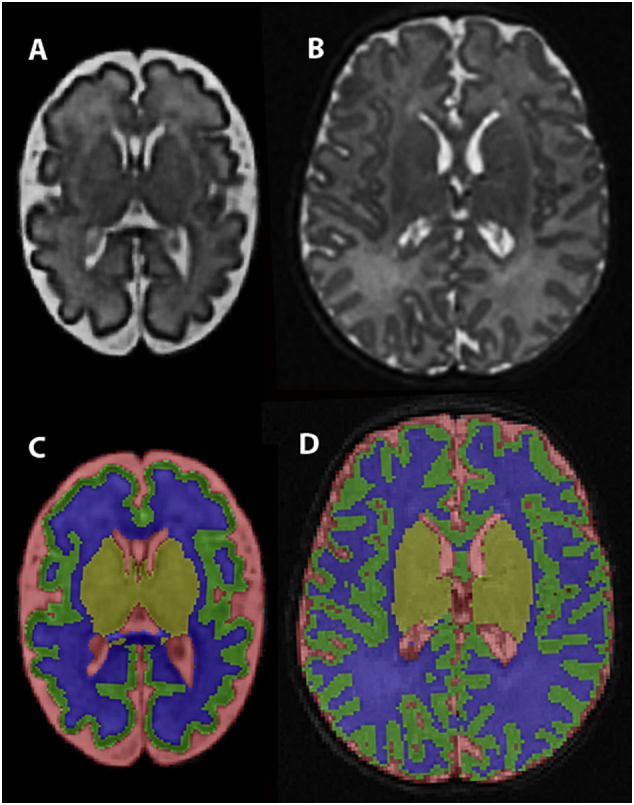
Segmentation for Volumetric Analysis. The T2-weighted fetal volumetric reconstruction (A) and the T2-weighted postnatal raw images (B) were used to generate tissue segmentations for the fetal and postnatal images, respectively (C and D). Green represents cortical gray matter (CGM), blue represents white matter (WM), yellow represents subcortical gray matter (SCGM), and red represents cerebrospinal fluid. The cerebellar segmentation is not shown in these images.
2.4. Statistical analysis
Statistical analyses were conducted with IBM SPSS Statistics for Windows, Version 25.0 (Armonk, NY: IBM Corp.) and SAS software, Version 9.4 (Cary, NC, USA). Demographic and clinical variables were compared between groups using Pearson's Chi-square or Fisher's Exact Test, as appropriate, for categorical variables and two-sample independent t-tests or Mann-Whitney U tests (for non-normally distributed data) for continuous variables. A random coefficients model was created to compare the growth of TBV from fetal to 3-month MRI between the CHD and control groups. Gestational/postmenstrual age at MRI (defined herein as GA at MRI for the statistical model), study group, and the interaction between the two were fixed effects, and subject and GA at MRI were the random coefficients. Because of a non-significant, but likely clinically important group difference in birth weight, this variable was included as a covariate in the model. Raw values, as opposed to z-scores, were used for anthropometric measures because the statistical model included GA at each MRI and brain volume had greater correlations with raw weight values than with z-scores (data not shown). Fetal/infant sex was not included in the model because this variable was matched between groups. The random coefficients model was repeated after removing infants with brain injury to determine the impact of injury on trajectory of brain growth.
Subanalyses were performed for each tissue-type using the same random coefficients model structure described above. To determine whether volumetric differences were present at each of the three MRI time points available for both groups, least square mean estimates of TBV were calculated and compared at the mean GA of all subjects at each time point.
Spearman correlations and Mann-Whitney U tests were performed to assess the association of clinical factors with TBV. Results are reported as the Spearman correlation coefficient (rs), which ranges from −1 to +1, with 0 representing no association. Mann-Whitney U tests are reported with group medians. These investigations were limited to the 3-month MRI because the greatest differences in TBV occurred at this time point and would be reflective of the entire clinical course. Because of the small sample size, a limited selection of clinical variables shown to affect brain volume in CHD patients or with biological plausibility were investigated. These variables included hospital length of stay, presence of brain injury, HLHS diagnosis, and weight measurements (Ortinau et al., 2012a; Peyvandi et al., 2018a; International Cardiac Collaborative on Neurodevelopment, 2016). Associations were only performed in the CHD group because the variables tested were all confounded by the diagnosis of CHD. Nonparametric methods were chosen for these analyses to be more conservative and relax the assumption of normality, given the sample size and distribution of the data. A multivariable linear regression model was explored using these variables; however, several variables were collinear and no model performed better than a univariate model. Due to the pilot nature of the study and the relatively small sample size, no experiment-wise correction for multiple tests was performed.
3. Results
3.1. Cohort characteristics
Thirty-four patients, including 16 CHD and 18 healthy controls, were enrolled and underwent at least one research MRI. One control subject was excluded from analysis for a postnatal diagnosis of a small ventricular septal defect, microcephaly, and hypotonia that prompted admission to the Neonatal Intensive Care Unit. Two control subjects underwent fetal MRI, but their imaging data could not be reconstructed for volumetric analysis, and both subjects subsequently withdrew from the postnatal study visits. Thus, the final cohort included 16 CHD and 15 control subjects who had volumetric measures (Fig. 2, Table 3). Pregnancy, delivery, and hospitalization characteristics of the cohort are displayed in Table 1. Compared to controls, CHD infants were more likely to have an earlier GA at birth, a smaller length and head circumference at birth, a longer hospital length of stay, an earlier postmenstrual age at preoperative and 3-month MRI, a lower weight at 3-month MRI, and a smaller change in weight from birth to 3-month MRI. One CHD infant was delivered preterm at 33 weeks gestation. All other CHD and control infants delivered at ≥37 weeks gestation. Cardiac diagnoses and surgical procedures for the CHD group are displayed in Table 2.
Fig. 2.
Flow Diagram of Study Participants. Numerators represent successful volumetric analysis, whereas the denominators represent participants eligible for each MRI.
Table 3.
Brain injury/abnormalities in CHD subjects.
| Subject | Fetal MRI | Preoperative MRI | Postoperative MRI | 3-month MRI | BIS score |
|---|---|---|---|---|---|
| 1d | None | – | Bilateral moderate WMI, bilateral mild ventriculomegaly, increased extra-axial spaces and open Sylvian Fissures (greatest on right) | Mild bilateral ventriculomegaly, increased extra-axial spaces and open Sylvian Fissures (greatest on right) | 3 |
| 2 | – | – | Open Sylvian Fissures | None | 0 |
| 3 | Nonea | – | None | None | 0 |
| 4 | None | None | Mild bilateral ventriculomegaly | Mild bilateral ventriculomegaly | 0 |
| 5 | None | Bilateral moderate WMI, bilateral basal ganglia infarcts, bilateral grade II IVH | Bilateral moderate WMI, bilateral ventriculomegaly, left basal ganglia infarct | Bilateral moderate WMI, bilateral ventriculomegaly, residual left basal ganglia hemorrhage | 3 |
| 6 | None | – | Died | Died | N/A |
| 7d | None | – | – | Left acute and chronic frontotemporal subdural hematoma with adjacent mass effect >5% of hemisphere, bilateral ventriculomegaly, open Sylvian Fissures | 3c |
| 8b | Nonea | None | – | None | 0 |
| 9 | None | – | – | – | N/A |
| 10b | None | None | – | Nonea | 0 |
| 11b | Nonea | None | – | None | 0 |
| 13 | Nonea | None | – | None | 0 |
| 14 | Nonea | – | Bilateral mild WMI, left grade II and right grade I IVH, and open Sylvian Fissures | – | 1 |
| 15 | Nonea | – | Small right frontal cortical infarct | Mild bilateral ventriculomegaly | 2 |
| 17 | None | – | – | None | N/A |
| 18d | None | None | None | None | 0 |
(−) Subjects unable to undergo MRI at that time point. BIS = brain injury severity, IVH = intraventricular hemorrhage, N/A = not applicable because perioperative MRI data not available, WMI = white matter injury.
MRIs with motion artifact precluding volumetric analysis.
Cardiac surgery was after the 3-month MRI for these subjects.
3-month MRI was used for BIS score because cardiac surgery was 15 days prior to the 3-month MRI for this subject.
Preoperative atrial septostomy occurred in these subjects. Subject 18 had the septostomy before the preoperative MRI.
Table 1.
Characteristics of the cohort.
| CHD (n = 16) | Control (n = 15) | P value | |
|---|---|---|---|
| Pregnancy characteristics | |||
| Maternal age | 27.2 (5.8) | 29.0 (5.7) | 0.39 |
| Maternal race | 0.33 | ||
| Caucasian | 15 (93.8) | 15 (100) | |
| African American | 1 (6.3) | 0 (0) | |
| Other | 0 (0) | 0 (0) | |
| Paternal race | 0.21 | ||
| Caucasian | 13 (81.2) | 15 (100) | |
| African American | 1 (6.3) | 0 (0) | |
| Other | 2 (12.5) | 0 (0) | |
| Maternal asthma | 3 (18.8) | 1 (6.7) | 0.60 |
| Pre-pregnancy or gestational DM | 4 (25.0) | 0 (0) | 0.10 |
| Gestational HTN or pre-eclampsia | 2 (12.5) | 0 (0) | 0.48 |
| Maternal Hypothyroidism | 1 (6.3) | 1 (6.7) | 1.00 |
| Delivery characteristics | |||
| Mode of delivery | 0.34 | ||
| Vaginal | 12 (80.0) | 13 (86.7) | |
| Non-emergent cesarean section | 1 (6.7) | 2 (13.3) | |
| Emergent cesarean section | 2 (13.3) | 0 (0) | |
| Apgar Score at 1 mina | 8 (7–8) | 8 (8–8) | 0.29 |
| Apgar score at 5 mina | 8 (8–9) | 9 (9–9) | 0.074 |
| Gestational age at birth, wksa | 38.9 (37.3–39.0) | 39.7 (38.9–40.3) | 0.002† |
| Birthweight, g | 3062 (722) | 3385 (470) | 0.16 |
| Birth length, cm | 48.4 (3.4) | 50.9 (2.4) | 0.03† |
| Birth head circumference, cm | 33.3 (1.4) | 34.4 (1.2) | 0.02† |
| Infant sex, male | 10 (62.7) | 8 (53.3) | 0.71 |
| Postnatal characteristics | |||
| Preoperative prostaglandins | 11 (73.3) | – | – |
| Preoperative atrial septostomy | 3 (20.0) | – | – |
| Age at surgery, daysa | 11 (3–83) | – | – |
| Cardiopulmonary bypass | 12 (80.0) | – | – |
| Cardiopulmonary bypass time, min | 135 (49.1) | – | – |
| Cross-clamp time, min | 82 (44.4) | – | – |
| Deep hypothermic circulatory arrest | 7 (46.7) | – | – |
| Deep hypothermic circulatory arrest time, min | 80 (57.6) | – | – |
| Extracorporeal life support | 2 (13.3) | – | – |
| Length of hospital stay, daysa | 32.0 (13.0–49.0) | 2.0 (2.0–3.5) | <0.001† |
| Died | 3 (20.0) | 0 (0) | 0.23 |
| MRI characteristics | |||
| GA at fetal MRI, wks | 32.7 (2.5) | 32.7 (3.7) | 0.99 |
| PMA at preoperative MRI, wks | 38.8 (0.7) | 40.3 (0.7) | 0.001† |
| Weight at preoperative MRI, g | 3570 (540) | 3574 (334) | 0.99 |
| PMA at postoperative MRI, wks | 42.6 (2.3) | – | – |
| Weight at postoperative MRI, g | 3352 (636) | – | – |
| PMA at 3-month MRI, wks | 51.0 (1.8) | 54.2 (1.2) | <0.001† |
| Weight at 3-month MRI, g | 4721 (698) | 5977 (557) | 0.001† |
| Change in weight, birth to 3-month MRI, g | 1650 (807) | 2637 (710) | 0.01† |
Data are presented as mean (SD) or number (percentage), unless otherwise noted. Delivery and hospitalization characteristics are reported for 15 CHD subjects, as one subject transferred care to her local hospital prior to delivery. MRI characteristics were for those subjects with usable volumetric data. (−) Represents variables that were not applicable to the control population and could not be compared between groups. DM = diabetes mellitus, GA = gestational age, HTN = hypertension, PMA = postmenstrual age.
Data are displayed as median (interquartile range).
p < 0.05.
Table 2.
Cardiac diagnoses and surgical procedures for CHD subjects.
| Cardiac diagnosis | Primary corrective or palliative procedure | |
|---|---|---|
| Subject 1 | HLHS, MS, AA, with LV sinusoids | Hybrid procedure with bilateral pulmonary artery banding and stenting of the PDA |
| Subject 2 | HLHS, MS, AS | Norwood with Sano modification |
| Subject 3 | HLHS, MA, AA, restrictive atrial septum | Norwood with Sano modification |
| Subject 4 | HLHS, MA, AA | Norwood with Sano modification |
| Subject 5 | d-TGA with restrictive atrial septum | Arterial switch operation and primary closure of muscular VSD |
| Subject 6 | HLHS, MA, AA | Norwood with Sano modification |
| Subject 7 | PA with small VSD, hypoplastic TV and RV with overriding aorta, moderate to large ASD, bicupsid aortic valve | Orthotopic heart transplant |
| Subject 8 | TOF with a small pulmonary valve annulus | Tetralogy of Fallot repair |
| Subject 9 | HLHS, MA, AA | Unknowna |
| Subject 10 | DORV with d-TGA, ASD, VSD | Rastelli Procedure |
| Subject 11 | Double-inlet left ventricle, hypoplastic right ventricle, L-TGA | Damus-Kaye-Stansel Procedure and modified bidirectional Glenn |
| Subject 13 | TOF/PA | Modified BT shunt |
| Subject 14 | PA/IVS with severe tricuspid stenosis | Modified BT shunt |
| Subject 15 | HLHS, MS, AS, restrictive atrial septum | Norwood with Sano modification |
| Subject 17 | TOF without pulmonary stenosis | Tetralogy of Fallot repair |
| Subject 18 | d-TGA/IVS with restrictive atrial septum | Arterial switch operation |
AA = aortic atresia, AS = aortic stenosis, ASD = atrial septal defect, BT = Blalock-Taussig, DORV = double outlet right ventricle, d-TGA = dextro-transposition of the great arteries, HLHS = hypoplastic left heart syndrome, IVS = intact ventricular septum, L-TGA = levo-transposition of the great arteries, LV = left ventricle, MA = mitral atresia, MS = mitral stenosis, PA = pulmonary atresia, PDA = patent ductus arteriosus, RV = right ventricle, TOF = tetralogy of Fallot, TV = tricuspid valve, VSD = ventricular septal defect.
This subject transferred care back to her local hospital prior to delivery.
3.2. Magnetic resonance imaging
3.2.1. Brain injury
There was no brain injury noted on any MRI scans for the control population. There was one control infant whose fetal and neonatal MRIs were normal, but the 3-month MRI showed mild ventricular prominence with mild enlargement of the subarachnoid spaces. All volumetric measures for this subject were within two standard deviations of the mean for the control population, therefore this patient was included in the cohort. For the CHD population, there was no evidence of brain injury or qualitative abnormalities in brain development on any fetal MRI. Of CHD infants who underwent postnatal imaging, preoperative or postoperative brain injury was present in 36% (5/14). Four of these subjects had a brain injury severity score of 2–3, two with moderate white matter injury, one with infarct, and one with hemorrhage causing mass effect of >5% of the hemisphere (Table 3). Qualitative abnormalities in brain development were noted in 29% (4/14) (Table 3). Representative longitudinal images of CHD patients with and without injury are displayed in Supplementary Fig. 1.
3.2.2. Volumetric analysis
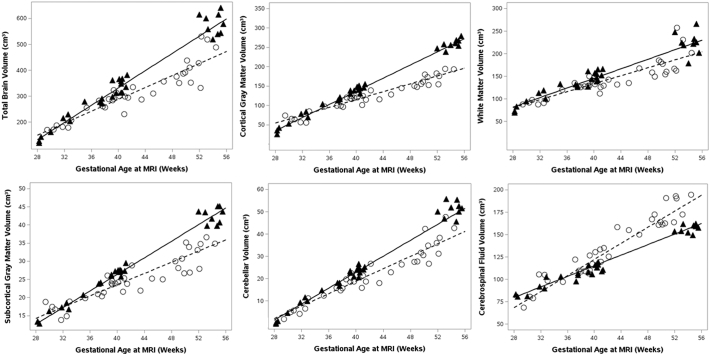
Of the 31 subjects included in the study, three had useable volumetric data at four time points, eight at three time points, eleven at two time points, and nine at one time point (Fig. 2, Table 3). The random coefficients model demonstrated an association between GA at MRI and TBV, where TBV increased as GA increased (p < 0.0001) (Fig. 3). Birth weight neared significance with an estimated slope of 0.02 cm3 per one gram increase (p = 0.06). Controlling for GA at MRI and birth weight, there was a significant interaction between group and GA at MRI, such that CHD infants had an 11.5 cm3 increase in TBV per week compared to a 16.7 cm3 increase for controls (p = 0.0002) (Fig. 3).
Fig. 3.
Trajectory of Brain Volumes. Data points and fit lines represent the random coefficients model results for the CHD (open circles, dashed line) and control (solid triangles, solid line) groups. The regression equations are as follows (BW = birth weight): TBV CHD = −388.08 + (0.02 × BW) + 162.82 + (11.49 × GA at MRI), TBV control = −388.08 + (0.02 × BW) + (16.66 × GA at MRI), CGM CHD = −248.97 + (0.01 × BW) + 115.21 + (5.07 × GA at MRI), CGM control = −248.97 + (0.01 × BW) + (8.47 × GA at MRI), WM CHD = −101.05 + (0.01 × BW) + 23.73 + (4.41 × GA at MRI), WM control = −101.05 + (0.01 × BW) + (5.30 × GA at MRI), SCGM CHD = −25.96 + (0.002 × BW) + 12.00 + (0.77 × GA at MRI), SCGM control = −25.96 + (0.002 × BW) + (1.14 × GA at MRI), cerebellum CHD = −52.03 + (0.001 × BW) + 11.70 + (1.40 × GA at MRI), cerebellum control = −52.03 + (0.001 × BW) + (1.79 × GA at MRI), CSF CHD = 33.15 – (0.01 × BW) – 54.5 + (1.41 × GA at MRI), CSF control = 33.15 – (0.01 × BW) + (2.95 × GA at MRI).
Subanalyses were performed for all tissues-types to determine which brain tissues were contributing to the difference in TBV over time and are reported here as the estimated slopes per week in CHD versus control infants. These analyses showed a significant interaction between group and GA at MRI, such that infants with CHD had a smaller slope, for the CGM (5.1 cm3 versus 8.5 cm3, p < 0.0001), the SCGM (0.8 cm3 versus 1.1 cm3, p = 0.002), and the cerebellum (1.4 cm3 versus 1.8 cm3, p = 0.005). There was no group difference in slope for the WM (4.4 cm3 versus 5.3 cm3, p = 0.30) or the CSF (4.5 cm3 versus 3.0 cm3, p = 0.085) (Fig. 3).
Specific to each MRI time point, total and tissue-specific volumes at fetal, preoperative, and 3-month MRIs were compared between groups using least square mean estimates from the random coefficients model at the mean GA of each MRI time point. There was no group difference in TBV at fetal MRI, but CHD infants did have smaller TBV at the preoperative (p < 0.001) and 3-month MRI (p = 0.0001) (Table 4). The CGM, SCGM, and cerebellum displayed a similar pattern. There was no difference in WM at any MRI time point. CSF volume was marginally greater in the CHD group compared to the control population on the 3-month MRI. (Table 4).
Table 4.
Estimated mean brain volumes in CHD subjects relative to controls.
| Tissue (cm3) | Fetal MRI |
Preoperative MRI |
3-month MRI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHD | Control | Percent difference | P value | CHD | Control | Percent difference | P Value | CHD | Control | Percent difference | P value | |
| TBV | 204 (190–218) | 211 (197–224) | −3 | 0.51 | 285 (269–301) | 327 (311–343) | −13 | <0.001 | 431 (398–463) | 539 (504–573) | −20 | <0.001 |
| CGM | 78 (68–89) | 75 (65–84) | +4 | 0.61 | 114 (106–121) | 134 (126–142) | −15 | <0.001 | 178 (167–189) | 242 (231–252) | −26 | <0.0001 |
| WM | 101 (90–113) | 107 (96–117) | −6 | 0.48 | 132 (123–142) | 144 (134–154) | −8 | 0.11 | 188 (168–208) | 211 (190–233) | −11 | 0.13 |
| SCGM | 18 (16–19) | 18 (17–19) | 0 | 0.89 | 23 (22–24) | 26 (25–27) | −12 | 0.003 | 33 (31–35) | 41 (38–43) | −20 | <0.001 |
| CER | 9 (8–10) | 10 (9–11) | −10 | 0.12 | 18 (17–20) | 22 (21–24) | −18 | 0.001 | 36 (33–40) | 45 (41–49) | −20 | 0.001 |
| CSF | 89 (72–106) | 94 (78–109) | −5 | 0.72 | 121 (109–133) | 114 (102–126) | +6 | 0.44 | 178 (161–195) | 152 (135–169) | +17 | 0.04 |
Data are displayed as the estimated mean (95% confidence interval) for the random coefficients model using the model estimates at the mean gestational age for each MRI time point. Mean gestational at fetal MRI was 32.7 weeks, at preoperative MRI was 39.7 weeks, and at 3-month MRI was 52.4 weeks. The percent difference is the difference in means relative to the control population. CER = cerebellum CGM = cortical gray matter, CSF = cerebrospinal fluid, SCGM = subcortical gray matter, TBV = total brain volume, WM = white matter.
Associations of 3-month TBV with clinical factors in the CHD group demonstrated that lower 3-month TBV was associated with longer hospital length of stay (rs = −0.80, p = 0.002) and brain injury (median TBV for infants with brain injury = 349 cm3, median TBV for infants without brain injury = 428 cm3, p = 0.02). The relationship of lower TBV with a diagnosis of HLHS did not reach significance (median 3-month TBV for HLHS = 367 cm3, median 3-month TBV for other CHD = 427 cm3, p = 0.27). To better delineate the impact of brain injury on the trajectory of TBV, the random coefficients model was repeated after removing infants with brain injury. An altered trajectory of brain growth persisted in the CHD population, where CHD infants without brain injury had a 13.6 cm3 increase in TBV per week compared to a 16.6 cm3 increase per week for controls (p = 0.006).
Given the potential for impairment in somatic growth in an intensive care environment, which may affect TBV and be confounded by diagnosis, brain injury, and hospital length of stay, analyses were undertaken to further define the relationship between somatic and brain growth. Lower 3-month TBV was associated with lower birth weight (rs = 0.75, p = 0.005) and lower weight at 3-month MRI (rs = 0.71, p = 0.02), but there was no association of TBV with change in weight from birth to 3-month MRI (rs = −0.08, p = 0.81). Infants with a lower birth weight were more likely to have brain injury (median birth weight in infants with brain injury = 2194 g, median birth weight in infants without brain injury = 3330 g, p = 0.01) and a longer hospital length of stay (rs = −0.64, p = 0.01).
4. Discussion
This study demonstrated an altered trajectory of brain growth in infants with CHD in a unique cohort of patients who underwent serial prenatal and postnatal MRI. CHD infants displayed a progressively smaller TBV over time, resulting in an 11.5 cm3 increase in TBV per week in CHD infants in comparison to a 16.7 cm3 increase per week in controls after adjusting for GA at MRI and birth weight. Tissue-specific analyses revealed that this altered trajectory of growth included prominent effects in the CGM, SCGM, and cerebellum. Importantly, the differences in growth trajectories remained evident even when CHD infants with brain injury were removed from the analyses, suggesting that injury is not the sole predictor of altered growth in this clinical population.
4.1. Trajectory of total and tissue-specific brain volumes
Studies in the CHD population have consistently shown reductions in brain volume prenatally and postnatally that appear to be global in nature(Limperopoulos et al., 2010; Sun et al., 2015; Rollins et al., 2017; von Rhein et al., 2015; Heye et al., 2018; Peyvandi et al., 2018a; Clouchoux et al., 2013; Owen et al., 2014; Olshaker et al., 2018). In fetuses with HLHS, specific involvement of the cortical plate and developing WM has been reported after 30 weeks gestation, with a less significant effect on SCGM (Clouchoux et al., 2013). Cerebellar volumes have also been shown to be reduced prenatally (Olshaker et al., 2018). Reductions in CGM, WM, subcortical structures, and the cerebellum have all been reported postnatally before cardiac surgery (von Rhein et al., 2015; Owen et al., 2014). These data demonstrated no differential effects between tissue-types, ranging from an 18% reduction for WM to a 29% reduction for CGM (von Rhein et al., 2015). However, data in infants beyond the neonatal period has demonstrated decreased brain volume to be largely driven by smaller WM measures in infants with biventricular circulation at one year of age (Rollins et al., 2017).
Our study adds to the existing literature by characterizing the trajectory of total and tissue-specific brain volumes from the prenatal environment through early infancy in a single cohort. To our knowledge, this study is the first to do so. The growth trajectory of 11.5 cm3 per week in the CHD group is not only smaller than controls, but is also similar to previous data reporting growth rates of 7–12 cm3 in the perioperative setting for CHD infants (Peyvandi et al., 2018a). In addition to identifying a slower rate of growth for TBV, our study also demonstrated regionally-specific alterations in growth trajectory for CGM, SCGM, and cerebellar volumes in CHD infants. Although our data did not find differences on fetal MRI, reductions were present across multiple tissue-types on the preoperative and 3-month MRI, coinciding with increased CSF volume over time. Further, though WM volume was not significantly different in our cohort, WM measures were consistently smaller in the CHD group across all time points. This collection of findings suggests diffuse disturbances in brain growth. Although the exact timing of these differential tissue effects has not been fully elucidated, it is becoming more clear that they are global in nature and likely occur secondary to multifactorial innate (i.e., genetic) and modifiable (i.e., brain injury) variables from both the prenatal and postnatal environment (Volpe, 2014; Hovels-Gurich, 2016; Chai, 2018).
Volumetric deficits in the CHD population are thought to begin prenatally secondary to cerebral hypoxia/hypoperfusion and, indeed, reduced fetal TBV has been associated with reduced ascending aortic oxygen saturation and cerebral oxygen consumption (Sun et al., 2015; Lauridsen et al., 2017). The pathophysiology of postnatal deficits are likely multifactorial. It has been suggested that processes are similar to those identified in preterm infants, such that pre- and post-natal cerebral hypoxia and hypoperfusion result in injury to the developing white matter (displayed as white matter volumetric reductions). This can result in interruption of thalamocortical connectivity, leading to volume reductions of cortical and subcortical gray matter. This process could occur independently or in combination with direct neuronal and axonal injury (Volpe, 2014; Morton et al., 2017). Our findings demonstrating less significant reductions in the WM of CHD infants, in comparison to more prominent disturbances in gray matter tissues, may support such a “two-hit” phenomenon of both direct and secondary neuronal and axonal effects. This may explain the progression of gray matter (and therefore TBV) deficits over time in our cohort. Alternatively, or in addition, there may be postnatal clinical factors that directly impact growth of gray matter tissues.
4.2. Trajectory of TBV and clinical factors
Repeated measures of postnatal brain volumes have been evaluated in two other studies, both of which have identified cardiac diagnosis as an important factor for brain volume. The first investigated acute perioperative changes from pre- to post-operative MRI in a large cohort of patients (n = 79) with two distinct cardiac physiologies – HLHS and d-TGA. Their data demonstrated poorer perioperative brain growth over approximately two weeks in infants with HLHS and in infants with moderate-severe brain injury, with HLHS a stronger predictor (Peyvandi et al., 2018a). These data may suggest lesion-specific effects, but also highlight the complexities of diagnosis, perioperative care, and brain injury. Similarly, repeated postoperative imaging at one and three years of age in 10 children with TGA and 23 with single ventricle physiology showed initial deficits in both groups that only persisted for the single ventricle patients, suggesting correction of hypoxemic conditions may improve brain growth (Ibuki et al., 2012). While our data did not demonstrate volumetric differences in HLHS infants, the cohort included a smaller, more heterogeneous sample and was not designed to investigate subgroup differences.
Regarding the impact of brain injury, we identified perioperative injury in 36% of infants with CHD in our cohort. The current literature suggest 26–55% of CHD infants have perioperative brain injury, 26–41% of which occurs preoperatively and 30–44% as new postoperative lesions. White matter injury is the most common pattern of injury across cohorts (Dimitropoulos et al., 2013; Beca et al., 2013; Claessens et al., 2018; Peyvandi et al., 2018b). Our rate of brain injury is consistent with rates previously described. Additionally, white matter injury was also common in our cohort. Of note, we did not identify any injury on fetal MRI, though it is possible that injury patterns related to chronic fetal hypoxia, such as white matter injury, fell below the threshold of resolution that could be detected on fetal imaging.
The exact relationships between brain injury and fetal and postnatal volumetric deficits have not been clearly defined. Conventional qualitative assessments of fetal and neonatal MRI have shown that over one-third of neonates with brain injury have markers of altered brain development on either fetal or neonatal imaging (Brossard-Racine et al., 2016). Postnatal, preoperative assessments have also suggested a relationship between brain development and subsequent postoperative brain injury, although diagnostic category was a stronger predictor of injury (Beca et al., 2013). We were able to identify an association between brain injury and TBV, with TBV being 79 cm3 smaller on 3-month MRI in infants with injury. When we excluded infants with brain injury, our model continued to demonstrate an altered trajectory of brain growth for infants with CHD. This would suggest that brain injury is not the only factor affecting the trajectory of brain growth. Given the divergence of TBV through early infancy, it is possible that other clinical factors in the perioperative and intensive care environment (i.e., analgesia and sedation management, nutritional factors beyond weight assessments, developmental care models) could be affecting this trajectory.
Another potential factor affecting the trajectory of brain growth is the prenatal environment. Our cohort demonstrated associations of smaller TBV with lower birth weight and lower 3-month weight. In contrast, there was no association with change in weight from birth to three months, suggesting that birth weight (reflective of prenatal growth) is perhaps more important than change in weight in early infancy. Of interest, lower birth weight was also associated with brain injury and longer hospital length of stay, both important factors associated with TBV in our cohort. Although these analyses were exploratory, these results could indicate prenatal somatic growth is an alternative or interactive pathway by which brain growth is associated with brain injury and hospital length of stay.
4.3. Prenatal volumetric deficits
While our cohort did not display significant reductions in TBV on fetal imaging, infants with CHD are known to have MRI deficits in brain volume that begin prenatally. Specifically, the seminal work of Limperopoulos and colleagues discovered fetuses with CHD to have decreased TBV that progressed during the third trimester of pregnancy, which was most prominent in fetuses with HLHS and reflected a reduction of approximately 13% at 32 weeks gestation (Limperopoulos et al., 2010). Subsequent prenatal studies have corroborated this finding, reporting deficits in brain volume as early as the late second trimester in fetuses with tetralogy of Fallot and a 13% reduction in TBV at 36 weeks gestation in fetuses with single and biventricular cardiac defects (Sun et al., 2015; Schellen et al., 2015).
It is unlikely that our inability to detect a difference in TBV at fetal MRI is due to methodologic variation in volumetric analysis, as this should result in systematic variations across CHD and control populations, which would not affect overall findings. More likely is that our sample size and/or differences in cohort characteristics are contributing to this result. To better delineate why our cohort did not show differences on fetal MRI, we compared our findings to those of Limperopoulos et al. using their regression equation. This comparison showed similar measurements in TBV between cohorts for CHD fetuses at 32.7 weeks (204 cm3 compared to 210 cm3), but TBV varied for controls (211 cm3 compared to 244 cm3) (Limperopoulos et al., 2010). Further, Sun et al reported TBV of 279 cm3 for CHD fetuses and 319 cm3 for controls at 36 weeks gestation (Sun et al., 2015). Our model estimates provide a TBV of 254 cm3 for CHD fetuses and 277 cm3 for controls at 36 weeks, reflecting a 9% and 13% difference in volume, respectively, compared to Sun et al. We matched our CHD and control subjects on clinical variables including GA at MRI, fetal sex, and maternal race and recruited both groups from a clinical setting of similar sociodemographic backgrounds. However, it is possible that the control population we selected, while matched to our infants with CHD, had smaller brain volumes than other cohorts, contributing to the lack of significance prenatally. This could reflect differences in sociodemographic or genetic factors for our controls, which may be important to consider for future investigations.
4.4. Postnatal volumetric deficits
Cerebral volumetric deficits have been reported on neonatal preoperative MRI scans in a heterogeneous group of cardiac diagnoses compared to controls (von Rhein et al., 2015), and several studies have shown reductions in brain volume during infancy and early childhood, well after the initial acute perioperative period (Rollins et al., 2017; Heye et al., 2018; Watanabe et al., 2009). In addition, a single study in adolescents with CHD and another in adults have also reported diminished brain volumes (Cordina et al., 2014; von Rhein et al., 2014). Our data demonstrated a significant reduction in TBV on both preoperative and 3-month MRIs, consistent with the existing postnatal imaging literature reporting differences after birth. However, our preoperative reductions in TBV were only 13% compared to previous data reporting a 20% reduction (von Rhein et al., 2015). These differences may be related to the timing of the neonatal MRIs, as ours occurred at earlier postmenstrual ages and there is a progression of reduced TBV over time in our cohort. Although, cohort variation may again play a role, as our controls had comparatively smaller TBV, similar to our prenatal findings.
4.5. Methodologic considerations
The “gold standard” for measuring brain growth is with volumetric methods, which were employed here. As established by our group and others, comparisons of volumetric measures between groups and methodologies in fetal and neonatal populations standardly utilize manually-corrected segmentations generated by blinded personnel as the optimal reference (Beare et al., 2016; Matthews et al., 2018; Habas et al., 2010; Makropoulos et al., 2014). Here, after an automated segmentation algorithm was used for the initial segmentation process, all data across all time points were subsequently manually modified in a blinded fashion and in a systematic manner to ensure the fidelity of results. This rigorous approach eliminates variations in procedures specific to group and mitigates the possibility of differences in methodology contributing significantly to the differences that we identified.
Our prior work took a more simplistic approach by using 2D biometric measurements on MRI, and identified smaller brain size across multiple regions preoperatively that persisted through the first three months of life. These data showed a similar rate of growth between the CHD and control groups (Ortinau et al., 2012a; 2012b). However, 2D biometrics methods are global regional measures, as opposed to the analyses reported here, which reflect tissue-specific changes beginning prenatally at approximately seven weeks before our previous 2D neonatal measures. Additionally, 2D biometric measures have limitations in their correlation with 3D volumetry for tissue measures. For example, bifrontal diameter (2Dregional measure) and cortical gray matter volume (3D tissue-type measure) only have a correlation coefficient of 0.482 (Nguyen The Tich et al., 2009). Thus, this study expands our previous data by defining total and tissue volumetric changes from prenatal to postnatal imaging, which provides complementary information to our prior work regarding brain growth.
4.6. Limitations
This study has several limitations. First, the sample size is relatively small, which impacted the ability to define the relative contributions of specific tissue-types to longitudinal TBV, as well as the variance explained by relevant clinical factors. While exploratory analyses were undertaken, future studies with larger samples are needed to rigorously evaluate these relationships. Second, our sample includes a heterogeneous cohort of CHD that may have variable effects on brain development at different gestational ages, which could influence the timing and severity of fetal and infant impairments in brain development. Finally, our cohort had missing data at each MRI time point. We addressed this limitation with a random coefficients model, but there may be systematic differences between those subjects who did and did not undergo imaging at each time point. For example, infants deemed too unstable for pre- or post-operative MRI may have had the greatest deficits in volumetric measurements at this time point. Their findings might be better represented on the 3-month MRI results when these infants were more clinically stable and a larger number were able to undergo MRI scans. Despite these limitations, these data are the first to evaluate longitudinal changes in brain volume from prenatal to postnatal imaging and provide a foundation for future work investigating the timing of tissue-specific involvement in altered brain development.
5. Conclusions
This study demonstrated that infants with CHD have an altered trajectory of brain growth from fetal through neonatal and 3-month MRI. Importantly, by three months of age multiple tissue-types were involved, suggesting global disturbances in brain development that are likely multifactorial.
The following is the supplementary data related to this article.
Longitudinal Imaging in Individual CHD Patients with and without Brain Injury. The top row represents the fetal MRI (A), neonatal MRI (B), and 3-month MRI (C) for Subject 5. The bottom row represents the fetal MRI (D), neonatal MRI (E), and 3-month MRI (F) for Subject 18. The white arrows demonstrate white matter injury, the white arrowhead demonstrates the left caudate infarct, the black arrows demonstrate bilateral intraventricular hemorrhage, and the black arrowhead demonstrates ventriculomegaly.
Acknowledgements
This work was supported by the National Institutes of Health (NIH) Institute of Clinical and Translational Sciences (UL1 TR000448 and KL2 TR 000450) (C.O.), National Institute of Neurological Disorders and Stroke (K02 NS089852) (C.S.), National Institute of Biomedical Imaging and Bioengineering(R01EB018988) (A.G.), and the Eunice Kennedy Shriver National Institute Of Child Health & Human Developmentunder Award Number U54 HD087011 to the Intellectual and Developmental Disabilities Research Center at Washington University (B.L.S.); the Children's Discovery Institute of Washington University and St. Louis Children's Hospital (C.O. and C.S); the McKnight Foundation Technological Innovations in Neuroscience Award (A.G.); and a Career Development Award from the Office of Faculty Development at Boston Children's Hospital (A.G.). The content of this work is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Jennifer Brockmeyer and Reginald Lee for their assistance with recruitment and MRI analysis.
References
- Avants B.B., Tustison N.J., Wu J., Cook P.A., Gee J.C. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 2011;9:381–400. doi: 10.1007/s12021-011-9109-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare R.J., Chen J., Kelly C.E. Neonatal brain tissue classification with morphological adaptation and unified segmentation. Front. Neuroinf. 2016;10:12. doi: 10.3389/fninf.2016.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beca J., Gunn J.K., Coleman L. New white matter brain injury after infant heart surgery is associated with diagnostic group and the use of circulatory arrest. Circulation. 2013;127:971–979. doi: 10.1161/CIRCULATIONAHA.112.001089. [DOI] [PubMed] [Google Scholar]
- Brossard-Racine M., du Plessis A., Vezina G. Brain injury in neonates with complex congenital heart disease: what is the predictive value of MRI in the fetal period? AJNR Am. J. Neuroradiol. 2016;37:1338–1346. doi: 10.3174/ajnr.A4716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai P.J. Apparently, size matters...in congenital heart disease and brain injury. J. Thorac. Cardiovasc. Surg. 2018;155:289–290. doi: 10.1016/j.jtcvs.2017.09.083. [DOI] [PubMed] [Google Scholar]
- Claessens N.H.P., Algra S.O., Ouwehand T.L. Perioperative neonatal brain injury is associated with worse school-age neurodevelopment in children with critical congenital heart disease. Dev. Med. Child Neurol. 2018;60:1052–1058. doi: 10.1111/dmcn.13747. [DOI] [PubMed] [Google Scholar]
- Clouchoux C., du Plessis A.J., Bouyssi-Kobar M. Delayed cortical development in fetuses with complex congenital heart disease. Cereb. Cortex. 2013;23:2932–2943. doi: 10.1093/cercor/bhs281. [DOI] [PubMed] [Google Scholar]
- Cordina R., Grieve S., Barnett M., Lagopoulos J., Malitz N., Celermajer D.S. Brain volumetric, regional cortical thickness and radiographic findings in adults with cyanotic congenital heart disease. Neuroimage Clin. 2014;4:319–325. doi: 10.1016/j.nicl.2013.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos A., McQuillen P.S., Sethi V. Brain injury and development in newborns with critical congenital heart disease. Neurology. 2013;81:241–248. doi: 10.1212/WNL.0b013e31829bfdcf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A., Estroff J.A., Warfield S.K. Robust super-resolution volume reconstruction from slice acquisitions: application to fetal brain MRI. IEEE Trans. Med. Imaging. 2010;29:1739–1758. doi: 10.1109/TMI.2010.2051680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A., Estroff J.A., Barnewolt C.E., Connolly S.A., Warfield S.K. Fetal brain volumetry through MRI volumetric reconstruction and segmentation. Int. J. Comput. Assist. Radiol. Surg. 2011;6:329–339. doi: 10.1007/s11548-010-0512-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gholipour A., Rollins C.K., Velasco-Annis C. A normative spatiotemporal MRI atlas of the fetal brain for automatic segmentation and analysis of early brain growth. Sci. Rep. 2017;7:476. doi: 10.1038/s41598-017-00525-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habas P.A., Kim K., Rousseau F., Glenn O.A., Barkovich A.J., Studholme C. Atlas-based segmentation of developing tissues in the human brain with quantitative validation in young fetuses. Hum. Brain Mapp. 2010;31:1348–1358. doi: 10.1002/hbm.20935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heye K.N., Knirsch W., Latal B. Reduction of brain volumes after neonatal cardiopulmonary bypass surgery in single-ventricle congenital heart disease before Fontan completion. Pediatr. Res. 2018;83:63–70. doi: 10.1038/pr.2017.203. [DOI] [PubMed] [Google Scholar]
- Hovels-Gurich H.H. Factors influencing neurodevelopment after cardiac surgery during Infancy. Front. Pediatr. 2016;4:137. doi: 10.3389/fped.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki K., Watanabe K., Yoshimura N. The improvement of hypoxia correlates with neuroanatomic and developmental outcomes: comparison of midterm outcomes in infants with transposition of the great arteries or single-ventricle physiology. J. Thorac. Cardiovasc. Surg. 2012;143:1077–1085. doi: 10.1016/j.jtcvs.2011.08.042. [DOI] [PubMed] [Google Scholar]
- International Cardiac Collaborative on Neurodevelopment Impact of operative and postoperative factors on neurodevelopmental outcomes after cardiac operations. Ann. Thorac. Surg. 2016;102:843–849. doi: 10.1016/j.athoracsur.2016.05.081. [DOI] [PubMed] [Google Scholar]
- Kainz B., Steinberger M., Wein W. Fast volume reconstruction from motion corrupted stacks of 2D Slices. IEEE Trans. Med. Imaging. 2015;34:1901–1913. doi: 10.1109/TMI.2015.2415453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuklisova-Murgasova M., Quaghebeur G., Rutherford M.A., Hajnal J.V., Schnabel J.A. Reconstruction of fetal brain MRI with intensity matching and complete outlier removal. Med. Image Anal. 2012;16:1550–1564. doi: 10.1016/j.media.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyriakopoulou V., Vatansever D., Davidson A. Normative biometry of the fetal brain using magnetic resonance imaging. Brain Struct. Funct. 2017;222:2295–2307. doi: 10.1007/s00429-016-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latal B., Patel P., Liamlahi R., Knirsch W., O'Gorman Tuura R., von Rhein M. Hippocampal volume reduction is associated with intellectual functions in adolescents with congenital heart disease. Pediatr. Res. 2016;80:531–537. doi: 10.1038/pr.2016.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauridsen M.H., Uldbjerg N., Henriksen T.B. Cerebral oxygenation measurements by magnetic resonance imaging in fetuses with and without heart defects. Circ. Cardiovasc. Imag. 2017;10 doi: 10.1161/CIRCIMAGING.117.006459. [DOI] [PubMed] [Google Scholar]
- Limperopoulos C., Tworetzky W., McElhinney D.B. Brain volume and metabolism in fetuses with congenital heart disease: evaluation with quantitative magnetic resonance imaging and spectroscopy. Circulation. 2010;121:26–33. doi: 10.1161/CIRCULATIONAHA.109.865568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makropoulos A., Gousias I.S., Ledig C. Automatic whole brain MRI segmentation of the developing neonatal brain. IEEE Trans. Med. Imaging. 2014;33:1818–1831. doi: 10.1109/TMI.2014.2322280. [DOI] [PubMed] [Google Scholar]
- Mathur A.M., Neil J.J., McKinstry R.C., Inder T.E. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr. Radiol. 2008;38:260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- Matthews L.G., Walsh B.H., Knutsen C. Brain growth in the NICU: critical periods of tissue-specific expansion. Pediatr. Res. 2018;83:976–981. doi: 10.1038/pr.2018.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuillen P.S., Barkovich A.J., Hamrick S.E. Temporal and anatomic risk profile of brain injury with neonatal repair of congenital heart defects. Stroke. 2007;38:736–741. doi: 10.1161/01.STR.0000247941.41234.90. [DOI] [PubMed] [Google Scholar]
- Morton P.D., Korotcova L., Lewis B.K. Abnormal neurogenesis and cortical growth in congenital heart disease. Sci. Transl. Med. 2017;9 doi: 10.1126/scitranslmed.aah7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen The Tich S., Anderson P.J., Shimony J.S., Hunt R.W., Doyle L.W., Inder T.E. A novel quantitative simple brain metric using MR imaging for preterm infants. AJNR Am. J. Neuroradiol. 2009;30:125–131. doi: 10.3174/ajnr.A1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olshaker H., Ber R., Hoffman D., Derazne E., Achiron R., Katorza E. Volumetric Brain MRI study in fetuses with congenital heart disease. AJNR Am. J. Neuroradiol. 2018;39:1164–1169. doi: 10.3174/ajnr.A5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinau C., Inder T., Lambeth J., Wallendorf M., Finucane K., Beca J. Congenital heart disease affects cerebral size but not brain growth. Pediatr. Cardiol. 2012;33:1138–1146. doi: 10.1007/s00246-012-0269-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinau C., Beca J., Lambeth J. Regional alterations in cerebral growth exist preoperatively in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2012;143:1264–1270. doi: 10.1016/j.jtcvs.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen M., Shevell M., Donofrio M. Brain volume and neurobehavior in newborns with complex congenital heart defects. J. Pediatr. 2014;164:1121–1127. doi: 10.1016/j.jpeds.2013.11.033. (e1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyvandi S., Kim H., Lau J. The association between cardiac physiology, acquired brain injury, and postnatal brain growth in critical congenital heart disease. J. Thorac. Cardiovasc. Surg. 2018;155:291–300. doi: 10.1016/j.jtcvs.2017.08.019. (e3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyvandi S., Chau V., Guo T. Neonatal brain injury and timing of neurodevelopmental assessment in patients with congenital heart disease. J. Am. Coll. Cardiol. 2018;71:1986–1996. doi: 10.1016/j.jacc.2018.02.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins C.K., Asaro L.A., Akhondi-Asl A. White matter volume predicts language development in congenital heart disease. J. Pediatr. 2017;181:42–48. doi: 10.1016/j.jpeds.2016.09.070. (e2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellen C., Ernst S., Gruber G.M. Fetal MRI detects early alterations of brain development in tetralogy of fallot. Am. J. Obstet. Gynecol. 2015:213–392. doi: 10.1016/j.ajog.2015.05.046. (e1–7) [DOI] [PubMed] [Google Scholar]
- Sun L., Macgowan C.K., Sled J.G. Reduced fetal cerebral oxygen consumption is associated with smaller brain size in fetuses with congenital heart disease. Circulation. 2015;131:1313–1323. doi: 10.1161/CIRCULATIONAHA.114.013051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe J.J. Encephalopathy of congenital heart disease- destructive and developmental effects intertwined. J. Pediatr. 2014;164:962–965. doi: 10.1016/j.jpeds.2014.01.002. [DOI] [PubMed] [Google Scholar]
- von Rhein M., Buchmann A., Hagmann C. Brain volumes predict neurodevelopment in adolescents after surgery for congenital heart disease. Brain. 2014;137:268–276. doi: 10.1093/brain/awt322. [DOI] [PubMed] [Google Scholar]
- von Rhein M., Buchmann A., Hagmann C. Severe congenital heart defects are associated with global reduction of neonatal brain volumes. J. Pediatr. 2015;167:1259–1263. doi: 10.1016/j.jpeds.2015.07.006. (e1) [DOI] [PubMed] [Google Scholar]
- Watanabe K., Matsui M., Matsuzawa J. Impaired neuroanatomic development in infants with congenital heart disease. J. Thorac. Cardiovasc. Surg. 2009;137:146–153. doi: 10.1016/j.jtcvs.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Yushkevich P.A., Piven J., Hazlett H.C. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31:1116–1128. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Longitudinal Imaging in Individual CHD Patients with and without Brain Injury. The top row represents the fetal MRI (A), neonatal MRI (B), and 3-month MRI (C) for Subject 5. The bottom row represents the fetal MRI (D), neonatal MRI (E), and 3-month MRI (F) for Subject 18. The white arrows demonstrate white matter injury, the white arrowhead demonstrates the left caudate infarct, the black arrows demonstrate bilateral intraventricular hemorrhage, and the black arrowhead demonstrates ventriculomegaly.