Abstract
‘Candidatus Chloroploca asiatica’ B7–9 and ‘Candidatus Viridilinea mediisalina’ Kir15-3F are mesophilic filamentous anoxygenic phototrophic bacteria from alkaline aquatic environments. Both bacteria became available in the last few years and only in stable enrichment culture. In this study, we report the draft genomic sequences of ‘Ca. Chloroploca asiatica’ B7–9 and ‘Ca. Viridilinea mediisalina’ Kir15-3F, which were assembled from metagenomes of their cultures with a fold coverage 86.3× and 163.8×, respectively. The B7–9 (5.8 Mb) and the Kir15-3F (5.6 Mb) draft genome harbors 4818 and 4595 predicted protein-coding genes, respectively. In this article, we analyzed the phylogeny of representatives of the Chloroflexineae suborder in view of the appearance of new genomic data. These data were used for the revision of earlier published group-specific conserved signature indels and for searching for novel signatures for taxons in the Chloroflexineae suborder.
Electronic supplementary material
The online version of this article (10.1186/s40793-018-0329-8) contains supplementary material, which is available to authorized users.
Keywords: Chloroflexi, Chloroflexales, Chloroploca asiatica, Viridilinea mediisalina, Anoxygenic phototrophic bacteria
Introduction
It is difficult to study the mesophilic representatives of filamentous anoxygenic phototrophic (FAP) bacteria (bacteriochlorophyll-based phototrophic Chloroflexota), as maintaining mesophiles in axenic culture and isolating them are challenging. In fact, over the course of four decades of study on FAP bacteria, stable axenic culture of only Oscillochloris trichoides DG-6 has been described [1]. Therefore, a description of the mesophiles in enrichment cultures are common in studies [2–6]. However, the approach based on studying the enrichment cultures limits research in frame of morphological observations and rough ecophysiological characterization. Nonetheless, enrichment culture allows for genome sequencing of a target bacterium with high efficiency. Recently, a new mesophilic FAP representative was described in stable highly enriched cultures [5]. Here, we report the results of a genomic study of ‘Candidatus Chloroploca asiatica’ B7–9 and a new bacterium, ‘Candidatus Viridilinea mediisalina’ Kir15-3F. A partial description of the latter one has been published for the first time. We have assembled high-quality draft genomes of both FAP bacteria. The extended examination into the genomic data was focused on the phylogeny of the Chloroflexineae suborder and its taxonomic implications. The new genomic data will help to extend our knowledge about the phylogenetic and functional diversity of FAB bacteria, which is highly limited to date.
Organism information
Classification and features
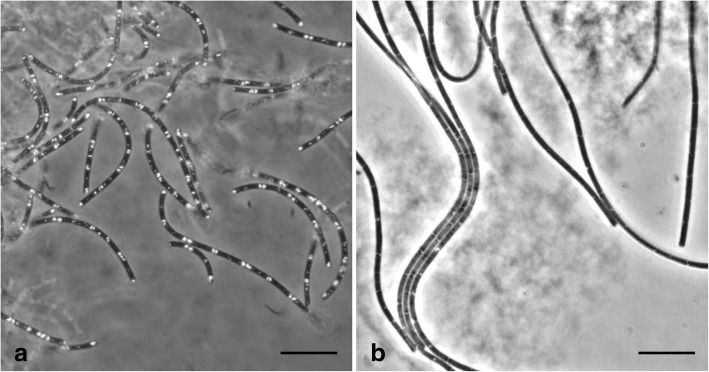
A description of the bacterium ‘Ca. Chloroploca asiatica’ was published in 2014 [5]. A partial description of bacterium the ‘Ca. Viridilinea mediisalina’ was published in this article. Both bacteria are FAP Chloroflexota bacteria isolated from alkaline environments in Eastern Siberia. The B7–9 was isolated from the Doroninskoe soda lake [5], and the Kir15-3F was isolated from the Kiran soda lake. The bacteria were described in stable enrichment cultures. A summary of the key features of ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’ is provided in Tables 1 and 2, respectively. Both bacteria have a multicellular filamentous morphology. However, ‘Ca. Chloroploca asiatica’ forms short filaments (Fig. 1a) whereas ‘Ca. Viridilinea mediisalina’ forms long typical Oscillochloris-like filaments (Fig. 1b). The common morphological properties of both bacteria are: a monoderm-type cell envelope, gas vesicles, chlorosomes, polyphosphate-like inclusions and motility (presumably gliding). Both bacteria are supposedly obligate anaerobic anoxygenic phototrophs because they do not grow in the upper part of the agar column and in the dark. Moreover, both bacteria are mesophiles and exhibit the best growth under alkaline conditions.
Table 1.
Classification and general characteristics of ‘Ca. Chloroploca asiatica’ B7–9 [25]
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Classification | Domain: Bacteria | TAS [26] | |
| Phylum: Chloroflexota | TAS [27–29] | ||
| Class: Chloroflexia | TAS [14, 30] | ||
| Order: Chloroflexales | TAS [14, 30] | ||
| Family: incertae sedis | IDA | ||
| Genus: ‘Ca. Chloroploca’ | TAS [5] | ||
| Species: ‘Ca. Chloroploca asiatica’ | TAS [5] | ||
| Strain B7–9 | TAS [5] | ||
| Gram stain | Negative | TAS [5] | |
| Cell shape | Filaments | TAS [5] | |
| Motility | Motile | TAS [5] | |
| Sporulation | Not reported | NAS | |
| Temperature range | Not determined | TAS [5] | |
| Optimum temperature | 25–32 °C | TAS [5] | |
| pH range; Optimum | Not determined; 8.0 | TAS [5] | |
| Carbon source | Not determined | TAS [5] | |
| MIGS-6 | Habitat | Soda lakes | TAS [5] |
| MIGS-6.3 | Salinity | 0.3–1.5% NaCl (w/v) | TAS [5] |
| MIGS-22 | Oxygen requirement | Anaerobic | TAS [5] |
| MIGS-15 | Biotic relationship | Free-living | TAS [5] |
| MIGS-14 | Pathogenicity | Non-pathogen | NAS |
| MIGS-4 | Geographic location | Russia/East Siberia | TAS [5] |
| MIGS-5 | Sample collection | September 2010–2012 | TAS [5] |
| MIGS-4.1 | Latitude | 51.235707 | TAS [5] |
| MIGS-4.2 | Longitude | 112.236169 | TAS [5] |
| MIGS-4.4 | Altitude | Not determined | TAS [5] |
aEvidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [31, 32]
Table 2.
Classification and general characteristics of ‘Ca. Viridilinea mediisalina’ Kir15-3F [25]
| MIGS ID | Property | Term | Evidence codea |
|---|---|---|---|
| Classification | Domain: Bacteria | TAS [26] | |
| Phylum: Chloroflexota | TAS [27–29] | ||
| Class: Chloroflexia | TAS [14, 30] | ||
| Order: Chloroflexales | TAS [14, 30] | ||
| Family: incertae sedis | IDA | ||
| Genus: ‘Ca. Viridilinea’ | IDA | ||
| Species: ‘Ca. Viridilinea mediisalina’ | IDA | ||
| Strain Kir15-3F | IDA | ||
| Gram stain | Not determined | IDA | |
| Cell shape | Filaments | IDA | |
| Motility | Motile | IDA | |
| Sporulation | Not reported | IDA | |
| Temperature range | Mesophile | IDA | |
| Optimum temperature | Not determined | IDA | |
| pH range; Optimum | Not determined | IDA | |
| Carbon source | Not determined | IDA | |
| MIGS-6 | Habitat | Soda lakes | IDA |
| MIGS-6.3 | Salinity | Halotolerant | IDA |
| MIGS-22 | Oxygen requirement | Anaerobic | IDA |
| MIGS-15 | Biotic relationship | Free-living | IDA |
| MIGS-14 | Pathogenicity | Non-pathogen | NAS |
| MIGS-4 | Geographic location | Russia/East Siberia | IDA |
| MIGS-5 | Sample collection | September 2015 | IDA |
| MIGS-4.1 | Latitude | 50.332958 | IDA |
| MIGS-4.2 | Longitude | 106.851128 | IDA |
| MIGS-4.4 | Altitude | Not determined | IDA |
aEvidence codes - IDA: Inferred from Direct Assay; TAS: Traceable Author Statement (i.e., a direct report exists in the literature); NAS: Non-traceable Author Statement (i.e., not directly observed for the living, isolated sample, but based on a generally accepted property for the species, or anecdotal evidence). These evidence codes are from the Gene Ontology project [31, 32]
Fig. 1.
Morphological features of ‘Candidatus Chloroploca asiatica’ B7–9 (a) and ‘Candidatus Viridilinea mediisalina’ Kir15-3F (b) as observed on phase-contrast micrographs (Scale bars = 10 μm)
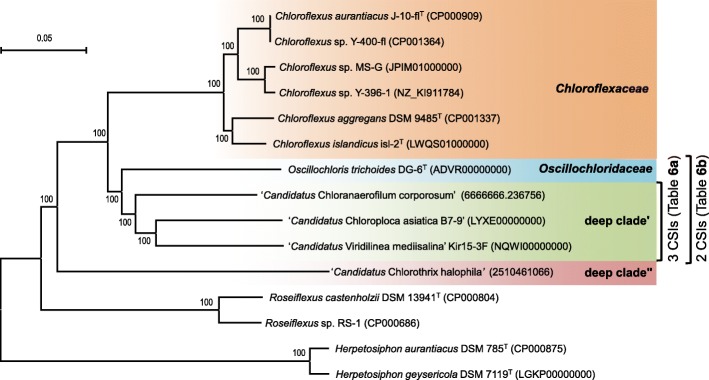
Phylogenetic analysis based on the concatenated amino acid sequences of the core proteins revealed that ‘Ca. Chloroploca asiatica’ B7–9 and ‘Ca. Viridilinea mediisalina’ Kir15-3F are closest relatives to each other (Fig. 2). The closest taxonomically defined representative for the clade of both bacteria is the mesophilic bacterium O. trichoides DG-6. However, the closest relative is ‘Ca. Chloranaerofilum corporosum’, whose population has been detected in the Mushroom hot spring [7]. All four bacteria were assigned to the Chloroflexales order, which encompasses all representatives of the FAP bacteria. However, the complete taxonomic position of ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’ as well as ‘Ca. Chloranaerofilum corporosum’ remains unclear.
Fig. 2.
Neighbor-joining phylogenetic tree inferred from the concatenated amino acid sequences of the core proteins showing the phylogeny of the representatives of the Chloroflexineae suborder. A total of 52,793 positions in the final dataset were analyzed. The bootstrap values obtained with the neighbor-joining methods are based on 1000 replicates. The scale bars represent a 5% amino acid sequence divergence. The accession number for ‘Ca. Chloranaerofilum corporosum’ corresponds to RAST, and the accession number for ‘Ca. Chlorothrix halophila’ corresponds to IMG database. The other accession numbers correspond to GenBank
Chemotaxonomic data
Bacteriochlorophyll c is the main phototrophic pigment of both ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’, and bacteriochlorophyll a is found in trace amounts [5].
Genome sequencing information
Genome project history
The study of ‘Candidatus Chloroploca asiatica’ B7–9 and ‘Candidatus Viridilinea mediisalina’ Kir15-3F was conducted as part of the collaborative project of the Laboratory of Molecular Diagnostics and Laboratory of Ecology and Geochemical Activity of Microorganisms at the Research Center for Biotechnology RAS (Moscow, Russian Federation). Attempts to isolate the axenic culture of both bacteria have not been successful. Therefore, to further study these bacteria, a strategy based on metagenomic sequencing of their highly enriched culture was used. We assembled a high-quality draft genome sequence of the target bacteria with a fold coverage of more than 86.3×. The draft genome sequences have been deposited in GenBank under the accession numbers LYXE00000000.1 and NQWI00000000.1 for B7–9 and Kir15-3F, respectively. The main project information is summarized in Table 3.
Table 3.
Project information
| MIGS ID | Property | ‘Ca. Chloroploca asiatica’ B7–9 | ‘Ca. Viridilinea mediisalina’ Kir15-3F |
|---|---|---|---|
| Term | Term | ||
| MIGS 31 | Finishing quality | Improved high-quality draft | Improved high-quality draft |
| MIGS-28 | Libraries used | Illumina Standard shotgun library | Illumina Standard shotgun library |
| MIGS 29 | Sequencing platforms | Illumina Hiseq 2500 | Illumina Hiseq 2500 |
| MIGS 31.2 | Fold coverage | 86.3× | 163.8× |
| MIGS 30 | Assemblers | SPAdes v. 3.11.1 | SPAdes v. 3.11.1 |
| MIGS 32 | Gene calling method | RAST, PGAP | RAST, PGAP |
| Locus Tag | A9Q02 | CJ255 | |
| Genbank ID | LYXE00000000.1 | NQWI00000000.1 | |
| GenBank Date of Release | 12-OCT-2017 | 12-OCT-2017 | |
| GOLD ID | Gp0236327 | Gp0236326 | |
| BIOPROJECT | PRJNA323704 | PRJNA398606 | |
| MIGS 13 | Source Material Identifier | B7–9 | Kir15-3F |
| Project relevance | Evolution of FAP bacteria | Evolution of FAP bacteria |
Growth conditions and genomic DNA preparation
‘Ca. Chloroploca asiatica’ B7–9 was grown in an agar medium described previously [5] in glass tubes at 27 °C in the light (3100 lx). The bacterium forms spherical colonies, which were used for isolation of the total genomic DNA. In the first step, the colonies were collected from the agar into a 2.0-ml screw-cap microcentrifuge tube containing 375 μl of TE buffer (containing 10 mM Tris and 1 mM EDTA) and 1-mm glass beads to make a total volume of about 500 μl. The microcentrifuge tube was treated using a Mini-Beadbeater (Biospec) until two to three cells were observed to have formed filaments under the microscope. The 475 μl suspension was transferred to a 1.5-ml microcentrifuge tube, to which 25 μl of 100 mM Dithiothreitol was added, mixed and incubated for 100 min at 65 °C. Following this, add 100 μl of 10% Sodium dodecyl sulfate and 5 μl Proteinase K (20 mg/mL). were added, mixed in, and incubated for 60 min at 37 °C. Next, 100 μl of 5 M NaCl and 5 μl RNAse (10 mg/mL) were added, mixed and incubated for 10 min at 65 °C. Finally, 160 μl of Cetrimonium bromide (CTAB) solution (containing 5% CTAB and 0.35 M NaCI) was added, mixed, and incubated for 10 min at 65 °C. The solution was allowed to cool down to room temperature, after which 700 μl of chloroform was mixed in carefully and the solution was spun for 10 min in a microcentrifuge. The upper phase was transferred to a fresh microcentrifuge tube and the interface was left behind. These procedures were repeated with chloroform, and then the upper phase was transferred to a fresh tube. Isopropanol (0.6 vol.) was added to precipitate the DNA. The precipitated DNA was washed with 70% ethanol, briefly dried, and redissolved in MQ water.
‘Ca. Viridilinea mediisalina’ Kir15-3F was grown on agar that covered the bottom of a 50-ml vial filled with liquid medium consisting of the following mix (per litre): KH2PO4 (0.20 g), NH4Cl (0.20 g), MgCl2·6H2O (0.20 g), KCl (0.30 g), NaCl (25.0 g), Na2S2O3 (0.30 g), Na2SO4 (0.30 g), CaCl2·2H2O (0.05 g), NaHCO3 (0.60 g), Na2S·9H2O (0.70 g), soytone (0.05 g), yeast extract (0.05 g), sodium acetate (0.10 g), trace element solution (1 mL) and Pfennig’s vitamin solution (1 mL). The final pH was adjusted to 9.0. The vial was incubated at 38 °C in the light (3800 lx). ‘Ca. Viridilinea mediisalina’ Kir15-3F was isolated from the biofilm at the bottom. The biofilm was collected for isolation of the genomic DNA, following the same protocol as described above for ‘Ca. Chloroploca asiatica’ B7–9.
Genome sequencing and assembly
The same method was used for sequencing of the total DNA from the cultures of both bacteria. The sequencing was performed at “I gene” LLC, Moscow, Russian Federation. A sequence library was constructed with the NEBNext DNA library prep reagent set for Illumina according to the manufacturer’s protocol. The 4,000,203 and 4,793,690 paired-end 150-bp reads were generated using Illumina Hiseq 2500 platforms for metagenomic sequences of the B7–9 and Kir15-3F culture, respectively. Raw sequences were assembled with SPAdes version 3.11.1 [8] and binned using MetaWatt version 3.5.3 [9]. The Chloroflexota genomes were uploaded to RAST [10] for overall characterization and were assessed for completeness and contamination using CheckM [11]. Finally, they were assembled into 166 and 291 contigs for ‘Ca. Chloroploca asiatica’ B7–9 (coverage, 86.3×) and ‘Ca. Viridilinea mediisalina’ Kir15-3F (coverage, 163.8×) bacterium, respectively.
Genome annotation
The assembled draft genomic sequences of ‘Ca. Chloroploca asiatica’ B7–9 and ‘Ca. Viridilinea mediisalina’ Kir15-3F were submitted to the NCBI Prokaryotic Genome Annotation Pipeline for annotation [12].
Genome properties
The properties of both genomes are summarized in Table 4. The draft genome of ‘Ca. Chloroploca asiatica’ B7–9 contained 5,817,919 bp with a G + C content of 58.81% and 4878 (100%) predicted genes: 4818 (98.77%) were protein-coding genes; 46 (0.94%), tRNA genes; and 9 (0.18%), rRNA genes. The genome was 98.74% complete. The draft genome of ‘Ca. Viridilinea mediisalina’ Kir15-3F contained 5,588,620 bp with a G + C content of 58.01% and 4657 (100%) predicted genes: 4595 (98.67%) were protein-coding genes; 46 (0.99%), tRNA genes; and 10 (0.21%), rRNA genes. This genome was 99.21% complete. The properties and statistics of both genomes are summarized in Table 4. The assignment of genes to COG functional categories is presented in Table 5.
Table 4.
Genome statistics
| Attribute | ‘Ca. Chloroploca asiatica’ B7–9 | ‘Ca. Viridilinea mediisalina’ Kir15-3F | ||
|---|---|---|---|---|
| Value | % of Total | Value | % of Total | |
| Genome size (bp) | 5,817,919 | 100.00 | 5,588,620 | 100.00 |
| DNA coding (bp) | 5,229,318 | 89.88 | 4,851,981 | 86.82 |
| DNA G + C (bp) | 3,421,494 | 58.81 | 3,241,714 | 58.01 |
| DNA scaffolds | 166 | 100.00 | 291 | 100.00 |
| Total genes | 4878 | 100.00 | 4657 | 100.00 |
| Protein coding genes | 4818 | 98.77 | 4595 | 98.67 |
| RNA genes | 60 | 1.23 | 62 | 1.33 |
| Pseudo genes | – | – | – | – |
| Genes in internal clusters | 1117 | 22.90 | 1081 | 23.21 |
| Genes with function prediction | 3518 | 72.12 | 3234 | 69.44 |
| Genes assigned to COGs | 2634 | 54.00 | 2356 | 50.59 |
| Genes with Pfam domains | 3550 | 72.78 | 3264 | 70.09 |
| Genes with signal peptides | 171 | 3.51 | 139 | 2.98 |
| Genes with transmembrane helices | 1371 | 28.11 | 1127 | 24.20 |
| CRISPR repeats | 95 | 445 | ||
Table 5.
Number of genes associated with general COG functional categories
| Code | ‘Ca. Chloroploca asiatica’ B7–9 | ‘Ca. Viridilinea mediisalina’ Kir15-3F | Description | ||
|---|---|---|---|---|---|
| Value | %age | Value | %age | ||
| J | 178 | 6.05 | 178 | 6.79 | Translation, ribosomal structure and biogenesis |
| A | – | – | – | – | RNA processing and modification |
| K | 163 | 5.54 | 144 | 5.49 | Transcription |
| L | 105 | 3.57 | 110 | 4.19 | Replication, recombination and repair |
| B | 2 | 0.07 | 1 | 0.04 | Chromatin structure and dynamics |
| D | 43 | 1.46 | 36 | 1.37 | Cell cycle control, Cell division, chromosome partitioning |
| V | 74 | 2.52 | 81 | 3.09 | Defense mechanisms |
| T | 215 | 7.31 | 248 | 9.45 | Signal transduction mechanisms |
| M | 225 | 7.65 | 207 | 7.89 | Cell wall/membrane biogenesis |
| N | 24 | 0.82 | 24 | 0.91 | Cell motility |
| U | 29 | 0.99 | 32 | 1.22 | Intracellular trafficking and secretion |
| O | 140 | 4.76 | 136 | 5.18 | Posttranslational modification, protein turnover, chaperones |
| C | 193 | 6.56 | 154 | 5.87 | Energy production and conversion |
| G | 198 | 6.73 | 135 | 5.03 | Carbohydrate transport and metabolism |
| E | 254 | 8.64 | 202 | 7.70 | Amino acid transport and metabolism |
| F | 83 | 2.82 | 69 | 2.63 | Nucleotide transport and metabolism |
| H | 191 | 6.50 | 163 | 6.21 | Coenzyme transport and metabolism |
| I | 128 | 4.35 | 104 | 3.96 | Lipid transport and metabolism |
| P | 150 | 5.10 | 142 | 5.41 | Inorganic ion transport and metabolism |
| Q | 61 | 2.07 | 39 | 1.49 | Secondary metabolites biosynthesis, transport and catabolism |
| R | 307 | 10.44 | 280 | 10.67 | General function prediction only |
| S | 127 | 4.32 | 110 | 4.19 | Function unknown |
| – | 2244 | 46.00 | 2301 | 49.41 | Not in COGs |
The total is based on the total number of protein coding genes in the genome
Insights from the genome sequence
The draft genomes reported here and the recently published partial genomic sequence of ‘Ca. Chloranaerofilum corporosum’ [13] provide a detailed picture of the evolutionary relationships among chlorosome-containing representatives. Recently, it was proposed that the Chloroflexales order of the class Chloroflexia be divided into two suborders: Roseiflexineae and Chloroflexineae. It was proposed that the first one encompasses chlorosome-lacking Roseiflexus spp., whereas the other one encompasses all the chlorosome-containing representatives of the order [14]. This suggestion based on the obvious morphophysiological differences is strongly supported by the results of genomic analysis [15]. However, the taxonomic hierarchy within Chloroflexineae is not so clear, and this is why it was a subject of the current analysis.
Group-specific conserved signature indels
In the first step, we analyzed the previously proposed specific conserved signature indels (CSIs) [14]. Analysis revealed that ‘Ca. Chloroploca asiatica’ B7–9, ‘Ca. Viridilinea mediisalina’ Kir15-3F and ‘Candidatus Chloranaerofilum corporosum’ have Chloroflexinea-specific insertions in a Phage SPO1 DNA pol-like protein, nucleoside diphosphate kinase, translation initiation factor-2, threonine synthase, ArsA and the acetolactate synthase large subunit, which have been reported previously [14]. Thus, this finding confirms that the bacteria belong to the Chloroflexinea suborder. However, the new chlorosome-containing FAP bacteria do not have specific inserts in the protein sequences of a nucleotide sugar dehydrogenase (Additional file 1: Figure S1a). Moreover, the new representatives have specific insertion in the magnesium-protoporphyrin IX monomethyl ester cyclase proteins (Additional file 1: Figure S1b) was earlier proposed to be Chloroflexus-specific CSIs [14]. Thus, the new genomic data indicate that the CSIs based on the nucleotide sugar dehydrogenase and magnesium-protoporphyrin IX monomethyl ester cyclase proteins must be eliminated from the taxonomic description.
In the second step, we identified new specific CSIs for the studied bacteria ‘Ca. Chloroploca asiatica’ B7–9, ‘Ca. Viridilinea mediisalina’ Kir15-3F and ‘Candidatus Chloranaerofilum corporosum’: specifically, CSIs for phosphoglycerate kinase, heat-inducible transcription repressor, and UMP kinase were identified (Additional file 1: Figure S2a-c). Moreover, some of the new CSIs were found to be common to both the new bacteria and O. trichoides DG-6: these were threonine synthase and glutamate 5-kinase (Additional file 1: Figure S3a and b). The new CSIs are shown in Table 6.
Table 6.
CSIs that are specific for O. trichoides, ‘Ca. Chloranaerofilum corporosum’, O. trichoides, ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’
| Protein name | GI number | Indel size | Indel position |
|---|---|---|---|
| (a) CSIs that are specific for the Viridilinea + Chloroploca + Chloranaerofilum | |||
| Phosphoglycerate kinase | WP_044200294.1 | 1 aa Ins | 54–55 |
| Heat-inducible transcription repressor HrcA | WP_006560707.1 | 1 aa Ins | 131–132 |
| UMP kinase | WP_006562130.1 | 1 aa Del | 23 |
| (b) CSIs that are specific for the Viridilinea + Oscillochloris + Chloroploca + Chloranaerofilum | |||
| Threonine synthase | WP_006561465.1 | 1 aa Del | 304–305 |
| Glutamate 5-kinase | WP_044201831.1 | 2 aa Ins | 65–66 |
Phylogeny of the Chloroflexineae suborder
The concatenated core protein tree has strong bootstrap support for the observed branching (Fig. 2), with the chlorosome-containing FAP bacteria represented as a monophyletic group relative to the chlorosome-lacking Roseiflexus spp. This core protein phylogeny, similarity in pigment composition and the presence of common CSIs indicate that the Chloroflexineae suborder encompasses the Chloroflexus strains, ‘Ca. Chloranaerofilum corporosum’, ‘Ca. Chlorothrix halophila’, O. trichoides, ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’ i.e., all the chlorosome-containing FAP bacteria. Bacteria from the Roseiflexineae suborder show ancestral relations to the representatives listed above [15]. The tree depicts three main clades within the Chloroflexineae suborder: the clade of closely related Chloroflexus species, the ‘Ca. Chlorothrix halophila’ clade and the clade containing the deeply branched lineages ‘Ca. Chloranaerofilum corporosum’, O. trichoides, ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’.
Strains of the Chloroflexus genus form a clade that is clearly separated from the other representatives of the suborder (Fig. 2). This branching has congruence with the morphological and ecophysiological uniformity of Chloroflexus strains, which are thermophilic photoheterotrophs capable of respiration in the dark [16–18]. Genomes of the Chloroflexus strains contain genes of the autotrophic 3-hydroxypropionate CO2 fixation cycle (3-OHP cycle), the activity of which was demonstrated in the OK-70-fl strain [19]. At the moment, only thermophilic Chloroflexus species form the Chloroflexaceae family. A mesophilic Chloroflexus-like bacterium, called ‘Cfl. aurantiacus var. mesophilus’, was identified based on its morphological properties [20]. However, since 16S rRNA gene sequence and other sequencing data are absent, it is highly likely that this bacterium does not belong to the 16S rRNA Chloroflexus clade.
The next two clades were formed by genera represented by a single species. The first clade, which is comprised of the halophilic bacterium ‘Ca. Chlorothrix halophila’, forms a deeply branched lineage within the chlorosome-containing group in accordance with the protein phylogenetic tree (Fig. 2). It was speculated earlier that significant deep branching of a protein tree can be the result of adaptation to halophilic conditions [15]. This led to the preferential use of the 16S phylogeny, but this created contradictions in the protein tree. This explains the difficulty with using the CSI approach for this bacterium. However, ‘Ca. Chlorothrix halophila’ clearly formed an external deeply branched lineage in the current core protein tree (Fig. 2). Moreover, this bacterium has a 14–18% dissimilarity, which represents is the greatest distance from other representatives of the Chloroflexineae suborder according to a comparison of the 16S rRNA sequences. The bacterium ‘Ca. Chlorothrix halophila’ shows preference for halophilic conditions, which is a unique characteristic among the described FAP bacteria [3]. The halophilic preference, combined with the results of phylogenetic analysis and cell ultrastructure, indicate that the bacterium is a candidate for a rank not below the family level.
The third clade was formed by O. trichoides and the recently described bacteria ‘Ca. Chloranaerofilum corporosum’, ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’. The bacterium O. trichoides DG-6 is a type genus and species for Oscillochloridaceae family [21]. Main specific features of the O. trichoides strains are mesophilic lifestyle, the presence of gas vesicles, autotrophic Calvin cycle CO2 fixation (the 3-OHP cycle is absent), and of nitrogen fixation. It was proposed that Chloronema species belonged to the Oscillochloridaceae family [14], but physiological and sequence data for this bacterium remain highly limited. The bacteria ‘Ca. Chloroploca asiatica’ and ‘Ca. Viridilinea mediisalina’ have some common features with O. trichoides, such as their mesophilic features, the presence of gas vesicles, motility, and inability for growth in aerobic conditions and in the dark [1, 5]. However, the closest relative to both new bacteria is the probably thermophilic bacterium ‘Ca. Chloranaerofilum corporosum’ (Fig. 2). The delineation of the subclades ‘Chloroploca+Viridilinea’ and ‘Ca. Chloranaerofilum corporosum’ from O. trichoides is supported by the CSIs identified (Additional file 1: Figure S2a-c). Additionally, the three recently described bacteria have 3-OHP cycle genes and lack Calvin cycle genes.
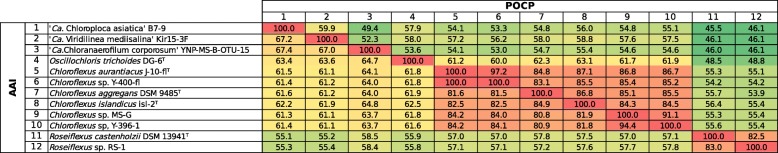
The deep divergence between the subclades O. trichoides and ‘Chloroploca+Viridilinea’ was supported by the results of an analysis of the average amino acid identity and percentage of conserved proteins. AAI was calculated using a web-based tool [22], and POCP was calculated using a script described previously with some modifications [23]. The modified script was published at figshare.com [24]. The results for ‘Ca. Chloranaerofilum corporosum’ should be considered carefully because of the low completeness of the genome (64%) [7], which could lead to misinterpretation, particularly with regard to POCP. The AAI values for ‘Ca. Chloranaerofilum corporosum’ could be overestimated due to the presence of ambiguous amino acids. The 2999 “X” residues were found in a set of all proteins from the genome. Therefore, we will further focus on AAI and PCOP in the comparison of the subclades O. trichoides and ‘Chloroploca+Viridilinea’.
On the one hand, the highest AAI value, about 67, was found for ‘Ca. Chloroploca asiatica’, ‘Ca. Viridilinea mediisalina’ and ‘Ca. Chloranaerofilum corporosum’ (Fig. 3). On the other hand, the values between the subclades O. trichoides and ‘Chloroploca+Viridilinea’ were about 63, which is close to the interfamily values for the Chloroflexaceae and Oscillochloridaceae families (61.6–62.5). Moreover, the POCP values were close to those between the subclades O. trichoides and ‘Chloroploca+Viridilinea’ (57.9–58.0) and between the clades of the Chloroflexaceae and Oscillochloridaceae families (60.0–63.1). These results provide evidence that the listed subclades have significant phylogenetic divergence which corresponds to family-level difference within Chloroflexineae suborder.
Fig. 3.
Results of the analysis of average amino acid identity (AAI) and percentage of conserved proteins (POCP)
The low genomic completeness of the bacterium ‘Ca. Chloranaerofilum corporosum’ limited the pan-genomic comparison and search for CSIs. Nonetheless, it is clear that this bacterium is the closest relative to the subclade ‘Chloroploca+Viridilinea’, based on the results of the phylogenetic analysis and the common CSIs identified (Fig. 2, Additional file 1: Figure S2a-c). The phylogenetic distance is significant according to both the core protein tree and 16S rRNA phylogeny. Importantly, ‘Ca. Chloranaerofilum corporosum’ has distinctive ecophysiological and morphological features. The bacterium forms a native population within the 52.5 °C temperature zone of the Mushroom hot spring [13]. Additionally, gas vesicles were not shown. However, the features were described using environmental observations, and therefore, experimental verification is required. At the moment it is difficult to make an exact taxonomic proposal: Does ‘Chloroploca+Viridilinea’ represent a new family within the Chloroflexineae suborder or not?
Conclusions
Comparative analysis of the new genome of recently described chlorosome-containing FAP bacteria exhibits a trend towards the segregation of new families within the Chloroflexineae suborder. If representatives of the Chloroflexaceae family show phylogenetic uniformity, other bacteria from the Chloroflexineae suborder significantly diverge from each other. The observed “phylogenetic jumps” among lineages within the Chloroflexineae suborder could reflect high underestimation of the genomic diversity of FAP bacteria.
Additional file
Figure S1. Previously reported CSIs: nucleotide sugar dehydrogenase (a) and magnesium-protoporphyrin IX monomethyl ester cyclase (AcsF) proteins (b). Figure S2. CSIs which specific for ‘Ca. Chloroploca asiatica’ B7–9, ‘Ca. Viridilinea mediosalina’ Kir15-3F and ‘Candidatus Chloranaerofilum corporosum’: phosphoglycerate kinase (a), heat-inducible transcription repressor (b), UMP kinase (c). Figure S3. CSIs which specific for ‘Ca. Chloroploca asiatica’ B7–9, ‘Ca. Viridilinea mediosalina’ Kir15-3F, ‘Candidatus Chloranaerofilum corporosum’ and O. trichoides DG-6: threonine synthase (a) and glutamate 5-kinase (b). (PDF 1652 kb)
Acknowledgements
We are grateful to Yulia Ahtitelnova and Alexander Mazur, “I gene” LLC, Moscow, Russian Federation, for their assistance with genome sequencing.
Funding
This study was supported by the Russian Foundation for Basic Research (research projects № 16–34-60071 and 16–34-00835).
Abbreviation
- FAP
filamentous anoxygenic phototrophic
Authors’ contributions
DSG analyzed the genomes and CSIs. MSR worked on sequence assembling and binning. IAB cultured the bacterium ‘Ca. Chloroploca asiatica’. VMG supervised and coordinated the entire project. GVA wrote the manuscript, isolated bacterial DNA and cultured the bacterium ‘Ca. Viridilinea mediisalina’. DSG, VMG and GVA discussed the results and implications reported here. All authors commented on the manuscript before submission. All authors have read and approved the final manuscript.
Authors’ information
You can address your queries about the technical details of the sequencing and analysis of genomic sequences to Denis Grouzdev (denisgrouzdev@gmail.com).
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Keppen OI, Baulina OI, Kondratieva EN. Oscillochloris trichoides neotype strain DG-6. Photosynth Res. 1994;41:29–33. doi: 10.1007/BF02184143. [DOI] [PubMed] [Google Scholar]
- 2.Gich F, Airs RL, Danielsen M, Keely BJ, Abella CA, Garcia-Gil J, et al. Characterization of the chlorosome antenna of the filamentous anoxygenic phototrophic bacterium Chloronema sp. strain UdG9001. Arch. Microbiol. 2003;180:417–426. doi: 10.1007/s00203-003-0608-6. [DOI] [PubMed] [Google Scholar]
- 3.Klappenbach JA, Pierson BK. Phylogenetic and physiological characterization of a filamentous anoxygenic photoautotrophic bacterium ‘Candidatus Chlorothrix halophila’ gen. Nov., sp. nov., recovered from hypersaline microbial mats. Arch. Microbiol. 2004;181:17–25. doi: 10.1007/s00203-003-0615-7. [DOI] [PubMed] [Google Scholar]
- 4.Bañeras L, Gich F, Martinez-Medina M, Miller M, Abella CA, Borrego CM. New phylotypes of mesophilic filamentous anoxygenic phototrophic bacteria enriched from sulfide-containing environments. Environ Microbiol Rep. 2009;1:86–93. doi: 10.1111/j.1758-2229.2008.00009.x. [DOI] [PubMed] [Google Scholar]
- 5.Gorlenko VM, Bryantseva IA, Kalashnikov AM, Gaisin VA, Sukhacheva MV, Gruzdev DS, et al. Candidatus “Chloroploca asiatica” gen. Nov., sp. nov., a new mesophilic filamentous anoxygenic phototrophic bacterium. Microbiology. 2014;83:838–848. doi: 10.1134/S0026261714060083. [DOI] [Google Scholar]
- 6.Pierson BK, Valdez D, Larsen M, Morgan E, Mack EE. Chloroflexus-like organisms from marine and hypersaline environments: distribution and diversity. Photosynth Res. 1994;41:35–52. doi: 10.1007/BF02184144. [DOI] [PubMed] [Google Scholar]
- 7.Thiel V, Hügler M, Ward DM, Bryant DA. The dark side of the mushroom spring microbial mat: life in the shadow of chlorophototorphs. II Metabolic functions of abundant community members predicted from metagenomics analyses. Front Microbiol. 2017;8:943. doi: 10.3389/fmicb.2017.00943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strous M, Kraft B, Bisdorf R, Tegetmeyer HE. The binning of metagenomic contigs for microbial physiology of mixed cultures. Front Microbiol. 2012;3:1–11. doi: 10.3389/fmicb.2012.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, et al. The RAST server: rapid annotations using subsystems technology. BMC Genomics. 2008;9:75. doi: 10.1186/1471-2164-9-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parks DH, Imelfort M, Skennerton CT, Hugenholtz P, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 2015;25:1043–1055. doi: 10.1101/gr.186072.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tatusova T, DiCuccio M, Badretdin A, Chetvernin V, Nawrocki EP. NCBI prokaryotic genome annotation pipeline. Nucleic Acids Res. 2016;44:6614–6624. doi: 10.1093/nar/gkw569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tank Marcus, Thiel Vera, Ward David M., Bryant Donald A. Modern Topics in the Phototrophic Prokaryotes. Cham: Springer International Publishing; 2017. A Panoply of Phototrophs: An Overview of the Thermophilic Chlorophototrophs of the Microbial Mats of Alkaline Siliceous Hot Springs in Yellowstone National Park, WY, USA; pp. 87–137. [Google Scholar]
- 14.Gupta RS, Chander P, George S. Phylogenetic framework and molecular signatures for the class Chloroflexi and its different clades; proposal for division of the class Chloroflexi class. Nov. into the suborder Chloroflexineae subord. Nov., consisting of the emended family Oscillochloridaceae and the family Chloroflexaceae fam. Nov., and the suborder Roseiflexineae subord. Nov., containing the family Roseiflexaceae fam. Nov. Antonie Van Leeuwenhoek. 2013;103:99–119. doi: 10.1007/s10482-012-9790-3. [DOI] [PubMed] [Google Scholar]
- 15.Grouzdev DS, Kuznetsov BB, Keppen OI, Krasil’nikova EN, Lebedeva NV, Ivanovsky RN. Reconstruction of bacteriochlorophyll biosynthesis pathways in the filamentous anoxygenic phototrophic bacterium Oscillochloris trichoides DG-6 and evolution of anoxygenic phototrophs of the order Chloroflexales. Microbiology. 2014;161:120–130. doi: 10.1099/mic.0.082313-0. [DOI] [PubMed] [Google Scholar]
- 16.Pierson BK, Castenholz RW. A phototrophic gliding filamentous bacterium of hot springs, Chloroflexus aurantiacus, gen. And sp. nov. Arch. Microbiol. 1974;100:5–24. doi: 10.1007/BF00446302. [DOI] [PubMed] [Google Scholar]
- 17.Hanada S. Hiraishi A, Shimada K, Matsuura K. Chloroflexus aggregans sp. nov., a filamentous phototrophic bacterium which forms dense cell aggregates by active gliding movement. Int. J Syst Bacteriol. 1995;45:676–681. doi: 10.1099/00207713-45-4-676. [DOI] [PubMed] [Google Scholar]
- 18.Gaisin VA, Kalashnikov AM, Grouzdev DS, Sukhacheva MV, Kuznetsov BB, Gorlenko VM. Chloroflexus islandicus sp. nov., a thermophilic filamentous anoxygenic phototrophic bacterium from geyser Strokkur (Iceland) Int J Syst Evol Microbiol. 2017;67:1381–1386. doi: 10.1099/ijsem.0.001820. [DOI] [PubMed] [Google Scholar]
- 19.Zarzycki J, Brecht V, Müller M, Fuchs G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc Natl Acad Sci U S A. 2009;106:21317–21322. doi: 10.1073/pnas.0908356106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gorlenko VM. Characteristics of filamentous phototrophic bacteria from freshwater lakes. Mikrobiologiia. 1975;44:756–758. [PubMed] [Google Scholar]
- 21.Keppen OI, Tourova TP, Kuznetsov BB, Ivanovsky RN, Gorlenko VM. Proposal of Oscillochloridaceae fam. nov. on the basis of a phylogenetic analysis of the filamentous anoxygenic phototrophic bacteria, and emended description of Oscillochloris and Oscillochloris trichoides in comparison with further new isolates. Int J Syst Evol Microbiol. 2000;50:1529–1537. doi: 10.1099/00207713-50-4-1529. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez-R LM, Konstantinidis KT. The enveomics collection : a toolbox for specialized analyses of microbial genomes and metagenomes. Peer J Prepr. 2016. 10.7287/peerj.preprints.1900v1.
- 23.Harris HMB, Bourin MJB, Claesson MJ, O’Toole PW. Phylogenomics and comparative genomics of Lactobacillus salivarius, a mammalian gut commensal. Microb Genomics. 2017. 10.1099/mgen.0.000115. [DOI] [PMC free article] [PubMed]
- 24.Pantiukh K, Grouzdev D. POCP-matrix calculation for a number of genomes. Figshare. 2017. 10.6084/m9.figshare.4577953.v1.
- 25.Field D, Garrity G, Gray T, Morrison N, Selengut J, Sterk P, et al. The minimum information about a genome sequence (MIGS) specification. Nat. Biotechnol. 2008;26:541–547. doi: 10.1038/nbt1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woese CR, Kandler O, Wheelis ML. Towards a natural system of organisms: proposal for the domains archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990;87:4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Validation List No. 85: Validation of publication of new names and new combinations previously effectively published outside the IJSEM. Int J Syst Evol Microbiol. 2002;52:685–90. [DOI] [PubMed]
- 28.Oren A, Da Costa MS, Garrity GM, Rainey FA, Rosselló-Móra R, Schink B, et al. Proposal to include the rank of phylum in the international code of nomenclature of prokaryotes. Int J Syst Evol Microbiol. 2015;65:4284–4287. doi: 10.1099/ijsem.0.000664. [DOI] [PubMed] [Google Scholar]
- 29.Whitman WB, Oren A, Chuvochina M, da Costa MS, Garrity GM, Rainey FA, et al. Proposal of the suffix–ota to denote phyla. Addendum to ‘proposal to include the rank of phylum in the international code of nomenclature of prokaryotes’. Int J Syst Evol Microbiol. 2018;68:967–969. doi: 10.1099/ijsem.0.002593. [DOI] [PubMed] [Google Scholar]
- 30.Oren A, Garrity GM. List of new names and new combinations previously effectively, but not validly, published. Int J Syst Evol Microbiol. 2013;63:1577–1580. doi: 10.1099/ijs.0.052571-0. [DOI] [PubMed] [Google Scholar]
- 31.Consortium TGO. Gene ontologie: tool for the unification of biology. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carbon S, Dietze H, Lewis SE, Mungall CJ, Munoz-Torres MC, Basu S, et al. Expansion of the gene ontology knowledgebase and resources: the gene ontology consortium. Nucleic Acids Res. 2017;45:D331–D338. doi: 10.1093/nar/gkw1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Previously reported CSIs: nucleotide sugar dehydrogenase (a) and magnesium-protoporphyrin IX monomethyl ester cyclase (AcsF) proteins (b). Figure S2. CSIs which specific for ‘Ca. Chloroploca asiatica’ B7–9, ‘Ca. Viridilinea mediosalina’ Kir15-3F and ‘Candidatus Chloranaerofilum corporosum’: phosphoglycerate kinase (a), heat-inducible transcription repressor (b), UMP kinase (c). Figure S3. CSIs which specific for ‘Ca. Chloroploca asiatica’ B7–9, ‘Ca. Viridilinea mediosalina’ Kir15-3F, ‘Candidatus Chloranaerofilum corporosum’ and O. trichoides DG-6: threonine synthase (a) and glutamate 5-kinase (b). (PDF 1652 kb)