Abstract
Novel treatments against migraine are an urgent medical requirement. α6 subunit-containing GABAA receptors (α6GABAARs) are expressed in trigeminal ganglia (TG), the hub of the trigeminal vascular system (TGVS) that is involved in the pathogenesis of migraine. Here we reveal an unprecedented role of α6GABAARs in ameliorating TGVS activation using several pharmacological approaches in an animal model mimicking pathological changes in migraine. TGVS activation was induced by intra-cisternal (i.c.) instillation of capsaicin in Wistar rats. Centrally, i.c. capsaicin activated the trigeminal cervical complex (TCC) measured by the increased number of c-Fos-immunoreactive (c-Fos-ir) TCC neurons. Peripherally, it elevated calcitonin gene-related peptide immunoreactivity (CGRP-ir) in TG and depleted CGRP-ir in the dura mater. Pharmacological approaches included a recently identified α6GABAAR-selective positive allosteric modulator (PAM), the pyrazoloquinolinone Compound 6, two α6GABAAR-active PAMs (Ro15–4513 and loreclezole), an α6GABAAR-inactive benzodiazepine (diazepam), an α6GABAAR-selective antagonist (furosemide), and a clinically effective antimigraine agent (topiramate). We examined effects of these compounds on both central and peripheral TGVS responses induced by i.c. capsaicin. Compound 6 (3–10 mg/kg, i.p.) significantly attenuated the TCC neuronal activation and TG CGRP-ir elevation, and dural CGRP depletion induced by capsaicin. All these effects of Compound 6 were mimicked by topiramate, Ro15–4513 and loreclezole, but not by diazepam. The brain-impermeable furosemide antagonized the peripheral, but not central, effects of Compound 6. These results suggest that the α6GABAAR in TG is a novel drug target for TGVS activation and that α6GABAAR-selective PAMs have the potential to be developed as a novel pharmacotherapy for migraine.
Keywords: trigeminovascular activation, α6GABAAR, positive allosteric modulator, trigeminal ganglia, calcitonin gene-related peptide
1. Introduction
GABAA receptors (GABAARs), the major inhibitory transmitter receptors in the brain, are composed of five subunits that form a central chloride channel. Nineteen GABAAR subunits (6α, 3β, 3γ, δ, ε, π, θ, 3ρ) have been identified (Olsen and Sieghart, 2008), and the majority of GABAA receptors in the mammalian brain is composed of 2α, 2β, and 1γ subunits (αβγGABAARs) (Olsen and Sieghart, 2008). Diazepam is a classical benzodiazepine exerting its anxiolytic, sedative, antiepileptic and muscle relaxant activities by acting as a positive allosteric modulator (PAM) of αβγGABAARs containing α1, α2, α3 or α5 subunits (Rudolph and Knoflach, 2011). The diazepam-insensitive α6 subunit-containing GABAA receptors (α6GABAARs), however, have been much less investigated due to a lack of a highly selective pharmacological ligand. Recently, we have identified several pyrazoloquinolinones (Treven et al., 2018; Varagic et al., 2013a; Varagic et al., 2013b) and their deuterated derivatives (Knutson et al., 2018) as PAMs highly selective for GABAARs consisting of α6β2/3γ2 subunits, and displayed their lack of sedative, ataxic and cytotoxic effects in rodents (Knutson et al., 2018). Particularly, Compound 6 (originally coded as PZ-II-029) (Chiou et al., 2018; Varagic et al., 2013a; Varagic et al., 2013b; Zhang et al., 1995), a highly selective α6GABAAR PAM, has recently been used to explore the functional role of cerebellar α6GABAARs in the regulation of sensorimotor gating in our previous study (Chiou et al., 2018).
α6GABAARs are mainly expressed in cerebellar granular cells (Gutierrez et al., 1996; Pirker et al., 2000), but also in some sensory neurons (Gutierrez et al., 1996) including trigeminal ganglia (TG) (Puri et al., 2012). Recently, it was demonstrated that the α6 subunit-expressing TG neurons project to the temporomandibular joint (TMJ) of rats (Puri et al., 2011), and that rats with a 30% reduction of TG α6GABAARs showed hyperalgesia to TMJ inflammation (Puri et al., 2012). This suggests that TG α6GABAARs are important for inhibiting primary sensory afferents in the trigeminal pathway, supporting previous evidence for the presence of functional GABAARs in TG (Hayasaki et al., 2006).
In addition to TMJ disorders, TG are also involved in the pathogenesis of migraine. TG neurons receive the peripheral input from dural vessels via the ophthalmic branch of trigeminal sensory nerves and send central projections to the trigeminal cervical complex (TCC) (Scheme 1) that subsequently projects to higher-order pain centers (Bae et al., 2004; Goadsby, 2006). The TCC includes the trigeminal nucleus caudalis (TNC) and the upper cervical spinal dorsal horn (C1/2) (Shigenaga et al., 1988). TG in combination with their peripherally innervated dural vessels and centrally-projected TCC form the trigemino-vascular system (TGVS). TGVS activation via both peripheral and central sensitizations and releasing calcitonin gene related peptide (CGRP) (Goadsby et al., 1988) is considered an essential neuropathogenic mechanism of migraine (Scheme 1). Peripheral sensitization of the TGVS is attributed to neurogenic inflammation in the meninges, which is characterized by vasodilation due to CGRP released from dural sensory nerve terminals, plasma extravasation secondary to capillary leakage, edema, and mast cell degranulation (Ramachandran, 2018). Central sensitization of the TGVS due to TNC activation is also involved in nociceptive processing and cerebrovascular regulation, and contributes to migraine (Lance et al., 1983).
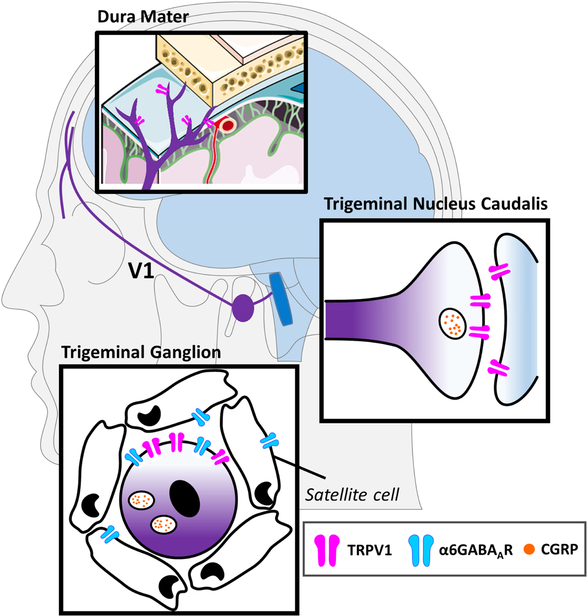
Scheme 1.
A scheme illustrating the location of peripheral and central sites of the trigeminal vascular system (TGVS) that are involved in migraine. The peripheral sites (purple) include the sensory nerve endings of the ophthalmic (V1) branch of the trigeminal nerve in the dura mater as well as the cell bodies of the trigeminal nerve fibers in the trigeminal ganglion (TG). The central site (blue) of the TGVS consists of the trigeminal nucleus caudalis (TNC) and the upper cervical spinal dorsal horn (not shown) that form the trigeminal cervical complex, which receive sensory inputs from the central terminals of TG neurons. From here, the sensory signal will be transmitted to higher brain centers for further integration and processing. TG neurons are surrounded by satellite cells. The peripheral structures of the TGVS are not enclosed by the blood-brain barrier (BBB). The image of meninges is adapted from a free medical image provider (Servier Medical Art, Servier). The images of human brain and neurons are adapted from Illustration Toolkit Neuroscience by Motifolio.
Capsaicin is an agonist of vanilloid 1 type of transient receptor potential channels (TRPV1) that are co-expressed with CGRP and substance P in small and medium sized unmyelinated TG neurons (Bae et al., 2004; Caterina and Julius, 2001). TRPV1 activation in TG causes the release of CGRP that induces vasodilation and neurogenic inflammation within the meninges in experimental animals, mimicking migraine (Meents et al., 2010). Given the possibility that TG α6GABAARs might be involved in inhibiting primary sensory afferents of the trigeminal system (Puri et al., 2012) we hereby investigated whether α6GABAAR PAMs can counteract both peripheral and central effects of capsaicin given by intracisternal (i.c.) injection in rats, an established rat model of TGVS activation to mimic migraine (Cutrer et al., 1999; Cutrer and Moskowitz, 1996; Fan et al., 2012).
In addition to the α6GABAAR-selective Compound 6, Ro 15–4513 (Hadingham et al., 1996) and loreclezole (Wafford et al., 1994), two PAMs of GABAARs including, but not selective to, α6GABAARs, were used as positive controls. Besides, diazepam, an α6GABAAR-inactive PAM (Hevers and Luddens, 2002; Whittemore et al., 1996), was used as a negative control. For comparison, we also examined the effects of topiramate, a clinical effective anti-migraine agent. Results obtained suggest that a positive modulation of α6GABAARs can ameliorate capsaicin-induced changes in the TGVS with an efficacy similar to topiramate, indicating that α6GABAARs are novel targets for the development of anti-migraine agents. Finally, we substantiated that the targeted α6GABAARs are located in TG by confirming that systemic administration of furosemide, an allosteric α6GABAAR antagonist that does not cross the blood-brain barrier (BBB) (Seelig et al., 1994), is able to antagonize effects of Compound 6.
2. Materials and Methods
2.1. Animals
All animal care and experimental protocols were approved by the Institution of Care and Use of Laboratory Animals of the College of Medicine of National Taiwan University. Male Wistar rats at 8–10 weeks (250–300 g) were used. They were housed in an animal room with a 12-h light/12-h dark cycle and free access to food and water.
2.2. Intra-cisternal instillation of capsaicin
The TGVS activation model induced by i.c. instillation of capsaicin is similar to our previous study (Fan et al., 2012). Briefly, rats were anesthetized by chloral hydrate (400 and 100 mg/kg, i.p. for inducing and maintaining anesthesia, respectively) and catheterized with a catheter (PE-10, SIMS Portex Ltd, Hythe, UK) inserted 3 mm deep into the cisterna magna. Rats were then placed in a prone position for 5.5 hours. The capsaicin solution (10 nmol, 100 μl) was instilled through the catheter into the cisterna magna over 1 min. The rats were then placed in a reverse Trendelenburg position (−30 degrees) for 30 min to facilitate capsaicin distribution within the subarachnoid space, which was followed by a prone position for another 90 min. Rats were pretreated with Compound 6 or its vehicle by i.p. (100 μl) injection 30 min before capsaicin instillation. Furosemide (20 mg/kg, i.p.) (Agunu et al., 2005) or its vehicle was co-administered with Compound 6. For the sham group, 100 μl of the vehicle of capsaicin was administered by i.c. instillation.
Two hours after capsaicin instillation, the rat was euthanized by an overdose of chloral hydrate and then perfused via the ascending aorta with paraformaldehyde (4%) as described previously (Fan et al., 2012) for further immunostaining measurements.
2.3. Immunohistochemistry of c-Fos protein in TCC sections
The preparation of TCC sections and c-Fos immunohistochemistry were conducted as described previously (Fan et al., 2012). Briefly, brainstem with attached cervical cord was serially sectioned (50 μm) using a cryostat (LEICA CM3050S, Nussloch, Germany) from 1 mm rostral to the obex to the C6 level of the spinal cord. The sections at +0.6, −1.2, and −9 mm from the obex of the rat were collected and subjected to c-Fos immunohistochemical staining. The total number of c-Fos immunoreactive (c-Fos-ir) TCC neurons was estimated based on the formula derived previously (Fan et al., 2012): 16(N1 + N2) /2 + 53(N2 + N3) /2, where N1, N2, and N3 were the c-Fos-ir neuronal numbers measured at the level of 0.6, −1.2, and −9 mm from the obex, respectively.
Free-floating immunohistochemistry of c-Fos protein was conducted using the avidin-biotin method as described previously (Fan et al., 2012) with an anti-c-Fos rabbit polyclonal antibody (1:7000 dilution, Calbiochem, San Diego, CA, USA), biotinylated anti-rabbit IgG (1:200, Vector Labs, Burlingame, CA, USA), and horseradish peroxidase avidin D (1:500, Vector Labs, Burlingame, CA, USA). Immunoreactions were visualized using the DAB Reagent kit (KPL, Gaithersburg, MD, USA).
c-Fos-ir neurons, i.e., neurons with stained nuclei, were counted under a microscope (Olympus BX51, Essex, UK). Data were reviewed by an investigator who was blinded to the treatment groups.
2.4. TG slice sections and CGRP immunofluorescence quantification
From each rat, two TG preparations were dissected and then serially sectioned at 50 μm thickness using a microtome (LEICA RM2245, Nussloch, Germany). Nine TG sections were dissected from the central part of either the left or the right ganglion of a rat. Then, every third section was sampled for CGRP immunofluorescence, i.e. the immunofluorescent data was taken from three TG sections from each rat.
TG sections were incubated in blocking solution (PBS containing 5 % normal goat serum, 0.2 % Triton X-100) for one hour at room temperature. Sections were then incubated overnight at 4°C with the rabbit primary antibody against CGRP (1:200; EMD Millipore, Burlington, MA, USA) and left overnight at 4°C in fluorescein-conjugated goat anti-rabbit IgG (1:50; Vector Labs, Burlingame, CA, USA) secondary antibody solution. Sections were then placed on microscope slides and mounted with Aqua Poly/Mount (Polyscience Inc., Warrington, PA, USA). The sliced tissues were examined using an inverted light microscope (Zeiss Axio Observer. D1; Carl Zeiss, Jena, Germany). All analyses were calculated under a field of TG at 100X magnification by the software of Image J. The expression intensity of CGRP was calculated from the immunoreactivity that displayed as the optical density multiplies activated areas in each image.
2.5. Dura mater preparations and quantification of CGRP density by immunohistochemistry
The dura mater dissected from the cranial cavity of the rat was prepared for CGRP immunohistochemical staining as described previously (Fan et al., 2012). In brief, identical areas in six similar locations of the dura for each animal were selected and examined under an inverted microscope (ZEISS Axio Observer.D1, Jena, Germany). For each animal, the total lengths of positive-stained segmented lines in the field (100X) were measured in pixels by the software of Image J.
Immunohistochemistry of CGRP was performed with the avidin-biotin method with a protocol similar to that for c-Fos staining except using a 30 min-blocking incubation, anti-CGRP rabbit polyclonal antibody (1:1000, Calbiochem, San Diego, CA, USA), and horseradish peroxidase avidin D (1:200 Vector Labs, Burlingame, CA, USA).
2.6. Double immunofluorecent staining of CGRP and α6GABAARs in TG
TG sections were prepared as described in Section 2.4. After incubation in donkey serum blocking solution for 1 hour, sections were incubated overnight at 4°C with the goat primary antibody against CGRP (1:100; Cat: ab36001, Lot: GR3186077–10, Abcam, Cambridge, UK) followed by Alexa Fluor 594-conjugated donkey anti-goat secondary antibody (1:300; Cat: 705–585-147, Lot: 130926, Life Technologies, Carlsbad, CA, USA) at room temperature for one hour. Next, sections were incubated with the rabbit primary antibody against the α6 subunit of GABAARs (1:100; Cat: ab92747, Lot: GR272612–5, Abcam, Cambridge, UK), whose specificity has been confirmed by negative data after blocking of the antibody by the immunizing peptide. (http://www.abcam.com/gaba-a-receptor-alpha-6-antibody-ab92747.html#description_images_1) (Yang et al., 2016). The secondary antibody used is Alexa Fluor 488-conjugated donkey anti-rabbit antibody (1:300; Cat: 406416, Lot: B243796, BioLegend, San Diego, CA, USA). The double stained sections were mounted on slides with ProLong Gold Antifade Mountant (Thermo Fischer Scientific, Waltham, MA, USA) and visualized under a confocal microscope (LSM 780; Carl Zeiss, Jena, Germany).
To confirm the specificity of the secondary antibody, we incubated TG preparations with the secondary antibody, only. This resulted in no staining of the tissue section (Figure S1A). To further confirm the expression of α6GABAARs in TG, we repeated the immunohistochemical staining in TG preparations using another primary antibody against the a6 subunit of GABAARs (1:100; NB300–196, Lot: kn205, Novus Biologicals, Littleton, CO). Its specificity has been confirmed by an absence of staining of the a6 subunit in a6-knock-out mice, as indicated by the supplier (https://www.novusbio.com/products/gaba-a-r-alpha-6-antibody_nb300-196).
2.7. Drugs
Capsaicin (Sigma Chemical, St. Louis, MO, USA) was dissolved in the vehicle containing 10% ethanol and 10% Tween 80, sonicated for 5 min, and then further diluted (1:100) in an artificial cerebrospinal fluid solution (aCSF) as a stock solution stored at 4°C. The aCSF consisted of the followings (in mm): 117 NaCl, 4.5 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.2 NaH2PO4, 25 NaHCO3, and 11.4 dextrose bubbled with 95% O2/5% CO2, pH 7.4. Compound 6 was synthesized as reported previously (Varagic et al., 2013a). Loreclezole, Ro15–4513, and diazepam (under the approval from Taiwan Food and Drug Administration, the Ministry of Health and Welfare, Taiwan) were purchased from Tocris Bioscience (Bristol, UK) and furosemide from Sigma-Aldrich (St. Louis, USA). All compounds were dissolved in a vehicle containing 20% DMSO, 20% Cremophor® EL (polyoxyethylene castor, Sigma-Aldrich) and 60% normal saline.
2.8. Drug treatments and statistical analysis
Rats were randomly divided into 11 groups. Among them, ten groups received i.c. capsaicin instillation and one group received i.c. saline instillation as the sham control group. Each one of the ten capsaicin-treated groups was treated differently; being pretreated with 1, 3, or 10 mg/kg (i.p.) of Compound 6 or its vehicle, or with topiramate, loreclezole, Ro 154513, diazepam, or 3 mg/kg compound 6 with and without furosemide, respectively, at 30 min before capsaicin instillation.
All statistical analyses were performed with IBM SPSS Statistics 20 for Windows. Data were expressed as mean±S.E. Differences among groups were compared using the Kruskal-Wallis test. Then, differences between the tested group versus the capsaicin-treated group (Family-1, Table S1) or versus the sham-control group (Family-2, Table S2) were compared using the Mann-Whitney U test followed by the Benjamini–Hochberg correction (BHC) , which was used to control the false positive rate due to multiple comparisons (McDonald, 2014). The p value of each comparing pair in a family was ranked. The difference was considered statistically significant when a p value < BHC value, which is the p value multiplied by i/m; m is the total number of comparisons in each family, and i is the rank of the p value in the family. The n numbers are the numbers of rats tested in each treatment group. From each rat, TCC, TG and dura tissue samples were prepared.
3. Results
As reported previously (Cutrer et al., 1995; Fan et al., 2012), i.c. capsaicin (10 nmol) instillation in rats significantly activated the TGVS in both central and peripheral sites. Centrally, it induced neuronal activation in the TCC, which can be measured by the increased number of c-Fos-ir TCC neurons (Fig. 1A, Cap vs. Sham). In the periphery, capsaicin led to increased CGRP-ir in TG (Fig. 2A, Cap vs. Sham) and CGRP release from TG peripheral terminals as indicated by the depletion of CGRP-ir in the dura mater (Fig. 3A, Cap vs. Sham).
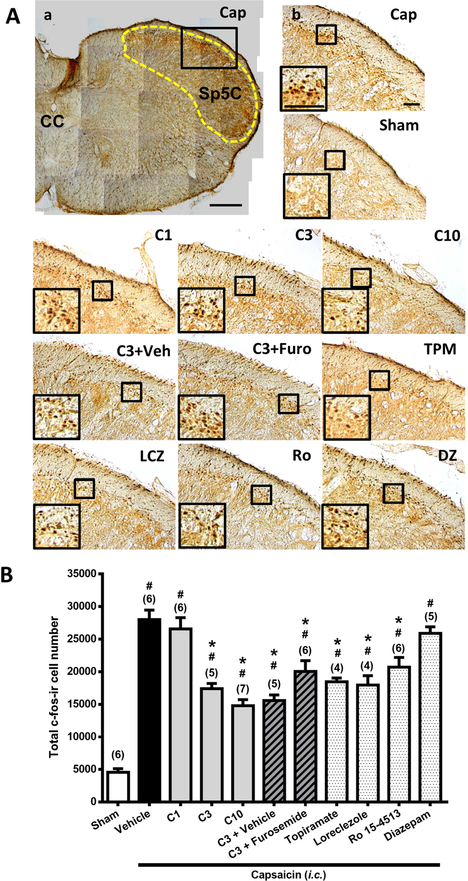
Figure 1. Effects of Compound 6, topiramate, loreclezole, Ro 15–4513, diazepam on capsaicin-induced neuronal activation and anti-α6GABAAR effects of furosemide in the trigemino-cervical complex (TCC).
Immunohistograms (A) and the total number of activated neurons (B), i.e. c-Fos-immunoreactive (c-Fos-ir) neurons, in the TCC of rats having received intracisternal (i.c.) instillation of 10 nmol capsaicin in the group pretreated with i.p. injection of Compound 6 at 1 (C1), 3 (C3) or 10 (C10) mg/kg, 30 mg/kg topiramate (TPM), 40 mg/kg loreclezole (LCZ), 5 mg/kg Ro 15–4513 (Ro) or 4 mg/kg diazepam (DZ), or their vehicle (a, b, Cap), as well as with 3 mg/kg Compound 6 plus 20 mg/kg furosemide (i.p.) (C3+Furo) or Compound 6 plus the vehicle of furosemide (C3+Veh). The sham group received i.c. instillation of the saline instead of capsaicin (Sham). A close-up image (inset ) of c-Fos-containing TCC neurons is shown in each treatment group. Note that Compound 6 at 3 or 10, but not 1, mg/kg significantly attenuates capsaicin-induced TCC neuronal activation. This effect of Compound 6 is comparable to topiramate, a clinically effective antimigraine drug, and mimicked by loreclezole and Ro 15–4513, two α6GABAAR-acting PAMs, but not by the α6GABAAR-inactive diazepam. The inhibitory effect of Compound 6 in the TCC was not significantly reversed by furosemide, a highly selective allosteric inhibitor for α6GABAARs. Scale bar: 500 μm (a); 100 μm (b). CC: central canal; Sp5C: spinal trigeminal nucleus caudalis. Data are mean+SE. The n number shown in parentheses is the number of tested rats. * p< 0.005* i, vs. the Vehicle (Capsaicin) group;# p< 0.005* i, vs. the Sham group; i: the rank of p value (Table S1 and S2). (Kruskal-Wallis test followed by post hoc Mann-Whitney U test with Benjamini–Hochberg correction).
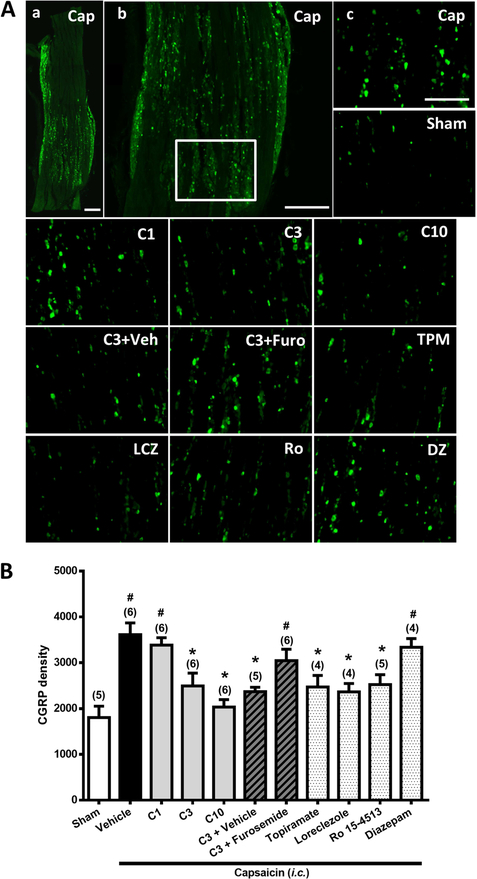
Figure 2. Effects of Compound 6, topiramate, loreclezole, Ro 15–4513, or diazepam on capsaicin-induced neuronal activation and anti-α6GABAAR effects of furosemide in the trigeminal ganglia (TG).
Immunohistograms (A) and the CGRP-ir (B) in TG of rats in i.c. capsaicin-treated group with Vehicle (a, b, Cap) and various pretreatments, respectively, as in Figure 1, as well as in the Sham group (Sham). Note that Compound 6 at 3 or 10, but not 1, mg/kg significantly prevents capsaicin-induced CGRP-ir in TG in a manner comparable to topiramate. The capsaicin-induced CGRP-ir inhibited by Compound 6 at 3 mg/kg is significantly reversed by furosemide. Loreclezole and Ro 15–4513 significantly prevent capsaicin-induced CGRP-ir in TG, but diazepam does not. Scale bar: 500 μm (a, b), 250 μm (c). In each rat, total CGRP-ir fluorescence in 3 TG sections was measured. Shown is the average CGRP-ir fluorescence of all tested rats. Data presentation and statistical analyses are the same as in Fig. 1.
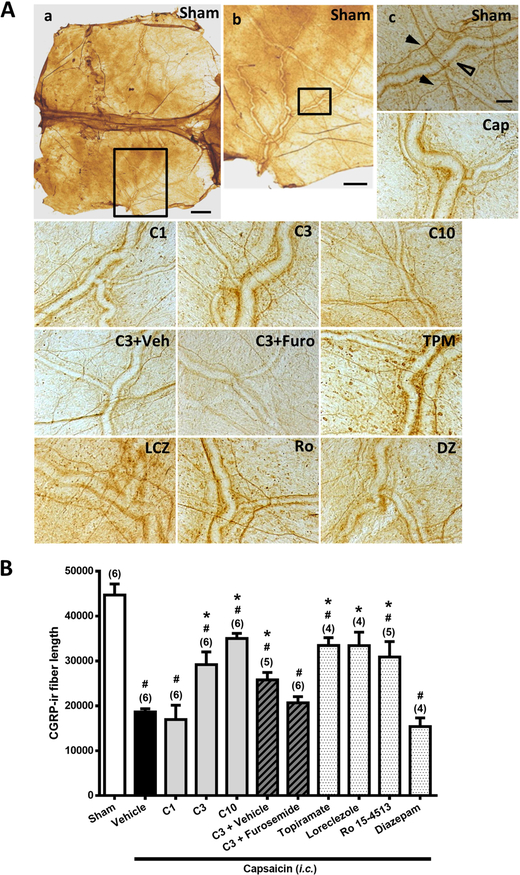
Figure 3. Effects of Compound 6, topiramate, loreclezole, Ro 15–4513, or diazepam on capsaicin-induced neuronal activation and anti-α6GABAAR effects of furosemide in the dura mater.
Immunohistograms (A) and the average of total length of CGRP-ir nerve fiber length (B) in the dura mater of rats in i.c. capsaicin-treated group with various pretreatments as in Figure 1, as well as in Sham group (a, b). Note that Compound 6 at 3 or 10, but not 1, mg/kg significantly suppressed capsaicin-induced depletion of dura CGRP-ir in a manner comparable to topiramate. The capsaicin-induced CGRP depletion inhibited by Compound 6 at 3 mg/kg is significantly reversed by furosemide. Loreclezole and Ro 15–4513 significantly inhibit capsaicin induced CGRP depletion in the dura, but diazepam does not. Scale bar: 1000 μm (a), 400 (b) and 100 μm (c). The length of CGRP-ir nerve fiber (arrowhead), stained by immunohistochemistry, in the dura mater was quantified by Image J. The total length of CGRP-ir fiber length (in pixel) in six fixed comparable areas of the dura mater in each rat was collected. Arrow head: CGRP-ir nerve fiber; Open arrow head: dural vessel. Data presentation and statistical analyses are the same as in Fig. 1.
3.1. Compound 6 attenuated capsaicin-induced TCC neuronal activation.
Figure 1B shows the total number of c-Fos-ir TCC neurons in the groups of rats that received i.c. instillation of capsaicin with various pre-treatments as well as the sham control group that received i.c. saline instillation only. The differences among groups were significant (p<0.001, Kruskal-Wallis test). The Mann-Whitney U test followed by the Benjamini–Hochberg correction was used to compare each treatment group with the capsaicin group (Family 1, Table S1) or with the sham group (Family 2, Table S2). In total, 10 comparisons in each family have been conducted.
The number of c-Fos-ir TCC neurons was significantly increased in rats receiving capsaicin instillation, compared to the sham group (p=0.004, Table S2, Sham vs. Vehicle-Cap, Fig. 1B ), as reported in our previous study (Fan et al., 2012). In rats pretreated with Compound 6, the number of capsaicin-induced c-Fos-ir TCC neurons was decreased significantly at the dose of 3 (p=0.006, Fig. 1A, C3 vs. Vehicle-Cap) and 10 (p=0.003, Fig. 1A, C10 vs. Vehicle-Cap) mg/kg, but not 1 mg/kg (p>0.05, Fig. 1A, C1 vs. Vehicle-Cap), compared to the vehicle-pretreated capsaicin group (Fig. 1A, Fig. 1B, Table S1). These results suggest that Compound 6 significantly attenuates capsaicin-induced neuronal activation in the TCC. However, even at 10 mg/kg, Compound 6 was unable to reduce the number of activated TCC neurons to the level as in the sham group (3 and 10 mg/kg Compound 6 vs. Sham, = C3 vs. Sham and C10 vs. Sham, p=0.006 and 0.003, respectively; Fig. 1A, Fig. 1B, Table S2).
Topiramate, a clinically effective anti-migraine agent, also attenuated capsaicin-induced TCC neuronal activation (p=0.011, Fig. 1A, Fig. 1B, TPM vs. Vehicle-Cap), at an effective dose (30 mg/kg, i.p.) reported previously in another migraine model (Andreou and Goadsby, 2011). Interestingly, topiramate reduced the activated TCC neuronal number to a level that is still higher than in the sham group (p=0.011, Fig. 1A, Fig. 1B, TPM vs. Sham). Therefore, topiramate, like Compound 6, did not completely abolish i.c. capsaicin-induced TCC neuronal activation.
3.2. Compound 6 suppressed capsaicin-induced CGRP-ir in TG.
Figure 2 shows the CGRP-ir in TG of rats in various groups as in Fig. 1. The differences among groups were significant (p<0.001, Kruskal-Wallis test). The Mann-Whitney U test followed by the Benjamini–Hochberg correction shows that the CGRP-ir in TG in the capsaicin-treated group was significantly higher than in the sham group (p=0.006, Fig. 2A, Fig. 2B, Vehicle-Cap vs. Sham), as reported in our previous study (Fan et al., 2012). Compound 6 dose-dependently reduced capsaicin-induced elevation of CGRP-ir in TG (Fig. 2B). The TG CGRP-ir was significantly suppressed by Compound 6 at the dose of 3 (p=0.018, Fig. 2A, C3 vs. Vehicle-Cap) and 10 mg/kg (p=0.004, Fig. 2A, C10 vs. Vehicle-Cap), but not 1 mg/kg (p>0.05, Fig. 2A, C1 vs. Vehicle-Cap), as compared to the vehicle-capsaicin group. The TG CGRP-ir level was suppressed to the level as in the sham group by 3 or 10 mg/kg of Compound 6 (3 and 10 mg/kg Compound 6 vs. Sham, = C3 vs. Sham and C10 vs. Sham, p=0.347 and 0.715, respectively; Fig. 2A, Fig. 2B, Table S2).
Similarly, topiramate completely suppressed capsaicin-induced elevation of CGRP-ir in TG (p=0.011, Fig. 2A, Fig. 2B, TPM vs. Vehicle-Cap). The CGRP-ir in the topiramate-pretreated group was significantly reduced to the level of the sham group (p=0.142, Fig. 2A, Fig. 2B, TPM vs. Sham).
3.3. Compound 6 rescued capsaicin-induced depletion of CGRP-ir in the dura mater.
Figure 3 shows the CGRP-ir, quantified by the CGRP-ir nerve fiber length, in the dura mater of rats in various treatment groups as in Fig. 1. The differences among groups were significant (p<0.001, Kruskal-Wallis test). The Mann-Whitney U test followed by the Benjamini–Hochberg correction shows that after i.c. capsaicin instillation, the CGRP-ir in the dura mater was significantly depleted (Fig. 3A, Fig. 3B, Vehicle-Cap vs. Sham) as reported previously (Fan et al., 2012), which was demonstrated by the reduction of CGRP-ir nerve fiber length in the dura mater of capsaicin-treated group (p=0.004, Fig. 3A, Vehicle-Cap vs. Sham), as compared to the sham group. Compound 6 significantly rescued capsaicin-induced CGRP depletion, demonstrated by the partially regained CGRP-ir fiber length in the dura mater in groups pretreated with Compound 6 at 3 (p=0.004, Fig. 3A, C3 vs. Vehicle-Cap; p=0.025, C3 vs. Sham) and 10 mg/kg (p=0.004, Fig. 3A, C10 vs. Vehicle-Cap; p=0.004, C10 vs. Sham), but not 1 mg/kg (p>0.05, Fig. 3A, C1 vs. Vehicle-Cap).
Interestingly, topiramate also significantly rescued capsaicin-induced CGRP depletion in the dura mater (p=0.011, Fig. 3A, TPM vs. Vehicle-Cap). However, there was still a significant difference between the TPM and the sham groups (p=0.011, Fig. 3A, TPM vs. Sham), suggesting only a partial recovery.
3.4. Loreclezole and Ro15–4513, two α6GABAAR-acting PAMs, mimicked effects of Compound 6 in capsaicin-induced migraine model.
Loreclezole is a PAM of GABAARs containing β2 or β3 subunits and any one of the six α subunits, including α6GABAARs (Wafford et al., 1994; Whittemore et al., 1996) acting through a binding site different from that for benzodiazepines (Wafford et al., 1994). Loreclezole, at 40 mg/kg (i.p.) that was effective in increasing GABAergic sensitivity in an alcohol-intolerant rat model (Wong et al., 1996), significantly reduced the number of capsaicin-increased c-Fos-ir TCC neurons (p=0.011, Fig. 1A, Fig. 1B, LCZ vs. Vehicle-Cap), prevented capsaicin-induced elevation of CGRP-ir in TG (p=0.011, Fig. 2A, Fig. 2B, LCZ vs. Vehicle-Cap), and significantly rescued capsaicin-induced depletion of dural CGRP-ir (p=0.011, Fig. 3A, Fig. 3B, LCZ vs. Vehicle-Cap).
Ro 15–4513 is an imidazobenzodiazepine acting as a PAM at the benzodiazepine binding site of α4- and α6-subunit-containing GABAARs but also as a negative allosteric modulator via the benzodiazepine site of α1-, α2-, α3- or α5-subunit-containing GABAARs (Hadingham et al., 1996; Whittemore et al., 1996; You et al., 2010). Ro15–4513 at 5 mg/kg (i.p.) that effectively modulated the GABAA receptor activity in ethanol-treated rats (Kuzmin et al., 2012), also significantly reduced the number of capsaicin-increased c-Fos-ir TCC neurons (p= 0.01, Fig. 1A, Fig. 1B, Ro vs. Vehicle-Cap), prevented capsaicin-induced CGRP-ir in TG (p= 0.011, Fig. 2A, Fig. 2B, Ro vs. Vehicle-Cap), and rescued capsaicin-induced depletion of dural CGRP-ir (p= 0.006, Fig. 3A, Fig. 3B, Ro vs. Vehicle-Cap).
Interestingly, similar to Compound 6 and topiramate, neither loreclezole nor Ro 15–4513 was able to reduce the number of capsaicin-activated TCC neurons to the baseline level as measured in the sham group (Fig. 1B, Fig. 3B).
3.5. Diazepam, an α6GABAAR-inactive benzodiazepine, did not affect capsaicin-induced central and peripheral responses in the TGVS.
Diazepam is the prototype benzodiazepine that binds at the benzodiazepine binding site (at the interface of α- and γ-subunits) of α1-, α2-, α3-, or α5-subunit-containing αβγGABAARs, but is inactive at α4- or α6-subunit-containing GABAARs (Hevers and Luddens, 2002; Whittemore et al., 1996). In contrast to loreclezole or Ro 15–4513, diazepam did not affect capsaicin-induced TGVS activation in both central and peripheral ends. It did not significantly affect the number of c-Fos-containing TCC neurons (p=0.273 Fig. 1A, Fig. 1B, DZ vs.Vehicle-Cap), the CGRP-ir in TG (p=0.522, Fig. 2A, Fig. 2B, DZ vs. Vehicle-Cap) or the CGRP-ir in the dura mater (p=0.136, Fig. 3A, Fig. 3B, DZ vs. Vehicle-Cap) induced by capsaicin instillation.
3.6. Furosemide (i.p.), an α6GABAAR antagonist, prevented the peripheral effects of Compound 6 in the capsaicin-induced migraine model.
In addition to TG (Puri et al., 2011; Puri et al., 2012) that is located outside the BBB (Eftekhari et al., 2015), the site of action of α6GABAAR PAMs may also be in other brain regions where α6GABAARs are located, such as cerebellar granular cells (Gutierrez et al., 1996; Pirker et al., 2000), the cochlea nuclei (Drescher et al., 1993), and the hippocampus (Yang et al., 2016), since all three tested α6GABAAR PAMs are BBB-permeable and were administered systemically. To further substantiate that Compound 6 targets α6GABAARs located in TG, we investigated whether systemic administration of a BBB-impermeable α6GABAAR antagonist would block the effects of Compound 6. Furosemide was used since it is BBB-impermeable (Seelig et al., 1994) and it is an α6GABAAR-selective antagonist that effectively blocked the effects of Compound 6, Ro 15–4513 and loreclezole in our previous study (Chiou et al., 2018).
We examined effects of systemic-administered furosemide (20 mg/kg, i.p.) on Compound 6 (3 mg/kg, i.p.)-induced inhibition of TCC neuronal activation (Fig. 1A, C3+Furo), TG CGRP-ir, (Fig. 2A, C3+Furo) and dural CGRP depletion (Fig. 3A, C3+Furo) induced by capsaicin. In the TCC, the c-fos-ir cell number in capsaicin-treated rat group pretreated with Compound 6 (3 mg/kg)+Furosemide was still significantly reduced (p=0.01, Fig. 1A, C3+Furo vs. Veh-Cap, Fig. 1B), as observed in the group pretreated with Compound 6 (3 mg/kg) plus the vehicle of furosemide (p=006, Fig. 1A, C3+Veh vs. Veh-Cap, Fig. 1B). This suggests that systemic administration of furosemide did not significantly affect Compound 6-induced inhibitory effect on TCC neuronal activation induced by capsaicin.
On the other hand, furosemide completely reversed Compound 6-induced inhibitory effects in TG. The CGRP density in TG in the Compound 6 (3 mg/kg)+Furosemide pre-treated group was significantly restored, as compared with the sham group (p=0.018, Fig. 2A, C3+Furo vs. Sham, Fig. 2B) to level as in capsaicin-treated group (p=0.2; Fig. 2A, C3+Furo vs. Vehicle-Cap). This is unlike the significant reduction observed in the Compound 6 (3 mg/kg)+Vehicle pretreated group (p=0.006; Fig. 2A, C3+Veh vs. Vehicle-Cap). Therefore, furosemide completely reversed the inhibitory effect of Compound 6 in TG. Similarly, furosemide also completely reversed the enhancing effect of Compound 6 on CGRP release in the dura mater (p=0.15, Fig. 3B, C3+Furo vs. Vehicle-Cap, whereas, p=0.004, Fig. 3A, C3+Furo vs. Sham, Fig. 3B).
3.7. The expression of α6GABAARs and CGRP in TG
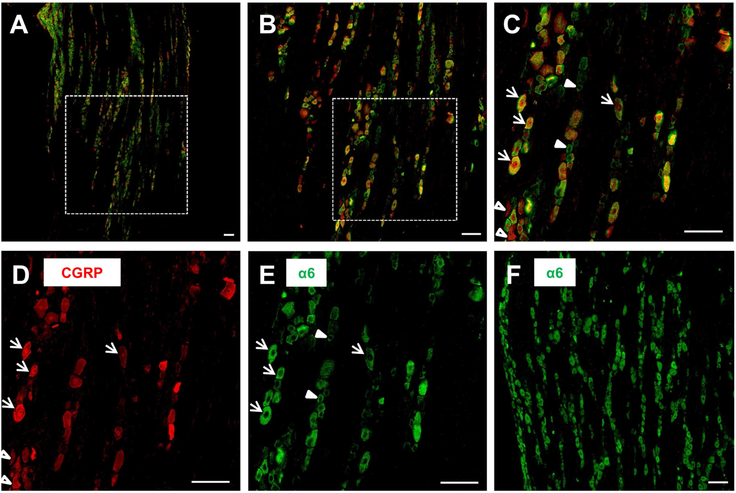
To investigate a possible co-localization of α6GABAARs and CGRP, we conducted double staining of the α6 subunit of GABAARs (α6) and CGRP in TG sections prepared from capsaicin-treated rats. Figure 4 demonstrates a section with double immunofluorescent staining of CGRP (red) and a6 (green) (Figure 4 A–C), and the monochrome micrographs of CGRP (Figure 4D) and a6 (Figure 4E), respectively, in a rat TG section. In agreement with the results in Figure 2, CGRP was densely stained in TG neurons of capsaicin-treated rats (Figure 4D). Interestingly, a6 was also densely stained in TG neurons of capsaicin-treated rats (Figure 4E). A single-immunoreactive staining of a6 in another TG section (Figure 4F) also shows dense distribution of the a6 protein in capsaicin-treated rat TG. Although there were neurons immunoreactive only to a6 (green, filled arrowheads, Fig. 4C, E) or to CGRP (red, open arrowheads, Fig. 4C,D), many TG neurons were double-immunoreactive to a6 and CGRP (Figure 4C, yellow color, arrows). These results suggest that α6GABAARs are co-localized with CGRP in many TG neurons.
Figure 4. Expression of CGRP and the α6 subunit of GABAARs in capsaicin-treated rat TG. A-C.
: Double immunofluorescent staining of CGRP (red, D) and the α6 subunit of GABAARs (α6, green; E) in a TG section prepared from a rat receiving i.c. capsaicin instillation. A-C: A shows a longitudinal section at a low (10X) magnification of a trigeminal ganglion. B: The inset from A at 20X magnification. C: The inset from B at 40X magnification. Note that there are neurons double-immunoreactive to CGRP and α6 (arrows), or only to CGRP (empty arrowheads) or α6 (filled arrowheads). F: Single immunostaining of the α6 protein in another TG section from a capsaicin-treated rat. Scale bars: 100 μm for all figures.
The dense expression of α6 in TG neurons of capsaicin-treated rats was further confirmed by a single immunofluorescent staining of a6 using a different primary antibody against the a6 subunit (NB300–196, Novus Biologicals). As shown in Figure S1B, α6 was densely distributed in a TG section of capsaicin-treated rats with a similar distribution pattern as seen in Figure 4F, where the a6 antibody (ab92747, Abcam, Cambridge, UK) was used for the double immunofluorecence.
4. Discussion
4.1. α6GABAAR PAMs ameliorate both peripheral and central responses in capsaicin-induced TGVS activation in a rat model mimicking migraine.
In this study, we used a rat model mimicking pathological changes in migraine (Fan et al., 2012) induced by i.c. instillation of capsaicin. This migraine model has previously been employed for evaluating anti-migraine agents, including the clinically effective valproic acid and a sumatriptan analogue (Cutrer et al., 1999; Cutrer and Moskowitz, 1996). It is in agreement with the peripheral CGRP hypothesis (Dodick et al., 2014; Sun et al., 2016) and the brain stem central sensitization hypothesis of migraine (Bae et al., 2004; Goadsby, 2006), and displays both central and peripheral activation in the TGVS as we reported previously (Fan et al., 2012).
Here, we found that Compound 6 attenuated capsaicin-induced elevation of TG CGRP-ir, TCC neuronal activation and depletion of dural CGRP-ir. Importantly, effects of Compound 6 in this migraine model were mimicked by two α6GABAAR-active PAMs, loreclezole and Ro 15–4513, but not by α6GABAAR-inactive diazepam. Compound 6 is a pyrazoloquinolinone and an α6GABAAR-selective PAM acting at the α6+β3- interface of GABAARs (Varagic et al., 2013a). At concentrations up to 1 mM, it only enhanced GABA currents at a6β2/3γ2GABAARs, but not at β2/3γ2GABAARs containing α1-, α2-, α3-, α4- or α5-subunits (Varagic et al., 2013a). Ro15–4513 is an imidazobenzodiazepine and a PAM at α4βγ2GABAARs and α6βγ2GABAARs but is also a negative allosteric modulator at α1-, α2-, α3- and α5-containing aβγGABAARs by acting via the benzodiazepine binding site at the α+γ- interface (Hadingham et al., 1996; Whittemore et al., 1996; You et al., 2010). Loreclezole is a triazole derivative and is a PAM at GABAARs containing β2 or β3 subunits (Wafford et al., 1994; Whittemore et al., 1996). These three compounds not only have distinct structural backbones but also exhibit their effects via different modulatory mechanisms on various GABAAR subtypes. However, they all attenuated i.c. capsaicin-induced peripheral and central effects in the rat TGVS. The only common feature is their PAM activity at α6GABAARs, which therefore may contribute to their effects in this migraine model.To the best of our knowledge, this is the first study providing evidence for the notion that a positive modulation of α6GABAARs can inhibit nociceptive activation of the TGVS.
4.2. Effects of Compound 6 are comparable to topiramate, a clinically effective antimigraine drug.
Topiramate is known for its antiepileptic activity with several proposed targets of action, including Na+ channels, Ca2+ channels, non-NMDA receptors and GABAARs (White, 2005). Topiramate is also a clinically effective preventive treatment for migraine (Bussone et al., 2005). In migraine animal models, topiramate can reduce superior sagittal sinus-evoked TCC neuronal firings (Storer and Goadsby, 2004), attenuate neurogenic dural vasodilation likely through inhibiting prejunctional CGRP release (Akerman and Goadsby, 2005a), and inhibit regional cerebral blood flow changes (Akerman and Goadsby, 2005b). The mechanism(s) of its antimigraine activity are unclear but blocking peripheral GluR5 kainate receptors may contribute to its inhibition of neurogenic dural vasodilatation (Andreou et al., 2009). Interestingly, topiramate was also able to enhance GABA currents at several cloned GABAARs, including the one consisting of α6β3γ2s subunits, when GABA was applied at low concentrations (0.1–1 μM) (Simeone et al., 2006). It remains to be elucidated whether topiramate at the effective dose in this study can potentiate GABA-induced inhibition via α6β3γ2s GABAA receptors. The present findings that both peripheral and central effects of Compound 6 (3–10 mg/kg) are comparable to those of topiramate (30 mg/kg) suggest a potential clinical effectiveness of Compound 6 in the treatment of migraine.
4.3. The α6GABAARs mediating TGVS inhibition are probably located in TG.
The fact that TG is located outside the BBB (Eftekhari et al., 2015), and the finding that systemic furosemide, a BBB-impermeable α6GABAAR antagonist, prevented the effects of Compound 6 in the peripheral (TG and dura mater), but not in the central (TCC), sites of the TGVS, suggest that the targets of action of Compound 6 are α6GABAARs in TG. This conclusion is consistent with the recent notion that TG are important peripheral targeting sites for anti-migraine drugs. For example, botulinum toxin A (Aoki, 2005; Dodick et al., 2010) and several antibodies against CGRP or CGRP receptors (Bigal et al., 2015; Dodick et al., 2014; Sun et al., 2016; Tepper et al., 2017), which are BBB-impermeable, are effective in the treatment of migraine.
In addition, several lines of evidence indicate that enhancing the GABAAR-mediated functions in TG may be an effective strategy for treating migraine. Valproic acid, a clinically used antimigraine agent that inhibits GABA degradation, can reduce capsaicin-induced TNC activation as well as CGRP- and substance P-induced dural plasma protein extravasation (Lee et al., 1995; Meents et al., 2010). The neuroactive steroid allopregnanolone (a progesterone metabolite), which is also a PAM of GABAARs, including α6GABAARs (Fodor et al., 2005), was also effective in a similar migraine model (Cutrer and Moskowitz, 1996). Effects of both valproic acid and allopregnanolone were attenuated by i.p. administration of the BBB-impermeable GABAAR antagonist, bicuculline methiodide, supporting the conclusion that both compounds exert their effects via GABAARs in TG.
4.4. The expression and possible functions of α6GABAARs in TG
In TG, GABA is synthesized and released mainly by neurons, but is accumulated by their surrounding satellite cells (Hayasaki et al., 2006). Cell bodies of mammalian sensory ganglia are generally devoid of synaptic contacts, but exogenous GABA can induce Cl− currents in all TG neurons examined (Hayasaki et al., 2006). It was thus hypothesized that frequently firing sensory neurons can lead to elevated extracellular K+ concentrations during repolarization of neurons in between action potentials that might induce GABA release from satellite cells and/or neuronal cells, providing an inhibitory feedback to firing sensory neurons (Hayasaki et al., 2006).
Immunohistochemical studies in 2–3 week-old rats demonstrated that the majority of TG neurons express a1, a3, a4, a5, β2/3, and γ1/2/3 subunits of GABAARs, while a6 and δ subunits were detected only in a subset of small neurons.(Hayasaki et al., 2006). a6GABAARs might thus be partially expressed in the same small unmyelinated sensory neurons that contain TRPV1, CGRP, and substance P (Bae et al., 2004).In addition, the extremely high GABA sensitivity of a6GABAARs make them ideally suited for becoming modulated by already small amounts of extracellular GABA (Karim et al., 2013; Mortensen et al., 2011).
On the other hand, in a subsequent immunofluorescent study performed in adult rats (Puri et al., 2012), it was demonstrated that a6 was expressed not only in TG neurons but also in satellite cells. Moreover, the population of a6-positive neurons was quite high in this study and comprised up to 86%, 74% and 74% of small, medium and large TG neurons, respectively (Puri et al., 2012). The difference in the age of rats investigated in these studies might have contributed to the different abundance of a6GABAARs observed.
TG neurons, however, are heterogeneous. While numerous small and medium sized neurons in TG stained for TRPV1, only 29% of these neurons co-stained for TRPV1, CGRP, and substance P, while 44% co-stained for TRPV1 and CGRP, and 54% stained for TRPV1 and neither CGRP nor substance P (Bae et al., 2004). In another study, CGRP was identified in about 50% of all TG neurons (Reuss et al., 1992). Together with the high percentage of a6GABAARs (~80%) in TG neurons (Puri et al., 2012), these data suggest a partial co-expression of a6GABAARs with TRPV1 and CGRP.
An abundant expression of a6GABAARs in TG was also suggested in our study (Fig. 4) that used adult capsaicin treated-rats to demonstrate a possible co-localization of a6GABAARs with CGRP in TG. Although our results are very similar to the observation of Puri et al. (2012) in rats that had received intra-TG injection of scrambled siRNA, it remains to be investigated whether capsaicin treatment might have changed the expression of a6GABAARs. In any case, here we for the first time demonstrate an abundant colocalization of a6GABAARs with CGRP in many, but not all, TG neurons, and that there are also neurons immunoreactive to only a6GABAARs or CGRP.
4.5. A tentative explanation for the action of α6GABAAR PAMs in the capsaicin-induced migraine model
In the migraine model we employed, intra-cisternal capsaicin might have directly activated the TRPV1 channels, which are located at central terminals of TG neurons (Evans et al., 2012; Oxford, 2013) (Scheme 1). The resulting terminal depolarization and the subsequent firing of action potentials might have back-propagated to TG and the dura mater and released CGRP within the meninges, causing vasodilation, plasma extravasation, edema, mast cell degranulation and inflammation (Ramachandran, 2018). Alternatively, capsaicin might have induced a noxious meningeal stimulation (Mitsikostas et al., 1998) possibly via activating TRPV1 channels in the sensory nerve terminals of the dura mater. The peripheral terminal depolarization might again have elicited action potentials and propagated to TG neurons . In any case,, rapid firing of TG neurons would have caused an elevation of the extracellular K+ concentration, and a subsequent release of GABA from TG neurons and/or satellite cells (Minchin, 1975). Subsequent activation of the highly GABA-sensitive a6GABAARs on the cell bodies of TG neurons would then have suppressed the firing of those TG neurons expressing a6GABAARs, and hence reduced CGRP release from peripheral terminals as well as synaptic transmission at central terminals of the trigeminal nucleus caudalis.
Activation of the trigeminal nucleus caudalis contributes to the central sensitization of the TGVS and is involved in nociceptive processing and cerebrovascular regulation and thus also contributes to the symptoms of migraine (Lance et al., 1983). The a6GABAAR-mediated reduction of this activation, and especially the enhancement of this reduction by a6GABAAR PAMs might thus have ameliorated the symptoms of migraine.
Such a tentative mechanism may explain the findings that all three α6GABAAR PAMs completely attenuated capsaicin-induced CGRP elevation in TG. However, α6GABAAR activation in TG neurons may not be able to inhibit the CGRP released from peripheral terminals that are activated by the locally released neuroinflammatory mediators. This may explain the observed incomplete inhibition of CGRP-release in the dura mater by α6GABAAR PAMs. In addition, capsaicin may also directly activate the TRPV1 channels on the TCC secondary neurons. This may explain the incomplete inhibition of capsaicin-induced c-Fos expression in TCC neurons by α6GABAAR PAMs.
4.6. Compound 6 seems to exert its effects via α6β2/3γ2 receptors
The abundantly expressed a6 in TG observed in the present study as well as in Puri et al. (2012) and the findings that a6GABAAR-active PAMs but not the a6GABAAR-inactive diazepam can reduce capsaicin-induced TGVS activation suggest that the a6 subunits expressed in TG are incorporated into GABAARs. However, the subunit composition of a6GABAARs within TG neurons is currently unclear because both a6β2/3γ2- and a6β2/3δ-containing GABAARs might be present in these neurons. In the absence of synapses at cell bodies of TG neurons (Hayasaki et al., 2006), all these receptors are located extrasynaptically. Compound 6 is a highly selective PAM of a6β2/3γ2GABAARs (Varagic et al., 2013a; Varagic et al., 2013b) although it also modulated a6β2/3δGABAARs at high concentrations (Chiou et al., 2018). In contrast, Ro 15–4513 is an antagonist of a6β2/3δGABAARs (Hanchar et al., 2006; Wallner et al., 2014), although being a PAM at a6β3γ2GABAARs (Adkins et al., 2001; Suzdak et al., 1986; Wallner et al., 2006). It is unlikely that Ro15–4513 exerts its anti-capsaicin effect via modulating a6β3δGABAARs. Therefore, Compound 6 as well as Ro15–4513 may exert their anti-capsaicin effects via modulating α6β2/3γ2GABAARs.
4.7. Limitations of the study
The present study for the first time demonstrates that α6GABAAR-selective PAMs significantly attenuated both central and peripheral responses of TGVS in a rat model of migraine induced by i.c. instillation of capsaicin. This in vivo model is a useful tool to study the histopathological changes in migraine-associated areas such as TG, TCC and dura mater, and has been employed to evaluate the pharmacological potency of various anti-migraine agents, including clinically effective valproic acid and a sumatriptan analogue (Cutrer et al., 1999; Cutrer and Moskowitz, 1996). However, there are some limitations to the present model. First, capsaicin was administered intracisternally but not directly on the dura mater. The TCC neurons that may be directly activated likely include those not innervated by TG. This may explain that even topiramate, a clinically-effective antimigraine agent, was unable to completely abolish the TCC neuronal activation. Nevertheless, both α6-GABAAR PAMs and topiramate effectively inhibited peripheral responses in the TGVS which represent a hallmark of migraine neuropathogenesis (Pietrobon and Striessnig, 2003). Second, all GABAAR PAMs were administered before i.c. instillation of capsaicin, supporting a future clinical applications as a preventive treatment for migraine. This notion is also in agreement with the effectiveness of topiramate, which is used clinically for a preventive, but not abortive, treatment for migraine. It remains to be further elucidated whether GABAAR PAMs can be used as abortive treatments. Third, in the present study the animals were under anesthesia throughout the study protocol. Further studies will have to be conducted to examine the effects of α6GABAAR-selective PAMs in migraine-associated behaviors such as allodynia, facial grimace and photophobia (Harris et al., 2017).
4.8. Future prospects
Novel treatments of migraine remain an unmet medical need since more than 20% migraineurs are refractory to current anti-migraine medications or intolerant to their side effects (Tfelt-Hansen, 2006). Migraine is one of the leading causes of disability with a prevalence of 12–15% (Lipton et al., 2001). Our results suggest that α6GABAAR-selective PAMs are potential novel antimigraine agents. The limited regional distribution of α6GABAARs and the absence of modulation of diazepam-sensitive GABAARs by Compound 6 (Chiou et al., 2018; Knutson et al., 2018; Varagic et al., 2013a) suggest that α6GABAAR-selective PAMs might have fewer side effects than current antimigraine agents. Since the non-BBB protected TG seems to be the site of the anti-migraine action of Compound 6, BBB-impermeable α6GABAAR PAMs may also have the potential to be novel anti-migraine agents.Their development thus represents another strategy for a migraine therapy with even less side effects.
Supplementary Material
Highlights.
α6GABAAR positive modulators inhibited central and peripheral trigeminal responses in a migraine model.
Diazepam, an α6GABAAR-inactive ligand, had no effect in this migraine model.
Systemic furosemide, an α6GABAAR blocker, inhibited peripheral responses only.
The α6GABAAR in trigeminal ganglia is a novel target for migraine treatment.
α6GABAAR positive allosteric modulators represent a potential treatment option for migraine.
Acknowledgements:
The authors would like to thank Prof Dr Shu-Hui Chang, Institute of Epidemiology and Preventive Medicine, National Taiwan University, for her advice in statistical analysis. This study was supported by the grants from the Ministry of Science and Technology, Taiwan (MOST 105–2314-B-002–150; MOST 104–2923-B-002–006-MY3; MOST 104–2745-B-002–004), National Taiwan University Hospital (NTUH 105-S3057), National Health Research Institutes, Taiwan (NHRI-EX107–10733NI), E-da Hospital, Taiwan (105-EDN11) and National Institutes of Health, USA (R01 NS076517; R01 MH096463).
Abbreviations
- α6GABAAR
α6 subunit-containing GABAA receptor
- aCSF
artificial cerebrospinal fluid
- BBB
blood-brain barrier
- c-Fos-ir
c-Fos-immunoreactive
- CGRP-ir
calcitonin gene-related peptide immunoreactivity
- GABAAR
GABAA receptor
- i.c.
intra-cisternal injection
- PAM
positive allosteric modulator
- TCC
trigeminal cervical complex
- TG
trigeminal ganglia
- TGVS
trigeminal vascular system
- TMJ
temporomandibular joint
- TNC
trigeminal nucleus caudalis
- TRPV1
transient receptor potential vanilloid type-1 channel
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors declare no conflicts of interest.
References
- Adkins CE, Pillai GV, Kerby J, Bonnert TP, Haldon C, McKernan RM, Gonzalez JE, Oades K, Whiting PJ, Simpson PB, 2001. alpha4beta3delta GABA(A) receptors characterized by fluorescence resonance energy transfer-derived measurements of membrane potential. J Biol Chem 276, 38934–38939. [DOI] [PubMed] [Google Scholar]
- Agunu A, Abdurahman EM, Andrew GO, Muhammed Z, 2005. Diuretic activity of the stem-bark extracts of Steganotaenia araliacea hochst [Apiaceae]. J Ethnopharmacol 96, 471–475. [DOI] [PubMed] [Google Scholar]
- Akerman S, Goadsby PJ, 2005a. Topiramate inhibits cortical spreading depression in rat and cat: impact in migraine aura. Neuroreport 16, 1383–1387. [DOI] [PubMed] [Google Scholar]
- Akerman S, Goadsby PJ, 2005b. Topiramate inhibits trigeminovascular activation: an intravital microscopy study. Br J Pharmacol 146, 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou AP, Goadsby PJ, 2011. Topiramate in the treatment of migraine: a kainate (glutamate) receptor antagonist within the trigeminothalamic pathway. Cephalalgia 31, 1343–1358. [DOI] [PubMed] [Google Scholar]
- Andreou AP, Holland PR, Goadsby PJ, 2009. Activation of iGluR5 kainate receptors inhibits neurogenic dural vasodilatation in an animal model of trigeminovascular activation. Br J Pharmacol 157, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki KR, 2005. Review of a proposed mechanism for the antinociceptive action of botulinum toxin type A. Neurotoxicology 26, 785–793. [DOI] [PubMed] [Google Scholar]
- Bae YC, Oh JM, Hwang SJ, Shigenaga Y, Valtschanoff JG, 2004. Expression of vanilloid receptor TRPV1 in the rat trigeminal sensory nuclei. J Comp Neurol 478, 62–71. [DOI] [PubMed] [Google Scholar]
- Bigal ME, Walter S, Rapoport AM, 2015. Therapeutic antibodies against CGRP or its receptor. Br J Clin Pharmacol 79, 886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussone G, Diener HC, Pfeil J, Schwalen S, 2005. Topiramate 100 mg/day in migraine prevention: a pooled analysis of double-blind randomised controlled trials. Int J Clin Pract 59, 961–968. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D, 2001. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci 24, 487–517. [DOI] [PubMed] [Google Scholar]
- Chiou LC, Lee HJ, Ernst M, Huang WJ, Chou JF, Chen HL, Mouri A, Chen LC, Treven M, Mamiya T, Fan PC, Knutson DE, Witzigmann C, Cook J, Sieghart W, Nabeshima T, 2018. Cerebellar alpha6 -subunit-containing GABAA receptors: a novel therapeutic target for disrupted prepulse inhibition in neuropsychiatric disorders. Br J Pharmacol 175, 2414–2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cutrer FM, Mitsikostas DD, Ayata G, Sanchez del Rio M, 1999. Attenuation by butalbital of capsaicin-induced c-fos-like immunoreactivity in trigeminal nucleus caudalis. Headache 39, 697–704. [DOI] [PubMed] [Google Scholar]
- Cutrer FM, Moskowitz MA, 1996. Wolff Award 1996. The actions of valproate and neurosteroids in a model of trigeminal pain. Headache 36, 579–585. [DOI] [PubMed] [Google Scholar]
- Cutrer FM, Schoenfeld D, Limmroth V, Panahian N, Moskowitz MA, 1995. Suppression by the sumatriptan analogue, CP-122,288 of c-fos immunoreactivity in trigeminal nucleus caudalis induced by intracisternal capsaicin. Br J Pharmacol 114, 987–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodick DW, Goadsby PJ, Silberstein SD, Lipton RB, Olesen J, Ashina M, Wilks K, Kudrow D, Kroll R, Kohrman B, Bargar R, Hirman J, Smith J, investigators A. L. D. s., 2014. Safety and efficacy of ALD403, an antibody to calcitonin gene-related peptide, for the prevention of frequent episodic migraine: a randomised, double-blind, placebo-controlled, exploratory phase 2 trial. Lancet Neurol 13, 1100–1107. [DOI] [PubMed] [Google Scholar]
- Dodick DW, Turkel CC, DeGryse RE, Aurora SK, Silberstein SD, Lipton RB, Diener HC, Brin MF, Group PCMS, 2010. OnabotulinumtoxinA for treatment of chronic migraine: pooled results from the double-blind, randomized, placebo-controlled phases of the PREEMPT clinical program. Headache 50, 921–936. [DOI] [PubMed] [Google Scholar]
- Drescher DG, Green GE, Khan KM, Hajela K, Beisel KW, Morley BJ, Gupta AK, 1993. Analysis of gamma-aminobutyric acidA receptor subunits in the mouse cochlea by means of the polymerase chain reaction. J Neurochem 61, 1167–1170. [DOI] [PubMed] [Google Scholar]
- Eftekhari S, Salvatore CA, Johansson S, Chen TB, Zeng Z, Edvinsson L, 2015. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood-brain barrier. Brain Res 1600, 93–109. [DOI] [PubMed] [Google Scholar]
- Evans MS, Cheng X, Jeffry JA, Disney KE, Premkumar LS, 2012. Sumatriptan Inhibits TRPV1 Channels in Trigeminal Neurons. Headache 52, 773–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan PC, Kuo PH, Hu JW, Chang SH, Hsieh ST, Chiou LC, 2012. Different trigemino-vascular responsiveness between adolescent and adult rats in a migraine model. Cephalalgia 32, 979–990. [DOI] [PubMed] [Google Scholar]
- Fodor L, Biro T, Maksay G, 2005. Nanomolar allopregnanolone potentiates rat cerebellar GABAA receptors. Neurosci Lett 383, 127–130. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ, 2006. brainstem mechanisms of ongoing pain In: Olesen J JT., (Ed), from basic pain mechanism to headache. Oxford University Press, New York, pp. 194–201. [Google Scholar]
- Goadsby PJ, Edvinsson L, Ekman R, 1988. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann Neurol 23, 193–196. [DOI] [PubMed] [Google Scholar]
- Gutierrez A, Khan ZU, De Blas AL, 1996. Immunocytochemical localization of the alpha 6 subunit of the gamma-aminobutyric acidA receptor in the rat nervous system. J Comp Neurol 365, 504–510. [DOI] [PubMed] [Google Scholar]
- Hadingham KL, Garrett EM, Wafford KA, Bain C, Heavens RP, Sirinathsinghji DJ, Whiting PJ, 1996. Cloning of cDNAs encoding the human gamma-aminobutyric acid type A receptor alpha 6 subunit and characterization of the pharmacology of alpha 6-containing receptors. Mol Pharmacol 49, 253–259. [PubMed] [Google Scholar]
- Hanchar HJ, Chutsrinopkun P, Meera P, Supavilai P, Sieghart W, Wallner M, Olsen RW, 2006. Ethanol potently and competitively inhibits binding of the alcohol antagonist Ro15–4513 to alpha4/6beta3delta GABAA receptors. Proc Natl Acad Sci U S A 103, 8546–8551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris HM, Carpenter JM, Black JR, Smitherman TA, Sufka KJ, 2017. The effects of repeated nitroglycerin administrations in rats; modeling migraine-related endpoints and chronification. J Neurosci Methods 284, 63–70. [DOI] [PubMed] [Google Scholar]
- Hayasaki H, Sohma Y, Kanbara K, Maemura K, Kubota T, Watanabe M, 2006. A local GABAergic system within rat trigeminal ganglion cells. Eur J Neurosci 23, 745–757. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H, 2002. Pharmacological heterogeneity of gamma-aminobutyric acid receptors during development suggests distinct classes of rat cerebellar granule cells in situ. Neuropharmacology 42, 34–47. [DOI] [PubMed] [Google Scholar]
- Karim N, Wellendorph P, Absalom N, Johnston GA, Hanrahan JR, Chebib M, 2013. Potency of GABA at human recombinant GABA(A) receptors expressed in Xenopus oocytes: a mini review. Amino Acids 44, 1139–1149. [DOI] [PubMed] [Google Scholar]
- Knutson DE, Kodali R, Divovic B, Treven M, Stephen MR, Zahn NM, Dobricic V, Huber AT, Meirelles MA, Verma RS, Wimmer L, Witzigmann C, Arnold LA, Chiou LC, Ernst M, Mihovilovic MD, Savic MM, Sieghart W, Cook JM, 2018. Design and Synthesis of Novel Deuterated Ligands Functionally Selective for the gamma-Aminobutyric Acid Type A Receptor (GABAAR) alpha6 Subtype with Improved Metabolic Stability and Enhanced Bioavailability. J Med Chem 61, 2422–2446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmin A, Liljequist S, Meis J, Chefer V, Shippenberg T, Bakalkin G, 2012. Repeated moderate-dose ethanol bouts impair cognitive function in Wistar rats. Addict Biol 17, 132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lance JW, Lambert GA, Goadsby PJ, Duckworth JW, 1983. Brainstem influences on the cephalic circulation: experimental data from cat and monkey of relevance to the mechanism of migraine. Headache 23, 258–265. [DOI] [PubMed] [Google Scholar]
- Lee WS, Limmroth V, Ayata C, Cutrer FM, Waeber C, Yu X, Moskowitz MA, 1995. Peripheral GABAA receptor-mediated effects of sodium valproate on dural plasma protein extravasation to substance P and trigeminal stimulation. Br J Pharmacol 116, 1661–1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton RB, Stewart WF, Diamond S, Diamond ML, Reed M, 2001. Prevalence and burden of migraine in the United States: data from the American Migraine Study II. Headache 41, 646–657. [DOI] [PubMed] [Google Scholar]
- McDonald JH, 2014. Handbook of Biological Statistics Sparky House Publishing, Baltimore, Maryland. [Google Scholar]
- Meents JE, Neeb L, Reuter U, 2010. TRPV1 in migraine pathophysiology. Trends Mol Med 16, 153–159. [DOI] [PubMed] [Google Scholar]
- Minchin MC, 1975. Factors influencing the efflux of [3H]gamma-aminobutyric acid from satellite glial cells in rat sensory ganglia. J Neurochem 24, 571–577. [DOI] [PubMed] [Google Scholar]
- Mitsikostas DD, Sanchez del Rio M, Waeber C, Moskowitz MA, Cutrer FM, 1998. The NMDA receptor antagonist MK-801 reduces capsaicin-induced c-fos expression within rat trigeminal nucleus caudalis. Pain 76, 239–248. [DOI] [PubMed] [Google Scholar]
- Mortensen M, Patel B, Smart TG, 2011. GABA Potency at GABA(A) Receptors Found in Synaptic and Extrasynaptic Zones. Front Cell Neurosci 6, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen RW, Sieghart W, 2008. International Union of Pharmacology. LXX. Subtypes of gamma-aminobutyric acid(A) receptors: classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev 60, 243–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxford GS, 2013. The Role of TRP Channels in Migraine. The Open Pain Journal 6, 37–49. [Google Scholar]
- Pietrobon D, Striessnig J, 2003. Neurobiology of migraine. Nat Rev Neurosci 4, 386–398. [DOI] [PubMed] [Google Scholar]
- Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G, 2000. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience 101, 815–850. [DOI] [PubMed] [Google Scholar]
- Puri J, Bellinger LL, Kramer PR, 2011. Estrogen in cycling rats alters gene expression in the temporomandibular joint, trigeminal ganglia and trigeminal subnucleus caudalis/upper cervical cord junction. J Cell Physiol 226, 3169–3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puri J, Vinothini P, Reuben J, Bellinger LL, Ailing L, Peng YB, Kramer PR, 2012. Reduced GABA(A) receptor alpha6 expression in the trigeminal ganglion alters inflammatory TMJ hypersensitivity. Neuroscience 213, 179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, 2018. Neurogenic inflammation and its role in migraine. Semin Immunopathol. [DOI] [PubMed] [Google Scholar]
- Rudolph U, Knoflach F, 2011. Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat. Rev. Drug Discov. 10, 685–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig A, Gottschlich R, Devant RM, 1994. A method to determine the ability of drugs to diffuse through the blood-brain barrier. Proc Natl Acad Sci U S A 91, 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigenaga Y, Sera M, Nishimori T, Suemune S, Nishimura M, Yoshida A, Tsuru K, 1988. The central projection of masticatory afferent fibers to the trigeminal sensory nuclear complex and upper cervical spinal cord. J Comp Neurol 268, 489–507. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Wilcox KS, White HS, 2006. Subunit selectivity of topiramate modulation of heteromeric GABA(A) receptors. Neuropharmacology 50, 845–857. [DOI] [PubMed] [Google Scholar]
- Storer RJ, Goadsby PJ, 2004. Topiramate inhibits trigeminovascular neurons in the cat. Cephalalgia 24, 1049–1056. [DOI] [PubMed] [Google Scholar]
- Sun H, Dodick DW, Silberstein S, Goadsby PJ, Reuter U, Ashina M, Saper J, Cady R, Chon Y, Dietrich J, Lenz R, 2016. Safety and efficacy of AMG 334 for prevention of episodic migraine: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Neurol 15, 382–390. [DOI] [PubMed] [Google Scholar]
- Suzdak PD, Glowa JR, Crawley JN, Schwartz RD, Skolnick P, Paul SM, 1986. A selective imidazobenzodiazepine antagonist of ethanol in the rat. Science 234, 1243–1247. [DOI] [PubMed] [Google Scholar]
- Tepper S, Ashina M, Reuter U, Brandes JL, Dolezil D, Silberstein S, Winner P, Leonardi D, Mikol D, Lenz R, 2017. Safety and efficacy of erenumab for preventive treatment of chronic migraine: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Neurol 16, 425–434. [DOI] [PubMed] [Google Scholar]
- Tfelt-Hansen P, 2006. Prioritizing prophylactic treatment of migraines. Lippincott, Williams &Wilkins, Philadelphia, PA. [Google Scholar]
- Treven M, Siebert DCB, Holzinger R, Bampali K, Fabjan J, Varagic Z, Wimmer L, Steudle F, Scholze P, Schnurch M, Mihovilovic MD, Ernst M, 2018. Towards functional selectivity for alpha6beta3gamma2 GABAA receptors: a series of novel pyrazoloquinolinones. Br J Pharmacol 175, 419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagic Z, Ramerstorfer J, Huang S, Rallapalli S, Sarto-Jackson I, Cook J, Sieghart W, Ernst M, 2013a. Subtype selectivity of alpha+beta- site ligands of GABAA receptors: identification of the first highly specific positive modulators at alpha6beta2/3gamma2 receptors. Br J Pharmacol 169, 384–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varagic Z, Wimmer L, Schnurch M, Mihovilovic MD, Huang S, Rallapalli S, Cook JM, Mirheydari P, Ecker GF, Sieghart W, Ernst M, 2013b. Identification of novel positive allosteric modulators and null modulators at the GABAA receptor alpha+beta- interface. Br J Pharmacol 169, 371–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wafford KA, Bain CJ, Quirk K, McKernan RM, Wingrove PB, Whiting PJ, Kemp JA, 1994. A novel allosteric modulatory site on the GABAA receptor beta subunit. Neuron 12, 775–782. [DOI] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW, 2006. Low-dose alcohol actions on alpha4beta3delta GABAA receptors are reversed by the behavioral alcohol antagonist Ro15–4513. Proc Natl Acad Sci U S A 103, 8540–8545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW, 2014. Alcohol selectivity of beta3-containing GABAA receptors: evidence for a unique extracellular alcohol/imidazobenzodiazepine Ro15–4513 binding site at the alpha+beta-subunit interface in alphabeta3delta GABAA receptors. Neurochem Res 39, 1118–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS, 2005. Molecular pharmacology of topiramate: managing seizures and preventing migraine. Headache 45 Suppl 1, S48–56. [DOI] [PubMed] [Google Scholar]
- Whittemore ER, Yang W, Drewe JA, Woodward RM, 1996. Pharmacology of the human gamma-aminobutyric acidA receptor alpha 4 subunit expressed in Xenopus laevis oocytes. Mol Pharmacol 50, 1364–1375. [PubMed] [Google Scholar]
- Wong G, Sarviharju M, Toropainen M, Matecka D, Korpi ER, 1996. Pharmacologic actions of subtype-selective and novel GABAergic ligands in rat lines with differential sensitivity to ethanol. Pharmacol Biochem Behav 53, 723–730. [DOI] [PubMed] [Google Scholar]
- Yang L, Xu T, Zhang K, Wei Z, Li X, Huang M, Rose GM, Cai X, 2016. The essential role of hippocampal alpha6 subunit-containing GABAA receptors in maternal separation stress-induced adolescent depressive behaviors. Behav Brain Res 313, 135–143. [DOI] [PubMed] [Google Scholar]
- You H, Kozuska JL, Paulsen IM, Dunn SM, 2010. Benzodiazepine modulation of the rat GABAA receptor alpha4beta3gamma2L subtype expressed in Xenopus oocytes. Neuropharmacology 59, 527–533. [DOI] [PubMed] [Google Scholar]
- Zhang P, Zhang W, Liu R, Harris B, Skolnick P, Cook JM, 1995. Synthesis of novel imidazobenzodiazepines as probes of the pharmacophore for “diazepam-insensitive” GABAA receptors. J Med Chem 38, 1679–1688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.