Abstract
Background
Toll-like receptor 4 (TLR4)-mediated signaling has been implicated in invasion, metastasis, and survival of various cancers. Activation of TLR4 can promote cyclooxygenase-2 (COX-2) and nuclear factor-κB (NF-κB). However, little is known about the effects of TLR4/COX-2 in prostate cancer (PCa).
Material/Methods
In our study, TLR4 and COX-2 expressions were detected by quantitative real-time reverse transcription PCR (qRT-PCR) in PCa tissues (n=34). Cell proliferation was measured by Cell Counting Kit-8 (CCK-8) and carboxyfluorescein succinimidyl ester (CFSE) assays. The migration and invasion abilities were detected by wound healing and Transwell assays. qRT-PCR and western blot assays were performed to detect TLR4, COX-2, matrix metalloproteinase (MMP)-2, MMP-9, tissue inhibitor of matrix metalloproteinases (TIMP)-1, epithelial-cadherin (E-cadherin), vimentin, NF-κB (p65), and p-p65 expressions.
Results
The results revealed that TLR4 and COX-2 were upregulated in PCa tissues; Silencing of TLR4 or COX-2 inhibited PCa cell proliferation, migration, and invasion, and TLR4 siRNAs combined with COX-2 siRNAs synergistically suppressed PCa cell proliferation, migration, and invasion. Silencing of TLR4 or COX-2 also downregulated MMP-2, MMP-9, and E-cadherin expressions, and upregulated TIMP-1 and vimentin expressions. In addition, silencing of TLR4 or COX-2 inhibited p65 phosphorylation and had a synergistic effect.
Conclusions
We demonstrated that TLR4/COX-2 inhibits PCa cell proliferation, migration, and invasion by regulating NF-κB.
MeSH Keywords: Cyclooxygenase 2, NF-kappa B, Prostatic Neoplasms, Toll-Like Receptor 4, Transcellular Cell Migration
Background
Prostate cancer (PCa) is the most common cancer in the world and is the leading cause of cancer-related death [1–3]. After the development of prostate-specific antigen (PSA) detection assays, the detection and diagnosis of PCa was significantly enhanced and reached a peak in the early 1990s [4–6]. 238 590 new cases and 29 720 deaths caused by PCa occurred in 2012 in the United States alone [7,8]. Despite the improvement of PSA testing in early detection, medical and scientific communities are still debating its benefits [9,10]. PSA is not a specific detection index of PCa and may be related to benign prostatic hyperplasia, inflammation, and infections [11–13]. Therefore, it is important for research to explore the mechanisms involved in the development of PCa. Recently, accumulating evidence has shown that the alteration of Toll-like receptor 4/cyclooxygenase-2 (TLR4)/COX-2) expression is involved in tumorigenesis.
TLR is a type I transmembrane protein, which contains an extracellular domain and intracellular domain. At present, 11 mammalian TLRs have been identified. Moreover, TLR4 is one of the most studied TLRs. TLR4 is a membrane protein expressed on immune cells and epithelial cells, and its activation by exogenous and endogenous ligands can initiate myeloid differentiation factor 88 (MyD88)-dependent and MyD88-dependent signaling, leading to downstream nuclear factor-κB (NF-κB) pathway and MAPK pathway activation, and inflammatory gene transcription [14–18]. TLR4 not only plays a critical role in cell confrontation with exogenous infections and wound healing, but also promotes the initiation and progression of tumors when aberrantly activated [19,20]. Studies also have demonstrated that the function of TLR4 in tumor cells may be partly through COX-2 [21–23]. Studies showed that the activation of NF-κB could upregulate the expression levels of inflammation-related genes including COX-2. The analysis revealed overexpression of COX-2 could increase cancer risk by promoting cell proliferation and neovascularization, and inhibiting cell apoptosis and immune function. Therefore, COX-2 also has been confirmed as the important prognostic indicators in various tumors.
NF-κB is a family of transcription factors that comprises 5 subunits: p50, p52, c-Rel, RelB, and p65. In the classic, or canonical pathway, NF-κB has the chief heterodimer consisting of p50 and p65. COX-2 contains NF-κB binding sites. Studies have found that NF-κB p50 antisense oligodeoxynucleotides inhibits the activity of NF-κB and the expression level of COX-2. Therefore, NF-κB interacts with COX-2 to regulate transcription. COX is a rate-limiting enzyme during the conversion of arachidonic acid to prostaglandin (PG) which contains 2 isoforms: COX-1 and COX-2 [24,25]. COX-1 is expressed constitutively in most tissues and plays a role in the production of PGs [26,27]. COX-1 maintains self-balancing functions, for instance, renal vascular expansion, gastric cell protection, and agglutination-promoting prostaglandin thromboxane production via blood platelets [28]. Several studies have supported the involvement of prostanoids in the pathogenesis of cancer [29,30]. The metabolism of arachidonic acid has been found to be enhanced via the COX pathway in various human tumors and it is now widely accepted that this is due to the induction of COX-2 enzymes [31–33]. COX-2 is an inducible enzyme, which is induced by various inflammatory and mitogenic stimuli [34]. These findings have been confirmed in many tumors including pancreas, gastric, bladder, and lung cancer [35–38]. Therefore, COX-2 plays a potential role in tumor formation and growth. However, it is not completely clear whether the molecular mechanisms are responsible for PCa.
In this study, we demonstrated the expression levels of TLR4 and COX-2 in PCa tissues. We also confirmed the roles of TLR4/COX-2 on PCa cell proliferation, migration, and invasion. In addition, we explored the mechanisms of TLR4/COX-2 in PCa including the regulatory effects of TLR4/COX-2 on invasion-associated genes (tissue inhibitor of matrix metalloproteinases (TIMP)-2, matrix metalloproteinase (MMP)-2, and MMP-9), and the regulatory effects of TLR4/COX-2 on NF-κB (p65) phosphorylation. Therefore, we suggest that TLR4/COX-2 may be a novel therapeutic target for the treatment of PCa.
Material and Methods
Clinical samples
PCa tissues and corresponding normal tissues (5 cm away from tumor) were obtained from 34 patients who were diagnosed with PCa in the Tiantai People’s Hospital from July 2014 to June 2015. Tissues were immersed into liquid nitrogen immediately after removal from patients and stored at –80°C until used. Informed consent was obtained from the study PCa patients. The ethics approval was granted by the Ethics Committee of the Tiantai People’s Hospital.
Cell culture
PC3 cells were purchased from the American Type Culture Collection (ATCC). Cells were maintained in sterile culture bottles containing RPMI-1640 medium (HyClone, Cat. No. SH30027), 10% heat inactivated fetal bovine serum (FBS, cat # SH30071.03), 100 U/mL penicillin G, and 100 g/mL streptomycin (Invitrogen, Carlsbad, CA, USA) at 37°C in a humidified 5% CO2 incubator.
siRNA interference
Control siRNAs and siRNAs for TLR4 and COX-2 were obtained from GenePharma Co., Ltd. (Shanghai, China). The sequence of control siRNAs was 5’-UUCUCCGAACGUGUCACGU-3’, the sequence of TLR4 siRNAs was 5’-GUCUCAGAUAUCUAGAUCU-3’, the sequence of COX-2 siRNAs was 5’-GGAUUUGACCAGUAUAAGUTT-3’. One day before transfection, PC3 cells (2 x 104 cells/well) in the logarithmic phase were seeded into 6-well plates in 2 mL of medium and incubated overnight. Next day, cells were transfected with 50 μM scramble siRNA (negative control, NC), TLR4-siRNAs or COX-2-siRNAs using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) following the manufacturer’s protocol.
RNA extraction and quantitative real-time PCR (qRT-PCR)
Total RNA was extracted from the cultured PC3 cells using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. RNA was reverse transcribed using High-Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol. The qRT-PCR assay was performed on ABI 9700 PCR Thermal Cycler with SYBR Green kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA) according to the manufacturer’s protocol. The relative mRNA expression levels of targeting genes were analyzed using 2-ΔΔCt method [39]. The primer sequences were as follows: TLR4, forward 5’-GTG CCT CCA TTT CAG CTC TG-3’, reverse 5’-CAA AGA TAC ACC AGC GGC TC-3’; COX-2, forward 5’-TAC CCT CCT CAA GTC CCT GA-3’, reverse 5’-ACT GCT CAT CAC CCC ATT CA-3’ (reverse); MMP-2, forward 5’-GAT ACC CCT TTG ACG GTA AGG A-3’, reverse 5’-CCT TCT CCC AAG GTC CAT AGC-3’ (reverse); MMP-9, forward 5’-TTC AGG GAG ACG CCC ATT TC-3’, reverse 5’-AAA CCG AGT TGG AAC CAC GA-3’; TIMP-1, forward 5’-CCT TCT GCA ATT CCG ACC TC-3’, reverse 5’-GTA TCC GCA GAC ACT CTC CA-3’; epithelial-cadherin (E-cadherin), forward 5’-AAT GCT GCA ACC ACT TCGG AT-3’, reverse 5’-CAT ATG TCA CGC CAG ACA TTC-3’; vimentin, forward 5’-GTT CCA ATG CAA CCG ACT CAA CTT-3’, reverse 5’-CCG AGG CCA TCT TAC CGA CAC-3’;GAPDH, forward 5’-GCC ATC ACA GCA ACA CAG AA-3’, reverse 5’-GCC ATA CCA GTA AGC TTG CC-3’.
Western blotting
Total proteins were lysed in radioimmunoprecipitation (RIPA) buffer (Beyotime Institute of Biotechnology, Haimen, China). The concentrations of proteins were detected using the Bicinchoninic Acid (BCA) protein assay kit (Thermo Fisher Scientific, Inc.). Equivalent proteins (40 μg) were separated by SDS-PAGE and transferred to polyvinylidene fluoride membranes (Merck KGaA, Darmstadt, Germany). Membranes were incubated with primary antibodies, and secondary antibody (cat no LK2003L, Sungene Biotech Co., Ltd, China). The protein was detected using the enhanced chemiluminescence (ECL) substrate kit (Thermo Scientific Pierce) and ImageJ software (NIH, Bethesda, MD, USA). The results were analyzed by densitometry using Quantity One V4.6.2 software (Bio-Rad, USA). The primary antibodies were anti-TLR4 antibody (1: 1000, Abcam, ab13556); anti-COX-2 antibody (1: 1000, Abcam, ab52237); anti-TIMP-1 antibody (1: 1000, Abcam, ab61224); anti-MMP-2 antibody (1: 1000, cat. no. PF023, Millipore, Darmstadt, Germany); anti-MMP-9 antibody (1: 50, Santa Cruz, cat no. sc-21733); anti-E-cadherin antibody (1: 500, Abcam, ab76055); anti-vimentin antibody (1: 800, Abcam, ab92547); anti-p-p65 antibody (1: 1000, Abcam, ab86299); anti-p65 antibody (1: 2000, Abcam, ab16502); anti-β-actin antibody (1: 1000, Abcam, ab8226).
Cell Counting Kit-8 (CCK-8) assay
PC3 cells were seeded into a 96-well plate at a density of 2×103 cells/well and transfected with TLR4siRNAs or COX-2-siRNAs, or both, at 37°C for 24 hours. Every day until day 5, CCK-8 (Beyotime, Haimen, China, 15 μL/well) was added to each well and incubated at 37°C for 3 hours. The optical density (OD) value was measured using a microplate reader (BIOTEK, VT, USA) at 450 nm.
Carboxyfluorescein succinimidyl ester (CFSE) assay
The proliferation capacity was detected by carboxyfluorescein succinimidyl ester (CFSE) dye (Life Technologies-Molecular Probes, Grand Island, NY, USA). PC3 cells were incubated with CFSE (10 nM) for 30 minutes, and then washed with complete medium. The labeled cells were incubated with antibody for 4 days, and then analyzed by flow cytometry.
Wound-healing assay
The cell migration ability of the treated PC3 cells was detected by using wound-healing assay. PC3 cells were seeded into a 6-well plate at a density of 1×105 cells/well and transfected with TLR4-siRNAs or COX-2-siRNAs, or both, at 37°C. A straight scratch was made by a pipette tip, and the suspending cells were gently washed off by cold PBS. The pictures of the scratch were captured at 0 and 24 hours.
Transwell assay
The cell invasion ability of the treated PC3 cells was detected by using Transwell assay. The Transwell chambers were coated with Matrigel for 30 minutes at 37°C. Then 200 μL of treated PC3 cells were seeded into the upper chamber at a density of 2×103 cells/mL, and 600 μL of the complete medium were added to the lower chamber. After 48 hours, the uninvaded cells were cleared up. The cells on the lower side were washed with PBS 3 times, fixed with 4% paraformaldehyde (cat no 158127) for 30 minutes, then stained with crystal violet (cat no 61135) for 10 minutes. Images were captured under microscopes.
Statistical analysis
All experimental data were expressed as mean ± standard deviation (SD), and all statistical analyses were carried out by the SPSS software version 20.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was considered statistically significant.
Results
TLR4 and COX-2 were high-expressed in PCa cells
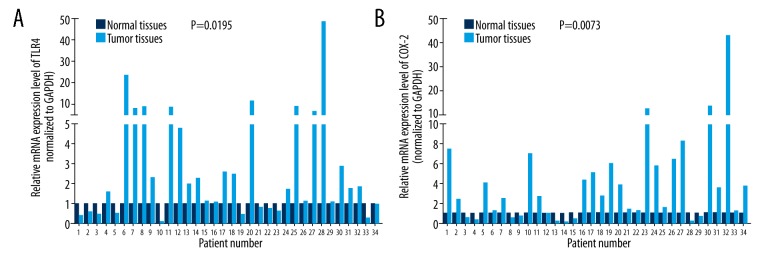
To explore the expression levels of TLR4 and COX-2 in PCa, we used qRT-PCR assays to analyze their expressions in 34 pairs of PCa (tumor tissues) and adjacent non-cancerous tissues (normal tissues). We found that the relative mRNA expression level of TLR4 was markedly higher in PCa tissues than normal tissues (n=34, P=0.0195, Figure 1A); COX-2 expression was also significantly increased in PCa tissues compared with normal tissues (n=34, P=0.0073, Figure 1B).
Figure 1.
TLR4 and COX-2 were high-expressed in PCa. (A) TLR4 expression was analyzed by qRT-PCR assays in PCa tissues and corresponding non-tumor tissues (n=34, P=0.0195). (B) COX-2 expression was detected by qRT-PCR assays in PCa tissues and corresponding non-tumor tissues (n=34, P=0.0073). TLR4 – toll-like receptor 4; COX-2– cyclooxygenase-2; PCa – prostate cancer.
Silencing of TLR4/COX-2 inhibits PCa cell proliferation
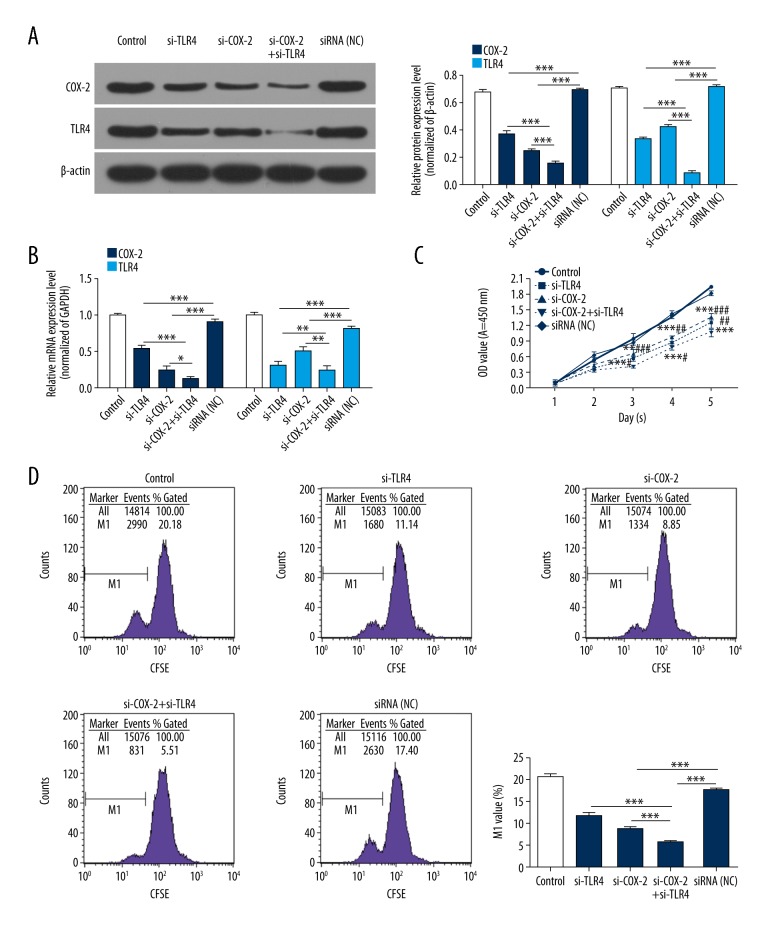
Based on the aforementioned observations, we constructed siRNA vectors targeting TLR4 and COX-2, namely si-TLR4 and si-COX-2, respectively. In addition, PCa3 cells were transfected with PBS (control), TLR4 siRNAs (si-TLR4), COX-2 siRNAs (si-COX-2), COX-2 siRNAs plus TLR4 siRNAs (si-COX-2+si-TLR4), negative control (NC), respectively. The analysis of TLR4 and COX-2 expressions were carried out in treated PCa3 cells using western blot and qRT-PCR assays. As shown in Figure 2A and 2B, the knockdown of TLR4 significantly decreased TLR4 and COX-2 expressions; the knockdown of COX-2 also dramatically decreased TLR4 and COX-2 expressions; more importantly, double-knockout of TLR4 and COX-2 more obviously inhibited TLR4 and COX-2 expressions than si-TLR4 group or si-COX-2 group in PCa3 cells (P<0.05, P<0.01, P<0.001, respectively).
Figure 2.
Silencing of TLR4/COX-2 inhibits PCa cell proliferation. PCa3 cells were transfected with PBS (control), TLR4 siRNAs (si-TLR4), COX-2 siRNAs (si-COX-2), COX-2 siRNAs plus TLR4 siRNAs (si-COX-2+si-TLR4), negative control (NC). (A) The protein expression levels of TLR4 and COX-2 were measured by western blot assays, and the results were analyzed according to the protein grayscales (*** P<0.001). (B) The mRNA expression levels of TLR4 and COX-2 were detected by qRT-PCR assays (* P<0.05, ** P<0.01, *** P<0.001). (C) The proliferation ability was analyzed by CCK-8 assays (** P<0.01, *** P<0.001 vs. NC group; # P<0.05, ## P<0.01, ### P<0.001 vs. si-COX-2+ si-TLR4 group). (D) The proliferation ability was also detected by CFSE assays (*** P<0.001). TLR4 – toll-like receptor 4; COX-2 – cyclooxygenase-2; PCa – prostate cancer.
To further demonstrate the potential roles of siRNA-mediated TLR4 and COX-2 silencing on PCa cell proliferation, CCK-8 and CFSE assays were performed after siRNA transfection. The results proved that TLR4 knockdown or COX-2 knockdown inhibited the proliferation capacity of PCa3 cells. Furthermore, double-knockout of TLR4 and COX-2 more observably inhibited PCa3 cell proliferation ability than si-TLR4 group or si-COX-2 group (P<0.05, P<0.01, P<0.001, respectively, Figure 2C, 2D). The results showed that TLR4 and COX-2 inhibits PCa cell proliferation, and there was a coordinated effect between TLR4 silence and COX-2 silence.
Silencing of TLR4/COX-2 suppressed PCa cell migration and invasion
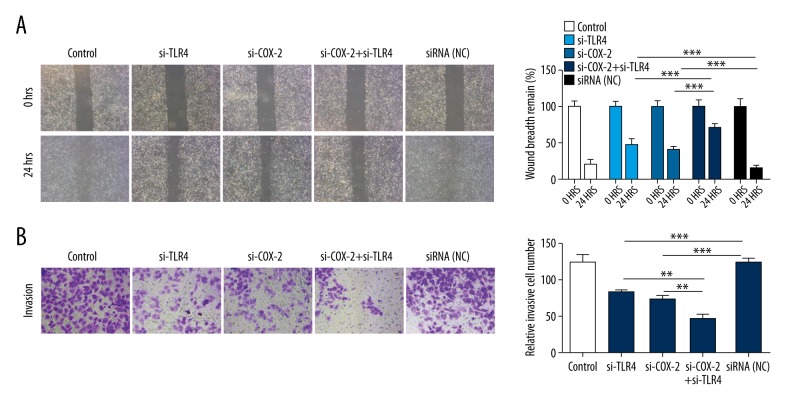
To further explore the roles of si-TLR4 and si-COX-2 on cell migration and invasion, si-TLR4, si-COX-2, and siRNA-NC were transfected into PCa3 cells. The migration capacity was detected by wound healing assay, and the results showed that TLR4 knockdown or COX-2 knockdown aggrandized the remnant wound breadth of PCa3 cells; joint-knockout of TLR4 and COX-2 obviously increased the remnant wound breadth of PCa3 cells compared with TLR4 knockdown group and COX-2 knockdown group (P<0.001, Figure 3A). In addition, the invasion ability was measured by Transwell assays, and the results indicated that TLR4 knockdown or COX-2 knockdown significantly decreased the number of the invasive PCa3 cells; joint-knockout of TLR4 and COX-2 further reduced the number of the invasive PCa3 cells compared with TLR4 knockdown group and COX-2 knockdown group (P<0.01, P<0.001, respectively, Figure 3B).
Figure 3.
Silencing of TLR4/COX-2 suppresses PCa cell migration and invasion. PCa3 cells were transfected with PBS (control), TLR4 siRNAs (si-TLR4), COX-2 siRNAs (si-COX-2), COX-2 siRNAs plus TLR4 siRNAs (si-COX-2+si-TLR4), negative control (NC). (A) Wound healing assays were performed to detect PCa cell migration capacity (*** P<0.001). (B) Transwell assays were used to measure PCa cell invasion ability (** P<0.01, *** P<0.001). TLR4 – toll-like receptor 4; COX-2 – cyclooxygenase-2; PCa – prostate cancer.
Silencing of TLR4/COX-2 decreases MMP-2, MMP-9, and E-cadherin expressions, and increases TIMP-1 and vimentin expressions
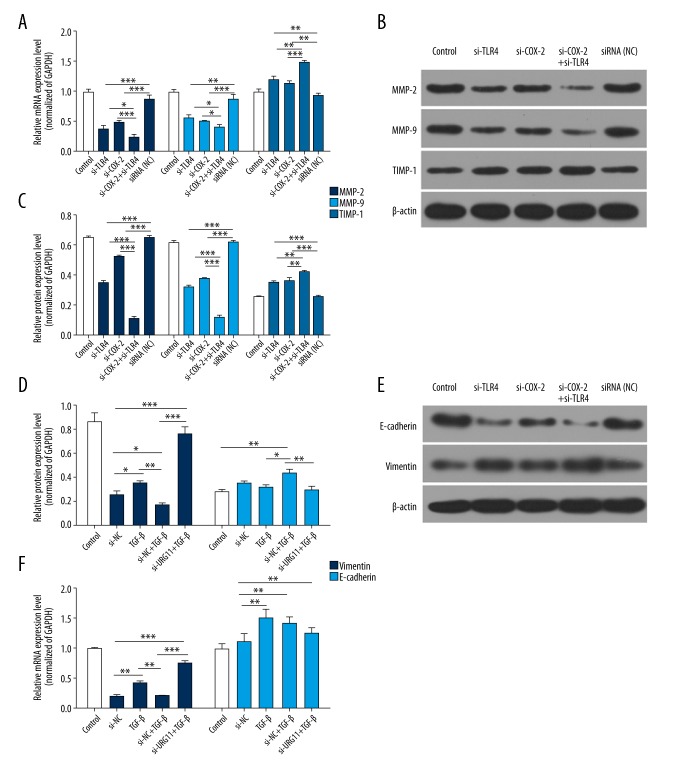
Furthermore, qRT-PCR and western blot assays were carried out to explore the functional mechanism of TLR4 and COX-2 in PCa3 cell migration and invasion. The results revealed that MMP-2, MMP-9, and E-cadherin expressions were downregulated in the si-TLR4 group and the si-COX-2 group compared with the siRNA-NC group, and were lower in the si-TLR4 plus si-COX-2 group than in the si-TLR4 group or the si-COX-2 group. Besides, TIMP-1 and vimentin expressions were upregulated in the si-TLR4 group and the si-COX-2 group compared with the siRNA-NC group, and were higher in the si-TLR4 plus si-COX-2 group than the si-TLR4 group or the si-COX-2 group (P<0.05, P<0.01, P<0.001, respectively, Figure 4). Therefore, we suggest that TLR4 combined with COX-2 affected MPP-related genes (MMP-2, MMP-9, TIMP-1, E-cadherin, and vimentin) in PCa.
Figure 4.
Silencing of TLR4/COX-2 decreases MMP-2, MMP-9, and E-cadherin expressions, and increases TIMP-1 and vimentin expressions. (A) qRT-PCR assays were performed to analyze the expression levels of MMP-2, MMP-9, TIMP-1, E-cadherin, and vimentin in treated PCa3 cells, GAPDH was used as an internal control, (* P<0.05, ** P<0.01, *** P<0.001). (B) Western blot assays were used to detect MMP-2, MMP-9, TIMP-1, E-cadherin, and vimentin expressions in treated PCa3 cells, β-actin was used as an internal control, (* P<0.05, ** P<0.01, *** P<0.001). TLR4 – toll-like receptor 4; COX-2– cyclooxygenase-2; PCa – prostate cancer; MMP – matrix metalloproteinase; TIMP – tissue inhibitor of matrix metalloproteinases; E-cadherin – epithelial-cadherin.
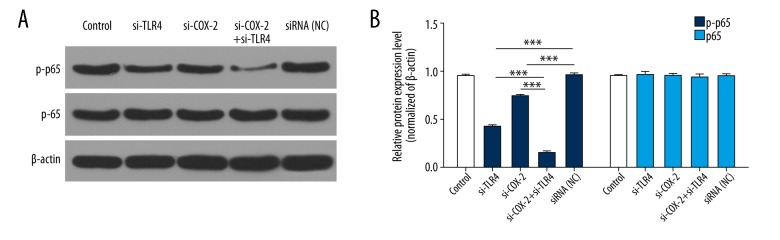
Silencing of TLR4/COX-2 inhibits p65 phosphorylation
Studies have shown that NF-κB (p65) is involved in the development of multiple human malignancies such as proliferation, migration, and invasion [40–42]. Therefore, we analyzed the effects of TLR4 and COX-2 on p65 expression and found that p-p65 expression was obviously downregulated in the si-TLR4 group or the si-COX-2 group compared with the siRNA-NC group; p-p65 expression was markedly decreased in the si-TLR4 plus si-COX-2 group compared with the si-TLR4 group or the si-COX-2 group (P<0.001, Figure 5).
Figure 5.
Silencing of TLR4/COX-2 inhibits p65 phosphorylation. (A) The protein expression levels of p65 and p-p65 were analyzed by western blot assays in treated PCa3 cells; β-actin was used as an internal control. (B) The protein grayscales were used to quantify the p65 and p-p65 expression (*** P<0.001).
Discussion
TLR4, a member of the family Toll-like receptors, can be widely activated in inflammatory environments, which not only mediates bacterial endotoxin reaction but can combine with non-bacterial heat-shock protein and hyaluronic acid [43,44]. TLR4 ligand lipopolysaccharide is in combination with LPS-binding protein (LBP) in serum, combined with CD14 on inflammatory cells, and then combined with myeloid differentiation protein-2 (MD-2) to initiate intracellular signaling [45]. MyD88 is a key connector in the process of MyD88-dependent signal transduction [46]. MyD88 is combined with downstream signal molecules, such as IL-1R associated kinase 4 (IRAK4) and release tumor necrosis factor associated factor 6 (TRAF6); the activated TRAF6 then releases NF-κB through a series of enzymatic reactions [47]. NF-κB can be combined with the promoters of inflammatory genes to regulate downstream gene expressions [48,49]. Studies have shown that NF-κB can inhibit cell apoptosis and prolong cell survival [50,51]. In our study, we found that TLR4 expression was markedly higher in PCa tissues than normal tissues, which suggested that TLR4 was upregulated in PCa.
COX-2, the internal oxidation synthetase of prostaglandin, is the rate-limiting enzyme of arachidonic acid converting to physiological activity eicosanoids such as prostaglandin, thromboxane, and prostacyclin [52]. Although the exact functions of COX-2 in tumorigenesis and development need to be further identified, it has been reported that the expression of COX-2 is upregulated in various tumor tissues [53]. In our study, we further revealed that COX-2 expression was markedly higher in PCa tissues than normal tissues, which suggested that COX-2 is highly expressed PCa.
MMP is involved in extracellular matrix degradation Zn2+-dependent protease family [54]. Studies have shown that MMPs, such as MMP-2 and MMP-9, play major roles in the processes of cell migration, and metastasis [55]. Tissue inhibitor of metalloproteinases (TIMPs) can inhibit the activity of MMPs [56]. Because the degradation of extracellular matrix (ECM) by secretory enzymes is evidence of epithelial mesenchymal transition (EMT) progression, and that EMT is regarded as a critical step in tumor metastasis and invasion [57]. Therefore, E-cadherin and vimentin as mark proteins of EMT were used for determining the degree of EMT. At present, metastasis is the major cause of tumor treatment failure. TLR4 is involved in tumor cell migration and invasion in various cancers. In this study, we investigated the biological roles of TLR4 and COX-2 on PCa cell proliferation, migration, and invasion through silencing TLR4 and COX-2 using small interfering RNA (siRNA). The results indicated silencing of TLR4 or COX-2 inhibited PCa cell proliferation, migration, and invasion, in addition, silencing of TLR4 or COX-2 decreased MMP-2 and MMP-9 expressions, and increased TIMP-1 expression. Therefore, we suggest that TLR4 and COX-2 are involved in the proliferation, migration, and invasion progresses of PCa cells.
Previous studies have shown that TLR4 affected proliferation and apoptosis by regulating COX-2 [21]; TLR4 can activate NF-κB and MAP kinase pathways to regulate COX-2 in renal medullary collecting duct cells [22]; COX-2 affected prostate epithelial cells by TLR4 and NF-κB [58]; TLR4 participated in gastric mucosal protection via COX-2 [59]. In our study, we found that joint-knockout of TLR4 and COX-2 inhibited PCa cell proliferation, migration and invasion, and there was a synergistic effect between TLR4 and COX-2.
NF-κB is one of the nuclear transcription factors which is found in eukaryotic cells and regulates DNA transcription [60]. Emerging research suggested that NF-κB plays critical roles in biological processes, such as proliferation, differentiation, migration, and metastasis [61,62]. Previous research has shown that NF-κB participates in the occurrence and development progresses of cancers [48]. In our study, we revealed that TLR4 knockdown or COX-2 knockdown significantly inhibited p65 phosphorylation; TLR4 and COX-2 showed a synergic effect.
Conclusions
TLR4 and COX-2 expressions are high-expressed in PCa, and TLR4 knockdown or COX-2 knockdown inhibits PCa cell proliferation, migration, and invasion progresses via inhibiting p65 phosphorylation. This study suggests that TLR4 and COX-2 may be novel prognostic markers and candidate drug targets for PCa.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Neppl-Huber C, Zappa M, Coebergh JW, et al. Changes in incidence, survival and mortality of prostate cancer in Europe and the United States in the PSA era: Additional diagnoses and avoided deaths. Ann Oncol. 2012;23:1325–34. doi: 10.1093/annonc/mdr414. [DOI] [PubMed] [Google Scholar]
- 2.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate cancer mortality: Results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35. doi: 10.1016/S0140-6736(14)60525-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lin YL, Li YL, Ma JG. Aberrant promoter methylation of Protocadherin8 (PCDH8) in serum is a potential prognostic marker for low Gleason score prostate cancer. Med Sci Monit. 2017;23:4895–900. doi: 10.12659/MSM.904366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook ED, Nelson AC. Prostate cancer screening. Curr Oncol Rep. 2011;13:57–62. doi: 10.1007/s11912-010-0136-x. [DOI] [PubMed] [Google Scholar]
- 5.Kitagawa Y, Namiki M. Prostate-specific antigen-based population screening for prostate cancer: Current status in Japan and future perspective in Asia. Asian J Androl. 2015;17:475–80. doi: 10.4103/1008-682X.143756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Deng QK, Lei YG, Lin YL, et al. Prognostic value of Protocadherin10 (PCDH10) methylation in serum of prostate cancer patients. Med Sci Monit. 2016;22:516–21. doi: 10.12659/MSM.897179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Womble PR, Montie JE, Ye Z, et al. Contemporary use of initial active surveillance among men in Michigan with low-risk prostate cancer. Eur Urol. 2015;67:44–50. doi: 10.1016/j.eururo.2014.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Cui Y, Li J, Zheng F, et al. Effect of SIRT1 gene on epithelial-mesenchymal transition of human prostate cancer PC-3 cells. Med Sci Monit. 2016;22:380–86. doi: 10.12659/MSM.895312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Labrie F. PSA screening for prostate cancer: Why so much controversy? Asian J Androl. 2013;15:603–7. doi: 10.1038/aja.2013.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qu Y, Zhang C, Du E, et al. Pim-3 is a critical risk factor in development and prognosis of prostate cancer. Med Sci Monit. 2016;22:4254–60. doi: 10.12659/MSM.898223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Norstrom MM, Radestad E, Sundberg B, et al. Progression of benign prostatic hyperplasia is associated with pro-inflammatory mediators and chronic activation of prostate-infiltrating lymphocytes. Oncotarget. 2016;7:23581–93. doi: 10.18632/oncotarget.8051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiaoli Z, Yawei W, Lianna L, et al. Screening of target genes and regulatory function of miRNAs as prognostic indicators for prostate cancer. Med Sci Monit. 2015;21:3748–59. doi: 10.12659/MSM.894670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu J, Sun P, Sun B, Wang C. Low LKB1 expression results in unfavorable prognosis in prostate cancer patients. Med Sci Monit. 2015;21:3722–27. doi: 10.12659/MSM.894847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chow A, Zhou W, Liu L, et al. Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci Rep. 2014;4:5750. doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim KH, Jo MS, Suh DS, et al. Expression and significance of the TLR4/MyD88 signaling pathway in ovarian epithelial cancers. World J Surg Oncol. 2012;10:193. doi: 10.1186/1477-7819-10-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu P, Li F, Qiu M, He L. Expression and cellular distribution of TLR4, MyD88, and NF-kappaB in diabetic renal tubulointerstitial fibrosis, in vitro and in vivo. Diabetes Res Clin Pract. 2014;105:206–16. doi: 10.1016/j.diabres.2014.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Mao SS, Hua R, Zhao XP, et al. Exogenous administration of PACAP alleviates traumatic brain injury in rats through a mechanism involving the TLR4/MyD88/NF-kappaB pathway. J Neurotrauma. 2012;29:1941–59. doi: 10.1089/neu.2011.2244. [DOI] [PubMed] [Google Scholar]
- 18.Wang W, Xia T, Yu X. Wogonin suppresses inflammatory response and maintains intestinal barrier function via TLR4-MyD88-TAK1-mediated NF-kappaB pathway in vitro. Inflamm Res. 2015;64:423–31. doi: 10.1007/s00011-015-0822-0. [DOI] [PubMed] [Google Scholar]
- 19.Suga H, Sugaya M, Fujita H, et al. TLR4, rather than TLR2, regulates wound healing through TGF-beta and CCL5 expression. J Dermatol Sci. 2014;73:117–24. doi: 10.1016/j.jdermsci.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 20.Dasu MR, Martin SJ. Toll-like receptor expression and signaling in human diabetic wounds. World J Diabetes. 2014;5:219–23. doi: 10.4239/wjd.v5.i2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukata M, Chen A, Klepper A, et al. Cox-2 is regulated by Toll-like receptor-4 (TLR4) signaling: Role in proliferation and apoptosis in the intestine. Gastroenterology. 2006;131:862–77. doi: 10.1053/j.gastro.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuper C, Beck FX, Neuhofer W. Toll-like receptor 4 activates NF-kappaB and MAP kinase pathways to regulate expression of proinflammatory COX-2 in renal medullary collecting duct cells. Am J Physiol Renal Physiol. 2012;302:F38–46. doi: 10.1152/ajprenal.00590.2010. [DOI] [PubMed] [Google Scholar]
- 23.Lin A, Wang G, Zhao H, et al. TLR4 signaling promotes a COX-2/PGE2/STAT3 positive feedback loop in hepatocellular carcinoma (HCC) cells. Oncoimmunology. 2016;5:e1074376. doi: 10.1080/2162402X.2015.1074376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agundez JA, Blanca M, Cornejo-Garcia JA, Garcia-Martin E. Pharmacogenomics of cyclooxygenases. Pharmacogenomics. 2015;16:501–22. doi: 10.2217/pgs.15.6. [DOI] [PubMed] [Google Scholar]
- 25.Fitzpatrick FA. Cyclooxygenase enzymes: Regulation and function. Curr Pharm Des. 2004;10:577–88. doi: 10.2174/1381612043453144. [DOI] [PubMed] [Google Scholar]
- 26.Adelizzi RA. COX-1 and COX-2 in health and disease. J Am Osteopath Assoc. 1999;99:S7–12. [PubMed] [Google Scholar]
- 27.Bondar TN, Kravchenko NA. [Cyclooxigenase-1 gene polymorphism and aspirin resistance]. Tsitol Genet. 2012;46:66–72. [in Russian] [PubMed] [Google Scholar]
- 28.Morita I. Distinct functions of COX-1 and COX-2. Prostaglandins Other Lipid Mediat. 2002;68–69:165–75. doi: 10.1016/s0090-6980(02)00029-1. [DOI] [PubMed] [Google Scholar]
- 29.Fujino H. The roles of EP4 prostanoid receptors in cancer malignancy signaling. Biol Pharm Bull. 2016;39:149–55. doi: 10.1248/bpb.b15-00840. [DOI] [PubMed] [Google Scholar]
- 30.Yang Y, Tang LQ, Wei W. Prostanoids receptors signaling in different diseases/cancers progression. J Recept Signal Transduct Res. 2013;33:14–27. doi: 10.3109/10799893.2012.752003. [DOI] [PubMed] [Google Scholar]
- 31.Marnett LJ, Rowlinson SW, Goodwin DC, et al. Arachidonic acid oxygenation by COX-1 and COX-2. Mechanisms of catalysis and inhibition. J Biol Chem. 1999;274:22903–6. doi: 10.1074/jbc.274.33.22903. [DOI] [PubMed] [Google Scholar]
- 32.Poorani R, Bhatt AN, Dwarakanath BS, Das UN. COX-2, aspirin and metabolism of arachidonic, eicosapentaenoic and docosahexaenoic acids and their physiological and clinical significance. Eur J Pharmacol. 2016;785:116–32. doi: 10.1016/j.ejphar.2015.08.049. [DOI] [PubMed] [Google Scholar]
- 33.Yui K, Imataka G, Nakamura H, et al. Eicosanoids derived from arachidonic acid and their family prostaglandins and cyclooxygenase in psychiatric disorders. Curr Neuropharmacol. 2015;13:776–85. doi: 10.2174/1570159X13666151102103305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu KK, Cheng HH, Chang TC. 5-methoxyindole metabolites of L-tryptophan: Control of COX-2 expression, inflammation and tumorigenesis. J Biomed Sci. 2014;21:17. doi: 10.1186/1423-0127-21-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aziz F, Qiu Y. The role of anti-LeY antibody in the downregulation of MAPKs/COX-2 pathway in gastric cancer. Curr Drug Targets. 2014;15:469–76. doi: 10.2174/1389450115666140217152042. [DOI] [PubMed] [Google Scholar]
- 36.Khan Z, Khan N, Tiwari RP, et al. Biology of Cox-2: An application in cancer therapeutics. Curr Drug Targets. 2011;12:1082–93. doi: 10.2174/138945011795677764. [DOI] [PubMed] [Google Scholar]
- 37.Misra S, Sharma K. COX-2 signaling and cancer: New players in old arena. Curr Drug Targets. 2014;15:347–59. doi: 10.2174/1389450115666140127102915. [DOI] [PubMed] [Google Scholar]
- 38.Yu T, Lao X, Zheng H. Influencing COX-2 activity by COX related pathways in inflammation and cancer. Mini Rev Med Chem. 2016;16:1230–43. doi: 10.2174/1389557516666160505115743. [DOI] [PubMed] [Google Scholar]
- 39.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 40.Jing H, Lee S. NF-kappaB in cellular senescence and cancer treatment. Mol Cells. 2014;37:189–95. doi: 10.14348/molcells.2014.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li F, Zhang J, Arfuso F, et al. NF-kappaB in cancer therapy. Arch Toxicol. 2015;89:711–31. doi: 10.1007/s00204-015-1470-4. [DOI] [PubMed] [Google Scholar]
- 42.Liu B, Sun L, Liu Q, et al. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–81. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Choi SY, Ryu HM, Choi JY, et al. The role of Toll-like receptor 4 in high-glucose-induced inflammatory and fibrosis markers in human peritoneal mesothelial cells. Int Urol Nephrol. 2017;49:171–81. doi: 10.1007/s11255-016-1430-9. [DOI] [PubMed] [Google Scholar]
- 44.Liu Y, Yin H, Zhao M, Lu Q. TLR2 and TLR4 in autoimmune diseases: A comprehensive review. Clin Rev Allergy Immunol. 2014;47:136–47. doi: 10.1007/s12016-013-8402-y. [DOI] [PubMed] [Google Scholar]
- 45.Ranoa DR, Kelley SL, Tapping RI. Human lipopolysaccharide-binding protein (LBP) and CD14 independently deliver triacylated lipoproteins to Toll-like receptor 1 (TLR1) and TLR2 and enhance formation of the ternary signaling complex. J Biol Chem. 2013;288:9729–41. doi: 10.1074/jbc.M113.453266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Laird MH, Rhee SH, Perkins DJ, et al. TLR4/MyD88/PI3K interactions regulate TLR4 signaling. J Leukoc Biol. 2009;85:966–77. doi: 10.1189/jlb.1208763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiang Q, Yi M, Guo Q, et al. Protective effects of polydatin on lipopolysaccharide-induced acute lung injury through TLR4-MyD88-NF-kappaB pathway. Int Immunopharmacol. 2015;29:370–76. doi: 10.1016/j.intimp.2015.10.027. [DOI] [PubMed] [Google Scholar]
- 48.Xia Y, Shen S, Verma IM. NF-kappaB, an active player in human cancers. Cancer Immunol Res. 2014;2:823–30. doi: 10.1158/2326-6066.CIR-14-0112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Han X, Zhang JJ, Yao N, et al. Polymorphisms in NFKB1 and NFKBIA genes modulate the risk of developing prostate cancer among Han Chinese. Med Sci Monit. 2015;21:1707–15. doi: 10.12659/MSM.893471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hoesel B, Schmid JA. The complexity of NF-kappaB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. doi: 10.1186/1476-4598-12-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bi D, Yang M, Zhao X, Huang S. Effect of Cnidium lactone on serum mutant P53 and BCL-2/BAX expression in human prostate cancer cells PC-3 tumor-bearing BALB/C nude mouse model. Med Sci Monit. 2015;21:2421–27. doi: 10.12659/MSM.893745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci. 2014;35:284–92. doi: 10.1016/j.tips.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 53.Roelofs HM, Te Morsche RH, van Heumen BW, et al. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014;14:1. doi: 10.1186/1471-230X-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yadav L, Puri N, Rastogi V, et al. Matrix metalloproteinases and cancer – roles in threat and therapy. Asian Pac J Cancer Prev. 2014;15:1085–91. doi: 10.7314/apjcp.2014.15.3.1085. [DOI] [PubMed] [Google Scholar]
- 55.Tan RJ, Liu Y. Matrix metalloproteinases in kidney homeostasis and diseases. Am J Physiol Renal Physiol. 2012;302:F1351–61. doi: 10.1152/ajprenal.00037.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arpino V, Brock M, Gill SE. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–54. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Mao XW, Xiao JQ, Xu G, et al. CUL4B promotes bladder cancer metastasis and induces epithelial-to-mesenchymal transition by activating the Wnt/beta-catenin signaling pathway. Oncotarget. 2017;8:77241–53. doi: 10.18632/oncotarget.20455. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 58.Liu J, Hu S, Cui Y, et al. Saturated fatty acids up-regulate COX-2 expression in prostate epithelial cells via toll-like receptor 4/NF-kappaB signaling. Inflammation. 2014;37:467–77. doi: 10.1007/s10753-013-9760-6. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Chen H, Yang L. Toll-like receptor 4 participates in gastric mucosal protection through Cox-2 and PGE2. Dig Liver Dis. 2010;42:472–76. doi: 10.1016/j.dld.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 60.Panday A, Inda ME, Bagam P, et al. Transcription factor NF-kappaB: An update on intervention strategies. Arch Immunol Ther Exp (Warsz) 2016;64:463–83. doi: 10.1007/s00005-016-0405-y. [DOI] [PubMed] [Google Scholar]
- 61.Jiang C, Lin X. Analysis of epidermal growth factor-induced NF-kappaB signaling. Methods Mol Biol. 2015;1280:75–102. doi: 10.1007/978-1-4939-2422-6_6. [DOI] [PubMed] [Google Scholar]
- 62.Liang B, Chen R, Wang T, et al. Myeloid differentiation factor 88 promotes growth and metastasis of human hepatocellular carcinoma. Clin Cancer Res. 2013;19:2905–16. doi: 10.1158/1078-0432.CCR-12-1245. [DOI] [PubMed] [Google Scholar]