Long-distance movement of tobacco NsCET1 and PEBP mRNAs suggests that acquisition of RNA mobility is an early evolutionary event.
Abstract
Photoperiodic floral induction is controlled by the leaf-derived and antagonistic mobile signals florigen and antiflorigen. In response to photoperiodic variations, florigen and antiflorigen are produced in leaves and translocated through phloem to the apex, where they counteract floral initiation. Florigen and antiflorigen are encoded by a pair of homologs belonging to FLOWERING LOCUS T (FT)- or TERMINAL FLOWER1 (TFL1)-like clades in the phosphatidylethanolamine-binding domain protein (PEBP) family. The PEBP gene family contains FT-, TFL1-, and MOTHER OF FT AND TFL1 (MFT)-like clades. Evolutionary analysis suggests that FT- and TFL1-like clades arose from an ancient MFT-like clade. The protein movement of the PEBP family is an evolutionarily conserved mechanism in many plants; however, the mRNA movement of the PEBP family remains controversial. Here, we examined the mRNA movement of PEBP genes in different plant species. We identified a tobacco (Nicotiana sylvestris) CENTRORADIALIS-like1 gene, denoted NsCET1, and showed that NsCET1 is an ortholog of the Arabidopsis (Arabidopsis thaliana) antiflorigen ATC. In tobacco, NsCET1 acts as a mobile molecule that non-cell-autonomously inhibits flowering. Grafting experiments showed that endogenous and ectopically expressed NsCET1 mRNAs move long distances in tobacco and Arabidopsis. Heterografts of tobacco and tomato (Solanum lycopersicum) showed that, in addition to NsCET1, multiple members of the FT-, TFL1-, and MFT-like clades of tobacco and tomato PEBP gene families are mobile mRNAs. Our results suggest that the mRNA mobility is a common feature of the three clades of PEBP-like genes among different plant species.
Cell-to-cell and interorgan communication are crucial in synchronizing plant developmental programs with external cues. To integrate environmental stimuli perceived in distal tissues, plants have evolved many elegant systems to sense and convert the stimuli into mobile molecules. These mobile molecules are translocated through phloem to the target tissues to exert their non-cell-autonomous functions (Lacombe and Achard, 2016; Ham and Lucas, 2017). In addition to hormones, proteins, and metabolites, many plants recruit mRNAs as mobile molecules for long-distance communication (Kim et al., 2001; Haywood et al., 2005; Banerjee et al., 2006; Huang et al., 2012; Lu et al., 2012). Upon translocating to target tissues, the mobile mRNAs are believed to serve as a template to translate into many proteins. Thus, this mRNA-based regulatory network provides an efficient and specific communication system to transmit environmental stimuli perceived in distal tissues. It has been demonstrated that many mobile mRNAs are trafficked through phloem, probably by forming an RNA-protein complex to allow the stable translocation of mRNA molecules (Lucas et al., 2001; Ham and Lucas, 2017). Transcriptome profiling of phloem sap or heterografting experiments identified several thousand mobile mRNAs (Ruiz-Medrano et al., 1999; Guo et al., 2013; Thieme et al., 2015; Yang et al., 2015; Xia et al., 2018), suggesting that the use of mobile mRNAs as systemic signals may be a prevalent mechanism in plants to cope with environmental dynamics.
Photoperiodic floral regulation is mediated by two counteracting mobile molecules, namely florigen and antiflorigen, which are encoded by a pair of functionally opposite homologs belonging to different clades in the phosphatidylethanolamine-binding domain protein (PEBP) gene family (Zeevaart, 1976, 2008; Lang et al., 1977; Corbesier et al., 2007; Tamaki et al., 2007; Huang et al., 2012; Matsoukas et al., 2012; Higuchi et al., 2013). In Arabidopsis (Arabidopsis thaliana), florigen and antiflorigen are encoded by FLOWERING LOCUS T (FT) and ARABIDOPSIS THALIANA CENTRORADIALIS homolog (ATC), respectively. The expression of FT or ATC is induced under long-day (LD) or short-day (SD) conditions, respectively (Kardailsky et al., 1999; Kobayashi et al., 1999; Huang et al., 2012). FT and ATC both regulate the expression of the same downstream meristem identity gene, APETALA1, by interacting with the transcription factor FD (Abe et al., 2005; Wigge et al., 2005; Huang et al., 2012). The antagonistic functions of FT and ATC may be attributed to competition between FT and ATC for the same binding site on FD, because the deletion of 40 amino acids at the C terminus of FD abolished the binding of FD with FT and ATC (Huang et al., 2012). The expression of FT and ATC is mainly in vascular tissues and is absent in the apex (Takada and Goto, 2003; Huang et al., 2012), whereas the expression of FD is exclusively in shoot and root apices (Abe et al., 2005; Wigge et al., 2005), which suggests that the movement of FT and ATC from the vasculature to the apex is required for their functions. Through grafting experiments and tissue-specific expression, the protein and mRNA movement of FT and ATC has been demonstrated (Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007; Huang et al., 2012; Lu et al., 2012). The movement of FT protein from companion cells (CCs) to sieve elements (SEs) is mediated through the endoplasmic reticulum-localized protein FT-INTERACTING PROTEIN1 and the heavy metal-associated domain-containing protein SODIUM POTASSIUM ROOT DEFECTIVE1 (Liu et al., 2012; Zhu et al., 2016). However, deletion analysis of the Cucurbita moschata FT protein suggested that the transport of FT protein in CCs-SEs also is governed by a diffusion-based system (Yoo et al., 2013). Although ATC protein is detected in grafted scions, direct evidence to support the long-distance movement of ATC protein is lacking (Huang et al., 2012). In addition to protein movement, it has been reported that Arabidopsis FT and ATC are phloem-mobile mRNAs (Li et al., 2009; Huang et al., 2012; Lu et al., 2012). After transcription in leaves, FT and ATC mRNA is targeted selectively to plasmodesmata for cell-to-cell movement (Luo et al., 2018). However, the mRNA movement of florigen and antiflorigen has been observed only in Arabidopsis, because previous tomato (Solanum lycopersicum) grafting experiments failed to support long-distance trafficking of SINGLE FLOWER TRUSS (SFT) mRNA, a tomato FT ortholog (Lifschitz et al., 2006). Thus, whether the mRNAs of florigen and antiflorigen are mobile in different plant species remains to be elucidated.
The PEBP gene family is an evolutionarily conserved gene family across different kingdoms. In angiosperms, PEBP genes are grouped into three clades, namely FT-, TERMINAL FLOWER1 (TFL1)-, and MOTHER OF FT AND TFL1 (MFT)-like clades (Chardon and Damerval, 2005). However, in gymnosperms, phylogenetic analysis revealed that only MFT- and FT/TFL1-like genes are present, with only MFT-like genes in bryophytes and lycopods, which suggests that the MFT-like clade is the ancestor of all PEBP genes (Hedman et al., 2009; Karlgren et al., 2011). Evolutionary analysis of the PEBP family indicated that two ancient PEBP duplication events occurred in the common ancestor after the angiosperms split from gymnosperms. The first duplication event gave rise to the MFT-like clade and the ancient lineage of the TFL1/FT-like clade, which was confronted subsequently with a second duplication event forming the TFL1-like and FT-like clades (Hedman et al., 2009; Karlgren et al., 2011; Wang et al., 2015). The analysis of florigen movement in different plants indicates that the protein movement of PEBP genes is evolutionarily conserved in many plants (Conti and Bradley, 2007; Zeevaart, 2008; Turnbull, 2011); however, little is known about the mRNA movement of PEBP genes in different plant species. In addition, how florigen and antiflorigen have evolved to acquire mobility remains to be investigated.
In tobacco (Nicotiana spp.), many PEBP genes belonging to FT- or TFL1-like clades have been identified (Amaya et al., 1999; Harig et al., 2012). Unlike Arabidopsis, in which all FT-like genes are floral activators, functional analysis of Nicotiana tabacum FT-like genes showed that three FT-like genes (NtFT1, NtFT2, and NtFT3) act as floral inhibitors, whereas NtFT4 is a tobacco florigen homolog (Harig et al., 2012). NtFT1, NtFT2, and NtFT3 are expressed in leaves under SD conditions, probably in phloem CCs (Harig et al., 2012). However, whether these FT-like genes and the members of TFL1- or MFT-like genes are mobile mRNAs remains unknown. In this study, we investigated the mRNA movement of PEBP genes in tobacco. We identified three Nicotiana sylvestris TFL1-like genes, NsCET1, NsCET2, and NsCET10, which act non-cell-autonomously to inhibit floral initiation. Functional analysis suggested that NsCET1 is an ortholog of the Arabidopsis antiflorigen ATC. Analysis of heterografts showed that NsCET1 mRNA is mobile in Arabidopsis and tobacco, which suggests that the mRNA movement of antiflorigen is a conserved mechanism across different plant species. Further heterografting experiments showed that the mRNA of multiple PEBP genes, including FT-, TFL1-, and MFT-like genes, is mobile in tobacco and tomato. Thus, the movement of PEBP gene mRNA may be a conserved mechanism across different plant species.
RESULTS
Tobacco NsCET Genes Act Non-Cell-Autonomously to Inhibit Flowering in Arabidopsis and Tobacco
To explore the mRNA movement of antiflorigen in different plant species, we identified tobacco ATC orthologs to examine their mRNA movement. By combining database searches and reverse transcription (RT)-PCR analysis, we identified five TFL1-like genes, namely NsCET1, NsCET2, NsCET5, NsCET9, and NsCET10, from an obligate LD variety N. sylvestris. Phylogenetic analysis showed that NsCET5 and NsCET9 were grouped with Arabidopsis TFL1 (Supplemental Fig. S1A), which is consistent with previous results that NsCET5 is an ortholog of TFL1 (Amaya et al., 1999). Because our previous experiments revealed that ectopic expression of Arabidopsis TFL1 in CCs is not sufficient to affect flowering (Huang et al., 2012), we investigated the remaining CET genes, specifically NsCET1, NsCET2, and NsCET10. Sequence comparison revealed that NsCET1, NsCET2, and NsCET10 contain the key amino acids required for the activity of floral inhibitors (His-88 and Asp-144; Ahn et al., 2006). The flowering time of Arabidopsis transformants harboring these CET transgenes was delayed as compared with that of wild-type plants (Table 1), which indicates that NsCET1, NsCET2, and NsCET10 are floral inhibitors. In addition, the expression of NsCET1, NsCET2, and NsCET10 by the SUCROSE TRANSPORTER2 (SUC2) promoter, a CC-specific promoter, showed that these CETs acted non-cell-autonomously to inhibit flowering in Arabidopsis (Table 1). Among CET transformants, ectopic expression of NsCET1 exhibited the most severe late-flowering phenotype (Supplemental Fig. S1, B–E). The flowers of NsCET1 transformants produced leaf-like bracts, which resembled the phenotypes of Arabidopsis ATC overexpression lines (Supplemental Fig. S1, C–E; Huang et al., 2012). Similarly, Arabidopsis transformants harboring NsCET2 or NsCET10 transgenes showed moderate late-flowering and leaf-like bract phenotypes (Table 1; Supplemental Fig. S1, F–I), which suggests that the functions of NsCET1, NsCET2, and NsCET10 are partially redundant, but NsCET1 functions similar to Arabidopsis ATC.
Table 1. Flowering time of Arabidopsis transformants expressing tobacco CET transgenes.
Data are means ± sd.
| Plant Line | Flowering Time |
|---|---|
| no. of rosette leaves | |
| Columbia | 8.6 ± 0.7 |
| 35S-NsCET1 | 16.8 ± 1.7 |
| SUC2-NsCET1 | 22.4 ± 2.9 |
| 35S-NsCET2 | 11.9 ± 1.0 |
| SUC2-NsCET2 | 14.0 ± 1.7 |
| 35S-NsCET10 | 11.3 ± 0.9 |
| SUC2-NsCET10 | 11.1 ± 0.7 |
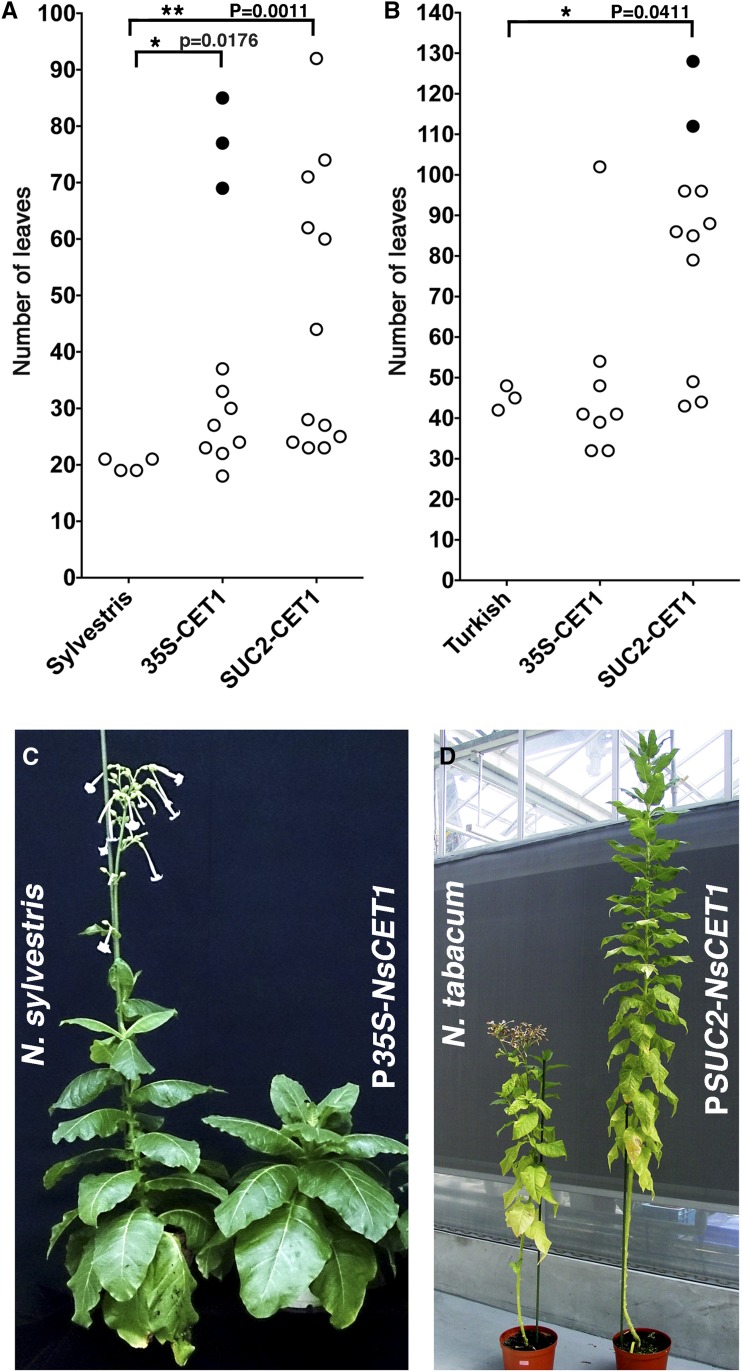
To further examine the function of NsCET1 in tobacco, we introduced P35S-NsCET1 or PSUC2-NsCET1 into tobacco. The obligate LD variety (N. sylvestris) or day-neutral variety (N. tabacum) tobacco harboring P35S-NsCET1 or PSUC2-NsCET1 transgenes showed a late-flowering phenotype (Fig. 1), which suggests that NsCET1 acts as a non-cell-autonomous floral inhibitor in tobacco. Tobacco transformants with extreme late-flowering phenotypes produced a substantial number of leaves. A number of transformants did not flower at 5 months after transfer to soil from rooting medium (Fig. 1, A and B, black circles). In addition to exhibiting a late-flowering phenotype, these tobacco transformants had a short-internode phenotype, which was easily recognized in N. tabacum transformants (Supplemental Fig. S2, A and B). However, unlike Arabidopsis P35S-NsCET1 or PSUC2-NsCET1 transformants, the floral organs of tobacco P35S-NsCET1 transformants were similar to those of wild-type plants (Supplemental Fig. S2, C and D), which indicates functional specificity for tobacco NsCET1.
Figure 1.
Tobacco NsCET1 acts non-cell-autonomously to inhibit flowering. A, Flowering time of wild-type N. sylvestris and N. sylvestris transformants harboring P35S-NsCET1 or PSUC2-NsCET1 transgenes under LD conditions. The black circles represent the leaf number of three transformants that did not flower at 5 months after transfer from rooting medium. Flowering time is represented by the number of leaves during flowering (*, P < 0.05 and **, P < 0.01, unpaired Student’s t test). B, Flowering time of wild-type N. tabacum cv Turkish and N. tabacum transformants harboring P35S-NsCET1 or PSUC2-NsCET1 transgenes under LD conditions. The black circles represent the leaf number of two transformants that did not flower at 5 months after transfer from rooting medium. (*, P < 0.05, unpaired Student’s t test). C, Wild-type N. sylvestris (left) and N. sylvestris P35S-NsCET1 transformants (right) under LD conditions. D, Wild-type N. tabacum cv Turkish (left) and N. tabacum PSUC2-NsCET1 transformants (right) under LD conditions.
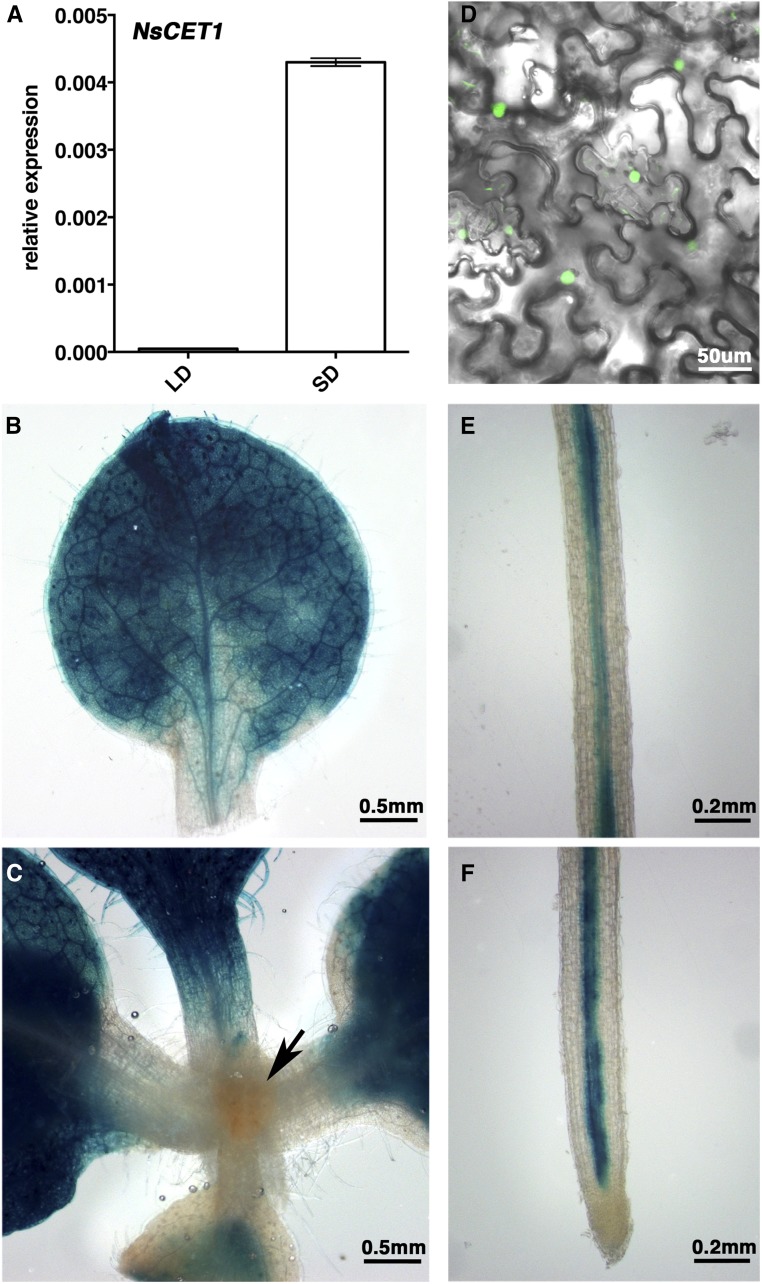
In N. sylvestris leaves, the mRNA accumulation of NsCET1, NsCET2, and NsCET10 was induced significantly under SD conditions (Fig. 2A; Supplemental Fig. S3), which is consistent with the production of antiflorigen under SD conditions (Lang et al., 1977; Huang et al., 2012). To examine the expression pattern of NsCET1 in tobacco, the NsCET1 promoter was PCR amplified and fused with a GUS reporter gene. Histochemical analysis showed that, in N. sylvestris and Arabidopsis transformants, GUS activity was detected mainly in leaves and in root vascular tissue (Fig. 2, B, C, E and F; Supplemental Fig. S4) but not in the shoot apex (Fig. 2C; Supplemental Fig. S4B), suggesting that the regulatory mechanism of NsCET1 expression is conserved in Arabidopsis and tobacco. In agreement with the non-cell-autonomous function of NsCET1, our bimolecular fluorescence complementation (BiFC) analysis revealed that NsCET1 interacted with FD (Fig. 2D), which is a bZIP transcription factor expressed specifically in the apex (Abe et al., 2005; Wigge et al., 2005). Thus, NsCET1 may translocate from the leaf to the shoot apex to inhibit flowering.
Figure 2.
Expression pattern of NsCET1 in N. sylvestris. A, Reverse transcription-quantitative PCR (RT-qPCR) analysis of NsCET1 mRNA levels in N. sylvestris under LD or SD conditions. The relative expression of NsCET1 was normalized to β-tubulin expression. B and C, Histochemical staining of leaf (B) and apex (C; indicated by the arrow) tissue of N. sylvestris transformants carrying a 5.5-kb fragment of NsCET1 promoter fused with a GUS reporter gene. Bars = 0.5 mm. D, BiFC assay of the interaction between NsCET1 and FD. VYNE(R)-CET1 and SCYCE(R)-FD were coinfiltrated in leaves of Nicotiana benthamiana by agroinfiltration. Bar = 50 μm. E and F, Histochemical staining of root (E) and root tip (F) tissue of N. sylvestris CET1pro-GUS transformants. Bars = 0.2 mm.
Knockdown of NsCET Expression Promotes Flowering in Tobacco
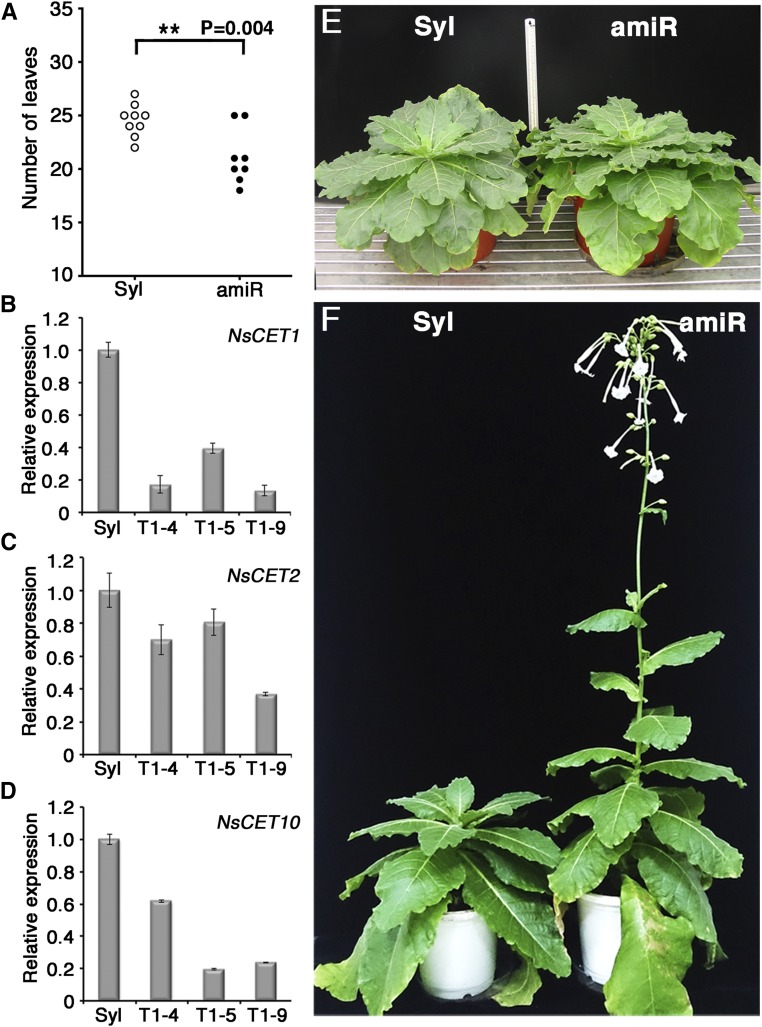
To investigate the contribution of CET genes to floral initiation in tobacco, we knocked down the expression of NsCET1, NsCET2, and NsCET10 using an artificial microRNA (amiR-CETs). Because the sequences of NtCET1, NtCET2, and NtCET10 in N. tabacum were identical to those of the respective CETs in N. sylvestris, the same amiR-CET construct with expression driven by a CaMV35S promoter was introduced into either N. tabacum or N. sylvestris. Similar to the Arabidopsis antiflorigen atc-2 mutant (Huang et al., 2012), the N. tabacum transformants harboring amiR-CETs displayed an early-flowering phenotype under SD but not under LD conditions (Supplemental Fig. S5, A and B), which indicates a conserved function of antiflorigen in day-neutral tobacco and Arabidopsis. In LD-grown N. sylvestris, the amiR-CET transformants flowered slightly earlier than wild-type N. sylvestris (Fig. 3A). RT-qPCR analysis revealed reduced levels of NsCET1, NsCET2, and NsCET10 mRNA in N. sylvestris 35S-amiR-CET transformants (Fig. 3, B–D). When these transformants were grown under SD conditions, floral induction was not observed in N. sylvestris 35S-amiR-CET transformants or wild-type N. sylvestris (Fig. 3E), suggesting that knocking down the expression of CET genes in obligate LD tobacco is not sufficient to induce flowering under nonfloral induction conditions. However, when N. sylvestris 35S-amiR-CET transformants were grown under SD conditions with dim light during the dark period to induce a low-level expression of NsFT4 (Supplemental Fig. S5, C and D), the N. sylvestris 35S-amiR-CET transformants flowered earlier than wild-type N. sylvestris plants (Fig. 3F), which is consistent with the notion that the function of CETs is to modulate flowering by antagonizing the activity of florigen.
Figure 3.
Knockdown of NsCET expression promotes flowering in tobacco. A, Flowering time of wild-type N. sylvestris (Syl; white circles) and N. sylvestris P35S-amiR-CET transformants (amiR; black circles) under LD conditions. Each circle represents the leaf number of individual wild-type or transformant plants during flowering (**, P < 0.01, unpaired Student’s t test). B to D, RT-qPCR analysis of NsCET1 (B), NsCET2 (C), and NsCET10 (D) mRNA levels in mature leaves of wild-type N. sylvestris (Syl) and three representative N. sylvestris P35S-amiR-CET T2 plants (T1-4, T1-5, and T1-9). The relative expression of CETs was normalized to β-tubulin expression. E, Representative images of wild-type N. sylvestris (left) and P35S-amiR-CET transformants (right) grown under SD conditions for 6 months. F, Representative images of wild-type N. sylvestris (left) and P35S-amiR-CET transformant (right) grown under SD conditions but with dim light (10 μmol m−2 s−1) during the dark period.
NsCET1 mRNA Is Mobile in Arabidopsis and Tobacco Heterografts
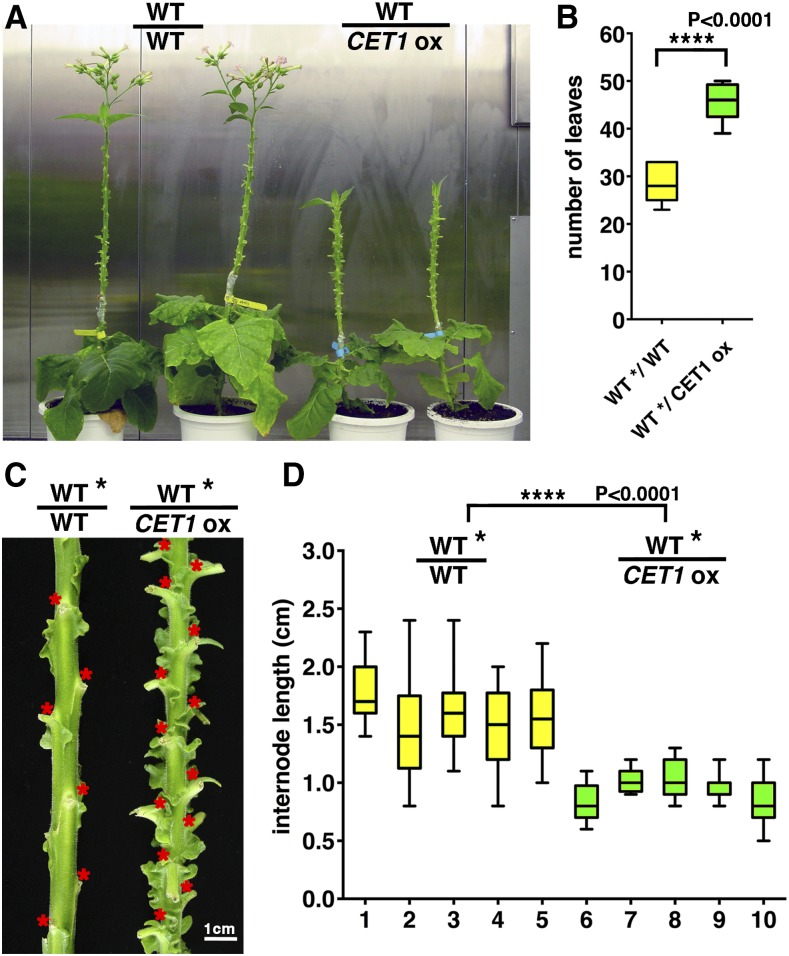
Given that the SUC2 promoter is a strong promoter expressed in most CCs, including CCs near the shoot apex, to further verify that the late-flowering phenotype in PSUC2-NsCET1 transformants is caused by long-distance signals associated with NsCET1, we grafted wild-type tobacco scions onto PSUC2-NsCET1 transformant stocks. Because N. sylvestris is a rosette-type plant, we used N. tabacum plants for grafting experiments. In control experiments, with wild-type N. tabacum scions grafted onto wild-type stocks, floral initiation occurred after a mean of 28.4 ± 3.8 leaves was produced on scions (Fig. 4, A and B). However, when wild-type scions were grafted onto PSUC2-NsCET1 transformant stocks, floral initiation occurred after a mean of 45.6 ± 3.7 leaves was produced (Fig. 4, A and B). In addition, the internodes of wild-type scions were shorter after grafting onto PSUC2-NsCET1 transformant stocks (Fig. 4, C and D). Therefore, the late-flowering and short-internode phenotypes observed in PSUC2-NsCET1 transformants were graft transmissible.
Figure 4.
Phenotypes of tobacco PSUC2-NsCET1 transformants are graft transmissible. A, Wild-type N. tabacum cv Turkish scions grafted onto wild-type Turkish stocks (WT/WT; left two plants) or onto PSUC2-NsCET1 transformant stocks (WT/CET1ox; right two plants). The mature leaves were removed regularly from scions to enhance sink strength. Note that the flowering time of the scions grafted onto PSUC2-NsCET1 transformant stocks was delayed significantly. B, Box-whisker plot of the flowering time of wild-type scions grafted onto wild-type stocks (WT*/WT; yellow; n = 7) or PSUC2-NsCET1 transformant stocks (WT*/CET1ox; green; n = 10). Flowering time is presented as the number of leaves of scions, which was calculated from the grafted junction to the first floral bud. WT* indicates that samples were calculated from wild-type scions (****, P < 0.0001, unpaired Student’s t test). C, Internodes of wild-type scions grafted onto wild-type stock or PSUC2-NsCET1 transformant stock. The nodes are indicated by red asterisks. Bar = 1 cm. D, Box-whisker plots of the internode length of five representative scions grafted onto wild-type stocks (yellow; 1–5) or PSUC2-NsCET1 transformant stocks (green; 6–10). Horizontal lines are the median, box edges are Q1 to Q3, and whiskers are highest and lowest values (****, P < 0.0001, unpaired Student’s t test).
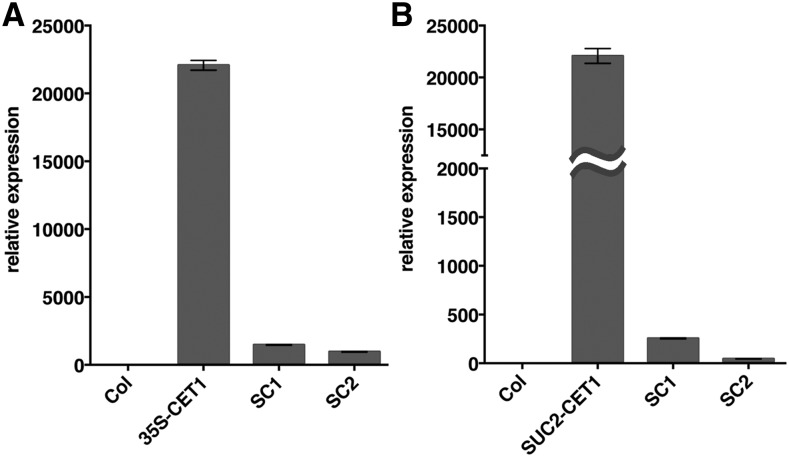
To examine whether tobacco NsCET1 mRNA is a mobile mRNA, we first used the Arabidopsis pin-fasten grafting method (Huang and Yu, 2015) to graft 10-d-old Arabidopsis wild-type scions onto Arabidopsis P35S-NsCET1 or PSUC2-NsCET1 transformant stocks. At 2 weeks after grafting, RT-qPCR analysis detected NsCET1 mRNA in wild-type scions grafted onto P35S-NsCET1 or PSUC2-NsCET1 transformant stocks but not onto wild-type controls (Fig. 5, A and B), which indicates that overexpressed NsCET1 mRNA was mobile in Arabidopsis. To investigate the long-distance movement of NsCET1 mRNA in tobacco, we grafted tobacco wild-type scions onto tobacco PSUC2-NsCET1 transformant stocks or control wild-type stocks. At 3 weeks after grafting, RT-PCR with NsCET1 transgene-specific primers resulted in PCR products from wild-type scions grafted onto PSUC2-NsCET1 stocks but not from controls of wild-type scions grafted onto wild-type stocks. Further sequencing of amplified DNA fragments confirmed the identity of PCR products (Supplemental Fig. S6), which indicates that overexpressed NsCET1 mRNA also is mobile in tobacco.
Figure 5.
Long-distance movement of NsCET1 mRNA in Arabidopsis. RT-qPCR analysis is shown for NsCET1 mRNA level in wild-type Arabidopsis scions grafted onto Arabidopsis P35S-NsCET1 (A) or PSUC2-NsCET1 (B) transformant stocks. RNA was extracted from mature leaves of wild-type (Col), P35S-NsCET1, or PSUC2-NsCET1 stocks or wild-type scions grafted onto P35S-NsCET1 or PSUC2-NsCET1 transformant stocks (SC1 and SC2). The relative mRNA level of NsCET1 was normalized to ubiquitin-conjugating enzyme expression.
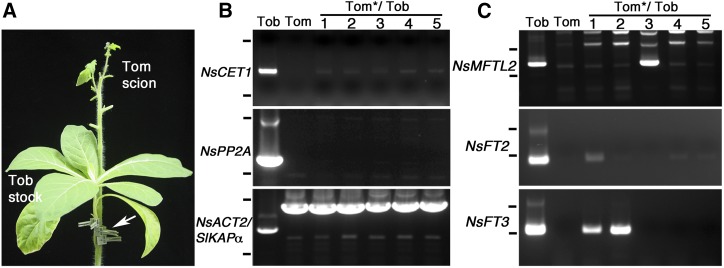
To further determine whether endogenous NsCET1 mRNA is a mobile mRNA in tobacco, we performed hetero-species grafting experiments. The sequences of the PEBP genes of tomato and tobacco exhibit significant variations, which allows for the differentiation of species-specific PEBP mRNA by RT-PCR and sequencing analysis. We grafted wild-type tomato scions with N. sylvestris stocks (Fig. 6A). At 3 weeks after grafting, RT-PCR with NsCET1 gene-specific primers resulted in PCR products from five independent tomato scions grafted onto N. sylvestris stocks but not from wild-type tomato plants (Fig. 6B). The PCR products from tomato scions verified by sequencing analysis showed that these products were indeed derived from tobacco NsCET1 mRNA (Supplemental Fig. S7). In contrast, control RT-PCR experiments with primers for tobacco PHLOEM PROTEIN2A (PP2A), a previously described nonmobile mRNA (Huang and Yu, 2009), revealed no tobacco PP2A mRNA in wild-type tomato plants and tomato scions grafted onto N. sylvestris stocks (Fig. 6B). These results indicate that endogenous NsCET1 mRNA can move from tobacco stocks to tomato scions.
Figure 6.
Long-distance movement of tobacco NsCET1 mRNA in tomato-tobacco heterografts. A, Representative image of tomato-tobacco heterografting experiments, depicting a wild-type tomato scion grafted with a wild-type N. sylvestris stock. The grafting union was secured by grafting clips (white arrow). Mature leaves of the tomato recipient were removed to enhance the sink strength. B and C, RT-PCR analysis of various mRNAs in mature leaves of wild-type N. sylvestris (Tob), wild-type tomato (Tom), or tomato scions (indicated by stars) grafted onto tobacco stocks (Tom*/Tob, 1–5). PCR (B) was performed with gene-specific primers for tobacco NsCET1 (top), tobacco NsPP2A (middle), and loading controls of tobacco ACTIN2 (NsACT2; first lane at bottom) or tomato IMPORTINα (SIKAPα; lanes 2–6 at bottom). Nested RT-PCR (C) was performed for tobacco NsMFTL2 (top), tobacco NsFT2 (middle), and tobacco NsFT3 (bottom). The positions of the 0.25- and 1-kb DNA markers (top and bottom black lines, respectively) are indicated for each gel.
The mRNA of MFT-Like Genes Is Mobile in Tomato-Tobacco Heterografts
In angiosperms, phylogenetic analysis shows that FT- and TFL1-like genes may arise from the ancestor in the MFT-like clade (Hedman et al., 2009; Karlgren et al., 2011). To investigate whether the mRNA movement of FT- and TFL1-like genes evolved from mobile MFT-like genes, we examined the mRNA movement of the leaf-expressed MFT-like genes in tomato-tobacco heterografting experiments. The literature and database search identified two tobacco MFT-like genes, namely NsMFTL1 and NsMFTL2, and two tomato MFT-like genes, namely SELF-PRUNING2G (SP2G) and SP3A (Supplemental Fig. S1A; Carmel-Goren et al., 2003). RT-PCR analysis showed weak expression of NsMFTL1 and NsMFTL2 in leaves of N. sylvestris, whereas SP2G but not SP3A was highly expressed in leaves of tomato (Supplemental Fig. S8). To examine the movement of NsMFTL1 and NsMFTL2 mRNA, RT-PCR analysis was conducted to analyze tomato scions grafted onto N. sylvestris stocks and revealed that NsMFTL2 but not NsMFTL1 mRNA was present in one of five independent tomato scions grafted onto N. sylvestris stocks (Fig. 6C). In addition, RT-PCR with primers for different tobacco PEBP genes detected NsFT2 and NsFT3 mRNA, which are two tobacco floral inhibitors belonging to the FT-like clade (Harig et al., 2012), in tomato scions grafted onto N. sylvestris stocks (Fig. 6C). These results suggest that, in tobacco, a number of members in the FT-, TFL1-, and MFT-like clades are mobile mRNAs.
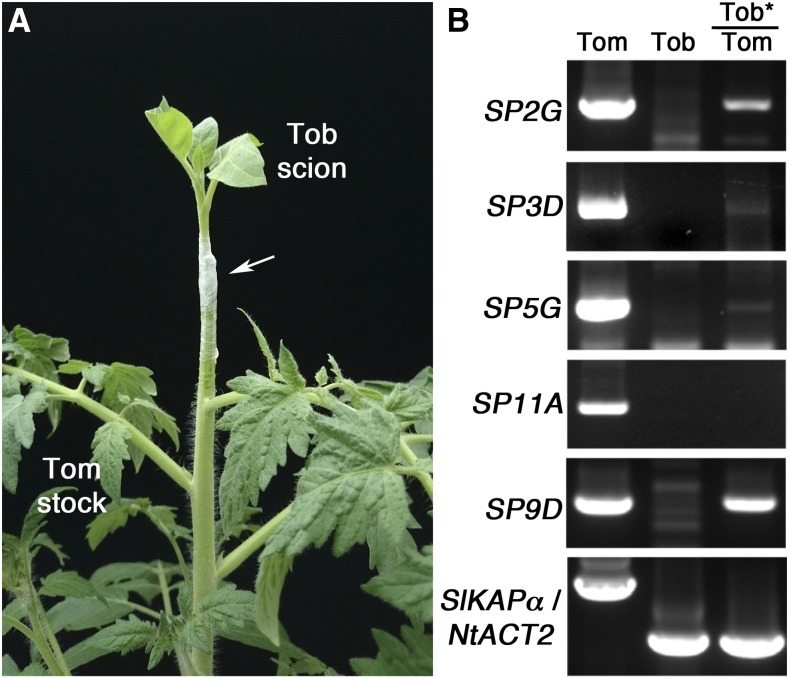
To further verify the mRNA movement of tomato PEBP genes, we grafted wild-type N. tabacum scions onto tomato stocks (Fig. 7A). At 3 weeks after heterografting, RT-PCR analysis detected SP2G mRNA in tobacco scions grafted onto tomato stocks (Fig. 7B), which suggests that the mRNA movement of MFT-like genes is conserved in tomato and tobacco. In addition, RT-PCR with the primers for different tomato PEBP genes detected SP3D, SP5G (FT-like clade), and SP9D (TFL1-like clade) mRNA in tobacco scions (Fig. 7B). However, RT-PCR with primers for SP11A (FT-like clade) detected SP11A mRNA in tomato stocks but not in tomato scions grafted onto tobacco stocks (Fig. 7B), which suggests that the mRNA movement of PEBP genes is transcript specific. Taken together, our results indicate that, in tobacco and tomato, multiple FT-, TFL1-, and MFT-like genes are mobile mRNAs.
Figure 7.
Long-distance movement of tomato PEBP gene mRNA in tobacco-tomato heterografting experiments. A, Representative image of tobacco-tomato heterografting experiments, depicting a wild-type tobacco scion grafted with a wild-type tomato stock. The grafting union was secured by Parafilm (white arrow). Mature leaves of the tobacco scions were removed to enhance the sink strength. B, RT-PCR analysis of mRNA from wild-type tomato (Tom), wild-type tobacco (Tob), or tobacco scions (indicated by stars) grafted onto tomato stocks (Tob*/Tom). PCR was performed with gene-specific primers for tomato SP2G (MFT-like clade); SP3D, SP5G, and SP11A (FT-like clade); SP9D (TFL-like clade); and loading controls of tomato, IMPORTINα (SIKAPα; first lane 1), or tobacco, ACTIN2 (NtACT2; lanes 2 and 3).
DISCUSSION
The PEBP gene family is an evolutionarily conserved gene family. In many plant species, the long-distance or cell-to-cell movement of different PEBP proteins in FT- and TFL1-like clades has been well established (Conti and Bradley, 2007; Zeevaart, 2008; Turnbull, 2011). However, whether the mRNA of PEBP genes is mobile remained controversial. Although previous Arabidopsis grafting experiments showed that the mRNA of FT and ATC can move long distance from stocks to scions (Huang et al., 2012; Lu et al., 2012), grafting experiments in tomato failed to detect the transport of SFT mRNA, which is a tomato FT ortholog (Lifschitz et al., 2006). Thus, questions remaining are whether the mRNA movement of PEBP genes occurs in other plant species and how florigen and antiflorigen evolved to acquire RNA mobility. In Arabidopsis, MFT is not involved directly in flowering control but rather is involved in seed development (Xi et al., 2010). A specific expression pattern of MFT in germinating seeds is inappropriate for examining RNA long-distance movement. In this study, we identified a tobacco antiflorigen, NsCET1, and showed that NsCET1 mRNA is mobile in Arabidopsis and tobacco (Figs. 5 and 6). Through the use of tobacco-tomato heterografts, we showed that many PEBP genes, including the genes in FT-, TFL-, and MFT-like clades, are mobile in tomato and tobacco (Figs. 6 and 7). The simplest explanation for these findings is that RNA mobility in the PEBP gene family has the same evolutionary lineage: the acquisition of mRNA mobility in FT- and TFL1-like genes may have evolved before the split of FT/TFL1-like clades from the more ancient MFT-like clade. Alternatively, individual PEBP genes in different clades may have evolved independently to access the RNA mobility. Our recent RNA live-imaging analysis showed that Arabidopsis FT and ATC mRNAs are targeted selectively to plasmodesmata for cell-to-cell movement (Luo et al., 2018), suggesting that the same mechanism may be used to direct the cell-to-cell movement of FT and ATC mRNA. This result is consistent with the hypothesis that the RNA mobility in the PEBP gene family may have evolved before the divergence of the FT/TFL1-like clade from the MFT-like clade. However, more experiments are required to verify whether the RNA movement of different PEBP genes is controlled by the same mechanism.
Whether the movement of mobile mRNAs is governed by specific or nonspecific mechanisms is uncertain (Kehr and Kragler, 2018; Morris, 2018). The detection of a significant number of mobile mRNAs in grafted plants (Thieme et al., 2015; Yang et al., 2015) and phloem exudates (Guo et al., 2013) indicates weak selection in regulating mRNA transport (Morris, 2018). A computational simulation indicated that the movement of mobile mRNAs is nonspecific and correlated with transcript abundance (Calderwood et al., 2016). In contrast, in Arabidopsis, grafting experiments with ectopic expression of nonmobile mRNAs, such as GFP, Arabidopsis dual-affinity nitrate transporter, or Arabidopsis ammonium transporter, showed that high mRNA abundance in CCs is not sufficient to trigger long-distance movement of these mRNAs (Huang and Yu, 2009; Xia et al., 2018). In addition, the RNA sequences involved in the long-distance movement of Arabidopsis GA-INSENITIVE and FT have been located (Huang and Yu, 2009; Lu et al., 2012). Recently, tRNA-like structures in some phloem-mobile mRNAs were found to be necessary and sufficient to trigger RNA movement (Zhang et al., 2016), which is consistent with the notion that the movement of mobile mRNAs operates by specific mobile RNA motifs. In our grafting experiments, tobacco NsCET1 mRNA could move long distance in both tobacco and Arabidopsis (Figs. 5 and 6). In addition, endogenous NsCET1 mRNA could move across the graft union from tobacco stocks to tomato scions (Fig. 6). These results suggest that the mechanism by which NsCET1 mRNA moves long distance is likely conserved in these plants. Our heterografting experiments failed to detect the translocated tomato SP11A mRNA in tobacco scions grafted onto tomato stocks (Fig. 7), which suggests that the movement of mobile mRNA is transcript specific. Thus, the transport of mobile mRNA may be controlled by a regulatory mechanism rather than by nonspecific diffusion. The cis-acting elements required for Arabidopsis FT RNA movement were localized to nucleotides 1 to 102 on the FT coding sequence (Li et al., 2009; Lu et al., 2012). Given that the sequences of NsCET1 and FT or other PEBP genes exhibit significant similarity, sequence comparison and deletion analysis may provide information to understand whether NsCET1 and FT or other PEBP genes share similar mobile RNA motifs.
The systemic spreading of many plant RNA viruses is mediated by movement proteins (MPs). These virus-encoded RNA-binding proteins (RBPs) have been shown to bind viral RNA for cell-to-cell movement through plasmodesmata (Heinlein, 2015). To elucidate the mechanisms underlying the long-distance transport of mobile mRNAs, the movement of plant mobile mRNAs was proposed to be mediated by the interaction of systemic RBPs and mobile RNA motifs (Lucas et al., 2001). The involvement of systemic RBPs in the delivery of plant phloem-mobile mRNAs was revealed by the identification of RBPs from phloem exudates. The analysis of pumpkin (Cucurbita maxima) phloem proteins that cross react with antiserum against the MP of red clover (Trifolium pratense) necrotic mosaic virus identified pumpkin PHLOEM PROTEIN16 (CmPP16), which acts as a paralog of viral MP in the long-distance movement of phloem-mobile mRNA (Xoconostle-Cázares et al., 1999). In pumpkin phloem sap, CmPP16 interacts with PHLOEM RNA BINDING PROTEIN50 (CmRBP50), encoding a polypyrimidine track-binding (PTB) protein, and other phloem proteins to form a protein complex that binds selectively with PTB motifs containing phloem-mobile mRNAs (Ham et al., 2009; Li et al., 2011). In agreement with these results, StPTB1 and StPTB6, two potato (Solanum tuberosum) homologs of CmRBP50, mediate tuber development by regulating the long-distance movement of mobile StBEL5 mRNA (Cho et al., 2015). Thus, the delivery of various mobile mRNAs may be regulated by the interaction of systemic RBPs with distinct mobile RNA motifs, such as PTB or tRNA-like structure motifs (Zhang et al., 2016). The identification of the RBPs involved in the long-distance trafficking of NsCETs or other mobile PEBP mRNAs may provide insights into the mechanism of mRNA movement.
Several lines of evidence demonstrate that plant mobile mRNAs play important roles in many developmental programs. This phloem-mediated RNA regulatory network is involved in leaf development, tuber formation, flowering, and many other developmental processes (Lucas et al., 1995; Kim et al., 2001; Haywood et al., 2005; Banerjee et al., 2006; Huang et al., 2012; Lu et al., 2012). Grafting experiments of tobacco (Lang et al., 1977), cucumber (Cucumis sativus), squash (C. maxima × C. moschata; Satoh, 1996), and soybean (Glycine max; Cober and Curtis, 2003) support the production of antiflorigen when plants are grown under nonfloral induction conditions. Although previous grafting experiments with early- or late-flowering mutants also suggested the presence of graft-transmissible floral inhibitors in pea (Pisum sativum; Paton and Barber, 1955), the effect is now more likely attributed to the lack of florigen in these mutants (Weller et al., 2009). Recently, an analysis of Arabidopsis and Chrysanthemum seticuspe revealed that TFL1-like genes act non-cell-autonomously to inhibit flowering (Huang et al., 2012; Higuchi et al., 2013), which agrees with our finding that NsCET1 is an antiflorigen in tobacco. In tobacco, the PEBP genes in both FT- and TFL-like clades may act as floral inhibitors. At least three FT-like floral inhibitors, specifically NtFT1, NtFT2, and NtFT3, are expressed in CCs (Harig et al., 2012). In addition, our heterografting experiments indicated that NsFT2 and NsFT3 are mobile mRNAs (Fig. 6C), which suggests that NtFT1, NtFT2, and NtFT3 probably also are mobile in tobacco. Thus, multiple members in both FT- and TFL-like clades of the tobacco PEBP gene family may act redundantly to contribute to antiflorigen activity.
The floral inhibition of antiflorigen is mediated by antagonizing florigen activity (Huang et al., 2012). In rice (Oryza sativa), florigen Hd3a may recruit 14-3-3 proteins and FD to form florigen activation complexes and induce the expression of downstream floral identity genes (Taoka et al., 2011). Analysis of Arabidopsis antiflorigen demonstrated that ATC can interact physically with FD to down-regulate similar floral identity genes (Huang et al., 2012). Similar to ATC, tobacco CET1 also interacted with FD in our BiFC assays (Fig. 2D). Therefore, antiflorigen may function to interfere in the binding of FD with florigen to form a florigen activation complex. However, in addition to displaying a late-flowering phenotype, CET1-overexpressed transformants also displayed other developmental alterations, including abnormal floral organs with leaf-like bracts (Supplemental Fig. S1) and shortened internodes (Supplemental Fig. S2). These phenotypes may not be attributed simply to the defective functions of FT or FD. Thus, in addition to antagonizing the activity of florigen, antiflorigen also may act independently of florigen to participate in other developmental regulation. In agreement with this notion, the interaction between Arabidopsis FT and BRANCHED1 is independent of FD (Niwa et al., 2013). Whether antiflorigen can interact with other factors remains to be investigated.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were obtained from the Arabidopsis Biological Resource Center and grown in growth chambers under LD (16 h of light/8 h of dark) or SD (8 h of light/16 h of dark) conditions, with a 22°C/20°C day/night cycle and white fluorescent light (light intensity of 100 μmol m−2 s−1). Tomato (Solanum lycopersicum), Nicotiana tabacum ‘Turkish’, and Nicotiana sylvestris were grown in a growth chamber with a 25°C/22°C day/night cycle and light intensity of 200 μmol m−2 s−1. To induce low-level expression of the tobacco florigen NsFT4, tobacco plants were grown under SD conditions but with dim light (10 μmol m−2 s−1) during the dark period.
Plasmid Construction
Full-length cDNA of tobacco NsCET1, NsCET2, or NsCET10 was amplified by RACE RT-PCR with gene-specific primers (sequences are shown in Supplemental Table S1). The cDNA was driven by a CaMV35S or Arabidopsis SUC2 promoter and transferred into Agrobacterium tumefaciens strain AGL1. The amiR-CETs were designed by using WMD3 Web MicroRNA Designer (http://wmd3.weigelworld.org/cgi-bin/webapp.cgi; Schwab et al., 2006) and confirmed by sequencing. The resulting amiR-CET constructs were driven by a CaMV35S promoter and transferred into A. tumefaciens strain AGL1 for plant transformation.
The tobacco NsCET1 5.5K promoter was amplified by PCR with forward (5′-AAGTGAAGTCGACTAATTCTTTTATATG-3′) and reverse (5′-CTTCACCCTTTTGTTTCTTCTTCTTTTTGG-3′) primers. The 5.5K promoter was fused with a GUS reporter gene in pCAMBIA1390 for plant transformation.
Arabidopsis and Tobacco Transformation
Arabidopsis transformation was performed by the floral dip method. The T1 transformants were selected on Murashige and Skoog medium containing 40 μg mL−1 hygromycin. At 10 d after selection, the resulting transformants were selected and transferred to soil for further analysis. For transformation of N. tabacum cv Turkish, tobacco seeds were surface sterilized and germinated on MS30 medium (Murashige and Skoog medium containing 30 g L−1 Suc) in growth chambers with 25°C LD conditions for 1 month until plants produced five to six fully expanded leaves. The midrib of developed leaves was removed, and the remaining blades were cut into 1-cm2 pieces and placed on plates with shooting medium I (SM I; MS30 medium containing 1 mg L−1 BAP and 0.1 mg L−1 NAA) at 25°C for 2 d. Explants were then cocultured with overnight A. tumefaciens cultures (diluted to OD600 = 0.8–1 in SM I solution) for 20 min and blotted dry on sterilized 3M filter paper, then transferred to SM I agar plates at 25°C under dark conditions for 2 d. The explants were transferred to shooting medium II (SM II; MS30 medium containing 1 mg L−1 BAP, 0.1 mg L−1 NAA, 200 mg L−1 cefotaxime, and 30 mg L−1 hygromycin) and incubated in growth chambers under LD conditions to produce callus. Every 2 weeks, calli were subcultured on a new SM II agar plate until new shoots developed. The well-developed shoots were transferred to rooting medium (MS30 medium containing 200 mg L−1 cefotaxime and 30 mg L−1 hygromycin) until roots were well developed. The successful transformants were transferred to soil. N. sylvestris transformation followed the N. tabacum transformation procedures, except that the hormone concentration in SM II medium was reduced to 0.5 mg L−1 BAP and 0.05 mg L−1 NAA.
BiFC Analyses
The cDNA of NsCET1 or NsCET2 was cloned into VenusN binary vector HygII-VYNE(R), and FD was cloned into the SCFP3AC vector KanII-SCYCE(R), under the control of a CaMV35S promoter (Waadt et al., 2008). The successful constructs were introduced into A. tumefaciens strain AGL1. For BiFC analysis, A. tumefaciens strains carrying individual BiFC constructs were cultured in Luria-Bertani medium containing 50 μg mL−1 kanamycin, 10 mm MES, pH 5.7, and 20 μm acetosyringone at 28°C overnight. The bacteria were pelleted and diluted in infiltration solution (10 mm MgCl2, 10 mm MES, pH 5.7, and 200 μm acetosyringone) to OD600 = 1. The bacteria solution was incubated at room temperature for 1 h. Coinfiltration was conducted with a 1:1 mix of HygII-VYNE(R)-CET and KanII-SCYCE(R)-FD strains. The mixed solution was infiltrated into the leaves of 3-week-old Nicotiana benthamiana using syringes. Three days after infiltration, tissue was visualized with a confocal laser-scanning microscope (Zeiss LSM 510 Meta).
Histochemical Analysis
The 40-d-old N. sylvestris transformants carrying NsCET1proGUS were incubated with GUS staining solution (50 mm sodium phosphate, pH 7, 10 mm EDTA, 0.5 mm potassium ferricyanide, 0.5 mm potassium ferrocyanide, 1 mm X-Gluc, and 0.01% (v/v) Triton X-100) at 37°C for 16 h. Plants were treated with 95% ethanol to remove chlorophyll and photographed with a Leica Z16 Apo microscope.
Grafting Experiments
Arabidopsis grafting was performed by pin-fasten grafting as described previously (Huang and Yu, 2015). At 2 weeks after grafting, mature leaves of the stocks and tissues from 0.2 cm above the graft union of the scions were collected for RNA extraction.
For tobacco grafting experiments, we used a simple cleft-grafting approach. The wild type or PSUC2-NsCET1 transformant of N. tabacum cv Turkish was grown in a growth chamber for 2 months. At this stage, plants usually produced 10 to 15 leaves. The wild-type scions were cut from 10 to 12 cm below the apex. Mature leaves on the scions were removed, and the base of scions was cut into a wedge shape to insert into the slit made by a vertical cut on apex-removed stocks. The graft junctions were secured with Parafilm and sealed in a Ziploc bag to retain humidity for 1 week. The mature leaves of the scions on successfully grafted plants were removed regularly to ensure sink strength.
For tomato and N. sylvestris heterografting, 1-month-old wild-type tomato was used as scions to graft with wild-type N. sylvestris tobacco plant stocks. The N. sylvestris plants with four to five expanding leaves were cut from the base of hypocotyls and shaped into a wedge cut, then inserted into slits made on the stem of tomato plants. The mature leaves of tomato were removed to ensure phloem transport from N. sylvestris to tomato. The graft junctions were secured with Parafilm and sealed in a Ziploc bag. Successfully grafted plants were grown in growth chambers with regular removal of mature leaves of tomato scions to ensure sink strength. At 3 weeks after grafting, the mature leaves of N. sylvestris stocks and the apex of tomato scions, which contains shoot meristem and young primordia, were collected for RNA extraction.
N. tabacum and tomato heterografting was done by the simple cleft-grafting method conducted with 1-month-old wild-type N. tabacum cv Turkish scions and tomato stocks. The N. tabacum scions were cut from 6 cm below the apex and shaped into a wedge to insert into the vertical cut of the apex-removed tomato stocks. The graft junctions were secured with Parafilm and sealed in a Ziploc bag to retain humidity for 1 week. The mature leaves of the scions on successfully grafted plants were removed regularly to ensure sink strength.
RNA Extraction and RT-PCR or RT-qPCR Analysis
Total RNA was extracted by using Trizol reagent according to the user’s manual with modifications (Invitrogen). In brief, 0.4 g of ground tissues was mixed with 1 mL of Trizol reagent and centrifuged at 4°C for 10 min at full speed to remove cell debrides. The solution was extracted with chloroform and phenol/chloroform and subjected to ethanol precipitation. RNA was vacuum dried and dissolved in DEPC-treated deionized water.
For RT-PCR analysis, 5 μg of total RNA was used in RT reactions performed with oligo(dT)20 and SuperScript III reverse transcriptase (Invitrogen). One microliter of cDNA was used for the PCR reaction with the following conditions: 1 min at 94°C for one cycle; 30 s at 94°C, 30 s at 60°C, and 1 min at 68°C for 35 cycles; and 10 min at 68°C for one cycle. An aliquot (5 μL) of PCR products was separated on agarose gels. For nested PCR, the PCR products were diluted 1:50 and subjected to a second round of PCR with nested primers.
For RT-qPCR analysis, RNA was treated with DNase I to remove potential DNA contamination. An amount of 5 μg of total RNA was used to synthesize first-strand cDNA using SuperScript III reverse transcriptase (Invitrogen) in a reaction volume of 20 μL. cDNA was diluted to 10 ng μL−1 with RNase-free water. An aliquot of 5 μL of diluted cDNA and 200 nm gene-specific primers was used for real-time PCR with the StepOnePlus Real-Time PCR System (Applied Biosystems). PCR was run at 95°C for 10 min, then 40 cycles of 95°C for 15 s, and 60°C for 1 min, with triplicate technical replicates included for each sample. The expression of β-tubulin was used as a normalization control. The sequences of primers are given in Supplemental Tables S1 and S2.
Accession Numbers
Sequence data from this article can be found in the GenBank/EMBL data libraries under the following accession numbers: NsCET1 (LOC104248269), NsCET2 (LOC104226905), NsCET5 (LOC104217580), NsCET9 (LOC104239376), NsCET10 (LOC104229471), NsFTL5 (LOC104234573), NsFTL6 (LOC104235396), NsFTL7 (LOC104225910), NsMFTL1 (LOC104210644), NsMFTL2 (LOC104210681), NsFT2 (XM_009770669), NsFT3 (XM_009773706), SP2G (AY186734), SP3D (AY186735), SP5G (XM_004239797), SP9D (AY186738), and SP11A (XM_004250027).
Supplemental Data
The following supplemental materials are available.
Supplemental Figure S1. Tobacco NsCET1, NsCET2, and NsCET10 act as floral inhibitors in Arabidopsis.
Supplemental Figure S2. N. tabacum cv Turkish PSUC2-NsCET1 transformants displayed phenotypes of short internodes and wild-type floral organs.
Supplemental Figure S3. Gene expression of N. sylvestris NsCET2 and NsCET10 under LD or SD conditions.
Supplemental Figure S4. GUS activity of NsCET1 promoters in tobacco and Arabidopsis.
Supplemental Figure S5. Suppression of CET expression in day-neutral tobacco promotes flowering under SD conditions.
Supplemental Figure S6. Long-distance movement of tobacco NsCET1 RNA in tobacco PSUC2-NsCET1 transformants.
Supplemental Figure S7. Sequencing analysis of mobile tobacco NsCET1 mRNA in tobacco-tomato heterografts.
Supplemental Figure S8. Expression pattern of MFT-like genes in tomato and tobacco.
Supplemental Table S1. Primers used in the identification of tobacco CET genes and RT-qPCR analysis.
Supplemental Table S2. Primers used in RT-PCR analysis.
Dive Curated Terms
The following phenotypic, genotypic, and functional terms are of significance to the work described in this paper:
Acknowledgments
We thank the Arabidopsis Biological Resource Stock Center for providing Arabidopsis seeds and Dr. Jörg Kudla for providing the BiFC vectors.
Footnotes
This work was supported by grants from the Ministry of Science and Technology (MOST), Taiwan.
Articles can be viewed without a subscription.
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Ahn JH, Miller D, Winter VJ, Banfield MJ, Lee JH, Yoo SY, Henz SR, Brady RL, Weigel D (2006) A divergent external loop confers antagonistic activity on floral regulators FT and TFL1. EMBO J 25: 605–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya I, Ratcliffe OJ, Bradley DJ (1999) Expression of CENTRORADIALIS (CEN) and CEN-like genes in tobacco reveals a conserved mechanism controlling phase change in diverse species. Plant Cell 11: 1405–1418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee AK, Chatterjee M, Yu Y, Suh SG, Miller WA, Hannapel DJ (2006) Dynamics of a mobile RNA of potato involved in a long-distance signaling pathway. Plant Cell 18: 3443–3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderwood A, Kopriva S, Morris RJ (2016) Transcript abundance explains mRNA mobility data in Arabidopsis thaliana. Plant Cell 28: 610–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmel-Goren L, Liu YS, Lifschitz E, Zamir D (2003) The SELF-PRUNING gene family in tomato. Plant Mol Biol 52: 1215–1222 [DOI] [PubMed] [Google Scholar]
- Chardon F, Damerval C (2005) Phylogenomic analysis of the PEBP gene family in cereals. J Mol Evol 61: 579–590 [DOI] [PubMed] [Google Scholar]
- Cho SK, Sharma P, Butler NM, Kang IH, Shah S, Rao AG, Hannapel DJ (2015) Polypyrimidine tract-binding proteins of potato mediate tuberization through an interaction with StBEL5 RNA. J Exp Bot 66: 6835–6847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cober ER, Curtis DF (2003) Both promoters and inhibitors affected flowering time in grafted soybean flowering-time isolines. Crop Sci 43: 886–891 [Google Scholar]
- Conti L, Bradley D (2007) TERMINAL FLOWER1 is a mobile signal controlling Arabidopsis architecture. Plant Cell 19: 767–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al. (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- Guo S, Zhang J, Sun H, Salse J, Lucas WJ, Zhang H, Zheng Y, Mao L, Ren Y, Wang Z, et al. (2013) The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat Genet 45: 51–58 [DOI] [PubMed] [Google Scholar]
- Ham BK, Lucas WJ (2017) Phloem-mobile RNAs as systemic signaling agents. Annu Rev Plant Biol 68: 173–195 [DOI] [PubMed] [Google Scholar]
- Ham BK, Brandom JL, Xoconostle-Cázares B, Ringgold V, Lough TJ, Lucas WJ (2009) A polypyrimidine tract binding protein, pumpkin RBP50, forms the basis of a phloem-mobile ribonucleoprotein complex. Plant Cell 21: 197–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harig L, Beinecke FA, Oltmanns J, Müth J, Müller O, Rüping B, Twyman RM, Fischer R, Prüfer D, Noll GA (2012) Proteins from the FLOWERING LOCUS T-like subclade of the PEBP family act antagonistically to regulate floral initiation in tobacco. Plant J 72: 908–921 [DOI] [PubMed] [Google Scholar]
- Haywood V, Yu TS, Huang NC, Lucas WJ (2005) Phloem long-distance trafficking of GIBBERELLIC ACID-INSENSITIVE RNA regulates leaf development. Plant J 42: 49–68 [DOI] [PubMed] [Google Scholar]
- Hedman H, Källman T, Lagercrantz U (2009) Early evolution of the MFT-like gene family in plants. Plant Mol Biol 70: 359–369 [DOI] [PubMed] [Google Scholar]
- Heinlein M. (2015) Plasmodesmata: channels for viruses on the move. Methods Mol Biol 1217: 25–52 [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Narumi T, Oda A, Nakano Y, Sumitomo K, Fukai S, Hisamatsu T (2013) The gated induction system of a systemic floral inhibitor, antiflorigen, determines obligate short-day flowering in chrysanthemums. Proc Natl Acad Sci USA 110: 17137–17142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NC, Yu TS (2009) The sequences of Arabidopsis GA-INSENSITIVE RNA constitute the motifs that are necessary and sufficient for RNA long-distance trafficking. Plant J 59: 921–929 [DOI] [PubMed] [Google Scholar]
- Huang NC, Yu TS (2015) A pin-fasten grafting method provides a non-sterile and highly efficient method for grafting Arabidopsis at diverse developmental stages. Plant Methods 11: 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang NC, Jane WN, Chen J, Yu TS (2012) Arabidopsis thaliana CENTRORADIALIS homologue (ATC) acts systemically to inhibit floral initiation in Arabidopsis. Plant J 72: 175–184 [DOI] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Karlgren A, Gyllenstrand N, Källman T, Sundström JF, Moore D, Lascoux M, Lagercrantz U (2011) Evolution of the PEBP gene family in plants: functional diversification in seed plant evolution. Plant Physiol 156: 1967–1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Kragler F (2018) Long distance RNA movement. New Phytol 218: 29–40 [DOI] [PubMed] [Google Scholar]
- Kim M, Canio W, Kessler S, Sinha N (2001) Developmental changes due to long-distance movement of a homeobox fusion transcript in tomato. Science 293: 287–289 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Lacombe B, Achard P (2016) Long-distance transport of phytohormones through the plant vascular system. Curr Opin Plant Biol 34: 1–8 [DOI] [PubMed] [Google Scholar]
- Lang A, Chailakhyan MK, Frolova IA (1977) Promotion and inhibition of flower formation in a dayneutral plant in grafts with a short-day plant and a long-day plant. Proc Natl Acad Sci USA 74: 2412–2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhang K, Zeng X, Jackson S, Zhou Y, Hong Y (2009) A cis element within flowering locus T mRNA determines its mobility and facilitates trafficking of heterologous viral RNA. J Virol 83: 3540–3548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Ham BK, Lucas WJ (2011) CmRBP50 protein phosphorylation is essential for assembly of a stable phloem-mobile high-affinity ribonucleoprotein complex. J Biol Chem 286: 23142–23149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifschitz E, Eviatar T, Rozman A, Shalit A, Goldshmidt A, Amsellem Z, Alvarez JP, Eshed Y (2006) The tomato FT ortholog triggers systemic signals that regulate growth and flowering and substitute for diverse environmental stimuli. Proc Natl Acad Sci USA 103: 6398–6403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Liu C, Hou X, Xi W, Shen L, Tao Z, Wang Y, Yu H (2012) FTIP1 is an essential regulator required for florigen transport. PLoS Biol 10: e1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KJ, Huang NC, Liu YS, Lu CA, Yu TS (2012) Long-distance movement of Arabidopsis FLOWERING LOCUS T RNA participates in systemic floral regulation. RNA Biol 9: 653–662 [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Bouché-Pillon S, Jackson DP, Nguyen L, Baker L, Ding B, Hake S (1995) Selective trafficking of KNOTTED1 homeodomain protein and its mRNA through plasmodesmata. Science 270: 1980–1983 [DOI] [PubMed] [Google Scholar]
- Lucas WJ, Yoo BC, Kragler F (2001) RNA as a long-distance information macromolecule in plants. Nat Rev Mol Cell Biol 2: 849–857 [DOI] [PubMed] [Google Scholar]
- Luo KR, Huang NC, Yu TS (2018) Selective targeting of mobile RNAs to plasmodesmata for cell-to-cell movement. Plant Physiol 177: 604–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Küttner F, Schmid M (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol 17: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Matsoukas IG, Massiah AJ, Thomas B (2012) Florigenic and antiflorigenic signaling in plants. Plant Cell Physiol 53: 1827–1842 [DOI] [PubMed] [Google Scholar]
- Morris RJ. (2018) On the selectivity, specificity and signalling potential of the long-distance movement of messenger RNA. Curr Opin Plant Biol 43: 1–7 [DOI] [PubMed] [Google Scholar]
- Niwa M, Daimon Y, Kurotani K, Higo A, Pruneda-Paz JL, Breton G, Mitsuda N, Kay SA, Ohme-Takagi M, Endo M, et al. (2013) BRANCHED1 interacts with FLOWERING LOCUS T to repress the floral transition of the axillary meristems in Arabidopsis. Plant Cell 25: 1228–1242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton DM, Barber HN (1955) Physiological genetics of Pisum. I. Grafting experiments between early & late varieties. Aust J Biol Sci 8: 231–240 [Google Scholar]
- Ruiz-Medrano R, Xoconostle-Cázares B, Lucas WJ (1999) Phloem long-distance transport of CmNACP mRNA: implications for supracellular regulation in plants. Development 126: 4405–4419 [DOI] [PubMed] [Google Scholar]
- Satoh S. (1996) Inhibition of flowering of cucumber grafted on rooted squash stock. Physiol Plant 97: 440–444 [Google Scholar]
- Schwab R, Ossowski S, Riester M, Warthmann N, Weigel D (2006) Highly specific gene silencing by artificial microRNAs in Arabidopsis. Plant Cell 18: 1121–1133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada S, Goto K (2003) Terminal flower2, an Arabidopsis homolog of heterochromatin protein1, counteracts the activation of flowering locus T by constans in the vascular tissues of leaves to regulate flowering time. Plant Cell 15: 2856–2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K (2007) Hd3a protein is a mobile flowering signal in rice. Science 316: 1033–1036 [DOI] [PubMed] [Google Scholar]
- Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, Yamaguchi M, Nakashima C, Purwestri YA, Tamaki S, et al. (2011) 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature 476: 332–335 [DOI] [PubMed] [Google Scholar]
- Thieme CJ, Rojas-Triana M, Stecyk E, Schudoma C, Zhang W, Yang L, Miñambres M, Walther D, Schulze WX, Paz-Ares J, et al. (2015) Endogenous Arabidopsis messenger RNAs transported to distant tissues. Nat Plants 1: 15025. [DOI] [PubMed] [Google Scholar]
- Turnbull C. (2011) Long-distance regulation of flowering time. J Exp Bot 62: 4399–4413 [DOI] [PubMed] [Google Scholar]
- Waadt R, Schmidt LK, Lohse M, Hashimoto K, Bock R, Kudla J (2008) Multicolor bimolecular fluorescence complementation reveals simultaneous formation of alternative CBL/CIPK complexes in planta. Plant J 56: 505–516 [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou Z, Liu Y, Liu T, Li Q, Ji Y, Li C, Fang C, Wang M, Wu M, et al. (2015) Functional evolution of phosphatidylethanolamine binding proteins in soybean and Arabidopsis. Plant Cell 27: 323–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller JL, Hecht V, Liew LC, Sussmilch FC, Wenden B, Knowles CL, Vander Schoor JK (2009) Update on the genetic control of flowering in garden pea. J Exp Bot 60: 2493–2499 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]
- Xi W, Liu C, Hou X, Yu H (2010) MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 22: 1733–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia C, Zheng Y, Huang J, Zhou X, Li R, Zha M, Wang S, Huang Z, Lan H, Turgeon R, et al. (2018) Elucidation of the mechanisms of long-distance mRNA movement in a Nicotiana benthamiana/tomato heterograft system. Plant Physiol 177: 745–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xoconostle-Cázares B, Xiang Y, Ruiz-Medrano R, Wang HL, Monzer J, Yoo BC, McFarland KC, Franceschi VR, Lucas WJ (1999) Plant paralog to viral movement protein that potentiates transport of mRNA into the phloem. Science 283: 94–98 [DOI] [PubMed] [Google Scholar]
- Yang Y, Mao L, Jittayasothorn Y, Kang Y, Jiao C, Fei Z, Zhong GY (2015) Messenger RNA exchange between scions and rootstocks in grafted grapevines. BMC Plant Biol 15: 251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo SC, Chen C, Rojas M, Daimon Y, Ham BK, Araki T, Lucas WJ (2013) Phloem long-distance delivery of FLOWERING LOCUS T (FT) to the apex. Plant J 75: 456–468 [DOI] [PubMed] [Google Scholar]
- Zeevaart JAD. (1976) Physiology of flower formation. Annu Rev Plant Physiol 27: 321–348 [Google Scholar]
- Zeevaart JAD. (2008) Leaf-produced floral signals. Curr Opin Plant Biol 11: 541–547 [DOI] [PubMed] [Google Scholar]
- Zhang W, Thieme CJ, Kollwig G, Apelt F, Yang L, Winter N, Andresen N, Walther D, Kragler F (2016) tRNA-related sequences trigger systemic mRNA transport in plants. Plant Cell 28: 1237–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Liu L, Shen L, Yu H (2016) NaKR1 regulates long-distance movement of FLOWERING LOCUS T in Arabidopsis. Nat Plants 2: 16075. [DOI] [PubMed] [Google Scholar]