Abstract
Vision is a highly rhythmic function adapted to the extensive changes in light intensity occurring over the 24-hour day. This adaptation relies on rhythms in cellular and molecular processes, which are orchestrated by a network of circadian clocks located within the retina and in the eye, synchronized to the day/night cycle and which, together, fine-tune detection and processing of light information over the 24-hour period and ensure retinal homeostasis. Systematic or high throughput studies revealed a series of genes rhythmically expressed in the retina, pointing at specific functions or pathways under circadian control. Conversely, knockout studies demonstrated that the circadian clock regulates retinal processing of light information. In addition, recent data revealed that it also plays a role in development as well as in aging of the retina. Regarding synchronization by the light/dark cycle, the retina displays the unique property of bringing together light sensitivity, clock machinery, and a wide range of rhythmic outputs. Melatonin and dopamine play a particular role in this system, being both outputs and inputs for clocks. The retinal cellular complexity suggests that mechanisms of regulation by light are diverse and intricate. In the context of the whole eye, the retina looks like a major determinant of phase resetting for other tissues such as the retinal pigmented epithelium or cornea. Understanding the pathways linking the cell-specific molecular machineries to their cognate outputs will be one of the major challenges for the future.
Keywords: circadian, retina, photoreceptor, rhythm, vision, melatonin, dopamine
The existence of daily rhythms in behavior and physiology is one of the major hallmarks of life on Earth. This characteristic has developed throughout evolution from bacteria or unicellular algae to vertebrates, in accordance with periodic variation of environmental factors such as the 24-hour day/night cycle. Not surprisingly, circadian rhythms (circadian = about 24 hours) are controlled by conserved, cell-autonomous, and self-sustaining mechanisms. These are mainly based on autoregulatory, negative feedback loops able to track time and to entrain, more or less directly, gene expression programs. Such timing systems have the property to anticipate predictable environmental changes and to control physiology and behavior, accordingly. A growing list of “clock genes” and of distinct interlocked transcriptional/translational regulatory loops has been described in mammals, suggesting diversity in molecular architecture between tissues or even cell types, but still conforming to the same general principle.1 Thus, a huge number of cellular clocks exist throughout the body. They are coordinated by a master clock located in the suprachiasmatic nuclei (SCN) within the hypothalamus and are themselves synchronized with the solar day by daily light signals transmitted through the retina. Genetic diversity as well as organization of oscillators into a network underlie the extreme robustness of clock systems, which are largely insensitive to minor environmental changes. The emergence of transcriptomic approaches in the field offered an opportunity for a systematic analysis of temporal gene expression that functions in a specific tissue or cell type. Using this technique, it was demonstrated that thousands of transcripts undergo circadian oscillations in various mouse organs and that about 43% of protein encoding genes are rhythmic in a tissue-specific manner.2–4 Recently, the first exhaustive analysis of rhythmic transcriptional expression profiles in >60 tissues/organs from a diurnal nonhuman primate was performed.5 This genome-wide transcriptome study uncovered a wide array (about 82%) of protein-encoding, ubiquitous, and tissue-specific genes undergoing rhythmic daily changes. These studies emphasize how pervasive the circadian clock system is, with extensive regulation of major, basic biologic processes such as metabolism, DNA repair, and gene expression but also tissue-specific functions,6 all intimately coordinated within the 24-hour period. The award of the 2017 Nobel Prize to the researchers who discovered the molecular mechanism governing daily rhythms (Jeffrey C. Hall, Michael Rosbash, and Michael W. Young) provided widespread acknowledgement of the fundamental importance of circadian clocks. It also underlines the emerging interest for biologic rhythms in today's society. Indeed, modern lifestyles with extensive use of artificial light throughout the 24-hour period, frequent jetlag, and sleep debt, challenge our circadian system, likely hasten aging and increase the risk of major health problems such as metabolic syndrome, neurobehavioral abnormalities, and cancer.7–9
The day/night cycle in ambient light is the major environmental factor able to entrain clocks, also called a zeitgeber (time-giver). In mammals, eyes constitute the only light input pathway to the SCN10 and entrainment of this central clock involves the three main light sensitive systems of the retina (i.e., rods, cones, and the intrinsically sensitive retinal ganglion cells [ipRGCs]). The latter were discovered about 20 years ago and express the photopigment melanopsin (OPN4). These ipRGCs send axons to the SCN as well as multiple other targets within the brain and integrate light information processed and transmitted by rods and cones.11,12 Both irradiance and wavelength serve as entraining factors to align the circadian system with the external light/dark cycle.13 Interestingly, eye physiology is also subject to circadian regulation, which adapts vision to the alternating day-night cycle (i.e., high and low light intensities), but also modulates the capacity of the retina to signal to the circadian system. In mammals, the retina was actually the first tissue outside of the SCN, described to display circadian clock properties, based on its capacity to synthesize and release melatonin in vitro with a ∼24-hour rhythm.14 This observation triggered extensive analysis of retinal physiology over the 24-hour cycle and many molecular and cellular processes are now known to be under clock-control.15,16 These extend from the expression of photopigments17,18 to visual sensitivity, as reflected in ERG by the amplitude of the photopic b-wave.19–21 They also include processes linked to retina survival such as rhythms in shedding of rod and cone outer segments and phagocytosis by the underlying RPE22,23 and the vulnerability to phototoxicity.24 In addition, rhythmic processes have been reported elsewhere in the eye, such as in the cornea (daily variation of thickness or mitotic rate25,26) and in the ciliary body (aqueous production contributing to intraocular pressure27,28). In line with these results, transcriptomics analysis of the eyes over the 24-hour period provided an extensive view of processes under cyclic control in ocular tissues.5,21 Although identified cycling genes appear linked mainly to the retina, these data provided potential insight into the rhythmic physiology of the cornea, lens, choroid, and sclera, among others. Conversely, disruption of the circadian clock as induced by Bmal1 knockout was described to dramatically alter corneal and lens structure.29,30 Likewise, photoreceptor viability during aging is significantly reduced in Bmal1−/− as well as in Npas2/Clock double knockout mice.31 Thus, alteration of clock signaling compromises cell viability and leads to accelerated aging in ocular tissues.
Circadian regulation of retinal functions is present in all the different classes of vertebrates but since significant differences exist in the circadian organization of the retina among these different systematic groups, and often among the different species within the same class, we decided to focus our review on mammals. This review presents general data about the molecular clock and its retinal target genes, updates the understanding of clock involvement in the regulation of vision, and provides information about the distinct synchronization mechanisms at play in the retina and within the eye.
Circadian Clocks in the Mammalian Retina
The Molecular Clock
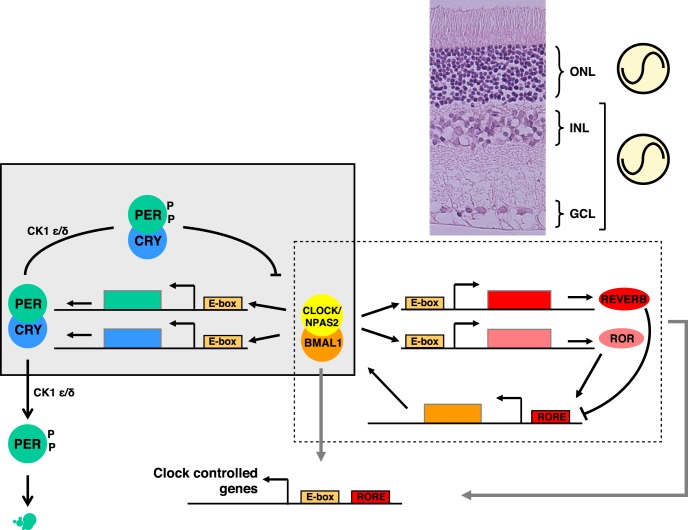
The central clock and most clocks throughout the organism share similar transcriptional architecture.1 This molecular time-keeping system is generated by interlocking transcriptional-translational feedback loops (TTFL; Fig. 1). The clock core loop involves the activator proteins complex CLOCK-BMAL1 (or NPAS2-BMAL1) which binds to the E-box sequences in the promoters of the repressor genes Period (Per) 1–3 and Cryptochrome (Cry) 1–2 to induce their transcription. PER proteins form complexes with CRY proteins, undergo phosphorylation by casein kinase CK1ε/δ and are translocated back to the nucleus to repress the transcriptional activity of the CLOCK-BMAL1 complex, thus repressing their own transcription. In addition, the CLOCK-BMAL1 complex activates the Ror and RevErb nuclear receptor genes also via the E-box enhancers. The ROR and REVERB proteins compete at the retinoic acid-related orphan receptor binding elements (RORE) sites and activate or, respectively, repress Bmal1 transcription. These interlocking feedback loops build a network of rhythmic clock genes and clock-controlled genes (CCGs) in which expression patterns are finely shaped by posttranslational processes such as proteasomal degradation, ubiquitination, sumoylation phosphorylation, and acetylation in order to set the phase coherence of cellular and tissue functions over a 24-hour cycle. In the mouse eye, one groundbreaking study reported that the steady-state levels of a large number of transcripts exhibit daily and circadian oscillations.21 These include genes involved in basic neural and cellular functions such as synaptic transmission, intercellular communication and photoreceptor signaling. Similar observations were made using proteomic approaches.32,33 In a nonhuman primate (Papio anubis), Mure and colleagues5 compared gene expression throughout a 24-hour cycle in several ocular tissues (retina, RPE, iris, and cornea) using RNA-Seq. The authors found that around 4% to 5% of the transcripts are rhythmic in the retina, the RPE, and the iris whereas the cornea exhibits 12% of rhythmic gene expression with peak phases occurring around the middle of the day and the end of the night. In this primate retina, clock genes were not found to cycle as previously reported in several studies in rodents.17,21,34–38 This may be related to the existence of several clocks in the retina, with differences in phasing and period between cells/layers that may render difficult the transcriptomic analysis at the whole tissue level. Indeed, ex vivo studies of clock gene expression demonstrated rhythms in photoreceptor layers on one hand and in the inner retina on the other39,40 (Fig. 1). Nevertheless, specific retinal ablation of the Bmal1 core clock gene led to abnormal retinal transcriptional responses to light21 while in retinal explants, Per1 (but not Per2 and Per3), Cry1 (but not Cry2), and Clock were found to be necessary for sustained circadian rhythms.41,42
Figure 1.
The molecular clock pathways and retinal clocks. Schematic representation of the transcriptional/translational feedback loops model for the molecular clock. The BMAL1/CLOCK (or BMAL1/NPAS2) dimer activates transcription of the Per and Cry genes upon binding to the Ebox sequences in their promoters. In turn, PER and CRY proteins form heterodimers able to inhibit transcriptional activity of BMAL1/CLOCK, thus turning down their own transcription. Meanwhile, these factors undergo posttranslational modifications, in particular, phosphorylation of PER proteins by the Casein Kinases 1δ or 1ε, signaling for ubiquitination and proteasomal degradation, and then allowing the cycle to restart. BMAL1/CLOCK likewise activates the expression of Rev-Erb and Ror genes, which products respectively repress and activate transcription of the Bmal1 gene at retinoic acid-related orphan receptor binding elements (RORE) sites. This generates an additional loop interlocked with the previous one, all together contributing to the robustness of the clockwork. The presence of Ebox and/or RORE sequences throughout the genome supports the rhythmic regulation of a set of target genes (CCG) for BMAL1/CLOCK, BMAL1/NPAS2, REV-ERB, and ROR transcription factors. Clock gene expression dynamics over the 24-hour cycle conforming to this model have been described in the ONL and in the inner retina (INL + GCL) in several ex vivo studies, as symbolized next to the eosin/hematoxylin stained transversal section of a rat retina shown in the upper-right corner of the figure.
Finally, it is worth mentioning that recent studies have shown the presence of a metabolic clock in red blood cells that is independent from the molecular clockwork above described.43 The role of this metabolic clock in the regulation of retinal circadian rhythmicity is not known and will require further investigation.
The exact cellular location of the retinal clock remains an area of ongoing research. However, it was demonstrated that the retina harbors a network of clocks localized in each cell layer. Strong evidence came from the bioluminescence recordings using the luciferase reporter coupled to Per1 and 2 clock genes.44,45 The mouse whole retinal explants as well as the isolated ganglion cell (GCL), inner nuclear (INL), and outer nuclear (ONL) layers exhibit autonomous PER2::LUC rhythmic expression with specific periods46 while Per1-Luc was shown to oscillate in the isolated rat ONL.47 GCL and INL are the major sites of PER2::LUC expression36,40,46 and the connectivity with the neighboring layers increases the robustness of the rhythms46 indicating dynamic network properties. Moreover, autonomous PER2::LUC oscillations were recorded in Müller cells.48 Hence, experimental evidence indicates the presence of a complex network of cell circadian oscillators throughout the retina and this might explain the rather divergent results that were previously obtained using in vivo and in vitro techniques with varying detection limits (in situ hybridization, whole retina/vibratome sections/microdissection/single cell qRT-PCR) on the expression patterns of circadian clock genes in the different retinal cell types.34,35,37,39,40,49–51
Immunohistochemistry studies confirmed the broad expression of core clock proteins. CLOCK, BMAL1, NPAS2, PER1, PER2, and CRY2 are expressed in ipRGCs, amacrine cells, bipolar cells, horizontal cells and cones, but clear diurnal and circadian rhythms were observed only in mouse cones.52 CRY1 is also expressed throughout all retinal layers, albeit within the ONL only the cones express CRY1.42 Taken together, these findings suggest that cones and not rods are cell-autonomous circadian clocks.40,52 Thus, further investigation is required to define the exact molecular clock make-up in each cell type.
Clock-Driven Gene Expression
Clock-dependent control of retinal physiology appears to involve transcriptional gene regulation. This follows from the observation that retinal clocks drive the expression of numerous genes21,53,54 including those that are: important for retinal health and genetically associated with severe retinal diseases (Esrrβ55; Rev-Erbα56; Nr2e357; Nxnl158; Rorβ59), important for vision (Arr160; Kcnv261; S-opsin and M-opsin17,18), and encoding key regulators of metabolism (Cpt-1α62; Pgc-1α63). Consistent with a significant role of clock-dependent gene regulation in retinal physiology, changes in transcript levels were seen to result in corresponding changes in protein amount.55,61,62,64
The retinal clock influences gene expression through various pathways. On the one hand, it directs transcription of target genes within the same cell. In this process, protein products of clock genes or those of so-called “CCGs” target gene transcription by binding to specific promoter sites. Thus, the protein products of Clock, Npas2, and Bmal1 form heterodimers (CLOCK/BMAL1 and NPAS2/BMAL1) that bind to the E-box sites of various genes65 in photoreceptors, including Aanat, which encodes arylalkylamine N-transferase, the key regulatory enzyme in melatonin synthesis.66–68 Consistent with a role of also CCGs in linking retinal clocks to cell-own target genes, Dbp—a CCG known to transactivate gene expression through the D-box element69—displays circadian changes in preparations of the whole retina and microdissected photoreceptors.21,62,70 On the other hand, the clock of a given retinal neuron appears to influence gene expression in other retinal cells, a process that requires the clock-dependent release of an extracellular messenger.15 Consistent with this concept, the clock-dependent release of dopamine from amacrine neurons50,71,72 appears to contribute to rhythmicity of gene expression in photoreceptors. This follows from studies in melatonin-proficient mouse strains in which dopamine levels show a circadian rhythm73: in mice deficient for D4 receptors, the periodicity of a subset of genes is attenuated in photoreceptors (Acadm, Cpt-1α62; Nr4a163; Gnaz64).
Similarly, the clock within photoreceptors may use melatonin signaling to influence gene expression in target cells expressing melatonin receptors.74 This becomes evident from the observations that photoreceptors (principally cones) display a clock-dependent release of melatonin15 and that the failure of functional MT1 receptors prevents (Pgc-1α63) or phase-advances (Acadm, Cpt-1α62; Drd4, Gnaz64; Rev-Erbα, Nr2e3, Rorβ63) the daily rhythmicity of circadian genes. Considering that melatonin released by photoreceptors might also feedback on photoreceptor MT1 receptors,15 autocrine signaling via melatonin may also participate in clock-dependent regulation of photoreceptor gene expression.
Circadian regulation of a subset of genes is abolished in the db/db mouse75—a mouse (melatonin-deficient) model of early diabetic retinopathy.62,64 Likewise, clock gene expression is strongly reduced in the retina of another model of type 2 diabetes and associated diabetic retinopathy.76 Interestingly, circadian disruption (Per2 knockout) recapitulates diabetic retinopathy in mice.77 Therefore, circadian regulation might also be impaired in diabetic retinopathy of humans—one of the most common causes of blindness in Europe and the United States.78 Interestingly, the genes affected by diabetic retinopathy are known to be under dopaminergic control. Consistent with this assumption, the diabetic retina displays disturbed dopamine signaling.34,79 Hence, altered circadian regulation of these genes may mirror dysfunction of the retinal dopaminergic system in diabetic retinopathy.
To conclude, control of retinal physiology by the circadian clock appears to be extensive in photoreceptors, in particular by targeting genes involved in phototransduction but also genes known to be important for their development. Whether circadian regulation of gene expression in the retina involves molecules distinct from dopamine and melatonin remains to be further investigated.
Circadian Clocks and Visual Function
A fundamental feature of sensory systems is their ability to change their responsiveness when confronted with a constant stimulus, a process called sensory adaptation.80 In the retina, light adaptation contributes to the tissue's ability to detect and process visual images at any time of the day or night, when ambient or background light intensity varies over more than a billion-fold range.81 This amazing capability depends on a specific functional architecture, including two classes of photoreceptors, rods, and cones. In addition, a wide variety of adaptive processes, collectively referred to as network or neural adaptation, are driven by ambient illumination and target cellular and synaptic elements of retinal circuits.81 Over the last 2 decades, it has become clear that circadian clock activity interacts with the effects of light to change signal processing in retinal circuits over the course of the day/night cycle (for reviews see Refs. 15 and 82 through 85). Although we know little about how such changes are implemented, the widespread expression of clock genes and proteins in retinal tissue15 suggests that clocks likely influence the functional organization in the retina at all levels, with the possibility that each cell type's clock acts in its direct vicinity through a restricted clock pathway or more remotly via diffusible signals such as dopamine and melatonin, able to diffuse through retinal layers, and act at larger distances. The challenge we are facing today is to identify these many putative clock pathways and establish their individual contributions to the overall daily changes in retinal function.
Organization of Rhythmic Visual Function at the Level of the Network of Photoreceptors
Little is known about how ambient illumination and circadian clocks implement changes in retinal circuit operation, but accumulating evidence indicates that gap junctions play a prominent role in neural adaptation in the retina.86,87 Gap junctions are intercellular channels made of connexins that permit direct cell-to-cell transfer of small molecules and electrical current and, thereby, constitute the anatomic substrate of electrical synapses.80 Each of the five major neuron classes in the retina is coupled by gap junctions that express a number of different connexins; connexin36 (Cx36), which is the predominant connexin in the central nervous system, is widely expressed in retinal neurons, including photoreceptors.86 It has become increasingly evident that gap junctions in neural networks are plastic, similar to chemical synapses.88 Consistent with this, most gap junctions in the retina show some degree of plasticity, generally in response to changes in lighting.86,87 The involvement of circadian clocks in the plasticity of gap junctions in retinal circuits remains largely untested, but a large body of evidence now indicates that electrical coupling between photoreceptors is modulated by ambient light and circadian clocks.
The presence of a dynamic regulation of photoreceptor electrical coupling is supported by indirect measures, such as tracer coupling patterns,89–92 the phosphorylation state of Cx3690,91,93 or Cx36 transcript expression,94 or light response properties of photoreceptors,92,95 as well as direct measures of junctional conductance.96 These different approaches predict that photoreceptor coupling is controlled by light/dark and circadian clocks: it is weak during the subjective day, much stronger in subjective night, and nearly abolished under bright light illumination, irrespective of the time in the daily cycle. These approaches also support a key role for the melatonin/dopamine pathway in the regulation of photoreceptor coupling (Fig. 2). Other clock effectors have been shown to contribute to the daily plasticity of photoreceptor coupling as well. In particular, adenosine, whose extracellular levels increase at night, acts as a coupling agent on photoreceptors.91,97,98 It is thus very likely that multiple clock pathways are at play to tightly regulate and set preferred states for junctional coupling at different times of the day.
Figure 2.
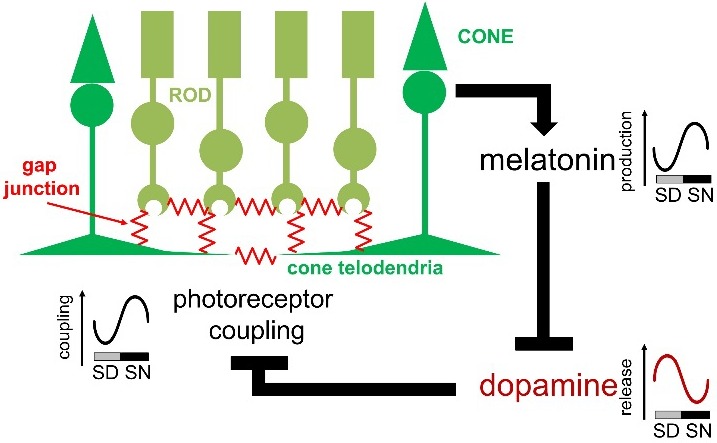
Overview of photoreceptor gap junction coupling and the melatonin/dopamine pathway. Gap junctions are located at photoreceptor terminals. Melatonin production is under the control of a clock within the photoreceptor layer and high at night. Melatonin suppresses dopamine release, and it is the nocturnal decrease in dopamine release and the subsequent decrease in activity of dopamine D2/4 receptors on photoreceptors that increases photoreceptor coupling. SD, subjective day; SN, subjective night.
The notion that electrical coupling between photoreceptors is dynamically regulated by circadian clocks suggests profound changes in downstream circuits in the process of rod and cone signals according to the time of day. Indeed, changes in the light response properties of rods and cones as a result of changes in photoreceptor coupling strength have been observed.92,95 Also, consistent with an increase in rod/cone coupling at night, signals that originate in rods are found to a greater extent in cones and cone-connected horizontal cells at night.92,99–103 Although the functional impact of the circadian modulation of photoreceptor coupling on the retinal output awaits further investigations, studies so far have established that photoreceptor gap junctions are the primary targets of the melatonin/dopamine clock pathway and that these gap junctions act as a neural circuit switch that controls signal flow between photoreceptors, and, thereby, downstream processing of rod- and cone-mediated signals in retinal circuits on a daily basis, at least in the outer retina.87,104
Our current knowledge offers a conceptual framework to explore the function of the reorganization of the photoreceptor network with the time of day in the retinal processing of visual information. The photoreceptor network alternates between a “daytime” state, where photoreceptor coupling must be weak to maintain the spectral purity of the cone-mediated responses, essential for high-acuity, color vision, and a “nighttime” state, where strong photoreceptor coupling maximizes processing of rod-mediated signals when vision is all about catching scarce photons and high sensitivity. Remarkably, circadian clocks modulate photoreceptor electrical coupling in anticipation of predictable changes in light intensity that occur at dawn and dusk, but the clocks also guarantee that the coupling strength is near optimal during day and night, thereby, conveying more reliability to this process than if it were solely controlled by ambient light.
Role of Melatonin in the Modulation of Retina Functions
The hormone/neurotransmitter melatonin is synthetized by the retinal photoreceptors during the night and its levels are tightly controlled by light/dark cycle and by the circadian clock.47,65,105,106 Melatonin acts by binding to two types of melatonin receptor (e.g., MT1 and MT2), which are present in many different retinal cell types.107 Consistently with this observation, several studies have shown that melatonin can indeed modulate a wide variety of retinal functions.108 In the mouse melatonin signaling is necessary for the circadian rhythm in the photopic ERG109 and in melatonin deficient strains (C57/BL6) the circadian rhythms in retinal dopamine content is no longer present. However, such a rhythm can be restored by cyclic administration of exogenous melatonin during the night,73 thus suggesting that the circadian rhythm in retinal dopamine levels is driven by the circadian rhythm in melatonin levels. Hence the circadian rhythm in rod-coupling observed in the mouse96 is also regulated by melatonin. Finally, melatonin drives the modulation of rod dark adaptation and thus it potentially protects the retina from light-induced damage during the day.110 Consistently with these experimental findings, several studies have shown that administration of exogenous melatonin can protect photoreceptors from apoptosis in mouse models of retinitis pigmentosa111,112 and that melatonin signaling is involved in the modulation of rod and cone photoreceptor viability during aging.113,114 A protective effect of melatonin on photoreceptor cells has been also observed in humans since administration of exogenous melatonin (3 mg) delayed the progression of age related macular degeneration (AMD) in a few patients.115 Additional studies have shown that low levels of circulating melatonin may correlate with the incidence of AMD116,117 and other retinal diseases.118
Clock Genes in Retinal Physiology and Health
Deletion of clock genes was shown, so far, to display mild effects in the retina,119 since only subtle morphologic alterations have been described in Per1/Per2 mutants up to 1 year of age, whereas none were seen in Cry1/Cry2 knockout mice.21,119,120 There is now evidence that changes occur in clock gene mutant mice at older ages.31 Alterations induced by the absence of these clock genes have been essentially linked to the absence of clock-regulated visual sensitivity.21,119 Developmental defects have been described in the Per1/Per2 mutant120 as well as in conditional photoreceptor-specific Bmal1 or Per2 knockout.121 These data correlate with the capacity of Bmal1 to regulate expression of the Dio2 gene, hence thyroid hormone signaling in the retina,121 but also indicate other developmental signaling pathways potentially under the control of clock gene expression.
Cryptochromes in the Retina
The role of Cry in the retina has been the subject of interest since the cloning of two human homologs in the 1990s.122 Given that CRY family proteins function as photoreceptors in plants and insects, this led to the suggestion that CRY was the elusive photopigment mediating circadian responses to light.123 Subsequent studies showed that this circadian photoreceptor was in fact melanopsin—an opsin/vitamin A–based photopigment expressed in ipRGCs.124–127 The demonstration that rods, cones, and melanopsin accounted for all retinal responses to light128 and that melanopsin expression alone was enough to make cells photoresponsive129–131 provided the key evidence that CRY did not function as a photoreceptor in mammals.
The initial evidence for a role of CRY as a photoreceptor in mammals came from studies showing that mice lacking both Cry1 and Cry2 (Cry1−/−;Cry2−/−) show attenuated nonimage forming responses to light.132–135 However, under constant conditions Cry1−/−;Cry2−/− double-knockout mice are arrhythmic.136 This is due to CRY playing a key role in the negative limb of the TTFL underlying molecular circadian rhythms. As such, CRY is also expected to play a role in the regulation of retinal circadian rhythms. As predicted, Cry1−/−;Cry2−/− mice show a loss of circadian rhythms in the photopic ERG b-wave amplitude.137 Furthermore, the attenuated pupillary responses described in Cry1−/−;Cry2−/− mice were found to be due to loss of circadian rhythms, with similar phenotypes in other clock mutants.119
While earlier studies had suggested that only CRY2 was expressed in the mammalian retina,52 recent data has shown that not only is CRY1 expressed in cones and amacrine cells, with some expression in retinal ganglion cells. Moreover, Cry1−/− mice show a loss of rhythms in photopic ERG b-wave amplitude, contrast sensitivity and pupillary light responses, with reduced robustness and stability of bioluminescent rhythms.42 By contrast, Cry2−/− mice show just an attenuated ERG b-wave amplitude. These data are consistent with previous data on PER2::LUC rhythms in Cry1−/−, Cry2−/− and Cry1−/−;Cry2−/− mice, showing a more important role of Cry1.41 Interestingly, Cry1−/− mice show retinal responses comparable to the “daytime” state at all phases, with a similar phenotype in Cry1−/−;Cry2−/− mice.137 By contrast, retinal conditional Bmal1−/− mice show physiology comparable to the “nighttime” state.21 These differences have been attributed to the roles of Bmal1 and Cry1 in the positive and negative limb of the TTFL, respectively. This model is consistent with data from Npas2−/− mice, which demonstrate contrast sensitivity responses comparable to the “nighttime” state.138
Rev-Erbα in the Retina
As outlined elsewhere in this article, it is thought that the principal driving force for retinal circadian clock function is to match light sensitivity to ambient light levels, which vary by ∼1010-fold between midnight and midday. In such a scheme, retinal light sensitivity can be simplistically thought as shifting along a continuum between at one end high sensitivity (necessary during nighttime when available light is very low) and at the other end low sensitivity corresponding to daytime (when ambient light levels are high and the retina must be protected from potential oxidative damage). As outlined earlier, these changes in light sensitivity are thought to be controlled in part by clock-driven physiologic mechanisms such as rod-cone coupling during the night,92,100 ion channel sensitivity,139 and visual pigment synthesis.18 REV-ERBα140 was well-placed to influence rod photoreceptor function because it was originally identified as being strongly linked with NR2E3, a rod-specific transcription factor involved in differentiation.141 Structural and immunochemical analyses did not reveal any significant differences between wild-type and Rev-Erbα−/− littermates, neither did noninvasive imaging by scanning laser ophthalmoscopy. Functional analyses using single flash ERG uncovered two distinct differences between control and mutant mice. First, under scotopic recording conditions, implicit times (response latencies) were significantly shorter in mutant mice, for both a and b-waves, over most stimulus intensities; second, the descending slope of the b-wave was abnormally shaped (“humped”) and large in mutant mice. These scotopic flash ERG data were complemented by analyses of the scotopic threshold response (STR), photopic flash ERG, and pupillary constriction. All these parameters were altered in Rev-Erbα−/− mice, with STR and photopic ERG also showing a hump-shaped distortion, and Rev-Erbα−/− mice exhibiting increased constriction and slower recovery compared to wild-type mice.142
These observations could be interpreted as a more rapid response to light stimulation in retinas of Rev-Erbα−/− compared to wild-type mice. The enhanced pupillary constriction suggested the involvement of ipRGCs. Immunohistochemical examination of whole flat-mounted retinas from the two mice strains revealed that melanopsin was more strongly expressed by individual cells in mutants, and that there was a large significant increase (62%) in total numbers of ipRGCs. To see whether this enhanced retinal response to light was translated into downstream locomotor activity, light sensitivity thresholds were explored as determined by behavioral tests. Using a two-step paradigm whereby mice were subjected simultaneously to experimental jetlag (delayed illumination of room lights by 6 hours) and reduced ambient light intensity,143,144 Rev-Erbα−/− mice were clearly still able to perceive and act upon light information at very low intensities to which wild-type mice no longer responded.142 Furthermore, negative masking responses could be elicited in Rev-Erbα−/− mice at low light intensities which no longer affected wild-type mice. Importantly, this latter behavior (negative masking) was abolished in double Rev-Erbα−/−;Opn4−/− mice, indicating that expression of melanopsin was necessary to convey the light information to the SCN.
Taken together, these data indicate that deletion of the Rev-Erbα gene enhances light detection, both at the retinal level (reduced implicit times in a and b-wave) and cognitive processing (increased light sensitivity). Figure 3 presents a hypothetical model to explain the different experimental data. In wild-type mice, output of ipRGCs in response to light information is an integrated product of all three photoreceptor types, rods, cones and ipRGCs. At sufficient light intensity, input from any/all of these sources can trigger firing. But at low light intensity, stimulation is insufficient to lead to firing. However, removal of Rev-Erbα leads to increased rod input (shortened a-wave latencies) and/or increased melanopsin input (higher expression levels, higher cell number), which allow ipRGCs to reach threshold depolarization. In double Rev-Erbα−/−;Opn4−/− mice, the melanopsin component is missing and even with higher rod signaling, the system is not able to attain threshold.
Figure 3.
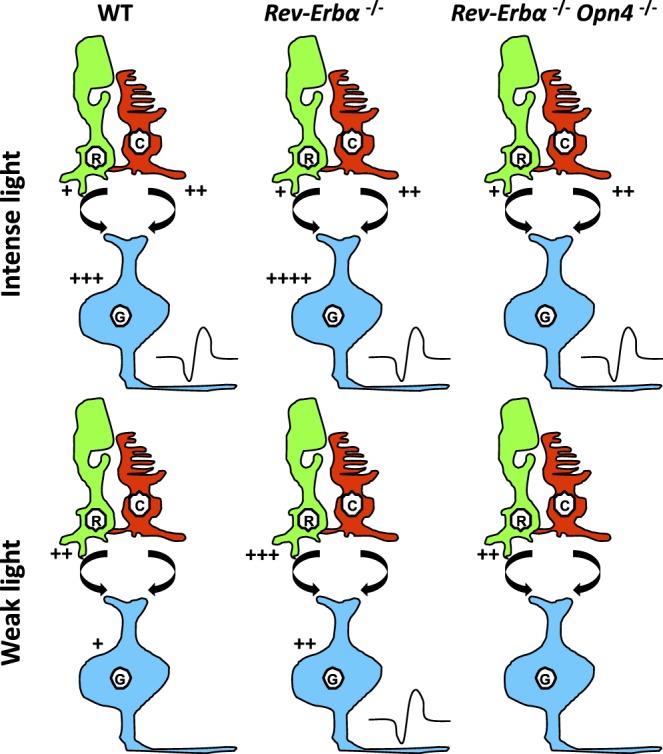
Hypothetical mechanism explaining the effect of Rev-Erbα knockout on nonvisual responses. In wild-type mice, output of ipRGCs (G, blue) in response to light information is an integrated product of all three photoreceptor types, rods (R, green), cones (C, red), and ipRGCs. At sufficient light intensity, input from any/all of these sources can trigger firing (symbolized by the black curve close to ipRGCs), whereas at low light intensity, stimulation is insufficient, although the cells are partly depolarized and exhibit resting potentials close to threshold. However, removal of Rev-Erbα leads to increased rod input (shortened a-wave latencies) and/or increased melanopsin input (higher expression levels, higher numbers), which allow iipRCGs (G, blue) to reach threshold depolarization. In double Rev-Erbα−/−;Opn4−/− mice, the melanopsin component is missing and even with higher rod signaling the system is not able to attain threshold. The number of “+” indicates the hypothetical level of stimulation. Curved arrow between rods and cones, and ipRGCs, indicates bipolar contact.
The data lead to several interesting lines of further investigation:
They suggest that the physiologic role of Rev-Erbα is to reduce light sensitivity, which by inference should correspond to a “daytime state” of visual processing. In other words, there should exist a circadian-dependent mechanism connecting Rev-Erbα to changes in retinal light sensitivity (e.g., through regulation of ion channel activity or cell-cell coupling).
Since Rev-Erbα−/− mice are “stuck” in a highly sensitive nighttime state, they could be more susceptible to intense light damage compared to normal littermates.24
Increased ipRGC numbers indicate that Rev-Erbα is possibly involved in developmentally programmed cell death, since total RGC numbers were not altered and it seems easier to imagine elevated ipRGC numbers arise through a faulty apoptotic mechanism145 rather than selective increase in this population. In this respect, it is interesting to note that the lack of other clock genes which negatively regulate Bmal1 lead to an increase in ipRGCs.119 Alternatively, perhaps the enhanced light sensitivity of the system makes the ipRGCs more responsive to early light history which changes melanopsin expression.146,147
These different possibilities are being actively investigated.
To conclude, the role of clock genes in the retina still requires further investigation, to understand both how they control rhythms in phototransduction and how they regulate retinal development.
The Eye: A Multioscillatory System With Diverse Entraining Mechanisms
Light Entrainment of the Retina Clock
The identification of an autonomous clock in the retina of Xenopus laevis brought circadian rhythms out of the brain and into individual “peripheral” tissues.148 Similar to previous observations in lizard and avian pineal cells, these clocks within Xenopus retinas could be photoentrained in a culture dish.149–151 In 1996, Tosini and Menaker demonstrated that melatonin rhythms of hamster retinas could also be entrained to light/dark cycles ex vivo, free of influence from the brain's “master” clock.14 While the notion of photoreceptive cells within a retina may not have been a surprise, their ability to directly set the phase of local clocks revealed a level of independence of retinal clocks not previously appreciated. It had previously been observed that the phases of rhythmic disk shedding in rod photoreceptors could be dissociated between the two eyes within the same animal through use of an eye patch.152 Also, in blind mice (Opn4−/−;rd1/rd1) which fail to synchronize behavioral rhythms to a light/dark cycle, the phase of the retinal circadian clocks remain synchronized to the light/dark cycle regardless of the phase of the SCN.153 Thus, such independence of the retina from the SCN can act in vivo, as well as in a dish. In vivo and in vitro studies showed that rhythmicity in the retina emerges from a network of coupled circadian oscillators located within distinct cellular layers.37,39,46 Thus, it might be that the phase shifting response of inner or outer layers involves different photoreceptors, known to encode distinct light parameters. For instance, light-induction and circadian rhythms of clock gene expression are abolished in the photoreceptor layer of Opn4−/− mice while, at the same time, these responses are maintained in the inner and ganglion cell layers,39 confirming the complexity of the clock system and entraining mechanisms in the retina.
The Roles of Dopamine and Melatonin
In the same work that demonstrated the local photoentrainability of the Xenopus retinal clocks, Cahill and Besharse154 identified dopamine as a key determinant of clock phase. In mammals, McMahon and colleagues36 blocked an exhaustive assortment of neurotransmitters and signaling modalities in the mouse retina, and they used the PER2::LUC reporter method discussed above as a measure of retinal clock amplitude and phase. Surprisingly, little effect was observed on oscillator amplitude by the blockade of many neurotransmitters; however, like frog retinas, dopamine agonists were capable of shifting the phase of the clock. Furthermore, inhibition of dopaminergic signaling (blockade of D1 receptors) diminished the phase shifting effects of light.36 This model fits with the observations that the activity of dopaminergic amacrine cells is both modulated by the circadian clock and, directly, by light.155,156 Both activation of D1 and D4 receptors has influence on retinal clock phase,36,157,158 but exactly how and where these signals are integrated is still being investigated.
While dopamine production and release is stimulated by light, the production of melatonin within the retina is observed at times of darkness and is gated by the circadian clock65 and melatonin levels are rapidly reduced in the presence of light via direct proteolysis of the AANAT enzyme.105,159 The regulation of melatonin synthesis in the rodent retina is similar to what has been described for the pineal gland85 and involves a well-defined biosynthetic pathway.160 However, it is important to note that only the retina Aanat transcription is under direct control of the circadian clock.66
The secretion of melatonin was measured as the circadian output from hamster retinas in tissue culture.14 Interestingly, melatonin likely also has a role in setting the phase of the retina clock. The retinas of mice that lack melatonin, either by natural mutation in genetic strain or by targeted loss of melatonin receptors, display phases of clock gene expression distinct from mice in which melatonin signaling is intact.70,161,162 In further support of the idea of the retinal clock being uniquely independent among peripheral oscillators, transcription of enzymes necessary for melatonin production remains rhythmic in retinas of behaviorally arrhythmic rats in which their SCN were surgically lesioned.163 While rhythmic melatonin expression is not dependent on light, it may be dependent on the presence of the cells of the outer nuclear layer in some species such as mice,47,106 but not in others such as rat.164 It is interesting to note that many common laboratory mouse strains are deficient in the enzymes to produce melatonin and thus lack melatonin signaling.165,166 However, the lack of melatonin in these mouse strains does not diminish the amplitude of the molecular circadian clock within the retina nor its ability to photoentrain.36,153 In these mice, synchronization of retinal oscillators was reported to involve GABA acting as a night signal, further relayed by casein kinase 1 epsilon (CK1ε) within cells.36 In retinas that express both dopamine and melatonin neuromodulators, it is likely that dopamine acts as a light signal, and melatonin signals darkness. It should be mentioned that melatonin regulation in the diurnal rodent Arvicanthis appears to be bimodal, with one source (cones) peaking in the night and a second source (ganglion cells) peaking in the day.167 Finally, it is worthwhile mentioning that although melatonin is present in the primate retina, it is not clear whether melatonin found in the eye is actually synthesized in situ or is of pineal origin.168
Photoreceptors Involved in Local Photoentrainment
In order for the retinal circadian clocks to photoentrain, one or more groups of photoreceptors must first capture the light and convey the information to the rest of the tissue. The landmark study by Ruan and colleagues36 clearly demonstrated that light is able to phase shift the retinal clock in vitro with respectively a phase delay/advance when light is applied at the beginning/end of the biologic night, but the use of broadband white light did not allow distinction of the putative roles of different photoreceptors. More recently, using monochromatic light, a typical phase response curve to light was described in the retina.153,169 Interestingly, the light-activated dopamine release from dopaminergic amacrine cells was shown to require the presence of rods and cones170 and ipRGCs.171 The respective contributions of the distinct photosensitive cells to dopaminergic amacrine cell activation by light are irradiance-dependent.172 The authors found that rods excite dopaminergic cells across a wide range of light intensities (from the scotopic to the photopic range) whereas middle wavelength-sensitive cones and ipRGCs present a higher threshold than rods (3 log units). In addition, several anatomic and functional studies suggest that ipRGCs occupy a central role in the retinal network and may contribute to the light response of the retinal clock. Specifically, synaptic contacts of ipRGCs with dopaminergic neurons have been reported in rodents, monkeys, and humans173–177 and provide sustained excitatory light responses to dopaminergic neurons with peak sensitivity near 480 nm, the maximum of sensitivity of melanopsin.171 In the absence of melanopsin this sustained dopaminergic light response is greatly diminished.39,177 Interestingly, a recent study in the rat retina showed that ipRGCs are able to transmit their tonic light response not only to dopaminergic cells but also to a variety of amacrine interneurons exclusively through gap junctions, suggesting a widespread intraretinal role of ipRGCs.175 Finally, in the Opn4 knockout mice, the circadian control of cone-light response, as assessed by ERG, is lost.19 All these data suggest a retrograde direction of signaling in the retina, distinct from the classical outer-to-inner layer pathways that represent a potential mechanism for transmitting irradiance information to the outer retina. Notwithstanding, at the level of transcription, the presence of rods and cones in the outer cellular layer influences the expression of Opn4 itself within the ganglion cells.147 Clearly, the ensemble of cells within the retinal system displays a level of interdependency.
In addition to the rod and cone photoreceptors in the outer layer and melanopsin in ganglion cells, neuropsin (Opn5) is yet another bistable opsin expressed within the mammalian retina.178,179 In birds, Opn5 is expressed in the retina as well as in neurons along the 3rd ventricle in the hypothalamus, and it acts as a deep brain photoreceptor regulating seasonality.180–182 In the mammalian retina, Opn5 expression is found primarily in a subset of ganglion cells, similar to melanopsin.169,178,183,184 Heterologous expression of human and mouse Opn5 shows a sensitivity to short-wavelength light with an absorbance maximum around 380 nm.178,184 In the absence of rods, cones and melanopsin, mouse retinas retain the ability to photoentrain in a culture dish and in vivo.153 The ability of mouse retinas to entrain to short-wavelength light/dark cycles in culture is lost in the absence of Opn5.169 This adds the influence of another opsin and ganglion cell-type to the regulation of the retinal clock's response to light. The fact that the mouse retina is able to be photoentrained by UV light is not surprising given the peak sensitivity of its short-wavelength opsin and the fact that its lens effectively transmits UV light.185 The role of neuropsin in the human retina remains to be investigated given its reduced UV transmittance.186,187
Circadian Regulation and Entrainment of Extra-Retinal Tissues Within the Eye
In addition to the retina, several structures within the eyes also contain circadian clocks that control several important physiologic functions. In this section, we will review the regulation of circadian rhythm and mechanisms by which these structures—that are not directly photosensitive—are entrained to the external light/dark cycle.
The RPE was the first extraretinal tissue in which a circadian rhythm was reported.23,188 This tissue plays a key role in the maintenance of photoreceptor health189 and, in addition to providing nutrients to the photoreceptors, the RPE cells are responsible for the phagocytosis of the disks that are shed by photoreceptor outer segments (see Ref. 190 for review). The shed ROS fragments are then engulfed, phagocytized, and degraded by the RPE. Lack of phagocytic activity by the RPE leads to the accumulation of membranous debris and subsequent photoreceptor degeneration.191 Another important aspect of RPE biology is the burst in disk shedding and phagocytosis that occur every day 1 to 2 hours after the onset of light for rod photoreceptors,23,188,192 and at the onset of or during the night for cones.193–195 The circadian rhythm in phagocytic activity is also present in a diurnal rodent (Arvicanthis ansorgei), but in this case, no difference was observed in the time of peak of phagocytic activity between rods and cones.22,196 These rhythms persist in constant conditions, thus demonstrating that they are controlled by circadian clocks.152,188,197,198
Earlier studies indicated that circadian clocks controlling these rhythmic events were located within the eye and possibly in photoreceptors.152,198 However, other studies indicate that the RPE is also involved in the control of disk shedding and phagocytosis since the diurnal rhythm in exposure of phosphatidylserine by rod outer segments is not entirely controlled by the photoreceptors, but RPE cells participate in the synchronization of this process.199 Additional studies have also revealed that the molecular mechanisms controlling the daily burst in phagocytosis activity by the RPE involves the activation of the FAK-MerTK signaling pathway200,201 and phosphoinositide signaling.202
Finally, recent experimental evidence has shown that the RPE cells contain an autonomous circadian clock, that is not influenced by the master circadian clock located in the brain and that the RPE is not directly photosensitive.203 Hence the entrainment of the RPE circadian clock must depend on a signal coming from the retinal photoreceptors. Indeed, a recent study has reported that dopamine—acting via D2R—can entrain the RPE circadian clock.204 Another study using human RPE cells has also shown that the activation of muscarinic receptors can induce a phase-shift in their circadian clock.205 Interestingly—although melatonin does not entrain the RPE circadian clock204—a recent study has reported that in mice lacking MT1 or MT2 receptors the peak of phagocytic activity by the RPE is advanced (about 3 hours) with respect to control mice.206 Hence these data suggest that the timing of the daily burst in phagocytic activity is the result of interactions between the retinal and the RPE circadian clocks.
The presence of a daily/circadian rhythm in the intraocular pressure (IOP) of many mammalian species—including humans—has been also demonstrated by several studies.207–210 The regulation of the circadian rhythm in IOP involves direct neural control via the sympathetic innervation211–213 and hormonal signaling via melatonin.214–218 In mice lacking a functional circadian clock—Cry1/Cry2-double knockout mice136—the circadian rhythm in IOP is no longer present regardless of environmental light conditions.219 However, it is important to note that this study did not provide any insight whether the master circadian clock in the brain or other ocular clocks control this rhythm. To address this question two recent studies investigated whether the iris/ciliary body contains a circadian clock. The first study reported that clock genes and proteins are expressed in the iris-ciliary body complex of mice220 and the second study demonstrated that cultured iris/ciliary body obtained from mPer2Luc mice could express a circadian rhythm in bioluminescence.28 Also in this case, the circadian rhythm in PER2::LUC bioluminescence from cultures of iris/ciliary body could not be entrained by light, thus suggesting that the entrainment of the circadian rhythm in these structures—and possibly the IOP—is mediated via neuronal or hormonal signaling outside the ciliary body and even from outside the eye.
Several studies have shown that a regular diurnal rhythm in the light/dark cycle is important for normal corneal growth and development (see Ref. 221 for review). The renewal of the corneal epithelium shows a daily rhythm222 and the mitotic rate of the corneal epithelial cells is highest during the night and lowest during the day.223 Finally, cultured corneas obtained from mPer2Luc mice show a robust circadian rhythm in bioluminescence45,224,225 that can be entrained by light via OPN5169 or by melatonin via activation of MT2 receptors.224 Interestingly it has been reported that the expression of clock genes in the corneal epithelium and their rhythmic profiles are altered in diabetic mice and such a rhythm can be restored by insulin administration.226 Finally, it is worthwhile mentioning that exposure to constant light, constant dark, or jetlag conditions can also alter the circadian pattern of the corneal epithelial mitosis and clock gene expression.26
Diurnal changes in corneal growth as well as in IOP might also be relevant to the daily changes in the eye axial length. Such changes in early life contribute to refractive development and quality of image formation. How these processes are regulated by the retinal circadian clock remains to be characterized (see Ref. 221 for review).
Thus, from what we have mentioned in this section, it is evident that many ocular structures contain circadian clocks; however, the cues that these different structures may use to entrain their circadian rhythms may vary (Fig. 4). Whether these clocks interact also remains to be investigated.
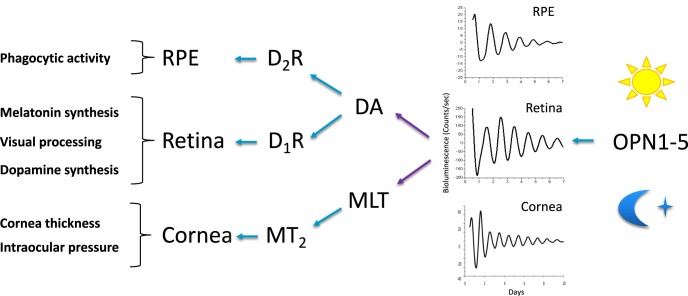
Figure 4.
Entrainment of circadian rhythms in ocular tissues. Many physiologic functions in eye tissues (left column) are regulated by circadian clocks and synchronized to the light/dark cycle. The retina, RPE, and cornea, among others, were shown to harbor autonomous clocks, as demonstrated by their capacity to display oscillating PER2::LUC activity (bioluminescence, counts/sec) in vitro (see representative recordings). One major site for the input of light information from the environment to the eye is the retina. OPN1-5 are opsin photopigments expressed at various levels in the retina: OPN1 (short and middle/long wavelength sensitive) in cones, OPN2 (rhodopsin) in rods, OPN4 in ipRGCs and OPN5 in a subpopulation of ganglion cells as well; localization of OPN3 expressing cells is not known. Most opsins are involved in the effects of light on retinal physiology. OPN4 and OPN5 are involved in the regulation of the retinal clock's response to light. The retina synthesizes (purple arrows) melatonin (MLT) in the night and dopamine (DA) during the day. These signals display phase regulatory properties (blue arrows), in the retina itself, but also in other ocular tissues such as the RPE and cornea.
Conclusions
Recent data have enriched our view of the diversity of photosensitive systems and downstream pathways, as well as of the complexity of the clock cellular network sustaining adaptation of eye physiology to the 24-hour cycle. They confirm that the eye stands apart in the circadian field, mainly by the capacity, within this network to integrate light information and in turn finely tune cellular function throughout the organ. Many questions are still currently unanswered, notably regarding the exact function of the molecular clock in the distinct constitutive cell types, in the adult and during development, as well as in nocturnal versus diurnal species. They also open stimulating perspectives regarding the variety of synchronization mechanisms at play, both in response to light and downstream within the eye. Similarly to what has been described in the SCN, the distinct photoreceptors present are likely to specifically contribute to shaping the visual response: how their signaling is integrated and communicated downstream within ocular tissues will be the focus of future studies.
Acknowledgments
Supported by grants from the National Institutes of Health: EY026291 (GT), EY018640 (CPR), and GM124246 (EDB).
Disclosure: M.-P. Felder-Schmittbuhl, None; E.D. Buhr, None; O. Dkhissi-Benyahya, None; D. Hicks, None; S.N. Peirson, None; C.P. Ribelayga, None; C. Sandu, None; R. Spessert, None; G. Tosini, None
References
- 1.Takahashi JS. Transcriptional architecture of the mammalian circadian clock. Nat Rev Genet. 2017;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hogenesch JB, Ueda HR. Understanding systems-level properties: timely stories from the study of clocks. Nat Rev Genet. 2011;12:407–416. doi: 10.1038/nrg2972. [DOI] [PubMed] [Google Scholar]
- 3.Panda S, Antoch MP, Miller BH, et al. Coordinated transcription of key pathways in the mouse by the circadian clock. Cell. 2002;109:307–320. doi: 10.1016/s0092-8674(02)00722-5. [DOI] [PubMed] [Google Scholar]
- 4.Zhang R, Lahens NF, Ballance HI, Hughes ME, Hogenesch JB. A circadian gene expression atlas in mammals: implications for biology and medicine. Proc Natl Acad Sci U S A. 2014;111:16219–16224. doi: 10.1073/pnas.1408886111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mure LS, Le HD, Benegiamo G, et al. Diurnal transcriptome atlas of a primate across major neural and peripheral tissues. Science. 2018;359:eaao0318. doi: 10.1126/science.aao0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaix A, Zarrinpar A, Panda S. The circadian coordination of cell biology. J Cell Biol. 2016;215:15–25. doi: 10.1083/jcb.201603076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fonseca Costa SS, Ripperger JA. Impact of the circadian clock on the aging process. Front Neurol. 2015;6:43. doi: 10.3389/fneur.2015.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tosini G, Ferguson I, Tsubota K. Effects of blue light on the circadian system and eye physiology. Mol Vis. 2016;22:61–72. [PMC free article] [PubMed] [Google Scholar]
- 9.West AC, Bechtold DA. The cost of circadian desynchrony: evidence, insights and open questions. Bioessays. 2015;37:777–788. doi: 10.1002/bies.201400173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamazaki S, Goto M, Menaker M. No evidence for extraocular photoreceptors in the circadian system of the Syrian hamster. J Biol Rhythms. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- 11.Paul KN, Saafir TB, Tosini G. The role of retinal photoreceptors in the regulation of circadian rhythms. Rev Endocr Metab Disord. 2009;10:271–278. doi: 10.1007/s11154-009-9120-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmidt TM, Do MT, Dacey D, Lucas R, Hattar S, Matynia A. Melanopsin-positive intrinsically photosensitive retinal ganglion cells: from form to function. J Neurosci. 2011;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walmsley L, Hanna L, Mouland J, et al. Colour as a signal for entraining the mammalian circadian clock. PLoS Biol. 2015;13:e1002127. doi: 10.1371/journal.pbio.1002127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 15.McMahon DG, Iuvone PM, Tosini G. Circadian organization of the mammalian retina: from gene regulation to physiology and diseases. Prog Retin Eye Res. 2014;39:58–76. doi: 10.1016/j.preteyeres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Felder-Schmittbuhl MP, Calligaro H, Dkhissi-Benyahya O. The retinal clock in mammals: role in health and disease. Chronophysiology Ther. 2017;7:33–45. [Google Scholar]
- 17.Bobu C, Sandu C, Laurent V, Felder-Schmittbuhl MP, Hicks D. Prolonged light exposure induces widespread phase shifting in the circadian clock and visual pigment gene expression of the Arvicanthis ansorgei retina. Mol Vis. 2013;19:1060–1073. [PMC free article] [PubMed] [Google Scholar]
- 18.von Schantz M, Lucas RJ, Foster RG. Circadian oscillation of photopigment transcript levels in the mouse retina. Brain Res Mol Brain Res. 1999;72:108–114. doi: 10.1016/s0169-328x(99)00209-0. [DOI] [PubMed] [Google Scholar]
- 19.Barnard AR, Hattar S, Hankins MW, Lucas RJ. Melanopsin regulates visual processing in the mouse retina. Curr Biol. 2006;16:389–395. doi: 10.1016/j.cub.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 20.Cameron MA, Barnard AR, Lucas RJ. The electroretinogram as a method for studying circadian rhythms in the mammalian retina. J Genet. 2008;87:459–466. doi: 10.1007/s12041-008-0068-5. [DOI] [PubMed] [Google Scholar]
- 21.Storch KF, Paz C, Signorovitch J, et al. Intrinsic circadian clock of the mammalian retina: importance for retinal processing of visual information. Cell. 2007;130:730–741. doi: 10.1016/j.cell.2007.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bobu C, Hicks D. Regulation of retinal photoreceptor phagocytosis in a diurnal mammal by circadian clocks and ambient lighting. Invest Ophthalmol Vis Sci. 2009;50:3495–3502. doi: 10.1167/iovs.08-3145. [DOI] [PubMed] [Google Scholar]
- 23.LaVail MM. Circadian nature of rod outer segment disc shedding in the rat. Invest Ophthalmol Vis Sci. 1980;19:407–411. [PubMed] [Google Scholar]
- 24.Organisciak DT, Darrow RM, Barsalou L, Kutty RK, Wiggert B. Circadian-dependent retinal light damage in rats. Invest Ophthalmol Vis Sci. 2000;41:3694–3701. [PubMed] [Google Scholar]
- 25.Kikkawa Y. Diurnal variation in corneal thickness. Exp Eye Res. 1973;15:1–9. doi: 10.1016/0014-4835(73)90183-8. [DOI] [PubMed] [Google Scholar]
- 26.Xue Y, Liu P, Wang H, et al. Modulation of circadian rhythms affects corneal epithelium renewal and repair in mice. Invest Ophthalmol Vis Sci. 2017;58:1865–1874. doi: 10.1167/iovs.16-21154. [DOI] [PubMed] [Google Scholar]
- 27.Lozano DC, Hartwick AT, Twa MD. Circadian rhythm of intraocular pressure in the adult rat. Chronobiol Int. 2015;32:513–523. doi: 10.3109/07420528.2015.1008135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsuchiya S, Buhr ED, Higashide T, Sugiyama K, Van Gelder RN. Light entrainment of the murine intraocular pressure circadian rhythm utilizes non-local mechanisms. PLoS One. 2017;12:e0184790. doi: 10.1371/journal.pone.0184790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kondratov RV, Kondratova AA, Gorbacheva VY, Vykhovanets OV, Antoch MP. Early aging and age-related pathologies in mice deficient in BMAL1, the core componentof the circadian clock. Genes Dev. 2006;20:1868–1873. doi: 10.1101/gad.1432206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang G, Chen L, Grant GR, et al. Timing of expression of the core clock gene Bmal1 influences its effects on aging and survival. Sci Transl Med. 2016;8:324ra316. doi: 10.1126/scitranslmed.aad3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba K, Ribelayga CP, Michael Iuvone P, Tosini G. The retinal circadian clock and photoreceptor viability. Adv Exp Med Biol. 2018;1074:345–350. doi: 10.1007/978-3-319-75402-4_42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moller M, Rath MF, Ludvigsen M, Honore B, Vorum H. Diurnal expression of proteins in the retina of the blind cone-rod homeobox (Crx(-/-)) mouse and the 129/Sv mouse: a proteomic study. Acta Ophthalmol. 2017;95:717–726. doi: 10.1111/aos.13429. [DOI] [PubMed] [Google Scholar]
- 33.Tsuji T, Hirota T, Takemori N, et al. Circadian proteomics of the mouse retina. Proteomics. 2007;7:3500–3508. doi: 10.1002/pmic.200700272. [DOI] [PubMed] [Google Scholar]
- 34.Lahouaoui H, Coutanson C, Cooper HM, Bennis M, Dkhissi-Benyahya O. Diabetic retinopathy alters light-induced clock gene expression and dopamine levels in the mouse retina. Mol Vis. 2016;22:959–969. [PMC free article] [PubMed] [Google Scholar]
- 35.Peirson SN, Butler JN, Duffield GE, Takher S, Sharma P, Foster RG. Comparison of clock gene expression in SCN, retina, heart, and liver of mice. Biochem Biophys Res Commun. 2006;351:800–807. doi: 10.1016/j.bbrc.2006.10.118. [DOI] [PubMed] [Google Scholar]
- 36.Ruan GX, Allen GC, Yamazaki S, McMahon DG. An autonomous circadian clock in the inner mouse retina regulated by dopamine and GABA. PLoS Biol. 2008;6:e249. doi: 10.1371/journal.pbio.0060249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandu C, Hicks D, Felder-Schmittbuhl MP. Rat photoreceptor circadian oscillator strongly relies on lighting conditions. Eur J Neurosci. 2011;34:507–516. doi: 10.1111/j.1460-9568.2011.07772.x. [DOI] [PubMed] [Google Scholar]
- 38.Tosini G, Kasamatsu M, Sakamoto K. Clock gene expression in the rat retina: effects of lighting conditions and photoreceptor degeneration. Brain Res. 2007;1159:134–140. doi: 10.1016/j.brainres.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dkhissi-Benyahya O, Coutanson C, Knoblauch K, et al. The absence of melanopsin alters retinal clock function and dopamine regulation by light. Cell Mol Life Sci. 2013;70:3435–3447. doi: 10.1007/s00018-013-1338-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruan GX, Zhang DQ, Zhou T, Yamazaki S, McMahon DG. Circadian organization of the mammalian retina. Proc Natl Acad Sci U S A. 2006;103:9703–9708. doi: 10.1073/pnas.0601940103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ruan GX, Gamble KL, Risner ML, Young LA, McMahon DG. Divergent roles of clock genes in retinal and suprachiasmatic nucleus circadian oscillators. PLoS One. 2012;7:e38985. doi: 10.1371/journal.pone.0038985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong JCY, Smyllie NJ, Banks GT, et al. Differential roles for cryptochromes in the mammalian retinal clock. FASEB J. 2018;32:4302–4314. doi: 10.1096/fj.201701165RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yamazaki S, Numano R, Abe M, et al. Resetting central and peripheral circadian oscillators in transgenic rats. Science. 2000;288:682–685. doi: 10.1126/science.288.5466.682. [DOI] [PubMed] [Google Scholar]
- 45.Yoo SH, Yamazaki S, Lowrey PL, et al. PERIOD2::LUCIFERASE real-time reporting of circadian dynamics reveals persistent circadian oscillations in mouse peripheral tissues. Proc Natl Acad Sci U S A. 2004;101:5339–5346. doi: 10.1073/pnas.0308709101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jaeger C, Sandu C, Malan A, Mellac K, Hicks D, Felder-Schmittbuhl MP. Circadian organization of the rodent retina involves strongly coupled, layer-specific oscillators. FASEB J. 2015;29:1493–1504. doi: 10.1096/fj.14-261214. [DOI] [PubMed] [Google Scholar]
- 47.Tosini G, Davidson AJ, Fukuhara C, Kasamatsu M, Castanon-Cervantes O. Localization of a circadian clock in mammalian photoreceptors. FASEB J. 2007;21:3866–3871. doi: 10.1096/fj.07-8371com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu L, Ruan G, Dai H, Liu AC, Penn J, McMahon DG. Mammalian retinal Muller cells have circadian clock function. Mol Vis. 2016;22:275–283. [PMC free article] [PubMed] [Google Scholar]
- 49.Buonfiglio DC, Malan A, Sandu C, et al. Rat retina shows robust circadian expression of clock and clock output genes in explant culture. Mol Vis. 2014;20:742–752. [PMC free article] [PubMed] [Google Scholar]
- 50.Dorenbos R, Contini M, Hirasawa H, Gustincich S, Raviola E. Expression of circadian clock genes in retinal dopaminergic cells. Vis Neurosci. 2007;24:573–580. doi: 10.1017/S0952523807070538. [DOI] [PubMed] [Google Scholar]
- 51.Schneider K, Tippmann S, Spiwoks-Becker I, et al. Unique clockwork in photoreceptor of rat. J Neurochem. 2010;115:585–594. doi: 10.1111/j.1471-4159.2010.06953.x. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Zhang Z, Ribelayga CP. Heterogeneous expression of the core circadian clock proteins among neuronal cell types in mouse retina. PLoS One. 2012;7:e50602. doi: 10.1371/journal.pone.0050602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bai L, Zimmer S, Rickes O, et al. Daily oscillation of gene expression in the retina is phase-advanced with respect to the pineal gland. Brain Res. 2008;1203:89–96. doi: 10.1016/j.brainres.2008.01.073. [DOI] [PubMed] [Google Scholar]
- 54.Kamphuis W, Cailotto C, Dijk F, Bergen A, Buijs RM. Circadian expression of clock genes and clock-controlled genes in the rat retina. Biochem Biophys Res Commun. 2005;330:18–26. doi: 10.1016/j.bbrc.2005.02.118. [DOI] [PubMed] [Google Scholar]
- 55.Kunst S, Wolloscheck T, Grether M, Trunsch P, Wolfrum U, Spessert R. Photoreceptor cells display a daily rhythm in the orphan receptor Esrrbeta. Mol Vis. 2015;21:173–184. [PMC free article] [PubMed] [Google Scholar]
- 56.Cruz NM, Yuan Y, Leehy BD, et al. Modifier genes as therapeutics: the nuclear hormone receptor Rev Erb alpha (Nr1d1) rescues Nr2e3 associated retinal disease. PLoS One. 2014;9:e87942. doi: 10.1371/journal.pone.0087942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mollema NJ, Yuan Y, Jelcick AS, et al. Nuclear receptor Rev-erb alpha (Nr1d1) functions in concert with Nr2e3 to regulate transcriptional networks in the retina. PLoS One. 2011;6:e17494. doi: 10.1371/journal.pone.0017494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolloscheck T, Kunst S, Kelleher DK, Spessert R. Transcriptional regulation of nucleoredoxin-like genes takes place on a daily basis in the retina and pineal gland of rats. Vis Neurosci. 2015;32:E002. doi: 10.1017/S0952523814000352. [DOI] [PubMed] [Google Scholar]
- 59.Andre E, Conquet F, Steinmayr M, Stratton SC, Porciatti V, Becker-Andre M. Disruption of retinoid-related orphan receptor beta changes circadian behavior, causes retinal degeneration and leads to vacillans phenotype in mice. EMBO J. 1998;17:3867–3877. doi: 10.1093/emboj/17.14.3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.McGinnis JF, Austin BJ, Stepanik PL, Lerious V. Light-dependent regulation of the transcriptional activity of the mammalian gene for arrestin. J Neurosci Res. 1994;38:479–482. doi: 10.1002/jnr.490380414. [DOI] [PubMed] [Google Scholar]
- 61.Holter P, Kunst S, Wolloscheck T, et al. The retinal clock drives the expression of Kcnv2, a channel essential for visual function and cone survival. Invest Ophthalmol Vis Sci. 2012;53:6947–6954. doi: 10.1167/iovs.12-10234. [DOI] [PubMed] [Google Scholar]
- 62.Vancura P, Wolloscheck T, Baba K, Tosini G, Iuvone PM, Spessert R. Circadian and dopaminergic regulation of fatty acid oxidation pathway genes in retina and photoreceptor cells. PLoS One. 2016;11:e0164665. doi: 10.1371/journal.pone.0164665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunst S, Wolloscheck T, Kelleher DK, et al. Pgc-1alpha and Nr4a1 are target genes of circadian melatonin and dopamine release in murine retina. Invest Ophthalmol Vis Sci. 2015;56:6084–6094. doi: 10.1167/iovs.15-17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vancura P, Abdelhadi S, Csicsely E, et al. Gnaz couples the circadian and dopaminergic system to G protein-mediated signaling in mouse photoreceptors. PLoS One. 2017;12:e0187411. doi: 10.1371/journal.pone.0187411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fukuhara C, Liu C, Ivanova TN, et al. Gating of the cAMP signaling cascade and melatonin synthesis by the circadian clock in mammalian retina. J Neurosci. 2004;24:1803–1811. doi: 10.1523/JNEUROSCI.4988-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen W, Baler R. The rat arylalkylamine N-acetyltransferase E-box: differential use in a master vs. a slave oscillator. Brain Res Mol Brain Res. 2000;81:43–50. doi: 10.1016/s0169-328x(00)00160-1. [DOI] [PubMed] [Google Scholar]
- 67.Haque R, Ali FG, Biscoglia R, et al. CLOCK and NPAS2 have overlapping roles in the circadian oscillation of arylalkylamine N-acetyltransferase mRNA in chicken cone photoreceptors. J Neurochem. 2010;113:1296–1306. doi: 10.1111/j.1471-4159.2010.06698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tosini G, Chaurasia SS. Michael Iuvone P. Regulation of arylalkylamine N-acetyltransferase (AANAT) in the retina. Chronobiol Int. 2006;23:381–391. doi: 10.1080/07420520500482066. [DOI] [PubMed] [Google Scholar]
- 69.Yamajuku D, Shibata Y, Kitazawa M, et al. Cellular DBP and E4BP4 proteins are critical for determining the period length of the circadian oscillator. FEBS Lett. 2011;585:2217–2222. doi: 10.1016/j.febslet.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 70.Hiragaki S, Baba K, Coulson E, Kunst S, Spessert R, Tosini G. Melatonin signaling modulates clock genes expression in the mouse retina. PLoS One. 2014;9:e106819. doi: 10.1371/journal.pone.0106819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gustincich S, Contini M, Gariboldi M, et al. Gene discovery in genetically labeled single dopaminergic neurons of the retina. Proc Natl Acad Sci U S A. 2004;101:5069–5074. doi: 10.1073/pnas.0400913101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Witkovsky P. Dopamine and retinal function. Doc Ophthalmol. 2004;108:17–40. doi: 10.1023/b:doop.0000019487.88486.0a. [DOI] [PubMed] [Google Scholar]
- 73.Doyle SE, Grace MS, McIvor W, Menaker M. Circadian rhythms of dopamine in mouse retina: the role of melatonin. Vis Neurosci. 2002;19:593–601. doi: 10.1017/s0952523802195058. [DOI] [PubMed] [Google Scholar]
- 74.Baba K, Benleulmi-Chaachoua A, Journe AS, et al. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci Signal. 2013;6:ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yang Q, Xu Y, Xie P, et al. Retinal neurodegeneration in db/db mice at the early period of diabetes. J Ophthalmol. 2015;2015:757412. doi: 10.1155/2015/757412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Busik JV, Tikhonenko M, Bhatwadekar A, et al. Diabetic retinopathy is associated with bone marrow neuropathy and a depressed peripheral clock. J Exp Med. 2009;206:2897–2906. doi: 10.1084/jem.20090889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhatwadekar AD, Yan Y, Qi X, et al. Per2 mutation recapitulates the vascular phenotype of diabetes in the retina and bone marrow. Diabetes. 2013;62:273–282. doi: 10.2337/db12-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stitt AW, Lois N, Medina RJ, Adamson P, Curtis TM. Advances in our understanding of diabetic retinopathy. Clin Sci (Lond) 2013;125:1–17. doi: 10.1042/CS20120588. [DOI] [PubMed] [Google Scholar]
- 79.Aung MH, Park HN, Han MK, et al. Dopamine deficiency contributes to early visual dysfunction in a rodent model of type 1 diabetes. J Neurosci. 2014;34:726–736. doi: 10.1523/JNEUROSCI.3483-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kandel E. Principles of Neural Science 5th ed. New-York, NY: McGraw-Hill Professional; 2012. p. 1760. [Google Scholar]
- 81.Dowling JE. The Retina: An Approachable Part of the Brain. Revised Edition. Cambridge, MA: The Belknap Press of Harvard University Press;; 2012. p. 355. [Google Scholar]
- 82.Barlow R. Circadian and efferent modulation of visual sensitivity. Prog Brain Res. 2001;131:487–503. doi: 10.1016/s0079-6123(01)31039-7. [DOI] [PubMed] [Google Scholar]
- 83.Cahill GM, Besharse JC. Circadian rhythmicity in vertebrate retinas - regulation by a photoreceptor oscillator. Prog Retin Eye Res. 1995;14:267–291. [Google Scholar]
- 84.Green CB, Besharse JC. Retinal circadian clocks and control of retinal physiology. J Biol Rhythms. 2004;19:91–102. doi: 10.1177/0748730404263002. [DOI] [PubMed] [Google Scholar]
- 85.Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog Retin Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 86.Bloomfield SA, Volgyi B. The diverse functional roles and regulation of neuronal gap junctions in the retina. Nat Rev Neurosci. 2009;10:495–506. doi: 10.1038/nrn2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ribelayga CP, O'Brien J. Circadian and light-adaptive control of electrical synaptic plasticity in the vertebrate retina. In: Jing J, editor. Network Functions and Plasticity: Perspectives from Studying Electrical Coupling in Microcircuits. Cambridge: Elsevier;; 2017. pp. 209–241. [Google Scholar]
- 88.O'Brien J. The ever-changing electrical synapse. Curr Opin Neurobiol. 2014;29:64–72. doi: 10.1016/j.conb.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Choi HJ, Ribelayga CP, Mangel SC. Cut-loading: a useful tool for examining the extent of gap junction tracer coupling between retinal neurons. J Vis Exp. 2012;59:3180. doi: 10.3791/3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li H, Chuang AZ, O'Brien J. Photoreceptor coupling is controlled by connexin 35 phosphorylation in zebrafish retina. J Neurosci. 2009;29:15178–15186. doi: 10.1523/JNEUROSCI.3517-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Li H, Zhang Z, Blackburn MR, Wang SW, Ribelayga CP, O'Brien J. Adenosine and dopamine receptors coregulate photoreceptor coupling via gap junction phosphorylation in mouse retina. J Neurosci. 2013;33:3135–3150. doi: 10.1523/JNEUROSCI.2807-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ribelayga C, Cao Y, Mangel SC. The circadian clock in the retina controls rod-cone coupling. Neuron. 2008;59:790–801. doi: 10.1016/j.neuron.2008.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhang Z, Li H, Liu X, O'Brien J, Ribelayga CP. Circadian clock control of connexin36 phosphorylation in retinal photoreceptors of the CBA/CaJ mouse strain. Vis Neurosci. 2015;32:E009. doi: 10.1017/S0952523815000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Katti C, Butler R, Sekaran S. Diurnal and circadian regulation of connexin 36 transcript and protein in the mammalian retina. Invest Ophthalmol Vis Sci. 2013;54:821–829. doi: 10.1167/iovs.12-10375. [DOI] [PubMed] [Google Scholar]
- 95.Jin NG, Chuang AZ, Masson PJ, Ribelayga CP. Rod electrical coupling is controlled by a circadian clock and dopamine in mouse retina. J Physiol. 2015;593:1597–1631. doi: 10.1113/jphysiol.2014.284919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin NG, Ribelayga CP. Direct evidence for daily plasticity of electrical coupling between rod photoreceptors in the mammalian retina. J Neurosci. 2016;36:178–184. doi: 10.1523/JNEUROSCI.3301-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li H, Chuang AZ, O'Brien J. Regulation of photoreceptor gap junction phosphorylation by adenosine in zebrafish retina. Vis Neurosci. 2014;31:237–243. doi: 10.1017/S095252381300062X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ribelayga C, Mangel SC. A circadian clock and light/dark adaptation differentially regulate adenosine in the mammalian retina. J Neurosci. 2005;25:215–222. doi: 10.1523/JNEUROSCI.3138-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ribelayga C, Mangel SC. Absence of circadian clock regulation of horizontal cell gap junctional coupling reveals two dopamine systems in the goldfish retina. J Comp Neurol. 2003;467:243–253. doi: 10.1002/cne.10927. [DOI] [PubMed] [Google Scholar]
- 100.Ribelayga C, Mangel SC. Identification of a circadian clock-controlled neural pathway in the rabbit retina. PLoS One. 2010;5:e11020. doi: 10.1371/journal.pone.0011020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ribelayga C, Wang Y, Mangel SC. Dopamine mediates circadian clock regulation of rod and cone input to fish retinal horizontal cells. J Physiol. 2002;544:801–816. doi: 10.1113/jphysiol.2002.023671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ribelayga C, Wang Y, Mangel SC. A circadian clock in the fish retina regulates dopamine release via activation of melatonin receptors. J Physiol. 2004;554:467–482. doi: 10.1113/jphysiol.2003.053710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wang Y, Mangel SC. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci U S A. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mangel SC, Ribelayga C. Comparative eye: the circadian clock in the retina regulates rod and cone pathways. In: Dartt DA, Besharse JC, Dana R, editors. Encyclopedia of the Eye. Oxford: Elsevier;; 2010. pp. 283–289. [Google Scholar]
- 105.Fukuhara C, Dirden JC, Tosini G. Photic regulation of melatonin in rat retina and the role of proteasomal proteolysis. Neuroreport. 2001;12:3833–3837. doi: 10.1097/00001756-200112040-00046. [DOI] [PubMed] [Google Scholar]
- 106.Tosini G, Menaker M. The clock in the mouse retina: melatonin synthesis and photoreceptor degeneration. Brain Res. 1998;789:221–228. doi: 10.1016/s0006-8993(97)01446-7. [DOI] [PubMed] [Google Scholar]
- 107.Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog Retin Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 108.Tosini G, Baba K, Hwang CK, Iuvone PM. Melatonin: an underappreciated player in retinal physiology and pathophysiology. Exp Eye Res. 2012;103:82–89. doi: 10.1016/j.exer.2012.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sengupta A, Baba K, Mazzoni F, et al. Localization of melatonin receptor 1 in mouse retina and its role in the circadian regulation of the electroretinogram and dopamine levels. PLoS One. 2011;6:e24483. doi: 10.1371/journal.pone.0024483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xue Y, Shen SQ, Corbo JC, Kefalov VJ. Circadian and light-driven regulation of rod dark adaptation. Sci Rep. 2015;5:17616. doi: 10.1038/srep17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liang FQ, Aleman TS, ZaixinYang, Cideciyan AV, Jacobson SG, Bennett J. Melatonin delays photoreceptor degeneration in the rds/rds mouse. Neuroreport. 2001;12:1011–1014. doi: 10.1097/00001756-200104170-00029. [DOI] [PubMed] [Google Scholar]
- 112.Xu XJ, Wang SM, Jin Y, Hu YT, Feng K, Ma ZZ. Melatonin delays photoreceptor degeneration in a mouse model of autosomal recessive retinitis pigmentosa. J Pineal Res. 2017;63:e12428. doi: 10.1111/jpi.12428. [DOI] [PubMed] [Google Scholar]
- 113.Baba K, Pozdeyev N, Mazzoni F, et al. Melatonin modulates visual function and cell viability in the mouse retina via the MT1 melatonin receptor. Proc Natl Acad Sci U S A. 2009;106:15043–15048. doi: 10.1073/pnas.0904400106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gianesini C, Hiragaki S, Laurent V, Hicks D, Tosini G. Cone viability is affected by disruption of melatonin receptors signaling. Invest Ophthalmol Vis Sci. 2016;57:94–104. doi: 10.1167/iovs.15-18235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yi C, Pan X, Yan H, Guo M, Pierpaoli W. Effects of melatonin in age-related macular degeneration. Ann N Y Acad Sci. 2005;1057:384–392. doi: 10.1196/annals.1356.029. [DOI] [PubMed] [Google Scholar]
- 116.Lv XD, Liu S, Cao Z, et al. Correlation between serum melatonin and aMT6S level for age-related macular degeneration patients. Eur Rev Med Pharmacol Sci. 2016;20:4196–4201. [PubMed] [Google Scholar]
- 117.Rosen R, Hu DN, Perez V, et al. Urinary 6-sulfatoxymelatonin level in age-related macular degeneration patients. Mol Vis. 2009;15:1673–1679. [PMC free article] [PubMed] [Google Scholar]
- 118.Schmid-Kubista KE, Glittenberg CG, Cezanne M, Holzmann K, Neumaier-Ammerer B, Binder S. Daytime levels of melatonin in patients with age-related macular degeneration. Acta Ophthalmol. 2009;87:89–93. doi: 10.1111/j.1755-3768.2008.01173.x. [DOI] [PubMed] [Google Scholar]
- 119.Owens L, Buhr E, Tu DC, Lamprecht TL, Lee J, Van Gelder RN. Effect of circadian clock gene mutations on nonvisual photoreception in the mouse. Invest Ophthalmol Vis Sci. 2012;53:454–460. doi: 10.1167/iovs.11-8717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Ait-Hmyed O, Felder-Schmittbuhl MP, Garcia-Garrido M, et al. Mice lacking period 1 and period 2 circadian clock genes exhibit blue cone photoreceptor defects. Eur J Neurosci. 2013;37:1048–1060. doi: 10.1111/ejn.12103. [DOI] [PubMed] [Google Scholar]
- 121.Sawant OB, Horton AM, Zucaro OF, et al. The circadian clock gene Bmal1 controls thyroid hormone-mediated spectral identity and cone photoreceptor function. Cell Rep. 2017;21:692–706. doi: 10.1016/j.celrep.2017.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hsu DS, Zhao X, Zhao S, et al. Putative human blue-light photoreceptors hCRY1 and hCRY2 are flavoproteins. Biochemistry. 1996;35:13871–13877. doi: 10.1021/bi962209o. [DOI] [PubMed] [Google Scholar]
- 123.Sancar A. Cryptochrome: the second photoactive pigment in the eye and its role in circadian photoreception. Annu Rev Biochem. 2000;69:31–67. doi: 10.1146/annurev.biochem.69.1.31. [DOI] [PubMed] [Google Scholar]
- 124.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 125.Hattar S, Liao HW, Takao M, Berson DM, Yau KW. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sekaran S, Foster RG, Lucas RJ, Hankins MW. Calcium imaging reveals a network of intrinsically light-sensitive inner-retinal neurons. Curr Biol. 2003;13:1290–1298. doi: 10.1016/s0960-9822(03)00510-4. [DOI] [PubMed] [Google Scholar]
- 128.Hattar S, Lucas RJ, Mrosovsky N, et al. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 130.Panda S, Nayak SK, Campo B, Walker JR, Hogenesch JB, Jegla T. Illumination of the melanopsin signaling pathway. Science. 2005;307:600–604. doi: 10.1126/science.1105121. [DOI] [PubMed] [Google Scholar]
- 131.Qiu X, Kumbalasiri T, Carlson SM, et al. Induction of photosensitivity by heterologous expression of melanopsin. Nature. 2005;433:745–749. doi: 10.1038/nature03345. [DOI] [PubMed] [Google Scholar]
- 132.Selby CP, Thompson C, Schmitz TM, Van Gelder RN, Sancar A. Functional redundancy of cryptochromes and classical photoreceptors for nonvisual ocular photoreception in mice. Proc Natl Acad Sci U S A. 2000;97:14697–14702. doi: 10.1073/pnas.260498597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Thresher RJ, Vitaterna MH, Miyamoto Y, et al. Role of mouse cryptochrome blue-light photoreceptor in circadian photoresponses. Science. 1998;282:1490–1494. doi: 10.1126/science.282.5393.1490. [DOI] [PubMed] [Google Scholar]
- 134.Van Gelder RN, Wee R, Lee JA, Tu DC. Reduced pupillary light responses in mice lacking cryptochromes. Science. 2003;299:222. doi: 10.1126/science.1079536. [DOI] [PubMed] [Google Scholar]
- 135.Vitaterna MH, Selby CP, Todo T, et al. Differential regulation of mammalian period genes and circadian rhythmicity by cryptochromes 1 and 2. Proc Natl Acad Sci U S A. 1999;96:12114–12119. doi: 10.1073/pnas.96.21.12114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.van der Horst GT, Muijtjens M, Kobayashi K, et al. Mammalian Cry1 and Cry2 are essential for maintenance of circadian rhythms. Nature. 1999;398:627–630. doi: 10.1038/19323. [DOI] [PubMed] [Google Scholar]
- 137.Cameron MA, Barnard AR, Hut RA, et al. Electroretinography of wild-type and Cry mutant mice reveals circadian tuning of photopic and mesopic retinal responses. J Biol Rhythms. 2008;23:489–501. doi: 10.1177/0748730408325874. [DOI] [PubMed] [Google Scholar]
- 138.Hwang CK, Chaurasia SS, Jackson CR, Chan GC, Storm DR, Iuvone PM. Circadian rhythm of contrast sensitivity is regulated by a dopamine-neuronal PAS-domain protein 2-adenylyl cyclase 1 signaling pathway in retinal ganglion cells. J Neurosci. 2013;33:14989–14997. doi: 10.1523/JNEUROSCI.2039-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ko GY, Ko ML, Dryer SE. Circadian regulation of cGMP-gated cationic channels of chick retinal cones. Erk MAP Kinase and Ca2+/calmodulin-dependent protein kinase II. Neuron. 2001;29:255–266. doi: 10.1016/s0896-6273(01)00195-7. [DOI] [PubMed] [Google Scholar]
- 140.Preitner N, Damiola F, Lopez-Molina L, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 141.Cheng H, Khanna H, Oh EC, Hicks D, Mitton KP, Swaroop A. Photoreceptor-specific nuclear receptor NR2E3 functions as a transcriptional activator in rod photoreceptors. Hum Mol Genet. 2004;13:1563–1575. doi: 10.1093/hmg/ddh173. [DOI] [PubMed] [Google Scholar]
- 142.Ait-Hmyed Hakkari O, Acar N, Savier E, et al. Rev-Erbalpha modulates retinal visual processing and behavioral responses to light. FASEB J. 2016;30:3690–3701. doi: 10.1096/fj.201600414R. [DOI] [PubMed] [Google Scholar]
- 143.Boudard DL, Mendoza J, Hicks D. Loss of photic entrainment at low illuminances in rats with acute photoreceptor degeneration. Eur J Neurosci. 2009;30:1527–1536. doi: 10.1111/j.1460-9568.2009.06935.x. [DOI] [PubMed] [Google Scholar]
- 144.Dkhissi-Benyahya O, Gronfier C, De Vanssay W, Flamant F, Cooper HM. Modeling the role of mid-wavelength cones in circadian responses to light. Neuron. 2007;53:677–687. doi: 10.1016/j.neuron.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen SK, Chew KS, McNeill DS, et al. Apoptosis regulates ipRGC spacing necessary for rods and cones to drive circadian photoentrainment. Neuron. 2013;77:503–515. doi: 10.1016/j.neuron.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hannibal J, Georg B, Hindersson P, Fahrenkrug J. Light and darkness regulate melanopsin in the retinal ganglion cells of the albino Wistar rat. J Mol Neurosci. 2005;27:147–155. doi: 10.1385/JMN:27:2:147. [DOI] [PubMed] [Google Scholar]
- 147.Sakamoto K, Liu C, Tosini G. Classical photoreceptors regulate melanopsin mRNA levels in the rat retina. J Neurosci. 2004;24:9693–9697. doi: 10.1523/JNEUROSCI.2556-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Besharse JC, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- 149.Robertson LM, Takahashi JS. Circadian clock in cell culture: II. In vitro photic entrainment of melatonin oscillation from dissociated chick pineal cells. J Neurosci. 1988;8:22–30. doi: 10.1523/JNEUROSCI.08-01-00022.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cahill GM, Besharse JC. Circadian clock functions localized in xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 151.Menaker M, Wisner S. Temperature-compensated circadian clock in the pineal of Anolis. Proc Natl Acad Sci U S A. 1983;80:6119–6121. doi: 10.1073/pnas.80.19.6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Teirstein PS, Goldman AI, O'Brien PJ. Evidence for both local and central regulation of rat rod outer segment disc shedding. Invest Ophthalmol Vis Sci. 1980;19:1268–1273. [PubMed] [Google Scholar]
- 153.Buhr ED, Van Gelder RN. Local photic entrainment of the retinal circadian oscillator in the absence of rods, cones, and melanopsin. Proc Natl Acad Sci U S A. 2014;111:8625–8630. doi: 10.1073/pnas.1323350111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Doyle SE, McIvor WE, Menaker M. Circadian rhythmicity in dopamine content of mammalian retina: role of the photoreceptors. J Neurochem. 2002;83:211–219. doi: 10.1046/j.1471-4159.2002.01149.x. [DOI] [PubMed] [Google Scholar]
- 156.Iuvone PM, Galli CL, Garrison-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- 157.Nir I, Harrison JM, Haque R, et al. Dysfunctional light-evoked regulation of cAMP in photoreceptors and abnormal retinal adaptation in mice lacking dopamine D4 receptors. J Neurosci. 2002;22:2063–2073. doi: 10.1523/JNEUROSCI.22-06-02063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Pozdeyev N, Tosini G, Li L, et al. Dopamine modulates diurnal and circadian rhythms of protein phosphorylation in photoreceptor cells of mouse retina. Eur J Neurosci. 2008;27:2691–2700. doi: 10.1111/j.1460-9568.2008.06224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Iuvone PM, Brown AD, Haque R, et al. Retinal melatonin production: role of proteasomal proteolysis in circadian and photic control of arylalkylamine N-acetyltransferase. Invest Ophthalmol Vis Sci. 2002;43:564–572. [PubMed] [Google Scholar]
- 160.Klein DC, Coon SL, Roseboom PH, et al. The melatonin rhythm-generating enzyme: molecular regulation of serotonin N-acetyltransferase in the pineal gland. Recent Prog Horm Res. 1997;52:307–357. discussion, 357–358. [PubMed] [Google Scholar]
- 161.Dinet V, Ansari N, Torres-Farfan C, Korf HW. Clock gene expression in the retina of melatonin-proficient (C3H) and melatonin-deficient (C57BL) mice. J Pineal Res. 2007;42:83–91. doi: 10.1111/j.1600-079X.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 162.Dinet V, Korf HW. Impact of melatonin receptors on pCREB and clock-gene protein levels in the murine retina. Cell Tissue Res. 2007;330:29–34. doi: 10.1007/s00441-007-0468-5. [DOI] [PubMed] [Google Scholar]
- 163.Sakamoto K, Oishi K, Shiraishi M, et al. Two circadian oscillatory mechanisms in the mammalian retina. Neuroreport. 2000;11:3995–3997. doi: 10.1097/00001756-200012180-00018. [DOI] [PubMed] [Google Scholar]
- 164.Sakamoto K, Liu C, Tosini G. Circadian rhythms in the retina of rats with photoreceptor degeneration. J Neurochem. 2004;90:1019–1024. doi: 10.1111/j.1471-4159.2004.02571.x. [DOI] [PubMed] [Google Scholar]
- 165.Goto M, Oshima I, Tomita T, Ebihara S. Melatonin content of the pineal gland in different mouse strains. J Pineal Res. 1989;7:195–204. doi: 10.1111/j.1600-079x.1989.tb00667.x. [DOI] [PubMed] [Google Scholar]
- 166.Roseboom PH, Namboodiri MA, Zimonjic DB, et al. Natural melatonin ‘knockdown' in C57BL/6J mice: rare mechanism truncates serotonin N-acetyltransferase. Brain Res Mol Brain Res. 1998;63:189–197. doi: 10.1016/s0169-328x(98)00273-3. [DOI] [PubMed] [Google Scholar]
- 167.Gianesini C, Clesse D, Tosini G, Hicks D, Laurent V. Unique regulation of the melatonin synthetic pathway in the retina of diurnal female Arvicanthis ansorgei (Rodentia) Endocrinology. 2015;156:3292–3308. doi: 10.1210/EN.2015-1267. [DOI] [PubMed] [Google Scholar]
- 168.Coon SL, Del Olmo E, Young WS, III,, Klein DC. Melatonin synthesis enzymes in Macaca mulatta: focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87) J Clin Endocrinol Metab. 2002;87:4699–4706. doi: 10.1210/jc.2002-020683. [DOI] [PubMed] [Google Scholar]
- 169.Buhr ED, Yue WW, Ren X, et al. Neuropsin (OPN5)-mediated photoentrainment of local circadian oscillators in mammalian retina and cornea. Proc Natl Acad Sci U S A. 2015;112:13093–13098. doi: 10.1073/pnas.1516259112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cameron MA, Pozdeyev N, Vugler AA, Cooper H, Iuvone PM, Lucas RJ. Light regulation of retinal dopamine that is independent of melanopsin phototransduction. Eur J Neurosci. 2009;29:761–767. doi: 10.1111/j.1460-9568.2009.06631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang DQ, Wong KY, Sollars PJ, Berson DM, Pickard GE, McMahon DG. Intraretinal signaling by ganglion cell photoreceptors to dopaminergic amacrine neurons. Proc Natl Acad Sci U S A. 2008;105:14181–14186. doi: 10.1073/pnas.0803893105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhao X, Wong KY, Zhang DQ. Mapping physiological inputs from multiple photoreceptor systems to dopaminergic amacrine cells in the mouse retina. Sci Rep. 2017;7:7920. doi: 10.1038/s41598-017-08172-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Joo HR, Peterson BB, Dacey DM, Hattar S, Chen SK. Recurrent axon collaterals of intrinsically photosensitive retinal ganglion cells. Vis Neurosci. 2013;30:175–182. doi: 10.1017/S0952523813000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Prigge CL, Yeh PT, Liou NF, et al. M1 ipRGCs influence visual function through retrograde signaling in the retina. J Neurosci. 2016;36:7184–7197. doi: 10.1523/JNEUROSCI.3500-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reifler AN, Chervenak AP, Dolikian ME, et al. All spiking, sustained ON displaced amacrine cells receive gap-junction input from melanopsin ganglion cells. Curr Biol. 2015;25:2878. doi: 10.1016/j.cub.2015.10.039. [DOI] [PubMed] [Google Scholar]
- 176.Wong KY, Dunn FA, Graham DM, Berson DM. Synaptic influences on rat ganglion-cell photoreceptors. J Physiol. 2007;582:279–296. doi: 10.1113/jphysiol.2007.133751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhang DQ, Belenky MA, Sollars PJ, Pickard GE, McMahon DG. Melanopsin mediates retrograde visual signaling in the retina. PLoS One. 2012;7:e42647. doi: 10.1371/journal.pone.0042647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kojima D, Mori S, Torii M, Wada A, Morishita R, Fukada Y. UV-sensitive photoreceptor protein OPN5 in humans and mice. PLoS One. 2011;6:e26388. doi: 10.1371/journal.pone.0026388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tarttelin EE, Bellingham J, Hankins MW, Foster RG, Lucas RJ. Neuropsin (Opn5): a novel opsin identified in mammalian neural tissue. FEBS Lett. 2003;554:410–416. doi: 10.1016/s0014-5793(03)01212-2. [DOI] [PubMed] [Google Scholar]
- 180.Nakane Y, Ikegami K, Ono H, et al. A mammalian neural tissue opsin (Opsin 5) is a deep brain photoreceptor in birds. Proc Natl Acad Sci U S A. 2010;107:15264–15268. doi: 10.1073/pnas.1006393107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Nakane Y, Shimmura T, Abe H, Yoshimura T. Intrinsic photosensitivity of a deep brain photoreceptor. Curr Biol. 2014;24:R596–R597. doi: 10.1016/j.cub.2014.05.038. [DOI] [PubMed] [Google Scholar]
- 182.Yamashita T, Ohuchi H, Tomonari S, Ikeda K, Sakai K, Shichida Y. Opn5 is a UV-sensitive bistable pigment that couples with Gi subtype of G protein. Proc Natl Acad Sci U S A. 2010;107:22084–22089. doi: 10.1073/pnas.1012498107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nieto PS, Valdez DJ, Acosta-Rodriguez VA, Guido ME. Expression of novel opsins and intrinsic light responses in the mammalian retinal ganglion cell line RGC-5. Presence of OPN5 in the rat retina. PLoS One. 2011;6:e26417. doi: 10.1371/journal.pone.0026417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yamashita T, Ono K, Ohuchi H, et al. Evolution of mammalian Opn5 as a specialized UV-absorbing pigment by a single amino acid mutation. J Biol Chem. 2014;289:3991–4000. doi: 10.1074/jbc.M113.514075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Henriksson JT, Bergmanson JP, Walsh JE. Ultraviolet radiation transmittance of the mouse eye and its individual media components. Exp Eye Res. 2010;90:382–387. doi: 10.1016/j.exer.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 186.Boettner EA WJ. Transmission of the ocular media. Invest Ophthalmol Vis Sci. 1962;1:776–783. [Google Scholar]
- 187.Najjar RP, Teikari P, Cornut PL, Knoblauch K, Cooper HM, Gronfier C. Heterochromatic flicker photometry for objective lens density quantification. Invest Ophthalmol Vis Sci. 2016;57:1063–1071. doi: 10.1167/iovs.15-18642. [DOI] [PubMed] [Google Scholar]
- 188.LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- 189.Bok D. The retinal pigment epithelium: a versatile partner in vision. J Cell Sci Suppl. 1993;17:189–195. doi: 10.1242/jcs.1993.supplement_17.27. [DOI] [PubMed] [Google Scholar]
- 190.Nguyen-Legros J, Hicks D. Renewal of photoreceptor outer segments and their phagocytosis by the retinal pigment epithelium. Int Rev Cytol. 2000;196:245–313. doi: 10.1016/s0074-7696(00)96006-6. [DOI] [PubMed] [Google Scholar]
- 191.Bok D, Hall MO. The role of the pigment epithelium in the etiology of inherited retinal dystrophy in the rat. J Cell Biol. 1971;49:664–682. doi: 10.1083/jcb.49.3.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Besharse JC, Hollyfield JG, Rayborn ME. Turnover of rod photoreceptor outer segments. II. Membrane addition and loss in relationship to light. J Cell Biol. 1977;75:507–527. doi: 10.1083/jcb.75.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Long KO, Fisher SK, Fariss RN, Anderson DH. Disc shedding and autophagy in the cone-dominant ground squirrel retina. Exp Eye Res. 1986;43:193–205. doi: 10.1016/s0014-4835(86)80087-2. [DOI] [PubMed] [Google Scholar]
- 194.Tabor GA, Fisher SK, Anderson DH. Rod and cone disc shedding in light-entrained tree squirrels. Exp Eye Res. 1980;30:545–557. doi: 10.1016/0014-4835(80)90039-1. [DOI] [PubMed] [Google Scholar]
- 195.Young RW. The daily rhythm of shedding and degradation of rod and cone outer segment membranes in the chick retina. Invest Ophthalmol Vis Sci. 1978;17:105–116. [PubMed] [Google Scholar]
- 196.Bobu C, Craft CM, Masson-Pevet M, Hicks D. Photoreceptor organization and rhythmic phagocytosis in the nile rat Arvicanthis ansorgei: a novel diurnal rodent model for the study of cone pathophysiology. Invest Ophthalmol Vis Sci. 2006;47:3109–3118. doi: 10.1167/iovs.05-1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Grace MS, Wang LM, Pickard GE, Besharse JC, Menaker M. The tau mutation shortens the period of rhythmic photoreceptor outer segment disk shedding in the hamster. Brain Res. 1996;735:93–100. doi: 10.1016/0006-8993(96)00600-2. [DOI] [PubMed] [Google Scholar]
- 198.Terman JS, Reme CE, Terman M. Rod outer segment disk shedding in rats with lesions of the suprachiasmatic nucleus. Brain Res. 1993;605:256–264. doi: 10.1016/0006-8993(93)91748-h. [DOI] [PubMed] [Google Scholar]
- 199.Ruggiero L, Connor MP, Chen J, Langen R, Finnemann SC. Diurnal, localized exposure of phosphatidylserine by rod outer segment tips in wild-type but not Itgb5-/- or Mfge8-/- mouse retina. Proc Natl Acad Sci U S A. 2012;109:8145–8148. doi: 10.1073/pnas.1121101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Finnemann SC. Focal adhesion kinase signaling promotes phagocytosis of integrin-bound photoreceptors. EMBO J. 2003;22:4143–4154. doi: 10.1093/emboj/cdg416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Nandrot EF, Kim Y, Brodie SE, Huang X, Sheppard D, Finnemann SC. Loss of synchronized retinal phagocytosis and age-related blindness in mice lacking alphavbeta5 integrin. J Exp Med. 2004;200:1539–1545. doi: 10.1084/jem.20041447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mustafi D, Kevany BM, Genoud C, Bai X, Palczewski K. Photoreceptor phagocytosis is mediated by phosphoinositide signaling. FASEB J. 2013;27:4585–4595. doi: 10.1096/fj.13-237537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Baba K, Sengupta A, Tosini M, Contreras-Alcantara S, Tosini G. Circadian regulation of the PERIOD 2::LUCIFERASE bioluminescence rhythm in the mouse retinal pigment epithelium-choroid. Mol Vis. 2010;16:2605–2611. [PMC free article] [PubMed] [Google Scholar]
- 204.Baba K, DeBruyne JP, Tosini G. Dopamine 2 receptor activation entrains circadian clocks in mouse retinal pigment epithelium. Sci Rep. 2017;7:5103. doi: 10.1038/s41598-017-05394-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Ikarashi R, Akechi H, Kanda Y, et al. Regulation of molecular clock oscillations and phagocytic activity via muscarinic Ca(2+) signaling in human retinal pigment epithelial cells. Sci Rep. 2017;7:44175. doi: 10.1038/srep44175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Laurent V, Sengupta A, Sanchez-Bretano A, Hicks D, Tosini G. Melatonin signaling affects the timing in the daily rhythm of phagocytic activity by the retinal pigment epithelium. Exp Eye Res. 2017;165:90–95. doi: 10.1016/j.exer.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Aihara M, Lindsey JD, Weinreb RN. Twenty-four-hour pattern of mouse intraocular pressure. Exp Eye Res. 2003;77:681–686. doi: 10.1016/j.exer.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 208.Moore CG, Johnson EC, Morrison JC. Circadian rhythm of intraocular pressure in the rat. Curr Eye Res. 1996;15:185–191. doi: 10.3109/02713689608997412. [DOI] [PubMed] [Google Scholar]
- 209.Nickla DL, Wildsoet C, Wallman J. The circadian rhythm in intraocular pressure and its relation to diurnal ocular growth changes in chicks. Exp Eye Res. 1998;66:183–193. doi: 10.1006/exer.1997.0425. [DOI] [PubMed] [Google Scholar]
- 210.Rowland JM, Potter DE, Reiter RJ. Circadian rhythm in intraocular pressure: a rabbit model. Curr Eye Res. 1981;1:169–173. doi: 10.3109/02713688109001822. [DOI] [PubMed] [Google Scholar]
- 211.Braslow RA, Gregory DS. Adrenergic decentralization modifies the circadian rhythm of intraocular pressure. Invest Ophthalmol Vis Sci. 1987;28:1730–1732. [PubMed] [Google Scholar]
- 212.Liu JH, Dacus AC, Bartels SP. Adrenergic mechanism in circadian elevation of intraocular pressure in rabbits. Invest Ophthalmol Vis Sci. 1991;32:2178–2183. [PubMed] [Google Scholar]
- 213.Yoshitomi T, Gregory DS. Ocular adrenergic nerves contribute to control of the circadian rhythm of aqueous flow in rabbits. Invest Ophthalmol Vis Sci. 1991;32:523–528. [PubMed] [Google Scholar]
- 214.Alarma-Estrany P, Crooke A, Mediero A, Pelaez T, Pintor J. Sympathetic nervous system modulates the ocular hypotensive action of MT2-melatonin receptors in normotensive rabbits. J Pineal Res. 2008;45:468–475. doi: 10.1111/j.1600-079X.2008.00618.x. [DOI] [PubMed] [Google Scholar]
- 215.Alcantara-Contreras S, Baba K, Tosini G. Removal of melatonin receptor type 1 increases intraocular pressure and retinal ganglion cells death in the mouse. Neurosci Lett. 2011;494:61–64. doi: 10.1016/j.neulet.2011.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Osborne NN, Chidlow G. The presence of functional melatonin receptors in the iris-ciliary processes of the rabbit eye. Exp Eye Res. 1994;59:3–9. doi: 10.1006/exer.1994.1076. [DOI] [PubMed] [Google Scholar]
- 217.Pintor J, Martin L, Pelaez T, Hoyle CH, Peral A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur J Pharmacol. 2001;416:251–254. doi: 10.1016/s0014-2999(01)00864-0. [DOI] [PubMed] [Google Scholar]
- 218.Wiechmann AF, Wirsig-Wiechmann CR. Melatonin receptor mRNA and protein expression in Xenopus laevis nonpigmented ciliary epithelial cells. Exp Eye Res. 2001;73:617–623. doi: 10.1006/exer.2001.1073. [DOI] [PubMed] [Google Scholar]
- 219.Maeda A, Tsujiya S, Higashide T, et al. Circadian intraocular pressure rhythm is generated by clock genes. Invest Ophthalmol Vis Sci. 2006;47:4050–4052. doi: 10.1167/iovs.06-0183. [DOI] [PubMed] [Google Scholar]
- 220.Dalvin LA, Fautsch MP. Analysis of circadian rhythm gene expression with reference to diurnal pattern of intraocular pressure in mice. Invest Ophthalmol Vis Sci. 2015;56:2657–2663. doi: 10.1167/iovs.15-16449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Stone RA, Pardue MT, Iuvone PM, Khurana TS. Pharmacology of myopia and potential role for intrinsic retinal circadian rhythms. Exp Eye Res. 2013;114:35–47. doi: 10.1016/j.exer.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Doughty MJ. Morphometric analysis of the surface cells of rabbit corneal epithelium by scanning electron microscopy. Am J Anat. 1990;189:316–328. doi: 10.1002/aja.1001890404. [DOI] [PubMed] [Google Scholar]
- 223.Sandvig KU, Haaskjold E, Refsum SB. Time dependency in the regenerative response to injury of the rat corneal epithelium. Chronobiol Int. 1994;11:173–179. doi: 10.3109/07420529409057237. [DOI] [PubMed] [Google Scholar]
- 224.Baba K, Davidson AJ, Tosini G. Melatonin entrains PER2::LUC bioluminescence circadian rhythm in the mouse cornea. Invest Ophthalmol Vis Sci. 2015;56:4753–4758. doi: 10.1167/iovs.15-17124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Evans JA, Suen TC, Callif BL, et al. Shell neurons of the master circadian clock coordinate the phase of tissue clocks throughout the brain and body. BMC Biol. 2015;13:43. doi: 10.1186/s12915-015-0157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Song F, Xue Y, Dong D, et al. Insulin restores an altered corneal epithelium circadian rhythm in mice with streptozotocin-induced type 1 diabetes. Sci Rep. 2016;6:32871. doi: 10.1038/srep32871. [DOI] [PMC free article] [PubMed] [Google Scholar]