Supplemental Digital Content is available in the text
Keywords: chemotherapy regimen, CLAG, overall survival, refractory or relapsed acute myeloid leukemia (R/R AML), treatment response
Abstract
To assess treatment response and overall survival (OS) in refractory or relapsed acute myeloid leukemia (R/R AML) patients treated by different common salvage chemotherapy regimens.
Medical records data from 142 R/R AML patients were reviewed in this retrospective study. Patients were treated with regimens based on the following drugs: cytarabine, granulocyte colony-stimulating factor (G-CSF), and fludarabine (FLAG) (n = 46); cytarabine and G-CSF in addition to aclarubicin or daunorubicin (CAG/DAG) (n = 30); cytarabine, G-CSF, and cladribine (CLAG) (n = 27); cytarabine, etoposide, and mitoxantrone (MEA) (n = 17); cytarabine plus idarubicin, daunorubicin, or mitoxantrone (IA/DA/MA) (n = 12); and homoharringtonine, cytarabine, and aclarubicin or daunorubicin (HAA/HAD) (n = 10).
A total of 43 (35.2%) patients achieved complete remission (CR), 60 (49.2%) patients achieved overall remission rate (ORR), and 18 (14.8%) patients received allogeneic hematopoietic stem cell transplantation (allo-HSCT) after CR. Median OS was 8.0 (95% CI 6.6–9.4) months with a 1-year OS rate of (29.9 ± 3.9)% and 3-year OS rate of (11.1 ± 3.6)%. No difference of CR (P = .621), ORR (P = .385), and allo-HSCT (P = .537) achievement was observed among different chemotherapy regimens. Interestingly, we observed that the CLAG-based regimen did not affect CR (P = .165), while it achieved a numerically higher ORR (P = .093) and was an independent factor for prolonged OS (P = .016). No other regimens were determined to be correlated with CR, ORR, or OS.
FLAG-, CAG/DAG-, CLAG-, MEA-, IA/DA/MA- and HAA/HAD-based regimens were found to achieve similar CR rates, while the CLAG-based regimen achieved numerically higher ORR rates and significant favorable OS. Therefore, CLAG-based regimens should be a prioritized treatment option for R/R AML patients.
1. Introduction
Acute myeloid leukemia (AML) is a form of cancer deriving from myeloid progenitor cells that has a rapid clinical course and infiltrates the bone marrow (BM), blood, and other tissues. AML affects approximately 19,950 individuals and led to 10,430 deaths in the United States in 2016.[1–3] Patients with AML who fail to respond to initial therapy (refractory AML) account for one quarter of AML patients. In addition, approximately 50% of patients that initially responded to treatment relapsed after a transient remission (relapsed AML). These patients with refractory or relapsed AML (R/R AML) have poor outcomes with a median survival of less than 6 months and a no more than 10% 3-year overall survival (OS) rate.[4–7] In light of the poor survival reported by previous studies, continued efforts to explore effective therapies is of great importance in R/R AML.
Cytarabine plays a crucial role in treating R/R AML patients, and it acts as a primary component of numerous chemotherapy regimens.[8] Once cytarabine enters into leukemic cells, it forms a triphosphate product (Ara-CTP), which inhibits DNA polymerase and ultimately induces apoptosis by terminating the chain elongation of DNA.[9] Intensive chemotherapy regimens, which are often composed of a combination of cytarabine with other cytotoxic agents or hematopoietic growth factors, are used as salvage therapies for R/R AML.[10,11] The commonly used salvage chemotherapy regimens include: regimens based on cytarabine, granulocyte colony-stimulating factor (G-CSF), and fludarabine (FLAG); regimens based on cytarabine and G-CSF in addition to aclarubicin or daunorubicin (CAG/DAG); regimens based on cytarabine, G-CSF, and cladribine (CLAG); regimens based on cytarabine, etoposide, and mitoxantrone (MEA); regimens based on cytarabine in addition to Idarubicin, daunorubicin, or mitoxantrone (IA/DA/MA); and regimens based on homoharringtonine, cytarabine, and aclarubicin or daunorubicin (HAA/HAD). All of these chemotherapy regimens are recommended by the 2017 Guidelines for Diagnosis and Treatment of Acute Myelogenous Leukemia (relapse/refractory) in China.[12] However, it is unclear if outcomes differ among these salvage chemotherapy regimens for R/R AML patients, and there is still no standard prioritized option. We therefore conducted this retrospective research into 142 R/R AML patients who were treated with several chemotherapies, including FLAG-, CAG/DAG-, CLAG-, MEA-, IA/DA/MA-, and HAA/HAD-based regimens, to assess the treatment response and OS by these regimens and to further explore the difference of outcomes among them.
2. Materials and methods
2.1. Patients
Medical records data from 142 R/R AML patients receiving treatment at Xiangyang Central Hospital between January 2013 and December 2016 were retrospectively reviewed and analyzed in this study. The screening criteria were as follows: diagnosed with AML confirmed by morphology, immunology, cytogenetics, and molecular biology; relapsed or refractory disease; received common salvage chemotherapy, including FLAG-, CAG/DAG-, CLAG-, MEA-, IA/DA/MA-, or HAA/HAD-based treatment regimens; and complete data for baseline features and treatment outcome information. In addition, if multiple lines of salvage therapies were used, only the 1st-line salvage therapy at our hospital was included and analyzed, which meant that no patients were included more than once.
This study was approved by the Ethics Committee of Xiangyang Central Hospital, the Affiliated Hospital of Hubei University of Arts and Science. Written informed consent or oral agreement by phone (with record) was received from each patient or family member.
2.2. Data collection
Baseline characteristics, salvage treatment regimen, and treatment outcomes were collected. Baseline characteristics included age, gender, disease status, de novo or secondary disease, risk stratification, Eastern Cooperative Oncology Group (ECOG) performance, BM blasts, complete remission (CR) status at first induction, previous allogeneic hematopoietic stem-cell transplantation (allo-HSCT), and lines of salvage therapy. The salvage treatment regimens were as follows:
-
(1)
an FLAG-based regimen of 30 mg/m2/day FLAG (days 1–5), 1 to 2 g/m2/day cytarabine (days 1–5), and 300 μg/m2/day G-CSF (days 0–5), with or without 10 mg/m2/day idarubicin for 3 days;
-
(2)
a CAG/DAG-based regimen, with a CAG regimen of 20 mg/day aclarubicin (days 1–4), 15 to 20 mg/m2/12 hour cytarabine (days 1–14) and 150 μg/m2/12 hour G-CSF (days 1–14), or a DAG regimen of 40 mg/m2/day daunorubicin (days 1–3), 15–20 mg/m2/12 hour cytarabine (days 1–7 or days 1–10), 300 μg/day G-CSF (days 1–7 or 1–10), with or without 20 mg/m2/day decitabine for 3 days;
-
(3)
a CLAG-based regimen of 5 mg/m2/day cladribine (days 1–5); 1 to 2 g/m2/day cytarabine (days 1–5) and 300 μg/m2/day G-CSF (days 0–5), with or without 10 mg/m2/day mitoxantrone (days 1–3);
-
(4)
an MEA-based regimen of mitoxantrone 10 mg/m2/day (days 1–5), etoposide 100 mg/m2/day (days 1–5), and cytarabine 100 to 150 mg/m2/day (days 1–7);
-
(5)
an IA/DA/MA-based regimen, with an IA regimen of 8 to 18 mg/m2/day idarubicin (days 1–3), 100 mg/m2/day cytarabine (days 1–7), a DA regimen of 45 to 60 mg/m2/day daunorubicin (days 1–3), 100 mg/m2/day cytarabine (days 1–7), or an MA regimen of 8 mg/m2/day mitoxantrone (days 1–3), 100 mg/m2/day cytarabine (days 1–7); and
-
(6)
an HAA/HAD-based regimen, with an HAA regimen of 2 mg/m2/day homoharringtonine (days 1–7), 100 to 200 mg/m2 cytarabine (days 1–7) and 20 mg/m2/day aclarubicin (days 1–7), or an HAD regimen of 2 mg/m2/day homoharringtonine (days 1–7), 100–200 mg/m2 cytarabine (days 1–7), and 40 mg/m2/day daunorubicin (days 1–7).
2.3. Definitions
CR was defined as BM with at least 20% cellularity and BM blasts <5% at steady state after chemotherapy, without cytological evidence of leukemia, no transfusion requirement, leucocyte count above 1 × 109/L and platelet count above 100 × 109/L, and without extramedullary disease. Partial remission (PR) was defined as either BM blasts 5% to 25%, or a 50% or better decrease in BM blasts, or BM blasts <5% but with Auer rods’ presence. Overall remission rate (ORR) was defined as patients with CR and PR. Refractory AML is defined as: the patient does not achieve CR after 2 courses of induction chemotherapy following a standard protocol; the patient relapses within 6 months after first CR; the patient relapses at 6 months or beyond after first CR and does not respond to any subsequent induction chemotherapy; the patient relapses more than 2 times; and the patient experiences extra-medullary infiltration of their leukemia. Relapsed AML is defined as leukemic cells reappear in peripheral blood or a percentage of BM blasts of above 10% with extra-medullary infiltration of the leukemia. The criteria for AML risk stratification were the NCCN Guidelines (NCCN Guidelines 2012 Acute Myeloid Leukemia) based on patients’ cytogenetics and molecular abnormalities. As to the time of risk stratification, there were 2 situations: for patients first diagnosed at our hospital, the risk stratification was applied at the diagnosis; for patients who are first diagnosed in other hospitals and presented with relapsed disease or failure to therapy in our hospital, the risk stratification was applied at the relapse or failure in our hospital.
2.4. Statistics
Statistical analysis was performed using SPSS 21.0 software (IBM, New York). Data were mainly presented as mean value ± standard deviation, medians with 1/4 and 3/4 quartiles, or count (percentage). Kaplan–Meier curves and log-rank test were used to evaluate OS. Univariate logistic regression analysis was performed to assess baseline factors and chemotherapy regimens predicting CR and ORR, and univariate Cox proportional hazard regression was used to evaluate predictive factors for OS. All factors with P value <.1 in the univariate logistic regression and the Cox regression were further analyzed by multivariate logistic regression and multiple Cox proportional hazard regression. P value <.05 was considered significant.
3. Results
3.1. Baseline characteristics
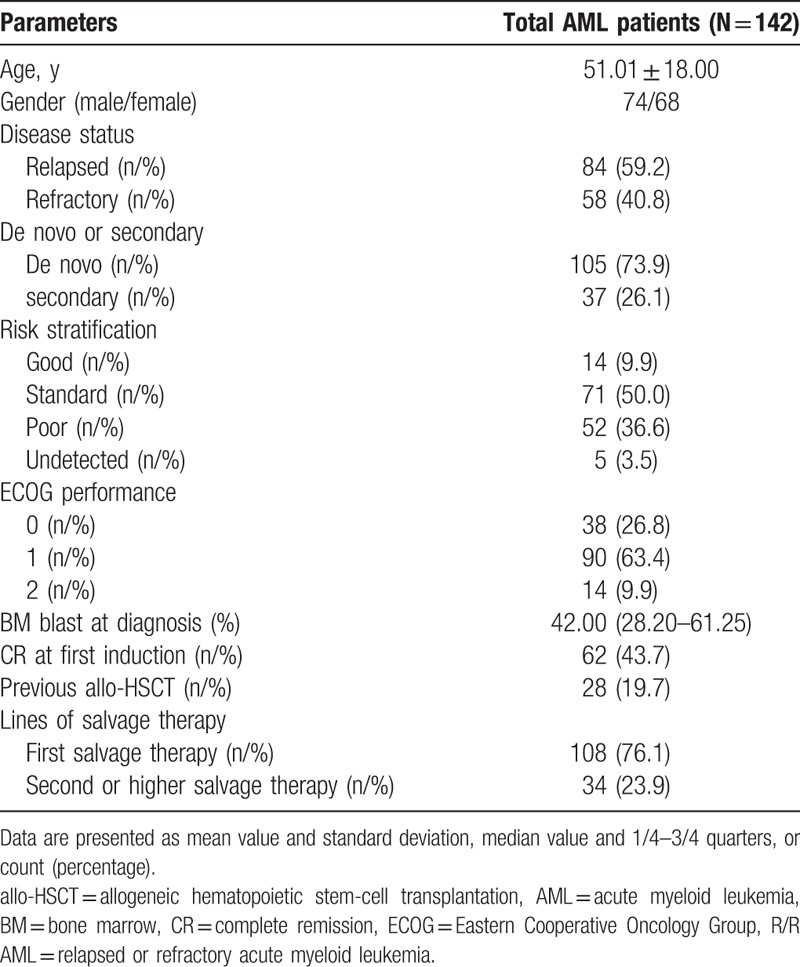
Our study included 142 R/R AML patients, of whom 74 were males and 68 were females, with a mean age of 51.01 ± 18.00 years. A total of 84 (59.2%) patients had relapsed AML and 58 (40.8%) had refractory AML, 105 (73.9%) patients had de novo AML, and 37 (26.1%) had secondary AML (Table 1). A total of 14 (9.9%), 71 (50.0%), 52 (36.6%), and 5 (3.5%) patients were classified into good, standard, poor, and undetected risk sets, while 38 (26.8%), 90 (63.4%), and 14 (9.9%) cases were categorized as ECOG performance score 0, 1, and 2, respectively. Median BM blast at diagnosis was 42.00 (28.20–61.25)% and 62 (43.7%) patients experienced CR at first induction. There were 28 (19.7%) patients who had previously undergone allo-HSCT, 108 (76.1%) patients were undergoing first salvage therapy and 34 (23.9%) patients were undergoing second salvage therapy or an even later round of salvage therapy.
Table 1.
Patients characteristics of total R/R AML patients.
3.2. Salvage chemotherapy regimens
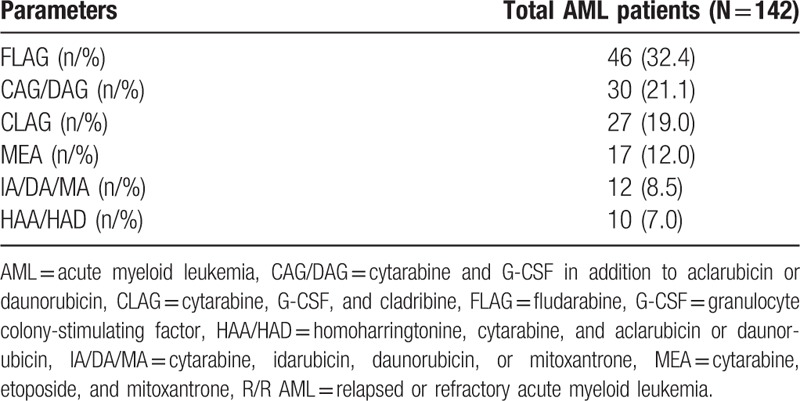
Salvage chemotherapy regimens for all patients are listed in Table 2. A total of 46 (32.4%) patients were treated by FLAG-based regimen, 30 (21.1%) patients were treated by CAG/DAG-based regimens, followed by 27 (19.0%), 17 (12.0%), 12 (8.5%), and 10 (7.0%) patients receiving CLAG, MEA, IA/DA/MA, and HAA/HAD-based regimens, respectively.
Table 2.
Salvage chemotherapy regimens in analyzed R/R AML patients.
3.3. Clinical efficacy by various salvage chemotherapies
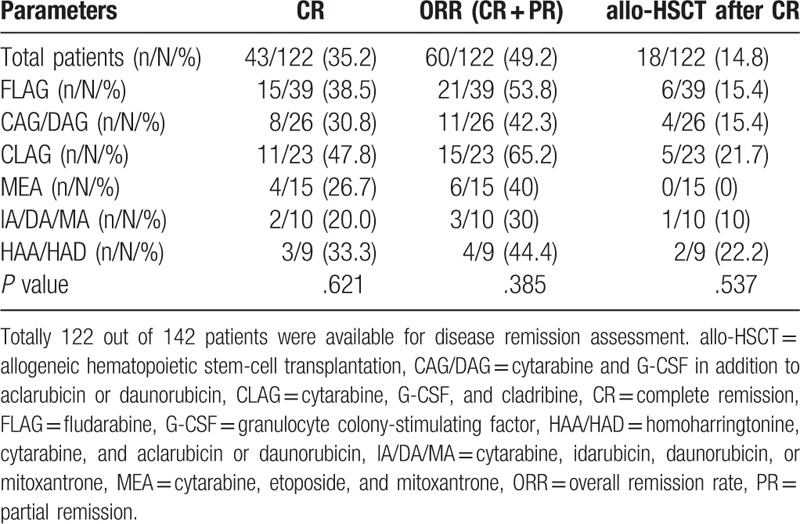
Among the 142 enrolled patients, 122 were available for assessment of disease remission. As displayed in Table 3, 43 (35.2%) patients achieved CR, 60 (49.2%) achieved ORR, and 18 (14.8%) received allo-HSCT after CR. No difference of CR (P = .621), ORR (P = .385), or allo-HSCT (P = .537) achievement was found among different chemotherapy regimens. As to the comparison of CR between CLAG and FLAG, no difference of CR between the 2 groups was found (P = .270) (Supplementary Fig. 1B). Moreover, we have classified the various regimens by cytarabine doses as follows: CAG/DAG = low-dose cytarabine; HAA/IA/DA/MA = intermediate-dose cytarabine; and CLAG/FLAG = high-dose cytarabine. And no difference of CR was observed among the low-dose cytarabine group (30.8%), intermediate-dose cytarabine group (26.9%), and high-dose cytarabine group (41.9%) (P = .274) (Supplementary Fig. 1A). When classified patients according to age, no difference in the rates of low-dose cytarabine, intermediate-dose cytarabine, and high-dose cytarabine between age >=60 years group and age <60 years group was found (P = .148) (Supplementary Fig. 2C), while CR in patients with age ≥60 years was lower than that in patients with age <=60 years (P = .003) (Supplementary Fig. 2A).
Table 3.
Treatment efficacy.
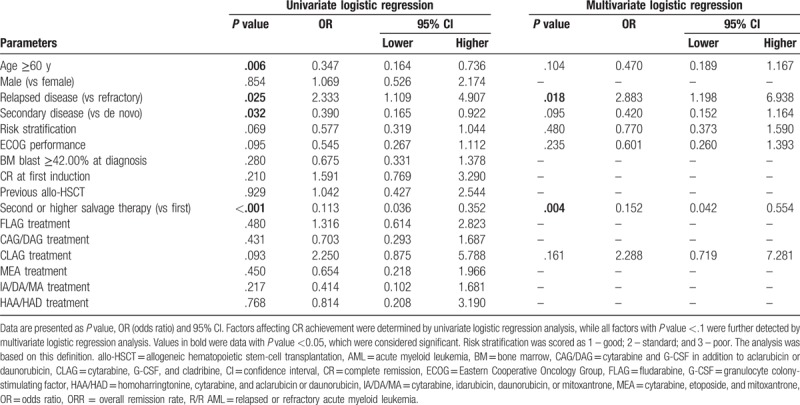
3.4. Analysis of factors affecting CR
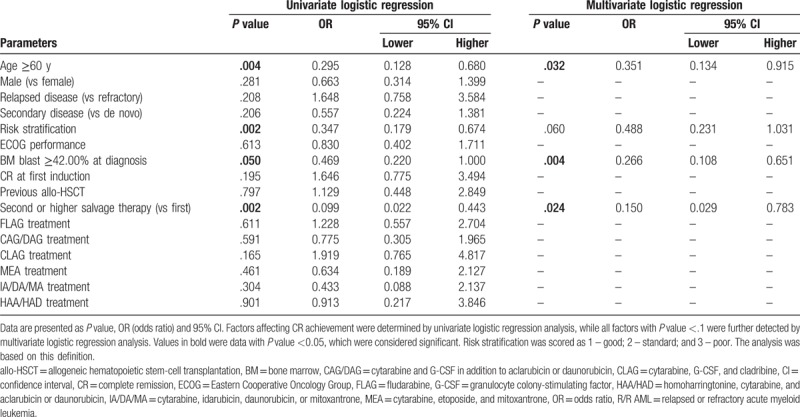
Univariate logistic regression was performed to analyze factors affecting CR. No chemotherapy regimen was associated with CR. Age ≥60 years (P = .004), membership in the poor risk set (P = .002), BM blast ≥42.00% at diagnosis (P = .050), and second or later round of salvage therapy (P = .002) were inversely associated with CR (Table 4). All factors with P value <.1 were subsequently assessed with multivariate logistic regression and age >=60 years (P = .032), BM blast ≥42.00% at diagnosis (P = .004), and second or higher salvage therapy (P = .024) were verified to be independent factors predicting absence of CR.
Table 4.
Logistic regression model analysis of factors predicting CR in R/R AML patients.
3.5. Analysis of factors affecting ORR
As shown in Table 5, patients on a CLAG-based regimen had greater odds of achieving ORR compared to patients on other regimens, but presented this effect was not significant (P = .093), while none of the other chemotherapy regimens was associated with ORR achievement. Age ≥60 years (P = .006), secondary disease (P = .032), and second or later round of salvage therapy (P < .001) were inversely associated with ORR, while relapsed disease (P = .025) was associated with ORR. All factors with P value less than .1 were assessed with multivariate regression which showed that second or later round of salvage therapy was an independent factor predicting lower odds of ORR (P = .004), while relapsed disease was verified to be an independent factor predicting higher odds of ORR (P = .018). No other difference of ORR was observed among groups divided by other factors.
Table 5.
Logistic regression analysis of factors predicting ORR in R/R AML patients.
3.6. Survival profiles
Kaplan–Meier curves analysis disclosed that median OS in total R/R AML patients was 8.0 (95% CI 6.6–9.4) months with a 1-year OS rate of (29.9 ± 3.9)% and a 3-year OS rate of (11.1 ± 3.6)% (Fig. 1A). In addition, the 30-day mortality rate of R/R AML patients in our study was 26.1%. As to survival among different therapy groups, FLAG, CAG/DAG, CLAG, MEA, IA/DA/MA, and HAA/HAD-based regimens achieved median OS of 9.0 (95% CI 6.6–11.4) months, 7.0 (95% CI 2.8–11.2) months, 10.0 (95% CI 3.4–16.6) months, 5.0 (95% CI 2.3–7.7) months, 5.0 (95% CI 0.0–11.8) months, and 8.0 (95% CI 6.6–9.4) months, respectively. No significant difference of OS among salvage chemotherapy regimens was observed (Fig. 1B) (P = .230). Regarding OS in CLAG group and FLAG group, no difference of OS was observed between the 2 groups (P = .151) (Supplementary Fig. 1D). Furthermore, no difference of OS was found among low-dose cytarabine group, intermediate-dose cytarabine group, and high-dose cytarabine group (P = .119) (Supplementary Fig. 1C), while OS in patients with age >=60 years was shorter compared to patients with age <60 years (P = .005) (Supplementary Fig. 2B).
Figure 1.
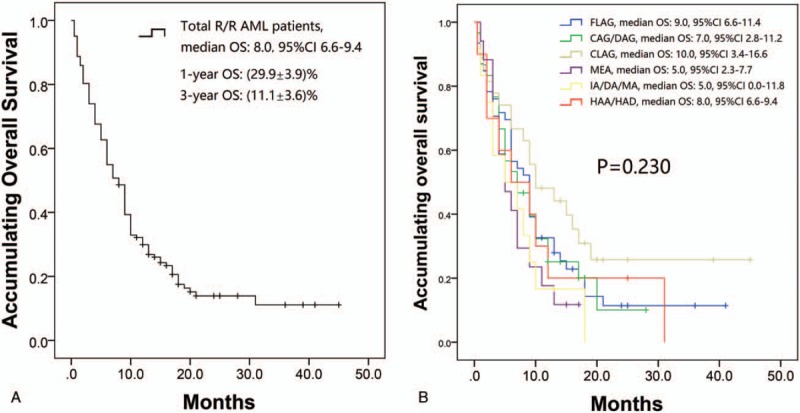
Accumulating OS by different treatments. (A) K-M curve analysis of OS in total R/R AML patients. Salvage chemotherapy regimens achieved a median OS of 8.0 (95% CI 6.6–9.4) months with 1-year OS rate of 29.9% ± 3.9% and 3-year OS of 11.1% ± 3.6%. (B) K-M analysis of OS by different treatments. The CLAG-based regimen achieved median OS of 10.0 (95% CI 3.4–16.6) months, and the FLAG-, CAG/DAG-, MEA-, IA/DA/MA-, and HAA/HAD-based regimens achieved 9.0 (95% CI 6.6–11.4) months, 7.0 (95% CI 2.8–11.2) months, 5.0 (95% CI 2.3–7.7) months, 5.0 (95% CI 0.0–11.8) months, and 8.0 (95% CI 6.6–9.4) months, respectively. No difference of OS among salvage chemotherapy regimens was found (P = .230). Comparison among groups was performed with log-rank test. P < .05 was considered significant. AML = acute myeloid leukemia, CAG/DAG = cytarabine and G-CSF in addition to aclarubicin or daunorubicin, CI = confidence interval, CLAG = cytarabine, G-CSF, and cladribine, FLAG = fludarabine, G-CSF = granulocyte colony-stimulating factor, HAA/HAD = homoharringtonine, cytarabine, and aclarubicin or daunorubicin, IA/DA/MA = cytarabine, idarubicin, daunorubicin, or mitoxantrone, K-M = Kaplan–Meier, MEA = cytarabine, etoposide, and mitoxantrone, OS = overall survival, R/R AML = relapsed or refractory acute myeloid leukemia.
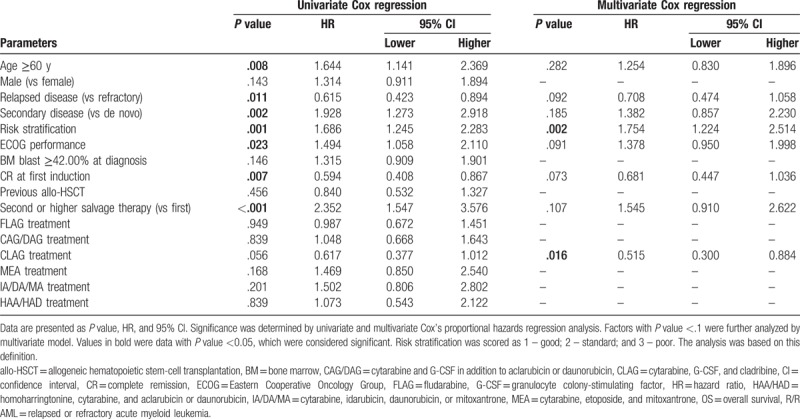
3.7. Analysis of factors affecting OS
As to factors affecting OS (Table 6), CLAG-based regimen showed a trend of better OS, but this association was not statistically significant (P = .056). Age >=60 years (P = .008), secondary disease (P = .002), poor risk set (P = .001), higher ECOG performance (P = .023), and second or later round of salvage therapy (P < .001) were assessed to be associated with shorter OS by univariate Cox's regression, while relapsed disease (P = .011) was associated with longer OS. Factors with P value < .1 were evaluated in the subsequent multivariate Cox regression, and CLAG-based regimen was an independent factor predicting prolonged OS (P = .016), while poor risk set was verified to be an independent predictive factor for worse OS (P = .002).
Table 6.
Cox's proportional hazards regression analysis of factors affecting OS in R/R AML patients.
3.8. Safety profiles
The data on toxicity are displayed in Supplementary Table 1, which reveals that febrile neutropenia and thrombocytopenia were the most 2 common hematological toxicities in each group. Numbers of patients with febrile neutropenia and thrombocytopenia were: 41 (89.1%) and 40 (87.0%) respectively in FLAG group; 25 (83.3%) and 26 (86.7%) in CAG/DAG group; 23 (85.2%) and 21 (77.8%) in CLAG group; 14 (82.4%) and 11 (64.7%) in MEA group; 10 (83.3%) and 8 (66.7%) in IA/DA/MA group; and 8 (80.0%) and 7 (70.0%) in HAA/HAD group. Other information of adverse events including infection, hemorrhage, ALT or AST elevation, cardiotoxicity, nausea/vomiting, and others are exhibited in the Supplementary Table 1. The infection rate in our study was 58.5%.
4. Discussion
In this study, we evaluated the efficacy of salvage chemotherapies, including FLAG-, CAG/DAG-, CLAG-, MEA-, IA/DA/MA-, and HAA/HAD-based regimens in R/R AML patients, as well as evaluating the other clinical factors that might be predictive of outcomes. This present research obtained following results:
-
(1)
R/R AML patients in our study achieved a CR of 35.2% and an ORR of 49.2%, and additionally, 14.8% of patients underwent allo-HSCT. Meanwhile, median OS was 8.0 (95% CI 6.6–9.4) months with 1-year OS rate of (29.9 ± 3.9)% and 3-year OS rate of (11.1 ± 3.6)%.
-
(2)
While the CLAG-based regimen did not affect CR, it exhibited a trend toward higher odds of ORR compared to other treatment regimens, and it was an independent factor for prolonged OS. No other regimens were observed to be correlated with CR, ORR, or OS.
-
(3)
As to other factors affecting outcomes, age >=60 years, BM blast >=42.00% at diagnosis, and second or later round of salvage therapy were independent predictive factors for lower odds of CR and the latter was also an independent factor predicting absence of ORR, while relapsed disease was an independent factor for achieving ORR, and poor risk stratification was associated with shorter OS.
Several cytotoxic agents are applied in salvage chemotherapy regimens for R/R AML. Aclarubicin, an oligosaccharide anthracycline as well as an antineoplastic antibiotic, inhibits replication and repair of DNA by interacting with topoisomerase I and II. It has been reported to have lower cardiac toxicity than doxorubicin and to be effective, regardless of gene status, for multidrug resistance.[13–15] Daunorubicin and idarubicin, which are also anthracyclines, are widely used with cytarabine in R/R AML due to their antileukemic effects through inhibiting repair of DNA in leukemia cells.[16] Etoposide, which is a topoisomerase II inhibitor, and mitoxantrone, which is an agent for intercalating DNA, are often used in salvage therapy for R/R AML patients.[17] Homoharringtonin is natural ester of alkaloid cephalotaxine originating in Cephalotaxus, and its cytotoxicity is due to several mechanisms, such as the inhibition of DNA synthesis as well as inhibition of proteins in leukemic cells.[18,19] FLAG and cladribine, both purine nucleoside analogues, possess similar structures and have the same functional mechanism: they increase the concentration of Ara-CTP, which is the active metabolite of cytarabine and cytotoxic to leukemic cells; moreover, they also present some difference of interaction with enzymes.[8,20] In contrast to the antineoplastic agents above, G-CSF is a cytokine that is usually incorporated into chemotherapy regimens due to its promotion of differentiation in myeloid cells.[21] According to the different properties of each drug, various combined chemotherapy regimens have been developed, including FLAG-, CAG/DAG-, CLAG-, MEA-, IA/DA/MA-, and HAA/HAD-based regimens.
A literature review performed by Valeriy Sedov and Robert K. Stuart has reported outcomes of various recent chemotherapy regimens that are frequently used in treating R/R AML, including FLAG, CLAG with or without mitoxantrone, high-dose cytarabine (HiDAC) with or without an anthracycline, etoposide together with cytarabine with or without mitoxantrone, and clofarabine-containing regimens. These researchers report a range of CR of 12% to 53% and a median OS of 3.3 to 9 months for all patients. In addition, they report CR and median OS in patients >60 years in the ranges of 12% to 35% and 3.3 to 6.6 months, respectively.[7] Furthermore, regimen with some new drugs have achieved relatively satisfactory treatment outcomes according to previous studies, for instance, the study that treats R/R AML patients with vosaroxin plus cytarabine in a randomized phase III trial (VALOR) displays OS of 7.5 (95% CI 6.4–8.5) months in vosaroxin plus cytarabine group and OS of 6.1 (5.2–7.1) months in placebo plus cytarabine group, and the Clofarabine and Cytarabine Studying Survival via Induction and Consolidation (CLASSIC I) study discloses median OS of 6.6 months in clofarabine plus Ara-C group and median OS of 6.3 months in cytarabine group.[22,23] In line with these previous studies, we found that the commonly used chemotherapy regimens achieved a CR rate of 35.2% and a median OS of 8.0 (95% CI 6.6–9.4) months in our patients, who had a mean age of 51.01 ± 18.00 years, and the results in our study were comparable to those in previous studies, including the studies applying new drugs. As to allo-HSCT achievements, the rate of allo-HSCT in our study was relatively lower than the previous VALOR study (14.8% vs 30%) due to the lack of donors.
As to analyses of the effects of different treatments on outcomes, a retrospective study performed by SL Price et al evaluated the difference of survival between CLAG and MEC in 162 R/R AML patients, and they report that patients treated with CLAG achieved longer OS compared to patients treated with MEC in refractory AML patients (P = .07).[24] Another retrospective research which treated R/R AML patients with cladribine-based and FLAG-based chemotherapy showed that cladribine-based therapy (CLAG and CLAG plus mitoxantrone) to be associated with better survival in some subsets, such as patients with de novo AML, CR at first induction, and not-poor risk.[25] In accordance with previous studies, we found that CLAG-based regimen is correlated with better OS and that it also shows a trend toward higher odds of ORR. The mechanisms of cladribine may explain these results:
-
(1)
As a purine analogue, cladribine inhibits ribonucleotide reductase (RNR), lowers the cellular deoxynucleotide pools, and interferes with DNA synthesis, subsequently reducing cell proliferation in leukemic blasts and leading to numerically higher ORR and better OS in R/R AML patients.[26,27]
-
(2)
Cytarabine forms its active metabolite Ara-CTP in leukemic blasts, which leads to terminate the extension of DNA stands, and cladribine increases the cellular uptake as well as accumulation of Ara-CTP in leukemic blasts. The bioactivations of cladribine and Ara-C both need the participation of deoxycytidine kinase (dCK). The activity of dCK is negatively regulated by deoxyadenosine (dAdo), which is formed from adenosine (Ado) under the activation of ribonucleotide reductase.[11] Cladribine is able to inhibit ribonucleotide reductase, thereby indirectly decreasing the level of dAdo, and further increasing the activity of dCK. Therefore, the level of Ara-CTP, which is the active metabolite of Ara-C, is raised due to the elevated dCK level. In brief, addition of cladribine results in loss of the negative feedback described above, thereby increasing uptake and accumulation of Ara-CTP and leading to the better outcomes by CLAG-based regimen.
-
(3)
Other than the common mechanisms of other purine analogues, cladribine also changes the membrane potential of mitochondria. It may result from 3 different ways as follows.[28,29] Firstly, cladribine causes loss of mitochondrial transmembrane potential via the activation of a Z-VAD-sensitive caspase; secondly, cladribine leads to the mitochondria translocation by action of a Bax-like protein, which further promotes the loss of Δψm; thirdly, cladribine may directly induce transition of mitochondrial permeability via an unidentified death signal. Therefore, all these processes lead to the loss of Δψm and the subsequent cells apoptosis.
-
(4)
Cladribine inhibits DNA methyltransferase and consumes methyl donors. As for the inhibition of DNA methyltransferase, cladribine presumably induces downregulation of DNA methyltransferase 1 as other adenosine analogues.[27] With regards to the effects of cladribine on methyl donors and DNA methyltransferase, they may be associated with the chemical structure of cladribine. As an adenosine analogue, cladribine suppresses DNA methylation through inhibiting S-adenosyl homocysteine hydrolase (SAHH), which hydrolyzes S-adenosyl homocysteine (SAH).[30] Subsequently, the accumulated SAH production consumes the S-adenosyl methionine (SAM), which is a methyl donor activated by methyltransferases, thereby inhibiting the methylation of DNA. As a consequence, the methylation of DNA has been remarkably impaired due to the reduction of methyl donors and repression of DNA methyltransferase, thereby increasing cell death in leukemic blasts.[11,31–34]
Our study also had several limitations:
-
(1)
Sample size was relatively small. In fact, most R/R AML patients experience rapid disease progression and have relatively short OS, which leads to a relatively small number of R/R AML patients in a single center. Therefore, in our study, 142 R/R AML patients (only 27 patients in CLAG group) were enrolled between January 2013 and December 2016, a relatively small sample size, which may have reduced the reliability of our results. To address this issue, we would recruit more R/R AML patients in the future, to investigate the efficacy of CLAG regimen with a larger sample size.
-
(2)
This study was a retrospective, single-center study, and future multicenter, prospective research is needed to verify outcomes under various salvage chemotherapy regimens in R/R AML patients.
In conclusion, FLAG-, CAG/DAG-, CLAG-, MEA-, IA/DA/MA-, and HAA/HAD-based regimens were observed to achieve similar CR rates, while the CLAG-based regimen achieved a trend towards higher odds of ORR, as well as a longer OS. Therefore, a CLAG-based regimen for treating R/R AML patients might be considered to be a prioritized option.
Author contributions
Conceptualization: Guolin Yuan.
Data curation: Jun Xu, Tingting Lv.
Formal analysis: Tingting Lv, Xiaofen Zhou, Ying Huang, Dongdong Liu.
Investigation: Jun Xu, Tingting Lv.
Methodology: Jun Xu, Tingting Lv, Xiaofen Zhou, Ying Huang.
Project administration: Guolin Yuan.
Resources: Guolin Yuan.
Software: Xiaofen Zhou, Ying Huang.
Supervision: Guolin Yuan.
Validation: Xiaofen Zhou, Dongdong Liu.
Writing – original draft: Ying Huang, Dongdong Liu.
Writing – review and editing: Dongdong Liu, Guolin Yuan.
Supplementary Material
Footnotes
Abbreviations: allo-HSCT = allogeneic hematopoietic stem cell transplantation, AML = acute myeloid leukemia, BM = bone marrow, CAG/DAG = cytarabine and G-CSF in addition to aclarubicin or daunorubicin, CLAG = cytarabine, G-CSF, and cladribine, CR = complete remission, ECOG = Eastern Cooperative Oncology Group, FLAG = fludarabine, G-CSF = granulocyte colony-stimulating factor, HAA/HAD = homoharringtonine, cytarabine, and aclarubicin or daunorubicin, IA/DA/MA = cytarabine, idarubicin, daunorubicin, or mitoxantrone, MEA = cytarabine, etoposide, and mitoxantrone, ORR = overall remission rate, OS = overall survival, R/R AML = refractory or relapsed acute myeloid leukemia.
JX and T-TL contributed equally to this work.
The authors have no funding and conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Niskanen L, Hedner T, Hansson L, et al. Reduced cardiovascular morbidity and mortality in hypertensive diabetic patients on first-line therapy with an ACE inhibitor compared with a diuretic/beta-blocker-based treatment regimen: a subanalysis of the Captopril Prevention Project. Diabetes Care 2001;24:2091–6. [DOI] [PubMed] [Google Scholar]
- [2].Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med 2015;373:1136–52. [DOI] [PubMed] [Google Scholar]
- [3].Ossenkoppele GJ, Janssen JJ, van de Loosdrecht AA. Risk factors for relapse after allogeneic transplantation in acute myeloid leukemia. Haematologica 2016;101:20–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Bose P, Vachhani P, Cortes JE. Treatment of relapsed/refractory acute myeloid leukemia. Curr Treat Options Oncol 2017;18:17. [DOI] [PubMed] [Google Scholar]
- [5].Estey E. Why are there so few randomized trials for patients with primary refractory acute myeloid leukemia? Best Pract Res Clin Haematol 2016;29:324–8. [DOI] [PubMed] [Google Scholar]
- [6].Mohty M, Malard F, Blaise D, et al. Sequential regimen of clofarabine, cytosine arabinoside and reduced-intensity conditioned transplantation for primary refractory acute myeloid leukemia. Haematologica 2017;102:184–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sedov V, Stuart RK. Vosaroxin in relapsed/refractory acute myeloid leukemia: efficacy and safety in the context of the current treatment landscape. Ther Adv Hematol 2017;8:185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Thiel A, Schetelig J, Ponisch W, et al. Mito-FLAG with Ara-C as bolus versus continuous infusion in recurrent or refractory AML--long-term results of a prospective randomized intergroup study of the East German Study Group Hematology/Oncology (OSHO) and the Study Alliance Leukemia (SAL). Ann Oncol 2015;26:1434–40. [DOI] [PubMed] [Google Scholar]
- [9].Van den Neste E, Cardoen S, Offner F, et al. Old and new insights into the mechanisms of action of two nucleoside analogs active in lymphoid malignancies: fludarabine and cladribine (review). Int J Oncol 2005;27:1113–24. [PubMed] [Google Scholar]
- [10].Nazha A, Kantarjian H, Ravandi F, et al. Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients </=60 years with newly diagnosed acute myeloid leukemia. Am J Hematol 2013;88:961–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Freyer CW, Gupta N, Wetzler M, et al. Revisiting the role of cladribine in acute myeloid leukemia: an improvement on past accomplishments or more old news? Am J Hematol 2015;90:62–72. [DOI] [PubMed] [Google Scholar]
- [12].Leukemia, Lymphoma Group CSoHCMA. [The guidelines for diagnosis and treatment of acute myelogenous leukemia (relapse/refractory) in China (2017)]. Zhonghua Xue Ye Xue Za Zhi 2017;38:183–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hajji N, Mateos S, Pastor N, et al. Induction of genotoxic and cytotoxic damage by aclarubicin, a dual topoisomerase inhibitor. Mutat Res 2005;583:26–35. [DOI] [PubMed] [Google Scholar]
- [14].Wei G, Ni W, Chiao JW, et al. A meta-analysis of CAG (cytarabine, aclarubicin, G-CSF) regimen for the treatment of 1029 patients with acute myeloid leukemia and myelodysplastic syndrome. J Hematol Oncol 2011;4:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lehne G, De Angelis P, Clausen OP, et al. Human hepatoma cells rich in P-glycoprotein are sensitive to aclarubicin and resistant to three other anthracyclines. Br J Cancer 1996;74:1719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Szmigielska-Kaplon A, Ciesielska E, Szmigiero L, et al. Anthracyclines potentiate activity against murine leukemias L1210 and P388 in vivo and in vitro. Eur J Haematol 2002;68:370–5. [DOI] [PubMed] [Google Scholar]
- [17].Abbi KK, Rybka W, Ehmann WC, et al. Phase I/II study of clofarabine, etoposide, and mitoxantrone in patients with refractory or relapsed acute leukemia. Clin Lymphoma Myeloma Leuk 2015;15:41–6. [DOI] [PubMed] [Google Scholar]
- [18].Wu L, Li X, Su J, et al. Effect of low-dose cytarabine, homoharringtonine and granulocyte colony-stimulating factor priming regimen on patients with advanced myelodysplastic syndrome or acute myeloid leukemia transformed from myelodysplastic syndrome. Leuk Lymphoma 2009;50:1461–7. [DOI] [PubMed] [Google Scholar]
- [19].Mai WY, Lin MF. Induction of apoptosis by homoharringtonine in G1 phase human chronic myeloid leukemic cells. Chin Med J (Engl) 2005;118:487–92. [PubMed] [Google Scholar]
- [20].Robak T. Purine nucleoside analogues in the treatment of myleoid leukemias. Leuk Lymphoma 2003;44:391–409. [DOI] [PubMed] [Google Scholar]
- [21].Liongue C, Ward AC. Granulocyte colony-stimulating factor receptor mutations in myeloid malignancy. Front Oncol 2014;4:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ravandi F, Ritchie EK, Sayar H, et al. Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 2015;16:1025–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol 2012;30:2492–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Price SL, Lancet JE, George TJ, et al. Salvage chemotherapy regimens for acute myeloid leukemia: Is one better? Efficacy comparison between CLAG and MEC regimens. Leuk Res 2011;35:301–4. [DOI] [PubMed] [Google Scholar]
- [25].Park H, Youk J, Kim I, et al. Comparison of cladribine- and fludarabine-based induction chemotherapy in relapsed or refractory acute myeloid leukaemia. Ann Hematol 2016;95:1777–86. [DOI] [PubMed] [Google Scholar]
- [26].Leist TP, Weissert R. Cladribine: mode of action and implications for treatment of multiple sclerosis. Clin Neuropharmacol 2011;34:28–35. [DOI] [PubMed] [Google Scholar]
- [27].Majda K, Kaufman-Szymczyk A, Lubecka-Pietruszewska K, et al. Influence of clofarabine on transcriptional activity of PTEN, APC, RARB2, ZAP70 genes in K562 cells. Anticancer Res 2010;30:4601–6. [PubMed] [Google Scholar]
- [28].Marzo I, Perez-Galan P, Giraldo P, et al. Cladribine induces apoptosis in human leukaemia cells by caspase-dependent and -independent pathways acting on mitochondria. Biochem J 2001;359:537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med 2000;6:513–9. [DOI] [PubMed] [Google Scholar]
- [30].Wyczechowska D, Fabianowska-Majewska K. The effects of cladribine and fludarabine on DNA methylation in K562 cells. Biochem Pharmacol 2003;65:219–25. [DOI] [PubMed] [Google Scholar]
- [31].Stumpel DJ, Schneider P, Pieters R, et al. The potential of clofarabine in MLL-rearranged infant acute lymphoblastic leukaemia. Eur J Cancer 2015;51:2008–21. [DOI] [PubMed] [Google Scholar]
- [32].Gandhi V, Estey E, Keating MJ, et al. Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood 1996;87:256–64. [PubMed] [Google Scholar]
- [33].Spurgeon S, Yu M, Phillips JD, et al. Cladribine: not just another purine analogue? Expert Opin Investig Drugs 2009;18:1169–81. [DOI] [PubMed] [Google Scholar]
- [34].Vahdat L, Wong ET, Wile MJ, et al. Therapeutic and neurotoxic effects of 2-chlorodeoxyadenosine in adults with acute myeloid leukemia. Blood 1994;84:3429–34. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


