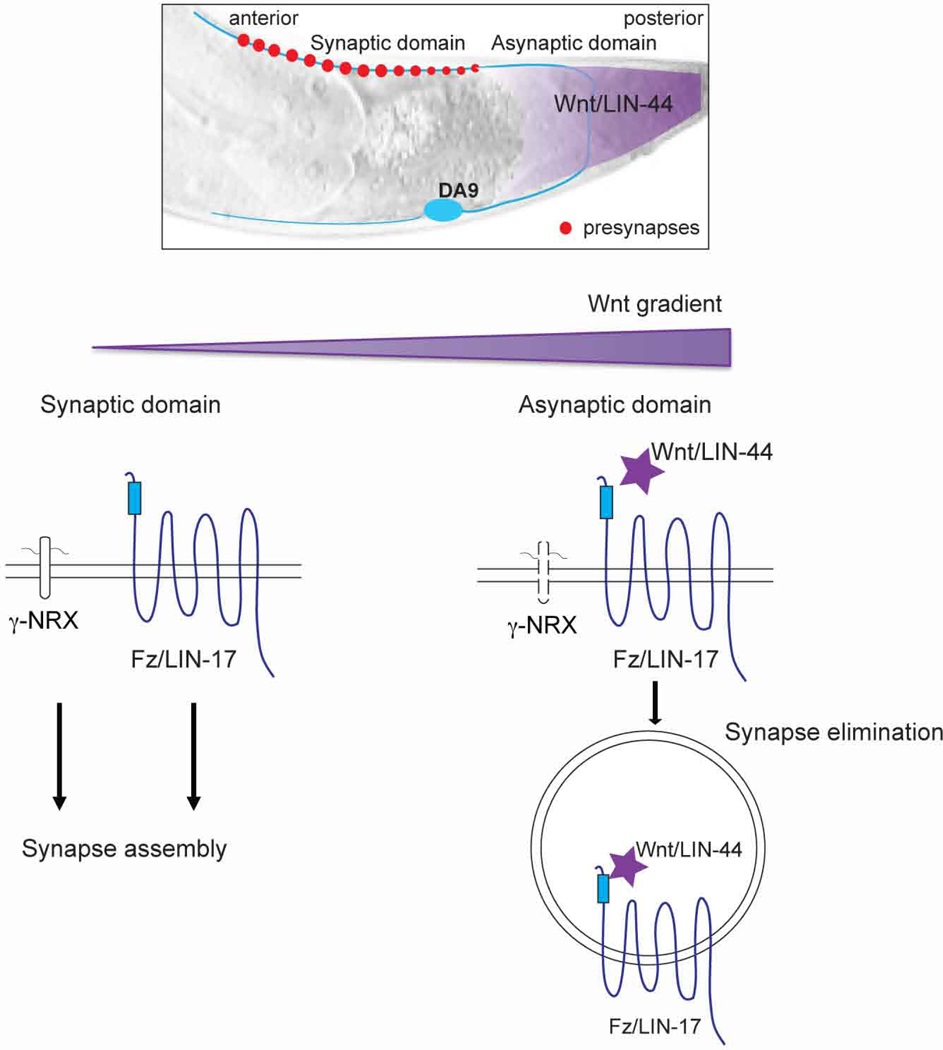
Summary
Synapse formation defines neuronal connectivity and is thus essential for neuronal circuit assembly. Trans-synaptic interactions of cell adhesion molecules are thought to induce synapse assembly. Here we demonstrate that a recently discovered and conserved short form of neurexin, γ-neurexin, which lacks canonical extracellular domains, is nonetheless sufficient to promote presynaptic assembly in the nematode C. elegans. γ- but not α-neurexin is required for assembling active zone components, recruiting synaptic vesicles, and clustering calcium channels at release sites to promote evoked synaptic transmission. Furthermore, we find that neurexin functions in parallel with the transmembrane receptor Frizzled, as the absence of both proteins leads to an enhanced phenotype - the loss of most synapses. Frizzled’s pro-synaptogenic function is independent of its ligand, Wnt. Wnt binding instead eliminates synapses by inducing Frizzled’s endocytosis and the downregulation of neurexin. These results reveal how pro- and anti-synaptogenic factors converge to precisely sculpt circuit formation in vivo.
eTOC blurb
Kurshan et al. show that a short version of the cell-adhesion molecule neurexin that lacks canonical extracellular domains can nonetheless drive presynaptic assembly. It functions in parallel with the Wnt receptor Frizzled. Wnt eliminates synapses via Frizzled endocytosis and neurexin downregulation.
Introduction
The formation of complex neuronal circuits is mediated by the recognition of correct synaptic partners followed by the assembly of the appropriate number and type of synapses. Synaptic cell adhesion molecules have been proposed to initiate this process and determine the specificity of synaptic connections (Bukalo and Dityatev, 2012; Siddiqui and Craig, 2011; Yogev and Shen, 2014). However, redundancy both within and between the different adhesion systems has complicated attempts to determine the precise role of specific molecules involved in mediating pre- and postsynaptic assembly. In mammals, the synaptic cell adhesion protein neurexin is alternatively spliced into thousands of isoforms that are differentially expressed throughout brain (Ullrich et al., 1995; Ushkaryov and Südhof, 1993), and may function as a transsynaptic “hub” by binding a host of postsynaptic partners (Südhof, 2017). Understanding the extent to which neurexin contributes to synapse assembly in vivo is particularly salient as mutations in neurexin have been linked to several neuropsychiatric disorders, including schizophrenia, autism, intellectual disability and Tourette’s syndrome (Südhof, 2008, 2017).
The synaptogenic activity of neurexin and its canonical binding partner neuroligin was initially demonstrated by showing that their reciprocal binding could induce the formation of hemi-synapses in vitro (Dean et al., 2003; Graf et al., 2004; Scheiffele et al., 2000), although whether neurexin-neuroligin interactions are required for synapse formation in mice in vivo has been disputed. While some evidence points to the contribution of neurexins and neuroligins to in vivo synapse formation at specific synapses (Banovic et al., 2010; Chen et al., 2017, 2012; Li et al., 2007; Liang et al., 2015; Stogsdill et al., 2017; Sun et al., 2011), other studies have concluded that their requirement is limited to synapse maturation or function (Missler et al., 2003; Varoqueaux et al., 2006). Recent studies have attempted to explain these conflicting results either by suggesting that the ratio rather than the absolute presence or absence of these proteins is important (Kwon et al., 2012), or by showing that their roles are synapse-specific (Chen et al., 2017). More clarity on precisely how neurexin and neuroligin are required for synapse assembly might help reconcile these findings.
Mammalian neurexins are encoded by three genes and expressed as α-, β-, and the recently identified yet largely uncharacterized γ-neurexin (Sterky et al., 2017; Ushkaryov and Südhof, 1993; Ushkaryov et al., 1992, 1994; Yan et al., 2015). α-neurexins contain extracellular laminin and EGF-like repeats. β-neurexins are extracellularly-truncated versions of α-neurexin that still include the neuroligin binding site, while γ-neurexins are truncated even further and lack all structured extracellular domains (Sterky et al., 2017; Yan et al., 2015). The functions of various neurexin isoforms appear to be cell and synapse specific, as different phenotypes can be obtained in different brain regions from manipulation of the same neurexin subtype (Aoto et al., 2015). Conditional triple knock outs that ablate expression of all α- and β-neurexins revealed dramatic differences in the functions of neurexin at different synapses, with some neurons exhibiting a dramatic decrease in synapse number while others displayed defects in synapse function or presynaptic calcium influx without affecting synapse number (Chen et al., 2017). Importantly, γ-neurexin was present in the α- and β-neurexin conditional knockouts, complicating the interpretation of these phenotypes, and leaving the role of γ-neurexin completely uncharacterized.
In C. elegans, a single neurexin gene (nrx-1) encodes long (α) and short (γ) isoforms that are expressed in the nervous system and localize to synapses (Haklai- Topper et al., 2011). The worm thus provides an excellent opportunity to dissect the contribution of each neurexin isoform in the context of a well-described nervous system. At inhibitory motor neuron synapses, presynaptic α-neurexin contributes to the neuroligin-dependent postsynaptic organization of GABA receptors (Maro et al., 2015; Tong et al., 2015), but how C. elegans α- or γ-neurexin functions in presynaptic assembly is not known. Indeed, while the canonical role for neurexin is the induction of postsynaptic assembly through trans-synaptic interactions (for example by binding neuroligin), how neurexin cell-autonomously mediates presynaptic assembly and function is less clear.
The many molecules implicated in synaptic induction and their relatively weak loss-of-function phenotypes suggests that synapse formation in vivo is likely to be mediated by parallel pathways. Even in the absence of the single Drosophila or C. elegans neurexin (Li et al., 2007; Maro et al., 2015), synapses still form. This suggests that there exist other molecules that function to induce presynaptic assembly. What other signaling molecules work in parallel with neurexin at a given synapse is unknown.
Here we demonstrate a novel role for C. elegans γ-neurexin in presynaptic assembly, independent of neuroligin. This short form of neurexin is necessary and sufficient to promote normal presynaptic development by clustering active zone proteins, synaptic vesicles and calcium channels at sites of release. Loss of γ- but not α-neurexin leads to a reduction in evoked synaptic transmission. Moreover, we identify a parallel pathway for presynaptic assembly mediated by the Frizzled receptor and show that Frizzled’s pro-synaptogenic function is independent of its ligand, Wnt. In fact, Wnt binding serves to abrogate Frizzled’s function by promoting its endocytosis, a process which also leads to the downregulation of neurexin within the asynaptic domain. These two unconventional synaptogenic pathways together mediate the large majority of synapse formation events in the C. elegans DA9 excitatory motor neuron.
Results
Neurexin is required for presynaptic assembly independent of neuroligin
C. elegans contains a single neurexin gene, nrx-1. It has previously been implicated in tuning synaptic strength as part of a retrograde signal (Hu et al., 2012; Tong et al., 2017), and in clustering postsynaptic GABA receptors at inhibitory synapses (Maro et al., 2015; Tong et al., 2015), both in conjunction with its canonical binding partner neuroligin. To understand whether nrx-1 plays a role in presynaptic development, we analyzed synapses in the DA9 excitatory motor neuron found in the tail of the worm.
DA9’s cell body and dendrite reside in the ventral cord, and its axon extends across to the dorsal side, where it elongates and forms synapses in a specific and stereotyped region (Figure 1A). Using a promoter specific for DA9 and its neighbor VA12 (whose axon remains on the ventral side), we can visualize the DA9 axon and synapses in the dorsal nerve cord with single-cell specificity. DA9 forms a row of en passant synapses along its axon. At each presynaptic bouton, active zones are marked by Clarinet-1 (CLA-1), a piccolo/bassoon-like protein that is involved in synaptic vesicle release (Xuan et al., 2017). Like other active zone proteins, it localizes to a discrete region at the ventral tip of the presynaptic varicosity where the axon is juxtaposed to postsynaptic cells (Figure 1B and Xuan et al., 2017). The synaptic vesicle-associated protein RAB-3 localizes to vesicle clusters and is more broadly distributed within the presynaptic bouton than CLA-1 (Figure 1B). Other than their size difference, puncta marked by these two proteins colocalize well at wild type synapses (Figure 1B’). Both proteins are only localized to the synaptic domain and form a highly stereotyped pattern in DA9.
Figure 1. Presynaptic neurexin is required for presynaptic assembly, independent of neuroligin.
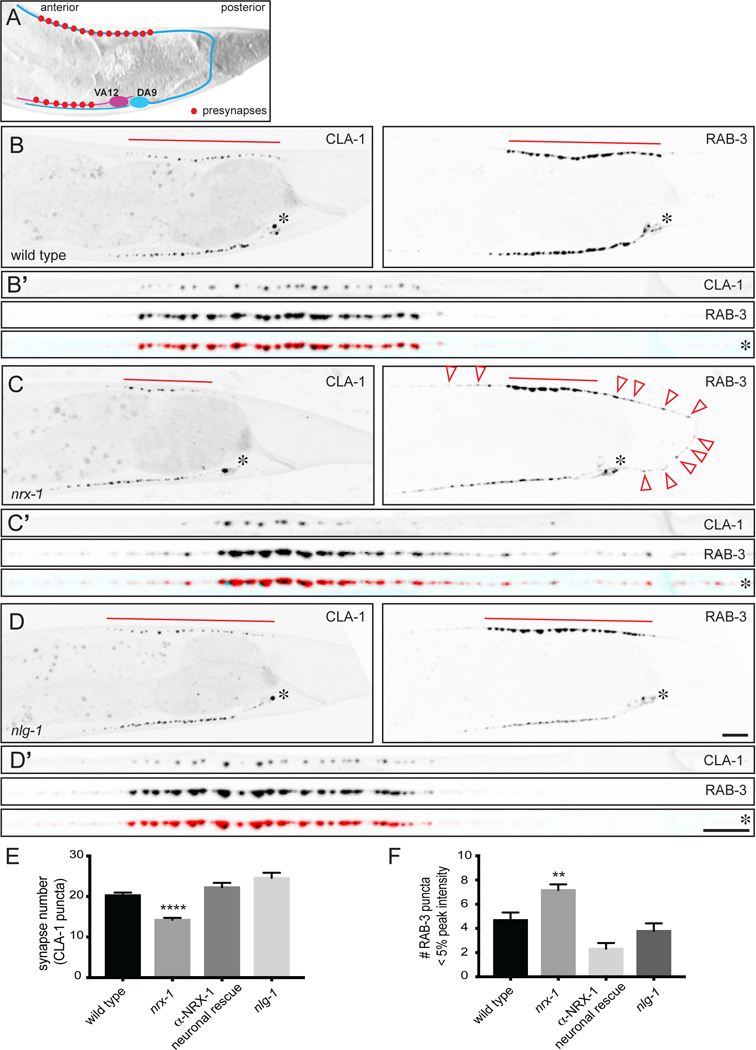
A. Schematic of the DA9 excitatory motor neuron, which forms presynapses along a portion of its axon in the tail of the worm. (The neighboring VA12 motor neuron also expresses the Pmig-13 promoter used in these studies.) B-D. Wild type (B), neurexin null mutant (wy1155) (C) and neuroligin null mutant (wy1190) (D) worms expressing the active zone marker clarinet (CLA-1::GFP) and the synaptic vesicle marker RAB-3::tdTomato in DA9 (and VA12) from the wyIs685 transgene. Insets are of straightened DA9 axons beginning from the cell body (marked with an asterisk). Note the reduced size of the synaptic domain (marked by a red line) and the increased number of asynaptic vesicle accumulations (marked by red arrowheads) in neurexin mutants (C). Scale bars = 5μm. E. Quantification of synapse number (as defined by CLA-1 puncta number). F. Quantification of the number of very small RAB-3 puncta (less than 5% the peak intensity of the largest puncta). For E-F, sample size = number of worms (the same worms, expressing both markers, were quantified) and is indicated on bars; statistical significance (ANOVA with Sidak correction) is compared to wild type.
We found that in nrx-1 mutants, the pattern of synaptic proteins was disrupted. The number of active zones (marked by CLA-1::GFP) was reduced (Figure 1C, E), and small, asynaptic vesicle accumulations (marked by RAB-3::tdTomato) that did not colocalize with CLA-1 appeared throughout the length of the axon (Figure 1 C, F). Both phenotypes could be fully rescued by expression of full-length NRX-1 cDNA presynaptically under a DA9-specific promoter, indicating that the requirement for NRX- 1 is cell-autonomous (Figure 1E, F and Supplemental Figure 1 related to Figure 1).
To determine whether the reduced number of CLA-1 puncta in DA9 was indicative of a more general defect in synapse assembly at other synapses, we analyzed the intensity of two endogenously-tagged active zone proteins, CLA-1 and SYD- 2/Liprin-α, within the nerve ring (Supplemental Figure 2 related to Figure 1). The C. elegans nerve ring comprises synapses of many head motor, sensory and interneurons. We measured the total intensity of the nerve ring as a proxy for synapse number and found that in nrx-1(null) mutants there was a dramatic decrease in total nerve ring intensity measured with either marker, indicating a broad role for NRX-1 in synapse assembly.
To determine whether the extra-synaptic RAB-3 puncta were a consequence of defective/reduced transport or, alternatively, defective synaptic capture, we performed live imaging of RAB-3::GFP in the dorsal asynaptic axonal region of DA9 (Supplemental Figure 3 related to Figure 1). In addition to the brighter clusters apparent from the static images, we could also detect individual RAB-3 transport packets moving along the axon. We found that the total frequency of movements is actually higher in nrx-1(null) mutants (in both the anterograde and retrograde directions), and the speeds are unchanged (data not shown), indicating that the synaptic defects are not due to reduced axonal transport. Indeed, even the brighter clusters can be seen to move sporadically (arrows). The loss of synaptic puncta and increased axonal mobile puncta are similar to the phenotype of presynaptic assembly mutants such as syd-2/liprin-α(Wu et al., 2013). We hypothesize that a defect in the ability of these cargoes to be captured at synaptic sites may be the cause of their ectopic accumulations.
C. elegans contains a single gene for neurexin’s canonical ligand, neuroligin (NLG-1). To determine whether neurexin’s effect on presynaptic assembly was mediated by binding to neuroligin, we analyzed synapses in nlg-1 mutants. nlg-1 mutants did not exhibit defects in presynaptic assembly (Figure 1D, E, F), indicating that the function of NRX-1 in presynaptic assembly was independent of NLG-1 binding.
The short γ-Neurexin isoform is sufficient to drive presynaptic assembly
Vertebrate neurexins are encoded by multiple genes and fall into three categories: full-length a-neurexin (Ushkaryov et al., 1992), which contains 6 laminin-like LNS domains interleaved with 3 EGF repeats (Figure 2A), a shorter β-neurexin (Ushkaryov et al., 1994) that only contains the 6th LNS domain (to which neuroligin binds), and a recently described even shorter γ-neurexin (Sterky et al., 2017; Yan et al., 2015), which does not contain any of the LNS/EGF domains. The single C. elegans nrx-1 gene encodes long isoforms homologous to α-neurexin, and short isoforms with homology to γ- neurexin (Figure 2A). To determine which isoforms of neurexin mediate presynaptic assembly, we made use of several large deletions of the nrx-1 genomic region (Figure 2A). The nu485 (“Δα”) allele specifically deletes only the α-neurexin isoforms (including all the LNS/EGF repeats). The wy778(“Δαγ”) allele is a complementary deletion that covers the common C-terminus of the two isoform classes (including the transmembrane and intracellular domains) and deletes the entire γ-neurexin region. To be certain that we were completely abrogating neurexin function, we generated a third allele by CRISPR, wy1155(“null”), which is a deletion of the entire nrx-1 open reading frame.
Figure 2. The short γ-Neurexin isoform is sufficient for presynaptic assembly.
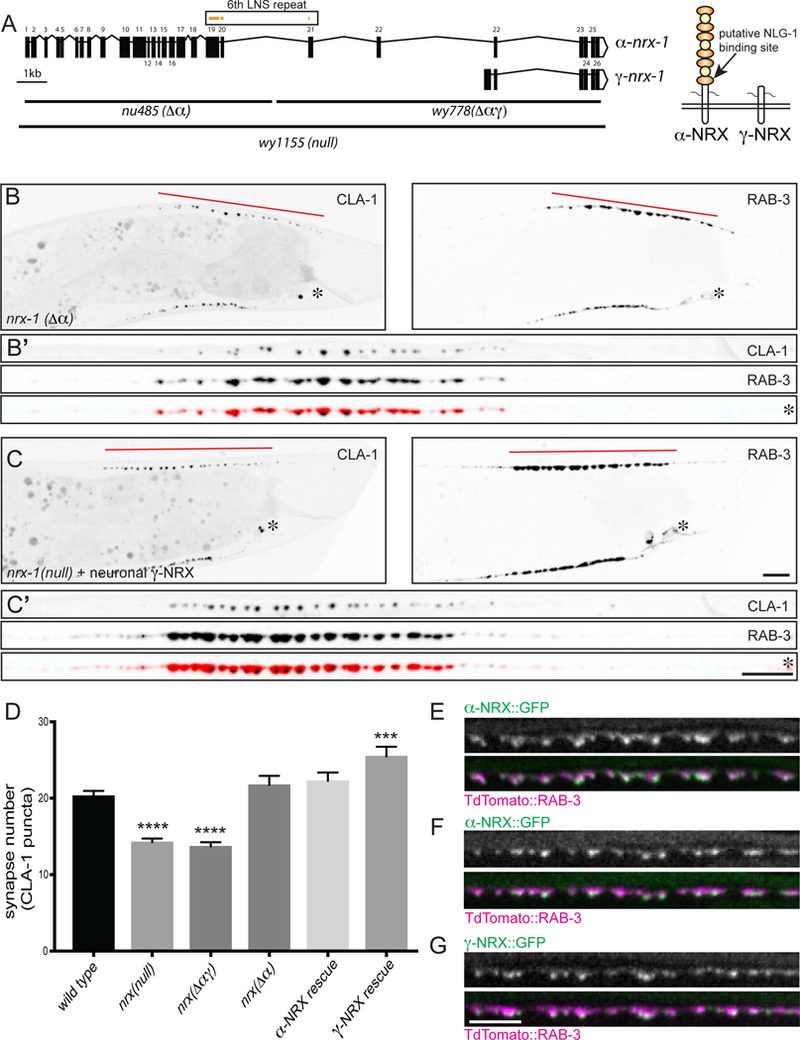
A. C. elegans neurexin-1 gene structure, showing the long α-nrx (nrx-1a) and short γ-nrx (nrx- 1m) isoforms. Regions deleted in alleles used in this study are marked below. The a- and γ-NRX predicted proteins are shown on the right: orange ovals indicate LNS repeats (including the 6th LNS repeat that binds neuroligin in mammals), yellow circles indicate EGF repeats. B, C. The Δα-neurexin-specific deletion allele (nu485) (B), and the null neurexin allele (wy1155) rescued with presynaptic γ-neurexin (wyEx9637) (C), expressing the active zone marker clarinet (CLA-1::GFP) and the synaptic vesicle marker RAB-3::tdTomato in DA9 (wyIs685). Synaptic domain size (marked by a red line) is normal (compare to Figure 1B). Insets are of straightened DA9 axons beginning from the cell body (marked with an asterisk). Scale bars = 5μm. D. Quantification of synapse number (as defined by CLA-1 puncta number). Sample size (number of worms) indicated on bars. Statistical significance (ANOVA with Sidak correction) is compared to wild type. E. α-nrx::GFP (wyEx9588) expressed in DA9 localizes to synapses (marked by RAB-3) and is concentrated at the active zone, at the ventral tip of the presynaptic varicosity. F. α-nrx::GFP localization is unchanged in neuroligin mutants. G. γ-nrx::GFP (wyEx9639) expressed in wild type DA9 localizes to the same synaptic regions as α- nrx::GFP. Scale bars = 5μm.
Both the wy1155 (null) and the wy778(Δαγ) alleles showed indistinguishable presynaptic assembly phenotypes in DA9 (Figure 2D). To our surprise, the α-neurexin- specific deletion allele nu485(Δα), in which only γ-neurexin remains, did not exhibit presynaptic assembly defects (Figure 2B, D). We verified that γ-neurexin expression was not dramatically upregulated in these mutants, using RT-PCR (Supplemental Figure S4 related to Figure 2). To further establish that γ-neurexin was sufficient for presynaptic assembly, we isolated a γ-neurexin cDNA (the nrx-1m transcript in wormbase.org) from a whole-worm cDNA library and expressed it under the DA9-specific promoter in the nrx- 1(null) mutant background. We found that presynaptic assembly was fully rescued, similar to the effect of expressing a-neurexin (Figure 2D). In fact, this level of overexpression of γ-neurexin led to the formation of even more CLA-1 puncta than in wild type (Figure 2C, D), confirming that the region of the neurexin protein encoded by γ-neurexin is sufficient to drive presynaptic assembly.
To investigate the subcellular and sub-synaptic localization of NRX-1, we tagged both α- and γ-neurexin cDNAs with a GFP inserted just upstream of the C-terminal PDZ- binding domain (putting the GFP directly at the C-terminus led to diffuse expression through the neuron, indicating that the PDZ-binding domain may be important for mediating NRX-1 localization) and compared their expression patterns to that of RAB-3. Both NRX-1 isoforms localized specifically to synapses in DA9 and accumulated at the ventral tip of the presynaptic varicosity, where active zone proteins reside (Figure 2E, G). These results confirm that NRX-1 is a presynaptically localized protein.
To determine whether the localization of α-neurexin, which has a putative neuroligin binding domain, is dependent on neuroligin binding, we crossed our α- neurexin::GFP strain into nlg-1 mutants. We could not detect any differences in α- neurexin localization in nlg-1 mutants (Figure 2 F), indicating that neuroligin is not required for either the synaptic or subsynaptic distribution pattern of NRX-1.
Presynaptic calcium channel clusters require γ-NRX-l
In addition to its role in synapse assembly, neurexin has also been shown to play a role in calcium channel function and calcium-dependent synaptic vesicle release in vivo (Chen et al., 2017; Li et al., 2007; Missler et al., 2003). Recently neurexin was shown to bind the α2δ−3 subunit of calcium channels and to reduce CaV2.2 currents in vitro (Tong et al., 2017), but a direct role for neurexin in localizing calcium channels in vivo has not been demonstrated.
In C. elegans the presynaptic CaV2-type calcium channel, encoded by the unc-2 gene, is required for normal synaptic transmission (Richmond et al., 2001). To localize the CaV2 calcium channel, we inserted a HALO tag (Encell et al., 2012; Peterson and Kwon, 2013) into the first intracellular loop of UNC-2 in the endogenous locus using CRISPR. Endogenously tagging UNC-2 in this manner did not disrupt its function, as worms incorporating this tag exhibited wild type locomotion, while unc-2 mutant worms display defects in locomotion (Supplemental Figure 5 related to Figure 3). We labeled the protein in living animals using a membrane-permeable dye that is a covalent substrate of the HALO tag (Grimm et al., 2015). To localize neurexin at synapses, we inserted the photoswitchable fluorescent protein Skylan-S (Zhang et al., 2015) into the endogenous nrx-1 locus by CRISPR, just upstream of the C-terminal PDZ-binding domain. Skylan-S did not disrupt NRX-1 function, since presynaptic assembly assessed in DA9 was wild type (Supplemental Figure 5 related to Figure 3). We performed dual-color super-resolution localization microscopy of endogenous neurexin and CaV2/UNC-2. In wild type worms, NRX-1 and CaV2 were colocalized at synapses in the dorsal cord of the worm (Figure 3A, D). The degree of colocalization between NRX-1 and CaV2 was higher than between another active zone protein, RIM-binding protein (RIMB-1), and CaV2 (Figure 3B, D), suggesting a potential functional interaction between NRX-1 and calcium channels.
Figure 3. γ-Neurexin is required for presynaptic calcium channel clustering.
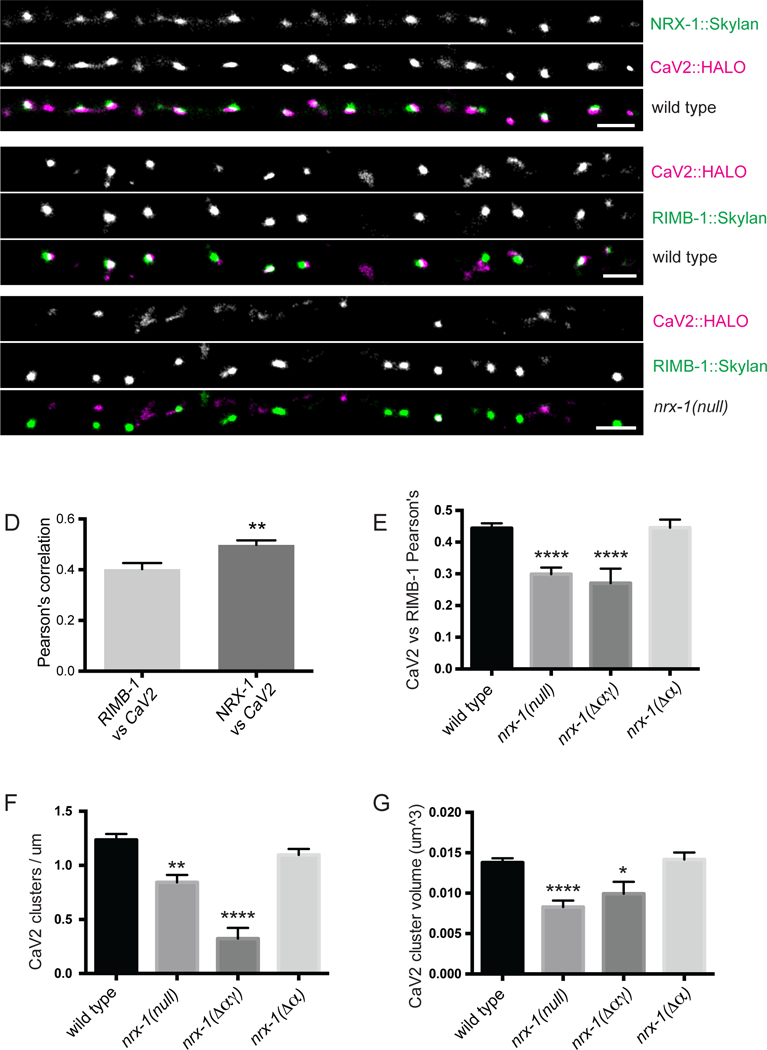
A. Single molecule localization microscopy (SMLM) super-resolution images of endogenously tagged neurexin (ox719) and CaV2 calcium channels (unc-2(ox672)). B-C. SMLM images of CaV2 calcium channels (unc-2(ox672)) and the active zone protein RIMB-1(ox704), in wild type (B) and nrx-1(wy1155) null mutants (C). Scale bar = 1µm. D. Pearson’s correlation coefficients indicate that NRX-1 colocalization with CaV2 is higher than RIMB-1 colocalization with CaV2. RIMB-1 vs CaV2, n = 23 images (from 6 worms); NRX-1 vs CaV2, n = 19 images (from 7 worms). Statistical significance determined by Student’s t-test. E. RIMB-1 colocalization with CaV2 is decreased in the nrx-1 null allele (wy1155) and in the Dag allele (wy778) but not in the Δα-specific allele (nu485). F-G. CaV2 channel cluster number per micron (F) and cluster volume (G) is reduced in the nrx-1 null allele (wy1155) and in the Dag allele (wy778) but not in the Δα- specific allele (nu485). For E-F, n = number of images and are as follows: wild type n = 28 (from 7 worms); nrx-1(null) n = 17 (from 3 worms); nrx-1(Δαγ) n = 13 (from 7 worms); nrx-1(Δα) n = 18 (from 7 worms). For G, n = number of clusters, with thresholds set to detect one cluster per synapse in wild type, and are as follows: wild type n = 597 (from 7 worms); nrx-1(null) n = 233 (from 3 worms); nrx-1(Δαγ) n = 71 (from 7 worms); nrx-1(Δα) n = 333 (from 7 worms). Statistical significance (ANOVA with Sidak correction in E and Kruskal-Wallis ANOVA in F,G) is compared to wild type.
To determine whether NRX-1 is required for localization of calcium channels, we examined CaV2 localization in nrx-1 mutants. In the absence of NRX-1, we used RIMB- 1 to mark active zones. In nrx-1 null mutants, CaV2 channels were greatly reduced at active zones (Figure 3C, E). Both CaV2 cluster number (Figure 3F) and cluster size (Figure 3G) were reduced in nrx-1 null mutants, which led to a reduction in colocalization between CaV2 and RIMB-1 (Figure 3E). Decreases in CaV2 were only observed in the null and Δαγ alleles. Specific loss of α-neurexin (Δα allele) did not affect calcium channel expression levels or clustering (Figure 3E, F, G), indicating that the γ- isoform of neurexin is sufficient for mediating proper calcium channel clustering.
γ-Neurexin is required for normal evoked neurotransmission
The disruption of calcium channel clustering in nrx-1 mutants suggested that synaptic transmission might be impaired. To test this, we used patch-clamp electrophysiology to record from the postsynaptic muscle cells of wild type and nrx-1 mutant worms. As expected, we found that the amplitudes of the evoked excitatory postsynaptic currents (eEPSCs) were significantly reduced in both the nrx-l(null) and nrx-1(Δαγ) alleles (Figure 4A, B). By contrast, and in agreement with previous reports (Hu et al., 2012; Tong et al., 2017), the eEPSC amplitudes remained unchanged in the nrx-1 (Δα) allele (Figure 4A, B). Consistent with a presynaptic role of γ-NRX-1, miniature current amplitudes were normal, indicating that postsynaptic receptors were likely fully functional and appropriately clustered (Figure 4C, D). Finally, the frequency of miniature currents was relatively normal in nrx-1 mutants (Figure 4C, E). This may be due to the fact that these currents reflect vesicle release from all types of motor neurons, while neurexin may play a larger role in synapse assembly at some neurons than in others. Alternatively, a reduction in synapse number may be compensated for by an increase in the frequency of spontaneous release at the remaining synapses.
Figure 4. γ-Neurexin is required for normal evoked release of synaptic vesicles.
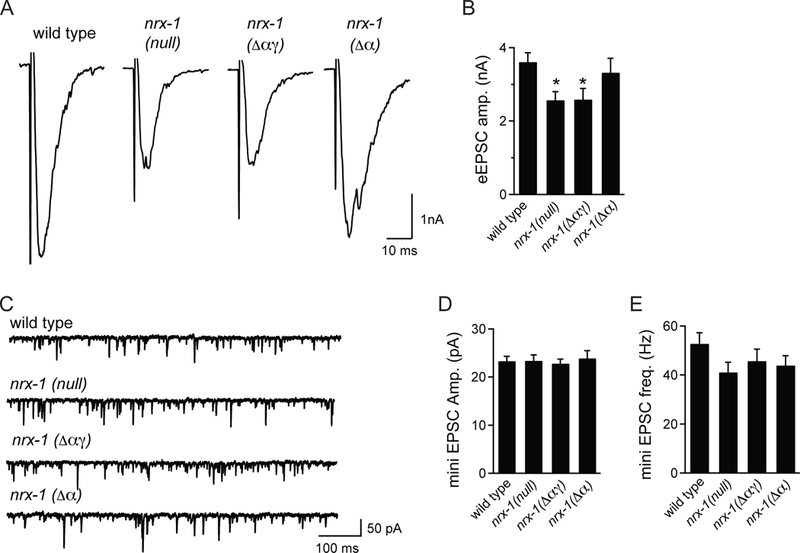
A.Example traces of evoked excitatory postsynaptic currents (eEPSCs) for the indicated genotypes. B. Evoked EPSCs are reduced in neurexin null allele (wy1155) and in the Δαγ allele (wy778) but not in the Δα-specific allele (nu485). Statistical significance (one-way ANOVA followed by Dunnett’s test) is compared to wild type; sample size (number of worms) indicated on bars. C. Example traces of miniature excitatory postsynaptic currents (mini EPSCs) for the indicated genotypes. D. Miniature EPSC amplitude is unchanged in neurexin mutants. E. Miniature EPSC frequency is unchanged in neurexin mutants.
To better understand how disrupted calcium channel localization in nrx-1(null) mutants affects release we measured evoked release in the presence of EGTA-AM, a cell- permeable slow Ca2+ chelator (Supplemental Figure 6 related to Figure 4). We found that EGTA did not inhibit the residual evoked response in nrx-1(null) mutants, suggesting that neurexin preferentially regulates synaptic vesicles that are EGTA-sensitive, and perhaps farther away from the calcium source.
Neurexin and Frizzled function in parallel to promote synapse assembly
Since many DA9 synapses remain in nrx-1 null mutants (Figure 1C), we reasoned that there must be additional pathways for presynaptic assembly. To uncover any parallel pathways, we created double mutants between nrx-1(null) and other synapse related genes and looked for enhanced synaptic phenotypes. We found that the seven-pass transmembrane receptor Frizzled (Fz)/LIN-17 was required for formation of the remaining synapses in nrx-1 mutants. Fz/Lin-17 single mutants had normal active zone numbers in DA9 (Figure 5A, B, E), although they did occasionally exhibit small ectopic RAB-3 puncta throughout the axon similar to nrx-1 mutants (data not shown). When combined with nrx-1(null) however, the resulting double mutants had a dramatic reduction in active zone number, with many worms having no or almost no remaining synapses (Figure 5C, E). Two different alleles of Fz/lin-17 mutants showed the same enhancement of synapse loss (Fig. 5E). Although Fz/LIN-17 is known to function postsynaptically to cluster acetylcholine receptors in the muscle (Jensen et al., 2012), the defect in presynaptic assembly was due to a cell-autonomous role for Fz/LIN-17 since it could be fully rescued (back to nrx-1(null) single mutant levels) by expression of Fz/LIN- 17 specifically in DA9 (Figure 5D, E).
Figure 5. Neurexin and Frizzled are parallel synapse assembly pathways.
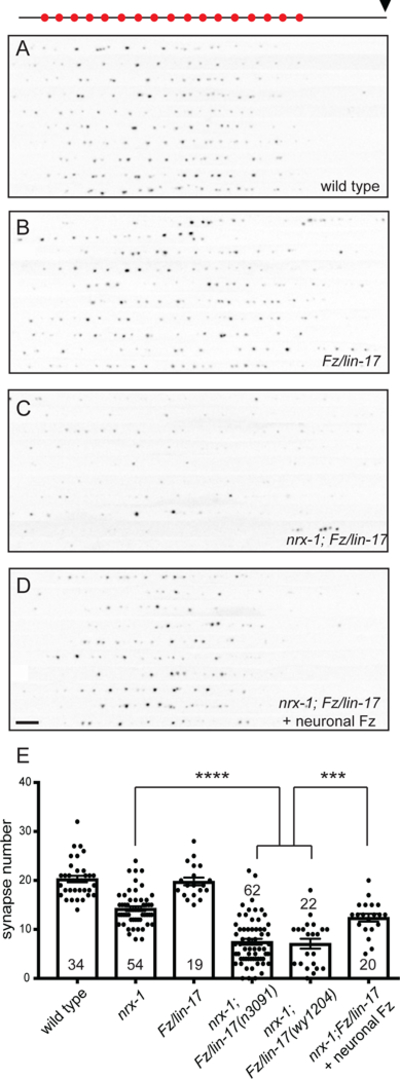
A-D. Dorsal axons of worms expressing the active zone marker CLA-1::GFP (wyIs685). Each panel is a compilation of the axons of 10 worms of the indicated genotype, aligned to the point at which the axon first reaches the dorsal cord (indicated by black arrowhead above panel A). Synapse number is normal in Fr/lin-17(n3091) mutants (B), but is markedly reduced in nrx-1(null);Fz/lin-17(n3091) double mutants (C). Presynaptic expression of Fz/LIN-17 (wyEx9376) can rescue synapse development (D). E. Quantification of synapse number (as defined by CLA-1 puncta number). Sample size (number of worms) indicated on bars. Statistical significance (ANOVA with Sidak correction) is compared as indicated.
The dramatic loss of CLA-1 puncta in nrx-1;Fz/lin-17 double mutants was accompanied by a loss of normal-sized presynaptic varicosities and RAB-3 clusters (Supplemental Figure S7A, B related to Figure 5). To test whether other active zone proteins were disrupted we analyzed worms expressing the active zone protein Liprin- α/SYD-2 endogenously tagged with GFP. In the posterior dorsal cord, where DA9 synapses normally form, both the number and intensity of SYD-2::GFP puncta were reduced (Supplemental Figure S7C-F related to Figure 5), suggesting a general disruption in active zone assembly.
To determine whether the loss of synapses in nrx-1;Fz/lin-17 double mutants had functional repercussions, we utilized an optogenetic behavioral assay to probe DA9- specific functional connectivity. The DA9 motor neuron is required for activation of the dorsal tail muscles, which when contracted lead to a dorsal tail bend. Expression of the red-shifted ChannelRhodopsin Chrimson (Klapoetke et al., 2014; Schild and Glauser, 2015) specifically in DA9 allows for temporally-precise DA9 activation along with visual analysis of dorsal tail bending in freely moving worms (Ding and Hammarlund, 2018). Wild type worms exhibit a highly synchronized and stereotyped dorsal tail bend upon green-light activation of DA9 Chrimson (Figure 6A). In contrast, nrx-1;Fz/lin-17 double mutants exhibited a weaker, more variable, unsynchronized, and at times completely absent response (Figure 6B), demonstrating that double mutants were more severely deficient in tail bending than either single mutant (Figure 6C, D). Together these data demonstrate that the loss of presynaptic specializations in nrx-1;Fz/lin-17 double mutants leads to a reduction in functional connectivity.
Figure 6. Frizzled and neurexin together promote functional synapse development in DA9.
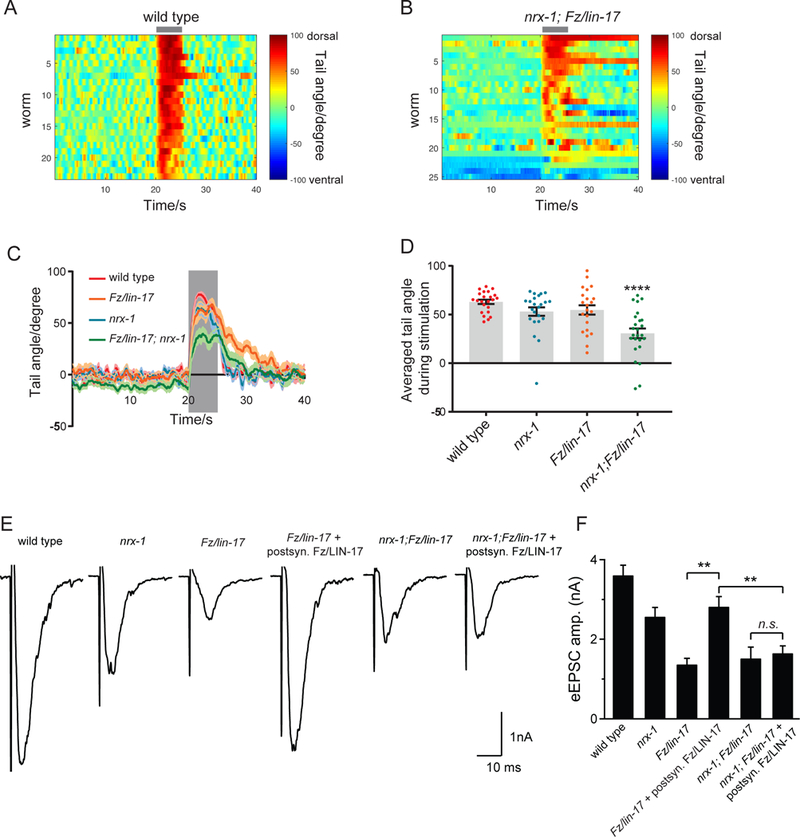
A,B. Compiled heat maps of tail bending in response to Chrimson stimulation in DA9 (wpIs98). Wild type worms (A) always exhibit a robust and stereotyped dorsal bending response upon light stimulation (indicated by gray bar above panel). Nrx-1(null);Fz/lin-17(n3091) double mutants (B) exhibit a weaker response of variable duration, and sometimes fail to respond at all. C. Combined responses for each genotype showing the enhancement of nrx-1;Fz/lin-17 double mutants compared to single mutants. D. Average tail angle during stimulation for each genotype. Statistical significance (ANOVA with Sidak correction) is compared to control. E. Example traces of evoked excitatory postsynaptic currents (eEPSCs) for the indicated genotypes. F. Evoked EPSCs are reduced in Fz/lm-17(n3091) single mutants but can be rescued by postsynaptic expression of Fz/LIN-17 under a muscle promoter (akEx2422). eEPSCs are similarly reduced in nrx-1(null);Fz/lin-17(n3091) double mutants, but can no longer be rescued by postsynaptic Fz/LIN-17 (αkEx2422), revealing the contribution of presynaptic Fz/LIN-17 in the absence of NRX-1. Sample size (number of worms) indicated on bars. Statistical significance (one-way ANOVA followed by Dunnett’s test) is compared as indicated; sample size indicated on bars.
To precisely understand the contribution of NRX-1 and Fz/LIN-17 to presynaptic release, we recorded evoked responses from ventral body wall muscles (Figure 6E, F). Fz/LIN-17 has been shown to be required for postsynaptic acetylcholine receptor clustering in these muscles (Jensen et al., 2012). To investigate the presynaptic function of Fz/LIN-17, we made use of a transgene that selectively expresses lin-17 in muscles and rescues Fz/lin-17 postsynaptically. In agreement with published results from these synapses (Jensen et al., 2012), we found that evoked responses in Fz/lin-17 single mutants were dramatically reduced compared to wild type, and this defect could be largely rescued by postsynaptic expression of Fz/LIN-17 (Figure 6E, F). In contrast, nrx- 1;Fz/lin-17 double mutants, which also exhibited reduced eEPSC responses, could not be rescued with postsynaptic Fz/LIN-17 (Figure 6E, F), revealing a presynaptic role of Fz/LIN-17 in the absence of NRX-1. Thus, comparing the elimination of either neurexin (Figure 6F, second bar), or presynaptic Frizzled (Figure 6F, fourth bar), with the elimination of both neurexin and presynaptic Frizzled together (Figure 6F, last bar) reveals a more severe deficit in the absence of both presynaptic proteins. Together, these data show that LIN-17 and NRX-1 act in parallel to regulate the formation and activity of presynaptic compartments.
Frizzled is known to signal through several downstream pathways (Li et al., 2005; Wang et al., 2016). To determine whether any of the known signaling pathways were involved in presynaptic assembly, we assessed mutants for several other genes (Dishevelled, dsh-1; Ryk, lin-18; Ror, cam-1; Celsr/Flamingo, fmi-1; Van Gogh, vang-1), both singly and in conjunction with nrx-1 null mutations. None of the genes investigated generated a phenotype comparable to the nrx-1;Fz/lin-17 double mutants (data not shown), suggesting that Frizzled’s role in presynaptic assembly might be mediated by an as yet unidentified downstream signaling pathway.
Frizzled ligand Wnt/LIN-44 antagonizes Neurexin- and Frizzled-mediated presynaptic assembly
The Frizzled receptor, a member of the G-protein coupled receptor (GPCR) family, is commonly activated by the binding of its ligand, Wnt (Wodarz and Nusse, 1998; Figure 7A). In DA9, however, Wnt mediates synapse elimination (Klassen and Shen, 2007). The Wnt LIN-44 is secreted by non-neuronal cells in the tail (Figure 7B) and sets the posterior boundary of the DA9 synaptic domain by inhibiting synapse formation in the most posterior region of the axon. In Wnt/lin-44 mutants, synapses form in the dorsal asynaptic region (red bar in Fig. 7C) of the DA9 axon (Figure 7C, D).
Figure 7. Loss of synapses in neurexin mutants is due to Wnt-mediated elimination.
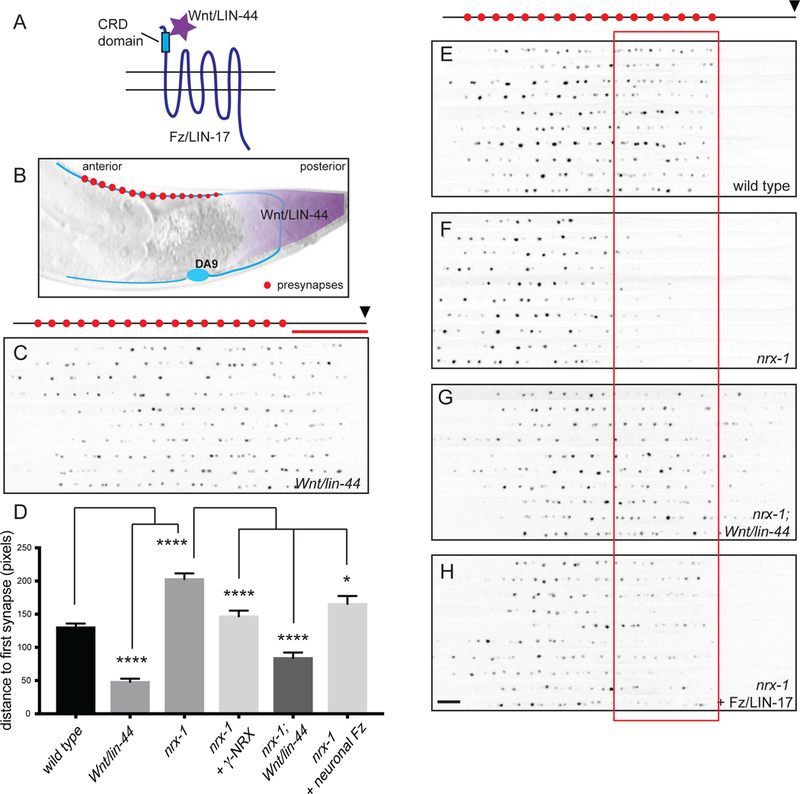
A. The Wnt ligand binds to the extracellular cysteine-rich domain (CRD) of Frizzled. B. Wnt/LIN-44 is secreted by cells in the tail of the worm and mediates synapse elimination in the dorsal asynaptic domain of DA9. C. Compilation of the dorsal axons of 10 Wnt/lin- 44 mutant worms expressing the active zone marker CLA-1::GFP (wyIs685), aligned to the point at which the axon first reaches the dorsal cord (indicated by black arrowhead above the panel). Note the appearance of synapses in the asynaptic domain, denoted by the red line above the panel. D. Quantification of the distance from the point at which the axon reaches the dorsal cord to the first CLA-1 puncta, measured in pixels. Sample size (number of worms) indicated on bars. Statistical significance (ANOVA with Sidak correction) is compared as indicated. E-H. Dorsal axons of worms expressing the active zone marker CLA-1::GFP, as in C. nrx-l(null) mutants (F) selectively lack synapses in the posterior half of the synaptic domain, indicated by the red box. Loss of Wnt/LIN-44 (n1792) (G) or presynaptic expression of Frizzled/LIN-17 (wyEx9374) (H) rescues this defect. Scale bar = 5μm.
A careful analysis of nrx-l(null) mutants revealed a spatial dimension to the loss of synapses, with a specific reduction in the posterior half of the synaptic domain (compare red boxed region in Figure 7 E, F, quantified in D). This defect could be fully rescued by expression of presynaptic γ-neurexin (Figure 7D) and was also apparent for other active zone proteins such as endogenously-tagged SYD-2/Liprin-α (Supplemental Figure 8 related to Figure 7). We wondered whether the loss of posterior synapses in nrx- 1 mutants was due to Wnt-mediated synapse elimination. To test this, we analyzed nrx- 1;Wnt/lin-44 double mutants. Indeed, we found that loss of Wnt/LIN-44 completely suppressed the loss of posterior synapses in nrx-1 mutants (Figure 7D, G). This double mutant phenotype is in stark contrast to the enhanced synapse loss in nrx-1;Fz/lin-17 double mutants (Figure 5C). nrx-1;Fz/lin-17;Wnt/lin-44 triple mutants were indistinguishable from nrx-1;Fz/lin-17 double mutants (data not shown), indicating that Frizzled signaling is downstream of Wnt. Moreover, over-expression of Fz/LIN-17 could rescue the loss of posterior synapses in nrx-1 mutants (Figure 7 D, H), indicating that increased levels of Frizzled receptor can compensate for the loss of neurexin. Together these data suggest that in the absence of neurexin-dependent synapse stabilization, Wnt- mediated synapse elimination can overwhelm Frizzled-mediated synapse assembly.
Wnt induces Frizzled endocytosis
The discovery that Frizzled promotes synapse assembly seemed to be in apparent conflict with the fact that its ligand Wnt functions via Frizzled to eliminate synapses (Klassen and Shen, 2007). It suggested that Wnt binding to Frizzled might act to shut down Frizzled’s otherwise pro-synaptogenic function. If this were true, we would predict that Wnt binding might lead to the removal of Fz from the cell surface (since GPCR signaling is often down-regulated through ligand-binding mediated endocytosis), and secondly that Frizzled’s pro-synaptogenic function should be independent of Wnt binding.
When expressed in DA9, YFP-tagged Fz/LIN-17 exhibits a striking localization pattern: most of the signal is found in intracellular vesicle-like puncta located exclusively to the posterior asynaptic domain, while the synaptic domain contains only a barely detectable diffuse component (Figure 8A and Klassen and Shen, 2007). This localization pattern is dependent on Wnt binding, as in Wnt/lin-44 mutants, the asynaptic Fz/LIN- 17::YFP vesicles are absent and the diffuse component is much brighter (Figure 8B and Klassen and Shen, 2007).
Figure 8. Frizzled promotes synapse assembly independent of Wnt binding, which instead mediates Frizzled endocytosis.
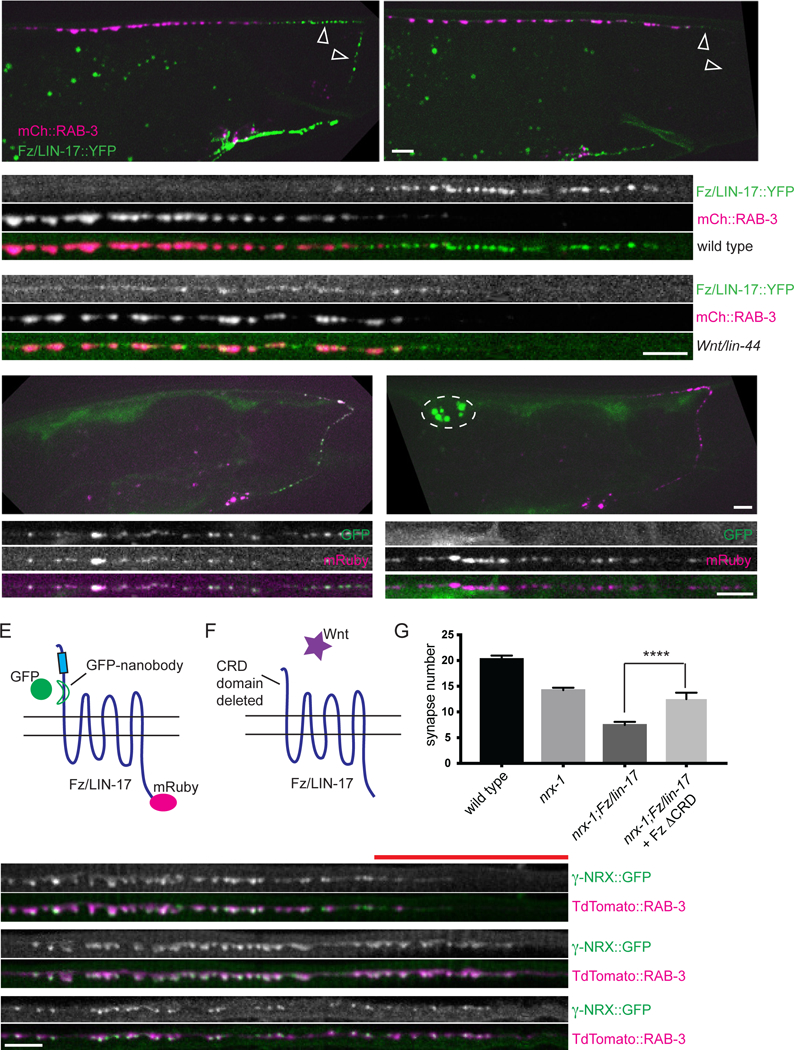
A. In wild type worms, Fz/LIN-17::YFP (wyEx677) localizes primarily to intracellular puncta in the asynaptic region of the DA9 axon (white arrowheads). A barely detectable diffuse component can sometimes be seen (A’) in the synaptic region (marked with RAB-3::tdTomato). B. In Wnt/lin-44 mutants, Fz/LIN-17::YFP puncta are gone from the asynaptic region (white arrowheads), and the diffuse component is much more apparent (B’). Insets are of straightened DA9 axons beginning from the start of the commissure (marked with an asterisk). Scale bar = 5μm. C. Worms expressing GFP secreted from the muscle and Fz/LIN-17 in DA9 tagged with a GFP nanobody on the extracellular side and mRuby on the intracellular side. D. Worms expressing GFP secreted from the muscle and Fz/LIN-17::mRuby in DA9. Dashed circle indicates secreted GFP accumulating in the coelemocyte scavenger cells. Insets are of straightened DA9 axons beginning from the start of the commissure (marked with an asterisk). Scale bar = 5μm. E. Schematic showing the location of the Frizzled GFP nanobody and mRuby tags used in C. F. Schematic showing a version of Frizzled with its Wnt-binding CRD domain deleted (Fz/LIN-17 ΔCRD). G. Fz/LIN-17 ΔCRD (wyEx9386) rescues synapse formation in nrx-l;Fz/lin-17 double mutants. Sample size (number of worms) indicated on bars. Statistical significance (ANOVA with Sidak correction) is compared as indicated. H-J. γ-NRX::GFP and RAB-3::tdTomato co-expressed in DA9 (wyEx9639). Note the expansion of γ-NRX::GFP localization into the asynaptic domain (marked by a red line above H) in Wnt/lin-44 and Fz/lin-17 mutants (I,J). Scale bar = 5μm.
The Fz/LIN-17-containing vesicles could either be secretory vesicles, destined for the cell surface, or endocytic vesicles, recovered from the cell surface. To differentiate between these two possibilities, we devised a strategy that would allow us to determine whether the Fz receptor located within the vesicle had ever resided on the cell surface. We swapped the Fz C-terminus YFP tag for a red mRuby tag, and then added a small GFP nanobody sequence (Ryckaert et al., 2010) to a flexible region just downstream of the Wnt-binding CRD domain (Figure 8E). We then expressed a secreted form of GFP from muscle cells, which could bind the Fz nanobody if the receptor were ever located on the cell surface. We found that all the Fz-containing vesicles were both red and green (Figure 8C), indicating that these were indeed endocytosed receptors. We saw no punctate intracellular GFP signal in worms expressing muscle secreted GFP and Fz/LIN- 17::mRuby without the extracellular GFP nanobody (Figure 8D), indicating that the GFP signal was specific to interaction with the nanobody.
If Wnt binding turns off Fz-mediated presynaptic assembly by causing its endocytosis, then Fz’s pro-synaptogenic function might be independent of Wnt binding. To test this we expressed a version of Fz/LIN-17 lacking the Wnt-binding CRD domain (Figure 8F) in nrx-1;Fz/lin-17 double mutants. We found that Fz/LIN-17ΔCRD could fully rescue the double mutants back to the nrx-1 single mutant synapse number (Figure 8G). Together these data support a model in which Frizzled promotes synapse assembly independent of Wnt binding, while Wnt functions to eliminate synapses by inducing the endocytosis of Frizzled.
To better understand how Wnt and Frizzled impact neurexin, we assessed γ-NRX localization in Wnt/lin-44 and Fz/lin-17 mutant backgrounds (Figure 8H-J). In the absence of either Wnt/LIN-44 or Fz/LIN-17, γ-NRX still clustered normally at active zones (underscoring its parallel role to Frizzled), but synapses containing γ-NRX clusters now formed in the asynaptic domain (marked by a red line above Figure 8H), indicating that γ-NRX elimination from the asynaptic domain in DA9 is dependent on Wnt- mediated Frizzled endocytosis. Together these data support a model in which neurexin and Frizzled mediate parallel pathways for synapse formation within the synaptic domain but are jointly modulated by Wnt-mediated synapse elimination in the asynaptic domain.
Discussion
The cell-autonomous function of cell adhesion molecules and other transmembrane receptors in presynaptic assembly in vivo is largely enigmatic. Here we describe an in vivo role for the newly identified gene product γ-neurexin and demonstrate that it is both necessary and sufficient for presynaptic assembly in excitatory motor neurons. Neurexin null mutants, but not α-neurexin-specific deletion alleles, exhibit defects in active zone assembly, synaptic vesicle recruitment, and calcium channel clustering. As a result, evoked synaptic transmission is impaired. However, many synapses still remain, suggesting the presence of parallel synapse assembly pathways. Indeed, we find that neurexin and the Wnt receptor Frizzled act in parallel to promote presynaptic assembly: in double mutants, synapses are dramatically reduced, and the function of this motor circuit is severely perturbed. This pro-synaptogenic function of Frizzled is Wnt-independent and is in contrast to the synapse-eliminating function of Wnt itself. We demonstrate that in the asynaptic domain, Wnt causes the endocytosis of Frizzled, a process that presumably both abrogates Frizzled’s own synapse assembly function and also serves to downregulate neurexin. Together these data reveal how multiple signaling pathways converge on the process of synapse assembly, and how both positive and negative regulators contribute to sculpting a functional synaptic circuit.
γ-Neurexin plays an important role in vivo
The presence of an additional alternative start site for a novel, short isoform of neurexin was first predicted computationally, using comprehensive analysis of deep RNA-Seq data of mouse cortex (Yan et al., 2015). The prediction was validated by the discovery that a mouse containing an HA-tag knocked in just before the transmembrane domain produced a short neurexin protein that corresponded in size to the predicted γ- isoform (Sterky et al., 2017). This short isoform was shown to be expressed differentially in various brain regions, and to be dynamically regulated during development, suggestive of a specific developmental function (Sterky et al., 2017). However, what specific role this isoform played in synaptic development remained unknown. Intriguingly, mouse mutants in which all α- and β-neurexins were deleted by conditional excision of their start sites still exhibited significant synapse assembly and function, although the extent was dependent on the specific brain region and cell type (Chen et al., 2017). Together these results raised the possibility that g-neurexin may be sufficient for many aspects of presynaptic assembly.
In cultured neurons, neurexin clustering has been shown to induce the recruitment of synaptic vesicles (Dean et al., 2003). The intracellular C-terminal PDZ-binding domain of neurexin binds the synaptic vesicle protein synaptotagmin as well as the scaffolding proteins Cask and Mint (Biederer and Südhof, 2000, 2001; Hata et al., 1996; Mukherjee et al., 2008; Sun et al., 2009). Drosophila neurexin has been shown to interact with the active zone protein SYD-1 (Owald et al., 2012) as well as the actin binding protein spinophilin (Muhammad et al., 2015). Our results now show that γ-neurexin is sufficient to mediate intracellular interactions required for properly clustering active zone proteins, synaptic vesicles and calcium channels in vivo.
The extracellular domain of mammalian γ-neurexin consists of a short region containing an O-linked sugar modification sequence and a short cysteine-loop domain. This region has been shown to bind C1q-like proteins (Matsuda et al., 2016), and to be a binding site within the secretory pathway for chaperone proteins of the carbonic anhydrase family (Sterky et al., 2017). Since C. elegans lack homologs for C1q, additional proteins may exist that act as extracellular ligands for this short isoform of neurexin.
Alternatively, γ-neurexin may function purely as a presynaptic organizer, independent of extracellular activation. This is in contrast to α-neurexin, which functions in these same neurons to trans-synaptically recruit and cluster postsynaptic receptors (Philbrook et al., 2018). The segregation of pre- versus postsynaptic functions of γ- and α-neurexin, respectively, may allow neurons to tightly regulate the expression of synaptic partner specificity genes such as α-neurexin, without losing the presynaptic assembly functions of their intracellular domains.
Parallel pathways mediate presynapse assembly
The severity of the phenotype in nrx-1;Fz/lin-17 double mutants underscores the importance of these two proteins for synapse assembly in this neuron. Very few mutations have been found, to date, that can lead to the complete loss of presynapses (i.e.- concomitant active zone protein and synaptic vesicle accumulations), with the exception of mutations in motors that transport synaptic material (Hall and Hedgecock, 1991). Among the most severe synapse assembly mutants described in C. elegans are those of the active zone protein SYD-2/Liprin-α (Zhen and Jin, 1999), yet many synapses still form in those mutants. Abrogating synapse formation completely may thus require eliminating all parallel pathways functioning in a given neuron.
Synapse elimination is achieved via endocytosis of pro-synaptogenic proteins
It is increasingly clear that synaptic circuits are sculpted by a plethora of both pro- and anti-synaptogenic cues. Synaptic adhesion molecules such as neurexin were thought to initiate this process by binding their synaptic partners, while proteins secreted by non-neuronal cells, such as Wnts or astrocyte-secreted SPARC in vertebrates (Kucukdereli et al., 2011), have been shown to antagonize synapse formation. However, exactly how positive and negative regulators of synapse assembly work in conjunction to sculpt a specific circuit in vivo is less clear.
Many studies have now implicated the Wnt signaling pathway in synaptogenesis (Dickins and Salinas, 2013; Rosso et al., 2013), as both a positive and negative regulator (Park and Shen, 2012). In fact, it has been proposed that its ability to both positively and negatively regulate synapse formation might be attributed to whether the canonical or non-canonical signaling pathway is activated (Davis et al., 2008). Our data suggest that Wnt and Frizzled can act antagonistically and thereby either inhibit or stimulate synapse formation. These data provide the first evidence that Wnt binding may serve to downregulate rather than activate Frizzled signaling in synaptogenesis. Moreover, the fact that mutants for other proteins known to be downstream of Wnt-mediated Frizzled signaling did not have a severe effect on synapse assembly suggests the Fz may signal through an as yet unidentified pathway.
Wnt binding is known to promote Frizzled’s endocytosis (Blitzer and Nusse, 2006), however this has been shown to result in activation rather than inhibition of signaling. In C. elegans, Wnt-mediated Frizzled endocytosis leads to the destabilization of neurexin, as exclusion of NRX-1 from the asynaptic domain is dependent on both Wnt and Frizzled (Figure 8H-J), and synapses generated by the over-expression of neurexin in the presence of Wnt do not form in the asynaptic zone (Figure 7D). Thus, in regions where Wnt is not present, either γ-neurexin or Frizzled can act redundantly to organize presynaptic assembly. By contrast, in asynaptic regions, Wnt binding to Frizzled causes its endocytosis and the elimination of both Frizzled and γ-neurexin, precisely removing both proteins capable of organizing synapse assembly.
STAR Methods
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Peri Kurshan (pkurshan@gmail.com).
Experimental Model and Subject Details
Strains and genetics
Worms were raised on NGM plates at 20°C using OP50 Escherichia coli as a food source. Transgenic animals were prepared by microinjection. Unless otherwise noted, animals were analyzed at the L4 larval stage. A full strain list is included in the Key Resources table.
Method Details
Molecular biology and transgenic lines
Expression clones were made in the pSM vector (Shen and Bargmann, 2003). The plasmids were generated using standard techniques and transgenic strains were prepared by microinjection (using 1–5 ng/μl of plasmid DNA) and coinjected with markers Podr-1::RFP (100 ng/µl) or Podr-1::GFP (100 ng/µl).
Generation of deletion alleles by CRISPR/cas9
To create nrx-1, Fz/lin-17 and nlg-1 null alleles, we chose sgRNAs designed to delete the entire open reading frame. sgRNAs were injected at 30ng/µl along with Cas9 plasmid at 50ng/µl and F2 worms were screened by PCR. The resulting deletions are flanked by the following sequences: nrx-1(wy1155): 5’: ttgcaacacctccctggtgt, 3’: tcaacggtgttcataagaag. nlg-1(wy1190): 5’: accatgtgatttatctaccc, 3’: tgaaatgtacacatccattg. Fz/lin-17(wy1204): 5’: ccaccacgaagttcataagt, 3’: atacatgcaaatggcgacga.
RT PCR
RNA from wild type (N2 Bristol) and nrx-l(nu485) worms was prepared using Trizol (Sigma Aldrich). A cDNA library was created by reverse transcription using SuperScript III First-Strand Synthesis SuperMix (Invitrogen). PCR amplification was conducted in triplicate using primers against the C-terminal intracellular domain of nrx-l, as well as against the housekeeping gene eif-3.C (a translation initiation factor), for variable cycle durations to assess expression levels.
Generation of CaV2::HALO by CRISPR/cas9
CaV2 was tagged by CRISPR-mediated insertion of HALO coding DNA into the unc-2 endogenous genomic locus. A DNA mix containing 1) PCR-generated DNA repair template that includes the HALO tag with an embedded unc-119(+) cassette flanked by loxP sites and 33bp homology arms to the cut site (Lo et al., 2013), 2) plasmid DNA that directs expression of Cas9 and an sgRNA (Schwartz and Jorgensen, 2016), and 3) an inducible negative selection plasmid directing expression of a histamine-gated chloride channel in neurons, pNP403 (Pokala et al., 2014) was injected into the gonads of young adult EG6207[unc-119(ed3)] animals (Maduro and Pilgrim, 1995). Transgenic animals were selected for expression of unc-119(+), and extrachromosomal-array bearing animals were selected against by addition of histamine to the media. The loxP::unc-119(+)::loxP region of the insertion was excised by injecting a CRE expressing plasmid and identifying unc-119(−) animals (Dickinson et al., 2013). The modified locus introduces HALO-tag within an unconserved region in the first intracellular loop of CaV2 encoding unc-2a. The resulting strain EG9823[unc-119(ed3); unc-2(ox672[HALO])] was subsequently used to generate CRISPR-mediated insertions of SKYLAN-S tags.
Generation of SKYLAN-S tags by CRISPR/cas9
NRX-1 and RIMB-1 were tagged 12 and 4 amino acids from the c-termini of each, respectively. A single plasmid containing sgRNA and the repair template, composed of 57bp homology arms and SKYLAN-S containing a loxP::unc-119(+)::loxP, was appended by SapTrap plasmid assembly (Schwartz and Jorgensen, 2016). Each assembled plasmid was mixed with plasmids to express Cas9 in the germline, and HisCL- in neurons, and injected into the gonads of young adult EG9823 animals. After selecting for unc-119(+) and selecting against extrachromosomal arrays by histamine application, animals were injected with pDD104[Peft-3::CRE], selected for excision of loxP::unc-119::loxP, and outcrossed once before analysis by super-resolution microscopy.
Behavior analysis of unc-2(ox672[HALO]) expressing animals.
The movement and behavior of wild type (N2), unc-2(lj1), and unc-2(ox672[HALO]) synchronized adult animals were analyzed 24 hours after the L4-stage molt.
Animals were raised at 18oC on NGM plates seeded with OP50 bacteria and adjusted to 22oC for 3 hours. Groups of 4 animals were transferred onto 10cm NGM plates without food by a glass pick and tracked for 5-minutes by a Stingray F-504 camera (Allied Vision) positioned with a 3.9cm field-of-view. Recordings were analyzed with WormLab v2018.1.1 (MBF Bioscience) to determine movement speed, the frequency of animal reversals, and sinusoidal movement wavelength.
Confocal microscopy
Images of fluorescently tagged fusion proteins were captured at room temperature in live C. elegans. Around 20 mid-L4 stage hermaphrodite animals were anesthetized using 10 mM levamisole (Sigma-Aldrich) in M9 buffer, mounted on 5% agar pads and imaged with a Zeiss Axio Observer Z1 microscope equipped with either a Plan-Apochromat 63x or 40× 1.4NA objective and a Yokagawa spinning-disk unit attached to an EM-CCD camera. Animals were selected for imaging based on the correct stage of vulval development using DIC optics, and then all images collected were included for analysis. Maximum-intensity projections and cropped, straightened images were generated using ImageJ (NIH) and composite images were generated in Adobe Photoshop. Brightness/contrast were adjusted in Adobe Photoshop to show relevant features, treating any images being compared in the same manner.
Single molecule localization super-resolution microscopy
Super-resolution images were recorded with a Vutara SR 350 (Bruker Nanosurfaces, Inc., Madison, WI) commercial microscope based on single molecule localization biplane technology (Juette et al., 2008; Mlodzianoski et al., 2009). Synchronized late L4 stage C. elegans expressing HALO-tagged proteins (Encell et al., 2012; Mollwitz et al., 2012) were stained in 50uM of HALO-JF646 (Gift of Luke Lavis, Janelia Farms; Grimm et al., 2015). Animals were recovered 12 hours at 15degC on agar seeded with OP50 bacteria. Live intact young adult animals were anesthetized in 25mM NaN3 and regions of their dorsal cords that were positioned directly against the cover glass and away from the intestine were imaged with 647nm excitation power of 8kW/cm2. SKYLAN-S was imaged by 488nm excitation at 1kW/cm2, while photoactivated by 405nm light. Images were recorded using a 60x/1.2 NA Olympus water immersion objective and Hamamatsu Flash4 V1 sCMOS camera with gain set at 50 and frame rate at 50 Hz. Data was analyzed by the Vutara SRX software (version 6.02). Single molecules were identified by their brightness frame by frame after removing the background. Identified molecules were localized in three dimensions by fitting the raw data in a 12×12-pixel region of interest centered around each particle in each plane with a 3D model function that was obtained from recorded bead data sets. Fit results were filtered by a density based denoising algorithm to remove isolated particles. The remaining localizations were classified into clusters by density-based spatial clustering of applications with noise (DBSCAN), a minimum of 10 localizations were connected around a 100nm search radius.
Localizations were rendered as 50nm points for analysis by Pearson’s correlation. The experimentally achieved image resolution of 40nm laterally (x,y) and 70 nm axially (in z) was determined by Fourier ring correlation.
Electrophysiology
Young adult worms were immobilized on Sylgard-coated coverslips with cyanoacrylate glue (Histoacryl Blue, Aesculap). N2 Bristol was used as the wild type reference strain. Animals were dissected in extracellular solution via a dorsolateral incision and removal of the gonad and intestines to reveal the underlying ventral nerve cord and body-wall- muscle quadrants as previously described (Dong et al., 2015; Richmond and Jorgensen, 1999). The worm prep was mounted onto a fixed stage upright microscope (BX51WI, Olympus) equipped with a 60x water-immersion objective lens. Whole-cell patch clamp recordings were carried out at 20°C. A body-wall muscle cell was voltage clamped at −60 mV to record postsynaptic currents. Evoked EPSC responses were induced by applying a 0.4 ms, 30 μΑ pulse, generated by a stimulus isolator (A365, WPI), though a borosilicate pipette (~2 ΜΩ) placed in close apposition to the ventral nerve cord. Series resistance was compensated to 70% for the evoked EPSC recording. The currents were amplified using EPC-10 (HEKA). The signals were sampled at 10 kHz using Patchmaster (HEKA), following low-pass filtering at 2 kHz. Patch pipettes (2–5 ΜΩ) were pulled using borosilicate glass and were fire polished. Extracellular solution contains (in mM) 150 NaCl, 5 KCl, 1 CaCl2, 5 MgCl2, 10 glucose and 10 HEPES, titrated to pH 7.3 with NaOH, 330 mOsm with sucrose. Internal solution contains 135 CH3O3SCs, 5 CsCl, 5 MgCl2, 5 EGTA, 0.25 CaCl2, 10 HEPES and 5 Na2ATP, adjusted to pH 7.2 using CsOH. For EGTA experiments, dissected worms were incubated in the external solution that contains 30 μΜ EGTA-AM for 10 minutes prior to electrophysiological recording. All chemicals were purchased from Sigma. Electrophysiological data were analyzed using Igor Pro 6 (Wavemetrics, OR) with custom-written software. Statistical analysis was performed using Igor Pro 6.
DA9 tail flip assay
Worms expressing Chrimson in DA9 were cultured with OP50 containing 10 mM all- trans-retinal. One day old adult progeny were transferred to a fresh plate without bacteria before the behavior assay. The plate was then placed on a Leica M165FC stereo scope. A Basler acA2440 camera mounted on the scope controlled by WormLab software was used to record movies. Continuous light stimulation was generated by a Prior lumen 200 light box coupled with a Leica mCherry filter set. Light intensity was about 300 W/m2. Bright field illumination was kept at low intensity to avoid any non-specific stimulation of Chrimson. The tail bending behavior was later analyzed using the Wormlab software. In brief, the animal was detected by thresholding, and then the body midline was segmented evenly with 14 points. The angle between the last three points was calculated as the tail angle for each frame.
Quantification and Statistical Analysis
Confocal image puncta quantification
Puncta number was quantified using a custom Matlab (Mathworks, Natick MA) script to count and measure peaks above threshold from maximum intensity plot profiles of segmented line scans. The threshold was determined by analyzing a distribution of all peaks to identify the mean amplitude of the background signal, then setting the threshold to be a defined number of sigmas above that mean. Quantification and threshold determination was thus independent of any variability in background intensity due to fluctuations in laser power between imaging sessions. Since quantification was automated and unbiased it therefore did not require blinding. For SYD-2::GFP analyses, a low signal-to-noise ratio and variable background intensity required the addition of a dynamic baselining algorithm that fit a smoothing spline to the background signal before thresholding.
Statistical analysis
Quantitation and statistical details can be found in the figure legends or Method Details section. Unless otherwise indicated, sample size refers to the number of worms, and is indicated on the bars of each graph. All data are reported as mean ± SEM. Data was tested for normalcy and statistical significance between means was calculated using one-way ANOVA with Tukey’s post-hoc analysis (for comparisons to wild type control), Dunnett’s post-hoc analysis (for comparisons within all groups) or Kruskal-Wallis test (for non-parametric data), as indicated in figure legends. * p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001.
Supplementary Material
Highlights.
γ-neurexin, which lacks canonical extracellular domains, promotes synapse assembly
γ-neurexin functions in parallel with Frizzled to promote synapse formation
Wnt eliminates synapses via Frizzled endocytosis and neurexin downregulation
Acknowledgements
The authors would like to thank the Caenorhabditis Genetics Center (CGC) for strains. We also thank J. Kaplan for the nrx-1(nu485) allele, X. Tong for nu485 super res imaging strains, A.V. Maricq for the Fz/LIN-17 postsynaptic rescue strain (akEx2422), L. Lavis for super resolution dyes, C. Richardson for input on the endocytosis assay and A. Draycott for technical assistance. Thanks to members of the Shen lab for discussions and critical review of the manuscript. This work was supported by the Howard Hughes Medical Institute (KS and EMJ) and the NIH (KS: R01-NS103037, R01-NS091144; EMJ: R01- NS034307; JB: R01-GM127857; MH: R01-NS094219 and R01-NS098817).
Footnotes
Declaration of Interests
EMJ is a consultant for Bruker Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aoto J, Földy C, Ilcus SMC, Tabuchi K, and Südhof TC (2015). Distinct circuit- dependent functions of presynaptic neurexin-3 at GABAergic and glutamatergic synapses. Nat. Neurosci. 18, 997–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banovic D, Khorramshahi O, Owald D, Wichmann C, Riedt T, Fouquet W, Tian R, Sigrist SJ, and Aberle H (2010). Drosophila neuroligin 1 promotes growth and postsynaptic differentiation at glutamatergic neuromuscular junctions. Neuron 66, 724–738. [DOI] [PubMed] [Google Scholar]
- Biederer T, and Südhof TC (2000). Mints as Adaptors. J. Biol. Chem. 275, 39803–39806. [DOI] [PubMed] [Google Scholar]
- Biederer T, and Südhof TC (2001). CASK and Protein 4.1 Support F-actin Nucleation on Neurexins. J. Biol. Chem. 276, 47869–47876. [DOI] [PubMed] [Google Scholar]
- Blitzer JT, and Nusse R (2006). A critical role for endocytosis in Wnt signaling. BMC Cell Biol. 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukalo O, and Dityatev A (2012). Synaptic Cell Adhesion Molecules. (Springer, Vienna: ), pp. 97–128. [DOI] [PubMed] [Google Scholar]
- Chen LY, Jiang M, Zhang B, Gokce O, and Sudhof TC (2017). Conditional Deletion of All Neurexins Defines Diversity of Essential Synaptic Organizer Functions for Neurexins. Neuron 94, 611–625.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y-C, Lin YQ, Banerjee S, Venken K, Li J, Ismat A, Chen K, Duraine L, Bellen HJ, and Bhat MA (2012). Drosophila neuroligin 2 is required presynaptically and postsynaptically for proper synaptic differentiation and synaptic transmission. J. Neurosci. 52, 16018–16030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EK, Zou Y, and Ghosh A (2008). Wnts acting through canonical and noncanonical signaling pathways exert opposite effects on hippocampal synapse formation. Neural Dev. 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, and Scheiffele P (2003). Neurexin mediates the assembly of presynaptic terminals. Nat. Neurosci. 6, 708–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickins EM, and Salinas PC (2013). Wnts in action: from synapse formation to synaptic maintenance. Front. Cell. Neurosci. 7, 162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson DJ, Ward JD, Reiner DJ, and Goldstein B (2013). Engineering the Caenorhabditis elegans genome using Cas9-triggered homologous recombination. Nat. Methods 10, 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding C, and Hammarlund M (2018). Aberrant Information Transfer Interferes with Functional Axon Regeneration. BioRxiv 347427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Gou Y, Li Y, Liu Y, and Bai J (2015). Synaptojanin cooperates in vivo with endophilin through an unexpected mechanism. Elife 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encell LP, Friedman Ohana R, Zimmerman K, Otto P, Vidugiris G, Wood MG, Los GV, McDougall MG, Zimprich C, Karassina N, et al. (2012). Development of a Dehalogenase-Based Protein Fusion Tag Capable of Rapid, Selective and Covalent Attachment to Customizable Ligands. Curr. Chem. Genomics 6, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin S-X, Linhoff MW, and Craig AM (2004). Neurexins Induce Differentiation of GABA and Glutamate Postsynaptic Specializations via Neuroligins. Cell 119, 1013–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JB, English BP, Chen J, Slaughter JP, Zhang Z, Revyakin A, Patel R, Macklin JJ, Normanno D, Singer RH, et al. (2015). A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 12, 244–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haklai-Topper L, Soutschek J, Sabanay H, Scheel J, Hobert O, and Peles E (2011). The neurexin superfamily of Caenorhabditis elegans. Gene Expr. Patterns 11, 144–150. [DOI] [PubMed] [Google Scholar]
- Hall DH, and Hedgecock EM (1991). Kinesin-related gene unc-104 is required for axonal transport of synaptic vesicles in C. elegans. Cell 65, 837–847. [DOI] [PubMed] [Google Scholar]
- Hata Y, Butz S, and Südhof TC (1996). CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J. Neurosci. 16, 2488–2494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Hom S, Kudze T, Tong X-J, Choi S, Aramuni G, Zhang W, and Kaplan JM (2012). Neurexin and neuroligin mediate retrograde synaptic inhibition in C. elegans. Science 337, 980–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M, Hoerndli FJ, Brockie PJ, Wang R, Johnson E, Maxfield D, Francis MM, Madsen DM, and Maricq AV (2012). Wnt signaling regulates acetylcholine receptor translocation and synaptic plasticity in the adult nervous system. Cell 149, 173–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juette MF, Gould TJ, Lessard MD, Mlodzianoski MJ, Nagpure BS, Bennett BT, Hess ST, and Bewersdorf J (2008). Three-dimensional sub-100 nm resolution fluorescence microscopy of thick samples. Nat. Methods 5, 527–529. [DOI] [PubMed] [Google Scholar]
- Klapoetke NC, Murata Y, Kim SS, Pulver SR, Birdsey-Benson A, Cho YK, Morimoto TK, Chuong AS, Carpenter EJ, Tian Z, et al. (2014). Independent optical excitation of distinct neural populations. Nat. Methods 11, 338–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klassen MP, and Shen K (2007). Wnt Signaling Positions Neuromuscular Connectivity by Inhibiting Synapse Formation in C. elegans. Cell 130, 704–716. [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, Chakraborty C, Workman G, Weaver M, Sage EH, et al. (2011). Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proc. Natl. Acad. Sci. U. S. A. 108, E440–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H-B, Kozorovitskiy Y, Oh W-J, Peixoto RT, Akhtar N, Saulnier JL, Gu C, and Sabatini BL (2012). Neuroligin-1-dependent competition regulates cortical synaptogenesis and synapse number. Nat. Neurosci. 15, 1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Kim Y, Lee S-J, Qiang Y, Lee D, Lee HW, Kim H, Je HS, Sudhof TC, and Ko J (2013). MDGAs interact selectively with neuroligin-2 but not other neuroligins to regulate inhibitory synapse development. Proc. Natl. Acad. Sci. U. S. A. 110, 336–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Chong ZZ, and Maiese K (2005). Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr. Neurovasc. Res. 2, 331–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Ashley J, Budnik V, and Bhat MA (2007). Crucial Role of Drosophila Neurexin in Proper Active Zone Apposition to Postsynaptic Densities, Synaptic Growth, and Synaptic Transmission. Neuron 55, 741–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Xu W, Hsu Y-T, Yee AX, Chen L, and Südhof TC (2015). Conditional knockout of Nlgn2 in the adult medial prefrontal cortex (mPFC) induces delayed loss of inhibitory synapses. Mol. Psychiatry 20, 793–793. [DOI] [PubMed] [Google Scholar]
- Lo T-W, Pickle CS, Lin S, Ralston EJ, Gurling M, Schartner CM, Bian Q, Doudna JA, and Meyer BJ (2013). Precise and heritable genome editing in evolutionarily diverse nematodes using TALENs and CRISPR/Cas9 to engineer insertions and deletions. Genetics 195, 331–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduro M, and Pilgrim D (1995). Identification and cloning of unc-119, a gene expressed in the Caenorhabditis elegans nervous system. Genetics 141, 977–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maro GS, Gao S, Olechwier AM, Hung WL, Liu M, Ozkan E, Zhen M, and Shen K (2015). MADD-4/Punctin and Neurexin Organize C. elegans GABAergic Postsynapses through Neuroligin. Neuron 86, 1420–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda K, Budisantoso T, Mitakidis N, Sugaya Y, Miura E, Kakegawa W, Yamasaki M, Konno K, Uchigashima M, Abe M, et al. (2016). Transsynaptic Modulation of Kainate Receptor Functions by C1q-like Proteins. Neuron 90, 752–767. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, and Sudhof TC (2003). α-Neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature 423, 939–948. [DOI] [PubMed] [Google Scholar]
- Mlodzianoski MJ, Juette MF, Beane GL, and Bewersdorf J (2009). Experimental characterization of 3D localization techniques for particle-tracking and super-resolution microscopy. Opt. Express 17, 8264–8277. [DOI] [PubMed] [Google Scholar]
- Mollwitz B, Brunk E, Schmitt S, Pojer F, Bannwarth M, Schiltz M, Rothlisberger U, and Johnsson K (2012). Directed evolution of the suicide protein O6-alkylguanine- DNA alkyltransferase for increased reactivity results in an alkylated protein with exceptional stability. Biochemistry 51, 986–994. [DOI] [PubMed] [Google Scholar]
- Muhammad K, Reddy-Alla S, Driller JH, Schreiner D, Rey U, Bohme MA, Hollmann C, Ramesh N, Depner H, Lützkendorf J, et al. (2015). Presynaptic spinophilin tunes neurexin signalling to control active zone architecture and function. Nat. Commun. 6, 8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee K, Sharma M, Urlaub H, Bourenkov GP, Jahn R, Südhof TC, and Wahl MC (2008). CASK Functions as a Mg2+-independent neurexin kinase. Cell 133, 328–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Khorramshahi O, Gupta VK, Banovic D, Depner H, Fouquet W, Wichmann C, Mertel S, Eimer S, Reynolds E, et al. (2012). Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nat. Neurosci. 15, 1219–1226. [DOI] [PubMed] [Google Scholar]
- Park M, and Shen K (2012). WNTs in synapse formation and neuronal circuitry. EMBO J. 31, 2697–2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson SN, and Kwon K (2013). The HaloTag: Improving Soluble Expression and Applications in Protein Functional Analysis. Curr. Chem. Genomics 6, 8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettem KL, Yokomaku D, Takahashi H, Ge Y, and Craig AM (2013). Interaction between autism-linked MDGAs and neuroligins suppresses inhibitory synapse development. J. Cell Biol. 200, 321–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philbrook A, Ramachandran S, Lambert CM, Oliver D, Florman J, Alkema MJ, Lemons M, and Francis MM (2018). Neurexin directs partner-specific synaptic connectivity in C. elegans. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokala N, Liu Q, Gordus A, and Bargmann CI (2014). Inducible and titratable silencing of Caenorhabditis elegans neurons in vivo with histamine-gated chloride channels. Proc. Natl. Acad. Sci. U. S. A. 111, 2770–2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, and Jorgensen EM (1999). One GABA and two acetylcholine receptors function at the C. elegans neuromuscular junction. Nat. Neurosci. 2, 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond JE, Weimer RM, and Jorgensen EM (2001). An open form of syntaxin bypasses the requirement for UNC-13 in vesicle priming. Nature 412, 338–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso SB, Inestrosa NC, and Rosso SB (2013). WNT signaling in neuronal maturation and synaptogenesis. Front. Cell. Neurosci. 7, 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryckaert S, Pardon E, Steyaert J, and Callewaert N (2010). Isolation of antigen-binding camelid heavy chain antibody fragments (nanobodies) from an immune library displayed on the surface of Pichia pastoris. J. Biotechnol. 145, 93–98. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, and Serafini T (2000). Neuroligin Expressed in Nonneuronal Cells Triggers Presynaptic Development in Contacting Axons. Cell 101, 657–669. [DOI] [PubMed] [Google Scholar]
- Schild LC, and Glauser DA (2015). Dual Color Neural Activation and Behavior Control with Chrimson and CoChR in Caenorhabditis elegans. Genetics 200, 1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz ML, and Jorgensen EM (2016). SapTrap, a Toolkit for High-Throughput CRISPR/Cas9 Gene Modification in Caenorhabditis elegans. Genetics 202, 1277–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui TJ, and Craig AM (2011). Synaptic organizing complexes. Curr. Opin. Neurobiol. 21, 132–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Trotter JH, Lee S-J, Recktenwald CV, Du X, Zhou B, Zhou P, Schwenk J, Fakler B, and Südhof TC (2017). Carbonic anhydrase-related protein CA10 is an evolutionarily conserved pan-neurexin ligand. Proc. Natl. Acad. Sci. 114, E1253–E1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stogsdill JA, Ramirez J, Liu D, Kim YH, Baldwin KT, Enustun E, Ejikeme T, Ji R-R, and Eroglu C (2017). Astrocytic neuroligins control astrocyte morphogenesis and synaptogenesis. Nature 551, 192–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2008). Neuroligins and neurexins link synaptic function to cognitive disease. Nat. 2008 4557215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2017). Synaptic Neurexin Complexes: A Molecular Code for the Logic of Neural Circuits. Cell 171, 745–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Liu L, Zeng X, Xu M, Liu L, Fang M, and Xie W (2009). Genetic interaction between Neurexin and CAKI/CMG is important for synaptic function in Drosophila neuromuscular junction. Neurosci. Res. 64, 362–371. [DOI] [PubMed] [Google Scholar]
- Sun M, Xing G, Yuan L, Gan G, Knight D, With SI, He C, Han J, Zeng X, Fang M, et al. (2011). Neuroligin 2 is required for synapse development and function at the Drosophila neuromuscular junction. J. Neurosci. 31, 687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X-J, Hu Z, Liu Y, Anderson D, and Kaplan JM (2015). A network of autism linked genes stabilizes two pools of synaptic GABAA receptors. Elife 4, e09648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X-J, López-Soto EJ, Li L, Liu H, Nedelcu D, Lipscombe D, Hu Z, and Kaplan JM (2017). Retrograde Synaptic Inhibition Is Mediated by α-Neurexin Binding to the α2δ Subunits of N-Type Calcium Channels. Neuron 95, 326–340.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, and Südhof TC (1995). Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron 14, 497–507. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, and Südhof TC (1993). Neurexin III alpha: extensive alternative splicing generates membrane-bound and soluble forms. Proc. Natl. Acad. Sci. U. S. A. 90, 6410–6414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov Y, Petrenko A, Geppert M, and Sudhof T (1992). Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science (80-. ). 257, 50–56. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Hata Y, Ichtchenko K, Moomaw C, Afendis S, Slaughter CA, and Sudhof TC (1994). Conserved domain structure of β-neurexins. Unusual cleaved signal sequences in receptor-like neuronal cell-surface proteins. J. Biol. Chem. 269, 11987–11992. [PubMed] [Google Scholar]
- Varoqueaux F, Aramuni G, Rawson RL, Mohrmann R, Missler M, Gottmann K, Zhang W, Südhof TC, and Brose N (2006). Neuroligins Determine Synapse Maturation and Function. Neuron 51, 741–754. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chang H, Rattner A, and Nathans J (2016). Frizzled Receptors in Development and Disease. In Current Topics in Developmental Biology, pp. 113–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, and Nusse R (1998). MECHANISMS OF WNT SIGNALING IN DEVELOPMENT. Annu. Rev. Cell Dev. Biol. 14, 59–88. [DOI] [PubMed] [Google Scholar]
- Wu YE, Huo L, Maeder CI, Feng W, and Shen K (2013). The Balance between Capture and Dissociation of Presynaptic Proteins Controls the Spatial Distribution of Synapses. Neuron 78, 994–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan Z, Manning L, Nelson J, Richmond JE, Colon-Ramos DA, Shen K, and Kurshan PT (2017). Clarinet (CLA-1), a novel active zone protein required for synaptic vesicle clustering and release. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q, Weyn-Vanhentenryck SM, Wu J, Sloan SA, Zhang Y, Chen K, Wu JQ, Barres BA, and Zhang C (2015). Systematic discovery of regulated and conserved alternative exons in the mammalian brain reveals NMD modulating chromatin regulators. Proc. Natl. Acad. Sci. U. S. A. 112, 3445–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogev S, and Shen K (2014). Cellular and Molecular Mechanisms of Synaptic Specificity. Annu. Rev. Cell Dev. Biol. 30, 417–437. [DOI] [PubMed] [Google Scholar]
- Zhang X, Chen X, Zeng Z, Zhang M, Sun Y, Xi P, Peng J, and Xu P (2015). Development of a reversibly switchable fluorescent protein for super-resolution optical fluctuation imaging (SOFI). ACS Nano 9, 2659–2667. [DOI] [PubMed] [Google Scholar]
- Zhen M, and Jin Y (1999). The liprin protein SYD-2 regulates the differentiation of presynaptic termini in C. elegans. Nature 401, 371–375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


