Abstract
Background:
In 2014, Ontario augmented its publicly funded multiple-marker screening program for prenatal aneuploidy by incorporating cell-free fetal DNA (cffDNA) analysis for high-risk pregnancies. We assessed trends in the use of multiple-marker screening, cffDNA screening and prenatal diagnostic testing before and after implementation of public funding.
Methods:
We conducted a descriptive study based on data from the Better Outcomes Registry & Network (BORN) Ontario. The study population included all pregnant women in Ontario with a singleton pregnancy and an expected date of delivery between July 1, 2012, and Mar. 31, 2016, with pregnancy data captured in BORN. Pregnancy losses and terminations before 20 weeks’ gestation not captured in BORN were excluded. We generated descriptive statistics to show trends and regional variations in use.
Results:
The study sample included 534 210 singleton pregnancies. After cffDNA screening was funded for specific indications, uptake of multiple-marker screening increased slightly, from 66.5% to 68.1% (p < 0.001). Uptake of cffDNA screening among women with a positive multiple-marker screening result increased substantially, from 3.2% to 48.8% (p < 0.001). In contrast, the rate of prenatal diagnostic testing in this group decreased from 54.8% to 30.8% (p < 0.001). Although women aged 40 years or older are eligible for primary cffDNA screening, only a small decrease in the use of multiple-marker screening was observed in this group. The greatest use of cffDNA screening and greatest decline in prenatal diagnostic testing were seen in women with a level of risk for trisomy 21 of 1:101–1:200 based on multiple-marker screening.
Interpretation:
After public funding of cffDNA screening was implemented in Ontario, there was a significant increase in cffDNA screening and a significant decrease in prenatal diagnostic testing among women with a positive multiple-marker screening result. These changing patterns show the significant impact of public policy and funding decisions on women’s choices regarding prenatal testing.
The offer of multiple-marker screening for aneuploidy became part of routine prenatal care in Ontario in the early 1990s.1 By combining maternal serum biochemical markers with, in most instances, an ultra-sonography marker of fetal nuchal translucency, measured between 11 and 13 weeks’ gestation, multiple-marker screening estimates a pregnant woman’s chance of having a fetus affected by a common type of aneuploidy. Several variations of multiple-marker screening tests have been used worldwide. Until recently, the most commonly employed tests in Ontario have been the integrated prenatal screen (nuchal translucency plus serum markers measured in the first and second trimesters) and the first-trimester screen (nuchal translucency plus first-trimester serum markers). Recently, the most common test in Ontario has shifted to the “enhanced” first-trimester screen, a variation of first-trimester testing with additional biochemical markers. The enhanced first-semester screen provides almost the same screening performance as the integrated prenatal screen but is completed by 14 weeks’ gestation.2–4 Although multiple-marker screening is routinely offered to all pregnant women in Ontario, around 67% of pregnant women were noted to have undergone prenatal screening between 2009 and 2010.5 There has been substantial variation in the overall uptake rate across the province, as well as variation in the multiple-marker screening test performed.6 Cell-free fetal DNA (cffDNA) screening, which became available in Ontario on a private-pay basis in late 2012, is based on sequencing of cffDNA in maternal plasma. The test provides a detection rate of about 99% with a false-positive rate of less than 0.1% for trisomy 21 (in contrast to the detection rate of 88% and the false-positive rate of 3% for enhanced first-trimester screening).3,7 Since early 2014, the Ontario Ministry of Health and Long-Term Care has funded cffDNA screening for pregnant women with pregnancies at increased risk for one of the common types of aneuploidy. The list of risk factors that meet funding criteria is outlined in Appendix 1 (available at www.cmajopen.ca/content/6/4/E436/suppl/DC1). Other jurisdictions have also incorporated cffDNA screening into traditional prenatal screening paradigms,8 although there is limited literature describing the effect of the systematic incorporation of cffDNA screening on use of prenatal screening and diagnostic tests at a population level. Because of its superior screening performance, cffDNA screening presented a welcome alternative to diagnostic testing by amniocentesis or chorionic villous sampling for pregnancies at increased risk for the common types of aneuploidy.9,10
The Better Outcomes Registry & Network (BORN) Ontario has prospectively collected data on pregnancies, including prenatal screening results, since 2012. We thus had an opportunity to assess the impact of publicly funded cffDNA screening on the use of multiple-marker prenatal screening, cffDNA screening and prenatal diagnostic testing. It was expected that public funding would result in increased use of cffDNA screening and decreased use of prenatal diagnostic testing among women at higher risk. Understanding these trends in use should aid and inform policy formation in Ontario, including policies that can address the potential inequities in offer of and access to prenatal screening and testing in different regions. The objective of this study was to assess trends in the use of multiple-marker screening, cffDNA screening and prenatal diagnostic testing before and after cffDNA screening was publicly funded for pregnancies at increased risk for the common types of aneuploidy.
Methods
Data source
This descriptive study was based on data collected by BORN, a prescribed registry under the Personal Health Information Protection Act.11 The BORN Information System houses data on pregnancy encounters with the prenatal care system, including multiple-marker screening, labour, birth and early newborn care, from all prenatal screening centres, midwifery practices and hospitals in Ontario, as well as from Newborn Screening Ontario. It has captured all live births, stillbirths and miscarriages/terminations before 20 weeks’ gestation that had multiple-marker screening since January 2012. It has also retrospectively collected data on all cffDNA screening provided to Ontario residents, as well as data on diagnostic testing from all but 1 cytogenetic laboratory in Ontario (data from this laboratory were unavailable owing to delays in data submission). At the time of this study, data on cffDNA screening and diagnostic testing were housed as stand-alone data sets and were not incorporated into the BORN Information System, thus requiring linkages to pregnancy records in the BORN Information System. Amniocentesis is the main form of prenatal diagnostic testing in Ontario. To maximize ascertainment of prenatal diagnostic testing, the number of amniocentesis procedures was captured through a combination of amniotic fluid α-fetoprotein records (as α-fetoprotein was measured in almost all amniotic fluid samples as a marker of fetal open neural tube defects) and cytogenetic records indicating amniocentesis as the tissue type. Chorionic villus sampling is performed in a small number of high-risk centres, and capture of this test was based on data housed in cytogenetic records. The study population included all pregnant women with pregnancy data captured in BORN with a singleton pregnancy and an expected date of delivery between July 1, 2012, and Mar. 31, 2016.
To link legacy data on cffDNA screening and cytogenetic testing with the BORN Information System records, each cffDNA screening and cytogenetic record was assigned a series of BORN Information System identifiers, including maternal person identification number, pregnancy identification number, birth identification number and infant person identification number, through record matching. The record matching was performed by BORN data analysts and was achieved through deterministic and probabilistic matching with the use of maternal and newborn health card number, date of birth, name, sex and postal code. Overall, 91.1% (12 755/13 999) of cffDNA screening records and 81.3% (12 279/15 107) of cytogenetic records were matched to a pregnancy record in the BORN Information System. In over 95% of cases, cffDNA screening and cytogenetic records were matched to a correct pregnancy. Unmatched records, which were excluded from the study, were mostly likely miscarriages or terminations before 20 weeks’ gestation, as these data points were not systematically captured in the BORN Information System during the study period. Alternatively, they may have been records from non-Ontario residents. Unlike information routinely collected in the BORN Information System, legacy records of cffDNA screening and prenatal diagnostic testing are not in a standard format. For this study, only relevant data elements were selected from these data sets and were combined after data cleaning. A single, cleaned data set linking the cffDNA screening data, the cytogenetic data and the pregnancy record in the BORN Information System was used in the study.
Statistical analysis
We used uptake rates to describe the use of multiple-marker screening, cffDNA screening and prenatal diagnostic testing. The numerator of uptake rate was the number of singleton pregnancies in Ontario that had multiple-marker screening, cffDNA screening or prenatal diagnostic testing. The denominator was singleton pregnancies in Ontario with a defined character (e.g., all pregnancies, pregnancies with a positive result of multiple-marker screening). We used expected date of delivery to describe date ranges. We described regional variations in the use of multiple-marker screening and cffDNA screening by census division and Local Health Integration Network (LHIN), and illustrated them using heat maps created with ArcMap (ArcGIS version 10.4.1, Esri). Census division describes provincially legislated geographic areas that are intermediate between the province/territory level and the municipality; they have been established in provincial law to facilitate regional planning and service provision. 12 Local Health Integration Networks were established under the Local Health System Integration Act, 2006 for planning, funding and management of health care services.13 There are 49 census divisions and 14 LHINs in Ontario. Each pregnancy in the study was assigned a census division and a LHIN based on the women’s postal code and home address, routinely collected by BORN. To assess how multiple-marker screening results are influencing next steps in testing, we also examined the use of cffDNA screening and prenatal diagnostic testing by risk level based on multiple-marker screening.
We generated descriptive statistics to describe the use of multiple-marker screening, cffDNA screening and prenatal diagnostic testing. We used the χ2 test to compare the uptake rates of these tests before and after the introduction of funded cffDNA screening for higher-risk pregnancies. Finally, we used the Cochran–Armitage test to examine temporal changes in the use of these tests.
Ethics approval
The study was approved by the Research Ethics Board of the Children’s Hospital of Eastern Ontario, Ottawa.
Results
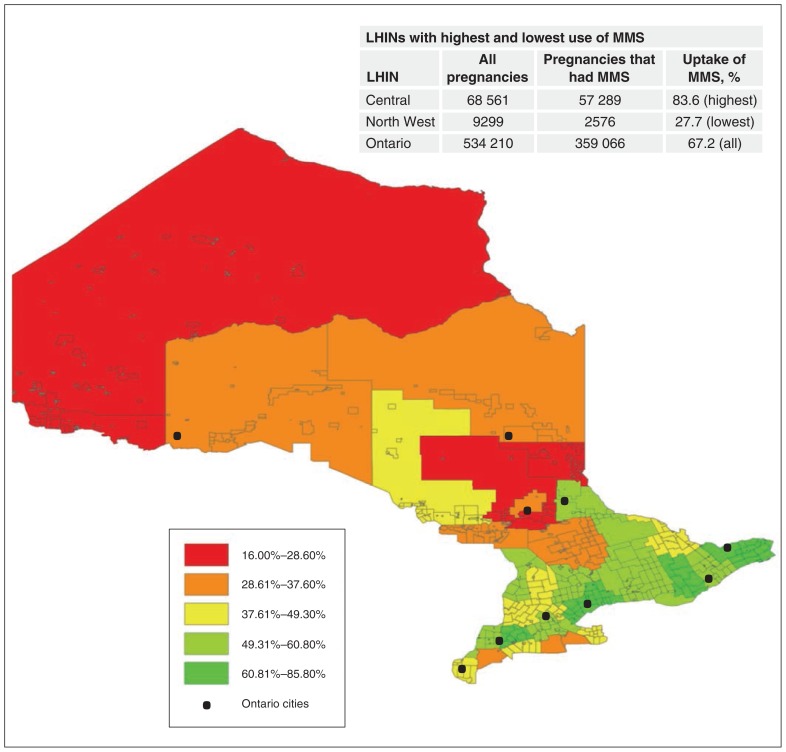
There were 534 210 singleton pregnancies with an expected date of delivery between July 1, 2012, and Mar. 31, 2016. Multiple-marker screening was performed in 359 066 pregnancies, yielding an overall uptake rate of 67.2%. Figure 1 illustrates the uptake of multiple-marker screening by census division via a heat map of Ontario, with an inlaid table illustrating the LHINs with the lowest and highest uptake rates. There were substantial variations in uptake of the test in different geographic areas in Ontario, from under 30% in the North West LHIN to over 83% in the Central LHIN.
Figure 1:
Use of multiple-marker screening (MMS) among all singleton pregnancies with an expected date of delivery between July 1, 2012, and Mar. 31, 2016, in Ontario, by census division. Note: LHIN = Local Health Integration Network.
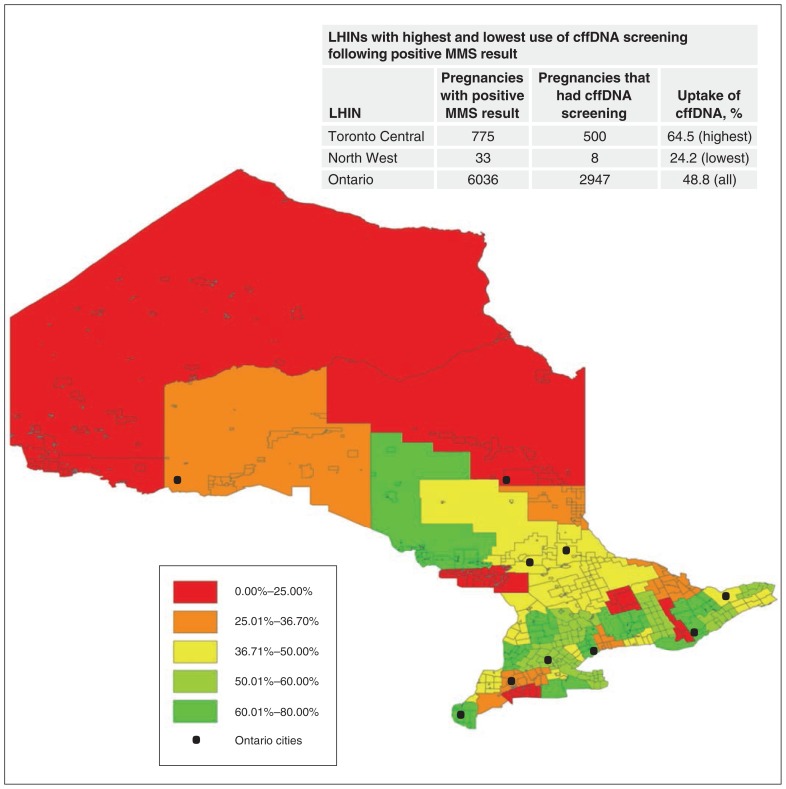
Over the study period, cffDNA screening was performed in 11 102 singleton pregnancies (2.1%), with a dramatic increase after implementation of public funding, from 854 in 2013 to 6298 in 2015. Figure 2 shows the rate of uptake cffDNA screening following implementation of public funding for high-risk pregnancies, by census division, with an inlaid table illustrating the LHINs with the lowest and highest uptake rates. Again, substantial regional variations in uptake were noted, with a 2.7-fold higher uptake rate in the Toronto Central LHIN (64.5%) compared to that for the North West LHIN (24.2%).
Figure 2:
Use of cell-free fetal DNA (cffDNA) screening among singleton pregnancies with an expected date of delivery between July 1, 2014, and Mar. 31, 2016, that had a positive result of multiple-marker screening (MMS), by census division. Note: LHIN = Local Health Integration Network.
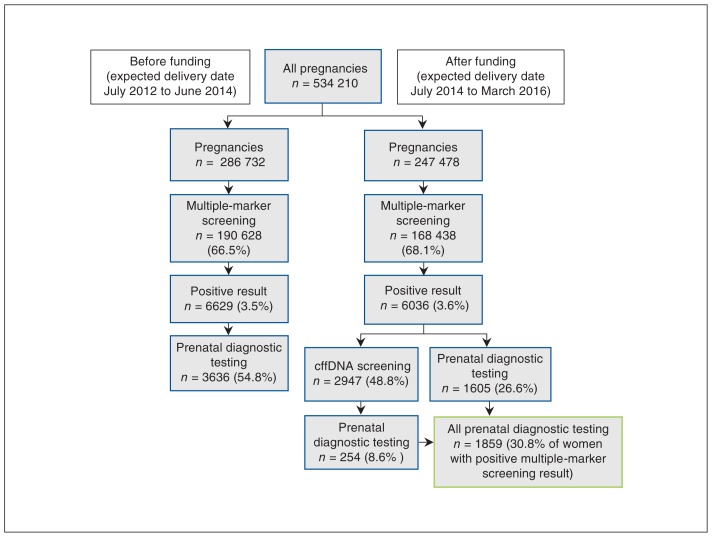
During the study period, a prenatal diagnostic test was done in 11 261 singleton pregnancies (2.1%). There were 10 312 amniocentesis procedures and 949 chorionic villus sampling procedures. Figure 3 shows key pathways of prenatal screening before and after public funding of cffDNA screening was introduced. The rate of uptake of multiple-marker screening increased slightly but significantly after implementation of funding (p < 0.001). The rate of uptake of prenatal diagnostic testing among women with a positive multiple-marker screening result decreased significantly after implementation of funding, from 54.8% to 30.8% (p < 0.001).
Figure 3:
Prenatal screening pathways before and after cell-free fetal DNA (cffDNA) screening was funded. Women who did not have follow-up testing following a positive result of multiple-marker screening are not shown.
After women aged 40 years or older became eligible for publicly funded primary cffDNA screening, in 2014, the rate of uptake of cffDNA screening in this age group increased from 2.4% to 33.0%, yet only 7.2% had primary cffDNA screening (data not shown). This was accompanied by a small but significant decrease (75.7% to 72.2%, p < 0.001) in the uptake of multiple-marker screening in this group.
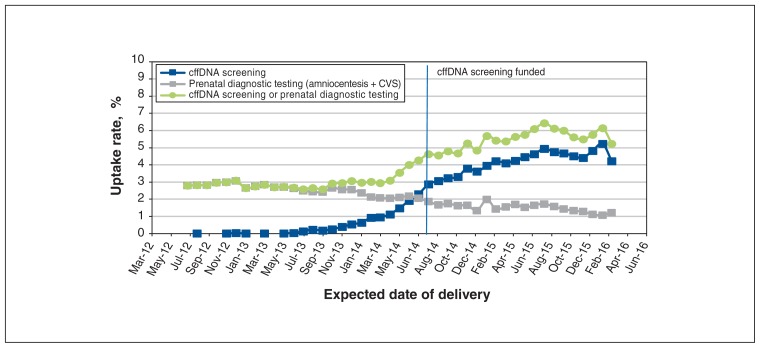
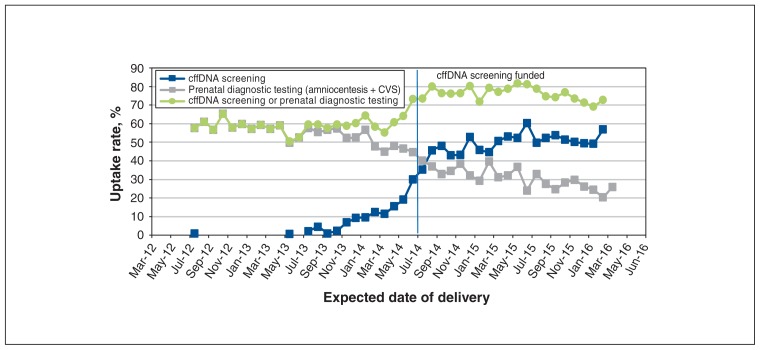
Rates of uptake of cffDNA screening and/or prenatal diagnostic testing among all pregnancies over the study period are depicted in Figure 4. Uptake of cffDNA screening increased from less than 1.0% in 2012 to more than 4.0% in 2016 (p < 0.001). The uptake of prenatal diagnostic testing decreased by more than 50%, and the proportion of women who had either cffDNA screening or prenatal diagnostic testing more than doubled (2.8% to 6.1%) (p < 0.001).
Figure 4:
Rates of uptake of cell-free fetal DNA (cffDNA) screening and prenatal diagnostic testing among all singleton pregnancies with an expected date of delivery between July 2012 and March 2016. Note: CVS = chorionic villous sampling.
Figure 5 shows the uptake of cffDNA screening and prenatal diagnostic testing among pregnancies with a positive multiple-marker screening result over the study period. There was a steady increase in uptake of cffDNA screening after implementation of funding of cffDNA screening, from 2.2% to 56.9%, with a concomitant decrease in uptake of prenatal diagnostic testing (p < 0.01). The proportion of women who had follow-up testing (either cffDNA screening or prenatal diagnostic testing) increased from about 60% to 75% after cffDNA screening was funded (p < 0.001).
Figure 5:
Rates of uptake of cell-free fetal DNA (cffDNA) screening and prenatal diagnostic testing among singleton pregnancies with an expected date of delivery between July 2012 and March 2016 that had a positive result of multiple-marker screening. Note: CVS = chorionic villous sampling.
Table 1 presents the use of cffDNA screening and prenatal diagnostic testing, stratified by level of risk for trisomy 21 based on multiple-marker screening, before and after implementation of funding of cffDNA screening. The highest rate of uptake of prenatal diagnostic testing was seen among pregnancies with a risk level of 1:10 or greater, with a relatively small decline in uptake after cffDNA screening was funded. The greatest decline (50.9% to 23.5%) in uptake of prenatal diagnostic testing following implementation of public funding was seen among pregnancies with a risk level of 1:101–1:200. This group also had the highest rate of uptake of cffDNA screening (55.1%) and the greatest increase in uptake of follow-up testing (56.3% to 76.3%).
Table 1:
Use of cell-free fetal DNA screening and prenatal diagnostic testing in singleton pregnancies by level of risk for trisomy 21 based on multiple-marker screening
| Multiple-marker screening risk for trisomy 21 | Before cffDNA screening funded*; rate of uptake, % | After cffDNA screening funded†; rate of uptake, % | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| cffDNA screening | Prenatal diagnostic testing | cffDNA screening or prenatal diagnostic testing | cffDNA screening | Prenatal diagnostic testing | cffDNA screening or prenatal diagnostic testing | |
| ≥ 1:10 | 4.0 | 67.5 | 70.2 | 30.5 | 54.1 | 76.1 |
|
| ||||||
| 1:11–1:50 | 6.1 | 63.1 | 67.8 | 44.3 | 40.5 | 78.8 |
|
| ||||||
| 1:51–1:100 | 8.0 | 55.9 | 62.6 | 51.1 | 29.2 | 77.3 |
|
| ||||||
| 1:101–1:200 | 6.4 | 50.9 | 56.3 | 55.1 | 23.5 | 76.3 |
|
| ||||||
| 1:201–1:350 | 3.1 | 13.1 | 15.9 | 25.2 | 6.0 | 29.9 |
|
| ||||||
| 1:351–1:500 | 1.4 | 6.4 | 7.5 | 12.5 | 2.3 | 14.6 |
|
| ||||||
| 1:501–1:1000 | 1.1 | 4.1 | 5.2 | 9.3 | 1.6 | 10.6 |
|
| ||||||
| 1:1000–1:5000 | 0.6 | 2.0 | 2.6 | 5.3 | 1.0 | 6.2 |
|
| ||||||
| < 1:5000 | 0.2 | 0.7 | 0.9 | 2.0 | 0.5 | 2.4 |
|
| ||||||
| All risk groups‡ | 0.6 | 3.3 | 3.8 | 5.0 | 1.9 | 6.6 |
Note: cffDNA = cell-free fetal DNA.
Expected date of delivery between July 2012 and June 2014.
Expected date of delivery between July 2014 and March 2016.
All risk groups plus no multiple-marker screening result.
Interpretation
We examined patterns of use of multiple-marker screening, cffDNA screening and prenatal diagnostic testing in Ontario from 2012 to 2016, a period spanning the introduction of cffDNA screening in the province, initially by self-pay and then publicly funded, in 2014. We specifically evaluated the impact of public funding of cffDNA screening for pregnancies at increased risk for aneuploidy on the use of prenatal screening and diagnostic testing. Over the study period, 67.6% of pregnant women had a prenatal screening test (whether multiple-marker screening, cffDNA screening or both). We observed substantial variation in overall rates of uptake of prenatal screening across the province, with higher uptake seen in large urban areas and lower uptake in northern Ontario. After public funding of cffDNA screening for high-risk pregnancies was implemented, there was a small increase in the overall uptake of multiple-marker screening, a decrease in prenatal diagnostic testing and an increase in the uptake of cffDNA screening among pregnancies with a positive result of multiple-marker screening. A possible explanation for the slight increase in multiple-marker screening uptake is heightened awareness of prenatal screening in general following advertising of cffDNA screening, which was widely marketed in Ontario, as elsewhere.14–16 Women who may previously have avoided a multiple-marker screening test because of the relatively high false-positive rate, with the offer of only a diagnostic test for follow-up, may have found cffDNA testing, with its superior performance, low false-positive rate and lack of risk associated with diagnostic testing, more appealing.
The patterns of uptake of cffDNA screening in the current study show a significant impact of public policy and funding decisions on prenatal testing patterns. The use of cffDNA testing increased markedly following implementation of funding among women whose pregnancies were at increased risk for aneuploidy. Interestingly, only 33% of women aged 40 years or older had any cffDNA screening, and only 7.2% had it as a primary screening test in spite of Ontario public funding for this indication alone. We were not able to assess details of counselling regarding prenatal testing options that may have directed use. Health care providers likely followed a 2011 Canadian guideline that advises the offer of a multiple-marker screening test to women of any age before diagnostic testing for aneuploidy.17 Alternatively, they may have been influenced by the fact that multiple-marker screening can provide additional information on pregnancy health that cffDNA screening alone cannot provide. As well, primary providers are challenged by rapidly changing information in this genomic era of prenatal testing, and some patterns of use observed over the study period may reflect delayed incorporation of these newer technologies into practice owing to inadequate knowledge dissemination. Last, there have been anecdotal reports of performance of 2 screens (multiple-marker and cffDNA screening) during the same pregnancy.
In the current study, provincial funding of cffDNA screening was accompanied by decreased use of prenatal diagnostic testing, with the largest decline among pregnancies with a risk for trisomy 21 lower than 1:50 (e.g., 1:100). The uptake of any form of follow-up testing following a positive multiple-marker screening result increased substantially across all risk groups but especially among pregnancies with a risk lower than 1:50, in which follow-up prenatal diagnostic testing was less likely before cffDNA screening was publicly funded. This suggests that cffDNA screening, as a noninvasive and highly accurate alternative to amniocentesis or chorionic villus sampling, is positively regarded by pregnant women or is more likely to be suggested by care providers in cases in which the multiple-marker screening test gives a moderately positive result.
There is a wide range in reported rates of uptake of multiple-marker screening in studies from different countries and screening programs, from 35.2% in the Netherlands to about 76.0% in the United Kingdom and 91.6% in Denmark. 8,18,19 In Ontario, the overall rate of uptake of prenatal screening increased from 63% to about 68% over the past 5–7 years,20,21 but regional variations remain. Publicly funded cffDNA screening has not substantially affected the overall prenatal screening uptake rate. Because multiple-marker screening is funded for all Ontario residents by the Ministry of Health and Long-Term Care, it is unlikely that the variation in regional uptake rates was cost-driven, as reported in the Netherlands.18 Lower rates of multiple-marker screening uptake were previously reported to be associated with living in a rural area, receiving first-trimester care from a family physician or midwife, and being in a lower income quintile in Ontario.20 Possible reasons for the regional variation in both multiple-marker and cffDNA screening observed in the current study include disparities in access and personal choices, as well as provider differences in adopting and incorporating new technologies and recommendations. We plan to analyze this further and, with the advent of a provincial prenatal screening program, to address the modifiable factors that may be barriers to prenatal screening.
Several investigators have assessed the impact of cffDNA screening on prenatal screening and diagnostic services.8–10,22,23 Because of the variations in prenatal screening tests and policies for cffDNA screening in those studies, it is difficult to directly compare the magnitude of the impacts. However, we observed similar patterns in the use of these tests in our population as in others. Chitty and colleagues8 noted that, after the implementation of funded cffDNA screening, the proportion of women having a prenatal diagnostic test decreased from 60% to 17.8%, and the rate of uptake of cffDNA screening was 74.4% following a positive first-trimester screen result. Chan and colleagues22 reported a decline of 45% in the rate of refusal of further testing and a reduction of one-third in prenatal diagnostic testing after a positive multiple-marker screening result following the introduction of self-pay cffDNA screening. A marked decline in the use of prenatal diagnostic testing following the implementation of cffDNA screening has also been shown in other studies, with both public and private funding models.9,10,23–25
Gil and colleagues26 examined the impact of risk level based on first-trimester screening on the uptake of cffDNA screening. Of women with a risk level of 1:100 or more, 40% opted for chorionic villus sampling, 57% opted for cffDNA screening, and 3% did not want any further testing. Of women with a risk of 1:101–1:1000, 91.7% opted for cffDNA screening. Similar results were reported by Manegold-Brauer and colleagues.27 In our population, we observed a reduction in prenatal diagnostic testing in all risk groups, with the greatest reduction seen in women with a risk level of 1:101–1:200.
Limitations
Our study has several limitations. Data from 1 of 9 cytogenetic laboratories were not available at the time of the study. There were unmatched legacy data on cffDNA screening and diagnostic tests, which would have led to underestimation of the absolute number of cffDNA screening and prenatal diagnostic tests performed. As a similar proportional change in test volumes over time were seen in all the other cytogenetic laboratories and the proportions of unmatched records of cffDNA screening and diagnostic testing were similar over the study period, inclusion of the missing/unmatched data likely would not have influenced the magnitude of the observed differences in uptake of cffDNA screening or prenatal diagnostic testing before and after implementation of public funding of cffDNA screening. In addition, data on pregnancies that ended as miscarriages or terminations were not systematically collected in BORN unless there was a multiple-marker screening or amniotic fluid α-fetoprotein test associated with the pregnancy. Therefore, the total number of pregnancies was underestimated to a small extent in the study. This may have slightly overestimated the overall rates of screening and testing uptake but would have affected these rates equally before and after implementation of public funding, thus not affecting our results.
Because our study included women with an expected date of delivery up to Mar. 31, 2016, we cannot comment on more recent trends in use that might reveal further impact of publicly funded cffDNA screening on patterns of use of prenatal screening and diagnostic testing. As detailed earlier, we cannot specifically delineate factors accounting for regional variations in uptake of these screening tests. Neither were we able to assess whether women were offered and declined prenatal screening tests, or the indications for cffDNA screening. We plan to perform further studies to evaluate referral and use patterns as they relate to geographic regions, as well as costs and performance of multiple-marker screening, cffDNA screening and prenatal diagnostic testing.
Conclusion
After public funding of cffDNA screening was implemented in Ontario, there was a significant increase in cffDNA screening and a significant decrease in prenatal diagnostic testing among women with a positive result of multiple-marker screening. These results should inform screening program planning and policy in Ontario and elsewhere. Further studies are warranted to investigate the factors that affect the use of multiple-marker screening, cffDNA screening and prenatal diagnostic testing in order to improve the adequacy, efficacy and performance of prenatal screening.
Supplementary Material
Acknowledgements
Farzana Yasmin performed record matching for this study. Qun Miao provided expert advice on record linkage and matching. Their expertise and assistance are gratefully acknowledged. The authors also thank Mari Teitelbaum and Ann Sprague for their administrative leadership and for facilitating the project.
Footnotes
Competing interests: None declared.
This article has been peer reviewed.
Contributors: Tianhua Huang, Nan Okun, Shelley Dougan and Christine Armour contributed to the study conception and design. Tianhua Huang analyzed the data and drafted the manuscript. Nan Okun, Shelley Dougan, Christine Armour and Mark Walker revised the manuscript critically for important intellectual content. All of the authors contributed to interpretation of the data, gave final approval of the version to be published and agreed to be accountable for all aspects of the work.
Supplemental information: For reviewer comments and the original submission of this manuscript, please see www.cmajopen.ca/content/6/4/E436/suppl/DC1.
References
- 1.Summers AM, Farrell SA, Huang T, et al. Maternal serum screening in Ontario using the triple marker test. J Med Screen. 2003;10:107–11. doi: 10.1177/096914130301000302. [DOI] [PubMed] [Google Scholar]
- 2.Okun N, Summers AM, Hoffman B, et al. Prospective experience with integrated prenatal screening and first trimester combined screening for trisomy 21 in a large Canadian urban center. Prenat Diagn. 2008;28:987–92. doi: 10.1002/pd.2084. [DOI] [PubMed] [Google Scholar]
- 3.Huang T, Dennis A, Meschino WS, et al. First trimester screening for Down syndrome using nuchal translucency, maternal serum pregnancy-associated plasma protein A, free-β human chorionic gonadotrophin, placental growth factor, and α-fetoprotein. Prenat Diagn. 2015;35:709–16. doi: 10.1002/pd.4597. [DOI] [PubMed] [Google Scholar]
- 4.Wald NJ, Rodeck C, Hackshaw AK, et al. SURUSS Research Group. First and second trimester antenatal screening for Down’s syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS) Health Technol Assess. 2003;7:1–77. doi: 10.3310/hta7110. [DOI] [PubMed] [Google Scholar]
- 5.2011–2012 BORN program report. Ottawa: BORN Ontario; 2013. Available: https://www.bornontario.ca/assets/BORN%202011-%202012%20Program%20Report.pdf. accessed 2018 Aug. 16. [Google Scholar]
- 6.2014–2016 biennial report. Ottawa: BORN Ontario; 2016. [accessed 2018 Aug. 16]. Facts from Ontario. Available: www.bornontario.ca/en/about-born/governance/annual-reports/2014-2016-annual-report/facts-from-ontario/ [Google Scholar]
- 7.Gil MM, Quezada MS, Revello R, et al. Analysis of cell-free DNA in maternal blood in screening for fetal aneuploidies: updated meta-analysis. Ultrasound Obstet Gynecol. 2015;45:249–66. doi: 10.1002/uog.14791. [DOI] [PubMed] [Google Scholar]
- 8.Chitty LS, Wright D, Hill M, et al. Uptake, outcomes, and costs of implementing non-invasive prenatal testing for Down’s syndrome into NHS maternity care: prospective cohort study in eight diverse maternity units. BMJ. 2016;354:i3426. doi: 10.1136/bmj.i3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams J, 3rd, Rad S, Beauchamp S, et al. Utilization of noninvasive prenatal testing: impact on referrals for diagnostic testing. Am J Obstet Gynecol. 2015;213:102.e1–6. doi: 10.1016/j.ajog.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 10.Larion S, Warsof SL, Romary L, et al. Uptake of noninvasive prenatal testing at a large academic referral center. Am J Obstet Gynecol. 2014;211:651.e1–7. doi: 10.1016/j.ajog.2014.06.038. [DOI] [PubMed] [Google Scholar]
- 11.BORN: Better Outcomes Registry & Network. Ottawa: BORN Ontario; 2018. [accessed 2018 Aug. 15]. Available: https://www.bornontario.ca/ [Google Scholar]
- 12.Census division. Ottawa: Statistics Canada; [accessed 2017 Jan 19]. [modified 2015]. Cat no 98-301-XWE. Available: http://www12.statcan.gc.ca/census-recensement/2011/ref/dict/geo008-eng.cfm. [Google Scholar]
- 13.Legislation: Local Health System Integration Act, 2006. Toronto: Ontario Ministry of Health and Long-Term Care; 2006. [accessed 2018 Jan. 19]. Available: www.health.gov.on.ca/en/common/legislation/lhins/default.aspx. [Google Scholar]
- 14.Learn important health information about your baby, earlier: Panorama™ non-invasive prenatal testing (NIPT) LifeLabs Genetics. [accessed 2018 Aug. 15]. Available: https://www.lifelabsgenetics.com/product/non-invasive-prenatal-testing/
- 15.Harmony prenatal test (NIPT), the proven option for safe, early, accurate prenatal testing. Brampton (ON): Dynacare; [accessed 2018 Aug. 15]. Available: https://www.dynacare.ca/news/harmony-prenatal-test-(nipt).aspx. [Google Scholar]
- 16.Michie M, Kraft SA, Minear MA, et al. Informed decision-making about prenatal cfDNA screening: an assessment of written materials. Ethics Med Public Health. 2016;2:362–71. doi: 10.1016/j.jemep.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chitayat D, Langlois S, Douglas Wilson R SOGC Genetics Committee; CCMG Prenatal Diagnosis Committee. Prenatal screening for fetal aneuploidy in singleton pregnancies. J Obstet Gynaecol Can. 2011;33:736–50. doi: 10.1016/S1701-2163(16)34961-1. [DOI] [PubMed] [Google Scholar]
- 18.Engels MA, Bhola SL, Twisk JW, et al. Evaluation of the introduction of the national Down syndrome screening program in the Netherlands: age-related uptake of prenatal screening and invasive diagnostic testing. Eur J Obstet Gynecol Reprod Biol. 2014;174:59–63. doi: 10.1016/j.ejogrb.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Miltoft CB, Wulff CB, Kjærgaard S, et al. Danish Fetal Medicine Study Group. Parental decisions about prenatal screening and diagnosis among infants with trisomy 21 in a national cohort with high uptake of combined first-trimester screening. Fetal Diagn Ther. 2017;41:209–14. doi: 10.1159/000448093. [DOI] [PubMed] [Google Scholar]
- 20.Hayeems RZ, Campitelli M, Ma X, et al. Rates of prenatal screening across health care regions in Ontario, Canada: a retrospective cohort study. CMAJ Open. 2015;3:E236–43. doi: 10.9778/cmajo.20140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Okun N, Teitelbaum M, Huang T, et al. The price of performance: a cost and performance analysis of the implementation of cell-free fetal DNA testing for Down syndrome in Ontario, Canada. Prenat Diagn. 2014;34:350–6. doi: 10.1002/pd.4311. [DOI] [PubMed] [Google Scholar]
- 22.Chan YM, Leung WC, Chan WP, et al. Women’s uptake of non-invasive DNA testing following a high-risk screening test for trisomy 21 within a publicly funded healthcare system: findings from a retrospective review. Prenat Diagn. 2015;35:342–7. doi: 10.1002/pd.4544. [DOI] [PubMed] [Google Scholar]
- 23.Robson SJ, Hui L. National decline in invasive prenatal diagnostic procedures in association with uptake of combined first trimester and cell-free DNA aneuploidy screening. Aust N Z J Obstet Gynaecol. 2015;55:507–10. doi: 10.1111/ajo.12380. [DOI] [PubMed] [Google Scholar]
- 24.Hui L, Muggli EE, Halliday JL. Population-based trends in prenatal screening and diagnosis for aneuploidy: a retrospective analysis of 38 years of state-wide data. BJOG. 2016;123:90–7. doi: 10.1111/1471-0528.13488. [DOI] [PubMed] [Google Scholar]
- 25.Hui L, Hutchinson B, Poulton A, et al. Population-based impact of noninvasive prenatal screening on screening and diagnostic testing for fetal aneuploidy. Genet Med. 2017;19:1338–45. doi: 10.1038/gim.2017.55. [DOI] [PubMed] [Google Scholar]
- 26.Gil MM, Giunta G, Macalli EA, et al. UK NHS pilot study on cell-free DNA testing in screening for fetal trisomies: factors affecting uptake. Ultrasound Obstet Gynecol. 2015;45:67–73. doi: 10.1002/uog.14683. [DOI] [PubMed] [Google Scholar]
- 27.Manegold-Brauer G, Berg C, Flöck A, et al. Uptake of non-invasive prenatal testing (NIPT) and impact on invasive procedures in a tertiary referral center. Arch Gynecol Obstet. 2015;292:543–8. doi: 10.1007/s00404-015-3674-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.