Abstract
Common wild rice (Oryza rufipogon Griff.) is invaluable genetic resource for rice resistance breeding. Whole-genome re-sequencing was conducted to systematically analyze the variations in two new inbred lines (Huaye 3 and Huaye 4) developed from a common wild rice. A total of 4,841,127 SNPs, 1,170,479 InDels, 24,080 structural variations (SVs), and 298 copy number variations (CNVs) were identified in three materials. Approximately 16.24 and 5.64% of the total SNPs and InDels of Huaye 3 and Huaye 4 were located in genic regions, respectively. Together, 12,486 and 15,925 large-effect SNPs, and 12,417 and 14,513 large-effect InDels, which affect the integrity of the encoded protein, were identified in Huaye 3 and Huaye 4, respectively. The distribution map of 194 and 245 NBS-LRR encoding homologs was constructed across 12 rice chromosomes. Further, GO enrichment analysis of the homologs with identical genotype variations in Huaye 3 and Huaye 4 revealed 67, 82, and 58 homologs involved in cell death, response to stress, and both terms, respectively. Comparative analysis displayed that 550 out of 652 SNPs and 129 out of 147 InDels were present in a widely used blast-susceptible rice variety (LTH). Protein-protein interaction analysis revealed a strong interaction between NBS-LRR candidates and several known R genes. One homolog of disease resistance protein (RPM1) was involved in the plant-pathogen interaction pathway. Artificial inoculation of disease/insect displayed resistance phenotypes against rice blast and brown planthopper in two lines. The results will provide allele-specific markers for rice molecular breeding.
Electronic supplementary material
The online version of this article (10.1007/s11105-018-1103-1) contains supplementary material, which is available to authorized users.
Keywords: Common wild rice (Oryza rufipogon Griff.), Genome re-sequencing, DNA variations, NBS-LRR
Introduction
Rice is one of the most important foods for over half of the world’s population. The increasing world population needs higher rice productivity. However, insects, pests and diseases, and abiotic stresses have become the major restrictions for rice productivity. Some new disease and insect resistance, and stress-tolerant cultivars need to be evolved to meet climate changing and pathogen plant co-evolution. The non-domesticated common wild rice (Oryza rufipogon Griff.) has been proved an invaluable resistance genetics resource for rice genetic improvement due to its high level of resistance against various biotic and abiotic stresses (Tanksley and McCouch 1997; Song et al. 2005).
Common wild rice accumulated valuable resistance genes and alleles during long-term exposure to the natural environment. Many insect and disease resistance genes and QTLs have been identified from wild rice lines, such as rice blast resistance genes (Pi9, Pi40(t), Pi-ta) (Qu et al. 2006; Jeung et al. 2007; Geng et al. 2008) and brown planthopper (BPH) resistance genes (Bph10(t), Bph18(t), Bph14) (Ishii et al. 1994; Jena et al. 2006; Du et al. 2009). Most of the cloned resistance genes (R genes) in rice are nucleotide-binding leucine-rich repeats (NBS-LRR) that involved in effector-triggered immunity (ETI) (Dangl et al. 1996). The host plant will get systemic acquired resistance (SAR) and significantly enhance the resistant after R protein regulated programmed cell death (PCD) at the site of infection during hypersensitive response and the ETI process (Fu and Dong 2013).
The achievements of the Nipponbare complete genome sequence by the International Rice Genome Sequencing Project (2005) provided a well-assembled reference genome for next-generation re-sequencing (NGR) technologies (Feuillet et al. 2011; Gao et al. 2012). NGR technologies largely simplified the process of DNA variation detection, made it easier to reveal the genetic diversity among different accessions, and provided opportunity to understand the correlation between the DNA polymorphisms and phenotype differentiation. Genome-wide DNA variations include single-nucleotide polymorphism (SNP), insertion/deletion (InDel), and large segment variations, such as structural variation (SV) and copy number variation (CNV) (Varshney et al. 2009; Huang et al. 2013). These DNA variations are responsible for the phenotype divergence among different rice germplasm. The abundant SNPs in the genome can be used for the determination of population structure, the measurement of linkage disequilibrium, and the bulked segregation analysis (Huang and Han 2014; Candela et al. 2015). InDels have been used for development of genome-wide markers for the specified phenotype (Hayashi et al. 2006; Liu et al. 2015; Yonemaru et al. 2015). SVs consist of large-scale insertions, deletions, and translocations, which are usually considered to be related with gain/loss of genes. CNVs revealed copy number gained and copy number loss in more than 1-kb length. These DNA variations present in the regulatory or coding regions can affect the gene expression or function.
Large amount of DNA sequence polymorphisms has been detected in rice and other plants by the whole-genome re-sequencing. The variation database between two Asian rice subspecies was established based on the draft genome of the japonica cultivar, Nipponbare, and the indica cultivar, 9311 (Feltus et al. 2004; Shen et al. 2004). The low-depth re-sequencing was used to detect the evolutionary relationship among large samples (McNally et al. 2009). High-depth re-sequencing provides a higher accuracy in variation detection and can be used for understanding the genetic basis underlying the divergence, and detecting candidate genes (Subbaiyan et al. 2012). An indica restorer line, 7302R, with ideal plant architecture was re-sequenced, and the genomic variants associated with ideal plant architecture were detected (Li et al. 2012). Re-sequencing of two rice accessions with low-phosphate-tolerant and -sensitive phenotypes exhibited several DNA polymorphisms in key phosphate starvation-responsive and root architecture genes (Mehra et al. 2015). Rice cultivars with contrasting drought and salinity stress response were re-sequenced and the variations in drought and salinity response QTLs were explored (Jain et al. 2014). Some NBS-LRR genes had been identified in two newly developed rice lines from common wild rice, which were indigenous to Dongxiang, Jiangxi Province, China, by using genome-wide re-sequencing (Liu et al. 2017). Recently, rice pan-genome data provided the information about thousands of newly identified genes, present/absent variations, and unique genes of cultivated and wild lines (Zhao et al. 2018).
In the present study, we reported two stable inbred lines, Huaye 3 and Huaye 4, with relatively high resistance to rice blast and brown planthopper, developed from a common wild rice, S24, which is indigenous to Suixi, Guangdong Province, China. In order to reveal the genome variation features of two inbred lines and their common wild rice progenitor and to get a preliminary understanding of the genetic basis lies in resistance phenotype, whole-genomes re-sequencing of S24, Huaye 3, and Huaye 4 was performed. We investigated the distribution features of four categories of DNA variations in whole genome, viz., SNPs, InDels, SVs, and CNVs in the three materials, and these variations were functionally annotated to reveal the functional candidates. Moreover, we also constructed a whole-genome variants map of NBS-LRR encoding genes of Huaye 3 and Huaye 4. This study will facilitate the utility of these variations and resistance alleles in molecular breeding and functional genomics.
Materials and Methods
Agronomic Traits Observation and Resistance Evaluation of Plant Materials
The common wild rice S24 is conserved at our wild rice germplasm center (South China Agricultural University), and the two inbred lines, Huaye 3 and Huaye 4, derived from S24, were planted at our research farm. Agronomic traits, including plant height, flag leaf length, flag leaf width, main panicle length, productive panicle number, seed setting, ratio of seed length to seed width, grain yield per plant, and 1000-grain weight, of Huaye 3 and Huaye 4 were measured at maturity during 2016 according to the protocols of the People’s Republic of China for the registration of a new plant variety DUS (distinctness, uniformity, and stability) test guidelines of rice (Oryza sativa L.) (Guidelines for the DUS test in China 2012; Guo et al. 2017). Single-factor variance analysis was conducted in Microsoft Excel 2010. Ten rice blast isolates, GD09-15, GD09-9, GW1, GW2, GW6, HB7, HN4, HN7, SH10, and SH5, were used to evaluate the blast resistance levels of Huaye 3 and Huaye 4 by single inoculation according to the method described by Mackill (1992). The brown planthopper resistance assay of Huaye 3 and Huaye 4 was conducted according to the method of Wang et al. (2015). The BPH-susceptible indica variety, Taichuang Native 1 (TN1), was used as control. When all the susceptible plants (control) died, six resistance levels (0, 1, 3, 5, 7, and 9) were used to score the BPH resistance according to the International Rice Research Institute (IRRI) guidelines (IRRI 1988). Moreover, resistance performance of Huaye 3 and Huaye 4 against BPH was also observed in disease epidemic years of 2015 and 2017.
Sample Preparation and Sequencing
Young leaves of the common wild rice progenitor S24 and two inbred lines Huaye 3 and Huaye 4 were collected and stored at − 80 °C for DNA extraction. Genomic DNA was extracted using a modified CTAB method (Cota-Sanchez et al. 2006). Genomic DNA quality was evaluated by Nanodrop 2000 and agarose gel electrophoresis. Sequencing library was prepared according to the standard protocol of Illumina. Then, pair-end sequencing was conducted by Illumina HiSeqTM 2500 and Hiseq X Ten platform. The re-sequencing data of LTH were downloaded and transformed to FASTQ format using an SRA Toolkit. The generated FASTQ file quality was evaluated using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Then, three types of low-quality reads, viz., reads with sequencing adapter, reads with more than 10% N content, and reads with more than 50% low-quality bases (< 10), were filtered.
Reads Mapping
The filtered high-quality reads were then mapped to the latest Nipponbare reference genome (MSU7.0, http://rice.plantbiology.msu.edu/) using BWA software (Li and Durbin 2009). MarkDuplicates in Picard (https://sourceforge.net/projects/picard/) were used to eliminate the PCR duplication. Base recalibration and realignment near insertion or deletion regions were conducted using a Genome Analysis Toolkit (GATK). Reference genome coverage was estimated using SAMtools (McKenna et al. 2010). Newly identified wild rice unique genes about PAV variations were selected from rice pan-genome data (Zhao et al. 2018). The files were downloaded from Rice Pan Genome website (http://202.127.18.228/RicePanGenome/index.php), and the sequencing reads were mapped onto these gene sequences using BWA software, and the coverage was calculated using SAMtools (McKenna et al. 2010).
Identification and Analysis of Variations
GATK was further used for the detection of SNPs and InDels after the filtration of alignment results. The following SNPs and InDels were filtered: two or more SNPs in a 5-bp or shorter window, SNPs near (5 bp or less) InDels, and two or more InDels in a 10-bp or shorter window. We further retained the SNPs and InDels with a coverage depth ranged from 11× to 100×. SVs were identified by using the BreakDancer software, and SVs with a coverage depth ranged from 21× to 100× were retained (Chen et al. 2009). CNVs were identified by using the FREEC software (Boeva et al. 2012). All the SNPs and InDels were annotated using the SNPEFF software, and SVs and CNVs were also annotated based on the GFF file of the Nipponbare reference genome (Cingolani et al. 2012). Distribution of these variations was further analyzed and the SNP/InDel-rich/-poor regions with high/low density in a 100-kb window were detected using a five-number summary boxplot.
Validation of Variations
The CDS sequences of randomly selected variation regions were used as template for primer design. Primer Premier 5 software was used for primer designing, and the primers were validated using NCBI Primer-BLAST (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). Polymerase chain reaction (PCR) was used to amplify the variation regions in a 20-μL volume containing 30 ng template, 0.15 μmol/L primer pairs, 1.0 μL dNTPs (2.0 mmol/L each), one 1 U Taq polymerase, and 1× PCR buffer (50 mmol/L KCl, 10 mmol/L Tris-HCl pH 8.3, 1.5 mmol/L MgCl2, 0.01% glutin). The PCR procedure was 94 °C for 5 min followed by 30 cycles of 94 °C for 45 s, 55 °C for 45 s, and 72 °C for 50 s, and a final extension at 72 °C for 5 min. PCR products were examined by agarose gel electrophoresis and further sequenced by Sanger sequencing method. The Sanger sequencing results were further assembled by using DNAMAN software. The assembled sequences were aligned to the reference genome sequences to validate the variations in all the selected regions using ClustalW software.
Annotation and Functional Prediction of Variant Genes
All the variant gene functions were annotated by gene ontology (GO), Swissprot protein database, Kyoto Encyclopedia of Genes and Genomes (KEGG), Clusters of Orthologous Groups (COG) of protein database, and NCBI non-redundant (NR) database using BLAST software (Altschul et al. 1997). Pfam database annotation was conducted using PfamScan (Li et al. 2015). We further analyzed the gene distribution across different chromosomes of all the annotated NBS-LRR genes in the two inbred lines by using MapDraw V2.1 software (Liu and Meng 2003). NBS-LRR gene clusters were identified based on the former definition of gene cluster in Arabidopsis (Holub 2001). GO enrichment analysis was conducted using agriGO (Du et al. 2010). RepeatMasker (http://www.repeatmasker.org/) was used to classify the transposon elements (TEs) in structure variations and copy number variations. The protein-protein interaction network was predicted using the STRING website tool (Szklarczyk et al. 2015).
Phylogenetic Relationship and Gene Structure Analysis of Candidate Genes and Homologs in Huaye 3 and Huaye 4
The homologs of candidate genes in the two inbred lines were obtained using SAMtools and VCFtools (https://github.com/vcftools/vcftools) based on the Nipponbare reference genome and the VCF files. All the nucleotide sequences were translated to amino acid sequences by using a rice ORF find program, Beijing Gene Finder website tool (BGF, http://bgf.genomics.org.cn/). MEME_4.11.3 program was used to identify the conserved ten motifs (Bailey et al. 2009). Further, all the conserved domains were identified by using NCBI CD-search (https://www.ncbi.nlm.nih.gov/cdd). Multiple sequence alignment was completed using ClustalW software, and neighbor-joining phylogenetic tree was constructed using MEGA7 software with 1000 bootstrap replications (Kumar et al. 2016).
Data Availability
Re-sequencing raw data have been deposited to the National Center for Biotechnology Information (NCBI) SRA database, and the accession number is “PRJNA396096.” The re-sequencing data of LTH were downloaded from the SRA database (accession numbers: ERX1442095, ERX1442095, and ERX1442095).
Results
Breeding Procedure of Huaye 3 and Huaye 4 Developed from Common Wild Rice
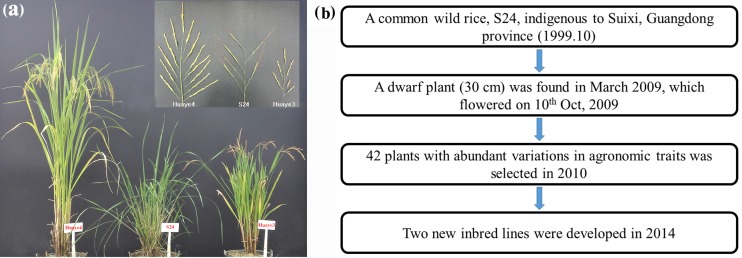
Two new inbred lines were developed from a common wild rice (S24) by our research group in 2014, which were designated as “Huaye 3” and “Huaye 4” in 2016 (Fig. S1). These two rice lines displayed significant differences in agronomic traits, including plant height, ratio of flag leaf length to width, main panicle length, productive panicle number, seed setting, ratio of seed length to width, grain yield per plant, and 1000-grain weight (Fig. 1, Table 1). The plant height and panicle number of Huaye 3 were 60.24 cm and 15.08, while Huaye 4 displayed 121.15 cm and 6.65, respectively (Table 1).
Fig. 1.
Phenotype and breeding procedure of common wild rice (S24) and its inbred lines (Huaye 3 and Huaye 4). (a) Whole-plant and panicle morphology of common wild rice (S24) and its inbred lines (Huaye 3 and Huaye 4) in the field. (b) Breeding procedure of Huaye 3 and Huaye 4
Table 1.
Agronomic traits data of Huaye 3 and Huaye 4 during 2016
| Agronomic traits | Early season | Late season | ||||
|---|---|---|---|---|---|---|
| Huaye 3 (average ± SE) | Huaye 4 (average ± SE) | Huaye 3 (average ± SE) | Huaye 4 (average ± SE) | |||
| Plant height (cm) | 64.23 ± 2.29 | 126.10 ± 6.38 | ** | 56.24 ± 2.80 | 116.20 ± 2.90 | ** |
| Flag leaf length (cm) | 12.52 ± 1.27 | 34.06 ± 4.70 | ** | 14.44 ± 2.05 | 46.16 ± 6.94 | ** |
| Flag leaf width (cm) | 1.24 ± 0.10 | 1.53 ± 0.10 | ** | 1.14 ± 0.11 | 1.76 ± 0.07 | ** |
| Ratio of flag leaf length to width | 10.10 ± 0.62 | 22.40 ± 3.70 | ** | 12.66 ± 1.76 | 26.18 ± 3.78 | ** |
| Main panicle length (cm) | 13.75 ± 0.65 | 27.69 ± 1.78 | ** | 13.21 ± 1.05 | 29.33 ± 1.65 | ** |
| Productive panicle number | 14.85 ± 2.58 | 5.80 ± 0.95 | ** | 15.30 ± 6.42 | 7.50 ± 2.40 | ** |
| Seed setting (%) | 89.43 ± 5.19 | 57.23 ± 5.95 | ** | 80.07 ± 5.38 | 76.41 ± 4.01 | * |
| Ten-grain length (cm) | 7.55 ± 0.12 | 8.68 ± 0.26 | ** | 7.20 ± 0.08 | 8.27 ± 0.12 | ** |
| Ten-grain width (cm) | 2.60 ± 0.06 | 2.65 ± 0.08 | * | 2.17 ± 0.06 | 2.03 ± 0.04 | ** |
| Ratio of grain length to width | 2.90 ± 0.09 | 3.27 ± 0.13 | ** | 3.32 ± 0.09 | 4.08 ± 0.08 | ** |
| Grain yield per plant (g) | 4.65 ± 1.11 | 7.36 ± 2.59 | ** | 3.21 ± 1.19 | 16.79 ± 5.16 | ** |
| 1000-grain weight (g) | 13.19 ± 1.51 | 15.42 ± 1.87 | ** | 13.35 ± 0.76 | 16.75 ± 0.41 | ** |
“*” represent significant difference (P < 0.05); “**” represent highly significant difference (P < 0.01)
Whole-Genome Re-sequencing and Reads Mapping
Whole-genome re-sequencing of S24, Huaye 3, and Huaye 4 generated 79,619,359, 95,853,301, and 77,152,550 raw reads, and about 97.85, 99.58, and 97.54% of these were high-quality reads. About 89.60, 87.98, and 86.85% of these bases were high-quality bases (quality score ≧ Q30). The G/C contents of S24, Huaye 3, and Huaye 4 were 43.46, 44.97, and 41.43%, respectively. The high-quality reads were further mapped onto the Nipponbare reference genome (MSU7.0) uniformly using BWA software (Fig. S2). Overall, 89.76, 90.34, and 91.17% of these reads were uniquely mapped and covered 91.71, 91.47, and 87.52% of reference genome at 10× coverage depth, and the average coverage depth was 44×, 65×, and 42× in S24, Huaye 3, and Huaye 4, respectively (Table 2).
Table 2.
Summary of re-sequencing data and reads mapping onto the Nipponbare genome
| S24 | Huaye 3 | Huaye 4 | |
|---|---|---|---|
| Total reads | 79,619,359 | 95,853,301 | 77,152,550 |
| High-quality reads | 77,907,543 | 95,453,868 | 75,256,129 |
| Clean bases | 19,629,347,386 | 28,611,001,160 | 18,960,007,854 |
| Percent of bases ≥ Q20 (%) | 96.55 | 94.67 | 92.47 |
| Percent of bases ≥ Q30 (%) | 89.6 | 87.98 | 86.85 |
| Mapped (%) | 96.54 | 97.71 | 97.83 |
| Properly mapped (%) | 89.76 | 90.34 | 91.17 |
| Coverage ratio 10× (%) | 91.71 | 91.47 | 87.52 |
| GC content (%) | 43.46 | 44.97 | 41.43 |
| Average coverage depth | 44 | 65 | 42 |
Detection and Validation of DNA Polymorphisms
A total of 2,018,840 (1,804,889 SNPs and 213,951 InDels), 1,505,317 (1,349,511 SNPs and 155,806 InDels), and 1,867,413 (1,686,727 SNPs and 180,686 InDels) DNA polymorphic sites were identified in S24, Huaye 3, and Huaye 4 compared to the Nipponbare reference genome, respectively (Tables 3 and 4). Venn analysis indicated that Huaye 3 and Huaye 4 shared more identical DNA polymorphic sites than S24/Huaye 3 and S24/Huaye 4 (Fig. S3a).
Table 3.
Number of total SNPs and different types of SNP variations
| Material | SNP number | Transition (Ti) | Transversion (Tv) | Ti/Tv | Heterozygosity (Het) | Homozygosity (Hom) | Het percentage |
|---|---|---|---|---|---|---|---|
| S24 | 1,804,889 | 1,274,152 | 530,737 | 2.4 | 987,563 | 817,326 | 54.71 |
| Huaye 3 | 1,349,511 | 964,498 | 385,013 | 2.5 | 157,094 | 1,192,417 | 11.64 |
| Huaye 4 | 1,686,727 | 1,209,376 | 477,351 | 2.53 | 191,451 | 1,495,276 | 11.35 |
Table 4.
Number of insertions and deletions in whole genome and coding regions
| Material | Insertions genome (CDS, percentage) | Deletions genome (CDS, percentage) | Total genome (CDS, percentage) |
|---|---|---|---|
| S24 | 213,951 (12,373, 5.78) | 231,017 (13,472, 5.83) | 444,968 (25,845, 5.81) |
| Huaye 3 | 155,806 (8665, 5.56) | 178,108 (10,126, 5.69) | 333,914 (18,791, 5.63) |
| Huaye 4 | 180,686 (9925, 5.49) | 210,911 (11,769, 5.58) | 391,597 (21,694, 5.54) |
In order to evaluate the accuracy of the variations detected by re-sequencing, 18, 13, and 13 genomic regions with abundant DNA variations in S24, Huaye 3, and Huaye 4 respectively were randomly selected for variation validation using Sanger sequencing. In total, 114, 88, and 85 DNA variation sites were validated in S24, Huaye 3, and Huaye 4, respectively. The results showed that the DNA variation sites detected by using Sanger sequencing were consistent with the re-sequencing data (Table S1).
Distribution of SNPs and InDels
The number and density distribution of DNA polymorphisms varied across different chromosomes. The highest SNP numbers were observed on Chr1, while Chr9, Chr12, and Chr8 had the lowest number of SNPs in S24, Huaye 3, and Huaye 4, respectively (Fig. S4a). Similarly, the highest InDel numbers were observed on Chr1, while Chr9, Chr9, and Chr8 had the lowest number of InDels in S24, Huaye 3, and Huaye 4, respectively (Fig. S4b). The highest SNP and InDel densities were found on Chr11, Chr10, and Chr10, and the lowest SNP and InDel densities were found on Chr5, Chr12, and Chr8 in S24, Huaye 3, and Huaye 4, respectively (Fig. S4c, d).
The average SNP densities were 483.57, 361.56, and 451.91 SNP/100 kb, and the average InDel densities were 119.01, 89.31, and 104.75 InDel/100 kb in S24, Huaye 3, and Huaye 4, respectively. However, the SNPs and InDels were not uniformly distributed along the chromosomes (Fig. S5). DNA polymorphism number in every 100-kb window represents the SNP and InDel density (number per 100 kb) along the chromosomes. Variation-rich and variation-poor regions were detected along the 12 rice chromosomes using a five-number boxplot. The outliers of the boxplot were considered as the variation-rich or variation-poor regions (Fig. S6). More variation-rich regions were detected than variation-poor regions in the three materials, and more variation-rich regions in S24 than in Huaye 3 and Huaye 4. In S24, Huaye 3, and Huaye 4, a total of 59, 14, and 23 SNP-rich regions and 178, 50, and 67 InDel-rich regions were detected. However, only 1, 10, and 37 SNP-poor regions and 1, 0, and 30 InDel-poor regions were detected (Table S2). Moreover, nine variation-rich regions (Chr01:12.5–12.6, Chr12:27.0–27.1, Chr10:20.3–20.4, Chr10:2.9–3.0, Chr09:13.9–14.0, Chr12:22.4–22.5, Chr05:19.4–19.5, Chr10:15.9–16.0, and Chr06:4.3–4.4) were jointly shared by S24, Huaye 3, and Huaye 4. These regions may contain the sequence with fast evolution speed between common wild rice and modern cultivated rice.
SNPs are abundant markers distributed across the whole genome, so the SNP zygosity can represent genome zygosity. Analysis on the distribution of SNP zygosity across the 12 rice chromosomes indicated that the wild rice progenitor S24 genome contained much more heterozygous SNPs than the two inbred lines on each chromosome (Table S3). In S24, the proportion of heterozygous SNPs on each chromosome ranged from 47.40 to 68.02%, and the average value was 54.75%. In Huaye 3 and Huaye 4, the proportion of heterozygous SNPs ranged from 8.10 to 25.47%, and the average values were 11.61 and 11.38%, respectively.
Analysis and Annotation of SNPs and InDels
Investigation on the nucleotide substitution type of SNPs indicated higher frequency of transitions (C/T and G/A, Ti) than transversions (C/A, G/T, C/G, and T/A, Tv), and the ratios of Ti to Tv were 2.40, 2.50, and 2.53 in S24, Huaye 3, and Huaye 4, respectively (Table 2). The frequency of G/C was lower than the other three types of Tv (Fig. S3b). The number of deletions was slightly higher than that on insertions, and only 5.5% of these InDels were in the CDS regions (Table 3). About 46.22, 46.28, and 47.05% of the total InDels were single-nucleotide InDels in S24, Huaye 3, and Huaye 4, respectively. Most of the InDels (91.00% in S24, 90.29% in Huaye 3, 90.21% in Huaye 4) ranged from 1 to 9 bp. Interestingly, we detected a relatively higher frequency of triple-nucleotide InDels in the CDS regions, almost 44.98, 43.85, and 42.74% InDels were triple-nucleotide in S24, Huaye 3, and Huaye 4, respectively (Fig. S3c).
SNPs and InDels of S24, Huaye 3, and Huaye 4 showed a similar distribution in different genomic regions. Approximately 64% SNPs and 70% InDels were distributed in the upstream regions (UPSTREAM, 2 kb), intergenic regions (INTERGENIC), and downstream regions (DOWNSTREAM, 2 kb). About 36% SNPs and 30% InDels were observed in the genic regions, and 668,533, 487,884, and 599,574 SNPs and 136,396, 98,221, and 113,176 InDels were detected in genic regions of S24, Huaye 3, and Huaye 4, respectively. The SNPs and InDels, which were distributed in the initiation/termination codon (START_LOST, NON_SYNONYMOUS_START, STOP_GAINED and STOP_LOST) and splice site regions (SPLICE_SITE_REGION, SPLICE_SITE_ACCEPTOR and SPLICE_SITE_DONOR), and the InDels, which cause frameshift (FRAME_SHIFT), were defined as the large-effect variations that have severe impact on the integrity of encoded products and the gene function. A total of 17,277, 12,486, and 15,925 large-effect SNPs, and 16,816, 12,417, and 14,513 large-effect InDels were identified spanning 16,692, 12,248, and 14,434 variant genes in S24, Huaye 3, and Huaye 4, respectively (Tables S4 and S5).
Detection and Functional Prediction of SVs and CNVs
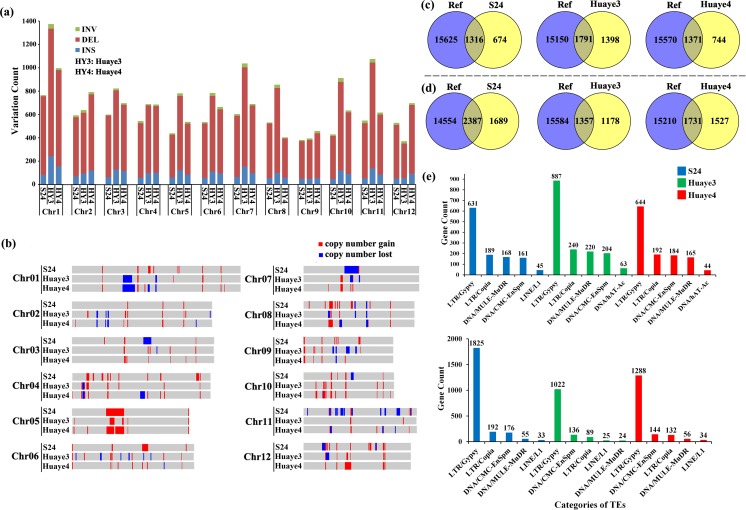
Large segment variations, such as structural variations (SVs) and copy number variations (CNVs), can affect the genome stability. Numerous SVs and CNVs were identified in the three materials. In the intergenic, exon, and intron regions, 6489, 9728, and 7863 SVs in S24, Huaye 3, and Huaye 4 were detected, respectively. Among these SVs, 2055, 3403, and 2169 were in the exon regions (Table S6). Moreover, 70, 75, and 91 copy number gained variations (CNGV) and 20, 24, and 18 copy number loss variations (CNLV) were identified in S24, Huaye 3, and Huaye 4, respectively (Table S7). Three types of SVs, viz., insertion (INS), deletion (DEL), and inversion (INV), were distributed across 12 chromosomes. The number of DELs was much more than INSs and INVs. The maximum SV number was detected on Chr1 in all the three materials, and the minimum SV number was detected on Chr9, Chr12, and Chr8 in S24, Huaye 3, and Huaye 4, respectively (Fig. 2a). Two types of CNVs were distributed across the 12 chromosomes, but only CNGVs were detected on Chr5 in all the three lines. The CNV length was different, and it ranged from 50 Kb to 4.15 Mb, 50 Kb to 2.35 Mb, and 50 Kb to 3.05 Mb in S24, Huaye 3, and Huaye 4, respectively. The longest CNGV (4.15 Mb) and CNLV (3.60 Mb) were detected on Chr05 of S24 (Fig. 2b).
Fig. 2.
Distribution and annotation of structural variations (SVs) and copy number variations (CNVs). Distribution of different types of SVs (a) and CNVs (b) across 12 rice chromosomes. Venn analysis of SV (c) and CNV (d) variant genes and rice genome transposon elements (TEs). Ref represents the TEs in the Nipponbare genome annotated by International Rice Genome Sequencing Project (2005). Classification of SV (e) and CNV (f) variant TEs
Venn analysis of all Nipponbare transposon elements (TEs) and SV/CNV variant genes indicated that more than half of these genes were TEs (Fig. 2c, d). These SV/CNV TEs were classified by RepeatMasker. Majority of these SV/CNV TEs were long terminal repeat (LTR) retrotransposons, and the number of LTR/Gypsy class was higher than that of other classes (Fig. 2e, f). GO enrichment analysis of the SV/CNV TEs shared by Huaye 3 and Huaye 4 exhibited some transposition or chromatin organization enriched GO terms, such as transposase activity (GO:0004803), chromatin assembly or disassembly (GO:0006333), chromatin organization (GO:0006325), and chromosome organization (GO:0051276) (Tables S8 and S9).
In order to identify the useful genomic regions that were specific to common wild rice lines, we compared our results to the newly released rice pan-genome data (Zhao et al. 2018). A total of 4001 unique genes related to common wild rice were selected and the sequencing reads of S24, Huaye3 and Huaye 4 were mapped onto these genes. The unique genes that fully covered by sequencing reads were considered as wild rice-specific homologs of S24, Huaye 3, and Huaye 4, which were lost from cultivated rice during selection and domestication. In total, 1302, 1118, and 994 wild rice unique homologs were detected in S24, Huaye 3, and Huaye 4 (Table S10), and 725 wild rice unique homologs were shared by the three materials, respectively (Fig. S7).
Detection of Genome-Wide NBS-LRR Variants in Huaye 3 and Huaye 4
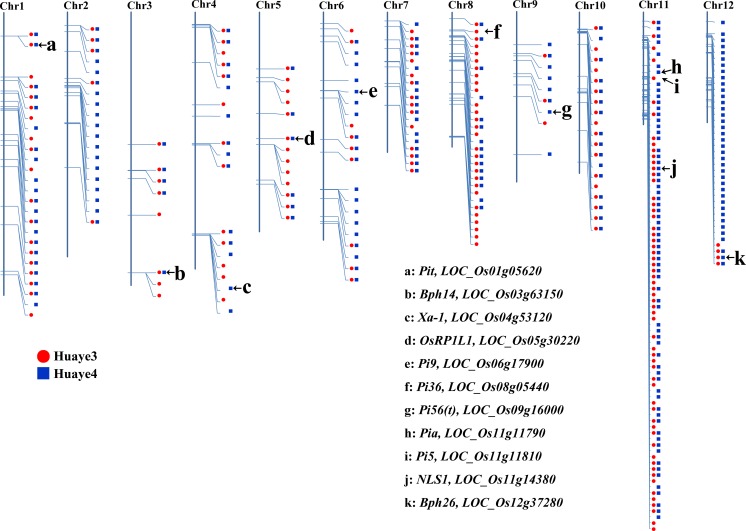
Sequence alignment with the susceptible control Nipponbare detected a total of 194 and 245 NBS-LRR encoding genes with large-effect genetic variations in Huaye 3 and Huaye 4, including six blast resistance genes (Pit, LOC_Os01g05620; Pi9, LOC_Os06g17900; Pi36, LOC_Os08g05440; Pi56(t), LOC_Os09g16000; Pia, LOC_Os11g11790; Pi5, LOC_Os11g11810), two BPH resistance genes (Bph14, LOC_Os03g63150; Bph26, LOC_Os12g37280), two bacterial blight resistance genes (Xa-1, LOC_Os04g53120; OsRP1L1, LOC_Os05g30220), and one defense response gene (NLS1, LOC_Os11g14380) (Table S11). These genes were distributed across the 12 chromosomes. We detected 59 NBS-LRR encoding genes with large-effect variations in Huaye 3 and 67 in Huaye 4 on Chr11, and the maximum number of NBS-LRR variants was located on Chr11. In Chr12, 38 NBS-LRR encoding genes with large-effect variations were detected in Huaye 4, but only four genes were detected in Huaye 3 (Fig. 3). Based on the definition of gene cluster in Arabidopsis (Holub 2001), gene clusters (more than three genes in less than 200 kb) of NBS-LRR encoding genes were detected in the present study. In total, 76 of Huaye 3 NBS-LRR variants were grouped into 20 gene clusters, and 101 of Huaye 4 NBS-LRR variants were distributed into 23 gene clusters.
Fig. 3.
Distribution of annotated NBS-LRR genes in Huaye 3 and Huaye 4. The red dots represent the variant NBS-LRR genes in Huaye 3, and the green boxes represent the variant NBS-LRR genes in Huaye 4. The serial number of homologs in Huaye 3 and Huaye 4 was named as “Or-Chr-NB-18/35-No.” “Or” represents Oryza rufipogon, “Chr” represents chromosome, “NB” represents NB-ARC, “18” represents Huaye 3, “35” represents Huaye 4, and “No.” represents serial number
Functional Enrichment of the Identical Genotype Variants in Huaye 3 and Huaye 4
In order to detect the functional variant genes with identical genotypes in the two inbred lines, similar large-effect SNPs and InDels between Huaye 3 and Huaye 4 were selected for further analysis. A total of 6128 and 4853 identical large-effect SNPs and InDels, which present on 2022 and 3203 variant genes in Huaye 3 and Huaye 4, were identified, respectively.
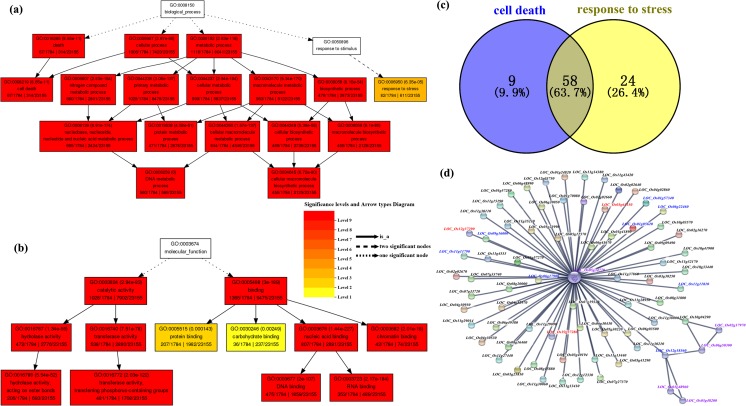
The SNPs were distributed in different Pfam domain families, and the ratios of non-synonymous to synonymous SNPs (Ns/Sy SNPs) ranged from 1.00 to 3.67. The ratios of Ns to Sy SNPs in the two conserved domains of NBS-LRR encoding genes NB-ARC and LRR_1 were 2.32 and 2.24, indicating that these two domains have more amino acid substitutions in Huaye 3 and Huaye 4 (Fig. S8). GO enrichment analysis of all the variant genes with similar genotypes were conducted in Huaye 3 and Huaye 4. In biological process category, the genes were enriched in cell death, response to stress, DNA metabolic process, and cellular macromolecule biosynthetic process. In molecular function category, the genes with the function of hydrolase activity, transferase activity, protein binding, carbohydrate binding, nucleic acid binding, and chromatin binding were significantly enriched (Fig. 4a, b). We identified 67 variant genes containing a BPH-resistant gene, LOC_Os12g37280 (Bph26), which were involved in cell death and 82 variant genes were involved in response to stress, while 58 variant genes were involved in both terms (Fig. 4c). All these 91 NBS-LRR homologs contain 652 and 147 homozygous large-effect SNPs and InDels. Comparative analysis indicated that 550 out of 652 SNPs and 129 out of 147 InDels were present in the Chinese widely used blast-susceptible rice variety LTH (Table S12).
Fig. 4.
Gene ontology (GO) annotation of the identical SNPs and InDels in Huaye 3 and Huaye 4 and protein interaction network of candidates. (a, b) GO enrichment analysis of identical variant genes in Huaye 3 and Huaye 4. (c) Venn analysis of variant genes involved in two plant resistance response-related GO terms. (d) Predicted protein-protein interaction network of 58 candidate genes and former reported rice resistant genes
These 58 candidate genes were used for predicted protein-protein interaction analysis and revealed a network with eight blast-resistant genes (Pi-d3, LOC_Os06g22460; Pi5, LOC_Os11g11810; Pi56(t), LOC_Os09g16000; Pi9, LOC_Os06g17900; Pia, LOC_Os11g11790; Pish, LOC_Os01g57340; Pit, LOC_Os01g05620; Pita, LOC_Os12g18360) and three BPH-resistant genes (Bph14, LOC_Os03g63150; Bph26, LOC_Os12g37280; BPH18, LOC_Os12g37290). A cytochrome C gene (LOC_Os05g34770) connected with the interaction network as a hub gene (Fig. 4d). KEGG pathway annotation revealed a disease resistance protein RPM1 homolog, LOC_Os09g09490, which is responsible for the hypersensitive response in plant-pathogen interaction pathway (Fig. S9).
Phylogenetic and Conserved Motif Analysis of Candidate Homologs in Huaye 3 and Huaye 4
Phylogenetic and MEME analysis of the protein sequences of 58 candidate genes and their homologs in Huaye 3 and Huaye 4 categorized these genes into four groups, including Group I, Group II, Group III, and Group IV (Fig. S10). We used ten motifs to illustrate the conserved region structure of the 174 putative NBS-LRR proteins (Fig. S11), and the details are as follow: 69 proteins were considered as Group I, and most of them were at least related to one NB-ARC domain motif, viz., motif 1, motif 9, or motif 10; 32 proteins were considered as Group II, and most of them were associated with at least one NB-ARC domain motif, viz., motif 1, motif 5, or motif 6; 67 proteins were included in Group III and related with P-loop (motif 4), NB-ARC (motif 6), and three other motifs (motifs 3, 7, 8); and Group IV was comprised of 6 proteins, and all of them were motif 5 and motif 6. The NBS-LRR proteins in different groups may involve in various functions.
NBS-LRR encoding genes and their homologs in Huaye 3 and Huaye 4 were predicted using NCBI CD search to evaluate the variation impact on protein domain structure (Table S13). We found that 28 domain changed NBS-LRR homologs in Huaye 3 and Huaye 4, which may have a significant impact on the gene function and resistant phenotype.
Preliminary Resistance Evaluation of Huaye 3 and Huaye 4
Artificial resistance inoculation further confirmed the resistance in wild rice lines. Ten races of Magnaporthe oryzae inoculation assay revealed that the average blast-resistant level of Huaye 3, Huaye 4, Nipponbare, and LTH were 2.0, 2.7, 3.9, and 5, which indicated higher blast resistance in the two wild rice inbred lines than two control cultivars, respectively. They also showed moderate resistant phenotype for BPH resistance assay, and the resistance scores of Huaye 3 and Huaye 4 were 3 and 5, respectively (Fig. S12, Table S14). Moreover, the resistance phenotypes of the two wild rice inbred lines were observed for 4 years at our farm, and Huaye 3 and Huaye 4 showed high-resistance phenotypes to the occurrence of brown planthopper in the epidemic years of 2015 and 2017.
Discussion
Common Wild Rice Lines Possess High DNA Variations
Wild rice showed relatively higher genetic diversity than cultivated rice (Sun et al. 2001, 2002; Tian et al. 2006; Zhu et al. 2007). The high-density DNA polymorphisms obtained by advanced next-generation genome re-sequencing offered us an opportunity to take deeper insights into the whole-genome diversity. The OryzaGenome database provides 11 deeply sequenced common wild rice accessions, and eight of them contained nearly or more than two million SNPs. About 2.5 times higher SNPs were detected in Australian wild rice than Asian wild rice, and O. sativa ssp. indica compared to Nipponbare reference genome (Krishnan et al. 2014). In the present study, we identified more than 1.5 million polymorphic sites in each material, and all the three samples exhibited higher variation rates compared to Nipponbare reference genome, which are consistent with the previous studies. The high variation rate in the two wild rice inbred lines with low genome heterozygosity rate is also meaningful towards the identification and utilization of favorable alleles.
The occurrence of variation-rich and variation-poor regions has been found in many organisms due to the non-uniform variation distribution (Smith and Lercher 2002; Nordborg et al. 2005; Ravel et al. 2006). This phenomenon may be caused by the artificial selection and selective sweeps during the domestication process of rice (Caicedo et al. 2007). The so-called SNP desert has already been detected in rice cultivars (Wang et al. 2009; Nagasaki et al. 2010), which may represent the existence of highly conserved regions in different rice subspecies (Arai-Kichise et al. 2011). In our study, a total of 201, 60, and 84 variation-rich regions, but only 2, 7, and 47 variation-poor regions were identified in S24, Huaye 3, and Huaye 4, respectively. The presence of variation-rich regions in higher number than that of variation-poor regions indicated a highly divergence and lack of conserved regions between these three common wild rice lines and Asian cultivar Nipponbare.
Large segment DNA variations are also important to genetic diversity and domestication study, especially in wild rice. The common wild rice lost transcripts and Nipponbare acquired genes, which is caused by large segment deletion and insertion in Dongxiang common wild rice (Zhang et al. 2016). Based on the wild rice unique genes of pan-genome data (Zhao et al. 2018), we identified important genes in S24, Huaye 3 and Huaye 4 that were lost from cultivated rice during selection and domestication. In the present study, about 58% of the SV/CNV variant genes are transposable elements (TEs), while the percentage in the Rice Genome Annotation Project was 30% (Kawahara et al. 2013). The high percentage of SV/CNV variant TEs is reported before in mouse genome (Quinlan et al. 2010). The transposons played a key regulatory role in plant stress response (Negi et al. 2016), which are responsible for gene expression change and phenotypic differentiations (Singh et al. 2014; Dhadi et al. 2015; Tan et al. 2017). These SV/CNV variant TEs may cause a dramatic genome rearrangement and have a strong influence on gene expression and resistance level of these three materials.
Abundant NBS-LRR Homologs May Improve Resistance Through Hypersensitive Response
A comprehensive understanding of the whole-genome variations about resistant genes is beneficial to get full usage of the elite genetic resources. NBS-LRR genes are the largest class of resistant genes in plants, and their variation patterns among different accessions have been studied extensively. A total of 535 NBS-coding genes were found in the Nipponbare genome, one fourth of these genes localized on chromosome 11, and 51% of these genes were in gene clusters (Zhou et al. 2004). Even though numerous resistance genes in rice have been mapped and cloned, and some of these different genes are subsequently proved allelic variations. Zhao et al. (2016) demonstrated that the eight BPH resistance genes on chromosome 12L are allelic with each other. Three rice blast resistance genes, Pi9, Pi2, and Piz-t, have been proved allelic with each other, and they were in one NBS-LRR gene cluster on Chr06 and only have several amino acid differentiations (Zhou et al. 2006). Pid3 and Pi25 were in the same locus on Chr06 and only have one amino acid differentiation (Chen et al. 2011). Pikm, Pikh, Pik-p, and Pi1 are also allelic variations in the same locus on Chr06 (Costanzo and Jia 2010; Ashikawa et al. 2012; Kumari et al. 2013). Therefore, the variation detection of resistance genes in highly resistant rice lines can be a good method to reveal the elite resistance alleles.
A previous study has shown that a single amino acid variation could change Pi-ta allele from resistant to susceptible (Bryan et al. 2000). There are abundant structural and genetic variations in different rice accessions, which indicate a highly differentiation in resistance performance (Bai et al. 2002; Yang et al. 2008). Here, investigation on whole-genome variants of NBS-LRR encoding genes of two common wild rice inbred lines with high resistance to rice blast and BPH revealed potentially favorable resistant alleles. A total of 194 and 245 NBS-LRR encoding genes exhibited large-effect variations, including 11 previously cloned R genes, and were explored in Huaye 3 and Huaye 4. Interestingly, only five NBS-LRR encoding genes have intersection with the genes recently identified in another two common wild rice lines (Liu et al. 2017). About 30% of these genes were located on chromosome 11, and 40% of these genes were distributed in gene clusters.
In the process of plant effector-triggered immunity (ETI), the programmed cell death (PCD) following the recognition of pathogen effector (Avr protein) by the R protein is a sign of plant disease resistance. Through PCD in the cell of pathogen infection site, the host plant can get non-specific resistance to other pathogens, known as systemic acquired resistance (SAR). Thus, the host plant can get broad-spectrum resistance (Tang et al. 1999; Spoel and Dong 2012). In the present study, identical SNPs and InDels were obtained in two inbred lines, which reside in 4583 variant genes. GO enrichment analysis indicated that these genes were significantly enriched in cell death, response to stress, hydrolase activity, and nucleic acid binding. A total of 58 annotated NBS-LRR genes involved in cell death and response to stress were considered as potential candidate genes. The gene function further explained by the predicted protein-protein interaction network among eight blast-resistant genes and three BPH-resistant genes. The variant alleles of these 58 candidate NBS-LRR genes were considered as main factors for highly resistant phenotypes in Huaye 3 and Huaye 4. The functional SNPs and InDels detected in our resistant materials could be used as molecular markers for the improvement of elite rice cultivars.
Electronic supplementary material
(PDF 4873 kb)
(XLSX 353 kb)
Acknowledgements
The authors thank Dr. Zhixiong Chen, Dr. Lan Wang, and Ms. Shuhong Yu for the technical assistance.
Author Contributions
XDL conceived and designed the experiments. HY, MQS, and XDL wrote the paper. HY, MQS, RBL, WL, WL, and FG performed the experiment and analyzed the data. XDL developed Huaye 3 and Huaye 4. All the authors read and approved the final manuscript.
Funding information
This work was financially supported by the Guangdong Provincial Key Natural Science Foundation (2014A030311042 to XD Liu) and the Guangzhou Science and Technology Key Program (201707020015 to XD Liu and MQ Shahid).
Contributor Information
Hang Yu, Email: yuhang5783@163.com.
Muhammad Qasim Shahid, Email: qasim@scau.edu.cn.
Rongbai Li, Email: lirongbai@126.com.
Wei Li, Email: liw703@126.com.
Wen Liu, Email: liuwen.fly@163.com.
Fozia Ghouri, Email: foziascau@yahoo.com.
Xiangdong Liu, Phone: +86 20 85280205, Email: xdliu@scau.edu.cn.
References
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai-Kichise Y, Shiwa Y, Nagasaki H, Ebana K, Yoshikawa H, Yano M, Wakasa K. Discovery of genome-wide DNA polymorphisms in a landrace cultivar of Japonica rice by whole-genome sequencing. Plant Cell Physiol. 2011;52(2):274–282. doi: 10.1093/pcp/pcr003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashikawa I, Hayashi N, Abe F, Wu J, Matsumoto T. Characterization of the rice blast resistance gene Pik cloned from Kanto51. Mol Breed. 2012;30(1):485–494. doi: 10.1007/s11032-011-9638-y. [DOI] [Google Scholar]
- Bai J, Pennill LA, Ning J, Lee SW, Ramalingam J, Webb CA, Zhao B, Sun Q, Nelson JC, Leach JE, Hulbert SH. Diversity in nucleotide binding site-leucine-rich repeat genes in cereals. Genome Res. 2002;12(12):1871–1884. doi: 10.1101/gr.454902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey TL, Boden M, Buske FA, Frith M, Grant CE, Clementi L, Ren J, Li WW, Noble WS. MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res. 2009;37:W202–W208. doi: 10.1093/nar/gkp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeva V, Popova T, Bleakley K, Chiche P, Cappo J, Schleiermacher G, Janoueix-Lerosey I, Delattre O, Barillot E. Control-FREEC: a tool for assessing copy number and allelic content using next-generation sequencing data. Bioinformatics. 2012;28(3):423–425. doi: 10.1093/bioinformatics/btr670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryan GT, Wu K, Farrall L, Jia Y, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B. A single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell. 2000;12(11):2033–2046. doi: 10.1105/tpc.12.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caicedo AL, Williamson SH, Hernandez RD, Boyko A, Fledel-Alon A, York TL, Polato NR, Olsen KM, Nielsen R, McCouch SR, Bustamante CD, Purugganan MD. Genome-wide patterns of nucleotide polymorphism in domesticated rice. PLoS Genet. 2007;3(9):1745–1756. doi: 10.1371/journal.pgen.0030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candela H, Casanova-Saez R, Micol JL. Getting started in mapping-by-sequencing. J Integr Plant Biol. 2015;57(7):606–612. doi: 10.1111/jipb.12305. [DOI] [PubMed] [Google Scholar]
- Chen K, Wallis JW, McLellan MD, Larson DE, Kalicki JM, Pohl CS, McGrath SD, Wendl MC, Zhang Q, Locke DP, Shi X, Fulton RS, Ley TJ, Wilson RK, Ding L, Mardis ER. BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat Methods. 2009;6(9):677–681. doi: 10.1038/nmeth.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Shi Y, Liu W, Chai R, Fu Y, Zhuang J, Wu J. A Pid3 allele from rice cultivar Gumei2 confers resistance to Magnaporthe oryzae. J Genet Genomics. 2011;38(5):209–216. doi: 10.1016/j.jgg.2011.03.010. [DOI] [PubMed] [Google Scholar]
- Cingolani P, Platts A, Wang LL, Coon M, Tung N, Wang L, Land SJ, Lu X, Ruden DM. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly(Austin) 2012;6(2):80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo S, Jia Y. Sequence variation at the rice blast resistance gene Pi-km locus: implications for the development of allele specific markers. Plant Sci. 2010;178(6):523–530. doi: 10.1016/j.plantsci.2010.02.014. [DOI] [Google Scholar]
- Cota-Sanchez JH, Remarchuk K, Ubayasena K. Ready-to-use DNA extracted with a CTAB method adapted for herbarium specimens and mucilaginous plant tissue. Plant Mol Biol Report. 2006;24(2):161–167. doi: 10.1007/BF02914055. [DOI] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH. Death don’t have no mercy: cell death programs in plant-microbe interactions. Plant Cell. 1996;8(10):1793–1807. doi: 10.1105/tpc.8.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhadi SR, Xu Z, Shaik R, Driscoll K, Ramakrishna W. Differential regulation of genes by retrotransposons in rice promoters. Plant Mol Biol. 2015;87(6):603–613. doi: 10.1007/s11103-015-0300-7. [DOI] [PubMed] [Google Scholar]
- Du B, Zhang W, Liu B, Hu J, Wei Z, Shi Z, He R, Zhu L, Chen R, Han B, He G. Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. Proc Natl Acad Sci U S A. 2009;106(52):22163–22168. doi: 10.1073/pnas.0912139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhou X, Ling Y, Zhang Z, Su Z. agriGO: a GO analysis toolkit for the agricultural community. Nucleic Acids Res. 2010;38:W64–W70. doi: 10.1093/nar/gkq310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltus FA, Wan J, Schulze SR, Estill JC, Jiang N, Paterson AH. An SNP resource for rice genetics and breeding based on subspecies indica and japonica genome alignments. Genome Res. 2004;14(9):1812–1819. doi: 10.1101/gr.2479404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuillet C, Leach JE, Rogers J, Schnable PS, Eversole K. Crop genome sequencing: lessons and rationales. Trends Plant Sci. 2011;16(2):77–88. doi: 10.1016/j.tplants.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Fu ZQ, Dong X. Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol. 2013;64:839–863. doi: 10.1146/annurev-arplant-042811-105606. [DOI] [PubMed] [Google Scholar]
- Gao Q, Yue G, Li W, Wang J, Xu J, Yin Y. Recent progress using high-throughput sequencing technologies in plant molecular breeding. J Integr Plant Biol. 2012;54(4):215–227. doi: 10.1111/j.1744-7909.2012.01115.x. [DOI] [PubMed] [Google Scholar]
- Geng X, Yang M, Huang X, Cheng Z, Fu J, Sun T, Li J. Cloning and analyzing of rice blast resistance gene Pi-ta+ allele from Jinghong erect type of common wild rice (Oryza rufipogon Griff) in Yunnan. Yi Chuan. 2008;30(1):109–114. doi: 10.3724/SP.J.1005.2008.00109. [DOI] [PubMed] [Google Scholar]
- Guo H, Mendrikahy JN, Xie L, Deng J, Lu Z, Wu J, Li X, Shahid MQ, Liu X. Transcriptome analysis of neo-tetraploid rice reveals specific differential gene expressions associated with fertility and heterosis. Sci Rep. 2017;7:40139. doi: 10.1038/srep40139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yoshida H, Ashikawa I. Development of PCR-based allele-specific and InDel marker sets for nine rice blast resistance genes. Theor Appl Genet. 2006;113(2):251–260. doi: 10.1007/s00122-006-0290-6. [DOI] [PubMed] [Google Scholar]
- Holub EB. The arms race is ancient history in Arabidopsis, the wildflower. Nat Rev Genet. 2001;2(7):516–527. doi: 10.1038/35080508. [DOI] [PubMed] [Google Scholar]
- Huang X, Han B. Natural variations and genome-wide association studies in crop plants. Annu Rev Plant Biol. 2014;65:531–551. doi: 10.1146/annurev-arplant-050213-035715. [DOI] [PubMed] [Google Scholar]
- Huang X, Lu T, Han B. Resequencing rice genomes: an emerging new era of rice genomics. Trends Genet. 2013;29(4):225–232. doi: 10.1016/j.tig.2012.12.001. [DOI] [PubMed] [Google Scholar]
- IRRI . Standard evaluation system for rice. Manila: International Rice Research Institute; 1988. [Google Scholar]
- Ishii T, Brar DS, Multani DS, Khush GS. Molecular tagging of genes for brown planthopper resistance and earliness introgressed from Oryza australiensis into cultivated rice, O. sativa. Genome. 1994;37(2):217–221. doi: 10.1139/g94-030. [DOI] [PubMed] [Google Scholar]
- Jain M, Moharana KC, Shankar R, Kumari R, Garg R. Genomewide discovery of DNA polymorphisms in rice cultivars with contrasting drought and salinity stress response and their functional relevance. Plant Biotechnol J. 2014;12(2):253–264. doi: 10.1111/pbi.12133. [DOI] [PubMed] [Google Scholar]
- Jena KK, Jeung JU, Lee JH, Choi HC, Brar DS. High-resolution mapping of a new brown planthopper (BPH) resistance gene, Bph18(t), and marker-assisted selection for BPH resistance in rice (Oryza sativa L.) Theor Appl Genet. 2006;112(2):288–297. doi: 10.1007/s00122-005-0127-8. [DOI] [PubMed] [Google Scholar]
- Jeung JU, Kim BR, Cho YC, Han SS, Moon HP, Lee YT, Jena KK. A novel gene, Pi40(t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice. Theor Appl Genet. 2007;115(8):1163–1177. doi: 10.1007/s00122-007-0642-x. [DOI] [PubMed] [Google Scholar]
- Kawahara Y, de la Bastide M, Hamilton JP, Kanamori H, McCombie WR, Ouyang S, Schwartz DC, Tanaka T, Wu J, Zhou S, Childs KL, Davidson RM, Lin H, Quesada-Ocampo L, Vaillancourt B, Sakai H, Lee SS, Kim J, Numa H, Itoh T, Buell CR, Matsumoto T. Improvement of the Oryza sativa Nipponbare reference genome using next generation sequence and optical map data. Rice. 2013;6(1):4. doi: 10.1186/1939-8433-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan SG, Waters DL, Henry RJ. Australian wild rice reveals pre-domestication origin of polymorphism deserts in rice genome. PLoS One. 2014;9(6):e988436. doi: 10.1371/journal.pone.0098843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7):1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumari A, Das A, Devanna BN, Thakur S, Singh PK, Singh NK, Sharma TR. Mining of rice blast resistance gene Pi54 shows effect of single nucleotide polymorphisms on phenotypic expression of the alleles. Eur J Plant Pathol. 2013;137(1):55–65. doi: 10.1007/s10658-013-0216-5. [DOI] [Google Scholar]
- Li H, Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics. 2009;25(14):1754–1760. doi: 10.1093/bioinformatics/btp324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Weizhong, Cowley Andrew, Uludag Mahmut, Gur Tamer, McWilliam Hamish, Squizzato Silvano, Park Young Mi, Buso Nicola, Lopez Rodrigo. The EMBL-EBI bioinformatics web and programmatic tools framework. Nucleic Acids Research. 2015;43(W1):W580–W584. doi: 10.1093/nar/gkv279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Shuangcheng, Xie Kailong, Li Wenbo, Zou Ting, Ren Yun, Wang Shiquan, Deng Qiming, Zheng Aiping, Zhu Jun, Liu Huainian, Wang Lingxia, Ai Peng, Gao Fengyan, Huang Bin, Cao Xuemei, Li Ping. Re-sequencing and genetic variation identification of a rice line with ideal plant architecture. Rice. 2012;5(1):18. doi: 10.1186/1939-8433-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Meng J. MapDraw: a Microsoft Excel macro for drawing genetic linkage maps based on given genetic linkage data. Yi Chuan. 2003;25(3):317–321. [PubMed] [Google Scholar]
- Liu J, Li J, Qu J, Yan S. Development of genome-wide insertion and deletion polymorphism markers from next-generation sequencing data in rice. Rice. 2015;8(1):63. doi: 10.1186/s12284-015-0063-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ghouri F, Yu H, Li X, Yu S, Shahid MQ, Liu X. Genome wide re-sequencing of newly developed rice lines from common wild rice (Oryza rufipogon Griff.) for the identification of NBS-LRR genes. PLoS One. 2017;12(7):e180662. doi: 10.1371/journal.pone.0180662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackill DJ. Inheritance of blast resistance in near-isogenic lines of rice. Phytopathology. 1992;82(7):746. doi: 10.1094/Phyto-82-746. [DOI] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally KL, Childs KL, Bohnert R, Davidson RM, Zhao K, Ulat VJ, Zeller G, Clark RM, Hoen DR, Bureau TE, Stokowski R, Ballinger DG, Frazer KA, Cox DR, Padhukasahasram B, Bustamante CD, Weigel D, Mackill DJ, Bruskiewich RM, Rätsch G, Buell CR, Leung H, Leach JE. Genomewide SNP variation reveals relationships among landraces and modern varieties of rice. Proc Natl Acad Sci U S A. 2009;106(30):12273–12278. doi: 10.1073/pnas.0900992106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehra P, Pandey BK, Giri J. Genome-wide DNA polymorphisms in low phosphate tolerant and sensitive rice genotypes. Sci Rep. 2015;5:13090. doi: 10.1038/srep13090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasaki H, Ebana K, Shibaya T, Yonemaru J, Yano M. Core single-nucleotide polymorphisms-a tool for genetic analysis of the Japanese rice population. Breed Sci. 2010;60:648–655. doi: 10.1270/jsbbs.60.648. [DOI] [Google Scholar]
- Negi P, Rai AN, Suprasanna P. Moving through the stressed genome: emerging regulatory roles for transposons in plant stress response. Front Plant Sci. 2016;7:1448. doi: 10.3389/fpls.2016.01448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordborg M, Hu TT, Ishino Y, Jhaveri J, Toomajian C, Zheng H, Bakker E, Calabrese P, Gladstone J, Goyal R, Jakobsson M, Kim S, Morozov Y, Padhukasahasram B, Plagnol V, Rosenberg NA, Shah C, Wall JD, Wang J, Zhao K, Kalbfleisch T, Schulz V, Kreitman M, Bergelson J. The pattern of polymorphism in Arabidopsis thaliana. PLoS Biol. 2005;3(7):e196. doi: 10.1371/journal.pbio.0030196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang GL. The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site-leucine-rich repeat protein and is a member of a multigene family in rice. Genetics. 2006;172(3):1901–1914. doi: 10.1534/genetics.105.044891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Clark RA, Sokolova S, Leibowitz ML, Zhang Y, Hurles ME, Mell JC, Hall IM. Genome-wide mapping and assembly of structural variant breakpoints in the mouse genome. Genome Res. 2010;20(5):623–635. doi: 10.1101/gr.102970.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel C, Praud S, Murigneux A, Canaguier A, Sapet F, Samson D, Balfourier F, Dufour P, Chalhoub B, Brunel D, Beckert M, Charmet G. Single-nucleotide polymorphism frequency in a set of selected lines of bread wheat (Triticum aestivum L.) Genome. 2006;49(9):1131–1139. doi: 10.1139/g06-067. [DOI] [PubMed] [Google Scholar]
- Shen YJ, Jiang H, Jin JP, Zhang ZB, Xi B, He YY, Wang G, Wang C, Qian L, Li X, Yu QB, Liu HJ, Chen DH, Gao JH, Huang H, Shi TL, Yang ZN. Development of genome-wide DNA polymorphism database for map-based cloning of rice genes. Plant Physiol. 2004;135(3):1198–1205. doi: 10.1104/pp.103.038463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Thakur S, Rathour R, Variar M, Prashanthi SK, Singh AK, Singh UD, Sharma V, Singh NK, Sharma TR. Transposon-based high sequence diversity in Avr-Pita alleles increases the potential for pathogenicity of Magnaporthe oryzae populations. Funct Integr Genomics. 2014;14(2):419–429. doi: 10.1007/s10142-014-0369-0. [DOI] [PubMed] [Google Scholar]
- Smith N, Lercher MJ. Regional similarities in polymorphism in the human genome extend over many megabases. Trends Genet. 2002;18(6):281–283. doi: 10.1016/S0168-9525(02)02659-8. [DOI] [PubMed] [Google Scholar]
- Song Z, Li B, Chen J, Lu B. Genetic diversity and conservation of common wild rice (Oryza rufipogon) in China. Plant Species Biol. 2005;20(2):83–92. doi: 10.1111/j.1442-1984.2005.00128.x. [DOI] [Google Scholar]
- Spoel SH, Dong X. How do plants achieve immunity? Defence without specialized immune cells. Nat Rev Immunol. 2012;12(2):89–100. doi: 10.1038/nri3141. [DOI] [PubMed] [Google Scholar]
- Subbaiyan GK, Waters DLE, Katiyar SK, Sadananda AR, Vaddadi S, Henry RJ. Genome-wide DNA polymorphisms in elite indica rice inbreds discovered by whole-genome sequencing. Plant Biotechnol J. 2012;10(6):623–634. doi: 10.1111/j.1467-7652.2011.00676.x. [DOI] [PubMed] [Google Scholar]
- Sun CQ, Wang XK, Li ZC, Yoshimura A, Iwata N. Comparison of the genetic diversity of common wild rice (Oryza rufipogon Griff.) and cultivated rice (O. sativa L.) using RFLP markers. Theor Appl Genet. 2001;102(1):157–162. doi: 10.1007/s001220051631. [DOI] [Google Scholar]
- Sun C, Wang X, Yoshimura A, Doi K. Genetic differentiation for nuclear, mitochondrial and chloroplast genomes in common wild rice (Oryza rufipogon Griff.) and cultivated rice (Oryza sativa L.) Theor Appl Genet. 2002;104(8):1335–1345. doi: 10.1007/s00122-002-0878-4. [DOI] [PubMed] [Google Scholar]
- Szklarczyk D, Franceschini A, Wyder S, Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos A, Tsafou KP, Kuhn M, Bork P, Jensen LJ, von Mering C. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Guan J, Ding S, Wu S, Saunders JW, Koch KE, McCarty DR. Structure and origin of the White Cap locus and its role in evolution of grain color in maize. Genetics. 2017;206(1):135–150. doi: 10.1534/genetics.116.198911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Xie M, Kim YJ, Zhou J, Klessig DF, Martin GB. Overexpression of Pto activates defense responses and confers broad resistance. Plant Cell. 1999;11(1):15–29. doi: 10.1105/tpc.11.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksley SD, McCouch SR. Seed banks and molecular maps: unlocking genetic potential from the wild. Science. 1997;277(5329):1063–1066. doi: 10.1126/science.277.5329.1063. [DOI] [PubMed] [Google Scholar]
- Tian F, Li DJ, Fu Q, Zhu ZF, Fu YC, Wang XK, Sun CQ. Construction of introgression lines carrying wild rice (Oryza rufipogon Griff.) segments in cultivated rice (Oryza sativa L.) background and characterization of introgressed segments associated with yield-related traits. Theor Appl Genet. 2006;112(3):570–580. doi: 10.1007/s00122-005-0165-2. [DOI] [PubMed] [Google Scholar]
- Varshney RK, Nayak SN, May GD, Jackson SA. Next-generation sequencing technologies and their implications for crop genetics and breeding. Trends Biotechnol. 2009;27(9):522–530. doi: 10.1016/j.tibtech.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Wang L, Hao L, Li X, Hu S, Ge S, Yu J. SNP deserts of Asian cultivated rice: genomic regions under domestication. J Evol Biol. 2009;22(4):751–761. doi: 10.1111/j.1420-9101.2009.01698.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cao L, Zhang Y, Cao C, Liu F, Huang F, Qiu Y, Li R, Lou X. Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J Exp Bot. 2015;66(19):6035–6045. doi: 10.1093/jxb/erv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Gu T, Pan C, Feng Z, Ding J, Hang Y, Chen JQ, Tian D. Genetic variation of NBS-LRR class resistance genes in rice lines. Theor Appl Genet. 2008;116(2):165–177. doi: 10.1007/s00122-007-0656-4. [DOI] [PubMed] [Google Scholar]
- Yonemaru J, Choi SH, Sakai H, Ando T, Shomura A, Yano M, Wu J, Fukuoka S. Genome-wide InDel markers shared by diverse Asian rice cultivars compared to Japanese rice cultivar ‘Koshihikari’. Breed Sci. 2015;65(3):249–256. doi: 10.1270/jsbbs.65.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Xu T, Mao L, Yan S, Chen X, Wu Z, Chen R, Luo X, Xie J, Gao S. Genome-wide analysis of Dongxiang wild rice (Oryza rufipogon Griff.) to investigate lost/acquired genes during rice domestication. BMC Plant Biol. 2016;16:103. doi: 10.1186/s12870-016-0788-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Huang J, Wang Z, Jing S, Wang Y, Ouyang Y, Cai B, Xin XF, Liu X, Zhang C, Pan Y, Ma R, Li Q, Jiang W, Zeng Y, Shangguan X, Wang H, Du B, Zhu L, Xu X, Feng YQ, He SY, Chen R, Zhang Q, He G. Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc Natl Acad Sci U S A. 2016;113(45):12850–12855. doi: 10.1073/pnas.1614862113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Feng Q, Lu H, Li Y, Wang A, Tian Q, Zhan Q, Lu Y, Zhang L, Huang T, Wang Y, Fan D, Zhao Y, Wang Z, Zhou C, Chen J, Zhu C, Li W, Weng Q, Xu Q, Wang ZX, Wei X, Han B, Huang X. Pan-genome analysis highlights the extent of genomic variation in cultivated and wild rice. Nat Genet. 2018;50(2):278–279. doi: 10.1038/s41588-018-0041-z. [DOI] [PubMed] [Google Scholar]
- Zhou T, Wang Y, Chen JQ, Araki H, Jing Z, Jiang K, Shen J, Tian D. Genome-wide identification of NBS genes in japonica rice reveals significant expansion of divergent non-TIR NBS-LRR genes. Mol Gen Genomics. 2004;271(4):402–415. doi: 10.1007/s00438-004-0990-z. [DOI] [PubMed] [Google Scholar]
- Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang GL. The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to Magnaporthe grisea. Mol Plant-Microbe Interact. 2006;19(11):1216–1228. doi: 10.1094/MPMI-19-1216. [DOI] [PubMed] [Google Scholar]
- Zhu Q, Zheng X, Luo J, Gaut BS, Ge S. Multilocus analysis of nucleotide variation of Oryza sativa and its wild relatives: severe bottleneck during domestication of rice. Mol Biol Evol. 2007;24(3):875–888. doi: 10.1093/molbev/msm005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 4873 kb)
(XLSX 353 kb)
Data Availability Statement
Re-sequencing raw data have been deposited to the National Center for Biotechnology Information (NCBI) SRA database, and the accession number is “PRJNA396096.” The re-sequencing data of LTH were downloaded from the SRA database (accession numbers: ERX1442095, ERX1442095, and ERX1442095).