Abstract
PTSD has been associated consistently with abnormalities in fear acquisition and extinction learning and retention. Fear acquisition refers to learning to discriminate between threat and safety cues. Extinction learning reflects the formation of a new inhibitory-memory that competes with a previously learned threat-related memory. Adjudicating the competition between threat memory and the new inhibitory memory during extinction may rely, in part, on cognitive processes such as working memory (WM). Despite significant shared neural circuits and signaling pathways the relationship between WM, fear acquisition, and extinction is poorly understood. Here, we analyzed data from a large sample of healthy Marines who underwent an assessment battery including tests of fear acquisition, extinction learning, and WM (N-back). Fear potentiated startle (FPS), fear expectancy ratings, and self-reported anxiety served as the primary dependent variables. High WM ability was associated with greater CS+ fear inhibition during the late block of extinction and greater US expectancy change during extinction learning. WM ability was not associated with magnitude of fear conditioning/expression. Attention ability was unrelated to fear acquisition or extinction supporting specificity of WM associations with extinction. These results support the conclusion that individual differences in WM may contribute to regulating fear responses.
Keywords: Fear extinction, Working memory, Fear-potentiated-startle, Cognition-emotion interactions, Individual differences
Introduction
The neuropsychiatric consequences of military combat, such as post-traumatic stress disorder (PTSD), are highly prevalent and debilitating (Baker et al., 2012; Hoge & Castro, 2006). Moreover, PTSD is difficult to treat, and current treatments are associated with high rates of relapse (Steenkamp, 2016; Yehuda & Hoge, 2016). The prevalence and chronic nature of these disorders represents a growing concern for public policy makers and health providers, underscoring the need to identify underlying neurocognitive and affective mechanisms that confer risk and promote resilience.
Aberrant fear learning processes may underlie vulnerability to PTSD (Acheson, Geyer, et al., 2015; Lissek & van Meurs, 2015; Rauch, Shin, & Phelps, 2006; VanElzakker, Kathryn Dahlgren, Caroline Davis, Dubois, & Shin, 2014; Zuj, Palmer, Lommen, & Felmingham, 2016). Fear acquisition is the process whereby a previously neutral cue becomes associated with threat whereas fear extinction is the process by which one learns that a cue that once signaled the occurrence of threat is no longer predictive of an aversive event (Bouton & Moody, 2004; LeDoux, 2000; Pavlov, 1927). Fear extinction is not an “unlearning” of the original fear-cue association, but a new type of learning in which an individual begins to inhibit fear responses to the cue (Barad, 2006; Bouton, Westbrook, Corcoran, & Maren, 2006; Craske, Treanor, Conway, Zbozinek, & Vervliet, 2014). The relative strength of this inhibitory memory compared to the old fear memory reflects extinction success (Bouton & Todd, 2014; Todd, Vurbic, & Bouton, 2014).
Fear learning is not just an emotional process, but relies on an individual’s cognitive abilities, such as working memory (WM) and attention (Hayes, VanElzakker, & Shin, 2012; Zuj et al., 2016). Cognition plays an important role in emotion regulation (Etkin, Buchel, & Gross, 2015; Okon-Singer, Hendler, Pessoa, & Shackman, 2015). For example, individuals with low WM ability have been shown to have more negative intrusive thoughts (Brewin & Smart, 2005; Klein & Boals, 2001) and have greater difficulty reappraising emotions (Schmeichel, Volokhov, & Demaree, 2008). This interplay between WM and emotion suggests that individual differences in WM, may also influence fear acquisition and extinction (Zuj et al., 2016). WM is a limited capacity workspace that evolved to support the internal maintenance and manipulation of task sets and other goals (Baddeley, 2012; Carruthers, 2013; Cowan, 2016). WM is critical for selecting relevant information for continued processing and preventing irrelevant information from being unnecessarily stored and manipulated (D’Esposito & Postle, 2015). A consequence of poor WM ability is that task-irrelevant information can compete for the same mental workspace and neural real estate where goal-relevant information is maintained (McNab & Klingberg, 2008; Vogel, McCollough, & Machizawa, 2005). Extending this framework to fear acquisition and extinction learning, individuals with high WM ability may be better at successfully discriminating between fear and safety cues, recalling the task-relevant inhibitory-memory, and preventing task-irrelevant and fear-related memory from being retrieved (Catarino, Küpper, Werner-Seidler, Dalgleish, & Anderson, 2015; Stout, Shackman, Pedersen, Miskovich, Larson, 2017; Stout, Shackman, Johnson, & Larson, 2015; Stout, Shackman, & Larson, 2013).
From a neuroscience perspective, both WM and extinction are neurobiologically instantiated in the frontal network (D’Esposito & Postle, 2015; Milad & Quirk, 2012; Sotres-Bayon & Quirk, 2010), with robust functional and structural connectivity with striatal regions involved in WM gating, via phasic dopaminergic signaling (Abraham, Neve, & Lattal, 2014; Badre, 2012; Chatham et al., 2011; D’Ardenne et al., 2012; Goldman-Rakic, 1995; McNab & Klingberg, 2008; O’Reilly & Frank, 2006). WM performance and frontostriatal activity is increased by DA agonists (Cools, Sheridan, Jacobs, & D’Esposito, 2007). Importantly, dopamine (DA) also plays a critical role in fear acquisition and extinction learning (Abraham et al., 2014; Pezze & Feldon, 2004; Tovote, Fadok, & Luthi, 2015). Mice with reduced DA signaling via genetic or pharmacological manipulations exhibit alterations in both fear learning and extinction (El-Ghundi, O’Dowd, & George, 2001; Fadok, Dickerson, & Palmiter, 2009; Risbrough, Ji, Hauger, & Zhou, 2014), while enhancement of DA has been shown to increase extinction learning and recall (Abraham, Cunningham, & Lattal, 2012; Abraham et al., 2014). Carriers of the human 9R DAT1 D1 transporter gene, which supports elevated DA release, display increased extinction learning (Raczka et al., 2011). These patterns raise the possibility that the neurocircuitry underlying WM is shared with fear extinction processes, particularly through mid-brain and frontostriatal DA signaling (Collins & Frank, 2012; Frank & Badre, 2012).
Despite the potential shared neural circuitry, the role that individual-variation in WM plays in acquisition and extinction learning is poorly understood. The aim of the current investigation was to leverage data from the Marine Resilience Study II (MRS II; Baker et al., 2012) to examine whether individual differences in WM ability influence fear acquisition and extinction. The MRS II study is a large prospective evaluation of active duty Marines with the aim of identifying neurocognitive predictors and biomarkers of risk and resilience for post-combat stress symptoms (Acheson et al., 2015). Here we focused on a healthy subset of the sample of healthy active duty Marines assessed prior to combat deployment that was reported in (Acheson, Geyer et al., 2015), where they performed a well-validated conditioned fear potentiated startle paradigm (FPS) (Acheson, Eyler, Resovsky, Tsan, & Risbrough, 2015; Acheson et al., 2013) and the Penn Web-Based Computerized Neurocognitive Battery (Penn WebCNB; Gur et al., 2010; Moore, Reise, Gur, Hakonarson, & Gur, 2015). The Penn WebCNB is a computerized neurocognitive assessment battery measuring a broad-spectrum of cognitive processes (Moore et al., 2015). Here we tested the hypothesis that WM performance is positively associated with measures of extinction learning and safety signal discrimination. To test our hypotheses we used the N-back task performance to assess WM ability (Ragland et al., 2002) and the FPS task to assess cue discrimination and fear extinction. Because of our specific hypothesis regarding the relationship between WM and fear learning, we focused on WM, and did not test all of the neurocognitive measures from the WebCNB.
Method
Participants
A total of 1031 infantry Marines and Navy Corpsmen (100% male) who completed both the fear-conditioning task and the Penn WebCNB were enrolled. We excluded participants if they met criteria for psychiatric symptoms (see Supplementary Information) including PTSD (n = 42), as diagnosed via the Clinician Administered PTSD Scale), clinically elevated levels of anxiety (as measured by > 15 on the Beck Anxiety Inventory, n=37) and depression (as measured by >19 on the Beck Depression Inventory 2, n= 12). A total of 21 subjects were removed due to fear conditioning technical problems. Participants who performed below 50% accuracy on the 2-back and 3-back conditions were excluded from further analyses (n=14). In the extinction analysis only, we removed 117 subjects for failure to show startle potentiation to the CS+ compared to baseline (NA trials) during fear acquisition (showed either identical or lower startle to the CS+ compared to baseline trials). Non-potentiators were removed because extinction learning cannot be assessed in subjects that show no measurable conditioned-fear response. Non-responders did have lower WM ability compared to responders, t = −2.89, p = .004. We report results of primary analyses including the non-responders in the Supplementary Materials. Participants provided written informed consent prior to assessment (see Table 1 for participant characteristics). The institutional review boards of the VA San Diego Research Service, the Naval Health Research Center, and the University of California San Diego approved all study procedures. For overall details of the Marine Resiliency Study see (Baker et al., 2012).
Table 1.
Participant Characteristics.
| Group
|
||
|---|---|---|
| High WM Ability | Low WM Ability | |
| N | 200 | 214 |
| Age (SD) | 22.27 (2.72) | 22.19 (3.03) |
| Education (SD)* | 3.48 (0.83) | 3.22 (0.80) |
| Race | ||
| American-Indian | 1.6% | 2.4% |
| African-American | 2.6% | 5.3% |
| Asian/Pacific Islander | 3.2% | 2.4% |
| White | 89.1% | 85.5% |
| Other | 3.5% | 4.4% |
| Ethnicity | ||
| Hispanic or Latino | 21.8% | 26.1% |
| Not Hispanic or Latino | 79.2% | 73.9% |
| Marital status | ||
| Single, never married | 68.8% | 67.6% |
| Married | 29.7% | 29.0% |
| Separated | 0.5% | 2.4% |
| Divorced | 1.0% | 1.0% |
| PTSD: CAPS- Total (SD) | 10.91(10.76) | 12.94(13.76) |
| Anxiety: BAI-Total (SD) | 3.06(3.65) | 3.46(4.00) |
| Depression: BDI-Total (SD) | 4.10(4.26) | 4.96(4.67) |
| N-back d-prime (SD)* | 3.36(.32) | 1.34(.44) |
| CPT Z-score (SD)* | 0.34(.73) | −0.28(1.05) |
p < .01
Measuring WM
The Penn Web-Based Computerized Neurocognitive Battery (Penn WebCNP; Gur et al., 2010; Moore et al., 2015) was used to measure WM (See Supplementary Figure 1). The Penn WebCNP is a validated battery aimed to provide a brief but comprehensive assessment of several neurocognitive domains using 13 sub-tests. Descriptions of the other sub-tests are provided elsewhere (Moore et al., 2017, 2015) and they were not used in this analysis.
WM ability
The Short Letter-N-Back task from the Penn WebCNP was used to assess WM ability. Participants were asked to pay attention to flashing letters that appeared, one at a time, in the middle of the computer screen. In this version of the task, subjects completed two WM load conditions: the 2-back and the 3-back. During the 2-back condition, participants were instructed to press the spacebar whenever the letter on the screen matched the one that appeared two letters prior (i.e. in the series “B”, “F”, “B”, the participant should press the spacebar on or immediately after the second “B”). During the 3-back, the participants were told to press the spacebar whenever the letter on the screen is the same as the one that appeared three letters prior (i.e. in the series “B”, “F”, “G”, “B”, the participant should press the spacebar on or immediately after the second “B”). Each letter appeared for 0.5 seconds with an ISI of 2.5 seconds. Participants were able to respond once the letter appeared or during the 2.5 second ITI. After participants passed practice trials for both N-back load conditions, they completed two blocks each of the N-back load condition (2-back: 10 targets + 20 foils; 3-back: 10 targets + 30 foils).
Fear acquisition and extinction task
Full details of the fear acquisition and extinction task (see Supplementary Figure 2) have been described elsewhere (Acheson, et al., 2015).
Apparatus
Startle pulses (108 dB, 40 ms) were delivered using a San Diego Instruments (SDI, San Diego, CA, USA) SR-HLAB Electromyography (EMG) system. The air puff was set at 250 psi and delivered via a plastic tube positioned 2.5 cm from the center of the throat. Air-puff onset was controlled by a solenoid system triggered by a laptop computer. Conditioned stimuli were presented via E-Prime software (Psychology Software Tools, Inc., Sharpsburg, PA, USA) run on a desktop computer with a 48 cm monitor positioned directly in front of the participant. Presentation of the stimuli was triggered by signals from the EMG system to control synchronization of visual, acoustic, and air-puff stimuli with EMG recording.
Eyeblink EMG responses to the acoustic pulses were recorded via Ag/Ag 3M Red Dot electrodes placed at the orbicularis oculi muscles at the left eye connected to the SDI SR-HLAB EMG system and laptop computer (Acheson, Eyler, et al., 2015; Acheson et al., 2012, 2013; Marshall, Acheson, Risbrough, Straus, & Drummond, 2014). A reference electrode was placed at the mastoid bone behind the left ear. Before electrode placement, skin was cleaned with alcohol and exfoliated with 3M electrode prep tape. All electrode resistances were <10 kΩ. EMG data were recorded at a sampling rate of 1 KHz, amplified (0.5 mV electrode input was amplified to 2500 mV signal output), band-pass filtered (100–1000 Hz), rectified, and smoothed with a 5-point rolling average. Expectancy responses were recorded trial-by-trial via responses on a key-pad linked to E-Prime. Self-report responses were recorded at the end of each experimental phase via the same keypad.
Eyeblink data were scored via SR-HLAB EMG Utilities software as previously described (Acheson, Geyer, et al., 2015). In brief, eyeblink responses were examined trial by trial at a window starting 100 ms before the startle pulse and ending 200 ms after the pulse. Only responses that peaked within 100 ms of pulse onset were scored. Trials with excessive baseline noise or artifact were removed (2.1% of trials) and imputed based on the average value of the immediately preceding and following trials.
Procedure
The fear acquisition and extinction protocol consisted of two testing “phases”: Acquisition and Extinction. Before acquisition, the participants were told that one colored symbol predicted the air-puff. Each phase began with 4 startle pulses in the absence of any other stimuli to stabilize startle responding. Acquisition consisted of 8 6-sec presentations of the conditioned stimulus (CS+; either a blue or yellow circle or square, balanced across subjects) that was paired with the air-puff in 75% contingency, 8 6-sec presentations of a non-reinforced conditioned stimulus (CS−; also either a blue or yellow circle or square) that was never paired with the air-puff, and 8 presentations of the startle stimulus in the absence of any stimuli (noise alone or “NA” trial) which measured baseline startle. The CS+ and air-puff co-terminated on reinforced trials. Startle pulses were presented ~4 sec following CS+ or CS− onset. Contingency awareness was measured using a numbered keypad to report at each CS+ and CS− trial whether or not they expected the air puff. Participants responded with a “1” if they expected the air puff, “2” if they were unsure, and “3” if they did not. After acquisition, participants were assessed for CS-US contingency awareness, self-reported anxiety to each CS, and subjective aversiveness of the air-puff.
After Acquisition, participants sat quietly for 5 min before beginning the Extinction phase. Participants were told to “remember what they learned” in the previous session. The Extinction phase consisted of 16 presentations of each stimulus type (CS+, CS−, and NA). No air puffs were presented. Startle pulses were delivered and ratings of expectancy were collected in the same fashion as in the Acquisition phase. After this phase, participants again rated their level of anxiety during the cues. Participants were then disconnected from the apparatus and went on to other assessment stations (see Baker et al., 2012 for full details of Marine Resiliency Study assessment battery).
Data Preparation
FPS data preparation followed our standard procedures for the fear acquisition and extinction as detailed elsewhere (Acheson, Geyer, et al., 2015; Acheson et al., 2013).
Fear acquisition
Raw startle responses to each stimulus type were averaged into two blocks of 4 trials. To adjust for startle habituation, potentiated startle responses to CS+ and CS− were then calculated for each block by subtracting NA averages from the CS+ and CS− averages. The second block, the last half of the acquisition phase or “late acquisition,” was then used as the operational measure of fear acquisition.
Fear extinction
To measure extinction, CS+ trials were averaged into 8 two-block trials and potentiated startle to the CS+ was calculated by subtracting the NA baseline responses for each block (CS+ - NA). To control for individual differences in fear learning (e.g. Acheson, Geyer, et al., 2015; Acheson, Eyler, et al., 2015; Milad et al., 2007; Norrholm et al., 2011), potentiated startle to the CS+ during extinction is normalized for the maximum CS+ potentiated responding observed during acquisition to create a “% conditioned fear” score [100*((CS+ - NA) response on extinction block/maximum CS+-NA response observed during acquisition)]1. %conditioned fear scores were then averaged into 4 blocks. To measure learning performance during extinction learning (Milad et al., 2007; Phelps, Delgado, Nearing, & LeDoux, 2004) we compared the %conditioned fear scores of the first block of extinction, to each subsequent block (e.g. Block 1 minus Block 2; Block 1 minus Block 3; and Block 1 minus Block 4). High scores signify a greater drop in % fear retention between the first block and later blocks of the extinction training session, therefore providing an index of extinction learning success compared to baseline (Milad et al., 2007; Phelps et al., 2004). Effects of extinction training on the safety signal (CS−) and baseline (NA alone trials) are presented in the Supplementary Materials.
Expectancy responses were re-coded as: expect air puff = 1, unsure = 0, do not expect air puff = −1. Expectancy responses over the first half of acquisition, and also the last half of the acquisition phase (4 trials/stimulus type) were averaged together as with the startle data. Expectancy responses for the extinction phase were analyzed in a similar manner as the FPS (i.e., extinction learning scores).
WM hypothesis Testing
Sensitivity d′ was computed on overall N-back performance to estimate WM ability. Sensitivity d′ is a measure of the ability to discriminate between targets and distractors, and is based upon signal detection theory (Macmillon, 2002). Using the method outlined by (Haatveit et al., 2010), d′ was calculated using the standard formula: Zhit-rate − Zfalse-alarm, where total hit-rates and false-alarms for the entire task (i.e., summing 2-back and 3-back performance) were first adjusted, and then Z-transformed using the Microsoft Excel algorithm NORMSINV (Haatveit et al., 2010). To maximize the ability to detect differences between high and low WM ability (detailed below), we created low and high WM groups by selecting the top and bottom 25% performers on the WM task. For fear acquisition, there was a final sample of 214 participants in the Low WM group, and 200 in the High WM group that had complete FPS and N-back data. For fear extinction, the sample was reduced to and 204 versus 192 for extinction. All significant findings using the extreme group approach were followed up with a multiple regression model using the entire sample (see confirmatory WM analysis below).
Fear acquisition
FPS and expectancy ratings were entered in to a Group (Low ability vs. High ability) × CS Type (CS+ vs. CS−) × Block (early vs. late) mixed factorial analysis of variance (ANOVA). CS Type and Block were entered as the within-subjects variables, and group was entered as the between-subjects variable. Analysis and results of baseline startle associations with WM group were not significant and are reported in the Supplementary Materials.
Fear extinction
FPS and expectancy extinction learning scores for CS+ and CS− were entered into a Group (Low vs. High ability) × Learning Block (Early, Middle, Late) repeated measures ANOVA (Greenhouse-Geisser corrected). Planned comparisons and post-hoc analyses were Bonferroni α-corrected at .0125 (.05/3). For violations of sphericity, Greenhouse-Geisser corrections are reported. Analysis and results of baseline startle during extinction were not significant and are reported in the Supplementary Materials.
Self-report analyses
For both fear acquisition and fear extinction, a CS Type (CS+ vs. CS−) X Group (Low ability vs. High ability) ANOVA was computed on anxiety ratings for the CS+ and CS−.
Confirmatory WM analyses
Following extreme group analyses, confirmatory analyses were conducted within the entire sample (n=905 for acquisition, 788 for extinction to ensure results are not an artifact of extreme group assignment (McClelland, Lynch John G., Irwin, Spiller, & Fitzsimons, 2015). For fear acquisition FPS, expectancy ratings, and self-report anxiety, CS Type (CS+ vs. CS−), and WM ability (N-back d′) were entered into a general linear model (GLM) where CS Type was entered as a within-subjects variable, and WM ability was entered as a continuous between-groups factor. Likewise, for fear extinction separate GLMs were computed for FPS and US expectancy using the extinction learning scores (Early, Middle, Late), and WM ability.
Individual differences in attention and fear learning
In order to understand if WM relationships with fear processes are specific to WM vs. generalized attention effects we conducted a follow-up analysis between attention performance and fear processes in the same subject. Participants completed the Short Penn Continuous Performance Test-Number and Letter Version (sPCPT-nl) from the Penn WebCNP (Gur et al., 2010; Moore et al., 2015). The sPCPT-nl measures basic attention and vigilance, independent of WM or perceptual factors. During each trial (1 sec), participants viewed a series of red vertical and horizontal lines flash for 300 ms in a digital frame (resembling a digital clock), followed by a blank screen (700 ms). Participants were instructed to press the spacebar when the lines formed complete numbers or complete letters. Lines forming letters or numbers were blocked into separate trials (2 blocks, 1.5 minutes each block). Practice trials for both sets of trials were conducted prior to the experimental trials (180 total trials). Overall accuracy across the CPT task (combing accuracy for letter and number trials) was used to calculate the ability to sustain attention. These scores were Z-transformed and used for analyses similar to those described for WM (see Supplementary Materials for details).
Results
WM ability does not facilitate fear discrimination performance during acquisition
FPS analysis
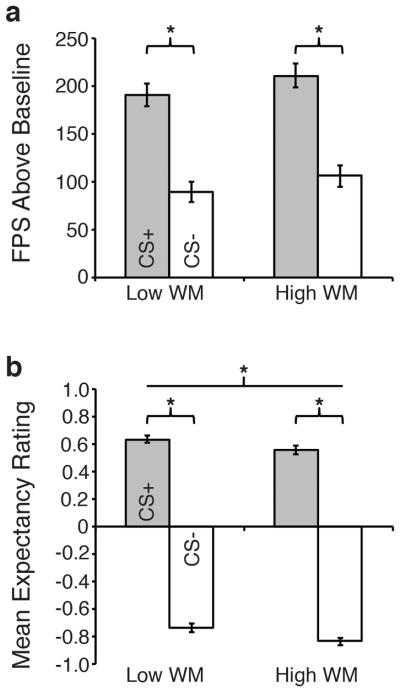
As expected, subjects showed strong cue discrimination with higher FPS to the CS+ than to the CS− [F(1, 458)=203.76, p<.0001]. However, individual differences in WM had no effect on cue discrimination [Group main effect and interaction Fs < 1.59, ps > .28], nor on overall FPS to the CS+ alone [F(1,458)=1.22, p = .25] (Figure 1a). GLM results using the full continuous sample (N=905) supported that fear discrimination was not modified by group (p = .85, N=905). However, there was a trend for increasing levels of WM ability to have overall larger FPS to both trials compared to baseline [F(1,918)=3.38, p=.07, η2 = .004, β = .061].
Figure 1.
Working memory associations with acquired fear and fear discrimination. Data represent an average of trials during the second half of fear acquisition. (a) Mean startle response reflects FPS to CS+ and CS− trials (FPS=raw startle to CS+ or CS− trials subtracted from baseline “NA” trials). (b) US expectancy ratings to the CS+ (grey bars) and CS− (white bars). The line denotes the significant main effect of WM group. Error bars reflect S.E.M. *p<.05.
Expectancy analysis
As with potentiated startle, expectancy ratings were higher for CS+ than for CS− trials [F(1, 402) = 1833.02, p < .0001, partial η2 = .82], with mean ratings of 0.59 (SE=.02) and −0.79 (SE=.02) for CS+ and CS− trials respectively. Individuals with high WM ability showed lower expectancy ratings for the US regardless of cue type (HighMean=−0.14, SE=.018) compared to individuals with low WM ability (LowMean=−0.06, SE=.018) [Group: F(1,402)=12.26, p=.001, partial η2 = .03]. The group × CS type interaction was not significant, F(1,402)=0.14, p=.71. GLM results across the whole sample were similar to the extreme group approach, the association between higher WM ability and reduced US expectancy remained significant, F(1, 821)=14.56, p<.001, and the group × CS type interaction was not significant, p=.40.
Self-report
Overall, self-reported anxiety was higher for the CS+ than for the CS− [F(1, 407) = 648.51, p < .0001, partial η2 = .61; CS+mean = 3.78, SE=.11; CS−mean = 1.00, SE= .08]. High and Low WM ability were similar in overall anxiety ratings [F(1, 407) = 0.88, p = .35, partial η2 = .002; Highmean = 2.47, SE = .11; Lowmean = 2.32, SE = .11]. There was a trend for individuals with high WM ability to report higher anxiety to the CS+ while showing similar anxiety levels to the CS− [group X type interaction, F(1, 407) = 4.55, p = .03, partial η2 = .01; Highmean = 3.97, SE = .16; Lowmean = 3.59, SE = .15; post hoc comparisons of groups: CS+ p = .09; CS− = .57].
WM ability is associated with CS+ fear extinction learning
FPS analysis
As expected, extinction learning scores increased across the training session, F(1.90, 749.08) = 20.67, p < .001, partial η2 = .05. WM ability moderated extinction learning scores across learning block, F(1.90, 749.08) = 3.93, p = .02, partial η2 = .01 (No main effect of Group, F(1, 394) = 1.61, p = .205, partial η2 = .004). As shown in Figure 2a, individuals in the high WM group displayed increased extinction learning scores during the last learning block compared to individuals in the low WM group, p = .01, but not in the early or middle learning blocks (ps > .65). This association remained after adding age, education, symptoms of anxiety, depression, and anxiety ratings as covariates, ps < .02. Moreover, individuals with low WM ability did not benefit from additional extinction trials as indicated by similar scores between the middle and late learning blocks (p = .353); whereas individuals with high WM did benefit (p < .001). GLM analyses using the full continuous sample (N=788) supported that differences in WM ability significantly influenced the magnitude of extinction learning across block [F(1.96, 1499.91) = 3.93, p = .02, partial η2 = .005, N=788]. WM ability was positively associated with extinction learning scores in the late block (p=.018). The effects of individual differences in WM ability effects on extinction learning was primarily driven by variation on the most difficult 3-back trials, although the performance across 2-back and 3-back was not significant (see Supplementary Materials). For WM effects on response to CS+ versus CS− during extinction training see Supplementary Materials.
Figure 2.
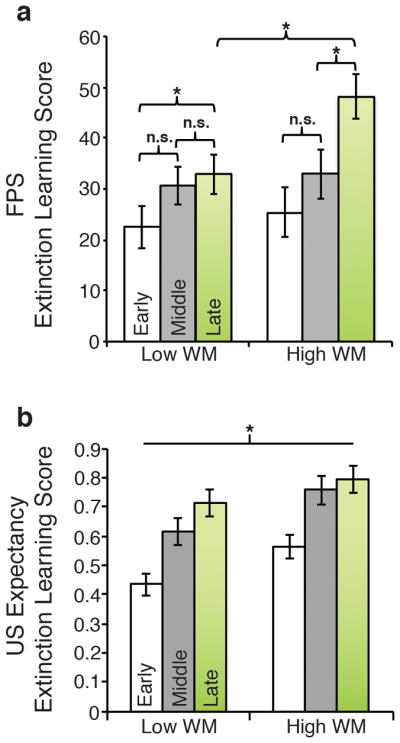
Individual differences in working memory ability moderate fear extinction learning (a) Mean FPS CS+ extinction learning scores (see Method) for low and high WM ability across the early, middle, and late training blocks. Learning scores reflect the difference in potentiated startle to CS+ responses normalized for individual differences in CS+ acquisition [(CS+ - NA)extinction/(CS+ - NA)acquisition)] between the first block of extinction and the remaining three blocks. Higher scores indicate greater extinction learning. Line with brackets denote the significant group × block interaction. (b) Mean US expectancy extinction learning scores during extinction plotted separately for low and high WM for each block. Scores reflect change in US expectancy from the first block of trials during extinction. Higher scores indicate greater reduction (i.e. extinction) in expectancy compared to US expectancy reported in block 1. The line denotes the significant main effect of WM group. Error bars reflect S.E.M. *p<.05.
Expectancy analysis
As expected, self-reported US expectancy to the CS+ was reduced over all blocks when compared to first block [F(8.51, 616.97) =76.33, p < .0001, partial η2 = .17]. Individuals with high WM ability reported a greater drop in US expectancy to the CS+ compared to individuals with low WM ability [main effect of group: F(1, 387) =4.10, p = .04, partial η2 = .01 (Figure 2B)]. However, the group × block interaction was not significant, F(1.59, 616.97)=1.02, p=.36. Similarly, using the whole sample, increasing levels of WM ability were associated with lower expectancy ratings during extinction (group main effect: F(1, 772) = 7.30, p = .007, partial η2 = .01). Likewise, the group × block interaction was not significant, F(1.62, 1251.44)=1.42, p=.24. For WM effects on response to CS+ versus CS− during trial level extinction training see Supplementary Materials.
Self-report
WM ability was unrelated to anxiety ratings to the CS+ or CS− during extinction, group main effect and interaction Fs < 0.73, ps > 0.39. Both groups reported more anxiety to the CS+ than to the CS− (p< .0001), although anxiety ratings were low overall (CS+mean = 1.99, SE=.12; CS−mean = 1.15, SE= .10).
Ability to sustain attention is unrelated to fear acquisition and extinction
Attention FPS analysis
Attention ability was not related to the ability to acquire fear (group main effect and interaction Fs < 0.43, ps > .52) or to extinguish learning (group main effect and interaction Fs < 2.30, ps > .10). GLM results with the full continuous sample (N=905) were consistent with the extreme approach, showing minimal influence of attention ability on FPS during acquisition (group main effect and interaction Fs < 1.35, ps > .24) or to extinction learning (group main effect and interaction Fs < 1.90, ps > .15).
Attention expectancy analysis
Individuals with high attention reported lower expectancy ratings compared to individuals with low attention ability [LowMean = −.05, SE = .014; HighMean = −.01, SE = .016; Main effect of group: F(1, 430) = 4.04, p = .045, partial η2 = .01]. In contrast, sustained attention ability was unrelated to changes in expectancy ratings during fear extinction, group main effect and interaction Fs < 1.18, ps > .29). GLM analyses on the full sample were similar to the extreme group approach finding a main effect of WM ability for fear acquisition, p = .006, but no effect on US expectancy during extinction learning, Fs < 0.45, ps >.55.
Attention self-report results
Attention ability was unrelated to anxiety ratings to the CS+ or CS− during fear acquisition (group main effect and interaction Fs < 0.96) or during fear extinction (group main effect and interaction Fs < 1.88, ps > 0.17).
Discussion
The results of the current investigation provide new evidence that individuals with high WM ability are better at extinguishing fear than individuals with low WM. Individuals with high WM ability showed decreased physiological CS+ responding during the late block of extinction and had greater US expectancy change to CS+ and CS− cues compared to individuals with low WM ability. These results were confirmed when WM ability was examined continuously using the full sample, and could not be explained by covarying individual differences in age, education, anxiety and depressive symptoms, CS+/CS− anxiety ratings. The pattern of results was highly consistent between the WM ability extreme group assignment and full sample analysis. In addition, WM ability was not associated with differences in acquiring fear discrimination (CS+ vs. CS− potentiation) nor baseline startle across fear acquisition and fear extinction learning, suggesting the results are not reflective of differences in generalized fear or habituation to the startle probe. Finally, we did not find evidence that individual differences in attention impact the ability to acquire or extinguish fear responses as measured through FPS. Although, we did find some evidence that attention ability was associated with US expectancy. This pattern suggests that our findings were relatively specific to WM, and not to poor attention (and thus encoding) of associative cues. Collectively, these data indicate that individual variation in the ability to control WM may contribute to extinction learning.
A number of recent studies have argued for the inclusion of the contribution of cognition to fear acquisition and extinction processes (Dunsmoor, Niv, Daw, & Phelps, 2015; Hofmann, 2008; Lovibond, 2004; Zuj et al., 2016). The results of the current investigation are consistent with this argument, and demonstrate an existence of a relationship between individual differences in WM ability and fear extinction performance. Yet, the mechanisms underlying how WM influences fear extinction is poorly understood. Here, we propose that WM is well positioned to influence extinction learning and highlight potential shared neurobiological circuits that will need to be tested in future studies.
One likely possibility is that WM and extinction learning share a frontostriatal gating circuit partially mediated by DA (Abraham et al., 2014; D’Ardenne et al., 2012). WM can be described as having a series of gating mechanisms that efficiently select relevant information for representation across time for the purpose of carrying out task-goals, and update WM by discarding LTM representations that are no longer relevant (Chatham & Badre, 2015; Chatham, Frank, & Badre, 2014). During extinction training, each CS presentation enters WM where it is compared to prior inhibitory and fear memory long term memory (LTM) representations (Bouton & Moody, 2004). As learning progresses, the new inhibitory memory is prioritized for selection, and reactivation of the obsolete fear memory is gated from WM, enabling appropriate inhibitory output responses. Thus, impaired extinction learning could reflect a breakdown in DA signaling important for input or output WM gating processes. For example, a failure to select the relevant inhibitory memory, or an inappropriate reactivation of the irrelevant fear-memory could result in indiscriminate output of a fear response (Catarino et al., 2015; Stout & Shackman et al., 2017; Stout et al., 2013). Future investigations will be needed confirm this prediction.
The relationship between WM and fear learning may also be through shared circuitry, specifically the medial PFC (mPFC) and hippocampus circuit. Although the DLPFC is traditionally thought to underlie WM, the mPFC is also important for WM (Liu et al., 2014). For example, synchronized mPFC-hippocampus theta oscillations (O’Neill, Gordon, & Sigurdsson, 2013) and mPFC-hippocampus structural connectivity are associated with improved WM performance (Spellman et al., 2015). Trial by trial persistent neural activity in the mPFC is associated with WM load (Kaminski et al., 2017) and pharmacological inactivation of the mPFC impairs WM (Yang, Shi, Wang, Peng, & Li, 2014). Likewise, the mPFC and hippocampus circuit is critical for extinction learning (Gilmartin, Balderston, & Helmstetter, 2014; Maren, Phan, & Liberzon, 2013). Lesions to the mPFC or hippocampus impair extinction recall (Giustino & Maren, 2015; Quirk, Garcia, & Gonzalez-Lima, 2006). In summary, WM and fear extinction have considerable overlap in circuit and signaling substrates supporting a neurobiological confluence between these important processes.
The results of the current investigation provide support for the influence of WM on the ability to extinguish fear. However, there are noteworthy limitations in our study. First, it will be necessary to replicate and extend these findings to a population other than young, active duty, male, Marine/Navy servicemen. With data indicating sex differences in fear learning (Day, Reed, & Stevenson, 2016; Jackson, Payne, Nadel, & Jacobs, 2006), and increased prevalence of anxiety and fear related disorders in female civilian and military populations (Levine & Land, 2014; Perrin et al., 2014), it will be important to investigate whether the results of the current investigation generalize to women and civilian populations. Second, the use of the N-back task to categorize individual differences in WM may be limited due to its modest relationship with complex WM span tasks (Jaeggi, Buschkuehl, Perrig, & Meier, 2010; Kane, Conway, Andrew, Miura, & Colflesh, 2007; Redick & Lindsey, 2013). However, the N-back task is widely used in cognitive neuroscience (Cohen et al., 1997; Gur et al., 2010; Moore et al., 2015), has been shown to discriminate between patient populations (Drummond et al., 2013; Matsuo et al., 2006; Philip et al., 2016), and is a reliable marker of PFC activation (Braver et al., 1997; Chatham et al., 2011), suggesting it has important clinical applications. Nevertheless, future research should utilize complex span tasks to determine whether the current results extend to multiple measures of WM ability.
A challenge for future work will be to determine whether the relationship between WM and fear extinction learning is associated with vulnerability to PTSD and other anxiety-related disorders. A potential fruitful application would be to investigate whether mechanistic interventions that target WM to ameliorate fear-based symptoms (Bomyea, Stein, & Lang, 2015; N. Cohen et al., 2016; Schweizer, Grahn, Hampshire, Mobbs, & Dalgleish, 2013) also improve extinction-learning performance. Indeed, cognition-enhancing pharmacological agents may enhance fear extinction or extinction recall (Singewald, Schmuckermair, Whittle, Holmes, & Ressler, 2015). Deficient fear extinction is a core facet of PTSD and other anxiety-related disorders (Acheson et al., 2012; Lissek & van Meurs, 2015; Zuj et al., 2016). Using WM as a framework to inform the understanding of fear learning may provide valuable insight into regulating fear, paving the way for a deeper understanding of vulnerability to PTSD and other anxiety disorders (Duits et al., 2015).
Supplementary Material
Highlights.
High working memory is associated with increased extinction learning
Working memory ability does not facilitate fear acquisition
Sustained attention ability is not associated with fear acquisition or extinction
Acknowledgments
The Navy Bureau of Medicine and Surgery (N62645-11-C-4037) (DGB, VBR) and the VA Center of Excellence supported this work for Stress and Mental Health (DMS, DTA, DGB, VBR). VBR is also supported by a VA Merit Award. TMM and RCG are partially supported by NIMH grants MH089983, MH019112, MH096891, MH042228; and the Dowshen Program for Neuroscience. Writing of this manuscript was partially supported by the Office of Academic Affiliations, Advanced Fellowship Program in Mental Illness Research and Treatment, Department of Veterans Affairs.
Footnotes
Some argue that % change scores can be biased and less reliable than using raw FPS or T-scores (Bradford et al., 2016; Lonsdorf et al., 2017). We therefore repeated our analyses using raw startle responses (subtracted from NA trials) without comparing it to maximum CS+ response during acquisition. Results were mostly consistent with our primary analyses. Individuals with high WM ability displayed an overall larger ‘fear extinction learning’ score, p=.047. Importantly, the WM group × block interaction remained significant, p=.015. A planned comparison supported the index-score method described in the primary analysis by showing that high WM ability was associated with larger extinction learning scores during the late block (p=.003). This pattern was similar when using the full continuous sample as well (non-extreme group approach): significant WM ability × block interaction (p=.029). Additional analyses for the T-scores are reported in the Supplementary Materials.
Conflict of Interest Statement
RCG received royalties from the Brain Resource Centre and, through Penn, from MindPrint Learning. MAG holds an equity interest in San Diego Instruments.
References
- Abraham AD, Cunningham CL, Lattal KM. Methylphenidate enhances extinction of contextual fear. Learning & Memory. 2012;19:67–72. doi: 10.1101/lm.024752.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham AD, Neve KA, Lattal KM. Dopamine and extinction: A convergence of theory with fear and reward circuitry. Neurobiology of Learning and Memory. 2014;108:65–77. doi: 10.1016/j.nlm.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Eyler LT, Resovsky J, Tsan E, Risbrough VB. Fear extinction memory performance in a sample of stable, euthymic patients with bipolar disorder. Journal of Affective Disorders. 2015;185:230–238. doi: 10.1016/j.jad.2015.06.053. [DOI] [PubMed] [Google Scholar]
- Acheson DT, Feifel D, de Wilde S, Mckinney R, Lohr J, Risbrough V. The effect of intranasal oxytocin treatment on conditioned fear extinction and recall in a healthy human sample. Psychopharmacology. 2013;229:199–208. doi: 10.1007/s00213-013-3099-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Geyer MA, Baker DG, Nievergelt CM, Yurgil K, Risbrough VB. Conditioned fear and extinction learning performance and its association with psychiatric symptoms in active duty Marines. Psychoneuroendocrinology. 2015;51:495–505. doi: 10.1016/j.psyneuen.2014.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acheson DT, Stein MB, Paulus MP, Ravindran L, Simmons AN, Lohr JB, Risbrough VB. Effects of anxiolytic treatment on potentiated startle during aversive image anticipation. Human Psychopharmacology: Clinical and Experimental. 2012;27:419–427. doi: 10.1002/hup.2243. [DOI] [PubMed] [Google Scholar]
- Baddeley A. Working Memory: Theories, Models, and Controversies. Annual Review of Psychology. 2012;63:1–29. doi: 10.1146/annurev-psych-120710-100422. [DOI] [PubMed] [Google Scholar]
- Badre D. Opening the gate to working memory. Proceedings of the National Academy of Sciences. 2012;109:19878–19879. doi: 10.1073/pnas.1216902109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker DG, Nash WP, Litz BT, Geyer MA, Risbrough VB, Nievergelt CM. Predictors of risk and resiliencefor posttraumatic stress disorder among ground combat Marines:methods of the Marine Resiliency Study. Preventing Chronic Disease. 2012;9:110134. doi: 10.5888/pcd9.110134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barad M. Is extinction of fear erasure or inhibition? Why both, of course. Learning & Memory. 2006;13:108–109. doi: 10.1101/lm.211306. [DOI] [PubMed] [Google Scholar]
- Bomyea J, Stein MB, Lang AJ. Interference control training for PTSD: A randomized controlled trial of a novel computer-based intervention. Journal of Anxiety Disorders. 2015;34:33–42. doi: 10.1016/j.janxdis.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Moody EW. Memory processes in classical conditioning. Neuroscience & Biobehavioral Reviews. 2004;28:663–674. doi: 10.1016/j.neubiorev.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Todd TP. A fundamental role for context in instrumental learning and extinction. Behavioural Processes. 2014;104:13–19. doi: 10.1016/j.beproc.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME, Westbrook RF, Corcoran KA, Maren S. Contextual and Temporal Modulation of Extinction: Behavioral and Biological Mechanisms. Biological Psychiatry. 2006;60:352–360. doi: 10.1016/j.biopsych.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A Parametric Study of Prefrontal Cortex Involvement in Human Working Memory. NeuroImage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Smart L. Working memory capacity and suppression of intrusive thoughts. Journal of Behavior Therapy and Experimental Psychiatry. 2005;36:61–68. doi: 10.1016/j.jbtep.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Carruthers P. Evolution of working memory. Proceedings of the National Academy of Sciences. 2013;110:10371–10378. doi: 10.1073/pnas.1301195110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catarino A, Küpper CS, Werner-Seidler A, Dalgleish T, Anderson MC. Failing to Forget. Psychological Science. 2015;26:604–616. doi: 10.1177/0956797615569889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Badre D. Multiple gates on working memory. Current Opinion in Behavioral Sciences. 2015;1:23–31. doi: 10.1016/j.cobeha.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, Badre D. Corticostriatal Output Gating during Selection from Working Memory. Neuron. 2014;81:930–942. doi: 10.1016/j.neuron.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Herd SA, Brant AM, Hazy TE, Miyake A, O’Reilly R, Friedman NP. From an Executive Network to Executive Control: A Computational Model of the n-back Task. Journal of Cognitive Neuroscience. 2011;23:3598–3619. doi: 10.1162/jocn_a_00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Perlstein WM, Braver TS, Nystrom LE, Noll DC, Jonides J, Smith EE. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386:604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- Cohen N, Margulies DS, Ashkenazi S, Schaefer A, Taubert M, Henik A, … Okon-Singer H. Using executive control training to suppress amygdala reactivity to aversive information. NeuroImage. 2016;125:1022–1031. doi: 10.1016/j.neuroimage.2015.10.069. [DOI] [PubMed] [Google Scholar]
- Collins AGE, Frank MJ. How much of reinforcement learning is working memory, not reinforcement learning? A behavioral, computational, and neurogenetic analysis. The European Journal of Neuroscience. 2012;35:1024–1035. doi: 10.1111/j.1460-9568.2011.07980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Sheridan M, Jacobs E, D’Esposito M. Impulsive personality predicts dopamine-dependent changes in frontostriatal activity during component processes of working memory. The Journal of Neuroscience. 2007;27:5506–5514. doi: 10.1523/JNEUROSCI.0601-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. The many faces of working memory and short-term storage. Psychonomic Bulletin & Review. 2016 doi: 10.3758/s13423-016-1191-6. [DOI] [PubMed]
- Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: An inhibitory learning approach. Behaviour Research and Therapy. 2014;58:10–23. doi: 10.1016/j.brat.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ardenne K, Eshel N, Luka J, Lenartowicz A, Nystrom LE, Cohen JD. Role of prefrontal cortex and the midbrain dopamine system in working memory updating. Proceedings of the National Academy of Sciences. 2012;109:19900–19909. doi: 10.1073/pnas.1116727109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR. The Cognitive Neuroscience of Working Memory. Annual Review of Psychology. 2015;66:115–142. doi: 10.1146/annurev-psych-010814-015031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day HLL, Reed MM, Stevenson CW. Sex differences in discriminating between cues predicting threat and safety. Neurobiology of Learning and Memory. 2016;133:196–203. doi: 10.1016/j.nlm.2016.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SPA, Walker M, Almklov E, Campos M, Anderson DE, Straus LD. Neural Correlates of Working Memory Performance in Primary Insomnia. Sleep. 2013;36:1307–1316. doi: 10.5665/sleep.2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duits P, Cath DC, Lissek S, Hox JJ, Hamm AO, Engelhard IM, … Baas JMP. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depression and Anxiety. 2015;32:239–253. doi: 10.1002/da.22353. [DOI] [PubMed] [Google Scholar]
- Dunsmoor JE, Niv Y, Daw N, Phelps EA. Rethinking Extinction. Neuron. 2015;88:47–63. doi: 10.1016/j.neuron.2015.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ghundi M, O’Dowd BF, George SR. Prolonged fear responses in mice lacking dopamine D1 receptor. Brain Research. 2001;892:86–93. doi: 10.1016/s0006-8993(00)03234-0. [DOI] [PubMed] [Google Scholar]
- Etkin A, Buchel C, Gross JJ. The neural bases of emotion regulation. Nature Reviews Neuroscience. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Fadok JP, Dickerson TMK, Palmiter RD. Dopamine Is Necessary for Cue-Dependent Fear Conditioning. The Journal of Neuroscience. 2009;29:11089–11097. doi: 10.1523/JNEUROSCI.1616-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Badre D. Mechanisms of hierarchical reinforcement learning in corticostriatal circuits: Supplementary modeling methods and analysis. Neuroscience. 2012;22:509–526. doi: 10.1093/cercor/bhr114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmartin MR, Balderston NL, Helmstetter FJ. Prefrontal cortical regulation of fear learning. Trends in Neurosciences. 2014;37:455–464. doi: 10.1016/j.tins.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Frontiers in Behavioral Neuroscience. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, … Gur RE. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. Journal of Neuroscience Methods. 2010;187:254–262. doi: 10.1016/j.jneumeth.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haatveit BC, Sundet K, Hugdahl K, Ueland T, Melle I, Andreassen OA. The validity of d prime as a working memory index: Results from the “Bergen n-back task. Journal of Clinical and Experimental Neuropsychology. 2010;32:871–880. doi: 10.1080/13803391003596421. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Weike AI. The neuropsychology of fear learning and fear regulation. International Journal of Psychophysiology. 2005;57:5–14. doi: 10.1016/j.ijpsycho.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Hayes J, VanElzakker M, Shin L. Emotion and cognition interactions in PTSD: a review of neurocognitive and neuroimaging studies. Frontiers in Integrative Neuroscience. 2012;6:89. doi: 10.3389/fnint.2012.00089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann SG. Cognitive processes during fear acquisition and extinction in animals and humans: Implications for exposure therapy of anxiety disorders. Clinical Psychology Review. 2008;28:199–210. doi: 10.1016/j.cpr.2007.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoge CW, Castro CA. Post-traumatic stress disorder in UK and US forces deployed to Iraq. Lancet (London, England) 2006;368:837. doi: 10.1016/S0140-6736(06)69315-X. author reply 837. [DOI] [PubMed] [Google Scholar]
- Jackson ED, Payne JD, Nadel L, Jacobs WJ. Stress Differentially Modulates Fear Conditioning in Healthy Men and Women. Biological Psychiatry. 2006;59:516–522. doi: 10.1016/j.biopsych.2005.08.002. [DOI] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- Kaminski J, Sullivan S, Chung JM, Ross IB, Mamelak AN, Rutishauser U. Persistently active neurons in human medial frontal and medial temporal lobe support working memory. Nature Neuroscience. 2017;20:590–601. doi: 10.1038/nn.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane MJ, Conway ARA, Andrew RA, Miura TK, Colflesh GJH. Working memory, attention control, and the n-back task: A question of construct validity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2007;33:615–622. doi: 10.1037/0278-7393.33.3.615. [DOI] [PubMed] [Google Scholar]
- Klein K, Boals A. The relationship of life event stress and working memory capacity. Applied Cognitive Psychology. 2001;15:565–579. [Google Scholar]
- LeDoux JE. Emotion Circuits in the Brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Levine B, Land H. Gender Disparities Among Veterans: The High Rate of Post-Traumatic Stress Disorder Among Women in the Military. Military Behavioral Health. 2014;2:59–63. [Google Scholar]
- Lissek S, van Meurs B. Learning models of PTSD: Theoretical accounts and psychobiological evidence. International Journal of Psychophysiology. 2015;98:594–605. doi: 10.1016/j.ijpsycho.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Gu X, Zhu J, Zhang X, Han Z, Yan W, … Li CT. Medial prefrontal activity during delay period contributes to learning of a working memory task. Science. 2014;346:458–463. doi: 10.1126/science.1256573. [DOI] [PubMed] [Google Scholar]
- Lovibond PF. Cognitive Processes in Extinction. Learning & Memory. 2004;11:495–500h. doi: 10.1101/lm.79604. [DOI] [PubMed] [Google Scholar]
- Macmillon NA. Signal detection theory. In: Pashler H, Wixted J, editors. Steven’s Handbook of Experimental Psychology: Methodology in Experimental Psychology. 3. Vol. 4. New York: John Wiley & Sons, Inc; 2002. pp. 43–90. [Google Scholar]
- Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nature Reviews Neuroscience. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall AJ, Acheson DT, Risbrough VB, Straus LD, Drummond SPA. Fear Conditioning, Safety Learning, and Sleep in Humans. The Journal of Neuroscience. 2014;34:11754–11760. doi: 10.1523/JNEUROSCI.0478-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuo K, Glahn DC, Peluso MAM, Hatch JP, Monkul ES, Najt P, … Soares JC. Prefrontal hyperactivation during working memory task in untreated individuals with major depressive disorder. Molecular Psychiatry. 2006;12:158–166. doi: 10.1038/sj.mp.4001894. [DOI] [PubMed] [Google Scholar]
- McClelland GH, Lynch John GJ, Irwin JR, Spiller SA, Fitzsimons GJ. Median splits, Type II errors, and false–positive consumer psychology: Don’t fight the power. Journal of Consumer Psychology. 2015;25:679–689. [Google Scholar]
- McNab F, Klingberg T. Prefrontal cortex and basal ganglia control access to working memory. Nature Neuroscience. 2008;11:103–107. doi: 10.1038/nn2024. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Fear Extinction as a Model for Translational Neuroscience: Ten Years of Progress. Annual Review of Psychology. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry. 2007;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Moore TM, Gur RC, Thomas ML, Brown GG, Nock MK, Savitt AP, … Stein MB. Development, Administration, and Structural Validity of a Brief, Computerized Neurocognitive Battery. Assessment. 2017 doi: 10.1177/1073191116689820. [DOI] [PMC free article] [PubMed]
- Moore TM, Reise SP, Gur RE, Hakonarson H, Gur RC. Psychometric properties of the Penn Computerized Neurocognitive Battery. Neuropsychology. 2015;29:235–246. doi: 10.1037/neu0000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological Psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill PK, Gordon JA, Sigurdsson T. Theta Oscillations in the Medial Prefrontal Cortex Are Modulated by Spatial Working Memory and Synchronize with the Hippocampus through Its Ventral Subregion. The Journal of Neuroscience. 2013;33:14211–14224. doi: 10.1523/JNEUROSCI.2378-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly RC, Frank MJ. Making working memory work: a computational model of learning in the prefrontal cortex and basal ganglia. Neural Computation. 2006;18:283–328. doi: 10.1162/089976606775093909. [DOI] [PubMed] [Google Scholar]
- Okon-Singer H, Hendler T, Pessoa L, Shackman AJ. The neurobiology of emotion-cognition interactions: fundamental questions and strategies for future research. Frontiers in Human Neuroscience. 2015;9:58. doi: 10.3389/fnhum.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes: An investigation of the physiological activity of the cerebral cortex. London: Oxford: University Press; 1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M, Vandeleur CL, Castelao E, Rothen S, Glaus J, Vollenweider P, Preisig M. Determinants of the development of post-traumatic stress disorder, in the general population. Social Psychiatry and Psychiatric Epidemiology. 2014;49:447–457. doi: 10.1007/s00127-013-0762-3. [DOI] [PubMed] [Google Scholar]
- Pezze MA, Feldon J. Mesolimbic dopaminergic pathways in fear conditioning. Progress in Neurobiology. 2004;74:301–320. doi: 10.1016/j.pneurobio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Philip NS, Sweet LH, Tyrka AR, Carpenter SL, Albright SE, Price LH, Carpenter LL. Exposure to childhood trauma is associated with altered n-back activation and performance in healthy adults: implications for a commonly used working memory task. Brain Imaging and Behavior. 2016;10:124–135. doi: 10.1007/s11682-015-9373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Raczka KA, Mechias ML, Gartmann N, Reif A, Deckert J, Pessiglione M, Kalisch R. Empirical support for an involvement of the mesostriatal dopamine system in human fear extinction. Translational Psychiatry. 2011;1:e12. doi: 10.1038/tp.2011.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragland JD, Turetsky BI, Gur RC, Gunning-Dixon F, Turner T, Schroeder L, … Gur RE. Working memory for complex figures: An fMRI comparison of letter and fractal n-back tasks. Neuropsychology. 2002;16:370–379. [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research—Past, Present, and Future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Redick TS, Lindsey DRB. Complex span and n-back measures of working memory: A meta-analysis. Psychonomic Bulletin & Review. 2013;20:1102–1113. doi: 10.3758/s13423-013-0453-9. [DOI] [PubMed] [Google Scholar]
- Risbrough V, Ji B, Hauger R, Zhou X. Generation and Characterization of Humanized Mice Carrying COMT158 Met/Val Alleles. Neuropsychopharmacology. 2014;39:1823–1832. doi: 10.1038/npp.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BJ, Volokhov RN, Demaree HA. Working memory capacity and the self-regulation of emotional expression and experience. Journal of Personality and Social Psychology. 2008;95:1526–1540. doi: 10.1037/a0013345. [DOI] [PubMed] [Google Scholar]
- Schweizer S, Grahn J, Hampshire A, Mobbs D, Dalgleish T. Training the Emotional Brain: Improving Affective Control through Emotional Working Memory Training. The Journal of Neuroscience. 2013;33:5301–5311. doi: 10.1523/JNEUROSCI.2593-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singewald N, Schmuckermair C, Whittle N, Holmes A, Ressler KJ. Pharmacology of cognitive enhancers for exposure-based therapy of fear, anxiety and trauma-related disorders. Pharmacology & Therapeutics. 2015;149:150–190. doi: 10.1016/j.pharmthera.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Current Opinion in Neurobiology. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellman T, Rigotti M, Ahmari SE, Fusi S, Gogos JA, Gordon JA. Hippocampal-prefrontal input supports spatial encoding in working memory. Nature. 2015;522:309–314. doi: 10.1038/nature14445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steenkamp MM. True evidence-based care for posttraumatic stress disorder in military personnel and veterans. JAMA Psychiatry. 2016;73:431–432. doi: 10.1001/jamapsychiatry.2015.2879. [DOI] [PubMed] [Google Scholar]
- Stout DM, Shackman AJ, Pedersen WS, Miskovich TA, Larson CL. Neural circuitry governing anxious individuals’ mis-allocation of working memory to threat. Scientific Reports. 2017;7:8742. doi: 10.1038/s41598-017-08443-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout DM, Shackman AJ, Johnson JS, Larson CL. Worry is associated with impaired gating of threat from working memory. Emotion. 2015;15:6–11. doi: 10.1037/emo0000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout DM, Shackman AJ, Larson CL. Failure to filter: Anxious individuals show inefficient gating of threat from working memory. Frontiers in Human Neuroscience. 2013;7:58. doi: 10.3389/fnhum.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd TP, Vurbic D, Bouton ME. Behavioral and neurobiological mechanisms of extinction in Pavlovian and instrumental learning. Neurobiology of Learning and Memory. 2014;108:52–64. doi: 10.1016/j.nlm.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovote P, Fadok JP, Luthi A. Neuronal circuits for fear and anxiety. Nature Reviews Neuroscience. 2015;16:317–331. doi: 10.1038/nrn3945. [DOI] [PubMed] [Google Scholar]
- VanElzakker MB, Kathryn Dahlgren M, Caroline Davis F, Dubois S, Shin LM. From Pavlov to PTSD: The extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiology of Learning and Memory. 2014;113:3–18. doi: 10.1016/j.nlm.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel EK, McCollough AW, Machizawa MG. Neural measures reveal individual differences in controlling access to working memory. Nature. 2005;438:500–503. doi: 10.1038/nature04171. [DOI] [PubMed] [Google Scholar]
- Yang ST, Shi Y, Wang Q, Peng JY, Li BM. Neuronal representation of working memory in the medial prefrontal cortex of rats. Molecular Brain. 2014;7:61. doi: 10.1186/s13041-014-0061-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yehuda R, Hoge CW. Treatment options for veterans with posttraumatic stress disorder—Reply. JAMA Psychiatry. 2016;73:758. doi: 10.1001/jamapsychiatry.2016.0572. [DOI] [PubMed] [Google Scholar]
- Zuj DV, Palmer MA, Lommen MJJ, Felmingham KL. The centrality of fear extinction in linking risk factors to PTSD: A narrative review. Neuroscience & Biobehavioral Reviews. 2016;69:15–35. doi: 10.1016/j.neubiorev.2016.07.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.