ABSTRACT
The problem of antimicrobial resistance (AMR) and the associated morbidity and mortality due to antibiotic resistant bacterial pathogens is not new. However, AMR has been increasing at an alarming rate with appearances of diseases caused by bacteria exhibiting resistance to not just one but multiple classes of antibiotics. The World Health Organization (WHO) supported by governments, health ministries and health agencies has formulated global action plans to combat the rise in AMR, supporting a number of proven initiatives such as antimicrobial stewardship, investments in development of new classes of antibiotics, and educational programs designed to eliminate inappropriate antibiotic use. Vaccines as tools to reduce AMR have historically been under-recognized, yet the positive effect in reducing AMR has been well established. For example Haemophilus influenzae type B (Hib) as well as Streptococcus pneumoniae (pneumococcal) conjugate vaccines have impressive track records in not only preventing life threatening diseases caused by these bacteria, but also reducing antibiotic use and AMR. This paper will describe the drivers of antibiotic use and subsequent development of AMR; it will make the case how existing vaccines are already participating in combatting AMR, describe future prospects for the role of new vaccines in development to reduce AMR, and highlight challenges associated with future vaccine development to combat AMR.
KEYWORDS: antimicrobial resistance, vaccines, Streptococcus pneumoniae, Staphylococcus aureus, Clostridium difficile, Group B streptococcus
AMR is an urgent health threat and large economic problem
The development of antibiotic resistances in bacteria is not a new phenomenon and is commonly observed after the introduction of new classes of antibiotics. For example, after introduction of penicillin in 1943 for the treatment of often fatal bacterial infections, resistance was observed for Staphylococcus aureus by 1948.1 S. aureus has several methods to resist the effect of penicillin. These include the alteration of the penicillin binding proteins that are the target of penicillin, as well as the production of beta-lactamases that inactivate the drug. Beta lactamases have also been effective at protecting S. aureus against new generations and classes of antibiotics including methicillin.2 Treatment of methicillin resistant S. aureus (MRSA) with vancomycin and increased empirical use of this antibiotic provided the antibiotic pressure associated with the emergence of vancomycin resistant enterococci and vancomycin intermediate resistant staphylococci.3 For other pathogens, resistance took longer to emerge. For example pneumococcal penicillin resistance was first observed only in the 1960s and subsequently spread globally due to continual changes to the antibiotic target, the bacterial penicillin binding proteins.2,4 Pneumoccoci with increasing resistance to third generation cephalosporins commonly used to treat meningitis were subsequently reported to the Centers for Disease Control (CDC) in the 1990s.5 In general, a trend is being observed that the time it takes bacteria to develop AMR after introduction of a new antibiotic is getting shorter.6 Additionally, given our global connectivity, antibiotic resistance can rapidly spread worldwide. A more recent example is metronidazole resistance in Clostridium difficile that developed quickly and led to a rapid spread of a quinolone resistant epidemic strains across the globe (Figure 1),7 causing high morbidity and mortality.8 Likewise genomic analysis of S. aureus clinical isolates has been able to map the development of fluoroquinolone resistance to the timeframe and region where the first fluoroquinolone clinical trials were conducted.9
Figure 1.
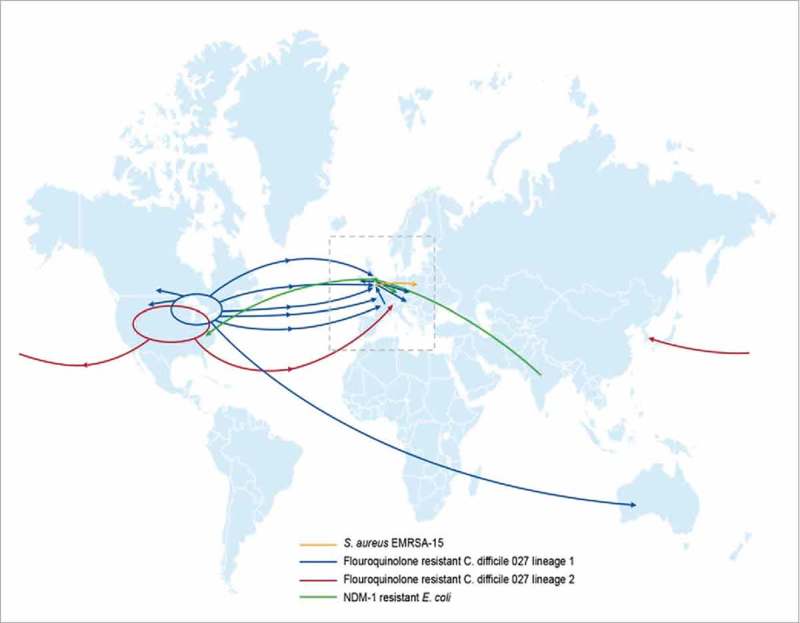
The rapid global spread of antibiotic resistant S. aureus,78 C. difficile8 and E. coli.79.
Multidrug resistant (MDR) Enterobacteriaceae are another example of rapid global spread of AMR initially due to the emergence of NDM-1 metallo-beta-lactamase producing strains. A resistant strain was isolated in patients in Europe who had previously traveled to India for surgical procedures10,11 (Figure 1) and NDM-1 subsequently spread to other Gram-negative species conferring resistance to all agents except colistin and tigecycline. MCR-1 plasmid-1 introduction into Escherichia coli isolates enabling resistance to the last line antibacterial agent colistin, now raises the concern that strains containing both MCR-1 and NDM-1 may emerge that may make routine outpatient urinary tract infections untreatable.12 And finally, Mycobacterium tuberculosis with the highest global burden of infected individuals of any single pathogen already shows resistance to many classes of antibiotics that make tuberculosis (TB) challenging to treat.13
Given the current trend of increasing AMR, it is estimated that by 2050, 10 million lives a year may be lost to AMR, exceeding the 8.2 million lives currently lost to cancer.14 To put this number in perspective, currently at least 700,000 people die of resistant infections every year globally, more than those caused by tetanus, cholera and measles combined. The AMR mortality burden is also not far behind mortality due to common afflictions such as diarrheal diseases or diabetes.15 Not only does AMR pose a significant health threat, it also represents a major economic burden on healthcare systems.14 In the EU, the economic burden associated with antibiotic resistant infections is estimated to be about €1.5 billion per year.16 In the US alone, at least two million infections a year are caused by bacteria that are resistant to at least first-line antibiotic treatments.17 Given that, the US health system spends 20 billion USD in excess each year to treat these infections.18 Organizations such as KPMG and RAND Europe have estimated that a continued increase in resistance could reduce world GDP by 2–3.5% by 2050.19,20
Vaccines as powerful tools to fight AMR
There are a number of well-established tools to reduce the global burden of AMR such as sanitation and hygiene, funding mechanisms to develop new classes of antibiotics, antibiotic stewardship, education to avoid inappropriate antibiotic use to treat viral infections for example and elimination of routine antibiotic use in livestock production. These interventions all have shown benefit when implemented (Box 1). However, it is less well known that prophylactic vaccines also are highly effective and valuable tools in the tool chest to fight AMR. Vaccines work by training the immune system to recognize and respond to a pathogen by mounting a rapid and effective immune defense, preventing the establishment of an infection/disease or decreasing disease severity.21 Many vaccines have an added benefit and can protect unvaccinated individuals or subjects that cannot be vaccinated in a given population by a process called herd immunity, which greatly reduces disease in the overall population. Disease prevention by vaccination lowers antibiotic use and reduces AMR. Both Haemophilus influenzae type B (Hib) and pneumococcal conjugate vaccines are instructive examples and success stories having demonstrated their effectiveness in reducing antibiotic use and reducing AMR. Before introduction of effective conjugate vaccines, Hib disease in children <5 years of age ranged from 3.5 to 601 per 100,000 in many countries of the world.22 A steady increase in Hib beta-lactam resistance was observed since the early 1970s, mediated by bacterial expression of beta-lactamases and/or to a lesser extent, modified penicillin binding proteins.23 One global surveillance study found that 16.6% of all Hib strains worldwide were beta lactamase positive with large variation between countries.24 This picture changed dramatically after the introduction of Hib conjugate vaccine. Disease cases dropped precipitously after the introduction of routine use of Hib conjugate vaccines25 also leading to a significant decrease in beta-lactamase positive strains.26
A similar picture emerged with the introduction of conjugate vaccines to prevent pneumococcal disease. In the 1990s, before the introduction of a 7-valent pneumococcal conjugate vaccine (PCV7) in children, ∼63,000 cases of invasive pneumococcal disease caused by vaccine serotypes (serotypes covered by PCV7) and non-vaccine serotypes (serotypes not covered by PCV7) pneumococcus occurred each year in the US.27 Importantly at this time, resistance to penicillin and other classes of antibiotics also spread in Str pneumoniae in the US, with invasive pneumococci becoming resistant to 3 or more drug classes.28,29 Similar to Hib vaccines, pneumococcal polysaccharide conjugate vaccines were highly effective with >90% efficacy against invasive pneumococcal disease (IPD) observed in the primary target populations of children <5 years of age. Not only was disease prevented, but significant reductions in bacterial colonization in US children was observed contributing to herd immunity in individuals initially not targeted for routine immunization, in particular adults.30 In 2000, 7 years after the introduction of PCV7, ∼211,000 cases of IPD caused by the 7 serotypes covered by PCV7 (including antibiotic-resistant strains) were prevented not only in children but in individuals of all ages29,30 Second generation pneumococcal conjugate vaccines with extended serotype coverage such as PCV10 (10-valent) and PCV13 (13-valent) have increased direct protection and herd immunity to the pneumococcal serotypes covered by these vaccines with similar efficacy.31,32 Very importantly, just as observed after introduction of PCV7, PCV13 also reduced antibiotic use and in parallel the prevalence of antibiotic non-susceptible strains also decreased (Figure 2).28,29,31
Figure 2.
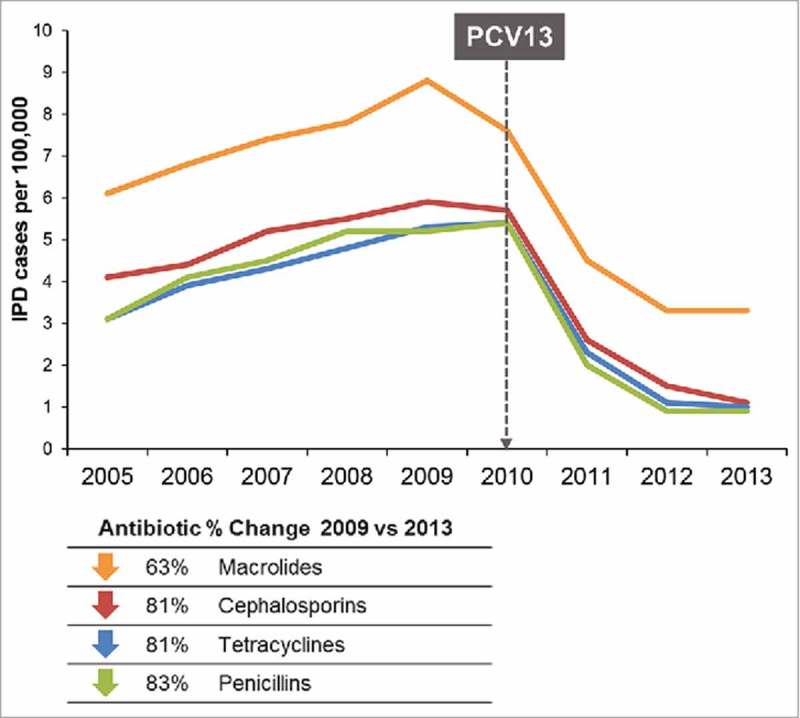
Rates of antibiotic non-susceptible invasive pneumococcal disease (<5 years) 2005–2013.28.
Impact of viral vaccines on AMR
Perhaps counter intuitive is the fact that viral vaccines are also very effective in reducing antibacterial resistance. Influenza vaccines for example do not only prevent influenza infections and disease but also decrease the likelihood of secondary bacterial infections, such as pneumonia and otitis media.33 There are two major mechanisms by which even moderately effective seasonal influenza vaccines can reduce antibiotic use. First, they prevent secondary bacterial infections and thus the use of antibiotics to treat these infections. In one study conducted in Turkey, influenza vaccination significantly reduced acute otitis media in children by 50.9% compared to unvaccinated controls and by inference antibiotic use in the vaccinated children.34 A study conducted in Canada demonstrated convincingly that influenza associated antibiotic prescriptions declined by ∼64% after introduction of universal influenza vaccination in Ontario compared to other Canadian provinces that limited the use of influenza vaccines to populations most at risk for disease.35 The second mechanism by which viral vaccines can reduce AMR is by prevention of inappropriate antibiotic prescriptions for respiratory tract infections caused by viral pathogens. For example, in the US half of all antibiotic prescriptions are inappropriately written for respiratory illnesses associated with pathogens such as influenza, respiratory syncytial virus (RSV) and other viruses that are not susceptible to antibiotics.36 These examples illustrate that even viral vaccines can play an important role in reducing antibiotic use that drives bacterial resistance.
Vaccines in late stage development with potential to reduce AMR
Clostridium difficile
C. difficile is anaerobic spore-forming Gram-positive bacillus and a main cause of antibiotic-associated nosocomial & community infectious diarrhea. The incidence and disease severity have increased over the last decade.37-39 In the US alone, approximately half a million cases of C. difficile infection (CDI) are diagnosed annually, along with 29,000 associated deaths. Given this burden of CDI and the rapid global spread of epidemic and antibiotic resistant strains, the CDC has classified C. difficile as an urgent threat.8 Once an infection occurs, the patient is often caught in a vicious cycle of antibiotic use, apparent cure but subsequent recurrence of the disease that then again has to be treated with antibiotics.
CDI is mediated by toxins, which can be inactivated by toxin neutralizing monoclonal or polyclonal vaccine elicited antibodies.40,41 The clear mechanism of action/pathogenesis of this toxin-based disease and an understanding that vaccine induced generation of toxin neutralizing antibodies has proven successful for a number of other toxin based diseases (such as tetanus, diphtheria or anthrax) further provided the rationale that a vaccine for protection against C. difficile should be technically feasible. Until recently, two C. difficile vaccines were in Phase 3 clinical development,41,42 yet one of the Phase 3 vaccine trials was terminated for futility; the cause of which has yet to be disclosed.43 Since CDI incidence increases with age, an efficacious vaccine to protect all adults 50 years and older would be highly desirable. Assuming the second vaccine in Phase 3 is successful and achieves licensure and recommendation, such vaccine would be an additional highly effective tool in reducing antibiotic use and associated AMR, as vaccinated individuals should not contract CDI thus reducing the spread of spores and bacteria, the associated antibiotic use and the generation of MDR bacterial species.
Staphylococcus aureus
S. aureus is a major cause of invasive disease including bacteremia, infectious endocarditis, osteomyelitis, pneumonia and various skin and soft tissue infections in hospital and community settings.44 Infections caused by MRSA are harder to treat than methicillin sensitive strains and associated with prolonged hospital stays and increased morbidity and mortality. MRSA contributes to over 70,000 cases of invasive disease per year in the US alone.45 Both the CDC and the WHO have recently placed MRSA on their AMR watch lists, highlighting the significance of AMR in this species. S. aureus has the propensity to develop resistance even to newer antibiotics that have been introduced such as linezolid and daptomycin44 and S. aureus glycopeptide resistance has emerged as a source of concern, as this class of antibiotics, including vancomycin, is one of the main resources for combating infections caused by MRSA and reduced susceptibility to vancomycin already has been described.46,47 With the often uncontrolled empirical vancomycin use particularly during elective surgeries, resistance rates could be further driven, which has already been documented by the emergence of vancomycin resistant enterococci and vancomycin-intermediate S aureus (VISA).2,48,49 Given the disease burden and adaptability of S. aureus to develop resistance to many classes of antibiotics, the development of effective vaccines would be highly desirable in curbing disease as well as reducing AMR.
To date, despite several attempts, there are no vaccines licensed to prevent S. aureus disease. Most notably two vaccines have failed in Phase 2/3 efficacy studies designed to prevent invasive disease either in an end stage renal disease population or in patients undergoing cardiothoracic surgery. Potential reasons for this failure have been comprehensively reviewed elsewhere.50
Vaccines that are currently in clinical development differ from these earlier vaccines in that they are designed to address more comprehensively the complex pathophysiology of S. aureus infection by eliciting antibodies that target multiple virulence factors. Furthermore, the antibody responses elicited are functional and either kill the bacteria or neutralize the virulence factors.51
The most advanced vaccine candidate, a 4 antigen S. aureus vaccine, SA4Ag is being evaluated in a phase 2/3 efficacy study for the prevention of invasive S. aureus disease in elective spinal surgery patients.52,53 SA4Ag was shown to be safe, well tolerated in early stage clinical trials, and induced high levels of bacterial killing antibodies in healthy adults, leading to a fast-track designation by the U.S. FDA.54 In addition, a separate 4 component S. aureus vaccine (4C-Staph) formulated with a TLR7-dependent adjuvant is under development.55
Vaccines in early stages of development with potential to reduce AMR
Streptococcus agalactiae, also known as Group B streptococcus (GBS) is an encapsulated Gram-positive bacterium that asymptomatically colonizes the vagina (women) and rectum (women and men) of approximately 25% of the population and can cause serious invasive infections including pneumonia, meningitis, bacteremia and sepsis across all age groups. Pregnant women and their babies are vulnerable to disease as asymptomatic maternal carriage is a prime risk factor for diseases in the mother, and the baby. Infant disease as a result of GBS infection presenting within the first 90 days of life is particularly devastating. In the US and some other high-income countries, GBS related disease incidence has been reduced by screening pregnant women for GBS recto-vaginal colonization and then treating carriers with antibiotics during labor, a process known as intrapartum antibiotic prophylaxis (IAP). In the US, approximately 25% of pregnant women are reported to undergo IAP and even higher rates are seen in other countries such as Canada.56 Although resistance to penicillin is low, penicillin sensitivity in 10% of pregnant females requires the administration of macrolide antibiotics. However, the prevalence of macrolide resistance in GBS has been rising in recent years, ranging from 7–25% in the US and Canada to over 60% in Asia, leaving physicians to use vancomycin for mothers with macrolide-resistant GBS who are allergic to penicillin.57,58
The period of risk for infant disease is within the first three months of life, with most disease occurring shortly after birth. For a vaccine to be successful in preventing most infant disease it would need to be administered to the mother during pregnancy as a maternal vaccine. The antibodies elicited in the mother, would be transferred to the fetus via the placenta before birth. The proof of concept that this approach could be successful was first described by Baker and Kasper who observed that there was a correlation between the mother's capsular polysaccharide antibody levels and the risk of their infant contracting GBS disease.59 Despite the concept being established nearly half a century ago, the concept of vaccine administration during pregnancy proved to be an unsurmountable hurdle for a long time. Attitudes have changed over the last 10 years due to the demonstration that tetanus, pertussis and flu vaccines can be safely administered to pregnant women and have had measurable positive effects on infant mortality and morbidity.60-62 Today, Tdap and influenza vaccines are successful maternal vaccines and are routinely recommended in pregnant women.63 Given these developments, GBS vaccines for use in maternal immunization are now under development. Current GBS vaccine development is focused on capsular polysaccharide conjugate vaccines (NCT03170609),64-66 as capsular serotype specific antibodies have been shown to kill GBS via complement mediated phagocytosis.
As discussed above, global disease burden and rising AMR rates in Gram-negative bacteria are very alarming so effective vaccines would be of great value.50 While some Gram-negative organisms present clinical development challenges with regard to identifying appropriate patient population with high enough disease incidence to perform prophylactic vaccine clinical studies, for a pathogen such as E coli however, patient populations can be identified and promising vaccine approaches focusing on proteins, capsular polysaccharides and lipopolysaccharides (or O antigens) are being evaluated preclinically and clinically.67-70
For M tuberculosis the hunt for effective vaccines has been challenging. The Bacille Calmette-Guerin (BCG) vaccine, in use for almost 100 years, is composed of an attenuated strain of Mycobacterium bovis and used to vaccinate >90% of newborns in endemic countries. This vaccination program is based on studies that have demonstrated that vaccination results in a reduction of disseminated disease and mortality in the youngest children. Re-administering BCG does not provide additional protection after a childhood dose and BCG will not prevent the reactivation of latent TB to pulmonary disease in the nearly one third of the human population that is already infected and at risk. Recent preliminary data presented by the South African Tuberculosis Vaccine Initiative (SATVI), at the Global Forum for TB at New Delhi (February 2018) suggest that reeexposure of BCG in adolescents may improve vaccine efficacy, thus administration of a booster dose in adolescents may help to curb the number of infections. Despite this, the shortcomings of BCG make the development of more modern vaccine approaches a priority.13,71
Many M tuberculosis vaccine approaches have been evaluated72-74 and as of today, none have been successful. Recent extensive studies into the immunophysiology of TB associated granulomas have shed new light on what could be considered a protective response.75 Vaccine development may also be advancing through the use of viral vectors; one promising approach that is still in preclinical stages of evaluation uses a Rhesus cytomegalovirus vector expressing multiple TB antigens (RhCMV/TB). By this approach, either complete protection against TB challenge and disease in some Rhesus macaques or partial protection was observed after immunization.76 The data are very exciting as the Rhesus TB challenges were conducted with a highly virulent TB strain (Erdman) and designed to also demonstrate long lasting protection in this model.77 If such RhCMV/TB-based vaccine constructs were to provide meaningful protection in humans in the future, the impact on AMR would be very significant given the high level of AMR exhibited by this organism and the difficulties treating patients with the disease requiring a cocktail of antibacterials.
Challenges and future prospects
As the global community is on high alert and gearing up to counteract AMR, much remains to be done to increase vaccine access globally for already licensed vaccines given their positive impact on reducing AMR. While it is unlikely that there will be vaccines that can protect against all bacteria that are contributing to the AMR problem, the prospects for vaccines in later stages of development are encouraging to do their part in fighting AMR, once shown to be effective and appropriately implemented. To provide maximal public health benefits, there must be efforts to steadily increase vaccine coverage as well as complete study and licensure proceedings of new vaccines addressing important bacterial and viral pathogens for which vaccines currently do not exist. Newer vaccines under development such as vaccines to prevent C. difficile or S. aureus, pneumococcal conjugate vaccines with extended serotype coverage or vaccines to prevent infections with Gram-negative bacteria and M. tuberculosis hold a profound promise to not only address these life threatening diseases but to help further curb antibiotic use and thereby prevent AMR. In addition, there is no limit to how many vaccines can be given to an individual as humans are exposed on a continuous basis to a myriad of infectious disease agents and the immune systems has been developed to deal with an almost infinitive number of pathogens. Of considerable importance will be an emphasis to reduce antibiotic use in animal and fish husbandry. The development of veterinary vaccines could help to stem the inappropriate widespread prophylactic use of antibiotics in animals. As a cautionary tale however, vaccine research and development organizations have seen dramatic consolidation over the last decade hence limiting the experienced human talent pool required and available to develop vaccines. The incentive to develop new vaccines would benefit also from innovation in regulatory sciences, to improve upon the speed of future vaccine development and new preventative measures to address AMR and to shorten licensure timing. In parallel, sustained investments in developing human talent would provide an experienced work force to innovate across vaccine research and development. Additionally, the importance of maintaining and creating new markets for vaccines cannot be understated. It is vital to provide incentives to vaccine companies to develop vaccines that are of public interest but may not be commercially viable. Given the importance of vaccines for global health and in reducing AMR, not addressing these trends could become a matter of national concern for many countries across the globe.
Box 1.
Approaches to reduce AMR burden
Continued improvements to sanitation and hygiene
Specific disease prevention guidelines from medical societies
Improved funding mechanisms for anti-infective and vaccine research and development
Incentives for manufacturers to invest in anti-infective and vaccine research and development
Continued education programs for prescribers on antibiotic stewardship
Continue initiatives globally to moderate/eliminate where possible the routine use of antibiotics in agriculture and aquiculture
Abbreviations
- AMR
antimicrobial resistance
- BCG
Bacille Calmette-Guerin
- CDC
Centers for Disease Control
- CDI
C. difficile infection
- GBS
Group B streptococcus
- Hib
Haemophilus influenzae type B
- IAP
intrapartum antibiotic prophylaxis
- IPD
invasive pneumococcal disease
- MDR
multidrug resistant
- MRSA
methicillin resistant S. aureus
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV10
10-valent pneumococcal conjugate vaccine
- PCV13
13- valent pneumococcal conjugate vaccine
- RSV
respiratory syncytial virus
- TB
tuberculosis
- VISA
Vancomycin-intermediate Staphylococcus aureus
- WHO
The World Health Organization
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to thank Robert Donald and Markay Hopps at Pfizer for editorial assistance and critical review of the manuscript.
References
- 1.Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. Lancet. 1948;2:641–4. doi: 10.1016/S0140-6736(48)92166-7. PMID:18890505. [DOI] [PubMed] [Google Scholar]
- 2.Neu HC. The crisis in antibiotic resistance. Sci (New York, NY). 1992;257:1064–73. doi: 10.1126/science.257.5073.1064. PMID:1509257. [DOI] [PubMed] [Google Scholar]
- 3.Sieradzki K, Roberts RB, Haber SW, Tomasz A. The development of vancomycin resistance in a patient with methicillin-resistant Staphylococcus aureus infection. N Engl J Med. 1999;340:517–23. doi: 10.1056/NEJM199902183400704. PMID:10021472. [DOI] [PubMed] [Google Scholar]
- 4.Kislak JW, Razavi LM, Daly AK, Finland M. Susceptibility of pneumococci to nine antibiotics. Am J Med Sci. 1965;250:261–8. doi: 10.1097/00000441-196509000-00003. PMID:4378532. [DOI] [PubMed] [Google Scholar]
- 5.Breiman RF, Butler JC, Tenover FC, Elliott JA, Facklam RR. Emergence of drug-resistant pneumococcal infections in the United States. JAMA. 1994;271:1831–5. doi: 10.1001/jama.1994.03510470035031. PMID:8196139. [DOI] [PubMed] [Google Scholar]
- 6.Pray L. Antibiotic R&D: resolving the paradox between unmet medical need and commercial incentive. Needham, MA: Cambridge Healthtech Institute;2008. [Google Scholar]
- 7.Peng Z, Jin D, Kim HB, Stratton CW, Wu B, Tang YW, Sun X. Update on Antimicrobial Resistance in Clostridium difficile: Resistance Mechanisms and Antimicrobial Susceptibility Testing. J Clin Microbiol. 2017;55:1998–2008. doi: 10.1128/JCM.02250-16. PMID:28404671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He M, Miyajima F, Roberts P, Ellison L, Pickard DJ, Martin MJ, Connor TR, Harris SR, Fairley D et al.. Emergence and global spread of epidemic healthcare-associated Clostridium difficile. Nat Genet. 2013;45:109–13. doi: 10.1038/ng.2478. PMID:23222960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holden M, Hsu LY, Kurt K, Weinert LA, Mather AE, Harris SR, Strommenger B, Layer F, Witte W et al.. A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013;23:653–44. doi: 10.1101/gr.147710.112. PMID:23299977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh TR, Weeks J, Livermore DM, Toleman MA. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. The Lancet Infect Dis. 2011;11:355–62. doi: 10.1016/S1473-3099(11)70059-7. PMID:21478057. [DOI] [PubMed] [Google Scholar]
- 11.Yong D, Toleman MA, Giske CG, Cho HS, Sundman K, Lee K, Walsh TR. Characterization of a new metallo-beta-lactamase gene, bla(NDM-1), and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother. 2009;53:5046–54. doi: 10.1128/AAC.00774-09. PMID:19770275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGann P, Snesrud E, Maybank R, Corey B, Ong AC, Clifford R, Hinkle M, Whitman T, Lesho E, Schaecher KE. Escherichia coli Harboring mcr-1 and blaCTX-M on a Novel IncF Plasmid: First Report of mcr-1 in the United States. Antimicrob Agents Chemother. 2016;60:4420–1. doi: 10.1128/AAC.01103-16. PMID:27230792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raviglione M, Marais B, Floyd K, Lönnroth K, Getahun H, Migliori GB, Harries AD, Nunn P, Lienhardt C, Graham S, et al.. Scaling up interventions to achieve global tuberculosis control: progress and new developments. Lancet (London, England). 2012;379:1902–13. doi: 10.1016/S0140-6736(12)60727-2. PMID:22608339. [DOI] [PubMed] [Google Scholar]
- 14.O'Neill J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations(The Review on Antimicroboal Resistance. 2015;1–84.
- 15.Ventola CL. The Antibiotic Resistance Crisis: Part 1: Causes and Threats. Pharm Ther. 2015;40:277–83. PMID:25859123. [PMC free article] [PubMed] [Google Scholar]
- 16.European Medicines Agency, European Centre for Disease Prevention and Control Joint technical report: the bacterial challenge—time to react. 2009.
- 17.Centers for Disease Control and Prevention ANTIBIOTIC RESISTANCE THREATS in the United States 2013. Available at: http://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf
- 18.The economic burden of antimicrobial resistance Why it is more serious than current studies suggest. 2013. Available at: www.lshtm.ac.uk/php/economics/assets/dh_amr_report.pdf.
- 19.KPMG LLP The Global Economic Impact of Anti-microbial Resistance. KPMG LLP. 2014. [Google Scholar]
- 20.Taylor J, et al.. Estimating the Economic Costs of Antimicrobial Resistance: Model and Results. Santa Monica, CA: RAND Corporation; 2014. [Google Scholar]
- 21.Fine PE. Herd immunity: history, theory, practice. Epidemiol Rev. 1993;15:265–302. doi: 10.1093/oxfordjournals.epirev.a036121. PMID:8174658. [DOI] [PubMed] [Google Scholar]
- 22.Peltola H, Rod TO, Jonsdottir K, Bottiger M, Coolidge JA. Life-threatening Haemophilus influenzae infections in Scandinavia: a five-country analysis of the incidence and the main clinical and bacteriologic characteristics. Rev Infect Dis. 1990;12:708–15. doi: 10.1093/clinids/12.4.708. PMID:2385772. [DOI] [PubMed] [Google Scholar]
- 23.Tristram S, Jacobs MR, Appelbaum PC. Antimicrobial resistance in Haemophilus influenzae. Clin Microbiol Rev. 2007;20:368–89. doi: 10.1128/CMR.00040-06. PMID:17428889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoban D, Felmingham D. The PROTEKT surveillance study: antimicrobial susceptibility of Haemophilus influenzae and Moraxella catarrhalis from community-acquired respiratory tract infections. J Antimicrob Chemother. 2002;50:49–59. doi: 10.1093/jac/dkf810. PMID:12239228. [DOI] [PubMed] [Google Scholar]
- 25.Adam HJ, Richardson SE, Jamieson FB, Rawte P, Low DE, Fisman DN. Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: evidence for herd effects and strain replacement due to Hib vaccination. Vaccine. 2010;28:4073–8. doi: 10.1016/j.vaccine.2010.03.075. PMID:20398617. [DOI] [PubMed] [Google Scholar]
- 26.Heilmann KP, Rice CL, Miller AL, Miller NJ, Beekmann SE, Pfaller MA, Richter SS, Doern GV. Decreasing prevalence of beta-lactamase production among respiratory tract isolates of Haemophilus influenzae in the United States. Antimicrob Agents Chemother 2005;49:2561–4. doi: 10.1128/AAC.49.6.2561-2564.2005. PMID:15917574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feldman C, Anderson R. Epidemiology, virulence factors and management of the pneumococcus. F1000Research. 2016;5:2320. doi: 10.12688/f1000research.9283.1. PMID:27703671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomczyk S, Lynfield R, Schaffner W, Reingold A, Miller L, Petit S, Holtzman C, Zansky SM, Thomas A, Baumbach J, et al.. Prevention of Antibiotic-Nonsusceptible Invasive Pneumococcal Disease With the 13-Valent Pneumococcal Conjugate Vaccine. Clin Infect Dis. 2016;62:1119–25. doi: 10.1093/cid/ciw067. PMID:26908787. [DOI] [PubMed] [Google Scholar]
- 29.Hampton LM, Farley MM, Schaffner W, Thomas A, Reingold A, Harrison LH, Lynfield R, Bennett NM, Petit S, Gershman K, et al.. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J Infect Dis. 2012;205:401–11. doi: 10.1093/infdis/jir755. PMID:22158567. [DOI] [PubMed] [Google Scholar]
- 30.Cohen R, Cohen JF, Chalumeau M, Levy C. Impact of pneumococcal conjugate vaccines for children in high- and non–high-income countries. Expert Rev Vaccines. 2017;1–16. [DOI] [PubMed] [Google Scholar]
- 31.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Holtzman C, Harrison LH, Zansky SM, Rosen JB, Reingold A, Scherzinger K, et al.. Effectiveness of 13-valent pneumococcal conjugate vaccine for prevention of invasive pneumococcal disease in children in the USA: a matched case-control study. Lancet Respir Med. 2016;4:399–406. doi: 10.1016/S2213-2600(16)00052-7. PMID:26987984. [DOI] [PubMed] [Google Scholar]
- 32.Knol MJ, Wagenvoort GH, Sanders EA, Elberse K, Vlaminckx BJ, de Melker HE, van der Ende A, et al.. Invasive Pneumococcal Disease 3 Years after Introduction of 10-Valent Pneumococcal Conjugate Vaccine, the Netherlands. Emerg Infect Dis. 2015;21:2040–4. doi: 10.3201/eid2111.140780. PMID:26488415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kash JC, Taubenberger JK. The role of viral, host, and secondary bacterial factors in influenza pathogenesis. Am J Pathol. 2015;185:1528–36. doi: 10.1016/j.ajpath.2014.08.030. PMID:25747532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ozgur SK, Beyazova U, Kemaloglu YK, Maral I, Sahin F, Camurdan AD, Kizil Y, Dinc E, Tuzun H. Effectiveness of Inactivated Influenza Vaccine for Prevention of Otitis Media in Children. Pediatr Infect Dis J. 2006;25:401–4. doi: 10.1097/01.inf.0000217370.83948.51. PMID:16645502. [DOI] [PubMed] [Google Scholar]
- 35.Kwong JC, Maaten S, Upshur REG, Patrick DM, Marra F. The Effect of Universal Influenza Immunization on Antibiotic Prescriptions: An Ecological Study. Clin Infect Dis. 2009;49:750–6. doi: 10.1086/605087. PMID:19624280. [DOI] [PubMed] [Google Scholar]
- 36.Fleming-Dutra KE, Hersh AL, Shapiro DJ, Bartoces M, Enns EA, File TM Jr, Finkelstein JA, Gerber JS, Hyun DY, Linder JA, et al.. Prevalence of inappropriate antibiotic prescriptions among us ambulatory care visits, 2010–2011. JAMA. 2016;315:1864–73. doi: 10.1001/jama.2016.4151. PMID:27139059. [DOI] [PubMed] [Google Scholar]
- 37.Lucado J, Could C, ELixhauser A. Clostridium difficile infections (CDI) in Hospital Stays, 2009. HCUP Statistical Brief 124. Rockville, (MD): Agency for Healthcare Research and Quality;2012. [PubMed] [Google Scholar]
- 38.Lessa FC, Mu Y, Bamberg WM, Beldavs ZG, Dumyati GK, Dunn JR, Farley MM, Holzbauer SM, Meek JI, Phipps EC, et al.. Burden of Clostridium difficile Infection in the United States. N Engl J Med. 2015;372:825–34. doi: 10.1056/NEJMoa1408913. PMID:25714160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med. 2015;372:1539–48. doi: 10.1056/NEJMra1403772. PMID:25875259. [DOI] [PubMed] [Google Scholar]
- 40.Wilcox MH, Gerding DN, Poxton IR, Kelly C, Nathan R, Birch T, Cornely OA, Rahav G, Bouza E, Lee C, et al.. Bezlotoxumab for Prevention of Recurrent Clostridium difficile Infection. N Engl J Med. 2017;376:305–17. doi: 10.1056/NEJMoa1602615. PMID:28121498. [DOI] [PubMed] [Google Scholar]
- 41.Sheldon E, Kitchin N, Peng Y, Eiden J, Gruber W, Johnson E, Jansen KU, Pride MW, Pedneault L. A phase 1, placebo-controlled, randomized study of the safety, tolerability, and immunogenicity of a Clostridium difficile vaccine administered with or without aluminum hydroxide in healthy adults. Vaccine. 2016;34:2082–91. doi: 10.1016/j.vaccine.2016.03.010. PMID:26993331. [DOI] [PubMed] [Google Scholar]
- 42.de Bruyn G, Saleh J, Workman D, Pollak R, Elinoff V, Fraser NJ, Lefebvre G, Martens M, Mills RE, Nathan R, et al.. Defining the optimal formulation and schedule of a candidate toxoid vaccine against Clostridium difficile infection: A randomized Phase 2 clinical trial. Vaccine. 2016;34:2170–8. doi: 10.1016/j.vaccine.2016.03.028. PMID:27013431. [DOI] [PubMed] [Google Scholar]
- 43.Sanofi (December 1). Sanofi ends development of Clostridium difficile vaccine [Press Release]. 2017. Retrieved from http://mediaroom.sanofi.com/sanofi-ends-development-of-clostridium-difficile-vaccine/
- 44.Dayan GH, Mohamed N, Scully IL, Cooper D, Begier E, Eiden J, Jansen KU, Gurtman A, Anderson AS. Staphylococcus aureus: the current state of disease, pathophysiology and strategies for prevention. Expert Rev Vaccines. 2016;15:1373–92. doi: 10.1080/14760584.2016.1179583. PMID:27118628. [DOI] [PubMed] [Google Scholar]
- 45.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–87. doi: 10.1128/CMR.00081-09. PMID:20610826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, Tenover FC. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–6. doi: 10.1093/jac/40.1.135. PMID:9249217. [DOI] [PubMed] [Google Scholar]
- 47.Hiramatsu K, Aritaka N, Hanaki H, Kawasaki S, Hosoda Y, Hori S, Fukuchi Y, Kobayashi I. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet (London, England). 1997;350:1670–3. doi: 10.1016/S0140-6736(97)07324-8. PMID:9400512. [DOI] [PubMed] [Google Scholar]
- 48.Caroom C, Tullar JM, Benton EGJ, Jones JR, Chaput CD. Intrawound Vancomycin Powder Reduces Surgical Site Infections in Posterior Cervical Fusion. Spine. 2013;38:1183–7. doi: 10.1097/BRS.0b013e31828fcfb5. PMID:23474597. [DOI] [PubMed] [Google Scholar]
- 49.Gaviola ML, McMillian WD, Ames SE, Endicott JA, Alston WKA. Retrospective Study on the Protective Effects of Topical Vancomycin in Patients Undergoing Multilevel Spinal Fusion. Pharmacother: The J Human Pharmacol Drug Therapy. 2016;36:19–25. doi: 10.1002/phar.1678. [DOI] [PubMed] [Google Scholar]
- 50.Jansen KU, Girgenti DQ, Scully IL, Anderson AS. Vaccine review: “Staphyloccocus aureus vaccines: problems and prospects”. Vaccine. 2013;7:2723–30. doi: 10.1016/j.vaccine.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 51.Anderson A, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, Jansen KU. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother. 2012;8:1585–94. doi: 10.4161/hv.21872. PMID:22922765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jansen KU, Jansen KU, Knirsch C, Anderson AS. The role of vaccines in preventing bacterial antimicrobial resistance. Nat Med. 2018;24:10–20. doi: 10.1038/nm.4465. PMID:29315295. [DOI] [PubMed] [Google Scholar]
- 53.Mohamed N, Wang MY, Le Huec JC, Liljenqvist U, Scully IL, Baber J, Begier E, Jansen KU, Gurtman A, Anderson AS. Vaccine development to prevent Staphylococcus aureus surgical-site infections. Br J Surgery. 2017;104:e41–54. doi: 10.1002/bjs.10454. [DOI] [PubMed] [Google Scholar]
- 54.Frenck RW Jr, Creech CB, Sheldon EA, Seiden DJ, Kankam MK, Baber J, Zito E, Hubler R, Eiden J, Severs JM, et al.. Safety, tolerability, and immunogenicity of a 4-antigen Staphylococcus aureus vaccine (SA4Ag): Results from a first-in-human randomised, placebo-controlled phase 1/2 study. Vaccine. 2017;35:375–84. doi: 10.1016/j.vaccine.2016.11.010. PMID:27916408. [DOI] [PubMed] [Google Scholar]
- 55.Mancini F, Monaci E, Lofano G, Torre A, Bacconi M, Tavarini S, Sammicheli C, Arcidiacono L, Galletti B, Laera D, et al.. One Dose of Staphylococcus aureus 4C-Staph Vaccine Formulated with a Novel TLR7-Dependent Adjuvant Rapidly Protects Mice through Antibodies, Effector CD4+ T Cells, and IL-17A. PLoS One. 2016;11:e0147767. doi: 10.1371/journal.pone.0147767. PMID:26812180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Simioni J, Hutton EK, Gunn E, Holloway AC, Stearns JC, McDonald H, Mousseau A, Schertzer JD, Ratcliffe EM, Thabane L et al.. A comparison of intestinal microbiota in a population of low-risk infants exposed and not exposed to intrapartum antibiotics: The Baby & Microbiota of the Intestine cohort study protocol. BMC pediatrics. 2016;16:183. doi: 10.1186/s12887-016-0724-5. PMID:27832763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lu B, Li D, Cui Y, Sui W, Huang L, Lu X. Epidemiology of Group B streptococcus isolated from pregnant women in Beijing, China. Clin Microbiol Infect. 2014:20:O370–3. doi: 10.1111/1469-0691.12416. PMID:24118553. [DOI] [PubMed] [Google Scholar]
- 58.Schrag S, Gorwitz R, Fultz-Butts K, Schuchat A. Prevention of Perinatal Group B Streptococcal Disease: revised Guidelines from CDC. Morb Mortal Wkly Rep (MMWR). 2002;51:1–22. [PubMed] [Google Scholar]
- 59.Baker CJ, Kasper DL. Correlation of maternal antibody deficiency with susceptibility to neonatal group B streptococcal infection. N Engl J Med. 1976;294:753–6. doi: 10.1056/NEJM197604012941404. PMID:768760. [DOI] [PubMed] [Google Scholar]
- 60.Thwaites CL, Beeching NJ, Netwon CR. Maternal and neonatal tetanus. Lancet. 2015;385:362–70. doi: 10.1016/S0140-6736(14)60236-1. PMID:25149223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omer SB. Maternal Immunization. N Engl J Med. 2017;376:1256–67. doi: 10.1056/NEJMra1509044. PMID:28355514. [DOI] [PubMed] [Google Scholar]
- 62.Amirthalingam G, Andrews N, Campbell H, Ribeiro S, Kara E, Donegan K, Fry NK, Miller E, Ramsay M. Eff ectiveness of maternal pertussis vaccination in England: an observational study. Lancet. 2014;384:1521–8. doi: 10.1016/S0140-6736(14)60686-3. PMID:25037990. [DOI] [PubMed] [Google Scholar]
- 63.CDC General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 2011;60. [PubMed] [Google Scholar]
- 64.Leroux-Roels G, Maes C, Willekens J, De Boever F, de Rooij R, Martell L, Bedell L, Wittke F, Slobod K, Dull P. A randomized, observer-blind phase Ib study to identify formulations and vaccine schedules of a trivalent group B Streptococcus vaccine for use in non-pregnant and pregnant women. Vaccine. 2016;34:1786–91. doi: 10.1016/j.vaccine.2016.02.044. PMID:26928074. [DOI] [PubMed] [Google Scholar]
- 65.Stålhammar-Carlemalm M, Waldemarsson J, Johnsson E, Areschoug T, Lindahl G. Nonimmunodominant regions are effective as building blocks in a streptococcal fusion protein vaccine. Cell Host Microbe. 2007;2:427–34. doi: 10.1016/j.chom.2007.10.003. PMID:18078694. [DOI] [PubMed] [Google Scholar]
- 66.Kobayashi M, Schrag SJ, Alderson MR, Madhi SA, Baker CJ, Sobanjo-Ter Meulen A, Kaslow DC, Smith PG, Moorthy VS, Vekemans J. WHO consultation on group B Streptococcus vaccine development: Report from a meeting held on 27–28 April 2016. Vaccine. 2016. Dec 22. doi: 10.1016/j.vaccine.2016.12.029. PMID:28017431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lundberg U, Senn BM, Schüler W, Meinke A, Hanner M. Identification and characterization of antigens as vaccine candidates against Klebsiella pneumoniae. Human Vaccines & Immunotherapeutics. 2013;9:497–505. doi: 10.4161/hv.23225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poolman JT, Wacker M. Extraintestinal Pathogenic Escherichia coli, a Common Human Pathogen: Challenges for Vaccine Development and Progress in the Field. J Infect Dis. 2016;213:6–13. doi: 10.1093/infdis/jiv429. PMID:26333944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mike LA, Smith SN, Sumner CA, Eaton KA, Mobley HL. Siderophore Vaccine conjugates protect against uropathogenic Escherichia coli urinary tract infection. PNAS. 2016;113:13468–73. doi: 10.1073/pnas.1606324113. PMID:27821778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huttner A, Hatz C, van den Dobbelsteen G, Abbanat D, Hornacek A, Frölich R, Dreyer AM, Martin P, Davies T, Fae K, et al.. Safety, immunogenicity, and preliminary clinical efficacy of a vaccine against extraintestinal pathogenic Escherichia coli in women with a history of recurrent urinary tract infection: a randomised, single-blind, placebo-controlled phase 1b trial. Lancet Infect dis. 2017;17:528–37. doi: 10.1016/S1473-3099(17)30108-1. PMID:28238601. [DOI] [PubMed] [Google Scholar]
- 71.Kaufmann SH, Lange C, Rao M, Balaji KN, Lotze M, Schito M, Zumla AI, Maeurer M. Progress in tuberculosis vaccine development and host-directed therapies–a state of the art review. Lancet Respir Med. 2014;2:301–20. doi: 10.1016/S2213-2600(14)70033-5. PMID:24717627. [DOI] [PubMed] [Google Scholar]
- 72.Gong W, Liang Y, Wu X. The current status, challenges, and future developments of new tuberculosis vaccines. Hum Vaccin Immunother. 2018;30:1–96. doi: 10.1080/21645515.2018.1458806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Floyd K, Glaziou P, Zumla A, Raviglione M. The global tuberculosis epidemic and progress in care, prevention, and research: an overview in year 3 of the End TB era. Lancet Respir Med. 2018;6:299–314. doi: 10.1016/S2213-2600(18)30057-2. PMID:29595511. [DOI] [PubMed] [Google Scholar]
- 74.Voss G, Casimiro D, Neyrolles O, Williams A, Kaufmann SHE, McShane H, Hatherill M, Fletcher HA. Progress and challenges in TB vaccine development. F1000Res. 2018;7. doi: 10.12688/f1000research.13588.1. PMID:29568497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Griffiths KL, Ahmed M, Das S, Gopal R, Horne W, Connell TD, Moynihan KD, Kolls JK, Irvine DJ, Artyomov MN. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nat Commun. 2016;7:13894. doi: 10.1038/ncomms13894. PMID:28004802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hansen SG, Zak DE, Xu G, Ford JC, Marshall EE, Malouli D, Gilbride RM, Hughes CM, Ventura AB, Ainslie E et al.. Prevention of tuberculosis in rhesus macaques by a cytomegalovirus-based vaccine. Nat Med. 2018;24:130–43. doi: 10.1038/nm.4473. PMID:29334373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Carpenter SM, Behar SM. A new vaccine for tuberculosis in rhesus macaques. Nat Med. 2018;24:124–6. doi: 10.1038/nm.4488. PMID:29414932. [DOI] [PubMed] [Google Scholar]
- 78.Aanensen DM, Feil EJ, Holden MT, Dordel J, Yeats CA, Fedosejev A, Goater R, Castillo-Ramírez S, Corander J, Colijn C et al.. Whole-Genome Sequencing for Routine Pathogen Surveillance in Public Health: a Population Snapshot of Invasive Staphylococcus aureus in Europe. MBio. 2016;7(3). pii:e00444–16. doi: 10.1128/mBio.00444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17:1791–8. doi: 10.3201/eid1710.110655. PMID:22000347. [DOI] [PMC free article] [PubMed] [Google Scholar]


