Abstract
Improving preparticipation screening of candidates for sports necessitates establishing the prevalence of high-risk cardiovascular conditions (hr-CVC) that predispose young people to sudden cardiac death (SCD). Our accurate, novel protocol chiefly involved the use of cardiac magnetic resonance (CMR) to estimate this prevalence.
Middle and high school students from a general United States population were screened by means of questionnaires, resting electrocardiograms, and CMR to determine the prevalence of 3 types of hr-CVC: electrocardiographic abnormalities, cardiomyopathies, and anomalous coronary artery origin from the opposite sinus with intramural coronary course (ACAOS-IM). We examined the range of normal left ventricular size and function in the main study cohort (schoolchildren 11–14 yr old). We defined diagnostic criteria for hr-CVC and compared the cardiac measurements of these younger participants with those of older children whom we examined (age, 15–18 yr).
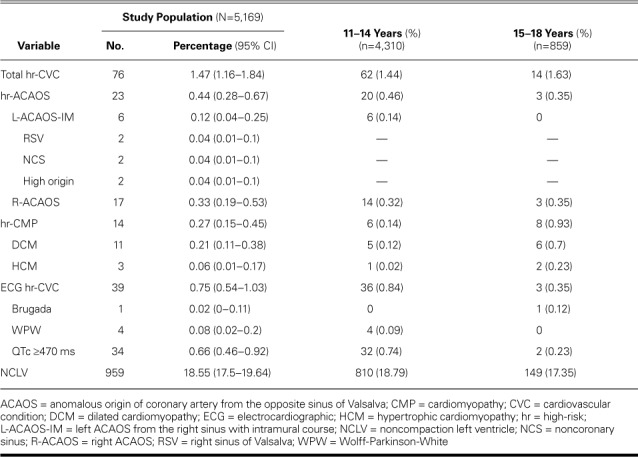
From 5,169 completed diagnostic studies (mean participant age, 13.06 ± 1.78 yr), CMR results revealed 76 previously undiagnosed cases of hr-CVC (1.47% of the total cohort): 11 of dilated cardiomyopathy (14.5%), 3 of nonobstructive hypertrophic cardiomyopathy (3.9%), 23 ACAOS-IM cases (30.3%; 6 left-ACAOS and 17 right-ACAOS), 4 Wolff-Parkinson-White patterns (5.3%), 34 prolonged QT intervals (44.7%), and 1 Brugada pattern (1.3%). Cardiomyopathies were significantly more prevalent in the older children. Of note, we identified 959 cases (18.5%) of left ventricular noncompaction.
If our estimate is accurate, only 1.47% of school-age sports participants will need focused secondary evaluations; the rest can probably be reassured about their cardiac health after one 30-minute screening study.
Keywords: Adolescent; death, sudden, cardiac/epidemiology/etiology/prevention & control; diagnostic imaging/methods; eligibility determination/standards; exercise/adverse effects; heart defects, congenital/diagnosis/diagnostic imaging/epidemiology/statistics & numerical data; magnetic resonance imaging/utilization; mass screening/economics/methods/statistics & numerical data; predictive value of tests; sports medicine/standards
Medical explanations for unexpected deaths of athletes during sports training or competition have not yet been adequately supported by objective evidence.1–14 The incidence, causes, and mechanisms of these sudden cardiac deaths (SCDs) are imprecisely understood. Published estimates lead to the assumption that structural heart disease underlies 84% of SCD cases in early adulthood, 44% of which are attributable to hypertrophic cardiomyopathy (HCM).1,11,13 However, no population-based, comprehensive surveys of SCD in the young have been performed to account for the important predisposing factors and events that occur in a cohort of adequate size and with known risk factors.
On the basis of limited data, promulgated guidelines state that medical history and physical examination are the only cost-effective methods of screening candidates for athletic competition or military service.4,15,16 Many experts agree, however, that these methods capture no more than 5% of the high-risk cardiovascular conditions (hr-CVC) that can cause SCD.3,12 Because current knowledge is inadequate, the first manifestation of an existing hr-CVC is frequently sudden death on the field, with the attendant dismal outcomes of improvised resuscitation efforts (usually associated with a mortality rate of 85%–95%).8,17
To characterize and minimize SCD in the young, it is necessary to perform accurate, prospective screening studies of populations entering the sports arena; evaluate the incidence and causes of SCD (during cardiovascular autopsy) in defined youthful populations; promote cost-efficient studies of available preventive measures; perform longitudinal studies of phenotypic manifestations of genetic causes of SCD, especially cardiomyopathies; and conduct appropriate pathophysiologic studies of mechanisms of SCD during athletic competition.3,4
Our study was designed primarily to determine the prevalence of hr-CVC in schoolchildren 11 to 18 years old, and to compare the prevalence in large groups of younger (11–14 yr) versus older (15–18 yr) children. We also compared our findings with prevalence estimated from history and physical examination and with electrocardiographic (ECG) or echocardiographic data in the medical literature. We hoped to develop a new paradigm for effective primary screening and secondary diagnostic evaluations to minimize SCD in athletes, military recruits, and others involved in strenuous exercise.
Patients and Methods
This study was approved by our Institutional Review Board (IRB). We used a focused questionnaire, a resting ECG, and cardiac magnetic resonance (CMR) for cardiovascular screening in a large cohort of unselected schoolchildren.4,18 Participation was voluntary and of no cost to the participants. Parental consent was required, as was the independent assent of participants younger than 18 years of age. Enrollment in middle or high school was the sole inclusion criterion.
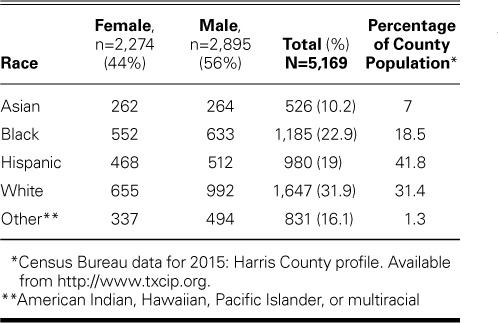
We initially studied middle-school children in Harris County (Houston), Texas (age range, 11–14 yr) and later included those in high school (age range, 15–18 yr). Approximately 17,000 invitations were sent through local communication channels, and 5,642 volunteer participants responded (~33%). Of these, the 5,169 who completed screening from December 2010 through January 2017 (4,310 younger children and 859 older children) composed our net study sample (Table I).
TABLE I.
Demographic Characteristics of the Study Population
When school was in session, screening was performed on school campuses. During school holidays, screening was provided at a location central to participants in the Houston area. Approximately one third of the participants enrolled and were screened during these holidays.
Screening
Participants and their family members completed a self-reported cardiovascular questionnaire that focused on personal and family history of hr-CVC, sudden cardiac arrest or SCD, dyspnea, angina pectoris, and syncope. Physical examination was limited to height, weight, and blood pressure measurements. A resting 16-lead ECG, obtained with use of a PageWriter TC70 cardiograph (Koninklijke Philips N.V.), underwent automatic computerized interpretation with use of the TraceMasterVue ECG Management System (Philips). An in-house electrophysiologist (AL) then confirmed or edited the results in accordance with established diagnostic criteria.16,19–21
All participants underwent cardiac-gated magnetic resonance imaging, without sedation or contrast agents, in a mobile Achieva 1.5T scanner (Philips); a 32-element surface coil was used for signal reception. Multiple-axis images were obtained in a steady-state free precession sequence. The subsequent screening protocol had 2 major components: evaluation of left ventricular (LV) structure and function with use of cine CMR, and evaluation of coronary artery origins and proximal courses.
The screening data were systematically reviewed by the electrophysiologist and 2 cardiac radiologists (BYC and VVLDR) and were evaluated for conclusive diagnosis by experienced cardiologists (PA and JTW) to determine whether the data were within normal limits or indicated probable hr-CVC warranting secondary evaluation.
For selective, quantitative evaluation of CMR images, contouring was performed in accordance with Society for Cardiovascular Magnetic Resonance guidelines for standardized image interpretation and postprocessing.22 The primary reader (CU) used cmr42® image-processing software (Circle Cardiovascular Imaging Inc.) under the supervision of the cardiac radiologists.
Criteria for Identifying High-Risk Conditions
We used 3 established categories of diagnostic results to justify referral of participants to secondary cardiac specialists for evaluation of potential hr-CVC:
-
1)
History markers: effort-related chest pain, syncope, or sudden cardiac arrest or SCD during exertion (in the children and first-degree family members).
-
2)
ECG findings: ≥2nd-degree atrioventricular block, Wolff-Parkinson-White patterns, arrhythmogenic right ventricular cardiomyopathy patterns, Brugada patterns, ventricular fibrillation or tachycardia, incessant ventricular tachycardia, or prolonged QT interval (QTc) (we considered a QTc of 470–489 ms to be abnormal and a QTc ≥490 ms to be severe). Expert opinion was sought in all suspected cases of prolonged QTc. Children who had right or left bundle branch block or ventricular hypertrophy identified only by ECG voltage and who had normal CMR findings did not undergo further evaluation.
-
3)
CMR findings: anomalous coronary arteries originating from an opposite sinus of Valsalva or with a high origin (ACAOS) and LV cardiomyopathies.
The ventricular image stack was analyzed by demarcating the endocardial and epicardial borders. Cross-referencing the ventricular short-axis images with long-axis images and observing wall motion in a cine fashion facilitated accurate determination of the atrioventricular and semilunar valve planes. Trabeculae and papillary muscles were excluded from LV mass calculation and were included in the blood pool. The epicardial and endocardial contours were drawn on the short-axis slices at end-diastole and end-systole; the contour slices covered the entire ventricle from apex to base.22
Coronary Anomalies. The term ACAOS, which refers to any ectopic origin of a coronary artery, succinctly describes an entity with many forms and adverse functional effects. The prefixes R- (right) and L- (left) indicate the coronary artery involved (Fig. 1). The suffix -IM specifies intramural course (inside the aortic tunica media), the only form associated with stenosis or ischemia. Other known forms—prepulmonic, intraseptal, and retro-aortic—are generally benign23,24 and were excluded from our hr-CVC totals.
Fig. 1.
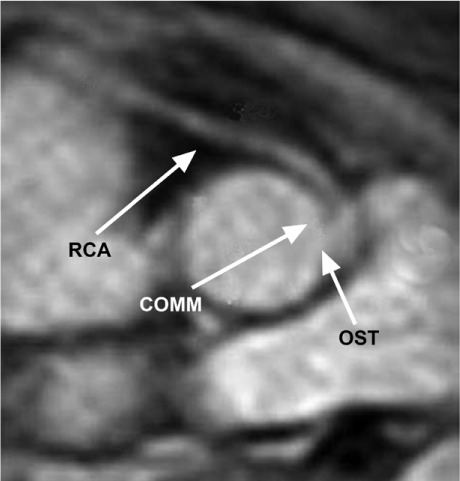
Anomalous coronary artery originates from the opposite sinus with intramural coronary course (R-ACAOS-IM). Tomographic section at the upper level of the aortic valve shows the right coronary artery (RCA) originating to the left of the anterior commissure (COMM), from the left sinus of Valsalva, next to the left coronary artery. OST = ostium
We identified ACAOS by finding the artery's location in the aortic root with respect to the closest aortic valve commissure. High coronary ostium was defined as any ostial position >5 mm above the sinotubular junction. We obtained a cross-sectional proximal view of the ACAOS-IM and determined the degree of possibly significant arterial compression (arteries whose maximum inner diameter exceeded the minimum diameter by a ratio >2:1).
Cardiomyopathies. Evaluations of LV structure and function (Fig. 2) were performed on the basis of the cardiac radiologists' qualitative impressions from the screening CMRs. Initial diagnosis of HCM was made after visual determination of an LV wall thickness >1.2 to 1.5 cm at any site, in longitudinal sections. Initial diagnosis of dilated cardiomyopathy (DCM) was made after visual characterization of LV function (LV ejection fraction [LVEF], <0.40–0.45) and size measurements made by the 3 radiology readers when they identified children whose LV dimensions—LV mass indexed to body surface area, myocardial wall thickness, LVEF, and LV end-systolic and end-diastolic volumes—deviated by more than one SD from the mean values of children without hr-CVC (Supplemental Table I).
Fig. 2.
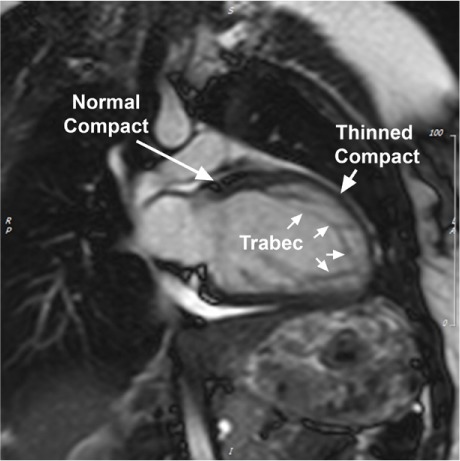
Magnetic resonance image (2-chamber view) of mild dilated cardiomyopathy with noncompaction left ventricle (LV) shows the LV during end-diastole. Note the typical structural changes of the thinned compact layer at the level of noncompaction architecture (“thinned compact”) in comparison with the normal LV segment (“normal compact”). This individual has morphologic signs of noncompaction for 50% of the LV circumference (indicated by a line in the accompanying motion image).
Trabec = trabeculations
Supplemental motion image is available for Figure 2 (3.4MB, mp4) .
Statistical Analysis
Means and percentages for the participants' baseline characteristics were computed. Prevalence of each high-risk condition was calculated by dividing the number of affected children by 5,169 (cohort total), and 95% CIs were computed. Associations of symptoms and ECG abnormalities with demographic characteristics and with CMR findings of hr-CVC were evaluated with use of the Fisher exact test; t tests were used to compare means. Fisher exact tests were used to compare the prevalence of ACAOS, cardiomyopathies, and noncompaction LV (NCLV) between age groups (11–14 vs 15–18 yr), by sex, and by race. Analyses were performed by using Stata® version 15.0 (StataCorp LLC). P <0.05 was considered statistically significant.
Results
The initial cohort of 5,642 adequately represented both sexes and area racial demographics, although the rate of participation by Hispanic children was slightly below what one would expect for the region (Table I). The completion rate was 91.6%. The chief reasons for noncompletion were declined assent and refusal of CMR (n=213; 3.77% of initial volunteers). Safety precautions related to metal implants, piercings, and tattoos excluded 72 children (1.27%) from CMR. Discomfort, excessive movement, or claustrophobia accounted for 78 incomplete cases (1.38%). We excluded from further study 4 children who had been treated for known cardiac conditions and 8 others in whom motion artifacts precluded image interpretation. Another 24 participants were excluded because their body habitus, their low heart rate, or equipment failure made their imaging data unusable. Finally, 74 participants were excluded because they were outside the targeted age range for the current analysis: 36 were 10 years old, and 38 were >18 years old. The resultant study sample comprised 5,169 children with completed, diagnostic-quality screening studies (Table II). Most were 11 to 14 years old; 859 (16.6%) were 15 to 18 years old. Screening-system availability was 99.77%; the 0.23% downtime was due to equipment problems. No child reported substantial negative clinical consequences from CMR.
TABLE II.
Prevalence of Potentially High-Risk Cardiovascular Conditions
Exercise Habits
Of children who reported their exercise habits (n=3,531; 68.3% of total cohort), most (n=2,012; 57%) exercised longer than 6 hours per week. The others reported weekly exercise of 3 to 6 hours (n=864; 24.5%) or 0 to 3 hours (n=655; 18.5%). Exercise time was quantified as time spent running or doing equivalent exercise.
High-Risk Cardiovascular Conditions
In our continuous series of 5,169 children 11 to 18 years of age, we found probable hr-CVC in 76 (1.47%) (Table II).
Symptoms
In our study sample, 926 questionnaire respondents reported symptoms: shortness of breath with exercise (572), chest pain (493), syncope at rest (99), and syncope with exercise (80); some reported more than one symptom. The rate of reported symptoms did not differ significantly between children identified as having hr-CVC (16 of 76; 21.1%) and those without hr-CVC (926 of 5,093; 18.2%) (P=0.55). Dyspnea upon exertion was reported by 569 of 5,158 children without DCM (11%) and by 3 of 11 who had DCM (27%) (P=0.11).
Electrocardiographic Anomalies
Potential hr-CVC was associated with the following ECG findings: Wolff-Parkinson-White pattern (in 4), Brugada type 1 pattern (in 1), and prolonged QTc ≥470 ms (in 34). Seven children had a QTc ≥490 ms, which typically indicates disqualification from sports participation.20 No child with a prolonged QTc reported syncope or had a history of cardiac arrest. More than twice as many boys than girls had a QTc ≥470 ms: 26 of 2,895 (0.9%) vs 8 of 2,274 (0.35%) (P=0.02). The prevalence of prolonged QTc did not differ significantly by race: Asian, 5 of 526 (0.95%); white, 14 of 1,647 (0.85%); Hispanic, 3 of 980 (0.31%); black, 7 of 1,185 (0.59%); and other, 5 of 831 (0.6%) (P=0.43).
Ventricular hypertrophy, identified only by voltage criteria (not strain pattern), was frequent (13.8%), as was early repolarization (14.7%) (Supplemental Table II). Neither ECG anomaly was found in the children who had structural hr-CVC.
All children whose CMR results suggested structural hr-CVC had a prevalence of mild ECG abnormalities similar to those without hr-CVC. No child had left bundle branch block or an arrhythmogenic right ventricular cardiomyopathy pattern. Right bundle branch block was found in 6 children, all of whom had normal CMR findings.
Anomalous Coronary Arteries
With use of CMR, ACAOS-IM was diagnosed in 23 of 5,169 children (0.44%): 6 had L-ACAOS-IM and 17 had R-ACAOS-IM (Fig. 1 and Table II). The type of ACAOS could be established in these cases; however, the severity of intramural stenosis could not be determined solely by using CMR. The potential L-ACAOS-IM cases were 2 instances each of intramural aortic origin from the right sinus of Valsalva, from the noncoronary sinus, and from a high origin (Table II).
Six children had anomalous origin of the left circumflex coronary artery from the right sinus of Valsalva with retro-aortic course. We suspected it in 7 others in whom this artery was not visible, but these cases could not be confirmed because of limitations on the height of the aortic root segment (3 cm) that the screening CMR program could evaluate. More boys and black children had ACAOS, but too few to determine statistical significance. We found no prepulmonic or retrocardiac L-ACAOS (benign variants). Adequate CMR views of the 3 main coronary arteries were obtained in all participants except for the 7 with suspected retro-aortic L-ACAOS (99.6% accuracy for magnetic resonance angiography in 15,729 coronary arteries).
Cardiomyopathies
Three children had nonobstructive HCM, and 11 were diagnosed to have DCM in accordance with the LV end-diastolic volume and LVEF criteria. The older children had significantly more cardiomyopathies (0.14% vs 0.93%; P=0.001), particularly DCM (0.12% vs 0.7%, P=0.004).
When we unexpectedly found numerous cases of NCLV during the study, we began using the Petersen criterion (noncompaction:compaction [NC:C] ratio >2.3)25 retrospectively and prospectively to identify NCLV quantitatively (Table II). We thus diagnosed 959 children (18.55%) to have NCLV. In a series of normal hearts studied with use of short-axis slices, we found normal LVEF in 90.1% of them and mildly diminished LVEF in the rest (Supplemental Table III). Minimal compact myocardial thickness averaged 2.6 ± 1 mm in NCLV segments versus 7 ± 1 mm in unaffected segments in the same children (difference, 4.4 mm; P <0.0001).
In a random sample of 1,439 children with normal hearts, the average LV wall thickness was 7.25 ± 1 mm (P <0.0001) in comparison with those who had NCLV at NC segments. Children with NCLV did not differ significantly from those without NCLV with regard to the prevalence of reported symptoms (18.5% vs 17.8%; P=0.58) and non–hr-CVC ECG abnormalities (21.1% vs 18.8%; P=0.11). In the absence of definitive guidelines, asymptomatic children with NCLV and LVEF >0.40 were assumed to be allowed participation in sports; however, we recommended repeat evaluation annually and a follow-up CMR study at age 18 to 20 years. In adherence to IRB restrictions, we gave only reports of the screening findings—not certifications for sports participation.
We report our incidental CMR findings of non–hr-CVC in Supplemental Table IV.
Interobserver Variance
The cardiac radiologists agreed in 197 of the 203 cases that were subjected to double-blinded reading. The other 6 involved the severity of cardiomyopathy. Qualitative diagnosis (mild vs normal) required initially unavailable quantitative normal ranges from comparable cohorts. Eventually, the evaluators and reviewer concluded that none of the 6 disputed instances were hr-CVC.
Discussion
Reported causes of SCD in athletes have varied, especially because of differing diagnostic criteria and unknown baseline population risks of hr-CVC.18,26,27 Normal values for adult and pediatric heart measurements by CMR have been suggested,28,29 and a report about the general accuracy of CMR for screening athletes18 was encouraging. Conversely, echocardiography performed poorly in identifying coronary anomalies and NCLV in 2 studies of more than 2,000 students,30,31 and computed tomographic angiography is impractical for screening children because of cost, ionizing radiation, discomfort, and the need for intravenous contrast agents.4
An understanding of baseline risks in athletes and military recruits is needed to justify routine population screening and interventions to prevent SCD. In sports-related SCD, death risk can probably be objectively established only by comparing the prevalence of hr-CVC in a general population at risk (like that in the current study) with the number of sports-related SCDs in a similar out-of-hospital SCD population. A similar comparison of persons with known L-ACAOS-IM (in autopsy data obtained from U.S. military recruits26) showed that the SCD risk associated with this condition was approximately 194 times that in the control population without L-ACAOS-IM.1
Experts have long debated whether exercise accelerates the development of cardiomyopathy in persons with a congenital predisposition to it. This possibility might be supported by the high reported incidence of cardiomyopathies, especially hypertrophic, in persons older than our current study population, at the age of peak SCD incidence (approximately 18–19 yr).4,6–9,28
Relevance of Our Findings
The 1.47% prevalence of hr-CVC that we report, obtained with use of the most accurate screening methods currently available (ECG and CMR) from a large, unselected general population, can be compared only with a tentative estimate of 0.1% in the 2007 American Heart Association guidelines.11 Prolonged QTc (≥470 ms)20 was the hr-CVC most frequently observed in our population, comprising 44.7% of hr-CVC cases—a finding that may help to explain the high frequency of normal autopsy findings in cases of SCD in athletes. Prevalence of R-ACAOS-IM (0.33%) and L-ACAOS-IM (0.12%) is first and reliably established in the current large population study. Our findings can be preliminarily compared with similar estimates derived from pathology data or clinical series; however, these data sources often have a pre-test bias. Both R- and L-ACAOS can be reliably identified by using CMR. Secondary, refined individual analysis should be done to determine the need for any intervention or disqualification from sports.23,24 Our findings suggest that if QTc of 470 to 489 ms were excluded from the hr-CVC category so that only QTc ≥490 ms was considered an hr-CVC,20 this longer QTc would be the second most prevalent hr-CVC after ACAOS-IM (Supplemental Table V).
Data used to evaluate cardiomyopathy-related risk in athletes should include measures of exercise intensity (in training and competition) and quantitative LV mass index and volume data.13,19 However, the relevant literature suggests that the phenotypic manifestations of genetic HCM develop in early to middle adolescence and that they worsen with age and possibly with athletic training, making the cause of hypertrophy (genetic vs acquired athlete's heart) an important consideration in preparticipation screening and counseling. Currently, we have too few data from older adolescents to yield definitive findings regarding HCM, but we plan to pursue this issue.
Coronary Artery Anomalies
In ACAOS,23,24 athletic exercise can affect intramural lateral compression of a coronary artery inside the aortic tunica media: acute and chronic exercise-related aortic root dilation and increased stroke volume32,33 exacerbate narrowing of the coronary cross-sectional area during periods of increased demand. Long-term prognosis in ACAOS-IM might depend on the variable phenomenon of aortic root expansion that has been reported in athletes.33
Electrocardiographic Detection of High-Risk Conditions
Questions persist about which cutoff for prolonged QTc better indicates high risk of SCD, especially given the reportedly high prevalence of QTc ≥470 ms (0.59%).13,34 In our cohort, the prevalence of QTc ≥490 ms was low (0.14%). We found no cases of arrhythmogenic right ventricular cardiomyopathy in these children on ECG or CMR. This entity is typically found in specific populations (for example, regions in Italy),35,36 suggesting a relationship with local genetic or environmental influences that are apparently not present in our geographic region.
Noncompaction Left Ventricle
The observed prevalence of NCLV in our cohort (18.6%) is substantially higher than that in previous echocardiographic25,37 and even CMR-based38 population screening studies. The largest previous CMR-based study was part of the Multi-Ethnic Study in the MESA trial, in which NCLV—identified by the criterion of more than 2 of 17 hypertrabecular LV segments—was observed in 6% of adults; however, clinical history of cardiac disease was an exclusion criterion.2,38
Noncompaction left ventricle is considered to be a myocardial structural anomaly of unclear and variable clinical severity and prognosis. We found a significantly higher incidence of DCM (but not of NCLV) in children 15 years of age or older than that in the younger children, possibly indicating the progressive nature of DCM in general and in individuals with NCLV.
Symptoms
In our study, the reported incidence of dyspnea, chest pain, and syncopal episodes was similar in children with and without hr-CVC (21.1% vs 18.2%; P=0.55), and these symptoms did not identify individual coronary artery anomalies or NCLV. Nevertheless, in the evaluation of individual carriers of hr-CVC, symptom characterization is often important in establishing severity, and the results may be used to indicate studies beyond screening. Physical examination was limited to measuring weight, height, and blood pressure and was noncontributory to hr-CVC diagnosis. The potential usefulness of an additional physical examination of limb pulses and heart murmurs was not evaluated; however, these and especially aortic coarctation would be evaluated adequately during CMR screening.
Study Limitations
Participation in this screening study was voluntary, so our findings are potentially subject to psychological, social, and economic pre-testing biases. For example, it is likely that children who were involved in sports or had suspicious symptoms were overrepresented in our cohort. Because our study involved only the first steps in screening, we acknowledge the need for follow-up clinical evaluations of potential hr-CVC that we identified. In addition, diagnoses of severity should be reevaluated periodically, because hr-CVC and especially cardiomyopathies can evolve over time.15
Despite the aforementioned limitations, CMR-based screening has the great advantage of accuracy. The small group carrying possible hr-CVC (approximately 1.5% of the study population) will still need secondary clinical evaluation that focuses on each child's potential issue. Our data suggest that fewer than half of the children with positive findings will need either intervention or disqualification from sports. This is an improvement over screening solely on the basis of symptoms and ECG abnormalities, because their high prevalence in our cohort suggests that they have little value in predicting structural hr-CVC.
To minimize time spent testing students, we chose not to perform detailed physical examinations. In addition, the chest CMR would have detected most of the relevant potential clinical findings from a physical examination.
Results of a preliminary analysis of these data were published previously.39 The inclusion and exclusion criteria were refined for the purpose of the present analyses, resulting in some differences in the data reported. However, the differences are small, and the conclusions remain the same: the number of participants with hr-CVC is still low, and the number with NCLV remains high.
Implications for Broader Application of CMR Screening
Our results suggest the worth of selective, routine screening of populations that are at high risk because of their profession (such as soldiers in basic training) or sports participation from middle school through professional levels.6 Relative to the current standard of care—medical history and physical examination—this screening method should be a safe, comfortable, and potentially cost-effective way to establish accurate diagnoses. Any proposed change to the current standard of care would have to be validated by a prospective, longitudinal multicenter study, including related changes in the incidence of SCD, by comparing the CMR and standard screening methods.
Crucial questions include the cost of the program5 and the savings achievable through a reliable screening approach that minimizes the referral of individuals with false-positive findings and essentially avoids missed cases (false negatives). In our experience, high usage of CMR screening facilities (>20 cases/d) might cost providers as little as $250 per case. On the basis of our estimate of the prevalence of hr-CVC and with use of our current method, the expected cost of identifying one case of hr-CVC in a general population of schoolchildren (the cost of screening divided by the prevalence, and assuming perfect sensitivity and specificity15) is approximately $18,750. Individual states in the U.S., military institutions, school systems, insurance companies, and charitable organizations will probably continue as the principal funding agents for pilot programs, pending an official change in the current standard of care. In addition, if sports and exercise are to be promoted for good health, reliable screening should be recognized as a condition of endorsing and safely engaging in them.
Conclusions
From data collected through ECG and CMR screening, this study provides the most precise estimate yet of the prevalence of hr-CVC in a young population. Electrocardiography inadequately identified structural hr-CVC but was useful in identifying electrical hr-CVC, especially prolonged QTc. Preventing SCD is possible and potentially effective and timely, especially in athletes and military recruits. In addition, because our school and health systems promote exercise to prevent disease, this study shows promise in identifying young people who are at high risk of life-threatening consequences from participating in sports.
If our estimate is accurate, only 1.47% of our participants will need specialized secondary evaluations. The remaining 98.53% can probably be reassured about their cardiac health on the basis of a single 30-minute screening study.
Supplementary Material
Acknowledgments
Stephen N. Palmer, PhD, ELS, contributed to the editing of the manuscript. Dr. Jonathan Tobis, Dr. Robert Paisley, and Dr. Julio Ivan Farjat Pasos helped to revise the text.
Supplementary Material
Supplemental tables for this article are available at 10.14503_thij-18-6645.s1.pdf.
References
- 1.Angelini P. Etiology of sudden cardiac death in athletes: can autopsy clarify all the issues? J Am Coll Cardiol. 2016;68(22):2495–6. doi: 10.1016/j.jacc.2016.07.787. [DOI] [PubMed] [Google Scholar]
- 2.Zemrak F, Ahlman MA, Captur G, Mohiddin SA, Kawel-Boehm N, Prince MR et al. The relationship of left ventricular trabeculation to ventricular function and structure over a 9.5-year follow-up: the MESA study. J Am Coll Cardiol. 2014;64(19):1971–80. doi: 10.1016/j.jacc.2014.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harmon KG, Asif IM, Klossner D, Drezner JA. Incidence of sudden cardiac death in National Collegiate Athletic Association athletes. Circulation. 2011;123(15):1594–600. doi: 10.1161/CIRCULATIONAHA.110.004622. [DOI] [PubMed] [Google Scholar]
- 4.Angelini P, Vidovich MI, Lawless CE, Elayda MA, Lopez JA, Wolf D, Willerson JT. Preventing sudden cardiac death in athletes: in search of evidence-based, cost-effective screening. Tex Heart Inst J. 2013;40(2):148–55. [PMC free article] [PubMed] [Google Scholar]
- 5.Wheeler MT, Heidenreich PA, Froelicher VF, Hlatky MA, Ashley EA. Cost-effectiveness of preparticipation screening for prevention of sudden cardiac death in young athletes. Ann Intern Med. 2010;152(5):276–86. doi: 10.1059/0003-4819-152-5-201003020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harmon KG, Asif IM, Maleszewski JJ, Owens DS, Prutkin JM, Salerno JC et al. Incidence, cause, and comparative frequency of sudden cardiac death in National Collegiate Athletic Association athletes: a decade in review. Circulation. 2015;132(1):10–9. doi: 10.1161/CIRCULATIONAHA.115.015431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maron BJ, Haas TS, Murphy CJ, Ahluwalia A, Rutten-Ramos S. Incidence and causes of sudden death in U.S. college athletes. J Am Coll Cardiol. 2014;63(16):1636–43. doi: 10.1016/j.jacc.2014.01.041. [DOI] [PubMed] [Google Scholar]
- 8.Meyer L, Stubbs B, Fahrenbruch C, Maeda C, Harmon K, Eisenberg M, Drezner J. Incidence, causes, and survival trends from cardiovascular-related sudden cardiac arrest in children and young adults 0 to 35 years of age: a 30-year review. Circulation. 2012;126(11):1363–72. doi: 10.1161/CIRCULATIONAHA.111.076810. [DOI] [PubMed] [Google Scholar]
- 9.Roberts WO, Stovitz SD. Incidence of sudden cardiac death in Minnesota high school athletes 1993–2012 screened with a standardized pre-participation evaluation. J Am Coll Cardiol. 2013;62(14):1298–301. doi: 10.1016/j.jacc.2013.05.080. [DOI] [PubMed] [Google Scholar]
- 10.Drezner JA, O'Connor FG, Harmon KG, Fields KB, Asplund CA, Asif IM et al. AMSSM position statement on cardiovascular preparticipation screening in athletes: current evidence, knowledge gaps, recommendations and future directions. Curr Sports Med Rep. 2016;15(5):359–75. doi: 10.1249/JSR.0000000000000296. [DOI] [PubMed] [Google Scholar]
- 11.Maron BJ, Thompson PD, Ackerman MJ, Balady G, Berger S, Cohen D et al. Recommendations and considerations related to preparticipation screening for cardiovascular abnormalities in competitive athletes: 2007 update: a scientific statement from the American Heart Association Council on Nutrition, Physical Activity, and Metabolism: endorsed by the American College of Cardiology Foundation. Circulation. 2007;115(12):1643–55. doi: 10.1161/CIRCULATIONAHA.107.181423. [DOI] [PubMed] [Google Scholar]
- 12.Paterick TE, Jan MF, Paterick ZR, Umland MM, Kramer C, Lake P et al. Cardiac evaluation of collegiate student athletes: a medical and legal perspective. Am J Med. 2012;125(8):742–52. doi: 10.1016/j.amjmed.2012.02.017. [DOI] [PubMed] [Google Scholar]
- 13.Pelliccia A, Fagard R, Bjornstad HH, Anastassakis A, Arbustini E, Assanelli D et al. Recommendations for competitive sports participation in athletes with cardiovascular disease: a consensus document from the Study Group of Sports Cardiology of the Working Group of Cardiac Rehabilitation and Exercise Physiology and the Working Group of Myocardial and Pericardial Diseases of the European Society of Cardiology. Eur Heart J. 2005;26(14):1422–45. doi: 10.1093/eurheartj/ehi325. [DOI] [PubMed] [Google Scholar]
- 14.Finocchiaro G, Papadakis M, Robertus JL, Dhutia H, Steriotis AK, Tome M et al. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol. 2016;67(18):2108–15. doi: 10.1016/j.jacc.2016.02.062. [DOI] [PubMed] [Google Scholar]
- 15.Kaltman JR, Thompson PD, Lantos J, Berul CI, Botkin J, Cohen JT et al. Screening for sudden cardiac death in the young: report from a National Heart, Lung, and Blood Institute working group. Circulation. 2011;123(17):1911–8. doi: 10.1161/CIRCULATIONAHA.110.017228. [DOI] [PubMed] [Google Scholar]
- 16.Yeo TJ, Sharma S. Using the 12-lead electrocardiogram in the care of athletic patients. Cardiol Clin. 2016;34(4):543–55. doi: 10.1016/j.ccl.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Khera R, Chan PS, Donnino M, Girotra S. Hospital variation in time to epinephrine for nonshockable in-hospital cardiac arrest. Circulation. 2016;134(25):2105–14. doi: 10.1161/CIRCULATIONAHA.116.025459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prakken NH, Velthuis BK, Cramer MJ, Mosterd A. Advances in cardiac imaging: the role of magnetic resonance imaging and computed tomography in identifying athletes at risk. Br J Sports Med. 2009;43(9):677–84. doi: 10.1136/bjsm.2008.054767. [DOI] [PubMed] [Google Scholar]
- 19.Drezner JA, Ackerman MJ, Anderson J, Ashley E, Asplund CA, Baggish AL et al. Electrocardiographic interpretation in athletes: the ‘Seattle criteria’. Br J Sports Med. 2013;47(3):122–4. doi: 10.1136/bjsports-2012-092067. [DOI] [PubMed] [Google Scholar]
- 20.Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol. 2009;53(11):992–1002. doi: 10.1016/j.jacc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Myerburg RJ, Vetter VL. Electrocardiograms should be included in preparticipation screening of athletes. Circulation. 2007;116(22):2616–26. doi: 10.1161/CIRCULATIONAHA.107.733519. [DOI] [PubMed] [Google Scholar]
- 22.Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER et al. Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson. 2013;15:51. doi: 10.1186/1532-429X-15-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Angelini P. Novel imaging of coronary artery anomalies to assess their prevalence, the causes of clinical symptoms, and the risk of sudden cardiac death. Circ Cardiovasc Imaging. 2014;7(4):747–54. doi: 10.1161/CIRCIMAGING.113.000278. [DOI] [PubMed] [Google Scholar]
- 24.Angelini P, Monge J. Coronary artery anomalies. In: Morsucci M, editor. Grossman & Baim's cardiac catheterization, angiography, and intervention. 8th ed. Philadelphia: Lippincott Williams & Wilkins; 2013. pp. 335–53. editor. p. [Google Scholar]
- 25.Petersen SE, Selvanayagam JB, Wiesmann F, Robson MD, Francis JM, Anderson RH et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J Am Coll Cardiol. 2005;46(1):101–5. doi: 10.1016/j.jacc.2005.03.045. [DOI] [PubMed] [Google Scholar]
- 26.Eckart RE, Scoville SL, Campbell CL, Shry EA, Stajduhar KC, Potter RN et al. Sudden death in young adults: a 25-year review of autopsies in military recruits. Ann Intern Med. 2004;141(11):829–34. doi: 10.7326/0003-4819-141-11-200412070-00005. [DOI] [PubMed] [Google Scholar]
- 27.Maron BJ, Henry WL, Roberts WC, Epstein SE. Comparison of echocardiographic and necropsy measurements of ventricular wall thicknesses in patients with and without disproportionate septal thickening. Circulation. 1977;55(2):341–6. doi: 10.1161/01.cir.55.2.341. [DOI] [PubMed] [Google Scholar]
- 28.Eikendal AL, Bots ML, Haaring C, Saam T, van der Geest RJ, Westenberg JJ et al. Reference values for cardiac and aortic magnetic resonance imaging in healthy, young Caucasian adults. PLoS One. 2016;11(10):e0164480. doi: 10.1371/journal.pone.0164480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawel-Boehm N, Maceira A, Valsangiacomo-Buechel ER, Vogel-Claussen J, Turkbey EB, Williams R et al. Normal values for cardiovascular magnetic resonance in adults and children. J Cardiovasc Magn Reson. 2015;17:29. doi: 10.1186/s12968-015-0111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grazioli G, Merino B, Montserrat S, Vidal B, Azqueta M, Pare C et al. Usefulness of echocardiography in preparticipation screening of competitive athletes. Rev Esp Cardiol (Engl Ed) 2014;67(9):701–5. doi: 10.1016/j.rec.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 31.Yim ES, Basilico F, Corrado G. Early screening for cardiovascular abnormalities with preparticipation echocardiography: utility of focused physician-operated echocardiography in pre-participation screening of athletes. J Ultrasound Med. 2014;33(2):307–13. doi: 10.7863/ultra.33.2.307. [DOI] [PubMed] [Google Scholar]
- 32.Angelini P, Uribe C, Monge J, Tobis JM, Elayda MA, Willerson JT. Origin of the right coronary artery from the opposite sinus of Valsalva in adults: characterization by intravascular ultrasonography at baseline and after stent angioplasty. Catheter Cardiovasc Interv. 2015;86(2):199–208. doi: 10.1002/ccd.26069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Angelini P, Uribe C. Anatomic spectrum of left coronary artery anomalies and associated mechanisms of coronary insufficiency. Catheter Cardiovasc Interv. 2018 Jul 26; doi: 10.1002/ccd.27656. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Pickham D, Hsu D, Soofi M, Goldberg JM, Saini D, Hadley D et al. Optimizing QT interval measurement for the preparticipation screening of young athletes. Med Sci Sports Exerc. 2016;48(9):1745–50. doi: 10.1249/MSS.0000000000000962. [DOI] [PubMed] [Google Scholar]
- 35.Corrado D, Basso C, Pavei A, Michieli P, Schiavon M, Thiene G. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA. 2006;296(13):1593–601. doi: 10.1001/jama.296.13.1593. [DOI] [PubMed] [Google Scholar]
- 36.Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376(1):61–72. doi: 10.1056/NEJMra1509267. [DOI] [PubMed] [Google Scholar]
- 37.Oechslin EN, Attenhofer Jost CH, Rojas JR, Kaufmann PA, Jenni R. Long-term follow-up of 34 adults with isolated left ventricular noncompaction: a distinct cardiomyopathy with poor prognosis. J Am Coll Cardiol. 2000;36(2):493–500. doi: 10.1016/s0735-1097(00)00755-5. [DOI] [PubMed] [Google Scholar]
- 38.Kawel N, Nacif M, Arai AE, Gomes AS, Hundley WG, Johnson WC et al. Trabeculated (noncompacted) and compact myocardium in adults: the multi-ethnic study of atherosclerosis. Circ Cardiovasc Imaging. 2012;5(3):357–66. doi: 10.1161/CIRCIMAGING.111.971713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Angelini P, Cheong BY, Lenge De Rosen VV, Lopez JA, Uribe C, Masso AH et al. Magnetic resonance imaging-based screening study in a general population of adolescents. J Am Coll Cardiol. 2018;71(5):579–80. doi: 10.1016/j.jacc.2017.11.051. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


