Knock-down of the carotenoid-cleavage dioxygenase OsCCD4a in rice enhances the content of carotenoids in leaves and seeds.
Keywords: Carotenoid, carotenoid-cleavage dioxygenase, rice, Oryza sativa, RNA interference
Abstract
Carotenoids of staple food crops have a high nutritional value as provitamin A components in the daily diet. To increase the levels of carotenoids, inhibition of carotenoid-cleavage dioxygenases (CCDs), which degrade carotenoids, has been considered as a promising target in crop biotechnology. In this study, suppression of the OsCCD1, OsCCD4a, and OsCCD4b genes using RNAi was verified in transgenic rice plants by quantitative RT-PCR and small RNA detection. Leaf carotenoids were significantly increased overall in OsCCD4a-RNAi lines of the T1 generation, and the highest accumulation of 1.3-fold relative to non-transgenic plants was found in a line of the T2 generation. The effects on seed carotenoids were determined via cross-fertilization between β-carotene-producing transgenic rice and one of two independent homozygous lines of OsCCD1-RNAi, OsCCD4a-RNAi, or OsCCD4b-RNAi. This showed that carotenoids were increased to a maximum of 1.4- and 1.6-fold in OsCCD1-RNAi and OsCCD4a-RNAi, respectively, with a different preference toward α-ring and β-ring carotenoids; levels could not be established in OsCCD4b-RNAi. In addition, the contents of four carotenoids decreased when OsCCD1, OsCCD4a, and OsCCD4b were overexpressed in E. coli strains accumulating phytoene, lycopene, β-carotene, and zeaxanthin. OsCCD1 and OsCCD4a had a similar high carotenoid degrading activity, followed by OsCCD4b without substrate specificity. Overall, our results suggest that suppresing OsCCD4a activity may have potential as a tool for enhancing the carotenoid content of seed endosperms and leaves in rice.
Introduction
Carotenoids, C40 terpenoid metabolites, play vital roles in plants as antenna pigments in photosynthesis, photo-protectants in the xanthophyll cycle, and precursors for the apocarotenoid hormones abscisic acid and strigolactone (Walter and Strack, 2011; Nisar et al., 2015). In addition, dietary uptake of carotenoids benefits animals by providing health-enhancing properties for development and immune systems, and can also enhance body pigmentation that can confer a sexual selective advantage (Mayer et al., 2008; Sommer and Vyas, 2012). In particular, humans require carotenoids as vitamin A precursors and antioxidants, and health problems are caused when they are deficient in the diet (Mayer et al., 2008; Nisar et al., 2015; Giuliano, 2017). As a consequence, biofortification of carotenoids has been a subject of ongoing interest in improvement of crop plants used for food and feed purposes, as summarized by Giuliano (2017). Diverse individual and combined techniques have been used to metabolically engineer the biosynthesis, degradation, sequestration, and stability of carotenoids by genetic modification (Zhai et al., 2016; Giuliano, 2017). Strategies to block carotenoid catabolism have increased the contents of nutritionally valuable β-carotene and zeaxanthin via gene-silencing of the downstream steps of β-carotene hydroxylase (BCH) in potato tubers and zeaxanthin epoxidase (ZEP) in orange fruit and the competing branch step of lycopene ε-cyclase (LCY-e) in potato tubers (Römer et al., 2002; Diretto et al., 2006; Pons et al., 2014). Wheat endosperm lacking carotenoids has been simultaneously engineered to produce carotenoids by ectopic expression of the bacterial phytoene synthase gene CrtB and to increase carotenoids by silencing the endogenous BCH-step gene TaHyd (Zeng et al., 2015).
Similar gene-silencing strategies could be considered for blocking the degradation step of carotenoids into apocarotenoids, which are generated by the action of carotenoid-cleavage dioxygenases (CCDs) encoded by a multigene family consisting of two groups of 9-cis-epoxycarotenoid dioxygenases (NCEDs) and CCDs (Giuliano et al., 2003; Auldridge et al., 2006; Walter and Strack, 2011; Frusciante et al., 2014; Hou et al., 2016). Homologous genes belonging to the CCD group from diverse plant sources include CCD1, CCD2, CCD4, CCD7, and CCD8. The negative correlation found between the expression of either CCD1 or CCD4 and carotenoid accumulation suggests that they have a role in modulating carotenoid content in diverse plant species and organs, including chrysanthemum petals, potato tubers, orchid flowers, peach fruit, and Arabidopsis seeds (Ohmiya et al., 2006; Campbell et al., 2010; Chiou et al., 2010; Brandi et al., 2011, Gonzalez-Jorge et al., 2013). Turning to staple crop plants, a strong up-regulation of ZmCCD1 transcripts in the white endosperm of maize correlates with a lack of carotenoid accumulation (Vallabhaneni et al., 2010). In contrast, no effect on carotenoid accumulation was reported in Golden rice when OsCCD1 was expressed in both sense and antisense directions, suggesting that OsCCD1 specifically degrades apocarotenoids rather than carotenoids (Ilg et al., 2010); however, transcript levels of OsCCD1 in antisense plants were not discriminatively examined from those of OsCCD1 in sense plants, which increased in the range of 50- to 240-fold. Recently, other CCD candidates have been used to boost carotenoid accumulation in common rice plants with white endosperm that fundamentally lacks carotenoid accumulation. Yang et al. (2017) found that OsCCD4a and OsCCD4b had no effects on carotenoid accumulation when they were knocked out using the RNA-guided targeted genome-editing technology known as CRISPR-CAS9. However, their abilities to enhance carotenoid content might still be possible in seeds of carotenoid-accumulating rice plants with a golden color phenotype.
In the current study, in order to fully elucidate the effects of OsCCD1, OsCCD4a, and OsCCD4b on carotenoid content, three genes were suppressed by RNAi in common rice plants, which were then conventionally cross-bred with a stPAC rice strain with a golden hue—this strain having previously been engineered to accumulate carotenoids in the endosperm (Jeong et al., 2017). The in vitro carotenoid-cleavage activities of OsCCD1, OsCCD4a, and OsCCD4b were assessed in four carotenoid-accumulating E. coli strains, and carotenoid content was enhanced in planta by blocking carotenoid degradation by OsCCD4a in the leaves of common rice and by OsCCD1 and OsCCD4a in seeds of carotenoid-accumulating rice.
Materials and methods
Rice plant material
Seeds of japonica-type Korean rice (Oryza sativa L. cv. Ilmi) were obtained from the National Institute of Crop Science, Rural Development Administration, Jeonju, Republic of Korea, and were used to analyse endogenous gene expression, to amplify the genes of interest, and to perform plant transformations. Seeds sterilized with 70% ethanol and 2% sodium hypochlorite were germinated on Murashige–Skoog agar medium for 1 week in a growth chamber, transplanted into soil, and were then grown in a greenhouse with natural light under a 16/8 h light/dark cycle at 28 °C. Transgenic plants of the T0 generation were acclimatized in a growth chamber and grown in a greenhouse until maturity. Rice plants were grown in the field during the summer season after T1 generation and interbreeding. Their seeds were harvested at 40 d after flowering (DAF), and the endosperm color was visually inspected after removing husks using a TR-200 Electromotion rice husker (Kett, Tokyo, Japan) followed by polishing with a Pearlest Polisher (Kett).
Analysis of endogenous expression and sequence information for plant CCD genes
Samples harvested from diverse tissues of rice plants at different developmental stages were used for total RNA extraction with a RNeasy Mini Kit (Qiagen) according to the manufacturer’s instructions. The cDNA synthesized from 1 μg of total RNA was used for quantitative real-time (qRT)-PCR in a final volume of 20 µl, which included 0.25 µl of cDNA, 10 µl ThunderbirdTM SYBR qPCR Mix (Toyobo, Osaka, Japan), and 200 nM of each gene-specific primer pair(see Supplementary Table S1 at JXB online), on a CFX ConnectTM Real-Time System (Bio-Rad Laboratories) under PCR conditions of 1 min at 95 °C and 40 cycles of 15 s at 95 °C and 1 min at 60 °C. After the specificity of the PCR amplification was verified with melting-curve analysis (60 °C to 95 °C), threshold cycles (Ct; the cycle at which the increase in fluorescence exceeded the threshold setting) were automatically determined. The expression level was analysed using CFX manager™ v.2.1 (Bio-Rad Laboratories) using the OsUbi5 gene (Os01g22490) as a reference.
The sequences and structures of the plant CCD genes were searched from the database of the National Center for Biotechnology Information (NCBI;https://ncbi.nlm.nih.gov/) and the Knowledge-based Oryza Molecular Biological Encyclopedia (KOME). Protein sequence alignment was performed using the ClustalW algorithm of MegAlign in the DNASTAR program (https://dnastar.com/), and transit peptide sequence for chloroplast targeting was predicted using the ChloroP algorithm version 1.1 (http://www.cbs.dtu.dk/services/ChloroP/).
Vector construction, transformation, and cross-fertilization of rice plants
OsCCD1 (Os12g44310), OsCCD4a (Os02g47510), and OsCCD4b (Os01g22490) gene fragments were amplified from rice leaf cDNAs using gene-specific primer pairs in the first PCR of a Gateway cloning strategy. After performing a second PCR using the common attB primer pair, three products were finally introduced into a pANDA-β vector (Miki and Shimamoto, 2004) to mediate the RNAi to suppress gene expression via recombination by Gateway cloning (Invitrogen). All primer pairs are listed in Supplementary Table S1.
The three final RNAi vectors were transformed into E. coli DH5α and then into Agrobacterium LBA4404 and co-cultivated with embryogenic calli induced from mature rice seeds (O. sativa cv. Iimi). Green calli generated on selection medium containing phosphinothricin (4 mg l–1) and cefotaxime (500 mg l–1) were regenerated into transgenic plants via shooting and rooting procedures.
To cross-breed the OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi lines with the stPAC rice strain that displays a golden color due to carotenoid production in the endosperm (Jeong et al., 2017), conventional interbreeding during field growth in the summer season was performed at the T3 generation between two independent homozygous lines of the three RNAi transgenic plants as female parents and the homozygous stPAC 25 with a single intact copy of the transgene as the male parent. The resultant F1 seeds were self-pollinated for two more generations in the field to obtain homozygosity for the transgene traits of both parents.
Molecular analysis of transgenic rice plants
The genomic DNA of transgenic rice plants was extracted from leaf tissues of the T0 and F3 generations using a modification of the CTAB extraction method. To confirm the introduction of the transgene, genomic DNA PCRs were performed with the transgene-specific primer pair and the common attB primer pair of the Gateway binary vector (Supplementary Table S1). For Southern blot analysis, 20 μg samples of each genomic DNA from leaf tissues of the T0 generation were used for EcoRI digestion and then separated on 1% agarose gel to make the Hybond N+ nylon membrane blot (Amersham Pharmacia). The membrane was hybridized with the NptII gene probe amplified with a specific primer pair (5′-GAAGGGACTGGCTGCTATTG-3′ and 5′-AATATCACGGGTAGCCAACG-3′) using a PCR DIG Probe Synthesis Kit (Roche). Hybridization and detection were carried out with a DIG High Prime DNA Labeling and Detection Starter Kit II (Roche). The signal bands were developed after exposure to Lumi-Film Chemiluminescent Detection film (Roche) for 12 h and photographed in an Image Analyzer (FLA 3000, Fujifilm).
To verify transgene homozygosity in the T3 and F3 generations, TaqMan PCRs were performed to detect Bar as a transgene and the α-tubulin gene as an internal reference in the rice genome. The Bar assay included a primer pair (5′-GCACGCAACGCCTACGA-3′ and 5′-CACCAGCGGACGGGA-3′) and a customized probe (5′-CCGTGTACGTCTCCC-3′) labeled with a 6-carboxyfluorescein (6-FAM) fluorescent reporter dye. The α-tubulin assay included a fluorescent reporter dye VIC-labeled probe that is commercially available (Assay ID: Os03643486_s1; Applied Biosystems). The PCR reaction was performed using a TaqMan Gene Expression Master Mix (Applied Biosystems) on a CFX ConnectTM Real-time System (Bio-Rad Laboratories) with one cycle of 95 °C for 10 min and 40 cycles of 94 °C for 30 s, 58 °C for 40 s, and 68 °C for 1 min, as described by Song et al. (2016). The copy number of the T3 leaf genomic DNA of stPAC 25 rice was calculated to have a value of 1 and verified to have a homozygous single-copy reference of the Bar transgene (Jeong et al., 2017) using CFX Manager Software (Bio-Rad Laboratories).
The qRT-PCRs of transgenic plants were performed with total RNAs extracted from 78-d-old leaf tissues of T1 plants according to the same PCR conditions and primer pairs that were used for analysis of endogenous gene expression described above. For detection of small interfering RNAs (siRNAs), 1-month-old leaf tissue of the T2 plant generation was used for the consecutive extraction of total RNAs and small RNAs according to the method of Martin et al. (2005). RNA samples (8 μg) were separated on a 15% (w/v) denaturing polyacrylamide gel (86 mm wide, 68 mm long, 1.5 mm thick) containing 7 M urea and 1× Tris-Borate-EDTA (TBE) buffer (pH 8.0) through a pre-run at 40 V for 1 h and a run at 150 V for 1 h. The RNAs were transferred onto a Hybond-N+ nylon membrane (GE Healthcare) using a Semi-Dry Transfer Unit (Bio-Rad Laboratories), UV cross-linked, and hybridized in Rapid-hybTM buffer (GE Healthcare). Gene-specific probes for OsCCD1, 4a, and 4b were prepared as 32P-dCTP-labeled PCR fragments using a Random Prime Labeling System Rediprime IITM (GE Healthcare). The membrane was exposed to a Phosphor Imaging Screen (GE Healthcare) for 3 d and then visualized using the Molecular Imager FX System (Bio-Rad).
Carotenoid and chlorophyll analyses of transgenic rice plants
Carotenoids were extracted from the same T1 and T2 leaf tissues used for qRT-PCR and siRNA detection, and from mature F4 seeds (40 DAF) that were harvested from F3 plants for TaqMan PCRs and genomic PCRs as described in two previous reports for leaf and seed carotenoids, respectively (Song et al., 2016; Jeong et al., 2017). For HPLC analysis, the extracted samples were prepared by dissolving in 50:50 (v/v) dichloromethane/methanol after the addition of β-apo-8′-carotenal (0.05 ml, 25 μg ml–1, Sigma-Aldrich) as an internal standard, extracted as separated layers with hexan (1.5 ml), and then desiccated under liquid nitrogen. Carotenoids were then separated in an YMC ODS C-30 column (3 µm, 4.6 × 250 mm; YMC Europe) using an Agilent 1100 Series HPLC system equipped with a photodiode array detector under elution conditions as described previously (Song et al., 2016). For quantitative analysis, the HPLC chromatograms generated at 450 nm were used for determination of the peak areas relative to those of the calibration curves, which plotted at four different concentrations of individual carotenoid standards based on the peak area ratios with β-apo-8′-carotenal. Carotenoid standards were purchased from CaroteNature (Lupsingen, Switzerland) and included α-carotene (β,ε-carotene), 13Z-β-carotene, (all-E)-β-carotene, 9Z-β-carotene, lutein (β,ε-carotene-diol), zeaxanthin (β,β-carotene-diol), β-cryptoxanthin (β,β-caroten-ol), antheraxanthin (dihydro-epoxy-β,β-carotene-diol), and violaxanthin (diepoxy-tetrahydro-β-carotene-diol). The contents of 13Z-β-carotene, (all-E)-β-carotene, and 9Z-β-carotene were combined as that of β-carotene.
Total chlorophylls were extracted from the same T2 leaf tissues for both siRNA detection and carotenoid analysis and quantified as chlorophyll a (666 nm) and b (653 nm) in an Optizen POP spectrophotometer (Mecasys Company, Daejeon, Republic of Korea) as previously described (Song et al., 2016).
For statistical analysis, all individual samples were analysed for metabolite quantification in triplicate. The relative differences between groups to non-transgenic plants were determined using a one-tailed Student’s t-test.
Vector construction, transformation, and carotenoid analysis for carotenoid-accumulating E. coli strains
Full-length genes that included the open reading frames of OsCCD1 (Os12g44310), OsCCD4a (Os02g47510), and OsCCD4b (Os01g22490) were amplified from rice leaf cDNA using gene-specific primer pairs (Supplementary Table S2). Common attB primer pairs were used to create three second PCR products that were introduced into a pDEST15 vector via the Gateway cloning procedure (Invitrogen). The resultant bacterial expression vectors pOsCCD1, pOsCCD4a, and pOsCCD4b (see Supplementary Fig. S5) were transformed into E. coli strain BL21-AITM (Invitrogen), which has a tightly regulated L-arabinose-inducible T7 RNA polymerase system.
To provide phytoene, lycopene, β-carotene, and zeaxanthin as substrates for the carotenoid-cleavage action of rice CCDs, four E. coli strains harboring either pPHYT, pLYC, pBETA, or pZEAX (see Supplementary Fig. S5) were kindly provided by Professor DellaPenna’s group at Michigan State University (Cunningham et al., 1996). After plasmid extraction using a Plasmid DNA Purification Kit (Macherey-Nagel), pPHYT, pLYC, pBETA, and pZEAX were individually transformed into competent BL21-AI E. coli cells harboring either pOsCCD1, pOsCCD4a, or pOsCCD4b vectors, and their transformations were verified by colony PCR with specific primer pairs (Supplementary Table S2) under PCR conditions of one cycle at 95 °C for 5 min and 25 cycles at 98 °C for 10 s, 65 °C for 30 s, and 72 °C for 2 min.
E. coli strains in a log-growth phase of OD600nm 0.5 were treated with 0.2% (w/v) arabinose to induce the overexpression of OsCCD1, 4a, and 4b. After overnight incubation with shaking followed by centrifugation, pellet colors of E. coli cells were visually inspected, and the carotenoid content was analysed by HPLC. In brief, wet E. coli cells were pelleted at 14000 g for 40 s at 4 °C, suspended in 300 µl of acetone and 0.2 ml of β-apo-8′-carotenal as an internal standard, vortex-mixed for 10 s, and sonicated for 5 min. After incubation at 55 °C for 15 min in the dark, vortexing for 10 s, and centrifugation at 14000 g for 10 min at 4 °C, the supernatants were transferred to a new tube for carotenoid analysis using HPLC by the same method described above for rice leaves and seeds.
Results
Expression of OsCCD1, OsCCD4a, and OsCCD4b among various tissues and developmental stages
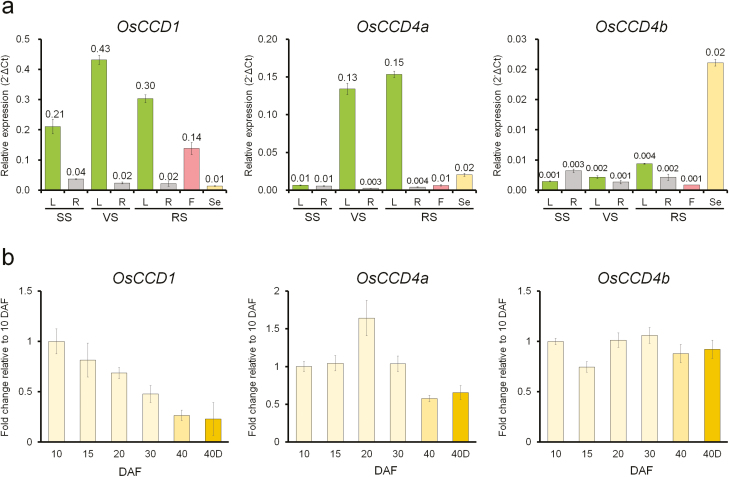
To profile the expression patterns of OsCCD1, OsCCD4a, and OsCCD4b, rice RNAs from various tissues (leaves, roots, florets, and seeds) at different development stages (seedling, vegetative, and reproductive) were examined by qRT-PCR. The results showed that the expression of OsCCD1 was ubiquitous, with higher levels in leaves than in other tissues (Fig. 1a). There was also higher expression of OsCCD4a in leaves after the seedling stage, but at lower levels relative to OsCCD1. The levels of expression of OsCCD4b were generally very low in all samples except for seeds at 40 DAF, which showed similar expression to OsCCD4a and slightly higher expression than OsCCD1.
Fig. 1.
Endogenous transcript levels of OsCCD1, OsCCD4a, and OsCCD4b in various tissues at different developmental stages in rice. (a) The relative expression levels of the genes quantified by qRT-PCRs in leaves (L), roots (R), flowers (F), and seeds (Se) harvested at 40 d after flowering (DAF), at the seedling stage (SS), vegetative stage (VS), and reproductive stage (RS). (b) The relative expression levels of the genes quantified by qRT-PCRs during the development of seeds at 10, 15, 20, 30, and 40 DAF and at an additional stage that was seeds at 40 DAF that had been desiccated for 1 week (40D). The mean (±SE) Ct values of triplicate measurements were used to calculate the expression of the target gene with normalization to an internal control (OsUbi5) using the ΔCt (a) and ΔΔCt (b) methods.
The expression of OsCCD1, OsCCD4a, and OsCCD4b was examined during five stages of seed development at 10, 15, 20, 30, and 40 DAF and at an additional stage that was seeds at 40 DAF that had been desiccated for 1 week (Fig. 1b). Interestingly, the expression of the three genes displayed different patterns as seeds matured. OsCCD1 was most highly expressed at the earliest stage (10 DAF) and then gradually decreased. The expression level of OsCCD4a was highest at the middle stage (20 DAF), whilst OsCCD4b was more or less the same across all stages. Desiccation did not affect the expression of the three genes after maturation.
Molecular characteristics of OsCCD1, OsCCD4a, and OsCCD4b based on protein sequence analysis
A phylogenetic analysis of CCD1 and CCD4 protein sequences among plants was performed using the sequence database from the NCBI and the ClustalW algorithm of MegAlign in DNASTAR (Supplementary Fig. S1a). Both the CCD1 and CCD4 groups, including an OsCCD1 and two OsCCD4 proteins, were positively distinguished among plants. One OsCCD1 and two OsCCD4 proteins had a closer relationship with those of the monocotyledonous plants Zea mays (ZmCCD1) and Crocus sativus (CsCCD1 and CsCCD4a, CsCCD4b, CsCCD4c, and CsZCD) than with other proteins of dicotyledonous plants.
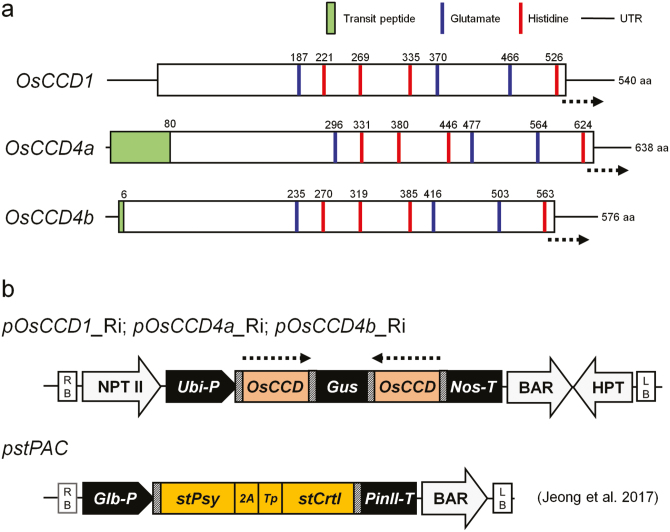
The protein structures of OsCCD1, CsCCD4a, and CsCCD4b were compared via sequence alignment among CCDs, including two Arabidopsis proteins (AtCCD1 and 4), a VP14 (Zea mays NCED), and three rice NCED proteins (OsNCED1, OsNCED2, and OsNCED3) (Supplementary Fig. S1b). The OsCCDs showed highly conserved characteristics as a CCD family, and these included NCED, a small CCD group that was distinct from NCED, and a distinct subgroup between CCD1 and CCD4 (Supplementary Fig. S1b). Key amino acid residues, including three glutamates and four histidines for CCD enzyme function, were also highly conserved in position and numbers (Supplementary Fig. S1b, Fig. 2a). The prediction of transit peptide (TP) sequences of OsCCDs using the ChloroP program indicated that OsCCD1 may not have a TP sequence, whereas two OsCCD4s have TP sequences of 80 and 6 amino acids (Fig. 2a), implying localization into the cytosol and chloroplasts, respectively.
Fig. 2.
Schematic representation of rice OsCCD1, OsCCD4a, and OsCCD4b gene structures and binary vectors used in this study. (a) The conserved position and numbers of key amino acids known for enzymatic action of CCDs and transit peptide regions predicted using the ChloroP program. (b) Diagram of RNAi-mediated suppression vectors of the three genes. The pstPAC vector used to endow the endosperm of stPAC rice with a carotenoid-intensifying golden color trait was developed in a previous study (Jeong et al., 2017) and is presented here because stPAC was used as a male parent in conventional interbreeding in this study. Dotted arrows in (a, b) indicate the gene region used in vector construction for RNAi-mediated suppression. In (b) the bacterial attachment attB sites needed for Gateway cloning are marked with hatched boxes. RB, right border; LB, left border; NPT II, neomycin-resistant gene cassette; BAR, bialaphos-resistant gene cassette; HPT, hygromycin-resistant gene cassette; Ubi-P, maize ubiquitin 1 promoter and 1st intron including splicing acceptor site; Nos-T, 3′-region from the nopaline synthase gene; Glb-P, rice globulin promoter; PinII-T, the 3′-region of the potato proteinase inhibitor II gene; stPsy, rice codon-optimized synthetic gene encoding Capsicum phytoene synthase (PSY); 2A, rice codon-optimized foot-and-mouth disease virus 2A peptide; Tp, transit peptide of rice Rubisco small subunit; stCrtI, rice codon-optimized synthetic gene encoding bacterial desaturase (CRTI).
Generation of RNAi-mediated suppression lines for OsCCD1, OsCCD4a, and OsCCD4b in rice plants
For gene-specific suppression of OsCCD1, OsCCD4a, and OsCCD4b in rice plants, three RNAi-mediated vectors were constructed using cDNA sequences that included the C-terminal and 3′-untranslated regions (Fig. 2b). Ten transgenic plants for OsCCD1-RNAi, nine for OsCCD4a-RNAi, and nine for OsCCD4b-RNAi were generated, and their T-DNA integrations into the rice genome were confirmed by transgene-specific PCRs displaying 258-, 177-, and 226-bp amplicons, respectively (Supplementary Fig. S2a). After Southern blot analysis using an NPT II probe within a vector backbone (Supplementary Fig. S2b), three independent lines with an RNAi construct for OsCCD1, OsCCD4a, and OsCCD4b at the T0 generation were selected and further developed for analyses of target gene suppression and carotenoid content.
RNAi-mediated suppression of the OsCCD1, OsCCD4a, and OsCCD4b genes, and their influences on leaf carotenoid content
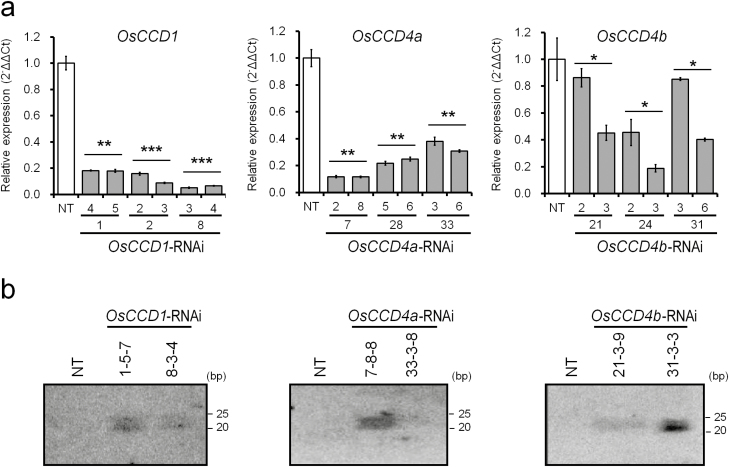
To determine whether the expression of OsCCD1, OsCCD4a, and OsCCD4b was suppressed by RNAi-mediated mechanisms, qRT-PCR was performed using mature leaves in two siblings of three independent T1 lines for OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi (Fig. 3a). All the transgenic lines showed reduced transcript levels of OsCCD1 (5.1–18.1%), OsCCD4a (8.2–23.2%), and OsCCD4b (18.81–86.81%) relative to non-transgenic (NT) plants. To further confirm the target gene suppression, RNA gel blot analysis was performed with mature leaves of two independent T2 plants for OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi (Fig. 3b). siRNAs corresponding to the OsCCD1, OsCCD4a, and OsCCD4b were detected in all the transgenic plants, suggesting that RNAi-mediated suppression was well maintained from one generation to the next in the RNAi plants.
Fig. 3.
Target gene suppression in the transgenic rice lines OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi. (a) Knock-down levels of each target gene were quantified by qRT-PCRs using T1 leaf tissues of two sibling lines from three independent transgenic plants for each construct. All data are the means (±SE) of triplicate measurements. The mean Ct values were used to calculate the expression of the target gene with normalization to an internal control (OsUbi5) using the ΔΔCt equation. The relative differences to non-transgenic (NT) plants (Oryza sativa cv. Ilmi) were determined using a one-tailed Student’s t-test: ***P<0.001, **P<0.01, *P<0.05. (b) The siRNA detection of each target gene by small RNA gel blot analysis was performed with T2 leaf tissues of two independent transgenic plants for each construct.
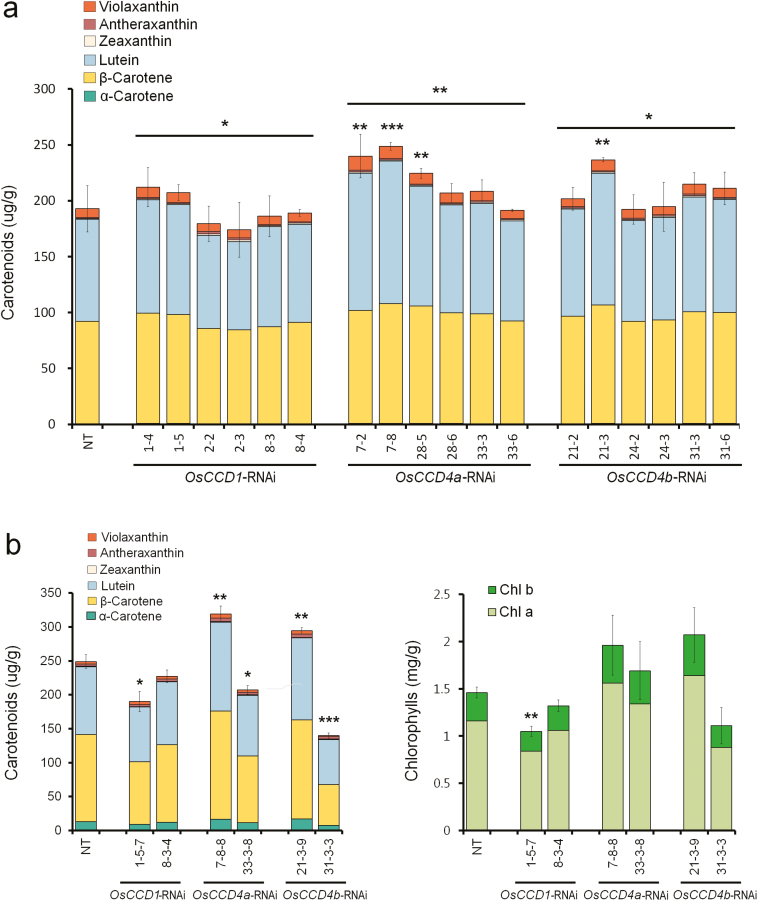
To assess the influence of OsCCD1, OsCCD4a, and OsCCD4b gene suppression on leaf carotenoids, the levels of carotenoids and chlorophylls were analysed by HPLC using the same mature leaf tissue as in the RNA analyses (Fig. 4). The total amount of carotenoids in the T1 generation was significantly increased overall in the OsCCD4a lines and in the individual lines OsCCD4a-RNAi-7 and -28 and OsCCD4b-RNAi-21 (Fig. 4a). Similar results were observed in the OsCCD4a-RNAi-7 and OsCCD4b-RNAi-21 lines in the T2 generation where, as well as total carotenoids, significant enhancements were found in five (α-carotene, β-carotene, lutein, antheraxanthin, and violaxanthin) and three (α-carotene, lutein, and antheraxanthin) components, respectively (Fig. 4b, Supplementary Fig. S3). The other four lines (OsCCD1-RNAi-1 and -8, OsCCD4a-RNAi-33, and OsCCD4b-RNAi-31) had decreased carotenoid levels in the T2 generation relative to NT plants (Fig. 4b). Levels of chlorophylls displayed similar patterns to those of carotenoids. Suppression of either OsCCD4a or OsCCD4b had the potential to affect the accumulation of leaf carotenoids, with the effect being dependent on the transgenic line.
Fig. 4.
Contents and composition of leaf carotenoids in the transgenic rice lines OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi. (a) Carotenoid levels by HPLC analysis in T1 leaf tissues of two sibling lines from three independent transgenic plants for each construct. (b) Levels of carotenoids, measured by HPLC, and chlorophylls, measured spectrophotometrically using absorbance, in T2 leaf tissues of two independent transgenic plants for each construct. NT, non-transgenic rice (Oryza sativa cv. Ilmi). Data are means (±SD) of three replicates. Differences relative to NT plants were determined using a one-tailed Student’s t-test: ***P<0.001, **P<0.01, *P<0.05.
Influence of OsCCD1, OsCCD4a, and OsCCD4b suppression on seed carotenoid content
To determine the effects of suppressing OsCCD1, 4a, and 4b on seed carotenoids, a strain of carotenoid-intensifying golden rice previously developed by Jeong et al. (2017) using a bicistronic recombinant gene stPAC (stPsy:2A:Tp:stCrtI), was used as a male parent for conventional breeding (Fig. 2b). With the homozygous stPAC rice having a single intact copy of the transgene, two independent lines each of OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi were cross-fertilized as female parents after verification of their homozygosity in the T3 plant generation by TaqMan-PCR (Supplementary Fig. S4a). After further propagation of the resultant F1 seeds to the F3 generation, homozygosity for two transgenes between stPAC and each of OsCCD1-RNAi, OsCCD4a-RNAi, or OsCCD4b-RNAi was verified using TaqMan-PCR (Supplementary Fig. S4b).
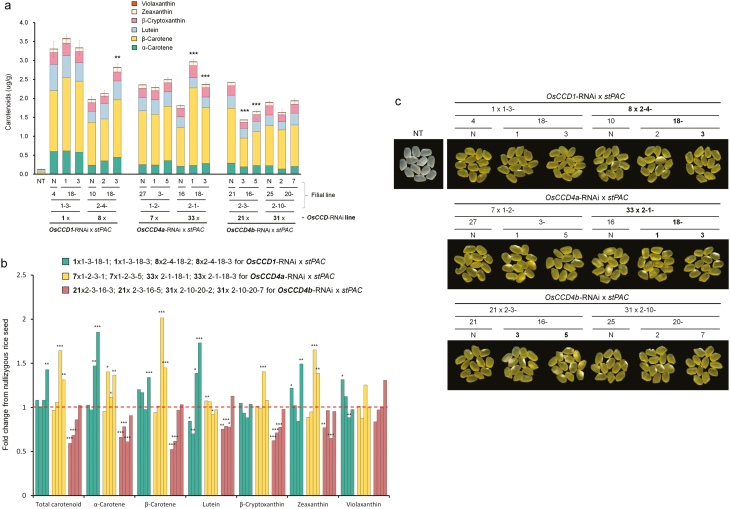
To evaluate more accurately the effects on carotenoid content in rice endosperms of knock-down of the OsCCD1, OsCCD4a, and OsCCD4b genes, control stPAC seeds without T-DNA for the suppression of OsCCD1, OsCCD4a, and OsCCD4b were segregated out as nullizygotes from each interbreeding line. This was confirmed by the detection of two different T-DNA fragments for stPAC and either OsCCD1-RNAi, OsCCD4a-RNAi, or OsCCD4b-RNAi, by genomic PCR (Supplementary Fig. S4c). HPLC analysis of filial lines from two independent lines of OsCCD1-RNAi and OsCCD4a-RNAi showed an increase in total carotenoids overall relative to nullizygous seeds (Fig. 5a, Supplementary Table S3). Of these, representative lines that were bred from OsCCD1-RNAi-8 and OsCCD4a-RNAi-33 respectively displayed significant enhancements in total carotenoids of up to 1.4- and 1.6-fold compared with each nullizygous lines, with a slightly more intense golden color (Fig. 5b, c). In contrast, the intercrossed lines from OsCCD4b-RNAi generally had decreased levels of total carotenoids and individual components, including α-carotene, β-carotene, lutein, zeaxanthin, and β-cryptoxanthin (Fig. 5b, Supplementary Table S3). Interestingly, OsCCD1-RNAi and OsCCD4a-RNAi showed differential patterns in the enhancement of α-ring carotenoids (maximum 1.9- and 1.7-fold increases in α-carotene and lutein, respectively) and β-ring carotenoids (maximum 2.0- and 1.7-fold increases in β-carotene and zeaxanthin, respectively) (Fig. 5b). This suggested that either OsCCD1 or OsCCD4a could significantly increase the levels of seed carotenoids when their expression was knocked-down, but this effect was not seen with OsCCD4b.
Fig. 5.
Carotenoid levels and polished color of interbred seeds of rice from two independent lines of OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi and a stPAC plant displaying carotenoid-accumulating golden colored seeds. (a) Carotenoid levels, measured by HPLC, of homozygous F4 seeds containing two transgenes: stPAC and one of the RNAi genes, compared with non-transgenic (NT) plants (Oryza sativa cv. Ilmi). Data are means (±SD) of three replicates. The relative difference compared with each nullizygous (N) rice seed was determined using a one-tailed Student’s t-test. The nullizygous rice line has only a stPAC gene and is without the RNAi gene for each of the OsCCDs after being segregated from interbreeding lines (b) Fold-changes in individual carotenoid components of homozygous F4 seeds relative to that of each N seed. Differences between groups relative to a value of 1 were also determined using a one-tailed Student’s t-test. All significant differences in (a, b) are indicated as ***P<0.001, * P<0.01, *P<0.05. (c) Phenotypes of endosperm colors in homozygous F4 seeds after polishing. Line numbers with significantly enhanced carotenoid contents in (a, b) and a slightly more intense golden color compared with each N line are highlighted in bold.
Carotenoid-cleavage activities of OsCCD1, OsCCD4a, and OsCCD4b in carotenoid-accumulating E. coli strains
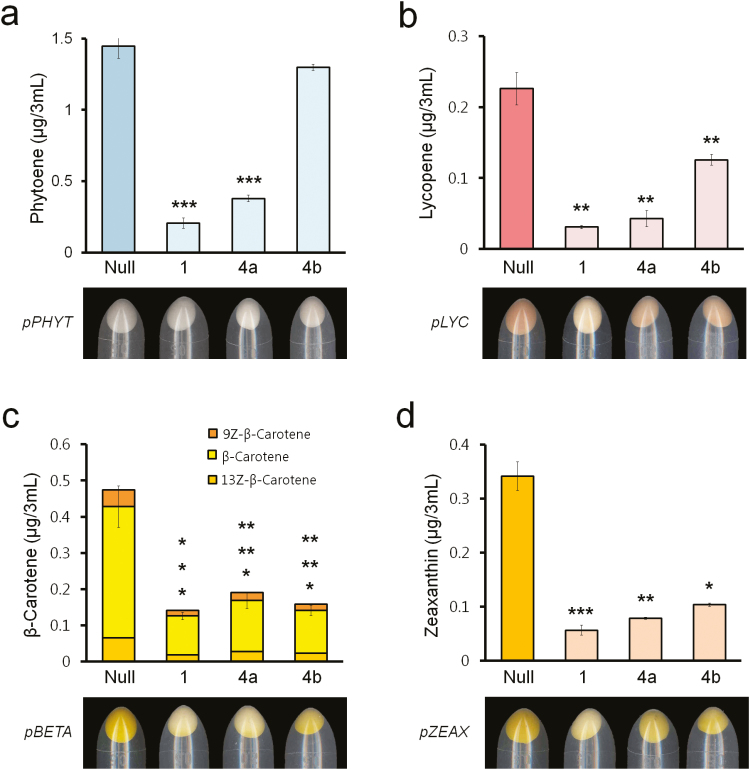
To determine whether OsCCD1, OsCCD4a, and OsCCD4b are involved in carotenoid degradation, three arabinose-inducible E. coli expression vectors were constructed with full-length cDNAs as pOsCCD1, pOsCCD4a, and pOsCCD4b (Supplementary Fig. S5a). They were used as competent cells for the individual transformation of four different vectors accumulating phytoene (pPHYT), lycopene (pLYC), β-carotene (pBETA), and zeaxanthin (pZEAX) (Supplementary Fig. S5b). The successful introduction of these four different carotenoid-accumulating plasmids into the E. coli strains harboring pOsCCD1, pOsCCD4a, and pOsCCD4b was confirmed by the detection of corresponding PCR fragments of OsCCD genes and carotenoid biosynthetic operon genes including CrtE, CrtB, CrtI, CrtY, and CrtZ (Supplementary Fig. S5c). The resultant E. coli strains were treated with arabinose to induce the transcription of OsCCD1, OsCCD4a, and OsCCD4b under the carotenoid-accumulating plasmids, which could display different colors depending on the production of phytoene (white), lycopene (pink), β-carotene (yellow), and zeaxanthin (yellow) as a null control (Fig. 6). Expression of the genes resulted in the display of paler colors in E. coli cells producing lycopene, β-carotene, and zeaxanthin except for colorless phytoene. The results showed more distinct differences in cell colors for OsCCD1 than the other genes. Further HPLC analysis showed that the overall contents of carotenoids, except for phytoene in the case of the OsCCD4b gene, were significantly decreased by the expression of OsCCD1, OsCCD4a, and OsCCD4b relative to the null control. Among the three rice CCDs, the cleavage activity resulting from the expression of OsCCD1 was highest in all four of the carotenoid substrates, with considerably reduced levels of phytoene (down to as low as 14%), lycopene (14%), β-carotene (30%), and zeaxanthin (16%), without substrate specificity. This was followed by OsCCD4a and OsCCD4b, except in the case of β-carotene, which showed a slightly lower cleavage efficiency in OsCCD4a than in OsCCD4b.
Fig. 6.
Changes in carotenoid levels and colony color according to the individual overexpression of OsCCD1 OsCCD4a, and OsCCD4b in four carotenoid-accumulating E. coli strains. (a) Phytoene contents measured by HPLC in a pPHYT-harboring E. coli strain. (b) Lycopene contents measured by HPLC in a pLYC-harboring E. coli strain. (c) β-Carotene contents measured by HPLC in a pBETA-harboring E. coli strain. (d) Zeaxanthin contents measured by HPLC in a pZEAX-harboring E. coli strain. Null represents each of the four vector-harboring E. coli strains without the rice CCD genes. The relative difference of each null E. coli strain was determined using a one-tailed Student’s t-test: ***P<0.001, **P<0.01, *P<0.05.
Discussion
Suppression of carotenoid catabolic metabolism by CCDs has been a notable strategy to increase carotenoid content in plants by blocking their degradation into small molecules called apocarotenoids or CCPs (Gonzalez-Jorge et al., 2013; Zhai et al., 2016). Individual CCDs across the different types that are found in plants specifically cleave targeted carotenoid substrates at different double-bond positions to yield diverse apocarotenoids, which function as pigments, volatiles, defense compounds, and signals (Walter and Strack, 2011; Hou et al., 2016). In order to increase nutritional value in crops by silencing carotenoid cleavage whilst having a minimal physiological effect on apocarotenoids, CCDs that are known to be associated with the biosynthesis of signal molecules (NCEDs for ABAs, and CCD7 and CCD8 for strigolactones) have been excluded as target candidates for genetic engineering. Thus, among the nine rice genes that are known to be orthologues of Arabidopsis and maize CCD gene families (three NCEDs: OsNCED2, OsNCED3, and OsNCED9; and six CCDs: OsCCD1, OsCCD4a, OsCCD4b, OsCCD7, OsCCD8a, and OsCCD8b; Vallabhaneni et al., 2010; Walter et al., 2010), we selected OsCCD1, OsCCD4a, and OsCCD4b as potential candidates for the improvement of carotenoid content in rice.
As target genes for knock-down in rice plants, transcript abundance for OsCCD1, OsCCD4a, and OsCCD4b was first compared in various tissues including leaves and seeds at different development stages (Fig. 1). The results indicated that OsCCD1 and OsCCD4a were preferentially expressed in the leaves, with the former having the highest level of expression, and the highest level of expression during seed development was at the early stages (10 DAF) for OsCCD1 and during the middle stages (20 DAF) for OsCCD4a, suggesting that these genes needed to be silenced in leaves and seeds. The expression of OsCCD4b was very low in leaves and showed similar levels throughout seed development.
Molecular characteristics based on protein sequence similarities effectively distinguished between OsCCD1 and the two OsCCD4s. The latter displayed a closer relationship within a monocotyledonous CCD1 subgroup including ZmCCD1 and CsCCD1, and a monocotyledonous CCD4 subgroup including CsCCD4a, CsCCD4b, CsCCD4c, and CsZCD (known as a truncated CsCCD4 form without cleavage activity; Rubio et al., 2008), than with dicotyledonous CCD1 and CCD4 subgroups (Supplementary Fig. S1a). The protein structures of OsCCD1, OsCCD4a, and OsCCD4b were analysed on the basis of comparative modeling results of crocus CsCCD4c via structural alignment with maize VP14 as the NCED form (Messing et al., 2010; Rubio-Moraga et al., 2014). All three rice CCDs had key residues recognized for VP14 activity from crystal structure studies, including two Phe residues of a broad CCD group, either Phe or Leu as a distinguishable residue between a narrow CCD and NCED group, and Trp and Leu, Ser, and Pro, and Ala and Pro residues between the CCD1 and CCD4 subgroups (Supplementary Fig. S1b). In addition, Supplementary Fig. S1b and Fig. 2a demonstrate good conservation in the position and numbers of four histidine residues that act as typical ligands of a non-heme iron co-factor that is required for oxygenase activity and three glutamate residues that fix the iron-ligating histidine by hydrogen bonds, supporting their enzymatic activities as either a CCD1 or CCD4 subgroup (Schwartz et al., 1997; Kloer and Schulz, 2006).
In previous studies, up-regulation of either CCD1 or CCD4 has been shown to lead to less pigmentation in flowers of Chrysanthemum (CmCCD4a), Oncidium (OgCCD1), and Lilium (LbCCD4), in fruits of peach (PpCCD4), in tubers of potato (StCCD4), in the endosperm of maize (ZmCCD1), and in the seed of Arabidopsis (AtCCD4). The reduced levels of carotenoids suggest that they are used as substrates for CCD activity (Ohmiya et al., 2006; Campbell et al., 2010; Chiou et al., 2010; Vallabhaneni et al., 2010; Brandi et al., 2011; Hai et al., 2012; Gonzalez-Jorge et al., 2013; Bai et al., 2016). They also influence flavors, pigments, or signals in flower petals of petunia (PhCCD1) and rose (RdCCD1 and RdCCD4), in flower stigmas of crocus (CsCCD1b, CsCCD2, CsCCD4a, CsCCD4b, and CsCCD4c), in fruits of tomato (LeCCD1), strawberry (FaCCD1), and citrus (CitCCD4 and CitCCD4b1), and in tubers of potato (StCCD4), implying that CCD action generates a diverse number of apocarotenoids (Simkin et al., 2004a, 2004b; García-Limones et al. 2008; Rubio et al. 2008; Huang et al., 2009a, 2009b; Ma et al., 2013; Rodrigo et al., 2013; Frusciante et al., 2014; Rubio-Moraga et al., 2014; Bruno et al., 2015).
To assess the ability of OsCCD1, OsCCD4a, and OsCCD4b in enhancing the nutritional value of crop plants by increasing carotenoid content, RNAi-mediated suppression was performed in transgenic rice plants (Supplementary Fig. S2, Fig. 3). Among these, the sequential generation of T1 and T2 of the OsCCD4a-RNAi-7 lines showed a reliable increase in the leaf carotenoids α-carotene, β-carotene, lutein, antheraxanthin, and violaxanthin (but not zeaxanthin) relative to non-transgenic (NT) plants (Fig. 4, Supplementary Fig. S3). The effects of the OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi lines on seed carotenoids were ascertained via conventional breeding with a rice strain that had previously been developed using a modified recombinant stPAC (stPsy:2A:Tp:stCrtI) gene via codon-optimization for rice on the basis of a bicistronic recombinant PAC (Psy:2A:Tp:CrtI) gene with the ability to produce carotenoids in the endosperm (Ha et al., 2010; Jeong et al., 2017). From homozygous F3 plants that were self-fertilized from F1 hybrid seeds between OsCCDs-RNAi lines and a stPAC 25 line (Supplementary Fig. S4), F4 seeds descended from the line OsCCD1-RNAi-8 displayed a 1.4-fold increase in total carotenoids, with an α-ring carotenoid preference in the 2-4-18-3 line relative to the 2-4-10-N (nullizygous) line. Filial seeds from OsCCD4a-RNAi-33 showed an even higher increase of 1.6-fold in total carotenoids in the 2-1-18-1 line relative to the 2-1-16-N line, with a β-ring carotenoid preference, including β-carotene, β-cryptoxanthin, and zeaxanthin (Fig. 5, Supplementary Table S3), suggesting that they had greater nutritional value as crop plants.
To assess the carotenoid cleaving activity of OsCCD1, OsCCD4a, and OsCCD4b, their genes were overexpressed in four E. coli systems supplying different substrates of phytoene, lycopene, β-carotene, and zeaxanthin by harboring pPHYT, pLYC, pBETA, and pZEAX, respectively (Supplementary Fig. S5). The results indicated that the expression of OsCCD1, OsCCD4a, and OsCCD4b lowered the carotenoid levels and lessened the color of the E. coli pellet, suggesting that carotenoids were used as substrates, and with similar relatively high activities of OsCCD1 and OsCCD4a compared to OsCCD4b without substrate specificity (Fig. 6). Our results and those of a previous study using several carotenoids and apocarotenoids as substrates suggest that OsCCD1 has cleaving activity at diverse double-bond sites, namely C5–C6 ⁄ C5′–C6′, C7–C8 ⁄ C7′–C8′ and C9–C10 ⁄ C9′–C10′ (Ilg et al., 2009). OsCCD1 might have strong carotenoid cleaving activity, and OsCCD4a could also be expected to have an equivalent capability (Fig. 6).
In studies using transgenic approaches, the overexpression of either CCD1 or CCD4 to produce apocarotenoid compounds functioning as signaling and volatile molecules has been reported in only a limited number of cases, for example LeCCD1A in petunia, OsCCD1 in Golden rice, VvCCD1 in grapevine, and AtCCD4 in rice plants, even though this could control plant physiology including development, biotic stress resistance, and abiotic stress tolerance, and enhance floral scents and fruit flavors (Simkin et al., 2004b;Ilg et al., 2010; Lashbrooke et al., 2013; Song et al., 2016). Only the heterologous AtCCD4 gene in rice leaves has resulted fewer carotenoids, with a reduction of 26% and a 2-fold increase of β-ionone. Both and have been noted as targets that could be blocked to improve the nutritional value in major food crops and the color intensity in fruit and flowering crops, for example via co-suppression of PhCCD1 through overexpression of LeCCD1A in petunia, antisense expression of LeCCD1 in tomato and OsCCD1 in Golden rice, RNAi of StCCD4 in potato and VvCCD1 in grapevine, virus-induced silencing of PpCCD4 in peach, gamma-irradiation of CmCCD4a in chrysanthemum, and CRISPR/Cas9-mediated mutagenesis of either OsCCD4a or OsCCD4b in rice and InCCD4 in Japanese morning glory (Simkin et al., 2004a, 2004b; Campbell et al., 2010; Ilg et al., 2010; Lashbrooke et al., 2013; Bai et al., 2016; Jo et al., 2016; Yang et al., 2017; Watanabe et al., 2018). Among these suppression studies, StCCD4 in potato tubers, PpCCD4 in peach fruits, and CmCCD4a and InCCD4 in chrysanthemum and in Japanese morning glory petals resulted in an enhanced yellow coloration due to the increase of carotenoid content, and PhCCD1 in petunia caused a maximum 76% decrease in β-ionone synthesis in flower corollas (Simkin et al., 2004b; Campbell et al., 2010; Bai et al., 2016; Jo et al., 2016; Watanabe et al., 2018). In contrast, suppression of LeCCD1 in tomato, OsCCD1 in Golden rice, and OsCCD4a and OsCCD4b in rice showed no correlation with enhanced carotenoid accumulation (Simkin et al., 2004a; Ilg et al., 2010; Yang et al., 2017). In our current study, even antisense expression of OsCCD1 seemed to slightly decrease the total content of carotenoids in a Golden rice background, but suppressed levels of OsCCD1 transcripts still need to be observed. In addition, OsCCD4a and OsCCD4b remain to be knocked-out in carotenoid-accumulating rice rather than in common rice, which is ineffectual for carotenoid biosynthesis.
The potential to enhance nutritionally valuable carotenoids by preventing their transition into apocarotenoids has been consistently proposed for major food crops including maize, rice, and wheat (Ilg et al., 2010; Gonzalez-Jorge et al., 2013; da Silva et al., 2014; Qin et al., 2016; Yang et al., 2017). Silencing of CCD genes in planta has already successfully resulted in affecting color traits in potato tubers, peach fruits, and chrysanthemum and Japanese morning glory flowers (Campbell et al., 2010; Bai et al., 2016; Jo et al., 2016; Watanabe et al., 2018).In this study, we used a knock-down strategy to demonstrate that OsCCD4a could enhance the contents of valuable provitamin A components in carotenoid-abundant rice leaves and carotenoid-accumulating rice seeds in the form of β-carotenoids. For practical use, a more powerful tool in the knock-out strategy is needed to completely turn off the function of OsCCD4a. In addition, the simultaneous silencing of OsCCD4a and OsCCD1 might be effective in further increasing the content of carotenoids in rice endosperm when accompanied with genetic engineering for carotenoid biosynthesis, as the potential of OsCCD1 as another promising target for crop biofortification remains considerable.
Supplementary data
Supplementary data are available at JXB online.
Table S1. Primer sequence information for experiments in planta including quantitative real-time PCR, binary vector construction, and genomic DNA PCR.
Table S2. Primer sequence information for experiments in vitro including expression vector construction and colony PCR.
Table S3. Carotenoid content and composition of interbred filial seeds as measured by HPLC.
Fig. S1. Phylogenetic tree and alignment among plant CCDs on the basis of amino acid sequence similarities.
Fig. S2. Verification of transgenic rice lines of OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi.
Fig. S3. Contents of individual carotenoid components in transgenic rice leaf tissues of OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi.
Fig. S4. Verification of transgene homozygosity in interbred rice lines between each of two independent lines for OsCCD1-RNAi, OsCCD4a-RNAi, and OsCCD4b-RNAi (female parent) and a stPAC line (male parent).
Fig. S5. In vitro expression of OsCCD1, OsCCD4a, and OsCCD4b in four carotenoid-accumulating E. coli strains to analyse carotenoid-cleavage activities.
Acknowledgments
This work was supported by grants from the Next-Generation BioGreen 21 Program (PJ01334601 and PJ01368801 to S-HH) funded by the Rural Development Administration. Our work was also supported by a research program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology (NRF-2016R1A2B4013485 to S-HH). The authors have no competing interests to declare.
Authors contributions
MRK, M-HS, and S-HL performed the molecular biological experiments in planta; JKK and S-AB analysed metabolites including carotenoids and chlorophylls; MRK and MKY performed the molecular biological experiments in E. coli; S-HH designed the experiments and wrote the paper with MRK. All authors reviewed and approved the final manuscript.
References
- Auldridge ME, McCarty DR, Klee HJ. 2006. Plant carotenoid cleavage oxygenases and their apocarotenoid products. Current Opinion in Plant Biology 9, 315–321. [DOI] [PubMed] [Google Scholar]
- Bai S, Tuan PA, Tatsuki M, Yaegaki H, Ohmiya A, Yamamizo C, Moriguchi T. 2016. Knockdown of carotenoid cleavage dioxygenase 4 (CCD4) via virus-induced gene silencing confers yellow coloration in peach fruit: evaluation of gene function related to fruit traits. Plant Molecular Biology Reporter 34, 257–264. [Google Scholar]
- Brandi F, Bar E, Mourgues F, Horváth G, Turcsi E, Giuliano G, Liverani A, Tartarini S, Lewinsohn E, Rosati C. 2011. Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biology 11, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno M, Beyer P, Al-Babili S. 2015. The potato carotenoid cleavage dioxygenase 4 catalyzes a single cleavage of β-ionone ring-containing carotenes and non-epoxidated xanthophylls. Archives of Biochemistry and Biophysics 572, 126–133. [DOI] [PubMed] [Google Scholar]
- Campbell R, Ducreux LJ, Morris WL, Morris JA, Suttle JC, Ramsay G, Bryan GJ, Hedley PE, Taylor MA. 2010. The metabolic and developmental roles of carotenoid cleavage dioxygenase4 from potato. Plant Physiology 154, 656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou CY, Pan HA, Chuang YN, Yeh KW. 2010. Differential expression of carotenoid-related genes determines diversified carotenoid coloration in floral tissues of Oncidium cultivars. Planta 232, 937–948. [DOI] [PubMed] [Google Scholar]
- Cunningham FX Jr, Pogson B, Sun Z, McDonald KA, DellaPenna D, Gantt E. 1996. Functional analysis of the beta and epsilon lycopene cyclase enzymes of Arabidopsis reveals a mechanism for control of cyclic carotenoid formation. The Plant Cell 8, 1613–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva Messias R, Galli V, Dos Anjos E Silva SD, Rombaldi CV. 2014. Carotenoid biosynthetic and catabolic pathways: gene expression and carotenoid content in grains of maize landraces. Nutrients 6, 546–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diretto G, Tavazza R, Welsch R, Pizzichini D, Mourgues F, Papacchioli V, Beyer P, Giuliano G. 2006. Metabolic engineering of potato tuber carotenoids through tuber-specific silencing of lycopene epsilon cyclase. BMC Plant Biology 6, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frusciante S, Diretto G, Bruno M, et al. . 2014. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proceeding of the National Academy of Sciences, USA 111, 12246–12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Limones C, Schnäbele K, Blanco-Portales R, Luz Bellido M, Caballero JL, Schwab W, Muñoz-Blanco J. 2008. Functional characterization of FaCCD1: a carotenoid cleavage dioxygenase from strawberry involved in lutein degradation during fruit ripening. Journal of Agricultural and Food Chemistry 56, 9277–9285. [DOI] [PubMed] [Google Scholar]
- Giuliano G. 2017. Provitamin A biofortification of crop plants: a gold rush with many miners. Current Opinion in Biotechnology 44, 169–180. [DOI] [PubMed] [Google Scholar]
- Giuliano G, Al-Babili S, von Lintig J. 2003. Carotenoid oxygenases: cleave it or leave it. Trends in Plant Science 8, 145–149. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Jorge S, Ha SH, Magallanes-Lundback M, et al. . 2013. Carotenoid cleavage dioxygenase4 is a negative regulator of β-carotene content in Arabidopsis seeds. The Plant Cell 25, 4812–4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha SH, Liang YS, Jung H, Ahn MJ, Suh SC, Kweon SJ, Kim DH, Kim YM, Kim JK. 2010. Application of two bicistronic systems involving 2A and IRES sequences to the biosynthesis of carotenoids in rice endosperm. Plant Biotechnology Journal 8, 928–938. [DOI] [PubMed] [Google Scholar]
- Hai NTL, Masuda J-I, Miyajima I, Thien NQ, Mojtahedi N, Hiramatsu M, Kim J-H, Okubo H. 2012. Involvement of carotenoid cleavage dioxygenase 4 gene in tepal color change in Lilium brownii var. colchesteri. Journal of the Japanese Society for Horticultural Science 81, 366–373. [Google Scholar]
- Hou X, Rivers J, León P, McQuinn RP, Pogson BJ. 2016. Synthesis and function of apocarotenoid signals in plants. Trends in Plant Science 21, 792–803. [DOI] [PubMed] [Google Scholar]
- Huang FC, Horváth G, Molnár P, Turcsi E, Deli J, Schrader J, Sandmann G, Schmidt H, Schwab W. 2009a. Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena. Phytochemistry 70, 457–464. [DOI] [PubMed] [Google Scholar]
- Huang FC, Molnár P, Schwab W. 2009b. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. Journal of Experimental Botany 60, 3011–3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilg A, Beyer P, Al-Babili S. 2009. Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. The FEBS Journal 276, 736–747. [DOI] [PubMed] [Google Scholar]
- Ilg A, Yu Q, Schaub P, Beyer P, Al-Babili S. 2010. Overexpression of the rice carotenoid cleavage dioxygenase 1 gene in Golden Rice endosperm suggests apocarotenoids as substrates in planta. Planta 232, 691–699. [DOI] [PubMed] [Google Scholar]
- Jeong YS, Ku H-K, Kim JK, You MK, Lim S-H, Kim J-K, Ha S-H. 2017. Effect of codon optimization on the enhancement of the β-carotene contents in rice endosperm. Plant Biotechnology Reports 11, 171–179. [Google Scholar]
- Jo YD, Kim Y-S, Ryu J, Choi HI, Kin SW, Kang HS, Ahn J-W, Kim J-B, Kang S-Y, Kim SH. 2016. Deletion of carotenoid cleavage dioxygenase 4a (CmCCD4a) and global up-regulation of plastid protein-coding genes in a mutant chrysanthemum cultivar producing yellow petals. Scientia Horticulturae 212, 49–59. [Google Scholar]
- Kloer DP, Schulz GE. 2006. Structural and biological aspects of carotenoid cleavage. Cellular and Molecular Life Sciences 63, 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lashbrooke JG, Young PR, Dockrall SJ, Vasanth K, Vivier MA. 2013. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biology 13, 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma G, Zhang L, Matsuta A, Matsutani K, Yamawaki K, Yahata M, Wahyudi A, Motohashi R, Kato M. 2013. Enzymatic formation of β-citraurin from β-cryptoxanthin and zeaxanthin by carotenoid cleavage dioxygenase4 in the flavedo of citrus fruit. Plant Physiology 163, 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin RC, Liu P-P, Nonogaki H. 2005. Simple purification of small RNAs from seeds and efficient detection of multiple microRNAs expressed in Arabidopsis thaliana and tomato (Lycopersicon esculentum) seeds. Seed Science Reserch 15, 319–328. [Google Scholar]
- Mayer JE, Pfeiffer WH, Beyer P. 2008. Biofortified crops to alleviate micronutrient malnutrition. Current Opinion in Plant Biology 11, 166–170. [DOI] [PubMed] [Google Scholar]
- Messing SA, Gabelli SB, Echeverria I, Vogel JT, Guan JC, Tan BC, Klee HJ, McCarty DR, Amzel LM. 2010. Structural insights into maize viviparous14, a key enzyme in the biosynthesis of the phytohormone abscisic acid. The Plant Cell 22, 2970–2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki D, Shimamoto K. 2004. Simple RNAi vectors for stable and transient suppression of gene function in rice. Plant & Cell Physiology 45, 490–495. [DOI] [PubMed] [Google Scholar]
- Nisar N, Li L, Lu S, Khin NC, Pogson BJ. 2015. Carotenoid metabolism in plants. Molecular Plant 8, 68–82. [DOI] [PubMed] [Google Scholar]
- Ohmiya A, Kishimoto S, Aida R, Yoshioka S, Sumitomo K. 2006. Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiology 142, 1193–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pons E, Alquézar B, Rodríguez A, Martorell P, Genovés S, Ramón D, Rodrigo MJ, Zacarías L, Peña L. 2014. Metabolic engineering of β-carotene in orange fruit increases its in vivo antioxidant properties. Plant Biotechnology Journal 12, 17–27. [DOI] [PubMed] [Google Scholar]
- Qin X, Fischer K, Yu S, Dubcovsky J, Tian L. 2016. Distinct expression and function of carotenoid metabolic genes and homoeologs in developing wheat grains. BMC Plant Biology 16, 155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo MJ, Alquézar B, Alós E, Medina V, Carmona L, Bruno M, Al-Babili S, Zacarías L. 2013. A novel carotenoid cleavage activity involved in the biosynthesis of Citrus fruit-specific apocarotenoid pigments. Journal of Experimental Botany 64, 4461–4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Römer S, Lübeck J, Kauder F, Steiger S, Adomat C, Sandmann G. 2002. Genetic engineering of a zeaxanthin-rich potato by antisense inactivation and co-suppression of carotenoid epoxidation. Metabolic Engineering 4, 263–272. [DOI] [PubMed] [Google Scholar]
- Rubio A, Rambla JL, Santaella M, Gómez MD, Orzaez D, Granell A, Gómez-Gómez L. 2008. Cytosolic and plastoglobule-targeted carotenoid dioxygenases from Crocus sativus are both involved in beta-ionone release. The Journal of Biological Chemistry 283, 24816–24825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio-Moraga A, Rambla JL, Fernández-de-Carmen A, Trapero-Mozos A, Ahrazem O, Orzáez D, Granell A, Gómez-Gómez L. 2014. New target carotenoids for CCD4 enzymes are revealed with the characterization of a novel stress-induced carotenoid cleavage dioxygenase gene from Crocus sativus. Plant Molecular Biology 86, 555–569. [DOI] [PubMed] [Google Scholar]
- Schwartz SH, Tan BC, Gage DA, Zeevaart JA, McCarty DR. 1997. Specific oxidative cleavage of carotenoids by VP14 of maize. Science 276, 1872–1874. [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Schwartz SH, Auldridge M, Taylor MG, Klee HJ. 2004a. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles beta-ionone, pseudoionone, and geranylacetone. The Plant Journal 40, 882–892. [DOI] [PubMed] [Google Scholar]
- Simkin AJ, Underwood BA, Auldridge M, Loucas HM, Shibuya K, Schmelz E, Clark DG, Klee HJ. 2004b. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of beta-ionone, a fragrance volatile of petunia flowers. Plant Physiology 136, 3504–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer A, Vyas KS. 2012. A global clinical view on vitamin A and carotenoids. The American Journal of Clinical Nutrition 96, 1204S–1206S. [DOI] [PubMed] [Google Scholar]
- Song M-H, Lim S-H, Kim JK, Jung ES, John KM, You M-K, Ahn S-N, Lee CH, Ha S-H. 2016. In planta cleavage of carotenoids by Arabidopsis carotenoid cleavage dioxygenase 4 in transgenic rice plants. Plant Biotechnology Reports 10, 291–300. [Google Scholar]
- Vallabhaneni R, Bradbury LM, Wurtzel ET. 2010. The carotenoid dioxygenase gene family in maize, sorghum, and rice. Archives of Biochemistry and Biophysics 504, 104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter MH, Floss DS, Strack D. 2010. Apocarotenoids: hormones, mycorrhizal metabolites and aroma volatiles. Planta 232, 1–17. [DOI] [PubMed] [Google Scholar]
- Walter MH, Strack D. 2011. Carotenoids and their cleavage products: biosynthesis and functions. Natural Product Reports 28, 663–692. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Oda-Yamamizo C, Sage-Ono K, Ohmiya A, Ono M. 2018. Alteration of flower colour in Ipomoea nil through CRISPR/Cas9-mediated mutagenesis of carotenoid cleavage dioxygenase 4. Transgenic Research 27, 25–38. [DOI] [PubMed] [Google Scholar]
- Yang X, Chen L, He J, Yu W. 2017. Knocking out of carotenoid catabolic genes in rice fails to boost carotenoid accumulation, but reveals a mutation in strigolactone biosynthesis. Plant Cell Reports 36, 1533–1545. [DOI] [PubMed] [Google Scholar]
- Zeng J, Wang X, Miao Y, et al. . 2015. Metabolic engineering of wheat provitamin A by simultaneously overexpressing CrtB and silencing carotenoid hydroxylase (TaHyd). Journal of Agricultural and Food Chemistry 63, 9083–9092. [DOI] [PubMed] [Google Scholar]
- Zhai S, Xia X, He Z. 2016. Carotenoids in staple cereals: metabolism, regulation, and genetic manipulation. Frontiers in Plant Science 7, 1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.