Abstract
Objective
Threat perceptions in the Emergency Department (ED) (e.g., patients’ subjective feelings of helplessness or lack of control) during evaluation for an acute coronary syndrome (ACS) are associated with the development of posttraumatic stress disorder (PTSD), and PTSD has been associated with medication nonadherence, cardiac event recurrence, and mortality. This study reports the development and validation of a 7-item measure of ED Threat Perceptions in English- and Spanish-speaking patients evaluated for ACS.
Methods
Participants were drawn from an observational cohort study of 1,000 patients evaluated for ACS between 2013–2016 in a large, New York City hospital. Participants reported on threat perceptions in the ED and during inpatient stay (using 12 items previously identified as predictive of PTSD) and reported on cardiac-induced PTSD one month post-discharge. Exploratory and confirmatory factor analyses were used to establish the factor structure and test measurement invariance. Validity and reliability were examined, as was the association of ED Threat Perceptions with cardiac-induced PTSD.
Results
Factor analyses identified a 7-item measure of ED Threat Perceptions (e.g., “I feel helpless,” “I am worried that I am going to die”) for both English- and Spanish-speaking patients. ED Threat Perceptions demonstrated convergent validity, correlating with ED stress and ED crowdedness (rs = .29, .14), good internal consistency (α = .82), and stability (r = .61). Threat Perceptions were associated with cardiac-induced acute stress at inpatient and PTSD symptoms at one month (rs = .43, .39).
Conclusions
This brief tool assessing ED Threat Perceptions has clinical utility for providers to identify patients at risk for developing cardiac-induced PTSD and is critical to inform research on whether threat may be modified in-ED to reduce PTSD incidence.
Patients’ experience of medical care received while in the emergency department (ED), both in terms of objective experience (e.g., crowding) and subjective experience (e.g., emotional reactions), has significant downstream consequences. For example, patients who report overcrowding in the ED during an evaluation for acute coronary syndrome (ACS) are more likely to develop subsequent cardiac-induced posttraumatic stress disorder (PTSD).1,2 Similarly, ACS patients’ self-reported perceptions of feeling threatened by the ACS event during ED evaluation (e.g., feeling vulnerable, feeling out of control, or feeling that the event will have a big impact on their life) are significantly associated with cardiac-induced PTSD symptoms during inpatient stay and one month after discharge.3–7 Cardiac-induced PTSD occurs in approximately 12 percent of ACS patients8 and is associated with a variety of negative outcomes. A recent meta-analysis found doubled risk for recurrent cardiovascular events and mortality in patients with cardiac-induced PTSD,8 perhaps in part due to the association of PTSD with nonadherence to secondary prevention medications.8–12 Collectively, this broad body of evidence suggests that patients’ ED experience can impact key psychological, behavioral, and cardiovascular outcomes long after they are discharged.
Indeed, a substantial body of literature suggests that feeling threatened—including feelings of helplessness and vulnerability (i.e., “threat perceptions”)—during a traumatic experience is one of the most powerful predictors of future PTSD.4–6,8,13,14 Usually, traumatic events occur in places where interventions to reduce individuals’ threat perceptions are unimaginable, such as combat zones or private homes. In the case of cardiac-induced PTSD, however, in which patients evaluated for ACS are often cared for in the ED, clinicians have the unique opportunity to identify patients with elevated threat perceptions who are at risk for adverse psychological outcomes and, potentially, intervene to modify experiences of the traumatic event.15 Yet, no instrument to date has been validated to help emergency providers assess patients’ threat perceptions during ED evaluation for acutely life-threatening cardiovascular events. To begin developing ED-based interventions that may prevent stress-related adverse psychological and cardiovascular outcomes, it is critical to identify patients who are at risk of developing cardiac-induced PTSD early in the care continuum. Identifying patients who are vulnerable to developing cardiac-induced PTSD early in the care continuum could inform theory-based interventions that target these patients at the point of contact.
This goal of this study was therefore to report on the development of a brief self-report measure that captures patients’ threat perceptions during ED evaluation for ACS (e.g., feeling vulnerable or out of control) that can be utilized in both English- and Spanish-speaking patients. We conducted exploratory factor analysis to identify a unidimensional scale and confirmatory factor analysis to establish measurement invariance across English- and Spanish-speaking patients. We then estimated internal consistency and stability (over a median of four days), and examined convergent validity by assessing the association of subjective ED threat perceptions with other ED factors and ED perceptions (both more objective and subjective; i.e., crowding, stress) and predictive validity by assessing its association with subsequent cardiac-induced acute stress and PTSD symptoms. We expected ED threat perceptions to be positively associated with, yet distinct from, patients’ perceptions of the ED as stressful and crowded. We further expected ED threat perceptions to be positively associated with cardiac-induced acute stress symptoms and cardiac-induced PTSD symptoms.
Methods
Procedure
Data were drawn from an observational cohort study of patients evaluated for ACS (non-ST elevation myocardial infarction [NSTEMI] or unstable angina [UA) between 2013–2016 at the New York Presbyterian Hospital (NYP) ED (REactions to Acute Care and Hospitalization; REACH). Trained research assistants were present in the ED for 70+ hours/week, including weekend and evening hours, to enroll participants and collect ED data. Potentially eligible participants were identified via the ED electronic track record and approached in the ED by trained research assistants. Upon confirming eligibility, patients provided informed consent and completed a brief interview at ED assessment. Specifically, patients were told “We are doing this research to test whether aspects of the emergency department (ED) are related to your level of stress during and after treatment, and whether your stress levels are related to your long term health after you leave the hospital.” Medical record numbers (MRN) were used to track transfer to inpatient care, where patients completed a second interview to report on acute stress disorder related to the acute cardiovascular event and recalled threat perceptions (this was completed by phone if discharged prior to inpatient interview; median four days after enrollment; 1st quartile = 2.0, 3rd quartile = 9.0, interquartile range = 7.0). All participants completed a phone interview for PTSD related to the acute cardiovascular event at one month post-discharge (median 45 days after enrollment; 1st quartile = 33.0, 3rd quartile = 72.0, interquartile range = 39.0). Study procedures were approved by the Columbia University Institutional Review Board.
Participants
Participants were a consecutive sample English- and Spanish-speaking patients 18+ years of age who presented to the NYP ED with an admitting diagnoses of non-ST-elevation myocardial infarction (NSTEMI) or unstable angina (UA). Exclusion criteria included: (1) terminal non-cardiovascular illness with life expectancy < 1 year; (2) severe mental illness requiring urgent psychiatric hospitalization or intervention; (3) significant cognitive impairment; (4) known alcohol or substance abuse that would impede ability to complete study protocol; and (5) unavailable for follow-up. Due to IRB restrictions regarding data collection, precise data on patients who did not enroll (i.e., those approached) were not collected. s
Measures
Item generation for assessment of ED experience
An initial pool of 12 items assessing patient perceptions of the ACS event were generated based on a meta-analysis of predictors of PTSD (see Table 2 for all items)14 (e.g., “I am worried that my symptoms are severe,” “I feel helpless”). All items were translated into Spanish and back-translated by a certified translator and were rated using a 4-point Likert scale: 1 (Not at all), 2 (A little bit), 3 (Moderately), and 4 (Extremely).
Table 2.
Factor matrix for Exploratory Factor Analysis.
| Item | ED Threat Perceptions | Item Mean (SD) |
|---|---|---|
| I feel vulnerable.a | .669 | 1.95 (1.07) |
| I am worried that I am not in control. | .665 | 1.99 (1.16) |
| I am worried that my symptoms are severe. | .665 | 2.19 (1.14) |
| I feel helpless. | .628 | 1.71 (1.05) |
| I am worried that I am going to die. | .617 | 1.49 (0.92) |
| I am afraid. | .579 | 1.52 (0.87) |
| I think this event will have a big impact on my life. | .563 | 2.30 (1.18) |
| I am worried that the doctors are not in control of my situation. | .457 | 1.51 (.93) |
| I am concerned about my family. | .454 | 2.54 (1.26) |
| I believe that my symptoms are connected to my heart. | .381 | 2.86 (1.08) |
| I am in pain. | .267 | 1.97 (1.03) |
| I feel like I can handle the symptoms myself. | −.133 | 1.59 (.93) |
Loadings for items that were retained are bolded in their respective columns.
Response options for items ranged from 1 to 4.
Analysis
Differences between patients completing inpatient/telephone follow up and one month assessments (vs. those who did not) were examined using independent samples t-tests and chi-square analyses, as appropriate.
Convergent validity and factor structure
To establish measurement structure, we first conducted an exploratory factor analysis (EFA) on the 12 items assessed at ED bedside. We specified principal axis factoring and oblimin rotation in SPSS, and explored one-, two-, and three-factor solutions. Oblimin rotation was selected because factors are often correlated in real-world settings, and this allows for a better and more reproducible representation of the factor structure.16 The EFA was followed up using a confirmatory factor analysis (CFA) specified using the lavaan package in R to examine measurement invariance across English- and Spanish-speaking participants. To test invariance, we examined fit indices to see how well the specified model fit the data when constraining scale items to be equal across groups (i.e., English- and Spanish-speaking participants). We chose to examine fit indices (which take into account total sample size) because, when the sample size is large, chi-square difference tests for model fit are often significant even when models are, in practice, equivalent across groups.17–19 A sample size of N = 1000 is sufficient for stable and valid results when conducting factor analysis.20
Both internal consistency and stability of ED threat perceptions were tested using reports at ED assessment and at inpatient follow up a median of four days later (1st quartile = 2.0, 3rd quartile = 9.0, interquartile range = 7.0). Internal consistency is reported using Chronbach’s α, and stability is reported as a correlation between scores at ED assessment and inpatient follow up. All available data were included for each analysis, resulting in slightly different N for different tests.
Validity
Convergent validity was assessed by estimating associations of the ED threat measure with patient perceptions of the ED itself as (1) crowded and (2) stressful, each a single-item measure assessed on a Likert scale ranging from 1 (Not at all) to 5 (Extremely). This was tested using Pearson correlations.
Predictive validity was assessed by estimating associations of the ED threat measure with subsequent acute stress disorder symptoms using the Acute Stress Disorder Scale21 (inpatient or telephone follow up, median four days later; 1st quartile = 2.0, 3rd quartile = 9.0, interquartile range = 7.0) and PTSD symptoms related to the ACS event (i.e., “cardiac-induced”) using the PTSD Checklist cued to a specific stressor22,23 (one month post-discharge, median 45 days later; 1st quartile = 33.0, 3rd quartile = 72.00, interquartile range = 39.0). PTSD was assessed one month post-discharge because a one-month period post-event is a criterion PTSD diagnosis.24
These associations were estimated using Pearson correlations. For cardiac-induced PTSD, we additionally tested a bivariate logistic regression model with ED threat predicting presence (vs. absence) of probable cardiac-induced PTSD diagnosis.
The Acute Stress Disorder Scale (ASDS) consists of 19 items designed to capture risk of PTSD development in the acute period following a traumatic event (i.e., prior to one month, at which time PTSD can be diagnosed).21 Participants reported on acute stress symptoms in relation to the “heart problem that brought you to the hospital” (e.g., “Do you feel very upset when you are reminded of the heart problem?”). Responses options ranged from 1 (Not at all) to 5 (Very much) and summed to compute a total symptom score. The ASDS demonstrated high internal consistency reliability in this sample, Chronbach’s α = .89.
Cardiac-induced PTSD (i.e., PTSD with respect to the “heart problem, ED visit, and hospitalization”) was measured using the PTSD Checklist cued to a specific stressor (PCL-S).22 The PCL-S is a reliable and valid tool for assessing PTSD symptoms secondary to medical events.7 During the course of the study, the DSM-V was released with updated PTSD criteria; these updates were reflected in the corresponding PCL-5.23 Study assessments were updated to reflect current criteria, and items were matched across these two instruments by two trained psychologists with experience in PTSD research to create a 17-item summary score of cardiac-induced PTSD over the previous month (see Appendix A for matched scale items). Response options ranged from 1 (Not at all) to 5 (Extremely) and total symptom severity score was obtained by summing all items. The PCL demonstrated high internal consistency reliability in this sample, Chronbach’s α = .95.
A dichotomous score indicating probably cardiac-induced PTSD was also created using a cutoff of ≥ 33. This cutoff was used in a recent meta-analysis examining the association between event-induced PTSD and cardiovascular outcomes.8 In addition, this cutoff falls in the middle of the range suggested by the National Center for PTSD for patients evaluated in medical settings (i.e., 30–35).25
Results
Characteristics of Study Subjects
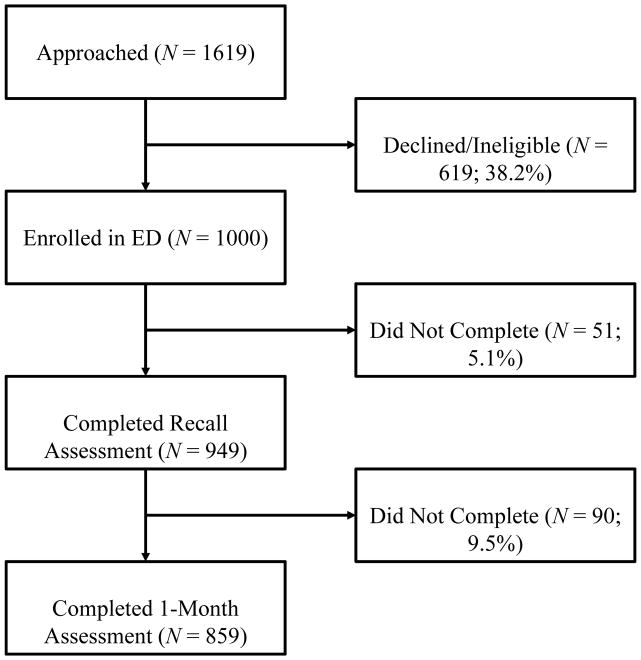
Participant flow is detailed in Figure 1. Because data were not collected on patients who were approached, estimates of refusal rates vs. those deemed ineligible are not available. Participants were more likely to complete the inpatient/telephone follow up if they had a confirmed ACS (i.e., NSTEMI or UA; 97.5% vs. 93.7%), χ2 = 6.43, p = .01, but did not differ by gender, race/ethnicity, age, or in-ED threat perceptions. Participants were more likely to complete the inpatient/telephone follow up if they had a confirmed ACS (i.e., NSTEMI or UA; 89.6% vs. 84.2%), χ2 = 5.34, p = .02, or were female (88.6% vs. 83.6%), χ2 = 5.03, p = .03, but did not differ by race/ethnicity, age, in-ED threat perceptions, or ED threat perceptions at inpatient/telephone follow up.
Figure 1.
Initial refusal rate was approximately 40%. Participant flow for N = 1000 participants.
Slightly more than half of the patients (N = 1000; mean age 60.8±13.2) were male (54.4%), Hispanic (54.4%), and spoke English as a first language (52.3%). Approximately half reported having a high school degree or less. Demographics for the full sample and separately by probable cardiac-induced PTSD diagnosis (score ≥ 33) are reported in Table 1.
Table 1.
Patient demographics (N = 1000).
| Mean (SD) or % | Cardiac-induced PTSDc < 33 (n = 678) | Cardiac-induced PTSD ≥ 33 (n = 139) | ||
|---|---|---|---|---|
| Gender | Female | 45.6% | 45.3% | 54.0% |
| Race/Ethnicity | Hispanic | 54.4% | 53.8% | 51.1% |
| Black | 19.9% | 19.8% | 24.5% | |
| White | 16.5% | 18.4% | 11.5% | |
| Other | 6.6% | 5.6% | 10.1% | |
| English as a first language | Yes | 52.3% | 52.2% | 58.3% |
| Education | Some High School or Less | 33.8% | 33.6% | 31.7% |
| High School or GEDa | 21.1% | 20.8% | 25.2% | |
| Some College or Trade School | 17.7% | 17.4% | 18.7% | |
| College Degree | 15.8% | 15.0% | 17.3% | |
| Graduate or Professional Degree | 11.5% | 13.1% | 7.2% | |
| Age | 60.8 (13.2) | 61.6 (13.3) | 58.8 (11.2) | |
| Confirmed ACSb | Yes | 31.8% | 34.4% | 30.2% |
General Education Degree
Acute Coronary Syndrome; non-ST elevation myocardial infarction (NSTEMI) or unstable angina (UA)
Posttraumatic Stress Disorder
Exploratory Factor Analysis
Exploratory factor analysis examines the underlying structure of the items, and tests how they “hang together’ (i.e., is there one underlying construct, or are there two or three? etc.) The one-factor solution resulted in a theoretically interpretable scale26 consisting of seven items (loadings ≥ .56). In contrast, the two-factor and three-factor solutions resulted in unclear distinctions between factors and few items (two/three in each) or factors with no items loading at .5 or higher; this could lead to less replicable and less meaningful scale scores. Thus, the one-factor solution was retained (seven items, with the single factor explaining 33.8% of the total item variance) representing ED Threat Perceptions (e.g., “I feel vulnerable,” and “I am worried that I am going to die”). When running the EFA separately for English- and Spanish-speaking patients, all seven items loaded above .5 in both groups; for Spanish-speaking patients, concerns about family and worrying that the doctors were not in control also loaded highly onto the latent factor (.56 and .50, respectively). To remain consistent across groups, however, only the initial seven items were retained. Full results for the factor matrix can be seen in Table 2.
Confirmatory Factor Analysis
Confirmatory factor analysis tests how well a hypothesized model (i.e., factor structure) represents the observed data (i.e., observed item scores). When examining English- and Spanish-speaking patients together, the CFA exhibited good fit, CFI = .93, TLI = .89, RMSEA = .10 [.08, .12], SRMR = .04 (though the chi-square was significant, χ2(14) = 115.86, p < .001, suggesting that there may be differences between English- and Spanish-speaking patients regarding the relationship between the scale items and the underlying construct. As noted in the analysis strategy, this is not surprising, given the large sample size).17–19 Loadings are displayed in Supplemental Figure S1. All loadings for ED Threat Perceptions were > .52.
Next, measurement invariance across English and Spanish versions was tested by conducting a multiple-groups CFA examining equality of factor loadings, item intercepts, and item residuals, and using effects coding to identify the model.27 This answers the question: does the scale function in effectively the same way when administered in English and Spanish? Fit statistics are reported in Table 3. Inspection of the unrestricted, baseline model revealed that the factor loadings and means were quite similar across groups (see Supplemental Figure S2).
Table 3.
Fit statistics for Confirmatory Factor Analysis models testing measurement invariance across English- and Spanish-speaking patients (N = 1000).
| χ (df) | CFIa | TLIb | RMSEA [CI]c | SRMRd | |
|---|---|---|---|---|---|
| Unrestricted Baseline Model | 172.906 (28) | .926 | .889 | .102 [.087, .117] | .044 |
| + Invariant Loadings | 186.878 (34) | .922 | .904 | .095 [.082, .108] | .053 |
| + Invariant Intercepts | 240.925 (40) | .898 | .893 | .100 [.088, .113] | .062 |
| + Invariant Residuals | 258.774 (47) | .892 | .904 | .095 [.084, .106] | .070 |
Comparative Fit Index
Tucker Lewis Index
Root Mean Square Error of Approximation [Confidence Interval]
Standardized Root Mean Square Residual
Comparing an unrestricted model that allowed loadings of the items on to the latent construct to differ across English- and Spanish-speaking patients to one that restricted all factor loadings equal produced a significant chi-square, Δχ2(6) = 13.97, p = .03, indicating a degradation in model quality. However, measurement invariance should also be examined using change in fit statistics, given the sensitivity of chi-square to sample size.17,18 When dealing with large samples (>300), Chen15 suggested a cutoff of change in CFI ≤ −.010, with ≥ .015 in RMSEA or ≥ .030 in SRMR, as indicating noninvariance of factor loadings. According to these criteria, metric invariance was tenable across groups (i.e., it is reasonable to conclude that the same latent factor, or meaning, is being captured by the items in both English- and Spanish-speaking patients). Further, inspection of the model in Supplemental Figure S2 revealed that the factor loadings were quite similar.
For testing invariance of intercepts (i.e., estimated mean item scores, wherein differences may indicate item-response bias across groups) or residuals (i.e., unexplained item variance once shared variance with the latent construct—ED Threat Perceptions—is accounted for, or the ability of the item to measure the construct rather than “noise”), Chen15 proposed cutoffs ≤ −.010 in CFI, supplemented with changes ≥ .015 in RMSEA or ≥ .010 in SRMR. Although the change in CFI exceeded this value when testing invariance of intercepts, the value was not exceeded for RMSEA or SRMR, supporting scalar invariance across English- and Spanish speaking patients. It is worth noting that testing intercept invariance is not always appropriate: if two groups can differ on the latent mean, then they will ostensibly differ on item means28 rather than simply indicating undesirable response biases that differ across groups. Bolstering this claim, partial invariance was confirmed via chi-square deviance tests constraining three intercepts equal, but leaving four free to vary (afraid, no control, helpless, and big event). Finally, examining fit indices provided support for invariance of residuals.
Descriptives
For ease of interpretation, we report ED Threat Perceptions scores using mean (SD) of items (scale sum/7 items), so that the score can be interpreted as an individual’s mean threat level. The mean of ED Threat Perceptions items was 1.9 at ED bedside (SD = .7) and 2.1 at inpatient recall (SD = .8), approximately corresponding to the anchor, “A little bit.” For English-speaking patients, means and standard deviations were 1.9±.7 and 2.2±.8, respectively; for Spanish-speaking patients, 1.8±.7 and 2.1±.8, respectively.
Internal Consistency and Stability
At ED bedside, overall reliability for ED Threat Perceptions was high, Chronbach’s α = .82. This was also true at inpatient recall, Chronbach’s α = .83.
ED Threat Perceptions from ED self-report to recall at inpatient/telephone follow up were relatively stable, as indicated by a strong, positive correlation, r = .61, p < .001 (this was slightly stronger for English-speaking patients, r = .68; for Spanish-speaking patients, r = .54).
Convergent Validity
As expected, greater perceptions of ED as stressful and crowded had weak positive associations with greater ED Threat Perceptions at ED assessment, rs = .29 and .18, respectively, and with ED Threat Perceptions at recall, rs = .25 and .14, respectively; all ps < .001.
For English-speaking patients, these correlations were (in the order above): .30, .21, .26, and .19; for Spanish speaking patients, these were: .29, .15, .23, and .07.
Predictive Validity
ED Threat Perceptions should be associated with poor psychological outcomes. Perceiving greater threat at ED assessment was associated with greater acute stress disorder symptoms at inpatient/telephone follow-up (median of four days later), r = .43, p < .001, and with PTSD symptoms specific to the cardiac event one month post-discharge (median of 45 days later, r = .38, p < .001. Similar associations were found between inpatient recall for ED Threat Perceptions with acute stress and PTSD symptoms, rs = .44 and .35, ps < .001. For English-speaking patients, these correlations were (in the order above): .48, .42, .47, and .38; for Spanish speaking patients, these were: .35, .33, .40, and .29.
To test the association of ED Threat Perceptions with a positive screen for PTSD at one month, we estimated an unadjusted logistic regression model with PCL-S score ≥ 33 as the binary outcome (positive screen = 1). For every 1-point increase in mean threat perception at ED assessment (e.g., from 1 [Not at all] to 2 [A little bit]) odds of a positive screen for PTSD were more than doubled, OR = 2.83, 95% CI [2.20, 3.65], p < .001. We found a similarly strong association with threat perceptions at recall, OR = 2.61, 95% CI [2.04, 3.33], p < .001. For English-speaking patients, these ORs were 2.82 [2.01, 3.96], and 2.72 [1.95, 3.81]. For Spanish speaking patients, ORs were 2.83 [1.93, 4.14], and 2.45 [1.71, 3.50] (all ps < .001).
Discussion
This study provides the initial validation of a brief, self-report measure of ED Threat Perceptions developed to identify English- and Spanish-speaking patients who are at risk for cardiac-induced PTSD one month after evaluation for ACS. Further, this study showed the feasibility of administering this instrument in the acute care setting. The 7-item measure takes approximately 2–3 minutes to administer (via RA interview or patient-completed checklist) with minimal RA training.
The validation of a brief, standardized instrument for threat perception has multiple implications for research in an acute care setting. Preliminary research examining the impact of threat perceptions on psychological outcomes has highlighted the importance of threat perceptions in the etiology of cardiac-induced PTSD. For example, previous examination of some of the items in this scale have demonstrated that threat perceptions—assessed both in the ED and inpatient—are associated with elevated acute stress symptoms, a precursor to PTSD, between three and 30 days post-hospitalization.3,6 The use of a broadly-adopted, validated assessment tool for assessing threat perceptions would permit rigorous evaluation of the relationship between threat perceptions and subsequent PTSD across multiple studies, various clinical protocols, and various patient populations (e.g., stroke, ACS). Indeed, threat perceptions are a cross-cutting experience in the ED, as evidenced by the fact that patients who have confirmed ACS and those who rule out experience similar levels of threat.29 Furthermore, a valid and standard instrument is needed for rigorous study of ED threat perceptions. This would pave the way for research to test whether ED threat perceptions might be modifiable and, thus, a target for clinical interventions to improve patient outcomes.
The link between cardiac-induced PTSD and medication nonadherence, ACS recurrence, and mortality highlights the urgency of developing a clinical tool that can identify patients early who are at elevated risk for cardiac-induced PTSD.8–11 If ED threat perceptions are in fact modifiable, clinical interventions in acute settings may buffer or attenuate the development of cardiac-induced PTSD15 and stress-related negative health outcomes (e.g., medication nonadherence). For example, simply reducing threat perceptions could reduce cardiac-induced PTSD incidence, and strong clinician-patient communication may block some of the negative effects of threat perceptions on psychological outcomes.6 In the present study, results suggest that an increase in threat perceptions by even one point (e.g., from “Not at all” to “A little bit”) more than doubled risk for cardiac-induced PTSD. In addition, the relative stability of the measure when assessing ED threat perceptions at recall suggests clinical utility, even if patients are unable to complete the measure immediately within the acute care environment.
Limitations
There were several limitations to our study. This was a single-site study limited to ED patients presenting with ACS symptoms in a New York City Hospital, so findings may not be generalizable to different patient populations or across different diseases or other types of medically-induced PTSD. Generalizability may also be limited by differences between the sample and patients who declined participation (i.e., selection bias), however, the direction of such bias may be toward greater distress in ED patients, as we would hypothesize that the most distressed patients may be most likely to decline enrollment. Similarly, patients were told the purpose of the study at enrollment, so it is possible that knowledge of the study’s purpose could have biased results. However, because the consent form only mentioned “aspects of the ED” in general, we believe this is somewhat unlikely.
Future research on ED quality and patient satisfaction should consider integrating this self-report measure, which is subject to reporting biases, with more, varied assessments of the ED environment (e.g., objective data on ED chaos, crowding, etc.). Parsing variance due to subjective perceptions vs. objectively measured environmental factors would illuminate impactful environment-based interventions. There may also be other self-report measures to capture threat (e.g., concurrent, momentary anxiety) that should be examined within this framework. Finally, at this initial stage of development, this continuous scale is primarily useful for research to estimate the association of ED threat perceptions with patient outcomes and early phase intervention development to test whether these perceptions are modifiable and, if so, whether changing patient threat perceptions results in improved psychological and cardiovascular outcomes. Identifying clinically significant levels of threat perceptions using a cutpoint would provide greater direction for emergency medicine providers. However, the mean of item means on the ED Threat Perceptions scale provides a quick indication of patients’ current level of threat perception, and in this study, each unit increase was associated with a more than doubled risk for subsequently screening positive for cardiac-induced PTSD. Future research that uses item response theory to identify the number of items necessary to identify at-risk patients or tests different cutpoints to improve the clinical utility of the scale (e.g., by establishing population norms) will be critical to aid clinicians in identifying patients with clinically elevated threat perceptions.
Conclusions
In sum, this validated, brief, self-report measure assessing patient perceptions of threat in an acute care setting informs both research and clinical efforts to understand the development of cardiac-induced PTSD. In addition to providing a foundation for standardized research programs across protocols and disease context, this brief assessment could inform clinical interventions and has practical implications: the ability to identify at-risk patients at an early point of contact in the ED and provide appropriate and targeted support to improve patient outcomes.
Supplementary Material
Acknowledgments
Grant Support: This work was supported by National Heart, Lung, and Blood Institute (NHLBI) grants to Dr. Edmondson [grant numbers R01HL117832, R01HL128310] and Dr. Kronish [R01HL123368]. Dr. Chang is supported by a mentored career development grant by the National Institute of Health/NCAT [KL2 TRANSFORM: KL2TR0001874] and the NY Empire Clinical Research Investigator Program.
Appendix A. PTSD Checklist cued to a specific stressor (PCL-S). Items on the PCL-5 (left column) matched to the PCL-S (right column)
| In the past month, how much were you bothered by… | |
| Repeated, disturbing, and unwanted memories of the stressful experience? | Repeated, disturbing memories, thoughts, or images of the experience? |
| Repeated, disturbing dreams of the stressful experience? | Repeated, disturbing dreams of the experience? |
| Suddenly acting or feeling as if the stressful experience were actually happening again (as if you were actually back there reliving it)? | Suddenly acting or feeling as if the experience were happening again (as if you were reliving it)? |
| Feeling very upset when something reminded you of the stressful experience? | Feeling very upset when something reminded you of the experience? |
| Having physical reactions when something reminded you of the stressful experience (e.g., heart pounding, trouble breathing, sweating)? | Having physical reactions (e.g., heart pounding, trouble breathing, or sweating) when something reminded you of the experience? |
| Avoiding memories, thoughts, or feelings related to the stressful experience? | Avoid thinking about or talking about the experience or avoid having feelings related to it? |
| Avoiding external reminders of the stressful experience (e.g., people, places, conversations, activities, objects, or situations)? | Avoid activities or situations because they remind you of the experience? |
| Trouble remembering important parts of the stressful experience? | Trouble remembering important parts of the experience? |
| Having strong negative beliefs about yourself, other people, or the world (e.g., having thoughts such as: I am bad, there is something seriously wrong with me, no one can be trusted, the world is completely dangerous)? | Feeling as if your future will somehow be cut short? |
| Blaming yourself or someone else strongly for the stressful experience or what happened after it? | |
| Having strong negative feelings such as fear, horror, anger, guilt, or shame? | |
| Loss of interest in activities that you used to enjoy? | Loss of interest in things that you used to enjoy? |
| Feeling distant or cut off from other people? | Feeling distant or cut off from other people? |
| Having trouble experiencing positive feelings (e.g., being unable to have loving feelings for those close to you, or feeling emotionally numb)? | Feeling emotionally numb or being unable to have loving feelings for those close to you? |
| Feeling irritable or angry or acting aggressively? | Feeling irritable or having angry outbursts? |
| Taking too many risks or doing things that cause you harm? | |
| Being “super alert” or watchful or on guard? | Being “super alert” or watchful on guard? |
| Feeling jumpy or easily startled? | Feeling jumpy or easily startled? |
| Having difficulty concentrating? | Having difficulty concentrating? |
| Trouble falling or staying asleep? | Trouble falling or staying asleep? |
Footnotes
Conflicts of Interest: All authors report no conflicts of interest.
This scale was presented as a poster at the 76th Annual Scientific Meeting of the American Psychosomatic Society, Louisville, KY.
Author Contributions: DE conceived and designed the study, obtained research funding, and contributed to manuscript revisions. TC drafted the manuscript and conducted data analysis. WC provided statistical advice and oversaw data analysis. BPC conducted manuscript revisions and contributed substantially to the conceptual design of the manuscript. SA contributed to manuscript revisions. OG enrolled participants, completed data collection, and contributed to manuscript revisions.
References
- 1.Edmondson D, Shimbo D, Ye S, Wyer P, Davidson KW. The association of emergency department crowding during treatment for acute coronary syndrome with subsequent posttraumatic stress disorder symptoms. JAMA internal medicine. 2013;173(6):472–475. doi: 10.1001/jamainternmed.2013.2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edmondson D, Kronish IM, Wasson LT, Giglio JF, Davidson KW, Whang W. A test of the diathesis-stress model in the emergency department: Who develops PTSD after an acute coronary syndrome? Journal of psychiatric research. 2014;53:8–13. doi: 10.1016/j.jpsychires.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Homma K, Chang B, Shaffer J, et al. Association of social support during emergency department evaluation for acute coronary syndrome with subsequent posttraumatic stress symptoms. Journal of behavioral medicine. 2016;39(5):823–831. doi: 10.1007/s10865-016-9748-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.White M, Edmondson D, Umland R, Sanchez G, Chang BP. Erratum to “Patient perceptions of stress during evaluation for ACS in the ED”, YAJEM 35/2 (2017) 351–352. The American Journal of Emergency Medicine. 2017 doi: 10.1016/j.ajem.2016.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.White M, Edmondson D, Umland R, Sanchez G, Chang BP. Patient perceptions of stress during evaluation for acute coronary syndrome in the emergency department. The American Journal of Emergency Medicine. 2017;35:351–352. doi: 10.1016/j.ajem.2016.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang BP, Sumner JA, Haerizadeh M, Carter E, Edmondson D. Perceived clinician–patient communication in the emergency department and subsequent post-traumatic stress symptoms in patients evaluated for acute coronary syndrome. Emerg Med J. 2016;33(9):626–631. doi: 10.1136/emermed-2015-205473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner JA, Kronish IM, Pietrzak RH, et al. Dimensional structure and correlates of posttraumatic stress symptoms following suspected acute coronary syndrome. Journal of affective disorders. 2015;186:178–185. doi: 10.1016/j.jad.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edmondson D, Richardson S, Falzon L, Davidson KW, Mills MA, Neria Y. Posttraumatic stress disorder prevalence and risk of recurrence in acute coronary syndrome patients: a meta-analytic review. PloS one. 2012;7(6):e38915. doi: 10.1371/journal.pone.0038915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Edmondson D, Horowitz CR, Goldfinger JZ, Fei K, Kronish IM. Concerns about medications mediate the association of posttraumatic stress disorder with adherence to medication in stroke survivors. British journal of health psychology. 2013;18(4):799–813. doi: 10.1111/bjhp.12022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kronish IM, Edmondson D, Goldfinger JZ, Fei K, Horowitz CR. Posttraumatic stress disorder and adherence to medications in survivors of strokes and transient ischemic attacks. Stroke. 2012;43(8):2192–2197. doi: 10.1161/STROKEAHA.112.655209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronish IM, Edmondson D, Li Y, Cohen BE. Post-traumatic stress disorder and medication adherence: results from the Mind Your Heart study. Journal of psychiatric research. 2012;46(12):1595–1599. doi: 10.1016/j.jpsychires.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vilchinsky N, Ginzburg K, Fait K, Foa EB. Cardiac-disease-induced PTSD (CDI-PTSD): A systematic review. Clinical Psychology Review. 2017 doi: 10.1016/j.cpr.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 13.Meli L, Kautz M, Julian J, Edmondson D, Sumner JA. The role of perceived threat during emergency department cardiac evaluation and the age-posttraumatic stress disorder link. Journal of behavioral medicine. 2017:1–7. doi: 10.1007/s10865-017-9904-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: a meta-analysis. Psychological bulletin. 2003;129(1):52. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- 15.Forneris CA, Gartlehner G, Brownley KA, et al. Interventions to prevent post-traumatic stress disorder: a systematic review. American Journal of Preventive Medicine. 2013;44(6):635–650. doi: 10.1016/j.amepre.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Costello AB, Osborne JW. Best practices in exploratory factor analysis: Four recommendations for getting the most from your analysis. Practical assessment, research & evaluation. 2005;10(7):1–9. [Google Scholar]
- 17.Chen FF. Sensitivity of goodness of fit indexes to lack of measurement invariance. Structural equation modeling. 2007;14(3):464–504. [Google Scholar]
- 18.Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Structural equation modeling. 2002;9(2):233–255. [Google Scholar]
- 19.Kline RB. Principles and practice of structural equation modeling. 4. The Guilford Press; 2016. [Google Scholar]
- 20.MacCallum RC, Widaman KF, Zhang S, Hong S. Sample size in factor analysis. Psychological methods. 1999;4(1):84. [Google Scholar]
- 21.Bryant RA, Moulds ML, Guthrie RM. Acute Stress Disorder Scale: a self-report measure of acute stress disorder. Psychological assessment. 2000;12(1):61. [PubMed] [Google Scholar]
- 22.Weathers F, Litz B, Herman D, Huska J, Keane T. The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. Paper presented at: annual convention of the international society for traumatic stress studies; San Antonio, TX. 1993. [Google Scholar]
- 23.Weathers F, Litz B, Keane T, Palmieri P, Marx B, Schnurr P. The PTSD checklist for DSM–5 (PCL-5) Boston. MA: National Center for PTSD; 2013. [Google Scholar]
- 24.Association AP. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. [DOI] [PubMed] [Google Scholar]
- 25.PTSD NCf. Using the PTSD Checklist for DSM-IV (PCL) 2014 Jan; http://www.ptsd.va.gov/professional/pages/assessments/assessment-pdf/PCL-handout.pdf.
- 26.Fabrigar LR, Wegener DT, MacCallum RC, Strahan EJ. Evaluating the use of exploratory factor analysis in psychological research. Psychological methods. 1999;4(3):272. [Google Scholar]
- 27.Little TD, Slegers DW, Card NA. A non-arbitrary method of identifying and scaling latent variables in SEM and MACS models. Structural Equation Modeling. 2006;13(1):59–72. [Google Scholar]
- 28.Vandenberg RJ, Lance CE. A review and synthesis of the measurement invariance literature: Suggestions, practices, and recommendations for organizational research. Organizational research methods. 2000;3(1):4–70. [Google Scholar]
- 29.Kronish IM, Edmondson D, Moise N, et al. Posttraumatic stress disorder in patients who rule out versus rule in for acute coronary syndrome. General hospital psychiatry. 2018 doi: 10.1016/j.genhosppsych.2018.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.