Abstract
The presence of myocilin is often used in the process of validating trabecular meshwork (TM) cells and tissue, but the antibody reagents used for detection are poorly characterized. Indeed, for over a century, researchers have been using antibodies to track proteins of interest in a variety of biological contexts, but many antibodies remain ill-defined at the molecular level and in their target epitope. Such issues have prompted efforts from major funding bodies to validate reagents and combat reproducibility issues across biomedical sciences. Here we characterize the epitopes recognized by four commercial myocilin antibodies, aided by structurally and biochemically characterized myocilin fragments. All four antibodies recognize enriched myocilin secreted from human TM cell media. The detection of myocilin fragments by ELISA and Western blot reveal a variety of epitopes across the myocilin polypeptide chain. A more precise understanding of myocilin antibody targets, including conformational specificity, should aid the community in standardizing protocols across laboratories and in turn, lead to a better understanding of eye physiology and disease.
Keywords: Myocilin, antibodies, trabecular meshwork, glaucoma, ELISA, Western blot, epitopes, antigen
Graphical Abstract
Antibodies, protein molecules derived from the immune response in animals, are powerful scientific and therapeutic reagents due to their ability to strongly and specifically bind a desired target antigen. Polyclonal antibodies have been used for over a century and monoclonal antibodies from hybridoma cell lines for decades (Kohler and Milstein, 1975). Although antibodies have been indispensable in addressing research questions across the biomedical sciences, these reagents are generally not rigorously characterized and are thus of variable quality (Bordeaux et al., 2010; Bradbury and Pluckthun, 2015b). Polyclonal antibodies are notorious for problems with cross-reactivity and batch-to-batch variability due to natural variation in animal titers (Hjelm et al., 2012). Only 0.5–5% of the antibodies in a polyclonal antibody sample bind their target protein (Bradbury and Pluckthun, 2015a; Ferrara et al., 2015; Nilsson et al., 2005), and even antibodies purified by using an antigen affinity step may include a variety of antibody isotypes targeting multiple epitopes with a variety of affinities (Ferrara et al., 2015). The use of hybridomas to express monoclonal antibodies overcomes some of this variability (Lipman et al., 2005). However, over time, hybridomas can undergo mutations that alter specificity, thus introducing a new source of variability (Meliopoulos and Schultz-Cherry, 2018). The rigorous validation of research reagents, such as antibodies, has become a high priority for NIH and other funding bodies due to the multitude of research studies that cannot be independently replicated (Begley and Ellis, 2012).
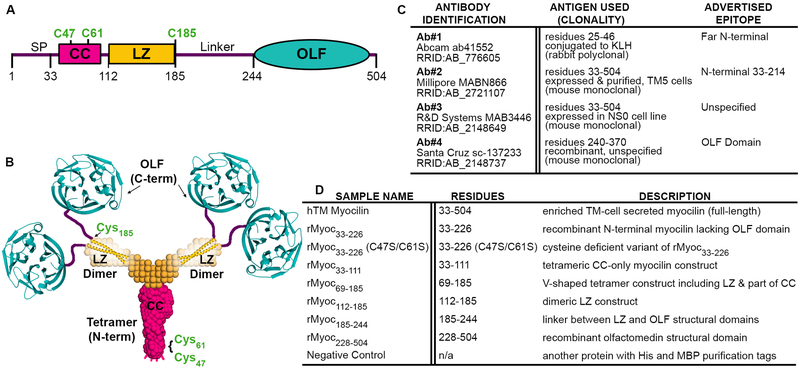
Vision researchers who study trabecular meshwork (TM) rely on antibodies to detect myocilin, a protein secreted from TM cells (Nguyen et al., 1998; Polansky et al., 1997) and associated with heritable glaucoma (Stone et al., 1997). Namely, myocilin expression is often used to distinguish TM cells and tissues from other anterior segment regions (Polansky et al., 2000; Stamer and Clark, 2017). Our lab recently characterized the structural regions of the myocilin protein (Figure 1A, B) including the N-terminal coiled-coil domains by solution X-ray scattering, chemical crosslinking, and X-ray crystallography (Hill et al., 2017), and the C-terminal β-propeller olfactomedin domain (OLF) by X-ray crystallography (Donegan et al., 2015). Our studies reveal that myocilin is a Y-shaped dimer-of-dimers (Figure 1B) (Hill et al., 2017): the N-terminal coiled-coil region (CC, residues 33–111) forms a tetrameric stem with disulfides at Cys47 and Cys61, and the leucine-zipper (LZ, residues 112–185), a special coiled-coil sub-type, forms two dimeric arms, each of which is stabilized by a C-terminal disulfide bond (Hill et al., 2017). Finally, a ~60 residue linker connects the LZ to two pairs of monomeric OLF domains (Figure 1B).
Fig. 1.
Introduction to myocilin structure, commercial antibodies, and protein constructs tested. (A) Gene structure depicting the domains of myocilin, including signal peptide (SP), location of key cysteine residues (C47, C61 and C185) and its coiled-coil (CC), leucine zipper (LZ) and olfactomedin (OLF) domains. (B) Myocilin quaternary structure based on solution X-ray scattering, X-ray crystallography and chemical cross-linking experiments. (C) Identifying information for commercial myocilin antibodies selected for this study, including antigen used for antibody development, clonality, and currently advertised epitope. (D) Myocilin constructs used in this study represent a combination of shorter and longer myocilin fragments. Each potential epitope for antibodies is represented at least twice.
Currently, there are several commercially-available antibodies against myocilin, but little is known about the epitope(s) recognized and specificity for detecting myocilin in the context of the (extra)cellular milieu. Knowledge of epitopes detected by these antibodies is important for several reasons. First, cleavage of myocilin has been reported in mammalian cells (Sanchez-Sanchez et al., 2007), like for other olfactomedin family members latrophilin (Silva et al., 2009) and gliomedin (Eshed et al., 2007; Maertens et al., 2007) where cleavage plays a functional role. Using a single antibody reagent to detect myocilin may not fully describe its whereabouts or function, especially if cleavage is functional. Related, not all epitopes may be accessible to myocilin in a biological context, for example, in the case of complexation with a binding partner or upon post-translational modification. Second, the OLF domain of myocilin can be driven to misfold, even in the absence of destabilizing disease-associated mutations, by selected environmental stressors (Hill et al., 2014; Orwig et al., 2012). Indeed, the overall oligomeric state of myocilin may be sensitive to its environment as higher-ordered states of myocilin, mediated by disulfide bonds, have been detected in several experiments (Fautsch et al., 2004; Gobeil et al., 2004). Detailed knowledge of epitope and conformational specificity of antibodies against myocilin could be transformative in our understanding of the functional role of myocilin processing, oligomerization, and misfolding.
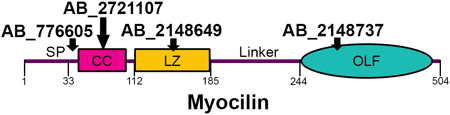
Here, we took advantage of the well-defined domain constructs our lab used for structural studies of myocilin to characterize four anti-myocilin antibodies from commercial vendors (Ab#1-Ab#4). We describe four distinct amino acid epitopes within myocilin recognized by the four reagents, the conformational specificity for the respective epitope, and, to the extent possible, cross-reactivity (Figure 1C). Ab#1 (Abcam Cat# ab41552, RRID:AB_776605) is a rabbit polyclonal antibody raised against recombinant myocilin residues 25–46 conjugated to keyhole limpet hemocyanin (KLH) and purified by protein A affinity chromatography. This antigen includes several residues within the signal peptide (residues 1–33), cleaved prior to secretion (Sohn et al., 2009), and far N-terminal residues without predicted structure. Ab#2 (Millipore Cat# MABN866, RRID:AB_2721107) is a mouse monoclonal antibody raised against myocilin residues 33–504 produced in TM5 cells with C-terminal V5 and hexa-histidine (6x-His) tags. This antibody was purified from mouse hybridoma culture supernatant by protein A/G affinity chromatography and its epitope was previously localized to residues 33–214 within N-terminal myocilin (Ezzat et al., 2008). Ab#3 (R&D Systems Cat# MAB3446, RRID:AB_2148649), is also an affinity-purified monoclonal mouse antibody, raised against recombinant myocilin (residues 33–504) that was produced in the mouse myeloma cell line NS0. Finally, Ab#4 (Santa Cruz Biotechnology Cat# sc-137233, RRID:AB_2148737) is a monoclonal mouse antibody raised against recombinant myocilin residues 240–370 (cell line unspecified), a predominantly internal sequence of the C-terminal OLF structural domain, which spans residues 245–504 (Donegan et al., 2015). The currently advertised epitopes for Ab#1, Ab#3 and Ab#4 are the antigen used for antibody development; Ab#2 was tested further against truncated myocilin constructs (Ezzat et al., 2008) prior to commercialization (Figure 1B).
Recombinant myocilin subdomains (Figure 1D) were cloned, expressed in E. coli, and purified as previously described (Burns et al., 2010; Hill et al., 2017). The linker region between the LZ and OLF domains (residues 185–244) was cloned into pET-28b vector with N-terminal 6x-His and S-tags (Genscript). The plasmid containing the linker region was transformed into E. coli BL21(DE3)pLysS, grown in Terrific Broth (Fisher) and expression was induced at OD600=0.8 with 1 mM isopropyl-β-D-thiogalactopyranoside for 3 hours at 37 °C. Cell lysates were prepared by gently resuspending 0.5 g cells in 2.5 mL 50 mM Hepes (pH 7.5), 200 mM NaCl, 10% glycerol (HBS) and 1/10 Complete EDTA-Free Protease Inhibitor Cocktail tablet (Roche), lysed by sonication, then clarified by centrifugation at ~20,000 × g. Human TM (hTM) cell medium was a kind gift from E. Snider and C. R. Ethier (Snider et al., 2017). To enrich endogenous, secreted myocilin from these samples, 20 mL of conditioned hTM medium was purified by heparin affinity (1-mL HiTrap Heparin HP, GE Healthcare) employing phosphate-buffered saline (10 mM Na2HPO4, 1.8 mM KH2PO4, 130 mM NaCl, 2.7 mM KCl, 10% glycerol, PBS) and an elution gradient to 1 M NaCl across 80 column volumes. Endogenous myocilin eluted from the heparin resin at approximately 0.4 M NaCl, along with many other proteins (not shown), and was detected by Western blot using the polyclonal anti-myocilin antibody N-15 (Santa Cruz Biotechnology Cat# sc-21243, RRID:AB_2148741, no longer commercially available). Corresponding fractions were pooled and concentrated approximately 36-fold using an Amicon Ultra centrifugal filter with 30 kDa molecular weight cut-off.
Western blots were conducted on 25–100x dilutions of clarified E. coli cell lysates or 5 μL heparin-enriched hTM myocilin, and transferred from SDS-PAGE to polyvinylidene difluoride membranes (Millipore) by standard electrophoresis methods. Primary antibodies were used at either 1 μg/mL or the manufacturer-recommended concentration, followed by 1:2000 dilution of secondary antibody: goat anti-mouse (Thermo Fisher Scientific Cat# 62–6520, RRID:AB_2533947) or goat anti-rabbit (KPL, Cat# 074–1506, RRID:AB_2721169). Blots were imaged using an Amersham Imager A600 (GE Healthcare). Western blot results represent at least two biological replicates per antibody tested. For both ELISA and Western Blots experiments employing clarified whole-cell lysate, we included a negative control cell sample expressing a protein which includes the hexahistidine and maltose-binding protein tags present in the recombinant myocilin constructs. For indirect ELISA, all protein samples were adsorbed in triplicate directly onto plastic, using one medium-binding 96-well Costar plates (Corning), by overnight 4 °C incubation of 50 μL/well 2x diluted cell lysate in HBS (with non-myocilin cell lysate as negative control), or 1–5 μM purified protein (Burns et al., 2010; Hill et al., 2017), employing antigen-free wells as a control. Samples were then treated with serial dilutions of primary antibody (starting from 5 μg/mL) and 1:2000 dilutions of appropriate secondary antibody. Binding of primary (and therefore secondary antibody) was detected via absorbance of HCl-quenched 3,3’,5,5’-tetramethylbenzidine (TMB, 450 nm), measured using a BioTek Synergy 2 plate reader. For each antibody tested, ELISA was conducted using one freshly prepared plate per antibody per biological replicate. ELISA results presented are an average of at least two biological replicates per antibody tested. Absorbance values for each ELISA plate were normalized to the maximum absorbance within that plate, then multiple, normalized plates were averaged and plotted in Origin Professional 2016. Error bars represent standard deviation across all samples.
The advertised epitope of Ab#1 lies within antigen residues 25–46. We found that Ab#1 primarily recognizes the far N-terminal residues of myocilin (residues 33–46), but a secondary epitope and other non-specific bands are also detected. In Western blot, Ab#1 recognizes recombinant myocilin comprising residues 33–226 (rMyoc33–226, Figure 2A) and secreted full-length myocilin from hTM media (Figure 2B). Over-exposure of Western blots results in detection of endogenous proteins from the E. coli cell lysate (not shown) as well as weak bands ~70 and 100 kDa in the hTM sample (Figure 2B). A “66 kDa myocilin isoform” has been detected by multiple myocilin antibodies (Cheng et al., 2002; Nguyen et al., 1998) but has fallen under suspicion due to poor reproducibility. The 66 kDa band has yet to be confirmed as myocilin by mass spectrometry (Anderssohn et al., 2011; Shepard et al., 2003), but it has been speculated that this band may instead comprise sequence-related proteins (Ezzat et al., 2008) or albumin (Shepard et al., 2003). In Western blot (Figure 2A) and ELISA (Figure 2C), Ab#1 also recognizes the smaller far-N-terminal tetrameric construct composed of residues 33–111 (rMyoc33–111). Thus, of the residues within the antigen for Ab#1 (25–46), residues 33–46 are sufficient for detecting larger myocilin constructs containing these residues. Ab#1 also weakly recognizes the construct composed of residues 69–185 (rMyoc69–185) in ELISA (Figure 2C) and in over-exposed Western blots (not shown). This could arise from two segments within this construct (residues 102–109 and 158–168), which share 75 and 81% sequence similarity, respectively, with the peptide antigen. This weak interaction likely benefits from the increased avidity of tetrameric rMyoc69–185 in ELISA.
Fig. 2.
Epitope mapping of commercial antibodies reveals four distinct epitopes. (A,D,G,J) Western blots demonstrate binding of antibodies against cell lysates containing recombinant myocilin fragments under denaturing conditions. Manufacturers’ recommended primary antibody concentrations are indicated with an asterisk, and standards (PageRuler™ Plus, Thermo Scientific) are indicated in kilodaltons (kDa). Note that the size of recombinant constructs is affected by expression tags, including maltose binding protein (~45 kDa) for rMyoc33–226 and rMyoc228–504 and hexahistidine/S-tags for the remaining constructs (~6 kDa). (B,E,H,K) All antibodies tested recognize myocilin enriched from hTM cell-conditioned medium in Western blots. ELISA conducted against clarified cell lysates (C,I) and purified protein (F,L) demonstrate antibody binding to recombinant myocilin constructs in native conditions. (M) Schematic representation of target regions of the four commercial antibodies on myocilin.
Ab#2 recognizes an epitope within rMyoc33–111, as compared to previous localization within residues 33–214 (Ezzat et al., 2008), and its detection is sensitive to the presence of disulfide bonds formed by C47 and C61. In Western blot, Ab#2 detects E. coli lysates containing rMyoc33–226 and rMyoc33–111 (Figure 2D) as well as secreted hTM myocilin (Figure 2E). In initial ELISA experiments against clarified cell lysates of all constructs, Ab#2 detected rMyoc33–226 and, to a lesser extent, rMyoc33–111 (not shown). These results were reproduced in a follow-up ELISA experiment conducted using equimolar concentrations of purified recombinant rMyoc33–226 and rMyoc33–111 (Figure 2F). Motivated by variations in disulfide bonding for rMyoc33–226 and rMyoc33–111 reported previously (Hill et al., 2017), we next tested the contribution of disulfide bond formation to the recognized epitope by Ab#2. ELISA was conducted with the purified disulfide-disrupted variant rMyoc33–226 C47S/C61S (rMyoc33–226(C47S/C61S), (Hill et al., 2017)), revealing apparent reduced affinity when compared to wild-type rMyoc33–226.
Our experiments narrow down the epitope recognized by Ab#3 from antigen residues 33–504 to the LZ region (residues 112–185) of myocilin. This antibody recognized rMyoc33–226 (Figure 2G) and endogenous hTM-secreted full-length myocilin (Figure 2H) in Western blots, and rMyoc33–226 in ELISA (Figure 2I). Both Western blot and ELISA yield robust recognition of smaller N-terminal constructs, corresponding to residues 69–185 (rMyoc69–185) and the LZ-containing subdomain 112–185 (rMyoc112–185). As expected for antibodies raised against lengthy target sequences, Ab#2 and Ab#3 each target different regions within myocilin.
Ab#4 recognizes the myocilin OLF domain (Figure 1C), as designed, but does not distinguish between natively folded and misfolded states. In Western blot, Ab#4 detects the purified OLF domain (Figure 2J) as well as full-length secreted endogenous hTM myocilin (Figure 2K). In ELISA (Figure 2L), Ab#4 recognizes both folded OLF monomer and amyloid-like misfolded OLF comprising residues 228–504 (rMyoc228–504), expressed and purified from E. coli as a maltose-binding protein (MBP) fusion (Orwig et al., 2012). Notably, we previously showed that the polyclonal precursor to this antibody (Santa Cruz H-130, no longer available) recognizes related domains from other olfactomedin family members (Hill et al., 2015), which share a conserved interior (Donegan et al., 2015), suggesting that Ab#4 may be similarly non-selective.
In sum, our study reveals that the commercial myocilin antibodies tested recognize amino acid sequence-derived epitopes across myocilin structural domains (Figure 2M), including far N-terminal (residues 25–46, Ab#1), CC (residues 33–111, Ab#2), LZ (residues 112–185, Ab#3) and C-terminal OLF domain (residues 240–370, Ab#4), and they are not dependent on the conformation of myocilin. All four antibodies recognize enriched endogenous myocilin secreted from hTM media; Ab#1 also exhibits non-specific binding to other proteins in hTM media and E. coli lysate at high exposures. The diversity of epitopes revealed by our in vitro ELISA and Western blot experiments suggest that biological insights may be gleaned from employing different antibodies to detect the same myocilin-containing tissue sample. Such comparative tissue studies could assess the accessibility of a given epitope, tying into protein function, processing, or misfolding. Conversely, by using a single myocilin antibody to a given epitope, functionally relevant myocilin fragments may remain undetected or non-myocilin contaminants may be inadvertently detected. Our library of recombinant myocilin constructs will be useful to monitor changes in epitope recognition by myocilin antibodies over time and from lot-to-lot. Finally, beyond the currently available myocilin antibodies, we anticipate that the TM research community may benefit from recombinant antibodies (Bradbury and Pluckthun, 2015b). Such antibodies can be held to higher quality control standards due to gene sequencing, and can be tailored by engineering for sensitivity, selectivity, and even bifunctionality.
Highlights.
-
*
Available commercial myocilin antibodies are not well validated
-
*
Recombinant myocilin constructs can identify epitopes recognized by commercial antibodies
-
*
Available antibodies recognize the three major structural regions of myocilin
Acknowledgements
This work was supported by the BrightFocus Foundation and National Institutes of Health R01EY021205. We thank I. Dominic, D. J. E. Huard, Y. Ku, for technical assistance, E. Snider and C.R. Ethier for hTM media, E. Nguyen for hTM myocilin enrichment, and J. Maynard for helpful discussions.
References
- Anderssohn AM, Cox K, O’Malley K, Dees S, Hosseini M, Boren L, Wagner A, Bradley JM, Kelley MJ, Acott TS, 2011. Molecular chaperone function for myocilin. Invest Ophth Vis Sci 52, 7548–7555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begley CG, Ellis LM, 2012. Drug development: Raise standards for preclinical cancer research. Nature 483, 531–533. [DOI] [PubMed] [Google Scholar]
- Bordeaux J, Welsh A, Agarwal S, Killiam E, Baquero M, Hanna J, Anagnostou V, Rimm D, 2010. Antibody validation. Biotechniques 48, 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury A, Pluckthun A, 2015a. Reproducibility: Standardize antibodies used in research. Nature 518, 27–29. [DOI] [PubMed] [Google Scholar]
- Bradbury AR, Pluckthun A, 2015b. Getting to reproducible antibodies: the rationale for sequenced recombinant characterized reagents. Protein Eng Des Sel 28, 303–305. [DOI] [PubMed] [Google Scholar]
- Burns JN, Orwig SD, Harris JL, Watkins JD, Vollrath D, Lieberman RL, 2010. Rescue of Glaucoma-Causing Mutant Myocilin Thermal Stability by Chemical Chaperones. ACS Chem Biol 5, 477–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng LE, Ueda J, Wentz-Hunter K, Yue BYJT, 2002. Age independent expression of myocilin in the human trabecular meshwork. Int J Mol Med 10, 33–40. [PubMed] [Google Scholar]
- Donegan RK, Hill SE, Freeman DM, Nguyen E, Orwig SD, Turnage KC, Lieberman RL, 2015. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet 24, 2111–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshed Y, Feinberg K, Carey DJ, Peles E, 2007. Secreted gliomedin is a perinodal matrix component of peripheral nerves. J Cell Biol 177, 551–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzat MK, Howell KG, Bahler CK, Beito TG, Loewen N, Poeschla EM, Fautsch MP, 2008. Characterization of monoclonal antibodies against the glaucoma-associated protein myocilin. Exp Eye Res 87, 376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fautsch MP, Vrabel AM, Peterson SL, Johnson DH, 2004. In vitro and in vivo characterization of disulfide bond use in myocilin complex formation. Mol Vis 10, 417–425. [PubMed] [Google Scholar]
- Ferrara F, D’Angelo S, Gaiotto T, Naranjo L, Tian H, Graslund S, Dobrovetsky E, Hraber P, Lund-Johansen F, Saragozza S, Sblattero D, Kiss C, Bradbury AR, 2015. Recombinant renewable polyclonal antibodies. MAbs 7, 32–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gobeil S, Rodrigue MA, Moisan S, Nguyen TD, Polansky JR, Morissette J, Raymond V, 2004. Intracellular sequestration of hetero-oligomers formed by wild-type and glaucoma-causing myocilin mutants. Invest Ophthalmol Vis Sci 45, 3560–3567. [DOI] [PubMed] [Google Scholar]
- Hill SE, Donegan RK, Lieberman RL, 2014. The glaucoma-associated olfactomedin domain of myocilin forms polymorphic fibrils that are constrained by partial unfolding and peptide sequence. J Mol Biol 426, 921–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SE, Donegan RK, Nguyen E, Desai TM, Lieberman RL, 2015. Molecular details of olfactomedin domains provide pathway to structure-function studies. Plos One 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill SE, Nguyen E, Donegan RK, Patterson-Orazem AC, Hazel A, Gumbart JC, Lieberman RL, 2017. Structure and misfolding of the flexible tripartite coiled-coil domain of glaucoma-associated myocilin. Structure 25, 1697–1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hjelm B, Forsstrom B, Lofblom J, Rockberg J, Uhlen M, 2012. Parallel immunizations of rabbits using the same antigen yield antibodies with similar, but not identical, epitopes. Plos One 7, e45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler G, Milstein C, 1975. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497. [DOI] [PubMed] [Google Scholar]
- Lipman NS, Jackson LR, Trudel LJ, Weis-Garcia F, 2005. Monoclonal versus polyclonal antibodies: distinguishing characteristics, applications, and information resources. ILAR J 46, 258–268. [DOI] [PubMed] [Google Scholar]
- Maertens B, Hopkins D, Franzke CW, Keene DR, Bruckner-Tuderman L, Greenspan DS, Koch M, 2007. Cleavage and oligomerization of gliomedin, a transmembrane collagen required for node of Ranvier formation. J Biol Chem 282, 10647–10659. [DOI] [PubMed] [Google Scholar]
- Meliopoulos VA, Schultz-Cherry S, 2018. Although it’s painful: The importance of stringent antibody validation. PLoS Pathog 14, e1006701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR, 1998. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem 273, 6341–6350. [DOI] [PubMed] [Google Scholar]
- Nilsson P, Paavilainen L, Larsson K, Odling J, Sundberg M, Andersson AC, Kampf C, Persson A, Szigyarto CAK, Ottosson J, Bjorling E, Hober S, Wernerus H, Wester K, Ponten F, Uhlen M, 2005. Towards a human proteome atlas: High-throughput generation of mono-specific antibodies for tissue profiling. Proteomics 5, 4327–4337. [DOI] [PubMed] [Google Scholar]
- Orwig SD, Perry CW, Kim LY, Turnage KC, Zhang R, Vollrath D, Schmidt-Krey I, Lieberman RL, 2012. Amyloid fibril formation by the glaucoma-associated olfactomedin domain of myocilin. J Mol Biol 421, 242–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Chen P, Chen H, LutjenDrecoll E, Johnson D, Kurtz RM, Ma ZD, Bloom E, Nguyen TD, 1997. Cellular pharmacology and molecular biology of the trabecular meshwork inducible glucocorticoid response gene product. Ophthalmologica 211, 126–139. [DOI] [PubMed] [Google Scholar]
- Polansky JR, Fauss DJ, Zimmerman CC, 2000. Regulation of TIGR/MYOC gene expression in human trabecular meshwork cells. Eye 14, 503–514. [DOI] [PubMed] [Google Scholar]
- Sanchez-Sanchez F, Martinez-Redondo F, Aroca-Aguilar JD, Coca-Prados M, Escribano J, 2007. Characterization of the intracellular proteolytic cleavage of myocilin and identification of calpain II as a myocilin-processing protease. J Biol Chem 282, 27810–27824. [DOI] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Sui RF, Steely HT, Lotery AJ, Stone EM, Clark AF, 2003. Characterization of rabbit myocilin: Implications for human myocilin glycosylation and signal peptide usage. BMC Genet 4, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva JP, Lelianova V, Hopkins C, Volynski KE, Ushkaryov Y, 2009. Functional cross-interaction of the fragments produced by the cleavage of distinct adhesion G-protein-coupled receptors. J Biol Chem 284, 6495–6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider EJ, Vannatta RT, Schildmeyer L, Stamer WD, Ethier CR, 2017. Characterizing differences between MSCs and TM cells: Toward autologous stem cell therapies for the glaucomatous trabecular meshwork. J Tissue Eng Regen Med, 1–10. [DOI] [PubMed] [Google Scholar]
- Sohn S, Joe MK, Kim TE, Im JE, Choi YR, Park H, Kee C, 2009. Dual localization of wild-type myocilin in the endoplasmic reticulum and extracellular compartment likely occurs due to its incomplete secretion. Mol Vis 15, 545–556. [PMC free article] [PubMed] [Google Scholar]
- Stamer WD, Clark AF, 2017. The many faces of the trabecular meshwork cell. Exp Eye Res 158, 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone EM, Fingert JH, Alward WLM, Nguyen TD, Polansky JR, Sunden SLF, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC, 1997. Identification of a gene that causes primary open angle glaucoma. Science 275, 668–670. [DOI] [PubMed] [Google Scholar]