Abstract
Background:
Individuals with chronic venous disease (CVeD) frequently experience associated leg pain that may influence disease management self-efficacy.
Objective:
To evaluate the influence of a cooling intervention on leg pain associated with more severe stages of CVeD and self-efficacy. This was a secondary aim of the trial.
Design:
Randomized, blinded, comparator-controlled, multisite trial.
Setting:
Three wound clinics and an academic medical research center in the United States of America.
Participants:
276 participants (54.3% female, 46.7% male) with stage 4 and 5 CVeD were randomly assigned by computer generated tables to the cooling intervention group (n=138) or control group (n=138).
Interventions:
Participants received either a cooling (intervention) leg cuff or placebo cuff (control) to apply topically over the affected skin area. Both groups performed standard of care including wearing compression wraps and elevating legs for 30 minutes during the intervention. Study visits occurred at baseline, and months 1, 3, 6, and 9.
Methods:
Visit measures included: Numeric Rating Scale (NRS) for short term pain; VEINES- QOL/Sym questionnaire for long-term pain; and, the Self Efficacy for Managing Chronic Disease Scale (SEMCD-6) for self-efficacy. Data were collected from September 2010 to December 2015 and analyzed using pooled t-tests, Chi-square tests, and mixed effects models. Observed 9-month patient retention rates were 94/138 (68.1%) in the intervention group and 91/138 (65.9%) in the control group. The primary analysis was based on the intention-to-treat principle.
Results:
Both the cooling intervention and control group experienced statistically significant decreases in unadjusted and adjusted mean NRS pain scores of 1.2 (95% CI: (−1.82, −0.64); p<0.000l) and 1.8 (95% CI: (−2.31, −1.24); p<0.000l) respectively from baseline, however, no statistically significant differences in change scores were observed between groups. The unadjusted mean VEINES-QOL/Sym pain scores had statistically significant decreases of 0.9 ((95% CI: (−1.07, −0.62) p<0.000l - cooling) and 0.8 (95% CI: (−1.09, −0.55) p<0.000l - control) points. When adjusting the scores for demographic and clinical features, both cooling and control groups maintained statistically significant decreases (p <0.001 for both). No statistically significant differences in change scores were observed between groups. The unadjusted and adjusted mean self-efficacy scores had no statistically significant improvements from baseline to month 9 within and between the cooling and control groups.
Conclusions:
Pain was reduced in both groups while self-efficacy did not change. Findings suggest that strictly implemented standard of CVeD care in each study group, with or without cooling, improved pain while there were no effects on self-efficacy.
Keywords: Chronic venous disease, Cooling therapy, Pain management, Self-efficacy, Symptom assessment
1. Introduction
Chronic venous disease (CVeD) of the lower limbs is a result of persistent venous hypertension and if left untreated can lead to vessel wall damage, varicosities, skin inflammation, skin changes including hardening, discoloration and ulceration, and delayed tissue healing (Raffetto and Mannello, 2014). CVeD is estimated to affect 1 – 2% of adults worldwide and is characterized by leg symptoms such as heaviness, swelling, cramping, burning, throbbing, itching, aching, and pain upon exertion (Eberhardt and Raffetto, 2014, Ruggiero et al., 2016, Wrona et al., 2015). Leg pain in particular is a common presenting complaint defined as a localized “hurting” and incessant sensation at the skin site most severely affected by CVeD (Kelechi et al., 2017a). This pain is different than other leg symptoms such as burning or piecing and has also been reported to be related to postural changes and foot/ankle static disorders (Perrin et al., 2016). While no one causative factor explains the pain mechanisms associated with CVeD, present-day hypotheses suggest local skin hypoxia activates endothelial cells, which induce a cascade of inflammatory mechanisms and biochemical changes including activation of phospholipase A2 and leukotriene B4 and the release of histamine and serotonin, causing adherence of neutrophils to the venous endothelium (Danziger, 2011). Nociceptors in venous system detect the noxious stimuli that are producing physical damage to the skin and causing pain (Dubin and Patapoutian, 2010). This nociceptive pain has high inter-individual variability, however when present it is a major debilitating symptom, occurring in 48%−81% of patients; approximately 90% of patients report pain as the major symptom for which they seek care (Radak et al., 2015). Pain is associated with decreased energy, depressed mood, decreased mobility, increased isolation, sleep disturbances and an inability to manage daily activities, and in the presence of venous ulcers, poor healing (Finlayson et al., 2017).
Guideline-driven medical and surgical therapies such as compression therapy, leg elevation, physical activity, medications such as Rutosides and endovascular procedures reduce negative symptoms such as swelling and cramps, slow disease progression, and prevent venous leg ulcers and their recurrence (Wittens et al., 2015). However, very few therapies have been studied for their effects specifically on pain, thus there is no standardised approach to pain management, the most common negative symptom (Gloviczki et al., 2011, Green and Jester, 2009, Kelechi et al., 2017,c, Nicolaides et al., 2014). Specifically, there are a lack of interventions that target the localized pain in the CVeD-affected leg.
Previous studies evaluating the effects of educational and behavioral interventions on venous leg ulcer healing and recurrent indicate that patient beliefs in compression therapy, skin care, activity and exercise, and proper nutrition are important for CVeD self-management (Kapp and Miller, 2015). While beliefs that a treatment will improve one’s condition may promote self-management behaviors, gaps remain in the understanding of whether one’s self-efficacy, or the belief or confidence one has in the ability to carry out specific tasks influences self-management approaches to improve symptoms associated with CVeD, and in particular CVeD pain. New approaches that augment guideline-driven care that target pain and enhance self-efficacy in the day-to-day management of disease symptoms are needed.
2. Objective
The overall purpose of this trial was to evaluate the efficacy of a self-administered home-based cooling “cuff’ intervention coupled with standard of care (compression wrap, leg elevation) compared to a placebo cuff and standard of care on skin physiological parameters including skin blood flow and temperature and the incidence of venous leg ulcers during the 9-month study period (Kelechi et al., 2017,b). No statistically significant differences were observed between groups for skin blood flow (p =0.619), temperature (p =0.540), or ulcer incidence (p =0.29). Based on the epidemiological venous leg ulcer literature (Shenoy, 2014) it was anticipated that approximately 40% of study participants would experience a new ulcer, however, the overall incidence of ulcers during the study period was 7%. A secondary aim of this trial was to report outcomes on quality of life and bothersome symptoms such as aching and itching. Findings suggest that cooling and control groups had significant improvements in quality of life from baseline (p <0.0001) but there were no differences between groups (p =0.58) while statistically significant improvements were noted for symptoms in the treatment group compared to the control group (p =0.015) (Kelechi, et al., 2017c). The purpose of this paper is to compare the effect of cooling versus control on leg pain and self-efficacy in patients with CVeD over a 9 month period. Pain and self-efficacy are secondary outcomes in the overall study.
2.1. Conceptual framework of the research
The study was guided by two conceptual frameworks: a physiologic pain theory and a self- efficacy/management model. Pain is defined as a “complex constellation of unpleasant sensory, emotional and cognitive experiences provoked by real or perceived tissue damage and manifested by certain pathophysiological, autonomic, psychological and behavioral reactions.” (Terman and Bonica, 2003). CVeD evokes tissue damage from an influx of inflammatory chemical mediators that attract more circulating inflammatory cells which increase the production of free radicals and damage the cellular membrane resulting in increased tissue injury (Freire, et al., 2016). This inflammatory process disrupts the peripheral nervous system, specifically the nociceptive pain pathway in the skin that is responsible for thermosensation. A group of specialized peripheral sensory neurons (nociceptors) A δ and C fibers respond to the thermosensation of cooling by detecting and coding this sensation and conveying it to the central nervous system (Dubin and Patapoutian, 2010). In addition, transient receptor proteins (TRP) are located in the free nerve endings of the skin. When innocuously or gently cooled, TRP modulate the transient receptor potential cation channel subfamily M member 8 (TRPM8) which stimulate the nociceptors, producing analgesic effects. Cold is a TRPM8 antagonist that has been shown to reverse pain in nociceptive pain models (Park and Kim, 2013) and may also act by decreasing velocity of nerve conduction and slowing the firing of muscle spindle afferents and reflex responses (Nadler et al., 2004). Cooling CVeD-inflamed skin is posited to slow the expression of the inflammatory substances and reduce the speed of nociceptive sensory pathway transmission, resulting in reduced pain (Freire, 2016). This process is similar to the therapeutic effects of cooling on inflammatory rheumatic diseases in which several mediators such as cytokines involved in joint inflammation and destruction are downregulated by cooling, resulting in a decrease in pain (Guillot et al., 2014).
Self-efficacy is defined as one’s belief in his or her ability to be successful in specific situations or accomplish a task in order to deal with life’s challenges (Bandura, 1986). Broadly defined, self-management is a complex set of various patient and disease characteristics and system-bound determinants that influence the day-to-day tasks or processes, beyond pure treatment adherence (e.g. self-monitoring) performed by the individual with chronic conditions over the course of an illness and that affect the cognitive, behavioral and emotional responses necessary to maintain a satisfactory quality of life (Barlow et al. 2002; Lorig and Holman, 2003; Schulman-Green et al. 2016). There is a substantial body of knowledge to support positive relationships between self-efficacy and self-management (Kapp et al., 2015; Greenberger et al., 2014).
Individuals with CVeD who believe they can improve their health by self-managing symptoms, will engage in and adhere to positive health behaviors such as interventions (cooling) that control or ameliorate those symptoms (pain), which in turn, raises self-efficacy. On the other hand, those with low levels of self-efficacy have little belief in their ability to improve their health and are less likely to engage in self-management behaviors. Results from the cryotherapy study suggest individuals who are not adherent to the cooling protocol were more than twice as likely to develop an ulcer (18%) compared to individuals who carried out the procedure ≥ 85% of the time (7%, p = 0.012) (Monsen et al., 2018). For this aim of the cryotherapy study, we sought to determine whether self-efficacy was related to self-management of lower leg pain, for both cooling and control treatments, in individuals with CVeD. The combined self-efficacy, self-management and pain symptom reduction model is shown in Figure 1.
Figure 1.
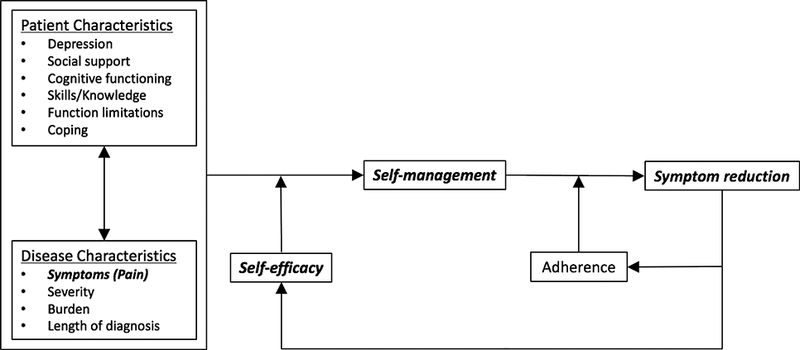
Conceptual Model
3. Methods and materials
3.1. Trial design
The cryotherapy study was designed as a multicenter randomized controlled trial that compared a 9-month graduated cooling intervention to a placebo control plus usual care among patients with the more severe forms of CVeD. Three wound care centers and an academic medical research center from the south-eastern region of United States (U.S.) participated in the study. The study complied with the Declaration of Helsinki and was approved by the university’s Institutional Review Board for Human Research (IRB). Data assessments were conducted at 5 different time points (baseline, and after 1, 3, 6 and 9 months) and data were collected between September 2010 and December 2015. Written informed consent was obtained from all participants. By the end of the trial, all study participants received a study compensation of $400 (U.S.). The cryotherapy study design is described in detail elsewhere (Kelechi et al., 2016) and registered at the Clinical Trial Registry (www.clinicaltrials.gov) with the identifier number NCT01509599. The 2017 CONSORT Statement for Randomized Trials of Nonpharmacological Treatments (Boutron et al., 2017) was used as a guide for the reporting of this study.
3.2. Participants and Recruitment
Potentially eligible individuals were identified through direct patient referrals from wound clinicians and general practitioners at participating study sites and indirectly from the general public through advertising on billboards and flyers posted in grocery stores and other commercial venues. Individuals who were interested in participation were instructed to contact the researchers by phone and were prescreened. If prescreened eligible, a baseline screening visit was scheduled. At the baseline visit, written informed consent was obtained, demographics and medical history were collected, and screening measures were conducted by the research study nurse. Eligibility criteria included: 1) age 21 years or older; 2) clinical-aetiologic-anatomic- pathophysiologic (CEAP) classification stages C4 (hyperpigmentation, eczema, atrophy blanche, lipodermatosclerosis) and C5 (healed ulcer within 2 years) (C4 and C5 represent the more severe stages of CVeD where skin is damaged); 3) ankle brachial index 0.8 – 1.3 (sufficient arterial flow); 4) intact skin and thermal sensation in the treatment leg and foot (to avoid frost injury); 5) agreement to wear compression wraps during waking hours; and, 6) the ability to speak and read English and provide consent. Exclusion criteria included: 1) lack of sensory discrimination and pressure perception suggestive of neuropathy (intact peripheral sensation was required to avoid possible skin injury from cooling); 2) impaired cognitive status (ability to follow directions); 3) chronic inflammatory or vascular disorders such as Lupus (cooling could exacerbate symptoms); and 4) recent surgery on affected leg (symptoms such as swelling could be as a result of surgery and not CVeD). Individuals with diabetes were included but if they tested positive for neuropathy, they were excluded. A screening log was maintained to track patient referrals and study enrollment outcomes. Study endpoints included completion of the 9-month study or the development or recurrence of a venous leg ulcer on the treatment leg.
3.3. Sample size
The sample size for the original study (138 participants per group) was designed to have 85% power to detect differences in efficacy of cooling versus control for the primary outcomes (skin blood flow, temperature and the incidence of venous leg ulcers); the sample size/power calculation did not take into account, a priori, the secondary outcomes of pain and self-efficacy. As a result, negative conclusions for the secondary outcomes are accompanied by a caution that the unknown power may be low resulting in failure to detect clinically relevant population treatment differences.
3.4. Randomization, allocation concealment, and blinding
At baseline, enrolled participants were allocated to the intervention or control group through use of a web-based centralized electronic research database Research Electronic Data Capture (REDCap) (Harris et al., 2009) according to a randomly generated 50/50 stratified block scheme developed by the study biostatistician so as to ensure equal allocation across all study sites. Due to the physical differences (weight and texture) between the intervention and placebo study materials, it was not possible for the enrolling study nurse to be blinded to the participant’s treatment allocation. However, the Principal Investigator and all study coordinators who collected subsequent visit study measures were blinded so as to minimize the potential for study bias.
3.5. Intervention
A 6-minute instructional study DVD was created that included a step-by-step actor demonstration of a patient performing the self-management intervention. This DVD was shown and given to all enrolled participants to take home at the baseline visit for the purpose of standardising the instruction of the delivery of the protocol across all participating study sites. After viewing the DVD, a 10-item post-study quiz was administered to assess participants’ understanding of expectations and to clarify any questions about the protocol. The intervention included taking the daily temperature of the affected leg skin area in the morning upon waking, and both before and immediately after performing the treatment (applying the cooling or control treatment over the affected skin area for 30 minutes while elevating the legs) in the evening. Based upon treatment allocation group, participants were given either a specially designed gel or placebo cotton-filled cuff that were identical in size (Southwest Technologies, Kansa City, Missouri, U.S.), and a long-handle skin thermometer (Diabetica Solutions, San Antonio, Texas, USA), leg elevator pillow, 30 mmHg compression wrap (JuxtaLite, Circaid by mediUSA, San Diego, California, USA), freezer thermometer, clock timer, and study logs and a clipboard for self-report as well as maintain a daily dairy of any problems encountered while performing the intervention. The cuffs were placed in a freezer set at −17.8°C (0°F) when not in use. The cotton placebo cuff did not freeze and warmed to room temperature within 2 minutes of removal from the freezer, while the gel intervention cuff retained its cooling properties for upwards of 1 hour to ensure the delivery of a sustained cooling effect. The cooling gel cuff is made of a predominantly glycerin based flexible sheet hydrogel of 1/2-inch thickness specifically designed to initiate low grade or “gentle” cooling - the goal was to reduce skin temperature no more than 5.55°C (10°F) below the individual’s baseline to avoid frost injury (Kelechi et al., 2011). To assess this, temperatures were taken before and after the treatment, and documented on study logs. On average, temperatures were reduced by 3.05°C (5.49°F) (Kelechi et al., 2015).
The treatments were titrated at varying doses during the study period. For the first month, daily doses of 30-minute cooling were reduced to three times per week for months 2–3, then weekly months 4–6, and finally whenever needed (PRN) in months 7–9. PRN was defined as a 1.1°C (2°F) elevation above baseline in morning temperature for 2 consecutive days in which case the participant resumed treatment for 5 consecutive days. This treatment regimen was based on our previous work (Kelechi et al., 2011). Morning skin temperatures during the non-treatment days were taken daily and recorded on the study logs throughout the entire study period. The researchers maintained weekly telephone contact with the participants during the first month of the study, bi-weekly contact during months 2 and 3, and monthly contact for the remainder of the study to assist with any problems that participants might have been experiencing while trying to perform or adhere to the intervention and to solicit any experienced adverse events. Additionally, logs were reviewed with the participant at month 1, 3 and 6 study visits to further promote and encourage adherence to intervention.
3.6. Outcomes measures
The outcome measures included two well-validated self-report instruments used extensively in research related to CVeD symptom management and a self-efficacy instrument used in chronic disease research.
Short Term Pain –
Numeric Rating Scale for Pain (NRS Pain) is a 3-item scale that measures average pain in the affected skin area in the last 24 hours, pain right now, and worst pain in the last 24 hours on a unilateral 11-point numeric scale. Higher scores indicate greater pain intensity, with ‘0’ representing one pain extreme (“no pain at all”) and ‘10’ representing the other pain extreme (“the worst pain imaginable”). Internal consistency is reported to be 0.99 (Hawker et al., 2011).
Long Term Pain –
VEINES-QOL/Sym is a 26-item disease specific instrument for chronic leg disorders. It includes questions about symptoms due to CVeD, limitations in daily activities, quality of life, psychological impact, and one question about the amount of change in the patient’s leg pain during the past 4 weeks, rated on a 6-point scale with “none” =0, “very mild” =1, “mild” =2, “moderate” = 3, “severe” =4, and “very severe” =5. Internal consistency is reported to be 0.87, and test-retest reliability is 0.86 (Lamping et al., 2003).
Self-efficacy –
The Self-Efficacy for Managing Chronic Disease Scale (SEMCD-6) is a 6-item scale that measures attitudes and beliefs towards the management of chronic diseases. Scores on this scale are computed as the mean of the six items, each measured on Likert scales ranging from 1 (“not at all confident”) to 10 (“totally confident”) with higher scores indicating greater self-efficacy. Internal consistency (Cronbach’s alpha) has been reported to be 0.88 to 0.95 (Freund et al., 2011, Lorig et al., 2001). Items 2 and 4 on this measure are specific to confidence in managing leg pain in the affected skin area.
3.7. Data collection
At the initial study visit, participant characteristics including demographics, medical history and the use of medications and topical creams to alleviate leg pain symptoms and baseline outcome data were collected in a question and answer interview format by the unblinded study nurse. Participants were given a copy of the measures to follow along with if they chose to do so. Data were collected in a similar manner at months 1, 3, 6 and 9 by the blinded study coordinators. All participant responses were directly entered into the study’s protected and secure electronic REDCap database using laptop computers.
3.8. Harms
A Data Safety and Monitoring Board (DSMB) met semi-annually to review cumulative participant adverse event data and to make recommendations regarding study modification and continuance. The DSMB was comprised of a Family Medicine physician, a Podiatrie Surgeon, the study Biostatistician and the Program Manager. Both physicians were independent of the academic institution and had no real or apparent conflicts of interest in their roles. Participants were given a phone number to self-report any suspected or experienced adverse events, and the coordinators solicited adverse events during supportive telephone calls and at each study visit. All gathered adverse events were first graded for seriousness, expectancy within the study protocol, and severity by the Principal Investigator in accordance with U.S. Code of Federal Regulations (CFR 312.32) and then reviewed by the DSMB. Reported adverse events were followed through to resolution up to 3 months post-study exit. A total of 132 adverse events were reported (65 Intervention group and 68 Control group), including 2 deaths; however, none of these involved Suspected Unexpected Serious Adverse Reactions (SUSARs).
3.9. Statistical analysis
Pooled t-tests and Chi-square tests were used for between treatment group comparisons for continuous and categorical demographic and clinical variables, respectively. Complete distributions of demographic and clinical measures are reported in Table 1, however, for subsequent mixed effects modeling some response categories were collapsed. For example, marital status was defined as married versus not married which included never married, widowed, separated, or divorced. Employment status was defined as employed (full or part-time) versus not employed (unemployed, retired, volunteer, student, or home-maker). For analyses that involved the assessment of the effect of race, two participants in the “other race” category were excluded and comparison was restricted to White versus Black/African American ethnicity. Statistical analyses were conducted using Statistical Analysis Software SAS 9.4 (SAS Institute, Cary, North Carolina, U.S., 2013). For the outcomes, NRS pain scores were the mean scores for the 3 questions. If any of the three questions were left unanswered, the score was not calculated. VEINES-QOL/Sym pain item was calculated directly from the item’s response coding values (‘0’ - “none” to ‘5’ - “very severe”). Self-efficacy scores were the means over the SEMCD-6 items. If two or more items were missing, the score was not calculated.
Table 1.
Patient Demographics and Baseli Characteristics
| Cooling Group n=138 |
Cooling Group n=138 |
P-Value | |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age (years) | 62.7 ± 11.5 | 60.9 ± 11.9 | 0.2068 |
| Sex | 0.468 | ||
| Male | 66 (47.8%) | 60 (43.5%) | |
| Female | 72 (52.2%) | 78 (56.5%) | |
| Race | 0.808* | ||
| White | 61 (44.2%) | 59 (42.8%) | |
| Black/African American | 76 (55.1%) | 78 (56.5%) | |
| Other | 1 (0.7%) | 1 (0.7%) | |
| Marital Status | 0.802 | ||
| Never Married | 23 (16.7%) | 24 (17.4%) | |
| Married | 69 (50.0%) | 67 (48.6%) | |
| Widowed | 17 (12.3%) | 19 (13.8%) | |
| Separated | 8 (5.8%) | 12 (8.7%) | |
| Divorced | 21 (15.2%) | 16 (11.6%) | |
| Employment | 0.616 | ||
| Employed | 41 (29.7%) | 42 (30.4%) | |
| Other | 4 (2.9%) | 8 (5.8%) | |
| Retired | 70 (50.7%) | 63 (45.7%) | |
| Unemployed | 23 (16.7%) | 25 (18.1%) | |
| Residence | 0.786 | ||
| Rural | 52 (38.2%)a | 53 (39.8%)b | |
| Urban | 84 (61.8%) | 80 (60.2%) | |
|
CLINICAL | |||
| Body mass index | 36.2 ± 9.3 | 36.4 ± 9.3 | 0.819 |
| Previous VLU | 84 (60.9%) | 76 (55.1%) | 0.329 |
| Surgery | 8 (5.8%) | 13 (9.4%) | 0.256 |
|
LEG PAIN | |||
| NRS Score | 3.9 ± 2.7e | 3.6 ± 2.7d | 0.5151 |
| Average Pain (past 24 hrs.) | 4.0 ± 2.7d | 3.9 ± 3.0c | 0.7294 |
| Pain Right Now | 2.5 ± 2.7c | 2.4 ± 2.6c | 0.8359 |
| Worst Pain (past 24 hrs.) | 5.1 ± 3.3c | 4.6 ± 3.4c | 0.2344 |
| VEINES QOL/Sym pain (past 4 weeks) | 3.7 ± 1.2c | 3.5 ± 1.3c | 0.2339 |
|
SELF-EFFICACY | |||
| SEMCD-6 Score | 6.9 ± 2.3c | 7.3 ± 2.3c | 0.1388 |
| Emotional Distress | 6.9 ± 3.0c | 7.7 ± 2.8c | 0.0244 |
| Fatigue | 6.7 ± 2.8c | 7.3 ± 2.6c | 0.0777 |
| Physical Discomfort | 6.5 ± 2.9c | 7.0 ± 2.9c | 0.2267 |
| Symptoms/Health problems | 7.0 ± 2.9c | 7.4 ± 2.9d | 0.2605 |
| Tasks to manage health | 7.3 ± 2.6c | 7.3 ± 2.8c | 0.9819 |
| Other than medication | 6.9 ± 3.0c | 7.3 ± 2.8c | 0.3326 |
|
MEDICAL CONDITIONS | |||
| Varicose Veins | 33 (23.9%) | 41 (29.7%) | 0.277 |
| Blood Clots | 17 (12.3%) | 19 (13.8%) | 0.721 |
| Vein Stripping | 8 (5.8%) | 10 (7.2%) | 0.626 |
| Cyst/Tumor Removal | 6 (4.3%) | 6 (4.3%) | 1 |
| Joint Replacement | 13 (9.4%) | 19 (13.8%) | 0.259 |
| Diabetes | 82 (59.4%) | 83 (60.1%) | 0.902 |
| Hypertension | 107 (77.5%) | 96 (69.6%) | 0.133 |
| Arthritis | 58 (42.0%) | 62 (44.9%) | 0.627 |
| Thyroid Problems | 20 (14.5%) | 18 (13.0%) | 0.727 |
| Kidney Problems | 13 (9.4%) | 13 (9.4%) | 1 |
|
MEDICATIONS | |||
| Psychotropic | 20 (14.5%) | 23 (16.7%) | 0.619 |
| Diabetes | 82 (59.4%) | 74 (53.6%) | 0.331 |
| Pain | 57 (41.3%) | 47 (34.1%) | 0.214 |
| Anticoagulants | 28 (20.3%) | 29 (21.0%) | 0.882 |
| Antibiotics | 8 (5.8%) | 3 (2.2%) | 0.124 |
| Diuretics | 30 (21.7%) | 36 (26.1%) | 0.397 |
| Cholesterol | 62 (44.9%) | 60 (43.5%) | 0.808 |
| Antihypertensive | 97 (70.3%) | 82 (59.4%) | 0.059 |
| Steroids | 0 (0.0%) | 3 (2.2%) | 0.082 |
| Topical Cream/Ointment | 72 (52.2%) | 66 (48.2%) | 0.507 |
Data are represented as mean ± SD for continuous variables and number (%) for categorical.
P-value is calculated excluding ‘other’ category (comparing only white vs. Black/African American ethnicity) due to small sample size.
Missing 2 values
Missing 5 values
Missing 6 values
Missing 7 values
Missing 8 values
To answer the two exploratory research questions, “Does the active treatment reduce pain compared to the control condition?” and “Do patients in the cooling intervention group experience increased self-efficacy compared to the control group?” mixed effects models (MEM) were used to analyze all longitudinal data using clustering by participant to control correlation among repeated measurements. MEM also allow for analysis of participants with missing data at various visits and clustering of repeated measures within participants. Group (cooling versus control) and a group-by-visit interaction term were included as fixed effects.
In exploratory analysis using the above MEM models, potential covariates included demographic and clinical characteristics, and potential effect modifiers based on a priori known relationships with CVeD as reported in the literature such as age, sex, body mass index (BMI), and co-morbid conditions (Vuylsteke et al., 2018). Covariates and potential effect modifiers with a bivariate p- value < 0.2 were included in the full model. Model selection was carried out by initially including all potential covariates and potential effect modifiers (interaction terms of covariates and treatment), then sequentially removing the covariate with the highest p-value until only the covariates with 0.05 level of significance remained. Effect modification was subsequently tested for all variables in the model, however, only those with significant p values indicating a differential effect are reported. Unadjusted and covariate-adjusted means for pain and self- efficacy outcomes at baseline, 9 month visit, the change from baseline to the 9 month visit and the difference in change from baseline between the two intervention groups are reported along with their 95% confidence intervals. As the examination of potential predictors was exploratory and therefore hypothesis-generating, rather than hypothesis confirming, p-values were not adjusted for multiple testing.
4. Results
4.1. Participants’ flow through the trial
An illustration of the study cumulative flow of participants across the three participating study sites through the trial is shown in Figure 2. Two hundred and seventy-six participants 276 were randomized, 138 per study arm (intervention and control). The total drop-out rate at the final month 9 (visit 5) was 29.0% (40/138) in the intervention group and 28.3% (39/138) in the control group and; 68.1% (94/138) in the intervention group and 65.9% (91/138) of participants in the control group completed all study measures by attending each of the 5 study visits, respectively. The mean and standard deviation (SD) follow-up time was 6.9 (3.5) and 7.0 (3.4) months for the cooling and control groups, respectively. All participant data were included in the analyses in accordance with the principles of intention to treat.
Figure 2.

CONSORT Participant Study Flowchart
4.2. Baseline patient characteristics
The mean age was 62.7 and 60.9 years for cooling and control groups (p=0.207), respectively (Table 1). Though not statistically significant, mean baseline Pain scores were observed to be slightly lower in the control group than the cooling group (NRS Total Score 3.7 vs 3.9, p =0.554; VEINES-QOL Pain Item score 3.5 vs. 3.7, p =0.234 for control and cooling groups, respectively). The overall mean baseline SEMCD-6 score (as well as individual item scores) for the control group was observed to be higher than that for the cooling group (7.3 vs. 6.9, p =0.139, respectively); however, these differences also did not reach statistical significance. Other demographic data reported in Table 1 have established relationships with CVeD such as higher BMI, previous venous leg ulcer, leg surgery, and medical conditions such as hypertension.
NRS Short Term Pain
To assess the effects of the cooling treatment on pain compared to the control treatment, changes in NRS pain score from baseline to month 9 were compared between the two groups (Table 2). Both cooling and control groups had experiences of statistically significant decreases in unadjusted mean NRS pain scores of 1.2 (95% CI: (−1.82, −0.64); p <0.0001) and 1.8 (95% CI: (−2.31, −1.24); p <0.0001) points, respectively. No statistically significant differences were observed at baseline, or month 9, or in change from baseline between the cooling and control groups. When the NRS pain score was adjusted for covariates, both cooling and control groups showed statistically significant decreases in mean pain scores (Δ=−1.5, p <0.001; Δ=−1.7, p <0.001 respectively) over the 9-month study. No statistically significant differences in scores at baseline or month 9 nor differences in change from baseline were observed between groups.
Table 2.
Unadjusted and adjusted pre- and post- leg pain and self-efficacy scores.
| Cooling | Control | Difference3 | P- Value |
|
|---|---|---|---|---|
| NRS SHORT TERM PAIN | ||||
| Unadjusted | ||||
| Baseline (Visit 1) | 3.86 (3.40, 4.33) | 3.67 (3.20, 4.14) | 0.20 (−0.46, 0.85) | 0.554 |
| Last (Visit 5) | 2.40 (1.87, 2.92) | 1.90 (1.45, 2.35) | 0.50 (−0.20, 1.19) | 0.159 |
| Difference1 | −1.23 (−1.82, −0.64) | −1.78 (−2.31, −1.24) | 0.55 (−0.25, 1.34) | 0.176 |
| P-Value | <.0001 | <.0001 | ||
| Adjusted4 | ||||
| Baseline (Visit 1) | 3.75 (3.31, 4.19) | 3.44 (3.00, 3.88) | 0.32 (−0.30, 0.93) | 1.0002 |
| Last (Visit 5) | 2.29 (1.78, 2.80) | 1.72 (1.20, 2.23) | 0.57 (−0.14, 1.29) | 0.5782 |
| Difference | −1.46 (−2.13, −0.80) | −1.72 (−2.38, −1.05) | 0.53 (−0.17, 1.23) | 0.690 |
| P-Value | <.0012 | <.0012 | ||
|
VEINES-QOL/Sym PAIN SCORE | ||||
| Unadjusted | ||||
| Baseline (Visit 1) | 3.68 (3.47, 3.89) | 3.50 (3.28, 3.72) | 0.18 (−0.12, 0.48) | 0.234 |
| Last (Visit 5) | 2.84 (2.61, 3.06) | 2.65 (2.42, 2.88) | 0.19 (−0.14, 0.51) | 0.260 |
| Difference1 | −0.85 (−1.07, −0.62) | −0.82 (−1.09, −0.55) | −0.02 (−0.37, −0.32) | 0.899 |
| P-Value | <.0001 | <.0001 | ||
| Adjusted5 | ||||
| Baseline (Visit 1) | 3.67 (3.47, 3.86) | 3.49 (3.30, 3.69) | 0.17 (−0.10, 0.44) | 1.0002 |
| Last (Visit 5) | 2.81 (2.59, 3.03) | 2.69 (2.47, 2.91) | 0.12 (−0.20, 0.44) | 1.0002 |
| Difference | −0.86 (−1.15, −0.56) | −0.81 (−1.10, −0.51) | 0.03 (−0.31, 0.37) | 1.000 |
| P-Value | <.0012 | <.0012 | ||
|
SEMCD-6 SELF-EFFICACY SCORE | ||||
| Unadjusted1 | ||||
| Baseline (Visit 1) | 6.90 (6.50, 7.30) | 7.32 (6.93, 7.72) | −0.42 (−0.98, 0.14) | 0.139 |
| Last (Visit 5) | 7.35 (6.89, 7.81) | 7.95 (7.55, 8.36) | −0.60 (−1.21, 0.01) | 0.055 |
| Difference1 | 0.34 (−0.19, 0.88) | 0.34 (−0.08, 0.76) | 0.005 (−0.57, 0.68) | 0.988 |
| P-Value | 0.203 | 0.113 | ||
| Adjusted6 | ||||
| Baseline (Visit 1) | 7.00 (6.65, 7.35) | 7.43 (7.07, 7.78) | −0.43 (−0.92, 0.06) | 0.4382 |
| Last (Visit 5) | 7.48 (7.07, 7.89) | 7.98 (7.57, 8.39) | −0.51 (−1.08, 0.07) | 0.4162 |
| Difference | 0.48 (−0.05, 1.01) | 0.56 (0.03, 1.09) | 0.07 (−0.50, 0.64) | 1.000 |
| P-Value | 0.3802 | 0.1992 | ||
Mean (95% CI)
Paired T-test
P-value adjusted for multiple comparisons (obtained p-value multiplied by 5- number of comparisons)
Difference = Cooling - Control
Covariates: age, race, marital status, employment status, diabetes, diabetes-by-treatment, arthritis, thyroid, thyroid-by-treatment, anti-hypertension medication, anti-hypertension medications-by-treatment, and pain medication.
Covariates: marital status, body mass index, arthritis, hypertension, hypertension-by-treatment, pain medication, anticoagulants, cholesterol medication, cholesterol meds-by-treatment, diabetes medication, and diabetes meds-by-treatment.
Covariates: sex, marital status, employment status, body mass index, diabetes, diabetes-by-treatment, arthritis, blood clots, vein stripping, anti-hypertension medication, anti-hypertension medications-by-treatment, psychotropic medication, anti-coagulants, and pain medication.
However, when regression analysis was performed, a statistically significant effect modifier for diabetes (p = 0.018) and thyroid (p = 0.006) indicated that the impact of cooling reduced pain scores for those with diabetes or thyroid more than for the control group. Conversely, a statistically significant effect modifier for those taking medication for hypertension indicated that the impact of cooling increased pain scores for those on antihypertensive medications more than the control group (p = 0.001; Supplement Table 1). The higher NRS pain scores (increased pain) were associated with those of Black African American ethnicity, those with arthritis, those taking pain medications, younger participants, those not married, and those not employed (Supplement Table 1).
VEINES-QOL/Sym Long Term Pain
The unadjusted mean VEINES-QOL/Sym pain item score was statistically significantly decreased by 0.9 (95% CI: (−1.07, −0.62); p <0.0001) and 0.8 (95% CI: (−1.09, −0.55); p <0.0001) points, for cooling and control groups respectively, from baseline to month 9 (Table 2). Similar to the NRS pain score, the decrease was statistically significant, however, no statistically significant differences were observed between the two groups at baseline or month 9. When adjusting the VEINES-QOL/Sym pain item scores for demographic and clinical features, both cooling and control groups maintained the statistically significant decreases in mean pain scores (both p <0.001), though differences in change scores observed between groups did not reach statistical significance.
Statistically significant effect modifiers for hypertension (p<0.0001) and cholesterol medication (p =0.032) indicated that the impact of cooling increased pain scores for those with hypertension and taking cholesterol medication more than for the control group. In contrast, participants in the cooling group taking diabetes medication had reduced pain compared to those in the control group (p <.0001), Supplement Table 1. The higher VEINES-QOL/Sym pain item scores were associated with higher BMI, arthritis, taking anticoagulants, and taking pain medications; whereas lower scores were associated with being married.
SEMCD-6 Self-Efficacy
The unadjusted mean SEMCD-6 self-efficacy scores (Table 2) had no statistically significant increases from baseline to month 9 nor observable differences at baseline or month 9 between the two groups. When adjusting self-efficacy for co-variables within and between group differences remained not statistically significant.
Interaction terms with treatment were statistically significant for diabetes (p =0.025) and anti-hypertensive medication (p =0.029) indicating that the impact of cooling increased selfefficacy scores for those with diabetes, however, decreased self-efficacy scores were noted among those taking medication for hypertension compared to the control group, Supplement Table 1. Higher self-efficacy scores were associated with being female, married, employed and having a history of vein stripping; whereas lower scores were associated with having a diagnosis of arthritis, a history of blood clots, higher BMI, and using psychotropic medication, pain medication, and anti-coagulants.
5. Discussion
To our knowledge, this is the first randomized trial to explore a non-pharmacologic intervention for pain and self-efficacy associated with CVeD. Among patients with CVeD, pain remains a common symptom that reduces function and quality of life and tends to worsen as the disease progresses. Pain and self-efficacy were evaluated over time in a group of patients who received standard of care (leg compression and elevation) and either a cooling cuff or a placebo cuff applied to the lower skin affected by CVeD. Overall the cooling self-management intervention did not demonstrate more beneficial effects on pain and self-efficacy than standard care combined with the control intervention. These exploratory data suggest there was no relationship between cooling and self-efficacy over standard of care.
Painful lower leg skin affected by CVeD has been reported in several studies (Radak et al., 2015) however, few nonpharmacological intervention studies have been conducted that specifically address CVeD localized pain. The rationale for using a cooling approach added to standard of care is that cooling the skin offers a quick analgesia that takes only minutes to exert a numbing effect (Kim et al., 2015). In this cryotherapy study the treatment cuff reduced skin temperature by 2 – 5°C, delivering a cooling sensation that affects afferent sensory perception in various skin neuronal pathways. The cooling effects have been found to relieve other CVeD related symptoms such as itch (Paul et al., 2011). One trial investigated the effect of a mixed Kinesio taping treatment in women with mild to moderate chronic venous insufficiency and pain after a 4-week period (Aguilar-Ferrandiz et al., 2014). However bodily pain, not extremity pain associated with CVeD, was measured with the McGill Pain Questionnaire, a multidimensional perception of pain that includes a self-report rating scale. The pain scores were statistically significantly reduced (p =0.001) after treatment.
In the present study, the short and long-term pain scores indicated that individuals experienced moderate pain (scores between 3 – 7) on the NRS and VEINES-QOL/Sym at baseline; pain decreased in both groups. The reductions might be explained by moderately high adherence to standard of care including the wearing the compression wraps and elevating the legs, the mainstay of treatment for CVeD. Participants in the study were contacted monthly by study staff, attended regularly scheduled clinic study visits, received study compensation, and were given the compression wraps and leg elevator pillow, at no cost. In the authors’ previously published work on adherence at the outset of the study, 100 individuals with CVeD receiving the cooling intervention (n = 54) or control (n = 46) were found to have moderately high adherence to the protocol (Kelechi et al., 2015). Adherence was defined as 85% or above in documentation of performance of the treatment and other evaluation metrics such as entry omissions on a self- reported log. Under intention-to-treat analysis, 38.4% were < 85% accurate, 25.0% were 85 −99% accurate and 36.6% were 100% accurate. There were no differences between groups on accuracy.
These factors could have contributed to higher than normal estimates of adherence, which is reported in the literature to be approximately 25% for compression stockings (Ziaga, et al., 2011) and as high as 75% for leg elevation (Miller et al., 2014). Leg elevation has been shown to reduce venous insufficiency related edema, improve the microcirculation of the skin (Abu-Own,et al. 1994), and is recommended in numerous clinical guidelines (Wittens et al., 2015; Gloviczki et al., 2011). Studies of low adherence to standard of care suggest that less than 50% of patients are ever prescribed compression, or are told to elevate their legs, and patients often discontinue wearing the compression garments due to high cost, sweating, itching, difficulty with application, poor cosmesis, lack of instruction on how to use compression and elevate the legs, pain, unaware of expected benefits, and difficulty putting on footwear (Sansal, et al., 2013; Weller at al., 2016).
Self-efficacy Outcomes
Self-efficacy, or the confidence to carry out certain behaviors in order to achieve a specific goal (Bandura, 1997) is well recognized as a prerequisite to effective chronic disease self-management. In this cryotherapy study, slight increases in self-efficacy in both groups were found, however, both groups in general had fairly high self-efficacy scores at baseline. There were no statistically significant improvements between groups, however, there were significant changes in certain subsets of individuals. Having diabetes or taking medications for hypertension were associated with improved scores. Perhaps individuals with certain chronic conditions may have become accustomed to personally managing their own disease and have the motivation to follow a behavior to achieve a goal (Sharifirad et al., 2013), which for this study, could have been better adherence to standard of care. While these findings were exploratory, future studies should consider these patient characteristics as co-variables.
5.1. Limitations
There are limitations in this study. The frequency of measuring pain was limited to only 4 time points, which did not provide an adequate representation of the wide variability of pain experienced over the course of the study. Other symptoms associated with CVeD could have exacerbated pain, such as having achy and heavy legs, or burning sensations. The analyses attempted to isolate pain from other symptoms, but this might not have been possible. Another limitation is that a preponderance of the self-efficacy scale questions was related to CVeD disease management as only two questions were specific to pain management. A number of patients were taking pain medications and had multiple comorbid conditions that made disease specific pain assessment problematic. A significant limitation was that we did not ask whether taking pain medications was specific to pain associated with CVeD or other conditions such as arthritis. The fact that data were self-reported is a limitation although the findings from the adherence aim of the study demonstrated that adherence was fairly high as evidenced by accuracy in documentation on study logs (Kelechi et al., 2015).
6. Conclusion
In general, in this exploratory aim of this cryotherapy study, there was no added benefit in pain reduction when cooling was added to standard of care including leg elevation and compression wraps in individuals with CVeD who were moderately adherent to the treatment protocol. Practitioners should inquire about the presence of leg pain and if present, have patients rate their pain on a standardised tool during clinic visits as pain remains a common problem associated with venous disorders especially in older individuals. The relatively high self-efficacy scores indicate patients were mostly confident in their ability to manage the disease, but whether patients feel confident in managing pain needs to be more clearly elucidated in future studies. For clinical care, we recommend every other day cooling with a non-solid gel pack encased in some type of thin cloth, and placed on the localized area of affected skin with legs elevated on at least 2 pillows for 30 minutes. The cooling cuff was easy to apply, comfortable to use, and a similar product is available over the counter in the form of cooling gel packs and pads. Compression stockings or wraps are to be worn when individuals are ambulatory and removed during the cooling treatment. Further research on cooling should be undertaken to determine effectiveness on pain and other bothersome CVeD symptoms. Cooling may offer an alternative or addition to pain management armamentarium including medications. Additional study of certain patient characteristics such as older age, married, in employment, and individuals with co-morbid conditions such hypertension, diabetes and arthritis are required to enhance the understanding of the associations between other chronic conditions, self-efficacy and pain.
Supplementary Material
What is already known about the topic?
Individuals with chronic venous disease (CVeD) experience multiple negative symptoms, such as leg pain.
Leg pain is infrequently assessed during clinical encounters and is poorly managed.
Few non-pharmacological self-management therapies have been evaluated to determine effectiveness on leg pain.
Topical and localized cooling of affected skin areas may provide an analgesic response that reduces pain and improves self-efficacy in the overall management of CVeD.
What this paper adds?
Adding a cooling treatment to usual care that included compression wraps and leg elevation reduced pain in individuals with CVeD, but strict adherence to usual care also reduced pain.
Cooling compared with usual care was more beneficial in subgroups of individuals such as those with concomitant conditions including diabetes.
Self-efficacy does not seem to be highly influenced by the self-cooling therapy but small improvements were noted.
Acknowledgments
Funding
This work was funded by the National Institute of Nursing Research (NINR) part of the National Institutes of Health (NIH) award #R01NR012237 and supported by the South Carolina Clinical & Translation Research (SCTR) Institute through NIH Grant Number UL1TR001450. The ideas and opinions expressed herein are those of the authors and not necessarily reflective of the NINR.
Abbreviation
- CONSORT
Consolidated Standards of Reporting Trials
Footnotes
Conflicts of interest
There are no conflicts of interest to declare in this work.
Trial Registration: Clinical Trials NCT01509599.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Teresa J. Kelechi, Medical University of South Carolina College of Nursing 99 Jonathan Lucas Street MSC 160 Charleston SC USA 29425.
Martina Mueller, Medical University of South Carolina College of Nursing 99 Jonathan Lucas Street MSC 160 Charleston SC USA 29425.
Mohan Madisetti, Medical University of South Carolina College of Nursing 99 Jonathan Lucas Street MSC 160 Charleston SC USA 29425.
Margie A. Prentice, Medical University of South Carolina College of Nursing 99 Jonathan Lucas Street MSC 160 Charleston SC USA 29425.
Mary J. Dooley, Medical University of South Carolina College of Nursing 99 Jonathan Lucas Street MSC 160 Charleston SC USA 29425.
References
- Abu-Won A, Scurr JH, Coleridge Smith PD, 1994. Effect of leg elevation on the skin microcirculation in chronic venous insufficiency. J Vasc Surg 20 (5), 705–710. [DOI] [PubMed] [Google Scholar]
- Aguilar-Ferrándiz ME, Moreno-Lorenzo C, Matarán-Peñarrocha GA, García-Muro F, García-Ríos MC, Cástro-Sanchez AM, 2014. Effect of a mixed Kinesio taping- compression technique on quality of life and clinical and gait parameters in postmenopausal women with chronic venous insufficiency: Double-blinded, randomized controlled trial. Arch Phys Med Rehabil 95 (7), 1229–1239. [DOI] [PubMed] [Google Scholar]
- Bandura A, 1997. Self-efficacy: The exercise of control. W H Freeman/Times Books/ Henry Holt & Co, New York, NY, US. [Google Scholar]
- Bandura A, 1986. Social foundations of thought and action : a social cognitive theory. Prentice- Hall, Englewood Cliffs, N.J. [Google Scholar]
- Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J, 2016. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns 48 (2), 177–187. [DOI] [PubMed] [Google Scholar]
- Boutron I, Altman DG, Moher D, Schulz KF, Ravaud P, Group CN, 2017. CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann Intern Med 167 (1), 40–47. [DOI] [PubMed] [Google Scholar]
- Danziger N, 2011. Pain in chronic venous disease: perspectives for research. Medicographia 33, 325–311. [Google Scholar]
- Dubin AE, Patapoutian A, 2010. Nociceptors: the sensors of the pain pathway. J Clin Invest 120 (11), 3760–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberhardt RT, Raffetto JD, 2014. Chronic Venous Insufficiency. Circulation 130 (4), 333–346. [DOI] [PubMed] [Google Scholar]
- Finlayson K, Miaskowski C, Alexander K, Liu W-H, Aouizerat B, Parker C, Maresco-Pennisi D, Edwards H, 2017. Distinct wound healing and quality-of-life outcomes in subgroups of patients with venous leg ulcers with different symptom cluster experiences. J Pain Symptom Manage 53 (5), 871–879. [DOI] [PubMed] [Google Scholar]
- Freire B, Beremia J, Baroni B, Vaz M, 2016. Effects of cryotherapy methods on circulatory, metabolic, inflammatory and neural properties: a systematic review. Physical Therapy in Movement. Retrieved http://www.scielo.br/scielo.php?script=sci_serial&pid=0103-5150&lng=en&nrm=iso. [Google Scholar]
- Freund T, Gensichen J, Goetz K, Szecsenyi J, Mahler C, 2011. Evaluating self-efficacy for managing chronic disease: psychometric properties of the six-item Self-Efficacy Scale in Germany. J Eval Clin Pract 19 (1), 39–43. [DOI] [PubMed] [Google Scholar]
- Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, Lohr JM, McLafferty RB, Meissner MH, Murad MH, Padberg FT, Pappas PJ, Passman MA, Raffetto JD, Vasquez MA, Wakefield TW, 2011. The care of patients with varicose veins and associated chronic venous diseases: Clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg 53 (5), 2S–48S. [DOI] [PubMed] [Google Scholar]
- Green J, Jester R, 2009. Health-related quality of life and chronic venous leg ulceration: part 1. Br J Community Nurs 14 (12), S12, S14,, S16–17. [DOI] [PubMed] [Google Scholar]
- Greenberger C, Freier DY, Lev I, Hazan HR, 2014. The inter-relationships between self-efficacy, self-management, depression and glycaemic control in Israeli people with type 2 diabetes. J Diabetes Nurs 18, 333–339. [Google Scholar]
- Guillot X, Tordi N, Mourot L, Demougeot c., Dugue B, Prati C, Wendling D 2014. Cryotherapy in inflammatory rheumatic diseases: a systemic review. Expert Rev Clin Immunol 10 (2), 281–294. [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG, 2009. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42 (2), 377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker GA, Mian S, Kendzerska T, French M, 2011. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale. Arthritis Care Res 63 (S11), S240–S252. [DOI] [PubMed] [Google Scholar]
- Kapp S, Miller C, 2015. The experience of self-management following venous leg ulcer healing. J Clin Nurs 24 (9–10), 1300–1309. [DOI] [PubMed] [Google Scholar]
- Kelechi TJ, Mueller M, Zapka JG, King DE, 2011. The effect of a cryotherapy gel wrap on the microcirculation of skin affected by chronic venous disorders. J Adv Nurs 67 (11), 2337–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelechi T, Mueller M, Dooley M, 2017a. Sex differences in symptom severity and clusters in patients with stage C4 and stage C5 chronic venous disease. Eur J Cardiovasc Nurs 16 (1), 28–36. [DOI] [PubMed] [Google Scholar]
- Kelechi T, Madisetti M, Mueller M, Dooley M, Prentice M, 2015. Self-monitoring of lower leg skin temperature: accuracy of self-reported data and adherence to a cooling protocol for the prevention of venous leg ulcers. Patient Prefer Adherence Volume 9, 1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelechi TJ, Mueller M, Madisetti M, Prentice MA, Dooley MJ, 2017b. Does cryotherapy improve skin circulation compared with compression and elevation in preventing venous leg ulcers? Int Wound J 14 (4), 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelechi T, Mueller M, Madisetti M, Prentice M, Dooley M, 2017c. Exploring the influence of a cooling treatment on quality of life in patients with chronic venous disease. Chronic Wound Care Management and Research 4, 65–76. [Google Scholar]
- Kim E-J, Choi Y-D, Lim C-Y, Kim K-H, Lee S-D, 2015. Effect of heating and cooling combination therapy on patients with chronic low back pain: study protocol for a randomized controlled trial. Trials 16, 285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamping DL, Schroter S, Kurz X, Kahn SR, Abenhaim L, 2003. Evaluation of outcomes in chronic venous disorders of the leg: Development of a scientifically rigorous, patient- reported measure of symptoms and quality of life. J Vasc Surg 37 (2), 410–419. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Holman H, 2003. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med 26 (1), 1–7. [DOI] [PubMed] [Google Scholar]
- Lorig KR, Sobel DS, Ritter PL, Laurent D, Hobbs M, 2001. Effect of a self-management program on patients with chronic disease. Eff Clin Pract 4 (6), 256–262. [PubMed] [Google Scholar]
- Monsen KA, Kelechi TJ, McRae ME, Mathiason MA, 2018. Nursing theory, terminology, and big data: Data-driven discovery of novel patterns in archival randomized clinical trial data. Nurs Res 67 (1), 122–132. [DOI] [PubMed] [Google Scholar]
- Miller C, Kapp S, Donohue L, 2014. Sustaining behavior changes following a venous leg ulcer client education program. Healthcare 2(3), 324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadler SF, Weingand K, Kruse RJ, 2004. The physiologic basis and clinical applications of cryotherapy and thermotherapy for the pain practitioner. Pain Physician 7 (3), 395–399 [PubMed] [Google Scholar]
- Nicolaides A, Kakkos S, Eklof B, Perrin M, Nelzen O, Neglen P, Partsch H, Rybak Z, 2014. Management of chronic venous disorders of the lower limbs - guidelines according to scientific evidence. Int Angiol 33 (2), 87–208. [PubMed] [Google Scholar]
- Park B, Kim SJ, 2013. Cooling the Skin: Understanding a specific cutaneous thermosensation. J Lifestyle Med 3 (2), 91–97. [PMC free article] [PubMed] [Google Scholar]
- Paul JC, Pieper B, Templin TN, 2011. Itch: association with chronic venous disease, pain, and quality of life. J Wound Ostomy Continence Nurs 38 (1), 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin M, Eklof B, A VANR, Labropoulos N, Vasquez M, Nicolaides A, Blattler W, Bouhassira D, Bouskela E, Carpentier P, Darvall K, M DEM, Flour M, Guex JJ, Hamel-Desnos C, Kakkos S, Launois R, Lugli M, Maleti O, Mansilha A, P NE, Rabe E, Shaydakov E, 2016. Venous symptoms: the SYM Vein Consensus statement developed under the auspices of the European Venous Forum. Int Angiol 35 (4), 374–398. [PubMed] [Google Scholar]
- Radak DJ, Tanaskovic SZ, Vlajinac HD, Marinkovic JM, Maksimovic MZ, 2015. Relationship between pain and CEAP C categories of chronic venous disease. Angiology 67 (7), 670–675. [DOI] [PubMed] [Google Scholar]
- Raffetto JD, Mannello F, 2014. Pathophysiology of chronic venous disease. Int Angiol 33 (3), 212–221. [PubMed] [Google Scholar]
- Ruggiero M, Grande R, Naso A, Butrico L, Rubino P, Placida GD, Cannistra M, Serra R, 2016. Symptoms in patients with skin changes due to chronic venous insufficiency often lead to emergency care service: an Italian observational study. Int Wound J 13 (5), 967–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman-Green D, Jaser S, Martin F, Alonzo A, Grey M, McCorkle R, Redeker NS, Reynolds N, Whittemore R, 2012. Processes of self-management in chronic illness. J Nurs Scholarsh 44 (2), 136–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifirad G, Azadbakht L, Feizi A, Kargar M, Mohebi S, 2013. Review the key role of self-efficacy in diabetes care. J Edu Health Promot 2 (1), 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenoy M 2014. Prevention of venous leg ulcer recurrence. Indian Dermatol Online J 5 (3), 386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stansal A, Lazareth I, Pasturel UM, Ghaffari P, Boursier V, Bonhomme S, Priollet P 2013. Compression therapy in 100 consecutive patients with venous leg ulcers. Journal des Maladies Vasculaires 38 (4), 252–258. [DOI] [PubMed] [Google Scholar]
- Terman GW, Bonica JJ, 2003. Spinal mechanisms and their modulation In: Loeser JD, Butler SH, Chapman CR, Turk DC, editors. Bonica’s management of pain. 3rd ed. Philadelphia: Lippincott Williams & Wilkins, p. 73–152. [Google Scholar]
- Vuylsteke ME, Colman R, Thomis S, Guillaume G, Van Quickenborne D, Staelens I 2018. An epidemiological survey of venous disease among general practitioner attendees in different geographicl regions on the the globe: The final results of the Vein Consult Program. Angiology doi: 10.1177/0003319718759834. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Weller CD, Buchbinder R, Johnston RV. 2016. Interventions for helping people adhere to compression treatments for venous leg ulceration. Cochrane Database Syst Rev 2 (3), doi 10.1002/14651858. CD00837.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittens C, Davies AH, Baekgaard N, Broholm R, Cavezzi A, Chastanet S, de Wolf M, Eggen C, Giannoukas A, Gohel M, Kakkos S, Lawson J, Noppeney T, Onida S, Pittaluga P, Thomis S, Toonder I, Vuylsteke M, Kolh P, de Borst GJ, Chakfe N, Debus S, Hinchliffe R, Koncar I, Lindholt J, de Ceniga MV, Vermassen F, Verzini F, De Maeseneer MG, Blomgren L, Hartung O, Kalodiki E, Korten E, Lugli M, Naylor R, Nicolini P, Rosales A, European Society for Vascular, S., 2015. Editor’s Choice - Management of Chronic Venous Disease: Clinical Practice Guidelines of the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg 49 (6), 678–737. [DOI] [PubMed] [Google Scholar]
- Wrona M, Jöckel KH, Pannier F, Bock E, Hoffmann B, Rabe E, 2015. Association of venous disorders with leg symptoms: Results from the Bonn Vein Study 1. Eur J Vasc Endovasc Surg 50 (3), 360–367. [DOI] [PubMed] [Google Scholar]
- Ziaja D, Kocełak P, Chudek J, & Ziaja K (2011). Compliance with compression stockings in patients with chronic venous disorders. Phlebology 26 (8), 353–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


